Abstract
Apigenin is a flavonoid with well-documented anti-cancer properties; however, its mechanisms of action are still unclear. We previously identified apigenin as a potential phytoprogestin, a natural product with a chemical scaffold that interacts with the progesterone receptor (PR). Our objective was to characterize the ability of apigenin to interact with PR through molecular docking studies, in vitro activity assays, and the ability of apigenin to elicit progestin-like effects in vivo. Molecular docking confirmed that apigenin could interact with PR, though with lower affinity than progesterone due to fewer van der Waals interactions. In Ishikawa cells stably expressing PR-B, apigenin significantly increased progesterone response element/luciferase (PRE/Luc) activity at 5 and 10 μM, but not in the parental Ishikawa cells that lack PR expression. In the presence of 100 nM of progesterone, 10 μM apigenin reduced PRE/Luc activity, indicative of mixed agonist activity. Apigenin also triggered degradation of PR in Ishikawa PR-B cells as measured by western blot. Apigenin reduced proliferation of Ishikawa cells, but through a PR-independent mechanism. In contrast, apigenin and progesterone both stimulated proliferation of T47D cells, an effect blocked by RU486. Apigenin activated other nuclear receptors evidenced by increased luciferase activity in MDA-MB-231 cells, which are PR negative. In vivo, apigenin blocked the genistein-stimulated increase in uterine epithelial cell height; stimulated endometrial expression of Hand2, a transcription factor stimulated by PR, and significantly reduced genistein-induced proliferation. In summary, apigenin is a phytoprogestin, with mixed agonist activity that demonstrates activity in vivo by hindering estrogen receptor-mediated uterine proliferation.
Electronic supplementary material
The online version of this article (10.1007/s12672-018-0333-x) contains supplementary material, which is available to authorized users.
Keywords: Ishikawa Cells, T47D Cells, Mixed-agonist Action, Epithelial Cell Height, Uterine Epithelium
Introduction
Apigenin is present in many fruits, vegetables, teas, and herbal supplements [1]. The use of herbal supplements is increasing in the general population [2] and is even higher among cancer patients [3, 4]. Therefore, interest in the role of apigenin (4′,5,7,-trihydroxyflavone) in cancer prevention and treatment has been increasing [5, 6]. Multiple studies have found an inverse correlation between flavone intake, of which apigenin is the most common, and a woman’s risk of breast cancer [7, 8]. In addition, apigenin has been shown to reduce the proliferation or increase apoptosis in numerous cancer cell lines [9–11]. However, the mechanisms of action for apigenin are still unclear.
In vitro apigenin has repeatedly been shown to inhibit the growth of breast cancer cell lines, an effect generally attributed to the ability of apigenin to interact with the estrogen receptor (ER) [12]. However, apigenin is a weak phytoestrogen, with relative binding affinities of 0.3 and 6% for estrogen receptor (ER) ɑ and ERβ, respectively [13]. Apigenin reduced proliferation in anti-estrogen-resistant MCF7 cell lines [11], and the estrogen antagonist ICI 182,780 did not reduce the incidence of apoptosis in prostate cancer cell lines (PWR-1E and PC-3) treated with apigenin [9]. In vivo apigenin slowed the growth of medroxyprogesterone acetate (MPA)-stimulated mammary tumors in mice perhaps hinting that it was able to block progesterone or androgen-like action typically induced by MPA [10, 14]. All of these data suggest that apigenin has effects independent of the estrogen receptor.
Apigenin has limited bioavailability, but is more available than other common flavonoids, such as quercetin and luteolin [15]. Apigenin was shown to reach steady state concentrations in the plasma of mice after 5 days on a diet with 1.1 mmol apigenin/kg [16], and radio-labeled apigenin could be detected up to 9 days after a single oral administration in rats [17]. Apigenin is primarily metabolized by glucuronidation and sulfation reactions, resulting in the majority of excretion occurring in the urine [17, 18]. In tumor studies, apigenin reduced the growth of malignant mesothelioma tumors after intraperitoneal treatment [19]. Oral gavage of apigenin slowed the growth of prostate cancer PC-3 cells [20] and reduced the number of implantation sites in mice [21]. Thus, apigenin reaches sufficient concentrations to have effects in vivo.
Progesterone plays a key role in development of breast and gynecological cancers [22]. The progesterone receptor (PR) has been used as a marker of ER activity, but increasing evidence suggests that PR signaling is also a key in the pathogenesis of breast cancer [23]. The Women’s Health Initiative (WHI) found that women who used hormone replacement therapy (HRT) containing both estrogen and progestin had increased risk of developing breast cancer relative to estrogen only HRT [24]. In endometrial and ovarian cancer, expression of PR is associated with longer overall survival [25, 26]. PR has been shown to regulate the expression of numerous genes associated with invasion and aggressiveness of endometrial cancer, and advanced endometrial cancer showed a loss of PR immunostaining [27]. In ovarian cancer, progesterone induced necroptosis in p53 defective cells [28]. Interestingly, different progestins differ in their PR-mediated biological effects. A recent meta-analysis found that women taking an HRT-containing synthetic progestins had an increased risk of breast cancer relative to women using progesterone [29]. The increased risk of breast cancer from HRT may be due to the fact that the synthetic progestins commonly used in HRT also activate the androgen and glucocorticoid receptors (AR and GR, respectively) [30].
While many flavonoids are known to interact with ER, it is also clear that some flavonoids can interact with PR [31]. Kaempferol activated a progesterone response element/luciferase (PRE/Luc) construct, was predicted to bind in the ligand binding domain of the progesterone receptor (PR) via computer modeling, and countered the effects of the phytoestrogen genistein in the uteri of rats [32, 33]. Apigenin blocked MPA-stimulated growth of mammary tumors in mice [10, 34] and reduced the MPA-stimulated secretion of VEGF from T47D and BT-474 cells [14], indicating that apigenin interferes with normal progestin signaling. We previously found that apigenin activated a progesterone response element/luciferase (PRE/Luc) construct in T47D cells [32], indicating that apigenin may interact directly with the PR. However, the effects of apigenin were not further characterized in that study. Therefore, our objectives were to determine if apigenin interacts with PR directly, to characterize apigenin’s agonist and antagonist effects for PR in a variety of progesterone sensitive cell lines, and to determine if apigenin acts as a progestin in vivo.
Material and Methods
Molecular Modeling of Apigenin and Progesterone with the Progesterone Receptor (PR)
Molecular Docking for Apigenin
Since there is no crystal structure for the human PR-apigenin complex, molecular docking was performed to predict the binding mode for apigenin with human PR. The Protein Preparation Wizard in Schrödinger Suite Release 2016-1 (www.schrodinger.com) was used to optimize the crystal structure of the human PR in complex with progesterone (PDB code 1A28 [35]). All hydrogens were added to the receptor and restrained minimization was first performed on those added hydrogens, and then, the root-mean-square deviation (RMSD) of heavy atoms was converged to less than 0.30 Å with the OPLS3 force field. The 3D structure of apigenin was created by LigPrep (Schrödinger Release 2016-1: ligprep, version 3.7). The OPLS3 force field was applied for ligand geometric optimization with all possible ionization and tautomeric forms created at pH 7.4 by EPIK (Schrödinger Release 2016-1: Epik, version 3.5) [36]. Default values were utilized for other parameters for protein and ligand preparations. Molecular dockings were performed using GOLD v5.2.2 [37] with the above prepared protein and apigenin. The active site for human PR was defined as being within 6 Å around progesterone in the crystal complex of 1A28.pdb. The best scoring pose for apigenin was selected for analysis.
