Abstract
In this work, a new extraction process using steam explosion at high temperature and pressure was developed, to drastically shorten the extraction time and improved extraction of the essential oil from citrus peels. In steam explosion process, the material is subjected to the high-pressure saturated steam following by substantially dropping the pressure through an angle valve to a vacuum tank. The optimum essential oil yield by the steam explosion was obtained at the 170 °C, 8 bar in 240 seconds duration time. The essential oil extraction of a certain amount of citrus peels by hydro-distillation took nearly eight times longer than explosion extraction process. The obtained citrus oil from hydro-distillation processes had 10 to 13 major components more than the steam explosion, as shown by gas chromatography (GC-MS). The maximum product yield of Limonene, a major favorable component, were 77% and 100% in hydro-distillation and steam explosion processes, respectively.
Keywords: Food analysis, Food science
1. Introduction
Extraction by the steam explosion is a relatively new and developing method that in through self-evaporation results in the displacement of a large part of the volatile molecules in the plant (Meng and Ragauskas, 2014). This process is based on the volatility of fugacious compounds where, the raw materials are exposed to saturated vapor for a short period of time, which varies by product type. Extraction of citrus essential oil performed by hydro-distillation with ambient pressure is traditionally used. Today, citrus fruit is extensively grown from temperate to tropical zones within the northern and southern. In the processing of citrus, the peels remain as a waste. One of the products from citrus peel is essential oil that is aromatic liquids characterized by an odor and produced by citrus peels (Guenther, 1948). Essential oil Citrus is intensively collected in oil glands of the peel (Boussaada et al., 2007), accounting for about 1–3% fresh weight on average (Njoroge et al., 2005). Essential oil Citrus is a complex combination of natural ingredients that can contain compounds with different concentrations and has significant applications in the pharmaceutical and food industries. Due to the attention of various industries to this valuable product, the presence of the essential substance of limonene (the major combination of essential oils in orange peel), which has antimicrobial, antioxidant, biological properties and herbal fragrance (Bauer et al., 2008). Recently, Citrus essential oil has been acknowledged no longer handiest for its fragrant features however additionally for its physiological homes, along with chemo-prevention in opposition to most cancers and aromatherapy consequences (Dorman and Deans, 2000; Murthy et al., 2013).
The main component of citrus essential oil is limonene. Its concentration in the oil differ between 30% and 99%, be depending on the variety: 30–40% in bergamot, 40–75% in lemon and 68–98% in sweet orange (Moufida and Marzouk, 2003). Limonene is a monoterpene whose empirical formula is C10H16. It is a liquid at room temperature. It exists as two optical isomers D- and L-limonene, and the racemic combination known as dipentene. The main industrial use of limonene is a precursor to carvone or a-terpineol (Boluda-Aguilar and López-Gómez, 2013). The extraction method modifies depending upon the final application. Thus, the preferred extraction methods are the steam distillation, cold expression and other methods inclusive of extraction with lipophilic solvents, supercritical fluids, and steam explosion are used (Allaf et al., 2013; Bakkali et al., 2008).
Through the use of steam to mediate the extraction, it is possible to hold little conditions and gain better extraction. Steam explosion is a valuable and important technology to open up the biomass fibers, to enhance the healing of waste and different useful compounds from plants. The steam contacts a admixture and targets the substance(s) which has the high boiling point. The steam carries the desired substance(s) into another vessel through a condenser. The product, i.e. the mixture of hot vapors will, if let to pass through a cooling system (vacuumed tank), condense to form a liquid in which the oil and water include two separate layers (Ozel and Kaymaz, 2004) (Aqueous phase & organic phase) or it will be as an emulsion. The organic part may be an essential oil and other volatile compounds. This process subjects the product to fast transition from high steam pressure to hoover. This transition induces a quick evaporation of water and unstable compounds. It is also discussed as pretreatment procedure for the production of solid biofuel pellets to increase the calorific value and to improve the pelletizing properties of the biomass (Jedvert et al., 2012).
In this work, we investigated that processing by steam explosion increase the total diffusivity of the liquid in peels and improve the supply of the liquid in the citrus peels. The essential oil removal based on this research is an appealing substitute to typical techniques of essential oil extraction, such as hydro-distillation or isolation with solvents. Moreover, in comparison to the steam explosion and hydro-distillation, the quick time contact between peels and the heated zones of equipment avoids the loss and degradation of unstable and thermolabile compounds (X.-M. Li et al., 2009). At the industrial scale, that is exciting regarding oil quality and energy saving.
The principle of this work was to get better an efficient process for extraction of essential oil from orange peel through a thermo-mechanical extraction technique developed in steam explosion process. This process is based by subjecting the product to a fast transition from the high steam strain chamber to a vacuum tank. For such products as citrus peels, the purpose became a upkeep of perfume coupled to an alveolate texture of the dried product. So, this technique used for the separation of essential oil from the solid material. An integrated technique for steam extraction accompanied via volatiles sampling and analysis from the essential oils of the citrus peels become explored. There have been two situations to overcome in the extraction from solid plant resources: that of releasing the essential oils from a solid matrix and letting it diffuse out efficiently in a manner that can be scaled-up to commercial volumes. In the direction of this give up, the impact of various parameters, such as pressure and extraction time at the extraction yield was investigated and the experimental outcomes display that all of these time, were significant parameters affecting yield. In addition, extraction of essential oil of drying peels was approved using hydro-distillation and their performance was compared to the steam explosion. The quality and comparison of its content were then based on the effect of test variables validated by GC-MS analysis.
