Abstract
Helicobacter pylori has a number of well-characterized carbohydrate-binding adhesins (BabA, SabA, and LabA) that promote adhesion to the gastric mucosa. In contrast, information on the glycoconjugates present in the human stomach remains unavailable. Here, we used MS and binding of carbohydrate-recognizing ligands to characterize the glycosphingolipids of three human stomachs from individuals with different blood group phenotypes (O(Rh−)P, A(Rh+)P, and A(Rh+)p), focusing on compounds recognized by H. pylori. We observed a high degree of structural complexity, and the composition of glycosphingolipids differed among individuals with different blood groups. The type 2 chain was the dominating core chain of the complex glycosphingolipids in the human stomach, in contrast to the complex glycosphingolipids in the human small intestine, which have mainly a type 1 core. H. pylori did not bind to the O(Rh−)P stomach glycosphingolipids, whose major complex glycosphingolipids were neolactotetraosylceramide, the Lex, Lea, and H type 2 pentaosylceramides, and the Ley hexaosylceramide. Several H. pylori-binding compounds were present among the A(Rh+)P and A(Rh+)p stomach glycosphingolipids. Ligands for BabA-mediated binding of H. pylori were the Leb hexaosylceramide, the H type 1 pentaosylceramide, and the A type 1/ALeb heptaosylceramide. Additional H. pylori-binding glycosphingolipids recognized by BabA-deficient strains were lactosylceramide, lactotetraosylceramide, the x2 pentaosylceramide, and neolactohexaosylceramide. Our characterization of human gastric receptors required for H. pylori adhesion provides a basis for the development of specific compounds that inhibit the binding of this bacterium to the human gastric mucosa.
Keywords: Helicobacter pylori, mass spectrometry (MS), glycolipid structure, adhesin, carbohydrate structure, glycoconjugate, virulence factor, gastric mucosa, glycosphingolipid characterization, H. pylori BabA adhesin, Human gastric glycosphingolipids, microbial adhesion
Introduction
Helicobacter pylori colonizes the human gastric mucosa, resulting initially in an acute inflammatory response and damage to epithelial cells and progressing to severe gastric diseases ranging from chronic gastritis and peptic ulcers to malignant neoplastic diseases (gastric cancer and mucosa-associated lymphoid tissue lymphoma). Over 50% of the world's population is infected by H. pylori (1, 2). The infection is acquired during childhood and persists throughout life unless treated with antibiotics.
An essential step in the initiation and establishment of infection is the attachment of a microbe to cell surface receptors on the target tissue. The majority of identified microbial receptors are glycoconjugates (3). For H. pylori, a number of different carbohydrate receptor candidates have been reported (reviewed by Teneberg (4)). Thus, the binding of this bacterium to such diverse compounds as sialic acid-containing glycoconjugates, phosphatidylethanolamine, gangliotetraosylceramide, the Leb blood group determinant and related carbohydrate antigens, heparan sulfate, sulfatide, lactosylceramide, neolacto sequences, and lactotetraosylceramide has been documented. The nonacid carbohydrate sequences recognized by H. pylori are summarized in Table 1. In contrast to the multitude of candidate H. pylori carbohydrate receptors, there are only three identified carbohydrate-binding adhesins, the blood group antigen–binding BabA2 adhesin, the sialic acid–binding SabA adhesin, and the LacdiNAc–binding LabA adhesin (5–7). In addition, HopQ has recently been shown to interact with human carcinoembryonic antigen-related cell adhesion molecules and is thus the first example of a protein-dependent H. pylori adhesin (8, 9). The adherence-associated lipoprotein A and B (AlpA/B) and the outer inflammatory protein A (OipA) are also involved in H. pylori adhesion (10–12).
Table 1.
Nonacid carbohydrate structures recognized by H. pylori
| Trivial name | Carbohydrate sequence | BabA bindinga | Reference |
|---|---|---|---|
| Gangliotetra | Galβ3GalNAcβ4Galβ4Glc | − | 57 |
| Leb | Fucα2Galβ3 (Fucα4)GlcNAcβ3Galβ4Glc | + | 5 |
| H type 1 | Fucα2Galβ3GlcNAcβ3Galβ4Glc | + | 5 |
| Lactoseb | Galβ4Glc | − | 25 |
| Lactotetra | Galβ3GlcNAcβ3Galβ4Glc | − | 17 |
| A type 1 hexa | GalNAcα3 (Fucα2)Galβ3GlcNAcβ3Galβ4Glc | + | 16 |
| B type 1 hexa | Galα3 (Fucα2)Galβ3GlcNAcβ3Galβ4Glc | + | 16 |
| A type 1 hepta/ALeb | GalNAcα3 (Fucα2)Galβ3 (Fucα4)GlcNAcβ3Galβ4Glc | + | 14 |
| B type 1 hepta/BLeb | Galα3 (Fucα2)Galβ3 (Fucα4)GlcNAcβ3Galβ4Glc | + | 14 |
| Neolactotetra | Galβ4GlcNAcβ3Galβ4Glc | − | 26 |
| Neolactohexa | Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc | − | 26 |
| Galα3-neolacto | Galα3Galβ4GlcNAcβ3Galβ4Glc | − | 26 |
| x2 | GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc | − | 26 |
| Globo H | Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc | + | 16 |
| Globo A | GalNAcα3 (Fucα2)Galβ3GalNAcβ3Galα4Galβ4Glc | + | 16 |
a Recognized by generalist BabA.
b Binding to lactosylceramide with phytosphingosine and/or hydroxy fatty acids.
The Leb (Fucα2Galβ3(Fucα4)GlcNAcβ-)-binding BabA was the first identified H. pylori adhesin (5). H. pylori strains expressing BabA together with the vacuolating cytotoxin A and the cytotoxin-associated antigen A (triple-positive strains) are highly associated with severe gastric diseases, such as peptic ulcer and gastric adenocarcinoma (13).
The initial observation that the fucosylated blood group antigens H type 1 (Fucα2Galβ3-GlcNAcβ-) and Leb are recognized by H. pylori BabA was followed by a division of BabA-producing H. pylori strains into specialist and generalist strains, depending on their mode of binding to Leb and related carbohydrate sequences (14). BabA of specialist strains binds only to glycoconjugates with a terminal Fucα2Gal sequence, as the H type 1 and Leb determinants, whereas the generalist BabA tolerates substitution with an αGal or an αGalNAc at the 3-position of the Gal, as in the A or B type 1 (GalNAcα3 (Fucα2)Galβ3GlcNAcβ- or (Galα3(Fucα2)Galβ3GlcNAcβ-) and ALeb or BLeb (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ-or Galα3(Fucα2)Galβ3(Fucα4)GlcNAcβ-) determinants. The structural determinants specifying these variant binding modes were recently characterized by X-ray crystallography of the adhesin domain of specialist and generalist BabA, alone and in complex with ABO/Leb-type oligosaccharides (15).
The structural requirements for carbohydrate recognition by the BabA adhesin of generalist and specialist strains have been redefined (16), demonstrating that BabA recognizes blood group O and A determinants on type 4 core chains in addition to blood group determinants on type 1 core chains.
In contrast to the wealth of information about H. pylori carbohydrate binding, the knowledge about the glycosylation of the human stomach (i.e. the target organ of H. pylori) is limited and mainly consists of immunohistochemistry studies. A few reports with characterization of selected human gastric glycosphingolipids are available (17, 18), but a thorough characterization with the methods available today has not been done. In a recent study, we have characterized the acid glycosphingolipids of human stomach (19) and identified two minor H. pylori SabA-binding gangliosides as Neu5Acα3-neolactohexaosylceramide (Neu5Acα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4 Glcβ1Cer) and Neu5Acα3-neolactooctaosylceramide (Neu5Acα3 Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer), whereas the other acid human stomach glycosphingolipids characterized (sulfatide and the gangliosides GM3, GD3, GM1, Neu5Acα3-neolactotetraosylceramide, GD1a, and GD1b) were not recognized by the bacteria. The aim of the present study was to characterize the nonacid glycosphingolipids of the human stomach, with particular interest in components with H. pylori-binding activity. Nonacid glycosphingolipids from three human stomachs from individuals with blood group O(Rh−)P, A(Rh+)P, and A(Rh+)p were tested for H. pylori binding, and the total nonacid glycosphingolipid fractions and isolated subfractions were characterized with MS and binding of antibodies, lectins, and bacteria.
Results
Binding of H. pylori to total nonacid glycosphingolipids of human stomach
Chemical staining of the human stomach nonacid glycosphingolipids showed that the major compounds in the fractions from the blood group O(Rh−)P and A(Rh+)P individuals migrated as mono- to tetraglycosylceramides (Fig. 1A, lanes 1 and 2), whereas the major glycosphingolipids in the fraction from the blood group A(Rh+)p individual migrated as mono- and diglycosylceramides, and no tri- and tetraglycosylceramides were visualized in this fraction (Fig. 1A, lane 3).
Figure 1.
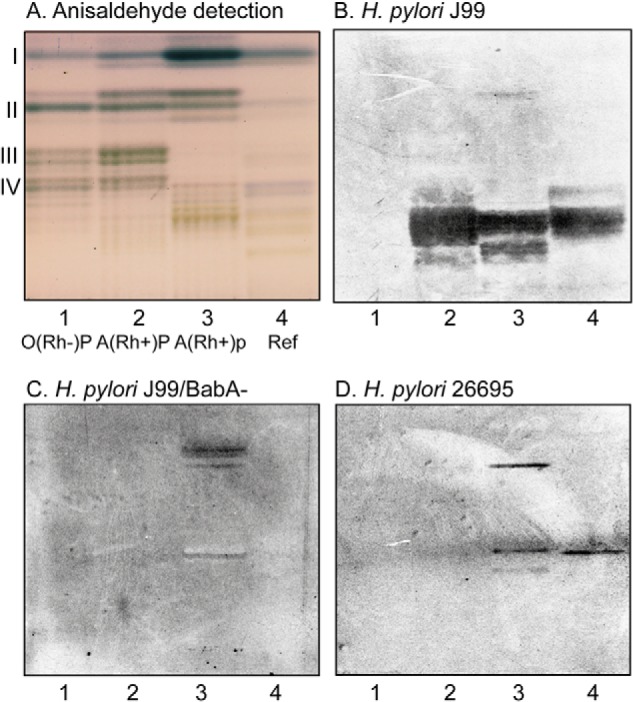
Binding of H. pylori to total nonacid glycosphingolipids of human stomach. A–D, thin-layer chromatogram detected with anisaldehyde (A) and autoradiograms obtained by binding of H. pylori strain J99 (B), H. pylori strain J99/BabA− (C), and H. pylori strain 26695 (D). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as the solvent system, and the binding assays were performed as described under “Experimental procedures.” Autoradiography was for 12 h. Lane 1, total nonacid glycosphingolipids of human stomach blood group O(Rh-)P, 40 μg; lane 2, total nonacid glycosphingolipids of human stomach blood group A(Rh+)P, 40 μg; lane 3, total nonacid glycosphingolipids of human stomach blood group A(Rh+)p, 40 μg; lane 4, reference total nonacid glycosphingolipids of human meconium, 40 μg. The Roman numerals to the left of the chromatogram in A denote the number of carbohydrate unit(s) in the bands.
The three nonacid glycosphingolipid fractions from human stomach separated on thin-layer chromatograms were tested for binding of the WT BabA–expressing H. pylori generalist strain J99, the deletion mutant strain lacking BabA (J99/BabA−), and the WT H. pylori strain 26695, which lacks Leb-binding activity (5). In this assay, the detection limit for binding of the generalist strain J99 to the A type 1/ALeb heptaosylceramide is ∼40 ng, whereas the detection limit for binding to the preferred ligand (i.e. the Leb hexaosylceramide) is below 40 ng (16).
Probing the stomach glycosphingolipids with the BabA-expressing strain J99 revealed an interaction with glycosphingolipids in the slow-migrating region in the stomach fractions from the blood group A(Rh+)P and A(Rh+)p individuals (Fig. 1B, lanes 2 and 3). The binding was over a rather broad area, suggesting recognition of several compounds. The interaction with these glycosphingolipids was BabA-dependent because binding to these glycosphingolipids was only observed with the J99 strain, and not with the two BabA-negative strains J99/BabA− and 26695 (Fig. 1 (C and D), lanes 2 and 3). The two non-BabA strains, on the other hand, bound to compounds migrating in the di- and tetraglycosylceramide regions in the A(Rh+)p stomach sample (Fig. 1 (C and D), lane 3), and the 26695 strain also bound weakly to a compound in the penta- to hexaosylceramide region. None of the strains bound to the glycosphingolipids of the blood group O(Rh−)P stomach (Fig. 1 (B–D), lane 1).
Characterization of the total nonacid glycosphingolipids of human blood group O(Rh−)P stomach by LC-ESI/MS
LC-ESI/MS of oligosaccharides using porous graphitized carbon columns gives resolution of isomeric oligosaccharides, and the carbohydrate sequence can be deduced from series of C-type fragment ions obtained by MS2 (20). Furthermore, MS2 spectra of oligosaccharides with a Hex or HexNAc substituted at C-4 have diagnostic cross-ring 0,2A-type fragment ions, which allow differentiation of linkage positions.
