Abstract
Intestinal hypoganglionosis or isolated hypoganglionosis is a rare entity with a clinical and radiologic presentation that can mimic Hirschsprung's disease in the neonatal period. The diagnosis of this entity can be challenging with suction rectal biopsies that are standard for diagnosing Hirschsprung's disease. We present this case of congenital intestinal hypoganglionosis detailing the neonatal course, due to its rarity and the conundrums faced before an eventual diagnosis could be rendered. This case also illustrates the role of full thickness rectal biopsy in selected cases such as ours where the radiologic features are typical of Hirschsprung's, despite negative suction biopsies.
Keywords: Intestinal hypoganglionosis, Hirschsprung's disease, Enteric neuropathy
Introduction
Intestinal hypoganglionosis encompasses enteric neuropathies with a decrease in ganglion cells which can be congenital or acquired. Congenital hypoganglionosis including its milder forms can account for up to 15% of non-Hirschsprung's related intestinal neuronal malformations [1]. Clinical features resemble Hirschsprung's disease. Both are a part of the Dysganglionoses or intestinal neuronal dysplasia [2]. A definite etiology for hypoganglionosis has not yet been elucidated. Intestinal hypoganglionosis is a variant of Hirschsprung's disease, given the clinical resemblance to Hirschsprung's disease despite the presence of ganglion cells in rectal suction biopsies [3].
Case report
A 4-day-old term infant, average for gestational age, presented with bilious emesis since birth. Patient had multiple stools since birth. Abdominal distention worsened on day of life 4 and patient was brought to radiology for an upper GI and contrast enema. Abdominal radiograph (Fig. 1) revealed diffuse distention of small and large bowel. Contrast enema with water soluble iodinated contrast demonstrated a transition zone in the distal descending colon with narrowed sigmoid and distal descending colon, “saw tooth” appearance of sigmoid colon and rounding of the flexures (Fig. 2a-c). Given the concern for Hirschsprung's disease on imaging, the patient was treated with rectal irrigations and a suction rectal biopsy was subsequently performed. The initial suction rectal biopsy demonstrated absence of submucosal ganglion cells and occasional hypertrophic submucosal nerves. However, calretinin staining was equivocal in that occasional small nerve fibers within the lamina propria were seen, which is not consistent with Hirschsprung's disease. Normal mucosa was noted on histopathology. Given the indeterminate results and concern for transition-zone bowel, repeat suction biopsy was performed more proximally which demonstrated the presence of submucosal ganglion cells, no neural hypertrophy, and normal calretinin staining, thereby excluding Hirschsprung's disease.
Fig. 1.

Diffuse bowel distention at day 4 of life associated with bilious vomiting. NG tube is in the stomach.
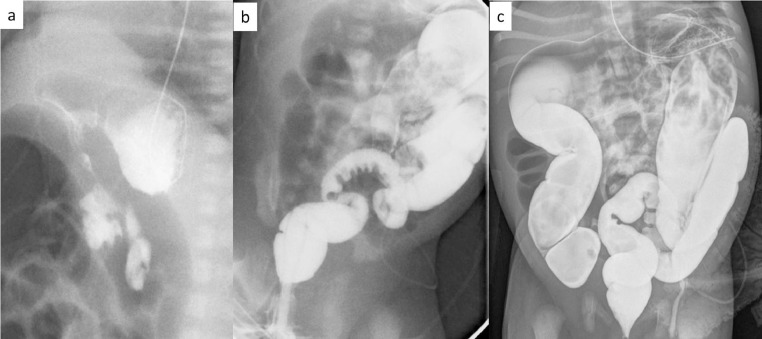
Fig. 2.
(a) Normal UGI exam with retroperitoneal duodenum, (b) narrowed rectum and sigmoid colon with a saw tooth sigmoid mucosa, (c) postevacuation film with narrow rectum, sigmoid and distal descending colon and more proximal dilated colon.
Meanwhile the patient stayed on total parenteral nutrition due to new concerns for pneumatosis intestinalis and necrotizing enterocolitis (Fig. 3). Daily rectal irrigations were started. A repeat contrast enema done at 4 weeks of life, (Fig. 4) after resolution of pneumatosis, demonstrated distal descending colon caliber change along with persistent dilatation of bowel proximally, which was again of concern for Hirschsprung's disease.
Fig. 3.

Necrotizing enterocolitis with pneumatosis intestinalis developed on day 12 of life.
Fig. 4.
Repeat contrast enema at 4 week of life demonstrates distal descending, sigmoid colonic and rectal narrowing and saw tooth mucosal pattern with dilatation of the ascending, transverse and proximal descending colon.
Hence decision was made to take the patient to surgery with the goal of identifying a stricture that correlated with the radiographic findings, or obtain full thickness tissue that could facilitate a definitive diagnosis. In the operating room no discrete narrowing or stricture was identified endoscopically and laparoscopically. A diverting colostomy was performed along with Hartmann's pouch and the sigmoid colon was removed for pathology as this correlated with the abnormal finding on the contrast study.
Histopathology revealed myenteric hypoganglionosis and/or aganglionosis (Fig. 5). Submucosal ganglion cells were seen. However myenteric plexus demonstrates variable areas of aganglionosis and hypoganglionosis with some areas of normal ganglion cells in up to 20% of the bowel wall. Subsequently biopsies of small bowel and rest of the colon were obtained. Given the histopathologic findings and nonfunctioning colostomy, he was taken back to the OR where an ileostomy was created and a gastrostomy tube was placed. Final pathology was consistent with myenteric hypoganglionosis in the ileum and splenic flexure, aganglionosis in the cecum and hepatic flexure and no neural hypertrophy. At the 6 month follow-up he was thriving on oral and occasional G tube feeds. Total parenteral nutrition had been stopped. The current surgical plan is an ileoanal pull through at 1-2 years of age if his ileostomy continues to function normally.
