Abstract
Many proteins in organelles of the secretory pathway, as well as secretory proteins, are translocated across and inserted into the endoplasmic reticulum membrane by the Sec61 translocon, a protein-conducting channel. The channel consists of 10 transmembrane (TM) segments of the Sec61α subunit and possesses an opening between TM2b and TM7, termed the lateral gate. Structural and biochemical analyses of complexes of Sec61 and its ortholog SecY have revealed that the lateral gate is the exit for signal sequences and TM segments of translocating polypeptides to the lipid bilayer and also involved in the recognition of such hydrophobic sequences. Moreover, even marginally hydrophobic (mH) segments insufficient for membrane integration can be transiently stalled in surrounding Sec61α regions and cross-linked to them, but how the Sec61 translocon accommodates these mH segments remains unclear. Here, we used Cys-scanned variants of human Sec61α expressed in cultured 293-H cells to examine which channel regions associate with mH segments. A TM segment in a ribosome-associated polypeptide was mainly cross-linked to positions at the lateral gate, whereas an mH segment in a nascent chain was cross-linked to the Sec61α pore-interior positions at TM5 and TM10, as well as the lateral gate. Of note, cross-linking at position 180 in TM5 of Sec61α was reduced by an I179A substitution. We therefore conclude that at least two Sec61α regions, the lateral gate and the pore-interior site around TM5, interact with mH segments and are involved in accommodating them.
Keywords: membrane biogenesis, endoplasmic reticulum (ER), protein translocation, protein import, transmembrane domain, membrane protein, protein sorting, protein synthesis, protein secretion, protein-protein interaction, membrane insertion, membrane integration, Sec translocon, signal sequence
Introduction
Many proteins in the secretory pathway and the plasma membrane, as well as secretory proteins, are cotranslationally translocated across and inserted into the endoplasmic reticulum (ER)3 membrane via a protein-conducting channel called a translocon and then transferred to their final destinations by vesicular transport. Nascent polypeptide chains containing hydrophobic signal sequences are targeted to the ER by a signal recognition particle and its receptor on the ER (1). Signal sequences are also recognized at the translocon, and translocation of the following parts is initiated by their membrane insertion (2–7). During cotranslational translocation, a transmembrane (TM) segment in a translocating nascent membrane protein stalls at the translocon and laterally moves into the lipid bilayer. The Sec61 complex, consisting of three subunits, α, β, and γ, is the core channel of the translocon. Structural analyses of the Sec61 complex (and their ortholog archaeal and bacterial SecY complexes) revealed that 10 TM segments of Sec61α form a polypeptide conduit (8–10). The channel can also open to the lipid environment between TM2b and TM7, which is termed the lateral gate. More recent structures of Sec61/SecY complexes with translocating substrates showed that signal sequences are sandwiched between these two TM segments (11, 12). These structures and cross-linking between Sec61α and signal sequences (13, 14) indicated that the lateral gate works as an exit for signal sequences to the lipid phase. In addition, prl mutants of SecY (15–17) and mutagenesis of yeast Sec61α (18–20) revealed that mutations at the lateral gate, as well as the inside regions including constricted positions of the pore (called a pore ring) and TM2a (called a plug segment), cause the channel to open even by insufficient signal sequences, suggesting that these regions are critical for recognizing signal sequences for opening the channel.
Structural (21) and mutagenesis (20, 22) analyses of the Sec61 channel indicated that the lateral gate and pore inside are also involved in recognition of hydrophobic (H) segments in translocating polypeptide chains. Moreover, the amino acid composition of H-segments required for translocation stalling at the translocon revealed that direct interaction with the lipid bilayer through the lateral gate mediates recognition and insertion of H-segments (23, 24). The majority of TM segments possess sufficient hydrophobicity, and they must be smoothly sorted into the lipid phase via these recognition steps. In contrast, marginally hydrophobic (mH) TM segments, whose hydrophobicity is insufficient for membrane integration, are frequently found in multipass membrane proteins. Membrane insertion of such mH segments is supported by the following TM segments forming an Nlumen/Ccyto orientation (25–28). Additionally, synthetic and natural mH segments can be stalled in the membrane (29, 30), and the stalling is enhanced by the following positively charged residues (31, 32). Site-specific cross-linking and chemical modification of stalling mH segments indicated that such segments are in an aqueous location and surrounded with Sec61α regions (30). How the Sec61 channel accommodates these mH segments, however, remained unclear.
In the present study, to explore which channel regions associate with stalling mH segments, we used Cys-scanned variants of human Sec61α expressed in cultured 293-H cells. Although a TM segment in a ribosome-associated polypeptide was mainly cross-linked to positions at the lateral gate, an mH segment in a nascent chain was cross-linked to the pore-interior positions in TM5 and TM10, as well as the lateral gate. Because the cross-linking at position 180 in TM5 was reduced by an I179A substitution, we conclude that at least two regions, the lateral gate and the pore-interior site around TM5, are interactive sites for the accommodation of mH segments.
Results
The FLAG-tagged Sec61 channel is functionally expressed in 293-H–derived microsomes
To determine the interactive sites in Sec61α with a translocating chain, eight endogenous Cys residues of human Sec61α (SEC61A1) were replaced with Ala, and 16 positions (eight original and eight new positions) were replaced with Cys (Fig. 1A). Several positions are the sites associated with a signal sequence (14), and others are in TM segments forming the pore. Stable cell lines of human 293-H cells expressing FLAG-tagged Sec61α proteins were established, and rough microsomal membranes (RMs) were prepared from the cells. Although expression levels varied, all FLAG-Sec61α proteins were expressed in RMs (Fig. 1B). When a secretory protein containing an N-glycosylation site was synthesized in a cell-free translation system, it was glycosylated in a manner dependent on the dose of 293-H–derived RMs containing Sec61α variants (data not shown); therefore, a sufficient amount of each RM was used in the following translation reactions.
Figure 1.
