Abstract
Background
Radiomics or computer-extracted texture features derived from MRI have been shown to help quantitatively characterize prostate cancer (PCa). Radiomics have not been explored depth in the context of predicting biochemical recurrence (BCR) of PCa.
Purpose
To identify a set of radiomic features derived from pretreatment biparametric MRI (bpMRI) that may be predictive of PCa BCR.
Study Type
Retrospective
Subjects
120 PCa patients from 2 institutions I1 and I2. Partitioned into training set D1 (N = 70) from I1 and independent validation set D2 (N = 50) from I2. All patients were followed for ≥ 3 years.
Sequence
3T, T2-weighted (T2WI) and apparent diffusion coefficient (ADC) maps derived from diffusion weighted sequences.
Assessment
PCa regions of interest (ROI) on T2WI were annotated by 2 experienced radiologists. Radiomic features from bpMRI (T2WI and ADC maps) were extracted from the ROI’s. A machine learning classifier (CBCR) was trained with best discriminating set of radiomic features to predict BCR (pBCR).
Statistical Tests
Wilcoxon rank-sum tests with P < .05 were considered statistically significant. Differences in BCR-free survival at 3 years using pBCR was assessed using the Kaplan-Meier method and compared with Gleason Score (GS), PSA and PIRADS-v2.
Results
Distribution statistics of co-occurrence of local anisotropic gradient orientation (CoLlAGe) and Haralick features from T2WI and ADC were associated with BCR (P < .05) on D1. CBCR predictions resulted in a mean AUC = 0.84 on D1 and AUC = 0.73 on D2. A significant difference in BCR-free survival between the predicted classes (BCR+ and BCR−) was observed (P = .02) on D2 compared to those obtained from GS (P = .8), PSA (P = .93) and PIRADS-v2 (P = .23).
Data Conclusion
Radiomic features from pretreatment bpMRI can be predictive of PCa BCR after therapy and may help identify men who would benefit from adjuvant therapy.
Keywords: Prostate cancer, Computer-Assisted Image Processing, Magnetic Resonance Imaging, Recurrence, Projections and Predictions
INTRODUCTION
Biochemical recurrence (BCR) affects a significant proportion of men after they undergo definitive treatment for prostate cancer (PCa). Approximately 20% to 40% men who undergo radical prostatectomy (RP) (1, 2) and 30% to 50% men who undergo radiation therapy (RT) (3) will develop BCR within 10 years after treatment, and nearly two-thirds of these cases occur within the first two years (4). Men with BCR have a higher risk of metastatic disease and death from PCa progression compared to those without BCR (2). Therefore, predicting which men will develop BCR could allow for early identification of patients who might benefit from adjuvant therapy.
There is growing evidence supporting the use of multiparametric magnetic resonance imaging (mpMRI) before and after treatment in men with PCa (PCa detection, staging and localization of sites of disease recurrence (5–8)). More recently, there has been interest in using information obtained from pretreatment mpMRI to predict the occurrence of BCR (9–11).
Radiomics refers to computer-based extraction of texture and image features from radiographic images. These features are used to characterize the underlying tumor microarchitecture and heterogeneity which is subtle and not immediately visible on routine imaging. Radiomic features derived from mpMRI have been shown to be useful in PCa detection and localization (12–16). In the context of BCR prediction using radiomic features, Gnep et al (17) found that Haralick features obtained from T2-weighted images (T2WI) were significantly associated with BCR occurrence. However, this inference was drawn from a limited cohort of 74 men and the study also lacked validation on an independent test set.
In our study, we set out to assess additional classes of features including Gabor features (18) (which capture filter responses at multiple scales and orientations), Laws texture energy descriptors (which capture filter responses that represent edges, spottiness, waves, and ripple patterns), and co-occurrence of local anisotropic gradient orientations (CoLlAGe) features (19) in the context of predicting PCa BCR and test using an independent validation dataset. The goal of our study was to identify a comprehensive set of radiomic features of PCa derived from pretreatment bpMRI using a machine learning classifier to predict the probability of PCa BCR occurrence.
MATERIALS AND METHODS
This retrospective study consisted of patients from two institutions I1 and I2 which were compliant with the Health Insurance Portability and Accountability Act (HIPAA) and approved by the Institutional Review Board (IRB), either with a waiver of informed consent (I1) or with written informed consent before enrollment (I2).
Patient Selection
From I1, information from 1865 male patients with suspected or confirmed PCa who underwent 3T mpMRI of the prostate between 2009 and 2013 was acquired. Of these, 874 men underwent mpMRI before radical prostatectomy (RP) or radiotherapy (RT). Patient records were reviewed and were included in the analysis if they 1) had documented BCR or 2) did not have BCR but followed for ≥ 3 years. BCR was defined as 2 consecutive readings of prostate specific antigen (PSA) > 0.2 ng/mL for men who underwent RP (20) and as an increase in PSA > 2 ng/mL compared to the initial PSA nadir value for men who underwent RT (with or without hormonal therapy) (21). Of the 113 cases identified through this process, 43 were excluded (Figure 1) prior to the study to obtain a balanced training cohort D1 consisting of 70 men (35 with BCR [BCR+] and 35 without BCR [BCR−]) (Table 1).
