Abstract
Background
Dentatorubral-pallidoluysian atrophy (DRPLA) is a rare, autosomal dominantly inherited disorder characterized by myoclonus, epilepsy, ataxia, and dementia. Diagnosis is challenging due to the heterogeneous presentation and symptomatic overlap with other spinocerebellar ataxias. Symptoms vary according to age of onset, with a mean age at onset of 31 years. A CAG repeat expansion in the ATN1 gene results in neuronal intranuclear inclusions, variable neuronal loss, and astrocytosis in the globus pallidus, dentate and red nuclei. No disease-modifying or curative treatments are currently available
Methods
We performed an online literature search using PubMed for all articles published in an English Language format on the topics of DRPLA or ATN1 over the last 10 years. Where these articles cited other research as support for findings, or statements, these articles were also reviewed. Contemporary articles from related research fields (e.g., Huntington’s Disease) were also included to support statements.
Results
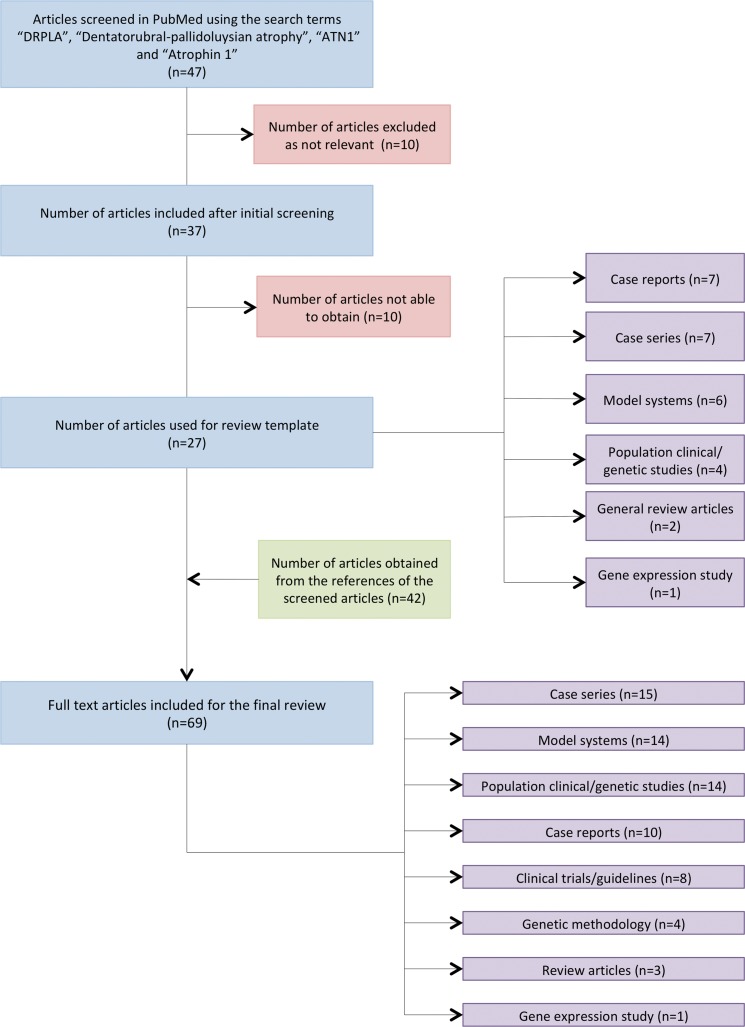
Forty-seven articles were identified, 10 were unobtainable and 10 provided no relevant information. The remaining 27 articles were then used for the review template: seven case reports, seven case series, six model system articles (one review article), four population clinical and genetic studies (one review article), two general review articles, and one human gene expression study. Other cited articles or research from related fields gave a further 42 articles, producing a total of 69 articles cited: 15 case series (including eight family studies), 14 model systems (one review article), 14 population clinical and genetic studies (two review articles), 10 case reports, eight clinical trials/guidelines, four genetic methodology articles, three general review articles, and one human gene expression study.
Discussion
DRPLA remains an intractable, progressive, neurodegenerative disorder without effective treatment. Early recognition of the disorder may improve patient understanding, and access to services and treatments. Large-scale studies are lacking, but are required to characterize the full allelic architecture of the disorder in all populations and the heterogeneous phenotypic spectrum, including neuroimaging findings, possible biomarkers, and responses to treatment.
Keywords: DRPLA, ATN1, trinucleotide, polyglutamine, myoclonus, ataxia, epilepsy, dementia
Introduction
Dentatorubral-pallidoluysian atrophy (DRPLA) (OMIM 125370) is characterized by symptoms such as myoclonus, epilepsy, ataxia, choreoathetosis, and dementia that are variable.1,2 DRPLA is one of a group of autosomal dominant, hereditary ataxias, and is caused by a CAG trinucleotide repeat expansion (≥48 tandem copies) in the Atrophin-1 (ATN1) gene.3–5 DRPLA was initially recognized in Asian populations, although wider genetic testing has increased diagnosis in other ethnic groups.6–8 Clinical presentation is frequently heterogeneous and typically varies dependent on age at onset: younger onset individuals often present with seizures, and older individuals more commonly present with ataxia and cognitive impairment. Clinical symptoms are progressive, with life expectancy typically 8–16 years from symptom onset.1,9 At present, no disease-modifying or curative therapies exist, with current treatment paradigms involving supportive care provided by multidisciplinary teams.3,10 As a rare disorder there are a lack of publications: only two general review articles (published in 2010 and 2016)1,2 and no systematic review articles. This systematic review provides an update of the understanding of the clinical phenotype, underlying disorder molecular and mechanistic pathophysiology, and future therapeutic direction.
Methods
We performed a systematic literature search of the PubMed database using the key words “DRPLA”, “dentatorubral-pallidoluysian atrophy”, “ATN1”, and “Atrophin 1” (Table 1). All articles published in English and in peer-reviewed journals, print or online books over the last 10 years were included. We also checked the reference lists of each relevant study that resulted from this search for further appropriate articles. Older articles and research articles from other fields were also cited where appropriate to support findings or statements. Forty-seven articles were identified of which we were able to access 37. Of the 37 articles, 10 were excluded due to lack of relevance to the clinical or molecular pathological aspects of DRPLA, resulting in 27 remaining articles. Supportive articles cited within these 27 core articles were also reviewed and included, together with contemporary research from DRPLA related fields (e.g., Huntington’s disease [HD]), giving a total of 69 articles included in this review (Figure 1). The articles are 15 case series (including eight family studies), 14 model systems (one review article), 14 population clinical and genetic studies (two review articles), 10 case reports, eight clinical trials/guidelines, four genetic methodology articles, three general review articles, and one human gene expression study.
