CrbS is a member of a unique family of sensor histidine kinases, as its structure suggests that it may link signaling to the transport of a molecule. However, mechanisms through which CrbS senses and communicates information about the outside world are unknown. In the Vibrionaceae, orthologs of CrbS regulate acetate metabolism, which can, in turn, affect interactions with host organisms. Here, we situate CrbS within a larger regulatory framework, demonstrating that crbS is regulated by nutrient-sensing systems. Furthermore, CrbS domains may play various roles in signaling during infection and growth in culture, suggesting a unique mechanism of host recognition. Finally, we define the roles of additional pathways in acetate flux, as a foundation for further studies of this metabolic nexus point.
KEYWORDS: Vibrio cholerae, acetate, two-component system, acetyl-CoA synthetase, sensor histidine kinase
ABSTRACT
Vibrio cholerae controls the pathogenicity of interactions with arthropod hosts via the activity of the CrbS/R two-component system. This signaling pathway regulates the consumption of acetate, which in turn alters the relative virulence of interactions with arthropods, including Drosophila melanogaster. CrbS is a histidine kinase that links a transporter-like domain to its signaling apparatus via putative STAC and PAS domains. CrbS and its cognate response regulator are required for the expression of acetyl coenzyme A (acetyl-CoA) synthetase (product of acs), which converts acetate to acetyl-CoA. We demonstrate that the STAC domain of CrbS is required for signaling in culture; without it, acs transcription is reduced in LB medium, and V. cholerae cannot grow on acetate minimal media. However, the strain remains virulent toward Drosophila and expresses acs similarly to the wild type during infection. This suggests that there is a unique signal or environmental variable that modulates CrbS in the gastrointestinal tract of Drosophila. Second, we present evidence in support of CrbR, the response regulator that interacts with CrbS, binding directly to the acs promoter, and we identify a region of the promoter that CrbR may target. We further demonstrate that nutrient signals, together with the cAMP receptor protein (CRP)-cAMP system, control acs transcription, but regulation may occur indirectly, as CRP-cAMP activates the expression of the crbS and crbR genes. Finally, we define the role of the Pta-AckA system in V. cholerae and identify redundancy built into acetate excretion pathways in this pathogen.
IMPORTANCE CrbS is a member of a unique family of sensor histidine kinases, as its structure suggests that it may link signaling to the transport of a molecule. However, mechanisms through which CrbS senses and communicates information about the outside world are unknown. In the Vibrionaceae, orthologs of CrbS regulate acetate metabolism, which can, in turn, affect interactions with host organisms. Here, we situate CrbS within a larger regulatory framework, demonstrating that crbS is regulated by nutrient-sensing systems. Furthermore, CrbS domains may play various roles in signaling during infection and growth in culture, suggesting a unique mechanism of host recognition. Finally, we define the roles of additional pathways in acetate flux, as a foundation for further studies of this metabolic nexus point.
INTRODUCTION
Vibrio cholerae causes global pandemics of the diarrheal disease cholera, but most strains are adapted for survival in marine environments. V. cholerae can multiply rapidly in response to an influx of dissolved organic carbon (1, 2), or it can thrive in close association with copepods, sea birds, fish, and midge larvae (3–7). V. cholerae may also inhabit the gastrointestinal (GI) tracts of terrestrial arthropods, including house flies (8, 9). Investigations of molecular mechanisms underlying V. cholerae infection in an arthropod (Drosophila melanogaster) model of infection revealed unexpected roles for bacterial metabolites, including short-chain fatty acids (SCFAs), in modulating the pathogenicity of these interactions (10–12). SCFAs produced by colonizing bacteria can alter the physiology of a variety of host organisms, affecting the development of the immune system, appetite, and overall body size (13–18). In Drosophila, levels of the SCFA acetate in the Drosophila midgut are controlled by commensal Acetobacter bacteria (18). During V. cholerae infection, the pathogen's molecular mechanisms of regulating both acetate excretion and consumption are important determinants of virulence (10, 19). V. cholerae removes acetate from the fly midgut, causing fats to amass not in the fat body, as they do in healthy flies, but in cells lining the fly GI tract, which sensitizes the flies to killing by this pathogen (10). Removal of acetate from the surrounding medium is controlled by the expression and activity of acetyl coenzyme A (acetyl-CoA) synthetase (Acs), which converts acetate to acetyl-CoA (10, 20). In V. cholerae and other members of the Vibrionaceae, acs transcription is positively regulated by the CrbS/R two-component system (10, 21, 22). Due to its role in controlling acs, the CrbS/R pathway is necessary for V. cholerae infection and virulence toward Drosophila; without it, V. cholerae is virtually avirulent (10, 21). By connecting acetate metabolism to the CrbS/R two-component system, V. cholerae and related pathogens may link this metabolic switch to additional environmental cues.
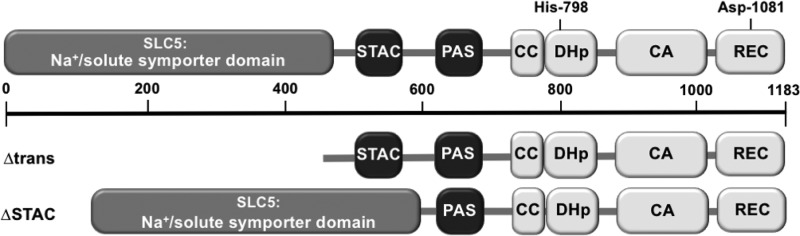
The structure of CrbS, with several domains of unknown function, suggests that it employs a novel mechanism of transmitting information across the bacterial cell envelope (Fig. 1). The N terminus consists of a membrane-localized domain with similarity to the solute carrier 5 (SLC5) transporter family and the sodium-proline symporter PutP from Escherichia coli, with 13 transmembrane regions (23, 24). Directly adjacent is a STAC (SLC5- and two-component signal transduction-associated component) domain, which is thought to regulate transport through the sodium/solute symporter domain, based on structural and bioinformatics analyses (25). CrbS also carries a PAS domain; these domains are often involved in signal sensing and transmission in two-component systems (26). CrbS includes both DHp (dimerization histidine phosphotransfer) and CA (catalytic ATP binding) domains involved in the autophosphorylation of a conserved histidine residue. Finally, CrbS carries a receiver (REC) domain, with the conserved aspartate residue that receives the phosphoryl group. The presence of a REC domain implies that CrbS functions as a hybrid histidine kinase that initiates a multistep phosphorelay, but our results indicate that the receiver domain is not necessary for signaling, and it may instead act as a negative regulator (21). We have shown, however, that the phosphor-accepting His residue located in the DHp domain is required for acs expression, indicating that phosphorylation is associated with the “on” state of this pathway (21).
FIG 1.
Structure of CrbS and deletions in crbS introduced into the V. cholerae genome. The CrbS protein consists of 1,183 amino acids, with the SLC5 Na+/solute symporter-like domain, the STAC domain, the PAS domain, a coiled-coil (CC) region, a DHp domain, the CA domain, and the receiver (REC) domain. The conserved His and Asp residues that may contribute to phosphotransfer are indicated. The Δtrans construct lacks the entire SLC5-like domain, while the ΔSTAC construct lacks the STAC domain.
Based on the arrangement of these domains, we hypothesize that CrbS and related proteins may activate a signaling mechanism that ties transport directly to gene expression (27). By linking the putative transporter domain with the signaling domains, the sensor kinase may regulate acs in response to a small molecule as it is transported into the cell (27). After transporting the signal, the pathway may then immediately convert this information into a response, by directly activating the expression of a suite of genes, including acs. This is borne out in other bacterial species; an ortholog of CrbR in Vibrio vulnificus, AcsR, directly binds to and activates the acs promoter (22), and a binding site has been defined in the promoters of genes targeted by CrbR homologs across the gammaproteobacteria (28). Thus, we hypothesize that a similar mechanism is operative in V. cholerae. Therefore, the first two goals of this study were to ascertain the effects of CrbS domains on signal detection and propagation and to characterize the nature of the interaction between CrbR and the acs promoter in V. cholerae.
CrbS is necessary for acs transcription, but it may function as one component of a larger network of regulators controlling acetate metabolism. If so, this could support the hypothesis that CrbS regulates acs under novel conditions, rather than supplanting information provided by these conserved regulators. Therefore, a third goal of this study was to determine whether other conserved signaling mechanisms contribute to acs transcription in V. cholerae. Here, we took a candidate approach, based on pathways important in E. coli for the regulation of acs and acetate metabolism (20). These include the cAMP receptor protein (CRP)-cAMP system, which regulates acs in response to preferred carbon availability; rpoS, the sigma factor required for the expression of stationary-phase genes; and the pta-ackA pathway, which is required for acetate excretion.
Altogether, our results suggest new mechanisms of acs regulation that intersect with CrbS. We also show that the in vivo environment may alter CrbS-dependent regulation of acs, supporting the hypothesis that additional in vivo signals modulate signaling.
RESULTS
Mutational analysis of the CrbS transporter domain.
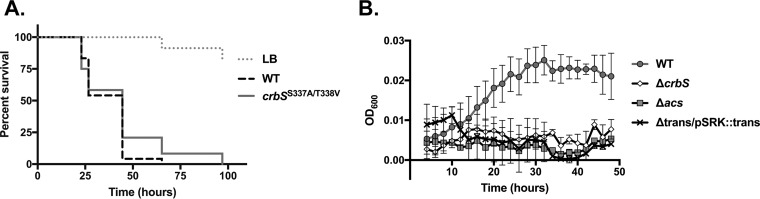
CrbS includes a transporter-like domain (Fig. 1), and we began by identifying specific residues, based on homology to PutP, that may mediate signaling (24). PutP utilizes a Na+ gradient to drive the transport of proline, and marine bacteria, including the Vibrionaceae, tie transport processes to Na+ gradients (23). We aligned the E. coli PutP protein sequence with the CrbS transporter domain and identified several conserved residues that contribute to Na+ transport in E. coli. These included Ser-340 and Thr-341 (29), which fall at residues 336 and 337 within transmembrane segment IX in V. cholerae. We introduced simultaneous mutations of these residues into the V. cholerae chromosome, and we tested the effects of these mutations on Drosophila survival. We observed no difference in fly survival relative to the wild-type (WT) strain, indicating that these residues are not required for signaling in vivo (Fig. 2A).
FIG 2.
The CrbS transporter-like domain is needed for CrbS function, but putative Na+ binding sites are not important. (A) Survival of Drosophila flies infected with V. cholerae strains carrying mutations in the putative CrbS Na+ binding site (S337A/T338V) does not differ from that of flies infected with the wild-type V. cholerae strain (P > 0.05 by a log rank test). Results of this assay are representative of data from two biological replicates. (B) The transporter domain is required for CrbS function, as the strain lacking the domain is unable to grow on minimal medium with acetate. Growth of V. cholerae strains carrying deletions in crbS, acs, or the transporter-like domain within crbS was observed in M63 minimal medium supplemented with acetate (10 mM). Complementation of the transporter domain in the pSRK-Km plasmid, with induction with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), does not restore growth.
We next tested the hypothesis that an intact transporter domain is necessary for signaling, by excising the entire transporter domain coding sequence, including codons for amino acids 4 to 504, from the gene in the chromosome. As expected, deletion of the transporter domain halted acetate catabolism both in culture and during infection. This deletion abrogated virulence in Drosophila (data not shown) and prevented growth on acetate minimal medium (Fig. 2B). These observations are consistent with one of two possible interpretations. First, the remaining protein may be unstable, as it has no means of being tethered in the membrane. Second, the protein may be blind to the presence of specific signaling molecules or conditions and therefore may be unable to initiate a signaling cascade. To test whether this phenotype can be rescued by the expression of the CrbS transporter domain alone, we overexpressed the transporter domain and observed the growth of this strain on acetate minimal medium. Overexpression of this domain was insufficient to rescue this deletion (Fig. 2B). This may be due to one of two reasons. First, the two domains may need to be physically linked in order for signaling to occur, as observed in CbrA (27). Alternatively, it is possible that the proteins are unstable or misfolded and are unable to function. These findings indicate that a functional transporter domain is necessary for CrbS signaling, due to its role in ensuring protein stability, membrane tethering, or signaling initiation and propagation.