2. Materials and methods
2.1. Raw material
Orange fruit used in this research were Citrus sinensis Osbeck CV. Valencia, grown in Ramsar. The orange peels were separated from the endocarp and cut into approximately 2 × 5 × 3 mm pieces giving a yield of 20 % (w/w) of peels with regard to total fruit. The peels were softly dried at 45 °C in a drying oven by a flowing air at 12 h. The moisture content reached 40% by weight on dry basis. They were stored in the refrigerator until treatments.
2.2. Extraction of essential oil
2.2.1. Hydro-distillation
Hydro-distillation is a variant of steam distillation, for the extraction of essential oil from dried samples in the laboratory. As opposed to the steam input, the samples materials in hydro-distillation are directly immersed in distilled water. This solid-liquid mixture is then heated until boiling under atmospheric pressure in an alembic, where the heat allows the release of odorous molecules in plant cells. This volatile aroma compounds and water forms an azeotropic (azeotropes) mixture, which can be evaporated together at the similar pressure and after that condensed and divided in a Florentine flask due to their density difference and immiscibility. Moreover, a cohobating system can recycle the distilled water through a siphon so as to improve the yield and quality of essential oil (Y. Li et al., 2014).
Samples, 100 g of dried peel were immersed in 2 L of distilled water. The extraction was carried out during 4h from the first drop of distillate until the amount of essential oils stabilized. Essential oils were stored in a refrigerator (+4 °C) until gas chromatography coupled to mass spectrometry (GC-MS) analysis.
2.2.2. Steam explosion apparatus
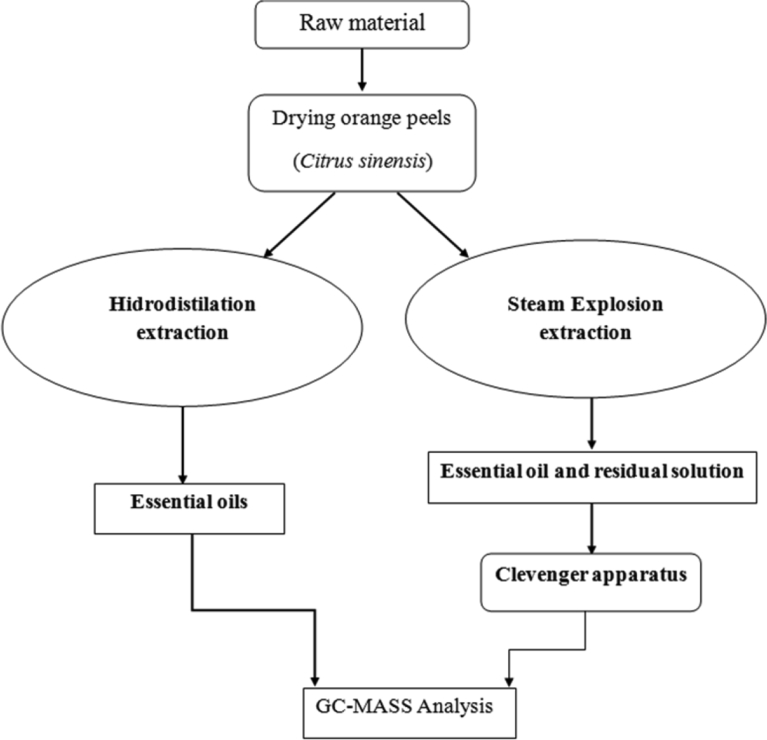
Steam explosion is a pretreatment process that opens up the essential oil pores, and abrupt the cell walls of plant organs more accessible for subsequent processes, i.e. extraction, fermentation, hydrolysis or densification processes, densification processes. Fig. 1 illustrates a representative diagram of pretreatment process used apparatus and the pressure profile is presented in Fig. 2.
Fig. 1.
Illustrates a representative diagram of pretreatment process.
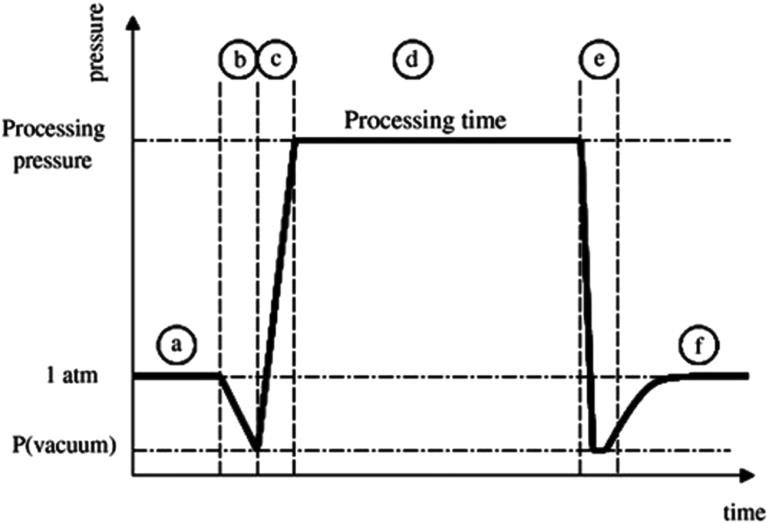
Fig. 2.
Typical pressure-time profile for steam explosion processing cycle. (a) sample at atmospheric pressure; (b) establishment of vacuum; (c) steam injection to reach selected pressure; (d) treatment time at selected processing pressure; (e) pressure drop; (f) atmospheric pressure for the sample recovery (Allaf et al., 2013).