Table 1. Clinical Characteristics of Cohort Studies and Case Series (N≥2) 2008–2018.
| Author | Year | Cohort Description (Male: Female) | Age at Symptom Onset (Range in Years) | CAG Repeat Length (Range) | Clinical Characteristics (No. of Cases with Each Reported Symptom) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ataxia | Choreoathetosis | Extrapyramidal Signs | Cognitive Impairment | Seizures | Psychiatric Symptoms | |||||
| Sugiyama et al.14 | 2018 | 10 (4:6) | 61–71 | 52–58 | 10 | 1 | – | 5 | 1 | 2 |
| Sone et al.53 | 2015 | 4 (1:3) | 9–23 | 60–67 | 4 | – | 1 | 3 | 4 | – |
| Maruyama et al.11 | 2012 | 9 (6:3) | 0.5–12 | 62–93 | 5 | 5 | 5 | – | 9 | 3 |
| Yoon et al.54 | 2012 | 3 (1:2) | 37–42 | 59–62 | 3 | – | – | 1 | 1 | – |
| Aridon et al.70* | 2012 | 10 (8:2) | 13–48 | 58–65 | 9 | 2 | – | – | 6 | 2 |
| Sunami et al.46* | 2011 | 2 (2:0) | 19–56 | 65–70 | 2 | 1 | – | 2 | 1 | 2 |
| Hasegawa et al.9 | 2010 | 183 (82:101) | 0–72 | 56–82 | 156 | 78 | – | 124 | 98 | 45 |
| Egawa et al.56 | 2008 | 17 (9:8) | 2–18 | 64–76 | – | – | – | ≥11 | 17 | – |
| Wardle et al.7 | 2008 | 17 (11:6) | 29–46 | 51–66 | 9 | 6 | 1 | 11 | 9 | 3 |
Single family study.
–, No Reported Cases.
Figure 1. Flow Diagram Summarizing the Steps Involved in the Literature Search.
Case descriptions
Here we provide two examples of clinically typical younger- and older-onset cases
Older-onset case
A 31-year-old Caucasian male presented to the neurology outpatient clinic with a 2-year history of progressive gait disturbance, principally manifesting as unsteadiness and difficulty with balance. More recently he had begun to fall and had started to use a single stick for support when outside the home. In addition to this, his partner had also commented that he was generally more fidgety than normal, with a tendency to move his hands and feet involuntarily when watching the television. He had no past medical history of note and was taking no regular medication. He drank occasionally, was a non-smoker, and had never used recreational drugs. There was a family history of similar symptoms, with both his father and paternal uncle developing atypical movements and memory impairment in their mid-30s, progressing to being wheelchair bound by their early 40s. On examination there was evidence of subtle choreiform movements involving the limbs and perioral region. There was a full range of pursuit eye movements, although saccades were impaired, and there was evidence of both vertical and horizontal nystagmus. Finger–nose, heel–shin, and tandem gait testing revealed clear evidence of cerebellar ataxia. Subsequent investigations included, among other tests, cerebral magnetic resonance imaging (MRI) that demonstrated atrophy of the cerebellum, and to a lesser extent the basal ganglia. Given the family history, genetic testing was undertaken, which initially included the spinocerebellar ataxias (SCA1, 2, 3, 6, 7, and 17) and HD. No mutation was identified with these tests, leading to subsequent testing of the ATN1 gene.
Younger-onset case
A 12-year-old female presented to her local emergency department with a sustained generalized seizure, having no prior reported seizure history, and was not taking any regular medication at the time. She was currently under long-term review with a local pediatrician because of some evidence of delayed developmental milestones during early childhood, and more recently the development of disruptive behavior in the classroom, as well as progressive difficulties with academic work. Her mother also described infrequent episodes that may have been consistent with visual hallucinations. The patient had no ongoing contact with her father, but her mother was aware of a paternal family history of dementia and progressive gait difficulties. Following post-ictal recovery, the patient was generally found to be withdrawn with a reluctance to engage in the examination. There were occasional brief jerks, consistent with a spontaneous myoclonus, predominantly visible with outstretched arms. The only other clinical finding of note was disrupted horizontal saccades during eye movement examination. Brain MRI demonstrated evidence of a few periventricular white matter hyperintensities, visible bilaterally, but no marked focal atrophy. Subsequent investigation of the family history found that her father had been found to have a CAG repeat expansion (65 repeats) of the ATN1 gene.
Results
Clinical findings
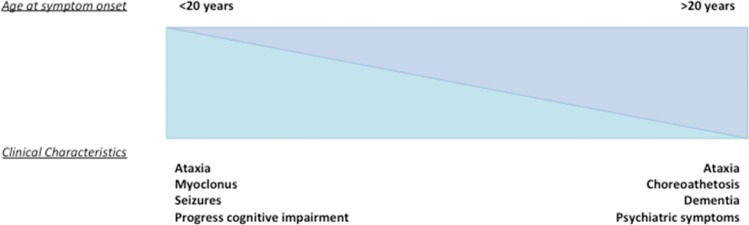
DRPLA has a median age at onset of 31 years of age and demonstrates no sex bias, affecting males and females equally. Ataxia and cognitive impairment are cardinal features of the disorder; however, age at onset affects clinical presentation.1,6,9 Typical clinical features of adult-onset disease (≥20 years of age) include ataxia, cognitive impairment, and choreoathetosis with median age at onset for these presentations of 38–43 years.1,6,9 Epilepsy and intellectual disability are more common in those with juvenile-onset symptoms, with epilepsy having a median age at onset of 15–19 years, dependent upon the study.7,9,11 This clinical separation is also observed in juvenile-onset and adult-onset HD, where the juvenile onset form tends to present with rigidity, seizures, and cognitive decline as opposed to the chorea that is the hallmark of the adult form.12 The length of the CAG repeat expansion in DRPLA within the ATN1 gene correlates strongly with age at onset (r2 = 0.62, r = 0.795, p<0.001), with altered clinical phenotype and life expectancy, with longer repeats being linked to earlier onset and more severe disease phenotype.1,6,9 Mean age at death is 49 years (range 18–80 years), median time from disease onset to death is 15 years, and life expectancy is inversely correlated with CAG repeat length (r = –0.89, p<0.001).9 Functional impairment is also inversely correlated with CAG repeat length, such as the age at which an individual becomes wheelchair bound (r = –0.87, p<0.001) or artificial feeding is required (r = –0.9118, p = 0.0013).6,9,11 In contrast, there is no direct relationship between CAG repeat length and the rate of disease progression.9 Pneumonia and less commonly status epilepticus represent the most common causes of death.9 A summary of common clinical characteristics and their frequencies in patient populations is shown in Table 1.