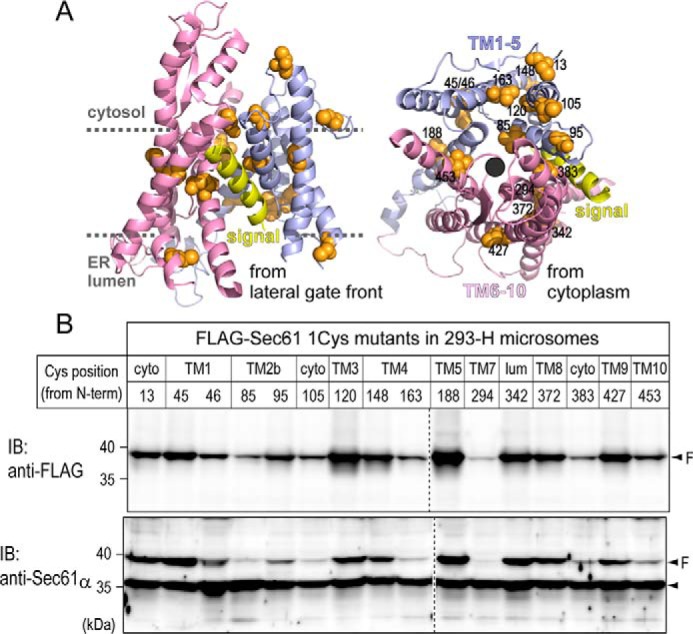
Preparation of RMs expressing Sec61α Cys-scanned mutants. A, the 3D structure of Sec61α with a nascent secretory protein (Protein Data Bank (PDB) code 3JC2) was illustrated using the PyMOL software (version 1.8.6.1). Original Cys positions (13, 45, 46, 148, 188, 342, 372, and 427) and newly Cys-substituted residues (85, 95, 105, 120, 163, 294, 383, and 453) are shown as orange spheres. B, endogenous (an arrowhead) and FLAG-tagged (arrowheads and “F”) Sec61α proteins in each RM were detected by immunoblotting (IB) using anti-Sec61α and anti-FLAG M2 antibodies. Dashed lines indicate where images of separate immunoblots were spliced together. Although the amounts varied, all Sec61α 1Cys proteins were expressed in 293-H–derived RMs. cyto, cytosol; lum, lumen.
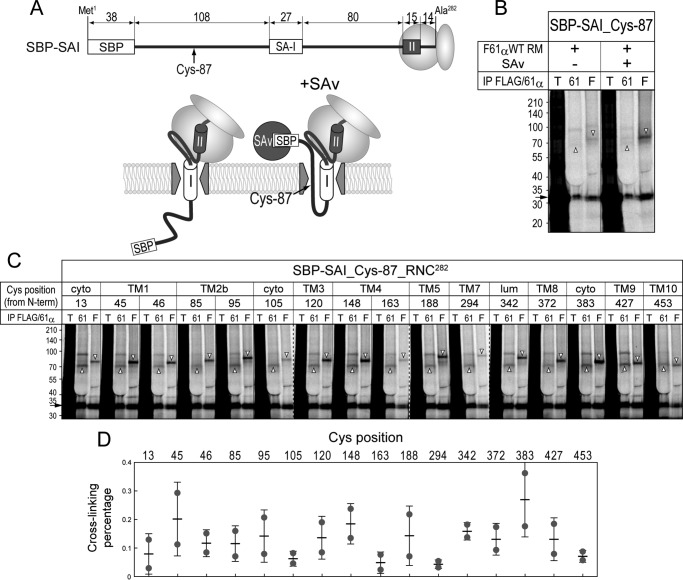
We first examined the cross-linking of Sec61α variants to a hydrophilic chain using a bifunctional cross-linker, bis(maleimido)ethane. Translocation of a streptavidin (SAv)-binding peptide–tagged N-terminal domain of a type I signal-anchor of synaptotagmin II was stalled in the presence of SAv, and the hydrophilic chain was cross-linked to Sec61α in canine pancreatic RMs (33). The FLAG-Sec61α was cross-linked to the stalled hydrophilic chain in an SAv-dependent manner (Fig. 2B), suggesting that the expressed Sec61α is functional in the RM. All 1Cys proteins were also cross-linked to the stalled chain as was endogenous Sec61α in the same RMs (Fig. 2C), but the percentages of cross-linking were diverse (Fig. 2D). This indicates that these Sec61α variants form a functional channel through which the hydrophilic chain passes.
Figure 2.
Cross-linking between Sec61α 1Cys proteins and a hydrophilic chain. A, the fusion construct consisting of an SAv-binding peptide tag (SBP), a type I signal-anchor (SA-I), and a type II TM segment (II), which possesses a single Cys at position 87, was used. TM II of the nascent chain was hidden within the ribosome due to truncation of the mRNA just after it (thus named SBP-SAI). In the presence of SAv, translocation of the N-terminal region of SA-I was stalled by trapping SBP. B, SBP-SAI was synthesized as a ribosome-nascent chain complex (RNC) in the presence of the RMs expressing FLAG-Sec61α WT protein. Where indicated, the translation was performed in the presence of SAv (+). After chemical cross-linking with bis(maleimido)ethane, cross-linked products of FLAG-tagged and endogenous Sec61α were sequentially immunoprecipitated (IP) with anti-FLAG (“F” lanes) and anti-Sec61α (“61” lanes) antibodies from the same lysates. The immunoisolated and 12.5% total products (“T” lanes) were analyzed by SDS-PAGE. Synthesized nascent chains and cross-linked products are indicated with arrows, upper (with FLAG-Sec61α), and lower (endogenous Sec61α) open triangles, respectively. C, the translation was carried out in the presence of RMs and SAv. Cross-linking and immunoisolation of the cross-linked products were performed as described in B. Dashed lines indicate where separate gel images were spliced together. All 1Cys proteins were cross-linked to the stalled hydrophilic chain. D, cross-linking percentages of the 1Cys proteins were quantified and are represented as individual data points (closed circles) with means ± S.D. (error bars; n = 2). cyto, cytosol; lum, lumen.