Mutational analysis of the STAC domain.
Next, we tested whether the STAC domain contributes to signaling. The STAC can comprise one of several domains in cytosolic or membrane-tethered proteins, or it can exist as a stand-alone domain (25). In proteins with SLC5-like transporter domains, the STAC is located directly adjacent to this domain. To identify CrbS residues conserved with STAC domains, we performed an alignment with other STAC-containing proteins in MUSCLE (30). The first of two G(X)XXA motifs, which likely falls at a hairpin turn in this protein family, includes the G residue alone, but the second motif is fully conserved (25). Hydrophobic amino acids, which are present at regular intervals in this domain, also align with the pattern of conservation observed in this domain family (25).
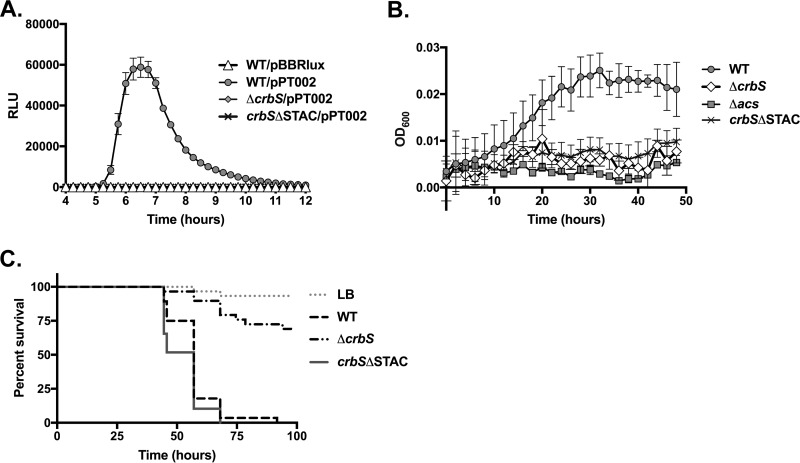
To test the effects of this domain on signaling, we engineered the removal of the STAC domain from the crbS gene in the chromosome, by excising amino acids 536 to 617, leaving behind no tag or scar in the protein sequence. We then introduced a plasmid carrying the acs promoter driving the expression of the lux genes (21), and we tested the effects of the STAC domain deletion on acs transcription. The expression level of acs was lower in the crbSΔSTAC strain than in the wild-type strain (Fig. 3A). To determine whether this deletion may have impacted growth on acetate minimal medium, the strain was inoculated into M63 medium supplemented with 10 mM acetate. This strain was unable to grow, suggesting that lower levels of acs expression correlated with reduced growth on acetate minimal medium (Fig. 3B). Next, we asked whether this deletion altered virulence in Drosophila. Surprisingly, flies fed the crbSΔSTAC strain died at a rate indistinguishable from that of flies fed the wild-type V. cholerae strain, in each of five independent biological replicates (Fig. 3C). This indicates that CrbS signaling is occurring in vivo, as a full deletion of crbS significantly impairs fly mortality (Fig. 3C). Furthermore, this result suggests that the deletion of a large domain of CrbS does not result in degradation of the protein during Drosophila melanogaster infection.
FIG 3.
Effects of the CrbS STAC deletion on acs transcription, acetate metabolism, and fly survival. (A) Deletion of the STAC domain prevents acs promoter activation, similarly to the ΔcrbS strain. For each strain, relative light units (RLU) (defined as luminescence/OD600 unit) from 8 wells of a 96-well plate were measured, with SIO strains carrying either the empty pBBRlux plasmid or the pPT002 plasmid into which the acs promoter was inserted. Error bars indicate standard deviations. (B) Deletion of the STAC domain prevents growth on acetate minimal medium. Growth of the SIO WT, Δacs, ΔcrbS, or crbSΔSTAC strain on M63 minimal medium supplemented with 10 mM acetate was monitored every 2 h for 48 h. Average growth in at least 3 wells of a 96-well plate is shown. Error bars indicate standard deviations. (C) Survival of Drosophila flies infected with the SIO WT, ΔcrbS, or crbSΔSTAC strain. The survival of flies fed the crbSΔSTAC strain did not differ from that of flies fed the WT strain in each of five biological replicates (P > 0.05 by log rank analysis), of which data from one representative experiment are depicted here.
The STAC domain is not required for CrbS-dependent signaling during infection of Drosophila.
We reasoned that the discrepancy between the lack of acetate metabolism of the crbSΔSTAC strain and its ability to cause Drosophila mortality could be explained in three ways. First, it is possible that this mutant, despite expressing acs at low levels, allows for consumption of acetate at rates similar to those of the wild-type strain. Second, this strain might reduce the expression of acs in vivo, but another (unknown) virulence factor that compensates for the lack of acs expression during infection may be upregulated. Third, signaling in vivo may be altered relative to in vitro conditions, such that the acs expression level in the flies is higher than our in vitro, culture-based assays had indicated. We tested each of these hypotheses in turn.
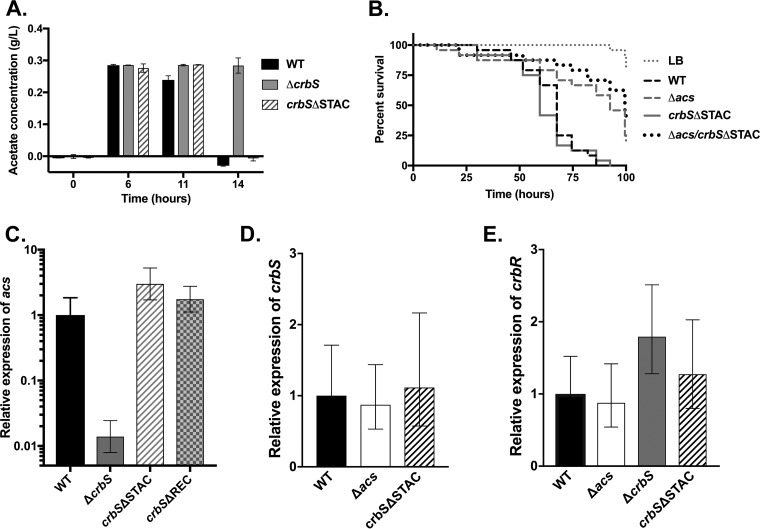
To first examine rates of acetate consumption in vitro, we measured acetate concentrations in cultures inoculated with wild-type V. cholerae, the crbS mutant, or the STAC mutant. The crbS mutant delayed acetate consumption, but deletion of the STAC domain did not affect acetate consumption relative to the WT (Fig. 4A). Thus, a reduction in acs expression may not result in a measurable difference in acetate levels under these conditions. This finding argues that a low threshold of acs transcription may be sufficient for the removal of acetate from the medium.
FIG 4.
The CrbS STAC domain deletion does not alter acetate uptake, other virulence mechanisms, or signaling in vivo. (A) Acetate concentrations in medium in the SIO WT, ΔcrbS, and crbSΔSTAC strains. Both the WT and crbSΔSTAC strains consumed acetate between 11 and 14 h, while the crbS strain was delayed. (B) Deletion of the STAC domain does not increase virulence in Drosophila when introduced into the Δacs background (P > 0.05 by log rank analysis). Results of this assay are representative of data from two biological replicates. (C) During Drosophila infection, the expression level of acs in the crbS mutant was lower than that in the WT, but the acs level was not reduced in the crbSΔSTAC strain. Averages and standard deviations of relative expression levels in each of three samples (with 6 to 10 flies in each sample) are shown. Expression is normalized to that of WT V. cholerae in flies. As an additional control, acs expression in the crbSΔREC strain was monitored, which did not reduce acs expression. An identical trend was observed in a second biological replicate of this assay. (D and E) In Drosophila, expression of crbS (D) or crbR (E) is not altered as a result of the introduction of the STAC domain deletion. As an additional control, expression in the Δacs strain was also monitored, and there was no difference, as expected.
Next, we tested whether the crbSΔSTAC strain may be upregulating a second virulence factor that can compensate for the lack of acs expression. We constructed a double-deletion strain that carries the Δacs and the crbSΔSTAC alleles. Introducing crbSΔSTAC into the Δacs background did not recover the virulence of this strain (Fig. 4B). Thus, a second, unknown virulence factor cannot explain the pathogenic phenotype of the crbSΔSTAC strain.
Finally, we tested whether this strain reduces acs expression during infection, by performing reverse transcription-quantitative real-time PCR (RT-qPCR) on RNA isolated from flies infected with the WT or crbSΔSTAC V. cholerae strain. As controls, we also tested acs expression in flies infected with the ΔcrbS strain, which should reduce acs expression, as well as a strain carrying a deletion in the crbS REC domain, which we expected to express acs similarly to the wild-type strain (21). We confirmed that the acs expression level is lower in flies carrying the crbS deletion (10), and we observed that acs expression was equivalent to that of the wild-type strain in both the STAC and REC domain deletions (Fig. 4C). Therefore, conditions in the fly gastrointestinal tract allow for acs expression regardless of the deletion of the CrbS STAC domain.
We reasoned that host-specific conditions could be affecting CrbS-dependent expression of acs via one of several mechanisms. First, the fly environment may be altering the function or stability of the CrbSΔSTAC protein. Second, it is possible that the in vivo environment reduces the threshold level of CrbS activity necessary for maximal acs transcription. Third, the fly environment may be inducing increased levels of transcription of crbS and crbR, such that acs is fully expressed despite a reduction in the activity of the protein. To examine the latter hypothesis, we measured the expression of crbS and crbR via RT-qPCR in V. cholerae within Drosophila. We observed that the crbS and crbR genes are expressed to similar levels in the WT and crbSΔSTAC strains (Fig. 4D and E). As a control, the acs deletion did not affect the expression of crbS or crbR. Therefore, we hypothesize that the Drosophila environment can alter the parameters of CrbS-dependent signaling relative to in vitro culture conditions.
CrbR interactions with the acs promoter.
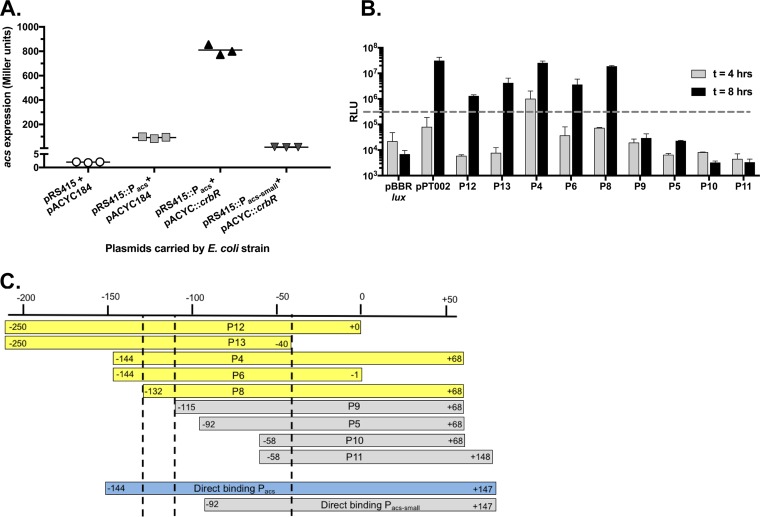
We hypothesize that CrbS directly couples transport to signal transduction in order to activate a maximally efficient pathway to regulate acs transcription. Thus, the CrbR response regulator likely binds directly to the acs promoter, without an intervening regulatory step. To test this hypothesis, we expressed the CrbR protein in E. coli together with the acs promoter fused to the lacZ gene (31). The presence of crbR significantly increased lacZ expression (Fig. 5A), consistent with crbR directly activating acs expression, as observed in V. vulnificus and Pseudomonas (22, 28).
