Abstract
Prevalence of cannabis use is increasing but many regular users do not develop cannabis use disorder (CUD); thus, CUD risk identification among current users is vital for targeted intervention development. Existing data suggest that high distress intolerance (DI), an individual difference reflective of the ability to tolerate negative affect, may be linked to CUD, but no studies have tested possible neurophysiological mechanisms. Increased motivated attentional processing of cannabis and negative emotional stimuli as indexed by neurophysiology (i.e., the late positive potential (LPP)), particularly during acute stress, may contribute to CUD among high DI users. Frequent cannabis users with high (n=61) and low DI (n=44) viewed cannabis, negative, and matched neutral images during EEG recording before and after a laboratory stressor. Cannabis cue-elicited modulation of the 1000–3000ms LPP was larger in high DI users at post-stressor only, though the effect was only robust in the 1000–2000ms window. Further, modulation magnitude in the high DI group covaried with stress-relief craving and some CUD indices in the 400–1000ms and 1000–3000ms windows, respectively. No significant effects of DI on negative stimuli-elicited LPP modulation were found, though inverse associations with some CUD indices were observed. Finally, exploratory analyses revealed some evidence for DI moderation of the relation between subjective stressor reactivity and negative stimuli-elicited LPP modulation such that greater stressor reactivity was associated with blunted vs. enhanced modulation in the high and low DI groups, respectively. Negative and cannabis stimuli-elicited LPP modulation appear to index distinct, CUD-relevant neural processes in high DI cannabis users.
Keywords: cannabis use disorder, cue reactivity, distress intolerance, late positive potential, mood induction
Introduction
The prevalence of cannabis use and cannabis use disorder (CUD) has increased in the United States over the past 20 years (Hasin et al., 2015). Although most users do not develop CUD (Cougle et al., 2016), national surveys from 2012–2013 suggest the lifetime prevalence of DSM-5 CUD is 6.3% (Hasin et al., 2016) and is expected to increase due to changes in cultural acceptance and legalization of cannabis (Hasin et al., 2017). Given the disability associated with CUD (Hasin et al., 2016), it is imperative to identify mechanisms that increase risk of CUD among current users to guide targeted intervention development.
Negative reinforcement processes are thought to be central to the maintenance of and progression to substance use disorders among regular users (Baker et al., 2004). Recent studies have demonstrated that high distress intolerance (DI), an individual difference reflective of the ability to tolerate and effectively manage negative affect, is linked with CUD indices in regular users (Bujarski et al., 2012; Farris et al., 2016). Further, these associations appear to be partially mediated by increased coping motives for cannabis use (i.e., self-administering cannabis to reduce negative affect), motives which have been linked with increased risk of developing CUD among regular users (van der Pol et al., 2013). High DI-related phenomena (i.e., low self-efficacy for resisting cannabis use while emotionally distressed) have also been linked with poorer CUD treatment outcome (Gullo et al., 2017), underscoring DI’s clinical relevance.
These data suggest that high DI may confer risk for CUD via increases in motivational salience/attentional processing of cannabis and negative emotional stimuli during acute stress. No studies have evaluated this specific hypothesis, but high DI has been prospectively linked to increased craving and relapse during acute stress in other substance-using populations (Abrantes et al., 2008; Banducci et al., 2012). Similarly, high DI has been associated with increased attention bias towards negative emotional stimuli (Macatee et al., 2017) and related psychiatric symptoms (e.g., negative intrusive thoughts; Macatee et al., 2016; Macatee et al., 2013) during acute stress. DI has not been evaluated as a moderator of laboratory stressor responding in cannabis users, though two studies have evaluated stressor-elicited craving and negative affect among frequent users, with mixed results (Cuttler et al., 2017; McRae-Clark et al., 2011), highlighting the possible relevance of individual differences (e.g., DI) to stressor responding among cannabis users. Further, these studies relied exclusively upon self-report/endocrine measures of craving and negative affect, whereas neural indices of these phenomena may be more sensitive to abnormalities in motivational salience/attentional processing of cannabis and negative emotional stimuli.
The late positive potential (LPP) is a neurophysiological marker of sustained, attentional processing of motivationally salient visual stimuli that begins approximately 400ms after stimulus onset, with larger amplitudes elicited by motivationally significant (e.g., threat, erotica, substance cues; Dunning et al., 2011; Hajcak et al., 2010) relative to neutral stimuli. The LPP elicited while viewing images depicting substance-related and negatively-valenced content has been linked with craving (see Field et al., 2009 for a meta-analysis) and negative emotionality (e.g., state rumination, anxiety psychopathology; Lewis et al., 2015; MacNamara et al., 2016), respectively. Taken together, this suggests that the LPP may be a suitable neural measure with which to test hypothesized mechanisms of CUD in regular cannabis users with high DI (i.e., increased motivated attentional processing of cannabis and negative emotional cues during acute stress).
Only one study known to the authors assessed cannabis cue-elicited modulation of the LPP and evaluated its relationship with self-reported craving (Wolfling et al., 2008). Wolfling and colleagues (2008) found enhanced cannabis cue-elicited modulation of the LPP in heavy cannabis users relative to controls, but the LPP effect was not significantly associated with self-reported positive or negative-reinforcement craving; no significant group effects were found on negative stimuli-elicited LPP modulation. However, these researchers utilized a small sample of cannabis users (n=15) with no history of psychiatric disorders, which likely resulted in a group of cannabis users different from those with high DI. Indeed, in contrast to Wolfling and colleagues’ (2008) findings, enhanced negative stimuli-elicited LPP modulation has been found in internalizing psychopathology linked to high DI (Allan et al., 2014; Macatee et al., 2015) in multiple studies (Lewis et al., 2015; MacNamara et al., 2016), suggesting that DI may moderate the effect of negative stimuli on the LPP among cannabis users. Relatedly, high DI’s positive associations with self-reported drug craving in the context of acute stress (Banducci et al., 2016) suggests that individual differences in DI among cannabis users may also moderate the effect of cannabis stimuli on the LPP, particularly during acute stress.
No studies known to the authors have employed a laboratory stressor to evaluate the effect of acute stress on negative or substance stimuli-elicited LPP modulation. Given the centrality of dysfunctional stressor-elicited salience attribution/attentional processing of substance and negative affective stimuli to prominent theoretical models of substance use disorders (e.g., Baker et al., 2004) and conceptually-related individual differences (e.g., DI, coping motives), this is a significant limitation. Measuring negative and cannabis stimuli-elicited LPP modulation before and after a laboratory stressor allows for an explicit test of the relations between exaggerated motivated attentional processing of negative and cannabis stimuli in a context (i.e., acute stress) that is theoretically most relevant to cannabis use-related problems among high DI users. Finally, by matching cannabis users with high and low DI on use frequency, differences in cannabis cue-elicited LPP modulation in the context of acute stress can be attributed to motivated attentional processes specific to DI and cannabis use-related problems as opposed to the mere degree of cannabis use, allowing for a rigorous test of exaggerated stressor-elicited attentional processing of negative and cannabis stimuli as mechanisms linking high DI and CUD.
In the present study, we sought to evaluate increased attentional processing of cannabis and negative emotional stimuli during acute stress as possible neural mechanisms of CUD among cannabis users high in DI. Towards this end, regular users with high and low DI were recruited and passively viewed cannabis and negative emotional stimuli before and after a laboratory stressor while EEG was recorded. We predicted that 1) cannabis and negative stimuli-elicited enhancement of the LPP would be greater in high relative to low DI users after the laboratory stressor, 2) post-stressor cannabis and negative stimuli-elicited enhancement of the LPP would be positively associated with state subjective stress-relief craving and negative affect in the high DI group, and 3) post-stressor cannabis and negative stimuli-elicited enhancement of the LPP would be positively associated with cannabis problem severity in the high DI group.
