Abstract
Bone metastasis is a leading cause of death in patients with breast cancer, but the underlying mechanisms are poorly understood. While much work focuses on the molecular and cellular events that drive breast cancer bone metastasis, it is mostly unclear what role bone extracellular matrix (ECM) properties play in this process. Bone ECM primarily consists of mineralized collagen fibrils, which are composed of non-stoichiometric carbonated apatite (HA) and collagen type I. Reduced bone mineral content is epidemiologically linked with increased risk of bone metastasis. Yet elucidating the potential functional impact of collagen mineralization on breast cancer cells has remained challenging because of a lack of model systems that allow studying tumor cell behavior as a function of physiological, intrafibrillar collagen mineralization. Here, we have developed cell culture substrates composed of mineralized collagen type I fibrils using a polymer-induced liquid-precursor (PILP) process. Intrafibrillar HA decreased breast cancer cell adhesion forces and accordingly reduced collagen fiber alignment relative to cells cultured on control collagen. The resulting mineral-mediated changes in collagen network characteristics and mechanosignaling correlated with increased cell motility, but inhibited directed migration of breast cancer cells. These results suggest that physiological mineralization of collagen fibrils reduces tumor cell adhesion with potential functional consequences on skeletal homing of disseminated tumor cells in early stages of breast cancer metastasis.
Key works: Bone metastasis, Intrafibrillar collagen mineralization, Hydroxyapatite, Mechanosignaling, Collagen fiber alignment
Introduction
Advanced breast cancer metastasizes to the skeleton in about 70% of patients causing disruption of physiological bone remodeling and ultimately poor clinical prognosis [1, 2]. According to the seed and soil theory, the homing of disseminated tumor cells to distinct organs is influenced by the present/predominant microenvironment, and extracellular matrix (ECM) plays a crucial role in this process [3, 4]. Given that the mineralized bone ECM is unique in its composition and structure relative to the ECM in other metastatic organs [5, 6], it is likely to play a regulatory role during early bone metastasis. Indeed, reduced bone mineral content has been associated with increased risk for bone metastasis [7]. Moreover, previous studies indicate that bone mineralization can vary as a function of localization, age and disease, and these variations, in turn, may modulate tumor progression [6, 8–10]. Despite these observations, relatively little is known about what role bone ECM plays in breast cancer cell adhesion and migration, two key steps during tumor cell homing to bone.
Mineralized collagen fibrils, the basic building block of bone ECM, consist of type I collagen fibrils with intrafibrillar nanocrystals of non-stoichiometric carbonated hydroxyapatite (HA) (Ca10(PO4,CO3)6(OH)2. HA crystals in bone grow as elongated platelets, oriented with respect to the long axis of the collagen fibrils, with a thickness of approximately 2–4 nm and with an average length and width of 50 × 25 nm [11, 12]. Recapitulating bone-like HA properties using synthetic approaches has been explored to guide osteogenic differentiation of mesenchymal stem cells, in the context of bone regeneration [13–15]. Increasing experimental evidence suggests that interactions with HA may also regulate tumor cell behavior. For example, the presence of HA in breast microcalcifications is linked with increased malignant and osteolytic potential of breast cancer cells, processes that further activate the vicious cycle of bone metastasis [16, 17]. The majority of studies testing the effects of mineral on breast cancer cells were performed with either synthetic HA in the absence of organic ECM components [18, 19] or with cell-deposited, mineralized matrices [20, 21] that do not allow identification of the isolated effect of physiologically mineralized vs. non-mineralized collagen. Yet understanding how collagen mineralization, a hallmark of osteogenesis, may influence tumor cell behavior is of critical importance given that bone-tropic breast cancer cells initially target osteogenic niches [22].
Various mineralization methods have been developed to produce mineralized collagen matrices for cell culture studies [23, 24]. However, most of these methods do not recapitulate the intrafibrillar mineral of bone. Recently, a polymer-induced liquid-precursor (PILP) process using acidic polypeptides has been introduced that robustly produces intrafibrillar mineralization of collagen in vitro [6, 25, 26]. For example, the introduction of polyaspartic acid (PAA) during mineralization of collagen promotes infiltration of mineral precursors into fibrillar collagen, where they subsequently transform into crystalline HA [6, 26, 27]. Despite the progress in the development of physiologically mineralized collagen matrices, to date, very few, if any, studies have used these substrates for functional cell studies [28]. Specifically, no previous studies have tested the suitability of the PILP process to generate cell culture substrates for analyzing breast cancer cell behavior as a function of collagen mineralization.
In the absence of mineral, changes in collagen network properties modulate tumor cell phenotype by altering adhesion-dependent changes in intracellular signaling. For example, changes in collagen microstructure, stiffness and chemical composition are known to govern breast cancer cell behaviors through altering mechanotransduction [29–31]. ECM stiffening due to increased collagen deposition and linearization enhances breast cancer cell malignant potential by increasing integrin signaling, activation of focal adhesion kinase and cell contractility ultimately causing transcriptional changes that promote tumor progression [31, 32]. Interestingly, tumor cell interactions with mineralized ECM are also dependent on integrin-mediated changes in cell adhesion. For example, breast cancer cell adhesion on osteoblast-derived, mineralized ECM depends on β1-integrins [20]. In addition, HA-containing 3D polymeric scaffolds as well as mineralized cell culture substrates mediate cell adhesion in an integrin-dependent manner by altering serum protein adsorption [18, 33]. While these studies highlight that inorganic components of the bone ECM can regulate tumor cell adhesion by varying integrin signaling, no studies have tested the effect of collagen fibril mineralization on adhesion-mediated cell signaling.
Here, we have adapted the PILP process to develop a cell culture platform that consists of physiologically mineralized collagen fibrils. We have utilized this model system to study the structure-function relationship of intrafibrillar collagen mineralization in regulating breast cancer cell adhesion and migration and to assess the role of mechanotransduction in this process. Our results indicate that intrafibrillar mineral incorporation alters collagen network properties with functional consequences on breast cancer cell phenotype that may affect homing during early stages of bone metastasis.
Materials and methods
Fabrication of mineralized collagen
Fibrillar collagen hydrogels for subsequent mineralization were fabricated as previously described [34–36]. Briefly, collagen type I stock solution (10 mg/mL in acetic acid) was prepared from rat tails and subsequently cast into poly(dimethylsiloxane) (PDMS) microwells after pH-adjustment. To generate the microwells, PDMS (Dow Corning, USA) was cured on a silicon wafer patterned with microscale circular features (diameter: 4mm, height: 250 μm) and subsequently punched out (Fig. 1a). To ensure binding of collagen to PDMS, the surface of each microwell was treated with 1% polyethyleneimine (PEI, Sigma-Aldrich, USA) and 0.1% glutaraldehyde (Fisher Scientific, USA) successively. After thorough washing, pH-adjusted collagen (1.5 mg/mL in PBS, pH = 7.4) was cast into each PEI/glutaraldehyde-treated microwell, flattened by temporarily placing a second layer of PDMS on top, and gelled under humidified conditions for 2 h at RT. To mineralize the collagen hydrogels using the polymer-induced liquid-precursor (PILP) process [25], the following solution was prepared in deionized distilled water (DDW) and adjusted to pH 7.4: 62.5μg/mL of poly aspartic acid (PAA, MW = 27k Da, Alamanda Polymers, USA), 1.67 mM CaCl2 (Fisher Scientific, USA), 1 mM (NH4)2HPO4 (Sigma-Aldrich, USA) and 0.85 × PBS. For mineralization, collagen-containing PDMS microwells were suspended in the mineralization solution with the collagen surface facing down and incubated in a humidified chamber for 3 days at 37°C (Fig. 1A). Mineralization solutions without PAA or without PAA and CaCl2 were used for control conditions.
Figure 1. Biomimetic mineralization enables intrafibrillar collagen mineralization with corresponding physicochemical changes of the collagen network.
