Summary
Obesity is prevalent among pregnant women in the United States; 15–20% of obese pregnant women have obstructive sleep apnea. The prevalence of obstructive sleep apnea increases along with body mass index, age and in the presence of other co-morbidities. Untreated obstructive sleep apnea in women is associated with a range of cardiovascular, pulmonary and metabolic co-morbidities; recent studies suggest that women with obstructive sleep apnea in pregnancy may be at significantly greater risk of entering pregnancy with chronic hypertension and/or of developing hypertensive disorders of pregnancy: gestational hypertension; preeclampsia; or eclampsia. This has serious public health implications; hypertensive disorders of pregnancy are a major cause of maternal and neonatal morbidity and mortality and are associated with a greater lifetime risk for cardiovascular disease. The mechanisms that associated obstructive sleep apnea with hypertensive disorders of pregnancy have not been defined, but several pathways are scientifically plausible. In this review, we will present a comprehensive literature review of the following: the associations between obstructive sleep apnea and hypertensive disorders of pregnancy; the proposed mechanisms that may connect obstructive sleep apnea and hypertensive disorders of pregnancy; and the effectiveness of treatment at mitigating these adverse outcomes.
Keywords: Obstructive sleep apnea, Sleep-disordered breathing, Preeclampsia, Pregnancy, Chronic hypertension, Eclampsia, Cardiovascular disease
Introduction
In the United States, obesity is highly prevalent among pregnant women.[1] Among obese pregnant women, 15–20% have obstructive sleep apnea (OSA); women with OSA have a significantly increased risk of entering pregnancy with chronic hypertension and/or developing hypertensive disorders of pregnancy (HDP), a spectrum of diseases that includes chronic hypertension, gestational hypertension, preeclampsia, and eclampsia.[2–7] Obesity is also more prevalent in African-American, Hispanic and Native American populations; this may explain the increased risk of OSA associated with black race seen in some studies of OSA in pregnant women (Table 2).[2, 6–8]
Table 2.
Selected studies of pregnancy-induced hypertension risk in women with obstructive sleep apnea that included objective testing to determine OSA status
| Adjusted Odds Ratio (95% Confidence Interval) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Authors, reference number |
Study type | Number of mothers studied |
Race/ethnicity & OSA status |
Inclusion criteria | OSA status of mother |
Gestational age at time of sleep testing (weeks) |
Gestational hypertension |
Preeclampsia | Hypertensive disorders of pregnancy* |
Eclampsia |
| Champagne K et al 2009 [29] | Case-control | 50 | Not reported | Gestational HTN; normotensive controls | PSG ; AHI ≥ 15 | Varied | 7.5 (3.5, 16.2) | -- | -- | -- |
| Louis J et al 2010 [33] | Retrospective cohort | 171 | No significant differences | OSA in pregnancy ; BMI-matched controls | PSG ; No PSG testing of controls | Varied | -- | 2.0 (0.8, 5.0) | -- | -- |
| Reid J et al 2011 [35] | Cross-sectional | 60 | Not reported | Gestational HTN; normotensive controls | PSG | ≥ 32 weeks | 8.31 (2.07, 33.43) | -- | -- | -- |
| Chen YH et al 2012 [30] | Retrospective, cross-sectional | 791 | Taiwanese cohort, race/ethnicity not reported | -- | ICD code assigned after PSG | Varied | 3.2 (2.1, 4.7) | 1.6 (2.2, 11.3)∫ | -- | -- |
| Facco F et al 2012 [31] | Retrospective cohort | 143 | No significant differences | PSG in hospital records | AHI ≥ 5 on PSG | Varied | -- | -- | No association | -- |
| Louis J et al 2012 [3] | Prospective cohort | 182 | No significant differences | BMI ≥ 30 kg/m2 | AHI ≥ 5 on level 3 home sleep test | All gestational ages | -- | 3.5 (1.3, 9.9) | -- | -- |
| Facco F et al 2014 [32] | Prospective cohort | 188 | No significant differences | high of risk preeclampsia¶ | AHI ≥ 5 on level 3 home sleep test | 6–20 | -- | No association | -- | -- |
| 22–31 | -- | 2.0 (1.2, 3.2) | 1.7 (1.2, 2.5) | -- | ||||||
| Louis J et al 2014 [2] | Retrospective, cross-sectional | 55,781,965 inpatient discharges | Non-hispanic, black women more likely to have OSA | -- | ICD-9-CM codes | -- | 1.3 (1.1, 1.5) | 2.5 (2.2, 2.9) | -- | 5.4 (3.3, 8.9) |
| O’Bren L et al 2014 [4] | Cross-sectional | 67 | Not reported | Hypertensive disorders§; normotensive controls | Portable PSG; AHI ≥ 5 | Varied | -- | -- | 2.0 (1.4, 2.8)§ | |
| Pamidi S et al 2014 [5] | Meta-analysis | 6 studies included | Not analyzed | -- | PSG | varied | -- | -- | 2.25 (1.13, 4.52) | -- |
| 12 studies included | -- | symptoms | varied | -- | -- | 3.11 (2.28, 4.25)† | -- | |||
| Pien G et al 2014 [34] | Prospective cohort | 105 | No significant differences | Stratified by BMI | In-lab PSG | 8–14 | -- | 0.44 (0.05, 3.74)f | -- | |
| repeated, 33–34 | -- | 0.50 (0.13, 1.90)† | -- | |||||||
| Xu T et al 2014 [36] | Meta-analysis | 5 studies included | Not analyzed | -- | In-lab PSG or home sleep test or symptom-based | Varied | -- | 1.96 (1.34, 2.86) | -- | -- |
| 3 studies included | Excluded symptom-based | Varied | -- | 2.31 (1.83, 3.23) | -- | -- | ||||
| Bourjeily G et al 2017 [7] | Retrospective, cross-sectional | 1,577,632 | Black mothers more likely to have OSA | OSA in pregnancy | ICD-9-CM codes | Varied | 1.7 (1.4, 2.0) | 2.22 (1.9, 2.5) | -- | 2.95 (1.1, 8.0) |
| Facco F et al 2017 [6] | Prospective cohort | 3705 | Non-hispanic, black women more likely to have OSA | Nulliparity, singleton | AHI ≥ 5 on level 3 home sleep test | 6–15 | -- | 1.94 (1.07, 3.5) | 1.5 (0.9, 2.3)* | -- |
| 28–37 | -- | No association | -- | -- | ||||||
Hypertensive disorders of pregnancy composite outcome included: mild, severe and superimposed preeclampsia and eclampsia, plus antepartum gestational hypertension.
Hypertensive disorders composite inclusion criteria: chronic hypertension, gestational hypertension or preeclampsia.
Composite outcome of preeclampsia and eclampsia.
High risk for preeclampsia defined as having either BMI ≥ 30 kg/m2, chronic hypertension, pregestational diabetes mellitus (type 1 or 2), prior history of preeclampsia, and/or twin gestation.
Composite outcome included gestational hypertension or preeclampsia.
AHI (Apnea-hypopnea index); BMI (body mass index); Hypertension (HTN); ICD-9-CM (International Classification of Diseases, Ninth Revision, Clinical Modification); OSA (obstructive sleep apnea); PSG (polysomnography)
HDP are classified by the American College of Obstetricians and Gynecologists’ (ACOG) Task Force on Hypertension in Pregnancy into four categories: 1) preeclampsia-eclampsia; 2) chronic hypertension (of any cause); 3) chronic hypertension with superimposed preeclampsia; and 4) gestational hypertension (Table 1).[9] HDP affect approximately 4–10% of pregnancies in high resource countries, and are more common in low resource countries.[10] They are a major cause of maternal morbidity and mortality worldwide from causes including, but not limited to postpartum hemorrhage, hemorrhagic stroke, cardiomyopathy, acute renal insufficiency, myocardial infarction, and pulmonary edema. [11, 12] Furthermore, several studies suggest that women with preeclampsia have a higher risk of developing cardiovascular disease later in life.[13–17]
Table 1.
