Abstract
We report on an elderly male patient with headache and right-side weakness. Imaging studies revealed multiple space-occupying lesions in the parietal and occipital cerebral regions. Biopsy revealed broad aseptate ribbon-like structures branching at right angles, suggestive of mucormycosis. Improvement was observed after medical therapy with 20 weeks of liposomal amphotericin B (5 mg/kg/day) combined with posaconazole, followed by posaconazole (400 mg twice a day) alone for 1 month. The patient recovered without neurological deficits; however, multidrug-resistant bacteraemia and hospital-acquired pneumonia occurred, resulting in death.
Nevertheless, our report shows that this lethal fungal infection can sometimes show favourable progress with drug therapy alone.
Keywords: Cerebral mucormycosis, Liposomal amphotericin B, Posaconazole, Fungal infection, Antifungal therapy
1. Introduction
Mucormycosis is a very aggressive disease characterised by vascular invasion, with subsequent thrombosis, endarteritis, and necrosis [1]. Necrosis and decreased perfusion reduce the access of the effector immune cells and antifungal therapy to the infection site. Further, penetration of the fungal hyphae into endothelial cells contributes to the haematogenous dissemination of the infection. Hence, mucormycosis is associated with very high mortality, ranging from 66% for rhino-cerebral infections to almost 100% for the disseminated disease [2]. Multiple conditions predispose a patient to mucormycosis, including haematological malignancies, organ transplantation, uncontrolled diabetes mellitus (DM), diabetic ketoacidosis, penetrating trauma, and iron overload and deferoxamine therapy. Iron is an important growth factor for mucormycosis [1]. In diabetic ketoacidosis and other states of acidosis, more iron is released from its binding proteins into blood circulation. Additionally, glucose-regulated protein 78 (GRP78), a virulence factor, allows the invasion of mucormycosis into endothelial cells in diabetes patients [3]. Moreover, impaired chemotaxis and killing mechanisms in neutrophils, due to hyperglycaemia, play a role in the progression of mucormycosis.
Inhalation of mould spores leads to the most common forms of the infection—the rhinocerebral disease in diabetes patients or pulmonary infection, which generally occurs in haematology patients [2]. Recently, an increasing number of cases of necrotising skin and soft-tissue mucormycosis following direct inoculation of the fungi have been reported, and these are common following natural disasters and war-inflicted wounds [4].
Central nervous system (CNS) mucormycosis is usually an extension of the infection from the sinuses to the eyes and brain [3]. Isolated cerebral mucormycosis is extremely rare. In a meta-analysis of 929 cases, CNS disease was described in 30% of the cases, and out of these cases, only 16% were confined to the CNS [5]. In most of these cases, cerebral mucormycosis resulted from the spread of a rhinocerebral infection, while isolated CNS cases tended to be a result of disseminated fungal infection and were particularly common among intravenous drug users. In such cases, the basal ganglia were the most common site involved. This predilection to the basal ganglia is interesting; the sporangiospore size is thought to facilitate the distribution of the fungus to the basal ganglia through the striatal arteries. Additionally, high levels of iron in the basal ganglia stimulate further fungal growth [6].
2. Case
65 years old male presented to the hospital with a left sided headache and left ear pain and discharge of 4 months duration DAY 0. On D −21 he presented to another hospital with ischemic stroke and right sided hemiparesis. He was found to have rupture tympanic membrane of the left ear.
The patient has history of nasal polypectomy in 1990. He achieved complete remission following treatment of naso- pharyngeal carcinoma in 2010. He is known diabetic with a poor control. The average HbA1C is 8.1 mmol/l. In addition, he is on regular treatment for hypertension and hypothyroidism.
His physical exam was significant for dysarthria and right sided weakness. The power was 3/5 in both right upper and lower limbs. He was fully conscious Glasgow coma scale 15/15. All cranial nerves were intact.