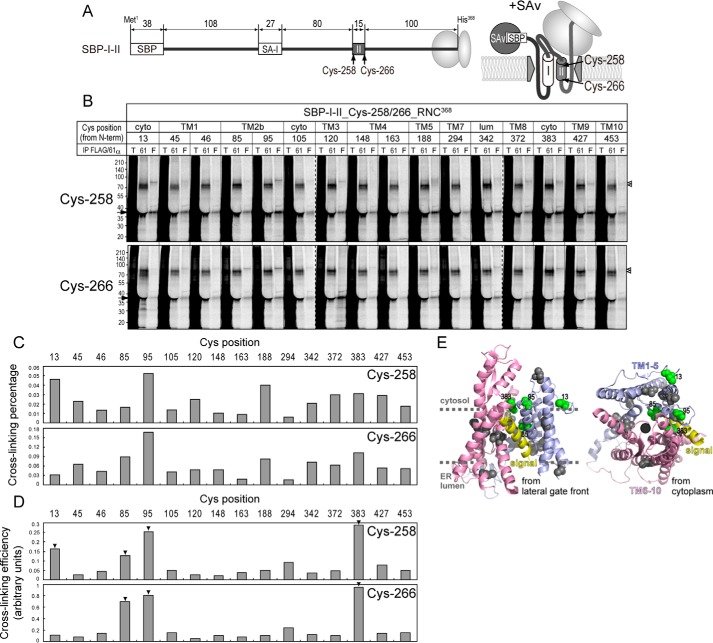
Next, we examined the cross-linking to a TM segment forming an Ncyto/Clumen orientation (Fig. 3) that was also cross-linked to canine Sec61α (34). The profile of cross-linking percentages was considerably different from that of the above hydrophilic chain (Fig. 3C versus 2D), and positions 13 (in the N-terminal region) and 95 (in TM2b) were well cross-linked to the TM segment. The efficiencies, normalized by expressing extents of Sec61α 1Cys proteins in supplementing RMs (see “Experimental procedures” for quantification and calculation), indicate that positions 13, 85 (in TM2b), 95, and 383 (at the C terminus of TM8) were efficiently cross-linked to the TM segment, consistent with previous data showing that positions 13, 95, and 383 are cross-linked to a signal sequence trapped in the channel (14). Our data suggest that the TM segment stands by the lateral gate before moving into the lipid bilayer.
Figure 3.
Cross-linking between Sec61α 1Cys proteins and a TM segment. A, the SBP-I-II nascent chain used in this figure possesses a single Cys at position 258 or 266 in TM II. B, cross-linking analyses using Sec61α 1Cys proteins were performed as described in Fig. 2C. Synthesized chains and cross-linked products of FLAG-tagged and endogenous Sec61α are indicated with arrows, upper, and lower open triangles, respectively. Dashed lines indicate where separate gel images were spliced together. C and D, cross-linking percentages were quantified (C) and normalized by the expression levels of the 1Cys proteins in supplementing RMs (D; see “Experimental procedures” for details of the calculation). Strongly cross-linked positions are indicated with arrowheads. E, efficiently cross-linked positions are shown as green spheres in the Sec61α 3D model. Positions 13, 85, 95, and 383 were well cross-linked to the TM II segment. cyto, cytosol; lum, lumen; T, total; 61, Sec61α; F, FLAG; IP, immunoprecipitation.
Stalling mH segments interact with the pore-interior site at TM5 and TM10 as well as with the lateral gate
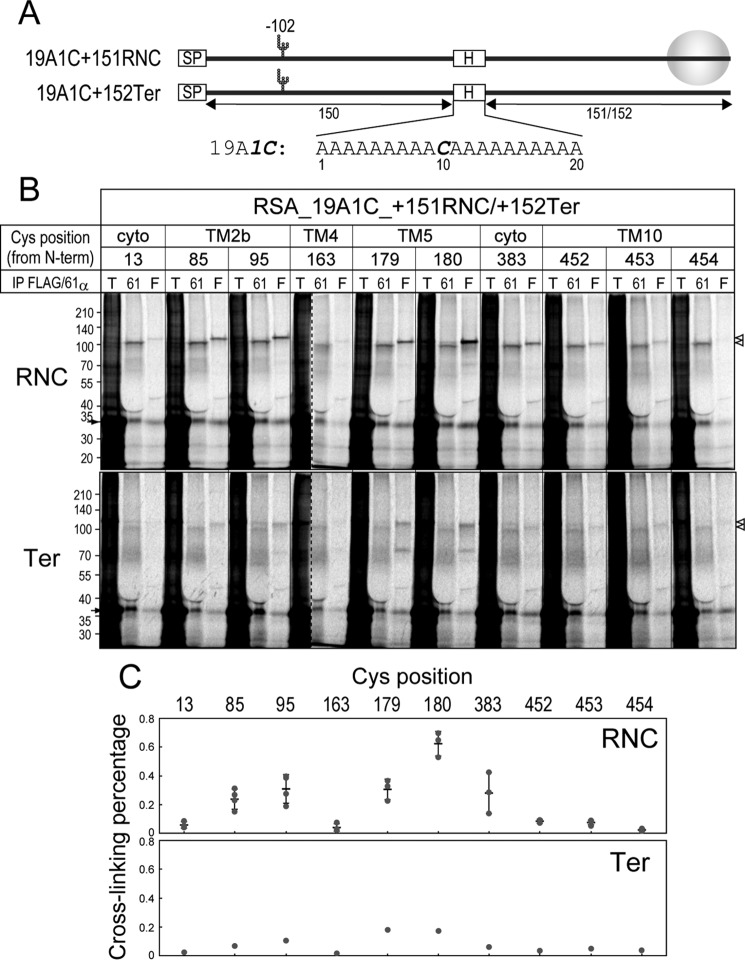
We previously demonstrated that designed mH segments in ribosome-bound nascent chains are stalled in surrounding Sec61α regions and cross-linked to them (30). Therefore, we next examined which Cys positions in Sec61α are cross-linked to mH segments inserted in a secretory protein, rat serum albumin (RSA). The 19A1C segment consists of 19 Ala and 1 Cys, and 2–4 Ala in the segment were replaced with either Leu or Ser to render it more or less hydrophobic (Fig. 4A). Cross-linked products between Sec61α (∼52 kDa) and nascent chains of RSA containing mH segments (∼35 kDa) revealed an unexpectedly large size of 100 kDa or more (Fig. 4B). Because the mobility of endogenous Sec61α and its recombinant 1Cys proteins was similarly shifted by the cross-linking and cross-linked products of substrate chains bound to and released from ribosomes showed a similar size (see Fig. 7), such a large size of the conjugates was due to neither further cross-linking nor attached tRNAs. Our previous study revealed that a substrate chain of the same length (321 residues without the signal peptide) also resulted in ∼100-kDa products cross-linked to canine Sec61α, whereas cross-linked products of a shorter chain (252 residues) showed the expected size, ∼70 kDa (30). Therefore, the region between residues 251 and 319 of RSA might possess an effect that decreases the mobility of the conjugates in SDS-PAGE. Although the 4L1C segment, which was the most hydrophobic segment here, was least cross-linked to Sec61α variants among the mH segments because of moving into the lipid phase, it was fairly cross-linked to position 95 at the lateral gate (Fig. 4C, bottom panel). The other segments were strongly cross-linked to positions 85, 95, and 383 at the lateral gate, indicating that the lateral gate is interactive even with the segments with lower hydrophobicity. Additionally, positions 13, 163 (in TM4), and 453 (in TM10) of the channel were slightly, but meaningfully cross-linked to the 2S1C and 19A1C segments but not the 2L1C segment possessing higher hydrophobicity (Fig. 4D). The normalized efficiency also reveals that position 294 in TM7 was fairly cross-linked to mH segments, whereas the cross-linking percentage was considerably low (Fig. 4C).
