Abstract
Introduction
The PI3K/AKT/mTOR pathway is an oncogenic driver in breast cancer (BC). In this multi-center, pre-surgical study, we evaluated the tissue effects of the AKT inhibitor MK-2206 in women with stage I–III BC.
Materials and Methods
Two doses of weekly oral MK2206 were administered at days -9 and -2 before surgery. The primary endpoint was reduction of pAktSer473 in breast tumor tissue from diagnostic biopsy to surgery. Secondary endpoints included changes in PI3K/AKT pathway tumor markers, tumor proliferation (ki-67), insulin-growth factor pathway blood markers, pharmacokinetics (PK), genomics, and MK-2206 tolerability. Paired-t tests were used to compare biomarker changes in pre- and post-MK-2206, and two-sample t-tests to compare with prospectively accrued untreated controls.
Results
Despite dose reductions, the trial was discontinued after 12 patients due to grade III rash, mucositis, and pruritus. While there was a trend to reduction in pAKT after MK-2206 (p=0.06), there was no significant change compared to controls (n=5, p=0.65). After MK-2206, no significant changes in ki-67, pS6, PTEN, or stathmin were observed. There was no significant association between dose level and PK (p=0.11). Compared to controls, MK-2206 significantly increased serum glucose (p=0.02), insulin (p<0.01), C-peptide (p<0.01), and a trend in IGFBP-3 (p=0.06).
Conclusion
While a trend to pAKT reduction after MK-2206 was observed, there was no significant change compared to controls. However, the accrued population was limited, due to toxicity being greater than expected. Pre-surgical trials can identify in vivo activity in early drug development, but side effects must be considered in this healthy population.
Keywords: phase 0, pre-surgical, MK-2206, AKT inhibitor, breast cancer
Introduction
The PI3K-AKT pathway is one of the most commonly altered pathways in cancer, including mutations, somatic copy number abnormalities, increased expression, and aberrant signaling1. Mutations in genes encoding for the subunits of PI3K represent some of the most commonly mutated genes in cancer, with kinase domain activating point mutations in the PIK3CA gene (which encodes for p110α), second only to p53 as the most commonly occurring mutations in all tumor specimens in the Cancer Genome Atlas2. In breast cancer, PIK3CA mutations occur in approximately one-third of human breast tumors3. A serine threonine kinase with 3 different isoforms, AKT (also known as protein kinase B) is an important signaling hub with more than 100 downstream target substrates, impacting cell metabolism, growth, survival and proliferation1. AKT represents an attractive therapeutic target.
MK-2206 is an oral allosteric inhibitor of the AKT kinase domain. It has nanomolar potency against recombinant human AKT1 and AKT2, with lower potency against AKT3. In breast cancer cell lines, MK-2206 inhibits cell cycle progression and induces apotptosis4. Tumor growth inhibition has been observed in xenograft models with a PIK3CA mutation or PTEN loss4. Phase I trials have been completed in patients with metastatic breast cancer combined with hormone therapy5, chemotherapy6, and HER2-directed therapy, including trastuzumab and lapatinib7–9. In these trials, anti-cancer activity, including tumor shrinkage, has been reported. In these metastatic trials, no specific marker has been identified as predictive of clinical response, including PIK3CA mutation status5 or PI3K activity score6.
Given that PI3K/AKT pathway activation, such as PIK3CA mutation, is detected early in breast tumor development and selected for in breast cancer progression, we performed a multi-center, “window of opportunity” or pre-surgical (phase 0) trial with MK-2206 in patients with operable breast cancer. The purpose of this study was to determine the effects of MK-2206 on tumor tissue among women with newly diagnosed, non-metastatic breast cancer during the interval between breast biopsy and surgery. The primary objective was to evaluate changes in pAKTSer473 levels in breast tissue after 2 doses of weekly MK-2206 given before surgery. Secondary objectives included assessing changes in the immunohistochemical (IHC) expression of other PI3K/AKT pathway markers and tumor proliferation in tumor tissue, molecular markers, pharmacokinetic (PK) and pharmacodynamics blood markers, and safety and tolerability of MK-2206.
Materials and Methods
Patients
We conducted an open-label, single-arm, pre-surgical trial with MK-2206 at Columbia University Medical Center (CUMC) and the Albert Einstein Cancer Center/Montefiore Medical Center (AECC) in New York, NY. Between October 2011 and March 2013, we enrolled patients with newly diagnosed clinical stage I–III (non-inflammatory) histologically confirmed operable invasive breast cancer. Patients were required to have undergone a core needle biopsy, with the plan of undergoing a surgical resection for residual disease after study enrollment. It was required that tumors were at least 1.0 cm by breast imaging or palpation in order to increase the likelihood of evaluable post-MK-2206 tissue. Additional inclusion criteria included women ≥ 18 years of age with appropriate hematologic, renal, and liver function, and performance status. Exclusion criteria included no prior chemotherapy, radiation therapy, or surgery within 6 months, and prior administration of agents that target the PI3K/Akt pathway, such as everolimus, was not allowed. As hyperglycemia is a known potential side effect of MK-2206, poorly controlled diabetes (hemoglobin A1C ≥ 8%) was an exclusion criterion. If patients were taking metformin, it was required that the patient had been receiving this medication for > 3 months, given metformin’s potential impact on PI3K/Akt signaling10. The study was approved by the CUMC and AECC institutional review boards (clinicaltrials.gov identifier: NCT01319539). In addition, we prospectively consented untreated controls and collected their tumor tissue and blood markers in the same manner as MK-2206-treated patients. The goal was to accrue in 2:1, non-randomized manner (MK-2206-treated: control). Eligiblity criteria were the same for MK-2206-treated patients and untreated controls. Patients were presented both options of MK-2206 or control, and they were provided an informed consent in English or Spanish prior to study participation.
