Abstract
This paper reports a case of Purpureocillium lilacinum infection in seven loggerhead sea turtle (Caretta caretta) hatchlings kept in an aquarium under inadequate condition. The fungus was isolated from skin and pulmonary lesions. Metilene blue and NaCl solutions, Schinus terebinthifolius and eucalyptus essential oils Minimum Inhibitory Concentrations were determined indicating new possibilities for treatment.
Keywords: Caretta caretta, Disease, Mycosis, Husbandry, Treatment
1. Introduction
Mycoses are considered important emerging diseases and threats to the conservation of many animal species such as bees, corals, amphibians, sea turtles and bats [1]. Dermatomycoses and systemic mycoses have been reported in reptiles more frequently and are mostly described as opportunistic, affecting immunosuppressed animals or under unsatisfactory captivity conditions as well as free-living animals [2]. The most commonly found fungi have been Aspergillus sp., Candida sp., Trichophyton sp., Chrysosporium sp., Geotrichum sp., Trichoderma sp., Penicillium sp. and Purpureocillium sp.[1], [3]. Some fungi that are considered saprophytic entomopathogens commonly found in soil and used in biological control of insects, have been reported as pathogenic for previously healthy or immunosuppressed reptiles, for example, Metarhizium anisopliae, Beauveria bassiana, and Purpureocilium lilacinum [2].
Clinical signs of superficial and systemic mycoses are usually spots on the skin, erosions or ulcerations, vesicles, granulomas, and necrosis that may culminate in the death of animals or in some cases, spontaneous remission [2], [4]. The treatment is usually difficult and time-consuming, leading to undesirable long periods of hospitalization that further compromise the animals complicating the outcome [2], [5]. Internal fungal infections require the use of systemic antifungals. In reptiles, few studies have reported the use of itraconazole, ketoconazole or voriconazole, which are ineffective when granulomas or abscesses are formed, and may be toxic [5], evidencing the need for new options of antifungal drugs. The use of synthetic drugs such as clorexidine, miconazole, ketoconazole and posaconazole together with formaldehyde or iodopovidine and dye-like malachite green and methylene blue stains are common in captive animals in addition to debridement when the lesion is localized and superficial [6].
Respiratory fungal diseases in reptiles are commonly diagnosed in captive animals and classified as multifactorial diseases, mainly related to inadequate management and generally caused by an infected host introduced into the environment [4]. In captive aquatic reptiles, low water temperature, low salinity and little water exchange in enclosures are predisposing factors for the occurrence of mycoses [7], especially when associated with stressful conditions due to overcrowding or free living animals kept in rehabilitation. Carettochelys insculpta turtles from an illegal seizure in the USA were diagnosed with Purpureocillium lilacinum-related mycoses and treated with malachite green, formaldehyde, and parenteral itraconazole [6]. In China, Trionyx sinensis turtles in commercial breeding systems also showed P. liliacinus mycosis associated with overcrowding, variations in water temperature and captivity stress [8]. In both cases above, P. lilacinus infections were characterized by circular white spots on the carapace or skin of turtles associated to anorexia and death.
The solitary habits of sea turtles may play a role in making them less vulnerable to fungal infections [3]. However, there are eventual reports of mycoses by Purpureocillium lilacinum, formerly called Paecilomyces lilacinum [9]. In Caretta caretta there are at least two reports of fungal pneumonia by the genus Purpureocillium sp. One of them was diagnosed by bronchoendoscopy in a juvenile C. caretta [10]. The other was a 12-year-old turtle found dead in its exhibition enclosure after a history of abnormal fluctuation and anorexia for weeks [4]. Pulmonary nodules were found at necropsy, where reproductive structures of the fungus were observed under microscopy, which, together with the cultural findings, aided in the confirmation of the diagnosis.
This work reports an outbreak of superficial and pulmonary mycosis by Purpureocillium lilacinus in Caretta caretta hatchlings kept in captivity and evaluates the sensitivity of the pathogen to non-conventional antifungal substances.
2. Case
Seven hatchlings of Caretta caretta turtles from artificial hatchery were kept in a 100 L tank with seawater exchanged weekly in a shaded environment, with no temperature control. They were fed with pieces of fish and shrimp every two days. Ten weeks after birth (day 0), they began to present whitish spot lesions (Fig. 1) and inappetence and died between age 12–20 weeks (day + 14 to + 70). The animals did not receive any treatment as well as the water. Postmortem examination revealed softening of the carapace and plastron, most of them presented low body weight with concavity of the plastron (Fig. 2). At necropsy, a caseous abscess was found in the lung of one of the turtles (Fig. 2). Unfortunately, the carcasses were frozen before being sent to the laboratory for necropsy and collection of material, preventing histopathological examinations.