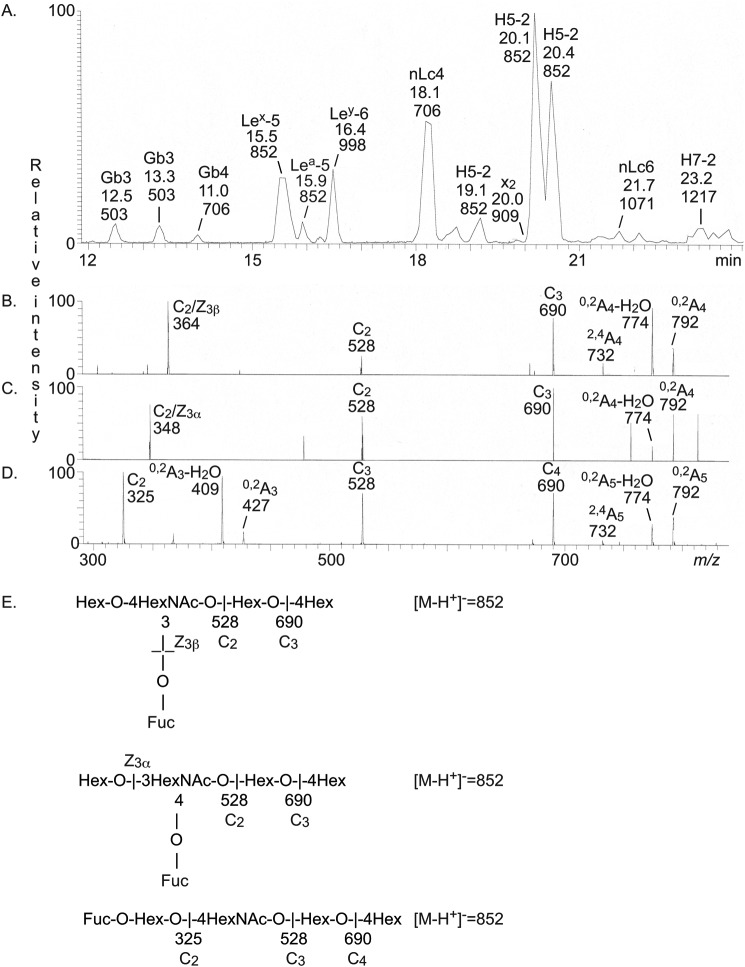
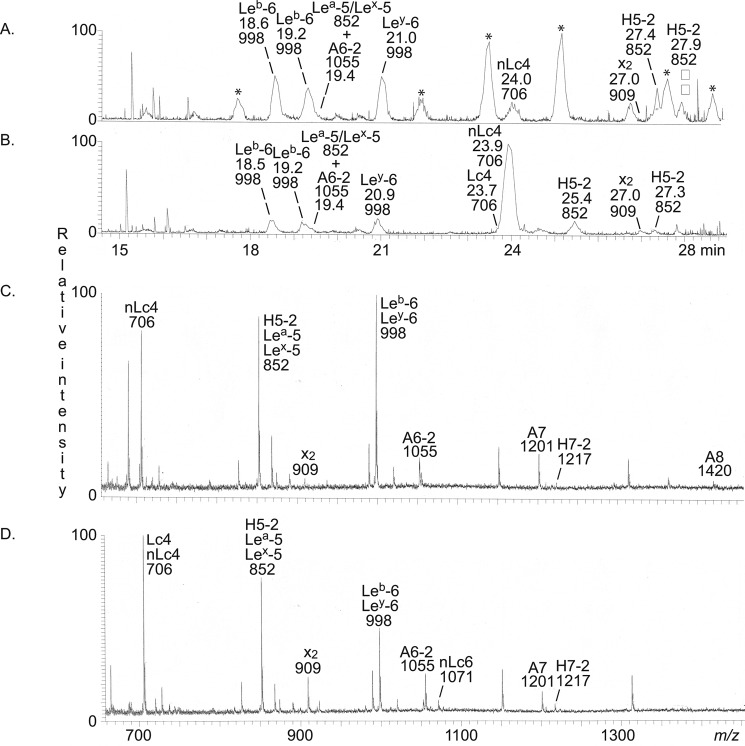
The base peak chromatogram obtained from LC-ESI/MS of the oligosaccharides obtained by hydrolysis of the nonacid glycosphingolipid fractions from human blood group O(Rh−)P stomach with Rhodococcus endoglycoceramidase II is shown in Fig. 2A. Molecular ions corresponding to oligosaccharides ranging from trisaccharides (detected as [M-H+]− ions at m/z 503) to heptasaccharides (detected as [M-H+]− ions at m/z 1217) were present. All molecular ions were subjected to MS2, and the oligosaccharides thereby identified are given in the chromatogram.
Figure 2.
LC-ESI/MS of the oligosaccharides obtained from the total nonacid glycosphingolipid fraction of human blood group O(Rh−)P stomach by hydrolysis with endoglycoceramidase II from Rhodococcus spp. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained from human stomach blood group O(Rh−)P. B, MS2 of the ion at m/z 852 (retention time 15.5 min). C, MS2 of the ion at m/z 852 (retention time 15.9 min). D, MS2 of the ion at m/z 852 (retention time 20.1 min). E, interpretation formulas showing the deduced oligosaccharide sequences. The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were Galα4Galβ4Glc (Gb3), GalNAcβ3Galα4Galβ4Glc (Gb4), Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Lex-5), Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Lea-5), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-6), Galβ4GlcNAcβ3Galβ4Glc (nLc4); Fucα2Galβ4GlcNAcβ3Galβ4Glc (H5-2), GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc (x2), Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (nLc6), and Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (H7-2).
The annotation procedure is exemplified in Fig. 2 (B–D) by the MS2 spectra of the [M-H+]− ions at m/z 852 eluting at 15.5, 15.9, and 20.1 min, respectively. A [M-H+]− ion at m/z 852 corresponds to an oligosaccharide with one Fuc, one HexNAc, and three Hex. The MS2 spectrum of the ion eluting at 15.5 min (Fig. 2B) had an intense ion at m/z 364. This fragment ion is obtained by double glycosidic cleavage of the 3-linked branch (C2/Z3β) and is characteristic for an internal 4-linked GlcNAc substituted with a Fuc at the 3-position (21). Taken together with the C2 ion at m/z 528 and the C3 ion at m/z 690, a Lex pentasaccharide (Galβ4(Fucα3)GlcNAcβ3Galβ4Glc) was thus tentatively identified.
A fragment ion at m/z 348 was present in the MS2 spectrum of the ion at m/z 852 eluting at 15.9 min (Fig. 2C). This ion is diagnostic for an internal 3-linked GlcNAc substituted with a Fuc at C-4 (21) and is due to a double glycosidic cleavage of the 3-linked branch (C2/Z3α). C-type fragment ions at m/z 528 (C2α) and m/z 690 (C3) were present, and taken together, this indicated a Lea pentasaccharide (Galβ3(Fucα4)GlcNAcβ3-Galβ4Glc).
The MS2 spectrum of the ion at m/z 852 eluting at 20.1 min was distinctly different (Fig. 2D) and had a series of C-type fragment ions (C2 at m/z 325, C3 at m/z 528, and C4 at m/z 690), identifying a pentasaccharide with a Fuc-Hex-HexNAc-Hex-Hex sequence. The 0,2A3-H2O fragment ion at m/z 409 and the 0,2A3 fragment ion at m/z 427 are characteristic for 4-substituted HexNAc (i.e. a type 2 carbohydrate chain) (20, 21). Thus, an H type 2 pentasaccharide (Fucα2Galβ4GlcNAcβ3 Galβ4Glc) was identified.
The oligosaccharides identified by LC-ESI/MS are summarized in Table 2. The majority of the characterized compounds had type 2 (Galβ4GlcNAc) core chains (i.e. neolactotetraosylceramide, the Lex, H type 2, and x2 pentaosylceramides, neolactohexaosylceramide, the Ley hexaosylceramide, and the H type 2 heptaosylceramide) (see Tables 2 and 3 for glycosphingolipid structures).3 The presence of a Lea pentasaccharide and the absence of saccharides with H type 1 and Leb determinants suggests that this stomach tissue was derived from a blood group OLe(a+b−) nonsecretor individual (22, 23).
Table 2.
Oligosaccharides obtained from the total nonacid glycosphingolipid fractions from human stomach by hydrolysis with endoglycoceramidase II from Rhodococcus spp. and characterized by LC-ESI/MS and MS2
| Abbreviation | Structure | O(Rh+)P | A(Rh+)P | A(Rh+)p | A(Rh+)P case 3a | A(Rh+)P case 4a |
|---|---|---|---|---|---|---|
| Gb3 | Galα4Galβ4Glc | + | + | − | − | − |
| Gb4 | GalNAcβ3Galα4Galβ4Glc | + | + | − | − | − |
| Leb-6b | Fucα2Galβ3 (Fucα4)GlcNAcβ3Galβ4Glc | − | + | + | + | + |
| Lex-5 | Galβ4 (Fucα3)GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| Lea-5 | Galβ3 (Fucα4)GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| Ley-6 | Fucα2Galβ4 (Fucα3)GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| H5-1 | Fucα2Galβ3GlcNAcβ3Galβ4Glc | − | + | − | − | − |
| Lc4 | Galβ3GlcNAcβ3Galβ4Glc | − | + | + | − | + |
| nLc4 | Galβ4GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| H5-2 | Fucα2Galβ4GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| x2 | GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc | + | + | + | + | + |
| nLc6 | Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc | + | + | + | − | − |
| H7-2 | Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc | + | + | + | − | − |
| A6-2 | GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc | − | + | + | + | + |
a Fractions from human gastric mucosa.
b The compounds in boldface type have been characterized as H. pylori binding in previous studies (summarized in Table 1).
Table 3.
Summary of glycosphingolipids characterized in the subfractions isolated from human stomachs
| Fraction | Amount | Migration | Glycosphingolipid | Abbreviation |
|---|---|---|---|---|
| mg | ||||
| P-I | 0.4 | Mono | Galβ1Cer | GalCer |
| Glcβ1Cer | GlcCer | |||
| P-II | 1.2 | Di + tri | Galβ4Glcβ1Cera | LacCer |
| Galα4Galβ1Cer | Galabia | |||
| Galα4Galβ4Glcβ1Cer | Gb3 | |||
| P-III | 1.3 | Tri + tetra | Galα4Galβ4Glcβ1Cer | Gb3 |
| GlcNAcβ3Galβ4Glcβ1Cer | Lc3 | |||
| GalNAcβ3Galα4Galβ4Glcβ1Cer | Gb4 | |||
| Galβ3GlcNAcβ3Galβ4Glcβ1Cer | Lc4 | |||
| Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc4 | |||
| Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Lex-5 | |||
| P-IV | 0.7 | ≥Tetra | GalNAcβ3Galα4Galβ4Glcβ1Cer | Gb4 |
| Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc4 | |||
| GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | x2 | |||
| Fucα2Galβ3GlcNAcβ3Galβ4Glcβ1Cer | H5-1 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H5-2 | |||
| Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | Lea-5 | |||
| Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Lex-5 | |||
| Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc6 | |||
| Fucα2Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | Leb-6 | |||
| Fucα2Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Ley-6 | |||
| GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | A6-2 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H7-2 | |||
| GalNAcα3 (Fucα2)Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | A7-1 | |||
| GalNAcα3 (Fucα2)Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | A7-2 | |||
| GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | A8-2 | |||
| p-I | 1.3 | Mono | Galβ1Cer | GalCer |
| Glcβ1Cer | GlcCer | |||
| p-II | 0.7 | Di | Galβ4Glcβ1Cer | LacCer |
| p-III | 0.3 | Tetra | GlcNAcβ3Galβ4Glcβ1Cer | Lc3 |
| Galβ3GlcNAcβ3Galβ4Glcβ1Cer | Lc4 | |||
| Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc4 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H5-2 | |||
| Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Lex-5 | |||
| GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | A6-2 | |||
| p-IVb | 1.2 | ≥Tetra | Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc4 |
| GalNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | x2 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H5–2 | |||
| Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | Lea-5 | |||
| Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Lex-5 | |||
| Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc6 | |||
| Fucα2Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | Leb-6 | |||
| Fucα2Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Ley-6 | |||
| GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | A6-2 | |||
| GalNAcα3 (Fucα2)Galβ3 (Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | A7-1 | |||
| GalNAcα3 (Fucα2)Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | A7-2 | |||
| Galβ3/4GlcNAcβ3Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Galβ3/4GlcNAcβ3- Lex-5 | |||
| Galβ4 (Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | Lex-7 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H7-2 | |||
| Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | nLc8 | |||
| GalNAcα3 (Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | A8–2 | |||
| Fucα2Galβ3/4GlcNAcβ3Galβ4 (Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | Fucα2Galβ3/4-GlcNAcβ3-Lex-5 | |||
| Fucα2Galβ4 (Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glclcβ1Cer | Ley-8 | |||
| Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | H9–2 | |||
| Fucα2Galβ4 (Fucα3)GlcNAcβ3Galβ4 (Fucα3)GlcNAcβ3Galβ4Glclcβ1Cer | Ley-9 |
a The compounds in boldface type have been characterized as H. pylori binding in previous studies (summarized in Table 1).
b The glycosphingolipids listed under fraction p-IV were characterized by LC-ESI/MS and MS2 of fractions p-IVa–f.
Separation of the total nonacid glycosphingolipids from human A(Rh+)P and A(Rh+)p stomachs
LC-ESI/MS of the oligosaccharides from the H. pylori-binding nonacid glycosphingolipid fractions from A(Rh+)P and A(Rh+)p stomachs showed that these fractions were complex mixtures (Fig. S1). To enrich the slow-migrating glycosphingolipids, the two stomach fractions (∼4 mg each) were separated on Iatrobeads columns (Iatron Laboratories). Thereby, four glycosphingolipid-containing fractions were obtained from each fraction (Fig. 3, A and B). These fractions were denoted fractions P:I-IV and p:I-IV, respectively. The amounts obtained for each fraction, and their migration level on thin-layer chromatograms, are summarized in Table 3.
Figure 3.
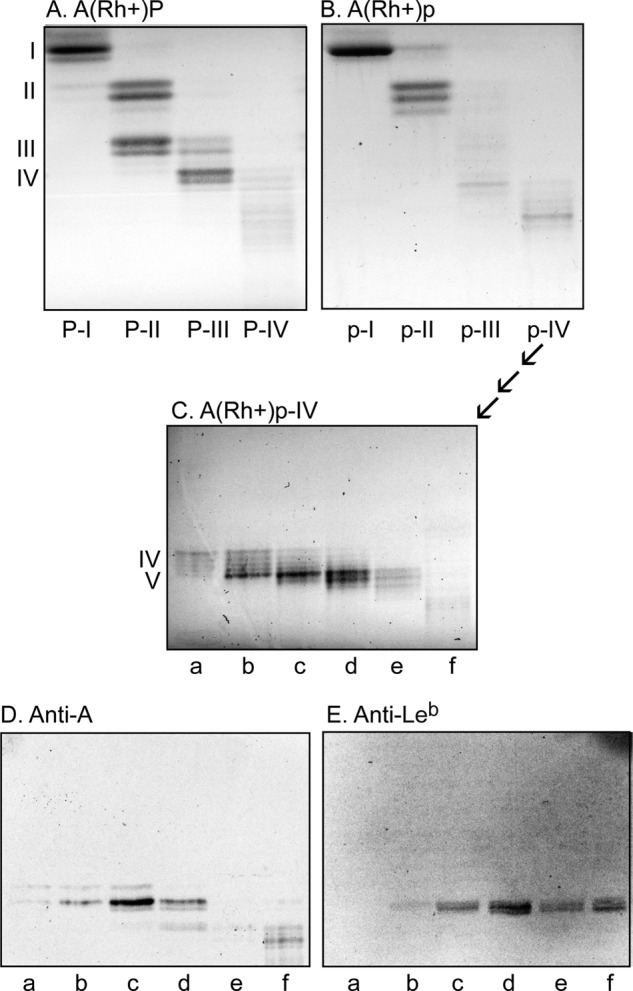
Glycosphingolipid subfractions isolated from human blood group A(Rh+)P and A(Rh+)p stomachs. A–E, thin-layer chromatograms after detection with anisaldehyde (A–C) and autoradiograms obtained by binding of monoclonal antibodies directed against the blood group A determinant (D) and the Leb determinant (E). The glycosphingolipids (4 μg/lane) were separated on glass or aluminum-backed silica gel plates, using chloroform/methanol/water (60:35:8 by volume) as the solvent system, and the binding assays were performed as described under “Experimental procedures.” Autoradiography was for 12 h. The Roman numerals to the left of A and C denote the number of carbohydrates in the bands.