Treatment
Enrolled patients received two weekly oral doses of MK-2206 prior to surgery. The timing of MK-2206 dosing was based upon the patient’s surgical date, with the first dose at day −9 (+/− 1 day) and the second dose at day −2 (+/− 1 day) from the date of surgery. MK-2206 was administered 2 hours before or after a meal. The starting dose was 200 mg. Due to observed toxicity, the study was amended on 12/6/11 to allow for a reduction of MK-2206 to 135 mg x 2 doses. It was further stipulated that, if a grade III or IV event occurred at that dose level, MK-2206 would be further lowered to 90 mg x 2 doses. If a grade III or IV event occurred at 90 mg, the trial would be terminated. Grading was based upon Common Terminology Criteria for Adverse Events (CTCAE), v4.0. The National Cancer Institute’s Cancer Therapy Evaluation Program Pharmaceutical Management Branch supplied MK-2206.
Tumor Biomarker Assessment
Formalin-fixed paraffin embedded (FFPE) samples were obtained from the pre-MK-2206 diagnostic core breast biopsies and post-MK-2206 surgical specimens, i.e., surgical excision or mastectomy. It was recommended that up to four cores be collected pre-MK-2206 with a 12-gauge or smaller gauge needle to increase the likelihood for evaluable pre-MK-2206 tissue. To maintain sample quality and ensure that phospho-markers evaluated on FFPE tissue were comparable across samples, all tissues were fixed in ice-cold formalin as soon as possible, given the short half-life of phospho-markers including pAKT11. While pre-biopsies were fixed immediately following biopsy in 10% formalin (<1 minute ischemic time), excisions experienced some delays despite efforts to fix them within 20 minutes of resection, with formalin diffusion rates also being a limiting factor. At the completion of the study, slides and/or blocks were cut at the same time as to ensure appropriate immunogenicity for tumor-based studies. Tissue from untreated prospectively accrued controls was collected in the same manner.
Estrogen receptor (ER), progesterone receptor (PR), and HER2 were assessed, per American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) guidelines12,13. The following immunostains were analyzed on pre- and post-MK-2206 4-μm slides, as previously described14–17: ki-67 (1:200 dilution; DAKO, Carpinteria, CA, M7240), pAKTSer473 (1:30; Cell Signaling, Danvers, MA, #4060), pS6Ser235/236 (1:30, Cell Signaling, #2211), PTEN (1:300, Cell Signaling, #9559), and stathmin (1:50, Cell Signaling, #3352). Tumor proliferation was assessed per the percentage of tumor cells that stained positive for ki-67 without regard to intensity, as per the International ki-67 Working Group recommendations for pre-surgical studies16. When evaluable, 500 malignant invasive cells were scored. Tumor and normal tissue were stained for pAKT (nuclei and cytoplasm), pS6 (cytoplasm, more pronounced at the periphery of tumor), stathmin (cytoplasm), and PTEN (cytoplasm). The stains were scored as: a) percentage of tumor cells stained and b) intensity of the staining (0, 1, 2, 3: no=0, 1=mild, 2=moderate, 3=strong staining intensity). The pathologists (HH and AA) were blinded to whether the tumor tissue was from MK-2206-treated or untreated controls.
We analyzed the surgical specimen for 48 commonly mutated genes using the TruSeq Targeted Cancer Panel, including AKT1, PIK3CA, and PTEN mutations (http://pathology.columbia.edu/diagnostic/PGM/pdf/TruSeq_Gene_List.pdf).
Blood-Based Biomarkers
Fasting serum and plasma samples were collected at baseline (pre-treatment) and the day prior to surgery (post-treatment). The following serum levels in the insulin growth factor (IGF) receptor pathway were evaluated: insulin [Radioimmunoassay (RIA); Millipore Linco Research, Billerica, MA], glucose [Cobas Integra 400 Plus Chemistry-analyzer; Roche Diagnostics, Indianapolis, IN], IGF binding protein-3 (IGFBP-3) [Immulite 1000, Siemens Healthcare Diagnostics, Deerfield, IL], C-reactive protein (CRP) [Cobas], and c-peptide (Immulite). At the same time points, plasma was extracted and MK-2206 quantified for PK analyses. PK assessments were performed using a Liquid Chromatography-Mass Spectrometry (LC-MS/MS) quantification method18.
Data Analysis
The primary endpoint was to assess for a decrease in pAktSer473 levels in tumor tissue after a pre-surgical intervention of MK-2206 in patients with operable invasive breast cancer. With the expectation that 20% of the matched pre- and post-treatment tissue samples would not be analyzable, we planned to enroll 30 patients to ensure that matched tumor samples from 24 patients were available for the primary analysis. A sample size of 24 patients would yield greater than 90% power to detect a difference in the mean score between pre-and-post treatment samples of 60 units (i.e., pre-mean score mean = 120 and post-mean score = 60), assuming the standard deviation of the differences is 60–80 points19, using a paired t-test with a 0.05 two-sided significance level.