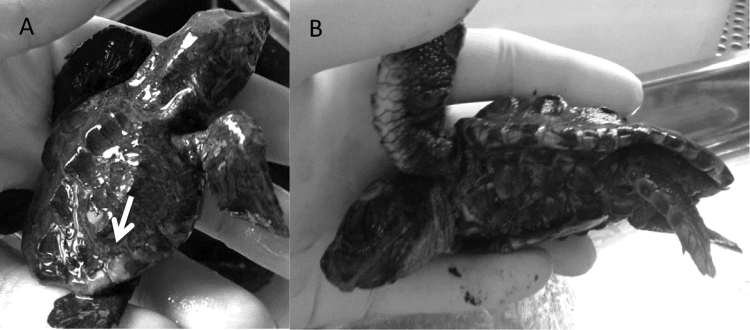
Fig. 1.
(A) Hatchling with whitish lesions in the carapace (arrow). (B) Puppy of Caretta caretta showing signs of cachexia.
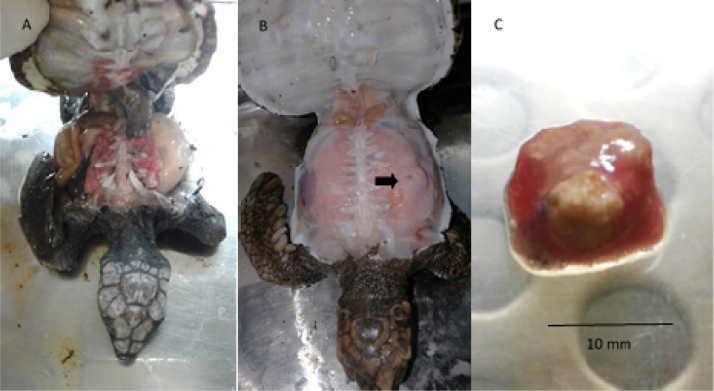
Fig. 2.
(A) Hatchling of Caretta caretta presenting poor body condition. (B) C. caretta hatchling with presence of pulmonary abscess (arrow) and (C) lung fragment with caseous abscess.
The aseptically collected samples of the carapaces and lungs of all seven turtles, as well as the caseous material from the pulmonary abscess of one of them, were inoculated into BHI broth (Brain Heart Infusion) and Sabouraud Dextrose agar with chloramphenicol and potato dextrose agar and incubated at 25 °C for fungal isolation.
The fungus Purpureocillium lilacinum (Fig. 3) was isolated from the material from the abscess and carapace of one of the turtles, identified by the microculture technique following the criteria described by Samson [11] and coded CC09–17.
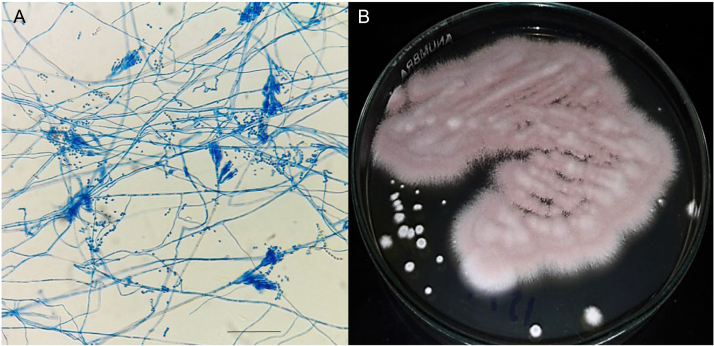
Fig. 3.
(A) Purpureocilium lilacinum isolated from Caretta caretta hatchling lung abscess. (B) P. lilacinum microscopy (400×) after microculture (scale bar 50 µm).
To confirm identification the fungal strain at the species level, the genomic DNA was extracted from the isolate (CC09–17). Partial sequencing of the internal transcribed spacer (ITS) region was evaluated using ITS5 (GGAAGTAAAAGTCGTAACAAGG) and ITS4 (TCCTCCGCTTATTGATATGC) [12], Briefly, the conditions were 100 ng DNA, 10pmol of each primer, and an annealing temperature of 48◦C. Automated sequencing was evaluated using the Sequencing Platform at Fundação Oswaldo Cruz PDTIS/FIOCRUZ, Brazil. The sequences were edited using Sequencher 4.9 software, compared using the BLAST and deposited in GenBank.
