Abstract
Heterotopic ossification (HO) occurs secondary to trauma, causing pain and functional limitations. Identification of the cells that contribute to HO is critical to the development of therapies. Given that innate immune cells and mesenchymal stem cells are known contributors to HO, we sought to define the contribution of these populations to HO and to identify what, if any, contribution circulating populations have to HO. A shared circulation was obtained using a parabiosis model, established between an enhanced green fluorescent protein–positive/luciferase+ donor and a same-strain nonreporter recipient mouse. The nonreporter mouse received Achilles tendon transection and dorsal burn injury to induce HO formation. Bioluminescence imaging and immunostaining were performed to define the circulatory contribution of immune and mesenchymal cell populations. Histologic analysis showed circulating cells present throughout each stage of the developing HO anlagen. Circulating cells were present at the injury site during the inflammatory phase and proliferative period, with diminished contribution in mature HO. Immunostaining demonstrated that most early circulatory cells were from the innate immune system; only a small population of mesenchymal cells were present in the HO. We demonstrate the time course of the participation of circulatory cells in trauma-induced HO and identify populations of circulating cells present in different stages of HO. These findings further elucidate the relative contribution of local and systemic cell populations to HO.
Heterotopic ossification (HO) is the pathologic formation of endochondral or intramembranous bone in soft tissue, which occurs after severe burn injury, musculoskeletal trauma, and spinal cord injury.1, 2, 3 Despite the various causes, these all share a common inflammatory stimulus. Recent studies have sought to elucidate the cells responsible for HO; however, the sources of these cells have not been fully explored. Foci of ectopic bone are consistently observed near connective tissue structures within myofascial planes, even when the site of inciting injury is spatially distinct from the HO lesion.4 The consistency of anatomic location would suggest a predominantly local source of contributing cells. Using a Prx-cre/ROSA26mTmG reporter mouse, our group has previously described mesenchymal lineage cells to be the primary contributor to HO formation; however, given the constraints of the models, we did not delineate if the paired related homeobox 1 (Prx) lineage cells were derived from local tissues or the circulation.2
Although many studies have defined these progenitor cells, they only undergo this transformation when exposed to an inflammatory stimulus and cross talk with inflammatory cells.2, 5, 6, 7, 8, 9 Current orthopedic treatment regimens to prevent HO attempt to target this inflammation with nonsteroidal anti-inflammatory drugs.10, 11, 12, 13, 14, 15 Recent studies have specifically identified the presence and role of macrophages, in particular, as playing a role in establishing the HO niche.16, 17, 18
Few studies have attempted to determine the timing of circulatory cell migration and whether circulating mesenchymal cells are also a source of progenitors for trauma-induced HO (tHO). Using a parabiosis mouse model, circulating osteogenic precursors, including mesenchymal cells, have previously been shown to contribute to fracture healing.19 In addition, previous and recent studies of HO in mouse models and in human HO have identified the presence of circulatory osteogenic cells.20, 21, 22, 23 Understanding the contributions of local and circulating osteogenic progenitor cells that contribute to HO would elucidate inflammatory and signaling mechanisms that can be targeted for therapies. In this study, we use a parabiosis model to better characterize the circulatory cells that participate in HO formation specifically, at which respective stages of HO development (fibroproliferative, chondrogenic, and osteogenic) they contribute. Herein, we hypothesize that circulating inflammatory cells play a role in establishing the niche necessary for HO using a parabiosis mouse model but that local cells provide the mesenchymal, chondrogenic, and osteogenic contributions.
Materials and Methods
Ethics Statement
Procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Michigan [University Committee on Use and Care of Animals (UCUCA); PRO0005909 and PRO0005742].
Animals
All animals were housed in standard conditions: 72°F ± 4°F, receiving 12 hours of light exposure each day, with no diet restrictions. Animal care was provided in accordance with the University of Michigan School of Medicine guidelines and policies for the use of laboratory animals. All mice used for burn/tenotomy were young-adult females of either FVB or C57BL/6 background between 8 and 10 weeks old. All animals intended for parabiosis were housed as a pair for 14 days before surgery to ensure tolerance of their partner. Wild-type animals and ubiquitous (Ubi)–green fluorescent protein (GFP), L2G85 mice were used for parabiosis experiments. Wild-type mice of an FVB background were matched to L2G85 reporters. Wild-type mice of a C57BL/6 background were matched to Ubi-GFP reporters. Mice were euthanized at time points indicated below.
Transgenic Animals
Transgenic mice used in this study were as follows: Ubi-GFP [CByJ.B6-Tg(UBC-GFP)30Scha/J] and L2G85 [FVB-Tg(CAG-luc,-GFP)L2G85Chco/J]. Ubi-GFP and L2G85 mice were purchased from the Jackson Laboratory (Bar Harbor, ME).
Parabiosis
Wild-type and transgenic reporter mice were surgically joined together at the lateral midline using 4-0 silk sutures at the elbow and knee joints and a 6-0 polyglactin 910 suture (Ethicon, Cincinnati, OH) along the skin edges of a full-thickness flank incision. Mice were monitored continuously for the first 3 days following joining, followed by twice-daily evaluation for the next 11 days. Pain management was achieved via s.c. buprenorphine (Reckitt Benckiser Pharmaceuticals Inc., Richmond, VA) every 12 hours for 3 days and as needed for the first 7 days after joining. After a period of 14 days after joining, blood chimerism was confirmed via bioluminescent analysis in L2G85 pairs, as described below.
