Abstract
Aim
The aim of this study is to: 1) develop and evaluate a model to predict severe pain during wound care procedures so that high-risk patients can be targeted for specialised dressings and preventive pain control; and 2) identify biological factors associated with severe pain during wound care procedures so that novel pain control strategies can be developed.
Background
Wound care procedures such as dressing changes can cause moderate to severe pain in 74% of patients, with nearly half (36%) of all patients experiencing severe pain (rated as 8-10 on a 10-point numeric rating scale) during dressing change. Additionally, clinicians have little direction with current guidelines regarding pain control during wound care procedures including the selection of the appropriate advanced wound dressings and the appropriate use of analgesics.
Design
This is a cross-sectional study.
Methods
The National Institute of Nursing Research approved and funded the study June of 2015 and the appropriate Institutional Review Board approved all study protocols prior to funding. Study enrolment is underway at the University of Iowa Hospitals and Clinics with a target of 525 subjects. Potential subjects must be adults (21+ years) and have a non-burn, non-diabetic foot, full-thickness wound. The research team performs a one-time study dressing change on enrolled subjects and collects all study data.
Discussion
This study will allow the development of a tool for clinicians to use to predict severe pain during wound care procedures and identify biological factors significantly associated with severe pain during wound care procedures.
Keywords: wound pain, wound care, nursing/nurses, microbiome, cytokines
INTRODUCTION
Wound care procedures (WCPs), such as dressing changes, cause moderate to severe pain in 74% of patients. Nearly half (36%) of all patients experience severe pain (rated as 8 to 10 on a 10-point numeric rating scale) (Gardner et al., 2014). Severe pain causes substantial stress for both patient and nurse performing the dressing change. Unfortunately, the high prevalence of severe pain during WCPs is under-recognised and under-studied. The objective of the study is to examine wound pain during dressing changes.
Background
Despite guidelines for administering preventive analgesics (World Union of Wound Healing Societies’ Initiative, 2004, 2007; Wounds UK, 2004), current practice sometimes involves performing WCPs without analgesia or chasing pain with opioids after the procedure has started when pain is severe (Stotts et al., 2004). When given, opioids are sometimes administered repeatedly or at doses resulting in prolonged, problematic, post-procedure sedation. Opioids can also delay healing (Martin et al., 2010; Rook, Hasan, & McCarson, 2009). In addition to analgesics, current practice guidelines recommend advanced wound dressings, such as foam, hydrofibers and alginate, but provide no guidance on which patients should be targeted for these advanced wound dressing materials (World Union of Wound Healing Societies’ Initiative, 2004, 2007; Wounds UK, 2004). As a result, clinicians may not consider advanced wound dressings based on the dressings’ ability to minimise wound pain (Vermeulen, Ubbink, de Zwart, Goossens, & de Vos, 2007).
Finally, organisations responsible for setting practice guidelines recognise the need to develop innovative pain control strategies that target specific mechanisms given the potential for adverse effects of analgesics, (World Union of Wound Healing Societies’ Initiative, 2007). Therefore, the mechanisms that lead to severe pain during WCPs need to be identified and targeted for novel pain control interventions.
THE STUDY
Aims
Aim 1 of this study is to develop and evaluate a clinical risk model to predict severe pain during WCPs so that high-risk patients can be targeted for specialised dressings and preventive pain control.
Aim 2 of this study is to identify biological factors associated with severe pain during WCPs so that novel pain control strategies can be developed.
Design/Methodology
Design
This is a cross-sectional study which began in December 2015. The research team performs a one-time study dressing change (i.e., WCP) on enrolled subjects and collects data to measure all study variables. Figure 1 depicts the study variables included for Aims 1 and 2. The development of the conceptual model of the study has been published (Gardner, Abbott, Fiala, & Rakel, 2017).
Figure 1.
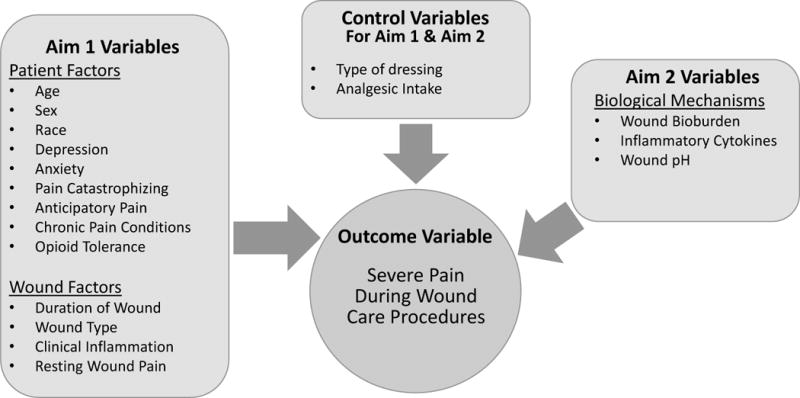
Study Variable Model
Setting and Sample
Participants are recruited from the inpatient units at large academic hospital in the Midwest using a customized report listing all hospital in-patients with wounds, generated through EPIC© (Epic Systems Corporation, Verona, WI), the electronic medical record. Table 1 lists inclusion and exclusion criteria. Only non-burn, non-diabetic foot, full-thickness wounds are included. Burn wounds are not included because the pain associated with burn wounds is substantially different than other types of wounds and people with diabetic foot ulcers often have significant neuropathy that alters the perception of pain in both the foot and the ulcer. Only open, full-thickness wounds are included because our interest is in the pain associated with packing and dressing wounds, which is not a component of care for wounds healing by primary intention. The sample includes only adults as wound care for children and adolescents are often performed in the operating room at UIHC.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria |
|
|
|
|
| Exclusion Criteria |
|
|
|
|
|
|
Of those patients that meet inclusion criteria, those that are non-English speaking or with a moderate to severe cognitive impairment are excluded because English and cognition abilities are essential to complete self-report tools in the study protocol (i.e., Generalised Anxiety Disorder-7 (GAD-7); Patient Health Questionaire-8 (PHQ-8); and Pain Catastrophizing Scale). Similarly, those with sensory impairment (e.g. spinal cord injuries, nerve blocks or other conditions resulting in lack of sensation) at the wound site are excluded because of their potential influence on pain at the wound site during the study dressing change; the primary outcome for both Aims. Wounds covered with 100% non-viable tissue (slough or eschar) are excluded because slough/eschar precludes the ability to collect wound specimens for bioburden and cytokine analyses. Wounds with a fistula are excluded because fistulous drainage may change the composition of wound specimens collected for bioburden and cytokine analyses. Malignant wounds are excluded because the pain associated with malignant wounds is substantially different than for other types of open wounds. Finally, wounds that require debridement during the study dressing change are excluded because debridement may cause higher levels of pain independent of the dressing change procedures.