Characterization of the mono-, di-, and triglycosylceramides
The glycosphingolipids in fractions P-I, p-I, P-II, and p-II were characterized by LC-ESI/MS and by binding of carbohydrate-recognizing ligands. The results are summarized in Table 3.
LC-ESI/MS of fractions P-I and p-I
On thin-layer chromatograms, fractions P-I and p-I migrated as monoglycosylceramides (Fig. 3 (A and B), lane 1), and LC-ESI/MS and subsequent MS2 of the native fractions identified monohexosylceramides, with d18:1-16:0 and d18:1-h16:0 as the major species (data not shown).
When analyzed by thin-layer chromatography on borate-impregnated silica gel plates, the major part (>90%) of the compounds in fractions P-I and p-I migrated as galactosylceramides (data not shown).
LC-ESI/MS of fractions P-II and p-II
Fraction P-II had compounds migrating as di- and triglycosylceramides (Fig. 3A, lane 2), whereas fraction p-II contained only compounds migrating as diglycosylceramides (Fig. 3B, lane 2). Dihexosylceramide with d18:1-16:0 was the major glycosphingolipid identified by LC-ESI/MS of both fractions, and there were also dihexosylceramide with d18:1-24:0 and dihexosylceramide with d18:1-h16:0. A trihexosylceramide with d18:1-16:0 was also characterized in fraction P-II (data not shown).
Binding of H. pylori and carbohydrate-recognizing ligands to the mono-, di-, and triglycosylceramides from human stomach
To further characterize the mono-, di-, and triglycosylceramides from human stomach, the binding of Solanum tuberosum lectin and P-fimbriated Escherichia coli to these fractions was examined.
The N-acetyllactosamine–binding lectin from S. tuberosum also binds to lactosylceramide (Galβ4Glcβ1Cer) with sphingosine and nonhydroxy fatty acids (24). Here, a binding to fraction P-II and p-II was obtained (Fig. 4B, lanes 3 and 5), confirming the presence of this variant of lactosylceramide in both fractions.
Figure 4.
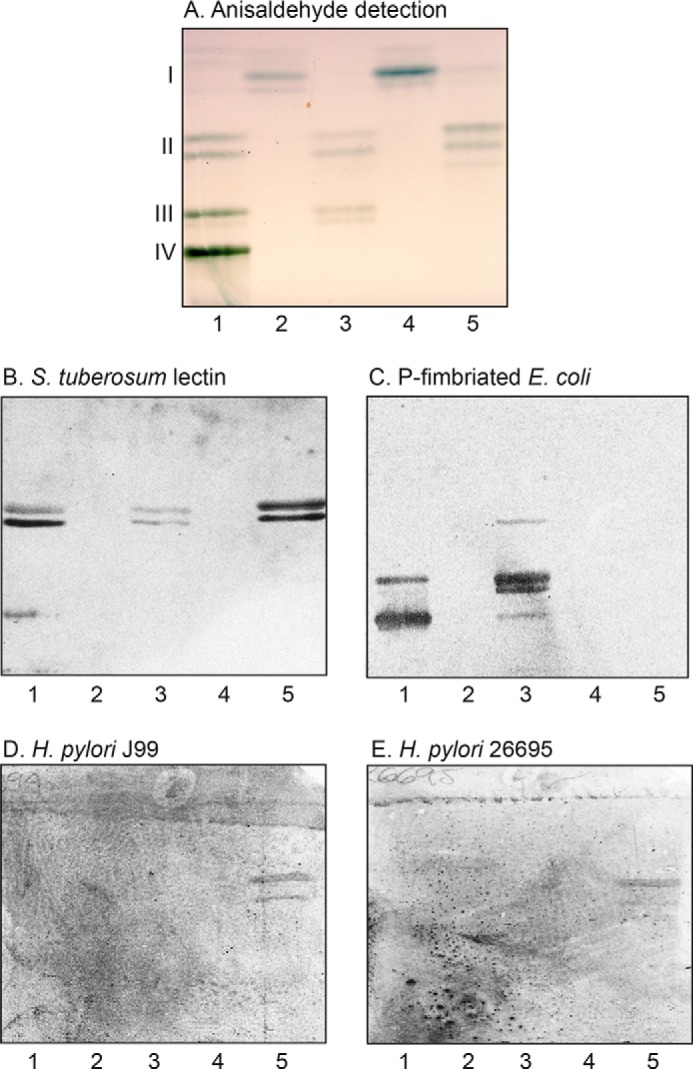
Binding of H. pylori, P-fimbriated E. coli, and S. tuberosum lectin to mono-, di-, and triglycosylceramides of human stomach. A–E, thin-layer chromatograms of the mono- to triglycosylceramide fractions isolated from human A(Rh+)P and A(Rh+)p stomachs (A) and autoradiograms obtained by binding of S. tuberosum lectin (B), P-fimbriated E. coli strain 291-15 (C), H. pylori strain J99 (D), and H. pylori strain 26695 (E). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 65:25:4 (by volume) as the solvent system, and the binding assays were performed as described under “Experimental procedures.” Autoradiography was for 12 h. Lane 1, reference nonacid glycosphingolipids of human blood group O erythrocytes, 40 μg; lane 2, fraction P-I, 4 μg; lane 3, fraction P-II, 4 μg; lane 4, fraction p-I, 4 μg; lane 5, fraction p-II, 4 μg. The Roman numerals to the left of the chromatogram in A denote the number of carbohydrate units in the bands.
We have previously reported that the major dihexosylceramide of the human stomach is galabiosylceramide (Galα4Galβ1 Cer) (18). In line with this, the Galα4Gal recognizing P-fimbriated Escherichia coli readily bound in the dihexosylceramide region in the glycosphingolipid fractions from the blood group P individual (Fig. 4C, lane 3), along with binding in the tri- and tetraosylceramide regions, indicating globotriaosylceramide (Galα4Galβ4Glcβ1Cer) and globotetraosylceramide (GalNAcβ3 Galα4Galβ4Glcβ1Cer).
There was no binding of the P-fimbriated E. coli to the diglycosylceramide fraction from the p individual (Fig. 4C, lane 5). However, binding of both the J99 and the 26695 H. pylori strain in the dihexosylceramide region of the p sample was obtained (Fig. 4 (D and E), lane 5). A preferential binding of H. pylori to lactosylceramide having sphingosine/phytosphingosine and 2-d-hydroxy fatty acids has been reported (25). According to mass spectrometry, fraction p-II contained a dihexosylceramide with d18:1-h16:0 ceramide, so most likely the H. pylori-binding compound in this fraction is lactosylceramide.
Characterization of the slow-migrating glycosphingolipids from human stomach
The glycosphingolipids in fractions P-III, p-III, P-IV, and p-IV:a–f were characterized by LC-ESI/MS and by binding of carbohydrate-recognizing ligands. The results are summarized in Table 3.
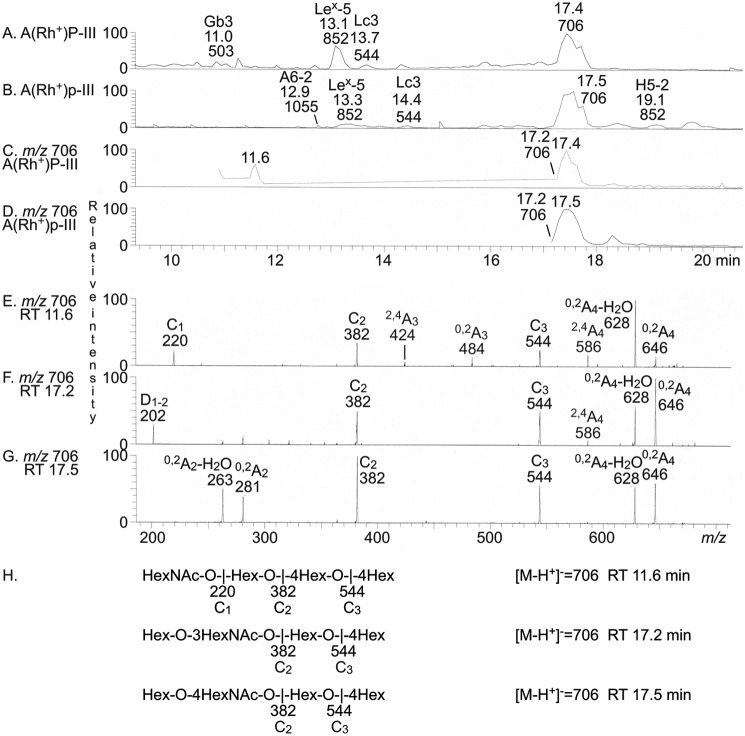
LC-ESI/MS of fractions P-III and p-III
TLC showed the presence of glycosphingolipids migrating in the tri- and tetraglycosylceramide region in fraction P-III (Fig. 3A, lane 3), whereas fraction p-III had a main compound that migrated in the tetraglycosylceramide region (Fig. 3B, lane 3).
The base peak chromatograms from LC-ESI/MS of the oligosaccharides obtained from fractions P-III and p-III after Rhodococcus endoglycoceramidase II digestion are shown in Fig. 5 (A and B). There are molecular ions corresponding to oligosaccharides ranging from trisaccharides (detected as [M-H+]− ions at m/z 503) to hexasaccharides (detected as [M-H+]− ions at m/z 1055). All molecular ions were subjected to MS2. Thereby, a lactotrisaccharide (m/z 544) and a Lex pentasaccharide (m/z 852) were identified in both fractions (not shown). A globotrisaccharide (m/z 503) was characterized in the fraction P-III, whereas in fraction p-III, a blood group A type 2 hexasaccharide (m/z 1055) and a blood group H type 2 pentasaccharide (m/z 852) were present.
Figure 5.
LC-ESI/MS of the oligosaccharides obtained by digestion of the slow-migrating nonacid glycosphingolipids of human blood group A(Rh+)P and A(Rh+)p stomachs with Rhodococcus endoglycoceramidase II. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction P-III. B, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction p-III. C, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from fraction P-III. D, mass chromatogram of m/z 706 from LC-ESI/MS of the oligosaccharides derived from fraction p-III. E, MS2 of the ion at m/z 706 (retention time 11.6 min) from LC-ESI/MS of the oligosaccharides derived from fraction P-III. F, MS2 of the ion at m/z 706 (retention time 17.2 min) from LC-ESI/MS of the oligosaccharides derived from fraction P-III. G, MS2 of the ion at m/z 706 (retention time 17.5 min) from LC-ESI/MS of the oligosaccharides derived from fraction P-III. H, interpretation formulas showing the deduced oligosaccharide sequences. The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were Galα4Galβ4Glc (Gb3), Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Lex-5), GlcNAcβ3Galβ4Glc (Lc3), GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc (A6-2), and Fucα2Galβ4GlcNAcβ3Galβ4Glc (H5-2).
To characterize the tetrasaccharide composition of the two fractions, we searched for molecular ions at m/z 706, corresponding to a tetrasaccharide with one HexNAc and three Hex. In fraction P-III, three sets of peaks at m/z 706 were present, eluting at 11.6, 17.2, and 17.4 min, respectively, whereas fraction p-III had two peaks eluting at 17.2 and 17.4 min (Fig. 5, C and D). The MS2 spectrum of the ion at m/z 706 at retention time 11.6 min in fraction P-III (Fig. 5E) had a C-type fragment ion series (C1 at m/z 220, C2 at m/z 382, and C3 at m/z 544), demonstrating a HexNAc-Hex-Hex-Hex sequence. The 0,2A3 and 0,2A3-H2O fragment ions at m/z 484 and 466 demonstrated a 4-substituted Hex (20, 21). Thus, a globotetrasaccharide (GalNAcβ3Galα4Galβ4Glc) was identified.
MS2 of the ion at m/z 706 at the retention time 17.2 min allowed a preliminary identification of a lactotetrasaccharide (Galβ3GlcNAcβ3Galβ4Glc) in both fractions (exemplified in Fig. 5F). This was concluded from the C-type fragment ions (C2 at m/z 382 and C3 at m/z 544) identifying a Hex-HexNAc-Hex-Hex sequence. This MS2 spectrum had a prominent D1–2 ion at m/z 202, which is obtained by a C2-Z2 double cleavage and diagnostic for a 3-substituted HexNAc (21). The MS2 spectrum also lacked a 0,2A2 fragment ion at m/z 281, further demonstrating that the HexNAc was substituted at the 3-position.
In contrast, the MS2 spectrum of the ion at m/z 706 at the retention time 17.5 min had a prominent 0,2A2 fragment ion at m/z 281 and a 0,2A2-H2O fragment ion at m/z 263, demonstrating a terminal Hex-HexNAc sequence with a 4-substituted HexNAc (i.e. a type 2 chain) (20, 21). In combination with the C2 ion at m/z 382 and the C3 ion at m/z 544, this demonstrated a neolactotetrasaccharide (Galβ4GlcNAcβ3Galβ4Glc) in both fractions (exemplified in Fig. 5G).
The three MS2 spectra all had 0,2A4 ions at m/z 646 and 0,2A4-H2O ions at m/z 628, which were derived from cross-ring cleavage of the 4-substituted Glc of the lactose unit at the reducing end. Thus, the globotetrasaccharide was present only in fraction P-III, whereas the lacto- and neolactotetrasaccharides were identified in both fractions P-III and p-III.