Frequency distributions and summary descriptive statistics were used for all biomarkers. All patients were considered evaluable for toxicity following the first MK-2206 dose. Paired t-tests were used to examine drug-related effects for each marker, including changes in tumor- and blood-based biomarkers. A probability of ≤5% was reported as significant. Ninety-five percent confidence intervals were calculated to assess the precision of the obtained estimates. All analyses were performed in SAS Version 9.2 (SAS Institute, Inc., Cary, North Carolina) and STATA Version 9.0 (Stata Corporation, College Station, Texas).
Results
Patient Population
Between September 2011 and March 2013, we accrued 12 patients with newly diagnosed invasive breast cancer to receive MK-2206 (Supplemental Figure 1: Consort Diagram). We prospectively enrolled 6 untreated controls from December 2013 to July 2014. The MK-2206 study was terminated early due to toxicity.
Of the 12 MK-2206 treated patients, 6 patients (50%) were post-menopausal, 7 were Hispanic (59%), 10 had an invasive ductal carcinoma (83%), 6 (50%) had a grade 2 breast tumor, and 7 (59%) underwent a mastectomy (Table 1). All but 1 had an ECOG performance status of 0. Of those who received MK-2206, 8 had hormone receptor (HR)+/HER2− tumors, 3 triple negative, and 1 HR+/HER2+ breast cancer. The 6 patients accrued to the prospective control arm had similar clinical and pathologic features, with the majority of tumors being invasive ductal histology (4 tumors), and all 6 having an HR+/HER2− immunophenotype.
Table 1.
Baseline Patient and Tumor Characteristics of Evaluable MK-2206-Treated Patients and Untreated Controls
| Patient Characteristics | MK-2206 (n=12) | Control (n=6) |
|---|---|---|
|
| ||
| Age (years), mean (SD) | 53.2 (11.2) | 58 (11.9) |
|
| ||
| Menopausal Status, n (%) | ||
| Pre-menopausal | 6 (50%) | 2 (33%) |
| Post-menopausal | 6 (50%) | 4 (67%) |
|
| ||
| Ethnicity, n (%) | ||
| Hispanic | 7 (59%) | 2 (33%) |
| Non-Hispanic White | 4 (33%) | 2 (33%) |
| Non-Hispanic Black | 1 (8%) | 1 (17%) |
| Asian | 0 (0%) | 1 (17%) |
|
| ||
| Tumor Characteristics | ||
|
| ||
| Initial Histology, n (%) | ||
| Invasive Carcinoma | ||
| Invasive Ductal Carcinoma | 10 (83%) | 4 (67%) |
| Invasive Lobular Carcinoma | 2 (17%) | 2 (33%) |
|
| ||
| Initial Size (cm) mean (SD) | ||
| Invasive Carcinoma | 2.5 (1.1) | 3.0 (1.4) |
|
| ||
| Immunophenotype, n (%) | ||
| HR+/HER2−1 | 8 (67%) | 6 (100%) |
| HR+/HER2+ | 1 (8%) | 0 (0%) |
| Triple Negative | 3 (25%) | 0 (0%) |
|
| ||
| Grade, n (%) | ||
| I | 2 (17%) | 1 (17%) |
| II | 6 (50%) | 4 (66%) |
| III | 4 (33%) | 1 (17%) |
|
| ||
| Type of surgery, n (%) | ||
| Lumpectomy | 5 (41%) | 2 (33%) |
| Mastectomy | 7 (59%) | 4 (67%) |
SD=Standard Deviation, HR=Hormone Receptor, HER2=Human Epidermal Growth Factor Receptor 2, TN=Triple Negative
Adverse Events
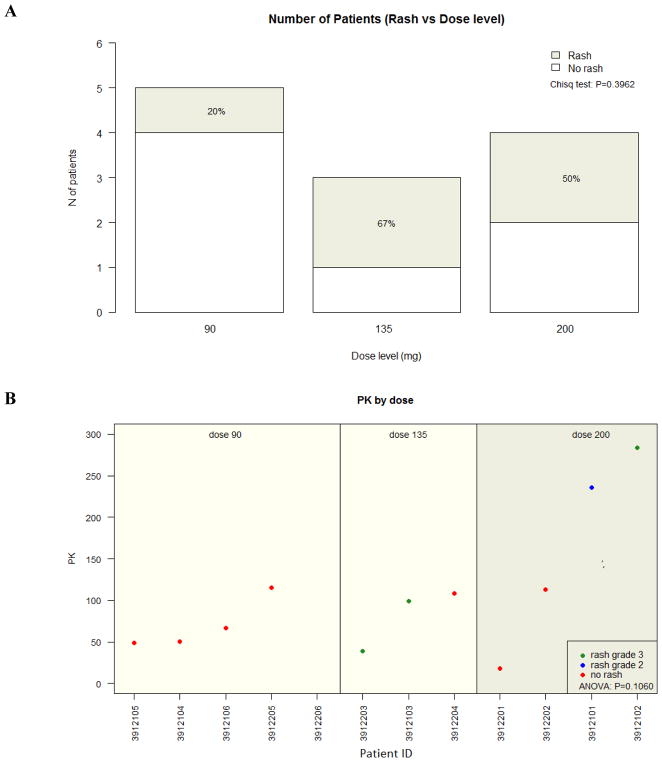
Of the MK-2206-treated patients, 4 patients received 200 mg doses of weekly MK-2206, 3 received 135 mg doses, and 5 received 90 mg doses. At the 200 mg dose, 1 patient experienced a grade III maculo-papular rash and 2 patients had grade III pruritus (Table 2). Other events at this dose include mucositis [grade I (n=1), grade II (n=2)], hyperglycemia [grade I (n=1) and grade II (n=1)], grade II rash (n=1), and grade II fever (n=2). Patient #4 experienced an 11-day delay to surgery due to rash and pruritus. All other MK-2206-treated patients went to surgery 2 days after the second MK-2206 dose, except for 2 patients unrelated to adverse events (patient #4 and #7: 4 and 2 days after MK-2206, respectively). As 2 patients experienced a grade III rash and 1 patient had grade III pruritus at 135 mg, the dose level was further reduced to 90 mg x 2 doses. Due to a grade III rash at the 90 mg dose, the trial was terminated to accrual. In total, 5 patients experienced mucositis (42%), 5 rash (42%), 4 pruritus (33%), and 3 hyperglycemia (25%). No grade IV events were observed. The distribution of skin rash by dose level is demonstrated in Figure 1A.