Molecular Dynamics (MD) Simulations for the Crystal Complex of PR with Progesterone and the Docked Complex of PR with Apigenin, and Determination of Binding Affinities by Molecular Mechanics - Poisson–Boltzmann Surface Area Method (MM-PBSA)
MD Simulations
Restrained electrostatic potential (RESP) atomic partial charges were assigned to apigenin and progesterone with geometry optimization, and the electrostatic potential calculations were performed using HF/6-31G* and Gaussian 09 in the R.E.D. online server [38]. The Amber FF14SB force field and the general AMBER force field (GAFF) parameters were assigned to the human PR and to apigenin and progesterone; a 10 Å octahedral TIP3P water molecule box was added to each of the complex systems along with Cl-counter-ions to neutralize the system. Then, both systems were subjected to 3 ns MD simulations by Amber14 with the PMEMD program [38]. The systems were first minimized using 10,000 steps of steepest descent minimization followed by another 10,000 steps of conjugate gradient minimization. After heating from 0 to 300 K over 100 ps, the systems were equilibrated over 100 ps at constant pressure (1 bar) and temperature (300 K). Then, 3 ns NPT production runs were respectively performed at 300 K and 1 bar for each of the two systems. The atomic coordinates were saved every 2.5 ps. During the MD simulation, all bonds involving hydrogen atoms were constrained to their equilibrium distance using the SHAKE algorithm [39], and a time step of 2 fs was adopted.
MM-PBSA Calculations for Binding Free Energy Calculations
MM-PBSA was used to calculate the binding affinity for progesterone and apigenin to human PR respectively based on the 3 ns MD simulations. In the MM-PBSA scheme, the binding free energy (ΔGbind) is computed using the following Eq. 1:
| 1 |
ΔEvdW, ΔEele, ΔGpol, and ΔGnonpol were calculated according to widely used methods [40–42]. The contribution of the entropy change to the binding affinity (− TΔS) was estimated by a computationally simple and efficient approach recently proposed by Duan et al. based on the fluctuations of protein-ligand interaction energies [43].
Cell Culture and Small Molecules
Ishikawa [44] cells stably expressing either PR-A or PR-B under the control of CMV were graciously donated by Dr. Leen J Blok, Department of Obstetrics and Gynecology, Erasmus Medical Center, Rotterdam, The Netherlands. Ishikawa cells were maintained in phenol red-free DMEM/F12, 5% charcoal-stripped FBS, and selection antibiotic (500 μg/ml G418 or 250 μg/ml hygromycin). Correct expression of PR was validated by western blot (Supplementary Fig. S1A). T47D cells were maintained in RPMI 1640 (11-835-030, Thermo Scientific, Waltham, MA) supplemented with 10% FBS, 4.5 g of glucose/l, and penicillin/streptomycin. MDA-MB-231 cells were maintained in DMEM/F-12 media (11330-032, Invitrogen) supplemented with 5% FBS, 20 ng/ml insulin (700112P, Gemini Bio-products, West Sacramento, CA), 2 mM L-glutamine, and penicillin/streptomycin. T47D and MDA-MB-231 cells were plated in steroid-free media 24 h before beginning any experiment, which lacked phenol red and used double charcoal-stripped FBS. All cells were maintained in a humidified incubator at 37 °C and 5% CO2 and passed at confluence.
Progesterone (P0130-25G, Sigma Aldrich, St. Louis, MO), RU486 (10006317, Cayman Chemical, Ann Arbor, MI), apigenin (A-002, INDOFINE Chemical Company, Hillsborough, NJ), and MPA (46412, Sigma Aldrich) were dissolved in DMSO at × 1000 before being added to media.
Progesterone and Androgen Response Element (PRE and ARE) Luciferase (Luc) Assay
Transcriptional activity was measured with a plasmid construct containing a thymidine kinase promoter with two steroid response elements (ACAAGA half site) that bind PR, GR, and AR [45] as previously described [32]. To measure PRE/Luc, 40,000 Ishikawa cells per well were plated in 24 well plates. The next day, cells were transfected with 200 ng/well of the luciferase construct and 100 ng/well of a construct containing β-galactosidase under the control of CMV. For activation of AR or GR Luc, the same construct was transfected into MDA-MB-231 cells which express AR and GR. Transfections were carried out with LT1 transfection reagent per the manufacturer’s instructions (MIR 2304, Mirus Bio, Madison, WI). Cells were treated with pure compounds at indicated concentrations. Twenty-four hours later, lysis buffer was added to each well and cells were frozen at − 80 °C. Luciferase activity was measured on a synergy BioTek plate reader. The first six determinations of relative luminance units (RLU) were averaged for each well. Luciferase activity was normalized to β-galactosidase and then normalized to vehicle control for each experiment.
Proliferation Assay
One thousand Ishikawa or T47D cells per well were seeded onto a 96-well plate. Two hours later, a day 0 plate was collected. Compounds (apigenin, RU486, or progesterone) were added at indicated concentrations on the remaining plates. For Ishikawa cells, a second plate was collected on day 7. For T47D cells, plates were collected on days 3, 6, 9, and 12. Half of the media was replaced at day 6 for day 9 and 12 plates. At collection, media was removed and cells were fixed with 20% tricholoroacetic acid (TCA). Proliferation was measured by sulforhodamine B (SRB) staining as previously described [46]. Values were normalized to day 0.
Western Blots
Ishikawa PR-B cells were treated with indicated concentrations of apigenin for 24 h on a 6-well plate. Cells were collected in RIPA lysis buffer containing protease and phosphatase inhibitors. Twenty-five micrograms of protein was separated on SDS-PAGE cells by electrophoresis and transferred to a nitrocellulose membrane [32]. Membranes were blocked for 1 h and probed overnight for AR, GR, PR, α tublin, or β actin (Supplementary Table 1). The next day, membranes were washed with TBS-T incubated with anti-rabbit secondary antibody and developed with SuperSignal West Femto Substrate (34095, Thermo Scientific, Rockford IL). Images were captured with a FluorChem C (Protein Simple, San Jose, CA).
Animal Study
Ovariectomized (OVX) Sprague Dawley rats (Harlan Laboratories, Madison, WI) were used for this study. Twenty-four animals (eight per treatment) were housed at 21 °C in 12-h light:12-h dark cycles and were fed 7% corn diet (Harlan Laboratories, Madison, WI) devoid of phytoestrogens. At least 2 weeks post OVX animals were treated by oral gavage with DMSO/corn oil mixture for eight consecutive days. Treatments consisted of oral gavage of corn oil/DMSO (control), or vehicle supplemented with 5.625 mg/animal/day of apigenin, 5.625 mg/animal/day genistein, or both compounds together. These doses were based on genistein intake in a previous study [47] and had been used previously by our laboratory to study kaempferol [33]. Twenty-four hours after last treatment, animals were euthanized by humane means and uteri were excised and fixed in 4% paraformaldehyde (PFA) for immunohistochemical analysis. All animal studies were approved by the UIC Institutional Animal Care and Use Committee.
Hematoxylin and eosin (H&E) and immunohistochemical staining for PR, Hand2, and Ki67 was carried out as previously described [33]. Reproductive tracts were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at 5 μm. Sections were deparaffinized, rehydrated, probed with primary antibody (Supplementary Table 1) ov ernight, washed, blocked, and probed with secondary antibody (1:200 biotinylated anti-rabbit; BA-1000, Vector Laboratory, Burlingame, CA) for 30 min. Slides were developed with 3,3′diaminobenzidine (SK-4100, Vector Laboratories, Burlingame, CA), counterstained with hematoxylin, and mounted. Images were captured at × 40 or × 100 with a Nikon Eclipse E600 microscope.
Statistical Analysis
All data is represented by the mean ± SEM and replicated at least three times. The data was analyzed by a one-way or two-way ANOVA followed by Dunnett’s post hoc analysis comparing each treatment to a control group as indicated. P < 0.05 was considered significant. All analyses were conducted in Prism version 7.0a.
