Abstract
In the brain, fast inhibitory neurotransmission is mediated primarily by the ionotropic subtype of the gamma-aminobutyric acid (GABA) receptor subtype A (GABAAR). It is well established that the brain’s GABAAR system mediates many aspects of neurobehavioral responses to alcohol (ethanol; EtOH). Accordingly, in both preclinical studies and some clinical scenarios, pharmacologically targeting the GABAAR system can alter neurobehavioral responses to acute and chronic EtOH consumption. However, many of the well-established interactions of EtOH and the GABAAR system have been identified at concentrations of EtOH ([EtOH]) that would only occur during abusive consumption of EtOH (≥40 mM), and there are still inadequate treatment options for prevention of or recovery from alcohol use disorder (AUD, including abuse and dependence). Accordingly, there is a general acknowledgement that more research is needed to identify and characterize: (1) neurobehavioral targets of lower [EtOH] and (2) associated brain structures that would involve such targets in a manner that may influence the development and maintenance of AUDs.
Nearly 15 years ago it was discovered that the GABAAR system of the cerebellum is highly sensitive to EtOH, responding to concentrations as low as 10 mM (as would occur in the blood of a typical adult human after consuming 1–2 standard units of EtOH). This high sensitivity to EtOH, which likely mediates the well-known motor impairing effects of EtOH, combined with recent advances in our understanding of the role of the cerebellum in non-motor, cognitive/emotive/reward processes has renewed interest in this system in the specific context of AUD. In this chapter we will describe recent advances in our understanding of cerebellar processing, actions of EtOH on the cerebellar GABAAR system, and the potential relationship of such actions to the development of AUD. We will finish with speculation about how cerebellar specific GABAAR ligands might be effective pharmacological agents for treating aspects of AUD.
Keywords: Addiction, Alcohol, AUD, Cerebellum, Ethanol, GABA
1 Introduction to GABAARs, Interactions with Alcohol, and Therapeutic Approaches to AUDs
1.1 Synaptic and Extrasynaptic GABAARs
The GABAAR is a plasma-membrane spanning, ligand-gated ionotropic channel that is primarily permeable to Cl− (Fig. 1a) (Lorenz-Guertin and Jacob 2017). Functional GABAARs are heteropentameric structures, comprised of varying subunit combinations from a family of 19 closely related subunit families (α1–6, β1–3 γ1–3, δ, ε θ, π, and ρ1–3; Fig. 1a, b). Most GABAAR channels in the brain are comprised of two α subunits, two β subunits, and either a γ or δ subunit, and the specific subunit makeup influences almost all biophysical/pharmacological properties of the receptor/channel complex, including channel conductance and kinetics, affinity for GABA and other agonists/antagonists, sensitivity to neuromodulators, modulation by phosphorylation, and subcellular location (for review, see Lorenz-Guertin and Jacob 2017).
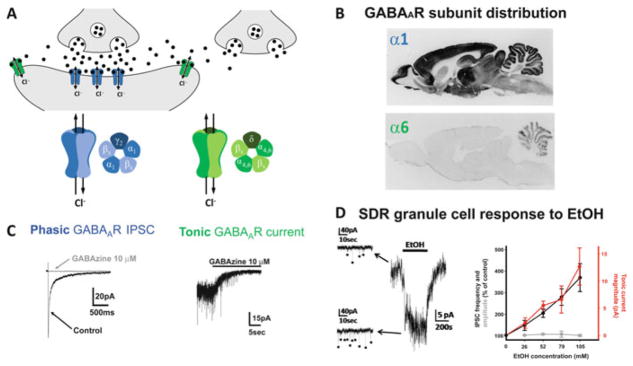
Fig. 1.
Phasic and tonic GABAAR currents and modulation by EtOH, as exemplified by cerebellar granule cells. (a) Schematic diagram showing GABAARs in the synaptic cleft (blue) and outside of the synaptic cleft (green). Synaptic GABAARs are typically comprised of two α subunits (with α1 dominating at most synapses), two β subunits, and a γ subunit. Extrasynaptic GABAARs replace the γ subunit with a δ subunit which is crucial for anchoring the receptor complex extrasynaptically, and at most synapses is paired with either the α4 (hippocampus and thalamus) or α6 (cerebellum) subunit [as in (b), bottom panel], although other permutations also exist. (b) Immunocytochemistry for the α1 (top) and α6 (bottom) subunit of the GABAAR receptor. Note, the α6 subunit is exclusively expressed in granule cells. (c) Phasic IPSCs (left) are mediated by synaptic GABAARs (as evidenced by their sensitivity to the GABAAR antagonist, GABAzine) that are rapidly activated by the high concentrations of vesicular GABA released into the synaptic cleft, and their decay time is dictated by receptor desensitization and inactivation as GABA is cleared from the synaptic cleft by diffusion and uptake by GABA transporters. Tonic GABAAR-mediated currents (right; steady state current blocked by GABAzine; downward deflections superimposed on the tonic current are phasic IPSCs that are also blocked by GABAzine) are mediated by extrasynaptic GABAARs that have a higher affinity for GABA and do not readily desensitize, and so generate a steady state current that varies in accordance with the concentration of ambient extracellular GABA. Note, because GABA released into the synaptic cleft diffuses out of the cleft where it can activate extrasynaptic GABAARs, the magnitude of the tonic GABAAR current increases or decreases in parallel with changes in vesicle release rate, either from the presynaptic neuron or from neighboring synapses not directly connected to the recorded cell. (d) Example voltage-clamp recording (left) showing that EtOH (52 mM) increases sIPSC frequency and tonic GABAAR current magnitude in a granule cell in a slice of cerebellum from a low EtOH consuming Sprague Dawley rat (SDR). EtOH dose–response plot (right) shows the mean enhancement of sIPSC frequency (black) and tonic GABAAR current magnitude (red), without affecting sIPSC amplitude (gray). Images are adapted with permission from Mohr et al. (2013) and Pirker et al. (2000)
Of the wide range of properties conferred by variations in GABAAR subunit composition, a major division is defined by whether their activity in situ is phasic or tonic (Fig. 1a, c) (Hamann et al. 2002; Brickley et al. 1996; Wall and Usowicz 1997; Mody and Pearce 2004; Stell and Mody 2002; Mody 2001; Lorenz-Guertin and Jacob 2017; Ye et al. 2013; Richardson et al. 2011). The nature of phasic, inhibitory postsynaptic currents (IPSCs) is dictated by the rapid activation of postsynaptic GABAARs by transiently high concentrations of GABA (due to the proximity of synaptic GABAARs to the vesicular release site), followed by receptor desensitization, and inactivation due to transmitter removal by plasma membrane GABA transporters (GAT1–4), which combined dictate the time course of IPSC decay (Cavelier et al. 2005; Rossi and Hamann 1998; Rossi et al. 2003; Banks and Pearce 2000; Bragina et al. 2008; Moldavan et al. 2017; Schousboe et al. 2014). In contrast, tonic GABAAR-mediated currents are mediated primarily by a specialized subset of GABAARs that are located outside of the synaptic cleft and generally have a higher affinity for GABA than synaptic GABAARs, and also are resistant to desensitization, two properties that enable extrasynaptic GABAARs to be tonically activated by the low ambient concentration of extracellular GABA (Rossi and Hamann 1998; Hamann et al. 2002; Stell et al. 2003; Glykys et al. 2008; Cavelier et al. 2005). Specifically, tonic currents are primarily mediated by GABAARs containing the δ subunit (rather than the more common γ subunit) combined with either the α4 or α6 subunit (depending on the brain region; Fig. 1b) which result in GABAARs that have a high affinity for GABA, do not easily desensitize, and are anchored close to, but outside of the synaptic cleft. Importantly, although the absolute magnitude of tonic GABAAR currents is small relative to the amplitude of IPSCs, because they are constantly active, tonic inhibition is significantly more powerful than phasic inhibition, mediating ~75% of total inhibition in cells that exhibit tonic inhibition (Richardson et al. 2011; Hamann et al. 2002). Thus, tonic GABAAR currents are potentially very powerful targets for neural modulation. Although tonic GABAAR currents have now been observed in numerous brain regions [mostly those expressing the δ subunit (Glykys et al. 2008; Richardson et al. 2011), although see (Lorenz-Guertin and Jacob 2017) for additional permutations], their properties and molecular makeup were first discovered and have been most thoroughly characterized in cerebellar granule cells (Brickley et al. 1996; Hamann et al. 2002; Stell et al. 2003; Wall and Usowicz 1997), which, given the topic of this chapter, will serve as the model for their role in the broader context of AUDs (Fig. 1c, d).
Since the ambient concentration of GABA is determined by the balance between vesicular GABA release [and possibly release from astrocytes (Rossi et al. 2003; Cavelier et al. 2005; Lee et al. 2010; Diaz et al. 2011)] and GABA removal by GATs, the magnitude of tonic GABAAR currents can be modulated by changes in (1) the rate of vesicular GABA release (whether it be into synapses on the recorded cell or from neighboring synapses not on the recorded cell), (2) the rate of GABA uptake, or (3) the density of extrasynaptic GABAARs or their affinity for GABA (Cavelier et al. 2005; Rossi et al. 2003). Thus, changes in vesicular GABA release rate manifest as changes in the frequency of phasic IPSCs and the magnitude of tonic GABAAR currents (in cells that express relevant extrasynaptic GABAARs).
Because in most neurons in the mature brain the extracellular concentration of Cl− is approximately 20-fold higher than the free concentration inside of cells, the reversal potential of Cl− (ECl−) is typically fairly hyperpolarized (−60 to −80 mV). Accordingly, activation of GABAARs typically results in an influx of Cl− ions, which is hyperpolarizing (i.e., inhibitory). Even if a neuron’s resting membrane potential is at or near ECl−, and thus activation of GABAARs produces little or no current, the opening of channels and associated increase in membrane conductance may inhibit responses to excitatory inputs via shunting inhibition (Mitchell and Silver 2003; Heigele et al. 2016). This effect is more efficacious when GABAAR currents are tonically active rather than phasic, as the ability for phasic currents to cause shunting inhibition is largely dependent on their coinciding temporally (within a few ms) with the occurrence of an excitatory current. Thus, in general, any action of EtOH on the GABAAR system will enhance or reduce the primary form of fast inhibitory neurotransmission in the brain. However, in developing neurons, when the Cl− gradient is not fully established, in subcellular compartments of mature neurons that have reduced Cl− gradients, or in some mature neurons that have had their Cl− gradient transiently reduced by exposure to hormones or peptides, GABAARs may be depolarizing (Ben-Ari et al. 2012; Eilers et al. 2001; Ostroumov et al. 2016; Pugh and Jahr 2011; Tyzio et al. 2006), and thus the same action of EtOH on GABAARs can have the opposite effect on overall cellular or subcellular excitation, although shunting inhibition can still occur even if activation of GABAARs does depolarize a given cellular compartment.
1.2 Pre- and Postsynaptic Actions of EtOH on GABAergic Transmission
EtOH has long been known to be a potent enhancer of GABAAR-mediated inhibition, but the mechanism(s) of action are complex, and vary across brain regions and GABAAR subtypes. First, in many brain regions, EtOH increases vesicular release of GABA (which can be triggered by EtOH actions in the presynaptic terminal itself, or other compartments of the presynaptic cell which induce increased action potential firing). Regardless of the site of EtOH action within the presynaptic cell that drives increased vesicular GABA release (hereafter referred to as presynaptic actions), the effect manifests as an increase in the frequency of spontaneous IPSCs (sIPSCs) and, in those brain regions that exhibit tonic GABAAR currents, an increase in its magnitude, due to spillover from the various activated synapses (Fig. 1d) (Hanchar et al. 2005; Carta et al. 2004; Liang et al. 2006; Kumar et al. 2009; Kelm et al. 2011, 2008; Criswell et al. 2008; Mohr et al. 2013; Kaplan et al. 2013). Although not the focus of this chapter, EtOH-induced changes in GABA release can also affect pre- and postsynaptic GABAB receptors (Silberman et al. 2009; Reilly et al. 2008), which are also known to influence a variety of EtOH-related phenotypes and processes (Enoch et al. 2016; Phillips and Reed 2014), including enhancing EtOH actions on GABAARs (Yang et al. 2000).
The ability of EtOH to increase GABA release varies across brain regions, and there is considerable variation in the underlying mechanisms across those synapses that do show EtOH-induced increased GABA release (Kelm et al. 2011). Indeed, EtOH-induced increased vesicular GABA release has been shown to be mediated by either increased action potential firing (Carta et al. 2004; Kaplan et al. 2013) or increased probability of release at the axon terminal, and the underlying molecular triggers vary from cell to cell, but often include presynaptic G-protein cascades, kinases, and a range of second messengers and effector proteins (Kaplan et al. 2013; Kelm et al. 2008, 2011; Criswell et al. 2008; Kumar et al. 2009; Nie et al. 2009).
While the evidence for EtOH increasing GABA release in various brain regions is very clear, and to our understanding not notably controversial, the more longstanding and commonly expressed thinking about EtOH actions on the GABAAR system [that it directly enhances GABAARs (Olsen et al. 2007; Wallner et al. 2003; Hanchar et al. 2004; Crews et al. 1996; Davies 2003; Mihic 1999)] is actually far more controversial (Korpi et al. 2007; Borghese and Harris 2007; Botta et al. 2007a, b). In particular, there are certainly some clear studies showing that EtOH can increase the amplitude or decay kinetics of IPSCs and the amplitude of tonic GABAAR currents, in many cases with action potentials blocked and in the absence of any clear increase in GABA release (i.e., no change in sIPSC frequency), which is compatible with postsynaptic mechanisms (Hanchar et al. 2005; Jia et al. 2007, 2008; Liang et al. 2009). In the context of AUD, some examples of EtOH direct modulation of GABAARs exhibit forms of adaptation to chronic EtOH exposure, which fits with a role in tolerance and dependence (Cagetti et al. 2003; Liang et al. 2006, 2009). Further, numerous studies of cloned GABAARs expressed in isolated cell preparations have demonstrated enhancement by EtOH of responses to exogenous GABA (Meera et al. 2010). However, the existence of these presumed postsynaptic actions have been somewhat controversial. In particular, often EtOH effects vary across cell types or species despite involving apparently similar GABAAR receptor subtypes, and not all groups have observed such effects, even when studying the same preparation and cell type (see below for a more detailed discussion) (Borghese and Harris 2007; Borghese et al. 2006; Botta et al. 2007b). While this controversy has yet to be fully explained, there is accumulating evidence that the phosphorylation state of GABAARs is a crucial determinant of whether EtOH affects postsynaptic responsivity of GABAARs to EtOH (Choi et al. 2008; Hodge et al. 1999; Kaplan et al. 2013; Qi et al. 2007; Trudell et al. 2014).
Another concern is whether the [EtOH] required to induce direct enhancement of GABAARs is commonly achieved in human clinical scenarios or rodent preclinical models. For example, EtOH enhancement of hippocampal and thalamic GABAAR currents doesn’t occur until ~50 mM EtOH (Jia et al. 2008; Liang et al. 2009), which may be achieved in some severe cases of AUD, but is not achieved during recreational consumption in humans or in any model of voluntary consumption in rodent models. Thus, while such actions, and associated adaptations may contribute to neural processes in the late stages of AUD (Cagetti et al. 2003; Liang et al. 2006, 2009), they are not likely to play a role in initial reactions to EtOH and thus predilection and early progression to AUD. A similar concern applies to most studies of EtOH action on recombinantly expressed GABAARs (Borghese et al. 2006).
Finally, even in cases where EtOH does alter postsynaptic GABAAR responsivity, it is not fully resolved whether such actions are due to direct interaction of EtOH with the GABAAR, or whether they are secondary to phosphorylation of GABAARs and/or GABAAR translocation to new locations within the plasma membrane or even out of the plasma membrane, all of which have also been observed in response to acute or chronic exposure to EtOH (Kumar et al. 2009; Lorenz-Guertin and Jacob 2017). Indeed, the best evidence for modulation of GABAARs by EtOH being mediated by direct interactions comes from modeling of the ρ subunit, based on crystallographic studies of the homologous GluCl subunit (a glutamate gated chloride channel found in insects), which is an uncommon GABAAR subunit in the brain, and whose activity is actually suppressed by EtOH (Borghese et al. 2016). In this regard, we recently discovered that low [EtOH] (9 mM) can directly suppress cerebellar granule cell tonic GABAAR currents in situ, but that such suppression is prevented by postsynaptic PKC activity (Kaplan et al. 2013). Importantly in the context of AUD, the level of postsynaptic PKC activity, and thus EtOH suppression of tonic GABAARs, varies across mammalian genotypes in a manner that suggests it is a key genetically controlled, molecular determinant of excessive EtOH consumption (see below for further detail) (Kaplan et al. 2013, 2016a; Mohr et al. 2013).
