Abstract
Background and Objectives
Both enchondroma and atypical cartilaginous tumors (ACT) are not considered malignant, so inactive and asymptomatic tumors might not need surgery. To the best of our knowledge, this is the first study that has been done to evaluate the natural course of conservative‐treated enchondroma and ACT in the long bones.
Methods
For this retrospective study, we analyzed the results of patients in whom we refrained from surgery and only regularly performed radiological follow‐up of the tumor. Minimal follow‐up after initial diagnosis was 24 months.
Results
Forty‐nine patients were included in this study. Eight out of forty‐nine cases received surgical treatment during follow‐up of the tumor. The reasons for this surgery were radiologic growth of the tumor in two cases, pain in one case, patient request in three cases, another indication for surgery in the same limb in two cases.
Conclusion
In this small series of conservatively treated enchondroma and ACT, only 6% of the patients had a medical indication for surgery. This study shows that indication for surgery should be discussed more thoroughly. Based on our results, we would recommend annual radiologic follow‐up for asymptomatic enchondroma or ACT in the long bones, irrespective of tumor size. J. Surg. Oncol. 2016;114:987–991. © 2016 The Authors. Journal of Bone and Mineral Research Published by Wiley Periodicals, Inc.
Keywords: chondroma, chondrosarcoma, watchful waiting, therapy
INTRODUCTION
Enchondroma and chondrosarcoma are common bone tumors that typically occur central in the medullary cavity in any bone originating from enchondral ossification. They are characterized by tumor cells producing cartilaginous matrix.
In the current 2013 World Health Organization (WHO) classification system, grade 1 chondrosarcoma has been renamed as “atypical cartilaginous tumors” (ACT) better describing its clinical behavior 1. ACT rarely metastasizes and therefore it is now classified as an intermediate type of tumor, not a malignancy 2, 3.
Due to more frequent imaging, for example, radiographs and MRI scans, accidental findings of enchondroma and ACT have become more common 4, 5. This often leads to referral of the patient to an orthopedic oncologic center for further diagnostics and treatment advice.
To distinguish enchondroma from ACT on conventional radiographs and MRI is often difficult, especially when the lesion is located in the long tubular bones 6, 7, 8, 9, 10, 11, 12. This may result in significant overtreatment of benign lesions in case of enchondroma being diagnosed as ACT. Or it results in under treatment in case of ACT being diagnosed as enchondroma and erroneously discharged from follow‐up. Histological differentiation between enchondroma and ACT depends on subtle criteria and malignant features could easily be missed by a biopsy due to the heterogeneity of cartilaginous tumors 9, 13.
Terms as borderline chondrosarcoma and chondrosarcoma grade 1/2 have been used for lesions with some radiographic malignant characteristics, but with insufficient histological signs to confirm the diagnosis chondrosarcoma 14. Dahlin used these terms to alert surgeons not to over treat this group of patients. Nowadays these terms have generally been abandoned.
The current surgical treatment for small, central enchondroma and ACT in long bones that are confined to the bone is intralesional curettage with local adjuvant therapy. Reported complications of curettage and local adjuvant treatment in enchondroma and ACT are postoperative fractures, infection, and local recurrence 15, 16. Enchondromas are benign lesions that do not need surgical treatment if inactive and symptomless 10, 17. It is estimated that approximately 4% of solitary enchondromas change into secondary chondrosarcoma, indicating that follow‐up is needed 18.
Many authors have proposed radiographic follow‐up instead of surgery for cartilaginous tumors in the long bones without signs of local aggressiveness 6, 7, 19, 20. Radiographic follow‐up instead of surgery may prevent over treatment of this group of patients, resulting in less morbidity and lesser costs.
However, to the best of our knowledge no study has yet been performed showing the results of radiological follow‐up.
The aim of our study was to evaluate the natural course of enchondroma and ACT through active surveillance. This study is approved by the medical ethical committee. To the best of our knowledge, this is the first study that has been done to evaluate the natural course of conservatively treated enchondroma and ACT in the long bones.
PATIENTS AND METHODS
To evaluate the natural course of enchondroma and ACT of the long bones, we analyzed the results of conservatively treated patients in which we refrained from surgery and only did regular follow‐up of the lesion.