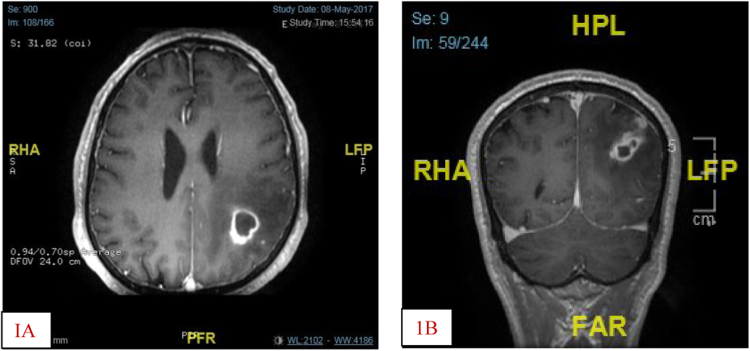
The pulse rate was 92beats/minute in sinus rhythm, blood pressure 130/65 mmhg and temperature 36.5 °C Respiratory, cardiovascular, gastrointestinal and genitourinary examinations were unremarkable. Laboratory findings showed a white blood cell count of 3.5/mm3, hemoglobin of 9.4 g/dL, platelet count of 323/mm3. Routine blood chemistry values were within normal limit except for HbA1C of 8.1, TSH 17.3 IU/ml (NR 0.27–4.2). An initial computerized tomography scan of the brain (day 0) showed a zone of abnormal density in left posterior temporal, occipital and parietal regions with few foci of hyper densities, could represent ischemic infarction with hemorrhagic transformation. A subtle low density on the right side of the pons could represent acute infarction. A gadolinium enhanced magnetic resonance imaging on day + 11 revealed Lobular fluid containing multiple lesions in the left parietal and occipital lobes which show interval increase in size suggestive of abscesses with pressure effect on left lateral ventricle and extension to the overlying dura (Fig. 1A&B).
Fig. 1.
A(Axial) and B(coronal) sections of gadolinium enhanced MRI showing brain abscess in the (L) parietal and occipital lobes with pressure effect on the (L) lateral ventricle.
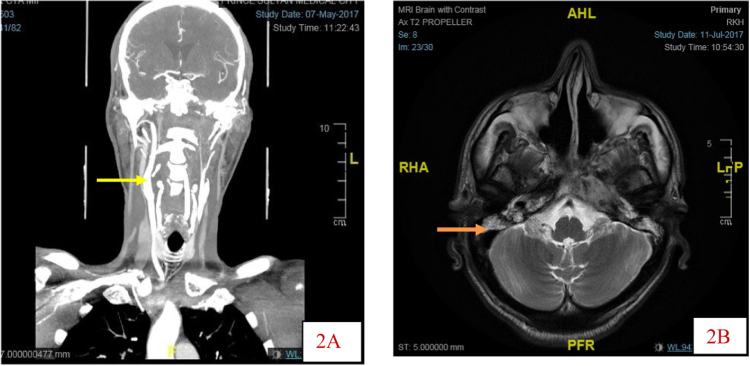
Differential diagnosis included multiple metastases from NPH tumour. The largest abscess in the parietal region measures 4.0 by 3.4 cm. Fluid was present in mastoid air cells bilaterally. A left internal carotid artery (ICA) pseudoaneurysm/aneurysm was also noted. CT angiogram confirmed occlusion of the left internal carotid artery from above its origin up to the cavernous segment where the artery is reconstituted with reduced calibre (Fig. 2A). However, the main intracranial arteries including anterior and middle cerebral arteries are filled, likely cross filling from collaterals. In addition, opacification of mastoid air cells and middle ear cavities indicating chronic otomastoiditis was detected (Fig. 2B).
Fig. 2.
A. Coronal section CTA showing patent ® ICA (yellow arrow) and Occlusion of (L) ICA from origin to the cavernous segment .2B MRI showing fluid in both mastoids. ( orange arrow).
He underwent left parietal craniotomy and aspiration biopsy D + 14. An initial report of the biopsy described multifocal vasculitis with focal wall necrosis (Fig. 3). No malignant cells seen.