(a) Schematic of the experimental setup for collagen mineralization. Collagen was cast into polyethyleneimine (PEI)- and glutaraldehyde-treated polydimethylsiloxane (PDMS) microwells. Mineralization of collagen was induced by immersing the microwells into a solution containing calcium, phosphate and polyaspartic acid (PAA) for 3 days. (b) Mineral formation was detected using Alizarin Red S (ARS) and von Kossa (VK) staining. Scale bar = 2 mm. (c) FT-IR spectra of collagen and mineralized collagen formed with PAA (MN-collagen). (d) pXRD patterns of collagen and collagen mineralized with and without PAA. (e) Representative SEM and TEM images visualizing the micro- and nano-structure of collagen and collagen mineralized with and without PAA. Collagen was stained with uranyl acetate to show the periodic banding pattern of collagen fibrils. White arrow: Mineral formation within collagen fibrils. Scale bar = 1 μm for SEM and 200 nm for TEM. (f) Young’s moudulus of collagen and MN-collagen measured by AFM-based nanoindentation (n ≥ 14 locations per condition). * indicates significant difference compared to collagen (p < 0.05).
Physicochemical characterization of mineralized collagen
To confirm the formation of mineral by Alizarin Red S and von Kossa staining, samples were fixed with 10% formalin for 30 min before staining. Samples were incubated with Alizarin Red S (40 mM, VWR, USA) for 1 hour followed by washing with distilled water. For von Kossa staining, fixed samples were incubated with 2% silver nitrate solution (Sigma-Aldrich, USA) and subsequently exposed to UV light for 1 hour. To remove un-reacted silver, samples were incubated with 2.5% sodium thiosulfate (Sigma-Aldrich, USA) for 5 min. To detect changes in materials composition following mineralization, samples were washed with distilled water, dried and analyzed by Fourier transform infrared (FT-IR) spectrometry (Hyperion 2000/Tensor 27, Bruker, USA) with a single reflection attenuated total reflectance (ATR) accessory (MIRacle ATR, PIKE Technologies, USA). After contacting on crystal plate, FT-IR spectra were measured in the range of 800–2000 cm−1 and baseline correction was performed using spectroscopy software (OPUS, Bruker, USA). The effect of PAA on the formation of mineral was analyzed using a powder X-ray diffractometer (pXRD) (D8 Advance ECO powder diffractometer, Bruker, USA) with Cu Kα radiation (λ = 1.54A), operating at 40 kV and 25 mA. To this end, samples were washed with distilled water, dried, mounted on a polymethyl methacrylate (PMMA) specimen holder and pXRD spectra were recorded in the range 2θ = 20–45°. Collagen was used as a reference. The morphology of samples was examined by scanning electron microscopy (SEM) (Mira3 LM, Tescan, Czech Republic) at 5 keV. Prior to SEM imaging, samples were dehydrated using an ethanol gradient followed by incubation in hexamethyldisilazane (Electron Microscopy Sciences, USA) and coating with gold/palladium alloy (Desk II, Denton Vacuum, USA). Sample nanostructure was examined by transmission electron microscopy (TEM) (T12 Spirit, FEI, USA) at 120 kV. To this end, carbon-coated nickel TEM grids (Electron Microscopy Sciences, USA) were treated with plasma and placed on droplets of collagen solution. After collagen formation, the grids were placed on droplets of mineralization solutions with or without PAA and incubated in a humidified chamber for 3 days at 37°C. Samples were air dried after rinsing with DDW and coated with amorphous carbon (DTT-IV, Denton Vacuum, USA). To confirm the typical periodic banding pattern of collagen, collagen samples were stained with 1.5% uranyl acetate solution; all other samples were imaged unstained, so all contrast results from the presence of HA mineral particles. To determine substrate-dependent changes in serum adsorption, tissue culture treated polystyrene (PS), collagen, and mineralized collagen formed with PAA (MN-collagen) were incubated in modified Eagle’s medium alpha (α-MEM, Thermo Fisher Scientific, USA) media containing 10% FBS and 1% P/S for 1 h. After washing with PBS, samples were incubated in RIPA lysis buffer (Thermo Fischer Scientific, USA) and the collected solution was used to measure adsorbed protein concentration with a micro BCA kit (Thermo Fisher Scientific, USA). For analyzing Ca2+ release from the different substrates, samples were incubated in serum free α-MEM and 25 mM HEPES solutions (pH 7.4), and solutions were collected after 24 h of incubation and measured with 0.4 mM Arsenazo III (MP Biomedicals, USA) in 0.02 M Tris-base (pH 7.4). Ca2+ levels were derived by converting absorbance intensity at 650 nm using standard curves with known Ca2+ concentrations. Absorbance was measured using a microplate reader (Biotek, Synergy HT, USA).
Mechanical characterization of mineralized collagen
The Young’s modulus of samples equilibrated in PBS solution was determined by AFM-based nanoindentation measurements using a scanning probe microscope (MFP-3D-AFM, Asylum Research, USA). Silicon nitride cantilever modified with a borosilicate glass sphere (sphere diameter: 5 μm; spring constant k: ~ 0.06 N/m, Novascan Tech, USA) were used for measurements. The nominal spring constant k of the cantilever was determined by thermal calibration prior to each measurement. Force-distance curves were acquired using 3 nN contact force and 20 nm/s approach/retract velocity. Each data set was generated by probing 14–18 randomly selected areas on one sample per condition. Force-distance curves were converted to force-indentation curves and the Young’s modulus was determined from approach force-indentation curves using the Hertz model in IGOR PRO software. Rheological measurements of substrate viscoelastic properties were performed on a rheometer (DHR3, TA instruments, USA) with built-in temperature and distance calibration. A 8-mm top and bottom-plate geometry was used for measurement of stress relaxation as a function of time. For the measurement, collagen was cast into a 8 mm diameter PDMS ring that was mounted on a PEI and glutaraldehyde coated glass coverslip and mineralization of collagen was performed as described above. After removing the PDMS ring, the glass coverslips were glued onto the bottom of the plate. Subsequently, the top plate was lowered onto the sample until the monitored axial forces turned into positive values. To prevent drying of samples measurements were performed in the presence of PBS. For stress-relaxation measurements, 20% strain was applied and sample relaxation was measured for 300 s.
Cell culture and cell adhesion experiments
Parental MDA-MB231 breast cancer cells (ATCC) and their bone metastatic subclone BoM1-2287 (kindly provided by Joan Massague) were routinely cultured on PS in α-MEM (Thermo Fisher Scientific, USA) supplemented with 10% (v/v) fetal bovine serum (FBS, Atlanta Biologicals, USA) and 1% penicillin/streptomycin (P/S, Thermo Fisher Scientific, USA). Cultures were maintained at 37 °C in a 5% CO2 incubator. To determine substrate-dependent changes in cell adhesion, MDA-MB231 and BoM1-2287 cells were trypsinized and seeded onto PS, collagen, and MN-collagen in α-MEM media/10% FBS. After 5 hours, non-adherent cells were removed by washing with PBS. Adherent cells were lysed in Caron’s buffer and DNA content was quantified using a QuantiFluor dsDNA kit (Promega, USA) according to manufacturer’s instructions. For SEM imaging, samples were fixed with 10% glutaraldehyde and prepared as described above. For analyzing the effect of integrin-mediated adhesion, collagen and MN-collagen were blocked with 1% bovine serum albumin (BSA, Fisher Scientific, USA) prior to cell seeding. PS was first incubated with α-MEM/10% FBS to ensure serum protein adsorption followed by incubation with 1% BSA. Before cell seeding, tumor cell suspensions were incubated with 10 μg/mL of anti-integrin β1 antibody or 10 μg/mL of anti-integrin α5β1 antibody (Millipore, USA) in serum-free media for 30 min at 37 °C. Tumor cell suspensions without antibody were used as controls. Cell adhesion was determined 1 hour after seeding as described above (n = 4).