In this study, we included conservatively treated patients with enchondroma or ACT, who were under radiologic follow‐up in Radboudumc between 2008 and 2013. Conservative‐treated patients, with enchondroma or ACT, were retrospectively selected by using a record of all patients seen in our hospital.
Inclusion criteria were conservatively treated patients with enchondroma or ACT, at least 18 years old, with lesions in the long bones of the extremities and a follow‐up time of minimal 24 months since initial diagnosis. Patients with Ollier disease, Maffucci syndrome, or high grade chondrosarcoma were excluded. Forty‐nine cases met the inclusion criteria and were included in our study.
All lesions were evaluated at diagnosis using physical examination, plain radiographs, and MR‐imaging. Whenever cases were referred to our hospital, all imaging was reviewed by our experienced musculoskeletal radiologist. Standardized techniques were used for plain radiographs in all cases.
In a few early cases, a trocar biopsy was performed, either in the referral hospital or in our hospital, before a treatment advice was given. Nowadays biopsies are no longer performed in our hospital for this cause since histological diagnosis is not reliable.
Due to the similarity of imaging characteristics of enchondroma and ACT on conventional radiographs and MRI, no difference could be made and all cases diagnosed based on only imaging methods were diagnosed as enchondroma/ACT.
Malignant radiologic characteristics used to indicate chondrosarcoma grade 2 or grade 3 were cortical destruction, presence of soft tissue mass, moth‐eaten or permeative osteolysis, pluri‐lamellar or speculated periosteal reaction 10, 19, 21. Based on these malignant characteristics, differentiation between high grade chondrosarcoma and enchondroma/ACT was made (see Figs. 1 and 2).
Figure 1.
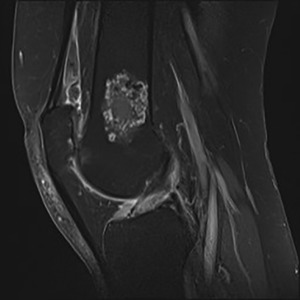
SE T1‐weighted contrast‐enhanced sagittal image with fat saturation of a 65‐year‐old woman shows a 4 cm intramedullary lobulated tumor with septal and rim enhancement abutting the posterior cortex. No surrounding edema or periosteal reaction. Histologically proven ACT.
Figure 2.
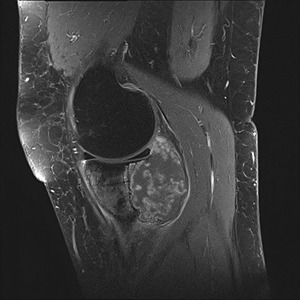
SE T1‐weighted contrast‐enhanced sagittal image with fat saturation of a 40‐year‐old woman shows predominantly irregular rim enhancement. There is vast cortical destruction with extensive soft tissue involvement as well as intra‐articular extension. Histologically proven chondrosarcoma grade 2.
When no invalidating pain and no radiographic signs of malignancy were seen, active surveillance was advised to the patient. Patients who approved through verbal consent, with active surveillance, were followed‐up with a MRI scan after 6 months. If the MRI scan showed no growth of the tumor or other radiological changes, radiologic follow‐up was continued every 1–2 years. Increased calcification was excluded as growth of the lesion. Radiologic follow‐up consisted of conventional radiographs or MRI depending on interpretability of the tumor on conventional radiographs.
All patients who were managed by active surveillance were instructed to contact our hospital in case of new or increased pain. In case of new or increased pain complaints, physical examination and radiologic assessment were performed to rule out other sources of pain.
Whenever invalidating pain or radiographic changes occurred or the patients revised their choice for conservative therapy, patients were treated with surgery. The following operating technique was used in all cases treated with surgery. An oval‐shaped cortical window was made with a high speed burr, after which the lesions were thoroughly curettaged followed by three cycles of cryosurgery with rapid freezing of at least −50°C and slow thawing. After cryosurgery, the bony defects were filled up with either bone graft or bone cement. In diaphyseal lesions, prophylactic plating was performed to prevent postoperative fractures. All operated cases stayed under follow‐up after surgery.
RESULTS
A total of 49 cases (27 female and 22 male patients) met the inclusion criteria for this study. Mean age at diagnosis was 49 years (range: 20–76 years). See Table I for patient demographics.
Table I.
