Abstract
A hexanucleotide GGGGCC repeat expansion in C9orf72 is the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal degeneration. Accurate determination and quantitation of the repeat length is critical in both clinical and research settings. However, because of the complexity of the C9orf72 expansion with high GC content, large size of repeats, and high rate of insertions/deletions (indels) and sequence variations in the flanking regions, molecular genetic analysis of the locus is challenging. To improve the performance characteristics for clinical testing, we evaluated a commercially available long-read C9orf72 PCR assay for research use only, AmplideX PCR/CE C9orf72 assay (AmplideX-C9), and compared its performance with our existing laboratory-developed C9orf72 expansion procedure. Overall, in comparison to the laboratory-developed C9orf72 expansion procedure, AmplideX-C9 demonstrated a more efficient workflow, greater PCR efficiency for sizing of repeat expansions, and improved peak amplitude with lower DNA input and higher analytic sensitivity. This, in turn, permitted detection of indels in the 3′ downstream of the repeat expansion region in expanded alleles, showed a higher success rate with formalin-fixed, paraffin-embedded tissue specimens, and facilitated the assessment of repeat mosaicism. In summary, AmplideX-C9 will not only help to improve clinical testing for C9orf72-associated amyotrophic lateral sclerosis and frontotemporal degeneration but will also be a valuable research tool to better characterize the complexity of expansions and study the effects of indels/sequence variations in the flanking region.
Frontotemporal degeneration (FTD) is an adult-onset presenile dementia that presents as heterogeneous clinical phenotypes, including progressive behavioral and/or language changes attributable to degeneration of the frontal and temporal cortex of the brain.1 Amyotrophic lateral sclerosis (ALS) is an adult-onset disease characterized by degeneration of upper and lower motor neurons; it often starts focally, then spreads rapidly, typically resulting in death in 3 to 5 years.2 The most common genetic cause of both FTD and ALS, two conditions that are now considered to be part of the same disease continuum, is a GGGGCC (G4C2) hexanucleotide repeat expansion in C9orf72. Expansions in C9orf72 are found in 26% of familial and 5% of sporadic FTD and 34% of familial and 6% of sporadic ALS and are inherited as an autosomal dominant disorder.3, 4 C9orf72 is located on chromosome 9p21.2 and contains 11 exons, including two alternate noncoding exons, 1a and 1b, between which the repeat region is located. Whereas most healthy individuals carry alleles between 2 and 20 repeats in length, the hexanucleotide repeat size in patients with C9orf72-associated ALS and FTD commonly ranges from hundreds to several thousand G4C2 repeats.5 The lower repeat size cutoff of 30 repeats is typically used to differentiate between normal and pathogenic repeat sizes; however, reports of familial segregation with disease of repeats in the intermediate size range between 21 and 29 repeats have been published.4, 6, 7, 8, 9 In addition, both small (32 to 35) and large (>400) repeat expansions have been reported in unaffected healthy individuals10, 11; however, these cases may represent incomplete disease penetrance of the repeat expansion. Penetrance of the C9orf72 is age dependent, with 19.4%, 50.6%, and 96.1% affected by the ages of 50, 57, and 72 years, respectively.5 Repeat alleles between 21 and 29 repeats have been reported in patients with ALS,12, 13 FTD,9 Parkinson disease,14, 15 and essential tremor,16 which suggests that intermediate-sized repeats may be a potential risk factor for a broad spectrum of neurodegenerative diseases.
The association between C9orf72 expansion size and clinical phenotypes has been widely studied. A significant association between longer C9orf72 expansion length in peripheral blood DNA and shorter disease duration in patients with FTD, but not ALS,5, 17 has been shown; and a significant association between C9orf72 expansion size in the frontal cortex of FTD patients and in ALS patients and age at onset has been shown.18, 19 However, no significant association with repeat size has been reported by others.11, 13 These conflicting results might be attributable to heterogeneity of the clinical phenotype of FTD and ALS,8, 20 incomplete penetrance,5, 21 and/or the possible existence of genetic modifiers.22 The method for sizing the expansion alleles may also be one of the variables that contributes to the conflicting results,5, 23 because the complexity of the C9orf72 expansion locus with GC-rich sequences as well as frequent insertions/deletions (indels) and sequence variations within the flanking sequences downstream of the expansion24, 25 may increase both false-negative results for an expansion and inaccuracy in estimation of the repeat length.