Results
Molecular Modeling of Apigenin with the Progesterone Receptor
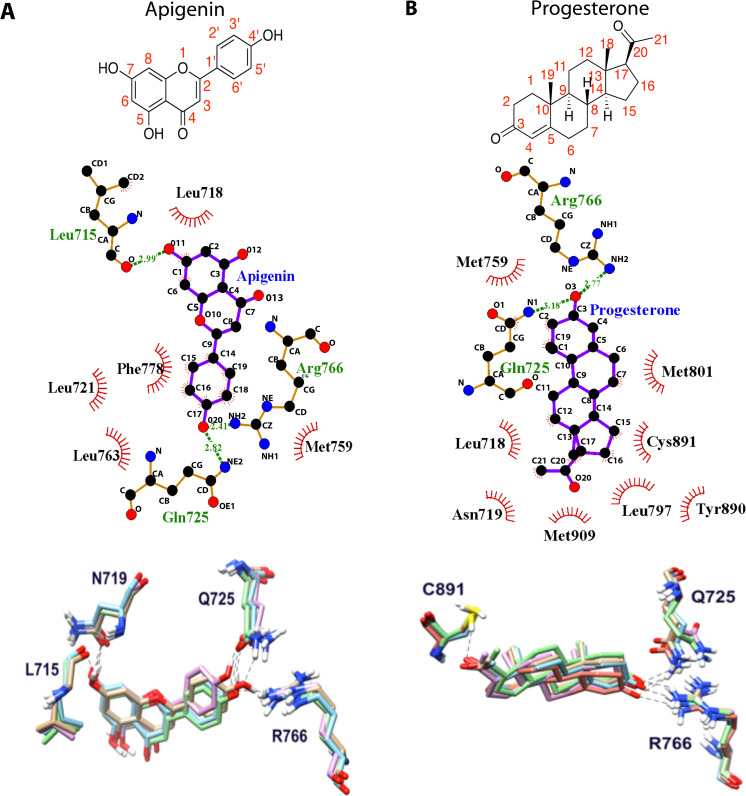
Previous studies suggest that apigenin alters progestin signaling [10, 14], potentially by interacting directly with the PR [32]. To test this hypothesis, the binding of apigenin and progesterone to the human PR was compared via molecular docking. Docking predicts that the 4′-OH of apigenin should form H bonds with Q725 and R766, and the 7-OH should form an H bond with the backbone of L715 as well as some van der Waals interactions with L718, L721, M759, L763, and F778 (Fig. 1a). Progesterone had similar interactions as apigenin (Fig. 1b), also forming H bonds with Q725 and R766 and van der Waals interactions with L718, N719, M759, L797, M801, C891, Y890, and M909 in the predicted crystal complex.
Fig. 1.
Modeling of apigenin (left) and progesterone (right) to the progesterone receptor. a–b H bonds (green lines) and van der Waals (red lines) between apigenin (a) and progesterone (b) with the progesterone receptor by LigPlot+ [65]
Both apigenin and progesterone were stable during the 3 ns MD simulations (Supplementary Fig. 1) with the RMSD less than 1 Å with respect to their initial conformation before MD simulation. However, much tighter binding was found for the native ligand of progesterone based on the MM-PBSA binding free energy calculation (Table 1). The van der Waals interactions contributed more to the predicted progesterone binding (− 52.9 kcal/mol) to its receptor than the apigenin binding (−39.1 kcal/mol). Although the electrostatic interactions attribute more to apigenin binding (− 30.0 kcal/mol), the electrostatic energy from solvation balanced this contribution. With a similar nonpolar solvation free energy (− 3.8 kcal/mol for progesterone and − 3.1 kcal/mol for apigenin) and entropy contributions (9.1 kcal/mol for progesterone and 9.9 kcal/mol for apigenin), the predicted binding affinity is stronger for progesterone (− 31.6 kcal/mol) than apigenin (− 15.5 kcal/mol), most of which comes from van der Waals difference between them. These results suggest that apigenin can readily bind to the PR, but at lower affinity than the native progesterone ligand. This agrees with our early report that apigenin bound to the progesterone receptor with an IC50 of 1.0 μM [32].
Table 1.
The energy component for binding free energy of ∆GMM-PBSA
| ∆EvdW a (kcal/mol) | ∆Eele b (kcal/mol) | ∆Gpol c (kcal/mol) | ∆Gnonpol d (kcal/mol) | -T∆S | ∆Gbind e (kcal/mol) | |
|---|---|---|---|---|---|---|
| Progesterone | − 52.9 | − 21.5 | 37.5 | − 3.8 | 9.1 | − 31.6 |
| Apigenin | − 39.1 | − 30.0 | 46.8 | − 3.1 | 9.9 | − 15.5 |
avan der Waals contribution
bElectrostatic energy
cPolar solvation free energy
dNonpolar solvation free energy
eBinding free energy
Expression of PR, AR, and GR in Ishikawa, MDA-MB-231, and T47D Cells
Synthetic ligands that activate PR typically also activate AR and GR, making it difficult to isolate the effect of a compound to a specific nuclear receptor. Therefore, we screened several cell lines using western blotting to determine PR, AR, and GR expression. Ishikawa cells are an endometrial adenocarcinoma cell line that does not express PR [44, 48, 49]. Therefore, we used Ishikawa cells engineered to stably express PR-A, PR-B, or neither receptor (PR null) [44, 49]. Western blot revealed very high PR-A and PR-B expression in T47D cells. Ishikawa PR-B cells expressed both PR-A and PR-B due to processing of the PR-B transcript. Ishikawa PR-A cells expressed only PR-A, while Ishikawa null and MDA-MD-231 cells lacked detectable PR. MDA-MD-231 and T47D cells expressed higher levels of AR-A and AR-B. Ishikawa and MDA-MB-231 cells expressed moderate levels of GR. T47D expressed very low levels of GR (Supplementary Fig. 2), while Ishikawa null was positive for GR.
Apigenin Has Mixed Agonist Effects
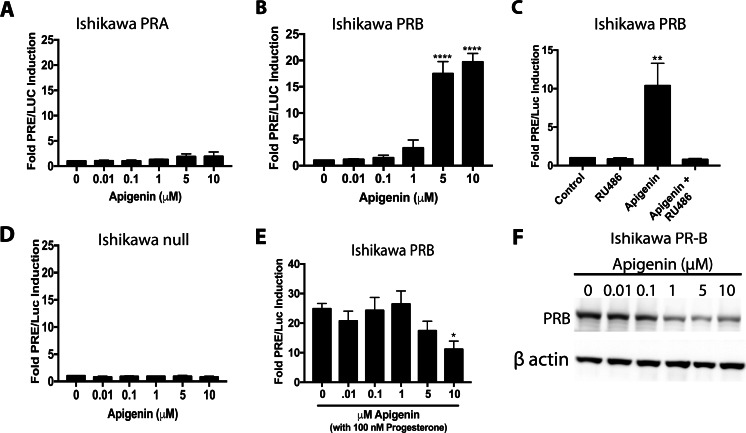
Apigenin had no effect on PRE/Luc activity in either Ishikawa null or PR-A (Fig. 2a). In contrast, 5 and 10 μM of apigenin increased PRE/Luc activity approximately 20-fold in Ishikawa PR-B cells (P < 0.0001; Fig. 2b). RU486 (which inhibits PR, GR, and AR) completely abrogated the effect of apigenin in Ishikawa PR-B cells (Fig. 2c). Confirming that apigenin specifically activates PR and not GR, apigenin had no effect on the parental Ishikawa cell line that lacks PR expression but retains GR (Fig. 2d). In the presence of 100 nM of progesterone, 10 μM of apigenin reduced the PRE/Luc activity induced by progesterone by 43% (P < 0.05, Fig. 2e), indicating that apigenin is a mixed agonist for PR in these cells. Confirming that apigenin interacts directly with the progesterone receptor to trigger ubiquitin-dependent degradation, apigenin induced a significant reduction in PR-B protein levels at concentrations ≥ 1 μM in Ishikawa PR-B cells (P < 0.05; Fig. 2f and densitometry in Supplementary Fig. S3A). These data all support that apigenin directly binds PR and alters transcriptional regulation.
Fig. 2.
