Abstract
The role of dietary fiber in chronic inflammatory disorders has been explored, but very little is known about its benefits in acute inflammation. Previously, we have demonstrated that dietary cellulose supplementation confers protection in a murine model of sepsis by promoting the growth of the gut microbiota that are linked to metabolic health. The survival benefit is associated with a decrease in serum concentration of pro-inflammatory cytokines, reduced neutrophil infiltration in the lungs, and diminished hepatic inflammation. Here we aim to understand if the benefit of manipulating the gut microbiome exerts a broader “systemic” influence on the immune system in a lethal murine endotoxemia model. We hypothesize that mice fed high fiber cellulose (HF) diet will demonstrate a reduction in activated macrophages and dendritic cells (DCs) and a concomitant increase in the suppressive capacity of T regulatory cells (Tregs) toward T cells responsiveness. We characterized the immunological profile and activation status of macrophages, DCs and T cells in mice on HF diet who were then subjected to endotoxemia. Supplementation with HF diet decreased the number and activation of splenic macrophages and DCs in mice after LPS administration. Similarly, HF diet amplified the suppressive function of Tregs and induced anergy in T cells as compared to mice on a regular diet. Our data suggest that the use of HF diet can be a simple, yet effective tool that decreases the hepatic DNA binding activity of NF-κB leading to a reduction in pro-inflammatory cytokine response in a murine endotoxemia model.
INTRODUCTION
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection and accounted for more than $20 billion (5.2%) of total US hospital costs in 2011[1]. The pathogenesis of sepsis is usually classified as an initial pro-inflammatory phase followed by an anti-inflammatory or immunosuppressive phase [2]. The initial pro-inflammatory or “cytokine storm” phase is responsible for the recruitment of innate immune cells at the site of the infection, e.g., macrophages and DCs, that secrete pro-inflammatory cytokines and chemokines. Over several days, the hyper-inflammatory response dissipates, and transitions to an anti-inflammatory and immunosuppressive phase characterized by increased Tregs, IL-10 secretion, anergy in T cells with a shift to Th2 phenotype and reduced MHC-II expression by DCs [3]. Contrary to the previously held opinion that the hyper-inflammatory and immunosuppressive phases are sequential, recent evidence suggests that these phases are concurrent and start early in the course of sepsis [4]. Moreover, classifying cytokines strictly as “pro-inflammatory” and “anti-inflammatory” may be oversimplifying the complex and dynamic pathophysiology of sepsis.
Over the last few decades, several immunotherapeutic approaches designed to abrogate the uncontrolled pro-inflammatory response in the early phases of sepsis have failed. Multiple trials that targeted the cascade of pro-inflammatory cytokines, e.g., tumor necrosis factor α (TNF-α), interleukin (IL)-1, and HMGB1 have demonstrated limited clinical benefit.
The mechanistic underpinnings of the anti-inflammatory effects of fermentable fiber have been previously studied [5, 6], but very little is known about the protective anti-inflammatory effects exerted by cellulose, a nonfermentable fiber. Previously, we have demonstrated that dietary supplementation of cellulose confers protection in two different murine models of sepsis (cecal ligation and puncture (CLP) and endotoxemia), and this survival benefit was associated with a decrease in serum concentration of pro-inflammatory cytokines, reduced neutrophil infiltration in the lungs, and diminished hepatic inflammation. Specifically, we noted that 24 hours after CLP, plasma concentrations of the pro-inflammatory cytokines TNF-α, IL-1α, β and monocyte chemoattractant protein1 (MCP-1) were significantly lower in mice that were fed cellulose-rich (HF) diet as compared to a normal diet (BF) [7]. The benefit of dietary cellulose was associated with enrichment of the gut microbiome taxon Akkermansia, a genus commonly associated with positive metabolic health. A criticism of using the CLP model is that fecal spillage in HF diet mice will likely contain less virulent micro-organisms which could potentially explain the amelioration in peritonitis, systemic inflammatory response and improved survival. Due to this potential confounder, we have selected the murine endotoxemia model for further experiments in this area of study.
Recently, it has been proven that the impact of the intestinal microbiota is not restricted to the intestine, but extends to maintain immune homeostasis in the systemic environment. For example, Schuijt et al. demonstrated that the gut microbiota acts as a protective factor in the host defense and enhances macrophage function in distal organ sites, e.g., the lung in a murine pneumococcal pneumonia model [8]. To determine if the benefit conferred by HF diet supplementation extends to systemic immune cells, we used flow cytometric analyses of innate and adaptive immune cells to identify, enumerate and characterize these populations in a murine endotoxemia model. The purpose of our study was to determine the effects of HF diet on macrophages, DCs, T and Treg cells, all critical components of the immune system. We hypothesize that mice fed HF diet will demonstrate a reduction in activated macrophages and DCs and a concomitant increase in the suppressive capacity of Treg cells toward T cells responsiveness.
MATERIALS AND METHODS
Animals care and animal use
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and All animal experiments were performed with approval from the University of Pittsburgh IACUC, and care of the animals was in accordance with National Institutes of Health guidelines for animal treatment. Twenty-four hours after arrival, mice (6–8 wk old) were randomized to receive chemically defined BF or HF diets (Dyets, Bethlehem, PA) with fiber concentration of 5% and 30% respectively. The source of fiber in the mouse chow is alpha cellulose obtained directly from natural vegetable fibers. Apart from the difference in the fiber concentration, the diets were otherwise similar [7]. The mice weight as well as the amount of food eaten per cage and was comparable in both groups. After 2 weeks of the dietary intervention, sepsis was induced by intraperitoneal injection of endotoxin (LPS) (30 mg/kg) (Sigma-Aldrich, St. Louis, MO). There were no antibiotics administrated after endotoxin injection. Animals were removed from the study (and euthanized before the study time points of 24 and 72 h) if they showed signs of distress, e.g., unable or unwilling to walk around their cage. Additionally, the animals were monitored for hunched posture, reduced movement, loss of body weight, and piloerection. Mice were anesthetized with inhalational isoflurane to collect blood via terminal cardiac puncture at 24 and 72 h post endotoxin injection, and tissues were collected. Plasma and tissue samples were stored frozen at −80 °C until further analysis. Fecal samples during the dietary intervention and at sacrifice were collected for microbiome profiling. The number of mice used per experiment are specified in the figure legends.