Thus, while there is much evidence supporting the idea that EtOH can enhance and in some cases suppress GABAAR transmission via postsynaptic mechanisms (Kaplan et al. 2013; Borghese et al. 2016), the details of such mechanisms are far from clear. Indeed, although the GABAAR inverse agonist, Ro 15-4513, which blocks many of the intoxicating effects of EtOH has been suggested to do so by blocking EtOH binding to GABAARs (Hanchar et al. 2006), other studies do not find any direct competitive molecular interaction between the two compounds (Korpi et al. 2007), and Ro 15-4513 activity at GABAARs could just as well counteract EtOH intoxication by functionally counteracting enhanced GABA release, or even simply by counteracting overall changes in network activity induced by EtOH in a given brain region.
Our overall thinking on the history of and current status of direct actions of EtOH on GABAARs is as follows. Similarities in the behavioral actions of EtOH and known GABAAR modulators (anesthetics and benzodiazepines) combined with numerous studies showing that modulating GABAARs (pharmacologically or genetically) affects EtOH-related behavioral or even cellular phenotypes correctly led to the conclusion that a primary target of EtOH is the GABAAR “system.” Parallel and/or consequent studies of recombinant GABAARs, combined with a limited number of in situ examples of EtOH modulating GABAARs (arguably directly), refined the thinking toward the notion that a major component of EtOH actions on the GABAAR system was via direct enhancement of GABAARs. However, many of the apparent examples of direct enhancement of GABAARs (both in situ and in recombinant systems) required higher [EtOH] than were likely involved in most behavioral actions of EtOH. Moreover, a general lack of reproducibility of some observations of direct enhancement of GABAARs, and the discovery that direct enhancement is tightly controlled by GABAAR receptor phosphorylation status, suggests that direct enhancement may not be as common as initially thought, although it may play a role in specific cellular/behavioral situations. Further, the discovery of EtOH-induced GABA release (often at more clinically typical concentrations) combined with the fact that the dominant role of GABAARs in all central neural processing, means that modulating GABAARs (pharmacologically or genetically) may, and often does, alter EtOH-related behaviors even if the relevant underlying neural actions of EtOH do not involve direct enhancement of GABAARs by EtOH at relevant concentrations. Finally, it is now clear that EtOH can actually directly suppress GABAARs, and that this process appears to be genetically regulated such that it correlates with and can drive high EtOH consumption phenotypes.
A final way in which EtOH may affect GABAAR-mediated transmission is via its effect on GABAAR-active neurosteroids, primarily deoxycorticosterone, progesterone, testosterone, and their respective metabolites (Helms et al. 2012; Finn et al. 2004; Porcu and Morrow 2014; Cook et al. 2014). In particular, both acute and chronic exposures to EtOH alter the local and systemic concentrations of these GABAAR-active neurosteroids, either by changes in their local or global synthesis or metabolism. Often such changes vary considerably across different brain regions and across different species or genetic lines that have divergent EtOH-related phenotypes, further implicating their interaction with GABAARs in AUDs (Jensen et al. 2017; Cook et al. 2014; Porcu and Morrow 2014; Snelling et al. 2014). It is important to note that while many such neurosteroids have been studied in the context of their ability to enhance GABAAR currents, in general the sulfated versions of otherwise GABAAR-enhancing neurosteroids actually suppress GABAAR-mediated currents (Helms et al. 2012; Snelling et al. 2014). Finally, it has been demonstrated that some neurosteroids can act on presynaptic GABAergic terminals to increase vesicular GABA release (Park et al. 2011). The concentration of neurosteroids required to induce such release is generally higher than the usual range detected in plasma, but it is conceivable that local neurosteroid synthesis could result in higher concentrations locally that could affect presynaptic GABA release. Regardless, potential AUD-related treatment options involving neurosteroid-GABAAR interactions could involve this process. Thus, regulation of GABAAR transmission by neurosteroids is complex on its own, and varied modulation by EtOH across different brain regions, EtOH-contexts (low versus high concentrations and acute versus chronic exposure), and species/genotypes adds another level of complexity for which considerably more research will be required to fully understand.
1.3 Preclinical Studies That Target GABAARs to Deter EtOH Consumption Have Not Translated into Clinical Treatment for AUDs
Because of the clear interactions of EtOH with GABAAR-mediated transmission, modulation of GABAARs has been a dominant focus of preclinical efforts to combat EtOH actions in a manner that might reduce AUDs. Such efforts tend to focus on blocking or replacing acute EtOH actions on GABAARs and on ameliorating GABAAR-related adverse reactions to chronic EtOH consumption and associated withdrawal (Anton et al. 2014). Specifically, in an early study, systemic administration of a GABAAR agonist (THIP/Gaboxadol) or antagonist (picrotoxin) increased and decreased, respectively, ongoing voluntary EtOH consumption by rats (Boyle et al. 1993). Similarly, knocking out specific GABAAR subunits globally generally reduces EtOH consumption across a range of EtOH consumption models and lines of mice (Crabbe et al. 2006b; Rewal et al. 2009, 2012; Nie et al. 2011). However, other studies have shown that systemic application of GABAAR agonists, including THIP, can reduce EtOH consumption, including binge EtOH consumption (Moore et al. 2007; Ramaker et al. 2012). Furthermore, detailed temporal analysis of EtOH bout patterns indicate that the effect of modulating GABAARs globally, with the synthetic GABAAR-enhancing neurosteroid ganaxolone, can be complex even within a single model and set of animals (Ramaker et al. 2011). Such variability with systemic application of GABAAR ligands likely reflects the widespread expression of GABAARs across the brain, with different brain regions playing different roles in various aspects of EtOH-induced responses (Ramaker et al. 2015; Kaplan et al. 2016b; Nowak et al. 1998; Nie et al. 2011; Pina et al. 2015; Rewal et al. 2009). However, even within a given brain region, such as the nucleus accumbens, the role of GABAARs in EtOH consumption is complex, with either blocking or activating extrasynaptic GABAARs able to reduce EtOH consumption (Rewal et al. 2009; Nie et al. 2011; Ramaker et al. 2015). An additional complication is that because GABAARs are widely distributed in most brain regions, many GABAAR modulators cause intolerable side effects, such as sedation, depression, and motor impairment, which preclude clinical use. Similarly, various GABAAR modulators have their own addictive potential. Thus, it is perhaps not surprising that despite the clear role of GABAARs in multiple aspects of EtOH responses, there are currently no GABAAR ligands that are clinically effective at reducing AUDs. Instead, clinically, the use of GABAAR modulators is primarily restricted to emergency care, in particular preventing life threatening EtOH withdrawal symptoms, and there is still inadequate pharmacotherapy for AUD treatment and recovery generally (Anton et al. 2014; Eastes 2010).
1.4 The Cerebellar GABAAR System May Provide a Missing Piece to the AUD Puzzle
A likely contributing factor to the inadequate clinical translation of the aforementioned preclinical studies of EtOH and the GABAAR system is that much of what has been learned about their interactions is based on the studies of relatively high [EtOH] (almost exclusively ≥20 mM, and often ≥40 mM). While such [EtOH] are achieved in the blood of humans after binge EtOH consumption by nonalcoholics, and may be common in advanced cases of AUD, such concentrations are not achieved by humans in their early experiences with recreational EtOH consumption. This discrepancy is problematic because individual variation in the sensitivity of various neural processes to low [EtOH] is a significant predictor of risk for developing AUD. Specifically, low sensitivity to the aversive effects and high sensitivity to the rewarding effects of low [EtOH] predict an increased risk for developing AUD (Crabbe et al. 2010; Quinn and Fromme 2011; Schuckit 1985; Schuckit and Smith 1996; Schuckit et al. 1996, 2003). Importantly, low [EtOH], such as those achieved when an adult human consumes 1–2 standard units of alcohol over a 1–2 h period (i.e., blood [EtOH] = ~10 mM), have profound impacts on affect, mood, and behavior. Effects include a sense of euphoria, social disinhibition, anxiolysis, and motor impairment (Gallaher et al. 1996; Gilman et al. 2008; Schuckit 1985; Schuckit et al. 2003, 2008; Spanagel 2009; Trudell et al. 2014). These behavioral manifestations are reflected by brain imaging studies showing low [EtOH] alters neural signaling in brain regions involved in executive function (prefrontal cortex), reward and anxiety (ventral tegmental area, striatum, and amygdala), and motor coordination (cerebellum) (Mitchell et al. 2012, 2013; Gan et al. 2014; Weber et al. 2014; Nikolaou et al. 2013a, b; Bjork and Gilman 2014; Gilman et al. 2008; Volkow et al. 2008). However, there is a significant gap in our understanding of specific cellular/molecular targets of low [EtOH], and the mechanisms by which they alter neural processing to influence behavior. In fact, although studies of isolated brain tissue have shown that higher [EtOH] (>20 mM) alter neuronal and synaptic function, the effects of low [EtOH] (≤ 10 mM) at this level have been reported to be minimal or absent (Choi et al. 2008; Jia et al. 2007, 2008; Liang et al. 2006; Morikawa and Morrisett 2010; Nie et al. 1994, 2000; Peris et al. 1992; Roberto et al. 2003; Theile et al. 2008, 2009; Weitlauf and Woodward 2008). Thus, it is not clear how low [EtOH] has such robust action on cognition, emotion, and behavior, and thus how variations in those actions contribute to risk for developing an AUD. Accordingly, a primary goal of the alcohol research field has become to identify the molecular mechanisms by which low [EtOH] alters neural processing and EtOH-associated subjective effects and behaviors, and to determine how individual differences affect risk for developing AUD.
In this context, the GABAAR system of the cerebellum is an appealing target. In particular, in studies of humans and animals, low [EtOH] clearly alters cerebellar neural processing and associated behaviors (Volkow et al. 2008; Gallaher et al. 1996; Schuckit 1985; Schuckit et al. 2003). Similarly, studies of cerebellar brain slices from low EtOH consuming Sprague Dawley rats have shown that 10 mM EtOH powerfully enhances GABAAR-mediated inhibition of cerebellar granule cells (Fig. 1d) (Botta et al. 2007a; Carta et al. 2004; Hanchar et al. 2005; Kaplan et al. 2013), which are the primary integrators of afferent information to the cerebellar cortex (Fig. 2). In the remainder of this chapter we will review recent advances in our understanding of the cerebellum and its potential relationship to AUDs, with a particular focus on the granule cell GABAAR system (including GABAARs and upstream mechanisms that affect GABA release).
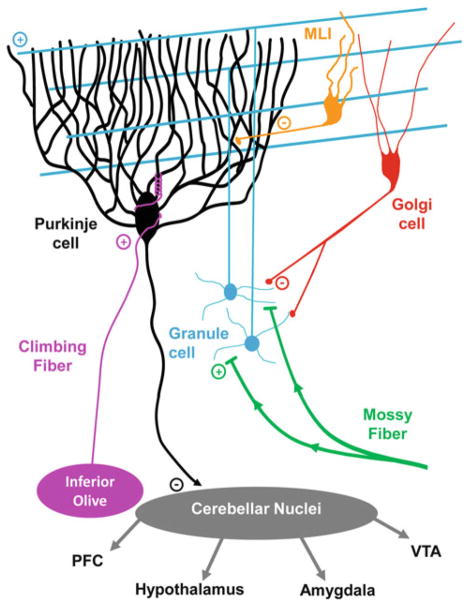
Fig. 2.
Circuitry of the cerebellar cortex. Circuit diagram of the cerebellum, showing the two excitatory/glutamatergic afferent inputs to the cerebellar cortex (mossy fibers and climbing fibers), the connectivity of the interneurons, which include the glutamatergic granule cells and GABAergic Golgi cells and Molecular Layer interneurons (MLIs), and the sole output of the cerebellar cortex, the GABAergic Purkinje cells. The Purkinje cells synapse onto a variety of cells distributed into three cerebellar nuclei, which in turn send mono- and polysynaptic efferents to most of the rest of the brain
2 Introduction to and Review of the Cerebellum and Its Relationship to AUDs
2.1 The Cerebellum and Genetic Risk for AUD
The importance of the cerebellum in motor control and balance has been known for nearly 200 years (Schmahmann 2010). However, over the last two decades it has been thoroughly established that the cerebellum plays a critical role in cognitive processes that had hitherto been overshadowed by its more obvious role in motor coordination. Compelling evidence from cerebellar specific lesion (Levisohn et al. 2000; Paulus et al. 2004; Schmahmann 2004; Tavano et al. 2007; Wolf et al. 2009), functional imaging (Stoodley and Schmahmann 2009, 2010; Stoodley et al. 2010, 2012), and anatomical tracing studies (Strick et al. 2009) indicate that the cerebellum contributes to attention, executive function, visual-spatial cognition, language, and emotion through reciprocal loops to and from the association areas of the parietal, frontal, temporal, and limbic cortices (Fig. 2) (Ito 2008; Schmahmann 2010; Strick et al. 2009). The cerebellum also communicates (see below for further details) with brain regions associated with EtOH reward [ventral tegmental area (Ikai et al. 1992, 1994), amygdala (Tomasi and Volkow 2011), and nucleus accumbens (Dempsey and Richardson 1987)], and with consummatory behavior (hypothalamus (Zhu et al. 2006; Zhu and Wang 2008)).
Adoption and twin studies suggest that predilection for developing AUD is 50–60% genetically determined (Hasin et al. 2007; Hill 2010). However, it is clear that there isn’t a single or even group of “AUD gene(s),” but rather that a wide range of genes lead to complex traits, and interactions amongst such traits engender a predilection for AUD. Consequently, the field of AUD researchers has tended to identify and characterize endophenotypes (genetically and mechanistically simpler heritable traits associated with genetic risk for developing an AUD), with the idea that understanding the molecular/genetic and neural substrates of such endophenotypes will be a more feasible approach to identifying potential targets for treatment of AUD. A common approach to identifying such AUD-related endophenotypes is to quantify differences in a given physical or behavioral trait between people with and without a family history of AUD (FH+ and FH−, respectively). Such studies have consistently identified cerebellar-related anatomical, neurological, and behavioral endophenotypes for which variation is tightly linked with AUD FH status.
First, in a series of MRI studies, Hill and colleagues determined that the cerebellum of FH+ offspring is significantly larger than in trait matched FH− offspring, due primarily to increased grey matter, potentially due to reduced synaptic pruning during development (Hill et al. 2007, 2011, 2016; Hill 2010). Importantly, this difference is separable from effects related to prenatal exposure to EtOH, which also affects the size of the cerebellum, but in the opposite direction and in distinct lobes (Sharma and Hill 2017). Also of interest, given the importance of GABAARs in EtOH actions, increased cerebellar volume in FH+ individuals is associated with an allelic variation in the GABAAR α2 subunit (Hill et al. 2011), which when knocked out in mice results in reduced EtOH consumption in females (Boehm et al. 2004).
In terms of cerebellar processing and communication with other brain regions, there are also considerable differences that correlate with AUD FH status. In a series of functional connectivity magnetic resonance imaging (fcMRI) studies, Nagel and colleagues determined that relative to FH− individuals, alcohol naïve FH+ offspring showed less functional connectivity between the cerebellum and two brain regions known to be involved in addictive behaviors, the prefrontal cortex (PFC) and nucleus accumbens (Cservenka et al. 2014; Herting et al. 2011). Further, using functional MRI, they also determined that FH+ individuals show reduced cerebellar activity during cognitive tasks (risky decision making and spatial working memory), despite not exhibiting any deficits in task performance (Cservenka and Nagel 2012; Mackiewicz Seghete et al. 2013). Thus, in addition to exhibiting reduced communication with addiction-associated brain regions, alcohol naïve, FH+ individuals exhibit altered cerebellar processing of behavioral tasks that likely play a role in addiction.
Another way in which genetic variation in cerebellar processing may influence predilection to AUD is via its role in neurological diseases/conditions that are risk factors for developing an AUD, possibly through self-medication with EtOH. The clearest example of self-medication with EtOH for a known cerebellar disease is a condition known as essential tremor, in which genetically determined cerebellar dysfunction leads to uncontrollable shaking, most frequently in the hands, but also in other body parts (Louis et al. 2017; Kuo et al. 2017). It is well established that consumption of EtOH ameliorates such tremors, and that patients use EtOH to self-medicate the condition (Rautakorpi et al. 1983; Mostile and Jankovic 2010). However, while some studies have suggested that essential tremor is a risk factor for AUD, others have not (Deik et al. 2012; Schroeder and Nasrallah 1982; Koller 1983). Similarly, tremor is a symptom of severe EtOH withdrawal, which also has a cerebellar etiology, and self-medication of the negative withdrawal symptoms may also contribute to the maintenance of AUD (Welsh et al. 2011; Deik et al. 2012).