Materials and Methods
Participants
Regular cannabis users (i.e. >= twice per week for at least one year) between 18 and 30 years old with high (i.e., >= 20) and low (i.e., <= 6) scores on the Distress Intolerance Index (DII; McHugh & Otto, 2012), a self-report measure of DI (see Supplemental material for more detail on this measure), were recruited from the psychology student subject pool at a large southeastern university as well as the surrounding community; participants were offered course credit or monetary compensation for study completion. Cut-off scores for high and low DI were determined based on the median score in a clinical sample (Macatee et al., 2018) and the bottom quartile score in a non-clinical young adult sample (Macatee et al., 2016), respectively. Among the 489 individuals screened, 63 high DI users were recruited as part of a larger RCT evaluating the efficacy of a computerized DI treatment in regular cannabis users reporting difficulty managing negative emotions; the high DI group’s baseline data were used in the present study. Among the 620 individuals screened, 44 were recruited as a low DI matched comparison group and only attended the baseline appointment. Participants were excluded if they endorsed history of psychotic symptoms, current suicidal ideation, or met criteria for Bipolar disorder and were not stabilized on medication for at least four weeks. Additional exclusion criteria for the high DI group included concurrent psychotherapy for stress/substance use and change in psychotropic medication in the past month. EEG data were not available for two high DI participants due to excessive drift and inadequate electrode contact, resulting in 61 high DI participants available for analyses.
Measures
Cannabis Use Descriptives and Problems
Cannabis Use Descriptives
Cannabis use history variables including typical method of administration and social context, number of prior serious quit attempts, and age at cannabis use initiation and onset of regular use were collected using the Marijuana Smoking History Questionnaire (Bonn-Miller & Zvolensky, 2009). Current cannabis use reduction intentions were measured with the Marijuana Ladder (Slavet et al., 2006), a 10-item visual analog ranging from pre-contemplation to maintenance stages of change. Number of cannabis use days in the past four weeks was assessed with the Timeline Follow-Back (Hjorthoj et al., 2012), and cannabis use motives were assessed with the Marijuana Motives Measure (Zvolensky et al., 2007). Past year frequency of other substance use was also collected with the Drug Use Questionnaire (Hien & First, 1991).
Cannabis Use Problems
Self-reported cannabis problem severity over the past four weeks was assessed with the Marijuana Problems Scale (MPS; Stephens et al., 2000), a 19-item measure with good psychometric properties (Buckner et al., 2011). Lifetime and past year CUD criteria were assessed using the Substance Use Disorder module of the SCID-5-RV (First et al., 2015); number of CUD criteria met in the past year was used to operationalize interviewer-assessed current CUD severity. Interviews were administered by a doctoral-level graduate student and post-baccalaureate research assistant. The research assistant received extensive diagnostic training and consulted with the graduate student when needed. In the current study, a subsample of subjects (n=17) were used for reliability coding which yielded excellent interrater reliability for past-year CUD criteria count (r = .98, p < .001).
State Variables
Negative affect was measured with the negative affect subscale of the Positive and Negative Affect Schedule (PANAS-NA; Mackinnon et al., 1999), a five-item self-report measure of state distress intensity. Cannabis craving was measured with the Marijuana Craving Questionnaire (MCQ), a 12-item self-report measure of four forms of state cannabis craving (i.e., compulsivity, emotionality, expectancy, and purposefulness), though the factor intercorrelation (0.78) and item cross-loadings suggests that emotionality and expectancy subscales both measure aspects of negative-reinforcement craving (Heishmen et al., 2009). Thus, these subscales’ items were averaged to measure stress-relief craving in the present study.1
Procedure
After interested participants provided informed consent, they were screened for eligibility and CUD criteria were assessed. Eligible participants were asked to abstain from drug use for 24 hours prior to the baseline appointment and to refrain from nicotine/caffeine use on the day of the appointment; upon arrival for the baseline, self-report was used to assess compliance and the appointment was rescheduled if the participant failed to abstain. In addition to demographic and substance use questionnaires, various measures of negative emotionality were also completed as part of the larger study (see Table S1 in Supplemental material). Following completion of the questionnaires, EEG recording was conducted in a dimly lit, sound-attenuated room. Stimuli were presented using a Dell OptiPlex 780 computer running E-Prime version 2.0.8.90. Stimuli were presented on a 21” CRT color monitor at a viewing distance of 100 cm, subtending a visual angle of 3.5°. The recording session consisted of several different tasks, including the Picture Viewing task which was completed before and after the stress induction. Participants completed state negative affect and cannabis craving measures after both Picture Viewing tasks (see Figure S1). Total recording time lasted between 1 and 1.5 hours.
Picture Viewing Task
Participants were instructed to view four categories of pictures (i.e., cannabis, negative, cannabis-neutral, negative-neutral) presented on the screen and allow themselves to respond naturally, but keep their eyes on the pictures for the entire presentation duration. A live camera feed was used to monitor compliance. Pictures were presented for 3000ms, followed by an ITI with a random duration of 500–3000ms (steps of 500ms). Each trial began with a fixation cross presented for 1000ms. Before each block, participants were informed of the upcoming content type. Pictures were randomly presented within each block and block order was randomized. Each block was composed of 20 pictures of a single content type (i.e., cannabis, negative, cannabis-neutral, negative-neutral), with each picture presented once in its block. The same picture set was used for the pre and post-stressor picture viewing task such that each picture was presented a total of two times to each participant. Negative and matched neutral pictures (see picture list in Supplemental material) were selected from the International Affective Picture System (IAPS; Lang et al., 1999) based upon prior work (Hajcak et al., 2009). Cannabis and matched neutral images were selected from online searches and stimuli sets used in prior cannabis studies (Asmaro et al., 2014; Cousijn et al., 2012; Vujanovic et al., 2016). Cannabis images included pictures of cannabis/paraphernalia as well as pictures of people smoking cannabis. Separate neutral picture categories were used for cannabis and negative images to match specific corresponding image features (e.g., size, color, presence of people). Task duration was approximately 10 minutes.
Stress Induction
The Mannheim Multicomponent Stress Test (MMST) is a five minute computerized task that involves simultaneous stressors in five modalities: 1) cognitive (i.e., increasingly difficult mental arithmetic), 2) emotional (i.e., presentation of images characterized by negative valence/high arousal), 3) acoustic (i.e., increasingly loud, continuous white noise), and 4) motivational (i.e., loss of monetary reward for each mistake on the mental arithmetic task). Significant MMST-elicited stress reactivity across subjective and physiological indices has been observed in multiple samples (Reinhardt et al., 2012). In the present study, self-reported stress reactivity was measured using the mean of five negative affect words (anxiety, frustration, irritability, difficulty concentrating, physical discomfort) scored on a 0–100 VAS scale (α = .88).
Stimulus Delivery and EEG Measurement
ERP data were collected using a Dell OptiPlex 780 computer and Neuroscan Acquire software. Two 64-channel Neuroscan SynAmps RT amplifiers and a BrainVision actiCap 64-channel cap were used to measure EEG responses (1000 Hz sampling rate, with an online analog bandpass filter of 0.05 – 100 Hz). The midline electrode AFz was used as the ground and FCz was used as an online reference electrode. Offline, the data were re-referenced to the averaged mastoids (electrodes TP9 and TP10). Horizontal electrooculogram (EOG) activity was recorded from electrodes placed lateral to the outer canthus of each eye, while vertical EOG activity was recorded from electrodes placed above and below the left eye. Electrodes were filled using high-chloride (10%) Abrasive Electrolyte-Gel (EasyCap). All impedance values were below 10 kohms throughout the recording session.