Figure 1.
Flowchart of patient selection from 2 institutions. Patients from the 2 classes were balanced in the training set to minimize bias. BCR = biochemical recurrence, bpMRI = biparametric magnetic resonance imaging, RP = radical prostatectomy, RT = radiotherapy.
Table 1.
Patient Specific Parameters
| Institution 1 | Institution 2 | |||
|---|---|---|---|---|
|
| ||||
| Parameter | BCR+ (N = 35) | BCR− (N = 35) | BCR+ (N = 7) | BCR− (N = 43) |
| MRI Receiver coils | ||||
| ERC | n = 20 | n = 35 | n = 0 | n = 0 |
| PPAC/Surface coil | n = 15 | n = 0 | n = 7 | n = 43 |
| Clinical parameters | ||||
| Mean age (range) | 62.4 (45–78) y | 58 (46–80) y | 62.6 (53 – 68) y | 62.9 (54 – 72) y |
| Mean pretreatment PSA (range) | 20.9 (0.37 – 48.5) ng/mL | 7.1 (0.03 – 21) ng/mL | 12.97 (6–26) ng/mL | 9.37 (3.5–35) ng/mL |
| Median Gleason score (range) | 9 (7 – 9) | 7 (6 – 9) | 7 (7 – 9) | 7 (5 – 9) |
| Mean follow-up time (range) | 17.2 (0–59) mo | 60 (25–85) mo | 17 (11 – 30) mo | 28.83 (3 – 67) mo |
| Median PIRADS (range) | 5 (2 – 5) | 4 (2 – 5) | 5 (4 – 5) | 5 (1 – 5) |
| Treatment type | ||||
| Radical prostatectomy | n = 22 | n = 34 | n = 7 | n = 43 |
| Radiotherapy | n = 10 | n = 0 | n = 0 | n = 0 |
| LHRH | n = 3 | n = 1 | n = 0 | n = 0 |
Note: ERC = Endo-rectal coil, PPAC = Pelvic-phased array coil, PSA = prostate-specific antigen, LHRH = luteinizing hormone-releasing hormone therapy
At I2, a total of 52 patients underwent mpMRI scan prior to RP between 2010 and 2013. The patients were followed for ≥ 3 years and time to recurrence was noted. Of these 52, 2 patients were excluded since no suspicious PCa lesions were identified by radiologist resulting in 50 PCa patients (7 BCR+ and 43 BCR−) which were used as an independent validation cohort D2 to robustly validate the findings across institutions (Table 1).
MRI Protocol and Annotations
All mpMRI examinations of D1 were performed using a 3T MR scanner with either a surface pelvic phase array coil (PPAC) or an endorectal coil (ERC). The pulse sequences analyzed in the current study included axial turbo spin-echo (TSE) T2WI, axial diffusion-weighted imaging (DWI) with creation of an apparent diffusion coefficient (ADC) map using vendor specific software. Patients in D2 underwent mpMRI examination performed using a 3T MR scanner and a PPAC. The pulse sequences analyzed in the study included turbo spin-echo T2WI, axial DWI with the creation of an ADC map.
Detailed MRI acquisition parameters are provided in Table 2. Additional sequences, such as dynamic contract enhanced MRI, proton MR spectroscopy and various other sequences were acquired but not analyzed in the current study.
Table 2.
MRI Parameters
| Institution 1 | Institution 2 | |||
|---|---|---|---|---|
|
| ||||
| Parameter | Scanner 1 | Scanner 2 | Scanner 1 | |
| Manufacturer | Philips Medical Systems, Best, Netherlands | Siemens Healthcare, Erlangen, Germany | Siemens Healthcare, Erlangen, Germany | |
| Model | 3T Achieva | 3T Skyra | 3T Verio | |
| Coils | ERC | PPAC | PPAC | |
| T2-weighted MR imaging | ||||
| Repetition time/echo time (TR/TE) | 3802-5151/105-115 | 3730/121 | 6400/101 | |
| Acquisition voxel size (mm3) | 0.4 × 0.4 × 3.0 | 0.6 × 0.7 × 3.0 | 0.6 × 0.6 × 3.0 | |
| Reconstruction voxel size (mm3) | 0.3 × 0.3 × 3.0 | 0.5 × 0.5 × 3.0 | 0.6 × 0.6 × 3.0 | |
| Acquisition time (min) | 5.36 | 2.53 | 2.27 | |
| Diffusion-weighted imaging | ||||
| Repetition time/echo time (TR/TE) | 3571-4880/50-74 | 4700/86 | 6400/101 | |
| Acquisition voxel size (mm3) | 1.3 × 1.3 × 3.0 | 1.5 × 1.8 × 3.0 | 2.0 × 2.0 × 3.0 | |
| Reconstruction voxel size (mm3) | 1.4 × 1.4 × 3.0 | 1.6 × 1.6 × 3.0 | 2.0 × 2.0 × 3.0 | |
| b-values (s/mm2) | 0, 500, 1000, 1500, 2000 | 0, 400, 900, 1500† | 0, 100, 200, 350, 500* | |
| Acquisition time (min) | 5.25 | 5.07 | 5.1 | |
b-value of 1500 was calculated;
1 patient was scanned with b-value up to 800 s/mm2.