The Minimum Inhibitory Concentration (MIC) test was performed with this isolate according to the protocol defined by Clinical & Laboratory Standards Institute (CLSI). Methylene blue (MB), aroeira essential oil, also named Brazilian peppertree (Schinus terebinthifolius), eucalyptus essential oil (Corymbia citriodora) and NaCl solution (SC) were tested in the initial concentrations according to Table 1. The essential oils were prepared from the calculation of their density and solubilized in distilled water and tween 80 according to Helal et al. [13].
Table 1.
Relation of MIC and fungicidal and fungistatic concentrations obtained for the fungus Purpureocillium lilacinum.
| Substances | Initial concentration | CIM | Fungicide concentration | Fungistatic concentration |
|---|---|---|---|---|
| Methylene blue | 0.01 g/L | 1.25% | 5% (diluted in 1 L) | |
| Aroeira essential oil (Schinus terebinthifolius) | 72 mg/mL | 9 mg/mL | 18 mg/mL | 9 mg/mL |
| Eucalyptus essential oil (Corymbia citriodora) | 72 mg/mL | 0.12 mg/mL | 0.48 mg/mL | 0.12 mg/mL |
| NaCl solution | 200 g/L | 200 g/L | – | 200 g/L |
| Methylene blue solution 5%, solubilized in NaCl 20% solution | 5 mg/mL of Methylene blue in NaCl solution (20 mg/mL) | 5 mg/mL | 5 mg/mL | 2.5 mg/mL |
The Minimum Inhibitory Concentration (MIC) methodology defined by the (CLSI) was performed using microdilutions in 96-well plates using Müller Hinton broth as culture media and Fluconazole 2 mg/mL as inhibition control. The results were measured by the addition of 0.015% resazurin [14]. After defining the minimum inhibitory concentration, a plaque test was made to verify if the concentration was fungicidal or fungistatic [15]. For this test, a new daily exposure of fungi to the tested substances was carried out for 5 days. All tests were performed in triplicates.
3. Discussion
The BLAST analysis comparing the ITS sequences obtained for isolate CC09–17 with sequences deposited in the NCBI GenBank database allowed the identification of this isolate as P. lillacinum with 99–100% similarity with other P.lillacinum sequences (KF367485, KC157741, KC157738, GU980015, HQ607796, HM242262, AB103380).
The isolation of the fungus Purpureocilium lilacinum in marine turtles is not novel, although, as far as we know, there are no reports of this infection in South America. The infection has been reported in pulmonary granulomas and pneumonia in free-living Caretta caretta [4], [10]. The conditions under which the turtles analyzed were kept predisposed them to fungal infections associated with captive stress (shading, poor water renewal, agglomeration and lack of water temperature control) and probably led to the mycotic infection in the skin and lungs, associated to the immunocompromising emaciation related to the nutritional deficit.
The fungus Purpureocillium lilacinum is described as an entomopathogen commonly found in soil and has been described as an opportunistic pathogen in humans and other vertebrates [8], [9]. Usually infections in humans and animals are described in association with immunosuppression [4], [9]. This fungus has been identified as clinically significant in cases of respiratory pathologies in reptiles, mainly chelonians and crocodilians [5].
All tested substances had fungicidal and fungistatic properties in the in vitro test, indicating that new treatments can be evaluated, with the advantage of not having the negative toxic effects observed with the conventionally used drugs and other substances. In addition, salinity of 20% was also fungicidal for P. lilacinum in vitro, and did not affect the MB activity when associated with it, indicating that it is not an important factor to be considered in the management of the animals against mycoses (Table 1).
Lafortune et al. [6] describe a successful treatment against P. lilacinus infection in freshwater turtles Carettochelys insculpta, using malachite green, formaldehyde and itraconazole injections, associated with frequent water changes, salinity elevation and parenteral nutrition. In our study we tested the sensitivity of P.lilacinus to salinities higher than the average found in seawater, which is 3.5%, and we verified that there is a need for a large increase in the NaCl concentration in order to promote some interference in the development of the microorganism, either temporarily or completely inhibiting its development. This excessive elevation of NaCl concentration may be detrimental to the animal that needs to undergo some therapeutic treatment. The finding of resistance to NaCl can be explained by the fact that P. lilacinus is a fungus with halotolerant characteristics, so its use as a therapeutic component itself or as therapeutic complement must have been overestimated or masked by the other substances. However, treatments with traditional antifungals and toxic substances such as formaldehyde can cause adverse effects and changes in the physiological and hematological parameters of reptiles [16]
Malachite green has carcinogenic, mutagenic and teratogenic effects, and causes chromosomal fractures and respiratory toxicity in fish and mammals. Lepidochelys kempii affected by cold-stunning were treated with itraconazole in a preventive way being clinically tolerated in doses of 5–25 mg/Kg sid to 5–15 mg/Kg every 72 h, orally, producing plasma concentrations equivalent to those recommended for humans and effective MIC of 0.5 mg/mL [17].