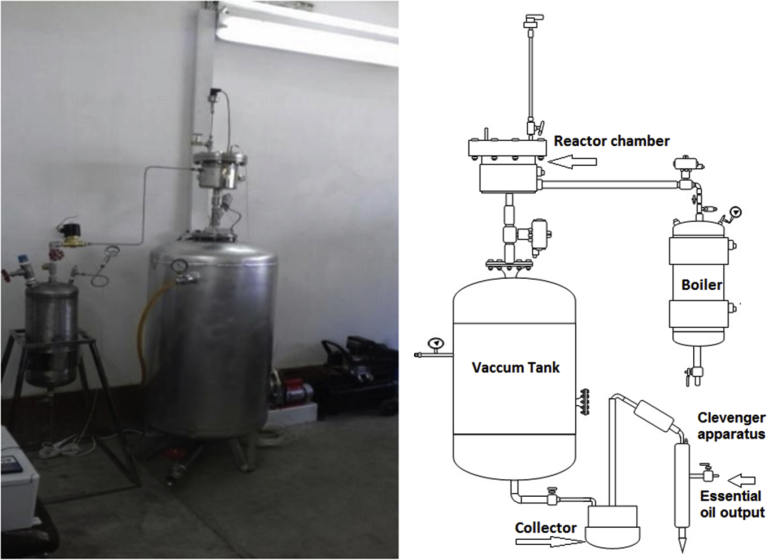
The reactor was designed and constructed (Fig. 3) with 2 L processing vessel with a steam jacket (Fig. 2, step a); where thermal treatment in this vessel is achieved using saturated steam with pressure varying from 3 up to 12 bars (Fig. 2, step b and c). Relative pressure of vacuum tank (130 L) maintained at −0.9 bar during process inside the vessel (Fig. 2, step e). After placing 100 g of dried orange peel in the vessel, the steam was injected in a vessel. Maximum pressure of saturated steam used in our case was 15 bars. This stage lasted few seconds (phase d) and ended by an abrupt pressure drop towards a vacuum (phase e) by opening the pneumatic valve between the treatment and vacuum thank. The essential oils are extracted as stable oil in water emulsion. Then it transferred to Clevenger apparatus, distillation operation was performed for 30 min. Afterwards, the orange peels were recovered and dried at room temperature. Steam explosion process' cycle can be divided into six steps as shown in Fig. 2.
Fig. 3.
Steam explosion apparatus schematic (right) and pilot (left).
2.3. Analysis of essential oils
A gas chromatograph-mass spectrophotometer (GC–MS) was used in this study to analyze the essential oils. The system had a gas chromatograph (6890 GC, Hewlett-Packard, USA) coupled to a mass spectrometer (5973A, Hewlett-Packard), by means of two columns as fused-silica-capillary with different phases. The non-symmetrical column was 30 m × 0.25 mm i.d., 0.25 μm film thickness (HP5MS) and the symmetrical column was a Stabilwax inclusive of Carbowax–PEG (60 m × 0.2 mm i.d., 0.25 mm film thickness). GC–MS spectra were taken by the following conditions: transporter gas, He; flow rate, 1 ml/min; split mode 1/20; injection volume, 1 μl; injection temperature, 250 °C; oven temperature program, 60 °C for 8 min, then increased at 2 °C/min to 250 °C and held at 250 °C for 15 min; ionization mode, electronic impact at 70 eV.v (Karakaya et al., 2014).
The additives of the oil have been identified by using the comparison of their linear retention indices on the two columns, determined with regards to a homologous collection of n-alkanes, with the ones from natural standards or reported in the literature (Davies, 1990). Evaluation of fragmentation templates in the mass spectra with these stored at the GC–MS databases (McLafferty et al., 1991) became additionally done. The odds of every issue were suggested as uncooked possibilities with our standardization. All of the components suggested in Tables 2 and 3 for the crucial oil extracted.
Table 2.
Hydro-distillation essential oil (essential oil yield: 1.2/100 g dm) composition via gas chromatography analysis.
| No. | Compound | HD Concentration (%) | Retention time (min) (RT) |
|---|---|---|---|
| 1 | alpha-Pinene | 1.17 | 14.49 |
| 2 | Sabinene | 1.41 | 15.34 |
| 3 | Beta-Myrcene | 6.08 | 15.61 |
| 4 | Limonene | 77.39 | 16.75 |
| 5 | 1-Octanol | 2.18 | 17.34 |
| 6 | L-Linalool | 5.13 | 18.09 |
| 7 | p-Menthon-8-thiol | 0.44 | 19.13 |
| 8 | Decanal | 2.92 | 20.27 |
| 9 | Z-Citral | 0.63 | 20.99 |
| 10 | E-Citral | 0.66 | 21.54 |
| 11 | 1-Cyclohexene-1-Carboxaldehyde | 1.03 | 21.80 |
| 12 | Dodecanal | 0.41 | 24.36 |
| 13 | Sinensal | 0.35 | 29.50 |
| Total compound | 100 | ||
Table 3.
