Abstract
Neuroinflammation has been observed in association with neurodegenerative diseases including Alzheimer's disease (AD). In particular, a positive correlation has been documented between neuroinflammatory cytokine release and the progression of the AD, which suggests these cytokines are involved in AD pathophysiology. A histological hallmark of the AD is the presence of beta-amyloid (Aβ) plaques and tau neurofibrillary tangles. Beta-amyloid is generated by the sequential cleavage of beta (β) and gamma (γ) sites in the amyloid precursor protein (APP) by β- and γ-secretase enzymes and its accumulation can result from either a decreased Aβ clearance or increased metabolism of APP. Previous studies reported that neuroinflammatory cytokines reduce the efflux transport of Aβ, leading to elevated Aβ concentrations in the brain. However, less is known about the effects of neuroinflammatory mediators on APP expression and metabolism. In this article, we review the modulatory role of neuroinflammatory cytokines on APP expression and metabolism, including their effects on β- and γ-secretase enzymes.
1. Introduction
The progression of neurodegenerative disorders including Alzheimer's disease (AD) leads to death and other negative health consequences [1–3]. The prevalence of AD increases significantly with increasing age in the United States (US) [4]. Importantly, it has been estimated that, by 2050, the population of AD patients in the US will increase threefold over the number in 2000 [5]. Moreover, studies have found that more than 4.5 million new cases of dementia arise per year, 70% of which are attributed to AD [6, 7]. In the US, AD costs over $170 billion every year [8]; reducing the progression of AD could provide beneficial consequences clinically and economically. Finding molecular therapeutic targets involved in the progression of AD is therefore highly important and studies exploring its causative factors are needed.
AD is accompanied by the formation of beta-amyloid (Aβ) plaques, neurodegeneration, and neuroinflammation [for review, see [11]]. Immune cells may produce autoimmune inflammation, which leads to the progression of neurodegenerative diseases including AD [for review, see [12]]. Triggering receptors expressed on myeloid cells 2 in microglial receptors for Aβ have been found to regulate the physiological and pathological functions of microglia [13]. A significant increase in neuroinflammatory cytokines release has been observed in animal models of AD [14]; these cytokines can cause neurodegeneration [15] and activation of microglia [16] that might further progress AD. The accumulation of Aβ in the brain also increases production of neuroinflammatory cytokines [14, 17]. Studies suggest that attenuation of AD symptoms is attributable at least in part to restored concentrations of neuroinflammatory cytokines, indicating that these molecules have a critical role in disease development [for review, see [18]]. Furthermore, the exposure of human neuronal and extraneuronal cells to an inflammatory cytokine such as interleukin-18 (IL-18) or a combination of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) has been shown to lead to increased Aβ production [19, 20]. This indicates that these cytokines modulate proteins that are responsible for generating Aβ [20]. The exposure to inflammatory cytokines also reduces Aβ transport [21, 22], which might lead to accumulation of Aβ in the brain. This was confirmed by a later study, which showed that an anti-inflammatory agent reduced the accumulation of Aβ through upregulating ATP-binding cassette-B1 (ABCB1) [23], a protein involved in the clearance of Aβ from the brain into the vascular system [24, 25]. A recent opinion article discussed the role of ABCB1 in AD progression through modulation of Aβ uptake [26].
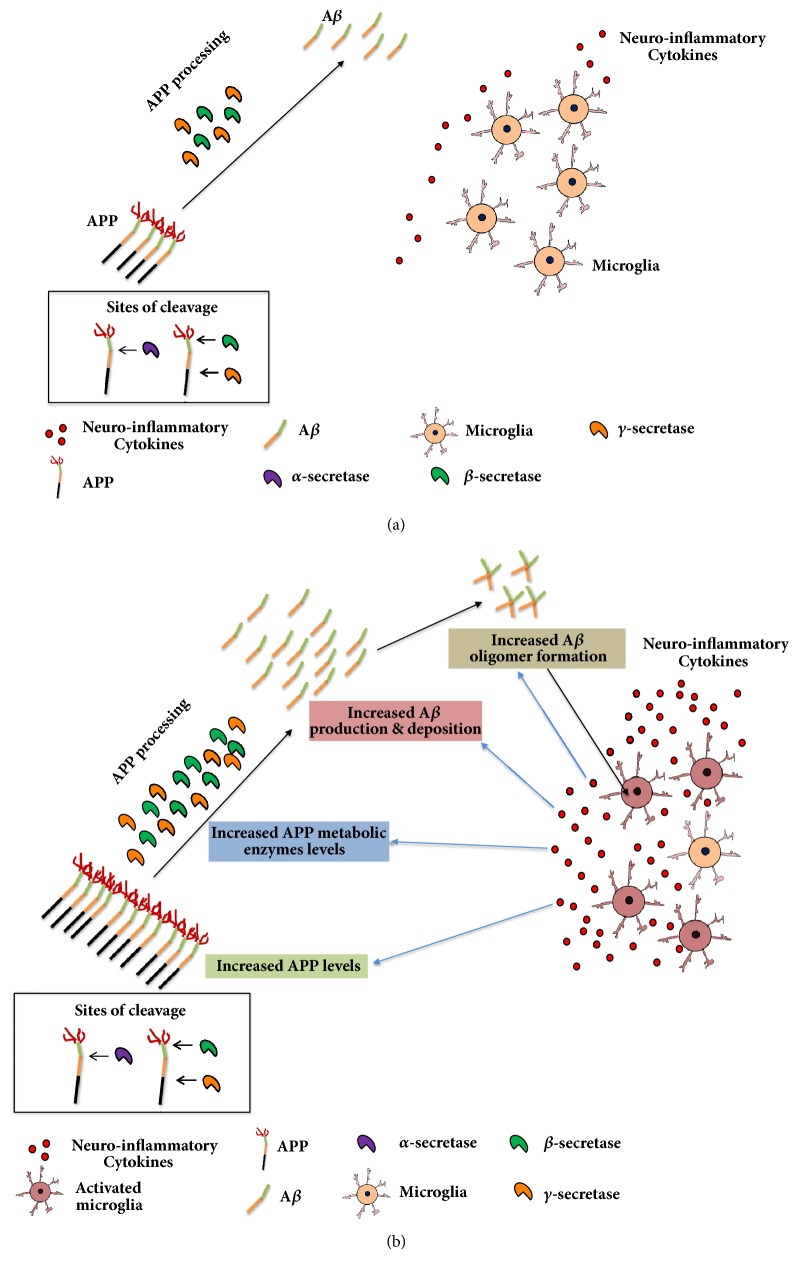
Aβ accumulation in the brain is one of the histological hallmarks associated with AD [for review, see [27]]. Aβ is formed through the sequential cleavage of the amyloid precursor protein (APP) by beta (β-) and gamma (γ-) secretase enzymes [28, 29]. Prior exposure to neuroinflammatory cytokines has been associated with significant increases in the expression of APP in neuronal and glial cells [30] (Figure 1(b)). The regulatory role of neuroinflammatory cytokines on secretase enzymes has been discussed in previous reviews [31, 32]. In this article, we highlighted the effects of neuroinflammatory cytokines on APP processing including APP secretases as well as Aβ production and accumulation.