Data Preprocessing
Data were first downsampled to 250 Hz, then high-pass (0.1 Hz; ripple = .05 dB, attenuation = 80 dB) and low-pass (30 Hz; ripple = .01 dB, attenuation = 40 dB) FIR filters were applied. Given that FCz was the online reference, it was regenerated offline using the average reference assumption so that it could be used in analyses. Epochs locked to picture onset were defined using the full 3000ms trial length with a 200ms pre-stimulus baseline correction. The Fully Automated Statistical Thresholding for EEG artifact Rejection algorithm (FASTER; Nolan et al., 2010), an EEGLAB plugin, was used for artifact detection and rejection (see Supplemental material for more detail). Of the 105 participants with EEG data, some did not have valid data for all four picture categories at both pre and post-stressor due to inattention (i.e., averting eyes from the pictures; n=20), technical errors (n=1), or voluntary withdrawal from the study (n=1).
Based upon prior substance and negative emotion LPP studies (Dunning et al., 2011; Foti & Hajcak, 2008), the entire 400–3000ms as well as separate 400–1000ms, 1000–2000ms, and 2000–3000ms time windows were used to measure average LPP amplitude locked to picture onset. Consistent with prior findings of a frontal-central maximum for substance cue-elicited LPP modulation using averaged mastoid reference (Dunning et al., 2011) and the present study’s scalp topographies (see Figure 1), cannabis and matched neutral LPP amplitudes were averaged over frontal-central sites (Fz, FCz, FC1, FC2, Cz). Consistent with prior findings of a central-parietal maximum for negative stimuli-elicited LPP modulation using averaged mastoid reference (Hajcak et al., 2009) and the present study’s scalp topographies (see Figure 2), negative and matched neutral LPP amplitudes were averaged over central-parietal sites (Cz, CPz, CP1, CP2, Pz). In line with recent recommendations (Hajcak, Meyer, & Kotov, 2017), split-half reliabilities (i.e., Spearman-Brown adjusted correlations between odd and even trial means) of the individual condition (see Tables S2 and S3 in Supplemental material) and difference score (see Table 1) amplitudes were computed. Consistent with a recent study (Luking et al., 2017), residualized difference scores were more reliable than subtraction-based scores; thus, residualized difference scores were used to test hypotheses two and three.
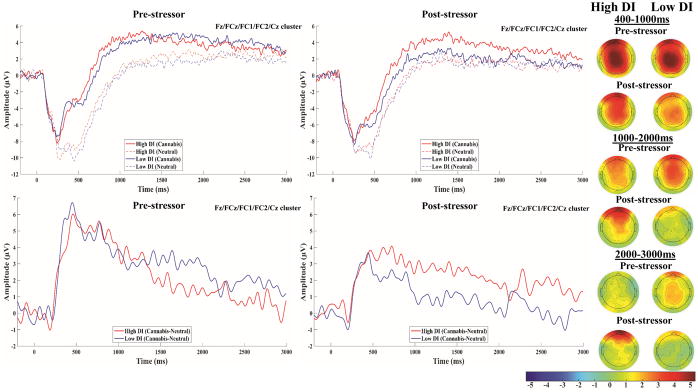
Figure 1.
Stimulus-locked ERPs to cannabis and matched neutral images at pre (top-left) and post-stressor (top-right) in the high and low DI groups are presented at the averaged frontal-central cluster (Fz, FCz, FC1, FC2, Cz). Stimulus-locked difference waves (i.e., cannabis – matched neutral) at pre (bottom-left) and post-stressor (bottom-right) in the high and low DI groups are presented at the averaged frontal-central cluster; a 15 Hz low-pass filter was applied to the difference waves for display purposes only. Topographical maps of the 400–1000ms, 1000–2000ms, and 2000–3000ms difference wave amplitudes at pre and post-stressor in high and low DI groups are presented on the right; the colorbar scale is in microvolts.
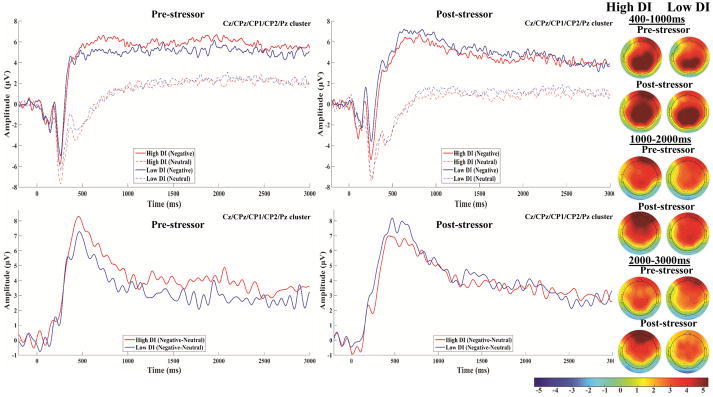
Figure 2.
Stimulus-locked ERPs to negative and matched neutral images at pre (top-left) and post-stressor (top-right) in the high and low DI groups are presented at the averaged central-parietal cluster (Cz, CPz, CP1, CP2, Pz). Stimulus-locked difference waves (i.e., negative – matched neutral) at pre (bottom-left) and post-stressor (bottom-right) in the high and low DI groups are presented at the averaged central-parietal cluster; a 15 Hz low-pass filter was applied to the difference waves for display purposes only. Topographical maps of the 400–1000ms, 1000–2000ms, and 2000–3000ms difference wave amplitudes at pre and post-stressor in high and low DI groups are presented on the right; the colorbar scale is in microvolts.
Table 1.
Picture Viewing Task Descriptives
| High DI (n=61) | Low DI (n=44) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| M | SD | Reliability | M | SD | Reliability | |
| Pre-Stress | ||||||
|
| ||||||
| Self-Report | ||||||
| PANAS-NA | 1.88 | 0.73 | .81 | 1.21 | 0.37 | .84 |
| MCQ-StressRelief | 5.02 | 1.31 | .84 | 2.98 | 1.39 | .84 |
| MCQ-Purposefulness | 5.34 | 1.41 | .81 | 4.21 | 1.81 | .87 |
| MCQ-Compulsivity | 2.76 | 1.47 | .75 | 1.29 | 0.70 | .67 |
| ERPs | ||||||
| 400–1000ms LPP | ||||||
| ΔCannabis | 4.90 | 3.94 | .28 (.46) | 4.88 | 4.37 | .46 (.66) |
| ΔNegative | 6.26 | 4.09 | .37 (.70) | 4.97 | 2.47 | .04 (.32) |
| 1000–2000ms LPP | ||||||
| ΔCannabis | 2.28 | 3.48 | −.12 (.44) | 3.17 | 3.67 | .29 (.65) |
| ΔNegative | 3.91 | 4.28 | .54 (.75) | 3.10 | 3.00 | .52 (.57) |
| 2000–3000ms LPP | ||||||
| ΔCannabis | 0.90 | 3.34 | −.27 (.34) | 1.83 | 3.67 | .62 (.73) |
| ΔNegative | 3.57 | 4.12 | .51 (.69) | 2.71 | 2.92 | .52 (.54) |
|
| ||||||
| Post-Stress | ||||||
|
| ||||||
| Self-Report | ||||||
| PANAS-NA | 1.80 | 0.72 | .77 | 1.22 | 0.49 | .80 |
| MCQ-StressRelief | 5.02 | 1.37 | .86 | 3.13 | 1.48 | .88 |
| MCQ-Purposefulness | 5.41 | 1.45 | .85 | 4.20 | 1.93 | .93 |
| MCQ-Compulsivity | 2.73 | 1.62 | .84 | 1.25 | 0.45 | .59 |
| ERPs | ||||||
| 400–1000ms LPP | ||||||
| ΔCannabis | 3.60 | 4.62 | .45 (.56) | 2.08 | 3.66 | .46 (.58) |
| ΔNegative | 6.12 | 4.31 | .73 (.81) | 6.69 | 4.40 | .44 (.49) |
| 1000–2000ms LPP | ||||||
| ΔCannabis | 2.68 | 4.70 | .21 (.38) | 0.52 | 3.89 | .41 (.50) |
| ΔNegative | 3.69 | 3.94 | .70 (.70) | 4.04 | 4.19 | .44 (.50) |
| 2000–3000ms LPP | ||||||
| ΔCannabis | 1.70 | 5.10 | .43 (.52) | −0.12 | 3.97 | .50 (.42) |
| ΔNegative | 3.05 | 4.25 | .58 (.61) | 2.98 | 4.35 | .49 (.57) |
Note. PANAS-NA=Positive and Negative Affect Schedule-Negative Affect (mean response); MCQ-StressRelief=Marijuana Craving Questionnaire-Emotionality and Expectancy subscales (mean response); MCQ-Purposefulness=Marijuana Craving Questionnaire-Purposefulness subscale (mean response); MCQ-Compulsivity=Marijuana Craving Questionnaire-Compulsivity subscale (mean response); ΔCannabis=mean cannabis LPP-mean matched neutral LPP; ΔNegative=mean negative LPP-mean matched neutral LPP.