Steam explosion essential oil (essential oil yield: 1.2 and 1.34 ± 0.09 ml/100 g dm) composition via gas chromatography analysis.
| No. | Compound | Steam explosion Concentration (%) | Retention time (min) (RT) |
|---|---|---|---|
| 1 | n-Nonane | 2.0 | 4.85 |
| 2 | alpha-Pinene | 0.72 | 5.72 |
| 3 | Sabinene | 0.55 | 6.77 |
| 4 | beta -Myrcene | 3.41 | 7.28 |
| 5 | n-Decane | 0.40 | 7.47 |
| 6 | n-Octanal | 0.35 | 7.67 |
| 7 | Limonene | 89.13 | 8.40 |
| 8 | p-Menthon-8-thiol | 0.22 | 10.36 |
| 9 | Dodecanal | 0.82 | 10.54 |
| 10 | Sinensal | 0.20 | 13.23 |
| Total compound | 97.8 | ||
2.4. Statistical analysis
The efficacy of independent variables (pressure: 3–15 bar, and time: 15–480 s) on extracted oils was investigated. Calculation of mean, standard deviation, and standard error were conducted by means of IBM SPSS Statistics version 18.0 (IBM Company, USA) was applied for statistical analysis of data and after passing factorial test, the one way ANOVAs was implemented to assess the significance of essential oil extraction between time and pressure while the Holm–Sidak method was used for multiple comparisons of mean value between groups. Variance (Balasubramaniam, Barbosa-Cánovas, & Lelieveld) was performed to determine significant differences between independent variables (P ≤ 0.05).
3. Results & discussion
In order to investigate the extraction yield of orange peels, they were crushed and treated by steam explosion processes in the range of 3–15 bars and 15–480 seconds. In addition, the extraction yield of essential oil by steam explosion is also compared with a hydro-distillation method. In this study, the pretreatment time, pressure and the concentration of the chemicals component are considered as treatment variable factors, and the effects of the treatment is measured using the produced essential oil.
3.1. Extraction of essential oil by hydro-distillation
Hydro-distillation is a traditional and widely used method for extraction of plants essential oils. Pressure, temperature and time are main factors in the design of an extraction method. One of the shortcomings observed in hydro-distillation experiments is the fact that during the process, temperature and pressure cannot be controlled. On the other hand, the amount of distillation of the essential oil extracted from the peels is highly dependent on the temperature and corresponding pressure of extraction. In order to compare the essential oil yield, hydro-distillation is considered as a reference treatment, and each experiment was carried out in five repetitions for four hours. The temperature was set to the boiling point of water and the pressure was slightly higher than the environment. The average yield of the extracted essential oil turns out to be 1.2 ± 0.1 ml/g dry material (dm) and in range of 1.1 ± 0.1 ml/g (dm) to 1.30 ± 0.1. In all the treatments, three separate intervals were observed. In the first interval, the extraction rate remained constant. In the next interval, this rate was increased significantly while it became constant in the third interval (Fig. 4). A reason for the existence of these time intervals may be explained as follows (Allaf and Allaf, 2016): During the first part of the heating, a breakdown of cell structures begins. As the heating continues, the rate of cell breakdown increased significantly until all the structure is degraded. This means that the penetration of solvent in the tissue, the rate at which oil was removed from the peel is solely dependent on the heating time. The result (Fig. 4), can be explained by the fact that the rate of oil separation from the plant material is affected by availability of the relative essential oils in pore what make up the oil; rather it is influenced by their solubility in the liquid phase. Therefore it is reasonable to conclude that it should take time that could still be trapped within the tissues of the plant (Allaf and Allaf, 2016) inaccessible to the water phase.
Fig. 4.
Hydro-distillation pretreatments with essential oil extraction yield (ml).
3.2. Extraction of essential oil by steam explosion
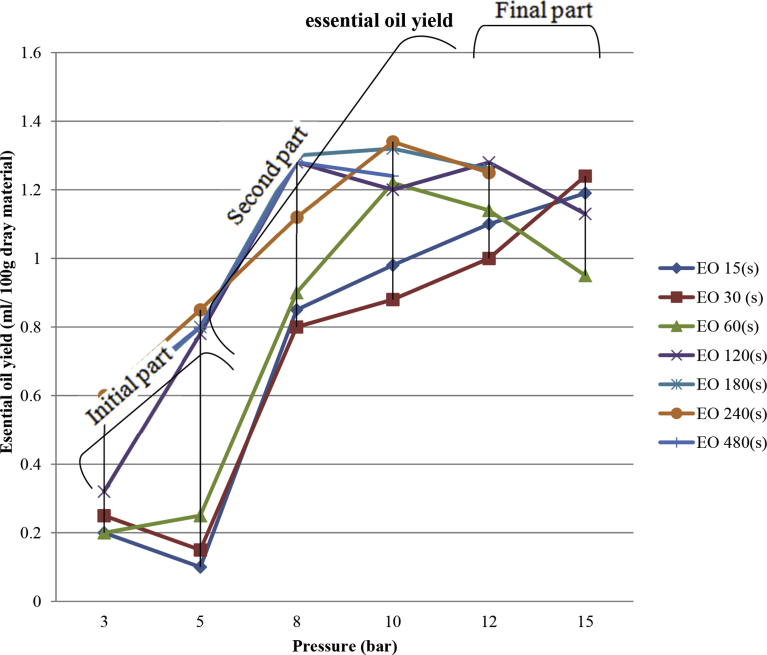
The steam explosion pretreatments of orange peels were performed in a pilot plant. The hypothesis was to reduce time and increase the yield of essential oil using higher pressure and also explosion process, compared to the traditional hydro-distillation method. In this work, a central composite experimental design was applied to investigate the effect of the total time (t) and pressure (P) as independent parameters as shown in Table 1. The essential oil extraction yields were considered as the main response parameter. These parameters were appropriate to represent respectively mechanical and thermal aspects of steam explosion process. Then, the performance of two methods was compared.
Table 1.