Table 2.
MK-2206 Adverse Events
| Dose Level (# of Patients) | Adverse Event | Grade I | Grade II | Grade III |
|---|---|---|---|---|
| 200 mg (4) | ||||
| Mucositis | 1 | 2 | ||
| Rash | 1 | 1 | ||
| Pruritus | 2 | |||
| Hyperglycemia | 1 | 1 | ||
| Fever | 1 | |||
| 135 mg (3) | ||||
| Rash | 2 | |||
| Pruritus | 1 | 1 | ||
| 90 mg (5) | ||||
| Mucositis | 1 | 1 | ||
| Rash | 1 | |||
| Hyperglycemia | 1 | |||
| Dry Skin | 2 | |||
| Total (12) | 6 | 7 | 7 | |
Figure 1.
MK-2206 Dose Level and Rash. (A) There was no association between the rate of any grade rash from MK-2206 and dose (p=0.40). All rash events were grade III, except 1 grade II event at the 200 mg dose. (B) There was no association between grade II or III rash and the pharmacokinetic (PK) level collected after the second MK-2206 dose (p=0.11). The PKs reflected for each patient were collected the day prior to the patient’s surgery.
Changes in PI3K/Akt pathway signaling and tumor proliferation
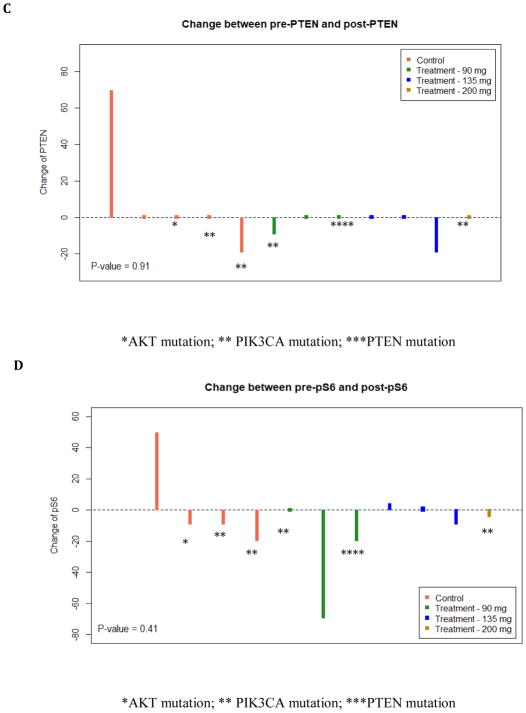
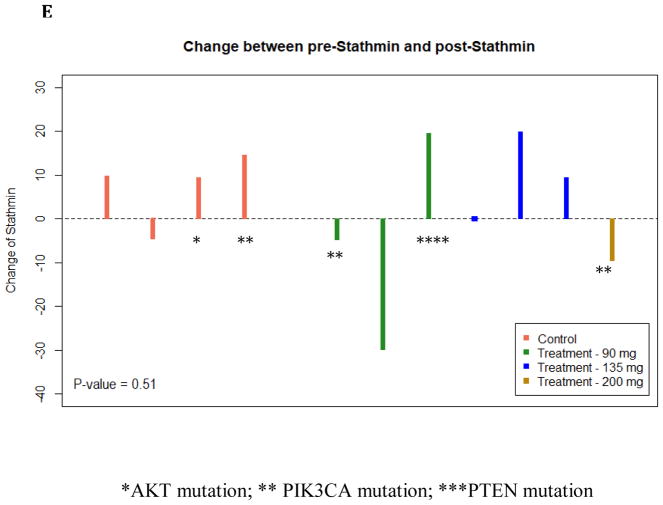
Of the 12 MK-2206 treated patients and 6 control patients, there were 7 MK-2206 and 5 control patients with evaluable matched core and surgical specimen tissue. While all breast tumor subtypes were eligible, all of the patients with evaluable tissue had HR+/HER2- breast cancer, except for 1 patient with a triple negative tumor treated with MK-2206. The primary reason for unevaluable tissue was limited matched core and surgical specimens with invasive cancer. For pS6 and stathmin, only 4 controls are available. There was a trend to a reduction in pAKT after MK-2206 (p=0.06): Table 3. However, a trend in pAKT reduction was also identified in the control group (p=0.09). Thus, compared to control, there was no significant difference change in pAKT in those treated with MK-2206 (p=0.65). There was no significant change for other tumor markers assessing expression before and after MK-2206: ki-67 (p=0.59), pS6 (p=0.20), PTEN (p=0.20), and stathmin (p=0.92). In addition, there was no significant difference in the following markers comparing the change in those treated with MK-2206 to untreated controls: ki-67 (p=0.47), pS6 (p=0.41), PTEN (p=0.91), and stathmin (p=0.51) (Figure 2).