Clinical diagnosis is frequently challenging, with case reports illustrating confounding factors such as family history of another neurodegenerative disease13 or non-specific age-associated neuroimaging findings.14 In childhood, features of delayed developmental milestones and seizures have a broad differential diagnosis, while in adolescence and early adulthood psychiatric symptoms such as irritability, depression, and psychosis may be the presenting features.15–19 Late-onset DRPLA presented as isolated cerebellar ataxia in 50% individuals and ataxia with or without dementia in 70% individuals in a recent elderly-onset (>60) case series.14 Differential diagnosis is therefore broad, making diagnosis of DRPLA difficult in the absence of a known family history of the disease. In addition, neuroimaging findings can be non-specific,14 with clinical mimics including HD and the genetically determined SCAs (e.g., SCA1, 2, 3, and 7).13,20
Longer CAG repeat lengths of ≥65 are often found in patients with a progressive myoclonus epilepsy (PME) phenotype of DRPLA consisting of generalized seizures, myoclonus, and progressive intellectual disability, and these individuals typically present before the age of 20 (juvenile-onset DRPLA).6,9,11 In a recent case series with partially complete phenotypic data for nine individuals with repeat lengths ≥62 and onset before 13 years of age, nine had epilepsy, eight had intellectual disability, and six exhibited myoclonus.11 Patients with the adult-onset (age ≥ 20) or non-PME phenotype invariably harbor shorter repeat lengths of <65 repeats and present with ataxia, choreoathetosis, dementia, and psychiatric features, with seizures being a rare presenting complaint as shown in reviews of DRPLA cases of non-Asian6 and supported by significant differences between the above clinical features between <65 and ≥65 repeat allele carriers of Asian ancestry9 (Figure 2). In addition to genetic testing to confirm diagnosis, a contemporary study found that the age at which hypoalbuminemia occurred in DRPLA patients inversely correlated with CAG length in eight of nine individuals (p<0.01), indicating a potentially useful biomarker for future clinical trials.21
Figure 2. Schematic Representation of the Differences in Clinical Symptomatology with Variation in Age at Onset. This is a schematic representation of the variation in more clinical phenotypic presentation between those with juvenile symptom onset (<20 years), and those with adult-onset disease (>20 years).
Epidemiology
DRPLA is most commonly recognized in populations of Japanese ancestry, with an incidence of 2–7 per million.7,8,22,23 DRPLA is thought to occur at lower rates in non-Asian populations, although these estimates are based on the evaluation of cohorts diagnosed with SCA and may therefore represent an underestimation of DRPLA prevalence.7 Worldwide the prevalence of SCAs is 2.7 per 100,000,24 and within these cohorts the highest frequency of DRPLA is seen among Japanese groups (7–20%), with lower rates in other Asian populations (Singapore, 6%; Korea, 3%).25 In European populations, DRPLA has a frequency of 2–4% in Portuguese SCA families,8 0.25–1% in other SCA samples of European descent,23,26,27, and 0.14–3.1% in Latin-American SCA cohorts.25,28 However, these estimates may be higher in some populations, potentially because of a higher proportion of “high-normal” repeat lengths or sampling variation.7 Longer repeat lengths appear more commonly in populations of Japanese origin than in Caucasians.29 There are no reported differences in disease characteristics between populations such as the incidence of adult-onset and juvenile-onset cases, although cohort sizes in some populations remain small.6
Genetics
The atrophin-1 gene (ATN1), located on chromosome 12p13.31, encodes a transcriptional co-repressor widely expressed in the central nervous system and elsewhere in the body.30 ATN1 mutations are inherited in autosomal dominant fashion, with the CAG repeat responsible for DRPLA present in exon 5, and is typically present at ≤35 repeats. Repeat length alleles of >20 are uncommon and considered mutable normal alleles, with one allele >20 repeats in a sample of 177 Caucasian families with mixed SCAs.1,11,29 Such alleles may be at higher risk for future intergenerational expansion.8,23,26 CAG repeat lengths differ between populations, such as a >40 repeat length allele frequency of 20% in families with SCA of Japanese ancestry versus 0% in Caucasian families (p<0.0001).29 Repeat lengths ≥48 demonstrate a fully penetrant clinical phenotype, while alleles of 35–47 repeat length are incompletely penetrant and have been associated with a milder DRPLA clinical phenotype in a small number of cases.1,6,11,31 There is one report of an asymptomatic individual aged 81 with a repeat length of 51.31
DRPLA, in common with other microsatellite repeat disorders, shows genetic anticipation whereby disease symptoms occur earlier and more severely generation on generation. Previous studies have provided estimates of CAG repeat length (median and range) dependent on age at onset of symptoms: <21 years (68 repeats; range 63–79), 21–40 years (64 repeats; range 61–69), >40 years (63 repeats; range 48–67). This phenomenon is thought to be driven by CAG repeat expansion in the gametes, particularly in spermatogenesis, as anticipation is more pronounced on paternal inheritance. A small number of DRPLA cases occur in the absence of any family history of the disease, suggestive of a de novo repeat expansion from the normal or intermediate range into the disease-causing range. The factors driving these cases are unknown.
Expanded polyglutamine (poly-Q) repeats are characteristic of other autosomal-dominant neurodegenerative disorders such as HD and six of the SCA disorders, which also demonstrate features of de novo expansion and genetic anticipation.30,32–34 CAG repeats can expand or contract during gametogenesis; therefore, a non-pathogenic sized CAG repeat may expand and become a de novo pathogenic allele in an offspring.6,7 Alternatively, a disease causing a CAG repeat may expand in subsequent generations leading to an earlier-onset and more severe disease: the phenomenon of anticipation,6,7,35 which is greatest during paternal transmission.4–6 Both phenomena may have a role, with a founder Asian haplotype either expanding to other population groups, or the CAG expansion within these groups arising due to the de novo phenomenon.6,7,23
Models