Figure 4.
Cross-linking between Sec61α 1Cys proteins and mH segments. A, an mH segment containing 19 Ala and 1 Cys (19A1C) was inserted in RSA. 2 or 4 Ala were replaced with Ser or Leu to render it less or highly hydrophobic. mRNAs were truncated before a termination codon so that the nascent chains formed as a ribosome-nascent chain complex (RNC). SP, signal peptide; H, hydrophobic region. B, cross-linking analyses between mH segments and Sec61α 1Cys proteins were performed as described in Fig. 2C. Dashed lines indicate where separate gel images were spliced together, and additional size markers were attached at the lanes of cross-linking between the 19A1C segment and the 1Cys294 protein. Smeared bands below the products cross-linked to endogenous Sec61α (open triangles) may be conjugates between Sec61α and shorter nascent chains caused by translational pausing. C and D, cross-linking percentages and normalized efficiencies are shown as in Fig. 3, C and D. Positions 85, 95, and 383 were strongly cross-linked to the mH segments except 4L1C (indicated with arrowheads). In addition, positions 13, 163, and 453 were slightly, but meaningfully cross-linked to the segments with lower hydrophobicity (2S1C and 19A1C; indicated with open triangles). E, positions strongly (85, 95, and 383) and fairly (13, 163, and 453) cross-linked to mH segments are shown as green and light blue spheres, respectively. cyto, cytosol; lum, lumen; T, total; 61, Sec61α; F, FLAG; IP, immunoprecipitation.
Figure 7.
Translational states affect the cross-linking. A, the cross-linking of the 19A1C mH segment in a polypeptide chain completed by translation termination (Ter) to reactive Sec61α Cys variants was examined. B and C, cross-linking analyses were performed and quantified as described in the above figure legends. Synthesized polypeptide chains and cross-linked products are indicated with arrows and open triangles, respectively. Dashed lines indicate where separate gel images were spliced together. Data of the cross-linking of the 19A1C segment in the ribosome-nascent chain complex (RNC) are the same as in Fig. 5. cyto, cytosol; IP, immunoprecipitation; T, total; 61, Sec61α; F, FLAG.
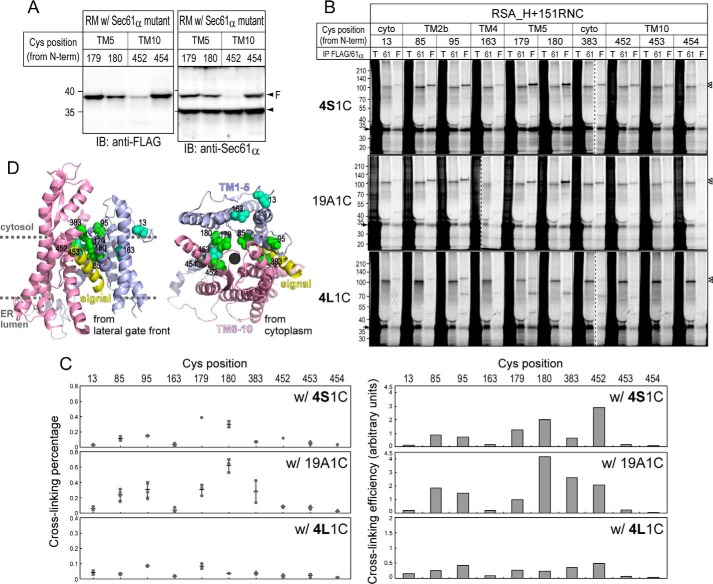
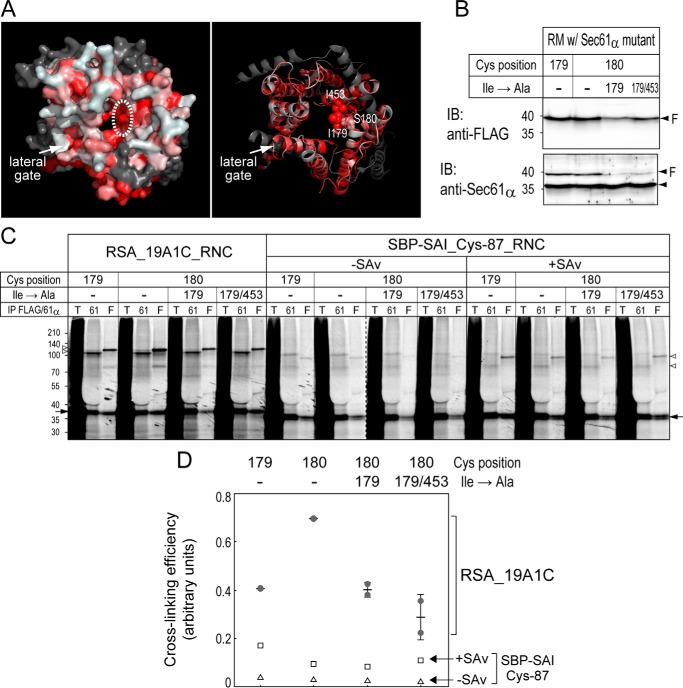
We next focused on position 453 in TM10 because it is located opposite to the lateral gate. Further Cys scanning at TM10 (positions 452 and 454) and TM5 (positions 179 and 180) near position 453 was performed (Fig. 5). Among them, positions 179 and 180 were similarly or more efficiently cross-linked to 19A1C and 4S1C segments than positions 85, 95, and 383 at the lateral gate. Also, position 452 was considerably cross-linked to the segments with lower hydrophobicity. These data suggest that these inside positions, as well as the lateral gate, are in contact with stalling mH segments.