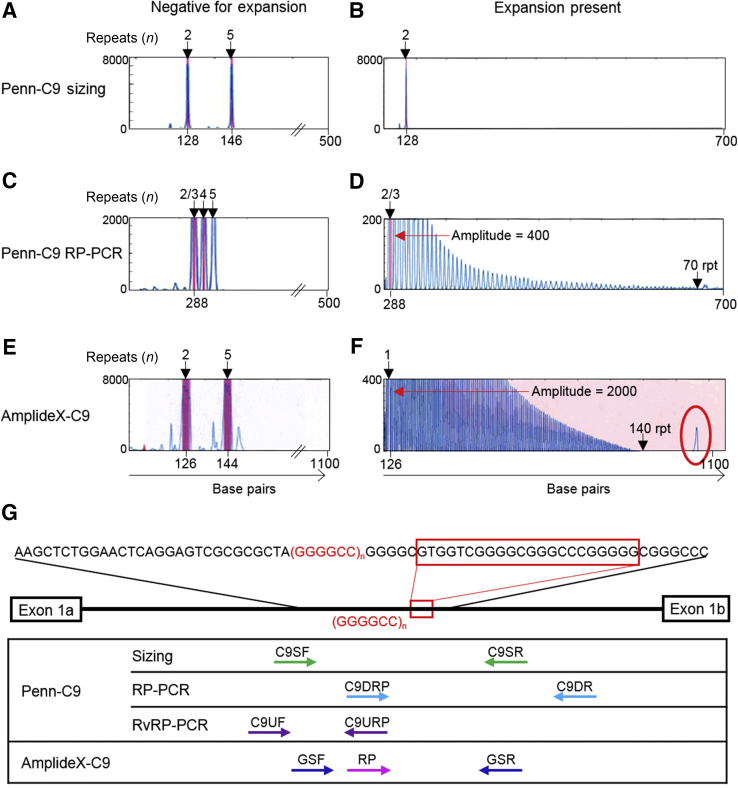
The high GC content of the G4C2 repeat region makes using PCR methods to amplify the C9orf72 repeat expansion region a challenge. Alleles within the normal and intermediate size range are easily amplifiable; however, an allele with, for example, 1500 G4C2 repeats (9000 G and C base pairs) does not result in an amplification product using standard PCR methods. Thus, using a fragment analysis PCR approach, an individual heterozygous for two different normal-sized alleles would have two peaks (Figure 1A), whereas an individual homozygous for a normal-sized allele would not be distinguishable from someone with one normal allele and one large expanded allele because the expanded allele would not produce a detectable product (Figure 1B). To overcome this difficulty, repeat-primed PCR is used, in which PCR is performed using a primer complimentary to three G4C2 repeats plus an anchor tail, an adjacent primer, and a third anchor primer. Amplification of the C9orf72 repeat region with this repeat-primed PCR method yields a characteristic saw-tooth pattern when an expansion is present, with the periodicity of the peaks equal to the size of the repeat (Figure 1, C and D).
Figure 1.
Example of electropherograms and primer schematic. A–F: Capillary electropherograms for a sample without (A, C, E) and with (B, D, F) a C9orf72 G4C2 repeat expansion using Penn-C9 sizing reaction (A and B), Penn-C9 repeat-primed PCR (RP-PCR) assays (C and D), and AmplideX-C9 (E and F). PCR products were separated on an ABI 3130xl Genetic Analyzer and visualized by GeneMapper software version 3.7. The vertical pink bars indicate off-scale peaks (in relative fluorescence units). The sample that is negative for an expansion is heterozygous for two and five repeat alleles, seen as peaks in A and E. Using the Penn-C9 RP-PCR method (which does not contain gene-specific primers, unlike AmplideX-C9, which does), peaks begin at three repeats because the primer incorporates three repeats into the product and then additional peaks are present at a periodicity of 6 bp up to the peak at the size of the second allele (in this case, five repeats). B: When a sample with a large G4C2 repeat expansion is amplified using the Penn-C9 sizing reaction, it only shows the normal allele (two repeats in this example). D and F: With both the Penn-C9 RP-PCR and AmplideX-C9, an expansion-containing sample demonstrates a characteristic saw-tooth pattern of peaks at 6-bp intervals that can be individually counted, up to 70 repeats (Penn-C9) or 140 repeats (AmplideX-C9). The horizontal red arrows label the four-repeat peak amplitude comparing the sensitivity of assay between AmplideX-C9 and Penn-C9 RP-PCR. A high-molecular-weight pile-up peak is highlighted by a red oval. G: Schematic diagram of the PCR primer binding sites in the intron between exons 1a and 1b of Penn-C9 and AmplideX-C9 assays relative to the C9orf72 hexanucleotide repeat region, (GGGGCC)n. A 22-bp region with frequent sequence variation downstream of the repeat region is shown (red boxed area). C9DR, downstream reverse primer; C9DRP, downstream repeat primer that contains an adapter primer tail (not shown); C9SF, sizing forward primer; C9SR, sizing reverse primer; C9UF, upstream forward primer; C9URP, upstream repeat primer that contains an adapter primer tail (not shown); GSF, gene-specific forward primer; GSR, gene-specific reverse primer; RP, AmplideX-C9 repeat primer; rpt, repeats; RvRP-PCR, reverse RP-PCR.
Our laboratory-developed procedure (LDP) for C9orf72 uses a combination of standard fragment PCR analysis (sizing PCR) to genotype normal and small expanded alleles plus two repeat-primed PCR (RP-PCR) assays, one downstream and one upstream of the repeat locus (Figure 1G), to qualitatively detect large expansion alleles.5 The upstream RP-PCR is included to improve clinical sensitivity for detection of expansions attributable to sequence variability, which can cause false-negative results. Southern blot analysis is performed as a research tool in C9orf72 expansion cases to quantify large expansions or to confirm PCR results as well as in homozygous cases with normal-sized alleles to confirm the absence of expansion.5 However, this approach requires a large input of genomic DNA and is time inefficient. In addition, a study of the reliability of testing for C9orf72 expansions in 14 international laboratories found poor concordance in testing results between the laboratories, with a high degree of false-positive and false-negative results, suggesting the need for improved methods.23 Therefore, we evaluated a new commercially available C9orf72 assay with the potential for a simpler workflow, a lower DNA input requirement, and an improved performance. In this study, we evaluated the performance characteristics of a novel single-tube long-read PCR technology for analysis of C9orf72 expansions, the commercially available AmplideX PCR/CE C9orf72 assay (AmplideX-C9; Asuragen, Inc., Austin, TX), including analytic sensitivity and specificity and repeat size detection limit for assessment of C9orf72 expansion locus, and compared the method with our LDP (Figure 1, E and F).