Although CAG repeat expansion disorders are all driven by similar expanded repeats in the DNA that result in central neurodegeneration, these repeats are in genes encoding different, unrelated proteins and so it is unsurprising that they induce variable clinical syndromes associated with different neuropathologies.30 Invertebrate models of neurodegeneration in DRPLA include the fruit-fly Drosophila melanogaster, where the introduction of a human disease allele causes age-dependent neuronal loss, potentially due to defects in lysosomal autophagy, and accumulation of the poly-Q repeat protein.30 Homozygous Atn1 knockout mice are viable, without obvious phenotype.36 There are several transgenic mouse models of DRPLA, one of the first involved introducing a single mutant copy of human ATN1 with a 65 CAG repeat-length human mutant allele.37 These mice showed tremor, ataxia, and seizures with premature death; however, although there was widespread distribution of the mutant protein with neuronal intranuclear inclusions (NIIs) there was no clear neuronal loss or macropathology.37
A distinct murine model, involved introduction of a 76 CAG repeat human disease allele into background wild-type lineage (Q76). However, in spite of widespread neuronal intranuclear accumulation (NIA) of mutant protein, initially in an anatomical pattern similar to DRPLA, these mice exhibited no clear neurological phenotype and did not form NIIs.38,39 They did however develop a cognitive phenotype with deficits in spatial learning and memory.40 The same background murine lineage was used to produce a model with a much longer repeat length, 129 CAG repeats (Q129), with a repeat of this length demonstrating features more akin to that seen in humans, including myoclonus, ataxia, epilepsy, and weight loss that progressed rapidly to death within 16-weeks.38,39 NIIs appeared, although some time after symptom onset, and although brain weight and cortical thickness of the Q129 mice was reduced there was no indication of astrocytosis or neuronal loss.38,39 Further models with varying repeat lengths have been generated from this lineage and show that CAG repeat length correlates with disease severity, as in DRPLA.40 These mouse models suggest that NIA forms an important pathological marker in DRPLA and occurs before symptom onset, that pathology is widespread within the nervous system, although DRPLA anatomical areas are affected preferentially, and that neuronal atrophy and dysfunction are the hallmarks of the disease.38,39
In vivo conditional knockdown of an atrophin-1 interacting gene, Lsd1, in mice has indicated a role for Atrophin-1 in regulating neuronal progenitor proliferation, a process that can be influenced by the LSD1 inhibitor tranylcypromine. In vitro cellular models have shown a neuronal accumulation of mutated cleaved products and that longer repeat length polyglutamine tracts are degraded more slowly than normal repeat lengths.41,42
Amelioration or reversal of pathology has not been shown in DRPLA models yet; however, there have been promising findings from HD.43 The growing field of allele specific gene-silencing technologies include allele-specific oligonucleotides (ASOs) and less-specific methods of RNA interference, such as small-hairpin RNA (shRNA). The latter has been shown to reduce levels of mutant huntingtin in vitro and in vivo in a transgenic mouse model of HD, with improvement of motor function.44,45 CRISPR/Cas technology, which allows in vitro or in vivo genome editing, has also been shown to reduce levels of both mutant and wild-type huntingtin in a transgenic mouse model of HD with improvement of motor function.43
Neuropathology
Post-mortem neuropathological findings in DRPLA include an overall smaller neuraxis, with neuronal loss and astrocytosis in the dentatorubral and pallidoluysian systems, with intrafamilial variability of neuroimaging findings being reported.46 The lateral segment of the globus pallidus is the area most greatly affected by neuronal loss, with the dentate nucleus and subthalamic nucleus affected to a lesser extent.2 In the red nucleus, astrocytosis is more prominent than neuronal loss, and in all affected areas neurons can appear swollen or shrunken.2 A single case report described severe cerebral white matter damage without axonal loss and mild atherosclerotic changes, although white matter findings have not been widely reproduced elsewhere.47 There are no published comparison studies of neuropathological findings between adult-onset and juvenile-onset DRPLA; however, a family study of a father and son that showed overall similar neuropathological findings for other cases of DRPLA exhibited marked frontal lobe and pontine atrophy in the juvenile-onset case that was postulated to contribute to the severe cognitive deterioration and epilepsy observed.46
DRPLA is one of a number of CAG repeat disorders that exhibit NIIs, with some debate as to whether these represent a key pathogenic feature, or are unrelated to the primary pathological process, potentially representing a failed neuroprotective process.48 Immunohistochemistry of DRPLA post-mortem brains using an antibody aimed at identifying polyglutamine stretches shows wide variation in the deposition of mutant protein, from diffuse intraneuronal accumulation (NIA) to densely packed areas of mutant protein within the nucleus of affected neurons (NII).38,49 NIIs are spherical, non-membrane bound structures that are eosinophilic, contain a mixture of granular and filamentous structures, are ubiquitinated, and contain the mutant ATN1 protein.49 Nuclear pathology and nuclear intranuclear inclusions are widespread in the CNS, although some regions, such as the hippocampus, appear to be spared, with some debate as to whether these inclusions are protective or pathological to the neuron.49,50
Neuroimaging
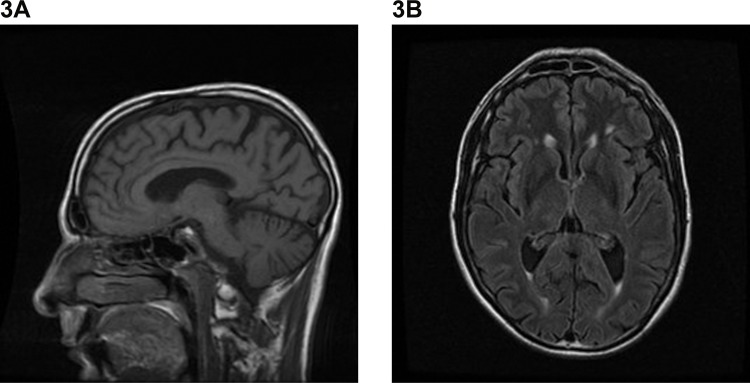
Brain MRI findings are variable and many case reports report only mild or non-specific changes in the early-stage of disease.10,18,19,51–53 There are only two small studies of DRPLA cohorts (n = 10, >60 years of age; n = 3, aged 39–46 years) and these have identified characteristic findings in later disease, including brainstem and cerebellar atrophy and high-intensity T2/fluid-attenuated inversion recovery weighted signal in the cerebral white matter, thalamus, and brainstem.14,54 Other imaging studies have used varying sequence protocols on individual cases10,17,20,47,52 or small pedigrees,16,46 with inconsistent findings ranging from small vessel disease to more typical cerebellar and brainstem atrophy, and cerebellar and supratentorial white matter changes with leuokoencephalopathy, in later disease. An individual case report highlighted the potential utility of susceptibility weighted imaging in DRPLA, with the possibility of iron or other cation deposition in susceptible areas. However, further and larger studies are required to determine whether this modality provides diagnostic value.52 Figure 3 shows the common MRI features of DRPLA.
Figure 3. Example of Magnetic Resonance Imaging Findings in Dentatorubral-pallidoluysian Atrophy. (A) Sagittal T1-weighted image demonstrating global cerebral atrophy, with more marked cerebellar atrophy. (B) Axial T1-weighted image demonstrating bilateral periventricular white-matter lesions.