FIG 5.
CrbR activates the acs promoter. (A) Evidence supporting a direct relationship between the CrbR response regulator and the acs promoter in V. cholerae. The gene encoding CrbR was introduced into the pACYC184 plasmid (pACYC::crbR) and cotransformed into E. coli carrying the empty pRS415 plasmid or the pRS415 plasmid with either of two segments of the acs promoter driving lacZ expression. The pRS415::Pacs segment extends from 144 bp upstream from the translational start site to 147 bp downstream, while pRS415::Pacs-small begins 92 bp upstream of the translational start site, as in panel C. The introduction of the crbR gene to the strain carrying pRS415::Pacs significantly increases acs expression (P < 0.0001 by a two-tailed t test). These results are representative of data from five biological replicates. (B) Light production from V. cholerae SIO transformed with pBBRlux carrying the promoters indicated in panel C, or the pPT002 plasmid, which includes a 660-bp fragment of the acs promoter, following growth in LB medium for either 4 or 8 h. The dashed line indicates the threshold above which a promoter is designated “on.” Error bars indicate standard deviations of data from duplicate samples, and the results are representative of data from two biological replicates. (C) Summary of the promoter fusion experiment indicating relative minimum promoter regions. Promoters in yellow initiated transcription in panel B, while those in gray did not. The promoter in blue initiated transcription in panel A. Numbering indicates the distance from the translational start site, as the transcriptional start site has not been defined.
Next, we defined the region of the acs promoter necessary for transcriptional activation, by taking a “promoter-bashing” approach. We fused various regions of the acs promoter to the pBBRlux plasmid and measured luminescence during exponential growth. We defined an ∼15-bp region necessary for expression (Fig. 5B and C), which may include the binding site targeted by CrbR. A previous study identified a putative CrbR binding site, with the primary consensus depicted as GAC(N4)GTC (28). Within the 15-bp region that we identified lies the sequence TCC(TAAA)GTCT (boldface indicates base pairs that match with the consensus CrbR binding site), which could act as the binding target. We also created a smaller version of a putative promoter for the CrbR activity assay that lacks this sequence and observed that CrbR did not increase expression (Fig. 5A and C). Together, these results strongly suggest that CrbR directly binds to and activates the acs promoter, and a specific region of the promoter is necessary for mediating this interaction.
Nutrients and cAMP-CRP regulate acs expression.
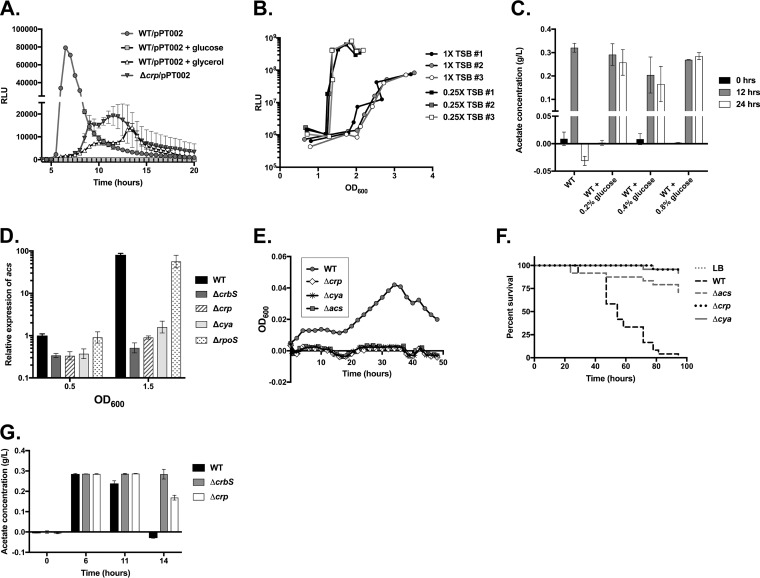
Acetate metabolism and acs transcription are controlled by multiple factors in E. coli. Closely related bacteria, including Vibrio fischeri, have also linked acetate metabolism to conditions that uniquely reflect their environmental settings, including the presence of chitin or glucose, and increased cell density (32, 33). In E. coli, glucose similarly regulates acs transcription. To determine whether nutrients affect acs transcription in V. cholerae, we examined acs transcription from the pBBRlux reporter plasmid in the presence of glucose or glycerol. Transcription of acs was suppressed to almost undetectable levels in the presence of glucose, while glycerol had a moderate effect (Fig. 6A). To further explore the role of nutrients in acs transcriptional activation, we grew the strains in different concentrations of tryptic soy broth (TSB) and measured the optical density (OD) at which expression was initiated. At lower concentrations of TSB, acs promoter activity was activated at earlier stages of growth, indicating that cell density is not the defining variable stimulating expression. Instead, nutrients, as well as other processes tied to nutrient levels, are an important determinant of acs promoter firing (Fig. 6B). We further observed that glucose prevented acetate uptake as well, demonstrating that nutrients repress acetate catabolism (Fig. 6C).
FIG 6.
Sugars and CRP regulate acs expression. (A) Glucose, glycerol, and CRP alter acs promoter activity. Luminescence (RLU) driven by the acs promoter in the pPT002 plasmid in WT SIO, the WT supplemented with either glucose (40 mM) or glycerol (40 mM), or the Δcrp strain was measured. Light production from an average of 4 wells in a 96-well plate, with standard deviations indicated by error bars, is depicted. Results are representative of data from at least three biological replicates. (B) Nutrient levels, and not cell density, alter acs promoter activity. Luminescence (RLU) driven by the acs promoter in the pPT002 plasmid in WT SIO in either 1× tryptic soy broth (TSB) or 0.25× TSB was measured in culture. Each line indicates measurements taken at the indicated optical densities from an individual culture. Results are representative of data from two biological replicates. (C) Glucose halts acetate uptake. Acetate concentrations were measured in duplicate samples after 0 h, 12 h, and 24 h of growth in LB medium or LB medium supplemented with glucose (0.2%, 0.4%, or 0.8%). Results are representative of data from at least two biological replicates. (D) CRP and adenylate cyclase, but not RpoS, are required for acs transcription. Levels of acs transcripts were quantified by RT-qPCR in the ΔcrbS, Δcrp, Δcya, and ΔrpoS strains in LB medium at OD600 values of 0.5 and 1.5, with relative expression normalized to the expression of the WT strain at an OD600 of 0.5. The average expression levels of three samples, with the standard deviations, are shown. This assay is representative of data from four biological replicates for the crp deletion and two replicates that include the cya deletion. (E) CRP and adenylate cyclase are required for growth on acetate minimal medium. Growth of the WT, Δcrp, Δcya, and Δacs strains in M63 minimal medium supplemented with acetate (10 mM) was measured every 2 h for 48 h. Average values from 3 wells of a 96-well plate are depicted. Standard deviations are indicated but are often smaller than the size of the symbol. Results are representative of data from two biological replicates. (F) CRP and adenylate cyclase are required for virulence in Drosophila. Survival of Drosophila flies infected with the Δcrp, Δcya, and Δacs strains was measured over 100 h. Survival of flies that ingested the Δcrp and Δcya strains was improved relative to those fed the WT (P < 0.0001 by a log rank test). For the Δcrp strain, results are representative of data from two biological replicates. (G) Acetate concentrations in culture were compared in the WT SIO, ΔcrbS, and Δcrp strains. Acetate consumption in the Δcrp strain was slightly delayed at 14 h (P = 0.0017 by a t test). However, this may be explained by a growth defect in LB medium for this strain (57) (data not shown). Acetate concentrations were measured in duplicate cultures, and results are representative of data from three biological replicates.
Regulation of gene expression by glucose can occur via its effects on the phosphoenolpyruvate-phosphotransferase (PTS) system, in which glucose alters concentrations of cAMP in the cell to affect CRP binding to targeted promoters. To determine whether CRP plays a role in regulating acs transcription, we constructed a deletion in crp and measured acs transcription from the same reporter plasmid. We observed a reduction in promoter activity, although some transcription was detectable (Fig. 6A). To confirm this finding, we also constructed an in-frame deletion in the gene encoding adenylate cyclase (cya). We then measured acs transcription via RT-qPCR and observed that acs transcription was similarly reduced in the both the Δcrp and Δcya strains when cells were grown to an OD at 600 nm (OD600) of 1.5 (Fig. 6D). Next, we examined growth on minimal medium supplemented with acetate and observed that neither the crp nor the cya deletion strain was capable of growing (Fig. 6E). Consistent with these findings, survival of flies fed the crp or cya mutant was significantly improved over that of flies provided the wild-type strain (Fig. 6F). Acetate consumption is either unaffected or slightly reduced in the Δcrp strain (Fig. 6G), indicating both that (i) a reduction in acs transcription is not sufficient to prevent the removal of acetate from media and (ii) CRP may control levels of another metabolite, in addition to acetate, to affect virulence in Drosophila (11, 12). Altogether, these findings collectively suggest that CRP regulates acs transcriptional activation.
Nutrients and cAMP-CRP regulate expression of crbS and crbR.
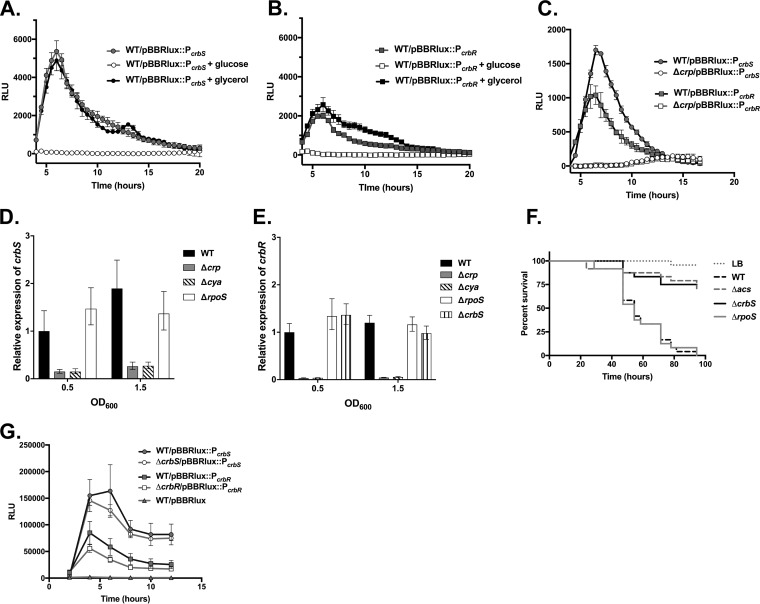
We next searched the acs promoter for a CRP binding site and identified multiple imperfect sites with Virtual Footprint (34). We reasoned instead that CRP may be regulating the transcription of the crbS and crbR genes, and in each of these promoters, we were able to identify stronger putative CRP binding sites, using the Virtual Footprint tool (34). The crbS gene carries a site, GCTGATTGAGTTCAAA (boldface type indicates nucleotides aligned with the consensus CRP binding site), centered at position −78.5 relative to the translational start site. The crbR gene carries a site, AGGGATACAGTTCAGA, at position −88.5 relative to the translational start site. To determine whether these promoters were subject to CRP regulation, we cloned both promoters into the pBBRlux plasmid and characterized nutrient-dependent responses. Levels of expression from the crbS and crbR promoters were each reduced in the presence of glucose but not glycerol (Fig. 7A and B). Next, we introduced the crbS and crbR promoter fusion plasmids into the Δcrp strain and examined expression. Both promoters were substantially suppressed (Fig. 7C). We further confirmed this result by RT-qPCR for both the Δcrp and the Δcya strains (Fig. 7D and E). Thus, the cAMP-CRP system regulates the transcription of the crbS and crbR genes.
FIG 7.