Steam explosion extraction treatment with essential oil extraction yield (ml).
| Time(s) | 15 | 30 | 60 | 120 | 180 | 240 | 480 | Standard Error |
|---|---|---|---|---|---|---|---|---|
| Pressure (bar) | ||||||||
| 3 | 0.2 | 0.25 | 0.2 | 0.32 | 0.55 | 0.6 | 0.58 | 0.039 |
| 5 | 0.1 | 0.15 | 0.25 | 0.78 | 0.8 | 0.85 | 0.8 | 0.075 |
| 8 | 0.85 | 0.8 | 0.9 | 1.28 | 1.3 | 1.12 | 1.28 | 0.050 |
| 10 | 0.98 | 0.88 | 1.22 | 1.2 | 1.32 | 1.34 | 1.24 | 0.037 |
| 12 | 1.1 | 1 | 1.14 | 1.28 | 1.26∗ | 1.25∗ | - | 0.032 |
| 15 | 1.19 | 1.24 | 0.95 | 1.13∗ | - | - | - | 0.036 |
Average of essential oil is show.
Samples were burned.
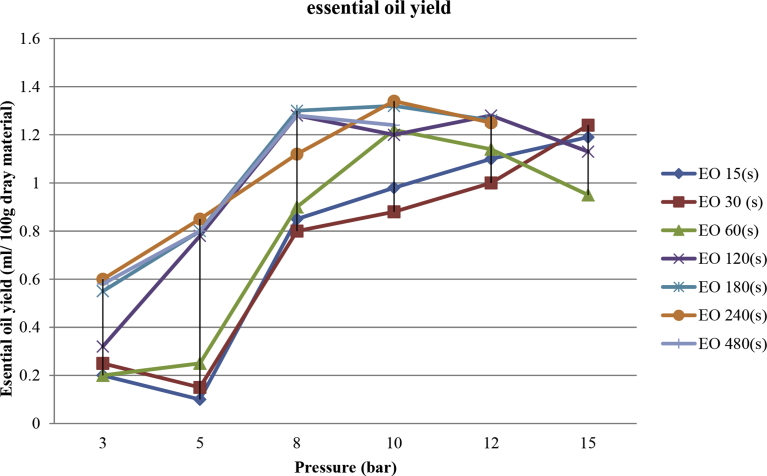
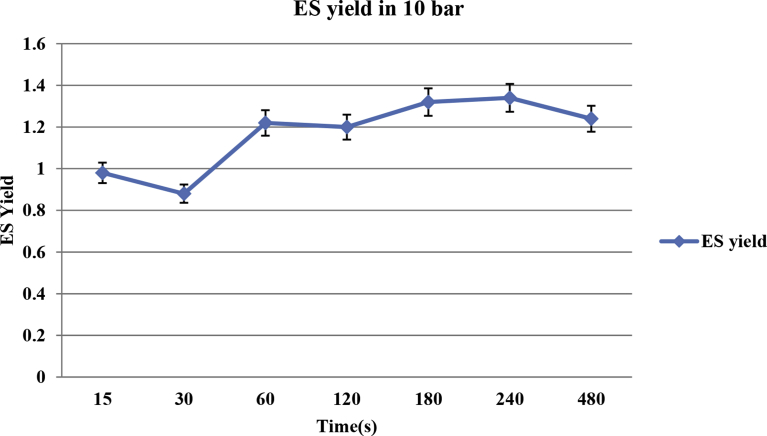
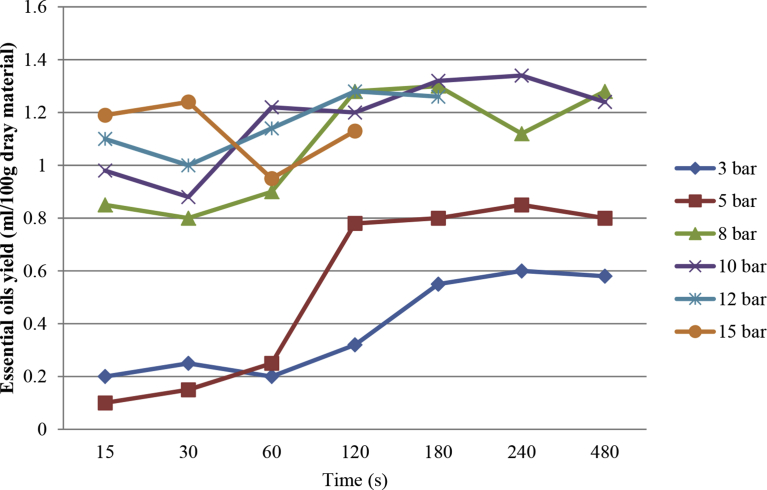
The improved and optimized conditions of extraction operation were determined with the goal to maximize and minimize the extraction yield. It was hence calculated as an optimum value of extraction yield of 1.34 ± 0.011 (ml), with the pressure of 10 bar and a total time t equal to 240 s. Higher values of time of treatment slightly reduced the value of extraction yields (0.58–1.24 ± 0.011 ml in 480 s) as shown in Fig. 5. An extraction yield was attenuated after a peak as a function of the total time. During the process time (240–480 second), in a constant pressure (10 bar), the essential oil is reversed from the pores to the extraction medium (Fig. 6). In other research, also is noted, thermal Excessive heat might imply a decreasing of the transfer of the volatile molecules within the structure (Allaf and Allaf, 2016). On the other hand, experimental results show that the sample burns at too high pressure and long time (Fig. 7), while the yield of extracted oil dropped sharply at too low pressure. It is probably because volatile component and essential oils did not have enough time to cross the cell wall and mass transfer (Karakaya et al., 2014). At low pressure, inflation and penetration of steam in the pores containing essential oil are also greatly reduced (Table 1). To justify this process behavior can be said that agitation of the surrounding solvent (vapor) normally allows the transport of solute from the product surface to the outside by convection (Balasubramaniam et al., 2016). By adding a vacuum step, the efficient exchange surfaces achieve a higher rate than the flat surface. Also, the decompression creates a larger temperature discrepancy between the two steps only before and after decompression. Indeed, the pressure difference is the dynamic force in the change in the pore size with time. The rate of change of pore size is thus proportional to the pressure discrepancy between the inside of the pore and the external medium.