Sequencing and analysis of bacterial 16S rRNA genes
Microbial DNA was extracted using MO BIO PowerSoil DNA Isolation kits using the manufacturer’s protocol with modifications. 16S rRNA amplicons were produced utilizing primers targeting the V4 region using 515F and 806R primers. Primers utilized either the Illumina adaptor, primer pad and linker (forward primer) or Illumina adaptor, Golay barcode, primer pad and linker (reverse primer) followed by a sequence targeting a conserved region of the bacterial 16S rRNA gene as described by Caporaso et al [9]. All PCR amplicons were purified, quantified, and pooled in equimolar ratios. Sequencing was performed on the Illumina MiSeq. QIIME (v1.9) was used to demultiplex raw sequence reads. UPARSE (v8.0) was used to quality filter the reads, cluster reads at a 0.97 operational taxonomic unit (OTU) threshold, and remove chimeric sequences (using the “gold database”). UCLUST was used to assign taxonomy to predicted OTUs, using the greengenes reference database (v13.8). Alpha and beta-diversity comparisons were performed using QIIME was and taxonomic summaries were generated with QIIME. Comparison of sample composition and identification of statistically significant differences was performed with LEfSe using correction for independent comparisons. Genera predicted to be biomarkers by LEfSe were excluded if their relative abundance was less than 0.01 on average in the sample set under consideration.
Multiplex analysis
Luminex-based multianalyte assays using commercially-purchased systems (Beadlyte Assay System, BioRad Biotechnology, Hercules, CA) were performed on plasma samples and culture supernatants (CD4 cells or MLR supernatants) to assess profile cytokine profile. All measurements were performed in duplicate.
Splenocyte preparation
Spleens were harvested from mice and transferred to a 50 ml conical tube containing PBS. Using sterile techniques, the mashed spleens were centrifuged at 1500 rpm for 5 minutes. The supernatant was discarded and the cell pellet resuspended in 5 ml of red blood cell lysis buffer (BD Biosciences, San Jose, CA). The cell suspension was then filtered, centrifuged, and the cell pellet resuspended in 10ml of media or buffer to be counted.
Flow cytometry analysis
Freshly isolated splenocytes were stained with the following antibodies: CD11b, CD11c, CD40, CD80, CD103, F4/80 for macrophages and DCs; B220, CD19, CD69, CD1d, CD5 for B cells; CD4, CD25, FOXP3 for T cells and Treg (eBioscience, San Diego, CA). Flow cytometry and flow-sorting were performed using a FACSCaria or a FACSVantage workstation (BD Biosciences, San Jose, CA) with non-overlapping fluorophores and appropriate isotype controls. At least 50,000 cells were counted for analysis. All experiments were performed in triplicate.
In vitro suppression assay
The conventional suppression assay was used herein [10] with the following modifications: Conventional Tregs (CD4+ CD25+) as well as CD4+ CD25− T cells were enriched from freshly-isolated splenocytes over commercial isolation columns (MACS column specific for CD4+ CD25+ T-cells; Miltenyi Biotec, Bergisch Gladbach, Germany). For the suppression assay, Tregs were added at increasing dilution ratios (1:1–1:16) to CD3/CD28-stimulated syngeneic freshly isolated splenic- 1 × 105 CD4+ CD25− T cells. CD3/CD28 stimulation was provided by the addition of Epoxy DynaBeads coated with anti-CD3 anti-CD28 monoclonal antibodies (Invitrogen, Carlsbad, CA). The incubation was carried out for 5 days in RPMI-1640 with 10% FBS. At the end of incubation, 1 mm of BrdU was added for the final 16 h to assess proliferation. Suppression was determined by the level of FITC fluorescence and percentage of cells fluorescent by FACS analysis using the FITC-BrdU flow cytometry kit (BD Biosciences, San Jose, CA). The experiment was performed in triplicate.
Protein preparation and Western blot
Protein extracts from the cytosol and nucleus were isolated using NE-PER Nuclear and Cytosolic Extraction Reagent (Thermo Fisher Scientific, Waltham, MA) at 24 and 72h. Protein concentration was determined by the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA). Nuclear (10μg), cytoplasmic (30μg) protein lysates were separated in 10% SDS-PAGE and transferred onto a PVDF membrane (BioRad Biotechnology, Hercules, CA). The membrane was then incubated with specific primary antibodies (NF-κB p65 (C20) and H3 (Santa Cruz Biotechnology, Dallas TX); β-actin, Sigma, St. Louis, MO) followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (HRP-conjugated goat anti-rabbit IgG, HRP-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Labs, West Grove, PA). Protein-antibody complexes were visualized by enhanced chemiluminescence with ECL system (GE Healthcare, Little Chalfont, UK).
NF-κB binding activity assay
The NF-κB binding activity assay was evaluated using a non-radioactive NF-κB p65 Transcription Factor Assay Kit (Abcam, Cambridge, UK) and the manufacturer’s instructions were followed. Nuclear extracts from BF and HF diet murine hepatic tissue were incubated in the presence of a specific double-stranded DNA sequence containing the NF-κB response element that was immobilized onto the bottom of a 96-well plate. The NF-κB (p65)-NF-κB response element complexes are detected by addition of a specific primary antibody directed against NF-κB (p65). A secondary antibody conjugated to HRP was added to provide a sensitive colorimetric readout at 450 nm. The experiment was performed in triplicate.