A board-certified radiologist with fellowship training in abdominal imaging and 7 years of experience in prostate imaging reviewed the mpMRI datasets and delineated contours of the dominant PCa lesions in D1 (using 3D Slicer (22)) using the histopathology template reports from RPs and biopsies as a reference while another radiologist with 8 years of experience in prostate imaging similarly reviewed the cases in D2. Prostate Imaging-Reporting and Data system version 2 (PIRADS-v2) scores were assigned to the annotated lesions (23). The whole prostate gland and dominant PCa lesions were delineated on axial T2WI data sets. DWI data sets were co-registered with T2WI using rigid deformation (24).
After treatment, all patients underwent periodic follow-up according to the established clinical protocol (3 – 6 months in the first year and 6 – 12 months the following years), which included serial measurements of PSA levels, recording of recurrence dates for BCR+ cases and date of last follow-up for BCR− cases. BCR− cases were censored at their last follow-up date for survival analysis. One patient from D1 died <36 months after RP and 5 patients from D2 at 4, 9, 23, 26, and 28 months after RP due to non-PCa-related causes without evidence of BCR.
MRI Preprocessing
T2WI obtained with an ERC are often affected by bias field artifacts which were corrected using a method described previously (25). With this method, the bias field is first estimated from acquired image data and is later subtracted from the acquired scan. Additionally, intensity drift artifacts arising from intra-patient variability in MRI can cause image intensities to lack in tissue-specific meaning. This was corrected using an intensity standardization method described previously (26) which normalizes the intensity histograms (within the delineated prostate) to a similar range providing tissue specific meaning to the intensities.
The images are scaled in a piecewise manner using histogram landmarks at every 10th percentile. These landmarks are automatically computed from the data. We have computed the standardization map (piecewise linear function) for just the prostate volume. All the prostates were standardized to a single template. This is illustrated in Figure 2 where the histograms of image intensities within the prostates of different patients are shown before and after standardization. The above pre-processing steps were aimed to minimize the differences related to the coil utilized (ERC or SC).
Figure 2.

A) Bias-field correction in MRI scanned with an ERC. ERC1 and ERC2 are prostates of 2 patients. The bias-field is estimated and then subtracted from the original images (top row). B) Effect of standardization on image intensities. ERC1 and ERC2 are prostates scanned with an endo-rectal coil (ERC) while SC1 and SC2 are scanned with a surface coil (SC). The top row shows images and the intensity histograms after standardization whereas the bottom shows after standardization. Scans with ERC are corrected for bias – field artifacts.
Radiomic Feature Extraction
Radiomic features employed in this study were extracted on a per-voxel basis from T2WI and ADC maps within the annotated cancerous regions. The choice of radiomic features for characterizing PCa lesions was motivated by their use in previous studies (12, 15, 18, 19, 27). We also included additional features that have recently been shown to be capable of distinguishing among subtly different disease types on imaging (19) (Table 3). Intensity-based tumor heterogeneity is characterized using Haralick, Gabor, and Laws features; gradient-based tumor heterogeneity is characterized using CoLlAGe and gradient features. A total of 150 (75 each from T2WI and ADC maps) radiomic features were extracted on a per-voxel basis from pretreatment T2WI and ADC maps (Figure 3). Mean, variance, skewness, and kurtosis were computed over the feature vectors within each lesion. Therefore, a total of 150 × 4 = 600 features were computed for each lesion.
Table 3.
Description and Significance of Radiomic Features Analyzed
| Feature | Description | Significance |
|---|---|---|
| First-order statistics | Mean, standard deviation, median, and range; first-order differentials computed using Sobel operators | Localize hypo- and hyperintense regions; gradients detect edges and quantify region boundaries |
| Co-occurrence features | Localization of regions with significant intensity changes; gradients detect edges and quantify region boundaries | Localizes regions based on underlying heterogeneity of voxel intensities |
| CoLlAGe features | Localization of regions with significant local oriented gradient changes; | Localizes regions based on underlying heterogeneity of oriented voxel gradients |
| Gabor features | Convolution of Gaussian function with a Fourier transform at different orientations and frequencies | Quantify the appearance of cancer lesions at multiple orientations and image scales |
| Laws texture features | Computed by convolution of the image with local masks obtained from vectors that capture local average, edge, spot, wave, and ripple patterns | Quantify the variation of pixel intensities within a fixed region of the image; regions of image containing cancer lesions typically contain lower texture energy |
Figure 3.

Experimental workflow illustrating segmentation of prostate cancer lesions on pretreatment mpMRI followed by radiomic feature extraction within the lesion and statistics of features computed for each lesion.