Bioluminescence Analysis
For L2G85 versus wild-type parabionts, blood chimerism was confirmed beginning at 2 weeks after parabiotic joining via D-luciferin injected intraperitoneally into the nonreporter mouse for bioluminescence imaging. Presence of a fluorescent signal in the reporter mouse (distal to injection of D-luciferin) served as confirmation of microvascular anastomoses between the two animals. All parabiotic pairs demonstrated blood chimerism by 2-week after joining. After injury, follow-up bioluminescent imaging was repeated after burn/tenotomy at 1, 4, 8, and 12 weeks.
All bioluminescence analyses were performed using the IVIS Spectrum system (Caliper Life Science, Hopkinton, MA). Peak luminescent values were represented via false-color heat map. Blanking for background luminescence was performed using a nonreflective/opaque cover. Live animals were maintained at a surgical plane of anesthesia during imaging via inhaled isoflurane provided through the IVIS system.
Mouse Burn/Tenotomy HO in Vivo Model
On the basis of the blood chimerism data from L2G85 parabionts, all parabiotic pairs received a burn/tenotomy at 14 days after joining. Parabionts receiving burn/tenotomy were anesthetized with inhaled isoflurane via a dual nozzle system. Dorsal hair was shaved, and the dorsum was exposed to a 60°C aluminum block for 18 seconds to achieve a 30% partial thickness burn injury.19 Each recipient mouse received a sterile tenotomy at the midpoint of the Achilles tendon with placement of a 5-0 vicryl suture to close the skin only.
Tissue Preparation
Hind limbs were fixed overnight in 10% neutral-buffered formalin and subsequently decalcified in 19% EDTA solution for 4 to 6 weeks at 4°C. Sections for immunohistochemistry (IHC) and immunofluorescence (IF) imaging were embedded in OCT compound (Tissue-Tek; Sakura Finetek, Torrance, CA), and sections (5 to 7 μm thick) were prepared. Samples for IF were post-fixed with ice-cold acetone for 20 minutes before blocking and were washed with phosphate-buffered saline (PBS) after acetone fixation. Samples for IHC were quenched for peroxidase activity with 3% hydrogen peroxide.
Histologic Staining for IHC
IHC samples were blocked with 5% bovine serum albumin in PBS. Staining was performed using the following primary antibody GFP (Abcam, Cambridge, UK), and appropriate biotinylated secondary antibodies were applied. 3,3′-Diaminobenzidine was used for visualization.
Histologic Staining for IF
IF samples were then blocked for 1 hour at room temperature (blocking: 1% bovine serum albumin, 2% normal donkey serum, 0.1% cold water fish skin gelatin, 0.1% Triton X-100, 0.05% Tween-20, and 0.3 mol/L glycine in PBS). After blocking, IF samples were incubated overnight at 4°C in 1:50 dilutions of the following primary antibodies: platelet-derived growth factor receptor α (PDGFRα; Santa Cruz Biotechnology, Dallas, TX; catalog number sc-338), sex determining region y (sry)-box 9 (Santa Cruz Biotechnology; catalog number 17341 or 20095), osterix (OSX; Bioss, Woburn, MA; catalog number bs-1110R), CD90/Thy-1 (Santa Cruz Biotechnology; catalog number sc-9163), CD105/endoglin (Santa Cruz Biotechnology; catalog number sc-101443), and CD31 (Abcam; catalog number ab28363). Samples were washed in PBS, and the following secondary antibodies were applied: donkey anti-rat Alexa Fluor 594 (Life Technologies, Carlsbad, CA), donkey anti-rabbit Alexa Fluor 594 (Life Technologies), donkey anti-goat Alexa Fluor 594 (Life Technologies), or donkey anti-rabbit Alexa Fluor 647 (Life Technologies). Tissues were incubated in secondary antibodies for 1 hour at room temperature and washed thoroughly. Samples were then incubated for 15 minutes at room temperature in a 1:10,000 dilution of Hoechst 33342 (Life Technologies) before being rinsed in PBS. IF samples were mounted with Prolong Gold Antifade Reagent (Life Technologies), and cover slips were applied. All stains were performed with appropriate secondary- and primary-only controls to evaluate for non-specific binding.
Microscopy
All fluorescently stained images were taken using a Leica Upright SP5X Confocal Microscope (Leica, Wetzlar, Germany) or an Olympus BX-51 upright light microscope (Olympus, Tokyo, Japan) equipped with standard DAPI, 488 nm, and tetramethylrhodamine isothiocyanate cubes attached to an Olympus DP-70 high-resolution digital camera. Each site was imaged in all channels and overlaid in DPViewer (Olympus, Tokyo, Japan) before examination in Adobe Photoshop (Adobe Systems, San Jose, CA).