Apigenin activates PR-B in Ishikawa cells. a–e Luciferase activity in Ishikawa cells stably expressing PR-A (a), PR-B (b, c, and e), PR null (d) transfected with a PRE/Luc construct and treated with a apigenin, progesterone, or RU486 as indicated. f Representative western blot for PR-B levels in Ishikawa PR-B cells treated with apigenin for 24 h. n ≥ 3. Significantly different from 0 μM or control, *P < 0.05, **P < 0.01. ****P < 0.0001
Apigenin Reduced Proliferation of Ishikawa Cells Independently of PR
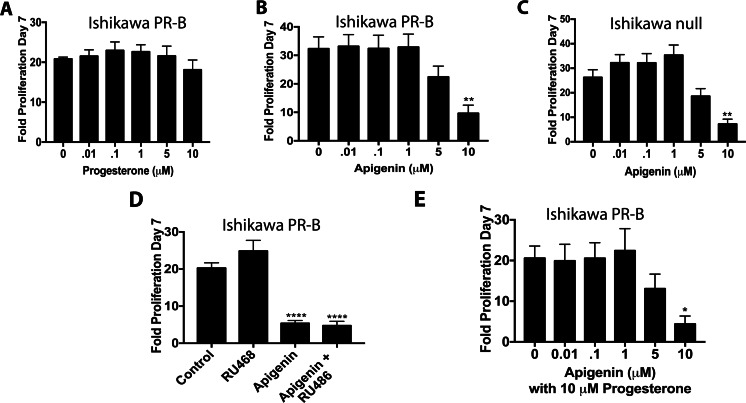
Progesterone typically represses the growth of endometrial cancer; therefore, the ability of apigenin and progesterone to reduce Ishikawa PR-B cell proliferation was tested. Interestingly, progesterone (0.01–10 μM) had no effect on proliferation (Fig. 3a), even though the highest concentration of apigenin (10 μM) reduced proliferation of Ishikawa PR-B cells (P < 0.01; Fig. 3b). The inability of progesterone to repress proliferation suggested that the effect of apigenin in Ishikawa PR-B cells was PR-independent. In confirmation, 10 μM of apigenin also suppressed proliferation of Ishikawa null cells (Fig. 3c). To determine if AR or GR was mediating the effect of apigenin on Ishikawa cells, we tested the ability of RU486, which inhibits AR, GR, and PR. However, RU486 was unable to block the anti-proliferative effects of apigenin (Fig. 3d). Finally, we tested for a potential interaction between progesterone and apigenin. But in the presence of 10 μM of progesterone, apigenin has similar effects as before, reducing proliferation of Ishikawa PR-B cells at 10 μM (P < 0.05; Fig. 3e). These results confirm that the effect of apigenin on Ishikawa cell proliferation was PR-independent, perhaps through inhibition of AKT signaling or production of reactive oxygen species (ROS) [6, 9].
Fig. 3.
Apigenin reduces proliferation of Ishikawa cells independently of progesterone receptor. a–b Proliferation of Ishikawa PR-B cells in response to progesterone (a) or apigenin (b). c Proliferation of Ishikawa null cells treated with apigenin. d Proliferation of Ishikawa PR-B cells treated with apigenin and Ru486 as indicated. e Proliferation of Ishikawa PR-B cells treated with apigenin in the presence of progesterone. n ≥ 3. Significantly different from 0 μM or control, *P < 0.05, **P < 0.01. ****P < 0.0001
Apigenin Stimulates Proliferation of T47D Cells Through PR
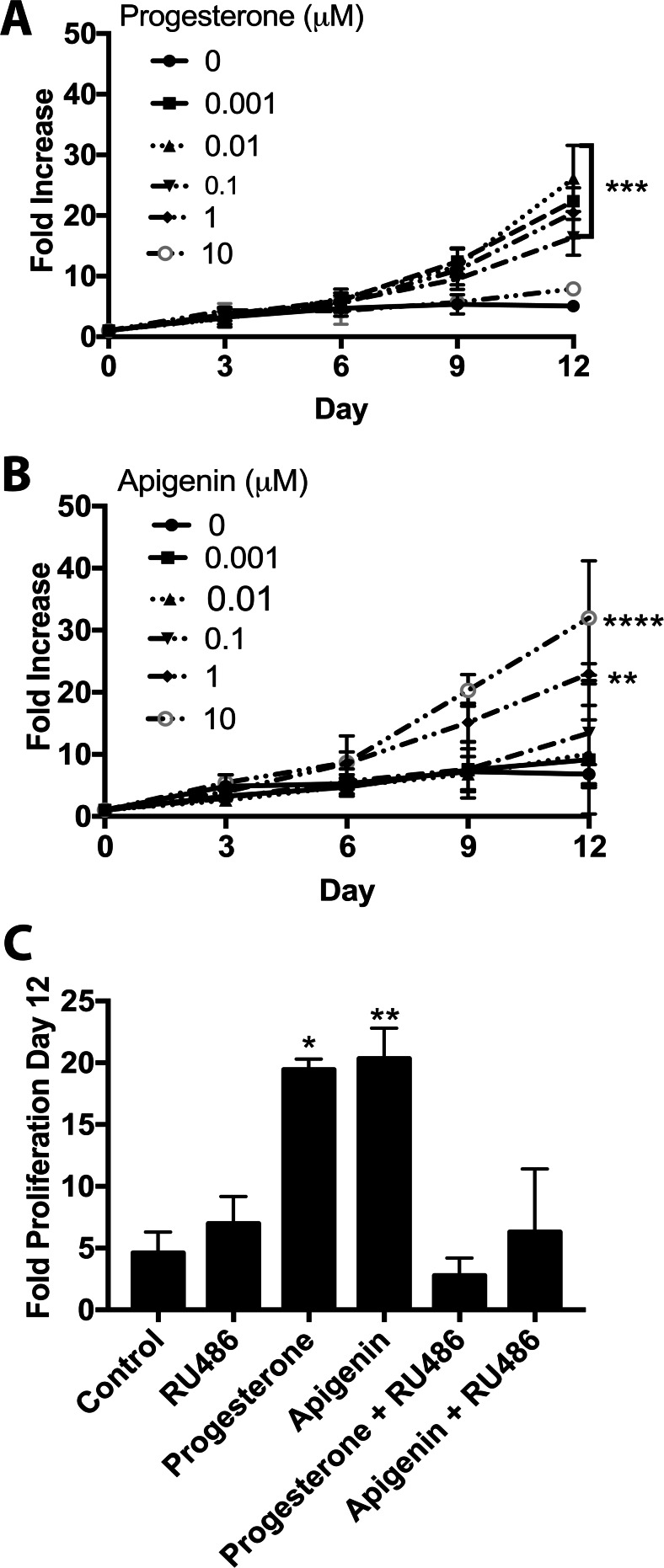
As the effects of apigenin on proliferation of Ishikawa cells were not mediated by PR, the ability of apigenin to alter proliferation of T47D cells was tested, which endogenously express high levels of PR and very low levels of GR (Supplementary Fig. 2). T47D cells were treated with apigenin (0.01–10 μM) or progesterone (0.001–10 μM) and allowed to proliferate for 12 days. Progesterone had a biphasic effect where concentrations ranging from 0.001–1 μM of progesterone significantly increased proliferation by day 12 (P < 0.05), but 10 μM had no effect (Fig. 4a). Apigenin demonstrated a similar pattern; however, ≥ 1 μM of apigenin was required to significantly increase proliferation of T47D cells (0.05; Fig. 4b). To confirm that PR mediated the effect, RU486 (1 μM) was added and completely blocked the proliferative effect of both 0.001 μM of progesterone and 1 μM of apigenin (Fig. 4c).
Fig. 4.
