Abstract
Cancer therapeutics-related cardiac dysfunction (CTRCD) is a well-established adverse effect resulting from a number of cancer therapeutics. Newer immunotherapy has been associated with cardiomyopathy and myocarditis making comprehensive imaging useful for early recognition. Cardiac MRI (CMR) offers a comprehensive evaluation to detect CTRCD. Established guidelines for monitoring left ventricular ejection fraction for potential cardiotoxicity have recently incorporated CMR. We will review the utility of CMR in contemporary evaluation for potential oncologic cardiotoxicity.
Introduction
Cardiac toxicity is a well-established adverse effect resulting from a number of cancer therapeutic agents. Cancer therapeutics-related cardiac dysfunction (CTRCD) was first recognized over 50 years ago following the introduction of anthracyclines.1 The risk of cardiomyopathy was found to increase with higher cumulative doses of anthracyclines. The recognition of this dose-dependent risk led to the development of protocols to perform baseline and serial assessments of left ventricular ejection fraction (LVEF), initially based on quantitative radionuclide angiocardiography.2 In the 1980s Schwartz et al. developed a protocol for screening and monitoring of cardiac toxicity based on the detection of an absolute decrease in LVEF of greater than 10% to an LVEF <50% in patients with a normal baseline LVEF.3 Since that time, various other cancer treatment agents have been identified to cause CTRCD. Notably Herceptin (Trastuzumab), which is used for adjuvant therapy in HER-2 positive breast cancer, has been associated with cardiac dysfunction and a higher risk of cardiomyopathy when given in combination with anthracyclines4 More recently, it has been recognized that certain immunotherapeutic agents can be associated with cardiac toxicity including immune-mediated myocarditis.5–7 Along with CTRCD, immune therapy related myocarditis, and radiation-induced cardiac disease have prompted the development of the rising field of “Cardio-Oncology”. Multi-modality imaging has taken a crucial role in diagnosing and monitoring these patients and cardiac magnetic resonance (CMR) has become an important modality incorporated into the 2014 Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy.8 As a comprehensive imaging modality, CMR offers gold standard assessment for LV function and volume assessment, is highly reproducible, and features myocardial strain assessment, characterization of early microstructural and microvascular changes, and assessment of pericardial disease.
CMR imaging protocol
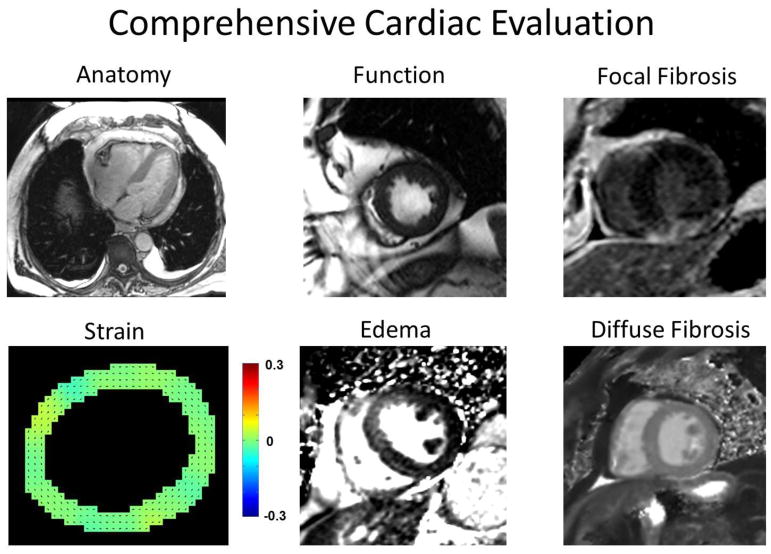
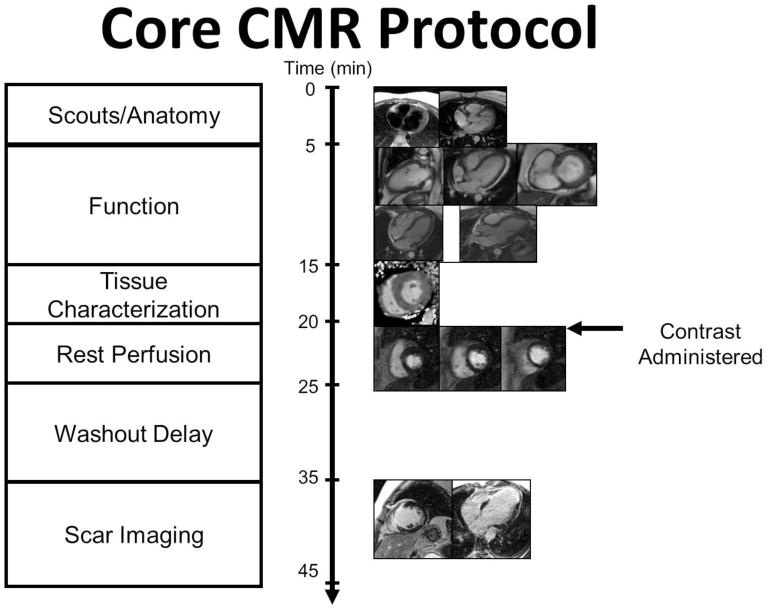
CMR has evolved into a versatile non-invasive modality with excellent spatial and temporal resolution that offers a comprehensive assessment of myocardial function and myocardial tissue characterization, including assessment of strain, edema, diffuse fibrosis, and focal fibrosis in a single study (Figure 1).9,10 Figure 2 illustrates the core of a contrast-enhanced CMR protocol which in most-cases can be acquired over a 30–45-minute examination. A shorter focused non-contrast protocol to assess LV function can be completed in <15 minutes. The basic contrast-enhanced imaging protocol includes scouts, cross-sectional anatomical images of the chest, cine imaging of myocardial function, T1 and T2 mapping to assess edema and diffuse fibrosis, resting first pass contrast-enhanced perfusion imaging, post-contrast T1 maps for assessment of the extracellular volume fraction (ECV), and late gadolinium enhanced (LGE) imaging for scar assessment. CMR has a number of advantages as compared to other non-invasive imaging modalities including being the gold standard for assessment of left ventricular volumes and function, the ability to visualize cardiac and pericardial structures, and the ability to detect myocardial edema and fibrosis.11 Below we will illustrate how CMR can be used for cardiotoxicity assessment.