Figure 1.
Schematic diagram for the effects of neuroinflammatory cytokines on amyloid precursor protein (APP) processing and beta-amyloid (Aβ) production. Amyloid precursor proteins (APP) are cleaved by beta (β) and gamma (γ) sites in the APP by β- and γ-secretase enzymes producing Aβ in the brain. (a) Normal levels and activity of APP, APP metabolic enzymes, and neuroinflammatory cytokines in control brain. (b) In Alzheimer disease (AD), Aβ is accumulated in the brain leading to formation of Aβ oligomers. This effect leads to activation of microglia, which increases the production of neuroinflammatory cytokines. These cytokines increase APP levels, upregulate β-secretase and γ-secretase, and decrease Aβ clearance in the brain. These effects result in further increase in Aβ concentrations and formation of Aβ oligomers and plaques.
Regarding the effects of neuroinflammation on APP processing in human brain samples, studies have found that APP levels and metabolism are altered in postmortem brain tissues from AD patients [33, 34]. A study reported that APP mRNA and protein expression level are increased in postmortem human temporal neocortex of AD patients [34]. Additionally, the activity and protein expression of β-secretase are increased in the neocortex of AD patients [33], suggesting that the formation of Aβ is further increased, leading to pathogenesis. For example, IL-1 levels are increased in the postmortem samples of hippocampus, thalamus, hypothalamus, and cortex of AD patients compared to those obtained from both individuals with vascular dementia and controls [35]. However, the effects of neuroinflammatory cytokines on APP cleaving enzymes merit further investigation. Since neuroinflammation is a common symptom associated with AD, we discuss herein the modulatory role of neuroinflammatory cytokines on APP expression and metabolism in AD models.
1.1. APP
APP is a protein expressed ubiquitously in human body and APP brain isoform is processed into Aβ and mainly localized in the neurons and synapses [36]. APP is the precursor of soluble APP-α and soluble APP-β through cleavage by α-secretase and β-secretase, respectively. Aβ is generated from the APP through sequential cleavage at the β and γ sites of the APP via β- and γ-secretase enzymes, respectively [28, 29]. Physiologically, it is involved in modulating neuronal development including differentiation, migration, and synaptogenesis, suggesting that APP is essential for maintaining homeostasis and synaptic plasticity [37–40]. In this article, we focus on the role of APP and its therapeutic implications in the AD.
1.1.1. Role of APP Expression/Metabolism in AD
The expression of APP cleaving enzymes is increased in the brain cortex of AD patients [41] and the mRNA expression of APP was also increased in AD rat models [42], and these effects are also associated with elevated brain Aβ concentrations [for review, see [43]]. It has been suggested that the activities of β- and γ-secretase enzymes are enhanced in the brains of AD individuals [33, 44], which could elevate brain Aβ levels leading to formation of senile plaques (Aβ deposit) [for review, see [45]]. Pharmacological targeting of β- and γ-secretase could, therefore, attenuate APP-increased Aβ concentrations [46, 47], which might reduce AD-associated symptoms in AD animal models. This hypothesis is supported by reviews indicating that β- and γ-secretase inhibitors attenuate the behavioral symptoms of AD in animals [48, 49]. Specifically, the expression and functions of these enzymes were altered in the brains of AD treated model [33, 41, 50]. Importantly, a γ-secretase enzyme modulator, CHF5074, has been found to attenuate the number and occupation area of Aβ plaques in the cortex and hippocampus of an AD model, and improved AD-associated behavioral symptoms were demonstrated using swimming path test [51]. This study found that CHF5074 also reduced plaques-occupied area in microglia suggesting that this compound can attenuate neuroinflammation associated with AD. However, studies are warranted to explore the effects of neuroinflammatory cytokines on the activity and expression of β- and γ-secretase enzymes in AD models.