Immunofluorescence and Western Blot analysis
For analysis of substrate-induced changes of cell morphology and adhesion-associated signaling molecules, cells were seeded onto the different matrices for 5 h in α-MEM/10% FBS and subsequently fixed with 10% formalin. Cell morphology was determined by image analysis of cells stained with DAPI and Alexa Fluor 568 phalloidin (Thermo Fisher Scientific, USA). Four randomly selected areas were imaged per sample and analyzed with built-in functions of Image J software to determine projected cell area and circularity (4π × area/perimeter2, index of 1 representing a circle) for a total of 3 samples. To detect phosphorylated focal adhesion kinase (pFAK) and myosin light chain (pMLC), fixed cells were permeabilized with PBS-X (0.05% Triton X-100 in PBS) and blocked with 1% BSA in PBS. Samples were incubated with anti-FAK (phospho Y397, 6 μg/mL, Abcam, UK) and anti-MLC (phospho S20, 5 μg/mL Abcam, UK) antibodies overnight at 4 °C and labeled with Alexa Fluor 568. Nuclei and F-actin were stained with DAPI and Alexa Fluor 488 phalloidin (Thermo Fisher Scientific, USA), respectively. Representative images were captured with a confocal microscope (710 LSM, Zeiss, Germany) using a 63× oil immersion objective. Confocal reflectance images of type I collagen were captured in reflectance mode using a laser at 488 nm. Protein levels of pFAK and pMLC were further quantified by Western Blot. Five hours after initial seeding, cells were lysed in RIPA buffer containing phosphatase and protease inhibitor cocktail (Thermo Fisher Scientific, USA) and 1 mM of phenylmethylsulfonyl fluoride (Calbiochem, USA). Equal amounts of protein measured by a BCA kit (Thermo Fisher Scientific, USA) were loaded onto gels, separated via SDS-PAGE, and transferred to PVDF membrane (Bio-Rad, USA). The membranes were blocked with 5% milk in washing buffer and incubated with primary antibodies against phospho-FAK (phospho Y397, 1 μg/mL, Abcam, UK), FAK (0.1 μg/mL, Santa Cruz Biotechnology, USA), phospho-MLC (phospho S20, 0.5 μg/mL, Abcam, UK), MLC (1:1000, Cell Signaling Technology, USA) and GAPDH (1 μg/mL, Ambion, USA) overnight at 4 °C. After probing with species-specific HRP-conjugated secondary antibodies (Thermo Fisher Scientific and Novus Biologicals, USA), the membranes were incubated with ECL Western blotting substrate (Thermo Fisher Scientific, USA) and chemiluminescence was detected using ChemiDoc Touch Imaging System (Bio-Rad, USA). Band intensity was quantified using the Image Lab software (Bio-Rad, USA) (n = 3).
Single cell force spectroscopy
A NanoWizard II atomic force microscope (AFM) equipped with a CellHesion module (JPK Instruments, Germany) mounted on an Axio Observer D1 (Zeiss, Germany) was used for single-cell force spectroscopy (SCFS). Measurements were performed in serum-free, CO2-independent measurement medium (α-MEM, Thermo Fisher Scientific; without sodium bicarbonate, containing 20 mM HEPES). The measurement temperature was adjusted to 37°C using a temperature-controlled sample holder (PetriDish Heater, JPK Instruments, Germany). Collagen matrices were immersed in the measurement medium either untreated or after a 1–2 hour pre-incubation period in serum containing growth medium (α-MEM, Thermo Fisher Scientific; containing 5% FBS). Tipless AFM cantilevers (PNP-TR-TL-Au, Nanoworld, Switzerland) were functionalized with wheat germ agglutinin (Sigma–Aldrich) as described previously [37]. Briefly, a single cell suspension of MDA-MB231 was pipetted into the AFM sample chamber. Under microscopic control, one single cell at a time was captured by pressing a wheat germ agglutinin functionalized cantilever onto an individual cell with a contact force of 1 nN for 10 seconds. The cell was lifted from the surface and allowed to establish firm adhesion to the cantilever for 5 minutes. For SCFS measurements, cantilevers were calibrated before measurements using the equipartition theorem [38]. MDA-MB231 were trypsinized, pelleted, and resuspended in measurement medium. Quantitative AFM-based SCFS experiments were performed as described previously [37]. The contact force was adjusted to 1 nN. The contact time was 3 secs. The approach and retract speed was set to 5 μm/sec. The maximum detachment force was extracted from the retract force–distance (F-D) curves using the data processing software provided by the AFM manufacturer (JPK Instruments, Germany). A minimum of eight randomly selected cells (immobilized on the AFM cantilever) were analyzed per sample from at least three samples per condition. Approximately 5–10 F-D curves were recorded for each individual cell.
Analysis of cell-mediated changes of collagen microarchitecture and migration
MDA-MB231 cells were cultured on collagen and MN-collagen for 10 hours in α-MEM/10% FBS, fixed, and stained with DAPI and Alexa Fluor 568 phalloidin (Thermo Fisher Scientific, USA). To detect collagen microarchitectural changes, confocal images of type I collagen were captured in reflectance mode using polarized laser at 488 nm (Zeiss 710 LSM, Germany). To detect cell-mediated changes in collagen fiber reflectance intensity, z-stacks of randomly selected locations containing a single cell were scanned at 512 × 512 frame size with a 2 μm step size (up to 20 μm deep) (n = 40 cells per condition). Averaged intensity projections of each z-stack were analyzed with Image J. Reflectance intensity of projected images was exported as pixel intensity and plotted using MATLAB (MathWorks) to yield pseudo-colored heat maps. To study the effect of cell contractility on collagen remodeling, cells were cultured in α-MEM media containing an inhibitor of myosin II ATPase (blebbistatin, 10 μM, Sigma-Aldrich, USA) or an inhibitor of Rho-associated protein kinase (Y-27632, 32 μM, Tocris, UK) prior to fixation. Subsequently, confocal reflectance images were captured and analyzed as described above (n = 20 cells per condition). For migration assays, MDA-MB231 were labeled with DiI Vybrant cell-labeling solution (Molecular Probes, USA) and incubated for 1 h in phenol red-free DMEM/F12 media with 10% FBS. Following 5 h adhesion, time lapse images were obtained over 10 hours using an inverted spinning disk confocal microscope in phenol red-free DMEM/F12 media with 10% FBS (Andor/Olympus IX-83, Olympus, Japan). 5 randomly selected areas per condition were captured every 11 mins with 2 μm distance between each slice of Z-stack. Cell speed (total travel distance/time) and directionality (distance between starting and final positions/total travel distance) of individual cells per condition were analyzed with Image J (n ≥ 38).
Statistical analysis
Statistical analysis was performed by student’s unpaired t-test for two conditions and one-way analysis of variance (ANOVA) followed by Tukey’s post test for multiple comparisons. Prism 5 (GraphPad, USA) and SPSS (IBM SPSS Statistics, USA) software programs were used to analyze standard deviation and statistical significance (p <0.05). Unless otherwise indicated a minimum of 3 independent samples was analyzed for each experiment. Data are represented as mean and error bars indicate standard deviations.
Results and discussion
Intrafibrillar mineralization of collagen results in the formation of bone-like collagen-HA composites
To develop matrices that allow studying cellular responses to mineral-mediated differences in collagen network characteristics, we adapted a previously established microwell-based culture platform. Type I collagen isolated from rat tails was cast into PDMS microwells (diameter: 4mm, depth: 250 μm) that were pre-treated with PEI and glutaraldehyde to prevent cell-mediated hydrogel detachment (Fig. 1a). Polymerization of collagen in PDMS microwells not only yielded 3D cell culture substrates, but also ensured that the collagen scaffolds can be floated upside down in mineral-forming solution. This geometry is critical for intrafibrillar mineralization and prevents gravity-assisted deposition of precipitates from the solution onto collagen. To achieve intrafibrillar mineralization of cast collagen, PAA was mixed with a solution containing calcium and phosphate ions. This leads to formation of PAA-stabilized nanophases of ACP that infiltrate the intrafibrillar space of collagen fibrils and subsequently transform into bone mineral crystals as demonstrated by prior in vitro studies of biomineralization mechanisms [25, 27]. To validate that this approach also leads to mineralization of 3D collagen substrates, mineral deposition was characterized at multiple levels. First, matrices were stained using Alizarin Red S and von Kossa to globally detect calcium and phosphate compounds indicative of mineral formation, respectively. Both protocols revealed positive staining when collagen matrices were incubated in calcium and phosphate containing solutions regardless of whether PAA was present or not. In contrast, no color change was observed when collagen matrices were incubated in a mineralization solution devoid of calcium ions, indicating that the staining depended on the presence of both calcium and phosphate ions. Interestingly, mineral formed in the presence of PAA was more uniformly distributed across the substrates relative to mineral deposited without PAA (Fig. 1b). This difference may be attributed to the inhibitory effect of PAA on mineral growth, while rapid mineral growth occurs in the absence of PAA [25].