Table 3.
Changes in Tumor-Based Markers in Evaluable MK-2206-Treated Patients (n=7) and Untreated Controls (n=5)
| Variable | Mean treatment Pre-MK-2206 Core (SD) | Mean treatment Post-MK-2206 Surgery (SD) | Paired t test p-valuea | Mean Control Core (SD) | Mean Control Surgery (SD) | Paired t test p-valuea | Overall t test p-valueb |
|---|---|---|---|---|---|---|---|
| Ki-67 (%) | 13 (23) | 12 (23) | 0.59 | 12 (16) | 8 (7) | 0.37 | 0.47 |
| PAKT (%) | 25 (29) | 0 (0) | 0.06** | 35 (35) | 1 (1) | 0.09** | 0.65 |
| pS6 (%) | 41 (36) | 26 (31) | 0.20 | 45 (24) | 48 (28) | 0.89 | 0.41 |
| PTEN (%) | 83 (11) | 79 (15) | 0.20 | 68 (33) | 78 (11) | 0.55 | 0.91 |
| Stathmin (%) | 41 (22) | 42 (26) | 0.92 | 46 (32) | 52 (31) | 0.18 | 0.51 |
Paired T-test comparing changes within group (example: pre-MK-2206-treated core versus post-MK-2206-treated surgical tissue)
Two-sided t-test comparing changes between MK-2206-treated samples versus control
Paired t-test, significance defined as p < 0.05
Paired t-test, trend toward significance
Figure 2.
Change in Tumor-Based Markers between MK-2206-treated patients and Untreated Controls. The vertical bar represents the protein expression change by immunohistochemistry (IHC) from surgical excision tissue to core biopsy in each treated patient. There was no difference observed in ki-67 (A), pAKT (B) PTEN (C), pS6 (D), and stathmin (E). Untreated control patients are illustrated (left) and MK-2206-treated patients (right) by dose. Mutations in the PI3K/AKT pathway are identified by asterix (*AKT mutation; ** PIK3CA mutation; ***PTEN mutation).
Changes in blood-based biomarkers
All 12 MK-2206 and 5 control patients had baseline and pre-surgical fasting serum collected. Compared to controls, there was a significant increase in the following levels in those treated with MK-2206 (Table 4): insulin (p < 0.01), increase in glucose (p=0.02), increase in C-peptide (p < 0.01), and a trend to an increase in IGFBP-3 (p=0.06). While there was a numerical increase in c-reactive protein after MK-2206 (+7 mg/L), there was also an increase observed in the control arm (+5 mg/L), resulting in no difference between the two arms (p=0.84). There was no association between change in any blood marker and development of rash, p>0.05 (data not shown).
Table 4.
Changes in Blood Markers (N=12) in MK-2206 Treated Patients and Prospectively Accrued Untreated Controls (n=5)
| Variable | Mean Treatment Pre-MK-2206 (SD) | Mean Treatment Post-MK-2206 (SD) | Paired t test p-valuea | Mean Control Baseline (SD) | Mean Control Level at Surgery (SD) | Paired t test p-valuea | Overall t test p-valueb |
|---|---|---|---|---|---|---|---|
| Insulin (uIU/mL) | 10 (7) | 41 (27) | <0.01* | 9 (6) | 7 (5) | 0.41 | <0.01* |
| Glucose (mg/dL) | 89 (19) | 116 (43) | <0.01* | 82 (19) | 80 (19) | 0.80 | 0.02* |
| C-Peptide (ng/mL) | 2 (1) | 6 (3) | <0.01* | 2 (1) | 2 (1) | 0.43 | <0.01* |
| C-Reactive Protein (mg/L) | 4 (4) | 11 (17) | 0.17 | 3 (3) | 8 (14) | 0.42 | 0.84 |
| IGFBP-3 (ug/mL) | 4 (1) | 5 (2) | 0.08** | 4 (1) | 4 (0.4) | 0.31 | 0.06** |
IGF BP-3=I nsul in-Like Gro wth Factor Binding Protein 3
Paired T-test comparing changes within group (example: pre-MK-2206-treated core versus post-MK-2206-treated surgical tissue)
Two-sided t-test comparing changes between MK-2206-treated samples versus control
Paired t-test, significance defined as p < 0.05
Paired t-test, trend toward significance
Mutation Detection
Of the 12 MK-2206-treated patients, 7 patients had enough available tissue to perform mutation analysis and had available matched phospho-marker data. Two patients had a PIK3CA mutant tumor: 1) H1047R and 2) E545K, along with a KRAS12G mutation. One evaluable patient had a PTEN mutation, along with ERBB2, KIT, and p53 mutations. Of the untreated controls, 3 had mutations in the PI3K/AKT pathway: 1) AKT1 E17K mutation, 2) H1047R PIK3CA mutation, and 3) Dual H1047R and E542K mutations. Given the small cohort, it was not possible to evaluate differences in phospho-marker changes between those with an activating mutation in the PI3K pathway vs. other mutations (highlighted in Figure 2).