Target Enrolment
Target enrolment (consent) is 525 subjects over a 3 ½ year period. Based on our data, we expect a 15% attrition rate leaving 445 subjects with complete study data. Previous data suggest 36% of inpatients at UIHC have severe pain (≥ 8/10) with WCPs (Gardner et al., 2014) so we expect 160 of 445 subjects to have severe pain during the WCP. With this sample, we will be able to estimate with 95% confidence the true accuracy of discriminating low/moderate from severe pain (i.e., the Receiver Operating Characteristic (ROC) area under the curve, or c-statistic) within a margin of error ≤ 0.058. Our variance estimate formulas are from Zhou, Obuchowski, & McClish (2002).
Methodology
Study Variables
Primary outcome variable
Pain intensity during the study dressing change is defined as the maximum pain intensity experienced during the dressing change, inclusive of removal, cleansing, specimen collection and re-dressing. The vertical 0-10 Numeric Rating Scale (NRS) with 0.5 increments is used to assess pain experienced in the wound during the study dressing change. The NRS pain scale is used in clinical practice, has established validity (correlation with the Visual Analog Scale r= 0.847, p< 0.001) (Paice & Cohen, 1997) and reliability (test-retest r= 0.93) (Jensen, 2003) for assessing acute pain and is associated with higher compliance and lower failure rates than the Visual Analogue Scales (Herr, Spratt, Mobily, & Richardson, 2004). For exploratory analyses, we are also assessing the quality of pain during the dressing change using the Short Form McGill Pain Questionnaire (SF-MPQ) (Melzack, 1987). This study will use the 15 pain quality descriptors from the SF-MPQ. The 15 items on the SF-MPQ have established validity (r= 0.77-0.88) when compared with the Long Form McGill Pain Questionnaire)(Dudgeon, Raubertas, & Rosenthal, 1993); and reliability (within patient correlation= 0.88-0.96 between sensory, affective and average pain scores)(Grafton, Foster, & Wright, 2005).
Aim 1 Study Variables (See Table 2 for a listing and brief explanation of the variables for Aim 1) The variables for this aim are based on the literature, described in an article by Gardner, Abbott, Fiala, & Rakel (2017).
Table 2.
Aim 1 Variables
| Variable | Method of Measurement | Operational Definition | Level of Measurement |
|---|---|---|---|
|
| |||
| Age | Medical Record/Patient Report | Age (in years) | Continuous |
|
| |||
| Sex | Medical Record/Patient Report | Male vs. Female | Dichotomous |
|
| |||
| Race | Medical Record/Patient Report | White vs. non-White | Dichotomous |
|
| |||
| Anxiety | Generalised Anxiety Disorder-7 (GAD-7) Scale | Score 0-21 | Continuous |
|
| |||
| Depressed | Patient Health Questionnaire-8 (PHQ-8) | Score 0-24 | Continuous |
|
| |||
| Pain Catastrophizing | Pain Catastrophizing Scale (PCS) | Score 0-52 | Continuous |
|
| |||
| Anticipatory Pain | Numeric Rating Scale (NRS) 0-10 | 0-10 on NRS | Continuous |
|
| |||
| Chronic Pain Conditions | Medical Record/Patient Report | No vs. Yes | Dichotomous |
|
| |||
| Opioid Tolerance | Medical Record/Patient Report | No vs. Yes | Dichotomous |
|
| |||
| Duration of Injury | Medical Record/Patient Report | Time from injury/tissue loss ≤ 7 days, 8-30 days, 31-90 days, 91-256 days, ˃257 days | Categorical |
|
| |||
| Type of Wound | Medical Record/Patient Report | Acute vs. Chronic | Dichotomous |
|
| |||
| Clinical Inflammation: Erythema | Clinical Signs and Symptoms Checklist (CSSC) | No vs. Yes | Dichotomous |
| AND | AND | ||
| Temperature | Infrared Thermometer | No vs. Yes | |
|
| |||
| Resting Wound Pain | 0-10 NRS | 0-10 on NRS | Continuous |
Patient Factors
Patient factors associated with high pain include younger age (Stotts et al., 2004), female sex (Morin, Lund, Villarroel, Clokie, & Feine, 2000) African-American race (Stotts et al., 2004; White, Asher, Lai, & Burton, 1999), high anxiety (Woo, Sadavoy, Sidani, Maunder, & Sibbald, 2008), depressed mood, pain catastrophizing (Baker, 2003), high levels of anticipatory pain (Woo et al., 2008), chronic pain condition(s) and opioid tolerance (Gardner et al., 2017).