Results
Establishment and Confirmation of Shared Circulation in a Parabiosis Mouse Model
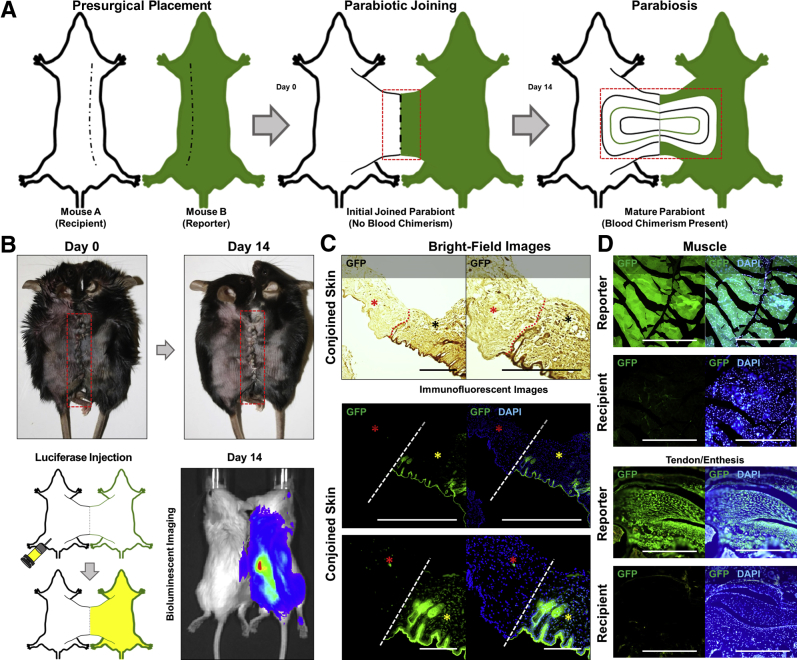
Currently, there is no marker, either singly or in combination, with sufficient sensitivity or specificity to differentiate circulating from tissue-resident cells across all lineages. Given this limitation, a parabiosis mouse model was established, by surgically joining two uninjured mice (Figure 1, A and B).24
Figure 1.
Establishment and confirmation of blood chimerism. A: Schematic of parabiotic mice on day of surgery and 14 days after surgery, with parabiont A (white) representing injured mouse and parabiont B (green) representing GFP-reporter mouse. Red boxed areas represent area of vascular interconnection. B: Image of parabiotic joining on day of surgery and 14 days after surgery. Red boxed areas indicate surgical joining with corresponding images of parabiotic mice. Schematic and images of conjoined mice illustrating injection of D-luciferin into nonreporter and subsequent bioluminescence of parabiosed luciferase mouse, indicating hematogenous transfer of D-luciferin from one parabiont to the other. C: Conjoined skin from the site of parabiotic joining with tissue from the recipient (red asterisks) and reporter (black asterisks and yellow asterisks). Skin imaged under immunofluorescence imaging for GFP (green) and DAPI (blue) or under bright-field imaging for anti-GFP (dark brown). Site of joining/demarcation marked with dotted red lines and dashed white lines. D: Histologic positive and negative controls from soft tissue sites in parabiotic mice demonstrating the presence (GFP+; GFP reporter) and absence (GFP−; wild-type mouse) of GFP signal in uninjured tissues. Scale bars = 200 μm (C and D).
Parabionts were surgically joined (Figure 1B) and survived for 2 weeks to allow for shared circulation before undergoing burn/tenotomy (Figure 1B). The anticipated outcome of parabiosis of two organisms is near equal contribution of each animal's circulatory system to a common blood circulation.25 Blood chimerism was confirmed by the presence of bioluminescence in the L2G85 reporter mouse secondary to injection of i.p. D-luciferin in the wild-type recipient animals (Figure 1B). Given the magnitude of difference in signal between the L2G85 reporter and the recipient, no luciferase signal was expected from the recipient in the absence of a localizing injury (Figure 1B). GFP signal was then examined at the site of conjoined skin under fluorescent imaging to confirm demarcation between reporter and recipient tissues (Figure 1C). Specificity of GFP signal was confirmed using an anti-GFP antibody for IHC under bright-field imaging (Figure 1C). These findings were confirmed using fluorescent imaging of soft tissue sites, including muscle and the Achilles enthesis (sites where HO commonly forms in trauma models) (Figure 1D).
Contribution of Circulating Cells to Early Stages of Developing HO
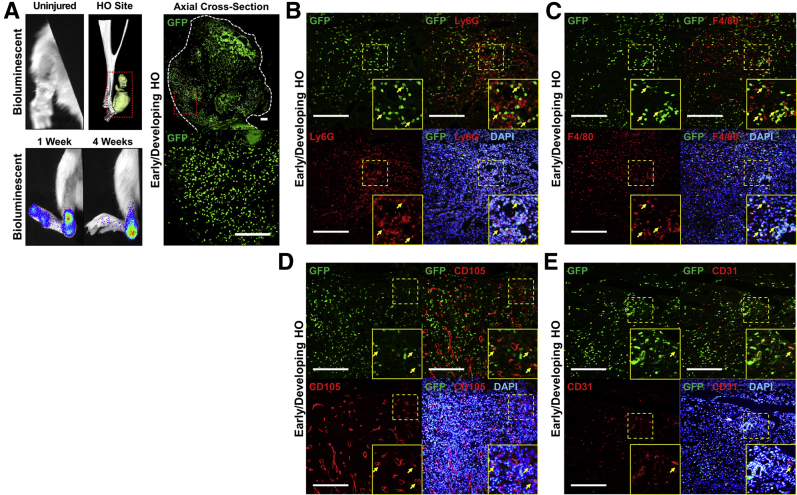
Burn/tenotomy was performed on the same-strain, nonreporter recipient mouse 2 weeks after parabiosis with the reporter mouse. D-luciferin was subsequently injected into the injured mouse at regular intervals to examine the immediate presence of donor-derived cells in the recipient mouse. Luminescence was observed at the injury site both 1 and 4 weeks after injury (Figure 2A). These findings at the injury site were confirmed histologically on the basis of GFP expression by donor-derived cells at the injury site present 4 weeks after injury (Figure 2A).