In addition to the role of APP metabolism in AD, the overexpression of APP has been used extensively to develop AD models [52, 53]. A previous study found that APP expression level was increased in AD models [34, 54] indicating that increase in APP expression may cause accumulation of Aβ in the brain. Interestingly, exposure to neuroinflammatory cytokines increased the expression of APP using neuronal and nonneuronal cell lines [20, 30, 55]. These findings suggest that neuroinflammatory cytokines could cause accumulation of Aβ in the brain either through increased expression of APP or by decreasing the transport of Aβ into the vascular system. It is important to note that both protein and mRNA expression of APP were upregulated [34], and other reports have also shown that apolipoprotein E4 (APOE4) secreted glia stimulated APP transcription and Aβ production in human cultured neurons [56]. This suggests that these inflammatory cytokines might upregulate APP through modulating the expression of its transcription factors; however, this hypothesis needs further investigation. In this article, we review the effects of proinflammatory cytokines produced in AD models on the mRNA and protein expression of APP.
1.1.2. APP Processing as a Therapeutic Target for Attenuating AD Pathology
As discussed earlier, APP metabolism is a process involved in the production of Aβ in the brain [28, 29]. Therefore, modulating enzymes that cleave APP into Aβ could attenuate the concentrations of Aβ [51, 57–59], which might lead to the attenuation of AD behavioral symptoms [51, 58]. The daily treatment of transgenic model of the AD with a γ-secretase enzyme modulator for seven months has been shown to reduce the concentrations of Aβ in the cortex and hippocampus [57]. In another study, mice with AD that were exposed to methylene blue for three months also had decreased productions and concentrations of Aβ1–40 and Aβ1–42 in the cortex via inhibitory effects on the β-secretase enzyme [58]. This effect was associated with the attenuation of AD behavioral symptoms such as cognition, locomotion, and rearing. Similarly, a three-month treatment of transgenic AD mice model with selenomethionine decreased the deposition and production of Aβ in the cortex and hippocampus through modulating β-secretase enzyme in the same brain regions [59]. Together, these findings indicate that modulating either secretase enzymes attenuates Aβ plaques and consequently the associated behavioral symptoms.
Targeting the expression of APP is another therapeutic strategy for attenuating Aβ deposition [60, 61] and therefore potentially limiting AD progression. In CHO APP751SW cells treated with 2-[(pyridine-2-ylmethyl)-amino]-phenol (2-PMAP), both APP level and the production of Aβx-40 and Aβx-42 were inhibited in a dose-dependent manner [60]. This study also found that the inhibitory effect of 2-PMAP on APP was realized through a mechanism affecting APP translation. In addition, 2-PMAP was able to reduce the length of APP and its fragmentation [60]. These effects were associated with improved memory performance in a transgenic mouse model. Another study found that treatment of an AD mouse model with icariin, a compound from Chinese herb Epimedium spp., reduced the burden and production of Aβ in the hippocampus through reducing the levels of APP and β-secretase [61]. These studies confirmed that APP level plays a crucial role in the deposition of Aβ in the brain and therefore in neurodegeneration and neuroinflammation. A novel approach would focus on targeting APP modifications. The selective inhibition of APP colocalization with BACE1 and γ-secretase could lead to a couple of therapeutic benefits. First, it would have minimal impact on substrates acting by binding to β- and γ-secretase. Second, it would directly affect APP trafficking leading to reducing in the colocalization of APP with β- and γ-secretase [62, 63]. Thus, modulating the levels of APP and secretase enzymes are promising therapeutic targets for attenuating AD pathogenesis.
1.2. Role of Neuroinflammatory Cytokines in AD
Increased production of neuroinflammatory cytokines is broadly associated with neurological disorders, including AD [20, 64, 65]. For instance, high concentrations of IL-1, IL-17, IFN-γ, and TNF-α are observed in the brains of AD models [35, 66, 67]. Moreover, increased secretion of IL-17 and IFN-γ from T cells has been demonstrated in a transgenic AD mouse model, and treatment with anti-IFN-γ antibody has been shown to attenuate Aβ deposition induced by CD4(+) T cells [66]. In addition, increased neuroinflammatory cytokines levels have been observed in human brain tissues [35]. Moreover, the combination of adeno-associated virus 1 expressing murine IL-6 and an Aβ suppressor induced further improvement in plaque clearance in transgenic mice [68]. Additionally, decoy receptor 3 (DcR3), a TNF soluble protein, has been found to reduce the accumulation of amyloid plaque, an effect associated with reduced Aβ-increased anti-inflammatory cytokines production from microglia [69]. It is important to note that overexpression of IL-17A, a marker around deposits of accumulated Aβ, reduced the level of Aβ in the hippocampus and cerebrospinal fluid. This effect was associated with increased ABCA1 expression; a protein transports Aβ from brain into the circulatory system [70]. High concentrations of TNF-α were observed in the hippocampus of a mouse AD model, which induced synaptic plasticity and transmission of glutamate [67]. These data provide evidence for targets of neuroinflammatory cytokines that may also provide pharmacological targets for attenuating the pathogenesis of AD.
