Abstract
Background:
Dibutyl phthalate (DBP) is an endocrine disruptor and used in some medication coatings, such as mesalamine for treatment inflammatory bowel disease (IBD).
Objectives:
To determine whether high-DBP from some mesalamine medications alters thyroid function.
Methods:
Seventy men with IBD, without thyroid disease or any radiation history participated in a crossover-crossback prospective study and provided up to 6 serum samples (2:baseline, 2:crossover, 2:crossback). Men on non-DBP mesalamine (background exposure) at baseline crossed-over to DBP-mesalamine (high exposure) then crossed-back to non-DBP mesalamine (B1HB2−arm) and vice versa for men on DBP-mesalamine at baseline (H1BH2−arm). Serum concentrations of total triiodothyronine (T3), total thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4), thyroid-stimulating hormone (TSH) and thyroid peroxidase antibody (TPOAb), and thyroglobulin antibody (TgAb).
Results:
After crossover in B1HB2−arm (26 men, 134 samples), T3 decreased 10% (95% confidence interval (CI):−14%,−5%), T3/T4 ratio decreased 8% (CI:−12%,−3%), TPOAb, and TgAb concentrations decreased, 11% (−20%, −2%) and 15% (−23%, −5%), respectively; after crossback, they increased. When men in the H1BH2−arm (44 men, 193 samples) crossed-over, T3 decreased 7% (CI: −11%, −2%) and T3/T4 ratio decreased 6% (CI: −9%, −2%). After crossback, only TgAb increased and FT4 decreased.
Conclusions:
High-DBP novel exposure or removal from chronic high-DBP exposure could alter elements of the thyroid system, and most probably alters the peripheral T4 conversion to T3 and thyroid autoimmunity, consistent with thyroid disruption. After exposure removal, these trends were mostly reversed.
Keywords: Phthalate, endocrine disruptor, thyroid, hormones, men
Introduction
Thyroid hormone regulates metabolism in practically all tissues of the human body. Differences in thyroid function, even within the clinical reference range, have been associated with a higher risk of adverse clinical outcomes including cardiovascular disease, dementia and diabetes1–3. Although recent experimental4–6 studies suggest that phthalate exposure can disrupt the hypothalamic-pituitary-thyroid axis, the limited human studies are inconsistent7–12. Most of the human studies were population-based on background exposure to phthalates among pregnant women and children9–16. The effects of exposure to high phthalate concentrations, specifically on thyroid function, has not been studied.
Ortho-phthalates (hereafter referred to as phthalates) are a commonly used high-production family of chemicals to which the general population are ubiquitously exposed17. Phthalates are present in personal care products18, medical equipment, foods, water and house dust17. Dibutyl phthalate (DBP)17 is also used for enteric coating in some specific mesalamine formulations19–23 despite the recent US Food and Drug Administration (FDA) recommendation against the use of phthalates in drug delivery vehicles24. Mesalamine or 5-aminosalicylic acid (5-ASA) medications are prescribed for inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD). Mesalamine is the active ingredient in Asacol® and Asacol®HD, with DBP acting as an excipient in their enteric coating25. Other mesalamine formulations such as Pentasa®, Lialda®, Apriso®, and Delzicol® do not contain DBP23,26. Asacol®, widely used to treat IBD in adults and children, was a first line of therapy for patients with UC and often used in pregnant women with IBD26,27.
Research including ours has shown that mesalamine medications with DBP-containing coatings contribute to high-DBP exposure as measured by concentrations of urinary monobutyl phthalate (MBP), the primary DBP metabolite28,29. Specifically, in individuals taking mesalamine medications that contain DBP, their MBP urinary concentrations were approximately 1,000 times higher than the median levels for men in the US general population (National Health and Nutrition Examination Survey (NHANES))30. Therefore, patients with IBD taking DBP-containing mesalamine have chronic high exposure to DBP because the medication is taken daily.
We recently found that sperm motility decreased in newly exposed men to high-DBP from mesalamine and continued to decrease even after a four month withdrawal31 and disrupted their reproductive hormones and perhaps the hypothalamic-pituitary-testis axis 18. However, to our knowledge, there are few studies that investigated the association between DBP and thyroid hormones, despite the observation that other phthalates appeared to affect the hypothalamic-pituitary-thyroid axis 7,8. Specifically, there are no studies on effects of high-DBP exposures from medications on hypothalamic-pituitary-thyroid axis. Therefore, as an extension of our previous work focusing on semen quality 31 and reproductive hormones 18, we took advantage of the marked difference in DBP-exposure from different mesalamine formulations using a crossover-crossback prospective cohort to assess the effects of high-DBP exposure from mesalamine medications on thyroid function and autoimmunity in adult men. We hypothesized that exposure to high-DBP is associated with a decrease in thyroid hormone concentrations and an increase in thyroid autoimmunity.
Methods
Participants
For this study, men aged 18 to 55 years old, with mild IBD, and on mesalamine for at least three months at enrollment, without history of steroid medication in the last three months, vasectomy, diabetes mellitus, hepatic, or renal diseases were eligible to participate. As previously described 18,31, 73 men were enrolled from gastroenterology clinics at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women’s Hospital (BWH) and Massachusetts General Hospital (MGH) in Boston into the Mesalamine And Reproductive health Study (MARS) (2010–2016). We excluded 3 men who had history of thyroid disease or radiation therapy leaving 70 men in our current analysis. The institutional review boards of Harvard T.H. Chan School of Public Health, BIDMC, BWH and MGH approved MARS. All men signed informed consents before participation.
Study design
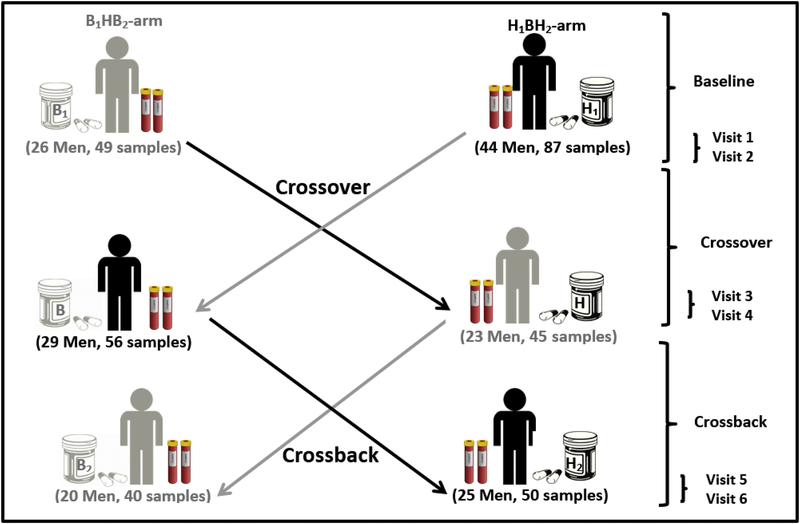
Men participated in up to six visits (2 baseline, 2 crossover, and 2 crossback). B1HB2−arm (Background1−High-Background2) represents men who started on non-DBP mesalamine (background-DBP exposure from other sources) then crossed-over to high-DBP mesalamine (high-DBP) then crossed-back to non-DBP mesalamine (background-DBP) and vice versa in H1BH2−arm (High1−Background-High2) men who started on high-DBP mesalamine (Figure 1). Because the major aim of this study was to investigate semen parameters 31, four month crossover and crossback periods were chosen a priori taking into account the spermatogenesis cycle 32. The duration between the two visits in the baseline, crossover and crossback phase were two weeks. At time 0 (T0), men reported their medications, demographics, lifestyle and health information and our research nurse measured their height and weight. At each visit, men reported their mesalamine medications and gave blood samples. Twelve out of the 44 men in the H1BH2−arm participated in a short protocol requiring only up to four visits that only required crossover as previously described and justified 31. These 12 men participated in 29 baseline and crossover visits and were also included in the analysis.