Analysis of the chemical composition of the different substrates by Fourier transform infrared (FT-IR) and mineral phase by powder X-ray diffraction (pXRD) also supports formation of mineralized collagen (Fig. 1c, d). FT-IR spectra from collagen mineralized with PAA showed collagen-associated amide peaks as well as strong PO43− peaks that can be attributed to mineral. PO43− peaks were also present in FT-IR spectra from collagen mineralized without PAA (data not shown), while they were absent in spectra collected from non-mineralized collagen matrices (Fig. 1c). To further assess the phase of mineral formed with and without PAA, the pXRD patterns were analyzed (Fig. 1d). The formation of HA was confirmed by the appearance of peaks at 26° and 32°, which are characteristic peak positions corresponding to the (002) and (112), (211), and (300) planes of HA, respectively. The broadness of both peaks indicates small crystal sizes and poorly crystalline HA, which is similar to the non-stoichiometric carbonated apatite found in bone. Taken together, chemical and phase analysis indicated that HA is the main mineral component formed in the collagen matrices.
To investigate the mineral arrangement within the collagen matrices, we examined the different collagen networks using electron microscopy. We analyzed global changes of collagen microstructure using scanning electron microscopy (SEM) and used transmission electron microscopy (TEM) to detect collagen fibril nanostructure and intrafibrillar mineral deposition (Fig. 1e). For non-mineralized collagen, SEM and TEM images confirmed the formation of a randomly oriented fibrous network that was composed of self-assembled fibrils with the characteristic banding pattern of collagen. SEM analysis after mineralization revealed microstructural changes of the composite networks that differed depending on whether PAA was utilized. In the absence of PAA, randomly distributed mineral clusters, with the characteristic “floret” morphology associated with solution-grown HA, were detected on the surface of collagen fibrous structures. In contrast, the addition of PAA to the mineralization solution impacted the morphology of the entire fibrillar network. More specifically, as seen in the SEM images, PAA-induced mineralization altered the topology of the collagen substrates in a manner that resembled the granular surface morphology of mineralized collagen fibrils in native bones [39, 40]. Importantly, no obvious clusters of mineral particles were observed on these substrates. To establish whether addition of PAA indeed enabled intrafibrillar mineralization of collagen, TEM analysis of unstained samples was performed. This technique confirmed that PAA-induced mineralization resulted in the formation of intrafibrillar, as well as interfibrillar HA nanocrystals. The intrafibrillar crystals are well-aligned along the longitudinal axis of the collagen fibrils, as previously reported for PILP and similar to mineralized collagen in vivo [41]. In contrast, in the absence of PAA in the mineralization solution, consistent with SEM images, the TEM images revealed randomly oriented assemblies of HA platelets deposited on top of collagen fibers, with no evidence of intrafibrillar mineral (Fig. 1e).
As expected, intrafibrillar mineralization also altered the mechanical properties of collagen as measured by AFM-based nanoindentation. The Young’s modulus of collagen mineralized with PAA was four-fold higher than that of non-mineralized collagen. This result is consistent with findings from other studies that mineralized collagen using other methods [23, 42] (Fig. 1f). Bone is significantly stiffer than the mineralized collagen in our studies, which may be explained by the fact that mineralized collagen only constitutes one level of bone hierarchical structure that determines the unique mechanical properties of the skeleton. Taken together, these findings suggest that PAA-induced intrafibrillar mineralization of collagen generates physiologically relevant composite materials to examine tumor cell behavior as a function of collagen mineralization.
Mineralized collagen mediates breast cancer cell adhesion in an integrin-dependent manner
Next, we utilized the developed bone-like collagen-HA composites to test whether mineralized collagen regulates adhesion of metastatic breast cancer cells (a key step of skeletal homing) differently than non-mineralized collagen. We seeded MDA-MB231 human breast cancer cells, a commonly utilized cell line representative of metastatic breast cancer, and BoM1-2287, a bone-metastatic MDA-MB231 subclone that was isolated by in vivo selection [43], onto the different substrates and quantified the number of adherent cells one hour after initial seeding. Both tumor cell types adhered significantly better on control and mineralized collagen substrates relative to PS, but no difference was noted between non-mineralized vs. mineralized collagen (Supplementary Fig. 1). However, analysis of cell morphology using SEM showed that both MDA-MB231 and BoM1-2287 cultured on mineralized collagen were less spread and more rounded when compared with cells on PS and non-mineralized collagen (Fig. 2a). Confocal image analysis of cells stained for nuclei and F-actin confirmed that breast cancer cells interacting with mineralized collagen spread less and were more circular relative to cells on non-mineralized matrices consistent with a reduction of stress fiber formation on mineralized collagen relative to all other conditions (Fig. 2b, c). Previous reports suggested that presence of HA significantly promotes serum protein adsorption onto cell culture substrates [18, 33]. Yet in our studies the detected changes in cell morphology did not correlate with serum protein adsorption, because cell morphology on PS and non-mineralized collagen was similar despite significantly greater serum protein adsorption on collagen (Fig 2a–c). In contrast, cell morphology on mineralized collagen, which adsorbed an intermediate amount of serum proteins, was distinct from that on other substrates (Supplementary Fig. 2). Consequently, these results suggest that cell-matrix interactions that induce morphological changes in adherent cells are not simply regulated by the quantity of adsorbed serum proteins, but potentially more directly regulated by cellular binding to collagen.
Figure 2. Intrafibrillar mineralization changes breast cancer cell morphology and adhesion onto collagen in a β1-integrin dependent manner.
(a) Representative SEM and confocal images of MDA-MB231 and BoM1-2287 breast cancer cells on tissue culture treated polystyrene (PS), collagen, and mineralized collagen formed with PAA (MN-collagen). Inset: magnified representative single cell image. Scale bar = 20 μm (Inset = 5 μm). (b) Projected cell area and (c) Circularity of F-actin stained cells (n = 3). (d) Breast cancer cell adhesion onto the different substrates in the presence and absence of a function-blocking β1-integrin antibody as measured by fluorimetric quantification of DNA content under the different experimental conditions. All measurements were performed 5 h after seeding (n = 4). &,* and # indicate significant difference compared to untreated, PS and collagen, respectively (p < 0.05).
Cells primarily utilize β1-integrins (i.e., α1β1, α2β1, α10β1 and α11β1) to bind to collagens [44]. Therefore, we next tested whether or not mineral incorporation changes cell binding to collagen via β1-integrins. Inhibition of β1 integrins with a function-blocking antibody reduced adhesion of both MDA-MB231 and BoM1-2287 breast cancer cells on all matrices when compared with their respective untreated conditions implying that cell adhesion on collagen was mediated by β1 integrins regardless of mineralization (Fig. 2d). This result is well-matched with previous studies showing that high expression of collagen-binding integrin subunits by MDA-MB231 cells is associated with increased adhesion on animal and cell-derived extracellular bone matrices [20, 45]. While blockade of β1 integrins affects collagen-binding receptors we cannot exclude potential contributions from other ECM receptors. In particular, inhibition of the fibronectin receptor α5β1 integrin may be relevant because MDA-MB231 cells express high levels of α5 and β1 subunits [20, 45] and because both HA and collagen promote adsorption of fibronectin [46, 47]. Nevertheless, blocking of α5β1 integrins in MDA-MB231 did not affect cell adhesion on the different matrices when compared with untreated conditions (Supplementary Fig. 3). This result indicates that tumor cells interact with mineralized collagen via integrins of the β1 family rather than through adhesion to adsorbed serum proteins including fibronectin consistent with previous studies of breast cancer cell adhesion on tissue and cell-derived bone matrix models [20, 45]. Nevertheless, mineralized collagen may affect integrin-mediated adhesion through alternative mechanisms. Given that integrin functions depend on divalent ions including calcium [48–50] and that HA can be dissolved [51] and thus, affect extracellular calcium levels during cell culture [52], we tested whether collagen and mineralized collagen differ in their release of Ca2+ into serum-free media. To control for pre-existing Ca2+ ions in serum-free media (1.8 mM Ca2+), HEPES buffer at physiological pH and temperature was used as a reference. Indeed, mineralized collagen released significantly more Ca2+ relative to all other conditions when incubated in HEPES buffer, but no difference was detected when incubated in serum-free media. These data suggest that the dissolution of HA from mineralized collagen can be affected by pre-existing Ca2+ levels in the aqueous solution to which the matrix is exposed, but that varied extracellular Ca2+ concentrations were unlikely to be responsible for the observed differences in cell adhesion between collagen and mineralized collagen (Supplementary Fig. 4).