Definitions of the four classes of hypertensive disorders of pregnancy
| Chronic hypertension | SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg or use of anti-hypertensive medications prior to pregnancy or before 20 weeks gestation |
| Gestational hypertension | SBP ≥ 140 mm Hg and/or DBP ≥ 90 mmHg in a woman not previously diagnosed with chronic hypertension after 20 weeks gestation |
| Preeclampsia-eclampsia | SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg on two occasions at least 4 hours apart if BP was previously normal, as well as proteinuria or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms. |
| Chronic hypertension with superimposed preeclampsia | proteinuria or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, and/or cerebral or visual symptoms. |
Systolic blood pressure (SBP); Diastolic blood pressure (DBP)
According to the American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy [9]
The underlying pathophysiologic mechanisms of HDP remain unclear, and treatments are limited. Because the most effective treatment for preeclampsia and eclampsia is delivery of the fetus and the placenta, these disorders are also a leading cause of preterm birth, which results in significant morbidity and mortality for the fetus.[18] The mechanisms that link OSA to HDP and other adverse outcomes in pregnant women have not been defined, but several pathways are plausible given data from non-pregnant populations and animal studies, and preliminary studies in pregnant women with OSA. The effect of OSA treatment on the risk of HDP remains unknown, and these studies have been limited by cost, feasibility and the narrow time window in pregnancy for diagnosis and treatment of OSA. Screening tools for OSA in pregnancy have not been reliable, and data are lacking regarding the optimal timing of screening.
Review question and purpose
In this review, we will present a comprehensive literature review of the following: 1) the association between OSA and HDP; 2) the proposed mechanisms that may connect OSA and HDP; and 3) the effectiveness of non-invasive positive airway pressure therapies at mitigating these adverse outcomes.
Methods
A systematic search was conducted for articles published between January 1, 1980 and May 1, 2017 using PubMed, and by searching bibliographies of relevant publications. Search terms in PubMed included the following MeSH terms for SDB exposure: sleep apnea, obstructive; sleep apnea syndromes; snoring. Search terms in PubMed included the following MeSH terms for HDP: hypertension, pregnancy-induced; hypertension; pregnancy; and preeclampsia. We focused on studies that included objective testing to determine OSA status (either in-lab polysomnography, home sleep testing or ICD codes) rather than symptom-based questionnaires, which have been shown by several studies to be unreliable in pregnant women.[19–21]
The associations between obstructive sleep apnea and hypertensive disorders of pregnancy
Chronic Hypertension
Chronic hypertension is part of the spectrum of HDP. It is defined by the ACOG as blood pressure ≥ 140 mm Hg systolic and/or 90 mm Hg diastolic or use of anti-hypertensive medications prior to pregnancy or before 20 weeks gestation.[9]
In both pregnant and non-pregnant women, chronic hypertension seems to be an important co-morbidity for OSA.[22, 23] The recent results of the prospective study of over 3000 women by Facco et al. (nuMoM2b Sleep Disordered Breathing Substudy) confirmed that chronic hypertension is significantly more common among pregnant women with OSA, and that this prevalence increases with OSA severity [2% vs. 8%, OSA negative vs. OSA positive; p < 0.001].[6] In this study, women underwent level 3 home sleep testing. According to the American Academy of Sleep Medicine, level 3 home sleep test devices do not record sleep stages or sleep disruption; they typically record airflow, respiratory movement, pulse oximetry and heart rate.[24] Home sleep testing was conducted twice during pregnancy: 6 –15 and 22–31 weeks gestation. The strength of this study was the number of women studied using objective testing at two time points in pregnancy. However, the cohort was relatively low risk, and only 114/3132 valid studies had an apnea hypopnea index (AHI) ≥ 5 events/hour.
In a smaller prospective cohort study of high-risk parturients by O’Brien et al., 51 pregnant women with a diagnosis of HDP underwent objective sleep testing and were compared with 16 normotensive pregnant controls of all gestational ages.[4] They found that the incidence of OSA in pregnant women with chronic hypertension was 43% compared to 19% of normotensive controls. In this cohort, a patient report of snoring along with HDP was strongly associated with more severe OSA with clinically significant oxygen desaturation. In particular, this study showed that chronic snoring was associated with chronic hypertension, while pregnancy-onset snoring was associated with gestational hypertension.
Louis et al. also tested another high-risk cohort of obese pregnant women [body mass index (BMI) > 30 kg․m−2] with home sleep studies and found that chronic hypertension was significantly more common among women with AHI ≥ 5 events/hour (58% vs. 33%, p = 0.02).[3] A prospective cohort study of 188 high-risk parturients by Facco et al. with a home sleep test conducted early in pregnancy study (6–20 weeks gestation) also found a significant difference in the incidence of chronic hypertension among the AHI ≥ 5/hour group compared to AHI < 5/hour (47% vs. 24%, p = 0.004).[25] A prospective study of 248 parturients in the third trimester that underwent home sleep testing to determine AHI status reported a combined incidence of chronic and gestational hypertension of 57% among women with AHI ≥ 5/hour compared to 23% among women with AHI < 5/hour (p < 0.001).[20]
Chronic hypertension in pregnancy is a known risk factor for preeclampsia; 17–29% of women with chronic hypertension will go on to develop superimposed preeclampsia.[26, 27] As in non-pregnant women, chronic hypertension seems to be an important risk factor for OSA in pregnancy.
Gestational hypertension and preeclampsia
According to the most recent ACOG task force, gestational hypertension is characterized by new blood pressure elevations after 20 weeks gestation without proteinuria in a woman that was not previously diagnosed with chronic hypertension. However, preeclampsia is diagnosed when a woman presents after 20 weeks gestation with blood pressure ≥ 140 mm Hg systolic and/or 90 mm Hg diastolic on two occasions at least 4 hours apart if her blood pressure was previously normal, as well as proteinuria. In the absence of proteinuria, preeclampsia is diagnosed when new-onset hypertension is accompanied by new onset of the following: thrombocytopenia; renal insufficiency; impaired liver function; pulmonary edema; and/or cerebral or visual symptoms.[9]
ACOG also differentiates between early- and late-onset preeclampsia; early preeclampsia is diagnosed prior to 34 weeks gestation, while late-onset is diagnosed after 34 weeks and up to 6 weeks into the postpartum period.[9] Early-onset preeclampsia has been associated with more severe disease, and worse maternal and fetal outcomes; some theories of the pathophysiology of preeclampsia suggest that the two phenotypes differ in their underlying mechanisms.[28]
Known risk factors for preeclampsia include: primiparity; chronic hypertension; chronic renal disease; thrombophilia; multiple gestation; family history; in vitro fertilization; diabetes mellitus; obesity; maternal age greater than 40 years; and systemic lupus erythematous. OSA shares multiple co-morbidities with HDP: chronic hypertension; diabetes mellitus; obesity and advanced maternal age.[9] However, only recently has OSA been considered a possible risk factor for HDP.