Training a Machine Learning Classifier
The training dataset D1 was used to extract 75 radiomic features. Distribution statistics (including mean, variance, skewness, and kurtosis) of these features were computed for each ROI, including all slices. Feature selection methods were employed to provide an optimal discriminating set of features (28, 29). Minimum redundancy maximum relevance (mRMR), joint mutual information (JMI), and conditional mutual information method (CMIM) were the 3 feature selection methods chosen for this study, as these approaches have been previously employed in radiomic studies involving PCa localization (15, 30, 31). The most discriminating features were then used to train a machine learning classifier in conjunction with class labels (BCR+ or BCR−). Linear discriminant analysis (LDA), support vector machines (SVM), and decision trees or random forests (RF) are widely used machine learning-based classification methods that have been employed by previous studies in the context of radiomic features-based classification (13, 15, 32). Each of these classification methods (LDA, SVM, and RF) was used in conjunction with the 3 feature selection methods to train a total of 9 different prediction models. Given a radiomic feature vector from a test case, the prediction models yield a probability score (pBCR) that the feature vector belongs to either the BCR+ or BCR− class.
A 3-fold cross-validation scheme with 150 runs for each of the 9 prediction models was performed, and a receiver operating characteristic (ROC) curve was generated by varying the threshold on the probability scores obtained from the classifier. The classifier that yielded a high area under the ROC curve (AUC) and a small standard deviation over the learning set was identified as the optimal classifier (CBCR). The set of radiomic features corresponding to CBCR that resulted in the best classification performance on D1 were evaluated on the independent validation set D2. A combination of clinical parameters (PSA, GS and PIRADS-v2) and radiomic features was also used to train a machine learning classifier and assess the performance.
Statistical Analysis
To anticipate a 70% incidence of BCR in the positive class and allowing for false detection in 30% of patients in the negative class (with an enrollment ratio of 1 between the classes), 46 patient studies were needed to obtain a power of 80% and α (type 1 error) = 0.05. A total of N = 70 patient studies were employed for classifier training.
A univariate Cox proportional hazards analysis was performed using the R software environment. This analysis was performed on D1 to determine individual radiomic features predictive of BCR. Hazard ratios (HRs) and the confidence interval (CI) of each feature associated with BCR were computed. Harrell’s C-index was also estimated to evaluate the correlation of each feature to BCR and to rank features in order of C-indices. A P value < .05 was used to assess statistical significance.
The predicted class probability pBCR obtained from CBCR on D2 was converted to a class label (BCR+ or BCR−) using a threshold of 0.5. Individual machine learning classifiers were trained using PSA, pre-treatment biopsy Gleason Score (GS) and PIRADS-v2 to predict class labels for comparison with pBCR. The class labels were then used to analyze the difference in survival using Kaplan-Meier analysis. A log-rank test was performed to assess statistical significance (P < .05).
RESULTS
Predicting BCR using a machine learning classifier trained with radiomic features
The top-discriminating 10 radiomic features that showed significant differences (P < .05) between BCR+ and BCR− lesions ranked according to C-index are listed in Table 4. A complete list of significant radiomic features is provided in supplementary section.
Table 4.
Top 10 Significant Radiomic Features Ranked According to the Concordance Index (C-Index)
| Statistic | MR Imaging | Radiomic Feature | C-Index | P Value | Hazard Ratio* | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| skewness | T2w | Haralick correlation | 0.74 | <0.0001 | 2.09 | 1.52 – 2.87 |
| kurtosis | ADC | CoLlAGe entropy | 0.72 | <0.0001 | 2.28 | 1.65 – 3.16 |
| variance | T2w | Haralick differential average | 0.72 | <0.0001 | 1.85 | 1.39 – 2.46 |
| variance | T2w | Haralick inertia | 0.71 | <0.0001 | 1.56 | 1.21 – 2.02 |
| kurtosis | ADC | CoLlAGe sum entropy | 0.71 | 0.0001 | 2.27 | 1.61 – 3.19 |
| skewness | ADC | CoLlAGe energy | 0.71 | <0.0001 | 2.20 | 1.57 – 3.09 |
| skewness | ADC | CoLlAGe info 1 | 0.70 | 0.0001 | 0.50 | 0.36 – 0.70 |
| variance | T2w | Haralick differential variance | 0.70 | <0.0001 | 1.51 | 1.17 – 1.95 |
| kurtosis | ADC | CoLlAGe energy | 0.70 | <0.0001 | 1.93 | 1.44 – 2.58 |
| kurtosis | T2w | Haralick sum variance | 0.69 | <0.0001 | 1.59 | 1.26 – 2.01 |
Note. —ADC = apparent diffusion coefficient; CoL1AGe = co-occurrence of local anisotropic gradient orientation; T2WI = T2-weighted imaging.
Obtained from Cox proportional hazards model univariate analysis.
On D1, SVM classifier was found to perform better than the LDA and RF classifiers in terms of AUC (Figure 4). The SVM classifier with the JMI feature selection scheme was chosen as the best predictive model (CBCR) and used for validation on D2. This model resulted in an AUC = 0.84 on D1 and AUC = 0.73 on D2 (Figure 5).