Pharmacokinetic analysis
PK assessment was performed on plasma samples from baseline and the day prior to surgery for MK-2206 treated patients (n=12) and controls (n=5, as 1 patient did not serum/plasma available for blood-based biomarker analysis). MK-2206 PK parameters, including elimination and absorption, have been previously described20,21. As expected, MK-2206 was not detected in any baseline samples for the MK-2206 cohort or baseline/pre-surgery plasma for the controls. One patient at the 90 mg dose did not have detectable post-MK2206 levels and was censored, as this may be due to insufficient quantity. Figure 1B demonstrates that there was no significant association between dose level and PK assessed after the second MK-2206 tablet (p=0.11).
Conclusion
In this pre-surgical trial, we observed a significant reduction in pAKT in breast primary tumor tissue after 2 doses of weekly MK-2206. A significant change was not identified in other PI3K/Akt pathway markers or tumor proliferation after MK-2206. Of note, we also identified a significant reduction in pAKT in prospectively enrolled controls, leading to no significant difference when comparing pAKT change in MK-2206-treated vs. untreated patients. However, compared to controls, we observed a significant increase in blood-based biomarkers, including insulin, glucose, C-peptide, and trend to an increase in IGFBP-3. Importantly, the trial was terminated early due to grade III toxicity, despite 2 dose reductions, demonstrating the lack of tolerability of MK-2206 in patients with operable breast cancer.
Window of opportunity trials with other inhibitors in the PI3K/AKT pathway have been published. In the OPPORTUNE trial, 70 patients with operable breast cancer were randomized 2 weeks of the PI3K inhibitor GDC-0941 plus anastrazole vs. anastrazole alone22. In this study, there was a significant greater reduction in ki-67, with the combination, particularly for luminal B tumors. This finding is notable, given that ki-67 modulation in pre-operative studies involving hormone therapies has predictive potential23–25. While we did not observe a difference in tumor proliferation with MK-2206, this may be due to the limited accrual due to the side effect profile. However, this is consistent with a neoadjuvant trial of MK-2206 plus endocrine therapy in PIK3CA mutant breast cancer, in which the combination did not suppress ki-67 more than endocrine therapy alone26. Of note, in the FERGI trial27, no significant benefit was identified when adding GDC-0941 to hormone therapy in the metastatic setting. While this may potentially be due to the dose adjustments and discontinuations with a longer duration of GDC-0941, the positive signal identified in the operable signal did not translate to clinical benefit in the metastatic setting.
Treating patients with two doses of weekly single-agent MK-2206 was not feasible in our pre-surgical trial. In a phase I trial of MK-2206 plus hormone therapy in metastatic endocrine-sensitive breast cancer, 5/30 patients discontinued weekly MK-2206 due to rash: 4 patients within the first 28 days of drug initiation5. After 9 patients, prophylactic prednisone was given the day before, on, and after MK-2206. Among the intent to treat population, the clinical benefit rate was 36.7%, including 2 with a partial response (PR) and 9 with stable disease (SD) for at least 6 months. Ultimately, the recommended phase II dose was 150 mg with prophylactic steroids. In a phase 1b trial with paclitaxel in metastatic breast cancer, MK-2206 was given weekly, the day after paclitaxel6. With paclitaxel, dexamethasone was given on week 1 and then dose reduced and discontinued if no infusion reaction occurred. Again, rash was the most concerning side effect. Of the 21 patients who received treatment, 5 had a PR and 9 SD. In another phase 1b of weekly MK-2206 plus paclitaxel plus trastuzumab in HER-amplified tumors, grade II or higher rash was seen in 4/16 patients7. Of the 16 patients, 3 achieved a complete response and 7 had a PR. In a neoadjuvant trial of MK-2206 plus endocrine therapy, 150 mg of MK-2206 was administered with prophylactic prednisone; however, toxicities, such as rash, were observed26. Though this trial included only those with a PIK3CA mutant tumor, no pathologic complete responses were observed. In our pre-surgical trial, prophylactic steroids were not given, and it is unclear whether tolerability would have been better in the pre-operative setting with steroid administration.