Concordance between AmplideX-C9 and our LDP was excellent for both normal allele sizing and qualitative detection of repeat expansions. However, in comparison to the LDP, the AmplideX-C9 had a more efficient workflow, demonstrated improved robustness for sizing of repeat expansions by PCR alone, and improved peak amplitude with lower DNA input and higher analytic sensitivity. These factors, in turn, permitted detection of indels in the 3′ downstream of the repeat expansion region in expanded alleles, showed a higher success rate for amplification of expansions from formalin-fixed, paraffin-embedded (FFPE) tissue, and facilitated the assessment of repeat mosaicism.
Materials and Methods
Samples and DNA Extraction
DNA samples used for this study were originally obtained from neurodegenerative disease patients and healthy controls under University of Pennsylvania (Philadelphia, PA) institutional review board–approved protocols as well as from brain tissue in the Center for Neurodegenerative Disease Research brain bank. The study includes 173 samples from 141 patients with ALS (n = 65), FTD (n = 50), or ALS-FTD (n = 20), or asymptomatic family members (n = 6) known to carry a C9orf72 expansion, as well as 83 cases without an expansion (41 with FTD, 10 with ALS, 9 with ALS-FTD, 16 with other neurodegenerative diseases, and 7 unaffected controls) and 4 FTD cases with one intermediate-sized allele. The sources of the DNA samples tested were as follows: peripheral blood (n = 139), fresh-frozen brain tissue (n = 45), saliva (n = 17), paired blood and fresh-frozen brain tissue (n = 27), and paired fresh-frozen brain and FFPE tissue (n = 11). DNA was extracted using commercial reagents, according to manufacturer protocols: QuickGene-610L (Autogen, Hollison, MA), Oragene DNA kit (DNA Genotek Inc., Ottawa, ON, Canada), or QIAamp DNA mini kit (Qiagen, Germantown, MD) for blood, saliva, or brain tissue, respectively. DNA from FFPE tissue was extracted using the QIAamp DSP DNA FFPE Tissue Kit (Qiagen), according to the manufacturer's protocol, with modifications. Briefly, xylene was added to three sections (10 μm thick) of fixed tissue to melt paraffin at 56°C for 3 minutes. Residual paraffin was removed by two washes of 100% ethanol. Tissue was lysed with Buffer ATL (Qiagen) and proteinase K at 56°C for approximately 48 hours, with intermittent mixing. Lysate was mixed with one volume of Buffer AL (Qiagen) and two volumes of 100% ethanol after the 90°C incubation. Each sample was applied to a single MinElute column, followed by two washes with each Buffers AW1 and AW2 (Qiagen). DNA was eluted in 200 μL Buffer ATE (Qiagen) and quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). For establishing the frequency of indels in C9orf72, data from ongoing C9orf72 testing on 2146 individuals of the Center for Neurodegenerative Disease Research cohort were reviewed and included in the analysis.5
Size Determination of C9orf72 Hexanucleotide Repeats and Repeat-Primed PCR Analysis
Laboratory-Developed C9orf72 Expansion Procedure
A sizing PCR assay was used to determine the number of repeats in normal and small expansion alleles, as described previously (Figure 1).5 Briefly, PCR was performed using 50 ng of DNA in a reaction containing 1× Amplitaq Gold buffer, 5% dimethyl sulfoxide, 1 mol/L betaine, dNTP mixture with 7-deaza-GTP instead of dGTP (0.25 mmol/L each), 0.9 mmol/L MgCl2, 1 μmol/L each of FAM-labeled forward (sizing forward primer, 5′-FAM-CAAGGAGGGAAACAACCGCAGCC-3′) and reverse (sizing reverse primer, 5′-GCAGGCACCGCAACCGCAG-3′) primers, and 0.5 U/reaction Amplitaq Gold polymerase (Life Technologies, Carlsbad, CA). Repeat-primed PCR (RP-PCR) toward the downstream side of the C9orf72 repeat expansion region was performed using 1 μg of DNA in a reaction containing 200 μmol/L each dNTP, 7.1% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO), 0.93 mol/L betaine (Sigma-Aldrich), 0.18 mmol/L deaza-GTP (Roche Life Science, Tucson, AZ), 0.9 mmol/L MgCl2 (Roche Life Science), 1.4 μmol/L FAM-labeled downstream reverse primer (C9DR: 5′-FAM-AGTCGCTAGAGGCGAAAGC-3′), 0.175 μmol/L forward repeat primer (C9DRP: 5′-TACGCATCCCAGTTTGAGACGGGGGCCGGGGCCGGGGCCGGGG-3′), and 1.4 μmol/L anchor primer (5′-TACGCATCCCAGTTTGAGACG-3′) matching the end of C9DRP, and 1.125 units FastStart polymerase (Roche Life Science). RP-PCR toward the upstream end of the C9orf72 repeat expansion region, reverse RP-PCR (RvRP-PCR), was performed under the same conditions as RP-PCR using 1.4 μmol/L FAM-labeled forward primer (upstream forward primer, 5′-CACTACTTGCTCTCACAGTACT-3′), 0.175 μmol/L reverse repeat primer (upstream repeat primer that contains an adapter primer tail, 5′-TACGCATCCCAGTTTGAGACGCCCCG-GCCCCGGCCCCGGCCCC-3′), and 1.4 μmol/L anchor primer. The three reactions comprising the laboratory-developed C9orf72 expansion procedure (Penn-C9) assay (sizing PCR, RP-PCR, and RvRP-PCR) were performed with touchdown PCR cycling conditions consisting of 4 minutes at 95°C, followed by cycles of 95°C for 30 seconds, annealing starting at 70°C for 1 minute, and extension at 72°C for 3 minutes, ending with a final extension step of 10 minutes at 72°C. The annealing temperature was decreased in 2°C steps as follows: 70°C for two cycles, 68°C for three cycles, 66°C for four cycles, 64°C for five cycles, 62°C for six cycles, 60°C for seven cycles, 58°C for eight cycles, and 56°C for five cycles. The ramp rate was set to 18%. PCR products, 2 μL sizing product diluted 1:10 in water or undiluted RP-PCR or RvRP-PCR products, were separated by capillary electrophoresis with 23 seconds' injection time using POP-7 polymer and a 36-cm 16-capillary array on an ABI PRISM 3130xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Fragment sizes were determined by size standard Genescan ROX-500 (Thermo Fisher Scientific) with GeneMapper software version 3.7 (Thermo Fisher Scientific). A repeat size of ≥30 repeats was used as the cutoff for an expansion.