Attention deficit hyperactivity disorder (ADHD) and schizophrenia are two genetically influenced disorders that have a strong cerebellar component to their etiology (Bledsoe et al. 2009; Mulder et al. 2008; Epstein et al. 2007; Mothersill et al. 2015; Baumann et al. 2015), and both conditions are risk factors for developing AUD (Daurio et al. 2017; Jones et al. 2011). In the case of ADHD, the relationship between risk for developing AUD is most tightly connected to the impulsivity aspects of ADHD (Daurio et al. 2017), which fits with the studies described above showing that AUD FH+ individuals exhibit reduced cerebellar processing during tests of impulsivity, i.e., risky decision making.
As discussed above, one consistent component to genetic risk for developing an AUD is increased sensitivity to the rewarding aspects of EtOH and reduced sensitivity to the aversive aspects of EtOH. One commonly studied manifestation of sensitivity to EtOH in humans is alcohol-induced static ataxia, which manifests as body sway, resultant from impaired vestibular and ocular feedback control of balance (a process that heavily depends on the cerebellum). Notably, studies of EtOH-induced body sway consistently find that the level of sensitivity to EtOH-induced body sway is heritably associated with AUD family history status and predictive of development of AUDs (Schuckit et al. 2005, 2011; Newlin and Thomson 1990; Newlin and Renton 2010). However, the nature of the relationship is complicated, with some studies finding a low level of response to EtOH-induced body sway in FH+ individuals, but others finding a high level of response in FH+ individuals (Newlin and Renton 2010; Quinn and Fromme 2011; Schuckit et al. 2005; Lex et al. 1988; Newlin and Thomson 1990; McCaul et al. 1991). These distinctions may result from differences in methodology or time point after EtOH exposure, or they may reflect two distinct sets of phenotypic risk (Quinn and Fromme 2011). This covariation also consistently occurs in animal models, albeit similar to humans, with varying polarity across studies. In particular, sensitivity to EtOH-induced ataxia shows an inverse relationship with EtOH consumption in some inbred strains of mice [e.g., DBA/2J (D2) and C57BL/6J (B6) mice] (Gallaher et al. 1996; Yoneyama et al. 2008) and lines of rodents selected for differences in alcohol consumption (e.g., Alko, Alcohol [AA]/Alko, Non-Alcohol [ANA] rats, and alcohol preferring (P)/alcohol non-preferring (NP) rats) (Bell et al. 2001, 2006; Malila 1978). Conversely, a recent study in high- and low-alcohol preferring mice (HAP and LAP) showed a positive relationship between EtOH consumption and sensitivity to EtOH-induced ataxia (Fritz et al. 2012). Thus, collectively, although the direction of the relationship varies across studies, there is nonetheless a consistent genetic relationship between cerebellar sensitivity to EtOH and risk for AUD in humans and level of EtOH consumption in rodent genotypes. In this context, it is also important to reiterate that although static ataxia in humans and rotorod performance in rodents are easily quantified measures of cerebellar sensitivity to EtOH, they do not have to be the only aspect of altered cerebellar processing that promotes or deters EtOH consumption and thus escalation to AUD. Instead, the relationship between cerebellar sensitivity to EtOH (quantified by measures of static ataxia and rotorod performance) and AUD risk could be mediated by cerebellar-dependent motor processes, cognitive processes, reward processes, or some combination. Thus, while motor impairment and disrupted balance are likely aversive (Damji et al. 1996; Hotson 1984; Ragge et al. 2003), whether or not the overall cerebellar response to EtOH promotes or deters excessive EtOH consumption likely depends on how motor aspects combine/interact with EtOH effects on the other, non-motor aspects of cerebellar processing.
In summary, there is a clear and consistent heritable relationship between genetic predisposition for developing an AUD and a variety of aspects of the cerebellum, including: (1) its size and white matter to grey matter ratios, (2) its communication with reward-associated brain regions (see below for further details), (3) its processing of behaviors that likely influence predilection for AUD, and (4) its sensitivity to EtOH. There are also a number of known cerebellar-related diseases that are also known risk factors for developing an AUD, and at least some of them are likely to provoke self-medication with EtOH.
2.2 The Neural Circuitry of Cerebellar Interactions with AUD-Associated Brain Regions
In order to consider how variable effects of EtOH on cerebellar cortical processing might influence EtOH consumption, we need to consider the outputs and function of the cerebellum. Purkinje cells are the sole output of the cerebellar cortex (Fig. 2), and they form GABAergic inhibitory synapses onto the glutamatergic/GABAergic/glycinergic neurons of the three cerebellar nuclei: the dentate (lateral), interpositus (intermediate), and fastigial (medial) (Ito et al. 1970; Billard et al. 1993; Jahnsen 1986; ten Bruggencate et al. 1972; Teune et al. 1998; De Zeeuw and Berrebi 1995; Chen and Hillman 1993; Monaghan et al. 1986). Efferents from these nuclei then project, directly and polysynaptically, to multiple other brain regions that are important for driving and/or regulating motor output, including motor cortex, thalamus, basal ganglia, red nucleus, inferior olive, and spinal cord. This dominant efferent distribution pattern, combined with the fact that cerebellar neurons respond to movement or changes in limb/body position (Barmack and Yakhnitsa 2008; Lisberger and Fuchs 1978; Thach 1968, 1970), and that damage to the cerebellum often results in motor control abnormalities like ataxias, dysmetria, or gaze control disorders (Schmahmann 2004), demonstrate that the cerebellum serves an important role in motor control and vestibular reflexes, including balance and ocular stabilization. Importantly, EtOH-induced ataxia and motor incoordination are largely mediated by EtOH actions in the cerebellar cortex that lead to disruption of cerebellar output to these motor areas (Dar 2015; Hanchar et al. 2005).
In addition to its well-established role in motor control, functional imaging studies in humans clearly indicate that changes in cerebellar activity are correlated with numerous non-motor behaviors, including various cognitive and emotional processing tasks (Ferrucci et al. 2012; Schraa-Tam et al. 2012; Stoodley and Schmahmann 2009, 2010; Stoodley et al. 2010, 2012). In parallel animal studies, clear mono- or polysynaptic functional connections between the cerebellum and non-motor brain regions have been identified (Harper and Heath 1973, 1974; Strick et al. 2009; Zhang et al. 2016). Accordingly, selective damage of the cerebellum in humans can result in non-motor, cognitive, or emotional processing abnormalities that may present with, or even without, motor function disruption (Schmahmann and Sherman 1998; Schmahmann 2004; Schmahmann et al. 2009). These studies indicate that the cerebellum is involved in many aspects of cognition, emotion, and overall behavior (Ito 2008; Schmahmann 2004, 2010; Schmahmann and Caplan 2006).
In the context of AUDs, some of the mono- and polysynaptic projections from cerebellar nuclei neurons target brain regions known to be involved in addiction and drug abuse, including the ventral tegmental area (VTA), nucleus accumbens, amygdala, PFC, hippocampus, and hypothalamus (Tomasi and Volkow 2011; Strick et al. 2009; Volkow et al. 2008). Therefore, EtOH-induced changes in the activity of these cerebellar efferents due to modulation of cerebellar cortical activity may be a mechanism by which EtOH alters reward processing, affect, memory, or consumption behavior. Thus, it is important to consider the nature of these connections in order to understand the direction and degree to which EtOH actions in the cerebellar cortex may alter synaptic activity and firing dynamics of neurons in these other brain regions.
The collection of dopaminergic neurons within the ventral tegmental area has long been known to be important in mediating reward responses or pleasurable feelings associated with specific stimuli (e.g., food, sex, drugs of abuse) by releasing dopamine in the nucleus accumbens of the ventral striatum (Koob and Volkow 2010). Unfortunately, the mechanisms by which EtOH at doses or concentrations that are within a range commonly experienced during recreational consumption (blood [EtOH] ≈ 45–150 mg/dl, 10–35 mM) modulate dopamine release through altering VTA dopaminergic neuron activity and dopamine release are unclear. In rodent models in vivo, low doses of systemically administered EtOH (0.5–2 g/kg ≈ 50–200 mg/dl) reduce firing of GABAergic (Steffensen et al. 2009; Stobbs et al. 2004) neurons and increase firing of dopaminergic neurons (Gessa et al. 1985; Ostroumov et al. 2016) in the VTA, but this approach cannot distinguish between sites of action for EtOH that are within the VTA or on VTA afferents, or on the cell bodies or synaptic inputs of such afferents. When directly administered into the posterior VTA, rats selectively bred to prefer EtOH will self-administer 50–75 mg/dl (11–16 mM) EtOH (Gatto et al. 1994; Hauser et al. 2011; Rodd et al. 2005). Although, in rat strains not bred selectively to prefer EtOH, 200 mg/dl (43.4 mM) appears to be the threshold dose to induce dopamine release in the nucleus accumbens (Ding et al. 2009), ventral pallidum, or PFC (Ding et al. 2011), and trigger self-administration (Rodd-Henricks et al. 2000). Importantly, EtOH in ex vivo brain slices typically needs to reach >40 mM before inducing any significant change in the activity of VTA dopaminergic neurons or synaptic activity in mice (Avegno et al. 2016; Brodie and Appel 2000; Okamoto et al. 2006) and rats (Xiao et al. 2009; Theile et al. 2011; Ostroumov et al. 2016; McDaid et al. 2008; Koyama et al. 2007; Brodie et al. 1990), although Mrejeru et al. (2015) recently indicated that a limited subset of VTA dopaminergic neurons may be sensitive to 20 mM EtOH. Together, these data suggest that VTA sensitivity to recreational levels of EtOH is likely not mediated entirely by mechanisms within the VTA, but rather may be dependent on modulation of VTA afferent activity from other brain regions that are only functionally intact in vivo and may induce excitation of VTA dopaminergic neurons.
One possible brain region that could mediate EtOH’s effects in the VTA may be the cerebellum, since it is exquisitely sensitive to low [EtOH] (see above). A functional synaptic connection between cerebellar nuclear neurons and the VTA was demonstrated by studies in which electrical stimulation of the dentate nucleus or Purkinje cells caused increased dopamine levels in the nucleus accumbens (Dempsey and Richardson 1987) or PFC in a manner that was blocked by local pharmacological inhibition of the VTA (Rogers et al. 2011, 2013; Mittleman et al. 2008). The as yet poorly understood cerebellar efferent pathway to VTA that increases dopamine release in the PFC, nucleus accumbens, and amygdala (Inglis and Moghaddam 1999; Oades and Halliday 1987; Loughlin and Fallon 1983; Beckstead et al. 1979; Fallon et al. 1978) may couple with the activity of a direct projections from the fastigial nucleus to the nucleus accumbens and amygdala which have been demonstrated in primate, cat, and rat (Harper and Heath 1973; Heath and Harper 1974; Oades and Halliday 1987). While these pathways are poorly understood, their clear existence provides a potential substrate for the translation of EtOH-induced changes in cerebellar cortical activity into EtOH-related modulation of reward and emotional processing.
Of the dopamine-related cerebellar efferent pathways, those that influence dopamine release in the PFC, a region involved in cognition, attention, and affect, is most thoroughly characterized. Cerebellar cortical or nuclear stimulation increases PFC dopamine, which peaks within 350–400 ms poststimulation (Mittleman et al. 2008). Specific nuclei/region inactivation studies using local application of lidocaine (a voltage-gated sodium channel blocker) or kynurenate (an ionotropic glutamate receptor antagonist) directly into the VTA alone reduced cerebellar stimulation-induced PFC dopamine levels by ~50%. Thus, half of the cerebellar-evoked PFC dopamine release is via excitation of the VTA. Further efforts to map the efferent pathway that drives such VTA excitation, again via local inactivation studies of potential intervening nuclei, revealed a dentate-reticulotegmental-peduncolopontine-VTA-PFC pathway (Rogers et al. 2011). Additional studies, blocking action potentials and glutamatergic transmission in putative complementary pathways (mediodorsal or ventrolateral thalamus) revealed that, together, pathways involving the thalamic nuclei mediate the remaining ~50% of the rise in PFC dopamine (Rogers et al. 2011). Collectively, these data suggest that electrical stimulation of the cerebellar cortex or dentate nucleus increases PFC dopamine release via parallel polysynaptic pathways that excite the VTA (dentate-reticulotegmental-peduncolopontine-VTA-PFC pathway) as well as VTA terminals (mediodorsal or ventrolateral thalamus) within the PFC (Rogers et al. 2011). However, recent exciting functional tract tracing studies have confirmed an older tract tracing study (Perciavalle et al. 1989) showing that there is also a direct projection from the cerebellar nuclei (dentate and interpositus) to the VTA (Kamran Khodakhah, personal communication; Richardson and Rossi, unpublished observations), but the function, behavioral relevance, and potential role of this pathway in mediating VTA responses to EtOH and reward have yet to be examined.
The increase in PFC dopamine levels upon cerebellar stimulation indicates the ability of dentate nuclei efferents to influence activity of both the VTA and the PFC (Rogers et al. 2011; Mittleman et al. 2008), but additional parallel cerebellar nuclear efferent pathways may also influence the PFC, albeit presumably in different contexts and time scales. In particular, electrical stimulation of the dentate nucleus evokes excitatory field potentials in the PFC in primates (Sasaki et al. 1979), and concurrent antero- and retrograde tracing techniques indicated that such potentials are mediated by dentate to thalamic nuclei to PFC pathways (Kelly and Strick 2003; Middleton and Strick 1994, 2001). Conversely, electrical stimulation of the fastigial nucleus typically induces a suppression or biphasic inhibition/excitation response of putative PFC pyramidal cells, indicating that fastigial efferents may primarily influence inhibitory synaptic activity in PFC (Kelly and Strick 2003; Middleton and Strick 1994, 2001; Watson et al. 2014), but does so after a much longer latency (10–13 ms) relative to dentate-evoked responses, suggesting that the connection is polysynaptic (Watson et al. 2014).
Thus, in addition to dentate driven VTA-derived dopamine release into the PFC, inhibitory fastigial and excitatory dentate projections to the PFC offer a mechanism by which differential EtOH-induced changes in cerebellar cortical output may be able to enhance and/or suppress PFC activity, although further work is needed to confirm the precise neuronal types that these cerebellar nuclei projections target to fully understand how the cerebellum may modulate or drive PFC activity to affect EtOH consumption.
In addition to cerebellar modulation of reward circuitry influencing the initial responses to EtOH, another way in which actions of EtOH in the cerebellum may influence abusive EtOH consumption is via an influence over cues and contexts associated with EtOH during the addiction cycle. In particular, learned associations between cues related to drugs of abuse (including EtOH) and pleasure are a key aspect of drug abuse and relapse. And, fMRI studies in abstinent alcoholic humans have shown that the cerebellum is strongly activated by cue-induced craving, and that such cue-induced activation of the cerebellum ceases to occur after cognitive therapy eliminates craving associated with the cues (Schneider et al. 2001). And a recent study in rodents found that a subset of granule cells in the cerebellar cortex is activated by cues that predict reward in a manner that suggests that they code expectation of reward (Wagner et al. 2017).
How might the cerebellum influence cue-induced craving and anticipation of reward? Given the role of the hippocampus in learning and memory, it has long been considered that the hippocampus is a key player in maintaining this association despite also contributing to AUD-associated cognitive impairment (Kutlu and Gould 2016), and evidence suggests that the cerebellum may powerfully influence the hippocampus, providing a potential mechanism by which the cerebellum influences craving for EtOH. For the purposes of this discussion, only the potential role of the cerebellum in mediating the association between context and reward will be discussed, not the multitude of deleterious effects EtOH has on hippocampal anatomy and function. Similarly, since the full range of pathways that may constitute polysynaptic connections between the cerebellum and hippocampus which shape aspects of spatial and temporal processing are vast, full coverage is also beyond the scope of this book chapter, but have been recently reviewed elsewhere (Yu and Krook-Magnuson 2015).
While there does not appear to be a direct connection between the dentate nucleus and hippocampus (Heath et al. 1978), field potential, single unit responses, and degenerating fiber tracing data from primate, cat, and rat indicate that there is a robust short latency bilateral direct projection from the fastigial nuclei to hippocampus (Newman and Reza 1979; Heath and Harper 1974; Heath et al. 1978). In line with the known expression of glycinergic projection neurons in the fastigial nucleus (Bagnall et al. 2009), this projection appears to be largely inhibitory (Heath and Harper 1974). However, these fastigial-evoked field potentials tended to be biphasic and also had a later excitatory component and generated action potentials within 12 ms of stimulation, likely due to a rebound from inhibition or activation of an additional long latency excitatory projection (Heath and Harper 1974; Newman and Reza 1979). This cerebellar-hippocampal projection is robust enough to block epileptiform activity when Purkinje cells are driven by activation of channelrhodopsin (Krook-Magnuson et al. 2014). PKC-dependent plasticity at the parallel fiber to Purkinje cell synapse in mice is essential for accurate coding of hippocampal place cells and performance on a navigation task, indicating the importance of cerebellar input to the hippocampus in maintaining spatial orientation (Rochefort et al. 2011). These data indicate that cerebellar nuclei neurons are capable of dramatically altering hippocampal activity and coding of spatial cues that may be relevant for shaping EtOH consumption behavior in the context of craving. Therefore, determining which nuclei, neurons, and synapses are involved in this pathway may provide further insight into the role of the cerebellum in AUDs.