Cronbach’s alpha was used to measure internal reliability of self-report measures; split-half reliabilities (even vs. odd trials) were computed for ERP difference scores; regression-based difference score reliabilities are presented in parentheses.
Data Analytic Plan
Due to unbalanced data (see Data Preprocessing section), multilevel marginal models were used to maximize inclusion of all valid data points. 400–3000ms as well as separate 400–1000ms, 1000–2000ms, and 2000–3000ms mean LPP amplitudes were entered as the dependent variable in separate models. Picture (i.e., cannabis/negative vs. neutral) and Stress (i.e., pre vs. post-stressor) were entered as within-subject factors and DI group was entered as a between-subjects factor. All main and interaction effects were included in each model. DI*Picture*Stress interactions were examined to test hypothesis one; significant interaction terms were probed with planned comparisons of DI*Picture interaction effects at pre vs. post-stressor. To test hypotheses two and three, bivariate correlations were used to examine associations between post-stressor ΔLPP (i.e., unstandardized residuals from cannabis/negative LPP regressed on matched neutral LPP) and cannabis problems, state negative affect, and state stress-relief craving in the high DI group. Spearman correlations were utilized for highly skewed variables in planned (i.e., state negative affect) and exploratory (state purposefulness and compulsivity craving, # of prior serious quit attempts) analyses. SPSS was used to conduct analyses.
Results
Descriptives
As expected, high relative to low DI cannabis users demonstrated greater cannabis use-related problems across multiple indices including self-reported past-month cannabis problem severity, # of CUD criteria, and # of prior serious quit attempts. Relatedly, high relative to low DI users reported substantially greater coping motives for cannabis use. In contrast, no significant differences were observed on demographics or substance use history variables, though high DI users reported greater interest in changing their cannabis use (see Table 2).
Table 2.
Demographics and Cannabis Use Descriptives
| High DI (n=61) | Low DI (n = 44) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Demographics | M | SD | M | SD | F/χ2 | p | hp2 |
| Age | 20.28 | 2.33 | 20.25 | 2.08 | F(1,103)=0.00 | .95 | <.001 |
| % Female | 68.90 | --- | 50.00 | --- | χ2 (1, N=105) = 3.82 | .051 | --- |
| % Non-Hispanic White | 60.66 | --- | 72.73 | --- | χ2 (3, N=105) = 4.22 | .24 | --- |
|
| |||||||
| Cannabis Use Descriptives | |||||||
|
| |||||||
| Method of cannabis administration (% bowl/bong) | 73.77 | --- | 63.64 | --- | χ2 (4, N=104) = 0.99 | .91 | --- |
| Cannabis use social context (% alone) | 31.15 | --- | 13.64 | --- | χ2 (2, N=105) = 5.47 | .065 | --- |
| Age at cannabis use initiation | 15.10 | 2.01 | 15.64 | 1.73 | F(1,103)=2.06 | .15 | .02 |
| Age at regular cannabis use onset | 17.15 | 1.68 | 17.67 | 1.86 | F(1,103)=2.26 | .14 | .021 |
| Intention to change cannabis use | 5.59 | 2.45 | 4.23 | 2.27 | F(1,103)=8.40 | .005 | .075 |
| # of use days in past month | 20.95 | 6.10 | 20.34 | 6.32 | F(1,103)=0.25 | .62 | <.001 |
|
| |||||||
| Other Substance Use Descriptives | |||||||
|
| |||||||
| Nicotine/Tobacco | 2.03 | 1.70 | 1.80 | 1.52 | F(1,103)=0.54 | .46 | .005 |
| Alcohol | 3.38 | 1.00 | 3.23 | 0.80 | F(1,103)=0.67 | .42 | .006 |
| Cocaine/Crack | 0.74 | 0.95 | 0.77 | 1.14 | F(1,103)=0.03 | .86 | <.001 |
| Amphetamines | 1.25 | 1.42 | 1.11 | 1.33 | F(1,103)=0.23 | .63 | .002 |
| Opioids | 0.38 | 0.73 | 0.16 | 0.48 | F(1,103)=2.96 | .088 | .028 |
| Hallucinogens | 0.97 | 1.00 | 0.98 | 0.93 | F(1,103)=0.00 | .96 | <.001 |
| Sedatives | 0.72 | 0.95 | 0.66 | 0.89 | F(1,103)=0.12 | .74 | .001 |
|
| |||||||
| Cannabis Use Problems | |||||||
|
| |||||||
| Number of serious quit attempts | 1.13 | 1.67 | 0.30 | 0.59 | F(1,103)=10.10 | .002 | .089 |
| MPS (total score) | 9.44 | 5.48 | 3.18 | 2.32 | F(1,103)=50.75 | <.001 | .33 |
| # of CUD criteria (past year) | 5.74 | 2.87 | 2.64 | 1.64 | F(1,103)=41.38 | <.001 | .287 |
| # of CUD criteria (lifetime) | 6.75 | 2.73 | 3.89 | 2.34 | F(1,103)=31.67 | <.001 | .235 |
|
| |||||||
| Cannabis Use Motives | |||||||
|
| |||||||
| Coping | 4.01 | 0.80 | 2.59 | 0.98 | F(1,103)=66.00 | <.001 | .391 |
| Conformity | 1.48 | 0.59 | 1.20 | 0.38 | F(1,103)=7.67 | .007 | .069 |
| Enhancement | 4.47 | 0.63 | 4.27 | 0.80 | F(1,103)=2.15 | .15 | .02 |
| Expansion | 3.40 | 1.19 | 2.70 | 1.13 | F(1,103)=9.23 | .003 | .082 |
| Social | 3.18 | 0.92 | 2.68 | 0.98 | F(1,103)=7.06 | .009 | .064 |
Note. Ms, SDs, and F/χ2 tests of the DI group effect for demographics, substance use descriptives, and cannabis problems/motives are presented above; χ2 tests are used for categorical variables (Sex: male, female; Race/Ethnicity: black, white, hispanic, asian, other; Typical cannabis use method: joint, bowl, bong, one-hitter, vaporizer; Typical cannabis use social context; alone, 2–3 people, 4+ people). Other substance use descriptives=typical use frequency in the past year (0=“never”,1=“once”,2=“monthly or less”,3=“2–4 times/month”,4=“2–3 times/week”,5=“4+ times/week”); MPS=Marijuana Problems Scale; CUD criteria=# of Cannabis Use Disorder criteria met in the past year; Cannabis use motives=Marijuana Motives Measure subscales (mean response). Significant group differences are bolded.