Fig. 5.
Steam explosion pretreatments with essential oil (EO) extraction yield (ml) in pressure 3–12 Bar.
Fig. 6.
Steam explosion extraction pretreatment with essential oil extraction yield (ml) in pressure 10 bar.
Fig. 7.
Essential oil extraction yield vs. time in pressure 3–12 Bar by steam explosion pretreatments.
The best temperature and pressure range for the steam explosion process was in the range of 8–12 bars and 260–240 s, respectively. The temperature increased due to increasing pressure, the process time must reduce because peels are burned. So the efficacy of temperature is as a variable depending on the pressure. Therefore, in comparison with the hydro-distillation method, the yield increased and the extraction time decreased (Fig. 7). This method has full control over the extraction parameters and extraction process has been made more uniform. In the following section, the comparison of the essential oils extracted from the two methods completely reveals the effect of the process parameters on the chemical compositions.
3.3. Essential oil analysis
Citrus peel oils are characterized by complex mixtures containing mostly terpenes as well as oxygen-containing compounds. For most citrus fruits, peel oils consist almost exclusively in the form of monoterpenes, sesquiterpenes, and other aliphatic hydrocarbons. Limonene is a clear, colorless liquid hydro-carbon classified as a cyclic monoterpene, and was the major component in oil of citrus fruit peels and it used as a functional index of ripeness in GC-MS analyses.
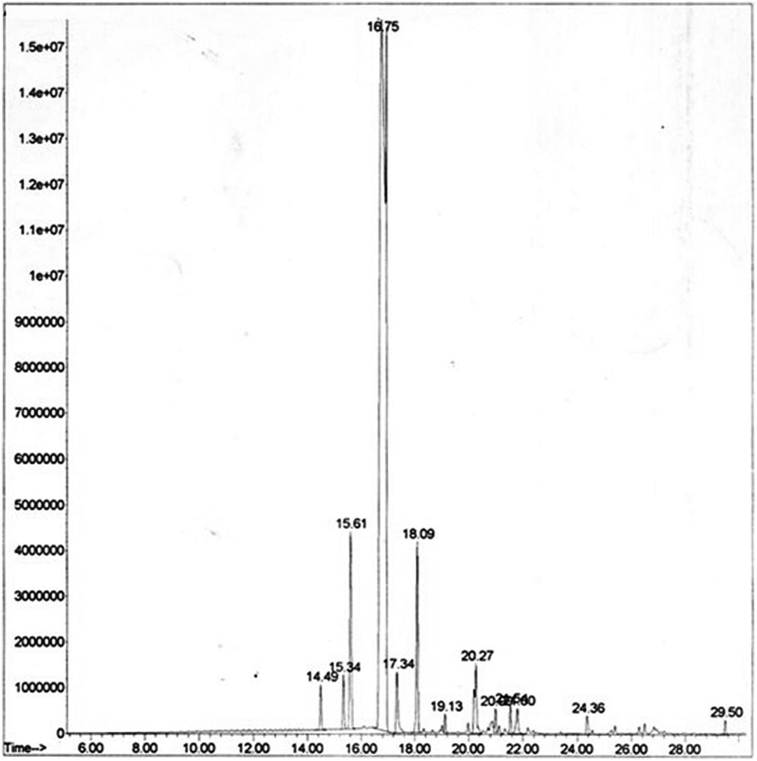
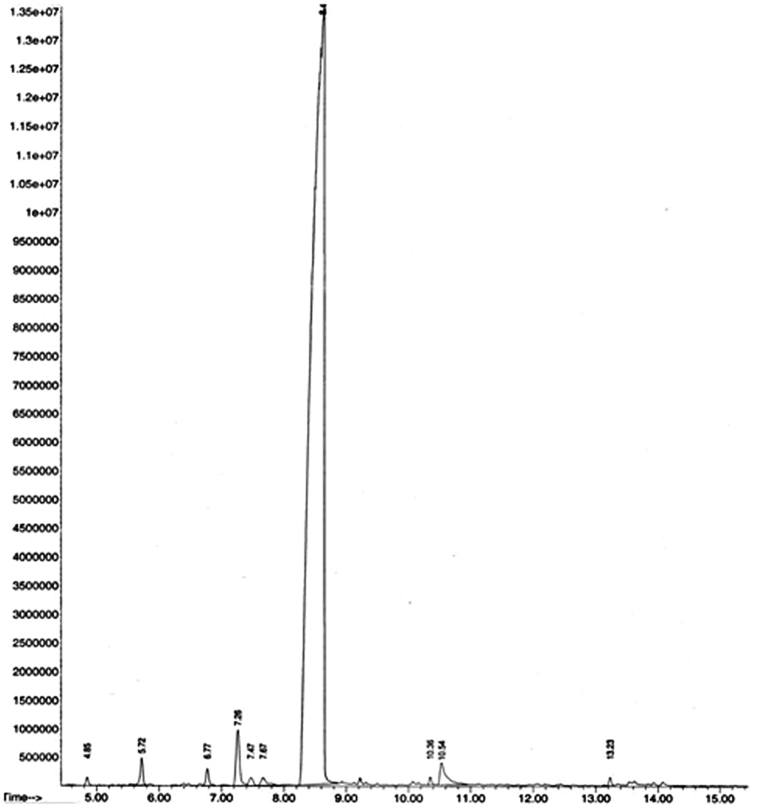
In a previous reports (Azar et al., 2011; Tao et al., 2009), limonene was observed as the main constituent (77.49 %) in the peel oil of sweet orange, followed by myrcene (6.27 %), α-farnesene (3.64 %) and γ-terpinene (3.34 %). The main compounds in C. sinensis from Uganda and Rwanda were limonene (87.9 and 92.5 %), myrcene (2.4 and 2.0 %), α-pinene (0.5 and 2.4 %) and linalool (1.2 and 0.9 %) (Njoroge et al., 2009). In every oil, limonene, α-pinene, sabinene and α-terpinene were the major compounds (Njoroge et al., 2005). As shown in Tables 2 and 3, in essential oil, extraction of hydro-distillation had 13 compounds, including 86.05% hydro-carbon monoeters and 0.2% non-terpene compounds such as aliphatic hydro-carbons, aldehydes, and esters. The main ingredient in this essential oil was limonene 77.39%, myrcene 6.08%, and linalool 5.13 % in 16.75, 15.61 and 18.09 (min) in RT (Retention time) respectively (Table 2). In essential oil, the extraction of steam explosion (10 bars, 34 min) had 10 compounds, which accounts for 97.8% of the total essential oil, was identified. This organic oil contains 94.49% hydrocarbon monoterpenes and 0.20% non-terpene compounds such as aliphatic hydrocarbons, aldehydes, and esters. The main ingredients in monoterpene: Limonene 89.13%, Myrcene 3.14%, and Nonane 2.0% in 8.40, 7.28, and 4.85 (min) in RT (Retention time), respectively (Table 2).
Therefore, in both processes, the amounts of chemical compounds in essential oils did not vary significantly with the values of the findings of other reports. Increasing pressure and reducing time had also no significant effect on Consists of chemical family and non-oxygenated and oxygenated fractions of orange peels essential oils obtained by both methods. Essential oils obtained whether by hydro-distillation or steam explosion of orange peel had a quality quite close. On the other hand, Limonene is the major component with a percentage of 77.39% and 100% respectively for hydro-distillation and steam explosion essential oils (Figs. 8 and 9).