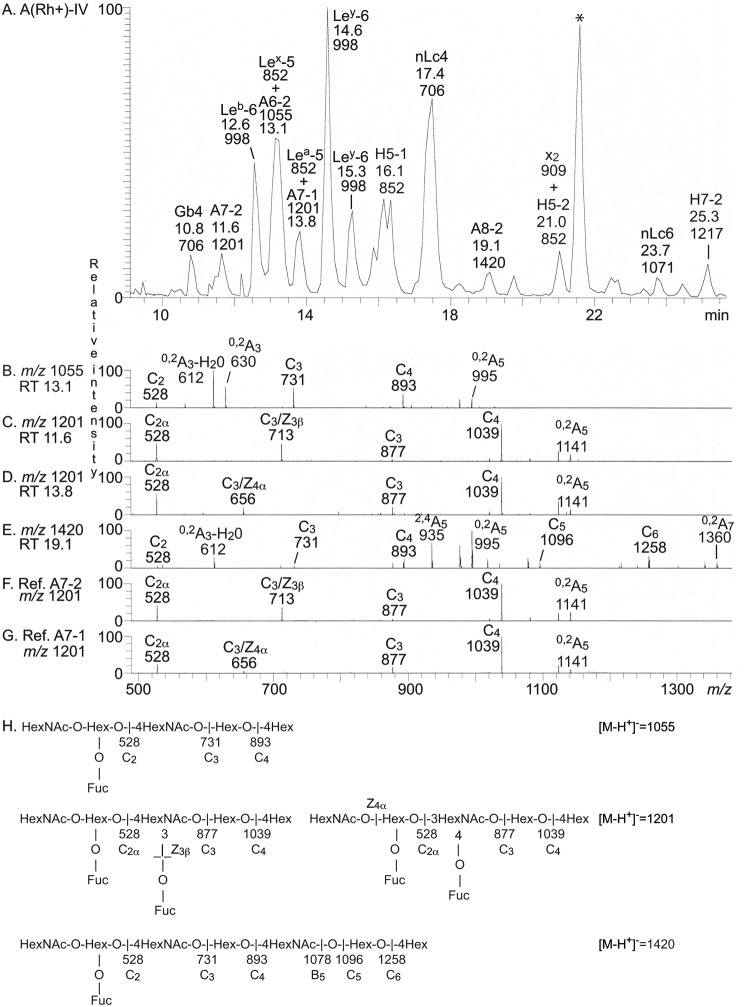
LC-ESI/MS of fractions P-IV and p-IV
Fraction P-IV and p-IV both contained a number of glycosphingolipids, migrating at the level of tetraglycosylceramide and below (Fig. 3 (A and B), lane 4). LC-ESI/MS of the oligosaccharides obtained from endoglycoceramidase hydrolysis of these fractions showed that both were complex mixtures (exemplified in Fig. 6A). All molecular ions were subjected to MS2, and the oligosaccharides thereby identified are given in the chromatogram (Fig. 6) and summarized in Table 3.
Figure 6.
LC-ESI/MS of the oligosaccharides obtained by Rhodococcus endoglycoceramidase II digestion of the slow-migrating nonacid glycosphingolipid fraction P-IV from human blood group A(Rh+)P stomach and of reference blood group A heptaosylceramides. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction P-IV. B, MS2 of the ion at m/z 1055 (retention time 13.1 min). C, MS2 of the ion at m/z 1201 (retention time 11.6 min). D, MS2 of the ion at m/z 1201 (retention time 13.8 min). E, MS2 of the ion at m/z 1420 (retention time 19.1 min). F, MS2 of the ion at m/z 1201 from LC-ESI/MS of the oligosaccharides derived from reference blood group A type 2/ALey heptaosylceramide (GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glc). G, MS2 of the ion at m/z 1201 from LC-ESI/MS of the oligosaccharides derived from reference blood group A type 1/ALeb heptaosylceramide (GalNAcα3(Fucα2)Galβ3(Fucα43)GlcNAcβ3Galβ4Glc). H, interpretation formulas showing the deduced oligosaccharide sequences. The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were GalNAcβ3Galα4Galβ4Glc (Gb4), GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (A7-2), Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Leb-6), Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Lex-5), GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc (A6-2), Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Lea-5), GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (A7-1), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-6), Fucα2Galβ3GlcNAcβ3Galβ4Glc (H5-1), Galβ4GlcNAcβ3Galβ4Glc (nLc4), GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (A8-2), GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc (x2), Fucα2Galβ4GlcNAcβ3Galβ4Glc (H5-2), Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (nLc6), and Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (H7-2). *, nonoligosaccharide contaminant.
By MS2, four oligosaccharides with terminal blood group A determinants were identified in fraction P-IV. First, MS2 of the molecular ion at m/z 1055 at retention time 13.1 min (Fig. 6B) gave a series of C-type fragment ions (C2 at m/z 528, C3 at m/z 731, and C4 at m/z 893) demonstrating a HexNAc-(Fuc-)Hex-HexNAc-Hex-Hex sequence. A type 2 core chain was identified by the 0,2A4 ion and 0,2A4-H2O ion at m/z 630 and 612. Taken together, these spectral features identified a blood group A type 2 hexasaccharide (GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc).
Second, the MS2 spectrum of the molecular ion at m/z 1201 at retention time 11.6 min (Fig. 6C) was very similar to the MS2 spectrum obtained from the reference blood group A type 2 heptasaccharide (Fig. 6F). Both spectra had a series of C-type fragment ions (C2 at m/z 528, C3 at m/z 877, and C4 at m/z 1039) identifying a HexNAc-(Fuc-)Hex-(Fuc-)HexNAc-Hex-Hex sequence. Both spectra also had a fragment ion at m/z 713, which, in analogy with the fragment ion at m/z 364 in the MS2 spectrum of the Lex pentasaccharide, is obtained by double glycosidic cleavage of the 3-linked branch (C3/Z3β) and thus characteristic for an internal 4-linked GlcNAc substituted with a Fuc at the 3-position (21). Taken together, this identified a blood group A type 2/ALey heptasaccharide (GalNAcα3(Fucα2)Galβ4(Fucα3)GlcNAcβ3Galβ4Glc).
Third, a blood group A type 1/ALeb heptasaccharide (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glc) was identified MS2 of the molecular ion at m/z 1201 at retention time 13.8 min (Fig. 6D). This spectrum had a high similarity with the MS2 spectrum obtained from the reference blood group A type 1 heptasaccharide (Fig. 6G). Again a C-type fragment ion series (C2 at m/z 528, C3 at m/z 877, and C4 at m/z 1039) identifying a HexNAc-(Fuc-)Hex-(Fuc-)HexNAc-Hex-Hex sequence was present in both spectra. In addition, both spectra had an ion at m/z 656, which, in analogy with the fragment ion at m/z 348 obtained by MS2 of the Lea pentasaccharide, is diagnostic for an internal 3-linked GlcNAc substituted with a Fuc at C-4 and is due to a double glycosidic cleavage of the 3-linked branch at C3 and Z4α (21).
Finally, the MS2 spectrum of the molecular ion at m/z 1420 (Fig. 6E) had B-type and C-type fragment ion series (C2 at m/z 528, B3 at m/z 713, C3 at m/z 731, C4 at m/z 893, B5 at m/z 1078, C5 at m/z 1096, and C6 at m/z 1258) demonstrating a HexNAc-(Fuc-)Hex-HexNAc-Hex-HexNAc-Hex-Hex sequence. The 0,2A4-H2O ion at m/z 612, along with the 0,2A5 ion at m/z 995, showed that both HexNAcs were substituted at C-4. Thereby, a blood group A type 2 octasaccharide (GalNAcα3(Fucα2)-Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) was identified.
The base peak chromatogram from LC-ESI/MS of the oligosaccharides from fraction p-IV was relatively weak. The largest oligosaccharide identified by MS2 was the A type 2 hexasaccharide, but information about the more complex oligosaccharides was not obtained. Therefore, after the binding assays described below, fraction p-IV was further separated on an Iatrobeads column in a final attempt to identify the complex glycosphingolipids. After pooling, six glycosphingolipid-containing fractions were obtained (denoted fractions p-IVa–f) (Fig. 3C). Each fraction was ∼0.1 mg.
Fractions p-IV:a–e were all complex oligosaccharide mixtures containing neolactotetra-, Lex penta-, Lea penta-, and Ley hexasaccharides, as found by LC-ESI/MS and MS2 (data not shown). The Leb hexasaccharide was present in fractions p-IV:c–e, and the x2 pentasaccharide and the A type 2 hexasaccharide were present in fractions p-IV:c and -d. In addition, the H type 2, the A type 1, and the A type 2 heptasaccharides were present in fractions p-IV:d and -e.
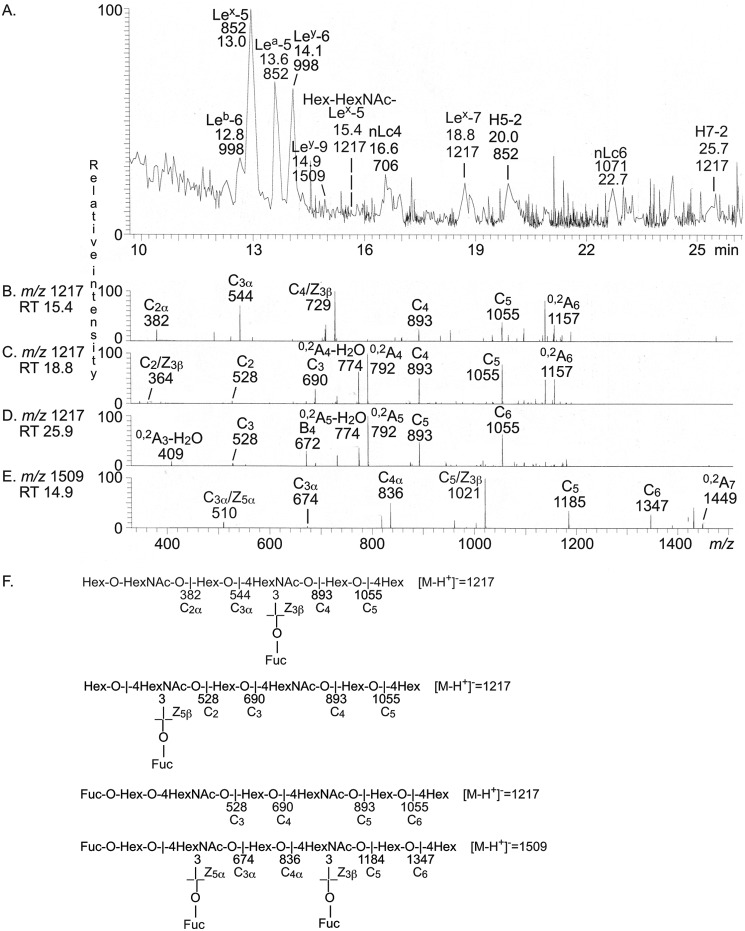
LC-ESI/MS of fraction p-IV-f
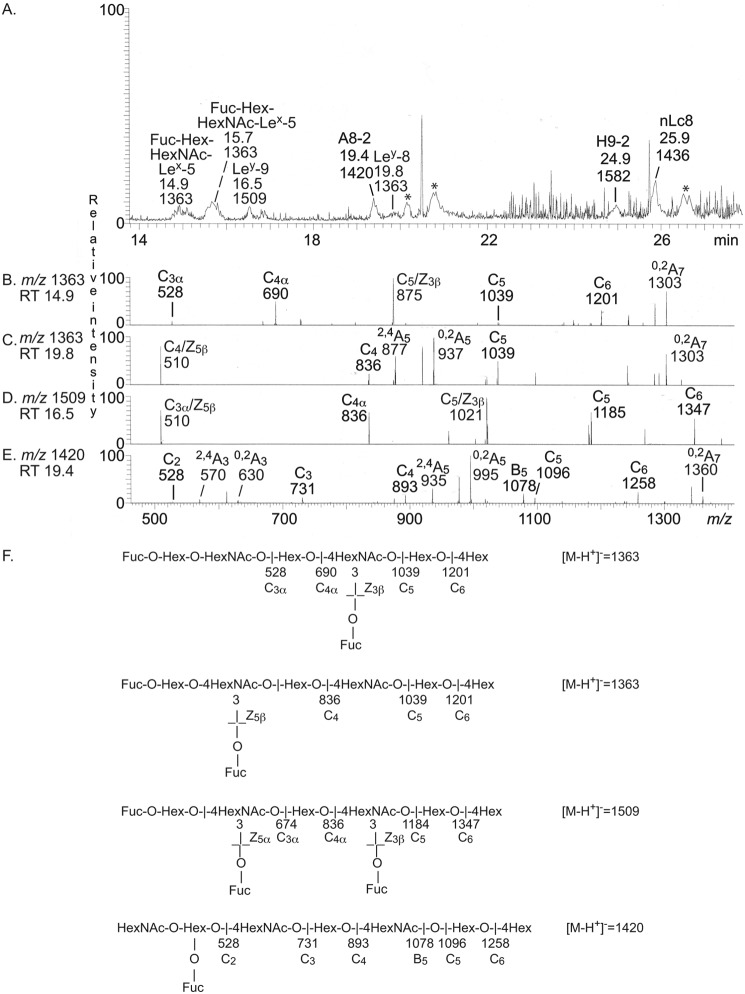
LC-ESI/MS of the oligosaccharides obtained from fraction p-IV-f gave a relatively weak base peak chromatogram (Fig. 7A). Again, MS2 of the major molecular ions identified the Leb and Ley hexasaccharides; the Lex, Lea, and H type 2 pentasaccharides; and the neolactotetra- and neolactohexasaccharides. The base peak chromatogram had also three minor molecular ions at m/z 1217 at retention times 15.4, 18.8, and 25.9 min, respectively, and a minor molecular ion at m/z 1509 eluting at 14.9 min.
Figure 7.
LC-ESI/MS of the oligosaccharides obtained by digestion of the slow-migrating nonacid glycosphingolipid fraction p-IV-f from human blood group A(Rh+)p stomach with Rhodococcus endoglycoceramidase II. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction p-IV-f. B, MS2 of the ion at m/z 1217 (retention time 15.4 min). C, MS2 of the ion at m/z 1217 (retention time 18.8 min). D, MS2 of the ion at m/z 1217 (retention time 25.9 min). E, MS2 of the ion at m/z 1509 (retention time 14.9 min). F, interpretation formulas showing the deduced oligosaccharide sequences. The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Leb-6), Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Lex-5), Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Lea-5), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-6), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-9), Galβ3/4GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc(Hex-HexNAc-Lex-5), Galβ4GlcNAcβ3Galβ4Glc (nLc4), Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (Lex-7), Fucα2Galβ4GlcNAcβ3Galβ4Glc (H5-2), Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (nLc6), and Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (H5-2).
The MS2 spectrum of the molecular ion at m/z 1217 eluting at 15.4 min (Fig. 7B) had a series of C-type fragment ions (C2α at m/z 382, C3α at m/z 544, C4 at m/z 893, and C5 at m/z 1055), which identified an oligosaccharide with Hex-HexNAc-Hex-(Fuc-)HexNAc-Hex-Hex sequence. The ion at m/z 729 was due to a double glycosidic cleavage of the 3-linked branch at C4 and Z3β (20). This fragmentation is characteristic for an internal 4-linked GlcNAc substituted with a Fuc at the 3-position and thus demonstrated an internal Lex determinant. Taken together, this indicated an oligosaccharide with Galβ3/4GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc sequence.