To support these findings, targeting neuroinflammatory cytokines has been shown to attenuate AD behavioral symptoms in animals [66, 67, 71, 72]. A study found that anti-IFN-γ attenuated impaired cognition in a transgenic AD mouse model [66]. Another model study showed that pretreatment with a TNF-α inhibitor prevented synaptic deficiency induced by increased secretion of TNF-α in the hippocampus [67]. Moreover, treatment with a TNF-α synthesis inhibitor, 3,6′-dithiothalidomide, attenuated impaired memory associated with neuroinflammation and also reduced neurogenesis in the hippocampus that had been induced by Aβ peptide [71]. Pretreatment with 3,6′-dithiothalidomide of an AD mouse model also attenuated Aβ peptide-induced memory deficiency, while chronic exposure reduced impaired cognition and memory dysfunction, effects that were so associated with reduced APP and Aβ plaque levels [73]. Furthermore, attenuation of memory dysfunction has been observed in mice treated with a TNF-α receptor inhibitor [72]. Taken together, these results suggest that neuroinflammatory cytokines in general and TNF-α, in particular, are implicated in AD pathogenesis.
2. Effects of Neuroinflammatory Cytokines on APP in AD Models
From our previous section, there is evidence that exposure to neuroinflammatory cytokines may modulate APP levels [73, 74] and metabolism [75, 76] (Figure 1(b)) which might be targeted for attenuating AD progression. These effects were not observed with the control group (Figure 1(a)). For example, increased APP expression has been found to lead to the increase of brain Aβ levels [60]. In addition, modulation of APP metabolism attenuated deposition of Aβ [57], which might lead to improved AD-behavioral symptoms.
2.1. Effects of Neuroinflammatory Cytokines on APP Expression/Level
Several studies found that APP expression is a key factor involved in the development of AD [34, 52]. Importantly, the expression/level of APP is positively correlated with the concentrations of Aβ in the brain [60, 61]. Studies have been performed to determine the causative factors involved in increased APP expression. In AD model, the neuroinflammatory cytokines have been found to modulate the expression level of APP [30]. This suggests that neuroinflammation associated with AD plays a substantial role in elevating the expression level of the APP which leads to increased Aβ production. This effect results in the formation of Aβ plaque and oligomers that lead to neurodegeneration, an effect involved in AD pathogenesis [for review, see [77]].
Supporting the hypothesis that neuroinflammation modulates AD development, the exposure to neuroinflammatory cytokines increases APP expression [30], which might modulate the level of Aβ in animals developing AD. A prior study reported that the exposure to IL-1β and IL-1 increased the mRNA expression of APP in endothelial and neuronal cells [30]. Similarly, the exposure to IL-1 increased the level of APP transcripts in endothelial cells from human umbilical veins [78]. We suggest here that the synthesis of APP is modulated by neuroinflammatory cytokines including IL-1, TNF-α, and IFN-γ [78–80]. A study found that the level of APP constitutive shedding is regulated by TNF-α converting enzyme [79]. Moreover, the exposure to TNF-α or IFN-γ upregulated APP in both neurons and astrocytes [80]. These findings provide evidence for the potential effects of neuroinflammation on the amyloid precursor system in the progression of the AD.
2.2. Neuroinflammatory Cytokines Modulate APP Metabolism
Neuroinflammatory cytokines have also been shown to modulate APP metabolic enzymes including β- and γ-secretase [75, 76, 80–82]. Interleukins, TNF-α, and IFN-γ are known to stimulate γ-secretase enzyme activity; this effect was associated with increased production of Aβ and the APP intracellular domain [75]. TNF-α and IFN-γ have been shown to enhance β-secretase enzyme expression in a transgenic mouse model of the AD, increasing Aβ deposition and reducing its uptake [76]. Treatment with sulindac sulfide, an anti-inflammatory agent, restored lipopolysaccharide (LPS)-induced β-secretase expression in neuron cells [80]. In addition, this study found that this compound could reduce the secretion of Aβ42 in neurons treated with LPS. Furthermore, genetic deletion of the TNF-α death receptor was associated with reduced β-secretase enzyme activity and expression in AD mice [81]. This effect was also associated with reduced microglia activation and reduced Aβ production and deposits. Further support comes from 5XFAD/TNF-α−/− mice, which have significantly decreased protein expression of β-secretase enzyme and Aβ deposition [82]. Finally, the TNF-α converting enzyme has been found to regulate γ-secretase enzyme activity [83]. These data shed light on the potential effects of inflammatory cytokines on β- and γ-secretase and Aβ system dysregulation in AD models. More studies are required to investigate whether these neuroinflammatory cytokines could be pharmacologically targeted to attenuate AD-associated symptoms.