The use of imaging to distinguish DRPLA from other clinically similar disorders is challenging. In late-onset disease, in particular, isolated atrophy of the brainstem and cerebellum increases the likelihood of more common diagnoses being made (e.g. alcohol-induced cerebellar degeneration), and imaging may show no supportive features in an elderly population.14 The SCAs and the cerebellar variant of progressive supranuclear palsy (PSP-C) often have similar imaging features to those with DRPLA; however, a single study (n = 15) identified increased signal in the brainstem and thalamus as a useful marker for identifying DRPLA.55 Few functional imaging studies have been undertaken, with no common disease-specific findings to date. However, striatal glucose hypometabolism was observed in two individuals with juvenile-onset DRPLA, which may warrant further investigation in a larger cohort.53
Neurophysiology
There are few reported electrophysiological studies performed on patients with DRPLA, with only one retrospective cohort study of 12 juvenile-onset individuals with video-electroencephalography (EEG)56 and the remainder being individual case studies.10,20,56 The seizure types observed in individuals with DRPLA vary widely and are not specific for the disorder. For example, a retrospective cohort study in juvenile-onset DRPLA found that half (six out of 12) exhibited generalized seizures and/or focal seizures, with slightly fewer atypical absence seizures (five out of 12), and myoclonic seizures (four out of 12), and one individual exhibited an atonic event.56 Reported EEG evidence so far indicates that myoclonus is likely to be cortical.10,56 Seizure semiology also typically evolves with early features of short-lived generalized seizures, with subsequent focal seizures and focal seizures with secondary generalization.10,56 EEG abnormalities have also been detected in the inter-ictal period, with evidence of frontal intermittent high-amplitude delta activity.13
Diagnosis
No established clinical diagnostic criteria have been established for DRPLA, with the genetic diagnosis typically made during the investigation of symptomatic individuals.1,51 Diagnostic genetic testing should be considered in any individual with an autosomal dominant pattern of family history involving cognitive impairment, dementia, or movement disorder.7 Consensus guidance on testing within adult-onset ataxia for DRPLA focuses on clinical findings, Asian ancestry, and family history as being important factors to consider.3 Family history can identify neurological presentations with overlapping clinical features, such as atypical Parkinsonism with cerebellar ataxia, Parkinsonism dementia, and cerebellar atrophy. 10,57
Diagnosis may be most difficult in an late-onset adult DRPLA where other differential diagnoses are more common, and the clinical presentation may be less typical;14 when isolated ataxia is the sole clinical finding, more common causes of ataxia are likely to be considered (e.g. a background of alcohol excess), potentially resulting in DRPLA not being considered during the diagnostic work-up.1 Genetic testing is typically via polymerase chain reaction amplification across the ATN1 CAG repeat region followed by gel or capillary electrophoresis, which identifies 100% of pathogenic expansions of ≥48 CAG repeats.1 Although next-generation sequencing technologies are promising they have not been widely used or validated for the ATN1 repeat expansion and diagnosis of DRPLA, and repetitive genomic elements remain problematic to assay via short-read next generation sequencing technologies.58–60
Management
The clinical management of those diagnosed with DRPLA is predominantly supportive within the context of a multidisciplinary team. Genetic counseling is ideally undertaken early to ensure families are aware of the autosomal dominant nature of the disease, to discuss predictive testing and potential means of family planning.1 EEG investigation is indicated in the context of the clinical suspicion of seizures, and neuropsychological assessment for evidence of dementia or psychiatric disturbance, particularly when deciding on psychological support or psychotropic medication.1
There are no clinical trials assessing the efficacy of different anti-epileptic medications in treating seizures in DRPLA. Myoclonic seizures can be managed with carbamazepine or phenytoin, unlike with other forms of myoclonic epilepsy, while sodium valproate, perampanel, and zonisamide have been used in the treatment of generalized seizures.10,51,56 Case reports describe the use of various combinations of anticonvulsants in specific circumstances, but it is unclear whether these regimens are more broadly useful. For example, a 17-year-old male diagnosed with juvenile-onset DRPLA exhibiting segmental myoclonus, gait ataxia, and several daily atonic falls improved with the addition of levetiracetam to sodium valproate, zonisamide, and taltirelin (2,500 mg, 1,000 mg, 200 mg, and 10 mg per day, respectively),10 and a bedbound, non-verbal 9 year old with frequent myoclonic and seizures and generalized tonic–clonic seizures showed gradually improved ambulation and communication (but no change in seizure frequency) with the addition of perampanel to lamotrigine, sodium valproate, clobazam, and phenobarbital (0.4 mg, 100 mg, 600 mg, 20 mg, and 90 mg per day respectively).51
Choreoathetoid and dystonic movements have been managed with tetrabenazine, risperidone, bromazepam, and gabapentin (no doses given).47 Consensus European guidelines advise that riluzole (100 mg/day) is likely effective in reducing ataxia symptoms for adult ataxias regardless of etiology, with a mean decrease of 7 points (>5 points was deemed clinically relevant) on the International Cooperative Ataxia Rating Scale after 8 weeks (Class II evidence).3,61,62 There is weaker evidence for the use of amantadine 300 mg/day for ataxia.3,63 Education, physiotherapy, occupational therapy, and environmental adaptation may also be needed at different disease stages. Specialist palliative care input is recommended throughout the disease course, with evidence suggesting particular importance later in disease course.1,3 A summary of case reports and clinical trials detailing response to treatments in DRPLA and cerebellar ataxia is shown in Table 2.
Table 2. Reported Treatment Outcomes in the Management of DRPLA and Other Forms of Degenerative Ataxia.
| Author | Year | No. of individuals managed | Treatment | Outcome |
|---|---|---|---|---|
| Shiraishi et al.51 | 2017 | DRPLA (1) | Perampanel 0.4 mg/day (added to lamotrigine, sodium valproate, clobazam and phenobarbital) | Myoclonic seizures and weekly generalized seizures resolved |
| Romano et al.62 | 2015 | SCA (19), FA (9) | Riluzole 100 mg/day | Improved SARA score after 12 months of treatment |
| Kobayashi et al.10 | 2012 | DRPLA (1) | Levetiracetam 2,500 mg/day (added to sodium valproate, zonisamide and taltirelin) | Reduced frequency of segmental myoclonus and cessation of falls |
| Ristori et al.61* | 2010 | Chronic cerebellar ataxia (19) | Riluzole 100 mg/day | Mean decrease of 7/100 points on the ICARS following 8 weeks of treatment |
| Botez et al.63† | 1996 | Degenerative cerebellar ataxia (30) | Amantadine maximum 300 mg/day | Improvement of visual and auditory reaction times and movement times at 3–5 months |
| Egawa et al.56 | 2008 | DRPLA (8) | Carbamazepine discontinuation | No change in seizure frequency or myoclonus |
| Egawa et al.56 | 2008 | DRPLA (5) | Phenytoin discontinuation | Phenytoin re-started in one patient owing to increased seizure frequency |
Abbreviations: DRPLA, Dentatorubral-pallidoluysian Atrophy; FA, Friedrich’s Ataxia; ICARS, International Cooperative Ataxia Rating Scale; SARA, Scale for the Assessment and Rating of Ataxia; SCA, Spinocerebellar Ataxia.
*Cited in van de Warrenburg et al, 2014.3
Cited in Veneziano and Frontali, 2016.1
Future directions
DRPLA forms one of a wider spectrum of CAG repeat expansion diseases, including HD, some SCAs, and spinal and bulbar muscular atrophy.32 DRPLA shares similarities with HD including that the clinical and genetic differences between juvenile-onset and adult-onset DRPLA remain intriguing and unexplained, although extrapolation from HD literature points towards a combined loss-of-function and increasingly toxic gain-of-function against the remaining normal allele with increasing repeat length.12 HD is the most widely studied CAG repeat expansion disorder and developments in gene silencing via RNA interference (RNAi) include an antisense oligonucleotide to huntingtin that alleviates disease in a mouse model.64 In vitro and in vivo studies have shown allele-specific RNA interference of multiple alleles is possible within the central nervous system.65
Intrathecal administration of an antisense oligonucleotide directed towards huntingtin is in phase 2 clinical trials for HD, demonstrating a dose-dependent reduction in the overall levels of the mutant protein in cerebrospinal fluid, although the full results of this initial phase of work are yet to be published.66 Similar approaches might be adopted to treat DRPLA. Previous success with intrathecal ASO therapy in neurodegenerative disease has been seen with spinal muscular atrophy, with higher levels of synthesis of the wild-type allele, and improved clinical motor function in phase 3 trials, resulting in an expanded access program for appropriate patients.67,68 Gene-replacement therapy via an adenovirus vector has also shown promise in this disorder.69 Given the rarity of DRPLA it is more likely that therapeutic advancements will arise through the understanding of other neurodegenerative and CAG repeat expansion disorders. At present, optimized clinical management includes a timely diagnosis and access to the multidisciplinary team, as well as the resources needed for ongoing care.