Figure 5.
Further Cys scanning around position 453. A, the expression of new 1Cys mutants (positions 179, 180, 452, and 454) in 293-H–derived RMs was confirmed by immunoblotting (IB). Endogenous and FLAG-tagged Sec61α proteins are indicated with an arrowhead and an arrowhead with “F,” respectively. B, cross-linking analyses of mH segments were performed as described in Fig. 2C. Dashed lines indicate where separate gel images were spliced together. C, cross-linking percentages (left panels; individual data points (closed circles) with means ± S.D. (error bars; n = 2–4)) and normalized efficiencies (right panels; mean values) are shown as in Fig. 3, C and D. Positions 179 and 180 in TM5 were quite strongly cross-linked to the 19A1C and 4S1C segments but not the 4L1C segment. D, positions strongly (85, 95, 179, 180, 383, and 452) and fairly (13, 163, and 453) cross-linked are indicated with green and light blue spheres, respectively. cyto, cytosol; IP, immunoprecipitation; T, total; 61, Sec61α; F, FLAG.
According to structural information (11), Ile-179 is a part of the pore ring and appears to protrude into the pore in the opened channel (Fig. 6A). We thus substituted Ile-179 and/or the flanking Ile-453 with Ala in the Sec61α 1Cys180 variant. These mutants were also cross-linked to the hydrophilic chain stalled with SAv (Fig. 6, C and D). The cross-linking to the 19A1C segment was reduced by the I179A substitution. Because the cross-linking efficiency of the I179A/I453A variant differed little from that of the I179A variant, the I179A substitution was critical in reducing the cross-linking. These findings suggest that the pore-interior site around TM5 interacts with the stalling mH segment and that Ile-179 has an important role in the interaction.
Figure 6.
Ile-179 is involved in the accommodation of mH segments. A, surface and ribbon models of the 3D structure of the opened Sec61 complex. Hydrophobic residues in Sec61α are indicated in red, and other chains are shown in gray. Ile-179, indicated with a dotted oval, appears to protrude into the pore. B, I179A and I179A/I453A substitutions were generated on the Sec61α 1Cys180 variant. The expression of new mutants was confirmed by immunoblotting (IB). C, cross-linking analyses between the new mutants and the 19A1C segment or a hydrophilic chain in SBP-SAI were performed as described in Fig. 2C. The dashed line indicates where separate gel images were spliced together. D, cross-linking efficiencies of the 19A1C segment (individual data points (closed circles) with means ± S.D. (error bars; n = 2)) and the hydrophilic chain with (open squares) or without SAv (open triangles) are shown as in Fig. 3D. IP, immunoprecipitation; T, total; 61, Sec61α; F, FLAG.
Translational states affect the accommodation of the mH sequence within Sec61α
The mH segment can be cross-linked to canine Sec61α only in a ribosome-bound state (30). We examined the cross-linking of the 19A1C segment in a nascent chain released from a ribosome to the Cys positions found in the present study (Fig. 7). In the released chain, the cross-linking to all positions was reduced (Fig. 7, B and C), suggesting that the mH segment left the Sec61 channel after translation termination. Because the mH segment is retained in an aqueous environment after translation termination (30), it might be associated with another protein(s) outside the channel.
Discussion
In the present study, we explored the interactive sites in Sec61α with translocating segments whose cross-linking was observed previously (30, 34). Site-specific cross-linking analyses using Sec61α Cys-scanned variants revealed the accessibility between stalling mH segments and the pore-interior site at TM5 and TM10, as well as the lateral gate.
According to several structures of Sec61/SecY with substrates, the lateral gate opens by TM2b movement away from TM7 and a signal sequence or a TM segment in translocating polypeptides is sandwiched between these TM segments (11, 12, 21). This led to the hypothesis that TM2b functions as a competitor of H-segments and that the hydrophobicity and length of TM2b create a threshold for exiting via the gate. Site-specific cross-linking analyses also indicated that signal sequences stand by the lateral gate just before moving into the lipid phase (13, 14). H-segments sufficient for integration into the lipid bilayer are presumably stalled at the lateral gate and immediately sorted into the lipid phase through the gate. Conversely, mH segments insufficient for membrane integration are also stalled at the translocon (29–31). Here, our findings indicate that stalling mH segments flank at least two regions, the lateral gate and the pore-interior site at TM5 and TM10, and the segments with lower hydrophobicity are apt to be well cross-linked to the pore interior. Structural and biochemical studies of the Sec61/SecY channel indicated that TM5 and TM10 face the path of translocating chains (12, 21, 35). It is thus suggested that stalling mH segments interact with the pore interior of the Sec61 channel. Furthermore, the pore ring of Sec61α in the closed state is formed with TM2b (Ile-81 and Val-85), TM5 (Ile-179 and Ile-183), TM7 (Ile-292), and TM10 (Leu-449), similar to archaeal and bacterial SecY (11). The hydrophobic patch, which is thought to be an initial binding site for signal sequences, is also formed with TM2b (Val-85 and Leu-89), TM5 (Ile-179), and TM7 (Ile-293). Because the I179A substitution reduced the cross-linking at position 180 to the 19A1C segment, there might be a “sticky” site around positions 179 and 180 that interacts with hydrophobic portions in translocating chains even after the channel opens. However, it is also possible that such pore-interior positions only flank mH segments binding the lateral gate and that a conformational change caused by mutation at Ile-179 only influences the accessibility of the region to passing polypeptide chains.