Several recent studies and two 2014 meta-analyses have suggested that women with OSA during pregnancy are at increased risk of developing preeclampsia, or that women with HDP are at increased risk for having SDB when identified and studied by objective testing.[2–7, 29–36] These studies are summarized in Table 2. Several important studies were published after the two 2014 meta-analyses that are presented in Table 2. [2, 4, 6, 7, 25, 34]
This field of study has been limited by a lack of large, prospective studies with objective sleep testing to define OSA. However, the first results of the Nulliparous Pregnancy Outcomes Sleep Disordered Breathing (nuMom2b-SDB) substudy were recently reported in 2017.[6] The nuMom2b-SDB was a substudy of the larger, prospective cohort nuMom2b study conducted across 8 clinical sites in the United States between 2011–2013.[37] The study of 3702 nulliparous women who underwent unattended, level 3 home sleep testing at two time points in pregnancy showed an increased risk of preeclampsia for women with both mild and severe OSA. In mid-pregnancy (22–31 weeks gestation), women with mild-moderate OSA (AHI 5–14.9/hour had an increased risk of developing preeclampsia during their pregnancy after adjusting for age, body mass index, pregnancy weight gain and chronic hypertension [adjusted odds ratio (aOR) = 1.98 (95% CI, 1.12–3.48)]. This risk was greater for women with severe OSA (AHI > 15/hour) [aOR = 4.27 (95% CI, 1.74 – 10.45)].[6] The number of women who developed preeclampsia in this cohort was within the range reported by population studies of the incidence of preeclampsia (approximately 3–6%).[38] While this was the largest, prospective cohort studied in this population to date, of the 114 women who had AHI ≥ 5/hour on home sleep testing early in pregnancy, only 16 women developed preeclampsia. One other prospective study also showed an association between OSA and preeclampsia, but two other prospective studies did not reproduce these findings. [3, 25, 34] However, none of these studies was powered to detect differences in adverse pregnancy outcomes, and the numbers of women with preeclampsia was small. Three of the studies included in Table 2 that showed an association between HDP and OSA specifically enrolled women with HDP and compared them to controls and found that the incidence of OSA was higher in the HDP groups.[4, 29, 35]
Three retrospective population-based dataset studies have been conducted to date in Taiwan and in the United States.[2, 7, 30] All found that pregnant women with OSA are at increased risk for preeclampsia. These differences remained significant after controlling for obesity. This is important because preeclampsia and OSA share many of the same risk factors: obesity; diabetes mellitus; chronic hypertension; and advanced maternal age.
Both meta-analyses published on this topic also showed an increased risk for gestational hypertension or preeclampsia in women with SDB.[5, 36] However, they both included studies that assessed OSA by symptom-based measures, as well as PSG. Studies based on symptom-based measures did not conduct objective testing for SDB, but rather classified subjects as having SDB or not by self-report of symptoms of SDB such as snoring, fatigue, and witnessed apneas or by using questionnaires validated in non-pregnant populations. These methods have not been shown to be reliable in pregnant subjects.[19–21, 39] However, when analyses were restricted to studies that used objective testing to classify OSA status, the associations continued to remain significant (Table 2).
Cardiovascular disease
Although preeclampsia is treated with the delivery of the placenta, mounting evidence suggests that the spectrum of HDP can have long-term health consequences for the mother, particularly from cardiovascular disease.[13–17] However, it is unknown whether HDP cause cardiovascular disease, or whether it is an early indicator of women predisposed to cardiovascular disease.[40]
Some have suggested that the interaction between OSA and preeclampsia may impart additional long-term cardiovascular risk.[41, 42] Certainly, OSA is associated with long-term cardiovascular disease in non-pregnant adults.[22, 43] Evidence from the large, national inpatient database study by Louis et al. showed that pregnant women with OSA were at significantly increased risk of having co-morbid cardiomyopathy [aOR = 9.0 (95% CI, 7.47–10.87)], congestive heart failure [aOR = 8.94, (95% CI, 7.45–10.73)], and pulmonary edema [aOR = 7.5, (95% CI, 4.63 – 12.15)] during their pregnancy or delivery admission. This study also showed that pregnant women with OSA were 5 times more likely to die in the hospital during a pregnancy or delivery admission than women without OSA.[2] These effects were unchanged after controlling for obesity, but there was no distinction made for OSA that pre-existed pregnancy. Data from the large, prospective nuMom2b Heart Health study will hopefully shed light on the impact of OSA in pregnancy on a woman’s future cardiovascular health.[44]
The pathogenesis of OSA and hypertensive disorders of pregnancy
As discussed previously, there are likely multiple phenotypes of SDB in pregnancy (snoring, chronic OSA and gestational-onset OSA) and the interaction of these phenotypes with HDP may be quite distinct. Certain hormonal, physiologic and pathophysiologic changes of pregnancy may increase a woman’s risk of worsening pre-existing OSA or developing SDB. The upper airway undergoes several dynamic changes as pregnancy progresses: mucosal hyperemia; oropharyngeal diameter narrowing; and increased Mallampati score.[45–47] Snoring is quite common in pregnancy, and there is evidence to suggest that AHI increases as pregnancy progresses.[6, 34] Increased upper airway resistance along with decreased functional residual capacity and increased oxygen consumption can exacerbate nocturnal hypoxemia.[46] Some of these airway changes are more pronounced in women who develop preeclampsia; the association between OSA and preeclampsia may be bidirectional.[48, 49]
However, other pregnancy-related factors may be protective, such as preference for the lateral sleep position and increased respiratory rate due to hormonal changes.[50, 51] The health consequences of the timing of the onset of OSA, either prior to or during pregnancy, are not well elucidated. O’Brien and colleagues have shown that pregnant women with chronic hypertension were more likely to report snoring prior to pregnancy, while pregnancy-induced hypertension is more common among women who report new onset snoring in pregnancy.[48]
Despite the significant impact of HDP on maternal and fetal health worldwide, their underlying pathophysiologic mechanisms are unclear. These mechanisms are likely to be multi-factorial, as HDP are a complex and heterogeneous group of diseases.[52] The pathophysiology of HDP is accepted as being related to the placenta, as the incidence of preeclampsia increases with multiple gestations and is seen in molar pregnancies.[52] The current predominant theories suggest that abnormally shallow placental invasion and a lack of spiral artery remodeling early in development lead to placental hypoperfusion that results in placental hypoxemia, ischemia, oxidative stress and inflammation.[53] The conditions and mechanisms that lead to abnormal placental implantation early in development remain unclear. These insults are thought to trigger the release of a variety of vasoactive factors from the placenta that cause systemic maternal endothelial dysfunction, and result in hypertension and proteinuria.[52]
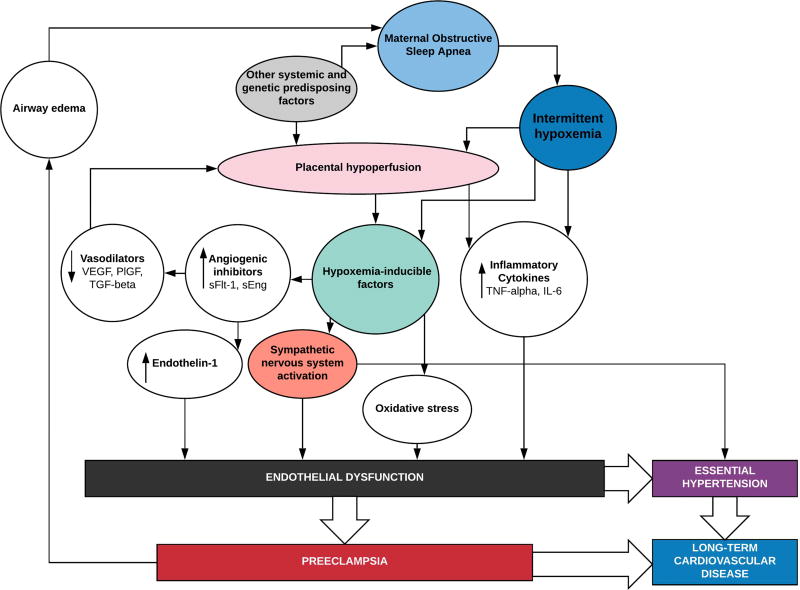
The mechanisms that link OSA to preeclampsia have also not been defined, although they share some common and potentially converging pathophysiologic pathways (Figure 1). Few studies have investigated these underlying pathways. The pathophysiologic consequences of OSA may interact with HDP in the following ways: 1) by potentiating abnormal placental development early in pregnancy as an upstream mediator of HDP; 2) by fueling endothelial dysfunction and sympathetic nervous system (SNS) imbalance further downstream; 3) by some combination of these; 4) or the conditions may simply co-exist in the same high-risk parturients.