Stress Induction Manipulation Check
A RM-ANOVA with Time (pre-MMST, post-MMST) entered as a within-subject factor and DI group entered as a between-subjects factor. As expected, the effect of Time was significant, F(1,103)=236.17, p<.001, such that subjective negative affect increased from pre, M=21.1, SE=1.5, to post-stressor, M=49.6, SE=2.1. The DI*Time interaction did not reach significance, F(1,103)=3.92, p=.050, revealing a trend towards greater subjective stress reactivity in the high relative to low DI group. In contrast, the main effect of DI was significant, F(1,103)=74.44, p<.001, revealing higher overall subjective stress in the high, M=49.0, SE=2.1, relative to low DI, M=21.7, SE=2.4, group.
Exploratory analyses testing relations between stressor reactivity and ΔCanLPP/ΔNegLPP revealed no significant effects for ΔCanLPP, whereas significant DI*StressorReactivity interactions emerged for ΔNegLPP such that greater subjective stressor reactivity was generally related to enhanced ΔNegLPP in low DI cannabis users but reduced ΔNegLPP in high DI cannabis users, with this pattern most robust for post-stressor ΔNegLPP (see Supplemental material).
Hypothesis One – Effect of DI on LPP modulation by picture type at pre vs. post-stressor
DI and cannabis cue-elicited LPP modulation (ΔCanLPP)
The main effect of Picture was significant across all time windows, Fs > 13.85, ps < .001, such that amplitudes were greater during cannabis relative to neutral images. The Stress main effect was non-significant at 400–1000ms, F(1,295.08) = 0.21, p = .65, but significant at the later windows, Fs > 6.19, ps < .014, such that overall amplitudes decreased from pre to post-stressor, whereas the Stress*Picture interaction was significant for the 400–1000ms, F(1,292.78) = 11.85, p = .001, but not later time windows, Fs < 3.54, ps > .060, revealing a decrease in cannabis cue-elicited modulation of the early LPP from pre to post-stressor. The DI*Stress*Picture interaction was non-significant for the entire 400–3000ms window, F(1,295.79)=3.61, p=.058, and 400–1000ms LPP, F(1,292.78) = 0.87, p = .35, but was significant for the 1000–2000ms, F(1,293.16) = 5.40, p = .021, and 2000–3000ms, F(1,294.90) = 3.95, p = .048, windows. Importantly, the DI*Stress*Picture interaction remained significant for the 1000–2000ms, F(1,290.55)=5.20, p=.023, but not 2000–3000ms, F(1,292.66)=3.83, p=.051, window after Sex was added to the model; further, sex did not moderate the interaction for either time window (see Supplemental material). As a final test of robustness, post-stressor ΔCanLPP outliers revealed by box plots were excluded and analyses rerun (see Figure S2); the DI*Stress*Picture interaction remained significant for the 1000–2000ms, F(1,287.53)=4.01, p = .046, but not 2000–3000ms, F(1,284.59) = 2.13, p = .15, window, after removal of post-stressor data from two and three high DI participants, respectively.
For 1000–2000ms, follow-up comparisons in the complete sample revealed significantly greater post-stressor amplitudes during cannabis relative to neutral images in the high, t(293.53) = 4.80, p < .001, but not low DI group, t(294.32) = 1.24, p = .22; in contrast, both high, t(293.57) = 4.27, p < .001, and low, t(291.31) = 5.24, p < .001, DI groups demonstrated greater pre-stressor amplitudes during cannabis relative to neutral images.
Because of the less robust DI*Stress*Picture interaction for 2000–3000ms, follow-up comparisons have been moved to the Supplemental material.
DI and negative stimuli-elicited LPP modulation (ΔNegLPP)
The main effect of Picture was significant across all time windows, Fs > 117.40, ps < .001, such that amplitudes were greater during negative relative to neutral images. The Stress main effect was non-significant at 400–1000ms, F(1,293.97) = 0.08, p = .78, but significant at the later windows, Fs > 9.57, ps < .003, such that overall amplitudes decreased from pre to post-stressor, whereas the Stress*Picture interaction was non-significant across all time windows, Fs < 2.22, ps > .13. The DI*Stress*Picture interaction was non-significant across all time windows, Fs < 2.74, ps > .098; inclusion of Sex in the model did not alter statistical significance nor was the Sex*DI*Stress*Picture interaction significant, though interactions emerged such that females generally demonstrated greater ΔNegLPP than males, particularly at post-stressor (see Supplemental material).
Hypothesis Two – Relations between post-stressor ΔCanLPP/ΔNegLPP and state stress-relief craving/negative affect in high DI users
Complete correlation tables are presented in Tables S4 and S5 in the Supplemental material.
As hypothesized, post-stressor 400–1000ms ΔCanLPP was positively associated with state stress-relief craving, r = .35, p = .009; however, correlations were non-significant for later windows, rs < .21, ps > .14. Contrary to hypotheses, correlations between post-stressor ΔCanLPP and state negative affect were non-significant across all time windows, rs < .12, ps > .38. Exploratory analyses suggested that post-stressor 400–1000ms ΔCanLPP was specifically related to stress-relief rather than compulsivity or purposefulness craving; further, the positive association with stress-relief craving was absent for pre-stressor ΔCanLPP.
Contrary to hypotheses, post-stressor ΔNegLPP was non-significantly associated with state stress-relief craving, rs < .23, ps > .11, and negative affect, rs < .26, ps > .06, across all time windows, though a significant inverse correlation with state compulsivity craving emerged in the 400–1000ms window.
Hypothesis Three - Relations between post-stressor ΔCanLPP/ΔNegLPP and cannabis problems in high DI users
Complete correlation tables are presented in Tables S4 and S5 in the Supplemental material.
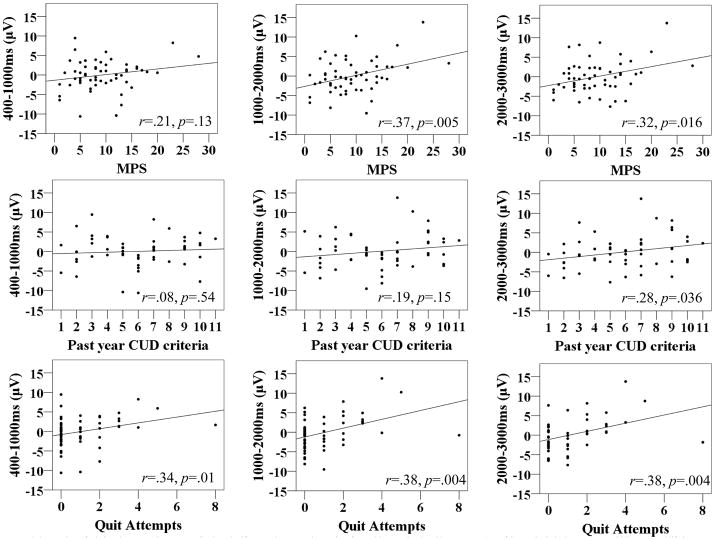
See Figure 3 for ΔCanLPP scatter plots and correlations. The MPS and ΔCanLPP were significantly positively correlated in later time windows only; however, the significant associations appeared to be driven by two participants with MPS scores approximately 2.5 SDs above the mean and became non-significant after they were excluded (1000–2000ms: r = .23, p = .092; 2000–3000ms: r = .17, p = .21). A significant correlation between CUD criteria and ΔCanLPP emerged in the 2000–3000ms window only, whereas no significant correlations with pre-stressor ΔCanLPP emerged. Exploratory correlations were conducted using number of prior serious quit attempts as an index of cannabis problem severity; number of prior quit attempts was significantly positively correlated with ΔCanLPP across all time windows, whereas correlations with pre-stressor ΔCanLPP were non-significant.