Figure 4.
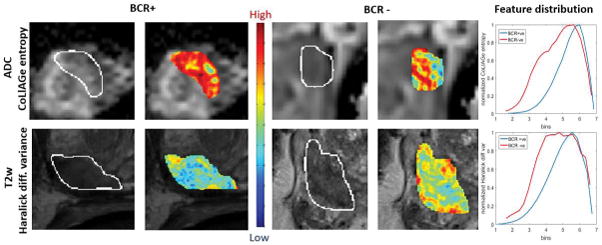
Radiomic feature maps showing differences between BCR+ and BCR− lesions on pretreatment MRI. There is a predominant overexpression of radiomic features (Haralick and CoLlAGe shown here) in BCR+ lesions compared to BCR− lesions. The distribution of the features shows significant differences between the 2 lesion categories; these differences are captured by the skewness statistic of the distribution.
Figure 5.
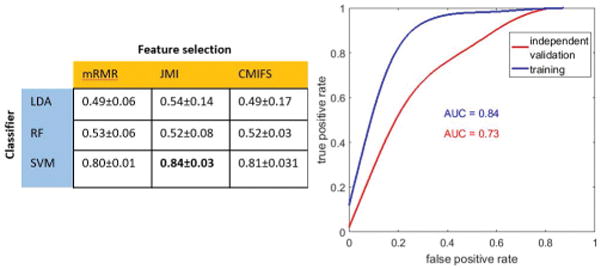
The AUC maps obtained from a pair-wise combination of 3 feature selection methods along with 3 machine learning-enabled classification strategies is summarized above (left). The best classification result was obtained with a combination of joint mutual information (JMI) feature selection and support vector machine (SVM) classification method, and the corresponding ROC on training set (n = 70) is illustrated (right). This classifier was tested on an independent validation set (n = 50).
The skewness and kurtosis statistics of CoLlAGe feature from ADC maps and Haralick features from T2WI were significantly associated with BCR (P < .05) on D1 (Table 4). The combination of clinical parameters and radiomic features resulted in an improved prediction on D1 (AUC = 0.91), however, only a marginal improvement in performance (AUC = 0.74) was observed on D2.
Survival Analysis
A significant separation in Kaplan-Meier survival curves between the 2 classes of patients (P = .02) was observed using pBCR on D2. In comparison, none of the clinical variables including Gleason Score (P = 0.81), PSA (P = 0.93) and PI-RADS-v2 (P = 0.23) resulted in a statistically significant separation (Figure 6). It may be noted that D2 is imbalanced and may not be sufficient to establish significant conclusions.
Figure 6.
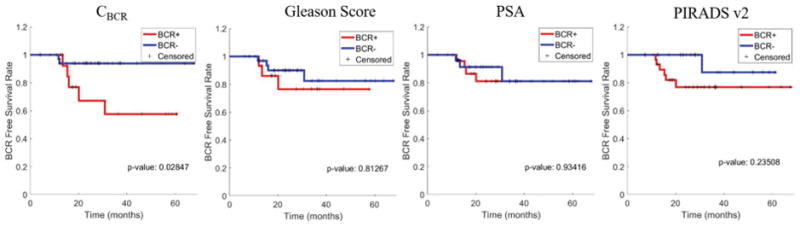
Kaplan-Meier survival curves using predicted BCR+ (red) and BCR− (blue) classes by machine learning classifiers trained using radiomic features (CBCR), Gleason Score, PSA and PIRADS-v2. Statistically significant separation in BCR free survival between the predicted BCR+ and BCR− patients is observed with CBCR (P = 0.02) as opposed to Gleason Score (P = 0.81), PSA (P = 0.93) and PIRADS-v2 (P = 0.23). Statistical significance (P < 0.05) was established using the log-rank test.
Effect of combining clinical parameters and radiomics in predicting BCR
Combining clinical variables with radiomics radiomic features improved prediction of BCR on training set D1 (AUC = 0.91) compared to radiomics alone (AUC = 0.84). However, a marginal improvement in performance (AUC = 0.74) was observed on the validation set D2 over just the radiomic features (AUC = 0.73). Our combination strategy involved integrating clinical variables with the best discriminating set of radiomic features. The results in terms of AUC on the training and validation set along with different combinations rank ordered in terms of performance on the validation set are presented in Table 5.
Table 5.
