Abstract
A 1976 chemical factory explosion near Seveso, Italy exposed residents to high levels of 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD or dioxin). Dioxin is a known human carcinogen and potent endocrine disruptor. It is highly lipophilic and has a long half-life in humans. Much of what we know and can learn about the risks of dioxin exposure on human health arose from the tragic circumstances of Seveso. This review aims to describe the Seveso accident, summarize the results of 40 years of research on the health of the Seveso population since the accident, and discuss next-stage research on the health of Seveso residents, their children, and grandchildren.
Keywords: Dioxin, TCDD, Seveso, epidemiology, disaster
Introduction
In July 1976, a chemical plant explosion near Seveso, Italy exposed locals to the highest known levels of 2,3,7,8-tetracholorodibenzo-p-dioxin (TCDD or dioxin) exposure to a residential population (Mocarelli 2001; Pesatori and Bertazzi 2012). Dioxin has been classified as a known human carcinogen by the International Agency for Research on Cancer (IARC 1997). It is a potent endocrine disruptor, highly lipophilic, and extremely stable with a long half-life in humans of 7 to 9 years (IARC 1997; Needhamet al. 1999; Needhamet al. 1997). Because it is a combustion byproduct, it is a widespread environmental contaminant in industrialized areas and background exposures, while declining, are ubiquitous. Much of what we know and can learn about the risks of dioxin exposure on human health arose from the tragic circumstances of Seveso. The purpose of this paper is to describe the Seveso accident, to summarize the results of 40 years of research on the health of the Seveso population after the accident, and to discuss next-stage research on possible health effects among the residents, their children, and grandchildren.
The History of the Seveso Accident in 1976
On Saturday, 10 July 1976 at 12:37 PM, a chemical reactor exploded at the ICMESA plant located in Meda near Seveso, Italy (25 km north of Milan) (see previous detailed descriptions (Mocarelli 2001; Pocchiariet al. 1979)). The plant was manufacturing 2,4,5-trichlorophenol, an intermediary for cosmetics and pharmaceuticals. A runaway chemical reaction resulted in the release of an aerosol cloud that included sodium hydroxide, ethylene glycol, sodium trichlorophenate, and an estimated 15 to 30 kg of TCDD over an 18-km2 area (di Domenicoet al. 1980). Area residents in the path of the aerosol cloud developed nausea, headaches, eye irritation, and 19 children were admitted to the local hospitals with skin lesions (Signoriniet al. 2000). In the ensuing weeks, the area experienced high animal and plant mortality, and nearly 200 cases of chloracne were reported among residents, mostly among children (Assennatoet al. 1989).
On 25 July, a multipronged study was launched under the sponsorship of the Regione Lombardia Italy. The bioclinical investigations team from the Hospital of Desio collected blood specimens from thousands of residents for immediate clinical chemistry tests. The researchers stored remaining serum for future analyses. Individuals who provided blood samples were followed up regularly until 1982 and periodically thereafter. Health effects assessed included neurological problems, infection, dermatologic lesions, and pregnancy and child health (Mocarelliet al. 1986).
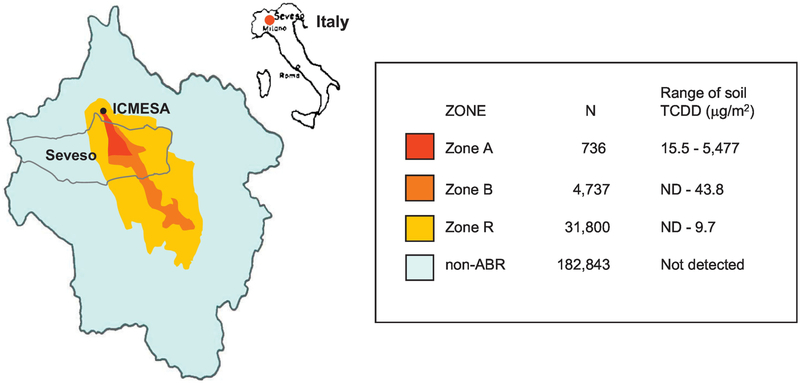
In the initial absence of individual biomarker-based exposure levels, health studies from Seveso relied upon zones of residence to classify levels of exposure. The zones of residence matrix was constructed based on systematic sampling and measurement of surface soil TCDD levels. The region with the highest surface soil TCDD levels (range: 15.5 – 5,477 μg/m2) was classified as zone A (Mocarelliet al. 1991b). The nearby area was divided into zone B, zone R, and zone non-ABR (soil TCDD not detected; considered to be unexposed) (see Figure 1) (Mocarelliet al. 1991a). Zone A housed 736 residents (212 families), all of whom were evacuated beginning two weeks (from July 26th to August 2nd) after the explosion; these residents underwent immediate medical examination and clinical laboratory tests. Those that lived in the most heavily exposed areas within zone A were not allowed to return to their homes, which were considered contaminated and later destroyed as part of the clean-up (Ghezziet al. 1982). Zone B, the area of next greatest contamination, housed almost 5,000 residents who were not evacuated but were warned against consuming locally-grown produce and poultry; these residents also received a medical examination and clinical laboratory tests. In addition, pregnant women and children less than 12 years from zone B were relocated out of the area on a daily basis. Zone R, the least contaminated area, housed about 32,000 residents who were warned not to consume local foods (Mocarelliet al. 1992).
Figure 1.
Map of Seveso, Italy indicating the location of Zones A, B, R, and Non-ABR, adapted from (Mocarelliet al. 1991a).
Although soil dioxin levels have been used as a proxy of exposure, these levels do not reflect variation in individual-level frequency and duration of contact with dioxin-contaminated media. Seveso residents are the only dioxin-exposed population for whom blood samples were collected near the time of exposure, enabling a more accurate exposure assessment of absorbed dose. In 1987, eleven years after the Seveso accident, the Centers for Disease Control and Prevention (CDC) developed an analytical method (using high-resolution gas chromatography/high-resolution mass spectrometry) to measure TCDD concentration in human serum (Pattersonet al. 1987). This new method and the nearly 30,000 stored samples at the University Laboratory of the Hospital of Desio opened the door for more precise measurements of individual exposure to be examined in relation to the health of the population.
Seveso Women’s Health Study (SWHS)
In 1996, 20 years after the explosion, a historical cohort study was initiated of the female residents (newborn to 40 years old in 1976) of zones A or B who had blood collected near the time of the explosion. The Seveso Women’s Health Study (SWHS), funded by the United States National Institutes of Health (NIH) and Regione Lombardia Italy, is the only comprehensive study to date of the health of a female population exposed to TCDD. It is unique in being a large cohort with a wide range of TCDD exposure documented by individual-level serum TCDD measurements. The methods for the SWHS have been summarized previously (Eskenaziet al. 2000
A total of 981 women participated in the first follow-up of the population (1996). The majority of women were from zone B (83%). At the time of the explosion, women in SWHS averaged 20.1 (±11.3) years of age, 71% were postmenarcheal, 38% were parous, and <1% were postmenopausal (see Table 1). The women were followed up again in 2008 (Warneret al. 2011) and 2014; participants did not differ from the full SWHS cohort with respect to characteristics at explosion (see Table 1). In 2014, the Seveso Second Generation Study was launched, with the goal of characterizing health impacts of in utero dioxin exposure in the children of SWHS participants. A total of 943 liveborn children (453 females, 490 males) who were born to 574 SWHS mothers after the explosion were enumerated and ranged in age from newborn to 39 years, A total of 611 children (66.4% of 920 alive and eligible) born to 402 mothers completed the study visit. The 611 child participants averaged 23.7 (range: 2–39) years of age and 51% were female.
Table 1.
Select characteristics of cohort participants by follow-up study date, Seveso Women’s Health Study, Seveso, Italy, 1976–2016
| SWHS 1996–1998 | Follow-up 2008–2009 | Follow-up 2014–2016 | |
|---|---|---|---|
| Characteristic | n (%) | n (%) | n (%) |
| Total | 981 (100) | 833 (84.9)a,b | 705 (71.9)a,c |
| Characteristics at explosion | |||
| Zone of residence | |||
| A | 167 (17.0) | 142 (17.0) | 123 (17.4) |
| B | 814 (83.0) | 691 (83.0) | 582 (82.6) |
| Age (years) | |||
| 0–10 | 232 (23.7) | 200 (24.0) | 181 (25.7) |
| 11–20 | 279 (28.4) | 252 (30.3) | 226 (32.1) |
| 21–30 | 241 (24.6) | 206 (24.7) | 175 (24.8) |
| 31–40 | 229 (23.3) | 175 (21.0) | 123 (17.4) |
| Menarche status | |||
| Premenarche | 284 (29.0) | 247 (29.7) | 221 (31.3) |
| Postmenarche | 697 (71.0) | 586 (70.3) | 484 (68.7) |
| Characteristics at interview | |||
| Age (years) | |||
| mean (SD) | 40.8 (11.7) | 52.2 (11.1) | 57.7 (11.0) |
| Range | 21 – 63 | 32 – 73 | 38 – 80 |
| Marital status | |||
| Never | 76 (7.7) | 47 (5.6) | 40 (5.7) |
| Ever | 905 (92.3) | 786 (94.4) | 665 (94.3) |
| Maternal education | |||
| ≤ Required | 651 (66.4) | 550 (66.0) | 382 (55.4) |
| Secondary school | 288 (29.4) | 249 (29.9) | 277 (40.2) |
| > Secondary school | 42 (4.3) | 34 (4.1) | 30 (5.4) |
| Menopause status | |||
| Premenopause | 703 (71.7) | 394 (47.3) | 223 (31.6) |
| Postmenopause | 278 (28.3) | 439 (52.7) | 482 (68.4) |
| Primary wage earner educationd | |||
| ≤ Required | 627 (63.9) | 529 (32.3) | 376 (54.7) |
| ≥ Secondary school | 354 (36.1) | 252 (32.1) | 311 (45.3) |
Percent of initial cohort (n=981)
16 (1.6%) deceased, 36 (3.7%) could not be located or contacted, 96 (9.8%) refused
33 (3.3%) deceased, 64 (6.5%) could not be located or contacted, 179 (18.3%) refused
Required (8 years of school), Secondary School (9–13 years), >Secondary School (>13 years)
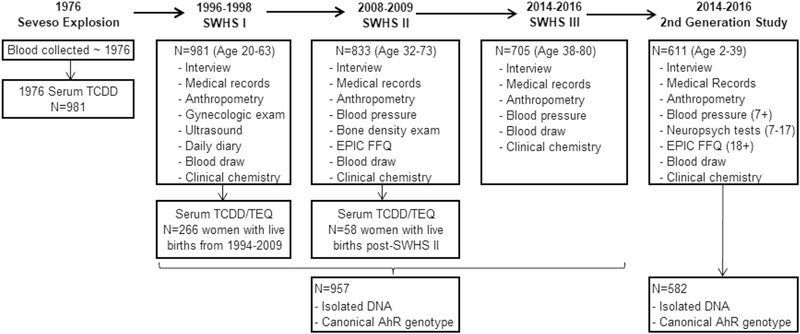
Details of data collected for the SWHS and the Second Generation cohorts at each followup study are summarized in Figure 2. For example, for the SWHS women the most recent data collection at the 2014 visit included a fasting blood draw for clinical chemistries and future studies, anthropometric and blood pressure measurements, a personal interview that included questions about her reproductive and medical history, and collection of medical records. For women with children less than 18 years, the interview also included questions about the health of their children as well as demographic and lifestyle factors and medical history. For children 2 to 6 years, participation included a fasting blood draw for clinical chemistries and future studies, and anthropometric measurements. Participation for children 7 to 17 years old included a fasting blood draw, anthropometric and blood pressure measurements, a battery of computerized neuropsychological tests (Rey’s Auditory Verbal Learning, Raven’s Progressive Matrices, Connor’s Continuous Performance, Wisconsin Card Sort) (Muriel Lezak 2012), and an online selfadministered questionnaire including smoking, alcohol, and caffeine habits (10 to 17 years only). Participation for children 18 years or older included fasting blood draw, anthropometric and blood pressure measurements, a personal interview that included the EPIC food frequency questionnaire (Pisaniet al. 1997), and collection of medical records.