Because MDA-MB231 and BoM1-2287 responded similar to mineral-induced changes of collagen in the above-described experiments, we concluded that our results may be broadly relevant and, therefore, performed all subsequent experiments with MDA-MB231.
Collagen mineralization reduces mechanosignaling
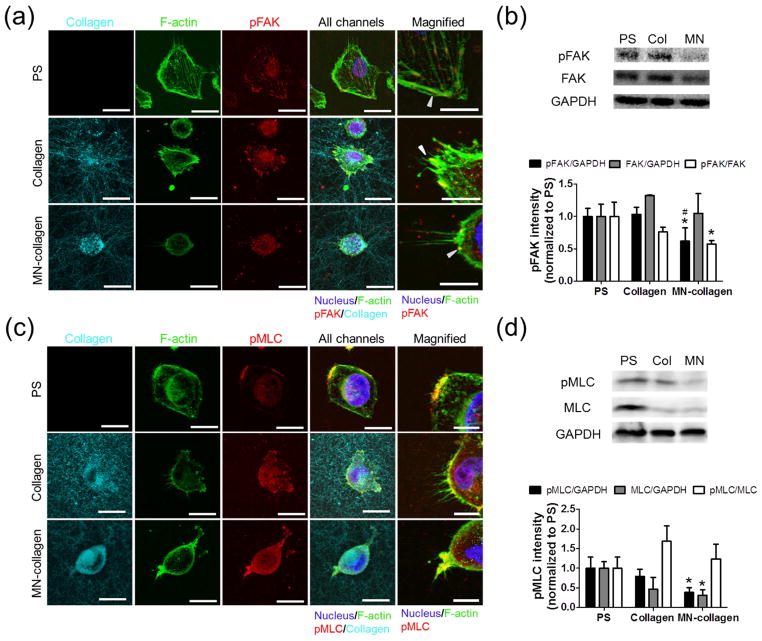
Since integrins play a key role in mechanotransduction and regulated cell adhesion on mineralized collagen in the studies above, we next examined if the detected substrate-dependent differences in cell morphology could be attributed to altered mechanotransduction. Key steps of mechanosignaling involve integrin-dependent focal adhesion formation and a consequential increase of cell contractility due to phosphorylation of focal adhesion kinase (FAK) and myosin light chain (MLC) [53, 54]. Hence, we determined pFAK and pMLC levels on mineralized and non-mineralized collagen using confocal microscopy and Western blot analysis (Fig. 3). Breast cancer cells on mineralized collagen had diffusely distributed pFAK staining throughout the cytoplasm, whereas pFAK co-localized with focal adhesions at the cell periphery on PS and non-mineralized collagen (Fig. 3a). Western blot analysis verified that pFAK levels on mineralized collagen were significantly lower than on PS and non-mineralized collagen, while no significant differences in total FAK levels were detected between the different matrices (Fig. 3b). Because focal adhesion formation positively correlates with cell spreading [55] these data suggest that the rounded morphology of tumor cells on mineralized vs. control collagen may be due to compromised focal adhesion formation. Based on the differences in pFAK levels and localization on the different matrices, we next examined whether these changes correlated with varied myosin light chain activity, which contracts actin fibers to regulate cell spreading and contractility [56]. Consistent with pFAK, we observed that pMLC was relatively homogenously distributed throughout the cytosol of cells cultured on mineralized matrices, while it co-localized with focal adhesions and actin stress fiber-rich areas on collagen and PS (Fig. 3c). Moreover, Western Blot analysis confirmed that when compared with PS, the levels of pMLC were similar on non-mineralized collagen but significantly lower on mineralized collagen (Fig. 3d). Collectively, these data suggest that intrafibrillar mineralization of collagen hinders the activation of focal adhesions with consequences on mechanotransduction.
Figure 3. Cells interacting with mineralized collagen exhibit reduced focal adhesion and myosin light chain activity.
(a,c): Representative MDA-MB231 breast cancer cell images of (a) phosphorylated focal adhesion kinase (pFAK) and (c) phosphorylated myosin light chain (pMLC) expression on tissue culture treated polystyrene (PS), collagen and mineralized collagen formed with PAA (MN-collagen) 5 h after seeding. Nucleus, F-actin, pFAK and pMLC were labeled and imaged using confocal microscopy. Collagen images were collected in confocal reflectance mode. Scale bar = 20 μm and 10 μm for magnified images. Arrow heads in (a) indicate pFAK co-localization with actin fibers in focal adhesions. (b,d): Western blot quantification of phosphorylated levels of (b) FAK and (d) MLC. MDA-MB231 cells were lysed 5 h after seeding on PS, collagen and MN-collagen. pFAK and pMLC levels were normalized to GAPDH or FAK and MLC respectively (n = 3). * and # indicate significant difference compared to PS and collagen, respectively (p < 0.05).
Focal adhesion assembly and consequential changes in actin polymerization and actomyosin-mediated cell contractility allow tumor cells to transmit forces against their underlying substrates, which promotes cell spreading and potential activation of tumorigenic pathways by supporting integrin clustering [4, 57]. Hence, we hypothesized that the detected decrease in FAK and MLC phosphorylation and cell spreading on mineralized vs. non-mineralized collagen may be directly related to reduced adhesion forces of tumor cells. To verify the effect of mineralization on the strength of collagen-receptor bonds, we measured the detachment force of single MDA-MB231 cells on the different matrices using AFM-based single-cell force spectroscopy (Fig. 4a; i and ii show a schematic of the detachment force measurement for a single cell and representative force-distance curves, respectively). Our data indicate that cells in contact with mineralized collagen exhibit decreased attachment forces as compared to cells on non-mineralized matrices (Fig. 4aiii) consistent with the detected reduction of focal adhesions and pFAK (Figs. 3a, b). This trend was not affected by the presence of serum proteins (data not shown), indicating that the effect of serum on cell adhesion is limited, and that mineralization of collagen reduces the strength of tumor cell adhesion by alternative mechanisms possibly including altered surface charge [27, 58]. Collectively, these results suggest that intrafibrillar mineralization reduces cellular adhesion forces possibly by altering adhesion-dependent signaling.
Figure 4. Cells interacting with mineralized collagen exhibit reduced adhesion forces, collagen remodeling, and altered migration.
(a) Quantification of cell-matrix interaction forces by AFM-based single cell force spectroscopy (SCFS): (i) Schematic of the SCFS measurements. Single MDA-MB231 cells, attached to a tipless AFM cantilever, were approached onto the different collagen matrices until a preset contact force was reached. After a contact time of 3 s, the cell was retracted until complete separation. During approach and retract, the cantilever deflection (that corresponds to the force acting on the cantilever) was recorded and plotted as force-distance curves. (ii) Representative retract force-distance curves of MDA-MB231 cells. The difference between the minimum of the curves and the baseline (complete detachment) corresponds to the maximum force detected during cell detachment i.e. the detachment force (iii) Detachment forces of MDA-MB231 cells tested in serum-free media (n α 8 cells per sample for at least three samples per condition). (b) Representative confocal images and pseudo-colored reflectance intensity maps indicating spatial variations in collagen alignment after 10 h of MDA-MB231 cell culture. Shown reflectance intensity maps are representative of data collected from single cells (n = 40 cells per condition). Circles indicate averaged, projected cell surface area per condition. Scale bar = 20 μm. (c) Representative pseudo-colored reflectance intensity of collagen and MN-collagen 5 h after cell seeding and treatment with blebbistatin. Circles indicate averaged, projected cell surface area for each condition. Scale bar = 20 μm (n = 20 cells per condition). (d) Migration paths of individual MDA-MB231 cells collected by 10 hours time-lapse imaging of MDA-MB231 (n ≥ 38 cells per condition). (e) Quantification of directionality and velocity of MDA-MB231 cells. * and # indicate significant difference compared to tissue culture treated polystyrene (PS) and collagen respectively (p < 0.05).