Glucose and CRP regulate transcription of crbS and crbR. (A and B) Luminescence driven by the crbS (A) or crbR (B) promoter is suppressed by glucose (40 mM) but not by glycerol (40 mM) in LB medium. The 461-bp crbS promoter fragment extended from 395 bp upstream to 66 bp downstream of the translational start codon. The crbR promoter fragment, 648 bp in length, extended 522 bp upstream and 126 bp downstream of the translational start codon. (C) Deletion of crp also impacts expression from the crbS and crbR promoters in the same assay. Average values for 3 or 4 wells of a 96-well plate with standard deviations are depicted, and results are representative of data from two biological replicates. (D and E) Both CRP and adenylate cyclase, but not RpoS, are required for expression of crbS (D) or crbR (E). crbS and crbR transcript levels were determined via RT-qPCR for the ΔcrbS, Δcrp, Δcya, and ΔrpoS strains in LB medium at OD600 values of 0.5 and 1.5 and normalized to the expression level of the WT strain at an OD600 of 0.5. The average expression levels for three samples, with the standard deviations, are shown. Expression levels in the Δcrp and Δcya backgrounds are representative of data from three and two independent replicates, respectively. (F) Survival of Drosophila flies infected with the rpoS mutant, together with the Δacs and ΔcrbS mutants as controls. Survival of flies provided the ΔrpoS mutant is not different from that of flies fed the WT (P > 0.05 by a log rank test). (G) Deletion of crbS or crbR has little, if any, effect on crbS or crbR promoter activity, respectively. Luminescence (RLU) driven by the crbS or the crbR promoter in the pBBRlux plasmid was measured in culture at the indicated time points for the WT, ΔcrbS, or ΔcrbR strain. Averages of triplicate values, with standard deviations, are indicated. This graph is representative of data from three biological replicates.
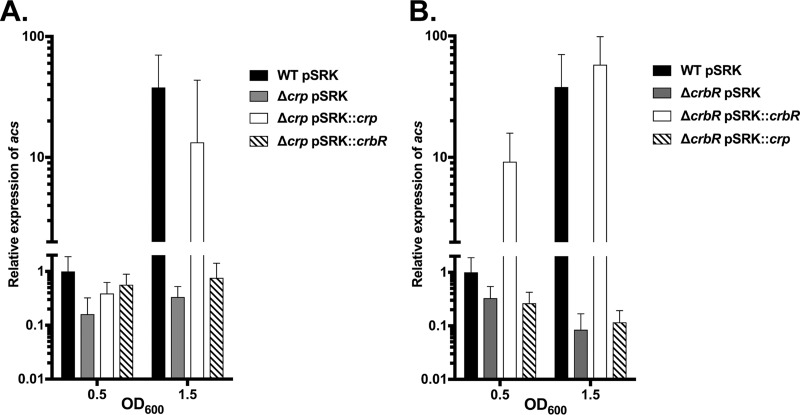
We next examined the relationship between crp and CrbS/R expression by complementing the crp and crbR genes and measuring the expression of acs in culture. First, we deleted crp and then overexpressed the crp or crbR gene in this background. Overexpression of crbR in the Δcrp background was not sufficient to restore expression (Fig. 8A). This confirms that crp may be regulating crbS and/or acs independently as well. As a control, overexpression of crp complemented the Δcrp phenotype. Similarly, the expression of crbR could complement the ΔcrbR phenotype. Interestingly, overexpression of crbR increased the expression of acs beyond wild-type levels, indicating that transcriptional regulation of crbS and crbR limits signaling through the pathway. Overexpression of CRP in this background has no effect on acs, indicating that expression requires crbR despite high levels of CRP (Fig. 8B).
FIG 8.
Expression of acs requires both CRP and CrbR. Expression of acs in strains overexpressing crp or crbR was quantified via RT-qPCR. Expression of acs was measured in the WT strain carrying the empty pSRK-Km (pSRK) complementation plasmid; in the Δcrp strain carrying pSRK, pSRK expressing crp, or pSRK expressing crbR (A); or in the ΔcrbR background (B). All experiments were performed in the presence of 1 mM IPTG.
CrbS and CrbR do not activate a positive-feedback loop.
We also examined whether the crbS and crbR genes activate their own transcription to initiate a positive-feedback loop that amplifies expression. We measured luminescence driven by the crbS and crbR promoters in the pBBRlux plasmid in strains lacking either the crbS or the crbR gene. We found that expression of crbS or crbR from the promoter was unaffected (Fig. 7G). We further confirmed this finding by RT-qPCR for the expression of the crbR gene (Fig. 7E). Therefore, transcriptional activation by CRP controls the expression of crbS, crbR, and acs, but there is no evidence that additional self-amplification occurs.
RpoS does not regulate acs transcription or virulence in Drosophila.
Finally, acetate catabolism and acs expression patterns correlate with the onset of late exponential and stationary phases in batch culture. Therefore, we reasoned that the stationary-phase sigma factor, encoded by rpoS, may be regulating acs transcription, as has been observed in E. coli (35). We constructed an in-frame deletion of rpoS and tested the expression of acs by RT-qPCR. We observed that rpoS has no effect on the expression of acs, crbS, or crbR (Fig. 6D and 7D and E). As expected, rpoS has no effect on Drosophila survival (Fig. 7F). Therefore, rpoS has no effect on acetate-dependent virulence or acs transcription in V. cholerae.
The pta-ackA system is required for excretion but not consumption of physiological concentrations of acetate in Vibrio cholerae.
In E. coli, multiple pathways mediate the interconversion of acetate and acetyl-CoA. Acs is thought to act as a scavenger of low concentrations of acetate, while the Pta-AckA system may assist with assimilation of higher concentrations (20). However, excretion of acetate is controlled by the Pta-AckA system in E. coli. The pta gene catalyzes the conversion of acetyl-CoA to acetyl-phosphate (acetyl-P), while ackA converts acetyl-P to acetate (20).
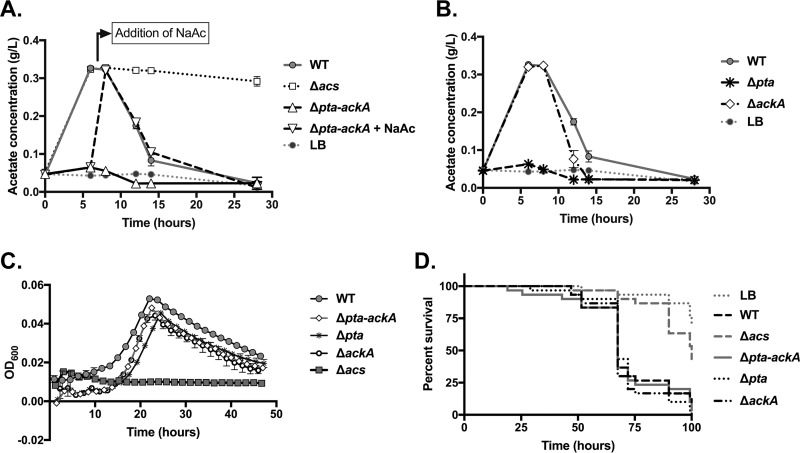
To define the contributions of the Pta-AckA system to acetate metabolism in V. cholerae, we constructed deletions in the pta and ackA genes individually, and we created a third deletion that removes the two genes together from the chromosome, as they are located directly adjacent to one another in an operon. When the Pta-AckA system is deleted, there is no detectable acetate accumulation in the medium (Fig. 9A). When acetate is added exogenously to this culture, the Δpta-ackA mutant consumes acetate similarly to the WT strain (Fig. 9A). Deletion of pta similarly abrogated acetate excretion, but deletion of ackA had no effect (Fig. 9B). The V. cholerae genome carries two genes annotated as acetate kinases; the second, VCA0235, may have a redundant function. Similarly, there is no effect of the pta-ackA system on growth on acetate or on Drosophila virulence (Fig. 9C and D). These results confirm that the Pta-AckA system is not required for consumption of acetate excreted by V. cholerae at these concentrations.
FIG 9.
The Pta-AckA system is required for acetate consumption in Vibrio cholerae. (A) Acetate was measured in duplicate cultures of the WT, Δacs, and Δpta-ackA strains at the designated times. The Δpta-ackA strain was incapable of excreting acetate but, upon supplementation with 5.6 mM acetate (NaAc) after the 6-h time point, was able to consume exogenous acetate. (B) In the same assay, acetate concentrations in cultures with the Δpta and ΔackA strains were measured. The Δpta strain was unable to excrete acetate, but the ΔackA strain both excreted and consumed acetate similarly to the WT strain. In both panels A and B, the concentration of acetate in uninoculated LB medium is also depicted, indicating the baseline level. Standard deviations are indicated and are often smaller than the size of the symbol. (C) Strains carrying deletions in the pta-ackA system are not impaired during growth on acetate minimal medium. Averages from at least 3 wells of a 96-well plate are shown; error bars depict standard deviations and are often smaller than the symbol. (D) Survival of Drosophila flies infected with the pta, ackA, or pta-ackA mutant. Survival of flies provided with these mutants does not differ from that of flies fed the WT (P > 0.05 by a log rank test).
DISCUSSION
Bacterial survival in a wide variety of environmental contexts requires efficient regulation of metabolic fluxes in response to changing nutrient availability. Vibrio cholerae, a waterborne pathogen, can experience fluctuations in both the abundance and composition of carbon sources as it grows in close association with host organisms, as a biofilm on aquatic particulate matter, or as single cells in the water column. In marine ecosystems, vibrios are uniquely attuned to dividing rapidly upon encountering dissolved organic carbon sources (1, 2, 36). However, mechanisms of controlling and responding to carbon catabolism in vibrios are complex and appear to differ in significant ways from those of E. coli (37–40). The regulation of acetate catabolism by the CrbS/R two-component system may further differentiate carbon metabolic pathways in the Vibrionaceae. In this study, we define additional pathways that regulate acs and acetate metabolism, and we provide evidence for novel roles for the STAC domain in CrbS signaling.
The domain structure of CrbS suggests a unique mechanism of signal sensing and transduction that links transport to signaling. We took a genetic approach to our investigation of these domains, a strategy that has informed previous studies of two-component regulators and their signaling pathways (41, 42). In a first analysis of the transporter-like domain, we demonstrate that conserved residues necessary for Na+ binding in PutP are not required for virulence, suggesting that they do not contribute to signaling in vivo. Deletion of the transporter domain is detrimental, though, indicating that the lack of the transporter disrupts signaling, protein function, or protein stability. This is not surprising but establishes a baseline from which future studies can investigate questions about the minimum components necessary. For example, can signaling occur if the protein is simply tethered in the membrane, or is transport necessary? Because the transporter-like domain has diverged relative to PutP, it may be more informative to take a random-mutagenesis approach to uncovering important residues for protein function. Furthermore, the substrate of the transporter domain remains elusive. This has been investigated in one homolog of CrbS, CbrA in Pseudomonas, which similarly carries a PutP-like transporter domain. Transport of proline, as well as other amino acids, was assessed, but no transport was detected, and no effect on signaling was observed (24). However, a link between histidine transport and signaling has been suggested (27).
We further demonstrate that the STAC domain is required for acs expression and in vitro growth on acetate, but it does not affect signaling when V. cholerae is infecting Drosophila. We considered three possible hypotheses to explain this unexpected result. In the first, we questioned whether the ΔSTAC deletion strain was upregulating a second, unknown virulence factor that may be sufficient to kill flies. We introduced the ΔSTAC deletion into the Δacs background, and this did not improve the virulence of the Δacs strain alone. Therefore, an unknown virulence factor is not increasing virulence in the absence of acetate-mediated pathogenesis mechanisms. Next, we showed that acetate consumption in these strains is not significantly affected by the STAC deletion, despite low levels of acs transcription. This alone could be sufficient to explain our finding, although it reveals an additional issue: a discrepancy between acs transcription levels and acetate consumption. Clearly, acetate consumption is dependent upon acs, because a deletion in acs entirely halts acetate uptake in these strains, and a deletion in pta-ackA has no effect on acetate removal from the medium at these concentrations. Therefore, low levels of acs transcription may be sufficient for the removal of acetate from the medium. We further observe that growth on minimal medium with acetate is reduced in the crbSΔSTAC strain, despite its ability to remove acetate from the medium. We hypothesize that CrbS may be further altering the expression of other genes involved in acetate metabolism that, when collectively expressed at low levels, prevent the assimilation of acetate.