Fig. 1.
Demonstration of the comprehensive myocardial evaluation offered by CMR.
Fig. 2.
Basic CMR imaging protocol.
CMR Tools for evaluation of CTRCD
A. Assessment of Left ventricular systolic function
The accuracy and reproducibility of left ventricular function assessment is crucial for identifying potential cardiotoxicity during administration of certain chemotherapeutics. Given that the definition of a significant change in LVEF suggesting cardiac toxicity is a reduction of >10% to an LVEF <53%,8 the technique would need to have a test-retest confidence interval considerably less than 10%.12 Echocardiography is widely utilized for baseline and follow-up LV function evaluation due to its wide availability, versatility, lack of radiation exposure, or contraindications for use. Importantly, 2DE is dependent on quality of the acoustic windows obtained for good endocardial border definition and on geometric assumptions that may not apply to patients with dilated or remodeled ventricles. Two decades ago, Otterstad et al. reported that two-dimensional echocardiography (2DE) is capable of recognizing differences in sequential measurements of LVEF of 8.9%.13 A more contemporary study in breast cancer patients undergoing chemotherapy followed the ASE recommendation for biplane calculation of LVEF, using two-chamber and apical four chamber views, found the upper limit of the 95% confidence interval for longitudinal variability was 9.8% (range 9.0–10.8%).14 Given that this reproducibility is on the order of the magnitude of change that it required to diagnose CTRCD, the sensitivity of 2DE has been questioned.8 This same study used three-dimensional echocardiography (3DE) and showed a lower but still significant temporal variability in EF of 6%.14 An additional caveat is that 3DE can only be quantified in patients with adequate echocardiographic windows. In a prospective study using 2DE and 3DE in an experienced echo lab to assess LV volumes compared to CMR, 6% of patients were excluded due to insufficient image quality for 3DE and 22% of the included patients had poor image quality.15 The poor image quality group had poor correlation with CMR volumes when using semi-automated (online) 3D analysis (R = 0.62).
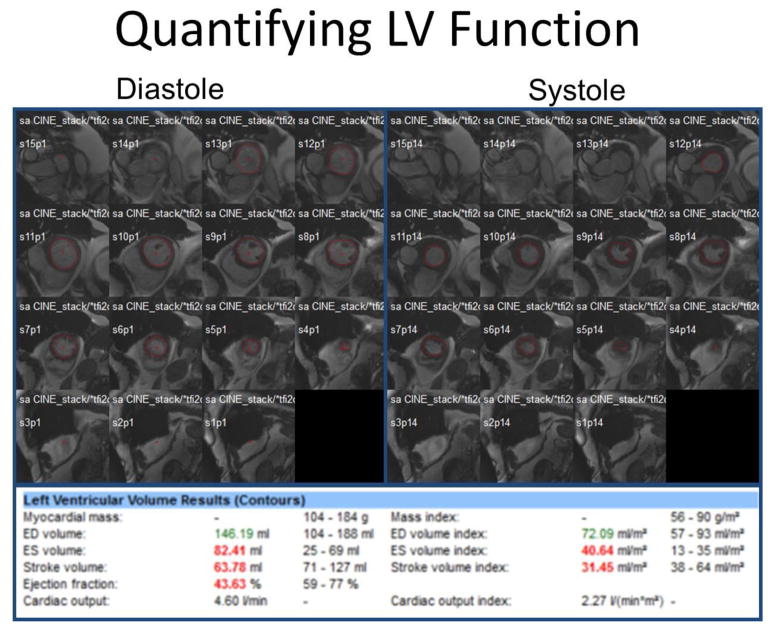
CMR is considered the gold standard for LV chamber size quantification and systolic function measurement, providing quantification of chamber size and LVEF which is free from geometric assumptions and independent of acoustic windows.16 LV chamber quantification is measured by evaluation of short axis images throughout the entire LV with computer-aided analysis packages as shown in Figure 3.17 In a study screening adult survivors of childhood cancer for cardiomyopathy, compared with CMR for detection of EF <50%, 2DE (biplane method) had a sensitivity and a false-negative rate of 25% and 75% respectively, 3DE 53% and 47%, respectively.18 11% had an EF <50% by CMR but were misclassified by ≥50% (range 50 % to 68%) by 2DE. Furthermore, CMR has been shown to have superior reproducibility with the interstudy reproducibility coefficient of variability LV EF 2.4% to 7.3% vs 8.6% to 19.4% in echo (p <0.001) in normal, dilated, and hypertrophic hearts.19
Fig. 3.