Fig. 3.
Showing vasculitis (Red arrow head).
Based on the biopsy result he was referred to the neurologist and rheumatologist services. Auto –antibodies including ANCA, ANA, and rheumatoid factor were all negative. Transoesophageal echocardiogram did not show vegetations. He was considered to have an atypical CNS vasculitis and started on 40 mg prednisolone.
The biopsy was reviewed by the pathologist and infectious diseases who requested further staining of the tissue for fungi on D18. In retrospect this disclosed 90-degree-branching hyphae; most consistent with mucormycosis (Fig. 4A&B).
Fig. 4.
A. H&E stain showing focal brain tissue necrosis and infiltration by thin walled broad, non-septated hyphae (black arrow) B. PAS stain showing multiple broad hyphae (black arrows).
The brain abscess culture did not yield any fungi. Similarly, the (L) ear culture showed skin type flora only. The nasopharyngeal biopsy histopathology and culture was negative for fungi. On day+ 20 the steroids were stopped, and he was put on liposomal amphotericin B 5 mg/kg for 20 weeks and posaconazole 400 mg twice daily. Debridement was advised by neurosurgeons, but the family refused. Repeat MRI at the end of treatment D+ 170 revealed almost complete regression in the sizes of all abscesses. (Fig. 5). He was then continued to posaconazole suspension 400 mg bid with meals for one month.
Fig. 5.
Follow up MRI showing resolution of brain abscess.
The patient continued to improve without any residual neurological deficits. MRI performed at the end of treatment D+ 187 revealed marked regressions in the size of all abscesses. However, the patient developed complications due to multidrug-resistant bacteraemia and hospital-acquired pneumonia, which resulted in his death on D+ 240.
3. Discussion
The patient described in this case report had multiple factors predisposing him to mucormycosis, including uncontrolled DM, nasopharyngeal disease, and otomastoiditis. However, the mould could not be isolated from sinus and nasopharyngeal cultures or identified on histopathological analyses. Additionally, the patient had chronic mastoiditis and a perforated eardrum, which are predisposing factors for contagious diseases, but the ear swab culture did not reveal the presence of the fungus. Nevertheless, the possibility of an extension could not be excluded since mucormycosis is isolated in < 50% of the cases.
The presentation of isolated cerebral mycosis varies. Headache and hemiparesis occur in 44% and 38% of patients, respectively. Additionally, other symptoms include fever and altered mental status, which are observed in 41% and 21% of patients, respectively [7]. The patient in the present report had a stroke and left-sided hemiplegia. The initial CT scan revealed a subtle low-density region in the right side of the pons, which was possibly an acute infarction.
Vessel occlusion due to hyphae growth in the vessel lumen and direct injury to the endothelium results in mycotic emboli, and infarction with or without haemorrhage in the brain. Major blood vessels such as the basilar and carotid arteries are commonly involved, and the dissemination of the hyphae can lead to cavernous sinus thrombosis and brain abscesses. In the present case, similar to previous reports, there was a total occlusion of the left internal carotid artery [8], [9]. Interestingly, the presence of good collaterals probably protected against a major stroke on that side. Another possible aetiology of stroke considered in this patient was vasculitis. The initial histopathology report was consistent with vasculitis and indicated that the patient required steroid treatment. Remarkably, fungal vasculitis followed by thrombotic occlusion of the major cerebral blood vessels has been previously described [10].
Brain CT and MRI revealed multiple abscesses in the left parietal and occipital lobes. Isolated brain abscesses secondary to mucormycosis are rare and are mostly an extension of an aggressive rhinocerebral disease [11]. Classically, intravenous drug abuse has been identified as an important risk factor for isolated cerebral abscesses. Verma et al. reported 30 cases of isolated cerebral mucormycosis, and 17 had a history of intravenous drug abuse [7].