Figure 3.
Scatterplots of relations between ΔCanLPP amplitude and self-reported past month severity of cannabis use-related problems (top row), # of CUD criteria in the past year (middle row), and lifetime # of serious cannabis quit attempts (bottom row). 400–1000ms, 1000–2000ms, and 2000–3000ms ΔCanLPP amplitude are presented from left to right. Pearson (MPS, CUD)/Spearman (Quit Attempts) correlation coefficients and associated significance values are presented in the bottom right of each scatterplot.
See Figure 4 for ΔNegLPP scatter plots and correlations. The MPS and ΔNegLPP were significantly inversely correlated in the 400–1000ms window only; the significant relation with early ΔNegLPP became non-significant when the MPS outliers were excluded, r = −.24, p = .088. CUD criteria and ΔNegLPP were significantly inversely correlated in the 1000–2000ms window only. Unexpectedly, pre-stressor early ΔNegLPP was significantly inversely correlated with CUD criteria and the MPS, with the latter association non-significant after exclusion of the two MPS outliers, r = −.23, p = .095. Additional exploratory analyses revealed that number of prior quit attempts was non-significantly correlated with post-stressor ΔNegLPP across all time windows, though inverse correlations with pre-stressor ΔNegLPP were significant in the 400–1000ms and 1000–2000ms windows.
Figure 4.
Scatterplots of relations between ΔNegLPP amplitude and self-reported past month severity of cannabis use-related problems (top row), # of CUD criteria in the past year (middle row), and lifetime # of serious cannabis quit attempts (bottom row). 400–1000ms, 1000–2000ms, and 2000–3000ms ΔNegLPP amplitude are presented from left to right. Pearson (MPS, CUD)/Spearman (Quit Attempts) correlation coefficients and associated significance values are presented in the bottom right of each scatterplot.
Discussion
As predicted, cannabis users with high relative to low DI demonstrated greater cannabis cue-elicited modulation of the LPP after the stress induction, though this effect was limited to the later time windows (i.e., >1,000ms) and only robust in the 1000–2000ms window; this finding suggests that cannabis cues elicit prolonged motivated attention to a greater extent in high relative to low DI users during acute stress. Further, greater cannabis cue-elicited modulation of the early LPP after the stressor was specifically related to enhanced subjective stress-relief craving but not state negative affect or stressor reactivity among high DI cannabis users, partially consistent with hypotheses and extant data on DI and coping motives for cannabis use (Bujarski et al., 2012; Farris et al., 2016). Somewhat consistent with predictions, cannabis cue-elicited modulation of the LPP during acute stress was significantly related to three indices of cannabis problem severity among high DI users, though these effects were generally limited to the later time windows and the relationship with the MPS was not robust to outlier exclusion. Further, with the exception of the MPS, the positive relations between cannabis problem indices and cannabis cue-elicited modulation of the LPP were more robust during acute stress in the high DI group.
Contrary to predictions, negative stimuli-elicited modulation of the LPP after the stressor was non-significantly different across high and low DI cannabis users. Further, negative stimuli-elicited modulation of the LPP was non-significantly related to state negative affect and stress-relief craving among high DI users. Relations between negative stimuli-elicited modulation of the LPP and cannabis problem severity were also inconsistent with hypotheses such that greater problem severity was generally associated with reduced rather than enhanced ΔNegLPP regardless of acute stress. Further, exploratory analyses revealed some evidence for DI moderation of the concordance between subjective stressor reactivity and ΔNegLPP such that greater perceived stressor reactivity was associated with reduced vs. enhanced ΔNegLPP in the high and low DI group, respectively. Overall, neurophysiologically-indexed, motivated attentional processing of cannabis and negative emotional stimuli appear to be generally orthogonal processes with differing sensitivity to DI and acute stress as well as opposing relations with cannabis problem severity among regular users with high DI.
Consistent with prior passive viewing LPP studies utilizing cannabis (Wolfling et al., 2008) and other drug cue stimuli (Dunning et al., 2011), significant enhancement of the LPP to cannabis relative to matched neutral cues was observed across all time windows at pre-stressor in the combined sample. Significant cannabis cue-elicited modulation of the later LPP (i.e., 1000–3000ms) was observed after the stress induction only in high DI users, whereas significant modulation was found in both groups for the early LPP (i.e., 400–1000ms). Interestingly, the cannabis cue effect on the early LPP significantly decreased from pre to post-stressor across both groups, whereas a stressor-elicited decrease in ΔCanLPP after 1000ms was only apparent among low DI cannabis users, indicating that the significant DI*Stress*Picture interactions were driven by a stressor-elicited decrease vs. maintenance of the 1000–3000ms ΔCanLPP among low and high DI users, respectively. However, it is important to note that this effect was less robust for the 2000–3000ms window compared to 1000–2000ms, which may be attributable to this window’s relatively worse reliability for the post-stressor cannabis and corresponding neutral LPP among low and high DI cannabis users, respectively (see Table S2). Among high DI cannabis users, the magnitude of post-stressor early but not later ΔCanLPP was significantly positively associated with subjective stress-relief craving, whereas post-stressor later ΔCanLPP was more consistently associated with CUD indices. This suggests that DI’s association with post-stressor ΔCanLPP is not attributable to conscious stress-relief craving but reflects distinct CUD-relevant motivated attentional processing.
Differential, time window-dependent relations between ΔCanLPP and DI, state stress-relief craving, and cannabis use-related problems suggests that the latent neural generators contributing to the LPP vary over time. PCA-based decomposition of the affective modulation of the LPP has revealed a number of time-varying, latent components sensitive to emotion (MacNamara et al., 2009). This study found that the early time window largely reflects a P3-like component thought to index initial attentional allocation to a salient stimulus. In contrast, the later time windows have been shown to be uniquely sensitive to semantic manipulations that involve elaborative engagement with stimulus meaning (Foti & Hajcak, 2008; MacNamara et al., 2009). Relatedly, emotion regulation strategies such as cognitive reappraisal do not appear to affect the early LPP, whereas covert distraction, a strategy that limits initial as well as sustained stimulus processing, reduces the early and later LPP (Thiruchselvam et al., 2011). Thus, state stress-relief craving’s association with the early but not later ΔCanLPP may reflect the initial salience of cannabis-related stimuli due to cannabis’s current perceived reward value, whereas the later ΔCanLPP may reflect the extent to which attentional processing of cannabis’s reward value is downregulated via cognitive control mechanisms. Indeed, the stress-relief craving items assess current appraisals of cannabis’s efficacy at reducing negative affect but not the extent to which craving is persistent or difficult to control. In contrast, CUD indices, which were generally only related to the later ΔCanLPP, directly measure loss of control over cannabis use and its associated negative consequences. Overall, DI’s specificity to the later ΔCanLPP may indicate that failure to downregulate elaborative processing of cannabis cues during acute stress is a mechanism of CUD in high DI users.