Statistical analyses
Statistical analyses were performed using GraphPad Prism version 4·0 software. Data are presented as mean ± standard error of the mean (SEM); * indicates the presence of a significant difference when P ≤ 0.05 between control and endotoxemic diet mice and # indicates P ≤ 0.05 between HF and BF-fed mice as determined by the Mann-Whitney rank-sum test or t-test.
RESULTS
Gut microbiome characterization and composition in mice fed with HF cellulose diet
Previously, we had demonstrated that there is a significant improvement in survival in HF diet-fed as compared to BF-fed mice during endotoxemia [7] and was associated with a sustained clear change in the gut microbiota. We noted significant differences in microbial community composition of the stool samples in HF diet group after 2 weeks of the prescribed diet regimen. Specifically, we found that samples collected after HF diet were highly enriched with bacteria from the family Lachnospiraceae, commonly found in the healthy colon, and Akkermansia, a bacterial genus with health-promoting properties (suppl. Fig. 1).
HF diet decreases serum pro-inflammatory cytokines and chemokines in endotoxemic mice
Using a commercially available cytokine assay, we analyzed the serum levels of the main pro- and anti-inflammatory cytokines, 24 and 72 h post LPS injection in BF and HF diet mice. At both time points, endotoxemic mice had significantly higher levels of five of the six measured cytokines as compared to controls; however, mice that were fed HF diet demonstrated a significant reduction in a majority of the pro-inflammatory cytokines in contrast to mice fed BF diet (Fig. 1). Thus, supplementation with HF diet results in a reduction in the serum pro-inflammatory cytokine response in a murine endotoxemia model.
FIGURE 1. Cellulose supplementation decrease severity of systemic inflammation after endotoxin injection.
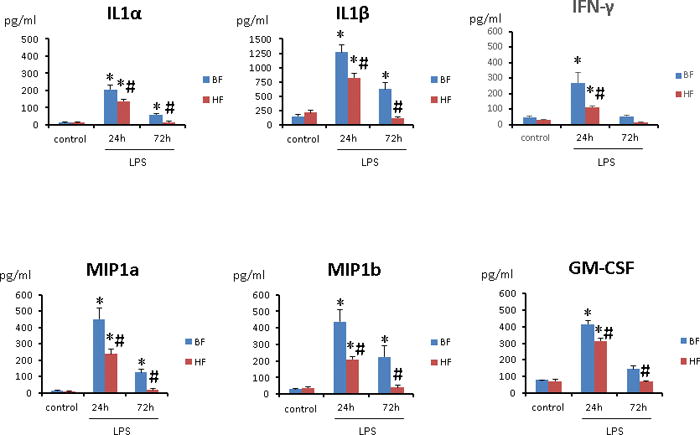
Multiplex analysis of cytokines and chemokines of serum from 8 survivor mice at 24 and 72 h post LPS injection was performed. Results are shown for mice fed BF and HF diets for two weeks prior to LPS injection. Each data point represents the mean ± S.E.M. of 8 mice for each group (*P ≤ 0.05 between control and endotoxemic diet mice; # P ≤ 0.05 between HF and BF-fed mice).
Decreased activation of macrophages and DCs in septic mice that were fed HF diet
To identify and characterize the macrophages and DCs in mice that were fed HF diet, we performed multi-color flow cytometry and sequential gating analysis. There was no difference in the mean percentage of macrophages, i.e., splenocytes that expressed CD11b in both groups of mice 24 h after endotoxin administration (14.2 ± 0.6% vs. 13.5 ± 0.5%; P>0.05) and were similar to control animals (Fig. 2A). Using gating strategy, we determined that markers of “classically activated” macrophages (CD40, CD80) were comparable in macrophages that were isolated from BF and HF diet mice (50 ± 2.4% vs. 48 ± 2.3%; P ≥ 0.05) as compared to control mice. However, 72 h after induction of endotoxemia, BF diet mice demonstrated a significantly higher number of macrophages along with a concomitant increase in the activation markers as compared to HF diet mice (P ≤ 0.05) (Fig. 2A). Also, expression of F4/80, a marker for mature macrophages, was significantly decreased in macrophages isolated from HF diet mice (4.5 ± 0.1% vs. 9.8 ± 1.2%; P ≤ 0.05) (Fig. 2A).
FIGURE 2. High fiber intake decreased the number and activation of splenic macrophages and DCs.