Finally, when considering the neurobiological underpinnings of EtOH consumption behaviors, it is essential to address the hypothalamus, a brain region responsible for a range of autonomic functions, regulating hormone secretion, and metabolic homeostasis. On its own, the hypothalamus has been shown to be important in mediating motivated behaviors to feed and seek out drugs of abuse or reward (Marchant et al. 2012). However, the hypothalamus also both sends and receives input to/from cerebellar nuclei neurons (Zhang et al. 2016; Zhu et al. 2006). In primate and rat, tract tracing suggests that neurons from all three cerebellar nuclei form projections that are broadly distributed across a number of posterior hypothalamic nuclei (Zhu et al. 2006; Zhang et al. 2016; Cavdar et al. 2001a, b; Haines et al. 1990), including the lateral hypothalamus which are known to be important in EtOH seeking behavior (Marchant et al. 2009; Dayas et al. 2008; Hamlin et al. 2007). However, outside of this tract tracing approach, little is known about the function of this pathway to influence behavior, or even the neurotransmitter systems and cell types in the hypothalamic nuclei that may be involved.
2.3 History of and Recent Controversies About Actions of EtOH Within the Cerebellum
Ultimately, fully understanding the actions of EtOH on human neurophysiology, and thus the etiology of and treatment for AUDs, requires determining the molecular and neural targets of EtOH. This sentiment is reflected in some of the first in vivo brain recording studies examining responses to systemic EtOH in various brain regions of the rat or rabbit (Klemm and Stevens 1974; Klemm et al. 1976). In this context, because of the clear adverse impact of EtOH on motor control and balance, many early in vivo studies of EtOH actions at a cellular level included or even focused on the cerebellum (Klemm and Stevens 1974; Klemm et al. 1976; Rogers et al. 1980; Deitrich et al. 1989), a presumed mediator of such EtOH-induced motor impairments. Such early studies determined that the cerebellum, along with the hippocampus and cerebral cortex, was one of the more EtOH-sensitive brain regions, generally responding to lower [EtOH] than other brain regions. In particular, most early in vivo studies used single unit recording from Purkinje cells (PCs), which are the sole output of the cerebellar cortex, and thus should reflect actions of EtOH anywhere in the cerebellar cortex. Such studies generally indicated that EtOH suppresses PC firing, although some studies also showed enhanced firing, potentially reflecting different doses of EtOH used and/or local direct versus secondary, upstream actions of EtOH (Deitrich et al. 1989). For example, Rogers et al. (1980) determined that systemic EtOH increased PC complex spike firing but simultaneously decreased their simple spike firing rates in an anesthetized preparation (Rogers et al. 1980). Given that PC complex spikes are synaptically driven by climbing fiber afferents from the inferior olive, whereas PCs fire simple spikes spontaneously, the opposite actions within the same cell were interpreted as reflecting actions of EtOH in the inferior olive and direct actions on the PCs, respectively. Interestingly, such acute responses were absent in rats that had been chronically exposed to EtOH prior to recording, and climbing fiber driven complex spike frequency was reduced during withdrawal (Rogers et al. 1980). Thus, in addition to being highly sensitive to EtOH (acute intoxication), the cerebellum exhibits neural correlates of behavioral tolerance and dependence.
Parallel in vivo studies examining PC responses to local cerebellar application of EtOH, combined with studies of isolated slices of cerebellum confirmed that acute suppression of PC simple spikes by EtOH is indeed due to direct actions within the cerebellum (George and Chu 1984; Siggins and French 1979). Importantly, while such impacts could be due to direct actions of EtOH on PCs, they could also be influenced by the underlying granule cells, which synaptically modulate PC spike firing and also appear sensitive to the actions of EtOH in vivo. For example, low dose EtOH dramatically suppressed sensory-evoked granule cell spiking activity in cat upon systemic administration of low dose EtOH (0.3 g/kg = 15–20 mM EtOH in CSF) (Huang and Huang 2007). Therefore, EtOH-induced changes observed at the level of PCs may also reflect actions elsewhere in the cerebellar cortex.
Given the above summarized establishment of the cerebellum as a sensitive target of EtOH, consequent studies began to focus on two crucial issues that have yet to be fully resolved: (1) identifying the molecular targets that mediate EtOH impacts on cerebellar signal propagation and (2) determining if there are genetic differences in such cerebellum-specific actions that may relate to predilection for excessive alcohol consumption and addiction. In terms of the latter interest, a series of studies by Hoffer and colleagues determined that the degree of EtOH-induced spike suppression in PCs correlated with genetic variation in sensitivity to the soporific effects of EtOH in vivo (Basile et al. 1983; Palmer et al. 1982, 1985; Sorensen et al. 1980, 1981). In particular, PCs in mouse lines bred to be sensitive (long sleep mice; LS mice) to the soporific effects of EtOH (i.e., duration of sleep, as assessed by a loss of the righting reflex) were significantly more sensitive to EtOH-induced suppression of spiking than PCs in mice bred to be insensitive to the soporific effects of EtOH (short sleep mice; SS mice). Such differences are somewhat specific to the cerebellum, as parallel recordings of similarly sensitive hippocampal neurons did not show any differences in sensitivity between LS and SS mice (Sorensen et al. 1981). Importantly, the observed cerebellar differences persisted in acutely isolated slices of cerebellum and when the cerebelli from LS/SS mice were transplanted into the ocular space of the opposite line of mice, collectively confirming that such genetic differences were inherent properties of the cerebellum (Basile et al. 1983; Palmer et al. 1982, 1985). Finally, when LS and SS mice were chronically exposed to EtOH in vivo, they both developed behavioral tolerance to the ataxic and soporific effects of EtOH, which was mirrored by the development of tolerance at the level of PC spiking, even in slices. This suggests again that crucial aspects of AUD (acute sensitivity and tolerance) are exhibited by the cerebellum at both the behavioral and cellular level (Palmer et al. 1985).
Interest in the cerebellum as a primary mediator of EtOH intoxication was further stimulated by the contemporarily developing appreciation that GABAARs were key mediators of EtOH intoxication (Allan et al. 1987; Harris et al. 1988; Palmer et al. 1988; Allan and Harris 1987), and that the newly developed GABAAR inverse agonist, Ro 15-4513, the so-called alcohol antagonist, had a high affinity for binding to the α6 subunit of GABAARs that are fairly exclusively expressed on cerebellar granule cells (Fig. 1b) (Luddens et al. 1990). In particular, studies of EtOH actions on GABAARs using membrane microsacs isolated from cerebellum determined that EtOH enhanced GABAAR-stimulated Cl− flux in LS mice, but not in SS mice (Allan and Harris 1986; Allan et al. 1987, 1988). And, similar to the cerebellar specificity that was observed with in vivo neuronal recordings, there were no differences in EtOH modulation of GABAAR-stimulated Cl− flux in membrane microsacs derived from the hippocampus of LS and SS mice. Lastly, EtOH suppression of Purkinje cell firing and enhancement of microsac Cl− flux was significantly reduced by Ro 15-4513 (Harris et al. 1988; Palmer et al. 1988). Collectively, the data suggest that genetic differences in behavioral sensitivity to EtOH intoxication are mediated in part by genetic differences in the sensitivity of the cerebellar GABAAR system to EtOH.
Despite the genetic relationship between behavioral intoxication (as assessed by sleep time) and cerebellar sensitivity to EtOH, relating such differences to actual genetic variation in EtOH consumption has been more complicated. In particular, early studies of EtOH consumption by LS and SS mice determined that SS mice consumed more EtOH than LS mice when the consumption options were sweetened EtOH versus tap water (Church et al. 1979). Importantly, when given the choice between sweetened EtOH and sweetened tap water, although SS mice still consumed more EtOH than LS mice, they both consumed significantly less EtOH. Together, these outcomes suggest that at least part of the limit on EtOH consumption was driven by aversive aspects of EtOH which can be overridden to an extent by sweetening. While these outcomes support the broad idea that genetic sensitivity to aversive aspects of EtOH, particularly those mediated by the cerebellum (reflected in this case by loss of righting reflex), is a deterrent to abusive EtOH consumption, parallel operant studies determined that only LS mice exhibited positive reinforcement by EtOH, and thus worked for pharmacologically active levels of EtOH (Elmer et al. 1990). Subsequent studies with a variety of inbred or selected lines have revealed similar discrepancies, with levels of EtOH consumption being greater in either higher or lower sensitivity rodent genotypes, or not being correlated at all (Kakihana et al. 1966; Malila 1978; Spuhler and Deitrich 1984; Tabakoff and Kiianmaa 1982; Riley et al. 1977; Erwin et al. 1980; Millard 1983; Daoust et al. 1987). Thus, while there is frequent genetic covariation between EtOH consumption and “sensitivity” phenotype, the relationship is not always negative, and the two behavioral phenotypes are, although frequently genetically linked, separable.
In this context, it is important to emphasize that few, if any rodent genotypes will voluntarily consume enough EtOH to induce sleep, and the high [EtOH] required to induce sleep will obviously affect multiple molecular and neural targets that may not influence early, nondependent voluntary consumption, which complicates interpretation of genetic risk for excessive EtOH consumption (Bell et al. 2001). Relatedly, although sleep duration and other measures of acute intoxication duration may reflect initial sensitivity, their duration is also affected by the rate of development of acute functional tolerance, which is a separable, genetically determined factor that presumably also influences the overall subjective initial “reaction” to EtOH (Crabbe et al. 2006a; Ponomarev and Crabbe 2002; Gallaher et al. 1996; Fritz et al. 2012). Further complicating interpreting the role of such genetic sensitivity in risk for abusive EtOH consumption, it is also possible that EtOH-induced sedation may be aversive to some genotypes and positive to others.
Collectively, the complications described above form part of the following rationale for focusing on responses to low [EtOH]. First, responses to low [EtOH] and genetic differences in such responses are key to determining the nature of initial reactions to EtOH in most nonhuman models and early non-abusive EtOH consumption by humans that may ultimately determine predilection to AUD. Second, the smaller number of molecular/neural targets of low [EtOH] will be more tractable and thus relatable to specific behavioral endophenotypes that influence development of AUD.
In this broad context, while most early in vivo studies of cerebellar responses to EtOH found PCs to be highly sensitive, parallel slice studies with synaptic transmission blocked found that the concentration of EtOH required to suppress PC firing directly were higher [30–100 mM (Basile et al. 1983)] than those required to induce motor incoordination (~10 mM). This discrepancy, combined with early evidence that low [EtOH] [10–15 mM (Allan and Harris 1986; Allan et al. 1988)] could enhance GABAAR-mediated Cl− flux in cerebellar-derived membrane microsacs prompted a shift in focus to other cellular targets in the cerebellar cortex that might underlie the higher in vivo sensitivity of the cerebellum to low [EtOH] (Carta et al. 2004; Freund et al. 1993). In particular, an early in vivo recording study determined that EtOH increased action potential firing of inhibitory Golgi cells that provide lateral and feedback inhibition to granule cells (Freund et al. 1993). Subsequently, a slice study confirmed that concentrations of EtOH as low as 10 mM increased Golgi cell spontaneous firing frequency, which increased GABA release onto granule cells, manifesting as increased phasic IPSC frequency and associated increase in magnitude of granule cell tonic GABAAR current (Carta et al. 2004).
While the initial slice study by Valenzuela and colleagues indicated that EtOH increased inhibition of granule cells by increasing Golgi cell firing (because EtOH did not affect granule cell inhibition in the presence of the sodium channel blocker tetrodotoxin) (Carta et al. 2004), a subsequent study by Olsen and colleagues argued that much of the enhancement of the granule cell tonic GABAAR current was mediated by direct actions of EtOH on the α6-δ-subunit containing extrasynaptic GABAARs that generate granule cell tonic GABAAR currents (Hanchar et al. 2005). Moreover, these authors argued that a single point mutation in the α6 subunit conferred increased sensitivity of the granule cell tonic GABAAR current to EtOH, and that the increased sensitivity resulted in increased behavioral sensitivity to the motor impairing effects of EtOH. This study, along with parallel studies of cloned GABAARs (Wallner et al. 2003) heralded an exciting moment in EtOH research in which the researchers concluded that the δ subunit, and its typical pairing with either the α4 (hippocampus and thalamus) or α6 (cerebellum) GABAAR subunits was the elusive “one glass of wine receptor” that mediated the well-established behavioral sensitivity to low [EtOH] (Olsen et al. 2007). While the authors also observed EtOH-induced increased release of GABA from Golgi cells, they have concluded that most of that increase is driven by EtOH directly enhancing δ-subunit-containing GABAARs on the axons of granule cells, which because they are excitatory (Pugh and Jahr 2011, 2013), actually enhance excitatory synaptic drive of Golgi cell-mediated feedback inhibition of granule cells (Santhakumar et al. 2013). Unfortunately, such direct actions on GABAARs of low [EtOH] have not been observed by most other researchers, ourselves included, either in situ or in cloned GABAARs, and the cause of such discrepancies remains unclear [for detailed discussions of this ongoing controversy see Santhakumar et al. (2007); Korpi et al. (2007); Borghese and Harris (2007); Botta et al. (2007a, b); Olsen et al. (2007); Valenzuela and Jotty (2015)].
While we predict that eventually the discrepancy will be discovered to stem from some subtle difference across labs in tissue health and/or intracellular milieu (such as intracellular [Ca2+], [NO], or phosphorylation status), we will provide a brief description of why we conclude that direct enhancement is not likely to be a dominant mediator of EtOH actions in the cerebellum or its role in AUD. First, similar to Valenzuela’s findings, in a range of mammalian species, including non-human primates, we have been unable to see any direct enhancement of granule cell tonic GABAAR currents by even high [EtOH] (9–105 mM) (Kaplan et al. 2013, 2016a; Mohr et al. 2013). Importantly, while concluding that there is direct enhancement in situ is dependent on ensuring that all possible sources of increased GABA are prevented, the converse is not true: a lack of enhancement cannot be explained by inadequate block of a potential source of GABA. Thus, we find the complete absence of EtOH enhancement of granule cell tonic GABAAR currents in the presence of tetrodotoxin in B6 and D2 mice, SD rats, prairie voles, and nonhuman primates to be compelling evidence that EtOH does not directly enhance granule cell tonic GABAAR currents (Kaplan et al. 2013, 2016a; Mohr et al. 2013). Similarly, we also reported that in a subset of granule cells in B6 and D2 mice, and nonhuman primates, EtOH did not affect the tonic GABAAR current even without blocking action potentials, which was associated with a lack of increase in sIPSC frequency, as well as low nNOS expression, which we have determined is a key mediator of EtOH excitation of Golgi cells (Kaplan et al. 2013). Importantly, the lack of enhancement in our hands cannot be due to a ceiling effect, because in all mammalian species we have tested, bath application of THIP [at concentrations that are absolutely selective for δ-subunit-containing GABAARs (Meera et al. 2011)] increases the magnitude of the tonic GABAAR current (Kaplan et al. 2013, 2016a; Mohr et al. 2013). Finally, as will be discussed in greater detail below, we recently discovered that EtOH actually directly suppresses tonic GABAAR currents in high EtOH consuming genotypes (B6 mice and prairie voles), and this suppression persists when granule cells are physically removed from the slice, thus precluding possible actions of EtOH-induced altered GABA release (Kaplan et al. 2013, 2016a). Similarly compelling, it was recently shown that EtOH increases tonic GABAAR currents in pre-weanling SD rats that do not yet express the δ-subunit of the GABAAR, with the cause of the enhancement again being increased GABA release (Diaz and Valenzuela 2016). This is particularly important because although germline deletion of the δ-subunit reduces responses to EtOH (Santhakumar et al. 2013), it is well known that germline deletion of GABAARs causes a variety of homeostatic adaptations in the cerebellum which could indirectly alter responses to EtOH (Valenzuela and Jotty 2015; Brickley et al. 2001).