Nevertheless, the findings in this study suggest that stalling mH segments are accommodated at the pore inside of the Sec61 channel besides at the lateral gate. The translocation stalling and partitioning into the lipid phase of H-segments occur gradually as the hydrophobicity of the H-segments increases (30, 36), and mH segments are not stably retained in the channel but rather fluctuate between the channel and the luminal space. The channel pore may act as an interchange between polar and apolar environments, and an mH TM segment in multipass proteins may be temporarily accommodated within the pore until its membrane integration is performed by interaction and sequestration with other TM segments (37). Also, the translocation pausing of apolipoprotein B occurs in the Sec61 channel (38, 39). The SecY structure in complex with a nascent secretory protein revealed that the translocating chain is stalled, and a part of the chain forms a loop on the cytoplasmic side (40). These findings indicate that the Sec61/SecY pore is not smooth but possesses a viscous property for translocating chains, and the pore-interior site around TM5 might be involved in such a property of the channel.
In the present study, we examined the molecular mechanism of the Sec61 channel using its Cys-scanned mutants and translocation-stalling segments as an interactive probe. Although the Sec61 mutants may possess local conformational perturbations caused by substitution of endogenous Cys residues to Ala and further introduction of Cys, they are expressed in the ER membrane and functional in polypeptide translocation (Fig. 2). Because detection of delicate associations between the channel and substrate polypeptide chains by structural analyses is considered difficult, our strategy is useful to explore the nature of the translocon pore in the active state. More detailed analyses using recombinant channels to understand the properties in an active state, in addition to structural and mutagenesis analyses, are required.
Experimental procedures
Constructs
Human Sec61α (SEC61A1) cDNA was obtained by RT-PCR of human fetal brain total RNA (Agilent) and subcloned into pBICEP-CMV-2 vector (Sigma; between EcoRI and EcoRV restriction sites) to create the pBICEP-Sec61α plasmid encoding N-terminally 3×FLAG-tagged Sec61α. Cys elimination from Sec61α by substitution with Ala was performed using the method of Kunkel (41). Further substitutions of target positions to Cys and/or Ala were performed using an inverse PCR–related method. The constructed DNAs were confirmed by DNA sequencing. DNA constructs encoding “S-I-II” fusion proteins of the SAv-binding peptide tag (S), type I signal-anchor from mouse synaptotagmin II (I), and TM3 of human NHE6 (II) were described previously (34). Also, constructs encoding truncated RSAs containing mH segments were described previously (30). Information for all of the oligo DNAs used in this study is available from the authors.
Cell culture and transfection
293-H cells (Sigma) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum under 5% CO2 at 37 °C. Plasmid transfection was performed using FuGENE® 6 transfection reagent (Promega) or PEI MAX (Polysciences) as recommended by the manufacturers. Stable cell lines transfected with pBICEP-Sec61α plasmids were selected in the presence of 1 mg/ml G-418.
Preparation of microsomes from cultured cells and immunoblotting
Cells stably expressing Sec61α recombinant proteins were collected, suspended in homogenization buffer (50 mm triethanolamine, pH 7.4, 250 mm sucrose, 50 mm potassium acetate, 1 mm magnesium acetate, 2 mm EDTA, 1 mm DTT, 0.5 mm phenylmethylsulfonyl fluoride, and cOmpleteTM EDTA-free protease inhibitor mixture (Merck)), and homogenized using a Potter–Elvehjem homogenizer. After centrifugation of the homogenate at 10,000 × g, the supernatant was placed on a cushion solution (1.2 m sucrose in homogenization buffer without EDTA) and ultracentrifuged at 150,000 × g for 3 h. The membrane precipitate was suspended in suspension buffer (50 mm triethanolamine, pH 7.4, 250 mm sucrose, and 1 mm DTT) using a Dounce homogenizer. The RM was treated with micrococcal nuclease (Merck) in the presence of 1 mm calcium chloride at 23 °C for 10 min and then extracted with 25 mm EDTA on ice. The RM was sedimented through a cushion (0.5 m sucrose in suspension buffer) by ultracentrifugation at 150,000 × g for 1 h and suspended in suspension buffer by pipetting and using a sample tube–shaped pestle. The dose of each RM for the translation reactions was determined to be sufficient for membrane targeting of RSA (>70%).
Proteins in 293-H–derived RMs were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membranes were blocked with blocking solution (2% nonfat dry milk in PBS and 0.2% Tween 20) and then incubated with primary (anti-FLAG M2 (Sigma) or anti-Sec61α (42)) and appropriate secondary antibodies. The blots were developed with ECL Western blotting detection reagents (GE Healthcare) and imaged using an ImageQuant LAS4000 mini (GE Healthcare).
In vitro transcription and translation
For the synthesis of S-I Cys-87 truncated mRNA (Fig. 2), the plasmid was linearized with NheI located just downstream of TM II. For the synthesis of S-I-II Cys-258 and Cys-266 truncated mRNAs (Fig. 3), plasmids were linearized with BspHI as described previously (34). For the synthesis of truncated and full-length mRNAs of mH segment–containing RSAs, plasmids were linearized with PmaCI (just upstream of the termination codon) and BamHI (downstream of the coding sequence), respectively. Template DNAs were transcribed with T7 RNA polymerase (TakaraBio). mRNAs were translated in a reticulocyte lysate cell-free system as described previously (30) except in the presence of 293-H–derived RMs instead of canine pancreatic RM.
Chemical cross-linking, immunoprecipitation, and image analysis
After the cell-free translation, chemical cross-linking using 1,2-bis(maleimido)ethane (Tokyo Chemical Industry) and sample preparation for immunoprecipitation were performed as described previously (30). The products conjugated to Cys-scanned Sec61α proteins were then sedimented from lysates with anti-FLAG M2 antibody and protein G–Sepharose (GE Healthcare). Subsequently, the same lysates were subjected to immunoprecipitation with anti-Sec61α antiserum to isolate the products conjugated to endogenous Sec61α proteins. Sedimented resins were extracted with SDS-PAGE sample buffer.