Age, Sex, Race
These variables are defined using the National Institute of Health categories and definitions. Sex is categorised as male vs. female. Race is categorised as white vs. non-white (i.e., African American/Black, American Indian or Alaskan native, Asian, multi-racial/two or more races, Native Hawaiian, or Pacific Islander).
Anxiety, Depression, Pain Catastrophizing
Standardised questionnaires with established validity and reliability are used to measure anxiety (Generalised Anxiety Disorder-7 (GAD-7))(Spitzer, Kroenke, Williams, & Löwe, 2006), depression (Patient Health Questionaire-8 (PHQ-8))(Kroenke, Spitzer, & Williams, 2001; Kroenke et al., 2009) and pain catastrophizing (Pain Catastrophizing Scale (PCS)(Sullivan, Bishop, & Pivik, 1995; Osman et al., 2000)). Table 3 summarises the measurement tools for Anxiety, Depression, Pain Catastrophizing and Anticipatory Pain; as well as their validity and reliability.
Table 3.
Validity and Reliability of Survey Instruments used to measure Patient Level Factors
| Variable | Instrument | Construct and General Information | Reliability | Validity | Study |
|---|---|---|---|---|---|
| Anxiety | GAD-7 | 7-item questionnaire Identifies likely cases of generalised anxiety disorder. Items are rated for past 2 weeks on 4-point scale for the frequency of anxiety symptoms. Scores can range from 0-21, with cut points at 5, 10, &15 representing mild, moderate, and severe levels of anxiety. | Cronbach’s α= 0.92 |
r= 0.72-0.74 correlated with the Beck Anxiety Inventory, anxiety subscale of the Symptom Checklist-90 Can be used among the general population |
Spitzer et al., 2006 |
| Depression | PHQ-8 | 8-item questionnaire identifies likely cases of depressive disorder(s). Items are rated for the past 2 weeks on a 4-point scale for the frequency of depression symptoms. Scores can range from 0-24, with <10 = negative for depression, ≥10 = major depression, and 20+ = indicates severe major depression. | Cronbach’s α= 0.86- 0.89 | AUC= 0.95 in discriminating between patients with and without major depression Can be used among the general population |
Kroenke et al., 2001; Kroenke et al., 2009 |
| Pain Catastrophizing | PCS | 13 item pain questionnaire which measures 3 domains (magnification, rumination, & helplessness) about pain. Items are rated on a 5-point scale with anchors being “not at all” and “all the time”. Scores can range from 0-52, with a score of 30 representing clinically relevant level of catastrophizing. | Cronbach’s α= 0.95 |
r= 0.80, p<0.001 when compared to the Fear of Pain Questionnaire Can be used in the general population |
Osman et al., 2000; Sullivan, Bishop, & Pivik, 1995 |
| Anticipatory Pain Intensity | NRS | Self-report on vertical 0-10 Numeric Rating Scale with 0.5 increments. The subject is asked how much pain they expect to have during the dressing change. | The NRS was not developed to assess Anticipatory Pain, thus there is no validity or reliability data for this indication. However, Woo et al. (2008) & Woo (2015) used the 11-point NRS (0-10) to assess anticipatory pain associated with dressing change procedures, and found the NRS is the best method for accurately and reliably determining the subject’s real-time pain experience and expectations. *The 0-10 NRS has been assessed for Reliability and Validity for Pain Intensity, this information can be found with the “Pain Intensity” variable. |
||
Chronic Pain Conditions are defined by any diagnosis documented in the medical record or reported by the subject that the subject reports is painful, such as migraine, fibromyalgia and rheumatoid arthritis. These data are validated with the subject prior to the study dressing change. The subject is asked if they have the condition and if so, if it is painful. Those responding affirmatively are categorised as having a chronic pain condition(s).
Opioid Tolerance is defined as a decrease in the response to the medication following the repeated and/or prolonged administration of the medication (Dumas & Pollack, 2008). Subjects who had the equivalent of 60 mg of morphine every day for the 5 days prior to the study dressing change are categorised as opioid tolerant. The type, route and dose of opioids in mg or mcg taken or administered to the subject are recorded. If/when the subject has not been hospitalised for the 5 days prior to the study dressing change, the subject is asked if and what pain medication they have taken during the designated time. This information is used to convert each to morphine equivalents in mg using established conversion factors (Dowell, Haegerich, & Chou, 2016). Equianalgesic calculation provides a point of reference when comparing two medications that differ in potency yet which are supposed to provide the same amount of pain relief (Gordon et al., 1999).
Wound Factors
Wound factors believed to be associated with high levels of pain during WCPs include shorter duration of injury (Meaume, Teot, Lazareth, Martini, & Bohbot, 2004), chronic wounds (Price et al., 2008), clinical inflammation (Gardner et al., 2014) and high levels of resting wound pain (Gardner et al., 2017).
Duration of Injury is defined as the time from injury/tissue loss to the time of enrolment. Time from injury/tissue loss is categorised as A) <=7 days; B) 8-30 days; C) 31-90 days; D) 91-256 days; or E) >257 days. Although this definition has face validity, in practice its reliability is unclear, especially for chronic wounds that have been present for weeks, months, or years. To enhance validity, we crosscheck duration of injury with the medical record and the subject.