Consistent with prior studies on emotional modulation of the LPP (Hajcak et al., 2010), significant enhancement of the LPP to negative relative to matched neutral stimuli was observed across all time windows at pre and post-stressor in both groups. In contrast to the cannabis LPP results, DI did not significantly moderate the effect of acute stress on ΔNegLPP nor was post-stressor ΔNegLPP significantly associated with state stress-relief craving or negative affect. However, exploratory analyses revealed some evidence for a moderation effect such that subjective stressor reactivity was inversely vs. positively related to ΔNegLPP in the high and low DI group, respectively. Overall, cannabis problem severity was inversely related to ΔNegLPP regardless of acute stress. Although not hypothesized, the inverse CUD/ΔNegLPP relationship is consistent with a growing literature on reduced biological and subjective response to laboratory stressors (Cuttler et al., 2017) and negative emotional stimuli (Somaini et al., 2012) among cannabis users. Taken together, the ΔNegLPP findings suggest that reduced motivated attentional processing of negative stimuli may be a CUD-related marker of aberrant stressor responding and/or avoidance of negative emotional processing in regular cannabis users with high DI.
There are some limitations of the present study. First, a non-stress induction control condition was not utilized, leaving open the possibility that the significant DI*Stress*Picture interaction effects could be due to other factors (e.g., fatigue); the relationship between post-stressor early ΔCanLPP and stress-relief craving argues against this possibility. Nevertheless, future studies should utilize a control condition to strengthen conclusions regarding causal associations. Second, internal reliabilities for ΔCanLPP and ΔNegLPP were generally below the acceptable threshold (< .70), consistent with prior data on difference scores for the LPP (Bondy et al., 2017) and other ERPs (Levinson et al., 2017), potentially limiting the possible magnitude of between-subjects effects (Hajcak et al., 2017). It is likely that the salience of individual cannabis and negative stimuli varied across participants (e.g., due to differential exposure history to particular cannabis administration methods or threats, respectively), negatively impacting internal reliability. Further, although the neutral stimuli sets were selected to match particular features of the corresponding cannabis and negative stimuli sets, the neutral stimuli were more heterogeneous, which may be why the neutral LPPs were generally less reliable. Future research is needed to maximize reliability of neutral, cannabis, and negative stimuli sets employed in LPP studies. Third, subjective negative affect scores measured after the picture viewing tasks were strongly skewed, resulting in a restricted range which may have limited correlations with the LPP; future studies should consider alternative measures of state negative affect. Fourth, it is possible that the null effects observed for some of the DI*Stress*Picture analyses are attributable to inadequate statistical power; future studies should recruit larger samples to rule out this possibility. Fifth, stressor efficacy was only assessed via self-reported negative affect assessed immediately before and after the stressor. Although a robust stressor-elicited increase in subjective negative affect was observed, future studies should repeatedly measure both subjective and biological (e.g., cortisol) indicators of acute stress to better assess the time course of the stress response and its influence on motivated attentional processing of cannabis and negative stimuli. Sixth, the same picture set was used at pre and post-stressor; future studies should use matched but novel sets to rule our practice effects.
The present study’s results have potential clinical implications. Both post-stressor ΔCanLPP and ΔNegLPP were sensitive to multiple CUD indices within a population of cannabis users known to be at high-risk for CUD, suggesting that these ERPs may be useful as part of a comprehensive risk profile, particularly if their internal reliabilities can be improved. Prospective studies examining the predictive utility and temporal stability of the ΔCanLPP/ΔNegLPP under baseline as well as stressful conditions are needed to determine if these components are risk factors for progression to and maintenance of CUD in high DI cannabis users.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Drug Abuse (NIDA) grant F31DA039644 (awarded to the first author). NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors have no additional acknowledgements and no potential conflicts of interest.
Footnotes
Item five was not included due to its trait-like nature (“Smoking marijuana would help me sleep better at night”).
Author Contributions
RM was the principal investigator of the study and developed the hypotheses and conducted analyses. RM and SO prepared the first draft of the manuscript. BA assisted in statistical analyses and interpretation of findings. NB and JC provided edits throughout the process. All authors critically reviewed manuscript content and approved the final version prior to submission.
References
- Abrantes AM, Strong DR, Lejuez CW, Kahler CW, Carpenter LL, Price LH, Niaura R, Brown RA. The role of negative affect in risk for early lapse among low distress tolerance smokers. Addictive behaviors. 2008;33(11):1394–1401. doi: 10.1016/j.addbeh.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan NP, Macatee RJ, Norr AM, Schmidt NB. Direct and interactive effects of distress tolerance and anxiety sensitivity on generalized anxiety and depression. Cognitive Therapy and Research. 2014;38(5):530–540. [Google Scholar]
- Asmaro D, Carolan PL, Liotti M. Electrophysiological evidence of early attentional bias to drug-related pictures in chronic cannabis users. Addictive behaviors. 2014;39(1):114–121. doi: 10.1016/j.addbeh.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological review. 2004;111(1):33. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Banducci AN, Bujarski SJ, Bonn-Miller MO, Patel A, Connolly KM. The impact of intolerance of emotional distress and uncertainty on veterans with co-occurring PTSD and substance use disorders. Journal of anxiety disorders. 2016;41:73–81. doi: 10.1016/j.janxdis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Bondy E, Stewart JG, Hajcak G, Weinberg A, Tarlow N, Mittal VA, Auerbach RP. Emotion processing in female youth: Testing the stability of the late positive potential. Psychophysiology. 2017 doi: 10.1111/psyp.12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ. An evaluation of the nature of marijuana use and its motives among young adult active users. The American Journal on Addictions. 2009;18(5):409–416. doi: 10.3109/10550490903077705. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Silgado J, Schmidt NB. Marijuana craving during a public speaking challenge: Understanding marijuana use vulnerability among women and those with social anxiety disorder. Journal of behavior therapy and experimental psychiatry. 2011;42(1):104–110. doi: 10.1016/j.jbtep.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski SJ, Norberg MM, Copeland J. The association between distress tolerance and cannabis use-related problems: the mediating and moderating roles of coping motives and gender. Addictive behaviors. 2012;37(10):1181–1184. doi: 10.1016/j.addbeh.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Cougle JR, Hakes JK, Macatee RJ, Zvolensky MJ, Chavarria J. Probability and correlates of dependence among regular users of alcohol, nicotine, cannabis, and cocaine: concurrent and prospective analyses of the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of clinical psychiatry. 2016;77(4):e444–50. doi: 10.4088/JCP.14m09469. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Grey matter alterations associated with cannabis use: results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Cuttler C, Spradlin A, Nusbaum AT, Whitney P, Hinson JM, McLaughlin RJ. Blunted stress reactivity in chronic cannabis users. Psychopharmacology. 2017;234(15):1199–2309. doi: 10.1007/s00213-017-4648-z. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Parvaz MA, Hajcak G, Maloney T, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Motivated attention to cocaine and emotional cues in abstinent and current cocaine users–an ERP study. European Journal of Neuroscience. 2011;33(9):1716–1723. doi: 10.1111/j.1460-9568.