Fig. 5.
Histopathologic findings in resected sigmoid. (a) Upper left. Myenteric plexus with no ganglion cells and no abnormal nerves (H&E; 100x magnification). (b) Upper right, Calretinin stain showing normal nerve twigs in lamina propria inconsistent with anganglionosis (Calretinin; 400x magnification). (c) Lower left-arrow showing abnormal intermyenteric ganglion with single ganglion cell and little neutrophil (H&E; 100x magnification). (d) Lower right-arrow shows rare normal cluster of ganglion cells present (H&E; 200x magnification).
Discussion
Hypoganglionosis is postulated to be due to inborn hypoplasia of parasympathetic myenteric plexus [4]. Acquired etiology has been speculated with adult onset hypoganglionosis [5]. Hypoganglionosis has been reported to have variable forms such as isolated, localized, disseminated forms and in combination with either Hirschsprung's disease or intestinal neuronal dysplasia [5].
A systematic review in 2010 studied 92 patients over 11 publications with intestinal hypoganglionosis, with an overall male to female ratio of 3:1. 32% of cases were diagnosed in the newborn period [6].
Clinical and radiological presentation of intestinal hypoganglionosis mimics Hirschsprung's disease. However, suction rectal biopsies are insufficient because the distribution and size of submucosal ganglion cells are variable in intestinal congenital hypoganglionosis. As in our case, the presence or absence of submucosal ganglion cell is irrelevant [1]. Interestingly the systematic review also found that failure to pass meconium was not a clinical sign of intestinal hypoganglionosis, similar to our case [6].
In a study which compared the imaging features of Hirschsprung's disease with intestinal hypoganglionosis, the transition zone ratio (dilated colon to narrowed colon diameter ratio) was found to be higher in Hirschsprung's disease as compared to hypoganglionosis. The transition zone in the patients with hypoganglionosis corresponded to the segment with the least number of ganglion cells [5].
Diagnosis depends on multiple full thickness biopsies of the colon [3]. Absence of acetylcholinesterase in the mucosa, reduction of nerve cells in the myenteric and submucosal plexus and reduction in the number of ganglion cells are characteristic findings on histology [4].
Hypoganglionosis has been described throughout the small intestine, colon, or isolated to the left colon or rectosigmoid [4]. Treatment involves resection of the most affected segments of bowel with colostomy or ileostomy formation. Jejunostomy, bowel transplant, and parenteral nutrition have an important role in patients with more extensive disease [7]. Mortality rates from neonatal enterocolitis, total parenteral nutrition associated complications, electrolyte imbalance have been reported [6]. Postoperative complications include recurrent enterocolitis, chronic constipation and need for redo of pull-through procedures for residual diseased segments.
Intestinal hypoganglionosis portends greater morbidity than Hirschsprung's disease and can have more extensive involvement as in this case. The purpose of this case report is to highlight the radiologic similarity of this variant to Hirschsprung's disease, which is difficult to diagnose in the neonatal period with routine suction biopsies that can be false negative. Therefore, a full thickness biopsy may be necessary. Awareness of such an entity and communication between the surgeon, pathologist and radiologist is vital, so that more extensive biopsies or resection of the bowel proximal to the radiographic transition zone could be planned.
Footnotes
No conflicts of interest to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2018.10.007.
Appendix. Supplementary materials
References
- 1.Kapur R.P. Intestinal motor disorders. In: Russo P., Ruchelli E.D., Piccoli D.A., editors. Pathology of pediatric gastrointestinal and liver disease. 2nd ed. Springer-Verlag; Berlin Heidelberg: 2014. p. 270. [Google Scholar]
- 2.Schärli A.F. Standardization of terminology of intestinal innervation disorders. Pediatr Surg Int. 1995;10:440. doi: 10.1007/BF00176383. [DOI] [Google Scholar]
- 3.Friedmacher F., Puri P. Classification and diagnostic criteria of variants of Hirschsprung's disease. Pediatr Surg Int. 2013;29:855. doi: 10.1007/s00383-013-3351-3. [DOI] [PubMed] [Google Scholar]
- 4.Schärli A.F., Sossai R. Hypoganglionosis. Semin Pediatr Surg. 1998;7(3):187–191. doi: 10.1016/s1055-8586(98)70016-2. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.J., Kim A.Y., Lee C.W., Yu C.S., Kim J.S., Kim P.N. Hirschsprung disease and hypoganglionosis in adults: radiologic findings and differentiation. Radiology. 2008;247:2:428–434. doi: 10.1148/radiol.2472070182. [DOI] [PubMed] [Google Scholar]
- 6.Dingemann J., Puri P. Isolated hypoganglionosis: Systematic review of a rare intestinal innervation defect. Pediatr Surg Int. 2010;26:1111–1115. doi: 10.1007/s00383-010-2693-3. [DOI] [PubMed] [Google Scholar]
- 7.Khalaf R., Karjoo S., Danielson P., Wilsey M., Shakeel F. Intestinal hypoganglionosis leading to intestinal failure and the compassionate use of Omegaven™. Pediatr Gastroenterol Hepatol Nutr. 2017;20(1):55–60. doi: 10.5223/pghn.2017.20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.