Figure 1. Mesalamine And Reproductive health Study (MARS) Design.
B1HB2−arm: B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline. H1BH2−arm: H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 44 men taking mesalamine containing DBP at baseline (24 men for less than 3 years and 20 men with 3 years or more).
Abbreviations: MARS, Mesalamine And Reproductive health Study; DBP, dibutyl phthalate.
Exposure assessment
We defined DBP exposure by medication type, i.e., high-DBP mesalamine or non-DBP mesalamine, in each visit as prescribed and self-reported which has been shown to be a reliable method for medication compliance 18,31,33. We a priori chose to define high-DBP exposure by the mesalamine medication type 18,31 rather than measuring urinary MBP concentrations that, due to the short DBP half-life34, only represent exposure over the past several hours.
Thyroid function assessment
At each visit, nurses collected non-fasting blood samples between 7 am and 2 pm. All samples were stored at −80° C and shipped in a single batch for analysis to the laboratory of clinical chemistry and haematology at the Máxima Medical Centre in Veldhoven, The Netherlands. We measured TSH, FT4, total thyroxine (T4), total triiodothyronine (T3), free triiodothyronine (FT3), thyroperoxidase antibodies (TPOAbs) and thyroglobulin antibodies (TgAbs) using a electrochemiluminescence immunoassay (Cobas e601, Roche Diagnostics, Mannheim Germany) in serum. The clinical reference values were 0.4 – 4.0 mU/L for TSH, 0.9 – 2.8 nmol/L for T3, 3.5 – 6.5 pmol/L for FT3, 58 – 161 nmol/L for T4, and 10 −24 pmol/L for FT4. Inter and intra-assay coefficients of variation were 2.1%, 3.5%, 3.8%, 3.8%, and 7.7% for TSH, FT4, T4, FT3 and T3, respectively. TPOAb and TgAb concentrations were considered positive if >35 IU/ml or >115 IU/ml (manufacturer cut-offs), respectively, and the coefficients of variation were 12.4% and 7.1% for TPOAbs at 33 or 100 IU/l, respectively, 10.9% and 8.6% for TgAbs at 76 and 218 IU/l, respectively. In addition, we calculated the ratio (%) of serum total T3 to serum total T4 (T3/T4) from the respective hormone concentrations (T3 (nmol/L)/T4 (nmol/L) * 100) as an index of the action of thyroid hormones in peripheral tissues 35 and employed previously by Johns et al. 36. Serum total T4 is maintained by a balance between T4 secretion from the thyroid gland and its clearance from blood (half-life of 7–10 days), whereas serum total T3 is largely controlled by peripheral deiodination in tissues as it becomes activated 37. Others have shown that the T3/T4 ratio is a good measure of thyroid state in health and disease 38. Therefore, when the ratio decreases, this suggests that deiodination decreased and vice versa.
Statistical Analysis
We defined the exposure as an indicator variable based on DBP-exposure (high versus background) at each phase (baseline, crossover and crossback) for the two study arms (H1BH2 and B1HB2). We modeled the thyroid function measurements as natural log-transformed continuous outcomes to satisfy model assumptions.
We performed descriptive statistics and tested for differences between men in the two arms using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. We selected the covariates based on directed acyclic graph (Supplementary Fig 1) and statistical considerations (>10% change in the effect estimate). The final model included age (years, continuous), body mass index (BMI) (Kg/m2, continuous), sample drawing time (hours, continuous, time varying), race (Caucasian or not), current smoking (binary), and duration on high-DBP mesalamine at T0 (years, continuous). Initially, we also considered adjustment for IBD severity, IBD diagnosis (UC/CD), duration since diagnosis, and education, but these variables were not confounders and thus not retained in the final models.
We estimated the crossover (crossover versus baseline), and crossback (crossback versus crossover) percent changes in the thyroid hormones within each arm separately. We also examined the cross-sectional differences at T0 between arms. We used linear mixed effect models (LMEM) with a random intercept to account for within-person correlation among longitudinal measures of a given hormone outcome arising from man-to-man heterogeneity across the study participants. We tested for effect modification by duration under high-DBP at T0 by adding an interaction term and we used the same alpha level as for the other analyses (0.05).
We applied fixed effect models (FEM) as a secondary analysis to estimate the terms as ordinary fixed regression coefficients, rather than assuming a random distribution for the person-specific intercepts. These models isolate the purely longitudinal within-person effect of exposure37. As a sensitivity analysis, we assessed LMEM model sensitivity to the covariance structure implied by the random intercept model, using robust empirical standard errors 39. In addition, we conducted the analysis after further adjusting for the antibodies concentrations, one at a time. We also conducted the analysis after excluding men with high observations of the antibodies, suspecting that they may have undiagnosed thyroid diseases. We considered two-sided alpha <0.05 as statistically significant. We conducted all statistical analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
LMEM equation:
Where Yit denotes the outcome for subject i at time t (baseline, crossover, or crossback), medit is an indicator denoting DBP/non-DBP mesalamine medication, Zit is a vector of time-varying variables e.g., sample drawing time, Wi is a vector of non-time varying variables e.g., race, β0 and β1 are scalar parameters, β2 and γ are vectors of coefficients, and ui is random subject-specific errors and εit is within-subject errors.
FEM equation:
Where ui is a fixed regression coefficient reflecting the constant effect for all measurements on a given subject. Note: ui controls for all fixed differences in outcome across subject, measured or unmeasured.