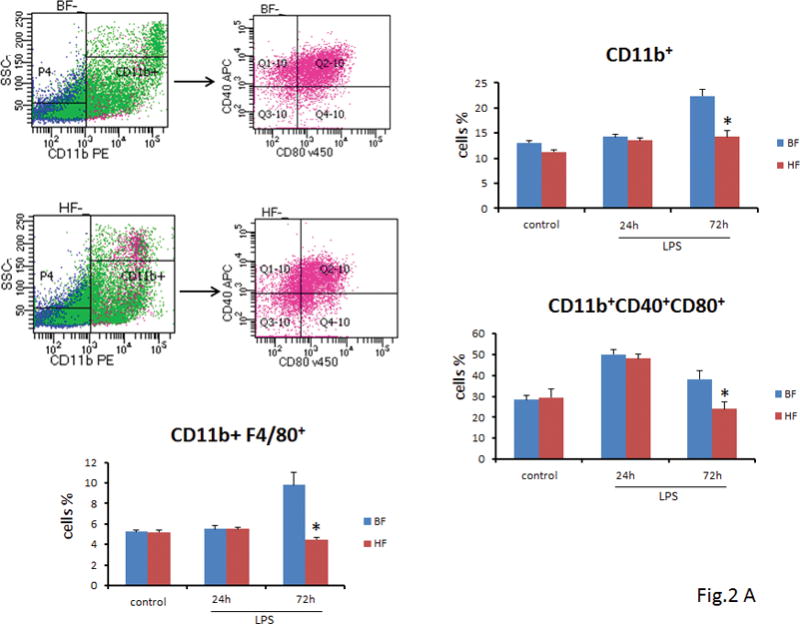
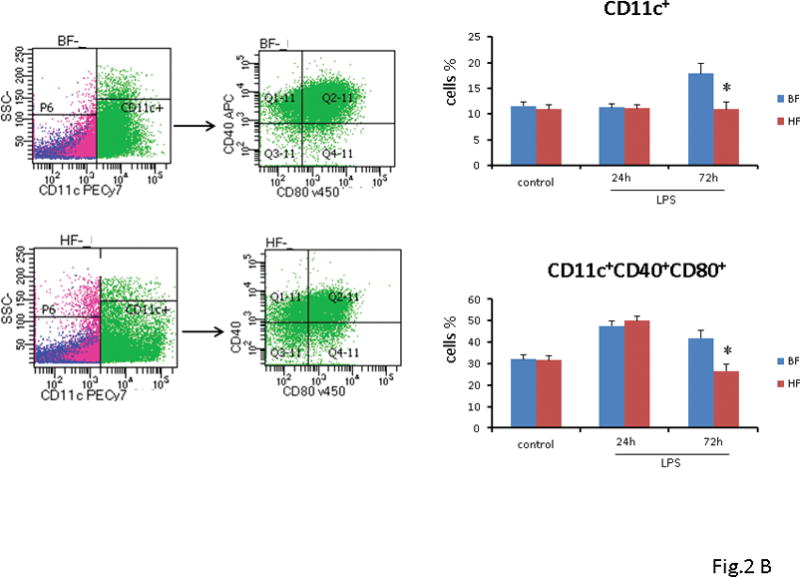
(A) Twenty-four h or 72 h after endotoxin injection, the percentage of splenic macrophages was analyzed by flow cytometry. Data show mean ± SEM of n=8 surviving mice per group at each time point and are representative of at least three experiments. Seventy-two h after induction of endotoxemia, BF diet mice demonstrated a significant increase in the number of macrophages (22.4 ± 1.4% vs. 14.2 ± 1.3%; P ≤ 0.05), and a concomitant increase in the activation markers as compared to mice that were fed HF diet (38.2 ± 4.2% vs. 24 ± 3.2%; P ≤ 0.05) (Fig. 2A) (B) Twenty-four h or 72 h after endotoxin injection, the percentage of splenic DCs was analyzed by flow cytometry. Data show mean + SEM of n=8 surviving mice per group at each time point and are representative of at least three experiments. At 72 h after LPS administration, the proportion of CD40+, CD80+ DC cells was significantly lower in the HF diet mice as compared to the BF-fed mice (26.5 ± 3% vs. 42 ± 3.8%; P ≤ 0.05) (Fig. 2B). (* indicates the presence of a significant difference when P ≤ 0.05 between HF and BF-fed mice).
Next, we examined the splenocytes that expressed CD11c+ 24 and 72 h after endotoxin administration. The percentage of CD11c+ DCs at 24 h were similar in HF and BF diet mice (11 ± 0.8% vs. 11.3 ± 0.7%; P ≥ 0.05) and comparable to DC isolated from control mice. However, by 72 h mice that were fed BF diet demonstrated a significantly higher CD11c+ DCs (18 ± 1.9% vs. 10.9 ± 1.3%; P ≤ 0.05) as compared to HF diet mice (Fig. 2B). To further elucidate the effect of HF diet on activation of DCs, we sought to determine the proportion of CD11c+ DC cells, which were positive for CD40+, CD80+, markers for activated DCs. Analogous to our results above, 72 h after endotoxin administration, the proportion of CD40+, CD80+ DC cells were significantly lower in the HF diet mice as compared to the BF-fed mice (26.5 ± 3% vs. 42 ± 3.8%; P ≤ 0.05) (Fig. 2B).
Taken together, these results suggest that supplementation with HF diet decreases the number and activation of both macrophages and DCs in the murine spleen.
HF diet is associated with hyporesponsiveness of T cells and the generation of Tregs with a strong suppressive activity in vitro
Having demonstrated a significant reduction in the number of DCs, we next wanted to ascertain if the effect of HF diet extended to DCs induced CD4+ T helper cell polarization. First, we assessed the number of CD4+ T cells in BF and HF-fed mice 24 and 72 h after endotoxemia. As shown in Fig. 3A, we noted a comparable decrease in the number of CD4+ T cells in both groups at 24 h after endotoxemia as compared to control animals. At 72 h after endotoxemia, both groups demonstrated a slight increase in the number of CD4+ T cells, although this increase was not significant (Fig. 3A). For analysis of their functional properties, splenic CD4+ cells were isolated from mice that were fed HF or BF diet for 2 weeks and had no prior exposure to LPS. At 16 h after exposure to LPS, the supernatant concentration of the pro-inflammatory cytokines, i.e., IL6, TNF-α, MIP-1a, and MIP-1b were significantly higher in the BF and HF diet mice as compared to their controls. As compared to the BF-fed mice, CD4+ T cells isolated from HF-fed mice that were treated with LPS ex vivo demonstrated a significant reduction in cytokines and chemokines secretion e.g., IL-6 (95 ± 0.3 vs. 50 ± 0.5 pg/ml) and TNF-α (200 ± 0.2 vs. 120 ± 1.3 pg/ml) (P ≤ 0.05) (Fig. 3B). These results extend and confirm our previous findings that HF diet induces anergy in a vital lymphocyte subset that influences the innate and the adaptive immune response.
FIGURE 3. HF diet is associated with hyporesponsivess of T cells ex vivo.