Finally, regarding the proposed role of excitatory, axonal δ-containing GABAARs in mediating EtOH-induced increased GABA release from Golgi cells (Santhakumar et al. 2013), we have done extensive immunocytochemistry for the δ-subunit in the cerebellar cortex, and we have seen no evidence for it being expressed in the molecular layer where the granule cell axons reside, despite robust expression within the granule cell layer (Kaplan et al. 2013; Mohr et al. 2013). Moreover, we were able to significantly reduce EtOH-increased Golgi cell firing and consequent GABA release onto granule cells by blocking nNOS, without blocking the glutamate receptors that were hypothesized to drive Golgi cell excitation (Kaplan et al. 2013). In this regard it is important to note that it is possible that blocking glutamate receptors will reduce basal Ca2+ influx and thus reduce nNOS activity, thereby circumventing EtOH from exciting Golgi cells via nNOS block, independent of EtOH-induced changes in glutamatergic synaptic transmission.
In summary, although there are clearly still some specific cross-lab conflicting results, many of the apparent conflicts can be explained by alternative interpretations of complex network and molecular interactions. Regardless, since the genotypic differences we will consider below were discovered in our lab using identical techniques and solutions, any differences in EtOH actions and associated impacts on EtOH consumption phenotypes should be specific to true genetic variation.
2.4 Genetic Variation in Cerebellar Cortical GABAAR Signaling Responses to EtOH Influences EtOH Consumption Phenotype
As discussed above, the cerebellum is exquisitely sensitive to EtOH, with concentrations as low as 10 mM significantly altering cerebellar neural signaling (Kaplan et al. 2013, 2016b; Welsh et al. 2011; He et al. 2013; Richardson and Rossi 2017) and, consequently, known cerebellar-dependent behaviors (Gallaher et al. 1996). Importantly in the context of genetic predilection to AUD, over the last several years we have discovered that the response of key cerebellar processes to low [EtOH] varies across mammalian genotypes in a manner that correlates with, and appears to influence EtOH consumption phenotype (Mohr et al. 2013; Kaplan et al. 2013, 2016a, b; Richardson and Rossi 2017). While there are many molecular targets of EtOH in the cerebellum, which collectively affect almost all cells and synapses within the cerebellar cortical circuit (Dar 2015; He et al. 2013; Kaplan et al. 2013; Richardson and Rossi 2017; Welsh et al. 2011; Valenzuela and Jotty 2015), only a few targets have been identified that are sensitive to 10 mM EtOH, including T-type Ca2+ channels in the Inferior Olivary neurons that provide climbing fiber inputs to Purkinje cells (Welsh et al. 2011), the NMDA receptors in the climbing fiber to Purkinje cell synapse (He et al. 2013; Zamudio-Bulcock et al. 2018), and the GABAAR synapse from Golgi cells to granule cells, which has both Golgi cell and postsynaptic targets (Kaplan et al. 2013). Indeed, we explicitly determined that 10 mM EtOH does not have any detectable impact on any of the other cells and synapses in the mouse cerebellar cortex (Kaplan et al. 2016b). And, while the two climbing fiber targets have been implicated respectively in EtOH withdrawal-induced tremors (Olivary neuron T-type Ca2+ channels) and cerebellar learning (Purkinje cell NMDA receptors), both of which may influence predilection to AUD, to our knowledge, only the Golgi cell to granule cell synapse is known to vary in its response to low [EtOH] in a manner that correlates with EtOH consumption phenotype across mammalian genotypes. Thus, for the remainder of this chapter, we will focus on the Golgi cell to granule cell synapse.
As introduced above, in low EtOH consuming Sprague Dawley rats (SDRs), low [EtOH] (starting at ~10 mM) enhances Golgi cell inhibition of granule cells (Carta et al. 2004). The enhancement manifests as both an increase in the frequency of sIPSCs and an increase in the magnitude of the tonic GABAAR current (Figs. 1d and 3a), which in granule cells is mediated by extrasynaptic α6 and δ subunit-containing GABAARs (Hamann et al. 2002). Despite the controversy described above (Hanchar et al. 2005; Santhakumar et al. 2007; Korpi et al. 2007; Borghese and Harris 2007; Botta et al. 2007a, b), we believe that the preponderance of data indicates that the primary mechanism is via EtOH-induced increased action potential firing by Golgi cells, which accounts for the increase in both sIPSC frequency and in tonic GABAAR current magnitude, due to elevated extracellular GABA. These fundamental observations have been replicated by several labs, including those that also see direct enhancement of GABAARs (Hanchar et al. 2005; Kaplan et al. 2013), and are part of the cornerstone of two widely accepted concepts: (1) a main target of recreational [EtOH] is the GABAAR system generally, and (2) EtOH-induced motor impairment is due to enhancement of Golgi cell inhibition of granule cells by EtOH (Hanchar et al. 2005; Dar 2015).
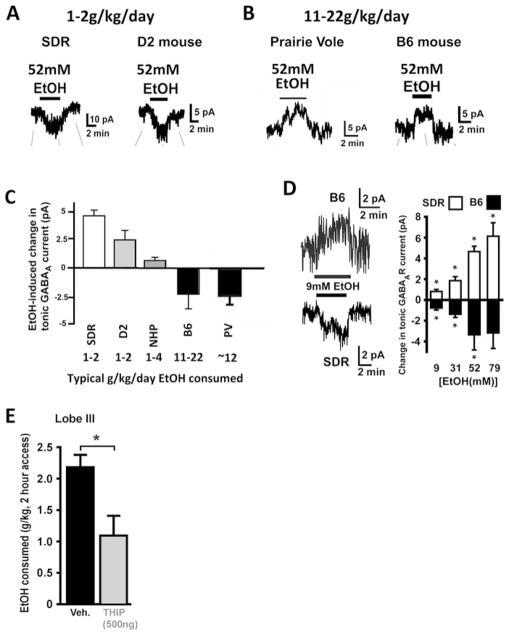
Fig. 3.
Response of granule cell tonic GABAAR current to EtOH varies in parallel with and influences EtOH consumption phenotype. (a, b) Example recordings showing that EtOH (52 mM) enhances the tonic GABAAR current in low EtOH consuming rodent genotypes (SDRs and D2 mice; a), but suppresses the tonic GABAAR current in high EtOH consuming rodent genotypes (Prairie Voles and B6 mice; b). (c) Plot of mean EtOH-induced change in magnitude of granule cell tonic GABAAR current across mammalian genotypes with divergent EtOH consumption phenotypes. Note, EtOH consumption values are rough estimates of average amount consumed across a 24 h period for each mammalian genotype, without consideration for consumption pattern across the day. (d) Example recordings and bar chart of mean responses to varying doses of EtOH in SDRs and B6 mice showing that opposite action of EtOH is preserved at low to high [EtOH]. (e) Bar chart depicts the mean amount of EtOH consumed by B6 mice during a 2 h 2 bottle choice (water and 10% EtOH) session, under control conditions and after a local injection of the GABAAR agonist, THIP, into lobe 3 of the cerebellum. Adapted with permission from Kaplan et al. (2013, 2016a, b)
The notably high sensitivity of the Golgi cell to granule cell GABAAR system to EtOH, combined with the clear role of this response in mediating at least one behavioral response to EtOH (motor impairment) makes it a potential mediator of genetic differences in response to low [EtOH] that influences initial subjective reactions to consumption of EtOH, and thus predilection for developing an AUD. In this context, we decided to determine if the action of EtOH on the Golgi to granule cell GABAAR system varied across rodent genotypes with differing EtOH consumption phenotypes (Fig. 3a–d) (Kaplan et al. 2013). Indeed, in our initial study we found that in stark contrast to the enhancement of inhibition induced by EtOH in low EtOH consuming SDRs, EtOH actually suppressed the magnitude of the granule cell tonic GABAAR current in the prototypical high EtOH consuming C57BL/6J mice (B6; Fig. 3b–d). This divergence is not simply a species difference, because EtOH enhanced sIPSC frequency and tonic GABAAR current magnitude in the low EtOH consuming DBA/2J mouse (D2; Fig. 3a–c). Parallel studies in high EtOH consuming prairie voles (PVs) and variable EtOH consuming nonhuman primates (NHPs) revealed a general pattern in which the impact of EtOH on granule cell tonic GABAAR currents varies in polarity and magnitude in parallel with the EtOH consumption phenotype of the mammal, with high and low EtOH consumption associated with suppression and enhancement of tonic GABAAR currents respectively (Fig. 3b, c). Crucially, this relationship persists across the dose–response range of EtOH concentrations tested, including 9 mM (Fig. 3d). To test whether this striking correlation actually played a role in EtOH consumption, we attempted to counteract the ability of EtOH to suppress the tonic GABAAR current in B6 mice in vivo. To do this, the GABAAR agonist THIP, which has an order of magnitude higher affinity for δ-subunit containing GABAARs, was focally injected into the cerebellum of B6 mice in vivo. This would effectively increase granule cell tonic GABAAR currents similar to what EtOH does in low EtOH consuming genotypes (Kaplan et al. 2013). Such injections effectively reduced EtOH consumption by B6 mice (Fig. 3e), without affecting water consumption or overall locomotion (Kaplan et al. 2016b). While more selective manipulations of the tonic GABAAR current will need to be tested, and ideally it should be determined if EtOH consumption can be increased or decreased based on the direction of manipulation, these studies suggest that either EtOH-induced suppression of granule cell tonic GABAAR currents promotes EtOH consumption or EtOH-induced enhancement of granule cell tonic GABAAR currents deters consumption, or both.
It is worth noting that although the cerebellar neurological differences that we discovered clearly correlate with and influence EtOH consumption (Fig. 3c, e) (Kaplan et al. 2016b), they are not the only factors that influence EtOH consumption. Of particular relevance, it is well established that a large factor in deterring EtOH consumption by D2 mice is their aversion to the taste of EtOH, and an apparent insensitivity to glucose, which undermines using it as a means to override taste aversion (Grahame and Cunningham 1997; McCool and Chappell 2012, 2014; Fidler et al. 2011). Thus, when taste is bypassed by intravenous or gastric cannulation, D2 mice will self-administer significant quantities of EtOH (Grahame and Cunningham 1997; Fidler et al. 2011). Similarly, D2 mice will also voluntarily orally consume EtOH if its taste is masked with monosodium glutamate (McCool and Chappell 2012, 2014). However, even with such aversive taste circumventions, D2 mice still consume significantly less EtOH than B6 mice, suggesting that aversive post-absorptive ethanol effects probably contribute to avoidance of oral consumption of ethanol by D2 mice (McCool and Chappell 2012, 2014; Fidler et al. 2011). In this regard, when normalized to the basal magnitude of tonic GABAAR current, the percent change induced by EtOH in D2 mice falls roughly between the percent change observed in B6 mice and SD rats, being significantly different from both (Kaplan et al. 2016a). This is interesting because unlike D2 mice, SD rats find EtOH aversive even when taste is bypassed (Fidler et al. 2004). Thus, EtOH actions on granule cell tonic GABAARs may contribute to deterrence of EtOH consumption in D2 mice relative to B6 mice, but not as strongly as it does in SD rats.
In these studies we also determined the molecular mechanisms that determine the overall response polarity. Specifically, we found that enhancement of tonic GABAAR currents is mediated by EtOH inhibition of neuronal nitric oxide synthase (nNOS), which excited Golgi cells enough to increase their action potential firing (Kaplan et al. 2013), possibly via NO actions on the Na+/K+-ATPase and/or K+ channels (Botta et al. 2010, 2012; Valenzuela and Jotty 2015). Genetic control of this process appears to be implemented by expression of nNOS, with low levels of expression in high EtOH consuming B6 mice and PVs, high levels of expression in low EtOH consuming SDRs and D2 mice, and intermediate and variable levels of expression across individual NHPs which also show variable levels of EtOH consumption (Kaplan et al. 2013, 2016a; Mohr et al. 2013). Conversely, the suppression of granule cell tonic GABAAR currents observed in B6 mice and PVs is mediated solely by postsynaptic actions on the GABAARs, as evidenced by the fact that suppression was observed in acutely isolated granule cells that had their GABAARs activated by exogenous GABA to circumvent any possible action of EtOH on endogenous GABA release (Kaplan et al. 2013). And, we determined that the ability of EtOH to postsynaptically suppress granule cell tonic GABAAR currents is genetically determined by the level of postsynaptic PKC activity. In particular, PKC activity appears to prevent EtOH from suppressing granule cell tonic GABAAR currents. Thus, activating PKC in B6 mouse granule cells eliminated EtOH suppression of tonic GABAARs, whereas blocking PKC in SDR granule cells enabled EtOH to suppress their tonic GABAAR currents (Kaplan et al. 2013).
Collectively, our data indicate that there are two genetically controlled molecular switches (nNOS expression and postsynaptic PKC activity), and that the balance of the two processes dictates the polarity and magnitude of the effect of EtOH on granule cell tonic GABAAR currents. High postsynaptic PKC activity and high nNOS expression result in EtOH enhancing granule cell tonic GABAAR currents, and low postsynaptic PKC activity and low nNOS expression result in EtOH suppressing tonic GABAAR currents. Importantly, the resultant direction of EtOH effects correlates with and influences EtOH consumption phenotype, wherein suppression and enhancement are associated with high and low EtOH consumption, respectively.
2.5 Is There Potential for Manipulating the Cerebellum to Deter EtOH Consumption with Fewer Side Effects?
Based on our recent studies, our overall contention is that EtOH suppression of granule cell tonic GABAAR currents promotes EtOH consumption in high EtOH consuming genotypes, whereas EtOH enhancement of such currents deters EtOH consumption in low EtOH consuming genotypes (Fig. 3a–d). Accordingly, we propose that pharmacological agents that selectively enhance granule cell tonic GABAAR currents, or can emulate the overall outcome of such enhancement on PC output (presumed to be the final mediator of any cerebellar behavior), should reduce EtOH consumption. And, given that granule cell tonic GABAAR currents are mediated by α6-subunit containing GABAARs, which are almost exclusively expressed by granule cells (Fig. 1c), any such pharmacotherapy might be achieved with fewer side effects than more broadly acting GABAAR modulators.
As discussed above, Ro 15-4513 strongly binds to the α6 containing GABAARs that generate tonic GABAAR currents in granule cells (Luddens et al. 1990). Although Ro 15-4513 is generally considered to be an inverse agonist of GABAARs, it has been shown to enhance currents generated by α6 containing GABAARs (Knoflach et al. 1996). In partial support of our broad contention, in at least one study of alcohol preferring (AA) rats, Ro 15-4513 did in fact decrease EtOH consumption (Wegelius et al. 1994). However, Ro 15-4513 is clearly not selective for the α6 subunit or the cerebellum. So, to more directly test the hypothesis, we have shown that a local injection of THIP into the cerebellum effectively reduces EtOH consumption by B6 mice without affecting water consumption or general locomotion (Fig. 3e) (Kaplan et al. 2016b). While this confirms the potential capacity of targeting the cerebellar GABAAR system to treat AUDs, as discussed above, it is unlikely that THIP will be a suitable therapeutic in the human clinic, due amongst other things to the widespread action of THIP and associated adverse side effects.
However, having determined that regulating granule cell excitability, and presumably therefore Purkinje cell output, can effectively reduce EtOH consumption without obvious adverse effects, it is reasonable to speculate that any drug that could specifically target cerebellar granule cell excitability or Purkinje cell output might be an effective treatment option. Accordingly, we will end this chapter by highlighting the fact that the cerebellum is an unusual brain structure in that there are a variety of subunits/subtypes of common receptor/channel/transporter families that are exclusively expressed in the cerebellum, many only on cerebellar granule cells. Importantly, many of these cerebellar, or even granule cell specific subunits are known to be key players in regulating signal transmission through the cerebellar cortex, including the α6 subunit of the GABAAR family (Fig. 1c) (Hamann et al. 2002), the EAAT4 subtype of the plasma membrane glutamate transporter (Kaplan et al. 2016b; Wadiche and Jahr 2005; Welsh et al. 2002), and the NR2C subunit of the NMDA receptor family (Cathala et al. 2000, 2003; Ebralidze et al. 1996). Thus, there are likely many potential targets for modulating cerebellar responses to EtOH that may not affect the rest of the brain. Indeed, GABAAR-positive allosteric modulators have already been developed that show orders of magnitude selective affinity for α6 containing GABAARs (Varagic et al. 2013). Given that enhancement of granule cell tonic GABAAR currents reduces EtOH consumption (Fig. 3e) (Kaplan et al. 2016b), we would predict that such ligands could reduce EtOH consumption, and given their selectivity for cerebellar granule cells, may have fewer side effects than drugs that target more widely expressed GABAAR subunits.
3 Summary
It is clear that the GABAAR system plays multiple important roles in mediating acute and chronic responses to EtOH and almost certainly plays a role in the development and maintenance of AUD. However, such information has not translated to effective treatment for AUD. Contributing factors to this failure likely include the inadequate understanding of: (1) the molecular/neural targets of low [EtOH] (~10 mM), (2) the neural systems that mediate initial reactions to low [EtOH], and (3) pharmaceuticals that can selectively target relevant neural systems in a manner that is not plagued by side effects.