Figure 2.
Contribution of circulating cells to early stages of developing heterotopic ossification (HO). A: Bioluminescent images illustrating high concentration of circulating cells at the site of injury at 1 and 4 weeks after burn tenotomy. Three-dimensional computed tomographic (CT) reconstruction indicates common site of HO formation relative to the ankle in these mice. Contribution of circulating GFP+ cells to the HO anlagen with representative images of areas of early/developing HO. B: Expression of neutrophil marker (Ly6G; red) by GFP+ (green) and DAPI+ (blue) circulating cells in early/developing HO. C: Expression of macrophage marker (F4/80; red) by GFP+ (green) and DAPI+ (blue) circulating cells in early/developing HO. D: Expression of pericyte marker (CD105; red) by GFP+ (green) and DAPI+ (blue) circulating cells in early/developing HO. E: Expression of endothelial marker (CD31; red) by GFP+ (green) and DAPI+ (blue) circulating cells in early/developing HO. Red dotted boxes outlines area of heterotopic ossification on CT. White dashed line outlines area of early, developing heterotopic ossification. Yellow dashed boxes mark areas that have been expanded for better visualization in insets. Yellow arrows highlight areas of costaining. Scale bars = 200 μm (A–E).
Given the timing, location, and prevalence of these circulating cells, immunostaining was next performed for Ly6G and F4/80 to confirm the presence of donor-derived neutrophils and macrophages, respectively (Figure 2, B and C). Additional analyses were performed to examine the wound site for CD105+ perivascular and CD31+ endothelial cells at the site of injury, but limited overlap was found between these markers and GFP (Figure 2, D and E). These data suggest that many of the circulating cells in the immediate postinjury period are of an immunologic nature, consistent with acute inflammation.
Contribution of Circulating Cells to Late Stages of Developing HO
Heterotopic ossification occurs in stages consistent with endochondral ossification occurring in successive waves of cellular proliferation, chondrogenic differentiation, and osteogenesis.1 Immunostaining demonstrated the presence of Sox9+GFP+ cells indicative of a circulating contribution of chondrogenic cells (Figure 3A). Similarly, GFP+ cells were noted to express Osx, a marker of mesenchymal cells capable of undergoing endochondral ossification (Figure 3B).26
Figure 3.
Contribution of circulating cells to late stages of developing heterotopic ossification (HO). A: Expression of chondrogenic differentiation marker [sex determining region y (sry)-box 9; red] by GFP+ (green) and DAPI+ (blue) circulating cells in developing HO. B: Expression of osteoprogenitor marker [osterix (OSX); red] by GFP+ (green) and DAPI+ (blue) circulating cells in developing HO. C: Bioluminescent images illustrating persistence of circulating cells at the site of injury at 8 weeks after burn tenotomy and changes in concentration of circulating cells at the site of injury between 8 and 12 weeks after burn tenotomy. Representative histology of demonstrating GFP+ cells in the later stages of the developing HO anlagen. D: Histologic evidence for persistence of circulating GFP+ in both remnant fibroproliferative and chondrogenic regions of developing HO as well as the changes in stromal cellularity expected as HO develops. Yellow dashed boxes mark areas that have been expanded for better visualization in insets. White arrows and yellow arrows highlight areas of costaining/coexpression. Scale bars = 200 μm (A–D).
Given these findings, it was determined whether circulating cells contribute to the ossified HO lesion. Parabiosis experiments showed reporter and nonreporter, same-strain mice were allowed to survive to 8 weeks after injury, a time point associated with presence of the ossified lesion in the burn/tenotomy model. Indeed, bioluminescence was observed at the injury site (Figure 3C). Histologic evaluation demonstrated a substantial number of GFP+ cells in the lesion (Figure 3, C and D). These cells were primarily present in the medullary cavity and stroma (Figure 3, C and D). On further evaluation, GFP+ cells were identified within fibroproliferative and cartilaginous regions of the late HO lesion, consistent with earlier time points when these states predominate the lesion (Figure 3D). When bioluminescent reporter pairs survived to 12 weeks after injury, a decrease in signal intensity was noted at the injury site (Figure 3C). Histologic evaluation demonstrated an ossified lesion with GFP+ cells relegated to the stroma (Figure 3D). These data, taken together, suggest a role for circulating cells in the development and proliferation of HO, but not within the osseous portions of the lesion.