MS2 of the molecular ion at m/z 1217, eluting at 18.8 min (Fig. 7C), gave a fragment ion at m/z 364, again identifying an internal 4-linked GlcNAc substituted with a Fuc at C-3 (21). C-type fragment ions at m/z 528 (C2), m/z 690 (C3), m/z 893 (C4), and m/z 1055 (C5) were present, as well as 0,2A4-H2O fragment ion at m/z 774 and a 0,2A4 fragment ion at m/z 792, demonstrating that the internal HexNAc was substituted at C-4. Taken together, this indicated a Lex heptasaccharide (Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc).
The MS2 spectrum obtained of the molecular ion at m/z 1217 eluting at 25.9 min (Fig. 7D) had a series of C-type fragment ions (C3 at m/z 528, C4 at m/z 690, C5 at m/z 893, and C6 at m/z 1055), which, together with the 0,2A3-H2O fragment ion at m/z 409, the 0,2A5-H2O fragment ion at m/z 774, and the 0,2A5 fragment ion at m/z 792, identified an oligosaccharide with Fuc-Hex-4HexNAc-Hex-4HexNAc-Hex-4Hex sequence (i.e. a blood group H type 2 heptasaccharide (Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc)).
A molecular ion at m/z 1509 corresponds to an oligosaccharide with three Fuc, two HexNAc, and four Hex. Here the MS2 spectrum obtained (Fig. 7E) had two C/Zβ ions at m/z 510 and m/z 1021, demonstrating that there were two internal 4-linked GlcNAcs substituted with a Fuc at the 3-position. There was also a series of C-type fragment ions at m/z 674 (C3α), m/z 836 (C4α), m/z 1185 (C5), and m/z 1347 (C6). Collectively, these spectral features allowed a tentative identification of a Ley nonasaccharide (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc).
In a final attempt to characterize the complex glycosphingolipids in fraction p-IV-f, this fraction was analyzed at m/z 1300–2000. Thereby, a number of molecular ions were detected at m/z 1363 (retention time 14.9–15.7 min and 19.8 min), m/z 1420, m/z 1436, m/z 1509, and m/z 1582 (Fig. 8A). MS2 of the ion at m/z 1420 identified a blood group A type 2 octasaccharide (GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) (Fig. 8E), and MS2 of the ion at m/z 1509 identified a Ley nonasaccharide (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc) (Fig. 8D), as above. A neolactooctasaccharide (Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) was characterized by MS2 of the ion at m/z 1436, and a blood group H nonasaccharide (Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc) was characterized by MS2 of the ion at m/z 1582 (data not shown).
Figure 8.
LC-ESI/MS (m/z 1300–2000) of the complex oligosaccharides obtained by digestion of the slow-migrating nonacid glycosphingolipid fraction p-IV-f from human blood group A(Rh+)p stomach with Rhodococcus endoglycoceramidase II. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from fraction p-IV-f. B, MS2 of the ion at m/z 1363 (retention time 14.9 min). C, MS2 of the ion at m/z 1363 (retention time 19.8 min). D, MS2 of the ion at m/z 1509 (retention time 16.5 min). E, MS2 of the ion at m/z 1420 (retention time 19.4 min). F, interpretation formulas showing the deduced oligosaccharide sequences. The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were Fucα2Galβ3/4GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Fuc-Hex-HexNAc-Lex-5), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-9), GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (A8-2), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (Ley-8), Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (H9-2), and Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (nLc8). *, nonoligosaccharide contaminants.
A molecular ion at m/z 1363 corresponds to an oligosaccharide with two Fuc, two HexNAc, and four Hex. Here, there were two ions at m/z 1363, eluting at retention times 14.9–15.7 min and 19.8 min, respectively. The MS2 spectrum of the ion at retention time 14.9 min (Fig. 8B) had a series of C-type fragment ions (C3α at m/z 528, C4α at m/z 690, C5 at m/z 1039, and C6 at m/z 1201) identifying an octasaccharide with Fuc-Hex-HexNAc-Hex-(Fuc-)HexNAc-Hex-Hex sequence. The ion at m/z 875 was obtained by a double glycosidic cleavage of the 3-linked branch at C5 and Z3β (21) (i.e. a fragmentation characteristic for an internal 4-linked GlcNAc substituted with a Fuc at 3-position) and thus again demonstrated an internal Lex determinant. Taken together, these spectral features thus indicated an octasaccharide with Fucα2Galβ3/4GlcNAcβ3Galβ4(Fucα3)GlcNAcβ3Galβ4Glc sequence.
MS2 of the ion at m/z 1363 eluting at 19.8 min (Fig. 8C) gave a C4/Z5β ion at m/z 510 demonstrating an internal 4-linked GlcNAc substituted with a Fuc at the 3-position, C-type fragment ions at m/z 836 (C4) and m/z 1039 (C5), and a 2,4A5 ion at m/z 877 along with a 0,2A5 ion at m/z 937. Taken together, this demonstrated a Ley octasaccharide (Fucα2Galβ4(Fucα3)-GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc).
Thus, several different complex Lex and Ley glycosphingolipids were characterized in fraction p-IV-f. Lex and Ley do not, however, belong to the reported spectrum of H. pylori-binding glycans. The only potential H. pylori-binding ligands present in this fraction were neolactotetra- and neolactohexaosylceramide, the Leb hexaosylceramide, and the A type 1 heptaosylceramide.
Binding of H. pylori and carbohydrate recognizing ligands to the slow-migrating glycosphingolipids from human stomach
To substantiate the data from MS, the binding of a number of antibodies, lectins, BabA adhesin, and bacteria to the slow-migrating glycosphingolipid subfractions from human stomachs was examined (Fig. 9).
Figure 9.
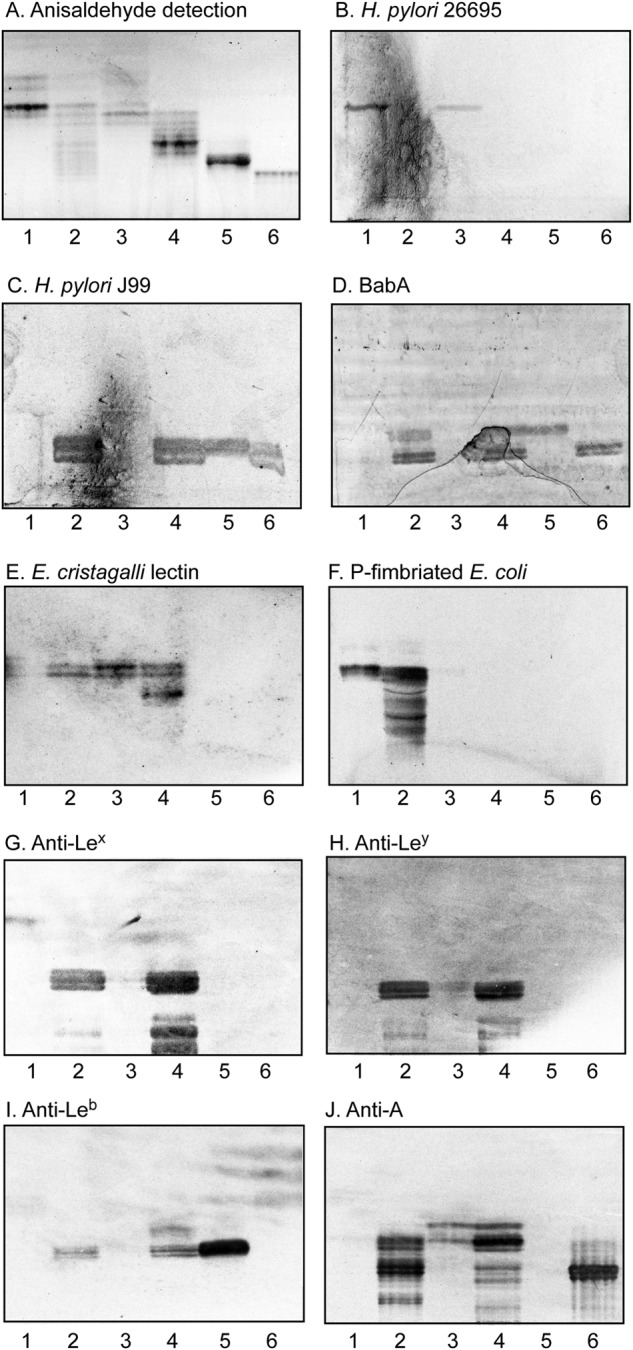
Characterization of the slow-migrating nonacid glycosphingolipids of human blood group A(Rh+)P and A(Rh+)p stomachs by binding of antibodies, lectins, adhesin, and bacteria. A–J, thin-layer chromatogram after detection with anisaldehyde (A) and autoradiograms obtained by binding of H. pylori strain 26695 (B), H. pylori strain J99 (C), BabA547 (D), E. cristagalli lectin (E), P-fimbriated E. coli strain 291–15 (F), and monoclonal antibodies directed against the Lex determinant (G), the Ley determinant (H), the Leb determinant (I), and the blood group A determinant (J). The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as the solvent system, and the binding assays were performed as described under ”Experimental procedures.” Autoradiography was for 12 h. Lane 1, fraction P-III, 4 μg; lane 2, fraction P-IV, 4 μg; lane 3, fraction p-III, 4 μg; lane 4, fraction p-IV, 4 μg; lane 5, reference Leb hexaosylceramide (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 4 μg; lane 6, reference ALeb/A type 1 heptaosylceramide (GalNAcα3(Fucα2)Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer), 4 μg.
The H. pylori strain 26695 bound in the tetraosylceramide region in fractions P-III and p-III (Fig. 9B, lanes 1 and 3). The structural characterization of fractions P-III and p-III demonstrated the presence of lactotetraosylceramide and neolactotetraosylceramide in these fraction, both of which have previously been shown to be recognized by H. pylori (17, 26).
H. pylori generalist strain J99, on the other hand, bound to slow-migrating compounds in fractions P-IV and p-IV (Fig. 9C, lanes 2 and 4). The same binding pattern was obtained with the recombinant truncated adhesin domain of BabA (BabA547) (Fig. 9D, lanes 2 and 4). Ligands for the BabA-mediated binding of the J99 strain to these slow-migrating glycosphingolipids were the H type 1 pentaosylceramide, the Leb hexaosylceramide, and the A type 1/ALeb heptaosylceramide identified in fraction P-IV and the Leb hexaosylceramide and A type 1/ALeb heptaosylceramide in fraction p-IV.
The Erythrina cristagalli lectin binds to glycoconjugates with terminal Galβ4GlcNAc and Fucα2Galβ4GlcNAc (27). Probing of the slow-migrating glycosphingolipid fractions with this lectin gave a binding double band in the tetraosylceramide region in fractions P-IV, p-III, and p-IV (Fig. 9E, lanes 2–4), confirming the presence of a Galβ4GlcNAc-terminated tetraosylceramide (i.e. neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer)). A more slow-migrating compound in fraction p-IV was also recognized (Fig. 9E, lane 4), most likely the blood group H type 2 heptaosylceramide (Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer). The binding of Galα4Gal-binding P-fimbriated E. coli in the tri- and tetraosylceramide region of the nonacid fractions of the stomach of the A(Rh+)P individual (Fig. 9F, lanes 1 and 2) supported the presence of globotriaosylceramide (Galα4Galβ4Glcβ1Cer) and globotetraosylceramide (GalNAcβ3Galα4-Galβ4Glcβ1Cer) in these fractions, whereas the binding of anti-Lex antibodies in the pentaosylceramide region in fractions P-IV and p-IV (Fig. 9G, lanes 2 and 4) was in line with the presence of Lex pentaosylceramide (Galβ4(Fucα3)GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer). Compounds in the hexaosylceramide region in fraction P-IV and p-IV were recognized by the anti-Ley and anti-Leb antibodies (Fig. 9 (H and I), lanes 2 and 4), supporting the presence of Ley (Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer) and Leb (Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer) hexaosylceramides. The anti-Lex and anti-Ley antibodies also bound to slow-migrating glycosphingolipids in fractions P-IV and p-IV, indicating complex Lex and Ley glycosphingolipids. Finally, the anti-A antibodies bound in the hexa-, hepta-, and octaosylceramide regions in the slow-migrating fractions from the blood group A(Rh+)P and A(Rh+)p stomachs (Fig. 9J, lanes 2 and 4).
LC-ESI/MS of native glycosphingolipids from human stomach
To characterize the ceramide composition, the native total nonacid glycosphingolipid fractions from human stomach were also analyzed by LC-ESI/MS. The base peak chromatograms from LC-ESI/MS of the blood group O(Rh−)P stomach and the blood group A(Rh+)P stomach (Fig. S2, A and B) were very similar, and both had [M-H+]− ions at m/z 1022, m/z 1225, m/z 1387, and m/z 1533, and the blood group A(Rh+)P sample also had a [M-2H+]2− ion at m/z 868. MS2 of these ions identified globotriaosylceramide with d18:1-16:0 (m/z 1022); globotetraosylceramide and neolactotetra/lactotetraosylceramide with d18:1-16:0 (m/z 1225); H, Lea, or Lex pentaosylceramide with d18:1-h16:0 (m/z 1387); and Leb or Ley hexaosylceramide with d18:1-h16:0 (m/z 1533), as exemplified in Fig. S2 (C–E). Dihexosylceramide with d18:1-h24:0 was demonstrated by MS2 of the ([M-H+]− ion at m/z 988 in the sample from the O(Rh−)P stomach, whereas MS2 of the ([M-H+]− ion at m/z 860 in the sample from the A(Rh+)P stomach identified a dihexosylceramide with d18:1-16:0. Finally, a blood group A heptaosylceramide with d18:1-h16:0 was characterized by MS2 of the [M-2H+]2− ion at m/z 868 in the nonacid fraction from the A(Rh+)P stomach (Fig. S2F).
The base peak chromatograms from LC-ESI/MS of the blood group A(Rh+)p stomach (data not shown) was dominated by [M-H+]− ions at m/z 860 and m/z 970, which upon MS2 identified dihexosylceramide with d18:1-16:0 and d18:1-24:1, respectively. No ions corresponding to globotria- or globotetraosylceramide were present. There was a minor ion at m/z 1225, and here MS2 gave a Hex-HexNAc-Hex-Hex carbohydrate sequence, as in neolactotetra- or lactotetraosylceramide. Minor ions at m/z 1387 and m/z 1533 identified H, Lea, or Lex pentaosylceramide and Leb or Ley hexaosylceramide, as above, in both cases with d18:1-h16:0.
Thus, the di-, tri-, and tetraosylceramides of human stomach had mainly sphingosine with nonhydroxy 16:0 fatty acid, whereas the predominant ceramide species among the pentaosylceramides and larger glycosphingolipids was sphingosine with hydroxy 16:0 fatty acid.