Apigenin stimulates proliferation of T47D cells through the PR. a–b Proliferation of T47D cells over 12 days in response to progesterone (a) or apigenin (b). c Proliferation of T47D cells treated with progesterone (0.01 μM), apigenin (1 μM), or RU486 (1 μM) as indicated. n ≥ 3. Significantly different from 0 μM or control, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001
Apigenin also Activates the Androgen Receptor (AR)
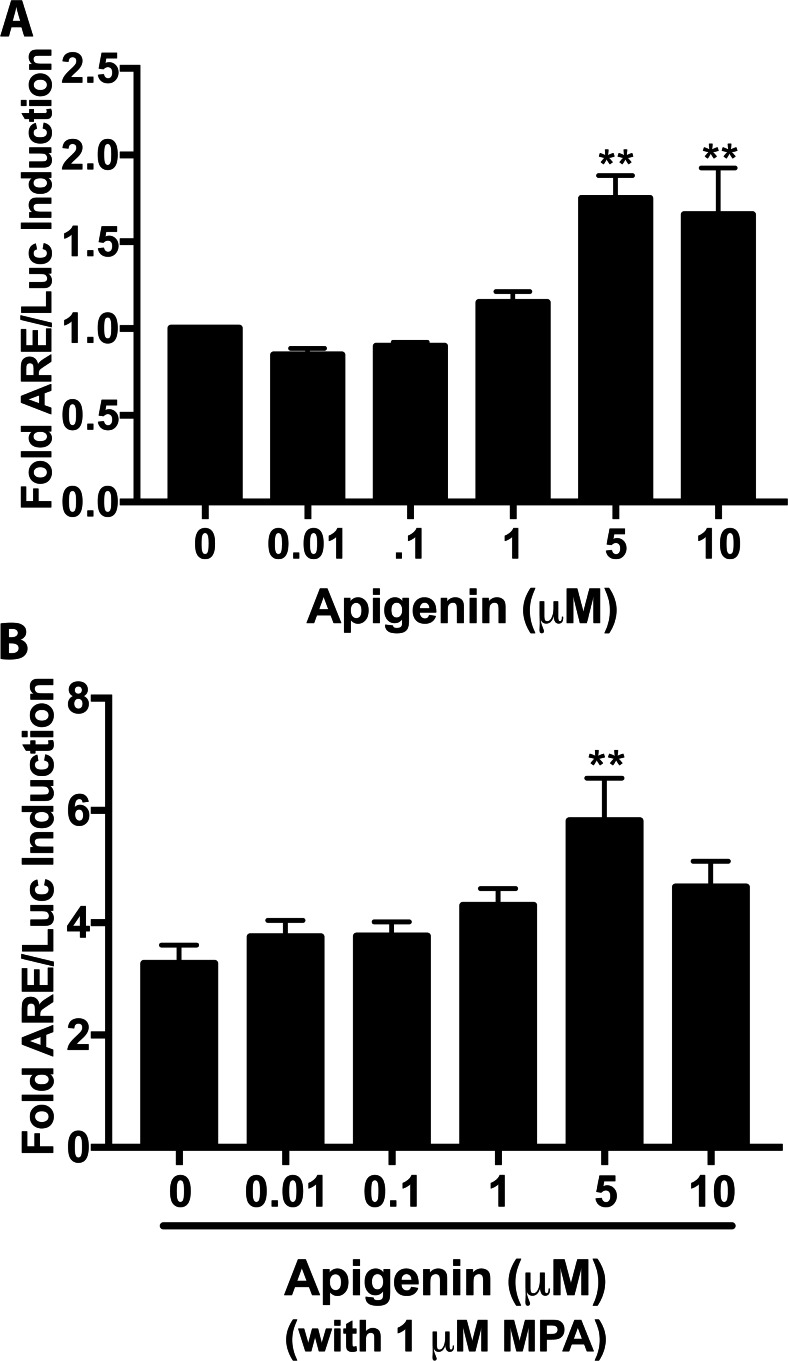
Natural and synthetic compounds that interact with the PR also frequently interact with AR and GR [50, 51]. To determine if apigenin interacted with other nuclear receptors, Luc activity was measured in MDA-MB-231 cells, which express AR and GR but not PR (Supplementary Fig. 2). When treated with apigenin alone, 5 and 10 μM of apigenin increased luciferase activity by approximately 75% (P < 0.01; Fig. 5a). To determine if apigenin interacted with a ligand for GR and AR, MDA-MB-231 cells were treated with a dose-response of apigenin in the presence of 1 μM of MPA (which activates PR, GR, and AR). MPA (1 μM) by itself increased luciferase activity 3-fold over DMSO treated cells. Interestingly, apigenin had an apparently additive effect, with 5 and 10 μM of apigenin in combination with 1 μM of MPA approximately doubling Luc activity over Luc activity due to MPA treatment alone control (Fig. 5b). These results indicate that apigenin also activates other steroid receptors, in addition to PR. However, since there was no induction of Luc in Ishikawa null cells that only express GR, the effect in MDA-MB-231 is most likely due to AR.
Fig. 5.
Apigenin activates the androgen receptor. a–b ARE/Luc activity in MDA-MB-231 cells (which express AR but not PR) treated with a dose-response of apigenin or apigenin, in the presence of 1 μM of MPA. n ≥ 4. Significantly different from 0 μM apigenin, **P < 0.01
Apigenin Demonstrated Progestin-Like Effects in the Uterus
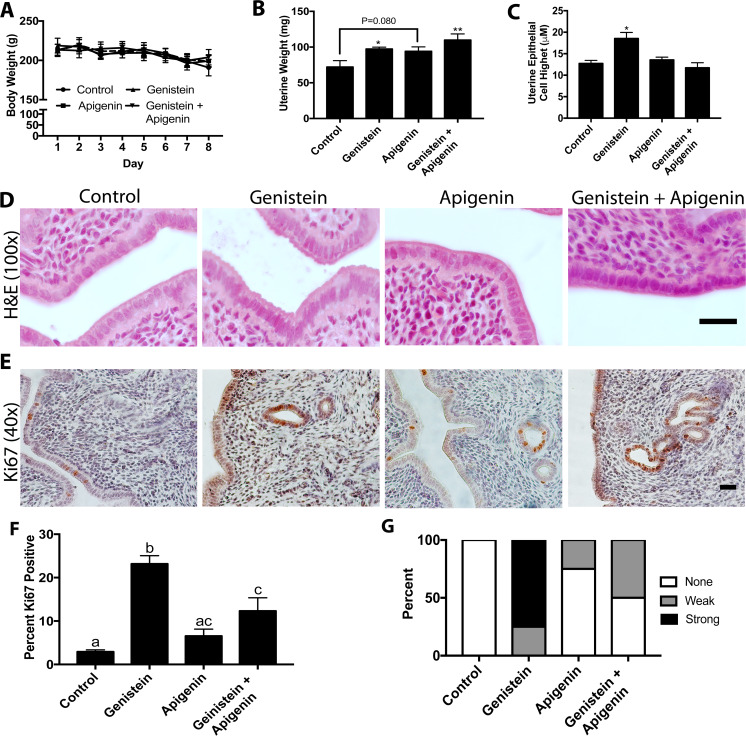
The results thus far indicated that apigenin acted a phytoprogestin in vitro. To confirm that apigenin had progestin-like effect in vivo, the ovariectomized rat model was tested [33]. Genistein is a well-established phytoestrogen [47], and it was used in combination with apigenin to determine if apigenin could block the estrogenic effects of genistein in the uterus. Genistein was also chosen due to its common presence in botanical formulations used for women’s health [52]. Rats were treated via oral gavage for 8 days with vehicle, genistein, apigenin, or genistein + apigenin as indicated [33]. No treatment had any effect on body weight over the 8-day treatment period (Fig. 6a). Genistein and genistein + apigenin treatments significantly increased uterine weight over control (P < 0.05). Apigenin tended to increase uterine weight, but this did not reach statistical significance (P = 0.08; Fig. 6b), which was similar to the effects we previously reported with kaempferol [33]. Genistein also increased epithelial cell height by 45%, (P < 0.05), a well-established effect of estrogens on the uterine epithelium [53]. Apigenin alone had no effect on epithelial cell height, and when given together, apigenin completely abrogated the effect of genistein on epithelia height (Fig. 6c, d).
Fig. 6.