For LV chamber quantification, endocardial (red) contours are delineated at end-diastole (left) and end-systole (right) in a stack of short axis slices that cover the entire left ventricle. Computer-aided analysis packages are used to calculate LV parameters as shown. This patient has a decrease in EF and increase in end-systolic volume which can be seen in CTRCD.
B. Myocardial Strain for Early Detection of LV Dysfunction
Cardiac dysfunction and injury from chemotherapy has recently been defined by >10% reduction in LV EF or >15% deterioration in myocardial strain as evidenced by speckle tracking strain analysis.8 A significant reduction in global longitudinal and radial strain rate at 3 months has been demonstrated after trastuzumab treatment.20 Similarly, decreases in LV longitudinal peak systolic strain (LPSS) preceded decreases in EF in patients receiving anthracycline therapy.21 Neghishi et al. recently studied the optimal myocardial deformation index to predict CTRCD at 12 months in breast cancer patients receiving trastuzumab with or without anthracyclines. They found that global longitudinal strain (GLS) at 6 months was the strongest predictor of CTRCD with an 11% (95% CI: 8.3% – 14.6%) reduction yielding a 65% sensitivity and 94% specificity.22 GLS appears to decrease on average between 10% to 20% depending on the duration of treatment, population, and chemotherapeutic agent studied and has been determined to be the optimal echocardiographic strain parameter for early detection of subclinical LV dysfunction.8
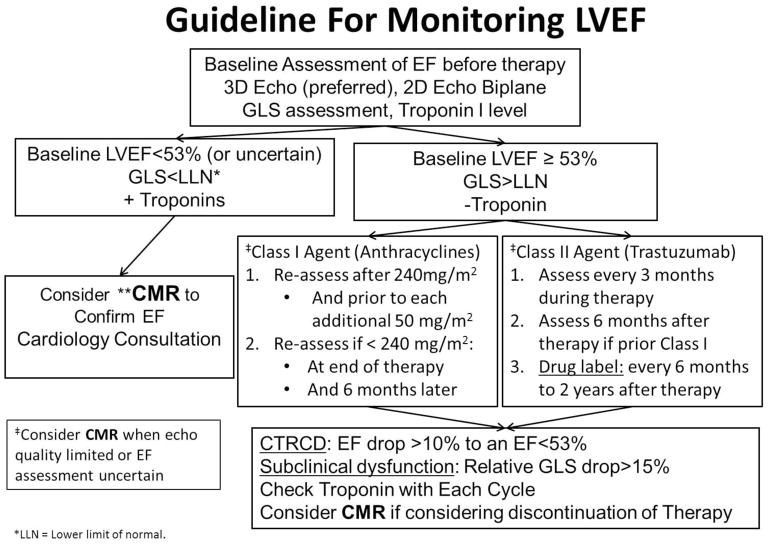
CMR myocardial tagging is a well-established technique for measuring myocardial strain, and was first described by Zerhouni et al. in 1988.23 Myocardial tagging has been extensively validated and has served as the gold-standard technique for validating other strain techniques including myocardial speckle tracking echocardiography. Drafts et al. studied CMR parameters on cancer patients receiving anthracyclines before and 1, 3, and 6 months after therapy. After 6 months, LV EF decreased 58 ± 1% to 53 ± 1% (p =0.0002) and mid-wall circumferential strain from −17.7 ± 0.4 to −15.1 ± 0.4 (p= 0.0003) without evidence of focal fibrosis as defined by late gadolinium enhancement.24 Jordan et al. demonstrated a deterioration in midwall eulerian circumferential strain (ECC) from −17.99 to −17.23 (p = 0.0052) in cancer patients after receiving three months of chemotherapy (71% anthracyclines).25 GLS assessed from 2- and 4-chamber cine views trended towards a decline −15.44 to −14.79 (p = 0.069). They observed an association between ECC and GLS strain deteriorations in patients with decrease in left ventricular end-diastolic volume (LVEDV) but no change in left ventricular end-systolic volume (LVESV). Their data suggested that LVEDV mediated relative strain changes of 9% may occur after chemotherapy causing a reduced preload. This illustrates the importance of reviewing LV volume changes with interpreting LV strain deteriorations after receipt of cardiotoxic chemotherapy, as decreases in LVEDV from poor oral intake, emesis, etc. are common. Recently feature tracking techniques for CMR have evolved and demonstrate the potential to be able to derive strain parameters from standard cine SSFP images.26,27 This may broaden the applicability of measuring strain by CMR without the need for additional myocardial tagging. Recently developed multi-modality consensus statements have begun to include CMR in the diagnostic algorithm.8 Figure 4 is a proposed guideline of how to incorporate CMR during chemotherapy monitoring adapted from Plana et al.8 If the baseline LV EF on echocardiogram is borderline low (EF <53%) or limited echo quality, then CMR can be used for a more reliable EF assessment. Furthermore, the consensus document suggests that CMR should be considered for a more accurate assessment of LVEF if there is consideration of discontinuing chemotherapy. A previous study in breast cancer patients on trastuzumab suggested that avoiding cardiotoxicity may have a greater impact on survival than needing to complete a full course of trastuzumab.28
Fig. 4.