LC-ESI/MS of nonacid glycosphingolipids from human gastric mucosa
Because the glycosphingolipids from human stomach were isolated from whole stomach tissue and potentially could be derived from the submucosal tissue, we also analyzed the nonacid glycosphingolipids from human gastric mucosal scrapings from two blood group A(Rh+)P individuals. LC-ESI/MS of the oligosaccharides derived from the nonacid glycosphingolipids of human gastric mucosa showed also in these cases a predominance of compounds based on type 2 core chains (Fig. 10). The base peak chromatograms of the two samples (Fig. 10, A and B) were very similar to the base peak chromatogram of the sample from A(Rh+)P stomach (Fig. S1A) but lacked ions corresponding to globotri-, globotetra-, Lea, and H type 1 oligosaccharides. The oligosaccharides identified in the nonacid glycosphingolipid fractions from human gastric mucosa are summarized in Table 2. Thus, the Leb, Ley, and blood group A type 2 hexasaccharides were the largest oligosaccharides characterized by MS2 in both samples. However, the molecular ion profiles (Fig. 10, C and D) indicated the presence of a blood group A heptasaccharide (ALeb and/or ALey) (m/z 1201) and an H type 2 heptasaccharide (m/z 1217) in both samples. The molecular ion profile from case 3 (Fig. 10C) also had an ion at m/z 1420, indicating the presence of a blood group A octasaccharide, and the ion at m/z 1071 in the molecular ion profile from case 4 (Fig. 10D) indicated a neolactohexasaccharide.
Figure 10.
LC-ESI/MS of the oligosaccharides obtained from the total nonacid glycosphingolipid fractions from human gastric mucosa by hydrolysis with endoglycoceramidase II from Rhodococcus spp. A, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained from human from human gastric mucosa blood group A(Rh+)P (case 3). Peaks marked with an asterisk are noncarbohydrate contaminants. B, base peak chromatogram from LC-ESI/MS of the oligosaccharides obtained from human from human gastric mucosa blood group A(Rh+)P (case 4). C, molecular ion profile from LC-ESI/MS of from human gastric mucosa blood group A(Rh+)P (case 3). Peaks marked with an asterisk are noncarbohydrate contaminants. D, molecular ion profile from LC-ESI/MS of from human gastric mucosa blood group A(Rh+)P (case 4). The identification of oligosaccharides was based on their retention times, determined molecular masses, and subsequent MS2 sequencing. The oligosaccharides identified in the chromatograms were Fucα2Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Leb-6), Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Lex-5), GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4Glc (A6-2), Galβ3(Fucα4)GlcNAcβ3Galβ4Glc (Lea-5), Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc (Ley-6), Galβ4GlcNAcβ3Galβ4Glc (nLc4), GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc (x2), Fucα2Galβ4GlcNAcβ3Galβ4Glc (H5-2), Fucα2Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (H7-2), Galβ3GlcNAcβ3Galβ4Glc (Lc4), GalNAcα3(Fucα2)Galβ3/4(Fucα4/3)GlcNAcβ3Galβ4Glc (A7), Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (nLc6), and GalNAcα3(Fucα2)Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc (A8).
Discussion
A high proportion of the world's population is infected by H. pylori, mainly in developing countries, where the infection occurs in up to 80% of the middle-aged adults (1, 2). The majority of infected individuals develop an acute gastritis, which evolves into a chronic active gastritis. Most cases remain asymptomatic, and only a subset of infected individuals develop severe gastric diseases, as peptic ulcers or gastric cancer. The virulence factors expressed by the infecting H. pylori strain is one known determinant factor of the outcome of the infection. Differences in the host genetic background, such as variability in host immune responses (see Mărginean et al. (28) and references therein) and differential expression of receptors in the gastric mucosa, are also involved in the final outcome of the infection. In this study, the nonacid glycosphingolipids of three human stomachs were characterized, demonstrating differences in the composition of glycosphingolipids, and also differences in H. pylori-binding compounds, between individuals.
No binding of H. pylori to the blood group O(Rh−)P stomach glycosphingolipids was obtained. LC-ESI/MS of the oligosaccharides obtained from the glycosphingolipids of this stomach demonstrated the presence of a Lea pentasaccharide, whereas oligosaccharides with H type 1 and Leb determinants were absent. This suggests that this stomach was derived from a blood group OLe(a+b−) nonsecretor individual (22, 23). Due to low amounts, this fraction was not further characterized.
Glycosphingolipids of the A(Rh+)P and A(Rh+)p stomachs were recognized by both the BabA-expressing H. pylori generalist strain J99 and the two strains without BabA (the J99/BabA− mutant strain and the 26695 strain), but with different binding patterns. The J99 strain and the recombinant truncated adhesin domain of BabA bound to compounds migrating as penta- to heptaosylceramides. Binding to these glycosphingolipids was not obtained with the BabA-negative strains, further demonstrating that this interaction was BabA-dependent. The candidate ligands for BabA-mediated binding identified by LC-ESI/MS and antibody binding were the Leb hexaosylceramide and the A type 1/ALeb heptaosylceramide in both stomach samples and also the H type 1 pentaosylceramide in the A(Rh+)P stomach.
The BabA-negative strains J99/BabA− and 26695, on the other hand, both bound to compounds migrating as di- and tetraosylceramides in the A(Rh+)p stomach sample, and the 26695 strain also bound in the penta- to hexaosylceramide region. The binding of H. pylori in the dihexosylceramide region of the A(Rh+)p sample is most likely due to recognition of lactosylceramide (25). The tetraosylceramides of the A(Rh+)p stomach were characterized as lactotetraosylceramide and neolactotetraosylceramide, both of which are recognized by H. pylori (17, 26). The penta- or hexaosylceramide recognized by the BabA-negative H. pylori 26695 strain is likely to be the x2 pentaosylceramide or neolactohexaosylceramide (26), which both were characterized in the A(Rh+)p stomach fraction.
The most obvious difference between the A(Rh+)P and A(Rh+)p stomachs was the absence of globoseries glycosphingolipids, such as globotriaosylceramide and globotetraosylceramide, in the A(Rh+)p stomach sample. This is due to lack of the α4-galactosyltransferase converting lactosylceramide to globotriaosylceramide (29). In a previous work, we found that the major dihexosylceramide in the human stomach is galabiaosylceramide (Galα4Galβ1Cer) (18). Here, there was no binding of Galα4Gal recognizing P-fimbriated E. coli in the dihexosylceramide region of the stomach sample from the blood group p individual, demonstrating that also galabiaosylceramide is absent in this case.
An unexpected finding was the predominance of nonacid glycosphingolipids with type 2 core chains in the human stomach. This is in contrast to the glycosphingolipids of human small intestine, which mainly have type 1 core (30, 31). Interestingly, several immunohistochemistry studies have demonstrated a differential distribution of ABH and Le blood group determinants with type 1 and type 2 core chains in the normal human gastric mucosa (32–36). Determinants with type 1 core (e.g. Leb and ALeb) are mainly found in the foveolar epithelium, whereas ABH and Le blood group determinants based on type 2 core (e.g. Ley and ALey) are found both in the foveolar epithelium and in deep gastric glands.
Binding of H. pylori to neolactoglycosphingolipids has been characterized, and here the optimal binding sequence is GlcNAcβ3Galβ4GlcNAc, whereas binding to neolactotetraosylceramide (Galβ4GlcNAcβ3Galβ4Glcβ1Cer), which lacks the terminal β3-linked GlcNAc, is relatively weak (26). This is in agreement with the nonbinding of H. pylori to several fractions containing neolacto compounds (neolactotetraosylceramide, neolactohexaosylceramide, and the x2 pentaosylceramide) characterized in this study (e.g. the nonacid glycosphingolipid fraction of the blood group O(Rh-)P stomach).
Several of the potential H. pylori receptors (lactotetraosylceramide, the x2 pentaosylceramide, and the Leb hexaosylceramide) were also characterized in the human gastric mucosal scrapings, although also these samples had a predominance of type 2 core glycosphingolipids. However, globotriaosylceramide and globotetraosylceramide, found in the analyses of glycosphingolipids from whole human stomach, were not present in these samples. An analogous situation has been reported for human small and large intestine, where the diglycosylceramides, globotriaosylceramide, and globotetraosylceramide are confined to the nonepithelial tissue, whereas monoglycosylceramides and glycosphingolipids with five or more carbohydrate residues are present in the epithelial cells (30, 31, 37, 38).
There was no binding of H. pylori to the nonacid glycosphingolipids from the blood group O(Rh−)P stomach that was deduced to be derived from a blood group OLe(a+b−) nonsecretor individual. Nonsecretor individuals have low amounts or no Leb antigens on their epithelial surfaces, because the precursor of the Leb sequence (i.e. the H type 1 sequence) is not formed due to lack of a functional FUT2 enzyme (39). However, in individuals with little or no Leb on the gastric epithelium, an enhanced H. pylori colonization density is obtained by interactions between the SabA adhesin and sialylated gastric glycoconjugates (40). In a recent study, we have characterized the acid glycosphingolipids of the human stomach (19) and identified two minor H. pylori SabA-binding gangliosides as Neu5Acα3-neolactohexaosylceramide (Neu5Acα3Galβ4GlcNAcβ3Galβ4-GlcNAcβ3Galβ4Glcβ1Cer) and Neu5Acα3-neolactooctaosylceramide (Neu5Acα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer), which thus might function as adhesion factors for SabA-expressing H. pylori.
The association between ABO blood groups and H. pylori infection has been examined in several studies (reviewed by Cooling (22) and Brandão de Mattos and de Mattos (42)). The results are, however, often discordant because some studies focus on H. pylori infection, whereas other focus on different H. pylori-related diseases (e.g. peptic ulcers or gastric cancer). One example is the nine studies that tested the association between ABO blood group and H. pylori infection in healthy subjects; two of them found a statistically significant association between the infection and blood group A status. However, in other studies, no association was detected. An increased risk for atrophic gastritis in individuals with blood group A has also been reported, but this was not confirmed in other studies on gastritis or gastric cancer patients.
Several studies have also examined the impact of Lewis and Secretor status on H. pylori infection or gastric diseases (reviewed by Cooling 22), also with contradictory results. In early studies, an increased incidence of peptic ulcers among nonsecretor individuals was found. However, later it was demonstrated that although nonsecretors are significantly more likely to have gastric disease, there is no difference in H. pylori infection rates by secretor status (i.e. secretor status and H. pylori infection are independent risk factors for gastroduodenal diseases).
In summary, the characterization of the nonacid glycosphingolipids of the three human stomachs demonstrated a high degree of structural complexity. Although type 2 chain was the dominating core chain of the complex glycosphingolipids of human stomach, there were several glycosphingolipids identified that are known to have roles in mediating binding of H. pylori to the human gastric epithelium. Identified ligands for BabA-mediated binding of H. pylori were the Leb hexaosylceramide, the H type 1 pentaosylceramide, and the A type 1/ALeb heptaosylceramide, and additional characterized H. pylori-binding compounds recognized by strains lacking BabA were lactosylceramide, lactotetraosylceramide, the x2 pentaosylceramide, and neolactohexaosylceramide.
The chromatogram binding assay is a powerful tool for defining ligands for carbohydrate recognizing proteins. However, the thin-layer plate is very far removed from the dynamic lipid bilayer in the biological cell membranes. In this bilayer, there are ordered and tightly packed microdomains, which can be viewed as floating lipid rafts composed of sphingolipids and cholesterol with sequestered glycosylphosphatidylinositol-linked proteins (43). Several microbes and microbial toxins selectively bind to lipid rafts due to the presence of their specific receptors, such as glycosphingolipids, in these domains, thereby gaining access to their host cells. Interaction with lipid rafts in gastric epithelial cells is important for intoxication by H. pylori vacuolating cytotoxin A (44, 45), and also type IV secretion system–dependent translocation of cytotoxin-associated antigen A is associated with lipid rafts (46). It has been demonstrated that the initial binding of H. pylori to AGS cells is independent of cellular rafts, whereas internalization in AGS cells requires the presence of sufficient cellular cholesterol on rafts (46). However, these studies were done using the H. pylori 26695 strain, which lacks Leb and sialic acid–binding capacities (6). Therefore, it would be of interest to investigate whether human gastric cell binding of BabA- or SabA-expressing H. pylori strains is raft-related and whether the different H. pylori-binding glycosphingolipids are present in lipid rafts.
Experimental procedures
Glycosphingolipid preparations
The studies were performed in accordance with the Declaration of Helsinki. The isolation of total acid and total nonacid glycosphingolipids from human stomach was done during the 1970s by the method described by Karlsson (47). The stomachs were collected in 1974 at Sahlgrenska University Hospital (Göteborg, Sweden) (before the hospital had an ethics committee). The blood group A(Rh+)P individual was an 84-year-old patient who died of heart failure due to a myocardial abscess, whereas the blood group O(Rh−)P individual was an 80-year-old patient who died of cerebral trauma. The stomachs were dissected out at autopsy and frozen at −70 °C. The tissues were lyophilized and thereafter extracted in two steps in a Soxhlet apparatus with chloroform and methanol (2:1 and 1:9 by volume, respectively). The extracts were subjected to mild alkaline hydrolysis and dialysis, followed by separation on a silicic acid column. Acid and nonacid glycosphingolipid fractions were obtained by chromatography on a DEAE-cellulose column. To separate the nonacid glycosphingolipids from alkali-stable phospholipids, the nonacid fractions were acetylated and separated on a second silicic acid column, followed by deacetylation and dialysis. Final purifications were done by chromatographies on DEAE-cellulose and silicic acid columns.
The isolation of glycosphingolipids from the stomach of a blood group A(Rh+)p individual was described previously (48). The tissue was in this case obtained after surgery for peptic ulcer disease. The glycosphingolipids were dissolved in chloroform/methanol (2:1 by volume) and kept at −20 °C
After initial tests for H. pylori binding and characterization by LC-ESI/MS (see below), the total nonacid glycosphingolipid fractions from the blood group A(Rh+)P and A(Rh+)p stomachs were separated on Iatrobeads (Iatrobeads 6RS-8060; Iatron Laboratories, Tokyo) columns eluted with increasing amounts of methanol in chloroform. The partly purified subfractions (denoted fractions P-I-IV and p-I-IV) obtained were further characterized by LC-ESI/MS and binding of antibodies, lectins, and bacteria. Finally, fractions P-IV and p-IV were again separated on Iatrobeads columns, and after pooling, five glycosphingolipid-containing fractions were obtained from fraction P-IV (denoted fractions P-IVa–e), and six fractions were obtained from fraction p-IV (denoted fractions p-IVa–f). Each fraction was ∼0.1 mg.
The nonacid glycosphingolipids from human gastric mucosa were from a previous study (cases 3 and 4, both blood group A(Rh+)P) (17). The materials used were stomach tissue (10 × 10-cm pieces) obtained from the fundus region from patients undergoing elective surgery for morbid obesity. After washing with 0.9% NaCl (w/v), the mucosal cells were gently scraped off and kept at −70 °C. The material was lyophilized, and acid and nonacid glycosphingolipids were isolated as described (17).