3. Conclusion
Neuroinflammation associated with AD is suggested to progress AD in part by increasing the accumulation of Aβ in the brain, which induces Aβ plaques and leads to neurodegeneration and microglial activation. Moreover, the release of neuroinflammatory cytokines is increased in AD models; these cytokines reduce the clearance of Aβ and increase its production, in part by increasing the expression/levels of APP (Figure 1(b)). The cytokines also modulate APP metabolism leading to AD pathogenesis. Studies are warranted to investigate the effects of compounds that have anti-inflammatory properties on AD progression.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research and College of Pharmacy Research Center at King Saud University for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Duckett L. Alzheimer's dementia: morbidity and mortality. Journal of insurance medicine (New York, N.Y.) 2001;33(3):227–234. [PubMed] [Google Scholar]
- 2.Ganguli M., Dodge H. H., Shen C., Pandav R. S., DeKosky S. T. Alzheimer disease and mortality: a 15-year epidemiological study. JAMA Neurology. 2005;62(5):779–784. doi: 10.1001/archneur.62.5.779. [DOI] [PubMed] [Google Scholar]
- 3.James B. D., Leurgans S. E., Hebert L. E., Scherr P. A., Yaffe K., Bennett D. A. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045–1050. doi: 10.1212/WNL.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocca W. A., Petersen R. C., Knopman D. S., et al. Trends in the incidence and prevalence of Alzheimer's disease, dementia, and cognitive impairment in the United States. Alzheimer’s & Dementia. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebert L. E., Scherr P. A., Bienias J. L., Bennett D. A., Evans D. A. Alzheimer disease in the US population: prevalence estimates using the 2000 census. JAMA Neurology. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 6.Hebert L. E., Weuve J., Scherr P. A., Evans D. A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitz C., Mayeux R. Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Biochemical Pharmacology. 2014;88(4):640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gooch C. L., Pracht E., Borenstein A. R. The burden of neurological disease in the United States: A summary report and call to action. Annals of Neurology. 2017;81(4):479–484. doi: 10.1002/ana.24897. [DOI] [PubMed] [Google Scholar]
- 9.McGeer P. L., McGeer E. G. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Research Reviews. 1995;21(2):195–218. doi: 10.1016/0165-0173(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 10.Eikelenboom P., Veerhuis R., Scheper W., Rozemuller A. J. M., Van Gool W. A., Hoozemans J. J. M. The significance of neuroinflammation in understanding Alzheimer's disease. Journal of Neural Transmission. 2006;113(11):1685–1695. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 11.Karran E., Mercken M., Strooper B. D. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nature Reviews Drug Discovery. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 12.Sommer A., Winner B., Prots I. The Trojan horse - Neuroinflammatory impact of T cells in neurodegenerative diseases. Molecular Neurodegeneration. 2017;12(1) doi: 10.1186/s13024-017-0222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao Y., Wu X., Li X., et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron. 2018;97(5):1023–1031.e7. doi: 10.1016/j.neuron.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel N. S., Paris D., Mathura V., Quadros A. N., Crawford F. C., Mullan M. J. Inflammatory cytokine levels correlate with amyloid load in transgenic mouse models of Alzheimer's disease. Journal of Neuroinflammation. 2005;2(1, article 9) doi: 10.1186/1742-2094-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith J. A., Das A., Ray S. K., Banik N. L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Research Bulletin. 2012;87(1):10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuno R., Wang J., Kawanokuchi J., Takeuchi H., Mizuno T., Suzumura A. Autocrine activation of microglia by tumor necrosis factor-α. Journal of Neuroimmunology. 2005;162(1-2):89–96. doi: 10.1016/j.jneuroim.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Liu S., Liu Y., Hao W., et al. TLR2 is a primary receptor for Alzheimer's amyloid beta peptide to trigger neuroinflammatory activation. The Journal of Immunology. 2012;188(3):1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 18.Bronzuoli M. R., Iacomino A., Steardo L., Scuderi C. Targeting neuroinflammation in Alzheimer’s disease. Journal of Inflammation Research. 2016;9:199–208. doi: 10.2147/JIR.S86958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasko I., Marx F., Steiner E., Hartmann T., Grubeck-Loebenstein B. TNFα plus IFNγ induce the production of alzheimer β-amyloid peptides and decrease the secretion of APPs. The FASEB Journal. 1999;13(1):63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- 20.Sutinen E. M., Pirttilä T., Anderson G., Salminen A., Ojala J. O. Pro-inflammatory interleukin-18 increases Alzheimer’s disease-associated amyloid-β production in human neuron-like cells. Journal of Neuroinflammation. 2012;9(1) doi: 10.1186/1742-2094-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evseenko D. A., Paxton J. W., Keelan J. A. Independent regulation of apical and basolateral drug transporter expression and function in placental trophoblasts by cytokines, steroids, and growth factors. Drug Metabolism and Disposition. 