Results
Seventy men (mean age 34.7 years) with no thyroid disease or any radiation history were enrolled and provided 327 blood samples (average: five samples/man) (Supplementary Fig.2). Among the 58 men who participated in the full-protocol, 49 men (85%) crossed-over and 45 (78%) crossed-back; 89% and 77%, respectively, in the B1HB2−arm, with 81% and 78%, respectively, in the H1BH2−arm. Men in the B1HB2−arm (26 men, 134 samples) were on non-DBP mesalamine medication at baseline for a median of 1 year. Men in the H1BH2−arm (44 men, 193 serum samples) were on their high-DBP mesalamine medication at baseline (H1) for a median duration of 3 years. Men’s characteristics did not differ significantly between the two arms except for smoking where 85% of the men in the B1HB2 arm and 75% of the men of H1BH2 arm never smoked (Table 1). Nine men were lost to follow-up, three in B1HB2−arm and six in H1BH2−arm (p-value= 0.64). All thyroid function distributions are shown in Table 2.
Table 1:
Demographics of 70 men contributing 327 blood samples in the MARS Study by arm
| B1HB2−arm (26 men, 134 visits) | H1BH2−arm (44 men, 193 visits) | Total (70 men, 327 visits) | ||
|---|---|---|---|---|
| N (%)/Mean (SD),[Range] | N (%)/Mean (SD),[Range] | N (%)/Mean (SD),[Range] | P-valueb | |
| Baseline characteristics (at Visit 1), men (N)a | ||||
| Age (Years) | 33.9(8.97), [20.2, 54.6] | 35.1 (9.69), [20.8, 55.7] | 34.7 (9.38), [20.2, 55.7] | 0.78 |
| Race | 0.23 | |||
| Caucasian | 25 (96) | 35 (80) | 60 (86) | − |
| Black/African American | 1(4) | 2(5) | 3(4) | − |
| Asian | 0 | 4(9) | 4(6) | − |
| Other | 0 | 3(7) | 3(4) | − |
| BMI (Kg/m2) | 25.3 (3.56), [19.4, 32.7] | 26.3 (6.53), [19.5, 54.0] | 26.0(5.61), [19.4, 54.0] | 0.95 |
| BMI-categories | 0.99 | |||
| Normal weight (18.5≤BMI<25) | 14(54) | 23 (52) | 37(53) | − |
| Overweight (25≤BMI<30) | 9(35) | 15 (34) | 24 (34) | − |
| Obese (BMI≥30) | 3(12) | 6(14) | 9(13) | − |
| Education | 0.69 | |||
| Below college | 3(14) | 4(10) | 7(12) | − |
| College graduate | 10(48) | 16(41) | 26 (43) | − |
| Graduate degree | 8(38) | 19(49) | 27 (45) | − |
| Smoking status | 0.007 | |||
| Never smoker | 22 (85) | 33 (75) | 55 (79) | − |
| Former smoker | 1(4) | 11(25) | 12(17) | − |
| Current smoker | 3(11) | 0 | 3(4) | − |
| Current Marijuana smoke | 6(46) | 6(27) | 12 (34) | 0.29 |
| Warm season at baselinec | 5(19) | 17(39) | 22(31) | 0.11 |
| IBD diagnosis | 0.80 | |||
| Ulcerative colitis | 16(62) | 29 (66) | 45 (64) | − |
| Crohn’s disease | 10(38) | 15(34) | 25 (36) | − |
| IBD scored | 1.65(1.38), [0,4] | 1.20(1.34), [0, 5] | 1.37(1.36), [0,5] | 0.15 |
| Duration since IBD | 9.00 (7.84), | 10.7(9.24), | 10.1 (8.73), | |
| diagnosis (years) | [0.28, 29.5] | [0.7, 39.0] | [0.28, 39.0] | |
| Alcohole | 0.18 | |||
| None or< 1 days/week | 7(32) | 17(45) | 24 (40) | − |
| 1 or 2 days/week | 13(59) | 13(34) | 26 (43) | − |
| ≥ 2 days/week | 2(9) | 8(21) | 10(17) | − |
| Time-varying characteristics, visits a | ||||
| Warm season of blood drawc | 60 (45) | 86 (45) | 146 (45) | 0.99 |
| Hour of the blood draw | 9.68(1.62), [7.25, 13.92] | 9.68(1.64), [7.17, 13.5] | 9.68(1.63), [7.17, 13.9] | 0.97 |
| Time of blood draw | 0.99 | |||
| 7 am to 10 am | 74 (55) | 106(55) | 180(55) | − |
| >10 am to 2 pm | 60 (45) | 87 (45) | 147 (45) | − |
Notes: B1HB2−arm, B1 represents background-exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background-exposure after crossback; included 26 men taking mesalamine that did not contain DBP at baseline; 16 men (62%) on Lialda®, 8 men (31%) on Pentasa®, one man (4%) on Apriso® and one man (4%) was not on mesalamine medication at the time of recruitment.
H1BH2−arm, H1 represents high-DBP exposure at baseline, B represents background-exposure after crossover and H2 represents high-DBP exposure after crossback; included 44 men taking mesalamine-containing DBP at baseline; 23 men (53%) on Asacol® and 21 men (48%) on Asacol®HD.
N (%) for categorical/binary variables and mean (SD), [Range] for continuous variables
P-values are based on Fisher exact test for categorical variables and Kruskal Wallis test for continuous variables.
Season of sample collection, Warm: April Through September
IBD score: included bowel frequency and urgency, presence of blood in the stool and general wellbeing. Mild IBD score: 5 or less on the simple clinical colitis activity index for UC and 4 or less on the Harvey-Bradshaw index for CD.
Abbreviations: MARS, Mesalamine And Reproductive health Study; BMI, body mass index; Kg, Kilogram; m, meter; SD, standard deviation; IBD, Inflammatory Bowel Disease; UC, ulcerative colitis; CD, Crohn’s disease; N, number of men; DBP, dibutyl phthalate; B1HB2, Background1−High-Background2 DBP exposure; H1BH2, High1−Background-High2 DBP exposure.
Table 2.
Hormone concentrations for 70 men (327 blood samples) in the MARS study
| Hormone Parameters | Arithmetic Mean | SD | Geometric means | Minimum | 5th Perc | 25th Perc | 50th Perc | 75th Perc | 95th Perc | Maximum | QR | Clinical normal range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TSH (mU/L) | 2.61 | 1.41 | 2.36 | 0.04 | 1.03 | 1.70 | 2.21 | 3.24 | 5.33 | 9.60 | 1.54 | 0.4–4.0 |
| FT4 (pmol/1) | 16.7 | 2.07 | 16.8 | 10.8 | 13.1 | 15.5 | 16.6 | 18.1 | 20.0 | 23.8 | 2.64 | 10.0–24.0 |
| FT3 (pmol/1) | 5.75 | 0.68 | 5.75 | 3.95 | 4.67 | 5.35 | 5.70 | 6.13 | 6.91 | 8.53 | 0.78 | 3.5–6.5 |
| T4 (nmol/1) | 105 | 16.2 | 105 | 62.8 | 79.4 | 94.5 | 104 | 116 | 132.8 | 162 | 21.6 | 58–161 |
| T3 (nmol/1) | 2.01 | 0.35 | 2.05 | 1.22 | 1.47 | 1.75 | 1.99 | 2.26 | 2.6 | 3.18 | 0.51 | 0.9–2.8 |
| T3/T4 Ratio, % | 1.92 | 0.28 | 1.95 | 1.37 | 1.56 | 1.73 | 1.87 | 2.06 | 2.44 | 2.90 | 0.33 | − |
| TPOAb (IU/ml) | 21.3 | 26.3 | 17.5 | 6.00 | 9.00 | 12.2 | 15.3 | 19.7 | 65.0 | 215.6 | 7.48 | ≤ 35 |
| TgAb (IU/ml) | 25.8 | 49.7 | 18.2 | 10.0 | 10.2 | 13.1 | 16.8 | 22.2 | 36.0 | 506.3 | 9.18 | ≤ 115 |
Abbreviations: MARS, Mesalamine And Reproductive health Study; SD, standard deviation; Perc, percentile; QR, quartile range; T3, total triiodothyronine; T4, total thyroxine; FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; TPOAb, thyroid peroxidase antibody; TgAb, thyroglobulin antibody.