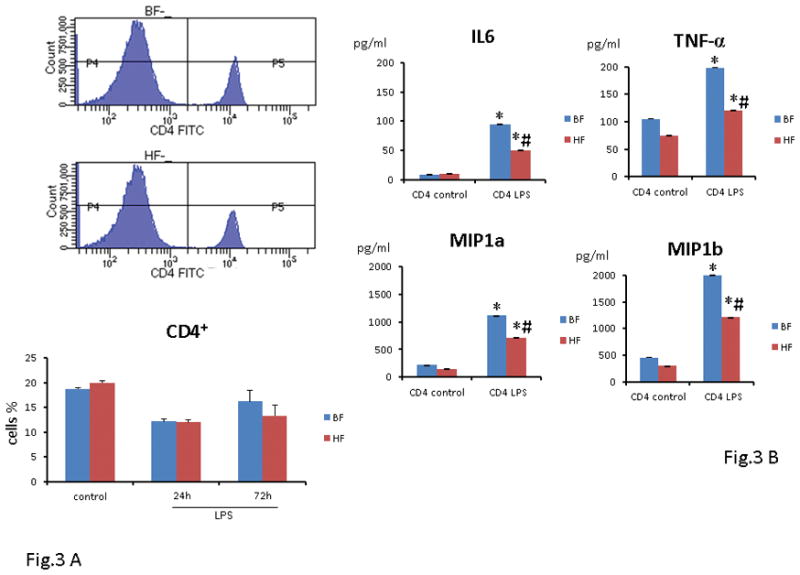
(A) Twenty-four h or 72 h after endotoxin injection, the percentage of splenic T cells was analyzed by flow cytometry. Data show mean ± SEM of n=8 surviving mice per group at each time point and are representative of at least three experiments. (B) In separate experiments, isolated splenic CD4 T cells from 8 mice that were fed either HF or BF diet were exposed overnight (16h) to LPS ex vivo. As compared to the BF diet mice, CD4T cells isolated from HF diet mice demonstrated a significant reduction in cytokines and chemokines secretion i.e. IL6, TNF-α, MIP-1a and MIP-1b. Each data point represents the mean ± S.E.M. of 8 mice for each group (*P ≤ 0.05 between control and endotoxemic diet mice; # P ≤ 0.05 between HF and BF-fed mice).
Tregs play a vital role in suppression of the immune response and are considered critical in the maintenance of immunologic homeostasis and tolerance [11]. The transcription factor forkhead box p3 (Foxp3) is specifically expressed in most CD4+CD25+ T cells and is required for the development of suppressive/regulatory T cells [12]. First, we determined if the Treg numbers are increased after induction of sepsis and examined the expression of the transcription factor Foxp3 as a more definitive marker of Tregs in cells that were CD4+CD25+. We observed a minimal change in the percentage of CD4+CD25+Foxp3+ T lymphocytes in both BF and HF diet mice 24 h after endotoxin administration as compared to control mice (7.6 ± 0.3% vs. 7.9 ± 0.2%; P ≥ 0.05). We observed a 2-fold increase in the percentage of CD4+CD25+Foxp3+ T cells in the spleen 72 h following induction of endotoxemia in both groups of mice as compared to control mice (13.5 ± 0.8 vs 12.6 ± 0.7%; P ≥ 0.05) (Fig. 4A). Thus, the type of diet had minimal impact on the number of Treg cells as both groups demonstrated an increase in the percentage of Tregs after endotoxin administration. We next examined the suppressor cell activity of Tregs as they are characterized as potent suppressors of T effector cell proliferation. Interestingly, the CD4+CD25+Foxp3+ cells from HF diet mice displayed significantly increased suppressive properties as compared to the CD4+CD25+Foxp3+ cells from the BF diet mice (Fig. 4B).
FIGURE 4. HF diet supplementation amplifies the suppressive activity of Tregs in vitro.
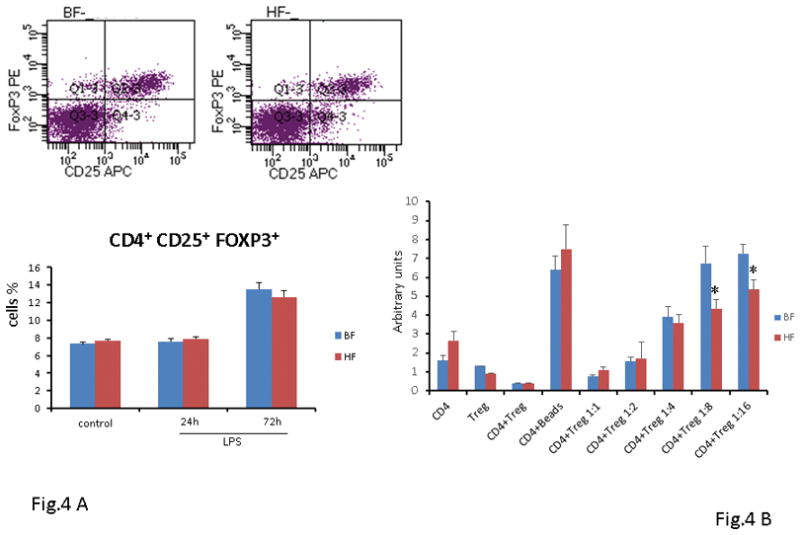
(A) Twenty-four h or 72 h after endotoxin injection, the percentage of splenic Tregs was analyzed by flow cytometry. Data show mean ± SEM of n=8 surviving mice per group at each time point and are representative of at least three experiments. (B) In vitro Tregs suppressive assay. Splenic Tregs were isolated from BF and HF diet-fed mice that were not exposed to LPS and co-cultured with CD3/CD28-stimulated syngeneic CD4 T cells at increasing dilution ratios (1:1–1:16) (* indicates the presence of a significant difference when P ≤ 0.05 between BF and HF-fed mice).