Proteins in lysates or immunoisolated were separated by SDS-PAGE and visualized on a bioimage analyzer (Typhoon FLA7000, GE Healthcare). Radiolabeled and immunodetected protein bands were quantified using ImageQuant TL software, version 7.0 (GE Healthcare). Cross-linking efficiencies were calculated as follows: products cross-linked to FLAG-Sec61α recombinants (a) and total radiolabeled products (the main band (∼32-kDa band of SBP-SAI; ∼37-kDa band of S-I-II; ∼35-kDa band of RSAs with mH segments) and all upper bands in 12.5% total cross-linked products) (b) were quantified, and the cross-linking percentage (X) was estimated using the following formula: X = a/8 × 100/b. These X values are presented in Figs. 2D, 3C, 4C, 5C, and 7C. Immunodetected FLAG-Sec61α proteins expressed in 293-H–derived RMs (c) were also quantified. The expression equivalents in the RMs added to the translation reactions (Y) were estimated as c × d/e (where d is the volume added to translation reaction and e is the volume applied to immunoblotting) and normalized to the largest amount, which was defined as 1. Finally, the cross-linking efficiencies were calculated as X/Y and are presented in Figs. 3D, 4D, 5C, and 6D.
Author contributions
Y. K. and M. S. conceptualization; Y. K. and M. S. resources; Y. K. and M. S. data curation; Y. K. formal analysis; Y. K. and M. S. funding acquisition; Y. K. investigation; Y. K. and M. S. methodology; Y. K. writing-original draft; Y. K. and M. S. project administration; Y. K. and M. S. writing-review and editing; M. S. supervision.
This work was supported by Japan Society for the Promotion of Science (Grant 17K07389 to Y. K., Grants 16H04766 and 16K14730 to M. S.) and MEXT (Grant 17H05673 to M. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- ER
- endoplasmic reticulum
- H
- hydrophobic
- mH
- marginally hydrophobic
- RM
- rough microsomal membrane
- RSA
- rat serum albumin
- SA
- signal-anchor
- SAv
- streptavidin
- TM
- transmembrane
- SBP
- SAv-binding peptide tag
- cyto
- cytoplasm.
References
- 1. Zhang X., and Shan S. O. (2014) Fidelity of cotranslational protein targeting by the signal recognition particle. Annu. Rev. Biophys. 43, 381–408 10.1146/annurev-biophys-051013-022653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cross B. C., Sinning I., Luirink J., and High S. (2009) Delivering proteins for export from the cytosol. Nat. Rev. Mol. Cell Biol. 10, 255–264 10.1038/nrm2657 [DOI] [PubMed] [Google Scholar]
- 3. Shao S., and Hegde R. S. (2011) Membrane protein insertion at the endoplasmic reticulum. Annu. Rev. Cell Dev. Biol. 27, 25–56 10.1146/annurev-cellbio-092910-154125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Park E., and Rapoport T. A. (2012) Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu. Rev. Biophys. 41, 21–40 10.1146/annurev-biophys-050511-102312 [DOI] [PubMed] [Google Scholar]
- 5. Mandon E. C., Trueman S. F., and Gilmore R. (2013) Protein translocation across the rough endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013342 10.1101/cshperspect.a013342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voorhees R. M., and Hegde R. S. (2016) Toward a structural understanding of co-translational protein translocation. Curr. Opin. Cell Biol. 41, 91–99 10.1016/j.ceb.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 7. Rapoport T. A., Li L., and Park E. (2017) Structural and mechanistic insights into protein translocation. Annu. Rev. Cell Dev. Biol. 33, 369–390 10.1146/annurev-cellbio-100616-060439 [DOI] [PubMed] [Google Scholar]
- 8. Van den Berg B., Clemons W. M. Jr., Collinson I., Modis Y., Hartmann E., Harrison S. C., and Rapoport T. A. (2004) X-ray structure of a protein-conducting channel. Nature 427, 36–44 10.1038/nature02218 [DOI] [PubMed] [Google Scholar]
- 9. Tsukazaki T., Mori H., Fukai S., Ishitani R., Mori T., Dohmae N., Perederina A., Sugita Y., Vassylyev D. G., Ito K., and Nureki O. (2008) Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature 455, 988–991 10.1038/nature07421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voorhees R. M., Fernández I. S., Scheres S. H., and Hegde R. S. (2014) Structure of the mammalian ribosome-Sec61 complex to 3.4 Å resolution. Cell 157, 1632–1643 10.1016/j.cell.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Voorhees R. M., and Hegde R. S. (2016) Structure of the Sec61 channel opened by a signal sequence. Science 351, 88–91 10.1126/science.aad4992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L., Park E., Ling J., Ingram J., Ploegh H., and Rapoport T. A. (2016) Crystal structure of a substrate-engaged SecY protein-translocation channel. Nature 531, 395–399 10.1038/nature17163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Plath K., Mothes W., Wilkinson B. M., Stirling C. J., and Rapoport T. A. (1998) Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94, 795–807 10.1016/S0092-8674(00)81738-9 [DOI] [PubMed] [Google Scholar]
- 14. Mackinnon A. L., Paavilainen V. O., Sharma A., Hegde R. S., and Taunton J. (2014) An allosteric Sec61 inhibitor traps nascent transmembrane helices at the lateral gate. Elife 3, e01483 10.7554/eLife.01483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emr S. D., Hanley-Way S., and Silhavy T. J. (1981) Suppressor mutations that restore export of a protein with a defective signal sequence. Cell 23, 79–88 10.1016/0092-8674(81)90272-5 [DOI] [PubMed] [Google Scholar]
- 16. Osborne R. S., and Silhavy T. J. (1993) PrlA suppressor mutations cluster in regions corresponding to three distinct topological domains. EMBO J. 12, 3391–3398 10.1002/j.1460-2075.1993.tb06013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith M. A., Clemons W. M. Jr., DeMars C. J., and Flower A. M. (2005) Modeling the effects of prl mutations on the Escherichia coli SecY complex. J. Bacteriol. 187, 6454–6465 10.1128/JB.187.18.6454-6465.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Junne T., Schwede T., Goder V., and Spiess M. (2007) Mutations in the Sec61p channel affecting signal sequence recognition and membrane protein topology. J. Biol. Chem. 282, 33201–33209 10.1074/jbc.M707219200 [DOI] [PubMed] [Google Scholar]