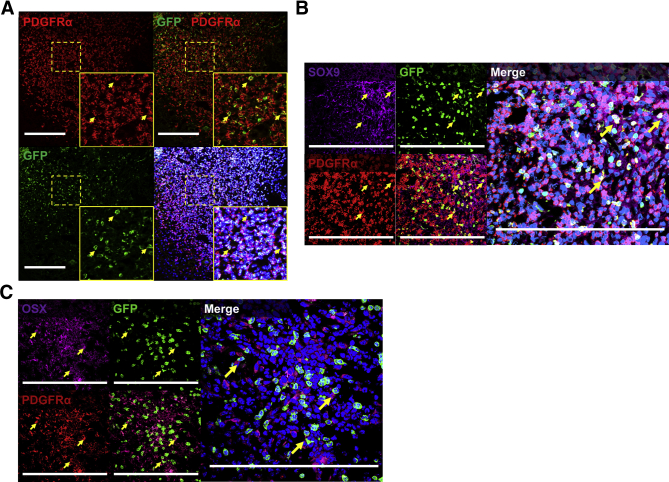
Contribution of Circulating Mesenchymal Cells to Developing HO
Although our results above suggest that chondrogenic or osteogenic cells can be systemically derived, it was unclear whether these cells were of a mesenchymal origin. A contribution of mesenchymal cells to HO lesions has previously been demonstrated2; however, an open question has been whether these cells are tissue-resident or circulating mesenchymal cells. To confirm the mesenchymal origin of GFP+ circulating cells contributing to HO, immunostaining for PDGFRα was performed. PDGFRα+GFP+ cells were identified in developing HO (Figure 4A). Because a single marker is insufficient to determine the mesenchymal nature of a population, the search was further restricted to those cells that had already demonstrated either osteogenic or chondrogenic differentiation. Thus, the population was further restricted out to those GFP+ cells that coexpressed PDGFRα and either SOX9 or OSX (Figure 4, B and C). Both subpopulations were present in a limited number of cells in the developing HO anlagen, consistent with the presence of GFP+ cells in chondrogenic regions of HO (Figure 3D and Figure 4, B and C).
Figure 4.
Contribution of circulating mesenchymal cells to developing heterotopic ossification (HO). A: Expression of mesenchymal marker [platelet-derived growth factor receptor α (PDGFRα); red] by GFP+ (green) and DAPI+ (blue) circulating cells in early/developing HO. B: Expression of PDGFRα (red) and sex determining region y (sry)-box 9 (SOX9) (purple) by GFP+ (green) and DAPI+ (blue) circulating cells in HO anlagen. C: Expression of PDGFRα (red) and osterix (OSX; purple) by GFP+ (green) and DAPI+ (blue) circulating cells in HO anlagen. Yellow dashed boxes mark areas that have been expanded for better visualization in insets. Yellow arrows highlight areas of costaining. Scale bars = 200 μm (A–C).
Discussion
Heterotopic ossification is a variant of pathologic wound healing involving the transition from postinjury inflammation to a series of ordered developmental processes.27 The final result of this process is heterotopic bone, causing significant and chronic morbidity. Because of similarities in the pathogenesis of HO and a related genetic condition of abnormal bone morphogenetic protein (BMP)-SMAD signaling called fibrodysplasia ossificans progressive, many studies evaluating the cellular and biochemical characteristic of HO have focused on the use of exogenous BMPs and/or genetic models of hyperactive type 1 BMP receptors.5, 8, 23, 28, 29, 30, 31, 32 These models have been used to identify several local and tissue-resident candidates that act as HO progenitors. However, it remains unclear whether these candidate cells are applicable to tHO, which typically occurs in the absence of exogenous BMP or mutation in type 1 BMP receptors.33 Herein, we demonstrate the validity of parabiosis as a technique to study the contribution of cells to HO caused by trauma.
This study focused on tHO using an Achilles tenotomy model. This is a model that our group has found to be both reliable and reproducible in prior studies. For our purposes, it has several benefits, including the following: i) As a trauma model, there is no application of exogenous material, BMP or otherwise. ii) No mutations are necessary for this model to be effective. iii) The model is simple, reliable, and reproducible, with a high volume and predictable pattern of bone formation in wild-type mice. Our group has published extensively on this model as a means of developing tHO. It has the benefit of a consistent and ordered progression of histologic stages, starting from inflammatory precursors, to mesenchymal condensate, chondrification, endochondral ossification, osseous maturation, and formation of a marrow cavity.2 These events are predictable in time, anatomic localization, and amount of bone formed, which allows us to closely monitor perturbation in the system. All of these factors led us to select it as the technique with which to induce tHO in our parabionts.
Circulating cells have been implicated in variants of both physiologic and pathologic wound healing.21, 34 Although several studies have previously examined the stem cell niche contributing to development of ectopic bone formation, they have been limited by one of two major confounders: use of models dependent on pathologic BMP-SMAD signaling or use of systems that incompletely differentiate between local/resident populations. Although mobilization of distant reservoir populations is commonly described in other inflammatory and immunologic processes,35 the contribution from these sources to various stages of HO remains unclear. By better understanding the contribution of these cells to normal and dysfunctional healing, we may identify new targets for disease therapy and prevention.
Parabiosis provides us with a powerful tool by which we can accurately separate populations that arise from the donor parabiont, populations that are exceedingly likely to have arrived via a shared circulatory system. This model has been successfully used to identify the role of circulating populations of bone marrow–derived mesenchymal progenitor cells in a diabetic ischemic flap model, to determine factors that recruit circulatory progenitors to the sites of chronic wounds, and to determine the contribution of hematopoietic cells to acute skin wounds.36, 37 Recent studies have suggested that circulating cells of hematopoietic origin can also serve as osteogenic precursors at remote sites of tissue inflammation.26, 31, 38 These cells were demonstrated in both early inflammatory and late marrow-repopulating stages of BMP4-induced HO, but had limited representation in fibroproliferative, chondrogenic, and osteogenic stages.23, 28, 29 Although a small population of circulating Osx+ osteoprogenitors was identified during early development of HO, a definitive structure in which these cells contributed could not be identified.