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Metrik J, Bonn-Miller MO, Kahler CW, Zvolensky MJ. Anxiety sensitivity and distress intolerance as predictors of cannabis dependence symptoms, problems, and craving: the mediating role of coping motives. Journal of studies on alcohol and drugs. 2016;77(6):889–897. doi: 10.15288/jsad.2016.77.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafò MR, Franken IH. A meta-analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychological bulletin. 2009;135(4):589. doi: 10.1037/a0015843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV) Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- Foti D, Hajcak G. Deconstructing reappraisal: Descriptions preceding arousing pictures modulate the subsequent neural response. Journal of Cognitive Neuroscience. 2008;20(6):977–988. doi: 10.1162/jocn.2008.20066. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dunning JP, Foti D. Motivated and controlled attention to emotion: time-course of the late positive potential. Clinical Neurophysiology. 2009;120(3):505–510. doi: 10.1016/j.clinph.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Olvet DM. Event-related potentials, emotion, and emotion regulation: an integrative review. Developmental neuropsychology. 2010;35(2):129–155. doi: 10.1080/87565640903526504. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Meyer A, Kotov R. Psychometrics and the Neuroscience of Individual Differences: Internal Consistency Limits Between-Subjects Effects. Journal of Abnormal Psychology. 2017;126(6):823–834. doi: 10.1037/abn0000274. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, Jung J, Zhang H, Grant BF. Prevalence and correlates of DSM-5 cannabis use disorder, 2012–2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions–III. American Journal of Psychiatry. 2016;173(6):588–599. doi: 10.1176/appi.ajp.2015.15070907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Saha TD, Kerridge BT, Goldstein RB, Chou SP, Zhang H, Jung J, Pickering RP, Ruan WJ, Smith SM, Huang B, Grant BF. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA psychiatry. 2015;72(12):1235–1242. doi: 10.1001/jamapsychiatry.2015.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Cerdá M, Keyes KM, Stohl M, Galea S, Wall MM. US adult illicit cannabis use, cannabis use disorder, and medical marijuana laws: 1991–1992 to 2012–2013. JAMA Psychiatry. 2017;24(6):579–588. doi: 10.1001/jamapsychiatry.2017.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and alcohol dependence. 2009;102(1):35–40. doi: 10.1016/j.drugalcdep.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, First M. Unpublished scale, Columbia College of Physicians and Surgeons. New York: State Psychiatric Institute; 1991. Drug use questionnaire. [Google Scholar]
- Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addictive behaviors. 2012;37(3):225–233. doi: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Technical manual and affective ratings. Gainesville, FL: The Center for Research in Psychophysiology, University of Florida; 1999. p. 2. [Google Scholar]
- Levinson AR, Speed BC, Infantolino ZP, Hajcak G. Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology. 2017;54(4):601–607. doi: 10.1111/psyp.12813. [DOI] [PubMed] [Google Scholar]
- Lewis KL, Taubitz LE, Duke MW, Steuer EL, Larson CL. State rumination enhances elaborative processing of negative material as evidenced by the late positive potential. Emotion. 2015;15(6):687–693. doi: 10.1037/emo0000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G. Internal Consistency of Functional Magnetic Resonance Imaging and Electroencephalography Measures of Reward in Late Childhood and Early Adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2(3):289–297. doi: 10.1016/j.bpsc.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Albanese BJ, Allan NP, Schmidt NB, Cougle JR. Distress intolerance as a moderator of the relationship between daily stressors and affective symptoms: Tests of incremental and prospective relationships. Journal of affective disorders. 2016;206:125–132. doi: 10.1016/j.jad.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Albanese BJ, Clancy K, Allan NP, Bernat EM, Cougle JR, Schmidt NB. Distress Intolerance Modulation of Neurophysiological Markers of Cognitive Control during a Complex Go/No-go Task. Journal of Abnormal Psychology. 2018;127(1):12–29. doi: 10.1037/abn0000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatee RJ, Capron DW, Guthrie W, Schmidt NB, Cougle JR. Distress tolerance and pathological worry: Tests of incremental and prospective relationships. Behavior therapy. 2015;46(4):449–462. doi: 10.1016/j.beth.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Macatee RJ, Capron DW, Schmidt NB, Cougle JR. An examination of low distress tolerance and life stressors as factors underlying obsessions. Journal of psychiatric research. 2013;47(10):1462–1468. doi: 10.1016/j.jpsychires.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Macatee RJ, McDermott KA, Albanese BJ, Schmidt NB, Cougle JR. Distress Intolerance Moderation of Attention to Emotion: An Eye-Tracking Study. Cognitive Therapy and Research 2017 [Google Scholar]
- Mackinnon A, Jorm AF, Christensen H, Korten AE, Jacomb PA, Rodgers B. A short form of the Positive and Negative Affect Schedule: Evaluation of factorial validity and invariance across demographic variables in a community sample. Personality and Individual Differences. 1999;27:405–416. [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9(4):531–43. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, Hajcak G. Diagnostic and symptom-based predictors of emotional processing in generalized anxiety disorder and major depressive disorder: an event-related potential study. Cognitive therapy and research. 2016;40(3):275–289. doi: 10.1007/s10608-015-9717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Otto MW. Refining the measurement of distress intolerance. Behavior Therapy. 2012;43(3):641–651. doi: 10.1016/j.beth.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae-Clark AL, Carter RE, Price KL, Baker NL, Thomas S, Saladin ME, Giarla K, Nicholas K, Brady KT. Stress-and cue-elicited craving and reactivity in marijuana-dependent individuals. Psychopharmacology. 2011;218(1):49–58. doi: 10.1007/s00213-011-2376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. Journal of Neuroscience Methods. 2010;192(1):152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Reinhardt T, Schmahl C, Wüst S, Bohus M. Salivary cortisol, heart rate, electrodermal activity and subjective stress responses to the Mannheim Multicomponent Stress Test (MMST) Psychiatry research. 2012;198(1):106–111. doi: 10.1016/j.psychres.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Slavet JD, Stein LAR, Colby SM, Barnett NP, Monti PM, Golembeske C, Lebeau-Craven R. The Marijuana Ladder: Measuring motivation to change marijuana use in incarcerated adolescents. Drug and Alcohol Dependence. 2006;83(1):42–48. doi: 10.1016/j.drugalcdep.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaini L, Manfredini M, Amore M, Zaimovic A, Raggi MA, Leonardi C, Gerra ML, Donnini C, Gerra G. Psychobiological responses to unpleasant emotions in cannabis users. European archives of psychiatry and clinical neuroscience. 2012;262(1):47–57. doi: 10.1007/s00406-011-0223-5. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Curtin L. Comparison of extended versus brief treatments for marijuana use. Journal of consulting and clinical psychology. 2000;68(5):898–908. [PubMed] [Google Scholar]
- Thiruchselvam R, Blechert J, Sheppes G, Rydstrom A, Gross, James J. The temporal dynamics of emotion regulation: An EEG study of distraction and reappraisal. Biological Psychology. 2011;87(1):84–92. doi: 10.1016/j.biopsycho.2011.02.009. [DOI] [PubMed] [Google Scholar]
- van der Pol P, Liebregts N, de Graaf R, Korf DJ, van den Brink W, van Laar M. Predicting the transition from frequent cannabis use to cannabis dependence: a three-year prospective study. Drug and Alcohol Dependence. 2013;133(2):352–359. doi: 10.1016/j.drugalcdep.2013.06.009. [DOI] [PubMed] [Google Scholar]
- Vujanovic AA, Wardle MC, Liu S, Dias NR, Lane SD. Attentional bias in adults with cannabis use disorders. Journal of addictive diseases. 2016;35(2):144–153. doi: 10.1080/10550887.2015.1116354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfling K, Flor H, Grüsser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. European Journal of Neuroscience. 2008;27(4):976–983. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Vujanovic AA, Bernstein A, Bonn-Miller MO, Marshall EC, Leyro TM. Marijuana use motives: A confirmatory test and evaluation among young adult marijuana users. Addictive behaviors. 2007;32(12):3122–3130. doi: 10.1016/j.addbeh.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.