AmplideX PCR/CE C9orf72 (RUO) Assay
A long-read PCR was performed, as described in the manufacturer's protocol (Asuragen, Inc.), consisting of a reaction with both gene-specific primers and repeat-primed amplification (Figure 1G). Briefly, 40 ng of DNA was mixed in a reaction containing 1× GC-rich amplification buffer, the gene-specific primer mix, repeat primer, and GC-rich polymerase mix; PCR was performed with PCR cycling conditions consisting of 5 minutes at 98°C, followed by 37 cycles of 97°C for 35 seconds, annealing starting at 62°C for 35 seconds, and extension at 72°C for 3 minutes, ending with a final extension step of 10 minutes at 72°C. PCR product (2 μL) was separated by capillary electrophoresis with a 20-second injection time using POP-7 polymer and a 36-cm 16-capillary array on an ABI PRISM 3130xl Genetic Analyzer. PCR fragment size was determined by ROX-1000 size standard (Asuragen, Inc.) with GeneMapper software version 3.7. The AmplideX-C9 assay includes the option of performing PCR with gene-specific primers only and running the products on an agarose gel; however, this option was not fully evaluated.
Southern Blot Analysis for Determination of Expanded C9orf72 Hexanucleotide Repeat Length
Southern blotting was performed as previously described.5 Briefly, 3 μg genomic DNA was digested at 37°C for 16 hours with AluI and DdeI (New England Biolabs, Ipswich, MA), denatured at 95°C for 5 minutes, and run on a 0.8% agarose gel at 100 V for 4 hours. DNA was transferred to a positively charged nylon membrane (GE Healthcare Life Sciences, Pittsburgh, PA) and cross-linked to the membrane via UV irradiation, and the blot was hybridized at 48°C overnight with 15 ng/μL of digoxigenin (DIG)-labeled (G4C2)5 oligonucleotide probe (Integrated DNA Technologies, Coralville, IA) per mL of DIG Easy Hyb buffer (Roche Life Science). After washing, the blot was incubated with anti-DIG antibody (1:10,000; Roche Life Science, Indianapolis, IN) and signals were developed with CSPD (Roche Life Science) and exposed in a ChemiDoc XRS system (Bio-Rad, Hercules, CA). C9orf72 hexanucleotide repeat length was determined as previously described.5 A Coriell lymphoblast cell line (Coriell Institute, Camden, NJ), ND11836, was used as a C9orf72 expansion-positive control and size ladder.
Sequencing of PCR Amplicons to Assess Indels in the Repeat Region
PCR products of interest were excised from 3% low-melt agarose gel, purified by the QIAquick PCR purification kit (Qiagen), and sequenced at the University of Pennsylvania Sequencing Core using the appropriate sequencing primers without FAM labels: sizing forward primer and sizing reverse primer for sizing PCR products or C9orf72 sequencing primer reverse 5(C9SeqR) (5′-CCAGCTTCGGTCAGAGAAAT-3′) for RP-PCR products.25
Results
Comparison of AmplideX-C9 and Penn-C9 Assays
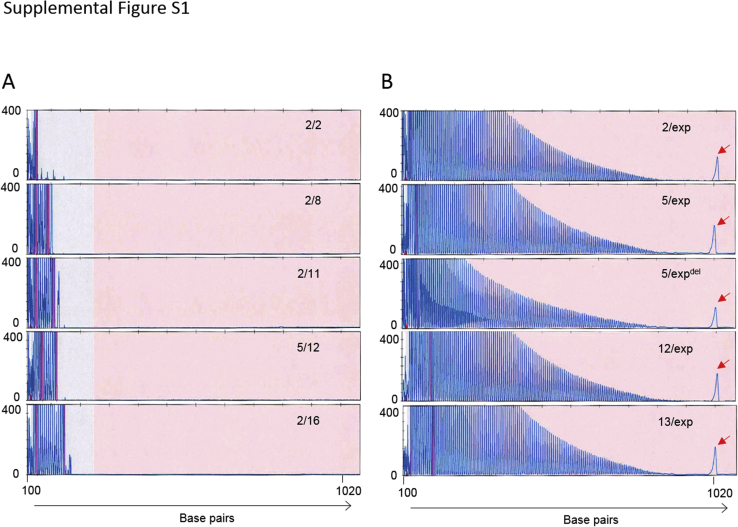
The AmplideX-C9 assay assesses the C9orf72 hexanucleotide repeat region with both gene-specific and repeat-primed primers in a single reaction, whereas the Penn-C9 assay is performed in three separate reactions: one for sizing normal and small expansion alleles and two RP-PCRs for assessing expansions from upstream and downstream directions (Figure 1G). The AmplideX-C9 assay requires 20 to 40 ng of DNA input and the completion time per 16 reactions, including PCR setup, capillary electrophoresis, and analysis (6 hours, including 1.5 hours of hands-on time, which is faster and more efficient compared with the Penn-C9 assay) (Table 1). In the Penn-C9 workflow, C9orf72 G4C2 repeat size is initially evaluated using the sizing assay; expansion status is determined by RP-PCR, whereas AmplideX-C9 determines both repeat size and expansion status in a single reaction (Figure 1G). An additional assay, RvRP-PCR, is included in the Penn-C9 workflow or done as a reflex in homozygous normal allele cases to improve clinical sensitivity for detection of an expansion in case of indels in the downstream region of the repeat. The accurate sizing range of the Penn-C9 RP-PCR is between 4 and 70 hexanucleotide (G4C2) repeats; two and three repeat alleles can be detected but are unable to be distinguished from each other, except on the sizing assay, because the repeat primer contains and incorporates three G4C2 repeats into the PCR product. In contrast, the AmplideX-C9 assay demonstrates reliable amplification and accurate sizing of between 1 and 140 G4C2 repeats at high resolution (Figure 1F). The sizing limit may represent the upper limit of capillary electrophoresis resolution on an ABI 3130xl rather than the upper limit of repeat amplification of the AmplideX-C9 assay, because a pile-up peak is seen with the AmplideX-C9 in large expansion cases, resulting from inability to separate larger PCR products. The presence of pile-up peaks is not unique to C9orf72 because this phenomenon is also observed with FMR1 PCR.26 A pile-up peak is not present in any normal or intermediate case tested (Supplemental Figure S1). Furthermore, the AmplideX-C9 method increases the peak amplitudes of C9orf72 repeat expansion amplification by fourfold or more compared with Penn-C9, despite using much less input DNA, demonstrating the greater PCR efficiency of the assay (Figure 1, D and F).