Apigenin acts as a phytoprogestin in the rat uterus. a Daily body weights of rats treated with vehicle (control), genistein (phytoestrogen), apigenin (phytoprogestin), or apigenin + genistein for 8 days. b Uterine weights of rats treated as indicated. c Height of the uterine luminal epithelial after treatment. d Representative H&E images of the endometrium in rats treated as indicated for 7 days (× 100). e Representative images of Ki67 immunostaining intensity in the uterine endometrium after 7 days of treatment. f Proliferative index (percent Ki67 positive) of the uterine epithelial. h Ki67 score in the ovarian stroma of rat treated as indicated. n ≤ 4. Significantly different from control, *P < 0.05, **P < 0.01. Bars without common letter are significantly different a–cP < 0.05. scale bar = 25 μm
Uterine proliferation was analyzed with Ki67 immunostaining. In untreated rats, 2.9% of epithelial cells were positive for Ki67. Genistein dramatically increased Ki67 immunoreactivity in the epithelium (23.17%, P < 0.05). Ki67 staining in apigenin treated rats was similar to control (6.5%). In apigenin treated rats, 12.32% of epithelia were positive for Ki67, which was significantly higher than control, but also less than genistein treatment (P < 0.05; Fig. 6e–f). Treatment with phytosteroids significantly altered (P < 0.05) Ki67 immunostaining in the uterine stroma, with 100% of control rats have undetectable Ki67 staining and 75% of genistein-treated rats having strong immunostaining and 25% have low immunostaining. In genistein + apigenin-treated rats, 50% had weak staining in the ovary stroma and 50% had no immunostaining.
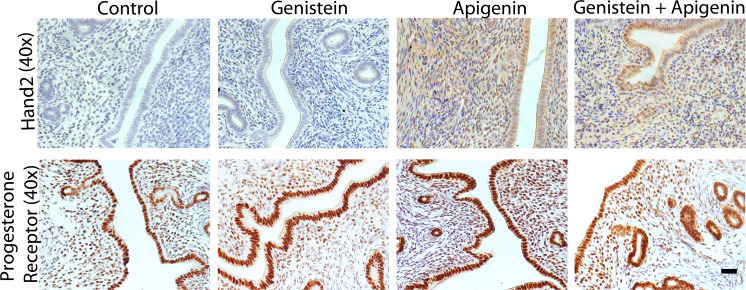
Next expression of Hand2, a transcription factor stimulated by progestins in the uterus [54] was analyzed. Hand2 was undetectable in the uteri of untreated rats, and genistein induced a small increase in immunostaining scattered throughout the endometrium. Supporting that apigenin is a phytoprogestin, apigenin induced a prominent increase in Hand2 expression in the uterine epithelial, with lighter staining in the endometrial stroma. Staining was dramatically reduced in the rat tissue from genistein combined with apigenin treatment relative to apigenin treatment alone (Fig. 7 top). Our previous results showed that apigenin reduced PR-B levels in Ishikawa PR-B cells; therefore, IHC for PR levels were performed. Immunostaining showed high PR levels in the uterine epithelial and in the sub-epithelial stroma of the control animals. No treatment had an observable effect on PR levels or distribution (Fig. 7 bottom). Collectively, these results show that apigenin is an orally available phytoprogestin that can counter the effects of phytoestrogens in the uterus as well as induce expression of known PR targets.
Fig. 7.
Apigenin induces Hand2 expression in the uterus but does not alter levels of the progesterone receptor. Immunohistochemistry for Hand2 (top) and progesterone receptor (bottom) in the uteri of rats treated with vehicle (control) genistein, apigenin, or genistein + apigenin as indicated. Scale bar = 25 μm
Discussion
Flavonoids are found in many foods and herbal supplements, with Americans consuming an estimated 189.7 mg/day [55]. There is an inverse relationship between intake of dietary flavonoids and a woman’s risk of epithelial cancer [56]. Among post-menopausal women, intake of flavonoids and flavones was associated with lower risk of breast cancer [8] and high flavonone intake tended to be associated with lower ovarian cancer risk [57]. These data suggest that flavonoids are potentially chemopreventive; however, their mechanisms of action remain an area of intense research. Apigenin is a flavone, a subclass of flavonoids, and is widely considered a phytoestrogen, though it has much lower potency than other phytoestrogens such as genistein and 8-prenylnaringenin [13]. The current research shows that apigenin is also a phytoprogestin, with mixed agonist effects. This agrees with previous studies showing that apigenin blocked MPA-stimulated growth of breast cancer [10, 14, 34]. Thus, one mechanism of action responsible for apigenin’s biological activity is altered PR signaling.
In vivo, apigenin is metabolized quickly into luteolin and to a lesser extent scutellarein and isoscutellarein [18]. Luteolin, as an apigenin metabolite, is also a phytoprogestin [58], potentially extending the biology activity of apigenin in vivo. Furthermore, people exposed to apigenin through diet or herbal supplements are also exposed to a wide range of flavonoids including apigenin, luteolin, and kaempferol, which all have phytoprogestin activity [32, 33, 58, 59]. Luteolin was shown to be a PR antagonist and did not cause PR downregulation [34]. Similarly, luteolin reduced medroxyprogesterone acetate (MPA)-induced alkaline phosphatase in T47D cells [58], and it reduced MPA-stimulated VEGF secretion from T47D and BT-474 cells [59]. Kaempferol did not increase uterine weight in vivo, inhibited uterine epithelial proliferation, and induced Hand2 protein expression [33]. Combined with the well-established phytoestrogens present in these same foods and supplements [52], there is potential for complex changes to steroid signaling [31].
Immunohistochemistry indicated that apigenin increased Hand2 expression in the uterine endometrium, further suggesting that apigenin is a phytoprogestin. Hand2 is a transcription factor that is well established to be stimulated by PR in the uterus [54]. Specifically, Li et al. [54] showed that Hand2 mediated the anti-proliferative effects of progesterone in the endometrium. This agrees with the current results that apigenin induced Hand2 expression and reduced the proliferative effect of genistein.
The fact that the flavones apigenin, kaempferol, and luteolin are all phytoprogestins [32, 33, 58], the flavanone naringenin and the isoflavone genistein and daidzein lack progestogenic activity [32] suggests an important structure/activity relationship. In flavones (apigenin, kaempferol, and luteolin), the B benzene ring is attached at the two carbons of the heterocyclic ring. In contrast, in isoflavones (genistein and daidzein), the B benzene ring is at the 3-carbon position. In daidzein, the B ring is attached at the 2-carbon position, but the lack of a double bond in the heterocyclic ring, relative to flavones, alters the stereochemistry at the 2-carbon position. This agrees with our molecular docking model of apigenin, which indicated that the B benzene ring contributed to PR binding through van der Waals interaction with Phe778 and hydrogen bonding with Gln725 and Arg776. The altered position of the B ring in isoflavones and flavanones would likely lead to loss of those interactions, though a more detailed analysis is necessary to confirm these potential changes.
Interestingly, apigenin increased luciferase in Ishikawa PR-B cells (with express both PR-A and PR-B) but did not increase PRE/Luc activity in PR-A cells. Given that both receptors are encoded by the same gene and hence have identical ligand binding domains (LBDs), apigenin likely binds to both receptors equally. PR activity is extensively controlled by phosphorylation, which differs between the receptor due to the extra N-terminal segment of the receptor. For example, phosphorylation at S294 (which is present in PR-A but typically not phosphorylated in intact cells) is important in controlling PR transcriptional activity [60, 61]. PR-B may be more active than PR-A on the simple PR promoter present in our PRE/Luc construct.
Apigenin displayed mixed agonist activity for PR, but has agonist activity for AR in MBA-MD-231 cells, increasing ARE/Luc activity in the presence of MPA. The reason for this difference is unclear but could be due to differences in how apigenin interacts with each steroid receptor or how each receptor recruits co-factors. In either case, the difference could be important in vivo if apigenin inhibits PR activity while increasing AR activity. For example, progesterone is well known to inhibit proliferation of endometrial cancer cells [44], but increasing evidence suggests that AR signaling contributes to endometrial cancer [62]. This suggests apigenin may have conflicting effects in some cancers.
Apigenin reduced proliferation of endometrial cells (Ishikawa) independently of PR. Apigenin could potentially be inhibiting AKT. Apigenin reduced levels of phospho-AKT in prostate and breast cancer cells [63, 64]. Another potential mechanism for reduced proliferation is via reactive oxygen species (ROS) production. Apigenin increased ROS production and stimulated mitochondria-mediated cell death in prostate cells [9]. Thus, apigenin may exhibit a multi-pronged effect on the proliferation of cancer cells, targeting PR, AKT, and ROS. Disrupting multiple pathways could be important in cancers that become resistant to PR-targeted therapy [27].