Figure 2.
Summary of data collection at each phase of the Seveso Women’s Health and Second Generation Studies, Seveso, Italy 1976–2016
Below, we review results from multiple studies on the exposure characteristics and health of the Seveso population, including for residents who resided in the Seveso area at the time of the explosion (denoted as F0) and the next generation of children born post-explosion (denoted as F1).
Dioxin Exposure in Seveso Residents (F0)
In 1988, the CDC measured TCDD in 30 archived serum samples collected in 1976 from 10 zone A residents who developed chloracne and 10 residents who did not develop chloracne, and 10 residents of zone non-ABR (CDC 1988; Mocarelliet al. 1991b). Results of these initial serum measurements confirmed overt exposure to TCDD in zone A residents. The TCDD levels were of similar magnitude to those reported in 2,4,5-trichlorophenol production workers and some were among the highest ever reported (Pattersonet al. 1989). Although some of the highest serum TCDD levels occurred in residents who developed chloracne, no TCDD threshold level for chloracne was apparent. Individual susceptibility in addition to dose likely played a role in who developed chloracne.
CDC performed additional TCDD measurements in archived serum from male and female residents of zone A (n=296), zone B (n=80), zone R (n=48), and zone non-ABR (11 pools, 4–10 individuals/pool) (Needhamet al. 1999; Needhamet al. 1997). Although no details on sample selection were provided, results indicated residents of zone A had higher median TCDD levels than zone B, who had higher levels than zone R. Within zones, serum TCDD levels were higher among those who were younger (<13 years of age) at time of explosion but did not differ by sex. The non-ABR pools (median = 15 ppt) were similar to background dioxin levels in 1976. Finally, serial TCDD measurements in serum collected between 1976 and 1982 for a sample of zone B residents suggested there was no additional TCDD exposure as a consequence of living in zone B after the explosion. The higher serum dioxin levels in Seveso could have resulted from inhalation of airborne fallout, dermal contact, ingestion of contaminated foods, or possibly other dioxin exposure (Needhamet al. 1999).
Of the 981 women in the SWHS cohort, 167 were from zone A and 814 were from zone B. Although blood had been collected and archived from area residents soon after the explosion, the CDC had previously measured TCDD in only a few hundred samples. As part of the SWHS, hundreds more were measured, providing important insight into the exposure of the Seveso population. Details of the serum sample selection and TCDD concentrations measured in 1976 serum have been presented elsewhere (Eskenaziet al. 2004a; Eskenaziet al. 2000).
The median lipid-adjusted serum TCDD concentration in blood samples from 1976 for the SWHS cohort was 55 parts per trillion (ppt) and ranged from 2.5 to 56,000 ppt (Eskenaziet al. 2004b). Median serum TCDD levels were significantly higher among residents of zone A than zone B (272 ppt vs. 47 ppt), but there was wide variation in TCDD exposure within zones (zone A, 3.2 to 56,000 ppt; zone B, 2.5 to 3,140 ppt) and considerable overlap in TCDD levels between zones. Thus, these results suggested that using zone of residence as the primary measure of TCDD exposure information could readily lead to exposure misclassification. Age at the time of the explosion was the only other factor related to initial serum TCDD levels. Those less than 10 years of age had higher serum levels.
In addition to determining the background levels of TCDD (as previously presented in (Needhamet al. 1999; Needhamet al. 1997), another objective was to determine the background exposure levels of other dioxins, furans, and dioxin-like polychlorinated biphenyls (PCB) unrelated to the accident. Serum samples collected in 1976 from female residents of the non-ABR zone were pooled (9 pools, 20–21 females/pool). The TCDD level in serum pools averaged 20.2 ppt, and the levels of other dioxin-like compounds (dioxins, furans, polychlorinated biphenyls) averaged 80.2 ppt, resulting in an average total dioxin toxic equivalency (TEQ) (Van den Berget al. 1998), of 100.4 ppt (Eskenaziet al. 2004b). (N.B.: It was not possible to measure other dioxinlike compounds in the very small volumes of archived 1976 serum samples in the SWHS cohort.) Thus, although the Seveso explosion resulted in exposure specifically to TCDD, the results of pooled serum measurements revealed background-level exposure to other dioxin-like chemicals in this population, similar to background levels reported in other European countries during this period (Papkeet al. 1994).
Individual TCDD and total TEQ (Van den Berget al. 2006), were measured in archived 1996–98 serum (referred to as “1996” samples) for the subset of SWHS women (n=266) who reported a live birth between 1994 and 2009 (Warneret al. 2014). This subset was younger at exposure (mean = 10.2 ± 5.8 years) and had higher 1976 TCDD levels than the full SWHS cohort, though levels ranged widely. For this subset, 1996 serum TCDD and TEQ levels remained elevated (median = 7.3 and 25.7 ppt, respectively) relative to those measured in serum collected from unexposed female residents in 1998–2000 (median = 1.6 and 18.1 ppt, respectively) (Warneret al. 2005). The elevated total TEQ found among SWHS participants was due primarily to elevated TCDD. In the SWHS cohort, TEQ concentrations for other congeners were not elevated above background-exposed female residents in 1996. Thus, 20 years after the initial exposure, TCDD concentrations in this SWHS sample, the majority of whom were children in 1976, remained relatively elevated. Initial 1976 serum TCDD levels and age at explosion were the strongest predictors of 1996 serum TCDD. The apparent TCDD elimination half-life was 7.1 years for those >10 years old in 1976, but shorter in younger age groups (<5 years old = 4.3 years; 6–10 years old = 5.2 years). The difference in elimination by age may result from dilution due to child growth and increased body mass, as well as hepatic elimination at the higher exposure doses (Warneret al. 2014).
There is some evidence the half-life of TCDD may be longer in women than men. Needham et al. reported a longer TCDD half-life in women (~9 years) than men (~7 years) based on serum TCDD concentrations measured in serial samples collected between 1976 and 1993 for 27 Seveso residents (12M, 15F) (Needhamet al. 1999; Needhamet al. 1997). Also, TCDD levels were measured in serum collected almost twenty years after the accident for 62 Seveso zone B residents (Landiet al. 1998); geometric mean serum TCDD levels were significantly higher in women (17.6 ppt) than men (6.7 ppt), even after adjustment for additional covariates. Taken together with findings from the SWHS, there is evidence that the half-life of TCDD in Seveso may be age- and sex-related.
Based on clinical and animal studies suggesting that the elimination rate of TCDD is dosedependent (Michaleket al. 2002), Emond et al. developed a physiologically based pharmacokinetic (PBPK) model in rodents, which uses body-burden-dependent elimination rates and includes physiologic compartments for liver, adipose tissue, rest-of-the-body (maternal), and two pregnancy-related compartments, the placenta (maternal/fetal) and fetus (Emondet al. 2004). The model was optimized for humans using data from U.S Air Force veterans who participated in Operation Ranch Hand, and a single volunteer who ingested a dose of 1.14 ng TCDD/kg and was followed for 40 days (Emondet al. 2005). Emond et al. modified the PBPK model to include pregnancy and lactation using repeated TCDD serum measurements from the SWHS data. The modified PBPK model accurately predicted blood TCDD levels during the 20 years post-exposure, including for periods of pregnancy and lactation; in fact, findings from the model’s validation suggested that gestation and lactation do not significantly impact the maternal elimination rate (Emondet al. 2016).
Health Effects of Dioxin Exposure in Seveso Residents (F0)
Health of F0 as Children
Several studies have focused on the health of the exposed children (see Table 2 for summary).
Table 2.
Summary of health studies in Seveso residents.
| Outcome | Author, Year | Study Population |
Sample | Exposure Measure |
Key Findings |
|---|---|---|---|---|---|
| CHILD HEALTH | |||||
| Chloracne | Caramaschi, 1981 | Surveillance of children <14 years |
146 cases, zones A, B, R 182 controls, zone nonABR |
Zone | ↑ % chloracne in Zone A |
| Baccarelli, 2005 | Case-control study | 101 cases 211 controls |
Plasma TCDD (1993–98) |
↑ >10 ppt vs ≤ 10 ppt | |
| Liver Function | Mocarelli, 1986 | Surveillance 1976 – 1982 |
1,500 children 6- 10 years, from zones A, B, R |
Zone | ↑ GGT (76–77) in Zone A boys ↑ ALT (76–77) in Zone A boys ↑ AST (8O–82) in Zone A girls |
| Ideo, 1982 | Surveillance 08/76-12/76 |
31 children, from Zone A 6–8 years |
Chloracne status |
↑ d-glucaric acid levels in children with chloracne |
|
| Ideo, 1985 | Surveillance of children 05/1981 |
34 zone A 61 zone B 121 zone R 61 zone nonABR |
Zone | ↔ d-glucaric acid levels | |
| Immune function | Mocarelli, 1991a | Surveillance 1976 – 1982 |
692 zone A 3,703 zone B 6,974 zone R 5,743 nonABR |
Zone | ↑ lymphocytes (76–77) in Zone A children 0 – 14 y ↔ lymphocyte response to PHA in Zone A children 0–14 y |
| Tooth development | Alaluusua, 2004 | Dental exam 2001 |
48 zone A, B, R 65 nonABR |
1976 serum TCDD |
↑ developmental defects of enamel in children <5 years in 1976 |
| REPRODUCTIVE HEALTH | |||||
| Male fertility | Mocarelli, 2008 | Sperm quality study (1997–1998) |
135 men, zone A, B, R and 1–26 y in 1976 184 men nonABR |
1976 serum TCDD |
1–9 y in 1976 ↓ sperm concentration ↓ motile sperm ↓ estradiol ↑ FSH 10–17 y in 1976 ↑ sperm concentration ↑ motile sperm ↓ estradiol ↑ FSH ↔ 17 – 26 years in 1976 |
| Female fertility | Eskenazi, 2010 | SWHS (1996–1998) |
463 women | 1976 serum TCDD, TCDD estimated to pregnancy |
↑ time to pregnancy ↑ infertility |
| Menstrual cycle | Baccarelli, 2005 | Chloracne case-control study |
45 cases 103 controls |
Chloracne | ↔ irregular cycle in cases vs controls |
| Menstrual cycle | Eskenazi, 2002b | SWHS (1996–1998) |
301 women | 1976 serum TCDD |
↑ cycle length only in women who were premenarche in 1976 |
| Ovarian function | Warner, 2007 | SWHS (1996–1998) |
363 women | 1976 serum TCDD |
↔ number or size of follicles ↔ ovulation ↔ progesterone, estradiol |
| Endometriosis | Eskenazi, 2002b | SWHS (1996–1998) |
601 women | 1976 serum TCDD |
↑endometriosis |
| Fibromas | Eskenazi, 2007 | SWHS (1996–1998) |
956 women | 1976 serum TCDD |
↔fibroids |
| Menopause | Eskenazi, 2005 | SWHS (1996–1998) |
616 women | 1976 serum TCDD |
nonmonotonic ↑ early menopause 20.1–100 ppt early menopause >100 ppt |
| CHRONIC HEALTH | |||||
| Cancer | Consonni, 2008 | Cancer Mortality Study (1976–2001) |
804 zone A 5,941 zone B 38,623 zone R 232,740 nonABR Total person- years: 6,192,864 |
Zone | ↔All cancer mortality (76–01) ↑All cancer morality after 20 years in Zone A (96–01) ↑Lymphatic-hematopoietic cancer mortality in Zone A ↑Lymphatic-hematopoietic cancer mortality in Zone B ↔Lymphatic-hematopoietic cancer mortality in Zone R |
| Pesatori, 2009 | Cancer Incidence Study (1976–1996) |
723 zone A 4,821 zone B 31,643 zone R 181,574 nonABR |
Zone | ↔All cancer incidence (76–96) ↑Breast cancer in Zone A (ns) ↑Lymphatic-hematopoietic cancer in Zone A (ns) ↑Lymphatic-hematopoietic cancer in Zone B |
|
| Warner, 2002 | SWHS (1976–1996) |
981 women | 1976 serum TCDD |