Taken together these results suggest that although HF diet failed to alter the number of Treg cells, it amplified the suppressive function of Tregs, thereby enhancing the regulatory capacity of Tregs after endotoxemia.
HF diet supplementation inhibits LPS-mediated activation of hepatic NF-κB
As NF-κB activation acts as a focal point for proinflammatory gene expression, we next determined the effect of HF diet on LPS-mediated activation of NF-κB using Western blot. We used an antibody directed to the p65 subunit of NF-κB to detect the transcription factor NF-κB in nuclear extracts prepared from samples of liver obtained from mice 24 and 72 h after LPS administration. After endotoxin administration, nuclear translocation of the p65 subunit of NF-κB was significantly increased in hepatic nuclear extracts isolated from BF-fed mice. In contrast, HF-diet mice demonstrated a marked reduction in nuclear p65 translocation as compared to BF-fed mice (Fig 5A). Further corroboration of the effect of HF diet on the DNA binding activity of NF-κB was obtained by using a non-radioactive binding assay. As shown in Figure 5B, supplementation of HF diet led to a significant decrease in the hepatic DNA binding activity of NF-κB on both study days as compared to mice that were fed BF diet. Taken together these findings illustrate the importance of HF diet-mediated effects on the immune cells and decrease of hepatic DNA binding activity of NF-κB leading to a reduction in pro-inflammatory cytokine response in endotoxemia.
FIGURE 5. NF-kB nuclear translocation and consequent activity is inhibited in liver of HF- fed mice.
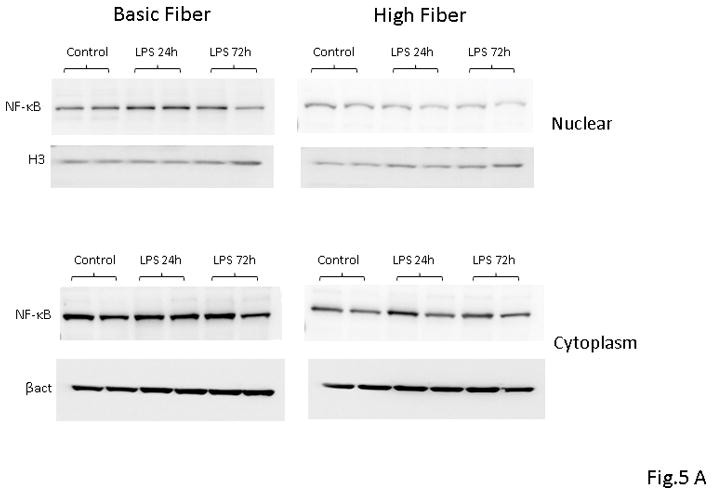
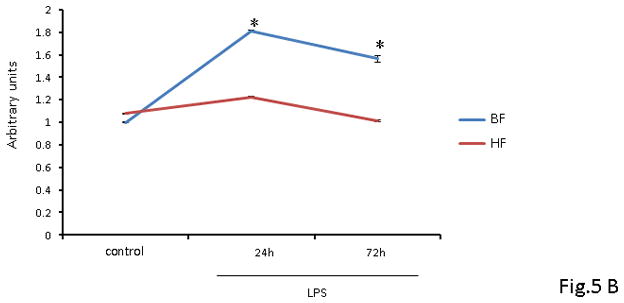
(A) Representative Western blot analysis of p65 nuclear translocation in liver protein extracts from BF and HF diet mice 24 h post LPS injection. Histone H3 and β-actin were used as loading controls. Proteins from 8 survivor mice were analyzed. (B) NF-kB DNA binding activity in liver nuclear extracts from BF and HF diet mice at 24 and 72 h post LPS injection. Each data point represents the mean ± S.E.M. of 8 mice for each group (* indicates the presence of a significant difference when P ≤ 0.05 between HF and BF-fed mice).
DISCUSSION
Fiber has long been recognized for its nutritional value, and as per the last iteration of the Dietary Reference Intakes (DRI), the daily value for fiber is ~14g/1000 Kcal. Despite these recommendations, the amount and type of fiber required to meet the recommended dietary allowances are unclear. In general, fiber represents a group of carbohydrates or carbohydrate-containing compounds that are neither digested nor absorbed in the small intestine [13, 14]. Based on the fermentation potential, the quantity and type of fibers will differentially modulate the evolution of the intestinal microbiome. The novelty of our work was the utilization of cellulose, an insoluble non-fermentable fiber that is a significant component of the vegetarian diet, as it is present in most plant tissues [15]. The term prebiotic has often been used to describe cellulose as it is a non-digestible carbohydrate that favors the growth of desirable microflora in the bowel. In contrast to fermentable fiber where the inflammatory benefits are secondary to the production of short chain fatty acids [16], the benefits of cellulose are due to the transformation it brings to the colonic microbial composition [17].
The resurgence of interest in the microbiome stems from its importance as a potential therapeutic target as well as its role in the modulation of host response to acute illness. Previously, we have demonstrated that gut microbiome constituents in this model are altered after HF diet supplementation, such that it confers survival protection and reduction of pro-inflammatory cytokines in a CLP and endotoxemia murine model of sepsis [7]. In continuation of our previous work, we note that the reduced pro-inflammatory response in HF diet mice can be ascribed to 1) the decrease in number and activation of the splenic macrophages and DCs, 2) the increase in the suppressive activity of Tregs, and 3) the development of T cell anergy.