In conclusion, the naturally occurring flavonoid apigenin is a phytoprogestin with mixed agonist activity. Apigenin stimulated PRE/Luc activity in Ishikawa cells and stimulated proliferation of T47D cells through PR. Furthermore, apigenin exerted progestin-like effects in vivo such as reduced induced Hand2 expression in the uterus. These progestogenic effects likely contribute to the anti-cancer effects seen in diets high in flavonoids and the anti-cancer properties of apigenin in culture. Researchers should consider PR as a potential mediator when studying the effects of apigenin in the future. More research is needed to understand the effects of complex combinations of flavonoids that someone on a diet high in flavonoids or taking herbal supplements would be exposed to.
Electronic Supplementary Materials
RMSD of all atoms of apigenin and progesterone with respect to their initial conformations before MD simulation. (GIF 24 kb)
Representative western blot for the progesterone receptor (PR), androgen receptor (AR), and glucocorticoid receptor (GR) in Ishikawa, MDA-MD-231, and T47D cells. (GIF 34 kb)
Densitometry for PR-B in Ishikawa PR-B cells treated with apigenin for 24 h. (GIF 7 kb)
(DOCX 31 kb)
Acknowledgements
This work was supported by R01 AT008824 to JEB and a Fellowship from T32 AT007533 supporting MD from the National Center for Complementary and Integrative Health.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 2.Smith, Kawa, Eckl, Stredney (2017) Herbal supplement sales in the US increase 7.7% in 2016. HerbalEGram 56–65
- 3.Zulkipli AF, Islam T, Mohd Taib NA, et al (2017) Use of complementary and alternative medicine among newly diagnosed breast cancer patients in malaysia: an early report from the MyBBC study. Integr Cancer Ther 1534735417745248 .10.1177/1534735417745248 [DOI] [PMC free article] [PubMed]
- 4.Song S, Youn J, Lee YJ, et al. Dietary supplement use among cancer survivors and the general population: a nation-wide cross-sectional study. BMC Cancer. 2017;17:891. doi: 10.1186/s12885-017-3885-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tong X, Pelling JC. Targeting the PI3K/Akt/mTOR axis by apigenin for cancer prevention. Anti Cancer Agents Med Chem. 2013;13:971–978. doi: 10.2174/18715206113139990119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosetti C, Spertini L, Parpinel M, et al. Flavonoids and breast cancer risk in Italy. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2005;14:805–808. doi: 10.1158/1055-9965.EPI-04-0838. [DOI] [PubMed] [Google Scholar]
- 8.Hui C, Qi X, Qianyong Z, et al. Flavonoids, flavonoid subclasses and breast cancer risk: a meta-analysis of epidemiologic studies. PLoS One. 2013;8:e54318. doi: 10.1371/journal.pone.0054318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrissey C, O’Neill A, Spengler B, et al. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 10.Mafuvadze B, Benakanakere I, López Pérez FR, et al. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz(a)anthracene-induced mammary tumors in Sprague-Dawley rats. Cancer Prev Res (Phila Pa) 2011;4:1316–1324. doi: 10.1158/1940-6207.CAPR-10-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long X, Fan M, Bigsby RM, Nephew KP. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-α-dependent and -independent mechanisms. Mol Cancer Ther. 2008;7:2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo H-S, DeNardo DG, Jacquot Y, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006;99:121–134. doi: 10.1007/s10549-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 13.Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 14.Mafuvadze B, Benakanakere I, Hyder SM. Apigenin blocks induction of vascular endothelial growth factor mRNA and protein in progestin-treated human breast cancer cells. Menopause. 2010;17:1055–1063. doi: 10.1097/gme.0b013e3181dd052f. [DOI] [PubMed] [Google Scholar]
- 15.Tang D, Chen K, Huang L, Li J. Pharmacokinetic properties and drug interactions of apigenin, a natural flavone. Expert Opin Drug Metab Toxicol. 2017;13:323–330. doi: 10.1080/17425255.2017.1251903. [DOI] [PubMed] [Google Scholar]
- 16.Cai H, Boocock DJ, Steward WP, Gescher AJ. Tissue distribution in mice and metabolism in murine and human liver of apigenin and tricin, flavones with putative cancer chemopreventive properties. Cancer Chemother Pharmacol. 2007;60:257–266. doi: 10.1007/s00280-006-0368-5. [DOI] [PubMed] [Google Scholar]
- 17.Gradolatto A, Basly J-P, Berges R, et al. Pharmacokinetics and metabolism of apigenin in female and male rats after a single oral administration. Drug Metab Dispos Biol Fate Chem. 2005;33:49–54. doi: 10.1124/dmd.104.000893. [DOI] [PubMed] [Google Scholar]
- 18.Gradolatto A, Canivenc-Lavier M-C, Basly J-P, et al. Metabolism of apigenin by rat liver phase I and phase II enzymes and by isolated perfused rat liver. Drug Metab Dispos. 2004;32:58–65. doi: 10.1124/dmd.32.1.58. [DOI] [PubMed] [Google Scholar]
- 19.Masuelli L, Benvenuto M, Mattera R, et al. In vitro and in vivo anti-tumoral effects of the flavonoid apigenin in malignant mesothelioma. Front Pharmacol. 2017;8:373. doi: 10.3389/fphar.2017.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandey M, Kaur P, Shukla S, et al. Plant flavone apigenin inhibits HDAC and remodels chromatin to induce growth arrest and apoptosis in human prostate cancer cells: in vitro and in vivo study. Mol Carcinog. 2012;51:952–962. doi: 10.1002/mc.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hiremath SP, Badami S, Hunasagatta SK, Patil SB. Antifertility and hormonal properties of flavones of Striga orobanchioides. Eur J Pharmacol. 2000;391:193–197. doi: 10.1016/S0014-2999(99)00723-2. [DOI] [PubMed] [Google Scholar]
- 22.Diep CH, Daniel A, Mauro L, et al. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54:R31–R35. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brisken C. Progesterone signalling in breast cancer: a neglected hormone coming into the limelight. Nat Rev Cancer. 2013;13:385. doi: 10.1038/nrc3518. [DOI] [PubMed] [Google Scholar]
- 24.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 25.Luo H, Li S, Zhao M, et al. Prognostic value of progesterone receptor expression in ovarian cancer: a meta-analysis. Oncotarget. 2017;8:36845–36856. doi: 10.18632/oncotarget.15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Zhao D, Gong C, et al. Prognostic role of hormone receptors in endometrial cancer: a systematic review and meta-analysis. World J Surg Oncol. 2015;13:208. doi: 10.1186/s12957-015-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanekamp EE, Gielen SCJP, Smid-Koopman E, et al. Consequences of loss of progesterone receptor expression in development of invasive endometrial cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2003;9:4190–4199. [PubMed] [Google Scholar]
- 28.Wu N-Y, Huang H-S, Chao TH, et al. Progesterone prevents high-grade serous ovarian cancer by inducing necroptosis of p53-defective fallopian tube epithelial cells. Cell Rep. 2017;18:2557–2565. doi: 10.1016/j.celrep.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 29.Asi N, Mohammed K, Haydour Q, et al. Progesterone vs. synthetic progestins and the risk of breast cancer: a systematic review and meta-analysis. Syst Rev. 2016;5:121. doi: 10.1186/s13643-016-0294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll JS, Hickey TE, Tarulli GA, et al. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer. 2017;17:54–64. doi: 10.1038/nrc.2016.116. [DOI] [PubMed] [Google Scholar]