Fig. 8.
GC-MS analysis for essential oil (hydro-distillation).
Fig. 9.
GC-MS analysis for essential oil (steam explosion).
3.4. Results of statistic analysis
The effect of independent variables (pressure (X1), Time (X2)) on the extraction yield (Y), Summary in statistic model shown in Table 4. The coefficients of determination R and R2 values of 0.821, 0.673 respectively, obtained for the essential oil extraction statistic model, display that more than 95 % of the overall system variability can be defined by the empirical models of Eq. (1) which is the specific case of the general predictive equation derived for the research from the multivariate regression analyses carried out on design expert.
Table 4.
Analysis of variance results in ANOVA table, Validity coefficients of linear regression equation for predicting essential oil content in analytical model.
| ANOVAb | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | R | R2 | Unstandardized Coefficients |
Standardized Coefficients |
t | df | F | Sig. | |
| B | Std. Error | Beta | |||||||
| 1 | 0.821a | 0.673 | 2 | 126.858 | .000a | ||||
| (Constant) | .159 | .052 | 3.054 | .003 | |||||
| P | .072 | .005 | .757 | 14.70 | .000 | ||||
| T | .001 | .000 | .316 | 6.130 | .000 | ||||
Predictors: (Constant), Time, Pressure.
Dependent Variable: Essential oil.
Analysis of variance results (Balasubramaniam et al.) and Coefficients of regression equation (Unstandardized and standard coefficients (B and Beta)) are shown in Table 4. The ANOVA results isolated from the predictive model display that the main linear effects due to individual control factors such as pressure, time coded as P, T respectively, was all significant variables, the observed P-values <0.05 in the numerical analysis. The regression equation for the accurate prediction of the values of the dependent variable is used as follows:
| Y = 0.159 + 0.757 X1 + 0.316 X2 | (1) |
This is equally true with the linear interaction effects pressure and essential oil yield, time and essential oil which these results showed the models were well adapted to the responses.
Analysis of correlation and standardized coefficients (Beta) between variables (essential oil yield, pressure and time) shown the effect of temperature and pressure on essential oil yield is significant. The pressure variable (70%) has more effect on the essential oil yield than the temperature variable (30%).
4. Conclusions
In order to recover wastes from citrus processing, a thorough study was carried out on dried orange peels. It is significant to note that the natural organization of some plants such as citrus peels prevents an easy essential oil extraction. We first extracted the essential oils hydro-distillation and steam explosion. The extraction performed by hydro-distillation showed that mechanical process, in this case grinding, was necessary. Therefore, in the steam explosion mechanical (illustrated by the pressure) and thermal (illustrated by the steaming time t) processes can be combined for this specific material. Pre-treatment by the steam explosion can improve extraction yields and kinetics. The swelling of the cells enabled a improved kinetic extraction in conditions of diffusivity and starting accessibility. Investigations with a steam explosion at industrial scale equipment could be realized for industry tests. This process can have a lot of potential as a supportable alternative for the industry since it allows recovering citrus by-products. In addition to this study compared a mathematic model based on single plate particle description to predict the behavior of steam explosion and hydro-distillation process.