Figure 1.
We propose plausible mechanisms by which intermittent hypoxemia due to maternal obstructive sleep apnea may converge with pathways implicated in the pathogenesis of preeclampsia. The factors that lead to the abnormal placental implantation and vascular development associated with preeclampsia are still under investigation, but several studies suggest that oxygen tension is an important modulating factor early in placental development. Inflammatory cytokines are released both in response to intermittent hypoxemia, and placental hypoperfusion and both may contribute to endothelial dysfunction. Hypoxemia-inducible factors released in response to intermittent hypoxemia and reoxygenation have been implicated as mediators of sympathetic activation, oxidative stress, and angiogenic factors that lead to endothelial dysfunction and subsequent essential hypertension or preeclampsia.
interleukin 6 (IL-6); placental growth factor (PlFG); soluble endoglin (sEng); soluble fms-like tyrosine kinase 1 (sFlt-1); transforming growth factor-beta (TGF-beta); tumor necrosis factor-alpha (TNF-alpha); vascular-derived endothelial growth factor (VEGF)
Hypoxemia as an upstream mediator of HDP
The intermittent hypoxemia that occurs during sleep is a defining feature of OSA. Some have suggested that hypoxemia may be an upstream mediator in the development of preeclampsia.[54] Pregnant rodents exposed to chronic hypoxia developed preeclampsia-like symptoms.[55, 56] Interestingly, pregnant women who live with chronically low arterial oxygen partial pressures (at altitudes above 2700 meters) have an increased risk of preeclampsia and intrauterine growth restriction. They also show less of a physiologic drop in blood pressure in response to pregnancy than women living at lower altitudes. A drop in systemic vascular resistance is a normal physiologic response to pregnancy and causes a decrease in blood pressure.[57, 58]
In vitro and animal studies suggest that oxygen tension plays a very specific role in the early development of the placenta, and that alterations in the oxygen tension may predispose to the pathologic placental development seen in preeclampsia.[55, 59, 60] Hypoxia-inducible factors-1 & 2 (HIF-1 & 2) are transcription factors that play a vital role in the cellular response to low oxygen tension.[61] They have been found to be overexpressed in the placentas of women living at high altitude.[62] Of note, HIFs have been studied for their role in connecting OSA with hypertension in non-pregnant adults, as mediators of inflammation, SNS activation, oxidative stress and endothelial dysfunction.[63]
One study has examined the placental tissues of women with OSA and habitual snoring compared to those of non-snoring controls, and found evidence of placental hypoxia in the placentas of women with SDB.[64] These SDB placentas manifested elevations in fetal normoblasts (nucleated red blood cells); normoblastemia is a marker of fetal hypoxia. Placentas of SDB mothers also manifested significantly increased expression of calcium-exchanger 1 (CAX1), an indirect cellular marker of hypoxia and HIF-1 activation. These differences remained significant after controlling for BMI, diabetes mellitus and hypertension. In another recent study, normoblastemia and evidence of chronic inflammation (chronic villitis) were also found in the placentas of obese gravidas (pregravid BMI = 38.2 ± 7.1 kg․m−2), compared to the placentas of women with lower BMIs (21.9 ± 1.5 kg․m−2).[65] This effect was more pronounced at higher BMIs. Interestingly, while this study controlled for the effects of parity, hypertension and diabetes mellitus, OSA status was not reported, but is likely to be much higher among the obese group and could be an important contributor to the differences seen in the placentas of obese vs. non-obese gravidas. Human and animal studies suggest there may be compensatory mechanisms that occur in the placenta and the fetus to adapt to the low oxygen tension, but these adaptive responses have not yet been studied in women with OSA.[52, 66]
Downstream pathways to endothelial dysfunction
The mechanisms that are thought to contribute to hypertension and cardiovascular disease in non-pregnant adults with OSA may contribute further downstream to the pathophysiology of preeclampsia via common pathways: 1) oxidative stress, 2) systemic inflammation, 3) SNS dysregulation and ultimately, 4) endothelial dysfunction.[51] The role of oxidative stress in mediating the association between OSA and cardiovascular disease has recently been questioned as CPAP therapy did not result in improvement in markers of oxidative stress in middle-aged adults.[67] This is supported by recent evidence from a cohort of pregnant women with OSA. Khan et al. conducted a case-controlled analysis of serum markers of oxidative stress and antioxidant capacity in women with OSA in pregnancy and, contrary to their hypotheses, found that the pregnant women with OSA had a significantly greater antioxidant capacity and lower oxidative and carbonyl stress markers than controls.[68]
Preeclampsia has also been associated with increased levels of pro-inflammatory cytokines [tumor necrosis factor-alpha (TNF-alpha); C-reactive protein (CRP); interlukin-6; and interleukin-8] that have also been described in OSA and may have synergistic pathways (Figure 1).[54, 69, 70] Several lines of evidence suggest that hypoxia-related signaling pathways in preeclampsia may be mediated by the immune system.[52, 54] Inflammatory pathways have been clearly implicated in the pathogenesis of cardiovascular disease, but studies supporting their role in OSA have produced mixed results.[71, 72] Inflammation is part of the pathophysiology of co-morbid disease states associated with OSA such as diabetes mellitus and obesity, and clinical studies have been confounded by the multiple, complex and interacting pathways.[73]
Repetitive nocturnal apneic episodes result in cycles of hypercapnia, intermittent hypoxia and reoxygenation that trigger chemoreflexes which stimulate the SNS.[74] These perturbations, combined with intrathoracic pressure changes and frequent arousals from sleep, lead to vascular endothelial dysfunction.[75, 76] In turn, these pathophysiologic processes can contribute to hypertension in adults with OSA, and could play a role in the pathogenesis of preeclampsia in women with OSA.[42] Increased blood pressure variability and decreased baroreceptor sensitivity have been observed in preeclamptic women as indicators of SNS over-activity.[77, 78] In non-pregnant, hypertensive and obese adults, increased nocturnal blood pressure variability is associated with long-term adverse cardiovascular outcomes, including increased mortality.[79, 80] Increased blood pressure variability and decreased baroreceptor sensitivity have also been observed in children and adults with OSA, and have improved with CPAP therapy [81–84] To our knowledge, there are no published studies on these indicators of SNS activation in pregnant women with OSA.