Discussion
Clinical diagnosis of DRPLA in the absence of known family history of the disease remains challenging within the context of neurodegenerative movement disorders, is currently intractable to medical treatment and causes significant disability for those diagnosed with the disorder. Cohort studies to date are underpowered to draw comprehensive conclusions of the true allelic spectrum that causes clinical symptoms, characteristic neuroimaging findings, and responses to current treatments. Larger scale, collaborative international studies are required, and would provide an opportunity for further understanding of this rare disorder, making use of next-generation genetic sequencing technologies, standardized neuroimaging pipelines, and fostering an environment for future clinical trials. As described above, there are promising developments in the discovery of disease-modifying therapies for other neurodegenerative disorders, which may be able to be translated to DRPLA in the future.
Footnotes
Funding: None.
Financial Disclosures: T.H.M. and K.J.P. are funded by the Medical Research Council with Post-Doctoral Clinical Research and Clinician-Scientist Fellowships respectively.
Conflict of Interests: The authors report no conflict of interest.
Ethics Statement: This study was performed in accordance with the ethical standards detailed in the Declaration of Helsinki. The authors’ institutional ethics committee has approved this study and all patients have provided written informed consent.
References
- 1.Veneziano L, Marina F. DRPLA. Seattle, WA: NCBI; 2016. GeneReviews. [Google Scholar]
- 2.Yamada M. Dentatorubral-pallidoluysian atrophy (DRPLA): The 50th anniversary of Japanese Society of Neuropathology. Neuropathology. 2010;30:453–457. doi: 10.1111/j.1440-1789.2010.01120.x. [DOI] [PubMed] [Google Scholar]
- 3.van de Warrenburg BP, van Gaalen J, Boesch S, Burgunder JM, Dürr A, Giunti P, et al. EFNS/ENS Consensus on the diagnosis and management of chronic ataxias in adulthood. Eur J Neurol. 2014;21:552–562. doi: 10.1111/ene.12341. [DOI] [PubMed] [Google Scholar]
- 4.Koide R, Ikeuchi T, Onodera O, Tanaka H, Igarashi S, Endo K, et al. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) Nat Genet. 1994;6:9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- 5.Nagafuchi S, Yanagisawa H, Sato K, Shirayama T, Ohsaki E, Bundo M, et al. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994;6:14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- 6.Wardle M, Morris HR, Robertson NP. Clinical and genetic characteristics of non-Asian dentatorubral-pallidoluysian atrophy: a systematic review. Mov Disord. 2009;24:1636–1640. doi: 10.1002/mds.22642. [DOI] [PubMed] [Google Scholar]
- 7.Wardle M, Majounie E, Williams NM, Rosser AE, Morris HR, Robertson NP. Dentatorubral pallidoluysian atrophy in south Wales. J Neurol Neurosurg Psychiatry. 2008;79:804–807. doi: 10.1136/jnnp.2007.128074. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho P, Ruano L, Loureiro JL, Cruz VT, Barros J, Tuna A, et al. Hereditary ataxia and spastic paraplegia in Portugal: a population-based prevalence study. JAMA Neurol. 2013;70:746–755. doi: 10.1001/jamaneurol.2013.1707. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa A, Ikeuchi T, Koike R, Matsubara N, Tsuchiya M, Nozaki H, et al. Long-term disability and prognosis in dentatorubral-pallidoluysian atrophy: a correlation with CAG repeat length. Mov Disord. 2010;25:1694–1700. doi: 10.1002/mds.23167. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi K, Takeuchi A, Oka M, Akiyama M, Ohtsuka Y. Amelioration of disabling myoclonus in a case of DRPLA by levetiracetam. Brain Dev. 2012;34:368–371. doi: 10.1016/j.braindev.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Maruyama S, Saito Y, Nakagawa E, Saito T, Komaki H, Sugai K, et al. Importance of CAG repeat length in childhood-onset dentatorubral-pallidoluysian atrophy. J Neurol. 2012;259:2329–2334. doi: 10.1007/s00415-012-6493-7. [DOI] [PubMed] [Google Scholar]
- 12.Latimer CS, Flanagan ME, Cimino PJ, Jayadev S, Davis M, Hoffer ZS, et al. Neuropathological comparison of adult onset and juvenile Huntington’s disease with cerebellar atrophy: a report of a father and son. J Huntingtons Dis. 2017;6:337–348. doi: 10.3233/JHD-170261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunc S, Tadic V, Zühlke C, Hellenbroich Y, Brüggemann N. Pearls & oy-sters: family history of Huntington disease disguised a case of dentatorubral-pallidoluysian atrophy. Neurology. 2018;90:142–143. doi: 10.1212/WNL.0000000000004833. [DOI] [PubMed] [Google Scholar]
- 14.Sugiyama A, Sato N, Nakata Y, Kimura Y, Enokizono M, Maekawa T, et al. Clinical and magnetic resonance imaging features of elderly onset dentatorubral-pallidoluysian atrophy. J Neurol. 2018;265:322–329. doi: 10.1007/s00415-017-8705-7. [DOI] [PubMed] [Google Scholar]
- 15.Uyama E, Kondo I, Uchino M, Fukushima T, Murayama N, Kuwano A, et al. Dentatorubral-pallidoluysian atrophy (DRPLA): clinical, genetic, and neuroradiologic studies in a family. J Neurol Sci. 1995;130:146–153. doi: 10.1016/0022-510X(95)00019-X. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz E, Milà M, Sánchez A, Latorre P, Ariza A, Codina M, et al. Dentatorubropallidoluysian atrophy in a Spanish family: a clinical, radiological, pathological, and genetic study. J Neurol Neurosurg Psychiatry. 1999;67:811–814. doi: 10.1136/jnnp.67.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver MR, Sethi KD, Mehta SH, Nichols FT, Morgan JC. Case report of optic atrophy in dentatorubropallidoluysian atrophy (DRPLA) BMC Neurol. 2015;15:260. doi: 10.1186/s12883-015-0520-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatano T, Okuma Y, Iijima M, Fujishima K, Goto K, Mizuno Y. Cervical dystonia in dentatorubral-pallidoluysian atrophy. Acta Neurol Scand. 2003;108:287–289. doi: 10.1034/j.1600-0404.2003.00150.x. [DOI] [PubMed] [Google Scholar]
- 19.Adachi N, Arima K, Asada T, Kato M, Minami N, Goto Yi, et al. Dentatorubral-pallidoluysian atrophy (DRPLA) presenting with psychosis. J Neuropsychiatry Clin Neurosci. 2001;13:258–260. doi: 10.1176/jnp.13.2.258. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi J, Nagao M, Kawata A, Matsubara S. A case of late adult-onset dentatorubral-pallidoluysian atrophy mimicking central pontine myelinolysis. J Neurol. 2009;256:1369–1371. doi: 10.1007/s00415-009-5111-9. [DOI] [PubMed] [Google Scholar]