Finally, we observed that the deletion of the STAC domain does not affect acs transcription during infection. Instead, acs levels are similar to those observed in the wild-type strain. This suggests that signals that activate acs in Drosophila can be detected, despite the lack of this domain. Deletion of the entire crbS gene reduces acs levels in Drosophila; therefore, the increase in acs levels cannot be ascribed to a CrbS-independent factor. We hypothesize that the STAC domain may be acting as a negative regulator of in vivo signals or that signaling proceeds independently of the STAC domain during infection. One alternative possibility is that the in vivo environment is regulating the stability of the CrbSΔSTAC proteins, providing a factor or condition that prevents degradation or maintains the membrane localization of the deletion constructs. It is also possible that the Drosophila environment simply lowers the threshold level of CrbS activity needed to reach wild-type levels of acs transcription. Thus, a future goal will be to distinguish between these alternative explanations. In each case, this finding suggests that the Drosophila gastrointestinal environment provides unique and specific signals to V. cholerae, which may be detected by CrbS.
Next, we examined whether acs, despite the presence of CrbS, responds to nutrient signals via conserved regulators important in other bacteria. We demonstrated that sugars reduce acs transcription and that deletion of CRP similarly lowered acs expression levels. However, our results suggest that CRP could be regulating acs indirectly; rather than binding directly to the acs promoter, CRP may instead regulate crbS and crbR transcription. Confirming that acs is not directly targeted by CRP will require further biochemical testing by an electrophoretic mobility shift assay and/or chromatin immunoprecipitation sequencing (ChIP-Seq). Whether or not CRP binds directly to acs, it is clear that the expression of crbS and crbR is subject to transcriptional control. This raises the question, then, regarding the nature of the primary signal sensed by CrbS. In one possible model, CrbS is expressed only in the absence of preferred carbon sources, and an additional signal is needed to activate the phosphorelay. Alternatively, CRP-cAMP could direct the transcription of CrbS proteins that are capable of constitutively activating CrbR, and a signal may instead suppress the phosphorelay. With either model, CRP is needed for acs expression via its role in controlling the expression of CrbS and CrbR. The advantage of creating a two-tiered regulatory scheme (i.e., CRP → CrbS/R → acs) is not yet clear but implies that additional information regarding the extracellular environment is needed to appropriately regulate acs.
The Pta-AckA system is the major pathway through which acetate is excreted (20). A second pathway involving pyruvate oxidase (PoxB) may contribute to acetate excretion in E. coli. However, the V. cholerae genome lacks a poxB homolog, which further establishes the importance of the Pta-AckA system in this bacterium. Appropriate regulation of acetate flux is critical for virulence in multiple mammalian pathogens. Pta and AckA are required for uropathogenic E. coli to colonize the bladder and kidneys (43). In toxigenic strains of V. cholerae, pta is required for full expression of the toxin-coregulated pilus (TCP) operon, which is necessary for colonization of the infant mouse (44). Both pta and ackA control the expression of toxT, a key regulator of both TCP and cholera toxin expression, via another metabolite involved in acetate metabolism (45). Pta and AckA can also affect levels of acetyl-P, which can control acetylation states of proteins and/or phosphorylate response regulators to alter two-component signaling (20, 46, 47). We demonstrate that the Pta-AckA pathway in V. cholerae functions similarly to that of E. coli, with Pta being required for acetate excretion. Our results suggest that there is redundancy, though, as the deletion of ackA did not prevent the conversion of acetyl-P to acetate. The Pta-AckA system does not affect virulence in flies, consistent with our observation that the system does not affect the removal of acetate from media.
In summary, we define roles for multiple pathways in the control of acetate metabolism in Vibrio cholerae. We suggest possible functions for the CrbS STAC domain during signaling, and we describe an additional layer of regulation that integrates nutrient availability into CrbS-dependent acs transcription. Altogether, these findings indicate that information funneled through CrbS is detectable to the bacterium only at times when preferred carbon sources are absent and that the environment may affect CrbS-dependent signaling mechanisms.
MATERIALS AND METHODS
Bacterial growth and storage.
Both E. coli and V. cholerae strains were routinely grown in LB-Miller (LB) medium and stored in 15% glycerol in LB medium at −80°C (Table 1). V. cholerae strains were derived from V. cholerae strain SIO, an environmental, non-O1/non-O139 nontoxigenic strain isolated from the coast of southern California (48). Antibiotics, purchased from Sigma, were added to a final concentration of 100 μg/ml unless otherwise noted.
TABLE 1.
Strains used in this study
| Strain | Description | Reference(s) |
|---|---|---|
| E. coli | ||
| MFDpir | MG1655 RP4-2-Tc::[ΔMu1::aac(3)IV-ΔaphA-Δnic35-ΔMu2::zeo] ΔdapA::(erm-pir) ΔrecA Aprar Zeor Ermr | 50 |
| DH5α λpir | F− Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 λpir | 58 |
| S17-1 λpir | RP4-2(Km::Tn7 Tc::Mu-1) pro-82 λpir recA1 endA1 thiE1 hsdR17 creC510 | 59 |
| AP09 | E. coli WM5406/pHC001B | 49 |
| AP302 | E. coli DH5α/pSRK-Km | 51, 60 |
| AP1033 | MFDpir/pHC001B::crbSS337A/T338V | This study |
| AP834 | MFDpir/pHC001B::crbSΔtrans | This study |
| AP1244 | MFDpir/pSRK-Km::trans | This study |
| AP1576 | MFDpir/pHC001B::crbSΔSTAC | This study |
| AP1021 | E. coli/pACYC184 | 31 |
| AP1294a | TOP10/pRS415 | 31 |
| AP1212 | TOP10/pACYC184::crbR | This study |
| AP1221 | TOP10/pRS415::Pacs | This study |
| AP1228 | TOP10/pRS415::Pacs-small | This study |
| AP1337a | TOP10/pACYC184 and pRS415 | This study |
| AP1298a | TOP10/pACYC184 and pRS415::Pacs | This study |
| AP1323a | TOP10/pACYC184::crbR and pRS415::Pacs | This study |
| AP1324a | TOP10/pACYC184::crbR and pRS415::Pacs-small | This study |
| AP1207a | S17-1/pBBRlux::Pacs-P12 | This study |
| AP1211a | S17-1/pBBRlux::Pacs-P13 | This study |
| AP1039 | S17-1/pBBRlux::Pacs-P4 | This study |
| AP1047 | S17-1/pBBRlux::Pacs-P6 | This study |
| AP1194a | S17-1/pBBRlux::Pacs-P8 | This study |
| AP1197a | S17-1/pBBRlux::Pacs-P9 | This study |
| AP1043 | S17-1/pBBRlux::Pacs-P5 | This study |
| AP1200a | S17-1/pBBRlux::Pacs-P10 | This study |
| AP1203a | S17-1/pBBRlux::Pacs-P11 | This study |
| AP547 | MFDpir/pHC001B::Δcrp | This study |
| AP1970 | MFDpir/pHC001B::Δcya | This study |
| AP1968 | MFDpir/pHC001B::ΔrpoS | This study |
| AP1719 | S17-1/pBBRlux::PcrbS | This study |
| AP1715 | S17-1/pBBRlux::PcrbR | This study |
| AP915 | MFDpir/pSRKKm::crp | This study |
| AP1900 | MFDpir/pSRKKm::crbR | This study |
| AP211 | MFDpir/pHC001B::Δpta-ackA | This study |
| AP1934 | MFDpir/pHC001B::Δpta | This study |
| AP1932 | MFDpir/pHC001B::ΔackA | This study |
| V. cholerae | ||
| AP95 | V. cholerae SIO wild type | 48 |
| AP27 | SIO ΔcrbS | 21 |
| AP218 | SIO Δacs | 57 |
| AP1161 | crbSS337A/T338V | This study |
| AP462 | SIO/pBBRlux | 21 |
| AP431 | SIO/pPT002 | 21 |
| AP1659 | SIO crbSΔSTAC | This study |
| AP1693 | SIO crbSΔSTAC/pPT002 | This study |
| AP847 | SIO crbSΔtrans | This study |
| AP1288a | SIO crbSΔtrans::pSRK-Km | This study |
| AP1293a | SIO crbSΔtrans::pSRK-trans | This study |
| AP1986 | SIO Δacs/crbSΔSTAC | This study |
| AP1268 | SIO/pBBRlux::Pacs-P12 | This study |
| AP1242 | SIO/pBBRlux::Pacs-P13 | This study |
| AP1059 | SIO/pBBRlux::Pacs-P4 | This study |
| AP1075 | SIO/pBBRlux::Pacs-P6 | This study |
| AP1264 | SIO/pBBRlux::Pacs-P8 | This study |
| AP1234 | SIO/pBBRlux::Pacs-P9 | This study |
| AP1067 | SIO/pBBRlux::Pacs-P5 | This study |
| AP1236 | SIO/pBBRlux::Pacs-P10 | This study |
| AP1238 | SIO/pBBRlux::Pacs-P11 | This study |
| AP555 | SIO Δcrp | This study |
| AP1974 | SIO Δcya | This study |
| AP1979 | SIO ΔrpoS | This study |
| AP601 | SIO Δcrp/pPT002 | This study |
| AP1739 | SIO/pBBRlux::PcrbS | This study |
| AP1723 | SIO/pBBRlux::PcrbR | This study |
| AP1980 | SIO Δcrp/pBBRlux::PcrbS | This study |
| AP1982 | SIO Δcrp/pBBRlux::PcrbR | This study |
| AP1763 | SIO ΔcrbS/pBBRlux::PcrbS | This study |
| AP1755 | SIO ΔcrbR/pBBRlux::PcrbR | This study |
| AP683 | SIO/pSRK-Km | 57 |
| AP937 | SIO Δcrp/pSRK-Km::crp | This study |
| AP2002 | SIO Δcrp/pSRK-Km::crbR | This study |
| AP1996 | SIO ΔcrbR/pSRK-Km | This study |
| AP1906 | SIO ΔcrbR/pSRK-Km::crbR | This study |
| AP2000 | SIO ΔcrbR/pSRK-Km::crp | This study |
| AP229 | SIO Δpta-ackA | This study |
| AP1988 | SIO Δpta | This study |
| AP1992 | SIO ΔackA | This study |
Construction of in-frame gene deletions, point mutations, complementation constructs, and transcriptional reporter fusion plasmids.
Deletions and mutations were constructed via a splicing by overlap extension (SOE)-PCR protocol, or via a Gibson protocol (New England BioLabs [NEB]), and introduced into the V. cholerae chromosome via conjugation and selection for double recombination events, as described previously (21). SOE constructs were generated by designing two sets of primers that amplify ∼1,000 bp upstream and downstream of the region to be deleted. Primers 1 and 4 were the “exterior” primers, while the interior primers, primers 2 and 3, were designed to fall within a few amino acids of the start codon and stop codon of the gene of interest. The primers amplified a construct that generated a complete, in-frame deletion of the gene, leaving behind just the overlapping tag incorporated into primers 2 and 3, together with a small number of codons at the 5′ and 3′ ends of the gene. When mutations rather than deletions were constructed, primers 2 and 3 were designed to overlap the region that includes the mutation. The STAC domain deletion primers do not include an exogenous tag and completely overlapped one another without additional base pairs. The Gibson protocol was used to delete the pta-ackA operon from V. cholerae, and primers were designed according to the manufacturer's instructions (NEB). Following SOE-PCR with Q5 high-fidelity DNA polymerase (NEB), the PCR product was then digested with appropriate restriction enzymes and ligated into the pHC001B plasmid (49). The plasmid was transformed into DH5α λpir cells, and the construct was verified by sequencing and then moved into MFDpir cells (50). The plasmid was then conjugated into V. cholerae, and transconjugants carrying the plasmid was selected for by plating on kanamycin, as described previously (21). Sucrose selection was used to identify clones that had lost the plasmid, and integration of the correct construct was verified by PCR or by sequencing.