Guideline for monitoring LVEF for Class I and Class II agents adapted from Plana JC et. al. J Am Soc Echocardiogr 2014;27:911–39.
** CMR should be considered in high risk patients for cardiotoxicity when echo quality is limited or EF is uncertain. A non-contrast abbreviated protocol for EF assessment can be used at baseline and follow-up at intervals directed above. CMR with gadolinium can be used when there are concerns for myocarditis or scar imaging is necessary.
C. Scar imaging with late gadolinium enhancement (LGE)
CMR imaging with LGE is the reference standard for the noninvasive detection of focal myocardial fibrosis.29 However, this is an uncommon finding in patients treated with anthracyclines. CMR assessment with delayed enhancement imaging of 62 long-term survivors of childhood cancer with anthracycline exposure did not show LGE despite the majority (79%) of patients having abnormal (EF < 45%) or subnormal (45% ≤ EF <55%) LV function. Another previously described study of anthracycline treated cancer patients had only an incidence of 7% LGE in a basal distribution but increase in ECV and native T1 mapping supporting diffuse fibrosis.30 Histologic findings have confirmed the diffuse nature of interstitial fibrosis31 and likely explains the low sensitivity of LGE, a marker of focal fibrosis, in identifying anthracycline-induced cardiomyopathies. LGE in a myocarditis-like pattern (focal subepicardial lateral wall) has been observed in 10 breast cancer patients diagnosed with CTRCD treated with trastuzumab and anthracycline.32
D. Tissue characterization with T1-Mapping, T2-Mapping, and ECV mapping
One major advantage of CMR for evaluation of potential cardiotoxicity is the use of non-contrast parametric mapping techniques such as native T1 and T2 mapping, which rely on the intrinsic magnetic relaxation properties of the myocardium to assess tissue characterization.33 In native T1 mapping, a quantitative map of T1 relaxation times of the myocardium and blood pool is acquired before the administration of gadolinium contrast agents (Figure 5).34 Native T1 values reflect signals from the intracellular and extracellular compartments as well as intrinsic variances in tissue properties. Increased native T1 is useful for detecting acute myocardial pathologies that can occur in cardiotoxicity, such as edema, infarction, or myocarditis and subacute processes such as diffuse fibrosis.34 T2-weighted imaging can identify presence of acute myocardial inflammation and injury as seen in myocarditis,35 myocardial infarction,36 or stress-induced cardiomyopathy37 but its utility in cardiotoxicity has not been thoroughly studied.
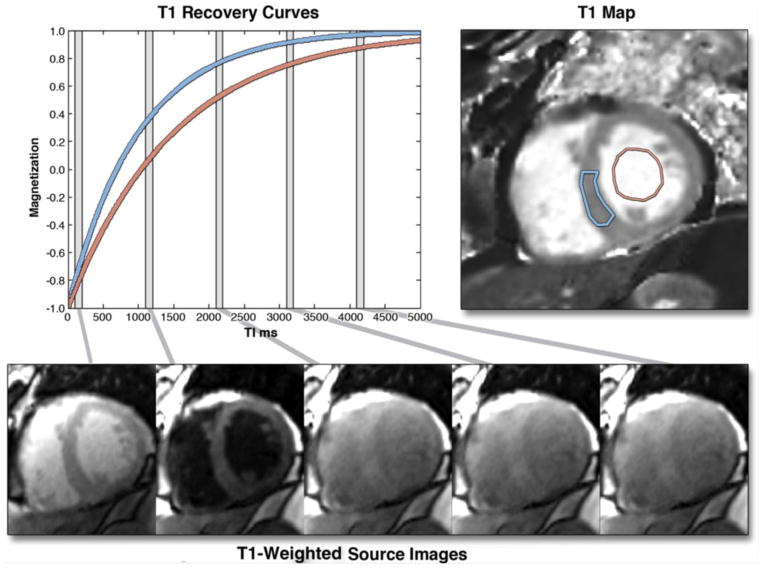
Fig. 5.