Classifiers trained with radiomics, clinical variables and a combination of both
| Classifier trained with | AUC (Training) | AUC (Validation) |
|---|---|---|
| Radiomics + GS + PSA + PIRADS | 0.91 | 0.74 |
| Radiomics alone | 0.84 | 0.73 |
| Radiomics + PSA + PIRADS | 0.85 | 0.7 |
| Radiomics + PSA | 0.83 | 0.7 |
| Radiomics + GS + PIRADS | 0.91 | 0.68 |
| Radiomics + PIRADS | 0.81 | 0.68 |
| GS + PSA + PIRADS | 0.92 | 0.62 |
| Radiomics + GS | 0.92 | 0.62 |
| PIRADS | 0.73 | 0.62 |
| Radiomics + GS +PSA | 0.89 | 0.6 |
| PSA | 0.78 | 0.57 |
| GS | 0.9 | 0.56 |
Note: GS – Gleason Score
Effect of treatment type on radiomic features for BCR prediction
Only patients who underwent surgery in the training set were used to train a different radiomic classifier. We obtained an AUC = 0.81 on D1 and AUC = 0.7 on D2 in predicting BCR. The top discriminating radiomic features when including patients who underwent RP or RT were skewness and kurtosis statistics of Haralick features obtained from T2WI and CoLlAGe features obtained from ADC maps. When including patients who underwent RP, the top discriminating features were again skewness and kurtosis statistics of Haralick features derived from T2WI, CoLlAGe features derived from ADC and Gabor feature derived from ADC. These are also summarized in Table 6.
Table 6.
Comparing radiomic classifiers: patients undergoing RP vs all patients
| Patients Undergoing RP | All patients | |
|---|---|---|
| Training Dataset | BCR+ = 24, BCR− = 24 | BCR+ = 35, BCR− = 35 |
| Validation Dataset | BCR+ = 7, BCR− = 43 | BCR+ = 7, BCR− = 43 |
| AUC (training) | 0.81 | 0.84 |
| AUC (validation) | 0.7 | 0.73 |
| Top 3 discriminating radiomic features | Skew(HaralickT2) | Skew(CoLlAGeADC) |
| Mean(GaborADC) | Mean(CoLlAGeADC) | |
| Skew(CoLlAGeADC) | Skew(HaralickT2) |
DISCUSSION
In this study, we identified a comprehensive set of radiomic features of PCa derived from T2WI and ADC maps that correlated with BCR. Additionally, using a machine learning classifier, we showed that these features could be used to predict BCR.
Gnep et al (18) previously demonstrated that Haralick features derived from T2WI are associated with BCR occurrence, suggesting that radiomic analysis may be capable of capturing the differences in BCR+ and BCR− lesions on pretreatment mpMRI. In our study, a more comprehensive set of radiomic features were analyzed and distribution statistics, specifically skewness and kurtosis, of T2WI-derived Haralick features and ADC-derived CoLlAGe entropy were found to be strongly predictive of BCR in men with PCa. Additionally, these results were tested on an independent validation dataset acquired from a different institution.
CoLlAGe features quantify local intensity gradient patterns in images and capture gradient-based tumor heterogeneity. The distribution of these gradients is captured by various statistics such as entropy, inertia, energy which were found to be different in BCR+ and BCR− lesions. There was a predominantly higher disorder of gradients in BCR+ lesions compared to BCR− lesions, and distributions of these features within the lesion were found to capture the differences between the two categories.
Similar to CoLlAGe features, we observed that distribution statistics of Haralick features derived from T2WI, captured by skewness and kurtosis metrics, were statistically significantly different between BCR+ and BCR− lesions. Haralick features are a result of various statistics computed over a matrix of spatial voxel-intensity relationships. An overexpression of Haralick features typically indicates more intensity-based tumor heterogeneity, which is observed in recurrent lesions. The distribution statistics quantified by skewness and kurtosis again demonstrated significant differences between BCR+ and BCR− lesions.
The set of top discriminating radiomic features demonstrated a high predictive accuracy on both D1 and D2. The result on D2 is particularly interesting given that these patients are from a different institution than what the radiomic classifier was trained on.
The study by Gnep et al (17) demonstrated that T2WI-derived Haralick features were associated with BCR. These features were extracted from the training dataset, and the same machine learning classification method (RF) was used to train a classification model. When the classifier was tested on D2, the resultant AUC was considerably lower than we found. Although our results indicated that Haralick features are associated with BCR, the additional features we assessed, including CoLlAGe and Gabor features, carry important complementary information that improved predictive accuracy.
The top ranked radiomic features also yielded a high C-index (~0.7 for the top radiomic features), showing a high correlation with BCR. This was also reflected in the Kaplan-Meier analysis, which showed significant differences in survival between BCR+ and BCR− patients predicted by the classifier on D2. On the other hand, none of the clinical parameters including Gleason Score, PSA and PIRADS-v2 were able to show significant differences in D2. This indicates that clinical variables suggesting aggressive disease may not always be predictive of BCR and radiomic features capturing subtle imaging signatures may add complementary and additional value in predicting BCR for these patients. However, D2 is imbalanced and additional experiments with a larger and balanced cohort are necessary to conclusively establish these findings.
We observe that combining clinical variables with radiomics almost always resulted in better performance on the training set. However, the AUC wasn’t consistently high in the validation set. The clinical variables of patients used in the training set were already predictive of BCR since the patients were carefully curated. And a combination of clinical variables with radiomics improved BCR prediction. With the validation set, the clinical variables were not strongly predictive of BCR by themselves. Combination of radiomics and clinical variables marginally improved the AUC in the validation set. None of the other combinations improved the AUC beyond radiomics alone.