↑Breast cancer incidence | |
| Warner, 2011 | SWHS (1976–2008) |
833 women | 1976 serum TCDD |
↑All cancer incidence | |
| Cardiovascular disease | Consonni, 2008 | Mortality Study (1976–2001) |
804 zone A 5,941 zone B 38,623 zone R 232,740 nonABR |
Zone | ↔All cause mortality ↑IHD mortality in Zone A males ↑Hypertension mortality in Zone A females |
| Warner, 2014 | SWHS (1976–2008) |
965 women | 1976 serum TCDD |
↑All CVD incidence (ns) ↑IHD incidence (ns) |
|
| Diabetes | Consonni, 2008 | Mortality Study (1976–2001) |
804 zone A 5,941 zone B 38,623 zone R 232,740 nonABR |
Zone | ↑Diabetes mortality in females in Zones A, B, R |
| Warner, 2013 | SWHS (1976–2008) |
981 women | 1976 serum TCDD |
↔Diabetes ↔BMI, Obesity ↑Metabolic syndrome in <12y in 1976 |
|
| Bone health | Eskenazi, 2014 | SWHS (2008–2009) |
340 women <20y in 1976 |
1976 serum TCDD |
↔BMD (spine, total hip, femoral neck) ↑Bone structure in women exposed before peak bone mass |
| Thyroid health | Chevrier, 2014 | SWHS (2008–2009) |
909 women | 1976 serum TCDD 1996 serum TCDD |
↓TT4 in 1996 ↔ TSH, FT3, FT4 in 1996 ↓TT4 in 2008 ↔ TSH, FT3, FT4 in 2008 |
| Immune function | Baccarelli, 2002 | Immunology study (1992–1994) |
62 zone A, B 58 zone nonABR |
1992 plasma TCDD |
→IgG w TCDD ↔IgM, IgA, C3, C4 w TCDD |
| Neurologic function | Pocchiari, 1979 | Surveillance 1977–1978 |
446 zone A, 255 zone B, R |
Zone | ↑ idiopathic clinical neurologic damage in Zone A |
| Assenato, 1989 | Surveillance 1985 |
193 chloracne cases |
↔Abnormal electrophysiologic or nerve conduction velocity measures |
||
| Ames, 2017a | SWHS (1996–1998) (2008–2009) |
154 women 459 women |
1976 serum TCDD |
↔Physical function↔Working memory | |
| SECOND GENERATION HEALTH | |||||
| Sex ratio | Mocarelli, 1996 | Municipality birth registry |
74 births (04/77 – 12/84) |
Zone A | ↓ Sex ratio (1977–84) ↔Sex ratio (1985–94) |
| Mocarelli, 2000 | Municipality birth registry |
674 births (04/77 – 12/96) |
1976 serum TCDD |
↓Sex ratio in fathers < 19 years in 1976 and TCDD >15 ppt ↔Sex ratio in fathers > 19 years in 1976 and TCDD >15 ppt |
|
| Neonatal thyroid | Baccarelli, 2008 | Neonatal Screening Registry (1994–2005) |
56 zone A 425 zone B 533 nonABR |
Zone | ↑b-TSH in Zone A ↑b-TSH in Zone B ↑b-TSH>5μU/mL in Zone A |
| Chloracne Study |
51 mother-child pairs |
TCDD extrapolated to pregnancy |
↑b-TSH w TCDD and TEQ extrapolated to pregnancy |
||
| Male fertility | Mocarelli, 2011 | Sperm quality study (2002–2003) |
39 Seveso sons born 1977–1984 58 unexposed male blood donors |
lactational exposure |
↓sperm concentration ↓progressive motility ↓FSH |
Chloracne.
While the exact number of identified chloracne cases has varied across reports (Assennatoet al. 1989; Caramaschiet al. 1981; Pocchiariet al. 1979), by most estimates nearly 200 cases were diagnosed among area residents, with the majority in children less than 15 years of age (Assennatoet al. 1989; Baccarelliet al. 2005; Caramaschiet al. 1981). The highest rate was found in children who resided in zone A, where 48.1% of children were diagnosed with chloracne. It is not clear whether the children were more susceptible to the chloracnegenic effects of TCDD or whether they had greater TCDD exposure (Sweeney and Mocarelli 2000). TCDD levels remained higher in 101 chloracne cases compared to 211 controls at the time of follow-up between 1993 and 1998, and chloracne risk was highest in those who were younger than 8 years at the time of the explosion and had TCDD concentrations at follow-up >10 ppt (OR = 7.4; 95% CI: 1.8, 30.3) (Baccarelliet al. 2005).
Liver function
In an early study of Seveso children with and without chloracne, 2.8% of children with chloracne had out-of-range serum γ-glutamyltransferase levels (a measure of detoxification activity), compared to none without chloracne (Caramaschiet al. 1981). Between July 1976 and June 1982, Mocarelli et al. performed 20 different clinical chemistry tests, including liver function and lipid metabolism in serial blood samples from 1,500 children who were 6 to 10 years of age at the time of the explosion and who resided in zones A, B or R (Mocarelliet al. 1986). Small but significant differences were found between zones A and B and zone R for serum γ-glutamyltransferase and alanine aminotransferase levels in boys between 1976 and 1977, but not in the following years. In girls, a difference in serum aspartate aminotransferase levels was found between 1980 and 1982. In 31 zone A children, 6 to 8 years old, children with chloracne had significantly elevated levels of urinary d-glucaric acid, a sensitive index for hepatic microsomal drug/xenobiotic-metabolizing enzyme induction, in samples collected between August and September 1976 (Ideoet al. 1982), By 1981, urinary d-glucaric acid levels for all children were in the normal range (Ideoet al. 1985).
Immune function.
Between 1976 and 1979, 48 zone A children and unexposed controls underwent repeated immunologic tests (Mocarelliet al. 1991a; Sirchia, 1982). Children from zone A exhibited higher values for lymphocyte responses to phytohemagglutinin (PHA) and to pokeweed mitogen (PWM), perhaps indicating dysregulated cellular immunity.
Tooth development.
In a sample of 48 children with a median serum TCDD of 476 ppt who were less than 9.5 years of age in 1976, high serum TCDD (>700 ppt) was related to developmental aberrations in the teeth, including hypoplastic enamel and missing lateral incisors (Alaluusuaet al. 2004).
Reproductive Health Outcomes in F0
Male fertility.
Twenty-two years after the explosion, Mocarelli et al. examined semen quality and reproductive hormone levels in 135 men who were 1 to 26 years of age in 1976 and lived in exposed zones at the time of the explosion as well as 184 unexposed controls (22–47 years at semen collection) (Mocarelliet al. 2008). Men who were youngest at the time of exposure (1 to 9 years old, median 1976 serum TCDD = 210 ppt) had significantly lower sperm concentration (mean = 53.6 ×106/mL vs. 72.5 ×106/mL; p = 0.03), percent progressive motility (mean = 33.2% vs. 40.8%; p < 0.001), and total motile sperm count (mean = 44.2 × 106 vs. 77.5 × 106; p = 0.02) than similar-aged unexposed men. In contrast, men who were older at the time of exposure (10 to 17 years, median 1976 serum TCDD = 164 ppt) had significantly higher total sperm count and total motile sperm count than the similar-age unexposed group; no significant differences were found in men who were over 17 years in 1976. In addition, men exposed at ages 1–17 years, but not in older age groups, had significantly lower serum estradiol levels and higher follicle stimulating hormone levels than unexposed men in similar age groups. These data suggest that men exposed to TCDD prior to puberty may have adverse effects on their semen quality while those exposed around or just after puberty may have better semen parameters.
Female fertility.
In the SWHS, Eskenazi et al. examined the relationship between TCDD exposure and time to pregnancy for the women’s first post-explosion conception resulting in a live birth (Eskenaziet al. 2010). TCDD exposure was examined as measured serum concentration circa 1976 and as estimated concentration at the time of “trying to become pregnant” extrapolated from 1976 concentrations. Fertility among women was reduced with increasing TCDD serum concentrations. A 10-fold increase in serum TCDD concentration in 1976 was associated with a 25% increase in time to pregnancy (adjusted fecundability OR = 0.75; 95% CI: 0.60, 0.95) and almost double the odds of infertility (≥12 months to become pregnant) (adjusted OR = 1.9; 95% CI: 1.1, 3.2). Similar results were found when TCDD serum concentrations were extrapolated to the time of pregnancy.
Menstrual cycle characteristics, age at menarche, and ovarian function.
In a follow-up case-control study of chloracne cases 20 years after the explosion, no significant difference in irregular menstrual cycle was noted, although the sample size was small (Baccarelliet al. 2005). Menstrual cycle characteristics were examined among the larger sample of pre-menopausal SWHS women (Eskenaziet al. 2002b). Stratification by menarcheal status in 1976 revealed that women who were premenarcheal at the time of the explosion experienced an almost 1 day increase in cycle length per 10-fold increase in 1976 TCDD concentrations (adjusted β = 0.93; 95% CI: –0.01, 1.86), while no such association was seen for women who were postmenarcheal when exposed (adjusted β = –0.03; 95% CI: -0.61, 0.54) (p-interaction = 0.08). Women who were premenarcheal at the time of the explosion also had reduced odds of scanty flow (adjusted OR = 0.33; 95% CI: 0.10, 1.06), whereas women who were postmenarcheal did not (adjusted OR = 1.36; 95% CI: 0.70, 2.64) (p-interaction = 0.03). There was no association with number of days of flow. In addition, TCDD serum levels were related to early menarche in those who were youngest at explosion, albeit nonsignificantly (Warner and Eskenazi 2005; Warneret al. 2004).
Although TCDD was associated with an elongation of the menstrual cycle in SWHS, there was no evidence that TCDD exposure was associated with ovarian dysfunction (Warneret al. 2007). TCDD was not associated with the number of follicles on ultrasound, odds of having any follicles > 10 mm, size of the dominant follicle, odds of ovulation, or estrogen or progesterone levels by TCDD (in women who had ovulated).
Endometriosis
In the SWHS, 601 women who were less than 30 years old at the time of explosion underwent a pelvic exam, transvaginal ultrasound and, if medically indicated, a laparoscopy. In addition, detailed information about pelvic pain, dysmenorrhea, and dyspareunia was collected. A total of 19 women (3.2%) were classified as cases (14 with surgically-confirmed diagnosis, and 5 diagnosed by ultrasound) (Eskenaziet al. 2002a). Compared to the lowest dose group (≤20 ppt), the relative risk ratio (RRR) of the moderate dose group (20.1–100 ppt) was 1.2 (90% CI: 0.3, 4.5) and the RRR of the highest dose group was 2.1 (90% CI: 0.5, 8.0) (p-trend = 0.25).
Fibromas.
At the 1996 SWHS follow-up, 251 (26.3%) had fibromas based on study ultrasound, medical record, or self-report (Eskenaziet al. 2007). The age-adjusted hazard ratio associated with a 10-fold increase in serum TCDD was 0.83 (95% CI: 0.65, 1.07), suggestive of an inverse association between the hazard of fibroid onset and increasing exposure. Age-adjusted hazard ratios for women with medium (20.1–75.0 ppt) and high (20.1–75.0 ppt) TCDD levels were 0.58 (95% CI: 0.41, 0.81) and 0.62 (95% CI: 0.44, 0.89), respectively, suggesting a lower hazard of having fibromas.
Age at onset of menopause.
In the SWHS, Eskenazi et al. examined the association between serum TCDD levels and age at menopause at the time of the 1996 follow-up. Of 616 eligible women who were 35 years or older at interview, 169 (27.4%) and 83 (13.5%) were in natural and surgical menopause, respectively (Eskenaziet al. 2005). In Cox proportional hazards modeling, risk of early menopause trended upward in the first four quintiles of TCDD, but not in the highest quintile. Compared with women in the lowest quintile (Q1), increased risk of early menopause was found for women in Q2 (adjusted hazard ratio (HR) = 1.1; 95% CI: 0.7, 1.8), Q3 (adjusted HR = 1.4; 95% CI: 0.9, 2.3), and Q4 (adjusted HR = 1.6; 95% CI: 0.9, 2.6), but not for women in Q5 (adjusted HR = 1.1 (95% CI: 0.6, 1.9). These results suggest a non-monotonic dose-related association between serum TCDD levels and earlier onset of natural menopause, up to a serum concentration of about 100 ppt TCDD, but not above.