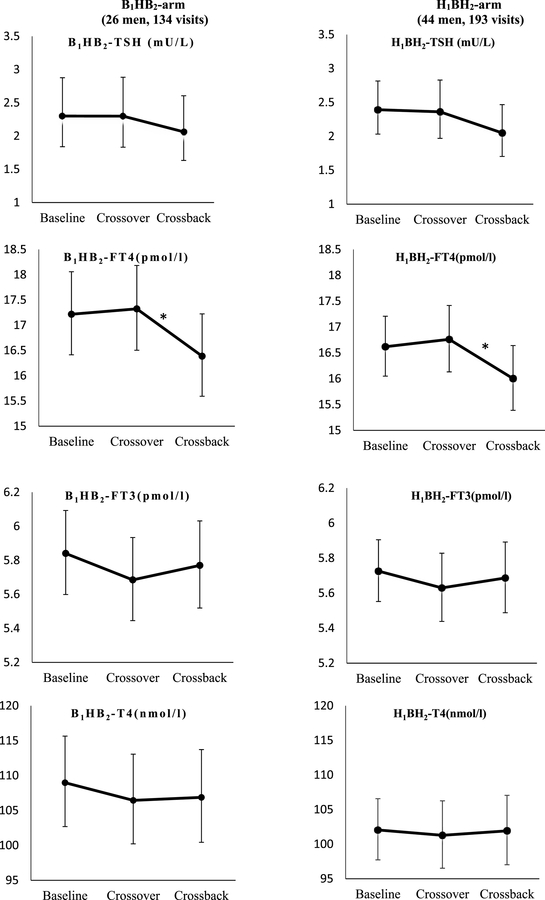
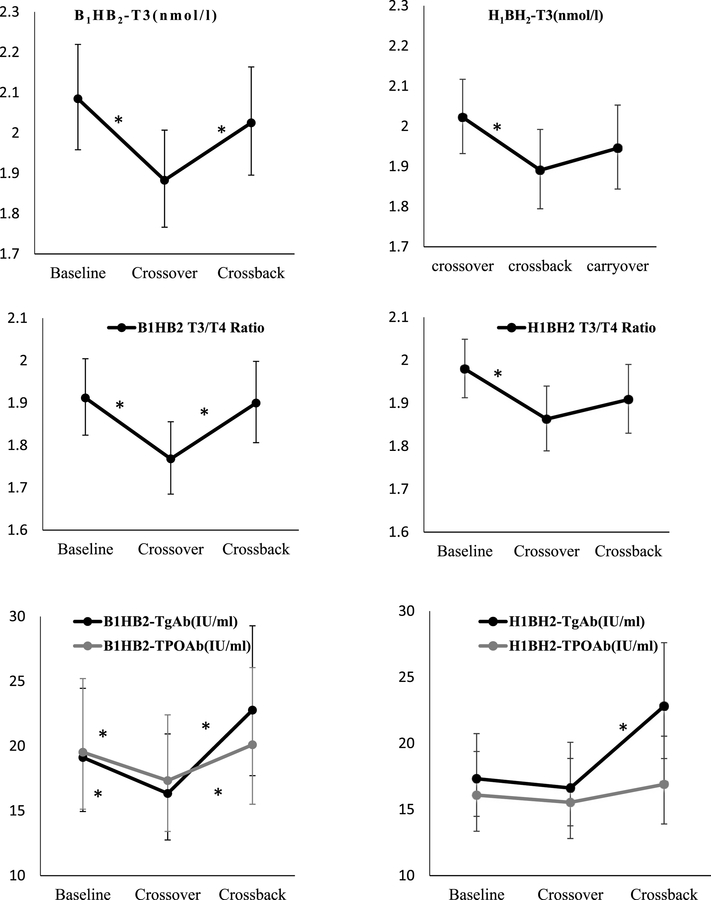
When the B1HB2−arm (26 men, 134 samples) (Table 3A and Figure 2) crossed-over, concentrations of T3 decreased 10% (95% confidence interval (CI):−14%,−5%), T3/T4 ratio decreased 8% (CI:−12%,−3%), TPOAb, and TgAb decreased 11% (CI: −20%, −2%) and 15% (CI: −23%, −5%), respectively. After crossback, T3 increased 8% (CI: 2%, 14%), T3/T4 ratio increased 7% (CI: 3%, 13%), and FT4 decreased 5% (CI: −8%, −2%). Both TPOAb, and TgAb increased 16% (CI: 4%, 29%) and 39% (CI: 24%, 57%) respectively. There was no significant change in T4, TSH, or FT3.
Table 3A.