Table 1.
Comparison of AmplideX-C9 and Penn-C9 Workflow
| Variable | Penn-C9 | AmplideX-C9 |
|---|---|---|
| PCRs, n | 3 | 1 |
| Assays | Sizing PCR | Gene-specific PCR and RP-PCR in a single reaction |
| Forward RP-PCR | ||
| Reverse RP-PCR | ||
| Input DNA, ng | 1000–2000 | 20–40 |
| Total time per 16 samples, hours | 8 | 6 |
| Hands-on time per 16 samples, hours | 2 | 1.5 |
| Detection range, bp | 128–680 | 121–840 |
| Sizing range, repeats | 2–70 | 1–140 |
| Ability to distinguish two- and three-repeat alleles | Sizing assay only | Yes |
RP-PCR, repeat-primed PCR.
Concordance of AmplideX-C9 and Penn-C9 Assays
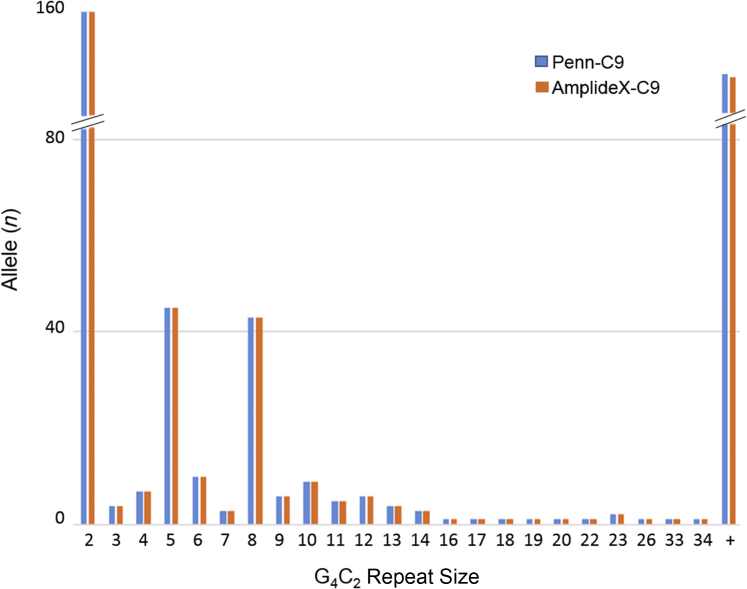
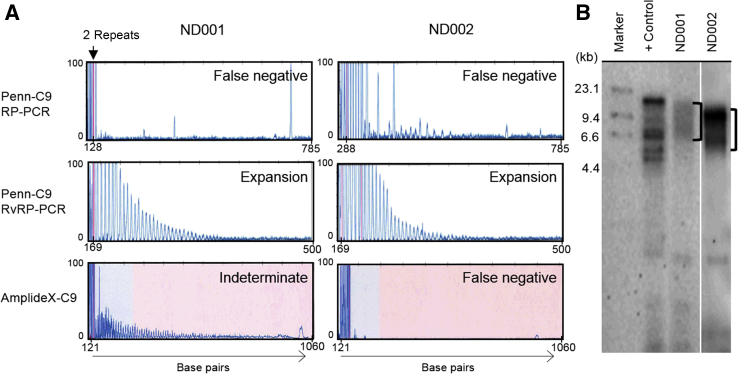
Performance of the AmplideX-C9 assay was evaluated by testing 255 DNA samples from 228 individuals for whom C9orf72 was previously assessed by the Penn-C9 method and evaluating the concordance of the results by both methods.5 The Penn-C9 results included genotypes with both normal alleles (≤20 repeats, n = 83), one normal allele plus one intermediate allele (21 to 29 repeats, n = 4), and one normal plus one C9orf72 expanded allele (≥30 repeats, n = 141). The size of all individual C9orf72 alleles up to 34 repeats was concordant between the two assays (Figure 2). As reported elsewhere, the most common normal allele sizes were of two, five, and eight repeats.5, 11 The concordance of the overall qualitative genotype interpretations is shown in Table 2. All Penn-C9 normal genotypes (83 of 83) were also interpreted as normal by the AmplideX-C9 assay; similarly, all cases with one intermediate-sized allele (four of four) were concordant by AmplideX-C9. Of 141 cases with a large C9orf72 expansion, as determined by Penn-C9 and confirmed by Southern blot, the concordance with AmplideX-C9 was 98.6% (139 of 141). However, the two discordant cases were only detectable by the Penn-C9 RvRP-PCR (Figure 3), designed to avoid the downstream region adjacent to the hexanucleotide repeat sequence, which is known to have a high rate of indels and other sequence variations that can affect the standard RP-PCR results.24, 25 Of the two discordant cases, one had a low-amplitude saw-tooth signal using AmplideX-C9, insufficient to interpret confidently as an expansion, so it was called indeterminate, whereas the other case was not detectable by the AmplideX-C9 assay (Figure 3A). The C9orf72 expansions in both discordant cases were verified by Southern blot (Figure 3B). Obvious somatic differences in size of the C9orf72 expansion were not observed in selected cases, in which pairs of DNA extracted from peripheral and frozen brain tissue from the same individual were tested by both Penn-C9 and AmplideX-C9 assays. DNA from all sample types tested (blood, saliva, and brain tissue) yielded concordant results (data not shown). The overall concordance of Penn-C9 and AmplideX-C9 was 99.1% (226 of 228 results). For the AmplideX-C9 assay, the clinical sensitivity was 99.3% (95% CI, 0.9552–0.9996), the clinical specificity was 100% (95% CI, 0.9473–1), the positive predictive value was 100% (95% CI, 0.9667–1), and the negative predictive value was 98.9% (95% CI, 0.9295–0.9994) (Table 2).