Type of Wound is categorised as acute vs. chronic. Wound type is defined by diagnosis in the medical record by physician or by the Certified Wound Ostomy Nurse (CWON) if the provider has not identified the type of wound in the medical record. Pressure injuries, venous ulcers and arterial ulcers are categorised as chronic while surgical, traumatic, mixed (traumatic wound with surgical fix) and other wounds are categorised as acute. The validity of medical record differentiation is enhanced by observation/assessment of CWON based on known differential criteria for wound type (i.e. subject and wound history, location, appearance) (Doughty & McNichol, 2015).
Clinical Inflammation is defined as the presence of erythema extending 2 cm from the wound edge and heat, defined as a positive temperature gradient at the wound margin compared with a control site. Erythema is measured using an item from the Clinical Signs and Symptoms Checklist (CSSC) that was developed and tested (kappa=0.70) by Gardner, Frantz, & Doebbeling (2001). The wound and a control site temperature is measured using a self-calibrating, portable, infrared thermometric probe (Exergen Model DT 1001, Exergen Products, Watertown, MA), that measures temperature in increments of 0.1°F and is accurate to within ± 0.2°F (Exergen Corporation, 2016). The wound perimeter is ‘outlined’ using the thermometric probe and the highest detected temperature displayed is recorded. This is repeated at a control site that is either at a contralateral location or on another site distant from the wound where the tissue appears normal (e.g. no erythema, wound, scaring). The use of two measures of inflammation, which is a cluster of physiological responses (i.e., heat, erythema, pain and oedema) to increase the validity of the presence of clinical inflammation because these signs and symptoms are subtle and have low reliability when assessed individually (Doughty & McNichol, 2015).
Resting Wound Pain Intensity is measured using a vertical 0 to 10 NRS with 0.5 increments as described under “pain during study dressing changes” above. The validity and reliability of the NRS was described in that section. The subject is asked how much pain they are having in their wound when resting quietly.
Aim 2 Study Variables (See Table 4 for a listing and brief explanation of the variables for Aim 2).
Table 4.
Aim 2 Variables
| Variable | Method of Measurement | Operational Definition | Level of Measurement |
|---|---|---|---|
| High Wound Bioburden | Swab specimen processed using 16 S rRNA sequencing | Microbial load (number of bacterial cells) Microbial Diversity Shannon index Faith’s index Relative Abundance of Staph Genre Anaerobic Genre |
Continuous Continuous Continuous Continuous Continuous Continuous |
| High Inflammatory mediators | Swab specimens processed to extract cDNA using qPCR | Cycle threshold (Ct) values | Continuous |
| Low Wound pH | pH Paper (6.5-13.0 pH with 0.5 incremental readings) | pH value | Continuous |
Biological Mechanisms
Several biological variables represent mechanisms linked to nociceptive sensitivity during WCPs (Brennan, 2002; Sluka, Deacon, Stibal, Strissel, & Terpstra, 1999; Sluka, Vance, & Lisi, 2005). These include wound bioburden (Tengvall, Björnhagen, Lindholm, Jonsson, & Wengström, 2006), inflammatory cytokines (Czeschik et al., 2008; Fukuoka, Kawatani, Hisamitsu, & Takeshige, 1994; Junger & Sorkin, 2000; Kawasaki, Zhang, Cheng, & Ji, 2008; Sorkin, Xiao, Wagner, & Myers, 1997) and wound pH (Kim, Freml, Park, & Brennan, 2007).
Wound bioburden is measured by assessing three dimensions of bioburden: microbial load, microbial diversity and relative abundance of potential pathogens. These dimensions are measured using swab specimens for high-throughput sequence analysis of the 16S rRNA gene yielding total microbial load, a variety of diversity metrics and the relative abundance of all microbes.
Wound specimens for the 16 S rRNA analysis are obtained using Levine’s technique, which has established validity compared with wound tissue specimens (Gardner et al., 2006). A pipeline for recovering, amplifying and sequencing bacterial 16S rRNA genes from wound swab specimens has been optimised (Figure 2). DNA is isolated from samples to maximise both Gram-positive and Gram-negative bacterial and fungal recovery, as described in Grice et al. (2010). Broad-range PCR primers, with barcodes unique for each sample, will amplify hypervariable regions 1-3 (V1-V3) of the bacterial 16S rRNA gene and sequenced on the Illumina MiSeq instrument (Caporaso et al., 2012). Sequences are processed in QIIME (Caporaso et al., 2010; Kuczynski et al., 2011), prior to downstream analyses. Briefly, sequences are first clustered into OTUs (operational taxonomic units, a proxy for ‘species’) using UCLUST (Edgar, 2010) at 97% sequence similarity.
Figure 2.

16S Workflow for analysing wound bioburden
Microbial load is measured using quantitative real-time PCR. Microbial load is extrapolated from real-time PCR data normalized to a well-characterised standard (i.e. E. coli); this allows estimation of 16S gene copy number and the number of bacterial cells.
Microbial diversity is calculated using the following alpha diversity indices: 1) Shannon diversity index, an ecological measure of diversity that incorporates the total number of different OTUs and the relative proportion of those OTUs; 2) Faith’s phylogenetic distance (PD), a measure of biodiversity that incorporates phylogenetic differences between species; and 3) number of observed OTUs. Beta-diversity, or “shared” diversity, is calculated using the UniFrac metric in forms both weighted and unweighted for relative abundance to identify association of microbial community structure with variables (e.g. level of pain). Taxonomic classification of sequences is made using BLAST, as implemented in QIIME (Caporaso et al., 2010).
Relative abundance of putative pathogens in the wounds (e.g., S. aureus and anaerobes) is calculated along with other bacterial taxa present, which allow us to identify additional, unrecognised putative pathogens amongst the wound microbiota.