Adjusted geometric means and percent changes (95%CI) of crossover and crossback on hormone concentrations among men starting on mesalamine medication not containing dibutyl phthalate (B1HB2−arm)
| Fitted Geometric Means (95%CI) | Comparisons | ||||||
|---|---|---|---|---|---|---|---|
| Hormone serum concentrations | Baseline (B1) | Crossover (H) | Crossback (B2) | Crossover effect (95%CI) H-B1 | P value | Crossback effect (95%CI) B2−H | P value |
| TSH(mU/L) | 2.30 (1.84, 2.88) | 2.30 (1.83, 2.88) | 2.06 (1.63, 2.61) | −0.07 (−14.3, 16.6) | 0.99 | −10.3 (−23.8, 5.68) | 0.19 |
| FT4(pmol/l) | 17.2 (16.4, 18.1) | 17.3 (16.5, 18.2) | 16.4 (15.6, 17.2) | 0.62 (−2.45, 3.78) | 0.70 | −5.40 (−8.45, −2.25) | 0.001 |
| FT3(pmol/l) | 5.84 (5.6, 6.09) | 5.68 (5.45, 5.93) | 5.77 (5.52, 6.03) | −2.67 (−5.66, 0.41) | 0.09 | 1.50 (−1.79, 4.91) | 0.37 |
| T4(nmol/l) | 109 (103, 116) | 106 (100, 113) | 107 (100, 114) | −2.33 (−6.23, 1.75) | 0.26 | 0.40 (−3.85, 4.84) | 0.86 |
| T3(nmol/l) | 2.08 (1.96, 2.22) | 1.88 (1.77, 2.01) | 2.03 (1.90, 2.16) | −9.69 (−14.2, −5.00) | <.0001 | 7.56 (1.93, 13.5) | 0.008 |
| T3/T4 Ratio | 1.91 (1.82, 2.00) | 1.77 (1.68, 1.86) | 1.90 (1.81, 2) | −7.52 (−11.5, −3.36) | 0.001 | 7.44 (2.54, 12.6) | 0.003 |
| TPOAb(IU/ml) | 19.5 (15.1, 25.2) | 17.3 (13.4, 22.4) | 20.1 (15.5, 26.1) | −11.2 (−19.6, −2.00) | 0.02 | 16.0 (4.42, 28.8) | 0.006 |
| TgAb(IU/ml) | 19.1 (14.9, 24.5) | 16.3 (12.8, 20.9) | 22.8 (17.7, 29.3) | −14.6 (.23.4, −4.61) | 0.005 | 39.4 (24.1, 56.6) | <.0001 |
Figure 2. Adjusted geometric means (95%CI) of thyroid hormone parameters at baseline, crossover and crossback for the 2 arms (B1HB2− arm, and H1BH2−arm).
a Adjusted for race (Caucasian or not), age (continuous), BMI (continuous), hour of sample draw (continuous), current smoking, and duration on DBP-containing mesalamine medication at baseline (in years) in the mixed effect model.
b Geometric means are presented for TSH, TPOAb, and TgAb.
c B1HB2−arm: B1 represents background low-DBP exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background low-exposure after crossback.
d H1BH2−arm: H1 represents high-DBP at baseline, B represents background-DBP exposure after crossover and H2 represents high-DBP after crossback.
* Indicates statistical significance of P-value< 0.05
Abbreviations: MARS, Mesalamine And Reproductive health Study; N, number of men; B1HB2, Background1−High-Background2 DBP exposure; H1BH2, High1−Background-High2 DBP exposure; 95% CI, 95% Confidence Interval; DBP, dibutyl phthalate; T3, Total Triiodothyronine; T4, Total thyroxine; FT3, Free Triiodothyronine; FT4.
When the men in the H1BH2−arm (44 men, 193 samples) (Table 3B and Figure 2) crossed-over, T3 decreased by 7% (CI: −11%, −2%) and T3/T4 ratio decreased by 6% (CI: −9%, −2%) while the changes in other thyroid parameters were not statistically significant. After crossback, FT4 decreased 5% (CI: −7%, −2%), TSH decreased 13.2% (CI: −25%, 0%) whereas TgAb increased 37% (CI: 24%, 52%).
Table 3B.
Adjusted geometric means and percent changes (95%CI) of crossover and crossback on hormone concentrations among men starting on mesalamine medication containing dibutyl phthalate (H1BH2−arm).
| Fitted Geometric Means (95%CI) | Comparisons | ||||||
|---|---|---|---|---|---|---|---|
| Baseline (H1) | Crossover (B) | Crossback (H2) | Crossover Effect (95%CI) B-H1 | P value | Crossback Effect (95%CI) H2−B | P value | |
| TSH(mU/L) | 2.39 (2.03, 2.82) | 2.36 (1.97, 2.83) | 2.05 (1.7, 2.47) | −1.31 (−13.7, 12.9) | 0.85 | −13.2 (−24.8, 0.26) | 0.05 |
| FT4(pmol/l) | 16.6 (16.0, 17.2) | 16.8 (16.1, 17.4) | 16.0 (15.4, 16.6) | 0.86 (−1.83, 3.63) | 0.53 | −4.53 (−7.25, −1.74) | 0.002 |
| FT3(pmol/l) | 5.73 (5.55, 5.91) | 5.63 (5.44, 5.83) | 5.69 (5.49, 5.89) | −1.67 (−4.30, 1.03) | 0.22 | 1.01 (−1.89, 3.99) | 0.50 |
| T4(nmol/1) | 102 (97.7, 107) | 101 (96.5, 106) | 102 (97.0, 107) | −0.76 (−4.23, 2.84) | 0.67 | 0.64 (−3.13, 4.55) | 0.74 |
| T3(nmol/1) | 2.02 (1.93, 2.12) | 1.89 (1.79, 1.99) | 1.95 (1.84, 2.05) | −6.51 (−10.5, −2.30) | 0.003 | 2.89 (−1.87, 7.88) | 0.24 |
| T3/T4 Ratio | 1.98 (1.91, 2.05) | 1.86 (1.79, 1.94) | 1.91 (1.83, 1.99) | −5.90 (−9.40, −2.26) | 0.002 | 2.44 (−1.70, 6.76) | 0.25 |
| TPOAb(IU/ml) | 16.1 (13.4, 19.4) | 15.5 (12.8, 18.9) | 16.9 (13.9, 20.5) | −3.42 (−11.5, 5.37) | 0.43 | 8.75 (−0.80, 19.2) | 0.07 |
| TgAb(IU/ml) | 17.3 (14.5, 20.7) | 16.6 (13.8, 20.1) | 22.8 (18.9, 27.6) | −4.06 (−12.9, 5.68) | 0.40 | 37.2 (23.9, 52.0) | <.0001 |
Notes:
aAdjusted for race (Caucasian or not), age (continuous), BMI (continuous), hour of sample draw (continuous), current smoking, and duration on DBP-containing mesalamine medication at baseline (in years) in the mixed effect model.
bPercentage changes are presented by exponentiating the beta coefficient. Negative sign means % decrease for the corresponding outcome compared to the measure in the previous period.
cB1HB2−arm: B1 represents background low-DBP exposure at baseline, H represents high-DBP exposure after crossover and B2 represents background low-exposure after crossback.
dH1BH2−arm: H1 represents high-DBP at baseline, B represents background-DBP exposure after crossover and H2 represents high-DBP after crossback.
Abbreviations: MARS, Mesalamine And Reproductive health Study; N, number of men; B1HB2, Background1-High-Background2 DBP exposure; H1BH2, High1−Background-High2 DBP exposure; 95% CI, 95% Confidence Interval; DBP, dibutyl phthalate; T3, Total Triiodothyronine; T4, Total thyroxine; FT3, Free Triiodothyronine; FT4, Free thyroxine; TSH, Thyroid-stimulating hormone; TPOAb, Thyroid peroxidase antibody; TgAb, Thyroglobulin antibody.