Intrafibrillar mineralization reduces collagen network remodeling and promotes migration of breast cancer cells
Tumor cell interactions with non-mineralized collagen are regulated by a complex interplay: integrin-dependent adhesion promotes actomyosin-mediated contractility, which, in turn, leads to collagen fiber alignment. This fiber alignment causes local strain stiffening of the collagen matrix that further promotes cell contractility via positive feedback control [59]. Since our results suggested that incorporation of mineral reduces cell forces by decreasing focal adhesion formation and thus, contractility, we hypothesized that these differences also reduce collagen network remodeling with potential functional consequences. Using confocal microscopy of F-actin-stained MDA-MB231 cells in combination with confocal reflectance imaging of collagen network architecture, we found that dense clusters of highly reflecting, aligned collagen fibers formed around the periphery of adhered cells on non-mineralized collagen. In contrast, the reflectance intensity and alignment of mineralized collagen fibers around the periphery of adhered cells was significantly reduced relative to non-mineralized collagen (Fig. 4b). To quantify these changes in collagen structure, we quantified the reflectance intensity of the collagen matrix that surrounded individual cells. This approach revealed a gradient of high reflectance intensity of collagen that decreased with increasing distance from the projected area of the adhered cell. In contrast, reflectance intensity of mineralized collagen was increased in areas immediately in contact with cells, but otherwise relatively uniformly distributed across each field of view. As cellular traction forces are necessary for collagen fiber alignment and stiffening [60, 61], we next verified that reduced adhesion forces due to decreased actomyosin-mediated contractility may have been responsible for the reduced alignment of mineralized collagen fibers in our studies. Indeed, inhibition of MLC activity using blebbistatin reduced collagen fiber alignment and clustering on non-mineralized collagen, whereas this treatment had no effect on mineralized collagen (Fig. 4c). Accordingly, inhibition of cell contractility using blebbistatin or the Rho-associated protein kinase (ROCK) inhibitor Y-27632 reduced spreading of MDA-MB231 on PS and collagen, but had a less pronounced effect on mineralized collagen (Supplementary Fig. 5).
These findings are interesting as nanoindentation measurements suggested that mineralized collagen networks are stiffer than their non-mineralized counterparts (Fig. 1f), and increased ECM stiffness is commonly associated with elevated mechanotransduction, cellular traction forces, and consequential changes in collagen remodeling [30, 55, 62, 63]. However, we have observed the opposite. To reconcile these observations, it has to be kept in mind that cell-derived ECMs including collagen are not purely elastic, but contain fibrous structures with viscoelastic properties that can relax stress in response to constant strain or stiffen matrices by strain-induced fiber remodeling [60, 61, 64]. Therefore, the limited ability of breast cancer cells to activate adhesion-dependent signaling may also be related to the mechanics of the collagen fiber network. To analyze whether mineralization affects the viscoelastic properties of collagen, we performed rheology in which we measured the stress relaxation rates of both substrates in response to constant strain. The rate of stress relaxation was retarded after mineralization of collagen, suggesting that mineralization interferes with the ability of collagen to release stress through matrix reorganization (Supplementary Fig. 6). These results indicate that tumor cells may be less able to remodel mineralized vs. non-mineralized collagen fiber networks, which reduces their ability to cluster integrin-binding sites, form focal adhesions, and activate mechanosignaling. Indeed, a previous study decoupling the bulk modulus of biomaterials from individual fiber stiffness showed that stiffer fibers are recruited less by cells, which, in turn, decreases focal adhesion assembly, cell spreading, and mechanotransduction [65]. Therefore, mineralization of collagen may impact tumor cell adhesion due in part to altered viscoelastic properties of the matrix network.
To determine whether the observed mineral-dependent differences in adhesion dynamics and matrix remodeling have functional consequences on tumor cell behavior, we compared the effect of mineralized vs. non-mineralized collagen on MDA-MB231 migration using live cell imaging (Fig. 4d). Individual cell tracking analysis indicated that cells cultured on mineralized collagen migrated faster, but with less directional persistence, than cells on non-mineralized collagen (Fig. 4e). These findings were consistent with previous studies showing that migration speed of anchorage dependent cells is inversely related to cell spreading [66] and that cells migrate faster at intermediate cell-surface attachment force [67, 68] when the spatiotemporal interplay between actomyosin and focal adhesion dynamics is optimized [69]. Furthermore, this finding may be physiologically relevant as others had previously shown that breast cancer cells migrate faster on osteoblast-derived ECM relative to collagen alone [20]. Therefore, mineralized collagen may increase cell migration relative to non-mineralized collagen by affecting the balance between cell spreading, contractile forces, and FA dynamics, but further experiments will be needed to confirm this potential relationship. The detected decrease in directionality may be attributed to reduced tumor cell-dependent alignment of collagen fibers that, in turn, plays a key role in guiding tumor cell migration and invasion in collagen matrices [59, 70]. Accordingly, migration of bone metastatic breast cancer cells on osteoblast-derived decellularized matrices also depends on ECM alignment [20]. While cell-derived mineralized matrices deposited by healthy osteoblasts are compositionally more complex and inherently more aligned than the mineralized matrices developed here, our results are still relevant as they provide insights into how collagen mineralization may affect tumor cell migration patterns. In summary, our results suggest that mineralization of fibrous collagen alters not only the chemical and physical properties of collagen, but also consequential interactions with breast cancer cells.
Summary and conclusions
We developed a biomimetic platform to investigate how intrafibrillar mineralization of collagen influences breast cancer cell behavior. In contrast to other mineral-containing collagen matrices, intrafibrillar mineral, achieved using an acidic polymer during the mineralization, more accurately recapitulates the physical and chemical properties of collagen in bone. For example, at the nanoscale, intrafibrillar mineral alters the stiffness of individual collagen fibrils and influences the chemistry accessible on the collagen surface. At the micro-to-macroscale, the bulk mechanical properties of the substrates are determined by mineral content, as well as the organization of the collagen fibrils over microns. All of these factors directly impact cell behavior on these substrates. Integrin-mediated adhesion of breast cancer cells on mineralized collagen led to limited spreading and rounded cell morphology when compared to non-mineralized collagen. This difference in adhesion was attributed to reduced cell attachment force, focal adhesion formation and contractility on mineralized collagen. In addition, these different phenotypes were associated with changes in integrin-dependent downstream signaling linked with mechanotransduction, which was regulated by feedback between cells and matrix. Cell-induced strain-stiffening of collagen fibrils was abrogated by altered viscoelastic properties of collagen after mineralization, which in turn influenced adhesion dynamics of breast cancer cells. As a result, we found apparent differences in cell migration patterns that might result from altered cell-matrix interactions and remodeling of the collagen matrix.
Our study highlights that intrafibrillar mineralization of collagen can alter the response of breast cancer cells through integrin-mediated mechanotransduction, but further experiments will be necessary to confirm in vivo relevance and potential implications for clinical decision-making. For example, the mineralized collagen substrates developed here do not recapitulate the full complexity of bone ECM and additional studies will be needed to validate our results in the context of enhanced ECM complexity, for example with cell-deposited native ECMs and/or with in vivo samples. Moreover, the mineral to matrix ratio may vary among patients as a function of age or disease [9, 71, 72], but we only tested one particular HA to collagen ratio. It is possible to adjust the utilized PILP-based mineralization protocol to control the degree of collagen mineralization by tuning both the concentration and molecular weight of anionic polymers as well as the incubation time [25, 28, 73]. Collagen mineralization may also alter bone cell behavior, which, in turn, may have independent or synergistic effects on tumor cells, for example by varying paracrine signaling between the different cell types. This possibility may be tested by co-culturing tumor cells with osteoblasts and/or osteoclasts on the different substrates. Finally, in vivo studies coupled with analysis of clinical specimens will ultimately be necessary to examine whether or not the reported mineral-mediated differences in tumor cell behavior may be functionally relevant to tumor cell homing to bone ECM during early stages of metastasis. Collectively, this study describes the development of a culture platform that can be used for further examination of the interactions between breast cancers and mineralized ECM, which may ultimately advance our understanding of the factors that regulate breast cancer bone metastasis.