To generate complementation constructs, the gene of interest was amplified with Q5 high-fidelity DNA polymerase (NEB) using a forward primer that incorporated an NdeI restriction site into the start codon of the gene and a reverse primer that fell after the last stop codon and incorporated a second restriction site. The resulting fragment was digested, ligated into the pSRK-Km plasmid (51), and then transformed into E. coli for conjugation into V. cholerae.
To generate reporter plasmid constructs in the pBBRlux plasmid (52), fragments of the respective promoters were amplified using Q5 high-fidelity DNA polymerase (NEB) with primers incorporating restriction sites, digested, and ligated into pBBRlux. The resulting plasmid was then introduced into V. cholerae by conjugation, followed by selection on ampicillin and chloramphenicol (5 μg/ml).
Measurement of transcription via luminescence assays.
Transcription driven by reporter fusions to the luxCDABE operon was determined by measuring luminescence in a 96-well multimode plate reader (Molecular Devices). Single colonies were inoculated into LB broth with chloramphenicol (5 μg/ml), which were grown overnight at 37°C with shaking. Cultures were diluted 1:500, and multiple wells of a 96-well plate (sterile, flat, clear-bottomed plate with black sides; Brand, Germany) were inoculated with 120 μl. Luminescence and the OD600 were measured over time, with incubation at 37°C and periodic shaking. Relative light units (RLU) were defined as luminescence units per OD600 unit.
Luminescence measured in culture was detected with a luminometer (GloMax; Promega) from aliquots of 12-ml cultures grown in 50-ml “bioreactor” conical tubes with lids containing 0.22-μm filters for gas exchange (Corning). Optical densities of cultures were measured with a spectrophotometer.
Measurement of transcription via RT-qPCR in culture.
Cultures grown overnight were diluted 1:500 into 12 ml of LB broth in a bioreactor conical tube and incubated at 37°C with shaking. The OD600 of the growing culture was monitored in a spectrophotometer; at an OD600 of either 0.5 or 1.5, a 1-ml sample was removed and spun down at 6,000 relative centrifugal force (rcf) for 2 min, and the pellets were frozen in a dry ice-ethanol bath before storage at −80°C. RNA was extracted with TRIzol (Sigma) according to the manufacturer's instructions. Briefly, samples were resuspended in 1 ml of TRIzol and incubated at room temperature for 5 min. Samples were then centrifuged at 12,000 rcf for 10 min, and the supernatant was transferred to a new tube, to which 200 μl of chloroform was added. The samples were incubated at room temperature for 3 min and centrifuged at 10,000 rcf for 15 min at 4°C. The nucleic acid was precipitated from the upper aqueous phase by the addition of 500 μl of isopropanol, followed by a 10-min incubation. The RNA was pelleted by centrifugation at 20,000 rcf for 10 min at 4°C. The pellets were washed with 75% ethanol twice, dried, and resuspended in RNase-free sterile water (Ambion). The nucleic acid was treated with Turbo DNase (Ambion), incubated for 1 h at 37°C, and then treated with 20 μl of DNase deactivation reagent (Ambion). After incubation for 2 min at room temperature, the samples were centrifuged at 12,000 rcf for 2 min, and the supernatant was transferred to a new tube. The RNA concentration and quality were assessed by measuring the concentration and purity on a Nanodrop instrument and by visualizing RNA on an agarose gel.
RNA was converted to cDNA using the SuperScript IV reverse transcription kit (Invitrogen) according to the manufacturer's instructions. Each reaction mixture included 1 μl of 50 μM random hexamers, 1 μl of 10 μM deoxynucleoside triphosphates (dNTPs), and 500 ng of the RNA template in a total volume of 13 μl. The reaction mixtures were heated at 65°C for 5 min, followed by 1 min on ice. A separate mixture of 4 μl of 5× SuperScript IV buffer, 1 μl of 100 mM dithiothreitol (DTT), 1 μl of an RNase inhibitor, and 1 μl of SuperScript IV reverse transcriptase was prepared. This was combined with the RNA-hexamer solution and incubated at 23°C for 10 min, 53°C for 10 min, and 80°C for 10 min. The cDNA concentration was measured with the Nanodrop instrument, and samples were stored at −20°C.
Preparation of samples for RT-qPCR.
The cDNA to be analyzed was diluted to 40 ng/μl in nuclease-free water. A solution consisting of 10 μl of iTaq SYBR green Super-Mix (Bio-Rad), 2 μl of forward and reverse primers (each at 10 μM), and 7 μl of nuclease-free water was prepared on ice. The solution was then added to a 96-well plate before 1 μl of the cDNA template was added to each well. The plate was centrifuged for 5 min at maximum speed at 4°C. Amplification was monitored with a quantitative PCR (qPCR) instrument (Agilent). Threshold cycle (CT) values for the gene of interest were normalized to that of clpX (21, 53). To calculate fold changes in expression, gene expression levels were normalized to that of the WT at an OD600 of 0.5. Relative gene expression and experimental error were determined by calculating the ratio between the wild-type and mutant strain mean gene expression levels (54). Primers for amplification of acs, crbS, and crbR were designed and are listed in Table 2, and primer efficiency calculations were performed to ensure linear amplification. Primers for amplification of clpX in V. cholerae MO10 O139 were previously described (37, 53) but were adjusted to account for a mismatch with the V. cholerae SIO genome.
TABLE 2.
Primers used in this study
| Primer | Sequence | Descriptiona |
|---|---|---|
| ED74_VC0303Na_P1 | GACAACTAGTTATCTCGGCGTGCTGTTTTT | SOE mutation of Na+ binding site S337A/T338V, P1 |
| ED75_VC0303Na_P2 | CATGATGGATAGTGCGATAACCGCCACAAT | SOE mutation of Na+ binding site S337A/T338V, P2 |
| ED76_VC0303Na_P3 | TCGGGGATGGTGATTGTGGCGGTTATCGCA | SOE mutation of Na+ binding site S337A/T338V, P3 |
| ED77_VC0303Na_P4 | CATTGGATCCAACCCTGGTGGAAACTCAAA | SOE mutation of Na+ binding site S337A/T338V, P4 |
| FD32_delTR_P1 | GACAACTAGTACTGTGGTCGGATTCTTGGT | SOE deletion of crbS transporter domain, P1 |
| ED59_trans_p2 | TAACGAGCGGCCGCAACCTTGCATCAGACATCCTTG | SOE deletion of crbS transporter domain, P2 |
| ED60_trans_p3 | TGCGGCCGCTCGTTAGCCAGCTTAAGTGAGCGTTT | SOE deletion of crbS transporter domain, P3 |
| ED61_trans_p4 | CATTGGATCCAAAAAGCGCGATTTGGATT | SOE deletion of crbS transporter domain, P4 |
| AEP221_CrbSStrF | GACACATATGCAAGGTTGGTTGGTAATTC | Complementation of transporter domain, F |
| AEP222_CrbSStrR1a | GTGATGGTGCAAACGCTCACTTAAGCTGGC | Complementation of transporter domain, R |
| AEP230delSTACSIO1 | GACAACTAGTCTGGCCGACCTTATTGATTC | SOE deletion of crbS STAC domain, P1 |
| AEP231delSTACSIO2 | AGACGCCTCCACAGTCACGCGACTTTGGTA | SOE deletion of crbS STAC domain, P2 |
| AEP232delSTACSIO3 | GTGACTGTGGAGGCGTCTGAGCTGTACG | SOE deletion of crbS STAC domain, P3 |
| AEP233delSTACSIO4 | CATTGGATCCCGAAACGCATTAGTCAGGAA | SOE deletion of crbS STAC domain, P4 |
| IM13_acsF4IDT | CAATGTCGCTTGGCATGAAC | RT-qPCR for acs, F |
| IM14_acsR4IDT | AGCCAGAGGTGTAGAGGATAAA | RT-qPCR for acs, R |
| IM25_Qpcr_CrbS1_F | TCAGCCGTCACTCACTCAGA | RT-qPCR for crbS, F |
| IM26_Qpcr_CrbS1_R | GCAAATCACGCATCCCAACC | RT-qPCR for crbS, R |
| IM31_Qpcr_CrbR2_F | AACGGCGAGCCTTATTTCCC | RT-qPCR for crbR, F |
| IM32_Qpcr_CrbR2_R | CCTTGTATTGCTGCGGAGTCA | RT-qPCR for crbR, R |
| IM5 _clpX_qRT_SIO_F | AGAGTTCATTGGTCGTCTGCCTGT | RT-qPCR for clpX, Fb |
| IM6_clpX_qRT_SIO_R | AACAACGCAGCATACTGTTTGGTC | RT-qPCR for clpX, Rb |
| AEP241_CrbR_F_EcoRV | CCTTCGATATCACCTGTTGTGACGTCATGGA | CrbR activity assay, crbR, F |
| AEP242_CrbR_R_SalI | CAGGAGTCGACAGCGCGAGTAAGCAGAAATG | CrbR activity assay, crbR, R |
| AEP238_Acs_F2_Bam | CATTGGATCCCACATATCCCATCAGGCTTTC | CrbR activity assay, Pacs, F |
| AEP237_acspromP2R | ACAGGGATCCAATCCAGTCGACGATTTTGC | CrbR activity assay, Pacs, R |
| AEP239_Acs_F3_Bam | CATTGGATCCTGCTCTGACTGAGAGTTATAAACG | CrbR activity assay, Pacs-small, F |
| AEP237_acspromP2R | ACAGGGATCCAATCCAGTCGACGATTTTGC | CrbR activity assay, Pacs-small, R |
| PT48_acs_prom_P1c | GACAACTAGTCGTTTAACCAAAGGCGATCT | Promoter bashing, promoter 12, F |
| AEP217_acs_R1 | CATTGGATCCTACGGGTTCTCCTTGTGAATT | Promoter bashing, promoter 12, R |
| PT48_acs_prom_P1c | GACAACTAGTCGTTTAACCAAAGGCGATCT | Promoter bashing, promoter 13, F |
| AEP235_acs_R5 | CATTGGATCCCGCGTTTCAAACACGAGA | Promoter bashing, promoter 13, R |
| AEP214_acs_F2 | GACAACTAGTCACATATCCCATCAGGCTTTC | Promoter bashing, promoter 4, F |