- 31.Dean M, Murphy BT, Burdette JE. Phytosteroids beyond estrogens: regulators of reproductive and endocrine function in natural products. Mol Cell Endocrinol. 2017;442:98–105. doi: 10.1016/j.mce.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toh MF, Sohn J, Chen SN, et al. Biological characterization of non-steroidal progestins from botanicals used for women’s health. Steroids. 2012;77:765–773. doi: 10.1016/j.steroids.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toh MF, Mendonca E, Eddie SL, et al. Kaempferol exhibits progestogenic effects in ovariectomized rats. J Steroids Horm Sci. 2014;5:136. doi: 10.4172/2157-7536.1000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mafuvadze B, Liang Y, Besch-Williford C, et al. Apigenin induces apoptosis and blocks growth of medroxyprogesterone acetate-dependent BT-474 xenograft tumors. Horm Cancer. 2012;3:160–171. doi: 10.1007/s12672-012-0114-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams SP, Sigler PB. Atomic structure of progesterone complexed with its receptor. Nature. 1998;393:392–396. doi: 10.1038/30775. [DOI] [PubMed] [Google Scholar]
- 36.Shelley JC, Cholleti A, Frye LL, et al. Epik: a software program for pK a prediction and protonation state generation for drug-like molecules. J Comput Aided Mol Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- 37.Verdonk ML, Cole JC, Hartshorn MJ, et al. Improved protein–ligand docking using GOLD. Proteins Struct Funct Bioinforma. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 38.Vanquelef E, Simon S, Marquant G, et al. R.E.D. Server: a web service for deriving RESP and ESP charges and building force field libraries for new molecules and molecular fragments. Nucleic Acids Res. 2011;39:W511–W517. doi: 10.1093/nar/gkr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kräutler V, van Gunsteren WF, Hünenberger PH. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J Comput Chem. 2001;22:501–508. doi: 10.1002/1096-987X(20010415)22:5<501::AID-JCC1021>3.0.CO;2-V. [DOI] [Google Scholar]
- 40.Kollman PA, Massova I, Reyes C, et al. Calculating structures and free energies of complex molecules: combining molecular mechanics and continuum models. Acc Chem Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Morin P, Wang W, Kollman PA. Use of MM-PBSA in reproducing the binding free energies to HIV-1 RT of TIBO derivatives and predicting the binding mode to HIV-1 RT of efavirenz by docking and MM-PBSA. J Am Chem Soc. 2001;123:5221–5230. doi: 10.1021/ja003834q. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Sun H, Li Y, et al. Assessing the performance of MM/PBSA and MM/GBSA methods. 3. The impact of force fields and ligand charge models. J Phys Chem B. 2013;117:8408–8421. doi: 10.1021/jp404160y. [DOI] [PubMed] [Google Scholar]
- 43.Duan L, Liu X, Zhang JZH. Interaction entropy: a new paradigm for highly efficient and reliable computation of protein–ligand binding free energy. J Am Chem Soc. 2016;138:5722–5728. doi: 10.1021/jacs.6b02682. [DOI] [PubMed] [Google Scholar]
- 44.Smid-Koopman E, Kuhne LCM, Hanekamp EE, et al. Progesterone-induced inhibition of growth and differential regulation of gene expression in pPRA- and/or PRB-expressing endometrial cancer cell lines. J Soc Gynecol Investig. 2005;12:285–292. doi: 10.1016/j.jsgi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 46.Dean M, Davis DA, Burdette JE. Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer Lett. 2017;391:114–124. doi: 10.1016/j.canlet.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santell RC, Chang YC, Nair MG, Helferich WG (1997) Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J Nutr 127:263–269 . 10.1093/jn/127.2.263 [DOI] [PubMed]
- 48.Lessey BA, Ilesanmi AO, Castelbaum AJ, et al. Characterization of the functional progesterone receptor in an endometrial adenocarcinoma cell line (Ishikawa): progesterone-induced expression of the α1 integrin. J Steroid Biochem Mol Biol. 1996;59:31–39. doi: 10.1016/S0960-0760(96)00103-3. [DOI] [PubMed] [Google Scholar]
- 49.Gielen SCJP, Hanekamp EE, Hanifi-Moghaddam P, et al. Growth regulation and transcriptional activities of estrogen and progesterone in human endometrial cancer cells. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2006;16:110–120. doi: 10.1111/j.1525-1438.2006.00279.x. [DOI] [PubMed] [Google Scholar]
- 50.Burris TP, Montrose C, Houck KA, et al. The hypolipidemic natural product guggulsterone is a promiscuous steroid receptor ligand. Mol Pharmacol. 2005;67:948–954. doi: 10.1124/mol.104.007054. [DOI] [PubMed] [Google Scholar]
- 51.Africander DJ, Storbeck K-H, Hapgood JP. A comparative study of the androgenic properties of progesterone and the progestins, medroxyprogesterone acetate (MPA) and norethisterone acetate (NET-A) J Steroid Biochem Mol Biol. 2014;143:404–415. doi: 10.1016/j.jsbmb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Dietz BM, Hajirahimkhan A, Dunlap TL, Bolton JL. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol Rev. 2016;68:1026–1073. doi: 10.1124/pr.115.010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato T, Wang G, Hardy MP, et al. Role of systemic and local IGF-I in the effects of estrogen on growth and epithelial proliferation of mouse uterus. Endocrinology. 2013;43:2673–2679. doi: 10.1210/endo.143.7.8878. [DOI] [PubMed] [Google Scholar]
- 54.Li Q, Kannan A, DeMayo FJ, et al. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science. 2011;331:912–916. doi: 10.1126/science.1197454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chun OK, Chung SJ, Song WO (2007) Estimated dietary flavonoid intake and major food sources of U.S. adults. J Nutr 137:1244–1252. 10.1016/j.nut.2014.04.012 [DOI] [PubMed]
- 56.Grosso G, Godos J, Lamuela-Raventos R, Ray S, Micek A, Pajak A, Sciacca S, D'Orazio N, Rio DD, Galvano F (2017) A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: level of evidence and limitations. Mol Nutr Food Res 61(4). 10.1002/mnfr.201600930 [DOI] [PubMed]
- 57.Cassidy A, Huang T, Rice MS, et al. Intake of dietary flavonoids and risk of epithelial ovarian cancer. Am J Clin Nutr. 2014;100:1344–1351. doi: 10.3945/ajcn.114.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nordeen SK, Bona BJ, Jones DN, et al. Endocrine disrupting activities of the flavonoid nutraceuticals luteolin and quercetin. Horm Cancer. 2013;4:293–300. doi: 10.1007/s12672-013-0150-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cook MT, Liang Y, Besch-Williford C et al (2015) Luteolin inhibits progestin-dependent angiogenesis, stem cell-like characteristics, and growth of human breast cancer xenografts. SpringerPlus 4. 10.1186/s40064-015-1242-x [DOI] [PMC free article] [PubMed]
- 60.Hagan CR, Daniel AR, Dressing GE, Lange CA. Role of phosphorylation in progesterone receptor signaling and specificity. Mol Cell Endocrinol. 2012;357:43–49. doi: 10.1016/j.mce.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clemm DL, Sherman L, Boonyaratanakornkit V, et al. Differential hormone-dependent phosphorylation of progesterone receptor A and B forms revealed by a phosphoserine site-specific monoclonal antibody. Mol Endocrinol Baltim Md. 2000;14:52–65. doi: 10.1210/mend.14.1.0413. [DOI] [PubMed] [Google Scholar]
- 62.Choi JP, Desai R, Zheng Y, et al. Androgen actions via androgen receptor promote PTEN inactivation induced uterine cancer. Endocr Relat Cancer. 2015;22:687–701. doi: 10.1530/ERC-15-0203. [DOI] [PubMed] [Google Scholar]
- 63.Erdogan S, Doganlar O, Doganlar ZB, et al. The flavonoid apigenin reduces prostate cancer CD44(+) stem cell survival and migration through PI3K/Akt/NF-κB signaling. Life Sci. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 64.Harrison ME, Power Coombs MR, Delaney LM, Hoskin DW. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Exp Mol Pathol. 2014;97:211–217. doi: 10.1016/j.yexmp.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RMSD of all atoms of apigenin and progesterone with respect to their initial conformations before MD simulation. (GIF 24 kb)
Representative western blot for the progesterone receptor (PR), androgen receptor (AR), and glucocorticoid receptor (GR) in Ishikawa, MDA-MD-231, and T47D cells. (GIF 34 kb)
Densitometry for PR-B in Ishikawa PR-B cells treated with apigenin for 24 h. (GIF 7 kb)
(DOCX 31 kb)