- 21.Nagai S, Saito Y, Endo Y, Saito T, Sugai K, Ishiyama A, et al. Hypoalbuminemia in early onset dentatorubral-pallidoluysian atrophy due to leakage of albumin in multiple organs. J Neurol. 2013;260:1263–1271. doi: 10.1007/s00415-012-6787-9. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji S, Onodera O, Goto J, Nishizawa M, Diseases SGoA. Sporadic ataxias in Japan—a population-based epidemiological study. Cerebellum. 2008;7:189–197. doi: 10.1007/s12311-008-0028-x. [DOI] [PubMed] [Google Scholar]
- 23.Le Ber I, Camuzat A, Castelnovo G, Azulay JP, Genton P, Gastaut JL, et al. Prevalence of dentatorubral-pallidoluysian atrophy in a large series of white patients with cerebellar ataxia. Arch Neurol. 2003;60:1097–1099. doi: 10.1001/archneur.60.8.1097. [DOI] [PubMed] [Google Scholar]
- 24.Ruano L, Melo C, Silva MC, Coutinho P. The global epidemiology of hereditary ataxia and spastic paraplegia: a systematic review of prevalence studies. Neuroepidemiology. 2014;42:174–183. doi: 10.1159/000358801. [DOI] [PubMed] [Google Scholar]
- 25.Braga-Neto P, Pedroso JL, Furtado GV, Gheno TC, Saraiva-Pereira ML, Jardim LB, et al. Dentatorubro-pallidoluysian atrophy (DRPLA) among 700 families with ataxia in Brazil. Cerebellum. 2017;16:812–816. doi: 10.1007/s12311-017-0862-9. [DOI] [PubMed] [Google Scholar]
- 26.Filla A, Mariotti C, Caruso G, Coppola G, Cocozza S, Castaldo I, et al. Relative frequencies of CAG expansions in spinocerebellar ataxia and dentatorubropallidoluysian atrophy in 116 Italian families. Eur Neurol. 2000;44:31–36. doi: 10.1159/000008189. [DOI] [PubMed] [Google Scholar]
- 27.Pujana MA, Corral J, Gratacòs M, Combarros O, Berciano J, Genís D, et al. Spinocerebellar ataxias in Spanish patients: genetic analysis of familial and sporadic cases. The Ataxia Study Group. Hum Genet. 1999;104:516–522. doi: 10.1007/s004390050997. [DOI] [PubMed] [Google Scholar]
- 28.Paradisi I, Ikonomu V, Arias S. Spinocerebellar ataxias in Venezuela: genetic epidemiology and their most likely ethnic descent. J Hum Genet. 2016;61:215–222. doi: 10.1038/jhg.2015.131. [DOI] [PubMed] [Google Scholar]
- 29.Takano H, Cancel G, Ikeuchi T, Lorenzetti D, Mawad R, Stevanin G, et al. Close associations between prevalences of dominantly inherited spinocerebellar ataxias with CAG-repeat expansions and frequencies of large normal CAG alleles in Japanese and Caucasian populations. Am J Hum Genet. 1998;63:1060–1066. doi: 10.1086/302067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charroux B, Fanto M. The fine line between waste disposal and recycling: DRPLA fly models illustrate the importance of completing the autophagy cycle for rescuing neurodegeneration. Autophagy. 2010;6:667–669. doi: 10.4161/auto.6.5.12433. [DOI] [PubMed] [Google Scholar]
- 31.Hattori M, Yuasa H, Takada K, Yamada T, Yamada K, Kamimoto K, et al. Genetic analysis of a dentatorubral-pallidoluysian atrophy family: relevance to apparent sporadic cases. Intern Med. 1999;38:287–289. doi: 10.2169/internalmedicine.38.287. [DOI] [PubMed] [Google Scholar]
- 32.Keo A, Aziz NA, Dzyubachyk O, van der Grond J, van Roon-Mom WMC, Lelieveldt BPF, et al. Co-expression patterns between ATN1 and ATXN2 coincide with brain regions affected in Huntington’s disease. Front Mol Neurosci. 2017;10:399. doi: 10.3389/fnmol.2017.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevanin G, Giunti P, Belal GD, Dürr A, Ruberg M, Wood N, et al. De novo expansion of intermediate alleles in spinocerebellar ataxia 7. Hum Mol Genet. 1998;7:1809–1813. doi: 10.1093/hmg/7.11.1809. [DOI] [PubMed] [Google Scholar]
- 34.Myers RH, MacDonald ME, Koroshetz WJ, Duyao MP, Ambrose CM, Taylor SA, et al. De novo expansion of a (CAG)n repeat in sporadic Huntington's disease. Nat Genet. 1993;5:168–173. doi: 10.1038/ng1093-168. [DOI] [PubMed] [Google Scholar]
- 35.Vinton A, Fahey MC, O’Brien TJ, Shaw J, Storey E, Gardner RJ, et al. Dentatorubral-pallidoluysian atrophy in three generations, with clinical courses from nearly asymptomatic elderly to severe juvenile, in an Australian family of Macedonian descent. Am J Med Genet A. 2005;136:201–204. doi: 10.1002/ajmg.a.30355. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Lee G, Choe Y, Zoltewicz JS, Peterson AS. Functional architecture of atrophins. J Biol Chem. 2007;282:5037–5044. doi: 10.1074/jbc.M610274200. [DOI] [PubMed] [Google Scholar]
- 37.Schilling G, Wood JD, Duan K, Slunt HH, Gonzales V, Yamada M, et al. Nuclear accumulation of truncated atrophin-1 fragments in a transgenic mouse model of DRPLA. Neuron. 1999;24:275–286. doi: 10.1016/S0896-6273(00)80839-9. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki K, Sato T, Yamada M, Takahashi H, Tsuji S. DRPLA: recent advances in research using transgenic mouse models. Methods Mol Biol. 2013;1010:277–292. doi: 10.1007/978-1-62703-411-1_18. [DOI] [PubMed] [Google Scholar]
- 39.Sato T, Miura M, Yamada M, Yoshida T, Wood JD, Yazawa I, et al. Severe neurological phenotypes of Q129 DRPLA transgenic mice serendipitously created by en masse expansion of CAG repeats in Q76 DRPLA mice. Hum Mol Genet. 2009;18:723–736. doi: 10.1093/hmg/ddn403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki K, Zhou J, Sato T, Takao K, Miyagawa T, Oyake M, et al. DRPLA transgenic mouse substrains carrying single copy of full-length mutant human DRPLA gene with variable sizes of expanded CAG repeats exhibit CAG repeat length- and age-dependent changes in behavioral abnormalities and gene expression profiles. Neurobiol Dis. 2012;46:336–350. doi: 10.1016/j.nbd.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Jin C, Yazawa I. Increased aggregation of polyleucine compared with that of polyglutamine in dentatorubral-pallidoluysian atrophy protein. Neurosci Lett. 2013;552:156–161. doi: 10.1016/j.neulet.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Nakayama K, Hashimoto N, Yazawa I. Proteolytic processing regulates pathological accumulation in dentatorubral-pallidoluysian atrophy. FEBS J. 2010;277:4873–4887. doi: 10.1111/j.1742-4658.2010.07893.x. [DOI] [PubMed] [Google Scholar]