Patient Demographics
| Variable | No (%) |
|---|---|
| Mean age (range, years) | 49 (20–76) |
| Mean follow‐up (range, months) | 67 (25–213) |
| Gender | |
| Male | 22 (45) |
| Female | 27 (55) |
| Location | |
| Distal femur | 33 (67) |
| Proximal femur | 3 (6) |
| Proximal humerus | 8 (16) |
| Proximal tibia | 3 (6) |
| Distal tibia | 1 (2) |
| Proximal fibula | 1 (2) |
| Size | |
| <2 cm | 7 (14) |
| 2–5 cm | 23 (47) |
| 5–10 cm | 17 (35) |
| 10–15 cm | 1 (2) |
| 15–20 cm | 1 (2) |
Thirty‐three of the forty‐nine cases (67%) were first diagnosed in another hospital and were referred to our hospital for further analysis.
In 5 of the 49 cases (10%), a trocar biopsy was performed to confirm the diagnosis. After biopsy, four of these five cases were diagnosed histopathologically as enchondroma and one case was diagnosed as ACT. In 44 out of 49 cases (90%), no biopsy was performed and the diagnosis was based on clinical examination and radiographic appearance, conventional radiographs, and MRI. All those 44 cases were diagnosed as enchondroma/ACT due to the similarity of those tumors on imaging methods.
Mean follow‐up time since initial diagnosis was 66 months (range: 25–213 months).
All patients are current on their surveillance imaging, no patients were lost to follow‐up.
Clinical Presentation
Forty‐three of the cases (88%) were incidental findings and two (4%) presented themselves with pain complaints. Of the remaining four cases (8%), the referral indication was unknown, all these cases were diagnosed more than 10 years ago.
Radiological Follow‐Up
In 8 of the 49 cases (16%), there was a radiologic change of the lesion noticed during follow‐up. All eight cases presented with growth of the lesion, none presented scalloping or cortical breakthrough. Mean time between initial diagnosis and change of the lesion was 41 months (range: 20–76 months). The only case histopathologically diagnosed as ACT showed no radiologic changes 2 years after biopsy.
Both cases that presented with pain complaints showed no radiologic changes.
Secondary Surgery
Eight of the forty‐nine cases (16%) underwent surgical treatment during follow‐up (Table II). Mean time between initial diagnosis and surgical treatment was 37 months (range: 21–57 months). The reasons for surgery were radiological change of the lesion in two cases, invalidating pain in one case, patient request in three cases, and total knee arthroplasty (TKA) due to osteoarthritis combined with curettage of the lesion in two cases.
Table II.
Cases Operated During Follow‐Up
| Referral indication | Location | Initial size in cm | Reason for surgery | Time in months from diagnosis to surgery | Prophylactic plating used | Radiologic diagnosis before surgery | Pathologic diagnosis after surgery |
|---|---|---|---|---|---|---|---|
| Incidental | Distal tibia | 3.2 | Radiologic growth (0.6 cm) | 53 | No | Ech/ACT | ACT |
| Incidental | Distal femur | 4.6 | Radiologic growth (1.0 cm) | 44 | Yes | Ech/ACT | ACT |
| Incidental | Distal femur | 20.0 | Pain | 10 | Yes | Ech/ACT | Ech |
| Incidental | Proximal tibia | 4.7 | Choice patient | 34 | Yes | ACT | Ech |
| Incidental | Proximal tibia | 6.0 | Choice patient | 21 | Yes | Ech/ACT | Ech |
| Incidental | Proximal tibia | 1.7 | Choice patient | 48 | No | Ech/ACT | Haemangioma |
| Incidental | Distal femur | 3.2 | Total knee arthroplasty | 57 | No | Ech/ACT | Ech |
| Incidental | Distal femur | 2.7 | Total knee arthroplasty | 30 | No | Ech/ACT | Ech |
Ech, enchondroma; ACT, atypical cartilaginous tumor.
All cases operated on have been under follow‐up after surgery. No recurrence of the tumor is seen in these cases. Mean time between surgery and last follow‐up was 12 months (range: 1–44 months).
Four out of eight cases (50%) that showed radiological change have been operated. Two cases were operated because of radiologic change during follow‐up and in two cases minimal growth (<5 mm) was detected and surgery was requested by the patient.