2007;35(4):595–601. doi: 10.1124/dmd.106.011478. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal M., Ho H. L., Petropoulos S., Moisiadis V. G., Gibb W., Matthews S. G. Pro-inflammatory cytokine regulation of P-glycoprotein in the developing blood-brain barrier. PLoS ONE. 2012;7(8) doi: 10.1371/journal.pone.0043022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qosa H., Batarseh Y. S., Mohyeldin M. M., El Sayed K. A., Keller J. N., Kaddoumi A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chemical Neuroscience. 2015;6(11):1849–1859. doi: 10.1021/acschemneuro.5b00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qosa H., Abuznait A. H., Hill R. A., Kaddoumi A. Enhanced brain amyloid-β clearance by rifampicin and caffeine as a possible protective mechanism against alzheimer's disease. Journal of Alzheimer's Disease. 2012;31(1):151–165. doi: 10.3233/JAD-2012-120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenn A., Grube M., Jedlitschky G., et al. St. John's Wort reduces beta-amyloid accumulation in a double transgenic Alzheimer's disease mouse model - Role of P-glycoprotein. Brain Pathology. 2014;24(1):18–24. doi: 10.1111/bpa.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alasmari F., Ashby C. R., Hall F. S., Sari Y., Tiwari A. K. Modulation of the ATP-Binding Cassette B1 Transporter by Neuro-Inflammatory Cytokines: Role in the Pathogenesis of Alzheimer's Disease. Frontiers in Pharmacology. 2018;9 doi: 10.3389/fphar.2018.00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy M. P., LeVine H., III Alzheimer's disease and the amyloid-β peptide. Journal of Alzheimer's Disease. 2010;19(1):311–323. doi: 10.3233/jad-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien R. J., Wong P. C. Amyloid precursor protein processing and alzheimer's disease. Annual Review of Neuroscience. 2011;34:185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y.-W., Thompson R., Zhang H., Xu H. APP processing in Alzheimer's disease. Molecular Brain. 2011;4(1, article 3) doi: 10.1186/1756-6606-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forloni G., Demicheli F., Giorgi S., Bendotti C., Angeretti N. Expression of amyloid precursor protein mRNAs in endothelial, neuronal and glial cells: modulation by interleukin-1. Brain Research. 1992;16(1-2):128–134. doi: 10.1016/0169-328X(92)90202-M. [DOI] [PubMed] [Google Scholar]
- 31.Sastre M., Walter J., Gentleman S. M. Interactions between APP secretases and inflammatory mediators. Journal of Neuroinflammation. 2008;5, article 25 doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heneka M. T., Carson M. J., Khoury J. El., et al. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14(4):388–405. doi: 10.1016/s1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. JAMA Neurology. 2002;59(9):1381–1389. doi: 10.1001/archneur.59.9.1381. [DOI] [PubMed] [Google Scholar]
- 34.Matsui T., Ingelsson M., Fukumoto H., et al. Expression of APP pathway mRNAs and proteins in Alzheimer's disease. Brain Research. 2007;1161(1):116–123. doi: 10.1016/j.brainres.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 35.Cacabelos R., Álvarez X. A., Fernandez-Novoa L., et al. Brain interleukin-1β in Alzheimer's disease and vascular dementia. Methods and Findings in Experimental and Clinical Pharmacology. 1994;16(2):141–151. [PubMed] [Google Scholar]
- 36.Hoe H.-S., Lee H.-K., Pak D. T. S. The upside of APP at synapses. CNS Neuroscience & Therapeutics. 2012;18(1):47–56. doi: 10.1111/j.1755-5949.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milward E. A., Papadopoulos R., Fuller S. J., et al. The amyloid protein precursor of Alzheimer's disease is a mediator of the effects of nerve growth factor on neurite outgrowth. Neuron. 1992;9(1):129–137. doi: 10.1016/0896-6273(92)90228-6. [DOI] [PubMed] [Google Scholar]
- 38.Qiui W. Q., Ferreira A., Miller C., Koo E. H., Selkoe D. J. Cell-surface β-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. The Journal of Neuroscience. 1995;15(3):2157–2167. doi: 10.1523/JNEUROSCI.15-03-02157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caillé I., Allinquant B., Dupont E., et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 40.Priller C., Bauer T., Mitteregger G., Krebs B., Kretzschmar H. A., Herms J. Synapse formation and function is modulated by the amyloid precursor protein. The Journal of Neuroscience. 2006;26(27):7212–7221. doi: 10.1523/jneurosci.1450-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holsinger R. M. D., McLean C. A., Beyreuther K., Masters C. L., Evin G. Increased expression of the amyloid precursor β-secretase in Alzheimer's disease. Annals of Neurology. 2002;51(6):783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 42.Li Q., Chen M., Liu H., Yang L., Yang G. Expression of APP, BACE1, AChE and ChAT in an AD model in rats and the effect of donepezil hydrochloride treatment. Molecular Medicine Reports. 2012;6(6):1450–1454. doi: 10.3892/mmr.2012.1102. [DOI] [PubMed] [Google Scholar]