- 43.Yang S, Chang R, Yang H, Zhao T, Hong Y, Kong HE, et al. CRISPR/Cas9-mediated gene editing ameliorates neurotoxicity in mouse model of Huntington's disease. J Clin Invest. 2017;127:2719–2724. doi: 10.1172/JCI92087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drouet V, Ruiz M, Zala D, Feyeux M, Auregan G, Cambon K, et al. Allele-specific silencing of mutant huntingtin in rodent brain and human stem cells. PLoS One. 2014;9:e99341. doi: 10.1371/journal.pone.0099341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci USA. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sunami Y, Koide R, Arai N, Yamada M, Mizutani T, Oyanagi K. Radiologic and neuropathologic findings in patients in a family with dentatorubral-pallidoluysian atrophy. AJNR Am J Neuroradiol. 2011;32:109–114. doi: 10.3174/ajnr.A2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muñoz E, Campdelacreu J, Ferrer I, Rey MJ, Cardozo A, Gómez B, et al. Severe cerebral white matter involvement in a case of dentatorubropallidoluysian atrophy studied at autopsy. Arch Neurol. 2004;61:946–949. doi: 10.1001/archneur.61.6.946. [DOI] [PubMed] [Google Scholar]
- 48.Saudou F, Finkbeiner S, Devys D, Greenberg ME. Huntingtin acts in the nucleus to induce apoptosis but death does not correlate with the formation of intranuclear inclusions. Cell. 1998;95:55–66. doi: 10.1016/S0092-8674(00)81782-1. [DOI] [PubMed] [Google Scholar]
- 49.Yamada M, Shimohata M, Sato T, Tsuji S, Takahashi H. Polyglutamine disease: recent advances in the neuropathology of dentatorubral-pallidoluysian atrophy. Neuropathology. 2006;26:346–351. doi: 10.1111/j.1440-1789.2006.00670.x. [DOI] [PubMed] [Google Scholar]
- 50.Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- 51.Shiraishi H, Egawa K, Ito T, Kawano O, Asahina N, Kohsaka S. Efficacy of perampanel for controlling seizures and improving neurological dysfunction in a patient with dentatorubral-pallidoluysian atrophy (DRPLA) Epilepsy Behav Case Rep. 2017;8:44–46. doi: 10.1016/j.ebcr.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simpson M, Smith A, Kent H, Roxburgh R. Neurological picture. Distinctive MRI abnormalities in a man with dentatorubral-pallidoluysian atrophy. J Neurol Neurosurg Psychiatry. 2012;83:529–530. doi: 10.1136/jnnp-2011-301612. [DOI] [PubMed] [Google Scholar]
- 53.Sone D, Sato N, Yokoyama K, Sumida K, Kanai M, Imabayashi E, et al. Striatal glucose hypometabolism in preadolescent-onset dentatorubral-pallidoluysian atrophy. J Neurol Sci. 2016;360:121–124. doi: 10.1016/j.jns.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Yoon WT, Youn J, Cho JW. Is cerebral white matter involvement helpful in the diagnosis of dentatorubral-pallidoluysian atrophy? J Neurol. 2012;259:1694–1697. doi: 10.1007/s00415-011-6401-6. [DOI] [PubMed] [Google Scholar]
- 55.Tomiyasu H, Yoshii F, Ohnuki Y, Ikeda JE, Shinohara Y. The brainstem and thalamic lesions in dentatorubral-pallidoluysian atrophy: an MRI study. Neurology. 1998;50:1887–1890. doi: 10.1212/WNL.50.6.1887. [DOI] [PubMed] [Google Scholar]
- 56.Egawa K, Takahashi Y, Kubota Y, Kubota H, Inoue Y, Fujiwara T, et al. Electroclinical features of epilepsy in patients with juvenile type dentatorubral-pallidoluysian atrophy. Epilepsia. 2008;49:2041–2049. doi: 10.1111/j.1528-1167.2008.01701.x. [DOI] [PubMed] [Google Scholar]
- 57.de Souza PV, Batistella GN, Pinto WB, Oliveira AS. Teaching neuroimages: leukodystrophy and progressive myoclonic epilepsy disclosing DRPLA. Neurology. 2016;86:e58–59. doi: 10.1212/WNL.0000000000002356. [DOI] [PubMed] [Google Scholar]
- 58.Klein CJ, Foroud TM. Neurology individualized medicine: when to use next-generation sequencing panels. Mayo Clin Proc. 2017;92:292–305. doi: 10.1016/j.mayocp.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Dunn P, Albury CL, Maksemous N, Benton MC, Sutherland HG, Smith RA, et al. Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet. 2018;9:20. doi: 10.3389/fgene.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang H, Kirkness EF, Lippert C, Biggs WH, Fabani M, Guzman E, et al. Profiling of short-tandem-repeat disease alleles in 12,632 human whole genomes. Am J Hum Genet. 2017;101:700–15. doi: 10.1016/j.ajhg.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ristori G, Romano S, Visconti A, Cannoni S, Spadaro M, Frontali M, et al. Riluzole in cerebellar ataxia: a randomized, double-blind, placebo-controlled pilot trial. Neurology. 2010;74:839–845. doi: 10.1212/WNL.0b013e3181d31e23. [DOI] [PubMed] [Google Scholar]
- 62.Romano S, Coarelli G, Marcotulli C, Leonardi L, Piccolo F, Spadaro M, et al. Riluzole in patients with hereditary cerebellar ataxia: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:985–991. doi: 10.1016/S1474-4422(15)00201-X. [DOI] [PubMed] [Google Scholar]
- 63.Botez MI, Botez-Marquard T, Elie R, Pedraza OL, Goyette K, Lalonde R. Amantadine hydrochloride treatment in heredodegenerative ataxias: a double blind study. J Neurol Neurosurg Psychiatry. 1996;61:259–264. doi: 10.1136/jnnp.61.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kordasiewicz HB, Stanek LM, Wancewicz EV, Mazur C, McAlonis MM, Pytel KA, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zalachoras I, Evers MM, van Roon-Mom WM, Aartsma-Rus AM, Meijer OC. Antisense-mediated RNA targeting: versatile and expedient genetic manipulation in the brain. Front Mol Neurosci. 2011;4:10. doi: 10.3389/fnmol.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Medicine NUSNLo . Safety, tolerability, pharmacokinetics, and pharmacodynamics of IONIS-HTTRx in patients with early manifest Huntington’s disease 2017. Available from. https://clinicaltrials.gov/ct2/show/NCT02519036. [Google Scholar]
- 67.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 68.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–35. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 69.Mendell JR, Al-Zaidy S, Shell R, Arnold WD, Rodino-Klapac LR, Prior TW, et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 70.Aridon P, Tarantino P, Ragonese P, D’Amelio M, Cinturino A, Salemi G, et al. Dentatorubral-pallidoluysian atrophy: haplotype of Asian origin in 2 Italian families. Mov Disord. 2012;27:460–461. doi: 10.1002/mds.24027. [DOI] [PubMed] [Google Scholar]