Four out of eight cases (50%) showed radiological change but were not operated on. All four cases showed minimal growth, ranging from 3 to 8 mm.
In 37 of the 49 cases (73%), there was no change observed during follow‐up, on conventional radiographs or MRI, or any other reason to perform surgery.
DISCUSSION
The purpose of this study was to evaluate the natural courses of enchondroma and ACT in the long bones by active surveillance. To the best of our knowledge, this is the first study that evaluates the natural course of enchondroma and ACT.
Performing radiographic monitoring for both enchondroma and ACT is in accordance with Crim et al. 6 who recommend serial follow‐up rather than curettage for non‐painful cartilaginous lesions of any size due to problematic imaging criteria for both enchondroma and ACT. Campanacci et al. 20 recommend that small (<5 cm), asymptomatic, intraosseous cartilaginous lesions of long bones without radiological signs of local aggressiveness should be observed as benign enchondroma, and no surgery or further investigations other than serial follow‐up is indicated.
Surgical treatment of ACT of the long bones has been a subject of debate for the last decades. In our hospital, curettage with cryosurgery has been the treatment of choice for ACT 16. Curettage and local adjuvant treatment is also considered as adequate treatment according to the 2013 WHO standard 1. Postoperative fractures, infection, and local recurrence have been reported to be complications after curettage and local adjuvant treatment. The low rate of transformation to higher grade of malignancy and rare metastases of enchondroma and ACT implicates that these lesions might not need surgery.
A malignant transformation rate of 4% for enchondroma was reported by Altay et al. 18. In their study, 6 out of 143 cases of enchondroma underwent malignant transformation, 5 had changed into ACT, and only 1 case transformed into grade 2 chondrosarcoma. Schwab et al. 22 reported a malignant transformation rate of ACT in the long bones in only 4 of 164 patients. Andreou et al. 23 reported a malignant transformation of ACT of 0% in the upper extremity and of 6% in the lower extremity. Only cases that transformed to grade 2 chondrosarcoma developed metastases. This low malignant transformation rate is in accordance with our study where none of the included lesions transformed into high grade chondrosarcoma.
In the present study, we found that only 3 of the 49 cases (6%) had a medically grounded indication for removal of the lesion, being invalidating pain or radiographic changes as described in Table II.
The longer follow‐up time before surgery had no consequences for the surgical procedure or the rehabilitation process. All cases operated on were treated with curettage and cryosurgery. None of the patients included in this study suffered from local complications, for example, pathologic fractures during follow‐up.
Selection of patients for conservative therapy is important. We were careful to exclude all tumors that were not clearly enchondroma or ACT, that is, showed radiological signs of high grade chondrosarcoma. Other studies showed that distinction between low and high‐grade chondral lesions can be safely determined based on MR‐imaging, without the need for pre‐operative biopsy 24, 25. We also excluded lesions of the axial skeleton because of the worse prognosis compared to lesions in the long bones 2, 23, 26, 27.
With only 6% of the studied cases needing surgery, this study shows that indication for surgery should be discussed more thoroughly. As the reported complications after curettage are considerable, surgery should be confined to tumors with substantial risk of malignant transformation or metastasis.
However, in case of conservative therapy, there is, to our knowledge, no evidence in the literature for follow‐up frequency and duration of follow‐up. In our study, mean time between initial diagnosis and radiologic change of the lesion was 41 months with a wide range of 20–76 months and the mean time between initial diagnosis and surgery was 37 months (range: 10–57 months).
The study of Herget et al. 28 showed that the time between the initial diagnosis of enchondroma and the diagnosis of malignancy varied between 6 months and up to 30 years. This indicates that enchondroma and ACT lesions might profit from a lifelong radiological follow‐up. Brien et al. 5 recommend a follow‐up of at least two decades for solitary enchondroma of the long bones if detected after age 25.
The frequency of skeletal imaging must be outweighed against the risk of cumulative radiation exposure.