Supplementary Material
Acknowledgments
The authors thank Alexander Boys for consultation on biomineralization methods and Aaron Chiou and Matthew Whitman for critical reading of this manuscript. Financial support was provided by the Human Science Frontiers Program (RGP0016/2017) and the National Cancer Institute through the Center on the Physics of Cancer Metabolism (1U54CA210184) and 1R01CA173083. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This work utilized the Cornell Center for Materials Research (CCMR) shared Facilities, which are supported through the NSF MRSEC program (DMR-1719875) and the Cornell University Biotechnology Resource Center (BRC) confocal microscopy (NIH-S10RR025502) and spinning disk confocal microscopy (NIH S10OD010605) facilities.
Footnotes
Author contributions: S. Choi performed all experiments unless otherwise noted; J. Friedrichs performed single cell force spectroscopy measurement; Y.H. Song performed spinning disk confocal microscope measurement; S. Choi, J. Friedrichs, Y.H. Song, C. Werner, L. Estroff and C. Fischbach analyzed the data; S. Choi, L. Estroff, and C. Fischbach designed the study and wrote the paper. All authors were involved in the discussion of results and critical reading of the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data and materials are available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–1633. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 2.Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–386. doi: 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature medicine. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. The Journal of Cell Biology. 2012;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byron A, Humphries JD, Humphries MJ. Defining the extracellular matrix using proteomics. International Journal of Experimental Pathology. 2013;94(2):75–92. doi: 10.1111/iep.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olszta MJ, Cheng X, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB. Bone structure and formation: A new perspective. Materials Science and Engineering: R: Reports. 2007;58(3):77–116. [Google Scholar]
- 7.Lipton A, Uzzo R, Amato RJ, Ellis GK, Hakimian B, Roodman GD, Smith MR. The Science and Practice of Bone Health in Oncology: Managing Bone Loss and Metastasis in Patients With Solid Tumors. Journal of the National Comprehensive Cancer Network: JNCCN. 2009;7(Suppl 7):S1–S30. doi: 10.6004/jnccn.2009.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donnelly E, Meredith DS, Nguyen JT, Boskey AL. Bone tissue composition varies across anatomic sites in the proximal femur and the iliac crest. Journal of Orthopaedic Research. 2012;30(5):700–706. doi: 10.1002/jor.21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boskey AL, Coleman R. Aging and Bone. Journal of Dental Research. 2010;89(12):1333–1348. doi: 10.1177/0022034510377791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, McDonald JM. Disorders of Bone Remodeling. Annual Review of Pathology: Mechanisms of Disease. 2011;6(1):121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reznikov N, Shahar R, Weiner S. Bone hierarchical structure in three dimensions. Acta Biomaterialia. 2014;10(9):3815–3826. doi: 10.1016/j.actbio.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 12.SW, Wagner HD. THE MATERIAL BONE: Structure-Mechanical Function Relations. Annual Review of Materials Science. 1998;28(1):271–298. [Google Scholar]
- 13.Wang P, Zhao L, Liu J, Weir MD, Zhou X, Xu HHK. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Research. 2014;2:14017. doi: 10.1038/boneres.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim BS, Kim JS, Chung YS, Sin YW, Ryu KH, Lee J, You HK. Growth and osteogenic differentiation of alveolar human bone marrow-derived mesenchymal stem cells on chitosan/hydroxyapatite composite fabric. Journal of Biomedical Materials Research Part A. 2013;101A(6):1550–1558. doi: 10.1002/jbm.a.34456. [DOI] [PubMed] [Google Scholar]
- 15.Murphy WL, Hsiong S, Richardson TP, Simmons CA, Mooney DJ. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26(3):303–310. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Cox RF, Morgan MP. Microcalcifications in breast cancer: Lessons from physiological mineralization. Bone. 2013;53(2):437–450. doi: 10.1016/j.bone.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 17.Baker R, Rogers KD, Shepherd N, Stone N. New relationships between breast microcalcifications and cancer. British Journal of Cancer. 2010;103:1034. doi: 10.1038/sj.bjc.6605873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pathi SP, Lin DDW, Dorvee JR, Estroff LA, Fischbach C. Hydroxyapatite nanoparticle-containing scaffolds for the study of breast cancer bone metastasis. Biomaterials. 2011;32(22):5112–5122. doi: 10.1016/j.biomaterials.2011.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu W, Wang M, Fu Y, Castro NJ, Fu SW, Zhang LG. Engineering a biomimetic three-dimensional nanostructured bone model for breast cancer bone metastasis study. Acta Biomaterialia. 2015;14:164–174. doi: 10.1016/j.actbio.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Taubenberger AV, Quent VM, Thibaudeau L, Clements JA, Hutmacher DW. Delineating breast cancer cell interactions with engineered bone microenvironments. Journal of Bone and Mineral Research. 2013;28(6):1399–1411. doi: 10.1002/jbmr.1875. [DOI] [PubMed] [Google Scholar]
- 21.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, Moretti M, Kamm RD. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35(8):2454–2461. doi: 10.1016/j.biomaterials.2013.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Yu C, Gao X, Welte T, Muscarella Aaron M, Tian L, Zhao H, Zhao Z, Du S, Tao J, Lee B, Westbrook Thomas F, Wong Stephen TC, Jin X, Rosen Jeffrey M, Osborne CK, Zhang Xiang HF. The Osteogenic Niche Promotes Early-Stage Bone Colonization of Disseminated Breast Cancer Cells. Cancer Cell. 2015;27(2):193–210. doi: 10.1016/j.ccell.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al-Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, Hammer J, O’Brien FJ. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;90B(2):584–591. doi: 10.1002/jbm.b.31320. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Van Manh N, Wang H, Zhong X, Zhang X, Li C. Synergistic intrafibrillar/extrafibrillar mineralization of collagen scaffolds based on a biomimetic strategy to promote the regeneration of bone defects. International Journal of Nanomedicine. 2016;11:2053–2067. doi: 10.2147/IJN.S102844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshpande AS, Beniash E. Bio-inspired Synthesis of Mineralized Collagen Fibrils. Crystal growth & design. 2008;8(8):3084–3090. doi: 10.1021/cg800252f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nudelman F, Lausch AJ, Sommerdijk NAJM, Sone ED. In vitro models of collagen biomineralization. Journal of Structural Biology. 2013;183(2):258–269. doi: 10.1016/j.jsb.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Nudelman F, Pieterse K, George A, Bomans PHH, Friedrich H, Brylka LJ, Hilbers PAJ, de With G, Sommerdijk NAJM. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nature materials. 2010;9(12):1004–1009. doi: 10.1038/nmat2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez DE, Thula-Mata T, Toro EJ, Yeh YW, Holt C, Holliday LS, Gower LB. Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomaterialia. 2014;10(1):494–507. doi: 10.1016/j.actbio.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guzman A, Ziperstein MJ, Kaufman LJ. The effect of fibrillar matrix architecture on tumor cell invasion of physically challenging environments. Biomaterials. 2014;35(25):6954–6963. doi: 10.1016/j.biomaterials.2014.04.086. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri O, Koshy ST, Branco da Cunha C, Shin J-W, Verbeke CS, Allison KH, Mooney DJ. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat Mater. 2014;13(10):970–978. doi: 10.1038/nmat4009. [DOI] [PubMed] [Google Scholar]