- 43.Vassar R., Kovacs D. M., Yan R., Wong P. C. The β-secretase enzyme BACE in health and Alzheimer's disease: Regulation, cell biology, function, and therapeutic potential. The Journal of Neuroscience. 2009;29(41):12787–12794. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kakuda N., Shoji M., Arai H., et al. Altered γ-secretase activity in mild cognitive impairment and Alzheimer's disease. EMBO Molecular Medicine. 2012;4(4):344–352. doi: 10.1002/emmm.201200214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J., Wang X., Li X., et al. Targeting the γ-/β-secretase interaction reduces β-amyloid generation and ameliorates Alzheimer’s disease-related pathogenesis. Cell Discovery. 2015;1:p. 15021. doi: 10.1038/celldisc.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strömberg K., Eketjäll S., Georgievska B., et al. Combining an amyloid-beta (Aβ) cleaving enzyme inhibitor with a γ-secretase modulator results in an additive reduction of Aβ production. FEBS Journal. 2015;282(1):65–73. doi: 10.1111/febs.13103. [DOI] [PubMed] [Google Scholar]
- 48.Imbimbo B. P., Giardina G. A. M. γ-secretase inhibitors and modulators for the treatment of Alzheimer's disease: disappointments and hopes. Current Topics in Medicinal Chemistry. 2011;11(12):1555–1570. doi: 10.2174/156802611795860942. [DOI] [PubMed] [Google Scholar]
- 49.Yan R., Vassar R. Targeting the β secretase BACE1 for Alzheimer's disease therapy. The Lancet Neurology. 2014;13(3):319–329. doi: 10.1016/s1474-4422(13)70276-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szaruga M., Veugelen S., Benurwar M., et al. Qualitative changes in human γ-secretase underlie familial Alzheimer's disease. The Journal of Experimental Medicine. 2015;212(12):2003–2013. doi: 10.1084/jem.20150892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Imbimbo B. P., Hutter-Paier B., Villetti G., et al. CHF5074, a novel γ-secretase modulator, attenuates brain β-amyloid pathology and learning deficit in a mouse model of Alzheimer's disease. British Journal of Pharmacology. 2009;156(6):982–993. doi: 10.1111/j.1476-5381.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X., Li H., Mao Y., et al. An Over Expression APP Model for Anti-Alzheimer Disease Drug Screening Created by Zinc Finger Nuclease Technology. PLoS ONE. 2013;8(11):p. e75493. doi: 10.1371/journal.pone.0075493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaguri H., Nilsson P., Hashimoto S., et al. APP mouse models for Alzheimer's disease preclinical studies. EMBO Journal. 2017;36(17):2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arai H., Lee V. M. ‐., Messinger M. L., Greenberg B. D., Lowery D. E., Trojanowski J. Q. Expression patterns of β‐amyloid precursor protein (β‐APP) in neural and nonneural human tissues from alzheimer's disease and control subjects. Annals of Neurology. 1991;30(5):686–693. doi: 10.1002/ana.410300509. [DOI] [PubMed] [Google Scholar]
- 55.Sommer G., Kralisch S., Lipfert J., et al. Amyloid precursor protein expression is induced by tumor necrosis factor α in 3T3-L1 adipocytes. Journal of Cellular Biochemistry. 2009;108(6):1418–1422. doi: 10.1002/jcb.22382. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y.-W. A., Zhou B., Wernig M., Südhof T. C. ApoE2, ApoE3, and ApoE4 Differentially Stimulate APP Transcription and Aβ Secretion. Cell. 2017;168(3):427–441.e21. doi: 10.1016/j.cell.2016.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kounnas M. Z., Danks A. M., Cheng S., et al. Modulation of γ-Secretase Reduces β-Amyloid Deposition in a Transgenic Mouse Model of Alzheimer's Disease. Neuron. 2010;67(5):769–780. doi: 10.1016/j.neuron.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mori T., Koyama N., Segawa T., et al. Methylene blue modulates β-secretase, reverses cerebral amyloidosis, and improves cognition in transgenic mice. The Journal of Biological Chemistry. 2014;289(44):30303–30317. doi: 10.1074/jbc.m114.568212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Z.-H., Chen C., Wu Q.-Y., et al. Selenomethionine reduces the deposition of beta-amyloid plaques by modulating β-secretase and enhancing selenoenzymatic activity in a mouse model of Alzheimer's disease. Metallomics. 2016;8(8):782–789. doi: 10.1039/c6mt00117c. doi: 10.1039/c6mt00117c. [DOI] [PubMed] [Google Scholar]
- 60.Asuni A. A., Guridi M., Pankiewicz J. E., Sanchez S., Sadowski M. J. Modulation of amyloid precursor protein expression reduces β-amyloid deposition in a mouse model. Annals of Neurology. 2014;75(5):684–699. doi: 10.1002/ana.24149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L., Shen C., Chu J., Zhang R., Li Y., Li L. Icariin decreases the expression of APP and BACE-1 and reduces the β-amyloid burden in an APP transgenic mouse model of Alzheimer's disease. International Journal of Biological Sciences. 2014;10(2):181–191. doi: 10.7150/ijbs.6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura A., Hata S., Suzuki T. Alternative selection of site APP-cleaving enzyme 1 (BACE1) cleavage sites in amyloid β-protein precursor (APP) harboring protective and pathogenic mutations within the Aβ sequence. The Journal of Biological Chemistry. 2016;291(46):24041–24053. doi: 10.1074/jbc.M116.744722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X., Zhou X., Li G., Zhang Y., Wu Y., Song W. Modifications and trafficking of APP in the pathogenesis of alzheimer’s disease. Frontiers in Molecular Neuroscience. 2017;10 doi: 10.3389/fnmol.2017.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chu K., Lee S.-T., Sinn D.-I., et al. Pharmacological induction of ischemic tolerance by glutamate transporter-1 (EAAT2) upregulation. Stroke. 2007;38(1):177–182. doi: 10.1161/01.STR.0000252091.36912.65. [DOI] [PubMed] [Google Scholar]