Herget et al. 28 recommend annual clinical and annual/biennial MRI examination for the follow‐up of asymptomatic enchondroma localized in the long bones, >5 to 6 cm. Annual clinical and bi‐/triennial radiological examination (plain radiographs, in any doubt MRI) follow‐up is recommended for asymptomatic enchondroma lesions <5 to 6 cm. Parlier‐Cuau et al. 19 recommend radiologic follow‐up once a year for inactive lesions. Based on our results, we would recommend conservative treatment for asymptomatic enchondroma or ACT in the long bones, irrespective of tumor size. Geinaerdt reports that only in the axial skeleton, tumors larger than 4–6 cm are generally malignant 10. Radiologic follow‐up is necessary, based on our experience we recommend annual MR‐imaging. MR‐imaging is recommended because it is better in correct tumor measurement compared to radiographs 10, 21. Since growth of the tumor is one of the criteria to decide for operation, the correct measurement of tumor size is of high importance. When no changes occur during follow‐up of at least 2 years, frequency of MR‐imaging can be reduced to every 2–3 years. More research should be done to make an international protocol for optimal radiological follow‐up of enchondroma an ACT.
The results of this study should be interpreted with some caution as this study had some limitations. Due to the rarity of these tumors, the size of the group studied was small and follow‐up in this study was relatively short. Considering the slow biological progression of echondroma and ACT, it is not possible to make definite conclusions about the oncological outcome.
In only 5 of the 49 cases, diagnosis was confirmed by biopsy, in the other cases diagnosis was made based on radiographic appearance, conventional radiographs, and MRI. In these 44 cases, no difference could be made between ACT or enchondroma. This means that the exact number of enchondroma and ACT cases included in this study is therefore not known.
CONCLUSION
In this small series of conservatively treated enchondroma and ACT, only 6% of the studied cases had a medically grounded indication for surgery. None of the surgically treated lesions was transformed into a high grade chondrosarcoma. This study shows that indication for surgery should be discussed more thoroughly. Based on our results, we would recommend annual radiologic follow‐up for asymptomatic enchondroma or ACT in the long bones, irrespective of tumor size.
Disclosures and funding sources: No financial support was used for this study.
REFERENCES
- 1. Fletcher CDM, Bridge JA, Hogendoorn PCW, et al.: World Health Organization classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. [Google Scholar]
- 2. Bindiganavile S, Han I, Yun JY, et al.: Long‐term outcome of chondrosarcoma: A single institutional experience. Cancer Res Treat 2015; 47:897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angelini A, Guerra G, Mavrogenis AF, et al.: Clinical outcome of central conventional chondrosarcoma. J Surg Oncol 2012; 106:929–937. [DOI] [PubMed] [Google Scholar]
- 4. Stomp W, Reijnierse M, Kloppenburg M, et al.: Prevalence of cartilaginous tumours as an incidental finding on MRI of the knee. Eur Radiol 2015; 25:3480–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brien EW, Mirra JM, Kerr R: Benign and malignant cartilage tumors of bone and joint: Their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I. The intramedullary cartilage tumors. Skeletal Radiol 1997; 26:325–353. [DOI] [PubMed] [Google Scholar]
- 6. Crim J, Schmidt R, Layfield L, et al.: Can imaging criteria distinguish enchondroma from grade 1 chondrosarcoma? Eur J Radiol 2015; 84:2222–2230. [DOI] [PubMed] [Google Scholar]
- 7. Ferrer‐Santacreu EM, Ortiz‐Cruz EJ, Gonzalez‐Lopez JM, et al.: Enchondroma versus low‐grade chondrosarcoma in appendicular skeleton: Clinical and radiological criteria. J Oncol 2012; 2012:437958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eefting D, Schrage YM, Geirnaerdt MJ, et al.: Assessment of interobserver variability and histologic parameters to improve reliability in classification and grading of central cartilaginous tumors. Am J Surg Pathol 2009; 33:50–57. [DOI] [PubMed] [Google Scholar]