Using these measures of bioburden, we found that wound duration was positively associated with the number of species-level OTUs (r=0.41; p = 0.022) and with a higher Shannon diversity index (r=0.32l; p=0.020). Wound depth was negatively associated with Staphylococcus (r= -0.47; p=0.0005) and positively associated with anaerobic bacteria relative abundance (r= 0.33; p=0.0182) (Gardner, Hillis, Heilmann, Segre, & Grice, 2013). These findings demonstrate concurrent validity. Strict quality control measures ensure the results are consistent and reproducible.
Inflammatory mediators (Cytokines)
Pro and anti-inflammatory cytokines (IL-1b, IL-6. IL-8 and TNF-a) are measured using gene expression. RNA is extracted from the swab specimen (Qiagen RNeasy Mini Kit, Valencia, CA) and reverse transcribed into cDNA (Invitrogen SuperScript IV VILO Kit, Carlsbad, CA). cDNA is amplified using quantitative PCR on the Viia7 real-time PCR platform (Applied Biosystems; Foster City, CA) and custom TaqMan Array Microfluidic cards (Thermo Fisher Scientific; Waltham, MA) to perform 384 reactions simultaneously. Cycle threshold (Ct) values will be measured and relative expression values will be calculated in the Viia7 software by normalising to an internal control/housekeeping gene to provide consistent results across samples and batches.
Wound pH is defined as the numeric value of acidity or alkalinity on a scale of 6.5 -13.0, measured in increments of 0.5 using Micro Essentials, Hydrion pH paper, which was quality tested by the manufacturer using NIST traceable standards. Validity and reliability of using litmus paper for measuring pH of wound fluid has not been reported in the literature. Shukla, Shukla, Tiwary, Agrawal, & Rastogi (2007) used litmus paper to assess pH of standard solutions of known pH and confirmed accuracy of the litmus strips.
Control Variables
Dressing and analgesic-intake Variables (See Table 5 for a listing and brief explanation of these variables. As previously noted, these variables will be used as additional covariates for estimating the Aim 1 risk model and as covariates when assessing associations in Aim 2).
Table 5.
Aim 1 & 2 Control Variables
| Variable | Method of Measurement | Operational Definition | Level of Measurement |
|---|---|---|---|
| Type of Dressing | Direct observation | Standard vs. Advanced dressing | Dichotomous |
| Analgesic intake Pre-procedural |
Medical record | Number of morphine equivalent opioids AND Number of non-opioid analgesic pills taken up to 1 hour prior to dressing change |
Continuous Continuous |
| Analgesic intake Procedure |
Medical record | Number of morphine equivalent opioids given during the dressing change | Continuous |
Type of Dressings
Dressings are categorised as standard (i.e., gauze- with or without solutions such as Normal Saline or Dakins, etc.) vs. advanced (i.e. hydrogel, negative pressure wound therapy, non-adherent (Mepitel®, KerraContact™), alginate, or hydrocolloid. Type of dressing is observed during the dressing change. Reliability of this method is strong because the same CWON completes the majority of the study dressing changes, identifying the type of dressings used.
Analgesic Intake
Preventive analgesic intake includes oral, intravenous or topical analgesics (opioids and non-opioids) given within 1 hour of study dressing change. Procedural analgesic intake includes those opioids and non-opioids given after the start of the study dressing change until it is completed. The type, route and dose (mg or mcg) of opioid and non-opioid analgesics taken or administered to the subject are recorded. This information is then used to convert opioids to morphine equivalents in mg using established conversion factors (Dowell et al., 2016) and non-opioids to number taken/dose. Equianalgesic calculation provides a point of reference when comparing two medications that differ in potency yet which are supposed to provide the same amount of pain relief (Gordon et al., 1999).
Data Collection Protocol
For consented subjects, a team of at least two research members, one who is a Certified Wound Ostomy Nurse (CWON) and one Registered Nurse (RN) Research Assistant (RA), complete the study dressing and collect study data/specimens. Using the same CWON to change the dressing, controls variability in dressing change technique that could have an impact on pain intensity.
Prior to the study dressing change the subject’s medical record is reviewed by the RA for demographic information, medical history, opioid medication history for the previous 5 days and up until 1 hour prior to the dressing change and adjuvant analgesic medication history. This information is verified with the subject before the dressing change. If the subject has any pain medications available, the team works with the subject’s nurse to have them administered as ordered before the study dressing change.
The RA then administers or assists the subjects with the GAD-7, PHQ-8 and PCS surveys. After survey completion, the RA asks the subject to rate their resting and anticipatory pain. If pain medications have been administered and have had appropriate time to work, the study dressing change begins. The CWON removes the old dressing, the time is recorded, and the wound is assessed for presence of sensation while cleansing the site for specimen collection. The wound is measured, photographed, inspected for erythema, peri-wound skin temperature (as well as a control site, as described above) and depth of tissue involvement. Then, two swab specimens are obtained. Swab specimens are collected using Levine’s technique. The wound is cleansed with non-bacteriostatic saline; and a swab (Puritan® Sterile Foam Tipped Applicators, Puritan Medical Products Co LLC; Guilford Maine) is rotated over a 1-cm2 area of viable, non-necrotic wound tissue for 5 seconds using sufficient pressure to mechanically disrupt the extra-polysaccharide matrix of a biofilm and extract wound tissue fluid. The time is recorded when the first swab is obtained, the swab is immediately placed in a 2-ml specimen tube (Eppendorf Biopur Safe-Lock, 2 ml; Hauppauge, NY) with 500 μl of RNA-Later and the other swab in an empty 2-ml specimen tube. Swabs are transported to the lab and stored at −80° Celsius within 30 minutes of collection. Cytokine and wound bio-burden swab specimens are batched for cytokine expression and16S gene sequencing, respectively. Lastly, a small strip of Micro Essentials Hydrion® pH paper is placed into the wound to absorb wound fluid, or fluid is aspirated into a sterile tuberculin syringe and placed on the pH paper to assess the pH.