Histologically, early GFP+ circulating populations found at the wound site were appropriately myeloid in origin, consistent with the immunologic response. These cells appeared to comprise most circulating cells contributing to early postinflammatory stages of HO development. Furthermore, the presence of circulating cells was demonstrated at each phase of developing HO. Specifically, the presence of a small population of circulating mesenchymal populations was identified in early developing HO. Also, GFP+ cells were found to be present in fibroproliferative and chondrogenic regions within the HO anlagen and to express PDGFRα.
Because of the spatially and temporally restricted nature of the chondrogenic phase of the heterotopic anlagen, histologic evidence of PDGFRα+/SOX9+/GFP+ in the recipient parabiont provides valuable evidence for incorporation of circulating cells into heterotopic ossification. It is important to recognize that presence of SOX9+/GFP+ cells in the chondrogenic phase of the anlagen does not guarantee that these cells will survive chondrocyte maturation and ossification to incorporate into the definitive osseous anlage; however, it demonstrates the presence and incorporation of these circulatory populations into the critical early stages of heterotopic ossification.
The presence of scattered GFP+/CD31+ cells and the near absence of GFP+/CD105+ cells were also identified. The limited presence of these cells was an interesting finding because mice carrying a cadherin 5 red fluorescent protein lineage marker, when in parabiosis with a wild-type mouse, lend a small portion of those cells to the site of injury. Our expectation was to likely find a more extensive correlation between vascular or perivascular markers and GFP+ cells. The absence of this correlation suggests two possibilities. An absence of contribution from these populations is an unlikely finding given the prior body of literature linking vascular lineages to the formation of heterotopic bone.5, 22 A significantly greater local contribution of vascular and perivascular cells is a finding that is, in and of itself, interesting and may be better explored in future studies using parabionts with two disparate reporters to track the local contribution directly.
Recent studies have suggested that circulating cells of hematopoietic origin can also serve as osteogenic precursors at remote sites of tissue inflammation.21, 24, 31 These cells were demonstrated in both early inflammatory and late marrow-repopulating stages of BMP4-induced HO, but had limited representation in fibroproliferative, chondrogenic, and osteogenic stages.17, 28, 29 A small population of circulating OSX+ osteoprogenitors was also identified during early development of HO; however, a definitive structure in which these cells contributed could not be identified. One hypothesis for the presence of these cells might be that their contribution is biochemical rather than physical. Given the well-described relationship between HO, its genetic counterpart fibrodysplasia ossificans progressive, and the BMP-SMAD signaling pathway, the use of parabionts carrying gain-of-function and loss-of-function mutations for BMP receptors is a logical next step.
One major limitation of our analysis, the use of the global reporter Ubi-GFP, serves to highlight other potential next steps in the use of parabiotic models for the evaluation of HO. Although there remains an incomplete understanding of the specific cell lineages that contribute to HO, substantial contributions from cells of a Prx-cre lineage have previously been demonstrated.2 Prx-cre marks those cells that derive from the lateral plate mesoderm, and in the context of HO, it defines most of the fibroproliferative, chondrogenic, and osseous anlagen. Further characterization of these implicated cell lineages revealed concurrence of phenotypic surface markers, including PDGFRα, stem cells antigen-1, and S100A4, which are consistent with mesenchymal stem cells shown to mediate HO and fibrogenesis in other injury models.5, 8, 30, 31, 32 Our findings indicate that these cells are, at least in part, circulating cells.
Parabiosis models are not without their own inherent limitation. Parabiosis in a chronic and systemic injury model compounds the initial trauma with sustained discomfort, psychosocial stress, and a heightened inflammatory response. Our model of combinatorial burn plus tenotomy presents a more acute combination of systemic and local inflammation, and it is feasible that in the chronic injury model, there may be differences in the cellular response to trauma compared with acute injury. A grossly similar progression of injury was seen through the stages of heterotopic ossification without deviation from what might have been predicted in a nonparabiotic model of tHO. Extrapolating the development of tHO seen previously to what we had identified in the parabiotic model seemed appropriate.
A further consideration in the use of parabiosis as a model is the possibility of a parabiotic barrier, or mechanism by which certain circulating cells are prevented from crossing between one parabiont and the other. This was explored previously by Gibney et al,25 whose group found that all tested circulatory subpopulations had reached equilibrium within 14 days. The fact that the cells tested were derived from peripheral blood studies is an important factor because the blood equilibrates in this model before other reservoir populations, such as those present in lymphoid organisms.25 Although true equilibration of all circulating populations may take >1 month, the 14-day mark, as described earlier, would be sufficient to identify those circulatory cells that would be readily available to home to the heterotopic anlagen.
This study did not address the signals that home mesenchymal cells to the wound site after trauma but rather identified the fact that these cells contribute to heterotopic ossification and all stages of developing ectopic cartilage and bone. Further studies will need to be performed to demonstrate the specific molecular signals/receptors for the recruitment and/or differentiation of circulating cell populations contributing to HO. Timely intervention on these processes may derail the development of the preosseous anlagen and ultimately prevent the transition to definitive bone. Ultimately, understanding the spectrum of cellular contributors to de novo ectopic bone and the shared cellular pathways driving pathogenesis will aid in the development of cell-specific therapeutic strategies to prevent trauma-induced HO.
Footnotes
Supported by NIH grants F32 AR066499, R01GM123069, 1R01AR071379, and F32 AR068902 (K.R.), the NIH Loan Repayment Program (S.A.), the Howard Hughes Medical Institute Medical Fellows Program (S.J.L.), NIH/National Institute of General Medical Sciences grant K08GM109105, an American Association of Plastic Surgery Academic Scholarship, the American College of Surgeons Clowes Award, and the International FOP Association (B.L.).