Because of these undesirable effects, testing for new medicinal possibilities for the treatment of mycosis in reptiles is of fundamental importance in order to reduce the risks inherent to the use of conventional drugs and substances with recognized toxicity.
Methylene blue is a dye with supposed antimicrobial properties popularly used in the treatment of fish by aquarists [18]. However, there is little information in the literature reporting its antimicrobial action. In fact, it presents a anti yeast action on Candida albicans mitochondria, causing dysfunction on membrane and homeostasis alterations [19], but for the best of our knowledge there are no other reports of antifungal action against filamentous fungi as we observed in this study. Studies related to pharmacological aspects in vivo and toxicity of methylene blue are necessary to validate its use in marine captive turtles, especially in hatchlings.
In a work carried out to verify the fungicidal action of eucalyptus essential oil against dermatophytes (Microsporum canis, M. gypseum, Tricophyton mentagrophytes and T. rubrum), its action was verified in concentrations ranging from 62.5 to 1000 μg/mL [20]. The essential oil of aroeira, is known for its antifungal action against many species, like Aspergillus sp., Candida albicans and Penicillium notatum [21].
This study demonstrates the occurrence of Purpureocillium lilacinum in superficial and pulmonary infection in hatchlings of Caretta caretta turtles kept in captivity.
Among the tested substances, methylene blue was effective as a fungicide for Purpureocillium lilacinum at 5% concentration diluted in one L of water for 4 days. We recommend that treatment with methylene blue should be tested on sea turtles immersed methylene blue solution at 0.05 g/L of water for 4 days.
The aroeira oil was effective in the concentration of 9 mg/mL with application for 3 days, whereas the eucalyptus oil was effective in the concentration of 0.12 mg/mL for 4 days, however, it is not known if there are harmful effects of the topical use of these oils on sea turtles and their evaluation for toxicity is recommended. It also should be tested an appropriate vehicle for topical application considering that the animals stay submerged for long periods.
Salinity elevation alone do not show to influence in P. lilacinus viability in vitro in concentrations below 20%, which would be inadequate to submit turtles to such water condition.
We recommend that in vivo studies should be conducted on the treatment of superficial and systemic mycoses with methylene blue, as well as broader in vitro and in vivo studies with eucalyptus and aroeira essential oils to verify their toxicity and clinical applicability.
Acknowledgements
The authors thank to Microbiology Laboratory, Biopractices Unity of Universidade Vila Velha (UVV), Brazil, for providing the material for the analysis. We also thank to Labpeixe (UVV) for the use of its facilities. Automated sequencing was done using the Genomic Platform-DNA Sequencing Platform at Fundação Oswaldo Cruz - PDTIS/FIOCRUZ (RPT01A), Brazil.
Acknowledgments
Conflict of interest
The authors have no conflicts of interest to declare and confirm that each one has made substantial contributions to the information or materials submitted for publication.
References
- 1.Sarmiento-Ramírez J.M., Abella-Pérez E., Phillott A.D., Sim J., Van West P., Martín M.P., Marco A., Diéguez-Uribeondo J. Global distribution of two fungal pathogens threatening endangered sea turtles. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt V. Fungal infections in reptiles - an emerging problem. J. Exot. Pet Med. 2015;24:267–275. [Google Scholar]
- 3.Orós J., Calabuig P., Arencibia A., Camacho M., Jensen H.E. Systemic mycosis caused by Trichophyton spp. in an olive ridley sea turtle (Lepidochelys olivacea): an immunohistochemical study. N. Z. Vet. J. 2011;59:92–95. doi: 10.1080/00480169.2011.552859. [DOI] [PubMed] [Google Scholar]