Declarations
Author contribution statement
M.J. Taherzadeh: Conceived and designed the experiments.
M. Golmohammadi: Wrote the paper and Performed the experiments.
A. Borghe: Analyzed and interpreted the data.
A. Zenouzi: Contributed reagents, materials, analysis tools or data.
N. Ashrafi: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The Authors gratefully acknowledge the support of Morteza Golmohammadi and Hormoz F. Nasiri in the Citrus and Subtropical Fruits Research Center (http://.icri.areeo.ac.ir).
References
- Allaf T., Allaf K. Springer; 2016. Instant Controlled Pressure Drop (DIC) in Food Processing. [Google Scholar]
- Allaf T., Tomao V., Besombes C., Chemat F. Thermal and mechanical intensification of essential oil extraction from orange peel via instant autovaporization. Chem. Eng. Process Process Intens. 2013;72:24–30. [Google Scholar]
- Azar A.P., Nekoei M., Larijani K., Bahraminasab S. Chemical composition of the essential oils of Citrus sinensis cv. valencia and a quantitative structure-retention relationship study for the prediction of retention indices by multiple linear regression. J. Serb. Chem. Soc. 2011;76(12):1627–1637. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam V., Barbosa-Cánovas G.V., Lelieveld H.L. Springer; 2016. High Pressure Processing of Food: Principles, Technology and Applications. [Google Scholar]
- Bauer K., Garbe D., Surburg H. John Wiley & Sons; 2008. Common Fragrance and Flavor Materials: Preparation, Properties and Uses. [Google Scholar]
- Boluda-Aguilar M., López-Gómez A. Production of bioethanol by fermentation of lemon (Citrus limon L.) peel wastes pretreated with steam explosion. Ind. Crop. Prod. 2013;41:188–197. [Google Scholar]
- Boussaada O., Skoula M., Kokkalou E., Chemli R. Chemical variability of flowers, leaves, and peels oils of four sour orange provenances. J. Essent. Oil Bear. Plants. 2007;10(6):453–464. [Google Scholar]
- Davies N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A. 1990;503:1–24. [Google Scholar]
- Dorman H., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88(2):308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Guenther E. Vol. I. D Van Nostrand Company. Inc.; New York: 1948. (The Essential Oils). [Google Scholar]
- Jedvert K., Wang Y., Saltberg A., Henriksson G., Lindström M.E., Theliander H. Mild steam explosion: a way to activate wood for enzymatic treatment, chemical pulping and biorefinery processes. Nord. Pulp Pap Res. J. 2012;27(5):828–835. [Google Scholar]
- Karakaya S., El S.N., Karagozlu N., Sahin S., Sumnu G., Bayramoglu B. Microwave-assisted hydrodistillation of essential oil from rosemary. J. Food Sci. Technol. 2014;51(6):1056–1065. doi: 10.1007/s13197-011-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-M., Tian S.-L., Pang Z.-C., Shi J.-Y., Feng Z.-S., Zhang Y.-M. Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and steam distillation. Food Chem. 2009;115(3):1114–1119. [Google Scholar]
- Li Y., Fabiano-Tixier A.-S., Chemat F. Essential Oils as Reagents in Green Chemistry. Springer; 2014. Essential oils: from conventional to green extraction; pp. 9–20. [Google Scholar]
- McLafferty F.W., Stauffer D.B., Loh S.Y. Comparative evaluations of mass spectral data bases. J. Am. Soc. Mass Spectrom. 1991;2(5):438–440. doi: 10.1016/1044-0305(91)85011-T. [DOI] [PubMed] [Google Scholar]
- Meng X., Ragauskas A.J. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr. Opin. Biotechnol. 2014;27:150–158. doi: 10.1016/j.copbio.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Moufida S. d., Marzouk B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry. 2003;62(8):1283–1289. doi: 10.1016/s0031-9422(02)00631-3. [DOI] [PubMed] [Google Scholar]
- Murthy K.N.C., Jayaprakasha G., Patil B.S. Citrus limonoids and curcumin additively inhibit human colon cancer cells. Food Funct. 2013;4(5) doi: 10.1039/c3fo30325j. [DOI] [PubMed] [Google Scholar]
- Njoroge S.M., Koaze H., Karanja P.N., Sawamura M. Essential oil constituents of three varieties of Kenyan sweet oranges (Citrus sinensis) Flavour Fragrance J. 2005;20(1):80–85. [Google Scholar]
- Njoroge S.M., Phi N.T.L., Sawamura M. Chemical composition of peel essential oils of sweet oranges (Citrus sinensis) from Uganda and Rwanda. J. Essent. Oil Bear. Plants. 2009;12(1):26–33. [Google Scholar]
- Ozel M.Z., Kaymaz H. Superheated water extraction, steam distillation and Soxhlet extraction of essential oils of Origanum onites. Anal. Bioanal. Chem. 2004;379(7-8):1127–1133. doi: 10.1007/s00216-004-2671-5. [DOI] [PubMed] [Google Scholar]
- Tao N. g., Liu Y. j., Zhang M. l. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck) Int. J. Food Sci. Technol. 2009;44(7):1281–1285. [Google Scholar]