Our study consisted of images from different scanners and coil that affect images in terms of resolution and intensity-bias. Radiomic features from higher resolution images may capture finer texture and be more descriptive of underlying heterogeneity. While correcting ERC related bias-field artifacts would not render it completely equivalent to PPAC, intensity standardization helps alleviate these issues to a large extent.
While a majority of patients in D1 and D2 underwent RP, there were a few patients who underwent RT/hormonal therapy. Training a machine learning classifier using radiomic features obtained from patients who underwent RP alone resulted in a good classification performance on D2 as indicated by the AUCs. Also, thetop discriminating features remained consistent. This suggests that controlling for treatment (surgery) did not significantly affect our results and conclusions. In other words, the radiomic features appeared to be picking up patterns of disease recurrence that transcended the treatment modality. For instance, it is worth noting that the same types of features were identified as predictive of BCR when controlling for treatment modality. However, we need additional patient data who were treated with radiotherapy, LHRH to draw strong conclusions about the effect of treatment modality on radiomic features for predicting BCR.
Our study has some limitations to be acknowledged. 1) The delineation of cancer lesions on T2WI and ADC was performed by a single radiologist at I1 and I2. Ideally, the correlation should be performed with slides from RP specimens. 2) The number (n = 7) of BCR+ patients in D2 were very small in comparison with BCR− patients (n = 43). However, we still obtain a reasonable AUC on D2 suggesting the robustness of radiomic features across institutions in predicting BCR given that AUC is a strong indicator of classification performance in skewed datasets. 3) Patients in D2 were all scanned with a surface coil while the majority of the patients in D1 were scanned with an endo-rectal coil. While this is not optimal, the effect of scanners and coils on the images was somewhat mitigated through bias-field and intensity standardization methods. 4) The two classes of patients (BCR+ and BCR−) had variable follow up times. BCR may thus have not been the appropriate end point and predicting time to recurrence may have been more appropriate. However, this would require a large number of patients spanning a range of recurrence times to train a regression model. This is an interesting avenue for future research. 5) ADC maps were acquired using different b-values between the institutions. While this did not significantly affect our top discriminating features, since they are based on relative statistics, as part of future work we plan to acquire data from multiple institutions with ADC maps acquired using identical DWI acquisition and post-processing parameters.
In conclusion, radiomic features derived from pretreatment bpMRI (T2WI and ADC maps) were predictive of prostate cancer BCR. These observations held on an independent validation set that was acquired from a different institution. The ability to identify men at elevated risk for BCR, before treatment, may potentially allow for the patients to benefit from alternative and/or aggressive treatment options including adjuvant therapy.
Supplementary Material
Acknowledgments
Grant Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers
1U24CA199374-01,
R01CA202752-01A1
R01CA208236-01A1
R01 CA216579-01A1
R01 CA220581-01A1
R21CA195152-01
National Center for Research Resources under award number
1 C06 RR12463-01
the DOD Prostate Cancer Idea Development Award;
the DOD Peer Reviewed Cancer Research Program W81XWH-16-1-0329
the Ohio Third Frontier Technology Validation Fund
the Wallace H. Coulter Foundation Program in the Department of Biomedical Engineering and the Clinical and Translational Science Award Program (CTSA) at Case Western Reserve University.
References
- 1.Roehl KA, Han M, Ramos CG, Antenor JAV, Catalona WJ. Cancer Progression and Survival Rates following Anatomical Radical Retropubic Prostatectomy in 3,478 Consecutive Patients: Long-term Results. J Urol. 2004;172:910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 2.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 3.Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology. 2006;68:593–598. doi: 10.1016/j.urology.2006.03.075. [DOI] [PubMed] [Google Scholar]
- 4.Walz J, Chun FK-H, Klein EA, et al. Nomogram Predicting the Probability of Early Recurrence After Radical Prostatectomy for Prostate Cancer. J Urol. 2009;181:601–608. doi: 10.1016/j.juro.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 5.Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010;20:1254–1266. doi: 10.1007/s00330-009-1647-4. [DOI] [PubMed] [Google Scholar]