The graph on the left shows 2 inversion recovery curves for a septal region of interest (blue) and the blood pool, generated from images, shown in the bottom row, taken at different times after an inversion pulse at time t = 0. Similar inversion recovery curves can be generated for each pixel location if the images are all acquired during a breath-hold and for the same cardiac phase. The T1 for each pixel location can be used to generate a T1 map, as shown in the top-right image. T1 maps represent arguably the most succinct and informative summary of the spatial and temporal changes during an inversion recovery. Borrowed with permission from Taylor et al. JACC: Cardiovascular Imaging. 2016. 34
Diffuse myocardial fibrosis seen in infarcts and non-ischemic cardiomyopathies results in increased collagen content which should change the myocardial extracellular volume fraction (ECV).38,39 CMR imaging with gadolinium can quantify the ECV, derived from pre-and-post-contrast T1 measurements, which correlates with histologic evidence of fibrosis.40 Anthracycline-treated patients had elevated myocardial ECV, a marker of diffuse fibrosis, which is associated with diastolic dysfunction and increased left atrial volumes.30 Jordan et al. further compared myocardial native T1 mapping and ECV pre- and mean duration 3 years post- anthracycline treatment. Myocardial ECV significantly increased (27.8 ± 0.7% to 30.4 ± 0.7%, p<0.01) in cancer patients 3 years after anthracycline therapy independent of underlying cancer or cardiovascular comorbidities. Native T1 was elevated in both pre- and post- anthracycline groups compared to controls as shown in Figure 6.41 In a cohort of 166 low-risk anthracycline treated breast cancer survivors, CMR with ECV imaging at a mean follow-up of 6.0 ± 1.2 years showed no difference in mean ECV compared with health volunteers (0.28 ± 0.028 vs 0.28 ± 0.029, p = 0.803). This corresponded with the finding that none of these patients met criteria for CTRCD and all had LV EF > 55%. This supports that ECV is useful for risk stratification in anthracycline therapy and that the majority of toxicity occurs early in the first year of therapy.42 However, given the uncertainty in current methods to measure ECV, whether this metric can be used to guide patient therapy requires further study.
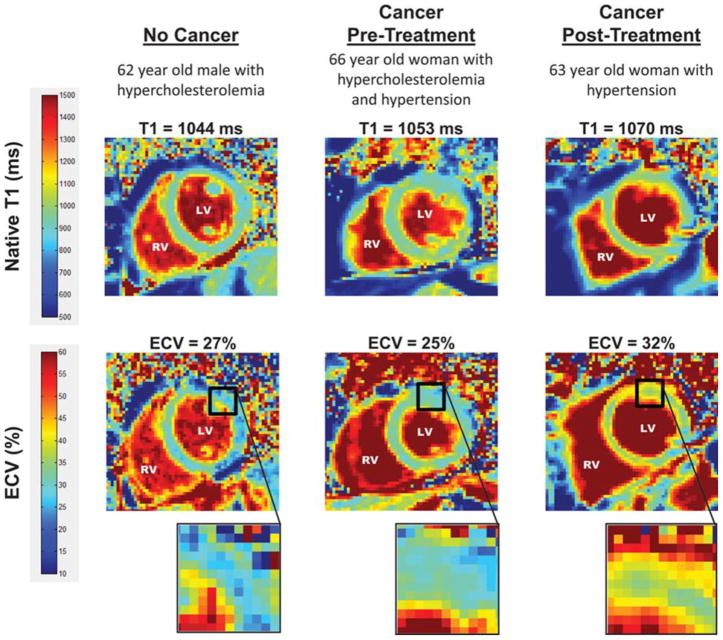
Fig. 6.