| AEP218_acs_R2 | CATTGGATCCGTGTCGTTATCGGCATGC | Promoter bashing, promoter 4, R |
| AEP214_acs_F2 | GACAACTAGTCACATATCCCATCAGGCTTTC | Promoter bashing, promoter 6, F |
| AEP217_acs_R1 | CATTGGATCCTACGGGTTCTCCTTGTGAATT | Promoter bashing, promoter 6, R |
| AEP232_acs_F2a | GACAACTAGTGGCTTTCATCCTAAAGTCTAATTG | Promoter bashing, promoter 8, F |
| AEP218_acs_R2 | CATTGGATCCGTGTCGTTATCGGCATGC | Promoter bashing, promoter 8, R |
| AEP233_acs_F2b | GACAACTAGTCTAATTGTAGAAATCCCTGCTCTG | Promoter bashing, promoter 9, F |
| AEP218_acs_R2 | CATTGGATCCGTGTCGTTATCGGCATGC | Promoter bashing, promoter 9, R |
| AEP215_acs_F3 | GACAACTAGTTGCTCTGACTGAGAGTTATAAACG | Promoter bashing, promoter 5, F |
| AEP218_acs_R2 | CATTGGATCCGTGTCGTTATCGGCATGC | Promoter bashing, promoter 5, R |
| AEP234_acs_F3a | GACAACTAGTTCTCGTGTTTGAAACGCG | Promoter bashing, promoter 10, F |
| AEP218_acs_R2 | CATTGGATCCGTGTCGTTATCGGCATGC | Promoter bashing, promoter 10, R |
| AEP234_acs_F3a | GACAACTAGTTCTCGTGTTTGAAACGCG | Promoter bashing, promoter 11, F |
| PT49_acs_prom_P2 | CATTGGATCCAATCCAGTCGACGATTTTGC | Promoter bashing, promoter 11, R |
| DN07_pVC2702_F | GACAACTAGTGCTAATCTAAGCGAGCTGCAA | CrbR promoter in pBBRlux F |
| DN08_pVC2702_R | CATTGGATCCAAAGCATCCAGCGAGTCC | CrbR promoter in pBBRlux R |
| DN15_PcrbS_3_F | GACAACTAGTTCAACTACTGCATGGGGTCA | CrbS promoter in pBBRlux F |
| DN16_pcrbS_3_R | CATTGGATCCCACGCAATCAAAAACAGCAC | CrbS promoter in pBBRlux R |
| MS37_P1 | GACAACTAGTCCAGATGCCCGGTACGTTTAC | SOE deletion of crp, P1c |
| MS33_P2 | GGTTTCGCGAGAACAGCCGTGAAAGAAACCACTCTAGTG | SOE deletion of crp, P2c |
| MS34_P3 | CACTAGAGTGGTTTCTTTCACGGCTGTTCTCGCGAAACC | SOE deletion of crp, P3c |
| MS39_P4 | CATTCTCGAGTGCATCAACTCCTACAAGAAG | SOE deletion of crp, P4c |
| CP12_delAClg_SOE_P1 | GACAGCGGCCGCTCGAGCTCAAGGCGTTTTAG | SOE deletion of cya, P1 |
| CP13_delAClg_SOE_P2 | TAACGAGCGCCCGCAAAATAGTGCGGGAATGAGGTG | SOE deletion of cya, P2 |
| CP14_delAClg_SOE_P3 | TGCGGGCGCTCGTTATATTCCAAGCCTTCGCAAGT | SOE deletion of cya, P3 |
| MS51_AClg_SOE4 | CATTGGATCCCGGTTTTACCCACACCAGAG | SOE deletion of cya, P4 |
| IM21_delRpos1 | GACAACTAGTCCAGCACCAGTCTCTGGATT | SOE deletion of rpoS, P1 |
| IM22_delRpos2 | TAACGAGCGGCCGCATTTGGTTACGGTATTGCTGACA | SOE deletion of rpoS, P2 |
| IM23_delRpos3 | TGCGGCCGCTCGTTAGCGCTGTTTAACGTCGAAT | SOE deletion of rpoS, P3 |
| IM24_delRpos4 | CATTGGATCCCGGGTATTCACATCCAAGGT | SOE deletion of rpoS, P4 |
| CP09_VC2702_SOEcomp_P1 | GACACATATGGACTCGACCTACACCATC | Complementation of crbR, F |
| ED92_VC2702_compR | CATTGGATCCGCAGAAATGAGCGAGTGGAT | Complementation of crbR, R |
| MS52_CRP_comp1 | GACACATATGGTTCTAGGTAAACCTCAAACC | Complementation of crp, F |
| MS53_CRP_comp2SIO | CATTGGATCCACGATACGGGTTATCGGG | Complementation of crp, R |
| CP15_ptadeletion_SOE_P1 | CATTGGATCCCGGTATTCGTCGCTACGGCATGC | SOE deletion of pta, P1 |
| CP16_ptadeletion_SOE_P2 | TAACGAGCGGCCGCAGAGACCCATGCTCACGCTGGTCA | SOE deletion of pta, P2 |
| CP17_ptadeletion_SOE_P3 | TGCGGCCGCTCGTTAACGGCTATCCAAGCGGGTCAAG | SOE deletion of pta, P3 |
| CP18_ptadeletion_SOE_P4 | GACAACTAGTGCAATTTGCTGCAGAATCTGGCCGT | SOE deletion of pta, P4 |
| CP19_acka_delSOE_P1 | CATTGGATCCGACAAATAGCCTACGCGGCAGCC | SOE deletion of ackA, P1 |
| CP20_acka_delSOE_P2 | TAACGAGCGGCCGCACTTAGACATGAGTGACTACCTGT | SOE deletion of ackA, P2 |
| CP21_acka_delSOE_P3 | TGCGGCCGCTCGTTAGATACTGCTCGTCTGACCAACAT | SOE deletion of ackA, P3 |
| CP22_acka_delSOE_P4 | GACAACTAGTGAGTACGGCCAGCTTCATCTACTGGT | SOE deletion of ackA, P4 |
| MMS17_pta-ackA_GP1 | CATTGGATCCCGAAACGCATTAGTCAGGAA | Gibson deletion of pta-ackA, P1 |
| MMS18_pta-ackA_GP2 | TTCTGCTCTTGACCCGCTTCGTCAACAACTGCGAACTT | Gibson deletion of pta-ackA, P2 |
| MMS19_pta-ackA_GP3 | AAGTTCGCAGTTGTTGACGAAGCGGGTCAAGAGCAGAA | Gibson deletion of pta-ackA, P3 |
| MMS20_pta-ackA_GP4 | GGCGGCCGCTCTAGAAGATGCAGGGTTTCGTGACTG | Gibson deletion of pta-ackA, P4 |
Measurement of V. cholerae transcription in Drosophila via RT-qPCR.
To measure transcription in Drosophila, flies were provided with V. cholerae or sterile LB medium, as described below. To collect flies, Drosophila flies were anesthetized with CO2, and live flies were transferred to an Eppendorf tube and immediately snap-frozen in a dry ice-ethanol bath, as described previously (53). Flies from each of three vials were placed into individual tubes so that each sample represents RNA taken from bacteria inside ∼6 to 10 live flies. Samples were stored at −80°C until Drosophila flies were homogenized with a sterile pestle in TRIzol, and RNA was isolated according to the procedure described above.
Measurement of growth on acetate minimal medium.
V. cholerae was streaked for single colonies and grown overnight in LB medium with appropriate antibiotics, if necessary. Next, 1 ml of each culture grown overnight was centrifuged at 15,000 rcf, and the pellet was resuspended in 1 ml of M63 minimal medium (Amresco). The resuspended cells were inoculated into M63 minimal medium supplemented with 10 mM sodium acetate, diluting the sample to reach an initial OD600 of 0.01 when measured in a standard 1-ml cuvette. Subsequently, 120 μl of each dilution was added to each of at least 3 wells of a sterile, flat, clear-bottomed, 96-well plate (Falcon) and incubated for 48 h at 37°C with periodic shaking inside a plate reader (Molecular Devices), during which the OD600 was monitored.
Determination of acetate concentrations in culture.
Single colonies were inoculated into 1.5-ml cultures grown overnight and incubated with shaking at 37°C. After 16 h, 0.5 ml of the bacterial culture was inoculated in 50 ml of LB broth in an Erlenmeyer flask, and flasks were inoculated in duplicate for each assay. Soon after inoculation, and at successive time points, a 1-ml sample from each flask was aliquoted into an Eppendorf tube and centrifuged at 10,000 rcf for 30 s. The supernatant was filtered with 0.2-μm syringe filters, heated at 80°C for 5 min to denature excreted enzymes or toxins, and stored at −20°C for future analysis. The OD600 associated with each time point was also recorded. The samples were analyzed with an acetic acid assay kit (catalog number K-ACETRM; Megazyme) according to the manufacturer's instructions, with either LB medium or buffer used as a blank. If LB medium was used as the reference point, the values are relative to LB medium, and if buffer was used as a blank, the values include a small amount of acetate present in LB medium.
Drosophila survival assays.
Drosophila survival assays were performed as previously described (10, 21, 55), with ∼10 male flies feeding on LB broth provided in a cellulose acetate matrix supplemented with a culture of V. cholerae grown overnight and diluted 1:10, in triplicate. Fly survival was monitored daily, and survival curves were analyzed in GraphPad Prism with the log rank test.
CrbR activity assay in E. coli.
To test whether CrbR regulates acs transcription by binding directly to the promoter of acs (Pacs), regions of the acs promoter were amplified with Q5 high-fidelity DNA polymerase (NEB) and ligated into pRS415, and the CrbR gene was amplified with Q5 high-fidelity DNA polymerase (NEB) and ligated into the pACYC184 vector, according to methods described previously (31). The plasmids were cotransformed in E. coli TOP10 cells (NEB). CrbR-dependent expression of the acs promoter was demonstrated with β-galactosidase assays. In preparation for these assays, E. coli was first streaked for single colonies onto LB medium with the appropriate antibiotics (chloramphenicol at 5 μg/ml and/or ampicillin at 100 μg/ml) and incubated at 37°C overnight for inoculation into cultures grown overnight. Following overnight growth, cultures were then diluted 1:500 into 3 ml of LB medium in triplicate with appropriate antibiotics and incubated at 37°C with aeration until an OD600 of between 0.4 and 0.6 was reached. β-Galactosidase assays were performed according to a protocol derived from methods described previously (56).
ACKNOWLEDGMENTS
We thank Elizabeth Daniele, Maureen Manning, and Lori Nichols (all of Amherst College) for additional technical assistance. We also thank Gabriela Kovacikova (Dartmouth), William Metcalf (Illinois), and Stephen Farrand (Illinois) for providing plasmids and Alan Wolfe (Loyola University Chicago) for assistance with characterizing the putative CRP binding sites.
This work was supported by funding from Amherst College and the National Science Foundation (MCB RUI 1715956) to A.E.P.