Macrophages and DCs are critical innate effector cells and are the first line of host defense to protect against bacterial infections. Most studies examining the microbiota and its regulation of immunity have examined it in a “local” context, i.e., maturation of the intestinal immune system and maintaining homeostasis [18, 19]. Recent data demonstrate that in the absence of signals from the commensal bacteria, certain bacterial pathogens, including Listeria monocytogenes and Klebsiella pneumoniae are lethal in mice [20–22]. Thus, the commensal microbiota can potentially regulate antibacterial immunity at extra intestinal sites or exert a broader “systemic” influence. Clarke et al. confirmed that the antibacterial activity of alveolar macrophages is compromised in the absence of the commensal microbiota, leading to defects in early bacterial clearance from the lung, which can be restored by administration of bacterial Nod-Like Receptor (NLR) ligands via the gastrointestinal tract [23]. In a murine model of endotoxemia we demonstrate that strategies like diet modification to an HF diet can alter commensal gut bacteria leading to a reduction in the number and activation of macrophages, thereby reducing the proinflammatory cytokine burden on the host.
Similar numbers of DCs in non-mucosal tissues in germ-free and conventional mice suggest that the microbiome does not regulate DCs differentiation [24]. This contrasts with our data as we demonstrate an increase in splenic DCs in mice that were fed BF diet and administered LPS. However, animals that were fed HF diet did not demonstrate an increase similar to the BF diet mice suggesting that diet may indeed modulate the immune response. Two possible explanations are: a) the change in splenic DCs is microbiome specific, i.e., it is the presence of certain constituents of the microbiome that leads to a reduction in the splenic DCs, b) in germ-free mice the temporal differentiation and activation of DCs may be different, requiring a longer duration. In any case, taken together, our work suggests that there may be a more robust role for microbiota in shaping the host immune response to bacterial infections. The next few steps entail comparison of DCs from germ-free mice, microbiome replete, and conventional mice, and examining the key genes involved in the regulation of cytokines and chemokines production.
Natural Tregs (CD4+CD25+/− ) are derived from the thymus and comprise 5%–10% of CD4+ T cells in the peripheral circulation and lymphoid compartment [25, 26]. We demonstrated that the use of HF diet was associated with an increase in the suppressive capacity of Tregs isolated from the spleen as compared to BF-fed mice. Corroborative data was also obtained from CD4 cells as CD4+ T cells isolated from spleens of mice on BF diet still retain the capacity to secrete high levels of both pro and anti-inflammatory cytokines and chemokines, e.g., IL-6, IL-10, TNF-α and RANTES in contrast to T cells isolated from HF diet mouse.
LPS signals via the TLR-4/CD14 complex and further downstream activates inhibitors of IκB Kinase (IKK). IKK mediates phosphorylation of IκB, an inhibitor of the transcription factor NF-κB, which leads to IκB degradation through the proteasome pathways and enables NF-κB to translocate to the nucleus, resulting in proinflammatory cytokine gene expression [27]. In its active DNA-binding form, NF-κB is a dimer composed of members of the Relish (Rel) family [28]. Several studies have demonstrated the role of NF-κB activation and the subsequent pro-inflammatory cytokine expression leading to the development of sepsis-induced organ failure [29]. Interventions that prevent NF-κB translocation to the nucleus or agents that block the nuclear localization sequence of the NF-κB dimer, not only decrease LPS induced expression of NF-κB–dependent pro-inflammatory genes but also result in diminished endotoxemia-induced organ dysfunction and death [28, 30]. Studies on animal models of sepsis-induced either by LPS injection or by CLP have demonstrated that inhibition of NF-κB protects the animals from sepsis lethality [31]. Furthermore, inhibition of NF-κB restored systemic hypotension and ameliorating septic myocardial dysfunction. In our study, use of a cellulose-rich diet was associated with a decrease in the nuclear accumulation, activation of NF-κB and expression of NF-κB-dependent pro-inflammatory cytokine expression, e.g., IL1-α, IL-β, MIP-1, and MIP-2. It is possible that the Tregs play a key role as their suppressive activity is amplified in HF diet mice as compared to BF diet mice.
The anti-inflammatory effect of fiber supplementation has been investigated in patients with inflammatory bowel disease, and specific benefits were noted in particular cohorts [32]. For example, patients with ulcerative colitis who received germinated barley foodstuff in addition to the anti-inflammatory medication demonstrated better control of their disease along with a decrease in the systemic inflammatory cytokines [33]. Currently, clinical studies of sepsis patients do not account for which species are present in the gut at the time of disease. This is likely to change with the awareness that the commensal microbiota exerts previously unrecognized influences on immunity and inflammation. Fiber supplementation, by promoting the growth of bacteria that are considered pro-health influences the immune system and thus modulates the inflammatory response [6]. Our data have important therapeutic implications as it suggests that the systemic host antibacterial response may be governed by the gut microbiota. Moreover, the novelty of our work was the utilization of cellulose, a non-fermentable fiber, as a daily source of fiber. The fermentable fiber, e.g., pectin, have been extensively studied and its beneficial effect on the immune system well documented but very little data exist on the effects of cellulose in the gut microbiome and the immune response [34].
Based on our findings, there are important questions that still need to be addressed about the role of fiber in modulating the inflammatory response in sepsis. How does the fostering of the pro-health gut microbiome lead to systemic effects on the immune system? Are these effects long-lasting or transient? Finally, will these strategies work if deployed after the onset of sepsis? In conclusion, our data suggest that the use of HF diet is a novel, effective tool that inhibits the hepatic DNA binding activity of NF-κB, leading to a reduction in the pro-inflammatory cytokine response in a murine endotoxemia model.
Supplementary Material
Acknowledgments
Funding: NIH grant R01GM098474 to RKA.