Beyond tumor proliferation, we evaluated whether there was a significant reduction in PI3K/AKT pathway markers after MK-2206 administration. Of the tumor biomarkers evaluated, we only identified a reduction in pAKT (p=0.09). However, a reduction trend was also seen in the control arm (p=0.06). This finding stresses the importance of including a control arm when assessing for biomarker modulation. In pre-clinical models, MK-2206 not only leads to a reduction in pAKT levels but also other targets in the PI3K pathway, including pS64. While there was a numerical decline in pS6 in our pre-surgical trial, this reduction did not reach statistical significance (p=0.20); however, this may be limited by the small sample size. Given the short half life of phospho-markers28, 29, including pAKT, careful pre-analytic considerations were made. We mandated that tumors be placed quickly in ice-cold formalin from the time of de-vascularization. Also, all tumor slides were cut within a few weeks of each other to ensure appropriate immunogenicity for tumor biomarkers. Differences in phosopho-marker expression between core needle biopsy tissue and surgical specimens have been reported, likely due to tissue handling during surgery and cold ischemia time after tissue resection28, 29. The fact that all post-MK-2206 tissue is from main surgical specimens may explain the pAKT reduction observed, including in the control arm. We suggest that future post-treatment core needle biopsies be obtained for comparison to pre-treatment core needle biopsies to avoid this potential limitation in future pre-surgical trials.
Compared to controls, we observed an increase in serum levels associated with the insulin growth factor pathway, including insulin, glucose, and a trend to IGFBP-3. We did not observe a relationship of these levels with rash. The sample size is too small to evaluate for tumor biomarker changes. Inhibitors of the PI3K/Akt pathway have been associated with hyperglycemia due to mechanisms including AKT2 mediating insulin-induced translocation of the glucose transporter isoform 4 (GLUT4) glucose transporter and modulation of the negative feedback loop with upstream insulin receptor substrate proteins1. As there a number of AKT inhibitors that remain in clinical development1, identifying predictors of response and resistance remains critical, including potentially blood-based markers such as changes in the insulin growth factor pathway.
The main limitations of this trial have been previously described: 1) small sample size due to drug toxicity and 2) comparison of pre-MK-2206 core biopsy tissue to post-MK-2206 surgical resection tissue, as opposed to core biopsy. In pre-clinical work, the presence of an activating PIK3CA mutation or PTEN loss associates with response to MK-22064. Clinical benefit has been reported in patients received the ATP-competitive pan-AKT kinase inhibitor AZD5363 in patients with AKT E17K mutated solid tumors30. In our pre-surgical trial, we performed mutation detection; however, due to the small sample size, we are unable to evaluate for differences in biologic response in these patients compared to those with PIK3CA wild type tumors.
While we identified that MK-2206 inhibited the proposed targeted, pAKT, we observed a reduction in pAKT in the prospectively accrued untreated controls as well. In future pre-surgical trials, pre-analytic concerns should be highly considered, including comparing pre-treatment core biopsies with post-treatment core tissue. In patients with operable breast cancer, the toxicity of MK-2206 was greater than expected. Window of opportunity trials remain an important way of identifying in vivo activity in early drug development; however, potential side effects should be monitored closely in this healthy patient population.
Supplementary Material
Consolidated Standards of Reporting Trials diagram for the Pre-Surgical MK-21106 Trial
Immunohistochemistry (IHC) at 200x magnification view: pAKT IHC with invasive carcinoma with nuclear positivity (brown stain) noted in pre-treatment specimen (A) but not in post treatment specimen (B). pAKT similarly stained biopsy control (C) and not excision control (D)suggesting degradation secondary to processing precluding evaluation. Ki-67 IHC shows nuclear (brown) staining in tumor cells in pre (E) with slight reduction in post control specimens (F) as well as in pre (G) and post treatment specimens (H) suggesting no significant change in Ki-67 between them.
Acknowledgments
Research Support:
Women at Risk funded this research. This work is also supported by contract N01-CM-62204 to the New York Cancer Consortium (PI: Joseph A. Sparano) from the National Institutes of Health. Additionally, this publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number KL2 TR000081. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors would like to report the following conflicts of interest: KK (Advisory: Biotheranostics, Lilly, Pfizer, Amgen, Novartis, Eisai), MM (Stock and Employment: Bristol-Myers Squibb)
Research Involving Human Participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Mundi PS, Sachdev J, McCourt C, et al. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol. 2016;82:943–56. doi: 10.1111/bcp.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kandoth C, McLellan MD, Vandin F, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalinsky K, Jacks LM, Heguy A, et al. PIK3CA mutation associates with improved outcome in breast cancer. Clin Cancer Res. 2009;15:5049–59. doi: 10.1158/1078-0432.CCR-09-0632. [DOI] [PubMed] [Google Scholar]