Recently, our understanding of non-motor roles of the cerebellum has evolved dramatically, and we now know that the cerebellar GABAAR system is highly sensitive to EtOH, responding to [EtOH] as low as 10 mM. Moreover, variability in the polarity and magnitude of the response of cerebellar GABAARs to EtOH correlates with and actually affects EtOH consumption phenotype across various mammalian genotypes. This suggests that the cerebellum generally, and the cerebellar GABAAR system specifically may be promising targets for AUD pharmacotherapy. Importantly, the cerebellum and its GABAAR system are unique in their pattern of expression of atypical subunits/subtypes of GABAARs and other transmitter systems that could be targeted, potentially without producing typical adverse side effects of drugs that affect more widely distributed targets.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01AA-012439 and R01AA-026078, and Washington State University Alcohol and Drug Abuse Research Program (ADARP) grant to D.J.R., and by an ADARP postdoctoral grant to B.D.R.
Contributor Information
David J. Rossi, Department of Integrative Physiology and Neuroscience, College of Veterinary Medicine, Washington State University, Pullman, WA, USA. Alcohol and Drug Abuse Research Program, Washington State University, Pullman, WA, USA
Ben D. Richardson, Department of Integrative Physiology and Neuroscience, College of Veterinary Medicine, Washington State University, Pullman, WA, USA. Alcohol and Drug Abuse Research Program, Washington State University, Pullman, WA, USA. Department of Biological Engineering, University of Idaho, Moscow, ID, USA
References
- Allan AM, Harris RA. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci. 1986;39:2005–2015. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- Allan AM, Harris RA. Involvement of neuronal chloride channels in ethanol intoxication, tolerance, and dependence. Recent Dev Alcohol. 1987;5:313–325. doi: 10.1007/978-1-4899-1684-6_12. [DOI] [PubMed] [Google Scholar]
- Allan AM, Huidobro-Toro JP, Bleck V, Harris RA. Alcohol and the GABA receptor-chloride channel complex of brain. Alcohol Alcohol Suppl. 1987;1:643–646. [PubMed] [Google Scholar]
- Allan AM, Spuhler KP, Harris RA. Gamma-aminobutyric acid-activated chloride channels: relationship to genetic differences in ethanol sensitivity. J Pharmacol Exp Ther. 1988;244:866–870. [PubMed] [Google Scholar]
- Anton RF, Schacht JP, Book SW. Pharmacologic treatment of alcoholism. Handb Clin Neurol. 2014;125:527–542. doi: 10.1016/B978-0-444-62619-6.00030-6. [DOI] [PubMed] [Google Scholar]
- Avegno EM, Salling MC, Borgkvist A, Mrejeru A, Whitebirch AC, Margolis EB, Sulzer D, Harrison NL. Voluntary adolescent drinking enhances excitation by low levels of alcohol in a subset of dopaminergic neurons in the ventral tegmental area. Neuropharmacology. 2016;110:386–395. doi: 10.1016/j.neuropharm.2016.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnall MW, Zingg B, Sakatos A, Moghadam SH, Zeilhofer HU, du LS. Glycinergic projection neurons of the cerebellum. J Neurosci. 2009;29:10104–10110. doi: 10.1523/JNEUROSCI.2087-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABA(A) receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barmack NH, Yakhnitsa V. Functions of interneurons in mouse cerebellum. J Neurosci. 2008;28:1140–1152. doi: 10.1523/JNEUROSCI.3942-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile A, Hoffer B, Dunwiddie T. Differential sensitivity of cerebellar purkinje neurons to ethanol in selectively outbred lines of mice: maintenance in vitro independent of synaptic transmission. Brain Res. 1983;264:69–78. doi: 10.1016/0006-8993(83)91121-6. [DOI] [PubMed] [Google Scholar]
- Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, Leggio M, Mattingley JB, Molinari M, Moulton EA, Paulin MG, Pavlova MA, Schmahmann JD, Sokolov AA. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Bell RL, Stewart RB, Woods JE, Lumeng L, Li TK, Murphy JM, McBride WJ. Responsivity and development of tolerance to the motor impairing effects of moderate doses of ethanol in alcohol-preferring (P) and -nonpreferring (NP) rat lines. Alcohol Clin Exp Res. 2001;25:644–650. [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–288. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Woodin MA, Sernagor E, Cancedda L, Vinay L, Rivera C, Legendre P, Luhmann HJ, Bordey A, Wenner P, Fukuda A, van den Pol AN, Gaiarsa JL, Cherubini E. Refuting the challenges of the developmental shift of polarity of GABA actions: GABA more exciting than ever! Front Cell Neurosci. 2012;6:35. doi: 10.3389/fncel.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billard JM, Vigot R, Batini C. GABA, THIP and baclofen inhibition of Purkinje cells and cerebellar nuclei neurons. Neurosci Res. 1993;16:65–69. doi: 10.1016/0168-0102(93)90010-n. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Gilman JM. The effects of acute alcohol administration on the human brain: insights from neuroimaging. Neuropharmacology. 2014;84:101–110. doi: 10.1016/j.neuropharm.2013.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe J, Semrud-Clikeman M, Pliszka SR. A magnetic resonance imaging study of the cerebellar vermis in chronically treated and treatment-naive children with attention-deficit/hyperactivity disorder combined type. Biol Psychiatry. 2009;65:620–624. doi: 10.1016/j.biopsych.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, Blednov YA, Harris RA. Gamma-aminobutyric acid A receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu S, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Ruiz CI, Lee US, Cullins MA, Bertaccini EJ, Trudell JR, Harris RA. Identification of an inhibitory alcohol binding site in GABAA rho1 receptors. ACS Chem Neurosci. 2016;7:100–108. doi: 10.1021/acschemneuro.5b00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Mameli M, Floyd KL, Radcliffe RA, Valenzuela CF. Ethanol sensitivity of GABAergic currents in cerebellar granule neurons is not increased by a single amino acid change (R100Q) in the alpha6 GABAA receptor subunit. J Pharmacol Exp Ther. 2007a;323:684–691. doi: 10.1124/jpet.107.127894. [DOI] [PubMed] [Google Scholar]
- Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol. 2007b;41:187–199. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, de Souza FM, Sangrey T, De Schutter E, Valenzuela CF. Alcohol excites cerebellar Golgi cells by inhibiting the Na+/K+ ATPase. Neuropsychopharmacology. 2010;35:1984–1996. doi: 10.1038/npp.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta P, Simoes de Souza FM, Sangrey T, De Schutter E, Valenzuela CF. Excitation of rat cerebellar Golgi cells by ethanol: further characterization of the mechanism. Alcohol Clin Exp Res. 2012;36:616–624. doi: 10.1111/j.1530-0277.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46:179–182. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Bragina L, Marchionni I, Omrani A, Cozzi A, Pellegrini-Giampietro DE, Cherubini E, Conti F. GAT-1 regulates both tonic and phasic GABA(A) receptor-mediated inhibition in the cerebral cortex. J Neurochem. 2008;105:1781–1793. doi: 10.1111/j.1471-4159.2008.05273.x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Misra C, Cull-Candy S. Developmental profile of the changing properties of NMDA receptors at cerebellar mossy fiber-granule cell synapses. J Neurosci. 2000;20:5899–5905. doi: 10.1523/JNEUROSCI.20-16-05899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala L, Brickley S, Cull-Candy S, Farrant M. Maturation of EPSCs and intrinsic membrane properties enhances precision at a cerebellar synapse. J Neurosci. 2003;23:6074–6085. doi: 10.1523/JNEUROSCI.23-14-06074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdar S, Onat F, Aker R, Sehirli U, San T, Yananli HR. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. J Anat. 2001a;198:463–472. doi: 10.1046/j.1469-7580.2001.19840463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavdar S, San T, Aker R, Sehirli U, Onat F. Cerebellar connections to the dorsomedial and posterior nuclei of the hypothalamus in the rat. J Anat. 2001b;198:37–45. doi: 10.1046/j.1469-7580.2001.19810037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol. 2005;87:3–16. doi: 10.1016/j.pbiomolbio.2004.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Colocalization of neurotransmitters in the deep cerebellar nuclei. J Neurocytol. 1993;22:81–91. doi: 10.1007/BF01181572. [DOI] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase Cdelta regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church AC, Fuller JL, Dann L. Alcohol intake in selected lines of mice: importance of sex and genotype. J Comp Physiol Psychol. 1979;93:242–246. doi: 10.1037/h0077563. [DOI] [PubMed] [Google Scholar]
- Cook JB, Dumitru AM, O’Buckley TK, Morrow AL. Ethanol administration produces divergent changes in GABAergic neuroactive steroid immunohistochemistry in the rat brain. Alcohol Clin Exp Res. 2014;38:90–99. doi: 10.1111/acer.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Ponomarev I, Prescott CA, Wahlsten D. Effects of genetic and procedural variation on measurement of alcohol sensitivity in mouse inbred strains. Behav Genet. 2006a;36:536–552. doi: 10.1007/s10519-006-9067-6. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addict Biol. 2006b;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Morrow AL, Criswell H, Breese G. Effects of ethanol on ion channels. Int Rev Neurobiol. 1996;39:283–367. doi: 10.1016/s0074-7742(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an fMRI study of youth at high risk for alcoholism. Alcohol Clin Exp Res. 2012;36(4):604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damji KF, Allingham RR, Pollock SC, Small K, Lewis KE, Stajich JM, Yamaoka LH, Vance JM, Pericak-Vance MA. Periodic vestibulocerebellar ataxia, an autosomal dominant ataxia with defective smooth pursuit, is genetically distinct from other autosomal dominant ataxias. Arch Neurol. 1996;53:338–344. doi: 10.1001/archneur.1996.00550040074016. [DOI] [PubMed] [Google Scholar]
- Daoust M, Lhuintre JP, Moore N, Saligaut C, Flipo JL, Boismare F. Is initial sensitivity to ethanol correlated with alcohol preference in alcohol-drinking and non-drinking rats? Alcohol Alcohol. 1987;22:409–414. [PubMed] [Google Scholar]
- Dar MS. Ethanol-induced cerebellar ataxia: cellular and molecular mechanisms. Cerebellum. 2015;14:447–465. doi: 10.1007/s12311-014-0638-4. [DOI] [PubMed] [Google Scholar]
- Daurio AM, Aston SA, Schwandt ML, Bukhari MO, Bouhlal S, Farokhnia M, Lee MR, Leggio L. Impulsive personality traits mediate the relationship between adult attention deficit hyperactivity symptoms and alcohol dependence severity. Alcohol Clin Exp Res. 2017;42(1):173–183. doi: 10.1111/acer.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- De Zeeuw CI, Berrebi AS. Postsynaptic targets of Purkinje cell terminals in the cerebellar and vestibular nuclei of the rat. Eur J Neurosci. 1995;7:2322–2333. doi: 10.1111/j.1460-9568.1995.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Deik A, Saunders-Pullman R, Luciano MS. Substance of abuse and movement disorders: complex interactions and comorbidities. Curr Drug Abuse Rev. 2012;5:243–253. doi: 10.2174/1874473711205030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- Dempsey CW, Richardson DE. Paleocerebellar stimulation induces in vivo release of endogenously synthesized [3H]dopamine and [3H]norepinephrine from rat caudal dorsomedial nucleus accumbens. Neuroscience. 1987;21:565–571. doi: 10.1016/0306-4522(87)90142-4. [DOI] [PubMed] [Google Scholar]
- Diaz MR, Valenzuela CF. Sensitivity of GABAergic tonic currents to acute ethanol in cerebellar granule neurons is not age- or delta subunit-dependent in developing rats. Alcohol Clin Exp Res. 2016;40:83–92. doi: 10.1111/acer.12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MR, Wadleigh A, Hughes BA, Woodward JJ, Valenzuela CF. Bestrophin1 channels are insensitive to ethanol and do not mediate tonic GABAergic currents in cerebellar granule cells. Front Neurosci. 2011;5:148. doi: 10.3389/fnins.2011.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, Rodd ZA. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastes LE. Alcohol withdrawal syndrome in trauma patients: a review. J Emerg Nurs. 2010;36:507–509. doi: 10.1016/j.jen.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Ebralidze AK, Rossi DJ, Tonegawa S, Slater NT. Modification of NMDA receptor channels and synaptic transmission by targeted disruption of the NR2C gene. J Neurosci. 1996;16:5014–5025. doi: 10.1523/JNEUROSCI.16-16-05014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers J, Plant TD, Marandi N, Konnerth A. GABA-mediated Ca2+ signalling in developing rat cerebellar Purkinje neurones. J Physiol. 2001;536:429–437. doi: 10.1111/j.1469-7793.2001.0429c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer GI, Meisch RA, Goldberg SR, George FR. Ethanol self-administration in long sleep and short sleep mice indicates reinforcement is not inversely related to neurosensitivity. J Pharmacol Exp Ther. 1990;254:1054–1062. [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Shen PH, Gorodetsky E, Marietta CA, Roy A, Goldman D. GABBR1 and SLC6A1, two genes involved in modulation of GABA synaptic transmission, influence risk for alcoholism: results from three ethnically diverse populations. Alcohol Clin Exp Res. 2016;40:93–101. doi: 10.1111/acer.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J. ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry. 2007;48:899–913. doi: 10.1111/j.1469-7610.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- Erwin VG, McClearn GE, Kuse AR. Interrelationships of alcohol consumption, actions of alcohol, and biochemical traits. Pharmacol Biochem Behav. 1980;13(Suppl 1):297–302. doi: 10.1016/s0091-3057(80)80045-1. [DOI] [PubMed] [Google Scholar]
- Fallon JH, Koziell DA, Moore RY. Catecholamine innervation of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol. 1978;180:509–532. doi: 10.1002/cne.901800308. [DOI] [PubMed] [Google Scholar]
- Ferrucci R, Giannicola G, Rosa M, Fumagalli M, Boggio PS, Hallett M, Zago S, Priori A. Cerebellum and processing of negative facial emotions: cerebellar transcranial DC stimulation specifically enhances the emotional recognition of facial anger and sadness. Cognit Emot. 2012;26:786–799. doi: 10.1080/02699931.2011.619520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler TL, Bakner L, Cunningham CL. Conditioned place aversion induced by intragastric administration of ethanol in rats. Pharmacol Biochem Behav. 2004;77:731–743. doi: 10.1016/j.pbb.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Freund RK, Wang Y, Palmer MR. Differential effects of ethanol on the firing rates of Golgi-like neurons and Purkinje neurons in cerebellar slices in vitro. Neurosci Lett. 1993;164:9–12. doi: 10.1016/0304-3940(93)90844-b. [DOI] [PubMed] [Google Scholar]
- Fritz BM, Grahame NJ, Boehm SL. Selection for high alcohol preference drinking in mice results in heightened sensitivity and rapid development of acute functional tolerance to alcohol’s ataxic effects. Genes Brain Behav. 2012;12(1):78–86. doi: 10.1111/j.1601-183X.2012.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallaher EJ, Jones GE, Belknap JK, Crabbe JC. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:604–612. [PubMed] [Google Scholar]
- Gan G, Guevara A, Marxen M, Neumann M, Junger E, Kobiella A, Mennigen E, Pilhatsch M, Schwarz D, Zimmermann US, Smolka MN. Alcohol-induced impairment of inhibitory control is linked to attenuated brain responses in right fronto-temporal cortex. Biol Psychiatry. 2014;76:698–707. doi: 10.1016/j.biopsych.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- George F, Chu NS. Effects of ethanol on Purkinje cells recorded from cerebellar slices. Alcohol. 1984;1:353–358. doi: 10.1016/0741-8329(84)90002-8. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABA(A) receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 1997;21:56–62. [PubMed] [Google Scholar]
- Haines DE, May PJ, Dietrichs E. Neuronal connections between the cerebellar nuclei and hypothalamus in Macaca fascicularis: cerebello-visceral circuits. J Comp Neurol. 1990;299:106–122. doi: 10.1002/cne.902990108. [DOI] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Heath RG. Anatomic connections of the fastigial nucleus to the rostral forebrain in the cat. Exp Neurol. 1973;39:285–292. doi: 10.1016/0014-4886(73)90231-8. [DOI] [PubMed] [Google Scholar]
- Harris RA, Allan AM, Daniell LC, Nixon C. Antagonism of ethanol and pentobarbital actions by benzodiazepine inverse agonists: neurochemical studies. J Pharmacol Exp Ther. 1988;247:1012–1017. [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Titley H, Grasselli G, Piochon C, Hansel C. Ethanol affects NMDA receptor signaling at climbing fiber-Purkinje cell synapses in mice and impairs cerebellar LTD. J Neurophysiol. 2013;109:1333–1342. doi: 10.1152/jn.00350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- Heath RG, Dempesy CW, Fontana CJ, Myers WA. Cerebellar stimulation: effects on septal region, hippocampus, and amygdala of cats and rats. Biol Psychiatry. 1978;13:501–529. [PubMed] [Google Scholar]