The diagnosis of mucormycosis depends on a high level of suspicion, supported by the identification of the fungus in culture and histopathology. New diagnostic approaches that include molecular testing and MALDI-TOF examinations of the serum and frozen sections have allowed the confirmation of the diagnosis [12]. Although identification at the genus/species level is of epidemiological importance, it is usually not required for optimal antifungal therapy. However, it is essential to differentiate between mucormycosis and other fungi since this has serious therapeutic consequences. Rhizopus spp., accounts for 69% of cases in India, and only 24% of the cases in Europe, as per a recent survey [1].
Optimal treatment involves prompt surgical debridement with antifungal therapy and the correction of any underlying predisposing condition. Vascular invasion with thrombosis leads to vascular occlusion and necrosis, markedly reducing drug penetration in the tissues, underscoring the importance of surgical debridement. The combination of surgery and medical treatment is superior to either therapy alone. Roden et al. reported, in a review of 929 cases, that patient survival rates were 61% (324 of 532) for cases treated with amphotericin B deoxycholate, 57% (51 of 90) for cases treated with surgery alone, and 70% (328 of 470) for cases treated with antifungal therapy and surgery [5].
Amphotericin B preparations, posaconazole, and isavuconazole are the standard medications for mucormycosis. Liposomal amphotericin B remains the primary therapeutic agent with a favourable side effect profile when compared to amphotericin B (AmB). Gleissner and colleagues reported that the survival rate with Liposomal AmB treatment (67%) was higher than that with AmB treatment (39%) [13]. Mucorales are inherently resistant to most antifungal drugs including echinocandins and some azoles and require a higher dose of Amphotericin B than other fungal infections. Although amphotericin lipid complex is as effective as AMB, inferior outcomes have been reported in CNS infections [12].
Posaconazole has been used for salvage therapy in patients who are difficult to treat or intolerant to amphotericin therapy. Currently, posaconazole is not recommended for primary treatment but can be used as stepdown medication [12]. Isavuconazole, a second-generation broad-spectrum triazole, is licensed by the FDA for the treatment of mucormycosis. Trials achieved a 32% response in patients when it was used as the primary treatment, and a 36% response in patients who were resistant to other antifungal therapy. Similar to posaconazole, the EMA recommends isavuconazole use in patients who are refractory or intolerant to other antifungals. Notably, unlike posaconazole, it does not require a specific timing or type of food for administration [12]. Additionally, both posaconazole and isavuconazole are available as oral preparations for step-down therapy.
In the present case, the patient's response to treatment was unusual despite the delay in treatment and lack of surgical debridement. Yohai et al. in their meta-analysis reported that survival rates decreased if there were delays > 6 days from diagnosis of mucormycosis to treatment [14]. Medical treatment alone is unreliable and is associated with high mortality. However, Gollard et al. reported the case of an intravenous drug abuser, with isolated brain lesions secondary to mucormycosis, who recovered after prolonged medical therapy [15].
Our present case illustrates the difficulty in diagnosis and treatment of cerebral mucormycosis. An initial diagnosis of vasculitis was made before the definitive identification of hyphae on histopathology examination. Although classical management relies on prompt surgical debridement and antifungals, medical treatment alone has been used successfully as salvage therapy or in patients with difficult-to-debride areas. A high level of suspicion is needed to consider this diagnosis since a delay in diagnosis can result in a grave outcome.