In non-pregnant adults with OSA, endothelial dysfunction and hypertension have been shown to result from repetitive cycles of hypoxemia and reoxygenation, frequent arousals during sleep, and changes in intrathoracic pressure.[75, 76] While the mechanisms that implicate OSA in the pathogenesis of cardiovascular disease in non-pregnant adults are complex, considerable evidence suggests that vascular endothelial dysfunction may play a role in the pathogenesis of these diseases and may share mechanistic pathways with preeclampsia (Figure 1).[75, 85, 86]
Vascular endothelial homeostasis depends on a complement of angiogenic and antiangiogenic proteins with complex roles in a number of physiologic and pathophysiologic processes throughout the body, including in the placenta. Vascular-derived endothelial growth factor (VEGF) and its cousin, placental growth factor (PlFG), are angiogenic proteins secreted by endothelial cells that promote vasodilation. The soluble circulating form of one of their receptors, soluble fms-like tyrosine kinase 1 (sFlt-1, also known as sVEGFR-1), is a potent VEGF/PlGF inhibitor. Soluble endoglin (sEng), a co-receptor for the transforming growth factor (TGF) β-1 and β-3 receptor complex, is released by endothelial cells in response to vascular injury, and may interfere with vasodilation by inhibiting the binding of transforming growth factor (TGF) β-1 & β-3 to endothelial cells.[87] Both sFlt-1 and sEng are released in response to hypoxia in experimental models; this may be mediated by HIF-1.[55, 61, 88, 89] In non-pregnant adults with OSA, up-regulation of sFlt-1 and s-Eng were associated with hypertension and endothelial dysfunction.[90, 91] This study suggested that differential release of these angiogenesis inhibitors may account for differences in individual responses to similar degrees of hypoxemia, and thus, differences in the progression of hypertension and cardiovascular disease.[91]
Interestingly, this protein imbalance is also implicated in the clinical manifestations of preeclampsia: vasoconstriction, hypertension, and proteinuria.[44, 55, 92–94] The role of these angiogenic and antiangiogenic proteins secreted by the placenta in the pathogenesis of preeclampsia has been studied extensively. Administration of excess levels of sFlt-1 has been associated with hypertension and clinical features of preeclampsia in animals.[95, 96] Blood pressure was reduced by administering PlGF to animals in an experimental model of preeclampsia.[97]
SFlt-1 and sEng levels are both associated with increased levels of endothelin-1, another potent vasoconstrictor that is elevated in preeclampsia.[98] Antagonism of its downstream receptor (endothelin Type A receptor) has been studied as a means of mitigating the hypertensive effects of these proteins in animal models, and as a potential future therapeutic target in humans.[56, 99] Others have explored removing the proteins from circulation; a case series reported improvement in blood pressure control, reduction of proteinuria, and prolongation of pregnancy in women with preterm severe preeclampsia after serial removal of plasma sFlt-1 using extracorporeal apheresis.[100]
One small, retrospective study of pregnant women with OSA provides preliminary evidence that elevated sFlt-1/PlGF ratios are associated with a diagnosis of OSA.[101] This study also detected lower serum levels of the placental peptide, pregnancy-associated plasma protein A (PAPP-A), in women with OSA, an alternation usually associated with preeclampsia. Women who subsequently developed preeclampsia were excluded, and the differences remained significant. The significance of these findings in the pathogenesis of OSA and HDP is not entirely clear. However, taken together, the preliminary evidence from studies in pregnant and non-pregnant subjects suggests that OSA-related nocturnal hypoxemia may affect the normal development and function of the placenta, and could be part of the pathway that leads to endothelial dysfunction in HDP.
The effect of OSA treatment on HDP
Treatment of OSA with continuous positive airway pressure (CPAP) has improved daytime sleepiness, mood, quality of life, hypertension, sympathetic dysregulation and endothelial function in a number of studies of non-pregnant adults.[102–105] However, while a large observational study of the impact of CPAP on cardiovascular morbidity and mortality in non-pregnant women suggested a positive effect of CPAP, particularly with good compliance, randomized controlled trials have produced mixed results.[43, 105, 106]
Additional studies are needed to determine if treatment of OSA with CPAP may play a role in the prevention of HDP.[107, 108] Previous studies by two research groups have investigated the role for CPAP in treating preeclampsia, but none have shown an effect of CPAP at preventing HDP. These studies provided preliminary evidence that CPAP may have a role in improving markers of fetal well-being in preeclamptic women, as well as improving blood pressure control and increasing cardiac output during sleep.[107, 109–111] Women with preeclampsia had greater mean AHI and flow limitation than gestational age and BMI-matched controls, but none of the women had diagnosed SDB prior to the study.[107]
A recent case report describes a parturient with untreated OSA who developed severe preeclampsia, and was treated with nightly CPAP.[112] She showed improved blood pressure control into the mild range, without medication, and a decrease in urinary protein and uric acid levels within a week of starting CPAP therapy, such that induction of labor was postponed for 30 days after diagnosis of severe preeclampsia. Interestingly, this case report also demonstrated a decrease in sFlt-1 and sEng levels, and in the sFlt-1/PlGF ratio after starting CPAP therapy. These findings suggest that OSA treatment with CPAP may play a role in stabilizing or reversing the endothelial dysfunction involved in the pathogenesis of preeclampsia. Further studies are needed to evaluate the efficacy of CPAP therapy to mitigate adverse pregnancy outcomes.
Discussion
Despite the public health importance of HDP and evidence that OSA is associated with these conditions, we have surprisingly limited data regarding the pathophysiologic mechanisms that may connect these diseases, and whether treatment of OSA might prevent or improve HDP. To better understand these mechanisms, it seems promising to build on the existing preliminary literature and to carry out rigorous studies to investigate the various pathways that may be converging in pregnant women with OSA: hypoxia-induced placental dysfunction; SNS dysregulation; systemic inflammation, and endothelial dysfunction.
Most importantly, there is a pressing need to determine if treatment of OSA may play a role in impacting these various pathways, and thereby mitigating the risk of developing HDP and other adverse pregnancy outcomes. Carrying out rigorous studies to address this need poses some methodologic challenges including: identifying a reliable and efficient means of screening women to identify those at risk for OSA as early as possible in pregnancy; identifying an optimal control condition that balances the need for scientific rigor with the ethics of not delivering effective treatment to pregnant women with OSA; and carrying out diagnosis and implementing effective treatment quickly enough to be able to have a significant impact during the limited time window of pregnancy.
There is a relative lack of studies in pregnant women with OSA that have investigated the underlying pathophysiology of these conditions. Existing studies are preliminary; the data presented here is largely based on the scientifically plausible intersection of pathways based on animal and human studies of the pathophysiology of OSA and cardiovascular disease and of HDP. No published studies to date have investigated the impact of OSA therapy on adverse pregnancy outcomes, and our discussion here is based on a few preliminary studies of the impact of CPAP on hemodynamics of women with preeclampsia, and on the existing literature from non-pregnant populations. Limitations of this review include the following: only papers written in English were reviewed; studies were identified using only the PubMed database and a review of relevant reference lists; and we did not review unpublished dissertations.
Despite these gaps in knowledge, all of these challenges can and must be addressed. A systematic effort is needed to develop a screening tool that is valid for use in pregnant women. The limited time window of pregnancy can be addressed by implementing streamlined procedures for rapidly carrying out diagnostic studies and CPAP titration in pregnant women coupled with a facilitated process for getting CPAP devices and related equipment to those women for whom this treatment is indicated. Health disparities related to access to diagnostic sleep studies and treatments are poorly described in the pregnant population, and warrant study so that these disparities can be addressed. Lastly, there is a need to develop study designs that allow for rigorous controlled trials in pregnant women with OSA without withholding CPAP therapy. Addressing these challenges and carrying out the needed studies, though difficult, promises to have a substantial positive impact on public health.
Practice Points.
The relationship between OSA and HDP:
is supported by several retrospective and prospective studies.
has unclear underlying mechanisms.
may contribute to one of the leading causes of maternal morbidity and mortality worldwide, as well as a leading cause of preterm birth.
once elucidated, may lead to identification of a treatable risk factor for HDP.
Research Agenda.