T1 and ECV map images. Representative left ventricular (LV) short-axis native T1 (top row) and extracellular volume (ECV, bottom row) maps are shown in similarly-aged participants. The LV and right ventricular (RV) blood pool cavities are noted. On each image, the color of pixels in the images (color scales on left) identifies the native T1 (milliseconds) and ECV (%). Insets on the ECV maps demonstrate the change in color intensity within the anterolateral wall of each ventricle. As shown, ECV is elevated in the cancer survivor previously treated with anthracycline-based chemotherapy. Borrowed with permission from Jordan et al. Circulation Imaging. 2016. 41
CMR imaging for Immunotherapy Related Myocarditis
Immune therapies can result in acute cardiotoxicities. Interleukin-2 (IL-2) is a primary cytokine released by CD4+ cells which through various mechanisms, including upregulation of proinflammatory cytokines, makes it suitable for treatment cancers such as melanoma and renal cell carcinoma. Cardiovascular toxicities such as myocarditis and/or myocardial infarction have been reported in 7% of cases in clinical trials.5 Immune checkpoint inhibitors have recently transformed the treatment of several cancers by upregulating antitumor immunity. Ipilimumab, an anti-cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody, and Nivolumab, an anti-programmed death-1 (PD-1) antibody, individually have improved survival in melanoma.6 The combination of Ipilimumab/Nivolumab was recently approved by the FDA for treatment of metastatic melanoma resulting in significantly longer progression-free survival compared to nivolumab alone. However, cases of fulminant myocarditis shortly after initiation of therapy are being recognized.6,7 We recently have seen two cases of fulminant myocarditis shortly after initiation of Ipilimumab/Nivolumab and confirmed the diagnosis in one of them. An elderly male with newly diagnosed metastatic melanoma and no known prior cardiac history presented to our emergency department with tachycardia, palpitations, and pre-syncope. He had just completed his first cycle of Ipilimumab/Nivolumab 11 days prior to presentation. Initial troponin I was 3.65 ng/ml. Transthoracic echocardiogram showed subtle inferior wall abnormality. Urgent CMR was obtained with findings consistent with acute myocarditis as demonstrated in Figure 7. Symptoms progressed to complete heart block, cardiogenic shock, with troponin I level elevation to 90 ng/ml, as well as new neurologic and respiratory symptoms consistent with myasthenia gravis. Despite aggressive immunosuppressive therapy the patient developed refractory pulseless ventricular tachycardia and expired.
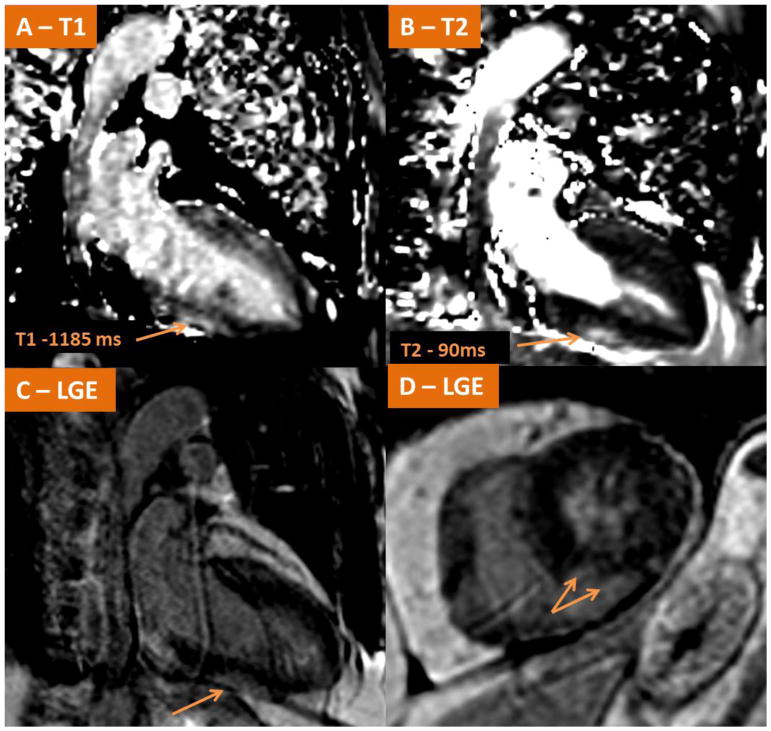
Fig. 7.
Demonstrates CMR findings in a case of acute fulminant myocarditis in a patient with metastatic melanoma shortly after receiving his first cycle of Ipilimumab/Nivolumab. (A–B) Native T1-mapping and T2 mapping respectively demonstrating elevated signals along the inferior wall consistent with myocardial edema. LGE imaging (C–D) demonstrate LGE present in the subepicardial mid inferior wall consistent with acute myocarditis.
CMR in Radiation-Induced Cardiac Disease
Radiation therapy (RT) is an integral part of cancer therapy occurring in more than half of cancer patients. RT is commonly used in intrathoracic malignancies such as breast, lung, esophageal, and thyroid cancers as well as Hodgkin lymphoma.43 RT-induced heart disease is typically a late complication commonly affecting heart valves and pericardium in approximately 11% and 5% of cases respectively with higher incidences in those exposed during childhood.44 Pericardial manifestations can result in acute pericarditis or constrictive pericarditis many years after therapy. CMR allows for various sequences such as tagged cine gradient, LGE, and free-breathing cine to help assess for fibrotic adhesion of pericardial layers, pericardial inflammation, and ventricular interdependence respectively.45 Figure 8 demonstrates CMR findings in a case of radiation-induced pericardial constriction. Mediastinal radiation can also result in restrictive cardiomyopathy which can be further evaluated with CMR. Currently, coronary angiography by MRI does not have the suitable spatial resolution to detect premature coronary atherosclerosis secondary to radiation exposure.