Figure 2.
Accurate sizing and concordance between AmplideX-C9 and Penn-C9 assays by repeat size. The repeat sizes of C9orf72 alleles were determined by Penn-C9, and AmplideX-C9 shows high concordance between the two assays at all G4C2 repeat allele sizes. n = 456 C9orf72 alleles. +, expansion.
Table 2.
Concordance of C9orf72 Genotypes of Patients with FTD and ALS between AmplideX-C9 and Penn-C9
| Assay method | AmplideX-C9 (n = 228) |
||||
|---|---|---|---|---|---|
| C9orf72 genotype | Expansion not detected | One intermediate allele present | Expansion detected | Indeterminate for expansion | |
| Penn-C9 (n = 228) | Expansion not detected, n | 83 | 0 | 0 | 0 |
| One intermediate allele present, n | 0 | 4 | 0 | 0 | |
| Expansion detected, n | 1∗ | 0 | 139 | 1∗ | |
ALS, amyotrophic lateral sclerosis; FTD, frontotemporal degeneration.
Discrepant resolution of these two cases is described in Figure 3.
Figure 3.
Result comparisons for two discordant samples. A: The electropherograms are shown for two samples (ND001 and ND002), tested by the Penn-C9 repeat-primed PCR (RP-PCR) assays, reverse RP-PCR (RvRP-PCR), and AmplideX-C9, with the result interpretation for each reaction indicated to the right of each panel. An expansion is not detected for either sample using the Penn-C9 RP-PCR (false negative), whereas using the Penn-C9 RvRP-PCR assay with upstream instead of downstream primers, the characteristic saw-tooth pattern of a repeat expansion is seen for both samples. Using AmplideX-C9, the expansion in ND002 is not detected; however, a low-amplitude peak pattern is seen for ND001, which is interpreted as indeterminate for an expansion. The sequence variation responsible for the false-negative downstream PCR results has not yet been fully characterized. B: Southern blot hybridization with a repeat probe confirms the C9orf72 expansion in ND001 and ND002. Brackets indicate the size ranges of C9orf72 repeat expansion with somatic mosaicism. +Control, DNA from a C9orf72 expansion-positive control lymphoblast cell line; Marker, DIG molecular-weight marker.
Limit of Detection and Analytic Sensitivity
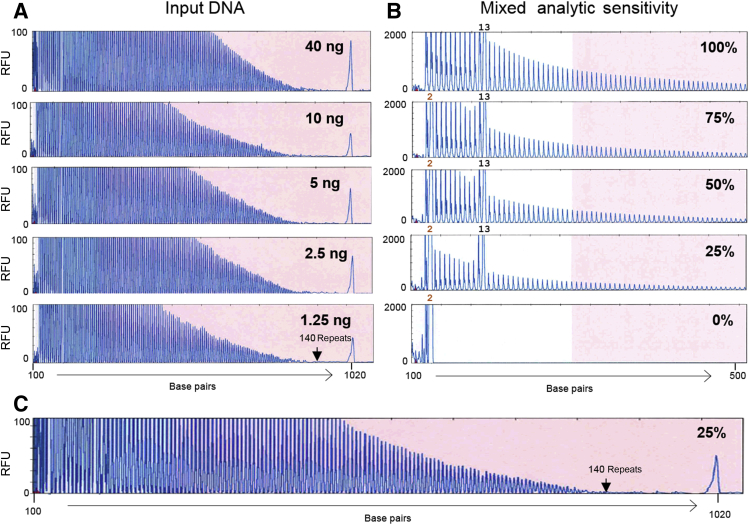
The Penn-C9 assay optimally requires a total of 1 o 2 μg DNA, with 50 ng used in the sizing PCR and 0.5 to 1 μg per reaction in each of the RP-PCRs, as validated for use in clinical testing. In the present study, the analytic sensitivity of AmplideX-C9 in the analysis of a sample with a C9orf72 expansion was tested using decreasing amounts of DNA input, ranging from 40 down to 1.25 ng. The AmplideX-C9 was able to easily detect the C9orf72 expansion, with as low as 1.25 ng of DNA input with no loss of robustness for sizing up to 140 repeats (Figure 4A). The ability to detect a low-level expanded sample (heterozygous for expansion and a 13-repeat allele) admixed with DNA from a nonexpanded case (homozygous for 2 repeat alleles), in ratios of 1:3, 1:1, and 3:1, respectively, was also assessed. The amplitude of the 25% of mass fraction of expanded sample was approximately 40% that of the expanded sample alone, without reduction in the ability to accurately size the PCR products up to 140 repeats (Figure 4, B and C).