- 4.Schumacher V.L., Mangold B., Lenzycki J., Hinckley L., Sutton D.A., Frasca S. Occurrence of fruiting structures allows determination of Purpureocillium lilacinum as an inciting agent of pleuritis and pneumonia in a loggerhead sea turtle (Caretta caretta) by histopathologic correlation to culture. Med. Mycol. Case Rep. 2014;6:42–45. doi: 10.1016/j.mmcr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaufrére H., Summa N., Le K. Respiratory system. In: Mitchell M.A., Tully T.N. Jr., editors. Current Therapy in Exotic Pet Practice. 1st ed. Elsevier Health Sciences; Saint Louis: 2016. pp. 100–101. [Google Scholar]
- 6.Lafortune M., Wellehan J.F.X., Terrell S.P., Jacobson E.R., Heard D., Kimbrough J.W. Shell and systemic hyalohyphomycosis in fly river turtles, Carettochelys insculpta, caused by Paecilomyces lilacinus. J. Herpetol. Med. Surg. 2005;15:15–19. [Google Scholar]
- 7.Miller D.L., Radi Z.A., Stiver S.L., Thornhill T.D. Cutaneous and pulmonary mycosis in green anacondas (Eunectes murinus) J. Zoo Wildl. Med. 2004;35:557–561. doi: 10.1638/03-096. [DOI] [PubMed] [Google Scholar]
- 8.Li X., Zhang C., Fang W., Lin F. White-spot disease of Chinese soft-shelled turtles (Trionyx sinens) caused by Paecilomyces lilacinus. J. Zhejiang Univ. Sci. B. 2008;9:578–581. doi: 10.1631/jzus.B0720009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luangsa-ard J., Houbraken J., van Doorn T., Hong S.-B., Borman A.M., Hywel-Jones N.L., Samson R.A. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol. Lett. 2011;321:141–149. doi: 10.1111/j.1574-6968.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 10.S.J. Hernandez-Divers, T.M. Norton, S.M. Hernandez-Divers, A. Strunk, S. Sanchez, P. Currin, P. Garcia, Endoscopic diagnosis of pulmonary granulomas due to Paecilomyces in a juvenile sea turtle., in: Proceedings Assoc. Reptil. Amphib. Vet., Reno, 2002, pp. 3–4.
- 11.Samson R.A. Paecilomyces and some allied Hyphomycetes. Stud. Mycol. 1974;6:1–119. [Google Scholar]
- 12.de Sequeira D.C.M., Menezes R.C., Oliveira M.M.E., ANTAS P.R.Z., de Luca P.M., de J., Oliveira-Ferreira B., de Moraes C. Experimental hyalohyphomycosis by Purpureocillium lilacinum: outcome of the Infection in C57BL/6 murine models. Front. Microbiol. 2017;8:1617. doi: 10.3389/fmicb.2017.01617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helal G.A., Sarhan M.M., Abu Shahla A.N.K., El-Khair E.K. Abou. Antimicrobial activity of some essential oils against microorganisms deteriorating fruit juices. Mycobiology. 2006;34:219. doi: 10.4489/MYCO.2006.34.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu M., Seidel V., Katerere D.R., Gray A.I. Colorimetric broth microdilution method for the antifungal screening of plant extracts against yeasts. Methods. 2007;42:325–329. doi: 10.1016/j.ymeth.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Batista J.M., Birman E.G., Cury A.E. Susceptibility to antifungal drugs of Candida albicans strains isolated from patients with denture stomatitis. Rev. Odontol. Da Univ. São Paulo. 1999;13:343–348. [Google Scholar]
- 16.Paré J.A. Update on fungal infections in reptiles. In: Mader D.R., Divers S., editors. Current Therapy in Reptile Medicine and Surgery. 1st ed. Elsevier Health Sciences; Saint Louis: 2014. pp. 53–56. [Google Scholar]
- 17.Manired C.A., Rhinehartb H.L., Pennickm G.J., Sutton D.A., Hunter R.P., Rinaldi M.G. Steady-state plasma concentrations of itraconazole after oral administration in kemp's ridley sea turtles, Lepidochelys kempii. J. Zoo Wildl. Med. 2003;34:171–178. doi: 10.1638/1042-7260(2003)034[0171:SPCOIA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Jepson L. Elsevier; Rio de Janeiro: 2010. Clínica de Animais Exóticos: Referência Rápida. [Google Scholar]
- 19.Ansari M.A., Fatima Z., Hameed S. Antifungal action of methylene blue involves mitochondrial dysfunction and disruption of redox and membrane homeostasis in. Open Microbiol. J. 2016;10:12–22. doi: 10.2174/1874285801610010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baptista E.B., Zimmermann-Franco D.C., Lataliza A.A.B., Raposo N.R.B. Chemical composition and antifungal activity of essential oil from Eucalyptus smithii against dermatophytes. Rev. Soc. Bras. Med. Trop. 2015;48:746–752. doi: 10.1590/0037-8682-0188-2015. [DOI] [PubMed] [Google Scholar]
- 21.Gundidza M., Gweru N., Magwa M.L., Mmbengwa V., Samie A. The chemical composition and biological activities of essential oil from the fresh leaves of Schinus terebinthifolius from Zimbabwe. Afr. J. Biotechnol. 2009;8:7164–7169. [Google Scholar]