Chronic Health Outcomes in F0
Cancer incidence and mortality.
Bertazzi and colleagues have published several papers examining cancer incidence and mortality in relation to zone of residence at the time of the accident (Bertazziet al. 1993; Bertazziet al. 2001; Bertazziet al. 1997; Bertazziet al. 1992; Bertazziet al. 1989; Consonniet al. 2008; Pesatoriet al. 2009; Pesatoriet al. 1993; Pesatoriet al. 1998). In the first 25 years following the explosion (1976–2001), overall cancer mortality was not elevated in any exposure zone. However, when the analysis was limited to deaths occurring 20 or more years after the explosion, cancer mortality was increased in zone A (n=18 deaths, rate ratio (RR) = 1.6; 95% CI: 1.0, 2.6) but not in zones B or R (Consonniet al. 2008). Increased mortality from lymphatichematopoietic cancers was noted in zones A (n=6 deaths, RR = 2.2; 95% CI: 1.0, 5.0) and B (n=28 deaths, RR = 1.6; 95% CI: 1.1, 2.3), with higher increases reported among females. In a recent update including deaths from 1976–2013, the authors reported that the observed increased mortality from lymphatic-hematopoietic cancers in zones A and B persisted, especially among women (Consonniet al. 2016; Consonniet al. 2008).
Though overall cancer incidence (as distinct from mortality) was not elevated in the first 20 years following the explosion (1976–1996), these researchers observed a non-significant increase in incidence of cancer at a wide range of cancer sites, including breast cancer (n=8 cases, RR = 1.4; 95% CI: 0.7, 2.9), among residents of zone A (Pesatoriet al. 2009). Consistent with the mortality studies, incidence of lymphatic-hematopoietic cancers was significantly increased in zone B (n=29 cases, RR = 1.6; 95% CI: 1.1, 2.3) and were also increased, albeit non-significantly, in zone A (n=4 cases, RR = 1.4; 95% CI: 0.5, 3.7).
In the SWHS, Warner et al. examined serum TCDD concentrations measured in the serum collected circa 1976 with overall cancer incidence and breast cancer incidence at 20 years postexplosion (Warneret al. 2002) and 32 years post-explosion (Warneret al. 2011). A significant increased risk for breast cancer incidence (HR = 2.1; 95% CI: 1.0, 4.8) per 10-fold increase in serum TCDD level was found at 20 years post-explosion and a significant, dose-related increased risk for all cancer incidence (HR = 1.8; 95% CI: 1.3, 2.5) was found at 32 years post-explosion. This relationship will be re-examined using the 2014–2016 data, when the majority of the cohort will have reached menopause and breast cancer incidence will likely have peaked.
Cardiovascular disease.
After 25 years (1976–2001), Consonni et al. (2008) reported increased mortality from ischemic heart disease (IHD) among male residents of zone A (n=7 deaths, RR = 2.5; 95% CI: 1.2, 5.2) and increased mortality from hypertension among female residents of zone A (n=4 deaths, RR = 2.6; 95% CI: 1.0, 6.9). Among residents of zone B, mortality from cerebrovascular disease was non-significantly increased in both females (n=50 deaths, RR = 1.2; 95% CI: 0.9, 1.6) and males (n=51 deaths, RR = 1.2; 95% CI: 0.9, 1.6). Among SWHS women, in 2008, a 10-fold increase in 1976 serum TCDD was non-significantly associated with increases in total cardiovascular disease (HR = 1.7; 95% CI: 0.9, 3.2) and IHD specifically (HR = 1.7; 95% CI: 0.8, 3.9) (Warneret al. 2014).
Diabetes, body mass index (BMI), and metabolic syndrome.
Using mortality data from the first 25 years of follow-up (1976–2001), Consonni and colleagues reported excess mortality from diabetes among female residents of all exposure zones, with more marked increases noted in zone B (n=20 deaths, RR = 1.78; 95% CI: 1.14, 2.77) than in zone A or zone R (Consonniet al. 2008). A recent update including deaths from 1976–2013 reported an increase in diabetes-related deaths, mainly among women, with an exposure zone-related gradient [zone A (n=5, RR = 2.1; 95% CI: 0.9, 5.0); zone B (n=24, RR = 1.7; 95% CI: 1.1, 2.5); zone R (n=161, RR = 1.4; 95% CI: 1.2, 1.6)] (Consonniet al. 2016).
In the SWHS, by the 2008 follow-up, 54 women (5.5%) were categorized as diabetic. Warner et al. observed a somewhat decreasing incidence of diabetes with increasing TCDD levels (adjusted HR = 0.8; 95% CI: 0.4, 1.3)(Warneret al. 2013). In line with these findings, a 10-fold increase in serum TCDD was associated with lower body mass index (adjusted β = -0.31; 95% CI: -0.88, 0.27) and a reduced odds ratio for obesity (adjusted OR = 0.8; 95% CI: 0.6, 1.1). However, an increase in metabolic syndrome was observed among women who were exposed early in life (newborn to 12 years of age) (adjusted OR = 2.0; 95% CI: 1.2, 3.3) but not older than age 12 (adjusted OR = 1.0; 95% CI: 0.7, 1.4). A similar difference was found for some but not all individual criteria for metabolic syndrome.
Bone health.
In 2008, 340 women from the SWHS who were <20 years old in 1976 underwent a dual-energy X-ray absorptiometry (DXA) bone scan (Eskenaziet al. 2014). Bone mineral density was measured at the lumbar spine and hip, and hip structural geometry was extracted from hip DXA scans using the Hip Structural Analysis method (Beck 2002). Eskenazi et al. observed no significant associations of TCDD levels with any measure of bone mass density (spine, total hip, femoral neck). However, among premenopausal women, serum TCDD levels were associated with better bone structure in those who were exposed before peak bone mass (considered at 2 years after menarche) (with somewhat stronger associations in those who were exposed before 5 years of age), and in postmenopausal women exposed after peak bone mass (sample sizes for this group however were small).
Thyroid health.
Levels of free and total thyroxine (T4), free triiodothyronine, and thyroidstimulating hormone (TSH) were measured in 909 SWHS women in 1996 and 724 SWHS women in 2008 (Chevrieret al. 2014). Chevrier et al. reported an inverse association of serum TCDD levels circa 1976 with total T4 levels in 1996, with strongest associations among women who were premenarcheal at exposure. By 2008, the association between TCDD levels circa 1976 and total T4, though still inverse, had weakened, and effect modification by menarche status at explosion was no longer evident. TCDD levels circa 1976 were not associated with levels of any other thyroid hormones, and 1996 serum TCDD levels were not associated with either 1996 or 2008 thyroid hormone levels.
Immune function.
A small study examined molecular markers of immune function and dioxin response using blood samples obtained in 1992 to 1994 from 62 randomly selected subjects from zones A and B and 58 from zone non-ABR (Baccarelli et al. 2004). Plasma TCDD measured nearly 20 years after the accident was negatively associated with plasma IgG levels, a marker of immune defense against microbial challenges, but not with other markers, including IgM, IgA, and complement component C3 and C4 levels (Baccarelli et al. 2002).
Neurologic function.
Initial neurologic screening of zone A residents in 1977 indicated signs of subclinical neurologic damage such as reduced nerve conduction velocity (Pocchiariet al. 1979). In addition, about 4% of 200 ICMESA plant workers were found to have damage to nerve fibers of multiple (unspecified) nerves (Pocchiariet al. 1979). Follow-up screening of the chloracne cases in 1985 found no increase in the prevalence of abnormal electrophysiological measures or conduction velocities (Assennatoet al. 1989).
In SWHS, in 1996, physical functioning was assessed in the oldest women (52 to 63 years of age, n=154). No associations were found between serum TCDD exposure and most measures of mobility and dexterity (walking speed, reach down test, coin flipping test); grip strength was the exception with an inverted U-shaped relationship with TCDD but this was examined in a smaller sample (n=98). In 2008, working memory was assessed by the Wechsler digit and spatial span tests in a randomly-selected subset of SWHS women (n=459). No associations were found between serum TCDD and any measure of working memory (Ameset al. 2017a).
Summary of adverse effects of TCDD exposure on F0.
Table 2 presents a summary of health studies in Seveso residents. Clearly, TCDD exposure resulted in chloracne, and children were most susceptible. The results of Seveso studies also suggest that early-life exposure to relatively high doses of TCDD may have effects on both male and female fertility. However, some of the most compelling evidence is that TCDD exposure increases risk for cancer, and within women, potentially breast cancer. There may be other individual cancers that are increased such as thyroid and blood cancers, but these results are limited by sample size or exposure assessment. TCDD seems to also affect cardiometabolic outcomes including metabolic syndrome, but results on diabetes are conflicting. Evidence for other endpoints examined are less compelling but follow-up may still be warranted of those who were youngest at the time of the accident as they are just beginning to enter middle age.
Dioxin Exposure in Children of Seveso Residents (F1)
As in research on the health of F0, TCDD exposure in F1 generation research also has been determined based on zones of residence and on TCDD concentrations in maternal samples collected circa 1976. In addition, for the Seveso Second Generation cohort, maternal TCDD and maternal total TEQ has been estimated at the time of pregnancy, which in many cases occurred years or decades post-explosion. Maternal TCDD at pregnancy was estimated by extrapolation from the nearest serum TCDD measure preceding the pregnancy (1976, 1996, 2008) using a firstorder kinetic model with a half-life that varied with initial dose, age, and other covariates, as described previously (Warner et al. 2014). For all mothers, the median time between the closest TCDD measure and pregnancy is 4.8 years (IQR: 2.2, 7.4 years), and 87% have a TCDD measurement within one TCDD half-life (9.0 years). Although 1976 specimen volumes were inadequate to assess total TEQ, 1996 and 2008 specimen volumes were adequate for total TEQ analysis; thus, we also examined total TEQ at pregnancy for the subset of births (n=275 livebirths, 343 total births) with 1996 or 2008 measurements. Analyses using maternal TCDD concentrations circa 1976 examine the hypothesis that the primary dose produces a permanent perturbation to the endocrine system or oocytes of exposed women, perhaps via epigenetic mechanisms, which could result in heritable or transmissible changes to offspring (Eskenaziet al. 2003). Analyses using either estimated TCDD or TEQ at time of pregnancy examine the hypothesis that the body burden during the pregnancy is the relevant dose.
Among the 943 liveborn children born to SWHS women between 1976 and 2016, the maternal TCDD level circa 1976 was high [median (IQR) = 60.2 (27.2, 153.0) ppt]. With birth years spanning from 1976 to 2016, estimated maternal TCDD at pregnancy was lower [median (IQR) = 11.9 (5.5, 30.5) ppt], but with a wide range (0.2, 5,730 ppt). For the subset of 275 live births with TEQ measurements, the median estimated maternal TEQ at pregnancy was 22.0 (IQR = 15.6, 29.6; range = 3.7, 634.1) ppt. All three of the second-generation dioxin exposure measures were significantly higher among children of mothers from zone A compared to children of mothers from zone B, but there was wide range of exposure within zones.
Health Effects of In Utero Dioxin Exposure (F1)
In the aftermath of the explosion, there was general concern among the population about the health of unborn children. Because of fear of teratogenicity of TCDD exposure, health authorities advised women to avoid conception (Mastroiacovoet al. 1988). Abortion was illegal at the time of the explosion and some unknown number of women chose to leave the country to abort (Mastroiacovoet al. 1988). Italy legalized abortion in May 1978 and women were able to receive one for psychological and/or social reasons (Tenchiniet al. 1983). Because of the unknown number of elected abortions, the risk for spontaneous abortion (SAB) and birth defects from TCDD exposure are potentially underestimated, since women living in highly exposed areas would presumably have been more likely to seek abortion.
Spontaneous abortion.