Figure 4.
Analytic sensitivity of the AmplideX-C9 assay. A: A C9orf72 expansion sample was tested on AmplideX-C9 using decreasing DNA input, starting with the recommended 40 ng. The results demonstrate a sensitive and robust saw-tooth amplification pattern down to 1.25 ng with the peak for 140 repeats still detectable, as well as the pile-up peak at 1020 bp. B: A sample heterozygous for a C9orf72 expansion and a normal allele of 13 repeats (100%) were admixed with a sample homozygous for two repeats (0%) at ratios of 3:1 expanded to normal (75%), 1:1 (50%), and 1:3 (25%) and amplified with AmplideX-C9. The zoomed-in electropherograms are shown, highlighting the locations of the 2- and 13-repeat normal alleles. In the 25% mixture, the amplitude of the expanded allele peaks is approximately 40% of the neat sample peak amplitude. C: Full range view of the 25% mixture electropherogram from B showing the ability to still count up to 140 repeats in this sample. RFU, relative fluorescence units.
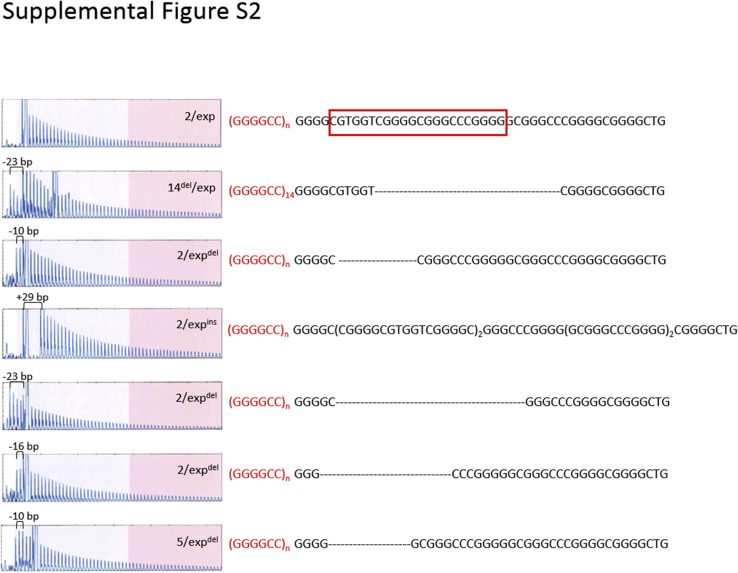
Detection of Indels and Sequence Variations in the Hexanucleotide Repeat Tract
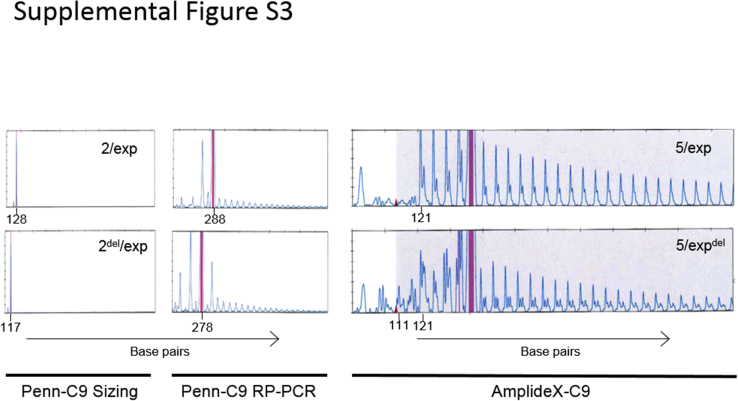
Precise determination of repeat number may be important for studying the potential functional impacts of the repeat length on clinical phenotypes. The region adjacent to the hexanucleotide repeat is known to have a high rate of indels (Figure 1G and Supplemental Figure S2), which can complicate accurate determination of the number of repeats in alleles with indels and may lead to a false-negative result for an expansion, as discussed above (Figure 3). The repeat number can be determined in the Penn-C9 assay by knowing the size of the expected products at approximately 6-bp intervals for both sizing and RP-PCRs. However, with the RP-PCR assay, a two-repeat allele cannot be distinguished from a three-repeat allele because the primer incorporates three repeats into all products. Suspicion for presence of an indel on a normal or expanded allele is based on seeing peaks that are of an unexpected size or that have an unusual saw-tooth peak pattern or larger than expected size gaps between some peaks in the RP-PCR electropherogram (Supplemental Figure S3). The robustness for detection of indels between Penn-C9 and AmplideX-C9 assays was compared. Of the 228 cases selected for this study, including the cases that demonstrated an atypical saw-tooth pattern by Penn-C9 RP-PCR, indels were detected in 6 nonexpansion alleles and 12 expansion alleles. Notably, the AmplideX-C9 assay detected indels in not only these 18 cases, but also in the expansion alleles of an additional 27 cases, demonstrating that the AmplideX-C9 method facilitated the detection of indels obscured by non-specific background peaks in expansion alleles and, therefore, were not detected with the Penn-C9 method (Figure 5). On the other hand, the scoring of indels in the nonexpanded alleles was concordant between Penn-C9 and AmplideX-C9. In the Center for Neurodegenerative Disease Research cohort (n = 2146), including cases with a variety of neurodegenerative diseases and healthy controls, the overall frequency of indels in expansion alleles was 27.46% (39 of 142 alleles), which was significantly greater than the frequency of 0.92% (38 of 4150 alleles) in the nonexpansion alleles (odds ratio = 40.9729, P < 0.0001) (Table 3), suggesting that indels are frequently found in expanded alleles within the immediate downstream region of the C9orf72 expansion site. AmplideX-C9 also demonstrated robustness in detection of potential mosaicism of the C9orf72 expansion compared with the Penn-C9 RP-PCR assay, as noted by the observation of heterogeneous saw-tooth patterns in some cases only in the AmplideX-C9 electropherograms (Figure 6).