- 65.Wei J., Pan X., Pei Z., et al. The beta-lactam antibiotic, ceftriaxone, provides neuroprotective potential via anti-excitotoxicity and anti-inflammation response in a rat model of traumatic brain injury. Journal of Trauma and Acute Care Surgery. 2012;73(3):654–660. doi: 10.1097/TA.0b013e31825133c0. [DOI] [PubMed] [Google Scholar]
- 66.Browne T. C., McQuillan K., McManus R. M., O'Reilly J.-A., Mills K. H. G., Lynch M. A. IFN-γ production by amyloid β-specific Th1 cells promotes microglial activation and increases plaque burden in a mouse model of Alzheimer's disease. The Journal of Immunology. 2013;190(5):2241–2251. doi: 10.4049/jimmunol.1200947. [DOI] [PubMed] [Google Scholar]
- 67.Cavanagh C., Tse Y. C., Nguyen H.-B., et al. Inhibiting tumor necrosis factor-α before amyloidosis prevents synaptic deficits in an Alzheimer's disease model. Neurobiology of Aging. 2016;47:41–49. doi: 10.1016/j.neurobiolaging.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 68.Verbeeck C., Carrano A., Chakrabarty P., Jankowsky J. L., Das P. Combination of Aβ Suppression and Innate Immune Activation in the Brain Significantly Attenuates Amyloid Plaque Deposition. The American Journal of Pathology. 2017;187(12):2886–2894. doi: 10.1016/j.ajpath.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y., Chen W., Lin Y., Lu P., Hsieh S., Cheng I. H. Amelioration of amyloid-β-induced deficits by DcR3 in an Alzheimer’s disease model. Molecular Neurodegeneration. 2017;12(1) doi: 10.1186/s13024-017-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang J., Kou J., Lalonde R., Fukuchi K.-I. Intracranial IL-17A overexpression decreases cerebral amyloid angiopathy by upregulation of ABCA1 in an animal model of Alzheimer's disease. Brain, Behavior, and Immunity. 2017;65:262–273. doi: 10.1016/j.bbi.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russo I., Caracciolo L., Tweedie D., et al. Erratum: 3,6'-Dithiothalidomide, a new TNF-α synthesis inhibitor, attenuates the effect of Aβ 1-42 intracerebroventricular injection on hippocampal neurogenesis and memory deficit (Journal of Neurochemistry (2012) 122 (1181-1192)) Journal of Neurochemistry. 2012;123(4):p. 645. doi: 10.1111/jnc.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Detrait E. R., Danis B., Lamberty Y., Foerch P. Peripheral administration of an anti-TNF-α receptor fusion protein counteracts the amyloid induced elevation of hippocampal TNF-α levels and memory deficits in mice. Neurochemistry International. 2014;72(1):10–13. doi: 10.1016/j.neuint.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Tweedie D., Ferguson R. A., Fishman K., et al. Tumor necrosis factor-α synthesis inhibitor 3,6'-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer's disease. Journal of Neuroinflammation. 2012;9 doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers J. T., Leiter L. M., McPhee J., et al. Translation of the Alzheimer amyloid precursor protein mRNA is up- regulated by interleukin-1 through 5'-untranslated region sequences. The Journal of Biological Chemistry. 1999;274(10):6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 75.Liaoi Y.-F., Wang B.-J., Cheng H.-T., Kuo L.-H., Wolfe M. S. Tumor necrosis factor-α, interleukin-1β, and interferon-γ stimulate γ-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. The Journal of Biological Chemistry. 2004;279(47):49523–49532. doi: 10.1074/jbc.m402034200. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto M., Kiyota T., Horiba M., et al. Interferon-γ and tumor necrosis factor-α regulate amyloid-β plaque deposition and β-secretase expression in Swedish mutant APP transgenic mice. The American Journal of Pathology. 2007;170(2):680–692. doi: 10.2353/ajpath.2007.060378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hardy J., Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 78.Goldgaber D., Harris H. W., Hla T., et al. Interleukin 1 regulates synthesis of amyloid β-protein precursor mRNA in human endothelial cells. Proceedings of the National Acadamy of Sciences of the United States of America. 1989;86(19):7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slack B. E., Ma L. K., Seah C. C. Constitutive shedding of the amyloid precursor protein ectodomain is up-regulated by tumour necrosis factor-α converting enzyme. Biochemical Journal. 2001;357(3):787–794. doi: 10.1042/0264-6021:3570787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J. W., Lee Y. K., Yuk D. Y., et al. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. Journal of Neuroinflammation. 2008;5, article no. 37 doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He P., Zhong Z., Lindholm K., et al. Deletion of tumor necrosis factor death receptor inhibits amyloid β generation and prevents learning and memory deficits in Alzheimer's mice. The Journal of Cell Biology. 2007;178(5):829–841. doi: 10.1083/jcb.200705042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paouri E., Tzara O., Zenelak S., Georgopoulos S. Genetic deletion of tumor necrosis factor-α attenuates amyloid-β production and decreases amyloid plaque formation and glial response in the 5xfad model of Alzheimer's disease. Journal of Alzheimer's Disease. 2017;60(1):165–181. doi: 10.3233/JAD-170065. [DOI] [PubMed] [Google Scholar]
- 83.Buxbaum J. D., Liu K.-N., Luo Y., et al. Evidence that tumor necrosis factor α converting enzyme is involved in regulated α-secretase cleavage of the Alzheimer amyloid protein precursor. The Journal of Biological Chemistry. 1998;273(43):27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]