H. pylori strains, culture conditions, and labeling
The H. pylori strain J99 and the construction of the J99/BabA− mutant babA::cam were described by Ilver et al. (5). The H. pylori strain 26695 was described previously (49). The J99 strain has a generalist BabA (i.e. in addition to the Leb determinant, this strain binds to the blood group A or B type 1 and ALeb or BLeb determinants) (14). The H. pylori strain 26695 lacks Leb-binding activity (5).
For chromatogram binding experiments, the bacteria were grown in a microaerophilic atmosphere at 37 °C for 48 h on Brucella medium (Fisher) containing 10% fetal calf serum (Fisher) inactivated at 56 °C and BBL IsoVitaleX Enrichment (Fisher). The mutant strain J99/BabA− was cultured on the same medium supplemented with chloramphenicol (20 μg/ml). Bacteria were radiolabeled by the addition of 50 μCi of [35S]methionine (PerkinElmer Life Sciences) diluted in 0.5 ml of PBS, pH 7.3, to the culture plates. After incubation for 12–72 h at 37 °C under microaerophilic conditions, the bacteria were harvested, centrifuged three times, and thereafter suspended to 1 × 108 cfu/ml in PBS. The specific activities of the suspensions were ∼1 cpm per 100 H. pylori organisms.
Recombinant BabA
A truncated BabA domain (BabA547), lacking the C-terminal β-barrel part, was expressed with a periplasmic leader sequence in E. coli and purified as described (50).
Reference glycosphingolipids
Total acid and nonacid glycosphingolipid fractions were isolated as described (47). Individual glycosphingolipids were isolated by repeated chromatography on silicic acid columns and by HPLC and identified by mass spectrometry (20, 51) and 1H NMR spectroscopy (52).
TLC
TLC was done on aluminum- or glass-backed silica gel 60 high performance TLC plates (Merck). Glycosphingolipid mixtures (40 μg) or pure glycosphingolipids (4 μg) were applied to the plates and eluted with chloroform/methanol/water (60:35:8 by volume) or chloroform/methanol/water (65:25:4 by volume) as the solvent system. Chemical detection was done with anisaldehyde (53).
Borate-impregnated plates (54) were prepared by spraying glass-backed silica gel 60 high-performance TLC plates with 1% (w/v) aqueous sodium tetraborate, followed by activation for 30 min at 120 °C. Chromatography on the borate-impregnated plates was done using chloroform/methanol/water (100:30:4 by volume) as the solvent system.
Chromatogram binding assays
Binding of 35S-labeled H. pylori to glycosphingolipids on thin-layer chromatograms was done as reported previously (17). Dried chromatograms were dipped for 1 min in diethylether/n-hexane (1:5 by volume) containing 0.5% (w/v) polyisobutylmethacrylate (Aldrich). After drying, the chromatograms were soaked in PBS containing 2% BSA (w/v), 0.1% NaN3 (w/v), and 0.1% Tween 20 (by volume) for 2 h at room temperature. The chromatograms were subsequently covered with radiolabeled bacteria diluted in PBS (2–5 × 106 cpm/ml). Incubation was done for 2 h at room temperature, followed by repeated washings with PBS. The chromatograms were thereafter exposed to XAR-5 X-ray films (Eastman Kodak Co.) for 12 h.
The mouse monoclonal antibodies tested for binding to the nonacid glycosphingolipids in the chromatogram binding assay are given in Table 4. Binding of antibodies to glycosphingolipids separated on thin-layer chromatograms was performed as described (55), using 125I-labeled monoclonal anti-mouse antibodies (Z0259; DakoCytomation Norden A/S) for detection.
Table 4.
Monoclonal antibodies used in chromatogram binding assays
| Antibodies | Clone | Manufacturer | Dilution | Isotype |
|---|---|---|---|---|
| Anti-Lewisx | P12 | Calbiochem | 1:200 | IgM |
| Anti-Lewisy | F3 | Calbiochem | 1:200 | IgM |
| Anti-Lewisb | BG-6/T218 | Signet/Covance | 1:100 | IgM |
| Anti-blood group A | HE-195 | Sigma-Aldrich | 1:1000 | IgM |
Binding of 125I-labeled E. cristagalli lectin (Sigma-Aldrich), 125I-labeled S. tuberosum lectin (Sigma-Aldrich), 125I-labeled BabA547, and 35S-labeled P-fimbriated E. coli strain 291-15 to glycosphingolipids on thin-layer chromatograms was done as described (18, 24, 27). The binding assays with BabA547 were done using Carbo-Free blocking solution (Vector Laboratories, Burlingame, CA) for blocking of the chromatograms.
Endoglycoceramidase digestion and LC-ESI/MS
Endoglycoceramidase II from Rhodococcus spp. (Takara Bio Europe S.A., Gennevilliers, France) was used for hydrolysis of the nonacid glycosphingolipids. The glycosphingolipids (50 mg) were resuspended in 100 ml of 0.05 m sodium acetate buffer, pH 5.0, containing 120 mg of sodium cholate and sonicated briefly. Thereafter, 1 milliunit of enzyme was added, and the mixture was incubated at 37 °C for 48 h. The reaction was stopped by the addition of chloroform/methanol/water to the final proportions 8:4:3 (by volume). The oligosaccharide-containing upper phase thus obtained was separated from detergent on a Sep-Pak QMA cartridge (Waters). The eluant containing the oligosaccharides was dried under nitrogen and under vacuum.
The glycosphingolipid-derived oligosaccharides were resuspended in 50 μl of water and analyzed by LC-ESI/MS as described (20). The oligosaccharides were separated on a column (100 × 0.250 mm) packed in-house with 5-μm porous graphite particles (Hypercarb, Thermo-Hypersil, Runcorn, UK). An autosampler, HTC-PAL (CTC Analytics AG, Zwingen, Switzerland) equipped with a Cheminert valve (0.25-mm bore) and a 2-μl loop was used for sample injection. An Agilent 1100 binary pump (Agilent Technologies, Palo Alto, CA) delivered a flow of 250 μl/min, which was split down in a -inch microvolume-T (0.15-mm bore) (Vici AG International, Schenkon, Switzerland) by a 50 cm × 50-μm inner diameter fused silica capillary before the injector of the autosampler, allowing ∼3–5 μl/min through the column. The oligosaccharides (3 μl) were injected onto the column and eluted with an acetonitrile gradient (A: 10 mm ammonium bicarbonate; B: 10 mm ammonium bicarbonate in 80% acetonitrile). The gradient (0–45% B) was eluted for 46 min, followed by a wash step with 100% B and equilibration of the column for 24 min. A 30 cm × 50-μm inner diameter fused silica capillary was used as a transfer line to the ion source.
The oligosaccharides were analyzed in negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer (Thermo Electron, San José, CA). The IonMax standard ESI source on the LTQ mass spectrometer was equipped with a stainless steel needle kept at −3.5 kV. Compressed air was used as nebulizer gas. The heated capillary was kept at 270 °C, and the capillary voltage was −50 kV. Full scan (m/z 380–2000, two microscans, maximum 100 ms, target value of 30,000) was performed, followed by data-dependent MS2 scans of the three most abundant ions in each scan (2 microscans, maximum 100 ms, target value of 10,000). The threshold for MS2 was set to 500 counts. Normalized collision energy was 35%, and an isolation window of 3 units, an activation q = 0.25, and an activation time of 30 ms were used. Selected fractions were also analyzed at m/z 1300–2000. Data acquisition and processing were conducted with Xcalibur software (version 2.0.7).
Manual assignment of glycan sequences was done on the basis of knowledge of mammalian biosynthetic pathways, with the assistance of the Glycoworkbench tool (version 2.1) and by comparison of retention times and MS2 spectra of oligosaccharides from reference glycosphingolipids (20).
LC-ESI/MS of native glycosphingolipids
The native nonacid glycosphingolipid fractions were analyzed by LC-ESI/MS as described (56). Aliquots of the glycosphingolipid fractions were dissolved in methanol/acetonitrile (75:25 by volume) and separated on a 100 × 0.250-mm column, packed in-house with 5-μm polyamine II particles (YMC Europe GmbH, Dinslaken, Germany). An autosampler, HTC-PAL (CTC Analytics AG, Zwingen, Switzerland), equipped with a Cheminert valve (0.25-mm bore) and a 2-μl loop, was used for sample injection. An Agilent 1100 binary pump (Agilent Technologies, Palo Alto, CA) delivered a flow of 250 μl/min, which was split down in a -inch microvolume-T (0.15-mm bore) (Vici AG International, Schenkon, Switzerland) by a 50 cm × 50-μm inner diameter fused silica capillary before the injector of the autosampler, allowing ∼2–3 μl/min through the column. Samples were eluted with an aqueous gradient (A (100% acetonitrile) to B (10 mm ammonium bicarbonate)). The gradient (0–50% B) was eluted for 40 min, followed by a wash step with 100% B, and equilibration of the column for 20 min. The samples were analyzed in negative ion mode on an LTQ linear quadrupole ion trap mass spectrometer (Thermo Electron, San José, CA), with an IonMax standard ESI source equipped with a stainless steel needle kept at −3.5 kV. Compressed air was used as nebulizer gas. The heated capillary was kept at 270 °C, and the capillary voltage was −50 kV. Full scan (m/z 500–1800, two microscans, maximum 100 ms, target value of 30,000) was performed, followed by data-dependent MS2 scans (two microscans, maximum 100 ms, target value of 10,000) with normalized collision energy of 35%, isolation window of 2.5 units, activation q = 0.25, and activation time 30 ms). The threshold for MS2 was set to 500 counts.
Data acquisition and processing were conducted with Xcalibur software (version 2.0.7). Manual assignment of glycosphingolipid sequences was done with the assistance of the Glycoworkbench tool (version 2.1), and by comparison of retention times and MS2 spectra of reference glycosphingolipids.
Author contributions
C. J., A. B., T. B., and S. T. data curation; C. J., A. B., T. B., and S. T. formal analysis; C. J., A. B., T. B., and S. T. investigation; C. J. and S. T. methodology; C. J., A. B., T. B., and S. T. writing-review and editing; S. T. conceptualization; T. B. and S. T. resources; T. B. and S. T. funding acquisition; T. B. and S. T. validation; S. T. writing-original draft; S. T. project administration.
Supplementary Material
This work was supported by the Swedish Cancer Foundation. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1 and S2.
The glycosphingolipid nomenclature follows the recommendations by the IUPAC-IUB Commission on Biochemical Nomenclature (41). It is assumed that Gal, Glc, GlcNAc, GalNAc, NeuAc, and NeuGc are of the d-configuration, that Fuc is of the l-configuration, and that all sugars are present in the pyranose form. In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length, and the number after the colon gives the total number of double bonds in the molecule. Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation (e.g. h16:0). For long-chain bases, d denotes dihydroxy and t trihydroxy. Thus, d18:1 designates sphingosine (1,3-dihydroxy-2-aminooctadecene) and t18:0 phytosphingosine (1,3,4-trihydroxy-2-aminooctadecane).
- BabA
- blood group antigen–binding adhesin
- ESI
- electrospray ionization
- LabA
- lacDiNAc-binding adhesin
- SabA
- sialic acid–binding adhesin
- Hex
- hexose
- HexNAc
- N-acetylhexosamine
- Fuc
- fucose
- Cer
- ceramide.