- 9.Sliced Study Group: Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am 2007; 89:2113–2123. [DOI] [PubMed] [Google Scholar]
- 10. Geirnaerdt MJ, Hermans J, Bloem JL, et al.: Usefulness of radiography in differentiating enchondroma from central grade 1 chondrosarcoma. AJR Am J Roentgenol 1997; 169:1097–1104. [DOI] [PubMed] [Google Scholar]
- 11. Scheitza P, Uhl M, Hauschild O, et al.: Interobserver variability in the differential diagnosis of benign bone tumors and tumor‐like lesions. RoFo: Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin 2016; 188:479–487. [DOI] [PubMed] [Google Scholar]
- 12. Flemming DJ, Murphey MD: Enchondroma and chondrosarcoma. Semin Musculoskelet Radiol 2000; 4:59–71. [DOI] [PubMed] [Google Scholar]
- 13. Welkerling H, Kratz S, Ewerbeck V, et al.: A reproducible and simple grading system for classical chondrosarcomas. Analysis of 35 chondrosarcomas and 16 enchondromas with emphasis on recurrence rate and radiological and clinical data. Virchows Arch 2003; 443:725–733. [DOI] [PubMed] [Google Scholar]
- 14. Unni KK, Inwards CY, Research MFME: Dahlin's bone tumors: General aspects and data on 10,165 cases. Philadelphia: Wollters Kluwer Health/Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 15. Meftah M, Schult P, Henshaw RM: Long‐term results of intralesional curettage and cryosurgery for treatment of low‐grade chondrosarcoma. J Bone Joint Surg Am 2013; 95:1358–1364. [DOI] [PubMed] [Google Scholar]
- 16. van der Geest IC, de Valk MH, de Rooy JW, et al.: Oncological and functional results of cryosurgical therapy of enchondromas and chondrosarcomas grade 1. J Surg Oncol 2008; 98:421–426. [DOI] [PubMed] [Google Scholar]
- 17. Muller PE, Durr HR, Wegener B, et al.: Solitary enchondromas: Is radiographic follow‐up sufficient in patients with asymptomatic lesions? Acta Orthop Belg 2003; 69:112–118. [PubMed] [Google Scholar]
- 18. Altay M, Bayrakci K, Yildiz Y, et al.: Secondary chondrosarcoma in cartilage bone tumors: Report of 32 patients. J Orthop Sci 2007; 12:415–423. [DOI] [PubMed] [Google Scholar]
- 19. Parlier‐Cuau C, Bousson V, Ogilvie CM, et al.: When should we biopsy a solitary central cartilaginous tumor of long bones? Literature review and management proposal. Eur J Radiol 2011; 77:6–12. [DOI] [PubMed] [Google Scholar]
- 20. Campanacci DA, Scoccianti G, Franchi A, et al.: Surgical treatment of central grade 1 chondrosarcoma of the appendicular skeleton. J Orthop Traumatol 2013; 14:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Murphey MD, Flemming DJ, Boyea SR, et al.: Enchondroma versus chondrosarcoma in the appendicular skeleton: Differentiating features. Radiographics 1998; 18:1213–1237; quiz 1244–1215. [DOI] [PubMed] [Google Scholar]
- 22. Schwab JH, Wenger D, Unni K, et al.: Does local recurrence impact survival in low‐grade chondrosarcoma of the long bones? Clin Orthop Relat Res 2007; 462:175–180. [DOI] [PubMed] [Google Scholar]
- 23. Andreou D, Gilg MM, Gosheger G, et al.: Metastatic potential of grade I chondrosarcoma of bone: Results of a multi‐institutional study. Ann Surg Oncol 2016; 23:120–125. [DOI] [PubMed] [Google Scholar]
- 24. Berber O, Datta G, Sabharwal S, et al.: The safety of direct primary excision of low‐grade chondral lesions based on radiological diagnosis alone. Acta Orthop Belg 2012; 78:254–262. [PubMed] [Google Scholar]
- 25. Brown MT, Gikas PD, Bhamra JS, et al.: How safe is curettage of low‐grade cartilaginous neoplasms diagnosed by imaging with or without pre‐operative needle biopsy? Bone Joint J 2014; 96‐b:1098–1105. [DOI] [PubMed] [Google Scholar]
- 26. Streitburger A, Ahrens H, Balke M, et al.: Grade I chondrosarcoma of bone: The Munster experience. J Cancer Res Clin Oncol 2009; 135:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Funovics PT, Panotopoulos J, Sabeti‐Aschraf M, et al.: Low‐grade chondrosarcoma of bone: Experiences from the Vienna Bone and Soft Tissue Tumour Registry. Int Orthop 2011; 35:1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herget GW, Strohm P, Rottenburger C, et al.: Insights into enchondroma, enchondromatosis and the risk of secondary chondrosarcoma. Review of the literature with an emphasis on the clinical behaviour, radiology, malignant transformation and the follow up. Neoplasma 2014; 61:365–378. [DOI] [PubMed] [Google Scholar]