- 4.Sangai T, Akcakanat A, Chen H, et al. Biomarkers of response to Akt inhibitor MK-2206 in breast cancer. Clin Cancer Res. 2012;18:5816–28. doi: 10.1158/1078-0432.CCR-12-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma CX, Sanchez C, Gao F, et al. A Phase I Study of the AKT Inhibitor MK-2206 in Combination with Hormonal Therapy in Postmenopausal Women with Estrogen Receptor-Positive Metastatic Breast Cancer. Clin Cancer Res. 2016;22:2650–8. doi: 10.1158/1078-0432.CCR-15-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Angulo AM, Krop I, Akcakanat A, et al. SU2C phase Ib study of paclitaxel and MK-2206 in advanced solid tumors and metastatic breast cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien AJ, Cockerill A, Fancourt C, et al. A phase 1b study of the Akt-inhibitor MK-2206 in combination with weekly paclitaxel and trastuzumab in patients with advanced HER2-amplified solid tumor malignancies. Breast Cancer Res Treat. 2016;155:521–30. doi: 10.1007/s10549-016-3701-7. [DOI] [PubMed] [Google Scholar]
- 8.Hudis C, Swanton C, Janjigian YY, et al. A phase 1 study evaluating the combination of an allosteric AKT inhibitor (MK-2206) and trastuzumab in patients with HER2-positive solid tumors. Breast Cancer Res. 2013;15:R110. doi: 10.1186/bcr3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisinski KB, Tevaarwerk AJ, Burkard ME, et al. Phase I Study of an AKT Inhibitor (MK-2206) Combined with Lapatinib in Adult Solid Tumors Followed by Dose Expansion in Advanced HER2+ Breast Cancer. Clin Cancer Res. 2016;22:2659–67. doi: 10.1158/1078-0432.CCR-15-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalinsky K, Zheng T, Hibshoosh H, et al. Proteomic modulation in breast tumors after metformin exposure: results from a “window of opportunity” trial. Clin Transl Oncol. 2016 doi: 10.1007/s12094-016-1521-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker AF, Dragovich T, Ihle NT, et al. Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res. 2005;11:4338–40. doi: 10.1158/1078-0432.CCR-05-0422. [DOI] [PubMed] [Google Scholar]
- 12.Hammond ME, Hayes DF, Wolff AC. Clinical Notice for American Society of Clinical Oncology-College of American Pathologists Guideline Recommendations on ER/PgR and HER2 Testing in Breast Cancer. Journal of Clinical Oncology. 2011;29:e458. doi: 10.1200/JCO.2011.35.2245. [DOI] [PubMed] [Google Scholar]
- 13.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 14.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 15.Saal LH, Johansson P, Holm K, et al. Poor prognosis in carcinoma is associated with a gene expression signature of aberrant PTEN tumor suppressor pathway activity. Proc Natl Acad Sci U S A. 2007;104:7564–9. doi: 10.1073/pnas.0702507104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dowsett M, Nielsen TO, A’Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–64. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalinsky K, Crew KD, Refice S, et al. Presurgical trial of metformin in overweight and obese patients with newly diagnosed breast cancer. Cancer Invest. 2014;32:150–7. doi: 10.3109/07357907.2014.889706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piovan E, Yu J, Tosello V, et al. Direct reversal of glucocorticoid resistance by AKT inhibition in acute lymphoblastic leukemia. Cancer Cell. 2013;24:766–76. doi: 10.1016/j.ccr.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guix M, de Granja NM, Meszoely I, et al. Short preoperative treatment with erlotinib inhibits tumor cell proliferation in hormone receptor-positive breast cancers. J Clin Oncol. 2008;26:897–906. doi: 10.1200/JCO.2007.13.5939. [DOI] [PubMed] [Google Scholar]
- 20.Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–95. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 21.Yap TA, Yan L, Patnaik A, et al. Interrogating two schedules of the AKT inhibitor MK-2206 in patients with advanced solid tumors incorporating novel pharmacodynamic and functional imaging biomarkers. Clin Cancer Res. 2014;20:5672–85. doi: 10.1158/1078-0432.CCR-14-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid P, Pinder SE, Wheatley D, et al. Phase II Randomized Preoperative Window-of-Opportunity Study of the PI3K Inhibitor Pictilisib Plus Anastrozole Compared With Anastrozole Alone in Patients With Estrogen Receptor-Positive Breast Cancer. J Clin Oncol. 2016;34:1987–94. doi: 10.1200/JCO.2015.63.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–70. doi: 10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 24.Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 25.Kalinsky K, Hershman DL. Cracking open window of opportunity trials. J Clin Oncol. 2012;30:2573–5. doi: 10.1200/JCO.2012.42.3293. [DOI] [PubMed] [Google Scholar]
- 26.Ma CX, Suman V, Goetz MP, et al. A Phase II Trial of Neoadjuvant MK-2206, an AKT Inhibitor, with Anastrozole in Clinical Stage II or III PIK3CA-Mutant ER-Positive and HER2-Negative Breast Cancer. Clin Cancer Res. 2017;23:6823–6832. doi: 10.1158/1078-0432.CCR-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krop IE, Mayer IA, Ganju V, et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–821. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinhel IF, Macneill FA, Hills MJ, et al. Extreme loss of immunoreactive p-Akt and p-Erk1/2 during routine fixation of primary breast cancer. Breast Cancer Res. 2010;12:R76. doi: 10.1186/bcr2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meric-Bernstam F, Akcakanat A, Chen H, et al. Influence of biospecimen variables on proteomic biomarkers in breast cancer. Clin Cancer Res. 2014;20:3870–83. doi: 10.1158/1078-0432.CCR-13-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyman DM, Smyth LM, Donoghue MTA, et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. J Clin Oncol. 2017;35:2251–2259. doi: 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Consolidated Standards of Reporting Trials diagram for the Pre-Surgical MK-21106 Trial
Immunohistochemistry (IHC) at 200x magnification view: pAKT IHC with invasive carcinoma with nuclear positivity (brown stain) noted in pre-treatment specimen (A) but not in post treatment specimen (B). pAKT similarly stained biopsy control (C) and not excision control (D)suggesting degradation secondary to processing precluding evaluation. Ki-67 IHC shows nuclear (brown) staining in tumor cells in pre (E) with slight reduction in post control specimens (F) as well as in pre (G) and post treatment specimens (H) suggesting no significant change in Ki-67 between them.