After specimens are collected, the wound is then cleansed and/or redressed as ordered by the physician. After completion of the dressing change procedure the subject is then asked what their worst pain was during the dressing change procedure (using the NRS) and which part of the procedure was the most painful (dressing removal, specimen collection, cleansing, or redressing). If the subject identifies that they had pain, they are asked about the quality of the pain using the SF-MPQ pain descriptors. After answering the questions about pain quality, the subject has completed study participation.
Data Analysis
Study data are collected and managed in REDCap™ (Research Electronic Data Capture) hosted at the University of Iowa Institute for Clinical and Translational Science (NIH CTSA Grant U54TR001356) (Harris et al., 2009). REDCap™ is a secure, web-based application designed to support data capture for research studies, providing 1) an interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures data downloads to statistical packages; and 4) procedures for importing data from external sources.
For Aim 1, clinically accessible wound and patient factors will be combined in a logistic regression model to produce a risk scale that predicts probability of severe pain during WCPs based on a patient’s levels of wound and patient factors. The ability of this model to distinguish between severe and not-severe pain will be assessed by the area under the ROC curve (AUC), which estimates the probability of correctly discriminating between a patient with severe pain and one without severe pain. For Aim 2, we will assess the ability of each biological mechanism to individually predict severe vs. not-severe pain during WCPs, as measured by the AUC. Additionally, a “best” model for predicting severe vs. not-severe pain will be determined using forward stepwise logistic regression, where all the biological mechanisms will be candidate predictors. The usefulness of this model will also be described by the AUC. Dressing factors and analgesic intake that may alter pain during WCPs are being measured and will be included as covariates in the Aim 1 logistic regression model and as control variables in the Aim 2 analysis. AUC estimated for the Aim 1 and Aim 2 multiple logistic regression models will be adjusted downward, using bootstrap validation methods as described by Harrell (2015), to account for overly optimistic AUC estimates due to estimating the regression model coefficients and AUC from the same data set (both aims), as well to account for performing variable selection (Aim 2) using the same data set.
Ethical Considerations
The appropriate Institutional Review Board (IRB) has approved all study protocols. The IRB approves all protocol modifications in addition to an annual continuing review to ensure compliance with Human Subject protections.
Briefly, the research team verbally describes all study procedures to eligible patients as well as their right to withdraw from the study at any time, the right to refuse to answer survey questions and that their decision to participate or not will not have an impact on their care. Subjects are then provided a written consent form to sign. All subjects who complete the study are reimbursed for their time.
Validity and Reliability/Rigor
The validity and reliability of all study measures are reported under Design/Methodology. We will also analyse and report the validity of the established survey tools (e.g., GAD-7, PHQ-8) in our population. The reliability of study data will be analysed through the use of consistency checks to identify inaccurate data and or recording. Although this is not a randomised clinical trial, we are using a CONSORT framework to describe recruitment and enrolment data according to inclusion/exclusion criteria and withdrawal from the study. Therefore, we will be able to fully describe the participants and nonparticipants in the study that may influence or bias study findings.
DISCUSSION
Based on experience, adjustments were made in this application that facilitate obtaining an adequate sample, including a 3-year plan for enrolment. If we encounter difficulty obtaining the needed numbers of subjects, or if enrolment of chronic wounds falls below 30% of the sample, we will seek to case-find, recruit, enrol and collect data from outpatient UIHC clinics, including the vascular clinic.
Type of wound may have an interaction effect on pain during WCPs. Alternative strategies will be used to examine separately wound and patient factors for acute vs. chronic wounds.
The proposed study addresses “severe” pain (8 to 10 on a 10-point NRS) because, in our view, predicting and controlling this level of pain would be a significant advance in wound care. We do recognise that moderate levels of pain (4-7) are also problematic so predictions of moderate pain will be examined in exploratory analyses and biological mechanisms associated with moderate pain will be explored by alternative analyses that treat pain during WCP as an interval level variable.
The use of 16 S rRNA gene sequencing to characterise wound bioburden may fail to identify significant relationships between bioburden and pain during WCPs. An alternative strategy will be to employ much more expensive and difficult metagenomic techniques that provide a much higher resolution of microbial characteristics, such as strain and pathogenicity factors.
Limitations
The cross-sectional study design precludes examining the role of pain during WCPs on wound outcomes, such as healing. A second limitation is recruitment of inpatients only, which may limit generalisation of study findings to outpatients.
CONCLUSION
Although previous studies have examined various aspects of pain during WCPs, the major innovation of this proposal over prior studies is that it is the first to systematically examine a comprehensive set of wound, patient and biological factors for their individual and collective associations with pain during WCPs using precisely defined and rigorously measured study variables. This study has the potential to make significant contributions because the predictive risk model developed in Aim 1 will enable clinicians to target those patients requiring preventive pain control, thereby (1) eliminating the spiralling impact of painful procedures on nociceptor sensitisation and development of anticipatory pain, (2), increasing appropriate use of advanced wound dressings to minimise painful dressing changes and (3) reducing the use of opioids and their undesirable side effects. This study will provide valuable information to begin development of a clinical tool to guide healthcare provider’s management of wound pain during painful dressing changes. In addition, this study will provide information on mechanisms associated with severe pain during WCPs so novel pain control strategies can be developed.
Why this study is needed.