- Heigele S, Sultan S, Toni N, Bischofberger J. Bidirectional GABAergic control of action potential firing in newborn hippocampal granule cells. Nat Neurosci. 2016;19:263–270. doi: 10.1038/nn.4218. [DOI] [PubMed] [Google Scholar]
- Helms CM, Rossi DJ, Grant KA. Neurosteroid influences on sensitivity to ethanol. Front Endocrinol. 2012;3:10. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. NeuroImage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY. Neural plasticity, human genetics, and risk for alcohol dependence. Int Rev Neurobiol. 2010;91:53–94. doi: 10.1016/S0074-7742(10)91003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, Tessner K, Holmes B, McDermott M, Zezza N, Stiffier S. Cerebellum volume in high-risk offspring from multiplex alcohol dependence families: association with allelic variation in GABRA2 and BDNF. Psychiatry Res. 2011;194:304–313. doi: 10.1016/j.pscychresns.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Lichenstein SD, Wang S, O’Brien J. Volumetric differences in cerebellar lobes in individuals from multiplex alcohol dependence families and controls: their relationship to externalizing and internalizing disorders and working memory. Cerebellum. 2016;15:744–754. doi: 10.1007/s12311-015-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hotson JR. Clinical detection of acute vestibulocerebellar disorders. West J Med. 1984;140:910–913. [PMC free article] [PubMed] [Google Scholar]
- Huang CM, Huang RH. Ethanol inhibits the sensory responses of cerebellar granule cells in anesthetized cats. Alcohol Clin Exp Res. 2007;31:336–344. doi: 10.1111/j.1530-0277.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–728. doi: 10.1016/0306-4522(92)90310-x. [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Mizuno N. Single neurons in the ventral tegmental area that project to both the cerebral and cerebellar cortical areas by way of axon collaterals. Neuroscience. 1994;61:925–934. doi: 10.1016/0306-4522(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshida M, Obata K, Kawai N, Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10:64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. Extracellular activation and membrane conductances of neurones in the guinea-pig deep cerebellar nuclei in vitro. J Physiol. 1986;372:149–168. doi: 10.1113/jphysiol.1986.sp016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JP, Nipper MA, Helms ML, Ford MM, Crabbe JC, Rossi DJ, Finn DA. Ethanol withdrawal-induced dysregulation of neurosteroid levels in plasma, cortex, and hippocampus in genetic animal models of high and low withdrawal. Psychopharmacology. 2017;234(18):2793–2811. doi: 10.1007/s00213-017-4671-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Harrison NL. GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity. Alcohol. 2007;41:177–185. doi: 10.1016/j.alcohol.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Jia F, Chandra D, Homanics GE, Harrison NL. Ethanol modulates synaptic and extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;326:475–482. doi: 10.1124/jpet.108.139303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Lichtenstein P, Grann M, Langstrom N, Fazel S. Alcohol use disorders in schizophrenia: a national cohort study of 12,653 patients. J Clin Psychiatry. 2011;72:775–779. doi: 10.4088/JCP.10m06320. [DOI] [PubMed] [Google Scholar]
- Kakihana R, Brown DR, McClearn GE, Tabershaw IR. Brain sensitivity to alcohol in inbred mouse strains. Science. 1966;154:1574–1575. doi: 10.1126/science.154.3756.1574. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, Rossi DJ. Opposite actions of alcohol on tonic GABA(A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci. 2013;16:1783–1793. doi: 10.1038/nn.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, Hostetler CM, Ryabinin AE, Finn DA, Rossi DJ. Alcohol suppresses tonic GABAA receptor currents in cerebellar granule cells in the prairie vole: a neural signature of high-alcohol-consuming genotypes. Alcohol Clin Exp Res. 2016a;40:1617–1626. doi: 10.1111/acer.13136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JS, Nipper MA, Richardson BD, Jensen J, Helms M, Finn DA, Rossi DJ. Pharmacologically counteracting a phenotypic difference in cerebellar GABAA receptor response to alcohol prevents excessive alcohol consumption in a high alcohol-consuming rodent genotype. J Neurosci. 2016b;36:9019–9025. doi: 10.1523/JNEUROSCI.0042-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–8444. doi: 10.1523/JNEUROSCI.23-23-08432.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. The role of protein kinase A in the ethanol-induced increase in spontaneous GABA release onto cerebellar Purkinje neurons. J Neurophysiol. 2008;100:3417–3428. doi: 10.1152/jn.90970.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm WR, Stevens RE., III Alcohol effects on EEG and multiple-unit activity in various brain regions of rats. Brain Res. 1974;70:361–368. doi: 10.1016/0006-8993(74)90327-8. [DOI] [PubMed] [Google Scholar]
- Klemm WR, Mallari CG, Dreyfus LR, Fiske JC, Forney E, Mikeska JA. Ethanol-induced regional and dose-response differences in multiple-unit activity in rabbits. Psychopharmacology. 1976;49:235–244. doi: 10.1007/BF00426822. [DOI] [PubMed] [Google Scholar]
- Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acidA receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- Koller WC. Alcoholism in essential tremor. Neurology. 1983;33:1074–1076. doi: 10.1212/wnl.33.8.1074. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, Rabe H, Bohme I, Aller MI, Wisden W, Luddens H. Does ethanol act preferentially via selected brain GABAA receptor subtypes? The current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. 2014;1:e.2014. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, Cortes EP, Vonsattel JG, Louis ED, Faust PL. Cerebellar pathology in early onset and late onset essential tremor. Cerebellum. 2017;16:473–482. doi: 10.1007/s12311-016-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutlu MG, Gould TJ. Effects of drugs of abuse on hippocampal plasticity and hippocampus-dependent learning and memory: contributions to development and maintenance of addiction. Learn Mem. 2016;23:515–533. doi: 10.1101/lm.042192.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science. 2010;330:790–796. doi: 10.1126/science.1184334. [DOI] [PubMed] [Google Scholar]
- Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- Lex BW, Lukas SE, Greenwald NE, Mendelson JH. Alcohol-induced changes in body sway in women at risk for alcoholism: a pilot study. J Stud Alcohol. 1988;49:346–356. doi: 10.15288/jsa.1988.49.346. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Spigelman I, Olsen RW. Tolerance to sedative/hypnotic actions of GABAergic drugs correlates with tolerance to potentiation of extrasynaptic tonic currents of alcohol-dependent rats. J Neurophysiol. 2009;102:224–233. doi: 10.1152/jn.90484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. II. Mossy fiber firing patterns during horizontal head rotation and eye movement. J Neurophysiol. 1978;41:764–777. doi: 10.1152/jn.1978.41.3.764. [DOI] [PubMed] [Google Scholar]
- Lorenz-Guertin JM, Jacob TC. GABA type a receptor trafficking and the architecture of synaptic inhibition. Dev Neurobiol. 2017;78(3):238–270. doi: 10.1002/dneu.22536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughlin SE, Fallon JH. Dopaminergic and non-dopaminergic projections to amygdala from substantia nigra and ventral tegmental area. Brain Res. 1983;262:334–338. doi: 10.1016/0006-8993(83)91029-6. [DOI] [PubMed] [Google Scholar]
- Louis ED, Kuo SH, Wang J, Tate WJ, Pan MK, Kelly GC, Gutierrez J, Cortes EP, Vonsattel JG, Faust PL. Cerebellar pathology in familial vs. sporadic essential tremor. Cerebellum. 2017;16:786–791. doi: 10.1007/s12311-017-0853-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luddens H, Pritchett DB, Kohler M, Killisch I, Keinanen K, Monyer H, Sprengel R, Seeburg PH. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Mackiewicz Seghete KL, Cservenka A, Herting MM, Nagel BJ. Atypical spatial working memory and task-general brain activity in adolescents with a family history of alcoholism. Alcohol Clin Exp Res. 2013;37:390–398. doi: 10.1111/j.1530-0277.2012.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malila A. Intoxicating effects of three aliphatic alcohols and barbital on two rat strains genetically selected for their ethanol intake. Pharmacol Biochem Behav. 1978;8:197–201. doi: 10.1016/0091-3057(78)90337-4. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Hamlin AS, McNally GP. Lateral hypothalamus is required for context-induced reinstatement of extinguished reward seeking. J Neurosci. 2009;29:1331–1342. doi: 10.1523/JNEUROSCI.5194-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, McNally GP. The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci. 2012;69:581–597. doi: 10.1007/s00018-011-0817-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul ME, Turkkan JS, Svikis DS, Bigelow GE. Familial density of alcoholism: effects on psychophysiological responses to ethanol. Alcohol. 1991;8:219–222. doi: 10.1016/0741-8329(91)90870-3. [DOI] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Using monosodium glutamate to initiate ethanol self-administration in inbred mouse strains. Addict Biol. 2012;17:121–131. doi: 10.1111/j.1369-1600.2010.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Persistent enhancement of ethanol drinking following a monosodium glutamate-substitution procedure in C57BL6/J and DBA/2J mice. Alcohol. 2014;48:55–61. doi: 10.1016/j.alcohol.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100:1202–1210. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Olsen RW, Otis TS, Wallner M. Alcohol- and alcohol antagonist-sensitive human GABAA receptors: tracking delta subunit incorporation into functional receptors. Mol Pharmacol. 2010;78:918–924. doi: 10.1124/mol.109.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Otis TS. Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAA receptors. J Neurophysiol. 2011;106(4):2057–2064. doi: 10.1152/jn.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Millard WJ. Self-administration of ethanol by genetically heterogeneous mice (RU:NCS): relationship to sensitivity and tolerance. Drug Alcohol Depend. 1983;12:333–338. doi: 10.1016/0376-8716(83)90004-2. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Janabi M, Marks SM, Jagust WJ, Fields HL. Alcohol consumption induces endogenous opioid release in the human orbitofrontal cortex and nucleus accumbens. Sci Transl Med. 2012;4:116ra6. doi: 10.1126/scitranslmed.3002902. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, O’Neil JP, Jagust WJ, Fields HL. Catechol-O-methyltransferase genotype modulates opioid release in decision circuitry. Clin Transl Sci. 2013;6:400–403. doi: 10.1111/cts.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittleman G, Goldowitz D, Heck DH, Blaha CD. Cerebellar modulation of frontal cortex dopamine efflux in mice: relevance to autism and schizophrenia. Synapse. 2008;62:544–550. doi: 10.1002/syn.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABA(A) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Mohr C, Kolotushkina O, Kaplan JS, Welsh J, Daunais JB, Grant KA, Rossi DJ. Primate cerebellar granule cells exhibit a tonic GABAAR conductance that is not affected by alcohol: a possible cellular substrate of the low level of response phenotype. Front Neural Circuits. 2013;7:189. doi: 10.3389/fncir.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldavan MG, Cravetchi O, Allen CN. GABA transporters regulate tonic and synaptic GABAA receptor-mediated currents in the Suprachiasmatic nucleus neurons. J Neurophysiol. 2017;118(6):3092–3106. doi: 10.1152/jn.00194.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan PL, Beitz AJ, Larson AA, Altschuler RA, Madl JE, Mullett MA. Immunocyto-chemical localization of glutamate-, glutaminase- and aspartate aminotransferase-like immuno-reactivity in the rat deep cerebellar nuclei. Brain Res. 1986;363:364–370. doi: 10.1016/0006-8993(86)91024-3. [DOI] [PubMed] [Google Scholar]
- Moore EM, Serio KM, Goldfarb KJ, Stepanovska S, Linsenbardt DN, Boehm SL. GABAergic modulation of binge-like ethanol intake in C57BL/6J mice. Pharmacol Biochem Behav. 2007;88:105–113. doi: 10.1016/j.pbb.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25:2274–2284. doi: 10.1002/mds.23240. [DOI] [PubMed] [Google Scholar]
- Mothersill O, Knee-Zaska C, Donohoe G. Emotion and theory of mind in schizophrenia-investigating the role of the cerebellum. Cerebellum. 2015;15(3):357–368. doi: 10.1007/s12311-015-0696-2. [DOI] [PubMed] [Google Scholar]
- Mrejeru A, Marti-Prats L, Avegno EM, Harrison NL, Sulzer D. A subset of ventral tegmental area dopamine neurons responds to acute ethanol. Neuroscience. 2015;290:649–658. doi: 10.1016/j.neuroscience.2014.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder MJ, Baeyens D, Davidson MC, Casey BJ, van den BE, van Engeland H, Durston S. Familial vulnerability to ADHD affects activity in the cerebellum in addition to the prefrontal systems. J Am Acad Child Adolesc Psychiatry. 2008;47:68–75. doi: 10.1097/chi.0b013e31815a56dc. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcohol Clin Exp Res. 2010;34:199–202. doi: 10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Newman PP, Reza H. Functional relationships between the hippocampus and the cerebellum: an electrophysiological study of the cat. J Physiol. 1979;287:405–426. doi: 10.1113/jphysiol.1979.sp012667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol inhibits glutamatergic neurotransmission in nucleus accumbens neurons by multiple mechanisms. J Pharmacol Exp Ther. 1994;271:1566–1573. [PubMed] [Google Scholar]
- Nie Z, Madamba SG, Siggins GR. Ethanol enhances gamma-aminobutyric acid responses in a subpopulation of nucleus accumbens neurons: role of metabotropic glutamate receptors. J Pharmacol Exp Ther. 2000;293:654–661. [PubMed] [Google Scholar]
- Nie Z, Zorrilla EP, Madamba SG, Rice KC, Roberto M, Siggins GR. Presynaptic CRF1 receptors mediate the ethanol enhancement of GABAergic transmission in the mouse central amygdala. Sci World J. 2009;9:68–85. doi: 10.1100/tsw.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Rewal M, Gill TM, Ron D, Janak PH. Extrasynaptic delta-containing GABAA receptors in the nucleus accumbens dorsomedial shell contribute to alcohol intake. Proc Natl Acad Sci U S A. 2011;108:4459–4464. doi: 10.1073/pnas.1016156108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou K, Critchley H, Duka T. Alcohol affects neuronal substrates of response inhibition but not of perceptual processing of stimuli signalling a stop response. PLoS One. 2013a;8:e76649. doi: 10.1371/journal.pone.0076649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaou K, Field M, Critchley H, Duka T. Acute alcohol effects on attentional bias are mediated by subcortical areas associated with arousal and salience attribution. Neuropsychopharmacology. 2013b;38:1365–1373. doi: 10.1038/npp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Blocking GABA(A) receptors in the anterior ventral tegmental area attenuates ethanol intake of the alcohol-preferring P rat. Psychopharmacology. 1998;139:108–116. doi: 10.1007/s002130050695. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostroumov A, Thomas AM, Kimmey BA, Karsch JS, Doyon WM, Dani JA. Stress increases ethanol self-administration via a shift toward excitatory GABA signaling in the ventral tegmental area. Neuron. 2016;92:493–504. doi: 10.1016/j.neuron.2016.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer MR, Sorensen SM, Freedman R, Olson L, Hoffer B, Seiger A. Differential ethanol sensitivity of intraocular cerebellar grafts in long-sleep and short-sleep mice. J Pharmacol Exp Ther. 1982;222:480–487. [PubMed] [Google Scholar]
- Palmer MR, Basile AS, Proctor WR, Baker RC, Dunwiddie TV. Ethanol tolerance of cerebellar purkinje neurons from selectively outbred mouse lines: in vivo and in vitro electro-physiological investigations. Alcohol Clin Exp Res. 1985;9:291–296. doi: 10.1111/j.1530-0277.1985.tb05752.x. [DOI] [PubMed] [Google Scholar]
- Palmer MR, van Horne CG, Harlan JT, Moore EA. Antagonism of ethanol effects on cerebellar Purkinje neurons by the benzodiazepine inverse agonists Ro 15-4513 and FG 7142: electrophysiological studies. J Pharmacol Exp Ther. 1988;247:1018–1024. [PubMed] [Google Scholar]
- Park HM, Choi IS, Nakamura M, Cho JH, Lee MG, Jang IS. Multiple effects of allopregnanolone on GABAergic responses in single hippocampal CA3 pyramidal neurons. Eur J Pharmacol. 2011;652:46–54. doi: 10.1016/j.ejphar.2010.10.097. [DOI] [PubMed] [Google Scholar]
- Paulus KS, Magnano I, Conti M, Galistu P, D’Onofrio M, Satta W, Aiello I. Pure post-stroke cerebellar cognitive affective syndrome: a case report. Neurol Sci. 2004;25:220–224. doi: 10.1007/s10072-004-0325-1. [DOI] [PubMed] [Google Scholar]
- Perciavalle V, Berretta S, Raffaele R. Projections from the intracerebellar nuclei to the ventral midbrain tegmentum in the rat. Neuroscience. 1989;29:109–119. doi: 10.1016/0306-4522(89)90336-9. [DOI] [PubMed] [Google Scholar]