- 31.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Acerbi I, Cassereau L, Dean I, Shi Q, Au A, Park C, Chen YY, Liphardt J, Hwang ES, Weaver VM. Human Breast Cancer Invasion and Aggression Correlates with ECM Stiffening and Immune Cell Infiltration. Integrative biology: quantitative biosciences from nano to macro. 2015;7(10):1120–1134. doi: 10.1039/c5ib00040h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi S, Coonrod S, Estroff L, Fischbach C. Chemical and physical properties of carbonated hydroxyapatite affect breast cancer cell behavior. Acta Biomaterialia. 2015;24(Supplement C):333–342. doi: 10.1016/j.actbio.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song YH, Shon SH, Shan M, Stroock AD, Fischbach C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integrative Biology. 2016;8(2):205–215. doi: 10.1039/c5ib00277j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-Controlled Three-Dimensional Cultures to Analyze Tumor Angiogenesis. Tissue Engineering Part A. 2010;16(7):2133–2141. doi: 10.1089/ten.tea.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Friedrichs J, Helenius J, Muller DJ. Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy. Nat Protocols. 2010;5(7):1353–1361. doi: 10.1038/nprot.2010.89. [DOI] [PubMed] [Google Scholar]
- 38.Hutter JL, Bechhoefer J. Calibration of atomic-force microscope tips. Review of Scientific Instruments. 1993;64(7):1868–1873. [Google Scholar]
- 39.Olszta MJ, Douglas EP, Gower LB. Scanning Electron Microscopic Analysis of the Mineralization of Type I Collagen via a Polymer-Induced Liquid-Precursor (PILP) Process. Calcified Tissue International. 2003;72(5):583–591. doi: 10.1007/s00223-002-1032-7. [DOI] [PubMed] [Google Scholar]
- 40.Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GAG, Stucky GD, Morse DE, Hansma PK. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nature materials. 2005;4:612. doi: 10.1038/nmat1428. [DOI] [PubMed] [Google Scholar]
- 41.McNally EA, Schwarcz HP, Botton GA, Arsenault AL. A Model for the Ultrastructure of Bone Based on Electron Microscopy of Ion-Milled Sections. PLOS ONE. 2012;7(1):e29258. doi: 10.1371/journal.pone.0029258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu L-n, Jiao K, Ryou H, Yiu CKY, Chen J-h, Breschi L, Arola DD, Pashley DH, Tay FR. Multiphase intrafibrillar mineralization of collagen. Angewandte Chemie (International ed in English) 2013;52(22):5762–5766. doi: 10.1002/anie.201210259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 44.Jokinen J, Dadu E, Nykvist P, Kapyla J, White DJ, Ivaska J, Vehvilainen P, Reunanen H, Larjava H, Hakkinen L, Heino J. Integrin-mediated Cell Adhesion to Type I Collagen Fibrils. Journal of Biological Chemistry. 2004;279(30):31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 45.van der P, Vloedgraven H, Papapoulos S, Lowick C, Grzesik W, Kerr J, Robey PG. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77(6):665–675. [PubMed] [Google Scholar]
- 46.Dolatshahi-Pirouz A, Jensen T, Foss M, Chevallier J, Besenbacher F. Enhanced Surface Activation of Fibronectin upon Adsorption on Hydroxyapatite. Langmuir. 2009;25(5):2971–2978. doi: 10.1021/la803142u. [DOI] [PubMed] [Google Scholar]
- 47.Gold LI, Pearlstein E. Fibronectin–collagen binding and requirement during cellular adhesion. Biochemical Journal. 1980;186(2):551–559. doi: 10.1042/bj1860551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mould AP, Akiyama SK, Humphries MJ. Regulation of Integrin α5β1-Fibronectin Interactions by Divalent Cations: EVIDENCE FOR DISTINCT CLASSES OF BINDING SITES FOR Mn2+, Mg2+, AND Ca2+ Journal of Biological Chemistry. 1995;270(44):26270–26277. doi: 10.1074/jbc.270.44.26270. [DOI] [PubMed] [Google Scholar]
- 49.Zhang K, Chen J. The regulation of integrin function by divalent cations. Cell Adhesion & Migration. 2012;6(1):20–29. doi: 10.4161/cam.18702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuhrmann A, Banisadr A, Beri P, Tlsty TD, Engler AJ. Metastatic State of Cancer Cells May Be Indicated by Adhesion Strength. Biophysical Journal. 112(4):736–745. doi: 10.1016/j.bpj.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JS, Suarez-Gonzalez D, Murphy WL. Mineral Coatings for Temporally Controlled Delivery of Multiple Proteins. Advanced Materials. 2011;23(37):4279–4284. doi: 10.1002/adma.201100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weisgerber DW, Caliari SR, Harley BAC. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomaterials science. 2015;3(3):533–542. doi: 10.1039/C4BM00397G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Provenzano PP, Keely PJ. Mechanical signaling through the cytoskeleton regulates cell proliferation by coordinated focal adhesion and Rho GTPase signaling. Journal of Cell Science. 2011;124(8):1195–1205. doi: 10.1242/jcs.067009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 55.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Meth. 2010;7(9):733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wakatsuki T, Wysolmerski RB, Elson EL. Mechanics of cell spreading: role of myosin II. Journal of Cell Science. 2003;116(8):1617–1625. doi: 10.1242/jcs.00340. [DOI] [PubMed] [Google Scholar]
- 57.Sun Z, Guo SS, Fassler R. Integrin-mediated mechanotransduction. The Journal of Cell Biology. 2016 doi: 10.1083/jcb.201609037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doostmohammadi A, Monshi A, Salehi R, Fathi MH, Karbasi S, Pieles U, Daniels AU. Preparation, chemistry and physical properties of bone-derived hydroxyapatite particles having a negative zeta potential. Materials Chemistry and Physics. 2012;132(2):446–452. [Google Scholar]
- 59.Ahmadzadeh H, Webster MR, Behera R, Jimenez Valencia AM, Wirtz D, Weeraratna AT, Shenoy VB. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proceedings of the National Academy of Sciences. 2017;114(9):E1617–E1626. doi: 10.1073/pnas.1617037114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall MS, Alisafaei F, Ban E, Feng X, Hui CY, Shenoy VB, Wu M. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proceedings of the National Academy of Sciences. 2016;113(49):14043–14048. doi: 10.1073/pnas.1613058113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Helvert S, Friedl P. Strain Stiffening of Fibrillar Collagen during Individual and Collective Cell Migration Identified by AFM Nanoindentation. ACS Applied Materials & Interfaces. 2016;8(34):21946–21955. doi: 10.1021/acsami.6b01755. [DOI] [PubMed] [Google Scholar]
- 62.Das RK, Gocheva V, Hammink R, Zouani OF, Rowan AE. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat Mater. 2016;15(3):318–325. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 63.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154(Supplement C):213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 64.Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H-p, Lippens E, Duda GN, Mooney DJ. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature materials. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baker BM, Trappmann B, Wang WY, Sakar MS, Kim IL, Shenoy VB, Burdick JA, Chen CS. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat Mater. 2015;14(12):1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webb K, Hlady V, Tresco PA. Relationships among cell attachment, spreading, cytoskeletal organization, and migration rate for anchorage-dependent cells on model surfaces. Journal of Biomedical Materials Research. 2000;49(3):362–368. doi: 10.1002/(sici)1097-4636(20000305)49:3<362::aid-jbm9>3.0.co;2-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. The Journal of Cell Biology. 1996;134(6):1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 69.Gupton SL, Waterman-Storer CM. Spatiotemporal Feedback between Actomyosin and Focal-Adhesion Systems Optimizes Rapid Cell Migration. Cell. 2006;125(7):1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 70.Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. The Journal of Cell Biology. 2013;201(7):1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. FTIR Microspectroscopic Analysis of Human Iliac Crest Biopsies from Untreated Osteoporotic Bone. Calcif Tissue Int. 1997;61:487–492. doi: 10.1007/s002239900372. [DOI] [PubMed] [Google Scholar]
- 72.Paschalis EP, Betts F, DiCarlo E, Mendelsohn R, Boskey AL. FTIR Microspectroscopic Analysis of Normal Human Cortical and Trabecular Bone. Calcified Tissue International. 1997;61(6):480–486. doi: 10.1007/s002239900371. [DOI] [PubMed] [Google Scholar]
- 73.Jee SS, Thula TT, Gower LB. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: Influence of polymer molecular weight. Acta Biomaterialia. 2010;6(9):3676–3686. doi: 10.1016/j.actbio.2010.03.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.