In the cross-sectional adjusted analysis at T0, on average men in B1HB2−arm had higher FT3, FT4, T3, and T4 and lower TSH concentrations compared to men in H1BH2−arm, but the difference was not statistically significant (Supplementary Table 1). The duration of high-DBP mesalamine intake at T0 modified the changes in T3, T3/T4 ratio and TgAb (p, interaction= 0.007, 0.006, 0.004) respectively (data not shown). For example, among men with a history of no high-DBP mesalamine at T0 (i.e., B1HB2−arm) or men with shorter duration of high-DBP mesalamine at T0 in the H1BH2−arm, high-DBP exposure was associated with lower T3, T3/T4 ratio and TgAb. This association was the opposite among the men in the H1BH2−arm with longer duration of high-DBP mesalamine at T0. The results were consistent in all sensitivity analyses (Supplementary Table 2 to 5) using the fixed effect models, robust empirical standard errors, after further adjusting for the antibodies concentrations one at a time and after excluding men with observation of high antibodies leaving 305 samples from 66 men (excluding two men from each arm).
Discussion
In the current study, men who were newly-exposed to high-DBP mesalamine in the B1HB2−arm exhibited a decrease in serum total T3, serum T3/T4 ratio, and both TPOAb and TgAb concentrations relative to T0. When these men crossed-back to non-DBP mesalamine, all of these measures returned toward their T0 concentrations while FT4 decreased. Because 80% of serum T3 is derived from peripheral T4 conversion to T3, these findings are consistent in part with the interpretation that DBP interferes with this conversion. Thus, DBP would decrease serum T3 and the T3/T4 ratio and would tend to increase serum T4 as a result, though increased T4 was not observed. Reducing DBP exposure among the newly-exposed men would be expected to reverse these trends. It is not immediately clear why antibodies for TPO and Tg decreased.
The decrease in T3 but not T4 in men newly exposed to high-DBP suggests that new high-DBP exposure could affect type 1 and/or type 2 deiodinase that is responsible for deiodination of T4 to T3. This may occur by reducing Dio1/Dio2 expression or by directly inhibiting Dio1/Dio2 activity. Another explanation could be that decrease in the T3/T4 ratio were mediated by changes in homeostasis of growth hormone and consequently insulin-like growth factor 1 (IGF-1). A decreased IGF-1 could lead to decrease in T4 to T3 conversion as hypothesized and observed before 40,41. Although we cannot confirm from this in our study, Boas et al., observed a negative association between concentrations of urinary MBP and serum IGF-1 and T3 in Danish children 11. Alternatively, DBP may alter thyroid function in subtle ways that we don’t currently understand.
Paradoxically, in men chronically exposed to high-DBP from mesalamine medications, removal of high-DBP mesalamine medication was also associated with a decrease in T3 and a decrease in the T3/T4 ratio while a subsequent restart of the high-DBP mesalamine was associated with a decrease in FT4 and an increase in TgAb concentrations. One potential interpretation is that long-term exposure to DBP could cause ‘adaptation’ in the thyroid system that may cause these paradoxical findings after removal of high-DBP exposure. This is supported by the observation of significant interactions of medication type (DBP exposure) and the duration on high-DBP mesalamine use at T0 on the response of T3, T3/T4 ratio and TgAb. For example, among men with history of no (B1HB2−arm) or shorter duration of high-DBP mesalamine at T0, high-DBP exposure was associated with decreased T3, T3/T4 ratio and TgAb concentrations. However, in the longer term exposed men in the H1BH2−arm, subsequent high-DBP exposure was associated with increased T3, T3/T4 ratio and TgAb concentrations. Overall, these results in both arms suggest that new high-DBP exposure or removal from chronic high-DBP exposure alters the thyroid system and thyroid autoimmunity, consistent with thyroid disruption. The seemingly similar changes in both arms may suggest that high-DBP exposure changes may have met the threshold for “change” in either direction.
In addition, in cross-sectional analysis, men exposed to high-DBP concentrations (H1BH2−arm) at T0 had lower T4, T3 and, FT4 concentrations and higher TSH concentrations compared to men not exposed to high-DBP concentrations (B1HB2−arm). It is important to note that the cross-sectional results may be due to chance sampling variability due to non-randomization at T0. Finally, although unlikely, we cannot exclude the possibility of a non-causal explanation for the similar trends in the two arms such as an unmeasured time-varying confounding or changes in health behaviors due to participation in the study.
Only a few studies have investigated the association of DBP exposure with thyroid hormones in adult men. These studies were limited by their cross-sectional study design 7,8,42 or by restricting to infertile/subfertile men 8, or Dirtu et al 43 who investigated the association in obese and control lean group. None of these studies measured thryoid antibodies. Consistent with our results for the newly exposed men, Park et al., 42 observed that urinary MnBP concentrations were significantly associated with lower T3 in adult Korean men. Janjua et al.,34 conducted the only human study with some similarity to our design, examining the association between dermal DBP-exposure and short term effects on thyroid hormones, concluding that their results were most likely due to chance. The dermal DBP-exposure was much lower compared to our study, and one week as washout period was likely too short compared to the four months in the current study. Clearly, more research is needed in the adult men.
Our study had some limitations. First, we did not randomize men to mesalamine formulations at T0. However, this should not influence the within person comparison of interest. Second, although our sample size was not very large due to the innovative study and length of participation, the unique design of MARS and the use of the men as their own control avoided the purely cross-sectional analysis that could be confounded by inter-individual variables. Third, we did not have data on the iodine status of our participants, which is an essential trace element for thyroid function 44. Although some studies observed correlations between urinary iodine and phthalate exposure, it is unclear whether an individual’s phthalate exposure directly influences iodine status (note: low iodine = elevated T3). In a study conducted among a representative sample of U.S. adults including men, iodine excretion had a negligible impact on the significant correlations observed between phthalate metabolites and thyroid hormone levels 45. In addition, iodine status is unlikely to be associated with the prescribed mesalamine medications and, most importantly, is expected to be constant within the same man. Therefore, iodine status is unlikely to confound our longitudinal results. Also, because mesalamine medications (with or without DBP coating) prescribed for treating IBD have the same active ingredient (mesalamine), confounding by indication was unlikely. Finally, there may be a concern of generalizing results from men with IBD to men in the general population due to the possible relation between IBD and thyroid disease 46. Results are still important at least for IBD patients as a growing disease especially in the developed world.
Our study had several strengths. First, the innovative prospective design is rarely conducted in environmental epidemiology studies. The design enabled us to compare the hormone concentrations within the same men during periods of high-DBP and background-DBP exposure while accounting by design for confounding by measured and unmeasured non-time-varying variables 18,31. Second, having two arms with different sequences of exposure and non-exposure allowed us to determine the reversibility of the changes after exposure removal and observe if the exposure history may change the hormonal response. Third, we had comprehensive measures for both free and total thyroid hormones, TSH and, in addition, thyroid antibodies. Finally, this is the first study to examine the thyroid hormone response to such high-DBP exposure (1,000 times higher than background) compared to environmental background exposure, and to examine the reversibility of the association when the source of high-DBP exposure was removed.