This study has the potential to make significant contributions to clinical care by:
Aiding clinicians in targeting patients in need of preventative pain control, resulting in the reduction of pain during wound care procedures
Improving the appropriate use of advanced wound care dressings and preventive analgesics including opioids
Acknowledgments
We thank Nicole L. Pierce, PhD(c), RN and Lynn Nakad, BSN, RN for their input to the study protocol.
Funding: This study received funding from NIH/NINR R01NR015642.
Footnotes
MRS CATHERINE FIALA (Orcid ID : 0000-0003-3492-5792)
DR SUE E GARDNER (Orcid ID : 0000-0001-6224-6944)
Conflict of Interest: No conflict of interest has been declared by the authors.
Author Contributions:
All authors have agreed on the final version and meet at least one of the following criteria (recommended by the ICMJE*):
1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data;
2) drafting the article or revising it critically for important intellectual content.
References
- Baker TA. Arthritis symptoms as indicators of pain in older African Americans. Ethnicity & Disease. 2003;13(4):513–520. [PubMed] [Google Scholar]
- Brennan TJ. Anesthesiology. United States: 2002. Sep, Frontiers in translational research: The etiology of incisional and postoperative pain. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7(5):335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Knight R. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal. 2012;6(8):1621–4. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeschik JC, Hagenacker T, Schafers M, Busselberg D, Schäfers M, Büsselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neuroscience Letters. 2008;434(3):293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- Doughty DB, McNichol LL. Wound, Ostomy and Continence Nurses Society core curriculum Wound management. Philadelphia: Wolters Kluwer; 2015. [Google Scholar]
- Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA. 2016;315(15):1624. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudgeon D, Raubertas RF, Rosenthal SN. The short-form McGill Pain Questionnaire in chronic cancer pain. Journal of Pain and Symptom Management. 1993;8(4):191–195. doi: 10.1016/0885-3924(93)90126-G. [DOI] [PubMed] [Google Scholar]
- Dumas EO, Pollack GM. Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. The AAPS Journal. 2008;10(4):537–51. doi: 10.1208/s12248-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26(19):2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Exergen Corporation. DermaTemp. Exergen Corporation; 2016. Retrieved September 15, 2017, from http://www.exergen.com/professional-medical-products/products/dermatemp. [Google Scholar]
- Fukuoka H, Kawatani M, Hisamitsu T, Takeshige C. Cutaneous hyperalgesia induced by peripheral injection of interleukin-1 beta in the rat. Brain Research. 1994;657(1–2):133–140. doi: 10.1016/0006-8993(94)90960-1. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7820610. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Abbott LI, Fiala CA, Rakel BA. Factors associated with high pain intensity during wound care procedures: A model. Wound Repair and Regeneration. 2017;25(4):558–563. doi: 10.1111/wrr.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner SE, Blodgett NP, Hillis SL, Borhart E, Malloy L, Abbott L, Rakel BA. HI-TENS reduces moderate-to-severe pain associated with most wound care procedures: A pilot study. Biological Research for Nursing. 2014;16(3):310–319. doi: 10.1177/1099800413498639. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Doebbeling BN. The validity of the clinical signs and symptoms used to identify localized chronic wound infection. Wound Repair and Regeneration. 2001;9(3):178–86. doi: 10.1046/j.1524-475x.2001.00178.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11472613. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Frantz RA, Saltzman CL, Hillis SL, Park H, Scherubel M. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 2006;14(5):548–57. doi: 10.1111/j.1743-6109.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Gardner SE, Hillis SL, Heilmann K, Segre JA, Grice EA. The neuropathic diabetic foot ulcer microbiome is associated with clinical factors. Diabetes. 2013;62(3):923–930. doi: 10.2337/db12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DB, Stevenson KK, Griffie J, Muchka S, Rapp C, Ford-Roberts K. Opioid Equianalgesic Calculations. Journal of Palliative Medicine. 1999;2(2):209–218. doi: 10.1089/jpm.1999.2.209. [DOI] [PubMed] [Google Scholar]
- Grafton KV, Foster NE, Wright CC. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. The Clinical Journal of Pain. 2005;21(1):73–82. doi: 10.1097/00002508-200501000-00009. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15599134. [DOI] [PubMed] [Google Scholar]
- Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, NISC Comparative Sequencing Program. Liechty KW, Segre JA. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14799–804. doi: 10.1073/pnas.1004204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. Regression modeling strategies : with applications to linear models, logistic and ordinal regression and survival analysis (Second) New York: Springer International Publishing; 2015. [DOI] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr KA, Spratt K, Mobily PR, Richardson G. Pain intensity assessment in older adults: Use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. The Clinical Journal of Pain. 2004;20(4):207–219. doi: 10.1097/00002508-200407000-00002. [DOI] [PubMed] [Google Scholar]
- Jensen MP. The validity and reliability of pain measures in adults with cancer. The Journal of Pain. 2003;4(1):2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1–2):145–151. doi: 10.1016/S0304-3959(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6 and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2008;28(20):5189–94. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Freml L, Park SS, Brennan TJ. Lactate concentrations in incisions indicate ischemic-like conditions may contribute to postoperative pain. The Journal of Pain : Official Journal of the American Pain Society. 2007;8(1):59–66. doi: 10.1016/j.jpain.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. Journal of Affective Disorders. 2009;114(1):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Kuczynski J, Stombaugh J, Walters WA, González A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. 2011 doi: 10.1002/0471250953.bi1007s36. Current Protocols in Bioinformatics/Editoral Board andreas D. Baxevanis … [et Al.], Chapter 10. Unit 10.7. [DOI] [PMC free article] [PubMed]