- 6.Vargas HA, Wassberg C, Akin O, Hricak H. MR Imaging of Treated Prostate Cancer. Radiology. 2012;262:26–42. doi: 10.1148/radiol.11101996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panebianco V, Barchetti F, Grompone MD, et al. Magnetic resonance imaging for localization of prostate cancer in the setting of biochemical recurrence. Urol Oncol. 2016;34:303–310. doi: 10.1016/j.urolonc.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Hegde JV, Mulkern RV, Panych LP, et al. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging. 2013;37:1035–1054. doi: 10.1002/jmri.23860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho R, Siddiqui MM, George AK, et al. Preoperative Multiparametric Magnetic Resonance Imaging Predicts Biochemical Recurrence in Prostate Cancer after Radical Prostatectomy. PLOS ONE. 2016;11:e0157313. doi: 10.1371/journal.pone.0157313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenna DA, Coakley FV, Westphalen AC, et al. Prostate Cancer: Role of Pretreatment MR in Predicting Outcome after External-Beam Radiation Therapy—Initial Experience. Radiology. 2008;247:141–146. doi: 10.1148/radiol.2471061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riaz N, Afaq A, Akin O, et al. Pretreatment endorectal coil magnetic resonance imaging findings predict biochemical tumor control in prostate cancer patients treated with combination brachytherapy and external-beam radiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:707–711. doi: 10.1016/j.ijrobp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Lemaître G, Martí R, Freixenet J, Vilanova JC, Walker PM, Meriaudeau F. Computer-Aided Detection and diagnosis for prostate cancer based on mono and multi-parametric MRI: a review. Comput Biol Med. 2015;60:8–31. doi: 10.1016/j.compbiomed.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg SB, Viswanath SE, Bloch BN, et al. Novel PCA-VIP scheme for ranking MRI protocols and identifying computer-extracted MRI measurements associated with central gland and peripheral zone prostate tumors. J Magn Reson Imaging JMRI. 2015;41:1383–1393. doi: 10.1002/jmri.24676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Litjens G, Debats O, Barentsz J, Karssemeijer N, Huisman H. Computer-Aided Detection of Prostate Cancer in MRI. IEEE Trans Med Imaging. 2014;33:1083–1092. doi: 10.1109/TMI.2014.2303821. [DOI] [PubMed] [Google Scholar]
- 15.Viswanath SE, Bloch NB, Chappelow JC, et al. Central gland and peripheral zone prostate tumors have significantly different quantitative imaging signatures on 3 Tesla endorectal, in vivo T2-weighted MR imagery. J Magn Reson Imaging JMRI. 2012;36:213–224. doi: 10.1002/jmri.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr D, Veeraraghavan H, Wibmer A, et al. Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proc Natl Acad Sci U S A. 2015;112:E6265–6273. doi: 10.1073/pnas.1505935112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gnep K, Fargeas A, Gutiérrez-Carvajal RE, et al. Haralick textural features on T2 -weighted MRI are associated with biochemical recurrence following radiotherapy for peripheral zone prostate cancer. J Magn Reson Imaging JMRI. 2016 doi: 10.1002/jmri.25335. [DOI] [PubMed] [Google Scholar]
- 18.Ginsburg SB, Rusu M, Kurhanewicz J, Madabhushi A. In: Computer extracted texture features on T2w MRI to predict biochemical recurrence following radiation therapy for prostate cancer. Aylward S, Hadjiiski LM, editors. 2014. p. 903509. [Google Scholar]
- 19.Prasanna P, Tiwari P, Madabhushi A. Co-occurrence of Local Anisotropic Gradient Orientations (CoLlAGe): A new radiomics descriptor. Sci Rep. 2016;6:37241. doi: 10.1038/srep37241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 21.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol. 2006;65:965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 22.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16–40. doi: 10.1016/j.eururo.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modat M, Cash DM, Daga P, Winston GP, Duncan JS, Ourselin S. Global image registration using a symmetric block-matching approach. J Med Imaging Bellingham Wash. 2014;1:024003. doi: 10.1117/1.JMI.1.2.024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juntu J, Sijbers J, Van Dyck D, Gielen J. Bias Field Correction for MRI Images. In: Kurzyński M, Puchała E, WoŸniak M, żołnierek A, editors. Comput Recognit Syst Proc 4th Int Conf Comput Recognit Syst CORES ’05; Berlin, Heidelberg: Springer Berlin Heidelberg; 2005. pp. 543–551. [Google Scholar]
- 26.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 27.Wang S, Burtt K, Turkbey B, Choyke P, Summers RM. Computer aided-diagnosis of prostate cancer on multiparametric MRI: a technical review of current research. BioMed Res Int. 2014;2014:789561. doi: 10.1155/2014/789561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown G, Pocock A, Zhao M, Lujan M. Conditional likelihood maximisation: a unifying framework for information theoretic feature selection. J Mach Learn Res. 2012;13:27–66. [Google Scholar]
- 29.Peng H, Long F, Ding C. Feature selection based on mutual information: criteria of max-dependency, max-relevance, and min-redundancy. IEEE Trans Pattern Anal Mach Intell. 2005;27:1226–1238. doi: 10.1109/TPAMI.2005.159. [DOI] [PubMed] [Google Scholar]
- 30.Yu S-N, Lee M-Y. Conditional mutual information-based feature selection for congestive heart failure recognition using heart rate variability. Comput Methods Programs Biomed. 2012;108:299–309. doi: 10.1016/j.cmpb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Tourassi GD, Frederick ED, Markey MK, Floyd CE. Application of the mutual information criterion for feature selection in computer-aided diagnosis. Med Phys. 2001;28:2394–2402. doi: 10.1118/1.1418724. [DOI] [PubMed] [Google Scholar]
- 32.Dietterich TG. An Experimental Comparison of Three Methods for Constructing Ensembles of Decision Trees: Bagging, Boosting, and Randomization. Mach Learn. 40:139–157. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