S.J.L. and S.A. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.07.014.
Supplemental Data
References
- 1.Shore E.M., Xu M., Feldman G.J., Fenstermacher D.A., Cho T.J., Choi I.H., Connor J.M., Delai P., Glaser D.L., LeMerrer M., Morhart R., Rogers J.G., Smith R., Triffitt J.T., Urtizberea J.A., Zasloff M., Brown M.A., Kaplan F.S. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S., Loder S., Brownley C., Cholok D., Mangiavini L., Li J., Breuler C., Sung H.H., Li S., Ranganathan K., Peterson J., Tompkins R., Herndon D., Xiao W., Jumlongras D., Olsen B.R., Davis T.A., Mishina Y., Schipani E., Levi B. Inhibition of Hif1alpha prevents both trauma-induced and genetic heterotopic ossification. Proc Natl Acad Sci U S A. 2016;113:E338–E347. doi: 10.1073/pnas.1515397113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potter B.K., Burns T.C., Lacap A.P., Granville R.R., Gajewski D.A. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 4.Shehab D., Elgazzar A.H., Collier B.D. Heterotopic ossification. J Nucl Med. 2002;43:346–353. [PubMed] [Google Scholar]
- 5.Kan L., Kessler J.A. Evaluation of the cellular origins of heterotopic ossification. Orthopedics. 2014;37:329–340. doi: 10.3928/01477447-20140430-07. [DOI] [PubMed] [Google Scholar]
- 6.Dey D., Bagarova J., Hatsell S.J., Armstrong K.A., Huang L., Ermann J., Vonner A.J., Shen Y., Mohedas A.H., Lee A., Eekhoff E.M., van Schie A., Demay M.B., Keller C., Wagers A.J., Economides A.N., Yu P.B. Two tissue-resident progenitor lineages drive distinct phenotypes of heterotopic ossification. Sci Transl Med. 2016;8:366ra163. doi: 10.1126/scitranslmed.aaf1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lounev V.Y., Ramachandran R., Wosczyna M.N., Yamamoto M., Maidment A.D., Shore E.M., Glaser D.L., Goldhamer D.J., Kaplan F.S. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wosczyna M.N., Biswas A.A., Cogswell C.A., Goldhamer D.J. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. J Bone Miner Res. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal S., Loder S.J., Cholok D., Peterson J., Li J., Breuler C., Cameron Brownley R., Hsin Sung H., Chung M.T., Kamiya N., Li S., Zhao B., Kaartinen V., Davis T.A., Qureshi A.T., Schipani E., Mishina Y., Levi B. Scleraxis-lineage cells contribute to ectopic bone formation in muscle and tendon. Stem Cells. 2017;35:705–710. doi: 10.1002/stem.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costopoulos C.L., Abboud J.A., Ramsey M.L., Getz C.L., Sholder D.S., Taras J.P., Huttman D., Lazarus M.D. The use of indomethacin in the prevention of postoperative radioulnar synostosis after distal biceps repair. J Shoulder Elbow Surg. 2017;26:295–298. doi: 10.1016/j.jse.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Rath E., Warschawski Y., Maman E., Dolkart O., Sharfman Z.T., Salai M., Amar E. Selective COX-2 inhibitors significantly reduce the occurrence of heterotopic ossification after hip arthroscopic surgery. Am J Sports Med. 2016;44:677–681. doi: 10.1177/0363546515618623. [DOI] [PubMed] [Google Scholar]
- 12.Beckmann J.T., Wylie J.D., Potter M.Q., Maak T.G., Greene T.H., Aoki S.K. Effect of naproxen prophylaxis on heterotopic ossification following hip arthroscopy: a double-blind randomized placebo-controlled trial. J Bone Joint Surg Am. 2015;97:2032–2037. doi: 10.2106/JBJS.N.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Y., Cai J., Li F., Liu S., Ruan H., Fan C. The efficacy of celecoxib in preventing heterotopic ossification recurrence after open arthrolysis for post-traumatic elbow stiffness in adults. J Shoulder Elbow Surg. 2015;24:1735–1740. doi: 10.1016/j.jse.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Yeung M., Jamshidi S., Horner N., Simunovic N., Karlsson J., Ayeni O.R. Efficacy of nonsteroidal anti-inflammatory drug prophylaxis for heterotrophic ossification in hip arthroscopy: a systematic review. Arthroscopy. 2016;32:519–525. doi: 10.1016/j.arthro.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Kan S.L., Yang B., Ning G.Z., Chen L.X., Li Y.L., Gao S.J., Chen X.Y., Sun J.C., Feng S.Q. Nonsteroidal anti-inflammatory drugs as prophylaxis for heterotopic ossification after total hip arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e828. doi: 10.1097/MD.0000000000000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torossian F., Guerton B., Anginot A., Alexander K.A., Desterke C., Soave S., Tseng H.W., Arouche N., Boutin L., Kulina I., Salga M., Jose B., Pettit A.R., Clay D., Rochet N., Vlachos E., Genet G., Debaud C., Denormandie P., Genet F., Sims N.A., Banzet S., Levesque J.P., Lataillade J.J., Le Bousse-Kerdilès M.C. Macrophage-derived oncostatin M contributes to human and mouse neurogenic heterotopic ossifications. JCI Insight. 2017;2:e96034. doi: 10.1172/jci.insight.96034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genêt F., Kulina I., Vaquette C., Torossian F., Millard S., Pettit A.R., Sims N.A., Anginot A., Guerton B., Winkler I.G., Barbier V., Lataillade J.J., Le Bousse-Kerdilès M.C., Hutmacher D.W., Levesque J.P. Neurological heterotopic ossification following spinal cord injury is triggered by macrophage-mediated inflammation in muscle. J Pathol. 2015;236:229–240. doi: 10.1002/path.4519. [DOI] [PubMed] [Google Scholar]