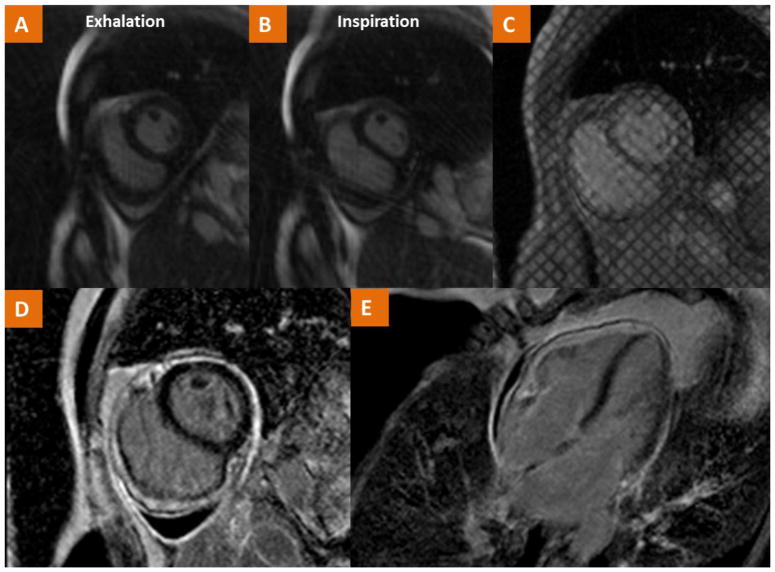
Fig. 8.
CMR findings in a case of a 54 year old female with history of Hodgkin’s Lymphoma treated with radiation at ages 20, 24, 27 and now with recurrent pericardial effusion and dyspnea. (A–B) Real-time cine imaging demonstrates flattening of the septum during inspiration consistent with ventricular interdependence as well as a small pericardial effusion. (C) Tagged cine gradient imaging used to detect fibrotic adhesion of pericardial layers. (D–E) LGE imaging demonstrates pericardial LGE consistent with pericardial inflammation. The consolidation of these findings is consistent with the diagnosis of pericardial constriction.
Conclusion
Cardio-oncology continues to evolve as newer chemotherapeutic agents enter the market with potential cardiotoxicities. Multi-modality imaging is at the core of management in high risk oncologic patients as detailed in recent consensus statements. Cardiac MRI can provide unique information in the assessment of patients undergoing potentially cardiotoxic chemotherapy including a detailed evaluation of left ventricular function and chamber sizes, myocardial strain, focal and diffuse fibrosis, inflammation, and edema.
CMR is a useful comprehensive supplemental modality to echocardiography when a more reliable EF measurement is needed as well as better tissue characterization. With advanced CMR techniques a comprehensive non-contrast assessment of cardiac toxicity can be acquired in less than 15 minutes. Furthermore, CMR allows for the diagnosis of other complications of cancer treatment such as radiation induced pericardial constriction and restrictive cardiomyopathy. Future studies are needed to better incorporate multi-modality cardiovascular imaging findings to decide when to discontinue potentially cardiotoxic chemotherapeutic agents.
Supplementary Material
Footnotes
Disclosures: MS receives grant support from Astra Zeneca and research support from Siemens. MS is supported in part by NIH R01 HL131919, and AL is supported by NIH 5T32EB003841
References
- 1.Tan C, Tasaka H, Yu KP, Murphy ML, Karnofsky DA. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. clinical evaluation with special reference to childhood leukemia. Cancer. 1967;20:333–53. doi: 10.1002/1097-0142(1967)20:3<333::aid-cncr2820200302>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J, Dainiak N, Berger HJ, Goldman L, Johnstone D, Reduto L, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med. 1979;300:278–83. doi: 10.1056/NEJM197902083000603. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RG, McKenzie WB, Alexander J, Sager P, D’Souza A, Manatunga A, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy: Seven-year experience using serial radionuclide angiocardiography. The American Journal of Medicine. 1987;82:1109–18. doi: 10.1016/0002-9343(87)90212-9. [DOI] [PubMed] [Google Scholar]
- 4.Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–21. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 5.Poust JC, Woolery JE, Green MR. Management of toxicities associated with high-dose interleukin-2 and biochemotherapy. Anticancer Drugs. 2013;24:1–13. doi: 10.1097/CAD.0b013e32835a5ca3. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–55. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the american society of echocardiography and the european association of cardiovascular imaging. Journal of the American Society of Echocardiography. 2014;27:911–39. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Salerno M, Kramer CM. Advances in parametric mapping with CMR imaging. JACC Cardiovasc Imaging. 2013;6:806–22. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw PW, Kramer CM. The case for CMR. J Nucl Cardiol. 2015;22:968–70. doi: 10.1007/s12350-015-0147-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–88. doi: 10.1007/s12350-012-9512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mor-Avi V, Lang RM. Is echocardiography reliable for monitoring the adverse cardiac effects of chemotherapy? J Am Coll Cardiol. 2013;61:85–7. doi: 10.1016/j.jacc.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Otterstad JE, Froeland G, St John Sutton M, Holme I. Accuracy and reproducibility of biplane two-dimensional echocardiographic measurements of left ventricular dimensions and function. Eur Heart J. 1997;18:507–13. doi: 10.1093/oxfordjournals.eurheartj.a015273. [DOI] [PubMed] [Google Scholar]