- 19. Trueman S. F., Mandon E. C., and Gilmore R. (2012) A gating motif in the translocation channel sets the hydrophobicity threshold for signal sequence function. J. Cell Biol. 199, 907–918 10.1083/jcb.201207163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reithinger J. H., Yim C., Kim S., Lee H., and Kim H. (2014) Structural and functional profiling of the lateral gate of the Sec61 translocon. J. Biol. Chem. 289, 15845–15855 10.1074/jbc.M113.533794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gogala M., Becker T., Beatrix B., Armache J. P., Barrio-Garcia C., Berninghausen O., and Beckmann R. (2014) Structures of the Sec61 complex engaged in nascent peptide translocation or membrane insertion. Nature 506, 107–110 10.1038/nature12950 [DOI] [PubMed] [Google Scholar]
- 22. Junne T., Kocik L., and Spiess M. (2010) The hydrophobic core of the Sec61 translocon defines the hydrophobicity threshold for membrane integration. Mol. Biol. Cell 21, 1662–1670 10.1091/mbc.e10-01-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hessa T., Kim H., Bihlmaier K., Lundin C., Boekel J., Andersson H., Nilsson I., White S. H., and von Heijne G. (2005) Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature 433, 377–381 10.1038/nature03216 [DOI] [PubMed] [Google Scholar]
- 24. Hessa T., Meindl-Beinker N. M., Bernsel A., Kim H., Sato Y., Lerch-Bader M., Nilsson I., White S. H., and von Heijne G. (2007) Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450, 1026–1030 10.1038/nature06387 [DOI] [PubMed] [Google Scholar]
- 25. Lu Y., Xiong X., Helm A., Kimani K., Bragin A., and Skach W. R. (1998) Co- and posttranslational translocation mechanisms direct cystic fibrosis transmembrane conductance regulator N terminus transmembrane assembly. J. Biol. Chem. 273, 568–576 10.1074/jbc.273.1.568 [DOI] [PubMed] [Google Scholar]
- 26. Ota K., Sakaguchi M., von Heijne G., Hamasaki N., and Mihara K. (1998) Forced transmembrane orientation of hydrophilic polypeptide segments in multispanning membrane proteins. Mol. Cell 2, 495–503 10.1016/S1097-2765(00)80149-5 [DOI] [PubMed] [Google Scholar]
- 27. Ojemalm K., Halling K. K., Nilsson I., and von Heijne G. (2012) Orientational preferences of neighboring helices can drive ER insertion of a marginally hydrophobic transmembrane helix. Mol. Cell 45, 529–540 10.1016/j.molcel.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yabuki T., Morimoto F., Kida Y., and Sakaguchi M. (2013) Membrane translocation of lumenal domains of membrane proteins powered by downstream transmembrane sequences. Mol. Biol. Cell 24, 3123–3132 10.1091/mbc.e13-04-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onishi Y., Yamagishi M., Imai K., Fujita H., Kida Y., and Sakaguchi M. (2013) Stop-and-move of a marginally hydrophobic segment translocating across the endoplasmic reticulum membrane. J. Mol. Biol. 425, 3205–3216 10.1016/j.jmb.2013.05.023 [DOI] [PubMed] [Google Scholar]
- 30. Kida Y., Ishihara Y., Fujita H., Onishi Y., and Sakaguchi M. (2016) Stability and flexibility of marginally hydrophobic-segment stalling at the endoplasmic reticulum translocon. Mol. Biol. Cell 27, 930–940 10.1091/mbc.e15-09-0672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuroiwa T., Sakaguchi M., Mihara K., and Omura T. (1991) Systematic analysis of stop-transfer sequence for microsomal membrane. J. Biol. Chem. 266, 9251–9255 [PubMed] [Google Scholar]
- 32. Fujita H., Kida Y., Hagiwara M., Morimoto F., and Sakaguchi M. (2010) Positive charges of translocating polypeptide chain retrieve an upstream marginal hydrophobic segment from the endoplasmic reticulum lumen to the translocon. Mol. Biol. Cell 21, 2045–2056 10.1091/mbc.e09-12-1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kida Y., Morimoto F., and Sakaguchi M. (2007) Two translocating hydrophilic segments of a nascent chain span the ER membrane during multispanning protein topogenesis. J. Cell Biol. 179, 1441–1452 10.1083/jcb.200707050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kida Y., Kume C., Hirano M., and Sakaguchi M. (2010) Environmental transition of signal-anchor sequences during membrane insertion via the endoplasmic reticulum translocon. Mol. Biol. Cell 21, 418–429 10.1091/mbc.e09-08-0738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cannon K. S., Or E., Clemons W. M. Jr., Shibata Y., and Rapoport T. A. (2005) Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J. Cell Biol. 169, 219–225 10.1083/jcb.200412019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gumbart J. C., Teo I., Roux B., and Schulten K. (2013) Reconciling the roles of kinetic and thermodynamic factors in membrane-protein insertion. J. Am. Chem. Soc. 135, 2291–2297 10.1021/ja310777k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. De Marothy M. T., and Elofsson A. (2015) Marginally hydrophobic transmembrane alpha-helices shaping membrane protein folding. Protein Sci. 24, 1057–1074 10.1002/pro.2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chuck S. L., and Lingappa V. R. (1992) Pause transfer: a topogenic sequence in apolipoprotein B mediates stopping and restarting of translocation. Cell 68, 9–21 10.1016/0092-8674(92)90202-N [DOI] [PubMed] [Google Scholar]
- 39. Kivlen M. H., Dorsey C. A., Lingappa V. R., and Hegde R. S. (1997) Asymmetric distribution of pause transfer sequences in apolipoprotein B-100. J. Lipid Res. 38, 1149–1162 [PubMed] [Google Scholar]
- 40. Park E., Ménétret J. F., Gumbart J. C., Ludtke S. J., Li W., Whynot A., Rapoport T. A., and Akey C. W. (2014) Structure of the SecY channel during initiation of protein translocation. Nature 506, 102–106 10.1038/nature12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kunkel T. A. (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. U.S.A. 82, 488–492 10.1073/pnas.82.2.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kida Y., Sakaguchi M., Fukuda M., Mikoshiba K., and Mihara K. (2000) Membrane topogenesis of a type I signal-anchor protein, mouse synaptotagmin II, on the endoplasmic reticulum. J. Cell Biol. 150, 719–730 10.1083/jcb.150.4.719 [DOI] [PMC free article] [PubMed] [Google Scholar]