- 18.Kan L., Liu Y., McGuire T.L., Berger D.M., Awatramani R.B., Dymecki S.M., Kessler J.A. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumagai K., Vasanji A., Drazba J.A., Butler R.S., Muschler G.F. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 20.Egan K.P., Duque G., Keenan M.A., Pignolo R.J. Circulating osteogentic precursor cells in non-hereditary heterotopic ossification. Bone. 2018;109:61–64. doi: 10.1016/j.bone.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 21.Pignolo R.J., Kassem M. Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res. 2011;26:1685–1693. doi: 10.1002/jbmr.370. [DOI] [PubMed] [Google Scholar]
- 22.Otsuru S., Tamai K., Yamazaki T., Yoshikawa H., Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 23.Otsuru S., Tamai K., Yamazaki T., Yoshikawa H., Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453–458. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 24.Peterson J.R., Agarwal S., Brownley R.C., Loder S.J., Ranganathan K., Cederna P.S., Mishina Y., Wang S.C., Levi B. Direct mouse trauma/burn model of heterotopic ossification. J Vis Exp. 2015;(102):e52880. doi: 10.3791/52880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibney B.C., Chamoto K., Lee G.S., Simpson D.C., Miele L.F., Tsuda A., Konerding M.A., Wagers A., Mentzer S.J. Cross-circulation and cell distribution kinetics in parabiotic mice. J Cell Physiol. 2012;227:821–828. doi: 10.1002/jcp.22796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaback L.A., Soung do Y., Naik A., Smith N., Schwarz E.M., O'Keefe R.J., Drissi H. Osterix/Sp7 regulates mesenchymal stem cell mediated endochondral ossification. J Cell Physiol. 2008;214:173–182. doi: 10.1002/jcp.21176. [DOI] [PubMed] [Google Scholar]
- 27.Chakkalakal S.A., Zhang D., Culbert A.L., Convente M.R., Caron R.J., Wright A.C., Maidment A.D., Kaplan F.S., Shore E.M. An Acvr1 R206H knock-in mouse has fibrodysplasia ossificans progressiva. J Bone Miner Res. 2012;27:1746–1756. doi: 10.1002/jbmr.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan F.S., Glaser D.L., Shore E.M., Pignolo R.J., Xu M., Zhang Y., Senitzer D., Forman S.J., Emerson S.G. Hematopoietic stem-cell contribution to ectopic skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 29.Suda R.K., Billings P.C., Egan K.P., Kim J.H., McCarrick-Walmsley R., Glaser D.L., Porter D.L., Shore E.M., Pignolo R.J. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–2219. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dey D., Wheatley B.M., Cholok D., Agarwal S., Yu P.B., Levi B., Davis T.A. The traumatic bone: trauma-induced heterotopic ossification. Transl Res. 2017;186:95–111. doi: 10.1016/j.trsl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kan L., Peng C.Y., McGuire T.L., Kessler J.A. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53:194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemos D.R., Eisner C., Hopkins C.I., Rossi F.M.V. Skeletal muscle-resident MSCs and bone formation. Bone. 2015;80:19–23. doi: 10.1016/j.bone.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal S., Loder S.J., Breuler C., Li J., Cholok D., Brownley C., Peterson J., Hsieh H.H., Drake J., Ranganathan K., Niknafs Y.S., Xiao W., Li S., Kumar R., Tompkins R., Longaker M.T., Davis T.A., Yu P.B., Mishina Y., Levi B. Strategic targeting of multiple BMP receptors prevents trauma-induced heterotopic ossification. Mol Ther. 2017;25:1974–1987. doi: 10.1016/j.ymthe.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Asakura A. Skeletal muscle-derived hematopoietic stem cells: muscular dystrophy therapy by bone marrow transplantation. J Stem Cell Res Ther. 2012;Suppl 11 doi: 10.4172/2157-7633.S11-005. pii:005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Januszyk M., Sorkin M., Glotzbach J.P., Vial I.N., Maan Z.N., Rennert R.C., Duscher D., Thangarajah H., Longaker M.T., Butte A.J., Gurtner G.C. Diabetes irreversibly depletes bone marrow-derived mesenchymal progenitor cell subpopulations. Diabetes. 2014;63:3047–3056. doi: 10.2337/db13-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song G., Nguyen D.T., Pietramaggiori G., Scherer S., Chen B., Zhan Q., Ogawa R., Yannas I.V., Wagers A.J., Orgill D.P., Murphy G.F. Use of the parabiotic model in studies of cutaneous wound healing to define the participation of circulating cells. Wound Repair Regen. 2010;18:426–432. doi: 10.1111/j.1524-475X.2010.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farahani R.M., Xaymardan M. Platelet-derived growth factor receptor alpha as a marker of mesenchymal stem cells in development and stem cell biology. Stem Cells Int. 2015;2015:362753. doi: 10.1155/2015/362753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.