References
- 1. Azevedo N. F., Huntington J., and Goodman K. J. (2009) The epidemiology of Helicobacter pylori and public health implications. Helicobacter 14, 1–7 10.1111/j.1523-5378.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 2. Wang F., Meng W., Wang B., and Qiao L. (2014) Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 345, 196–202 10.1016/j.canlet.2013.08.016 [DOI] [PubMed] [Google Scholar]
- 3. Pieters R. J. (2011) Carbohydrate mediated bacterial adhesion. Adv. Exp. Med. Biol. 715, 227–240 10.1007/978-94-007-0940-9_14 [DOI] [PubMed] [Google Scholar]
- 4. Teneberg S. (2009) The multiple carbohydrate binding specificities of Helicobacter pylori. Top. Curr. Chem. 288, 121–138 [DOI] [PubMed] [Google Scholar]
- 5. Ilver D., Arnqvist A., Ogren J., Frick I. M., Kersulyte D., Incecik E. T., Berg D. E., Covacci A., Engstrand L., and Borén T. (1998) Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279, 373–377 10.1126/science.279.5349.373 [DOI] [PubMed] [Google Scholar]
- 6. Mahdavi J., Sondén B., Hurtig M., Olfat F. O., Forsberg L., Roche N., Angstrom J., Larsson T., Teneberg S., Karlsson K.-A., Altraja S., Wadström T., Kersulyte D., Berg D. E., Dubois A., et al. (2002) Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297, 573–578 10.1126/science.1069076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossez Y., Gosset P., Boneca I. G., Magalhães A., Ecobichon C., Reis C. A., Cieniewski-Bernard C., Joncquel Chevalier Curt M., Léonard R., Maes E., Sperandio B., Slomianny C., Sansonetti P. J., Michalski J. C., and Robbe-Masselot C. (2014) The LacdiNAc-specific adhesin LabA mediates adhesion of Helicobacter pylori to human gastric mucosa. J. Infect. Dis. 210, 1286–1295 10.1093/infdis/jiu239 [DOI] [PubMed] [Google Scholar]
- 8. Königer V., Holsten L., Harrison U., Busch B., Loell E., Zhao Q., Bonsor D. A., Roth A., Kengmo-Tchoupa A., Smith S. I., Mueller S., Sundberg E. J., Zimmermann W., Fischer W., Hauck C. R., and Haas R. (2016) Helicobacter pylori exploits human CEACAMs via HopQ for adherence and translocation of CagA. Nat. Microbiol. 2, 16188 [DOI] [PubMed] [Google Scholar]
- 9. Javaheri A., Kruse T., Moonens K., Mejías-Luque R., Debraekeleer A., Asche C. I., Tegtmeyer N., Kalali B., Bach N. C., Sieber S. A., Hill D. J., Königer V., Hauck C. R., Moskalenko R., Haas R., et al. (2016) Helicobacter pylori adhesin HopQ engages in a virulence-enhancing interaction with human CEACAMs. Nat. Microbiol. 2, 16189 [DOI] [PubMed] [Google Scholar]
- 10. Odenbreit S., Till M., Hofreuter D., Faller G., and Haas R. (1999) Genetic and functional characterization of the alpAB gene locus essential for the adhesion of Helicobacter pylori to human gastric tissue. Mol. Microbiol. 31, 1537–1548 10.1046/j.1365-2958.1999.01300.x [DOI] [PubMed] [Google Scholar]
- 11. Odenbreit S., Kavermann H., Püls J., and Haas R. (2002) CagA tyrosine phosphorylation and interleukin-8 induction by Helicobacter pylori are independent from alpAB, HopZ and bab group outer membrane proteins. Int. J. Med. Microbiol. 292, 257–266 10.1078/1438-4221-00205 [DOI] [PubMed] [Google Scholar]
- 12. Dossumbekova A., Prinz C., Mages J., Lang R., Kusters J. G., Van Vliet A. H., Reindl W., Backert S., Saur D., Schmid R. M., and Rad R. (2006) Helicobacter pylori HopH (OipA) and bacterial pathogenicity: genetic and functional genomic analysis of hopH gene polymorphisms. J. Infect. Dis. 194, 1346–1355 10.1086/508426 [DOI] [PubMed] [Google Scholar]
- 13. Gerhard M., Lehn N., Neumayer N., Borén T., Rad R., Schepp W., Miehlke S., Classen M., and Prinz C. (1999) Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc. Natl. Acad. Sci. U.S.A. 96, 12778–12783 10.1073/pnas.96.22.12778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aspholm-Hurtig M., Dailide G., Lahmann M., Kalia A., Ilver D., Roche N., Vikström S., Sjöström R., Lindén S., Bäckström A., Arnqvist A., Mahdavi J., Nilsson U. J., Velapatiño B., Gilman R. H., et al. (2004) Functional adaptation of BabA, the Helicobacter pylori blood-group antigen binding adhesin. Science 305, 519–522 10.1126/science.1098801 [DOI] [PubMed] [Google Scholar]
- 15. Moonens K., Gideonsson P., Subedi S., Bugaytsova J., Romaõ E., Mendez M., Nordén J., Fallah M., Rakhimova L., Shevtsova A., Lahmann M., Castaldo G., Brännström K., Coppens F., Lo A. W., et al. (2016) Structural insights into polymorphic ABO glycan binding by Helicobacter pylori. Cell Host Microbe 19, 55–66 10.1016/j.chom.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benktander J., Ångström J., Breimer M. E., and Teneberg S. (2012) Re-definition of the carbohydrate binding specificity of Helicobacter pylori BabA adhesin. J. Biol. Chem. 287, 31712–31724 10.1074/jbc.M112.387654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Teneberg S., Leonardsson I., Karlsson H., Jovall P.-A., Angstrom J., Danielsson D., Naslund I., Ljungh Å., Wadström T., and Karlsson K.-A. (2002) Lactotetraosylceramide, a novel glycosphingolipid receptor for Helicobacter pylori, present in human gastric epithelium. J. Biol. Chem. 277, 19709–19719 10.1074/jbc.M201113200 [DOI] [PubMed] [Google Scholar]
- 18. Roche N., Ilver D., Ångström J., Barone S., Telford J. L., and Teneberg S. (2007) Human gastric glycosphingolipid receptors for Helicobacter pylori vacuolating cytotoxin VacA. Microbes Infect. 9, 605–614 10.1016/j.micinf.2007.01.023 [DOI] [PubMed] [Google Scholar]
- 19. Benktander J., Barone A., Madar Johansson M. M., and Teneberg S. (2018) Helicobacter pylori SabA binding gangliosides of human stomach. Virulence 9, 738–751 10.1080/21505594.2018.1440171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Karlsson H., Halim A., and Teneberg S. (2010) Differentiation of glycosphingolipid-derived glycan structural isomers by liquid chromatography-mass spectrometry. Glycobiology 20, 1103–1116 10.1093/glycob/cwq070 [DOI] [PubMed] [Google Scholar]
- 21. Chai W., Piskarev V., and Lawson A. M. (2001) Negative-ion electrospray mass spectrometry of neutral underivatized oligosaccharides. Anal. Chem. 73, 651–657 10.1021/ac0010126 [DOI] [PubMed] [Google Scholar]
- 22. Cooling L. (2015) Blood groups in infection and host susceptibility. Clin. Microbiol. Rev. 28, 801–870 10.1128/CMR.00109-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Mattos L. C. (2016) Structural diversity and biological importance of ABO, H, Lewis and secretor histo-blood group carbohydrates. Rev. Bras. Hematol. Hemoter. 38, 331–340 10.1016/j.bjhh.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ciopraga J., Angström J., Bergström J. Larsson T., Karlsson N., Motas C., Gozia O., and Teneberg S. (2000) Isolectins from Solanum tuberosum with different detailed carbohydrate binding specificities: unexpected recognition of lactosylceramide by N-acetyllactosamine-binding lectins. J. Biochem. 128, 855–867 10.1093/oxfordjournals.jbchem.a022824 [DOI] [PubMed] [Google Scholar]
- 25. Ångström J., Teneberg S., Milh M. A., Larsson T., Leonardsson I., Olsson B.-M., Halvarsson M. O., Danielsson D., Näslund I., Ljungh A., Wadström T., and Karlsson K.-A. (1998) The lactosylceramide binding specificity of Helicobacter pylori. Glycobiology 8, 297–309 10.1093/oxfordjournals.glycob.a018839,10.1093/glycob/8.4.297 [DOI] [PubMed] [Google Scholar]
- 26. Miller-Podraza H., Lanne B., Angström J., Teneberg S., Milh M. A., Jovall P.-Å., Karlsson H., and Karlsson K.-A. (2005) Novel binding epitope for Helicobacter pylori found in neolacto carbohydrate chains: structure and cross-binding properties. J. Biol. Chem. 280, 19695–19703 10.1074/jbc.M412688200 [DOI] [PubMed] [Google Scholar]
- 27. Teneberg S., Jovall P.-A., Angström J., and Karlsson K.-A. (1994) Characterization of binding of Galβ4GlcNAc-specific lectins from Erythrina christagalli and Erythrina corallodendron to glycosphingolipids: detection, isolation, and characteriztion of a novel glycosphingolipid of bovine buttermilk. J. Biol. Chem. 269, 8554–8563 [PubMed] [Google Scholar]
- 28. Mărginean M. O., Mărginean C. O., Melit L. E., Voidăzan S., Moldovan V., and Bănescu C. (2017) The impact of host's genetic susceptibility on Helicobacter pylori infection in children. Medicine (Baltimore) 96, e7612 10.1097/MD.0000000000007612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marcus D. M., Naiki M., and Kundu S. K. (1976) Abnormalities in the glycosphingolipid content of human Pk and p erythrocytes. Proc. Natl. Acad. Sci. U.S.A. 73, 3263–3267 10.1073/pnas.73.9.3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Björk S., Breimer M. E., Hansson G. C., Karlsson K. A., and Leffler H. (1987) Structures of blood group glycosphingolipids of human small intestine: a relation between the expression of fucolipids of epithelial cells and the ABO, Le, and Se phenotype of the donor. J. Biol. Chem. 262, 6758–6765 [PubMed] [Google Scholar]
- 31. Breimer M. E., Hansson G. C., Karlsson K. A., Larson G., and Leffler H. (2012) Glycosphingolipid composition of epithelial cells isolated along the villus axis of small intestine of a single human individual. Glycobiology 22, 1721–1730 10.1093/glycob/cws115 [DOI] [PubMed] [Google Scholar]
- 32. Mollicone R., Bara J., Le Pendu J., and Oriol R. (1985) Immunological pattern of type 1 (Lea, Leb) and type 2 (X, Y, H) blood group-related antigens in the human pyloric and duodenal mucosae. Lab. Invest. 53, 219–227 [PubMed] [Google Scholar]
- 33. Mollicone R., Le Pendu J., Bara J., and Oriol R. (1986) Heterogeneity of the ABH antigenic determinants expressed in human pyloric and duodenal mucosae. Glycoconj. J. 3, 187–202 10.1007/BF01049376 [DOI] [Google Scholar]
- 34. Sakamoto S., Watanabe T., Tokumaru T., Takagi H., Nakazato H., and Lloyd K. O. (1989) Expression of Lewisa, Lewisb, Lewisx, Lewisy, siayl-Lewisa, and sialyl-Lewisx blood group antigens in human gastric carcinoma and in normal gastric tissue. Cancer Res. 49, 745–752 [PubMed] [Google Scholar]
- 35. Torrado J., Blasco E., Cosme A., Gutierrez-Hoyos A., and Arenas J. I. (1989) Expression of type 1 and type 2 blood group-related antigens in normal and neoplastic gastric mucosa. Am. J. Clin. Pathol. 91, 249–254 10.1093/ajcp/91.3.249 [DOI] [PubMed] [Google Scholar]
- 36. Murata K., Egami H., Shibata Y., Sakamoto K., Misumi A., and Ogawa M. (1992) Expression of blood group-related antigens, ABH, Lewis(a), Lewis(b), Lewis(x), Lewis(y), CA19-9, and CSLEX1 in early cancer, intestinal metaplasia, and uninvolved mucosa of the stomach. Am. J. Clin. Pathol. 98, 67–75 10.1093/ajcp/98.1.67 [DOI] [PubMed] [Google Scholar]
- 37. Falk K. E., Karlsson K. A., Leffler H., and Samuelsson B. E. (1979) Specific pattern of glycosphingolipids enriched in a mucosa scraping of human small intestine. FEBS Lett. 101, 273–276 10.1016/0014-5793(79)81024-8 [DOI] [PubMed] [Google Scholar]
- 38. Holgersson J., Jovall P. A., and Breimer M. E. (1991) Glycosphingolipids of human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J. Biochem. 110, 120–131 10.1093/oxfordjournals.jbchem.a123530 [DOI] [PubMed] [Google Scholar]
- 39. Kelly R. J., Rouquier S., Giorgi D., Lennon G. G., and Lowe J. B. (1995) Sequence and expression of a candidate for the human Secretor blood group α(1,2)fucosyltransferase gene (FUT2): homozygosity for an enzyme-inactivating nonsense mutation commonly correlates with the non-secretor phenotype. J. Biol. Chem. 270, 4640–4649 10.1074/jbc.270.9.4640 [DOI] [PubMed] [Google Scholar]
- 40. Sheu B. S., Odenbreit S., Hung K. H., Liu C. P., Sheu S. M., Yang H. B., and Wu J. J. (2006) Interaction between host gastric Sialyl-Lewis X and H. pylori SabA enhances H. pylori density in patients lacking gastric Lewis B antigen. Am. J. Gastroenterol. 101, 36–44 10.1111/j.1572-0241.2006.00358.x [DOI] [PubMed] [Google Scholar]
- 41. Chester M. A. (1998) IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN): nomenclature of glycolipids–recommendations 1997. Eur. J. Biochem. 257, 293–298 10.1046/j.1432-1327.1998.2570293.x [DOI] [PubMed] [Google Scholar]
- 42. Brandão de Mattos C. C., and de Mattos L. C. (2017) Histo-blood group carbohydrates as facilitators for infection by Helicobacter pylori. Infect. Genet. Evol. 53, 167–174 10.1016/j.meegid.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 43. Sezgin E., Levental I., Mayor S., and Eggeling C. (2017) The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 18, 361–374 10.1038/nrm.2017.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schraw W., Li Y., McClain M. S., van der Goot F. G., and Cover T. L. (2002) Association of Helicobacter pylori vacuolating toxin (VacA) with lipid rafts. J. Biol. Chem. 277, 34642–34650 10.1074/jbc.M203466200 [DOI] [PubMed] [Google Scholar]
- 45. Gupta V. R., Patel H. K., Kostolansky S. S., Ballivian R. A., Eichberg J., and Blanke S. R. (2008) Sphingomyelin functions as a novel receptor for Helicobacter pylori VacA. PLoS Pathog. 4, e1000073 10.1371/journal.ppat.1000073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lai C. H., Chang Y. C., Du S. Y., Wang H. J., Kuo C. H., Fang S. H., Fu H. W., Lin H. H., Chiang A. S., and Wang W. C. (2008) Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA-induced responses in AGS cells. Infect. Immun. 76, 3293–3303 10.1128/IAI.00365-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Karlsson K. A. (1987) Preparation of total non-acid glycolipids for overlay analysis of receptors for bacteria and viruses and for other studies. Methods Enzymol. 138, 212–220 10.1016/0076-6879(87)38018-8 [DOI] [PubMed] [Google Scholar]
- 48. Breimer M. E., Cedergren B., Karlsson K. A., Nilson K., and Samuelsson B. E. (1980) Glycolipid pattern of stomach tissue of a human with the rare blood group A.p. FEBS Lett. 118, 209–211 10.1016/0014-5793(80)80220-1 [DOI] [PubMed] [Google Scholar]
- 49. Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A., Nelson K., Quackenbush J., Zhou L., Kirkness E. F., Peterson S., et al. (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388, 539–547 10.1038/41483 [DOI] [PubMed] [Google Scholar]
- 50. Fei Y. Y., Schmidt A., Bylund G., Johansson D. X., Henriksson S., Lebrilla C., Solnick J. V., Borén T., and Zhu X. D. (2011) Use of real-time, label-free analysis in revealing low-affinity binding to blood group antigens by Helicobacter pylori. Anal. Chem. 83, 6336–6341 10.1021/ac201260c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Samuelsson B. E., Pimlott W., and Karlsson K. A. (1990) Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 193, 623–646 10.1016/0076-6879(90)93442-N [DOI] [PubMed] [Google Scholar]
- 52. Koerner T. A. W. Jr., Prestegard J. H., Demou P. C., and Yu R. K. (1983) High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry 22, 2676–2687 10.1021/bi00280a014 [DOI] [PubMed] [Google Scholar]
- 53. Waldi D. (1962) in Dünnschicht-Chromatographie (Stahl E., ed) pp. 496–515, Springer-Verlag, Berlin [Google Scholar]
- 54. Karlsson K. A., Samuelsson B. E., and Steen G. O. (1973) Separation of monoglycosylceramides (cerebrosides) of bovine kidney into subgroups and characterization by mass spectrometry. Biochim. Biophys. Acta 306, 317–328 10.1016/0005-2760(73)90237-3 [DOI] [PubMed] [Google Scholar]
- 55. Barone A., Benktander J., Ångström J., Aspegren A., Björquist P., Teneberg S., and Breimer M. E. (2013) Structural complexity of non-acid glycosphingolipids in human embryonic stem cells grown under feeder-free conditions. J. Biol. Chem. 288, 10035–10050 10.1074/jbc.M112.436162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johansson M. M., Dedic B., Lundholm K., Branzell F. B., Barone A., Benktander J., and Teneberg S. (2015) Characterization of moose intestinal glycosphingolipids. Glycoconj. J. 32, 393–412 10.1007/s10719-015-9604-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lingwood C. A., Huesca M., and Kuksis A. (1992) The glycerolipid receptor for Helicobacter pylori (and exoenzyme S) is phosphatidylethanolamine. Infect. Immun. 60, 2470–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.