- Peris J, Coleman-Hardee M, Burry J, Pecins-Thompson M. Selective changes in GABAergic transmission in substantia nigra and superior colliculus caused by ethanol and ethanol withdrawal. Alcohol Clin Exp Res. 1992;16:311–319. doi: 10.1111/j.1530-0277.1992.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Reed C. Targeting GABAB receptors for anti-abuse drug discovery. Expert Opin Drug Discovery. 2014;9:1307–1317. doi: 10.1517/17460441.2014.956076. [DOI] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL. The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology. 2015;99:627–638. doi: 10.1016/j.neuropharm.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Ponomarev I, Crabbe JC. A novel method to assess initial sensitivity and acute functional tolerance to hypnotic effects of ethanol. J Pharmacol Exp Ther. 2002;302:257–263. doi: 10.1124/jpet.302.1.257. [DOI] [PubMed] [Google Scholar]
- Porcu P, Morrow AL. Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: relevance for human studies. Psychopharmacology. 2014;231:3257–3272. doi: 10.1007/s00213-014-3564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. Axonal GABAA receptors increase cerebellar granule cell excitability and synaptic activity. J Neurosci. 2011;31:565–574. doi: 10.1523/JNEUROSCI.4506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh JR, Jahr CE. Activation of axonal receptors by GABA spillover increases somatic firing. J Neurosci. 2013;33:16924–16929. doi: 10.1523/JNEUROSCI.2796-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi ZH, Song M, Wallace MJ, Wang D, Newton PM, McMahon T, Chou WH, Zhang C, Shokat KM, Messing RO. Protein kinase C epsilon regulates gamma-aminobutyrate type A receptor sensitivity to ethanol and benzodiazepines through phosphorylation of gamma2 subunits. J Biol Chem. 2007;282:33052–33063. doi: 10.1074/jbc.M707233200. [DOI] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: a quantitative review. Alcohol Clin Exp Res. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragge NK, Hartley C, Dearlove AM, Walker J, Russell-Eggitt I, Harris CM. Familial vestibulocerebellar disorder maps to chromosome 13q31–q33: a new nystagmus locus. J Med Genet. 2003;40:37–41. doi: 10.1136/jmg.40.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABA(A) receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35(11):1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong MN, Ford MM, Finn DA. Effect of ganaxolone and THIP on operant and limited-access ethanol self-administration. Neuropharmacology. 2012;63:555–564. doi: 10.1016/j.neuropharm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong-Kaufman MN, Ford MM, Phillips TJ, Finn DA. Effect of nucleus accumbens shell infusions of ganaxolone or gaboxadol on ethanol consumption in mice. Psychopharmacology. 2015;232:1415–1426. doi: 10.1007/s00213-014-3777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautakorpi I, Marttila RJ, Rinne UK. Alcohol consumption of patients with essential tremor. Acta Neurol Scand. 1983;68:177–179. doi: 10.1111/j.1600-0404.1983.tb05345.x. [DOI] [PubMed] [Google Scholar]
- Reilly MT, Milner LC, Shirley RL, Crabbe JC, Buck KJ. 5-HT2C and GABAB receptors influence handling-induced convulsion severity in chromosome 4 congenic and DBA/2J background strain mice. Brain Res. 2008;1198:124–131. doi: 10.1016/j.brainres.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewal M, Donahue R, Gill TM, Nie H, Ron D, Janak PH. Alpha4 subunit-containing GABAA receptors in the accumbens shell contribute to the reinforcing effects of alcohol. Addict Biol. 2012;17:309–321. doi: 10.1111/j.1369-1600.2011.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Rossi DJ. Recreational concentrations of alcohol enhance synaptic inhibition of cerebellar unipolar brush cells via pre- and postsynaptic mechanisms. J Neurophysiol. 2017;118:267–279. doi: 10.1152/jn.00963.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BD, Ling LL, Uteshev VV, Caspary DM. Extrasynaptic GABA(A) receptors and tonic inhibition in rat auditory thalamus. PLoS One. 2011;6:e16508. doi: 10.1371/journal.pone.0016508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, Worsham ED, Lester D, Freed EX. Selective breeding of rats for differences in reactivity to alcohol. An approach to an animal model of alcoholism. II. Behavioral measures. J Stud Alcohol. 1977;38:1705–1717. doi: 10.15288/jsa.1977.38.1705. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, Arabo A, Andre M, Poucet B, Save E, Rondi-Reig L. Cerebellum shapes hippocampal spatial code. Science. 2011;334:385–389. doi: 10.1126/science.1207403. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li TK, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacology. 2005;30:330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rogers J, Siggins GR, Schulman JA, Bloom FE. Physiological correlates of ethanol intoxication tolerance, and dependence in rat cerebellar Purkinje cells. Brain Res. 1980;196:183–198. doi: 10.1016/0006-8993(80)90725-8. [DOI] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, Heck DH, Goldowitz D, Mittleman G, Blaha CD. Connecting the dots of the cerebro-cerebellar role in cognitive function: neuronal pathways for cerebellar modulation of dopamine release in the prefrontal cortex. Synapse. 2011;65:1204–1212. doi: 10.1002/syn.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TD, Dickson PE, McKimm E, Heck DH, Goldowitz D, Blaha CD, Mittleman G. Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum. 2013;12:547–556. doi: 10.1007/s12311-013-0462-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–795. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol. 2003;548:97–110. doi: 10.1113/jphysiol.2002.036459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol. 2007;41:211–221. doi: 10.1016/j.alcohol.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Meera P, Karakossian MH, Otis TS. A reinforcing circuit action of extrasynaptic GABAA receptor modulators on cerebellar granule cell inhibition. PLoS One. 2013;8:e72976. doi: 10.1371/journal.pone.0072976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Jinnai K, Gemba H, Hashimoto S, Mizuno N. Projection of the cerebellar dentate nucleus onto the frontal association cortex in monkeys. Exp Brain Res. 1979;37:193–198. doi: 10.1007/BF01474266. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev. 2010;20:236–260. doi: 10.1007/s11065-010-9142-x. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Caplan D. Cognition, emotion and the cerebellum. Brain. 2006;129:290–292. doi: 10.1093/brain/awh729. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, MacMore J, Vangel M. Cerebellar stroke without motor deficit: clinical evidence for motor and non-motor domains within the human cerebellum. Neuroscience. 2009;162:852–861. doi: 10.1016/j.neuroscience.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K. Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry. 2001;158:1075–1083. doi: 10.1176/appi.ajp.158.7.1075. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Madsen KK, Barker-Haliski ML, White HS. The GABA synapse as a target for antiepileptic drugs: a historical overview focused on GABA transporters. Neurochem Res. 2014;39:1980–1987. doi: 10.1007/s11064-014-1263-9. [DOI] [PubMed] [Google Scholar]
- Schraa-Tam CK, Rietdijk WJ, Verbeke WJ, Dietvorst RC, van den Berg WE, Bagozzi RP, De Zeeuw CI. fMRI activities in the emotional cerebellum: a preference for negative stimuli and goal-directed behavior. Cerebellum. 2012;11:233–245. doi: 10.1007/s12311-011-0301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder D, Nasrallah HA. High alcoholism rate in patients with essential tremor. Am J Psychiatry. 1982;139:1471–1473. doi: 10.1176/ajp.139.11.1471. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J Stud Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Isacescu V. Level of response to alcohol measured on the self-rating of the effects of alcohol questionnaire in a group of 40-year-old women. Am J Drug Alcohol Abuse. 2003;29:191–201. doi: 10.1081/ada-120018846. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Danko GP. A cross-generational comparison of alcohol challenges at about age 20 in 40 father-offspring pairs. Alcohol Clin Exp Res. 2005;29:1921–1927. doi: 10.1097/01.alc.0000187154.94681.65. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Heron J, Horwood J, Davis J, Hibbeln J. The self-rating of the effects of alcohol questionnaire as a predictor of alcohol-related outcomes in 12-year-old subjects. Alcohol Alcohol. 2008;43:641–646. doi: 10.1093/alcalc/agn077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Allen RC, Fukukura T, Knight EE, Cesario EM, Kreikebaum SA. A prospective evaluation of how a low level of response to alcohol predicts later heavy drinking and alcohol problems. Am J Drug Alcohol Abuse. 2011;37:479–486. doi: 10.3109/00952990.2011.598590. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Hill SY. Differentiating the effects of familial risk for alcohol dependence and prenatal exposure to alcohol on offspring brain morphology. Alcohol Clin Exp Res. 2017;41:312–322. doi: 10.1111/acer.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggins GR, French E. Central neurons are depressed by iontophoretic and micropressure application of ethanol and tetrahydropapaveroline. Drug Alcohol Depend. 1979;4:239–243. doi: 10.1016/0376-8716(79)90004-8. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Ariwodola OJ, Weiner JL. Differential effects of GABAB autoreceptor activation on ethanol potentiation of local and lateral paracapsular GABAergic synapses in the rat basolateral amygdala. Neuropharmacology. 2009;56:886–895. doi: 10.1016/j.neuropharm.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snelling C, Tanchuck-Nipper MA, Ford MM, Jensen JP, Cozzoli DK, Ramaker MJ, Helms M, Crabbe JC, Rossi DJ, Finn DA. Quantification of ten neuroactive steroids in plasma in withdrawal seizure-prone and -resistant mice during chronic ethanol withdrawal. Psychopharmacology. 2014;231:3401–3414. doi: 10.1007/s00213-014-3618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen S, Palmer M, Dunwiddie T, Hoffer B. Electrophysiological correlates of ethanol-induced sedation in differentially sensitive lines of mice. Science. 1980;210:1143–1145. doi: 10.1126/science.7444444. [DOI] [PubMed] [Google Scholar]
- Sorensen S, Dunwiddie T, McClearn G, Freedman R, Hoffer B. Ethanol-induced depressions in cerebellar and hippocampal neurons of mice selectively bred for differences in ethanol sensitivity: an electrophysiological study. Pharmacol Biochem Behav. 1981;14:227–234. doi: 10.1016/0091-3057(81)90248-3. [DOI] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Spuhler K, Deitrich RA. Correlative analysis of ethanol-related phenotypes in rat inbred strains. Alcohol Clin Exp Res. 1984;8:480–484. doi: 10.1111/j.1530-0277.1984.tb05707.x. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. An fMRI study of intra-individual functional topography in the human cerebellum. Behav Neurol. 2010;23:65–79. doi: 10.3233/BEN-2010-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Kiianmaa K. Does tolerance develop to the activating, as well as the depressant, effects of ethanol? Pharmacol Biochem Behav. 1982;17:1073–1076. doi: 10.1016/0091-3057(82)90496-8. [DOI] [PubMed] [Google Scholar]
- Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, Borgatti R. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- ten Bruggencate G, Teichmann R, Weller E. Neuronal activity in the lateral vestibular nucleus of the cat. 3. Inhibitory actions of cerebellar Purkinje cells evoked via mossy and climbing fibre afferents. Pflugers Arch. 1972;337:147–162. doi: 10.1007/BF00587837. [DOI] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, De Zeeuw CI, Voogd J, Ruigrok TJ. Single Purkinje cell can innervate multiple classes of projection neurons in the cerebellar nuclei of the rat: a light microscopic and ultrastructural triple-tracer study in the rat. J Comp Neurol. 1998;392:164–178. doi: 10.1002/(sici)1096-9861(19980309)392:2<164::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968;31:785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. II. Purkinje cell output and input. J Neurophysiol. 1970;33:537–547. doi: 10.1152/jn.1970.33.4.537. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. GABAergic transmission modulates ethanol excitation of ventral tegmental area dopamine neurons. Neuroscience. 2011;172:94–103. doi: 10.1016/j.neuroscience.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Association between functional connectivity hubs and brain networks. Cereb Cortex. 2011;21:2003–2013. doi: 10.1093/cercor/bhq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudell JR, Messing RO, Mayfield J, Harris RA. Alcohol dependence: molecular and behavioral evidence. Trends Pharmacol Sci. 2014;35:317–323. doi: 10.1016/j.tips.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyzio R, Cossart R, Khalilov I, Minlebaev M, Hubner CA, Represa A, Ben-Ari Y, Khazipov R. Maternal oxytocin triggers a transient inhibitory switch in GABA signaling in the fetal brain during delivery. Science. 2006;314:1788–1792. doi: 10.1126/science.1133212. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Jotty K. Mini-review: effects of ethanol on GABAA receptor-mediated neurotransmission in the cerebellar cortex – recent advances. Cerebellum. 2015;14:438–446. doi: 10.1007/s12311-014-0639-3. [DOI] [PubMed] [Google Scholar]
- Varagic Z, Ramerstorfer J, Huang S, Rallapalli S, Sarto-Jackson I, Cook J, Sieghart W, Ernst M. Subtype selectivity of alpha+beta-site ligands of GABAA receptors: identification of the first highly specific positive modulators at alpha6beta2/3gamma2 receptors. Br J Pharmacol. 2013;169:384–399. doi: 10.1111/bph.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162:205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544:96–100. doi: 10.1038/nature21726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci. 1997;9:533–548. doi: 10.1111/j.1460-9568.1997.tb01630.x. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TC, Becker N, Apps R, Jones MW. Back to front: cerebellar connections and interactions with the prefrontal cortex. Front Syst Neurosci. 2014;8:4. doi: 10.3389/fnsys.2014.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AM, Soreni N, Noseworthy MD. A preliminary study on the effects of acute ethanol ingestion on default mode network and temporal fractal properties of the brain. MAGMA. 2014;27:291–301. doi: 10.1007/s10334-013-0420-5. [DOI] [PubMed] [Google Scholar]
- Wegelius K, Honkanen A, Korpi ER. Benzodiazepine receptor ligands modulate ethanol drinking in alcohol-preferring rats. Eur J Pharmacol. 1994;263:141–147. doi: 10.1016/0014-2999(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Woodward JJ. Ethanol selectively attenuates NMDAR-mediated synaptic transmission in the prefrontal cortex. Alcohol Clin Exp Res. 2008;32:690–698. doi: 10.1111/j.1530-0277.2008.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Welsh JP, Han VZ, Rossi DJ, Mohr C, Odagiri M, Daunais JB, Grant KA. Bidirectional plasticity in the primate inferior olive induced by chronic ethanol intoxication and sustained abstinence. Proc Natl Acad Sci U S A. 2011;108:10314–10319. doi: 10.1073/pnas.1017079108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf U, Rapoport MJ, Schweizer TA. Evaluating the affective component of the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2009;21:245–253. doi: 10.1176/jnp.2009.21.3.245. [DOI] [PubMed] [Google Scholar]
- Xiao C, Shao XM, Olive MF, Griffin WC, III, Li KY, Krnjevic K, Zhou C, Ye JH. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Ethanol modulation of gamma-aminobutyric acid (GABA)-mediated inhibition of cerebellar Purkinje neurons: relationship to GABAb receptor input. Alcohol Clin Exp Res. 2000;24:682–690. [PubMed] [Google Scholar]
- Ye Z, McGee TP, Houston CM, Brickley SG. The contribution of delta subunit-containing GABAA receptors to phasic and tonic conductance changes in cerebellum, thalamus and neocortex. Front Neural Circuits. 2013;7:203. doi: 10.3389/fncir.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Krook-Magnuson E. Cognitive collaborations: bidirectional functional connectivity between the cerebellum and the hippocampus. Front Syst Neurosci. 2015;9:177. doi: 10.3389/fnsys.2015.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio-Bulcock PA, Homanics GE, Woodward JJ. Loss of ethanol inhibition of NMDAR-mediated currents and plasticity of cerebellar synapses in mice expressing the GluN1(F639A) subunit. Alcohol Clin Exp Res. 2018 doi: 10.1111/acer.13597. [DOI] [PMC free article] [PubMed]
- Zhang XY, Wang JJ, Zhu JN. Cerebellar fastigial nucleus: from anatomic construction to physiological functions. Cerebellum Ataxias. 2016;3:9. doi: 10.1186/s40673-016-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol. 2008;28:469–478. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JN, Yung WH, Kwok-Chong CB, Chan YS, Wang JJ. The cerebellar-hypothalamic circuits: potential pathways underlying cerebellar involvement in somatic-visceral integration. Brain Res Rev. 2006;52:93–106. doi: 10.1016/j.brainresrev.2006.01.003. [DOI] [PubMed] [Google Scholar]