Early Seveso data, published prior to availability of individual TCDD blood levels, suggested a possible link between zone of residence and SAB (Fara and Del Corno 1985). Rates of SAB were elevated in zones A and B relative to zones R and non-ABR from the last quarter of 1976 through 1977, although variation in rates was hard to interpret due to the small number of pregnancies and that similar variations observed in years before 1977. A cytogenetic study of aborted fetuses from 19 women from the Seveso area and 16 women from outside the area, found a significant increase in the frequencies of aberrant cells and the average number of aberrations per damaged cell of exposed fetal tissues (Tenchini et al., 1983). Another study of placental morphology noted an unusual ultrastructural appearance in seven of 22 cases, but little information was provided about case selection (Remotti et al., 1981).
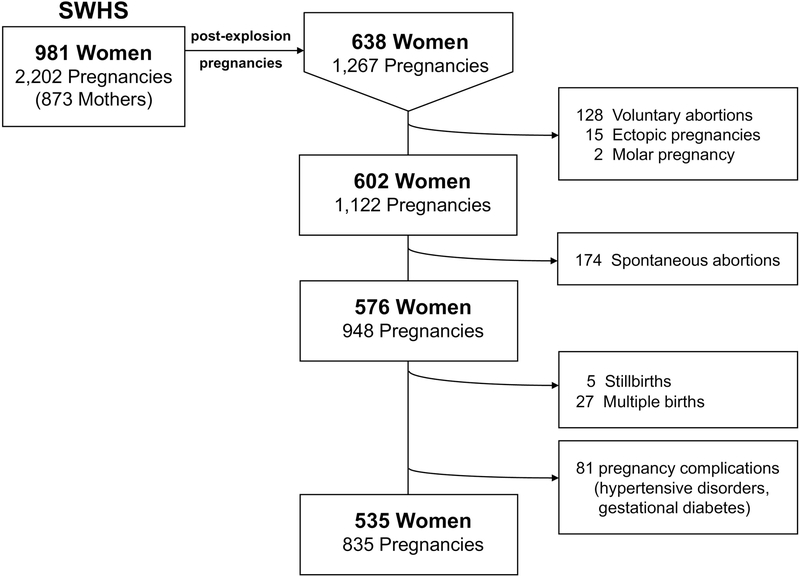
In the SWHS, however, maternal TCDD has not been associated with SAB (Eskenaziet al. 2003; Wesselinket al. 2014). Through the most recent follow-up (2014–2016), SWHS women reported 174 post-explosion SABs between July 10, 1976 and 2016 (see Figure 3 for postexplosion birth outcomes summary). As presented in Table 3, a 10-fold increase in maternal initial serum TCDD was not associated with SAB (adjusted Relative Risk = 0.85; 95% CI: 0.69, 1.05). Results were similar for maternal TCDD estimated at pregnancy (adjusted Relative Risk = 0.90; 95% CI: 0.72, 1.12). Results were also similar when the analysis was limited to first post-explosion pregnancies only.
Figure 3.
Post-explosion birth outcomes in the Seveso Women’s Health Study cohort, Seveso, Italy, 1976–2016
Table 3.
Results of relative risk regression modelsa showing the relationship between maternal TCDD exposure and spontaneous abortion, SWHS, Seveso, Italy, 1976–2016.
| Spontaneous abortion |
|||
|---|---|---|---|
| Exposure | Post-explosion pregnancies | n / Total d | Adj.RR (95% CI)b, c |
| 1976 Serum TCDD | All First |
174/ 1,122 79/ 573 |
0.85 (0.69, 1.05) 0.86 (0.63, 1.17) |
| Estimated TCDD at Pregnancy | All First |
174/ 1,122 79/ 573 |
0.90 (0.72, 1.12) 0.79 (0.56, 1.13) |
| Estimated TEQ at Pregnancy | All First |
69/ 344 21/ 118 |
1.27 (0.50, 3.23) 1.13 (0.32, 4.05) |
Results are for a 10-fold increase in serum TCDD or TEQ.
Adjusted for maternal height, pre-explosion history of spontaneous abortion, parity, and maternal age.
In TEQ model, pre-explosion history of spontaneous abortion and parity are omitted by collinearity.
Total excludes ectopic pregnancies (n=15), molar pregnancies (n=2), and voluntary abortions (n=128). However, first pregnancy is defined as the first pregnancy out of the 1,267 postexplosion pregnancies.
Sex ratio.
Mocarelli et al. examined the sex ratio of children born to zone A residents after the explosion (Mocarelliet al. 1996). Between April 1977 and December 1984, within about one TCDD half-life (~9 years), an excess of female births was reported (26 males versus 48 females, p<0.001). In the subset of 13 couples in which both parents resided in zone A, the nine couples with the highest serum TCDD levels all had daughters (sex ratio: observed 0.76 versus expected 0.514; p<0.001). There was no longer a significant difference in sex ratio for births in the ensuing decade (1985 to 1994). In a larger investigation, based on 674 children born between April 1977 and December 1996, no overall difference in observed versus expected sex ratio (0.487 versus 0.514) was found. However, a decrease in sex ratio (higher proportion of female births) was reported with increasing paternal serum TCDD measures, though only for fathers who were younger than 19 years at the time of explosion (sex ratio = 0.38) (Mocarelliet al. 2000). In contrast, an investigation of chloracne cases (n=101) and controls (n=211) reported more sons than daughters in chloracne cases for the period up to 20 years after the accident, although the sex ratio was not significantly different for cases and controls (Baccarelliet al. 2005).
Birth defects.
Early Seveso data suggested no association of zone of residence with birth defects (Fara and Del Corno 1985; Mastroiacovoet al. 1988). A congenital malformation registry was established in 1977. Between 1977 and 1983, no increased risk for all birth defects was observed in any of the exposed zones, although the number of births was small, limiting study power, and no infant was diagnosed with a major malformation in zone A (Mastroiacovoet al. 1988). SWHS research has likewise not produced evidence of any positive association of TCDD exposure and birth defects. As of the most recent follow-up (2014–2016), 52 children were born with any birth defect and 13 children were born with a major birth defect (hypospadias/epispadias (n=2), anencephaly (n=1), anomalies of the heart (n=5), anomalies of the vascular system (n=3), cleft palate/lip (n=2)). While the small number of cases limits statistical power and there is a lack of medical record confirmation, in unadjusted analyses, a 10-fold increase in maternal initial serum TCDD was not associated with having a child with a birth defect (Relative Risk = 1.18; 95% CI: 0.72, 1.91). Results were similar for maternal TCDD estimated at pregnancy (Relative Risk = 1.30; 95% CI: 0.84, 2.02).
Fetal Growth and Length of Gestation.
Among the 943 liveborn children born to 574 SWHS mothers between 1976 and 2016, the average birthweight was 3, 241 (±548) grams, with 7.4% of children born at low birthweight (<2,500 grams). Mean gestational age was 39.1 (±1.9) weeks with 7.5% of children born preterm (<37 weeks gestation). Table 4 presents descriptive characteristics, pregnancy-related factors and initial maternal serum TCDD levels for the 574 SWHS women and their 943 post-explosion livebirths.
Table 4.
Sociodemographic and pregnancy characteristics by initial maternal 1976 serum TCDD levels for 574 women with 943 post-explosion live births, SWHS, Seveso, Italy, 1976–2014.
| Characteristic | N (%) | Initial 1976 Maternal Serum TCDD (ppt) Median (IQR) |
|---|---|---|
| Total Women | 574 (100.0) | 61.2 (28.6, 162.0) |
| Zone of residence* | ||
| A | 100 (17.4%) | 264.5 (102.1, 919.0) |
| B | 474 (82.6%) | 49.7 (24.7, 111.0) |
| Age at explosion (years)* | ||
| 0–10 | 166 (28.9) | 157.5 (52.9, 322.0) |
| 11–20 | 241 (42.0) | 52.8 (25.4, 102) |
| 21–30 | 145 (25.3) | 40.6 (22, 77.0) |
| 31–40 | 22 (3.8) | 41.5 (27.7, 110) |
| Menarche status at explosion* | ||
| Premenarche | 213 (37.1) | 131.0 (50.6, 286.0) |
| Postmenarche | 361 (62.9) | 44.4 (22.5, 86.7) |
| Pre-explosion parity* | ||
| 0 | 458 (79.8) | 70.6 (31.4, 191.0) |
| 1 | 74 (12.9) | 36.6 (21.6, 70.4) |
| ≥2 | 42 (7.3) | 35.7 (25.1, 70.3) |
| Total livebirths | 943 (100.0) | 60.2 (27.2, 153.0) |
| Age at pregnancy (years)* | ||
| <25 | 157 (16.7) | 45.1 (21.3, 102.0) |
| 25–39 | 320 (33.9) | 54.7 (27.2, 125.0) |
| 30–34 | 281 (29.8) | 66.2 (27.7, 176.0) |
| ≥35 | 185 (19.6) | 64.1 (33.2, 180.0) |
| Smoking during pregnancy* | ||
| No | 850 (90.1) | 61.1 (28.6, 163.0) |
| Yes | 93 (9.9) | 45.4 (20.7, 98.3) |
| Weight gain during pregnancy (kg) a | ||
| <10 | 197 (20.9) | 67.5 (36.6, 141.0) |
| 10–14 | 437 (46.3) | 53.7 (26.3, 136.0) |
| 15–19 | 179 (19.0) | 62.8 (22.1, 223.0) |
| ≥20 | 103 (10.9) | 63.2 (28.8, 153.0) |
| Infant sex | ||
| Male | 490 (52.0) | 54.3 (26.7, 129.0) |
| Female | 453 (48.0) | 64.4 (28.8, 176.0) |
Anova p-value <0.05
Missing data (n=26)
Table 5 presents the relationship between TCDD exposure and length of gestation and fetal growth. Consistent with previous findings (Eskenaziet al. 2003; Wesselinket al. 2014), a 10-fold increase in maternal serum TCDD circa 1976 was not associated with gestational duration (adjusted β = -0.37; 95% CI: -1.84, 1.10) or preterm delivery (adjusted OR = 1.18; 95% CI: 0.71, 1.94), or with birthweight (adjusted β per 10-fold increase in TCDD = -38.2; 95% CI: -100.2, 23.7), small-for-gestational-age (adjusted OR = 1.33; 95% CI: 0.92, 1.94), or low birthweight (adjusted OR = 1.40; 95% CI: 0.88, 2.21). When estimated TCDD or TEQ at pregnancy was considered, results were similarly null. For fetal growth measures, limiting the analysis to term births also did not change the results (adjusted β = -37.8; 95% CI: -96.2, 21.0 for maternal TCDD circa 1976 and adjusted β = -5.1; 95% CI: -72.4, 62.1 for estimated TCDD at pregnancy). However, there was stronger evidence albeit non-significant of a negative association between maternal TCDD circa 1976 and infant birthweight when analyses were limited to first post-explosion pregnancy (adjusted β = -60.5; 95% CI: -130.4, 9.5).
Table 5.
Results of linear and logistic regression modelsa showing the relationship between maternal TCDD exposure and length of gestation and fetal growth, SWHS, Seveso, Italy, 1976–2014.
| Gestational age (weeks) |
Preterm |
Birthweight (grams) |
Small-for-Gestational-Age |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure | Pregnancies | N | Adj. β (95% CI)b | n/Total | Adj. OR (95% CI)b | n | Adj. β (95% CI) | n/Total | Adj. β (95% CI) |
| 1976 Serum | All | 834 | −0.37 (−1.84, 1.10) | 47/834 | 1.18 (0.71, 1.94) | 834 | −38.2 (−100.2, 23.7)c | 74/834 | 1.33 (0.92, 1.94)c |
| TCDD | First | 516 | −0.86 (−2.61, 0.90) | 32/516 | 1.17 (0.66, 2.08) | 516 | −60.5 (−130.4, 9.5)c | 52/516 | 1.39 (0.90, 2.15)c |
| TCDD at | All | 834 | 0.94 (−0.73, 2.60) | 47/834 | 0.68 (0.35, 1.36) | 834 | 28.4 (−41.7, 98.5)d | 74/834 | 1.21 (0.75, 1.94)d |
| Pregnancy | First | 516 | 0.44 (−1.42, 2.30) | 32/516 | 0.69 (0.33, 1.45) | 516 | −5.5 (−87.5, 76.6)d | 52/516 | 1.40 (0.82, 2.37)d |
| TEQ at | All | 228 | 9.6 (3.3, 15.8) | 17/228 | 0.09 (0.01, 1.06) | 228 | 235.1 (−17.6, 487.7)d | 15/228 | 1.53 (0.10, 22.85)d |
| Pregnancy | First | 113 | 8.5 (−1.8, 18.8) | 12/113 | 0.10 (0.01, 1.68) | 113 | 295.7 (−102.3, 693.8)d | 7/113 | 0.96 (0.01, 154.2)d |
Results are for a 10-fold increase in serum TCDD or TEQ.