DBP and other phthalates are still used in the enteric coatings of some licensed and over-the-counter medications including specific mesalamine formulations in the US23,28,29, Canada19, United Kingdom47, Germany20 and China48. Mesalamine, the active ingredient in Asacol®, Asacol®HD, Pentasa®, Lialda®, Apriso®, and Delzicol®, is commonly prescribed for IBD patients. The enteric coating of Asacol®, and Asacol®HD contains DBP as an excipient 49,50, whereas it is not used in other mesalamine formulations 23. Although manufacturers are required to disclose the inactive ingredients in the drug product labeling, they are not required to do so when the composition has a patented delivery mechanism (U.S. Pharmacopeial Convention 2009). The FDA has information on the type and amount of phthalates used in specific approved drug products, however this information is generally not publically available 23.
There are no guidelines for use of mesalamine or any other medications that contain phthalate coatings in men. Recently, there was a consensus that women with IBD who are pregnant or trying to get pregnant should avoid high-DBP mesalamine 19. Our results are of a concern because thyroid function is critical for body functions, such as energy level, heart rate and fertility 51 and, on a population level, the observed changes would be important. Undiagnosed thyroid disease may put patients at risk for certain serious conditions, such as cardiovascular diseases, osteoporosis and infertility 51,52. Therefore, our results support the need to reconsider the use of DBP-containing mesalamine and other DBP-containing medications for men, as well as facilitated education and access to information for physicians and patients.
Conclusion
Some medications are considered a source of high-DBP exposure including mesalamine. These medications are prescribed with little attention given to the potential health effects from such high overlooked exposure source. Our study is the first to examine the association between high-DBP exposure from mesalamine and thyroid hormones using the crossover-crossback design. Our results suggest that high-DBP exposure for four months or removal from chronic high-DBP exposure for four months could alter elements of the thyroid system most probably due to alteration of the peripheral T4 conversion to T3 and thyroid autoimmunity, consistent with thyroid disruption. After four months of exposure removal, these trends were mostly reversed. More research is needed to determine the underlying mode of action of high-DBP and the effect of exposure duration on thyroid functions.
Supplementary Material
Acknowledgments
The authors gratefully thank the study participants, all members of the MARS team especially Ramace Dadd, Pat Morey, and Myra Keller and the clinical staff.
Support:
a pilot grant for this work from NIEHS center at the Harvard T. H. Chan School of Public Health and NIH: R01ES017285 & P30 ES000002.
Disclosure Statement
J.R.K., receives research support from Abbvie, Transparency Life Sciences and Takeda, consults for Abbvie, and is a founder of a company called ColonaryConcepts. A.C.M, has no conflict currently and had previously received prior research grants from Shire, Salix and Aptalis, manufacturers of mesalamine medications. F.L.N, T.I.M.K, B.A.C, N.E.S, S.A.K, M.E, E.J.H, J.B.F, R.A.DP, M.A.B, T.R.Z, and R.H. have nothing to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaker L, Ligthart S, Korevaar TI, et al. Thyroid function and risk of type 2 diabetes: a population-based prospective cohort study. BMC medicine. 2016;14(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaker L, Wolters FJ, Bos D, et al. Thyroid function and the risk of dementia: The Rotterdam Study. Neurology. 2016;87(16):1688–1695. [DOI] [PubMed] [Google Scholar]
- 3.Chaker L, Heeringa J, Dehghan A, et al. Normal Thyroid Function and the Risk of Atrial Fibrillation: the Rotterdam Study. The Journal of clinical endocrinology and metabolism. 2015;100(10):3718–3724. [DOI] [PubMed] [Google Scholar]
- 4.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Molecular and cellular endocrinology. 2012;355(2):240–248. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Zhao L, Wei L, Li L. DEHP reduces thyroid hormones via interacting with hormone synthesis-related proteins, deiodinases, transthyretin, receptors, and hepatic enzymes in rats. Environmental science and pollution research international. 2015;22(16):12711–12719. [DOI] [PubMed] [Google Scholar]
- 6.Zhai W, Huang Z, Chen L, Feng C, Li B, Li T. Thyroid endocrine disruption in zebrafish larvae after exposure to mono-(2-ethylhexyl) phthalate (MEHP). PloS one. 2014;9(3):e92465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environmental health perspectives. 2011;119(10):1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) Phthalate Metabolites May Alter Thyroid Hormone Levels in Men. Environmental health perspectives. 2007;115(7):1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environmental health perspectives. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang PC, Kuo PL, Guo YL, Liao PC, Lee CC. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Human reproduction (Oxford, England). 2007;22(10):2715–2722. [DOI] [PubMed] [Google Scholar]
- 11.Boas M, Frederiksen H, Feldt-Rasmussen U, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environmental health perspectives. 2010;118(10):1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung H, Hong Y, Lee D, Pang K, Kim Y. The association between some endocrine disruptors in human plasma and the occurrence of congenital hypothyroidism. Environmental toxicology and pharmacology. 2013;35(2):278–283. [DOI] [PubMed] [Google Scholar]
- 13.Brucker-Davis F, Ferrari P, Boda-Buccino M, et al. Cord Blood Thyroid Tests in Boys Born With and Without Cryptorchidism: Correlations with Birth Parameters and In Utero Xenobiotics Exposure. Thyroid. 2011;21(10):1133–1141. [DOI] [PubMed] [Google Scholar]
- 14.Tsai HJ, Wu CF, Tsai YC, et al. Intake of Phthalate-tainted Foods and Serum Thyroid Hormones in Taiwanese Children and Adolescents. Scientific reports. 2016;6:30589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang PC, Tsai CH, Liang WY, Li SS, Huang HB, Kuo PL. Early Phthalates Exposure in Pregnant Women Is Associated with Alteration of Thyroid Hormones. PloS one. 2016;11(7):e0159398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang HB, Chuang CJ, Su PH, et al. Prenatal and Childhood Exposure to Phthalate Diesters and Thyroid Function in a 9-Year Follow-up Birth Cohort Study: Taiwan Maternal and Infant Cohort Study. Epidemiology. 2017;28 Suppl 1:S10–s18. [DOI] [PubMed] [Google Scholar]
- 17.CDC. (Centers for Disease Control and Prevention).National Report on Human Exposure to Environmental Chemicals. 2018; https://www.cdc.gov/exposurereport/. Accessed July 2018.