Future research studies should be aimed at:
building on preliminary evidence to better understand if OSA-induced hypoxemia may play a role in the placental pathology that leads to preeclampsia.
elucidating the pathophysiologic mechanisms that connect OSA and HDP along shared pathways of sympathetic dysregulation, inflammation and endothelial dysfunction.
determining if treatment of OSA may play a role in the prevention and treatment of HDP and other adverse pregnancy outcomes.
developing better screening tools and algorithms focused on the early diagnosis and treatment of OSA in pregnant women.
Acknowledgments
Funding sources: Dr. Dominguez’s work is supported in part by the NIH 5T32GM008600-20.
Abbreviations
- aOR
adjusted odds ratio
- AHI
apnea-hypopnea index
- ACOG
American College of Obstetricians and Gynecologists
- BMI
Body mass index
- CRP
C-reactive protein
- HDP
hypertensive disorders of pregnancy
- HIF-1
hypoxia-inducible factor-1
- IL-6
interleukin 6
- CPAP
continuous positive airway pressure ventilation
- OSA
obstructive sleep apnea
- PlFG
placental growth factor
- PSG
polysomnography
- SDB
sleep-disordered breathing
- sEng
soluble endoglin
- sFlt-1
soluble fms-like tyrosine kinase 1
- TGF
transforming growth factor
- TNF-alpha
tumor necrosis factor-alpha
- VEGF
vascular-derived endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement:
Devices used in our research have been loaned by ResMed and Itamar Medical, Ltd.
References
- 1.Branum AM, Kirmeyer SE, Gregory EC. Prepregnancy Body Mass Index by Maternal Characteristics and State: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65:1–11. [PubMed] [Google Scholar]
- 2.Louis JM, Mogos MF, Salemi JL, Redline S, Salihu HM. Obstructive sleep apnea and severe maternal-infant morbidity/mortality in the United States, 1998–2009. Sleep. 2014;37:843–9. doi: 10.5665/sleep.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–92. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien LM, Bullough AS, Chames MC, Shelgikar AV, Armitage R, Guilleminualt C, et al. Hypertension, snoring, and obstructive sleep apnoea during pregnancy: a cohort study. BJOG. 2014;121:1685–93. doi: 10.1111/1471-0528.12885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pamidi S, Pinto LM, Marc I, Benedetti A, Schwartzman K, Kimoff RJ. Maternal sleep-disordered breathing and adverse pregnancy outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;210:52 e1–e14. doi: 10.1016/j.ajog.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 6.Facco FL, Parker CB, Reddy UM, Silver RM, Koch MA, Louis JM, et al. Association Between Sleep-Disordered Breathing and Hypertensive Disorders of Pregnancy and Gestational Diabetes Mellitus. Obstet Gynecol. 2017;129:31–41. doi: 10.1097/AOG.0000000000001805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourjeily G, Danilack VA, Bublitz MH, Lipkind H, Muri J, Caldwell D, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;38:50–7. doi: 10.1016/j.sleep.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley KA, Patel SR. Disparities and genetic risk factors in obstructive sleep apnea. Sleep Med. 2016;18:96–102. doi: 10.1016/j.sleep.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CL, Ford JB, Algert CS, Antonsen S, Chalmers J, Cnattingius S, et al. Population-based trends in pregnancy hypertension and pre-eclampsia: an international comparative study. BMJ Open. 2011;1:e000101. doi: 10.1136/bmjopen-2011-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–33. doi: 10.1016/S2214-109X(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 12.Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36:56–9. doi: 10.1053/j.semperi.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol. 2013;28:1–19. doi: 10.1007/s10654-013-9762-6. [DOI] [PubMed] [Google Scholar]
- 14.Fraser A, Nelson SM, Macdonald-Wallis C, Cherry L, Butler E, Sattar N, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125:1367–80. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freibert SM, Mannino DM, Bush H, Crofford LJ. The association of adverse pregnancy events and cardiovascular disease in women 50 years of age and older. J Womens Health (Larchmt) 2011;20:287–93. doi: 10.1089/jwh.2010.2097. [DOI] [PubMed] [Google Scholar]
- 16.Smith GN, Pudwell J, Walker M, Wen SW. Ten-year, thirty-year, and lifetime cardiovascular disease risk estimates following a pregnancy complicated by preeclampsia. J Obstet Gynaecol Can. 2012;34:830–5. doi: 10.1016/S1701-2163(16)35381-6. [DOI] [PubMed] [Google Scholar]
- 17.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shih T, Peneva D, Xu X, Sutton A, Triche E, Ehrenkranz RA, et al. The Rising Burden of Preeclampsia in the United States Impacts Both Maternal and Child Health. Am J Perinatol. 2016;33:329–38. doi: 10.1055/s-0035-1564881. [DOI] [PubMed] [Google Scholar]
- 19.Facco FL, Ouyang DW, Zee PC, Grobman WA. Development of a pregnancy-specific screening tool for sleep apnea. J Clin Sleep Med. 2012;8:389–94. doi: 10.5664/jcsm.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lockhart EM, Ben Abdallah A, Tuuli MG, Leighton BL. Obstructive Sleep Apnea in Pregnancy: Assessment of Current Screening Tools. Obstet Gynecol. 2015;126:93–102. doi: 10.1097/AOG.0000000000000848. [DOI] [PubMed] [Google Scholar]
- 21.Olivarez SA, Maheshwari B, McCarthy M, Zacharias N, van den Veyver I, Casturi L, et al. Prospective trial on obstructive sleep apnea in pregnancy and fetal heart rate monitoring. Am J Obstet Gynecol. 2010;202:552 e1–7. doi: 10.1016/j.ajog.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–36. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 23.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–95. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 24.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 25.Facco FL, Ouyang DW, Zee PC, Strohl AE, Gonzalez AB, Lim C, et al. Implications of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2014;210:559 e1–6. doi: 10.1016/j.ajog.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milne F, Redman C, Walker J, Baker P, Bradley J, Cooper C, et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ. 2005;330:576–80. doi: 10.1136/bmj.330.7491.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shmueli A, Meiri H, Gonen R. Economic assessment of screening for pre-eclampsia. Prenat Diagn. 2012;32:29–38. doi: 10.1002/pd.2871. [DOI] [PubMed] [Google Scholar]
- 28.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544 e1–e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 29.Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33:559–65. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 30.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206:136 e1–5. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Facco FL, Liu CS, Cabello AA, Kick A, Grobman WA, Zee PC. Sleep-disordered breathing: a risk factor for adverse pregnancy outcomes? Am J Perinatol. 2012;29:277–82. doi: 10.1055/s-0031-1295658. [DOI] [PubMed] [Google Scholar]