Conflict of interest
Authors have no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Ashraf S. Ibrahim, Brad Spellberg, Thomas J. Walsh, Dimitrios P. Kontoyiannis; Pathogenesis of Mucormycosis, Clin. Infect. Dis., 54, suppl_1, 1 February 2012, Pages S16–S22. [DOI] [PMC free article] [PubMed]
- 2.A. Skiada, F. Lanternier, A.H. Groll, L. Pagano, S. Zimmerli, R. Herbrecht, O. Lortholary, G.L. Petrikkos, Diagnosis and treatment of mucormycosis in patients with haematological malignancies: guidelines from Proceedings of the 3rd European Conference on Infections in Leukemia (ECIL 3). haematologica Sep 14:haematol-2012, 2012. [DOI] [PMC free article] [PubMed]
- 3.Binder U., Maurer E., Lass-Flörl C. Mucormycosis–from the pathogens to the disease. Clin. Microbiol. Infect. 2014;20:60–66. doi: 10.1111/1469-0691.12566. (MatchedISSN: 1198-743X) [DOI] [PubMed] [Google Scholar]
- 4.Neblett Fanfair R., Benedict K., Bos J., Bennett S.D., Lo Y.C., Adebanjo T., Etienne K., Deak E., Derado G., Shieh W.J., Drew C. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012;367(23):2214–2225. doi: 10.1056/NEJMoa1204781. (MatchedISSN: 0028-4793) [DOI] [PubMed] [Google Scholar]
- 5.Roden M.M., Zaoutis T.E., Buchanan W.L., Knudsen T.A., Sarkisova T.A., Schaufele R.L., Sein M., Sein T., Chiou C.C., Chu J.H., Kontoyiannis D.P. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 2005;41(5):634–653. doi: 10.1086/432579. (MatchedISSN: 1058-4838) [DOI] [PubMed] [Google Scholar]
- 6.Hazama A., Galgano M., Fullmer J., Hall W., Chin L. Affinity of mucormycosis for basal ganglia in intravenous drug users: case illustration and review of literature. World Neurosurg. 2017;98 doi: 10.1016/j.wneu.2016.11.130. (872-e1.0) [DOI] [PubMed] [Google Scholar]
- 7.Verma A., Brozman B., Petito C.K. Isolated cerebral mucormycosis: report of a case and review of the literature. J. Neurol. Sci. 2006;240(1–2):65–69. doi: 10.1016/j.jns.2005.09.010. (MatchedISSN: 0022-510X) [DOI] [PubMed] [Google Scholar]
- 8.Al-Otaibi F., Albloushi M., Alhindi H., Timms M.S. Carotid artery occlusion by rhinoorbitocerebral mucormycosis. Case Rep. Surg. 2012;2012 doi: 10.1155/2012/812420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu K.A., Nguyen P.L., Sanossian N. Basilar artery territory stroke secondary to invasive fungal sphenoid sinusitis: a case report and review of the literature. Case Rep. Neurol. 2015;7(1):51–58. doi: 10.1159/000380761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma R.R. Fungal infections of the nervous system: current perspective and controversies in management. Int. J. Surg. 2010;8(8):591–601. doi: 10.1016/j.ijsu.2010.07.293. (Matched ISSN: 1743-9191) [DOI] [PubMed] [Google Scholar]
- 11.Han S.R., Choi C.Y., Joo M., Whang C.J. Isolated cerebral mucormycosis. J. Korean Neurosurg. Soc. 2007;42(5):400. doi: 10.3340/jkns.2007.42.5.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tissot F., Agrawal S., Pagano L., Petrikkos G., Groll A.H., Skiada A., Lass-Flörl C., Calandra T., Viscoli C., Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102(3):433–444. doi: 10.3324/haematol.2016.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleissner B., Schilling A., Anagnostopolous I., Siehl I., Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma. 2004;45(7):1351–1360. doi: 10.1080/10428190310001653691. (MatchedISSN: 1042-8194) [DOI] [PubMed] [Google Scholar]
- 14.Yohai R.A., Bullock J.D., Aziz A.A., Markert R.J. Survival factors in rhino-orbital-cerebral mucormycosis. Surv. Ophthalmol. 1994;39(1):3–22. doi: 10.1016/s0039-6257(05)80041-4. (MatchedISSN: 0039-6257) [DOI] [PubMed] [Google Scholar]
- 15.Gollard R., Craig R., Larsen R., Chandrasoma P. Isolated cerebral mucormycosis: case report and therapeutic considerations. Neurosurgery. 1994;34(1):174–177. doi: 10.1097/00006123-199401000-00026. (MatchedISSN: 0148-396X) [DOI] [PubMed] [Google Scholar]