Adjusted for maternal height, pre-explosion history of low birthweight, year of pregnancy, parity, and maternal age.
Adjusted for maternal height, pre-explosion history of low birthweight, year of pregnancy, parity, maternal age, maternal smoking.
Adjusted for maternal height, pre-explosion history of low birthweight, year of pregnancy, parity, maternal age, maternal smoking, gestational weight gain.
Given these borderline significant results that have been consistent at each point of followup, investigations are beginning to focus on whether there might be a particularly susceptible subpopulation within the larger cohort. Current research efforts include examining genetic differences in SNPs along the AhR pathway in the Seveso cohort to determine whether TCDD in a genetically-susceptible subpopulation could result in lowered birthweight (Ameset al. 2017b).
Neonatal thyroid hormone.
Since 1994, TSH has been routinely measured in newborn infant blood as part of the Italian Newborn Screening Program. In the Seveso population, Baccarelli et al. reported that children born between 1994 and 2005 to women from zones A (n = 56, geometric mean b-TSH = 1.66 μU/mL) and B (n = 425, geometric mean b-TSH = 1.35 μU/mL) had significantly higher geometric mean b-TSH levels at birth than the children of those living in zone non-ABR (n=533, geometric mean b-TSH = 0.98 μU/mL) (p-trend < 0.001) (Baccarelliet al. 2008). Similarly, the proportion of neonates with b-TSH levels > 5 μU/mL was higher among zone A (16.1%) and B (4.9%) mothers than non-ABR (2.8%) mothers. In addition, in a small study of 51 mother-child pairs with individual TCDD and TEQ levels measured in blood collected in 1994, a positive association between maternal plasma TCDD and TEQ extrapolated to time of pregnancy and neonatal TSH (β = 0.45, p<0.01) was observed. However, when women with serum TCDD greater than 50 ppt were excluded, the association between maternal TCDD levels and b-TSH was no longer significant (Baccarelliet al. 2008). Through data collected in the Second Generation Study, reevaluation of this association of maternal serum TCDD with neonatal TSH in a larger sample (over 250 births since 1994) is currently underway.
Male fertility
Mocarelli et al. (Mocarelliet al. 2011) measured sperm quality and hormone concentrations in 39 in utero exposed sons born between 1977 and 1984 to mothers who resided in Seveso at the time of the accident and 58 unexposed age-matched male blood donors whose mothers had not lived in the exposed area. In utero exposed and breast-fed sons (n=21) had lower sperm concentration (36.3 ×106/mL vs. 86.3 ×106/mL, p = 0.002), progressive motility (35.8% vs. 4.2%, p = 0.03), and total motile count (38.7 ×106 vs. 98 ×106, p = 0.01) than the unexposed comparison group (n=39). The in utero exposed group also had higher follicle stimulating hormone and lower inhibin B than the comparison group. The results support an effect of in utero exposure to dioxin on Sertoli Cell proliferation that could lead to permanently reduced sperm quality.
Looking to the Future
Some of the research on the health of the first generation of residents in Seveso (F0) suggests that those who were youngest at exposure may be more susceptible to the effects of TCDD. Evidence from animal studies concur that early exposure may pose greater effects, which may persist into adulthood. An association with adult-onset diseases, including polycystic ovarian disease, prostate disease, kidney disease (Manikkamet al. 2012b; Nilssonet al. 2012) and mammary cancer (Brownet al. 1998; Fentonet al. 2002) has been reported in rodents exposed to TCDD in utero. In addition, TCDD exposure has been shown in animal models to have multigenerational effects. Reduced sperm quality, fertility and increased incidence of premature births have been reported in three generations of offspring of mice exposed to a single dose of TCDD during pregnancy (Bruner-Tranet al. 2014; Bruner-Tran and Osteen 2011; Sanabriaet al. 2016). Other multigenerational effects include endometriosis, earlier onset of puberty, increased ovarian cysts, polycystic ovarian disease, and decreased pool size of ovarian primordial follicles in females; increased spermatogenic cell apoptosis and prostate disease in males; and kidney disease (Bruner-Tranet al. 2016; Manikkamet al. 2012a; Manikkamet al. 2012b; Nilssonet al. 2012). In some cases endpoints were noted in the unexposed third generation but not in the exposed first (Manikkamet al. 2012b). Such animal evidence of multi- and trans-generational effects are likely due to epigenetic changes (Bruner-Tran and Osteen 2011; Manikkamet al. 2012b) and demand examination in humans.
As we look forward, we will first examine many of the same outcomes in the children (F1) as we did in their SWHS mothers to determine whether in utero exposure may have resulted in the same or even more profound effects. Specifically, we will examine in the second generation whether in utero TCDD exposure is associated with obesity and metabolic syndrome, thyroid function, age at menarche and menstrual cycle characteristics, neuropsychologic function, and fertility. The children are still young, and thus few will have developed chronic diseases such as cancer, diabetes or cardiovascular disease. However, we feel this initial study lays the foundation for ongoing investigation of the long-term health of the Seveso second generation and generations beyond, including elucidation of genetic, epigenetic, and non-genetic mechanisms underlying associations between TCDD exposure and health. Although our research is focused on the Seveso women and their children, the Seveso males and their children should also be followed.
The SWHS provides the only opportunity to look at multigenerational effects of TCDD with biomarkers of exposure. Based on the results of animal studies, we will continue to follow not only the children but the grandchildren of the TCDD-exposed women. We have initiated a preliminary examination of the Seveso third generation (F2, i.e., the SWHS grandchildren). Of the 431 SWHS children (F1) who were 18 years or older, 76 daughters have reported 134 pregnancies and 45 sons have reported 76 pregnancies at interview (2014–2016) (see Table 6 for the outcomes of these pregnancies thus far).
Table 6.
Summary of pregnancy outcomes of SWHS daughters and sons, Seveso Second Generation Study, Seveso, Italy, 2014–2016.
| Daughters (n=76) n (%) |
Sons (n=45) n (%) |
|
|---|---|---|
| Total pregnancies | 134 (100.0) | 76 (100.0) |
| Currently pregnant | 0 (0.0) | 7 (9.2) |
| Live birtha | 96 (71.6) | 53 (69.7) |
| Spontaneous abortion | 32 (23.9) | 10 (13.2) |
| Voluntary abortion | 4 (3.0) | 3 (4.0) |
| Ectopic pregnancy | 2 (1.5) | 1 (1.3) |
| Stillbirth | 0 (0.0) | 1 (1.3) |
| Unknown | 0 (0.0) | 1 (1.3) |
| Live birtha | 96 | 53 |
| Birthweight (grams)b | 3,188 (±560) | --- |
| Preterm birth (<37 weeks)c | 9 (8.6) | 5 (9.4) |
| Low birthweight (<2,500 grams)c | 7 (6.8) | 7 (13.2) |
Live births include: 94 singletons and 2 twins (daughters); 49 singletons and 4 twins (sons).
mean (± standard deviation).
n (%) of live births.
Early molecular studies suggest that highly exposed Seveso residents may have altered AhR activity decades after the accident and that genetic variation in P450 enzymes could contribute to susceptibility to TCDD exposure (Landiet al. 2005; Landiet al. 2003). These variants and others in the AHR pathway have been linked to susceptibility to xenobiotics such as PAHs in other populations as well, though few have examined dioxin-like compounds (Brokken et al. 2014; Hung et al. 2013; Sasakiet al. 2006). Because AhR pathway genetics may shape susceptibility, we have begun to examine whether dioxin sensitivity differs by genetic subgroups in SWHS women and their children. As mentioned above, a gene-dioxin study of birthweight is underway with future analyses of additional health endpoints in both the SWHS and their children to follow. Such research may further our understanding of the biological underpinnings not only of TCDD but of other endocrine-disrupting compounds that activate the AhR pathway.
Lessons Learned
An environmental disaster, such as the one in Seveso, is clearly devastating to the local population and introduces potential long-term ecologic, economic, and health consequences both physical and psychological. Unfortunately, a number of environmental disasters, both man-made and natural, have followed the one in Seveso and others will undoubtedly occur in the future. The accident in Seveso provides an important example of the steps epidemiologists and related health professionals can take in the aftermath of a disaster to document its health effects. Epidemiologic research only became possible in Seveso because of the rapid establishment of a long-term health surveillance program of the population, which included - critically - collection and storage of biosamples from a large number of affected individuals despite the fact that methods to analyze exposure in those samples had not yet been developed. Other important steps included careful follow-up of individuals with acute responses to the exposure, in this case, children with chloracne, and development or augmentation of health registries, such as birth defect and cancer registries, to capture information on health effects that could present themselves years after initial exposure. The great degree to which our understanding of the human health effects of dioxin exposure has evolved since 1976 is directly attributable to the foresight of the health professionals who took these critical first steps in Seveso and to the dedicated participation of Seveso families in research studies over the last four decades.
Highlights.
A 1976 explosion in Seveso, Italy exposed residents to high levels of dioxin.
Many chloracne cases were diagnosed in Seveso residents, especially in children.
TCDD levels were associated with decreased male and female fertility in Seveso.
Increased risk for cancers and cardiometabolic outcomes has been noted.
Acknowledgments:
We gratefully acknowledge our collaborators at CDC including Donald G. Patterson, Jr., Wayman Turner, and Andreas Sjodin and the late Larry Needham and Vernon Houk, for their significant contributions to exposure assessment and sample analysis in the Seveso Women’s Health and Second Generation Studies, our field and laboratory staff at Hospital of Desio including study coordinators (Stefania Casalini, Aliza Parigi, Claudia Siracusa) who have contributed to the data collection, Natalie Slama, Lauren Hunter, and Sookee An for their assistance with the literature review, and Katherine Kogut for her editorial assistance. Lastly, we would like to thank our SWHS collaborators over the years: Steven Samuels, Paolo Vercellini, David Olive, Luigi Bonsignori, Pier Mario Gerthoux, Alessandro Rubinacci, Marcella Sirtori, Thomas Fuerst, Carlo Corbetta, Claude Emond, and Linda Birnbaum. We would especially like to acknowledge the families who were involved in the Seveso accident and who have participated over these last 40 years.
Funding: This study was supported by Grant Numbers F06 TW02075–01 from the National Institutes of Health, R01 ES07171 and 2P30-ESO01896–17 from the National Institute of Environmental Health Sciences, R82471 from the U.S. Environmental Protection Agency, and #2896 from Regione Lombardia and Fondazione Lombardia Ambiente, Milan, Italy.
Abbreviations:
- BMI
body mass index
- CDC
Centers for Disease Control and Prevention
- DXA
dual-energy X-ray absorptiometry
- EPIC
European Prospective Investigation into Cancer and Nutrition
- IARC
International Agency for Research on Cancer
- IHD
ischemic heart disease
- NIH
National Institutes of Health
- PBPK
physiologically-based pharmacokinetic
- PHA
phytohemagglutinin (PHA)
- PWM
pokeweed mitogen
- SWHS
Seveso Women’s Health Study
- SNP
single nucleotide polymorphism
- SAB
spontaneous abortion
- TCDD
2,3,7,8-tetracholorodibenzo-p-dioxin
- TSH
thyroid-stimulating hormone
- TT4
total thyroxine
- TEQ
toxic equivalent
Footnotes
Competing Financial Interest Statement: The authors declare they have no actual or potential competing financial interests.