- 32.Facco FL, Ouyang DW, Zee PC, Grobman WA. Sleep disordered breathing in a high-risk cohort prevalence and severity across pregnancy. Am J Perinatol. 2014;31:899–904. doi: 10.1055/s-0033-1363768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010;202:261 e1–5. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 34.Pien GW, Pack AI, Jackson N, Maislin G, Macones GA, Schwab RJ. Risk factors for sleep-disordered breathing in pregnancy. Thorax. 2014;69:371–7. doi: 10.1136/thoraxjnl-2012-202718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reid J, Skomro R, Cotton D, Ward H, Olatunbosun F, Gjevre J, et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011;34:1033–8. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu T, Feng Y, Peng H, Guo D, Li T. Obstructive sleep apnea and the risk of perinatal outcomes: a meta-analysis of cohort studies. Sci Rep. 2014;4:6982. doi: 10.1038/srep06982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Facco FL, Parker CB, Reddy UM, Silver RM, Louis JM, Basner RC, et al. NuMoM2b Sleep-Disordered Breathing study: objectives and methods. Am J Obstet Gynecol. 2015;212:542 e1–127. doi: 10.1016/j.ajog.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tantrakul V, Numthavaj P, Guilleminault C, McEvoy M, Panburana P, Khaing W, et al. Performance of screening questionnaires for obstructive sleep apnea during pregnancy: A systematic review and meta-analysis. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Romundstad PR, Magnussen EB, Smith GD, Vatten LJ. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation. 2010;122:579–84. doi: 10.1161/CIRCULATIONAHA.110.943407. [DOI] [PubMed] [Google Scholar]
- 41.Reid J, Glew RA, Skomro R, Fenton M, Cotton D, Olatunbosun F, et al. Sleep disordered breathing and gestational hypertension: postpartum follow-up study. Sleep. 2013;36:717–21B. doi: 10.5665/sleep.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunietz GL, Chervin RD, O'Brien LM. Sleep-disordered breathing during pregnancy: future implications for cardiovascular health. Obstet Gynecol Surv. 2014;69:164–76. doi: 10.1097/OGX.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 44.Haas DM, Ehrenthal DB, Koch MA, Catov JM, Barnes SE, Facco F, et al. Pregnancy as a Window to Future Cardiovascular Health: Design and Implementation of the nuMoM2b Heart Health Study. Am J Epidemiol. 2016;183:519–30. doi: 10.1093/aje/kwv309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pilkington S, Carli F, Dakin MJ, Romney M, De Witt KA, Dore CJ, et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638–42. doi: 10.1093/bja/74.6.638. [DOI] [PubMed] [Google Scholar]
- 46.Izci B, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J. 2006;27:321–7. doi: 10.1183/09031936.06.00148204. [DOI] [PubMed] [Google Scholar]
- 47.Zaremba S, Mueller N, Heisig AM, Shin CH, Jung S, Leffert LR, et al. Elevated upper body position improves pregnancy-related OSA without impairing sleep quality or sleep architecture early after delivery. Chest. 2015;148:936–44. doi: 10.1378/chest.14-2973. [DOI] [PubMed] [Google Scholar]
- 48.O'Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, et al. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207:487 e1–9. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izci B, Riha RL, Martin SE, Vennelle M, Liston WA, Dundas KC, et al. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167:137–40. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 50.Kapsimalis F, Kryger MH. Gender and obstructive sleep apnea syndrome, part 1: Clinical features. Sleep. 2002;25:412–9. [PubMed] [Google Scholar]
- 51.Bourjeily G, Ankner G, Mohsenin V. Sleep-disordered breathing in pregnancy. Clin Chest Med. 2011;32:175–89. x. doi: 10.1016/j.ccm.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Ilekis JV, Tsilou E, Fisher S, Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215:S1–S46. doi: 10.1016/j.ajog.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–70. doi: 10.1053/j.ackd.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma S, Norris WE, Kalkunte S. Beyond the threshold: an etiological bridge between hypoxia and immunity in preeclampsia. J Reprod Immunol. 2010;85:112–6. doi: 10.1016/j.jri.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lai Z, Kalkunte S, Sharma S. A critical role of interleukin-10 in modulating hypoxia-induced preeclampsia-like disease in mice. Hypertension. 2011;57:505–14. doi: 10.1161/HYPERTENSIONAHA.110.163329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J, Xiao D, Hu Y, Wang Z, Paradis A, Mata-Greenwood E, et al. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62:599–607. doi: 10.1161/HYPERTENSIONAHA.113.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol. 1999;180:1161–8. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 58.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54:20–5. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- 59.Genbacev O, Joslin R, Damsky CH, Polliotti BM, Fisher SJ. Hypoxia alters early gestation human cytotrophoblast differentiation/invasion in vitro and models the placental defects that occur in preeclampsia. J Clin Invest. 1996;97:540–50. doi: 10.1172/JCI118447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adelman DM, Gertsenstein M, Nagy A, Simon MC, Maltepe E. Placental cell fates are regulated in vivo by HIF-mediated hypoxia responses. Genes Dev. 2000;14:3191–203. doi: 10.1101/gad.853700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87:134. doi: 10.1095/biolreprod.112.102723. [DOI] [PubMed] [Google Scholar]
- 62.Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, et al. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007;170:2171–9. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanduri J, Peng YJ, Yuan G, Kumar GK, Prabhakar NR. Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med (Berl) 2015;93:473–80. doi: 10.1007/s00109-015-1274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ravishankar S, Bourjeily G, Lambert-Messerlian G, He M, De Paepe ME, Gundogan F. Evidence of Placental Hypoxia in Maternal Sleep Disordered Breathing. Pediatr Dev Pathol. 2015;18:380–6. doi: 10.2350/15-06-1647-OA.1. [DOI] [PubMed] [Google Scholar]
- 65.Leon-Garcia SM, Roeder HA, Nelson KK, Liao X, Pizzo DP, Laurent LC, et al. Maternal obesity and sex-specific differences in placental pathology. Placenta. 2016;38:33–40. doi: 10.1016/j.placenta.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 66.Rosario GX, Konno T, Soares MJ. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev Biol. 2008;314:362–75. doi: 10.1016/j.ydbio.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paz YMHL, Hazen SL, Tracy RP, Strohl KP, Auckley D, Bena J, et al. Effect of Continuous Positive Airway Pressure on Cardiovascular Biomarkers: The Sleep Apnea Stress Randomized Controlled Trial. Chest. 2016;150:80–90. doi: 10.1016/j.chest.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan N, Lambert-Messerlian G, Monteiro JF, Hodosy J, Tothova L, Celec P, et al. Oxidative and carbonyl stress in pregnant women with obstructive sleep apnea. Sleep Breath. 2017 doi: 10.1007/s11325-017-1475-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Lima FF, Mazzotti DR, Tufik S, Bittencourt L. The role inflammatory response genes in obstructive sleep apnea syndrome: a review. Sleep Breath. 2016;20:331–8. doi: 10.1007/s11325-015-1226-7. [DOI] [PubMed] [Google Scholar]
- 70.Parchim NF, Wang W, Iriyama T, Ashimi OA, Siddiqui AH, Blackwell S, et al. Neurokinin 3 receptor and phosphocholine transferase: missing factors for pathogenesis of C-reactive protein in preeclampsia. Hypertension. 2015;65:430–9. doi: 10.1161/HYPERTENSIONAHA.114.04439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jullian-Desayes I, Joyeux-Faure M, Tamisier R, Launois S, Borel AL, Levy P, et al. Impact of obstructive sleep apnea treatment by continuous positive airway pressure on cardiometabolic biomarkers: a systematic review from sham CPAP randomized controlled trials. Sleep Med Rev. 2015;21:23–38. doi: 10.1016/j.smrv.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Baessler A, Nadeem R, Harvey M, Madbouly E, Younus A, Sajid H, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers - a meta-analysis. J Inflamm (Lond) 2013;10:13. doi: 10.1186/1476-9255-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. 2015;16:25–34. doi: 10.1007/s11154-014-9304-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kara T, Narkiewicz K, Somers VK. Chemoreflexes--physiology and clinical implications. Acta Physiol Scand. 2003;177:377–84. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- 75.Atkeson A, Yeh SY, Malhotra A, Jelic S. Endothelial function in obstructive sleep apnea. Prog Cardiovasc Dis. 2009;51:351–62. doi: 10.1016/j.pcad.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mansukhani MP, Wang S, Somers VK. Chemoreflex physiology and implications for sleep apnoea: insights from studies in humans. Exp Physiol. 2015;100:130–5. doi: 10.1113/expphysiol.2014.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Logue OC, George EM, Bidwell GL., 3rd Preeclampsia and the brain: neural control of cardiovascular changes during pregnancy and neurological outcomes of preeclampsia. Clin Sci (Lond) 2016;130:1417–34. doi: 10.1042/CS20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faber R, Baumert M, Stepan H, Wessel N, Voss A, Walther T. Baroreflex sensitivity, heart rate, and blood pressure variability in hypertensive pregnancy disorders. J Hum Hypertens. 2004;18:707–12. doi: 10.1038/sj.jhh.1001730. [DOI] [PubMed] [Google Scholar]