REFERENCES
- 1.Mouriño-Pérez RR, Worden AZ, Azam F. 2003. Growth of Vibrio cholerae O1 in red tide waters off California. Appl Environ Microbiol 69:6923–6931. doi: 10.1128/AEM.69.11.6923-6931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worden AZ, Seidel M, Smriga S, Wick A, Malfatti F, Bartlett D, Azam F. 2006. Trophic regulation of Vibrio cholerae in coastal marine waters. Environ Microbiol 8:21–29. doi: 10.1111/j.1462-2920.2005.00863.x. [DOI] [PubMed] [Google Scholar]
- 3.Senderovich Y, Izhaki I, Halpern M. 2010. Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607. doi: 10.1371/journal.pone.0008607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirschner AKT, Schauer S, Steinberger B, Wilhartitz I, Grim CJ, Huq A, Colwell RR, Herzig A, Sommer R. 2011. Interaction of Vibrio cholerae non-O1/non-O139 with copepods, cladocerans and competing bacteria in the large alkaline lake Neusiedler See, Austria. Microb Ecol 61:496–506. doi: 10.1007/s00248-010-9764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. 1983. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broza M, Gancz H, Kashi Y. 2008. The association between non-biting midges and Vibrio cholerae. Environ Microbiol 10:3193–3200. doi: 10.1111/j.1462-2920.2008.01714.x. [DOI] [PubMed] [Google Scholar]
- 7.Laviad-Shitrit S, Lev-Ari T, Katzir G, Sharaby Y, Izhaki I, Halpern M. 2017. Great cormorants (Phalacrocorax carbo) as potential vectors for the dispersal of Vibrio cholerae. Sci Rep 7:7973. doi: 10.1038/s41598-017-08434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fotedar R. 2001. Vector potential of houseflies (Musca domestica) in the transmission of Vibrio cholerae in India. Acta Trop 78:31–34. doi: 10.1016/S0001-706X(00)00162-5. [DOI] [PubMed] [Google Scholar]
- 9.El-Bassiony GM, Luizzi V, Nguyen D, Stoffolano JG, Purdy AE. 2016. Vibrio cholerae laboratory infection of the adult house fly Musca domestica. Med Vet Entomol 30:392–402. doi: 10.1111/mve.12183. [DOI] [PubMed] [Google Scholar]
- 10.Hang S, Purdy AE, Robins WP, Wang Z, Mandal M, Chang S, Mekalanos JJ, Watnick PI. 2014. The acetate switch of an intestinal pathogen disrupts host insulin signaling and lipid metabolism. Cell Host Microbe 16:592–604. doi: 10.1016/j.chom.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamareddine L, Wong ACN, Vanhove AS, Hang S, Purdy AE, Kierek-Pearson K, Asara JM, Ali A, Morris JG Jr, Watnick PI. 2018. Activation of Vibrio cholerae quorum sensing promotes survival of an arthropod host. Nat Microbiol 3:243–252. doi: 10.1038/s41564-017-0065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanhove AS, Hang S, Vijayakumar V, Wong AC, Asara JM, Watnick PI. 2017. Vibrio cholerae ensures function of host proteins required for virulence through consumption of luminal methionine sulfoxide. PLoS Pathog 13:e1006428. doi: 10.1371/journal.ppat.1006428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. 2011. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 14.Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S, Carling D, Swann JR, Gibson G, Viardot A, Morrison D, Thomas EL, Bell JD. 2014. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy M, Thaiss CA, Elinav E. 2016. Metabolites: messengers between the microbiota and the immune system. Genes Dev 30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Lin S-H, Ren F, Li J-T, Chen J-J, Yao C-B, Yang H-B, Jiang S-X, Yan G-Q, Wang D, Wang Y, Liu Y, Cai Z, Xu Y-Y, Chen J, Yu W, Yang P-Y, Lei Q-Y. 2016. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun 7:11960. doi: 10.1038/ncomms11960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arpaia N. 2014. Keeping peace with the microbiome: acetate dampens inflammatory cytokine production in intestinal epithelial cells. Immunol Cell Biol 92:561–562. doi: 10.1038/icb.2014.40. [DOI] [PubMed] [Google Scholar]
- 18.Shin SC, Kim S-H, You H, Kim B, Kim AC, Lee K-A, Yoon J-H, Ryu J-H, Lee W-J. 2011. Drosophila microbiome modulates host developmental and metabolic homeostasis via insulin signaling. Science 334:670–674. doi: 10.1126/science.1212782. [DOI] [PubMed] [Google Scholar]
- 19.Kamareddine L, Robins WP, Berkey CD, Mekalanos JJ, Watnick PI. 2018. The Drosophila immune deficiency pathway modulates enteroendocrine function and host metabolism. Cell Metab 28:449.e5–462.e5. doi: 10.1016/j.cmet.2018.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfe AJ. 2005. The acetate switch. Microbiol Mol Biol Rev 69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacob K, Rasmussen A, Tyler P, Servos MM, Sylla M, Prado C, Daniele E, Sharp JS, Purdy AE. 2017. Regulation of acetyl-CoA synthetase transcription by the CrbS/R two-component system is conserved in genetically diverse environmental pathogens. PLoS One 12:e0177825. doi: 10.1371/journal.pone.0177825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim MJ, Kim J, Lee HY, Noh HJ, Lee K-H, Park S-J. 2015. Role of AcsR in expression of the acetyl-CoA synthetase gene in Vibrio vulnificus. BMC Microbiol 15:86. doi: 10.1186/s12866-015-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hase CC, Fedorova ND, Galperin MY, Dibrov PA. 2001. Sodium ion cycle in bacterial pathogens: evidence from cross-genome comparisons. Microbiol Mol Biol Rev 65:353–370. doi: 10.1128/MMBR.65.3.353-370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung H, Hilger D, Raba M. 2012. The Na+/L-proline transporter PutP. Front Biosci (Landmark Ed) 17:745–759. doi: 10.2741/3955. [DOI] [PubMed] [Google Scholar]
- 25.Korycinski M, Albrecht R, Ursinus A, Hartmann MD, Coles M, Martin J, Dunin-Horkawicz S, Lupas AN. 2015. STAC—a new domain associated with transmembrane solute transport and two-component signal transduction systems. J Mol Biol 427:3327–3339. doi: 10.1016/j.jmb.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Henry JT, Crosson S. 2011. Ligand binding PAS domains in a genomic, cellular, and structural context. Annu Rev Microbiol 65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X-X, Gauntlett JC, Oldenburg DG, Cook GM, Rainey PB. 2015. Role of the transporter-like sensor kinase CbrA in histidine uptake and signal transduction. J Bacteriol 197:2867–2878. doi: 10.1128/JB.00361-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepulveda E, Lupas AN. 2017. Characterization of the CrbS/R two-component system in Pseudomonas fluorescens reveals a new set of genes under its control and a DNA motif required for CrbR-mediated transcriptional activation. Front Microbiol 8:2287. doi: 10.3389/fmicb.2017.02287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hilger D, Böhm M, Hackmann A, Jung H. 2008. Role of Ser-340 and Thr-341 in transmembrane domain IX of the Na+/proline transporter PutP of Escherichia coli in ligand binding and transport. J Biol Chem 283:4921–4929. doi: 10.1074/jbc.M706741200. [DOI] [PubMed] [Google Scholar]
- 30.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hulbert RR, Taylor RK. 2002. Mechanism of ToxT-dependent transcriptional activation at the Vibrio cholerae tcpA promoter. J Bacteriol 184:5533–5544. doi: 10.1128/JB.184.20.5533-5544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Studer SV, Mandel MJ, Ruby EG. 2008. AinS quorum sensing regulates the Vibrio fischeri acetate switch. J Bacteriol 190:5915–5923. doi: 10.1128/JB.00148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan M, Schwartzman JA, Dunn AK, Lu Z, Ruby EG. 2015. A single host-derived glycan impacts key regulatory nodes of symbiont metabolism in a coevolved mutualism. mBio 6:e00811-15. doi: 10.1128/mBio.00811-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Münch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, Jahn D. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187–4189. doi: 10.1093/bioinformatics/bti635. [DOI] [PubMed] [Google Scholar]
- 35.Shin S, Song SG, Lee DS, Pan JG, Park C. 1997. Involvement of iclR and rpoS in the induction of acs, the gene for acetyl coenzyme A synthetase of Escherichia coli K-12. FEMS Microbiol Lett 146:103–108. doi: 10.1111/j.1574-6968.1997.tb10178.x. [DOI] [PubMed] [Google Scholar]
- 36.Beardsley C, Pernthaler J, Wosniok W, Amann R. 2003. Are readily culturable bacteria in coastal North Sea waters suppressed by selective grazing mortality? Appl Environ Microbiol 69:2624–2630. doi: 10.1128/AEM.69.5.2624-2630.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houot L, Chang S, Pickering BS, Absalon C, Watnick PI. 2010. The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J Bacteriol 192:3055–3067. doi: 10.1128/JB.00213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colton DM, Stabb EV. 2015. Rethinking the roles of CRP, cAMP, and sugar-mediated global regulation in the Vibrionaceae. Curr Genet 62:39–45. doi: 10.1007/s00294-015-0508-8. [DOI] [PubMed] [Google Scholar]
- 39.Colton DM, Stoudenmire JL, Stabb EV. 2015. Growth on glucose decreases cAMP-CRP activity while paradoxically increasing intracellular cAMP in the light-organ symbiont Vibrio fischeri. Mol Microbiol 97:1114–1127. doi: 10.1111/mmi.13087. [DOI] [PubMed] [Google Scholar]
- 40.Houot L, Chang S, Absalon C, Watnick PI. 2010. Vibrio cholerae phosphoenolpyruvate phosphotransferase system control of carbohydrate transport, biofilm formation, and colonization of the germfree mouse intestine. Infect Immun 78:1482–1494. doi: 10.1128/IAI.01356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geszvain K, Visick KL. 2008. The hybrid sensor kinase RscS integrates positive and negative signals to modulate biofilm formation in Vibrio fischeri. J Bacteriol 190:4437–4446. doi: 10.1128/JB.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tischler AH, Lie L, Thompson CM, Visick KL. 2018. Discovery of calcium as a biofilm-promoting signal for Vibrio fischeri reveals new phenotypes and underlying regulatory complexity. J Bacteriol 200:e00016-18. doi: 10.1128/JB.00016-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anfora AT, Halladin DK, Haugen BJ, Welch RA. 2008. Uropathogenic Escherichia coli CFT073 is adapted to acetatogenic growth but does not require acetate during murine urinary tract infection. Infect Immun 76:5760–5767. doi: 10.1128/IAI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang SL, Mekalanos JJ. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol 27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 45.Minato Y, Fassio SR, Wolfe AJ, Hase CC. 2013. Central metabolism controls transcription of a virulence gene regulator in Vibrio cholerae. Microbiology 159:792–802. doi: 10.1099/mic.0.064865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhn ML, Zemaitaitis B, Hu LI, Sahu A, Sorensen D, Minasov G, Lima BP, Scholle M, Mrksich M, Anderson WF, Gibson BW, Schilling B, Wolfe AJ. 2014. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS One 9:e94816. doi: 10.1371/journal.pone.0094816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinert BT, Iesmantavicius V, Wagner SA, Schölz C, Gummesson B, Beli P, Nyström T, Choudhary C. 2013. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol Cell 51:265–272. doi: 10.1016/j.molcel.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Purdy A, Rohwer F, Edwards R, Azam F, Bartlett DH. 2005. A glimpse into the expanded genome content of Vibrio cholerae through identification of genes present in environmental strains. J Bacteriol 187:2992–3001. doi: 10.1128/JB.187.9.2992-3001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Borisova SA, Christman HD, Metcalf MEM, Zulkepli NA, Zhang JK, van der Donk WA, Metcalf WW. 2011. Genetic and biochemical characterization of a pathway for the degradation of 2-aminoethylphosphonate in Sinorhizobium meliloti 1021. J Biol Chem 286:22283–22290. doi: 10.1074/jbc.M111.237735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrieres L, Hemery G, Nham T, Guerout A-M, Mazel D, Beloin C, Ghigo J-M. 2010. Silent mischief: bacteriophage Mu insertions contaminate products of Escherichia coli random mutagenesis performed using suicidal transposon delivery plasmids mobilized by broad-host-range RP4 conjugative machinery. J Bacteriol 192:6418–6427. doi: 10.1128/JB.00621-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan SR, Gaines J, Roop RM, Farrand SK. 2008. Broad-host-range expression vectors with tightly regulated promoters and their use to examine the influence of TraR and TraM expression on Ti plasmid quorum sensing. Appl Environ Microbiol 74:5053–5062. doi: 10.1128/AEM.01098-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69–82. doi: 10.1016/j.cell.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 53.Purdy AE, Watnick PI. 2011. Spatially selective colonization of the arthropod intestine through activation of Vibrio cholerae biofilm formation. Proc Natl Acad Sci U S A 108:19737–19742. doi: 10.1073/pnas.1111530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 55.Blow NS, Salomon RN, Garrity K, Reveillaud I, Kopin A, Jackson FR, Watnick PI. 2005. Vibrio cholerae infection of Drosophila melanogaster mimics the human disease cholera. PLoS Pathog 1:e8. doi: 10.1371/journal.ppat.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY. [Google Scholar]
- 57.Liimatta K, Flaherty E, Ro G, Nguyen DK, Prado C, Purdy AE. 2018. A putative acetylation system in Vibrio cholerae modulates virulence in arthropod hosts. Appl Environ Microbiol 84:e01118-18. doi: 10.1128/AEM.01113-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Merrell DS, Camilli A. 2000. Regulation of Vibrio cholerae genes required for acid tolerance by a member of the “ToxR-like” family of transcriptional regulators. J Bacteriol 182:5342–5350. doi: 10.1128/JB.182.19.5342-5350.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat Biotechnol 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 60.Fan F, Liu Z, Jabeen N, Birdwell LD, Zhu J, Kan B. 2014. Enhanced interaction of Vibrio cholerae virulence regulators TcpP and ToxR under oxygen-limiting conditions. Infect Immun 82:1676–1682. doi: 10.1128/IAI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]