This paper was based on a presentation at the 25 Years of Endocrine Disruption Research: Past Lessons and Future Directions meeting on September 18–20, 2016 as part of the National Institute of Environmental Health Sciences 50th year celebration.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaluusua S; Calderara P; Gerthoux PM; Lukinmaa PL; Kovero O; Needham L; Patterson DG Jr.; Tuomisto J; Mocarelli P Developmental dental aberrations after the dioxin accident in Seveso. Environ Health Perspect 2004;112:1313–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames J; Warner M; Brambilla P; Mocarelli P; Satariano WA; Eskenazi B Neurocognitive and physical functioning in the Seveso Women's Health Study. Environ Res 2017a;162:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames J; Warner M; Holland N; Mocarelli P; Brambilla P; Signorini S; Eskenazi B Gene-dioxin interactions and birthweight in the Seveso Second Generation Health Study. 29th Annual Conference of the International Society of Environmental Epidemiology (ISEE) Sydney, Australia; 2017b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assennato G; Cervino D; Emmett EA; Longo G; Merlo F Follow-up of subjects who developed chloracne following TCDD exposure at Seveso. Am J Ind Med 1989;16:119–125 [DOI] [PubMed] [Google Scholar]
- Baccarelli A; Giacomini SM; Corbetta C; Landi MT; Bonzini M; Consonni D; Grillo P; Patterson DG; Pesatori AC; Bertazzi PA Neonatal thyroid function in Seveso 25 years after maternal exposure to dioxin. PLoS medicine 2008;5:e161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A; Mocarelli P; Patterson DG Jr.; Bonzini M; Pesatori AC; Caporaso N; Landi MT Immunologic effects of dioxin: new results from Seveso and comparison with other studies. Environ Health Perspect 2002;110:1169–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A; Pesatori AC; Consonni D; Mocarelli P; Patterson DG Jr.; Caporaso NE; Bertazzi PA; Landi MT Health status and plasma dioxin levels in chloracne cases 20 years after the Seveso, Italy accident. Br J Dermatol 2005;152:459–465 [DOI] [PubMed] [Google Scholar]
- Baccarelli A; Pesatori AC; Masten SA; Patterson DG; Needham LL; Mocarelli P; Caporaso NE; Consonni D; Grassman JA; Bertazzi PA; Landi MT Aryl-hydrocarbon receptor-dependent pathway and toxic effects of TCDD in humans: a population-based study in Seveso, Italy. Toxicol Lett 2004;149:287–293 [DOI] [PubMed] [Google Scholar]
- Beck TJ Hip structural analysis (HSA) program. Baltimore, MD: Johns Hopkins School of Medicine; 2002 [Google Scholar]
- Bertazzi A; Pesatori AC; Consonni D; Tironi A; Landi MT; Zocchetti C Cancer incidence in a population accidentally exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. Epidemiology 1993;4:398–406 [DOI] [PubMed] [Google Scholar]
- Bertazzi PA; Consonni D; Bachetti S; Rubagotti M; Baccarelli A; Zocchetti C; Pesatori AC Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol 2001;153:1031–1044. [DOI] [PubMed] [Google Scholar]
- Bertazzi PA; Zocchetti C; Guercilena S; Consonni D; Tironi A; Landi MT; Pesatori AC Dioxin exposure and cancer risk: a 15-year mortality study after the "Seveso accident". Epidemiology 1997;8:646–652 [PubMed] [Google Scholar]
- Bertazzi PA; Zocchetti C; Pesatori AC; Guercilena S; Consonni D; Tironi A; Landi MT Mortality of a young population after accidental exposure to 2,3,7,8-tetrachlorodibenzodioxin. Int J Epidemiol 1992;21:118–123 [DOI] [PubMed] [Google Scholar]
- Bertazzi PA; Zocchetti C; Pesatori AC; Guercilena S; Sanarico M; Radice L Ten-year mortality study of the population involved in the Seveso incident in 1976. Am J Epidemiol 1989;129:1187–1200 [DOI] [PubMed] [Google Scholar]
- Brokken LJS; Lundberg PJ; Spanò M; Manicardi GC; Pedersen HS; Struciński P; Góralczyk K; Zviezdai V; Jönsson B.a.G.; Bonde JP; Toft G; Lundberg Giwercman Y; Giwercman A Interactions between polymorphisms in the aryl hydrocarbon receptor signalling pathway and exposure to persistent organochlorine pollutants affect human semen quality. Reprod Toxicol 2014;49C:65–73 [DOI] [PubMed] [Google Scholar]
- Brown NM; Manzolillo PA; Zhang JX; Wang J; Lamartiniere CA Prenatal TCDD and predisposition to mammary cancer in the rat. Carcinogenesis 1998;19:1623–1629 [DOI] [PubMed] [Google Scholar]
- Bruner-Tran KL; Ding T; Yeoman KB; Archibong A; Arosh JA; Osteen KG Developmental exposure of mice to dioxin promotes transgenerational testicular inflammation and an increased risk of preterm birth in unexposed mating partners. PLoS One 2014;9:e105084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL; Duleba AJ; Taylor HS; Osteen KG Developmental Toxicant Exposure Is Associated with Transgenerational Adenomyosis in a Murine Model. Biol Reprod 2016;95:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL; Osteen KG Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol 2011;31:344–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramaschi F; del Corno G; Favaretti C; Giambelluca SE; Montesarchio E; Fara GM Chloracne following environmental contamination by TCDD in Seveso, Italy. Int J Epidemiol 1981;10:135–143 [DOI] [PubMed] [Google Scholar]
- CDC. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans--Seveso, Italy. MMWR Morb Mortal Wkly Rep; 1988 [PubMed] [Google Scholar]
- Chevrier J; Warner M; Gunier RB; Brambilla P; Eskenazi B; Mocarelli P Serum dioxin concentrations and thyroid hormone levels in the Seveso Women's Health Study. Am J Epidemiol 2014;180:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consonni D; Pesatori A; Cavalieri D'Oro L; Rognoni MT; Bertazzi PA Cohort study of the population exposed to dioxin after the Seveso, Italy accident: mortality results, 1976–2013. ISEE; Rome, Italy; 2016 [Google Scholar]
- Consonni D; Pesatori AC; Zocchetti C; Sindaco R; D'Oro LC; Rubagotti M; Bertazzi PA Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-up. Am J Epidemiol 2008;167:847–858 [DOI] [PubMed] [Google Scholar]
- di Domenico A; Silano V; Viviano G; Zapponi G Accidental release of 2,3,7,8tetrachlorodibenzo-p-dioxin (TCDD) at Seveso, Italy. II. TCDD distribution in the soil surface layer. Ecotoxicol Environ Saf 1980;4:298–320 [DOI] [PubMed] [Google Scholar]
- Emond C; Birnbaum LS; DeVito MJ Physiologically based pharmacokinetic model for developmental exposures to TCDD in the rat. Toxicol Sci 2004;80:115–133 [DOI] [PubMed] [Google Scholar]
- Emond C; DeVito M; Warner M; Eskenazi B; Mocarelli P; Birnbaum LS An assessment of dioxin exposure across gestation and lactation using a PBPK model and new data from Seveso. Environ Int 2016;92–93:23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond C; Michalek JE; Birnbaum LS; DeVito MJ Comparison of the use of a physiologically based pharmacokinetic model and a classical pharmacokinetic model for dioxin exposure assessments. Environ Health Perspect 2005;113:1666–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Mocarelli P; Warner M; Chee WY; Gerthoux PM; Samuels S; Needham LL; Patterson DG Jr. Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ Health Perspect 2003;111:947–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Mocarelli P; Warner M; Needham L; Patterson DG; Samuels S; Turner W; Gerthoux PM; Brambilla P Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect 2004a;112:22–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Mocarelli P; Warner M; Samuels S; Vercellini P; Olive D; Needham L; Patterson D; Brambilla P Seveso Women's Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere 2000;40:1247–1253 [DOI] [PubMed] [Google Scholar]
- Eskenazi B; Mocarelli P; Warner M; Samuels S; Vercellini P; Olive D; Needham LL; Patterson DG Jr.; Brambilla P; Gavoni N; Casalini S; Panazza S; Turner W; Gerthoux PM Serum dioxin concentrations and endometriosis: a cohort study in Seveso, Italy. Environ Health Perspect 2002a;110:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Warner M; Marks A; Samuels S; Mocarelli P; Gerthoux PM; Needham L; Patterson DJ Serum dioxin levels and age at menopause in women of Seveso. Dioxin '04: 24th International Symposium on Halogenated Environmental Organic Pollutants and POPs Berlin; 2004b [Google Scholar]
- Eskenazi B; Warner M; Marks AR; Samuels S; Gerthoux PM; Vercellini P; Olive DL; Needham L; Patterson D Jr.; Mocarelli P Serum dioxin concentrations and age at menopause. Environ Health Perspect 2005;113:858–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Warner M; Marks AR; Samuels S; Needham L; Brambilla P; Mocarelli P Serum dioxin concentrations and time to pregnancy. Epidemiology 2010;21:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B; Warner M; Mocarelli P; Samuels S; Needham LL; Patterson DG Jr.; Lippman S; Vercellini P; Gerthoux PM; Brambilla P; Olive D Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol 2002b;156:383–392 [DOI] [PubMed] [Google Scholar]
- Eskenazi B; Warner M; Samuels S; Young J; Gerthoux PM; Needham L; Patterson D; Olive D; Gavoni N; Vercellini P; Mocarelli P Serum dioxin concentrations and risk of uterine leiomyoma in the Seveso Women's Health Study. Am J Epidemiol 2007;166:79–87 [DOI] [PubMed] [Google Scholar]
- Eskenazi B; Warner M; Sirtori M; Fuerst T; Rauch SA; Brambilla P; Mocarelli P; Rubinacci A Serum dioxin concentrations and bone density and structure in the Seveso Women's Health Study. Environ Health Perspect 2014;122:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fara GM; Del Corno G Pregnancy outcome in the Seveso area after TCDD contamination. Prog Clin Biol Res 1985;163B:279–285 [PubMed] [Google Scholar]
- Fenton SE; Hamm JT; Birnbaum LS; Youngblood GL Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol Sci 2002;67:63–74. [DOI] [PubMed] [Google Scholar]
- Ghezzi I; Cannatelli P; Assennato G; Merlo F; Mocarelli P; Brambilla P; Sicurello F Potential 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure of Seveso decontamination workers: a controlled prospective study. Scand J Work Environ Health 1982;8 Suppl 1:176–179 [PubMed] [Google Scholar]
- Hung W-T; Lambert GH; Huang P-W; Patterson DG; Guo YL Genetic susceptibility to dioxin-like chemicals' induction of cytochrome P4501A2 in the human adult linked to specific AhRR polymorphism. Chemosphere 2013;90:2358–2364 [DOI] [PubMed] [Google Scholar]
- IARC. Polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. IARC Monogr Eval Carcinog Risks Hum 1997;69:33–342 [PMC free article] [PubMed] [Google Scholar]
- Ideo G; Bellati G; Bellobuono A; Bissanti L Urinary D-glucaric acid excretion in the Seveso area, polluted by tetrachloro-dibenzo-p-dioxin (TCDD): five years of experience. Environ Health Perspect 1985;60:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideo G; Bellati G; Bellobuono A; Mocarelli P; Marocchi A; Brambilla P Increased urinary D-glucaric acid excretion by children living in an area polluted with tetrachlorodibenzoparadioxin (TCDD). Clin Chim Acta 1982;120:273–283 [DOI] [PubMed] [Google Scholar]