- Martin JL, Koodie L, Krishnan AG, Charboneau R, Barke RA, Roy S. Chronic morphine administration delays wound healing by inhibiting immune cell recruitment to the wound site. The American Journal of Pathology. 2010;176(2):786–799. doi: 10.2353/ajpath.2010.090457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaume S, Teot L, Lazareth I, Martini J, Bohbot S. The importance of pain reduction through dressing selection in routine wound management: the MAPP study. Journal of Wound Care. 2004;13(10):409–413. doi: 10.12968/jowc.2004.13.10.27268. https://doi.org/10.12968/jowc.2004.13.10.27268. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Morin C, Lund JP, Villarroel T, Clokie CML, Feine JS. Differences between the sexes in post-surgical pain. Pain. 2000;85(1–2):79–85. doi: 10.1016/S0304-3959(99)00248-1. [DOI] [PubMed] [Google Scholar]
- Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittmann L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. Journal of Behavioral Medicine. 2000;23(4):351–65. doi: 10.1023/A:1005548801037. [DOI] [PubMed] [Google Scholar]
- Paice JA, Cohen FL. Validity of a verbally administered numeric rating scale to measure cancer pain intensity. Cancer Nursing. 1997;20(2):88–93. doi: 10.1097/00002820-199704000-00002. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9145556. [DOI] [PubMed] [Google Scholar]
- Price PE, Fagervik-Morton H, Mudge EJ, Beele H, Ruiz JC, Nystrøm TH, Harding KG. Dressing-related pain in patients with chronic wounds: an international patient perspective. International Wound Journal. 2008;5(2):159–71. doi: 10.1111/j.1742-481X.2008.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Hasan W, McCarson KE. Morphine-induced early delays in wound closure: involvement of sensory neuropeptides and modification of neurokinin receptor expression. Biochemical Pharmacology. 2009;77(11):1747–1755. doi: 10.1016/j.bcp.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla VK, Shukla D, Tiwary SK, Agrawal S, Rastogi A. Evaluation of pH measurement as a method of wound assessment. Journal of Wound Care. 2007;16(7):291–294. doi: 10.12968/jowc.2007.16.7.27062. https://doi.org/10.12968/jowc.2007.16.7.27062. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Deacon M, Stibal A, Strissel S, Terpstra A. Spinal blockade of opioid receptors prevents the analgesia produced by TENS in arthritic rats. The Journal of Pharmacology and Experimental Therapeutics. 1999;289(2):840–846. [PubMed] [Google Scholar]
- Sluka KA, Vance CGT, Lisi TL. High-frequency, but not low-frequency, transcutaneous electrical nerve stimulation reduces aspartate and glutamate release in the spinal cord dorsal horn. Journal of Neurochemistry. 2005;95(6):1794–1801. doi: 10.1111/j.1471-4159.2005.03511.x. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81(1):255–262. doi: 10.1016/s0306-4522(97)00147-4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9300418. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine. 2006;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Stotts NA, Puntillo K, Bonham Morris A, Stanik-Hutt J, Thompson CL, White C, Reitman Wild L. Wound care pain in hospitalized adult patients. Heart and Lung: Journal of Acute and Critical Care. 2004;33(5):321–332. doi: 10.1016/j.hrtlng.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychological Assessment. 1995;7(4):524–532. doi: 10.1037/1040-3590.7.4.524. [DOI] [Google Scholar]
- Tengvall OM, Björnhagen VC, Lindholm C, Jonsson CE, Wengström Y. Differences in pain patterns for infected and noninfected patients with burn injuries. Pain Management Nursing : Official Journal of the American Society of Pain Management Nurses. 2006;7(4):176–82. doi: 10.1016/j.pmn.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Vermeulen H, Ubbink DT, de Zwart F, Goossens A, de Vos R. Preferences of patients, doctors and nurses regarding wound dressing characteristics: a conjoint analysis. Wound Repair and Regeneration : Official Publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15(3):302–7. doi: 10.1111/j.1524-475X.2007.00230.x. [DOI] [PubMed] [Google Scholar]
- White SF, Asher MA, Lai SM, Burton DC. Patients’ perceptions of overall function, pain and appearance after primary posterior instrumentation and fusion for idiopathic scoliosis. Spine. 1999;24(16) doi: 10.1097/00007632-199908150-00011. 1693-9-700. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10472104. [DOI] [PubMed] [Google Scholar]
- Woo KY, Sadavoy J, Sidani S, Maunder R, Sibbald RG. The relationship between anxiety, anticipatory pain and pain during dressing change in the older population. Ostomy Wound Management. 2008;54(4):77. [Google Scholar]
- World Union of Wound Healing Societies’ Initiative. Principles of Best Practice: Minimising pain at wound dressing-related procedures, A consensus document. London: Medical Education Partnership Ltd; 2004. Retrieved from http://www.woundsinternational.com/media/issues/79/files/content_39.pdf. [Google Scholar]
- World Union of Wound Healing Societies’ Initiative. Principles of Best Practice: Minimising pain at wound dressing-related procedures, A consensus document. Toronto, Ontario, Canada: WoundPedia, Inc.; 2007. Retrieved from http://www.woundsinternational.com/media/issues/79/files/content_39.pdf. [Google Scholar]
- Wounds UK. Best Practice Statement: Minimising Trauma and Pain in Wound Management. 2004 Retrieved August 11, 2010, from http://www.wounds-uk.com/best-practice-statements/best-practice-statement-minimising-trauma-and-pain-in-wound-management-1.
- Zhou XH, Obuchowski N, McClish D. Statistical methods in diagnostic medicine. New York, NY: John Wiley; 2002. [Google Scholar]