- 18.Nassan FL, Coull BA, Skakkebaek NE, et al. A crossover-crossback prospective study of dibutylphthalate exposure from mesalamine medications and serum reproductive hormones in men. Environmental research. 2018;160:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen GC, Seow CH, Maxwell C, et al. The Toronto Consensus Statements for the Management of Inflammatory Bowel Disease in Pregnancy. Gastroenterology. 2016;150(3):734–757.e731. [DOI] [PubMed] [Google Scholar]
- 20.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Molecular nutrition & food research. 2011;55(1):7–31. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez-Diaz S, Mitchell AA, Kelley KE, Calafat AM, Hauser R. Medications as a potential source of exposure to phthalates in the U.S. population. Environmental health perspectives. 2009;117(2):185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seckin E, Fromme H, Volkel W. Determination of total and free mono-n-butyl phthalate in human urine samples after medication of a di-n-butyl phthalate containing capsule. Toxicology letters. 2009;188(1):33–37. [DOI] [PubMed] [Google Scholar]
- 23.Kelley KE, Hernandez-Diaz S, Chaplin EL, Hauser R, Mitchell AA. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environmental health perspectives. 2012;120(3):379–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA. (U.S. Food and Drug Administration) Limiting the use of certain phthalates as excipients in CDER-regulated products. 2012; http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm330792.htm. Accessed March 2016.
- 25.FDA. Summary Review for Regulatory Action, Division Director Review NDA 204412, Division of Gastroenterology and Inborn Errors Products, CENTER FOR DRUG EVALUATION AND RESEARCH 2012; http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/204412Orig1s000SumR.pdf Accessed January, 2016.
- 26.Gallinger ZR, Nguyen GC. Presence of phthalates in gastrointestinal medications: is there a hidden danger? World J Gastroenterol. 2013;19(41):7042–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan N, Abbas AM, Koleva YN, Bazzano LA. Long-term mesalamine maintenance in ulcerative colitis: which is more important? Adherence or daily dose. Inflamm Bowel Dis. 2013;19(6):1123–1129. [DOI] [PubMed] [Google Scholar]
- 28.Hauser R, Duty S, Godfrey-Bailey L, Calafat AM. Medications as a source of human exposure to phthalates. Environmental health perspectives. 2004;112(6):751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hait EJ, Calafat AM, Hauser R. Urinary phthalate metabolite concentrations among men with inflammatory bowel disease on mesalamine therapy. Endocr Disruptors (Austin). 2014;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. Centers for Disease Control and Prevention, Fourth National Report on Human Exposure to Environmental Chemicals,Updated Tables, February 2015 2015; http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf Accessed February 2016.
- 31.Nassan FL, Coull BA, Skakkebaek NE, et al. A crossover-crossback prospective study of dibutylphthalate exposure from mesalamine medications and semen quality in men with inflammatory bowel disease. Environment international. 2016;95:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heller CG, Clermont Y. Spermatogenesis in man: an estimate of its duration. Science (New York, NY). 1963;140(3563):184–186. [DOI] [PubMed] [Google Scholar]
- 33.Gifford AE, Berg AH, Lahiff C, Cheifetz AS, Horowitz G, Moss AC. A random urine test can identify patients at risk of mesalamine non-adherence: a prospective study. Am J Gastroenterol. 2013;108(2):249–255. [DOI] [PubMed] [Google Scholar]
- 34.Janjua NR, Mortensen GK, Andersson AM, Kongshoj B, Skakkebaek NE, Wulf HC. Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environmental science & technology. 2007;41(15):5564–5570. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich JW, Landgrafe G, Fotiadou EH. TSH and Thyrotropic Agonists: Key Actors in Thyroid Homeostasis. Journal of thyroid research. 2012;2012:351864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johns LE, Ferguson KK, McElrath TF, Mukherjee B, Meeker JD. Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environmental health perspectives. 2016;124(11):1808–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jameson JL. Endocrinology : adult & pediatric. 7th edition. ed. Philadelphia, PA: Elsevier Saunders; 2016. [Google Scholar]
- 38.Mortoglou A, Candiloros H. The serum triiodothyronine to thyroxine (T3/T4) ratio in various thyroid disorders and after Levothyroxine replacement therapy. Hormones (Athens, Greece). 2004;3(2):120–126. [DOI] [PubMed] [Google Scholar]
- 39.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. 2nd ed 2011. [Google Scholar]
- 40.Huang HB, Pan WH, Chang JW, et al. Does exposure to phthalates influence thyroid function and growth hormone homeostasis? The Taiwan Environmental Survey for Toxicants (TEST) 2013. Environmental research. 2017;153:63–72. [DOI] [PubMed] [Google Scholar]
- 41.Jorgensen JO, Pedersen SA, Laurberg P, Weeke J, Skakkebaek NE, Christiansen JS. Effects of growth hormone therapy on thyroid function of growth hormone-deficient adults with and without concomitant thyroxine-substituted central hypothyroidism. The Journal of clinical endocrinology and metabolism. 1989;69(6):1127–1132. [DOI] [PubMed] [Google Scholar]
- 42.Park C, Choi W, Hwang M, et al. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population - Korean National Environmental Health Survey (KoNEHS) 2012–2014. The Science of the total environment. 2017;584–585:950–957. [DOI] [PubMed] [Google Scholar]
- 43.Dirtu AC, Geens T, Dirinck E, et al. Phthalate metabolites in obese individuals undergoing weight loss: Urinary levels and estimation of the phthalates daily intake. Environment international. 2013;59:344–353. [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann MB, Kohrle J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: biochemistry and relevance to public health. Thyroid. 2002;12(10):867–878. [DOI] [PubMed] [Google Scholar]
- 45.Mendez W, Jr., Eftim SE. Biomarkers of perchlorate exposure are correlated with circulating thyroid hormone levels in the 2007–2008 NHANES. Environmental research. 2012;118:137–144. [DOI] [PubMed] [Google Scholar]
- 46.Shizuma T Concomitant Thyroid Disorders and Inflammatory Bowel Disease: A Literature Review. BioMed research international. 2016;2016:5187061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jamieson L, McCully W. Review: UK medicines likely to be affected by the proposed European Medicines Agency’s guidelines on phthalates. BMC pharmacology & toxicology. 2015;16:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jia LL, Lou XY, Guo Y, Leung KS, Zeng EY. Occurrence of phthalate esters in over-the-counter medicines from China and its implications for human exposure. Environment international. 2016. [DOI] [PubMed] [Google Scholar]
- 49.FDA. ASACOL® (mesalamine) delayed-release tablets, for oral use - HIGHLIGHTS OF PRESCRIBING INFORMATION. 2015; http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm215476.htm, http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/019651s025lbl.pdf Accessed April, 2017.
- 50.FDA. Asacol® HD (mesalamine) delayed-release tablet for oral administration-HIGHLIGHTS OF PRESCRIBING INFORMATION. 2010; http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021830s005lbl.pdf Accessed April, 2017.
- 51.(ATA) ATA. Prevalence and Impact of Thyroid Disease. 2017; http://www.thyroid.org/media-main/about-hypothyroidism/. Accessed November, 2017.
- 52.Patel N, Kashanian JA. Thyroid Dysfunction and Male Reproductive Physiology. Seminars in reproductive medicine. 2016;34(6):356–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.