- Landi MT; Bergen AW; Baccarelli A; Patterson DG; Grassman J; Ter-Minassian M; Mocarelli P; Caporaso N; Masten SA; Pesatori AC; Pittman GS; Bell DA CYP1A1 and CYP1B1 genotypes, haplotypes, and TCDD-induced gene expression in subjects from Seveso, Italy. Toxicology 2005;207:191–202 [DOI] [PubMed] [Google Scholar]
- Landi MT; Bertazzi PA; Baccarelli A; Consonni D; Masten S; Lucier G; Mocarelli P; Needham L; Caporaso N; Grassman J TCDD-mediated alterations in the AhRdependent pathway in Seveso, Italy, 20 years after the accident. Carcinogenesis 2003;24:673–680 [DOI] [PubMed] [Google Scholar]
- Landi MT; Consonni D; Patterson DG Jr.; Needham LL; Lucier G; Brambilla P; Cazzaniga MA; Mocarelli P; Pesatori AC; Bertazzi PA; Caporaso NE 2,3,7,8-Tetrachlorodibenzo-p-dioxin plasma levels in Seveso 20 years after the accident. Environ Health Perspect 1998;106:273–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M; Guerrero-Bosagna C; Tracey R; Haque MM; Skinner MK Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One 2012a;7:e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M; Tracey R; Guerrero-Bosagna C; Skinner MK Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 2012b;7:e46249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroiacovo P; Spagnolo A; Marni E; Meazza L; Bertollini R; Segni G; Borgna-Pignatti C Birth defects in the Seveso area after TCDD contamination. JAMA 1988;259:1668–1672 [PubMed] [Google Scholar]
- Michalek J; Pirkle JL; Needham L; Patterson D; Caudill SP; Tripathi RC; Mocarelli P Pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin in Seveso adults and veterans of operation Ranch Hand. J Expo Anal Environ Epidemiol 2002;12:44–53 [DOI] [PubMed] [Google Scholar]
- Mocarelli P Seveso: a teaching story. Chemosphere 2001;43:391–402 [DOI] [PubMed] [Google Scholar]
- Mocarelli P; Brambilla P; Gerthoux PM; Patterson DG Jr.; Needham LL Change in sex ratio with exposure to dioxin. Lancet 1996;348:409. [DOI] [PubMed] [Google Scholar]
- Mocarelli P; Gerthoux PM; Ferrari E; Patterson DG Jr.; Kieszak SM; Brambilla P; Vincoli N; Signorini S; Tramacere P; Carreri V; Sampson EJ; Turner WE; Needham LL Paternal concentrations of dioxin and sex ratio of offspring. Lancet 2000;355:1858–1863 [DOI] [PubMed] [Google Scholar]
- Mocarelli P; Gerthoux PM; Needham LL; Patterson DG Jr.; Limonta G; Falbo R; Signorini S; Bertona M; Crespi C; Sarto C; Scott PK; Turner WE; Brambilla P Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect 2011;119:713–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P; Gerthoux PM; Patterson DG Jr.; Milani S; Limonta G; Bertona M; Signorini S; Tramacere P; Colombo L; Crespi C; Brambilla P; Sarto C; Carreri V; Sampson EJ; Turner WE; Needham LL Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect 2008;116:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P; Marocchi A; Brambilla P; Gerthoux P; Beretta C; Colombo L; Bertona M; Sarto C; Tramacere P; Mondonico A; Crespi C; Brivio R Human data derived from the Seveso accident- relevance for human risk assessment. Toxic Subst J 1992;12:51–73 [Google Scholar]
- Mocarelli P; Marocchi A; Brambilla P; Gerthoux P; Colombo L; Mondonico A; Meazza L Effects of dioxin exposure in humans at Seveso, Italy in: Gallo M, Scheuplein R, Van der Heijden K, eds. Biological Basis for Risk Assessment of Dioxins and Related Compounds - Banbury Report 35. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1991a [Google Scholar]
- Mocarelli P; Marocchi A; Brambilla P; Gerthoux P; Young DS; Mantel N Clinical laboratory manifestations of exposure to dioxin in children. A six-year study of the effects of an environmental disaster near Seveso, Italy. JAMA 1986;256:2687–2695 [PubMed] [Google Scholar]
- Mocarelli P; Needham LL; Marocchi A; Patterson DG Jr.; Brambilla P; Gerthoux PM; Meazza L; Carreri V Serum concentrations of 2,3,7,8-tetrachlorodibenzo-p-dioxin and test results from selected residents of Seveso, Italy. J Toxicol Environ Health 1991b;32:357–366. [DOI] [PubMed] [Google Scholar]
- Muriel Lezak DH, Bigler Erin, Tranel Daniel. Neuropsychological Assessment.Fifth ed^eds. New York, New York: Oxford University Press; 2012 [Google Scholar]
- Needham LL; Gerthoux PM; Patterson DG Jr.; Brambilla P; Smith SJ; Sampson EJ; Mocarelli P Exposure assessment: serum levels of TCDD in Seveso, Italy. Environ Res 1999;80:S200–S206 [DOI] [PubMed] [Google Scholar]
- Needham LL; Gerthoux PM; Patterson DG Jr.; Brambilla P; Turner WE; Beretta C; Pirkle JL; Colombo L; Sampson EJ; Tramacere PL; Signorini S; Meazza L; Carreri V; Jackson RJ; Mocarelli P Serum dioxin levels in Seveso, Italy, population in 1976. Teratog Carcinog Mutagen 1997;17:225–240 [PubMed] [Google Scholar]
- Nilsson E; Larsen G; Manikkam M; Guerrero-Bosagna C; Savenkova MI; Skinner MK Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One 2012;7:e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke O; Ball M; Lis A PCDD/PCDF in humans, a 1993-update of background data. Chemosphere 1994;29:2355–2360 [DOI] [PubMed] [Google Scholar]
- Patterson DG; Hampton L; Lapeza CR; Belser WT; Green V; Alexander L; Needham LL High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem 1987;59:2000–2005 [DOI] [PubMed] [Google Scholar]
- Patterson DG Jr.; Fingerhut MA; Roberts DW; Needham LL; Sweeney MH; Marlow DA; Andrews JS Jr.; Halperin WE Levels of polychlorinated dibenzo-p-dioxins and dibenzofurans in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Am J Ind Med 1989;16:135–146 [DOI] [PubMed] [Google Scholar]
- Pesatori AC; Bertazzi PA The Seveso Accident in: Schecter A, ed. Dioxins and Health: Including Other Persistent Organic Pollutants and Endocrine Disruptors. New Jersey: John Wiley & Sons, Inc.; 2012 [Google Scholar]
- Pesatori AC; Consonni D; Rubagotti M; Grillo P; Bertazzi PA Cancer incidence in the population exposed to dioxin after the "Seveso accident": twenty years of follow-up. Environ Health 2009;8:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesatori AC; Consonni D; Tironi A; Zocchetti C; Fini A; Bertazzi PA Cancer in a young population in a dioxin-contaminated area. Int J Epidemiol 1993;22:1010–1013 [DOI] [PubMed] [Google Scholar]
- Pesatori AC; Zocchetti C; Guercilena S; Consonni D; Turrini D; Bertazzi PA Dioxin exposure and non-malignant health effects: a mortality study. Occup Environ Med 1998;55:126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani P; Faggiano F; Krogh V; Palli D; Vineis P; Berrino F Relative validity and reproducibility of a food frequency dietary questionnaire for use in the Italian EPIC centres. Int J Epidemiol 1997;26 Suppl 1:S152–160 [DOI] [PubMed] [Google Scholar]
- Pocchiari F; Silano V; Zampieri A Human health effects from accidental release of tetrachlorodibenzo-p-dioxin (TCDD) at Seveso, Italy. Ann N Y Acad Sci 1979;320:311–320 [DOI] [PubMed] [Google Scholar]
- Sanabria M; Cucielo MS; Guerra MT; Dos Santos Borges C; Banzato TP; Perobelli JE; Leite GA; Anselmo-Franci JA; De Grava Kempinas W Sperm quality and fertility in rats after prenatal exposure to low doses of TCDD: A three-generation study. Reprod Toxicol 2016;65:29–38 [DOI] [PubMed] [Google Scholar]
- Sasaki S; Kondo T; Sata F; Saijo Y; Katoh S; Nakajima S; Ishizuka M; Fujita S; Kishi R Maternal smoking during pregnancy and genetic polymorphisms in the Ah receptor, CYP1A1 and GSTM1 affect infant birth size in Japanese subjects. Mol Hum Reprod 2006;12:77–83 [DOI] [PubMed] [Google Scholar]
- Signorini S; Gerthoux PM; Dassi C; Cazzaniga M; Brambilla P; Vincoli N; Mocarelli P Environmental exposure to dioxin: the Seveso experience. Andrologia 2000;32:263–270 [DOI] [PubMed] [Google Scholar]
- Sirchia CG and the Group for Immunological Monitoring. Exposure to TCDD: Immunologic effects. in: Dardanoni L and Miller RW, ed. Plans for Clinical and Epidemiological Follow-up After Area-Wide Chemical Contamination: Proceedings of an International Workshop Washington, DC: National Academy Press, National Academy of Sciences; 1982 [Google Scholar]
- Sweeney MH; Mocarelli P Human health effects after exposure to 2,3,7,8-TCDD. Food Addit Contam 2000;17:303–316. [DOI] [PubMed] [Google Scholar]
- Tenchini ML; Crimaudo C; Pacchetti G; Mottura A; Agosti S; De Carli L A comparative cytogenetic study on cases of induced abortions in TCDD-exposed and nonexposed women. Environ Mutagen 1983;5:73–85 [DOI] [PubMed] [Google Scholar]
- Van den Berg M; Birnbaum L; Bosveld AT; Brunstrom B; Cook P; Feeley M; Giesy JP; Hanberg A; Hasegawa R; Kennedy SW; Kubiak T; Larsen JC; van Leeuwen FX; Liem AK; Nolt C; Peterson RE; Poellinger L; Safe S; Schrenk D; Tillitt D; Tysklind M; Younes M; Waern F; Zacharewski T Toxic equivalency factors (TEFs) for PCBs, PCDDs, PCDFs for humans and wildlife. Environ Health Perspect 1998;106:775–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg M; Birnbaum LS; Denison M; De Vito M; Farland W; Feeley M; Fiedler H; Hakansson H; Hanberg A; Haws L; Rose M; Safe S; Schrenk D; Tohyama C; Tritscher A; Tuomisto J; Tysklind M; Walker N; Peterson RE The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci 2006;93:223–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Eskenazi B TCDD and Puberty: Warner and Eskenazi respond. Environ Health Perspect 2005;113:A18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Eskenazi B; Mocarelli P; Gerthoux PM; Samuels S; Needham L; Patterson D; Brambilla P Serum dioxin concentrations and breast cancer risk in the Seveso Women's Health Study. Environ Health Perspect 2002;110:625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Eskenazi B; Olive DL; Samuels S; Quick-Miles S; Vercellini P; Gerthoux PM; Needham L; Patterson DG; Mocarelli P Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environ Health Perspect 2007;115:336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Eskenazi B; Patterson DG; Clark G; Turner WE; Bonsignore L; Mocarelli P; Gerthoux PM Dioxin-Like TEQ of women from the Seveso, Italy area by IDHRGC/HRMS and CALUX. J Expo Anal Environ Epidemiol 2005;15:310–318 [DOI] [PubMed] [Google Scholar]
- Warner M; Mocarelli P; Brambilla P; Wesselink A; Patterson DG; Turner WE; Eskenazi B Serum TCDD and TEQ concentations among Seveso women, 20 years after the explosion. J Expo Sci Environ Epidemiol 2014;24:588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Mocarelli P; Brambilla P; Wesselink A; Samuels S; Signorini S; Eskenazi B Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso women's health study. Environ Health Perspect 2013;121:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Mocarelli P; Samuels S; Needham L; Brambilla P; Eskenazi B Dioxin exposure and cancer risk in the Seveso Women's Health Study. Environ Health Perspect 2011;119:1700–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M; Samuels S; Mocarelli P; Gerthoux PM; Needham L; Patterson DG Jr.; Eskenazi B Serum dioxin concentrations and age at menarche. Environ Health Perspect 2004;112:1289–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselink A; Warner M; Samuels S; Parigi A; Brambilla P; Mocarelli P; Eskenazi B Maternal dioxin exposure and pregnancy outcomes over 30 years of follow-up in Seveso. Environ Int 2014;63:143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]