Footnotes
Disclosure: The authors have no financial conflicts of interest.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) Jama. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. The New England journal of medicine. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annual review of pathology. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedlake L, Slack N, Andreyev HJ, Whelan K. Fiber in the treatment and maintenance of inflammatory bowel disease: a systematic review of randomized controlled trials. Inflamm Bowel Dis. 2014;20(3):576–586. doi: 10.1097/01.MIB.0000437984.92565.31. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Shi L, Pang W, Liu W, Li J, Wang H, Shi G. Dietary Fiber Intake Regulates Intestinal Microflora and Inhibits Ovalbumin-Induced Allergic Airway Inflammation in a Mouse Model. PloS one. 2016;11(2):e0147778. doi: 10.1371/journal.pone.0147778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morowitz MJ, Di Caro V, Pang D, Cummings J, Firek B, Rogers MB, Ranganathan S, Clark RSB, Aneja RK. Dietary Supplementation With Nonfermentable Fiber Alters the Gut Microbiota and Confers Protection in Murine Models of Sepsis. Crit Care Med. 2017;45(5):e516–e523. doi: 10.1097/CCM.0000000000002291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJ, de Boer JD, Hoogendijk AJ, de Beer R, de Vos A, Belzer C, et al. The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut. 2016;65(4):575–583. doi: 10.1136/gutjnl-2015-309728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. Isme J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164(1):183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 11.Wan YY. Regulatory T cells: immune suppression and beyond. Cell Mol Immunol. 2010;7(3):204–210. doi: 10.1038/cmi.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishioka T, Shimizu J, Iida R, Yamazaki S, Sakaguchi S. CD4+CD25+Foxp3+ T cells and CD4+CD25-Foxp3+ T cells in aged mice. J Immunol. 2006;176(11):6586–6593. doi: 10.4049/jimmunol.176.11.6586. [DOI] [PubMed] [Google Scholar]
- 13.Kuo SM. The interplay between fiber and the intestinal microbiome in the inflammatory response. Adv Nutr. 2013;4(1):16–28. doi: 10.3945/an.112.003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernazza CL, Gibson GR, Rastall RA. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J Appl Microbiol. 2006;100(4):846–853. doi: 10.1111/j.1365-2672.2006.02832.x. [DOI] [PubMed] [Google Scholar]
- 15.Cummings JH. Cellulose and the human gut. Gut. 1984;25(8):805–810. doi: 10.1136/gut.25.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20(2):159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 17.Wolin MJ. Fermentation in the rumen and human large intestine. Science. 1981;213(4515):1463–1468. doi: 10.1126/science.7280665. [DOI] [PubMed] [Google Scholar]
- 18.Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317(5834):124–127. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- 19.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Khosravi A, Mazmanian SK. Disruption of the gut microbiome as a risk factor for microbial infections. Curr Opin Microbiol. 2013;16(2):221–227. doi: 10.1016/j.mib.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagundes CT, Amaral FA, Vieira AT, Soares AC, Pinho V, Nicoli JR, Vieira LQ, Teixeira MM, Souza DG. Transient TLR activation restores inflammatory response and ability to control pulmonary bacterial infection in germfree mice. J Immunol. 2012;188(3):1411–1420. doi: 10.4049/jimmunol.1101682. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki H, Suzuki T, Nomoto K, Yoshikai Y. Increased susceptibility to primary infection with Listeria monocytogenes in germfree mice may be due to lack of accumulation of L-selectin+ CD44+ T cells in sites of inflammation. Infect Immun. 1996;64(8):3280–3287. doi: 10.1128/iai.64.8.3280-3287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun. 2014;82(11):4596–4606. doi: 10.1128/IAI.02212-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, Lienenklaus S, Weiss S, Staeheli P, Aichele P, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Ohkura N, Sakaguchi S. Regulatory T cells: roles of T cell receptor for their development and function. Semin Immunopathol. 2010;32(2):95–106. doi: 10.1007/s00281-010-0200-5. [DOI] [PubMed] [Google Scholar]
- 26.Cao C, Ma T, Chai YF, Shou ST. The role of regulatory T cells in immune dysfunction during sepsis. World J Emerg Med. 2015;6(1):5–9. doi: 10.5847/wjem.j.1920-8642.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnalich F, Garcia-Palomero E, Lopez J, Jimenez M, Madero R, Renart J, Vazquez JJ, Montiel C. Predictive value of nuclear factor kappaB activity and plasma cytokine levels in patients with sepsis. Infection and immunity. 2000;68(4):1942–1945. doi: 10.1128/iai.68.4.1942-1945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham E. Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis. 2003;187(Suppl 2):S364–369. doi: 10.1086/374750. [DOI] [PubMed] [Google Scholar]
- 29.Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. American journal of physiology Lung cellular and molecular physiology. 2006;290(4):L622–L645. doi: 10.1152/ajplung.00477.2005. [DOI] [PubMed] [Google Scholar]
- 30.Liu SF, Ye X, Malik AB. In vivo inhibition of nuclear factor-kappa B activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159(8):3976–3983. [PubMed] [Google Scholar]
- 31.Sheehan M, Wong HR, Hake PW, Zingarelli B. Parthenolide improves systemic hemodynamics and decreases tissue leukosequestration in rats with polymicrobial sepsis. Crit Care Med. 2003;31(9):2263–2270. doi: 10.1097/01.CCM.0000085186.14867.F7. [DOI] [PubMed] [Google Scholar]
- 32.Benjamin JL, Hedin CR, Koutsoumpas A, Ng SC, McCarthy NE, Hart AL, Kamm MA, Sanderson JD, Knight SC, Forbes A, et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut. 2011;60(7):923–929. doi: 10.1136/gut.2010.232025. [DOI] [PubMed] [Google Scholar]
- 33.Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and -8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48(Pt 3):233–237. doi: 10.1258/acb.2010.010093. [DOI] [PubMed] [Google Scholar]
- 34.Tian G, Wu X, Chen D, Yu B, He J. Adaptation of gut microbiome to different dietary nonstarch polysaccharide fractions in a porcine model. Molecular nutrition & food research. 2017 doi: 10.1002/mnfr.201700012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