- 79.Palatini P, Reboldi G, Beilin LJ, Casiglia E, Eguchi K, Imai Y, et al. Added predictive value of night-time blood pressure variability for cardiovascular events and mortality: the Ambulatory Blood Pressure-International Study. Hypertension. 2014;64:487–93. doi: 10.1161/HYPERTENSIONAHA.114.03694. [DOI] [PubMed] [Google Scholar]
- 80.Palatini P, Reboldi GP, Beilin L, Casiglia E, Eguchi K, Imai Y, et al. 7a.01: Increased Risk of Mortality in Obese Patients with High Nocturnal Blood Pressure Variability. Results from the Abp-International Study. J Hypertens. 2015;33(Suppl 1):e89. [Google Scholar]
- 81.Steinhorst AP, Goncalves SC, Oliveira AT, Massierer D, Gus M, Fuchs SC, et al. Influence of sleep apnea severity on blood pressure variability of patients with hypertension. Sleep Breath. 2014;18:397–401. doi: 10.1007/s11325-013-0899-z. [DOI] [PubMed] [Google Scholar]
- 82.Amin RS, Carroll JL, Jeffries JL, Grone C, Bean JA, Chini B, et al. Twenty-four-hour ambulatory blood pressure in children with sleep-disordered breathing. Am J Respir Crit Care Med. 2004;169:950–6. doi: 10.1164/rccm.200309-1305OC. [DOI] [PubMed] [Google Scholar]
- 83.Crisalli JA, McConnell K, Vandyke RD, Fenchel MC, Somers VK, Shamszumann A, et al. Baroreflex sensitivity after adenotonsillectomy in children with obstructive sleep apnea during wakefulness and sleep. Sleep. 2012;35:1335–43. doi: 10.5665/sleep.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pengo MF, Ratneswaran C, Berry M, Kent BD, Kohler M, Rossi GP, et al. Effect of Continuous Positive Airway Pressure on Blood Pressure Variability in Patients With Obstructive Sleep Apnea. J Clin Hypertens (Greenwich) 2016;18:1180–4. doi: 10.1111/jch.12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266–74. doi: 10.1378/chest.14-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 87.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 88.Nevo O, Soleymanlou N, Wu Y, Xu J, Kingdom J, Many A, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–93. doi: 10.1152/ajpregu.00794.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yinon Y, Nevo O, Xu J, Many A, Rolfo A, Todros T, et al. Severe intrauterine growth restriction pregnancies have increased placental endoglin levels: hypoxic regulation via transforming growth factor-beta 3. Am J Pathol. 2008;172:77–85. doi: 10.2353/ajpath.2008.070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohsenin V, Urbano F. Circulating antiangiogenic proteins in obstructive sleep apnea and hypertension. Respir Med. 2011;105:801–7. doi: 10.1016/j.rmed.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 91.Jafari B, Mohsenin V. Endothelial dysfunction and hypertension in obstructive sleep apnea - Is it due to intermittent hypoxia? J Cardiovasc Dis Res. 2013;4:87–91. doi: 10.1016/j.jcdr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 94.Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541–50. doi: 10.1152/ajpheart.01113.2007. [DOI] [PubMed] [Google Scholar]
- 95.Lu F, Longo M, Tamayo E, Maner W, Al-Hendy A, Anderson GD, et al. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am J Obstet Gynecol. 2007;196:396 e1–7. doi: 10.1016/j.ajog.2006.12.024. discussion e7. [DOI] [PubMed] [Google Scholar]
- 96.Makris A, Thornton C, Thompson J, Thomson S, Martin R, Ogle R, et al. Uteroplacental ischemia results in proteinuric hypertension and elevated sFLT-1. Kidney Int. 2007;71:977–84. doi: 10.1038/sj.ki.5002175. [DOI] [PubMed] [Google Scholar]
- 97.Spradley FT, Tan AY, Joo WS, Daniels G, Kussie P, Karumanchi SA, et al. Placental Growth Factor Administration Abolishes Placental Ischemia-Induced Hypertension. Hypertension. 2016;67:740–7. doi: 10.1161/HYPERTENSIONAHA.115.06783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nova A, Sibai BM, Barton JR, Mercer BM, Mitchell MD. Maternal plasma level of endothelin is increased in preeclampsia. Am J Obstet Gynecol. 1991;165:724–7. doi: 10.1016/0002-9378(91)90317-k. [DOI] [PubMed] [Google Scholar]
- 99.Bakrania B, Duncan J, Warrington JP, Granger JP. The Endothelin Type A Receptor as a Potential Therapeutic Target in Preeclampsia. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18030522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124:940–50. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]
- 101.Bourjeily G, Curran P, Butterfield K, Maredia H, Carpenter M, Lambert-Messerlian G. Placenta-secreted circulating markers in pregnant women with obstructive sleep apnea. J Perinat Med. 2015;43:81–7. doi: 10.1515/jpm-2014-0052. [DOI] [PubMed] [Google Scholar]
- 102.Ip MS, Tse HF, Lam B, Tsang KW, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–53. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 103.Ciccone MM, Favale S, Scicchitano P, Mangini F, Mitacchione G, Gadaleta F, et al. Reversibility of the endothelial dysfunction after CPAP therapy in OSAS patients. Int J Cardiol. 2012;158:383–6. doi: 10.1016/j.ijcard.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 104.Montesi SB, Edwards BA, Malhotra A, Bakker JP. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587–96. doi: 10.5664/jcsm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. 2016;375:919–31. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 106.Abuzaid AS, Al Ashry HS, Elbadawi A, Ld H, Saad M, Elgendy IY, et al. Meta-Analysis of Cardiovascular Outcomes With Continuous Positive Airway Pressure Therapy in Patients With Obstructive Sleep Apnea. Am J Cardiol. 2017;120:693–9. doi: 10.1016/j.amjcard.2017.05.042. [DOI] [PubMed] [Google Scholar]
- 107.Blyton DM, Skilton MR, Edwards N, Hennessy A, Celermajer DS, Sullivan CE. Treatment of sleep disordered breathing reverses low fetal activity levels in preeclampsia. Sleep. 2013;36:15–21. doi: 10.5665/sleep.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O'Brien LM. Positive airway pressure as a therapy for preeclampsia? Sleep. 2013;36:5–6. doi: 10.5665/sleep.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blyton DM, Sullivan CE, Edwards N. Reduced nocturnal cardiac output associated with preeclampsia is minimized with the use of nocturnal nasal CPAP. Sleep. 2004;27:79–84. doi: 10.1093/sleep/27.1.79. [DOI] [PubMed] [Google Scholar]
- 110.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162:252–7. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 111.Guilleminault C, Palombini L, Poyares D, Takaoka S, Huynh NT, El-Sayed Y. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings. Sleep Med. 2007;9:9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 112.Whitehead C, Tong S, Wilson D, Howard M, Walker SP. Treatment of early-onset preeclampsia with continuous positive airway pressure. Obstet Gynecol. 2015;125:1106–9. doi: 10.1097/AOG.0000000000000508. [DOI] [PubMed] [Google Scholar]