- 14.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: Application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. 2013;61:77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins C, Chan J, Hanekom L, Marwick TH. Accuracy and feasibility of online 3-dimensional echocardiography for measurement of left ventricular parameters. J Am Soc Echocardiogr. 2006;19:1119–28. doi: 10.1016/j.echo.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong AC, Gidding S, Gjesdal O, Wu C, Bluemke DA, Lima JA. LV mass assessed by echocardiography and CMR, cardiovascular outcomes, and medical practice. JACC Cardiovasc Imaging. 2012;5:837–48. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for cardiovascular magnetic resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35,429X-15-35. doi: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grothues F, Smith GC, Moon JCC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. The American Journal of Cardiology. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 20.Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II–Positive breast cancer treated with adjuvant trastuzumab therapy. Journal of the American College of Cardiology. 2011;57:2263–70. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 21.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. Journal of the American Society of Echocardiography. 2012;25:733–40. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–8. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: Tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 24.Drafts BC, Twomley KM, D’Agostino R, Jr, Lawrence J, Avis N, Ellis LR, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–85. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan JH, Sukpraphrute B, Melendez GC, Jolly MP, D’Agostino RB, Jr, Hundley WG. Early myocardial strain changes during potentially cardiotoxic chemotherapy may occur as a result of reductions in left ventricular end-diastolic volume: The need to interpret left ventricular strain with volumes. Circulation. 2017;135:2575–7. doi: 10.1161/CIRCULATIONAHA.117.027930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vo HQ, Marwick TH, Negishi K. MRI-derived myocardial strain measures in normal subjects. JACC Cardiovasc Imaging. 2018;11:196–205. doi: 10.1016/j.jcmg.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Salerno M. Feature tracking by CMR: A “double feature”? JACC Cardiovasc Imaging. 2018;11:206–8. doi: 10.1016/j.jcmg.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Wang SY, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP, et al. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. 2014;146:411–9. doi: 10.1007/s10549-014-3029-0. [DOI] [PubMed] [Google Scholar]
- 29.Kim RJ, Wu E, Rafael A, Chen E, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 30.Neilan TG, Coelho-Filho OR, Shah RV, Feng JH, Pena-Herrera D, Mandry D, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. The American Journal of Cardiology. 2013;111:717–22. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovascular Pathology. 2010;19:308–11. doi: 10.1016/j.carpath.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. 2008;10:5,429X-10-5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salerno M, Sharif B, Arheden H, Kumar A, Axel L, Li D, et al. Recent advances in cardiovascular magnetic resonance: Techniques and applications. Circ Cardiovasc Imaging. 2017 doi: 10.1161/CIRCIMAGING.116.003951. doi:10:10.1161/CIRCIMAGING.116.003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping: Basic techniques and clinical applications. JACC: Cardiovascular Imaging. 2016;9:67–81. doi: 10.1016/j.jcmg.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Bonner F, Spieker M, Haberkorn S, Jacoby C, Flogel U, Schnackenburg B, et al. Myocardial T2 mapping increases noninvasive diagnostic accuracy for biopsy-proven myocarditis. JACC Cardiovasc Imaging. 2016;9:1467–9. doi: 10.1016/j.jcmg.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Aty H, Zagrosek A, Schulz-Menger J, Taylor AJ, Messroghli D, Kumar A, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109:2411–6. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Aty H, Cocker M, Friedrich MG. Myocardial edema is a feature of tako-tsubo cardiomyopathy and is related to the severity of systolic dysfunction: Insights from T2-weighted cardiovascular magnetic resonance. Int J Cardiol. 2009;132:291–3. doi: 10.1016/j.ijcard.2007.08.102. [DOI] [PubMed] [Google Scholar]
- 38.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–58. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 39.Ugander M, Oki AJ, Hsu LY, Kellman P, Greiser A, Aletras AH, et al. Extracellular volume imaging by magnetic resonance imaging provides insights into overt and sub-clinical myocardial pathology. Eur Heart J. 2012;33:1268–78. doi: 10.1093/eurheartj/ehr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, et al. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: Preliminary validation in humans. Circulation. 2010;122:138–44. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 41.Jordan JH, Vasu S, Morgan TM, D’Agostino RB, Jr, Melendez GC, Hamilton CA, et al. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen KM, Offersen BV, Nielsen HM, Vaage-Nilsen M, Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol. 2017;40:255–61. doi: 10.1002/clc.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maraldo MV, Giusti F, Vogelius IR, Lundemann M, van der Kaaij MA, Ramadan S, et al. Cardiovascular disease after treatment for hodgkin’s lymphoma: An analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol. 2015;2:e492–502. doi: 10.1016/S2352-3026(15)00153-2. [DOI] [PubMed] [Google Scholar]
- 45.Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson. 2009;11:14,429X-11-14. doi: 10.1186/1532-429X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.