Figure 5.
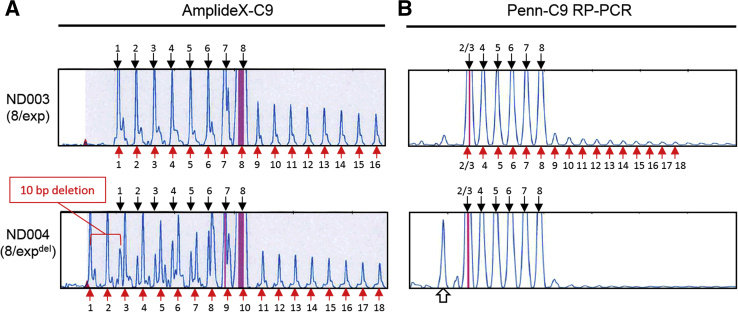
Detection of a deletion on the expansion allele. The repeat-primed peak patterns for AmplideX-C9 (A) and Penn-C9 repeat-primed PCR (RP-PCR) assays (B) for two samples (ND003 and ND004), each with an eight-repeat normal allele and a C9orf72 expansion (8/exp); however, ND004 has a 10-bp deletion on the expansion allele (8/expdel), which is clearly visible on the AmplideX-C9 peak profile, but not with the Penn-C9 RP profile. The numbered arrows correspond to the number of amplified G4C2 repeats for each peak from the normal allele (black, labels on top) and expansion allele (red, labels on bottom). For ND003, with no deletion, the normal and expanded allele peaks are indistinguishable; the shoulder peak, which is evident, is typically seen. The AmplideX-C9 detects and distinguishes the peaks down to one repeat, whereas the Penn-C9 assay does not distinguish between a two- and three-repeat allele. For ND004, a heterogeneous pattern can be seen with an offset of 10 bp to the left from the one repeat normal allele peak (red bracket). Around the normal seven-repeat allele peak, the two alleles become indistinguishable; and after the normal eight-repeat allele, only the expansion allele peaks are seen. The 10-bp deletion was confirmed by Sanger sequencing (data not shown). In contrast, with the Penn-C9 RP-PCR reagents, a peak consistent with the deletion is seen (open arrow); however, because non-specific peaks are frequently seen in this region of variable amplitudes, a deletion cannot be definitively identified with this method alone.
Table 3.
Frequency of Indels in C9orf72 Alleles in the Neurodegenerative Disease Cohort at University of Pennsylvania
| C9orf72 allele | Total alleles, n | Alleles with indel, n | Allele frequency, % | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|---|
| Nonexpanded allele∗ | 4150 | 38 | 0.92 | |||
| Expanded allele | 142 | 39 | 27.46 | 40.9729 | 25.159–66.725 | <0.0001 |
Indel, insertion/deletion.
Data for nonexpanded alleles were obtained from Center for Neurodegenerative Disease Research cohort patients with a variety of neurodegenerative diseases and healthy controls tested with the Penn-C9 assay, which is sensitive for detection of indels on nonexpanded alleles.
Figure 6.
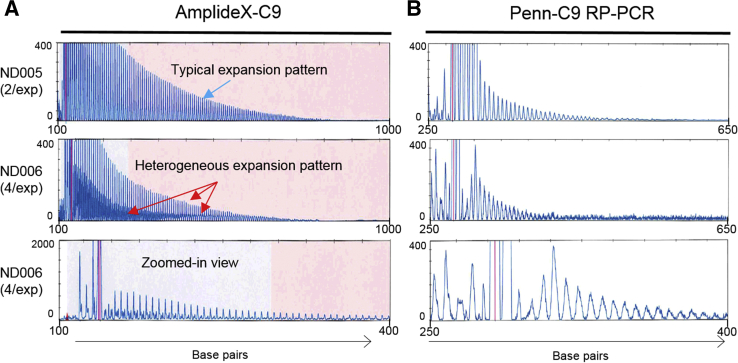
Mosaicism of the expansion allele pattern. The repeat-primed peak patterns for AmplideX-C9 (A) and Penn-C9 repeat-primed PCR (RP-PCR) assays (B) for two samples. One (ND005) demonstrates a typical saw-tooth peak pattern for both assays, whereas the pattern for ND006 demonstrates a heterogeneous expansion peak pattern (red arrows) with the AmplideX-C9 assay suggestive of mosaicism of the C9orf72 expansion, which is not observed with the Penn-C9 assay. The view of the x axis for ND006 is zoomed in on the bottom row, showing additional peaks between each repeat peak. Red lines indicate off-scale peaks. This heterogeneous expansion peak pattern is not obvious with the Penn-C9 assay. 2/exp, 2 repeats and expansion; 4/exp, 4 repeats and expansion.
Testing for C9orf72 Expansion FFPE DNA Samples
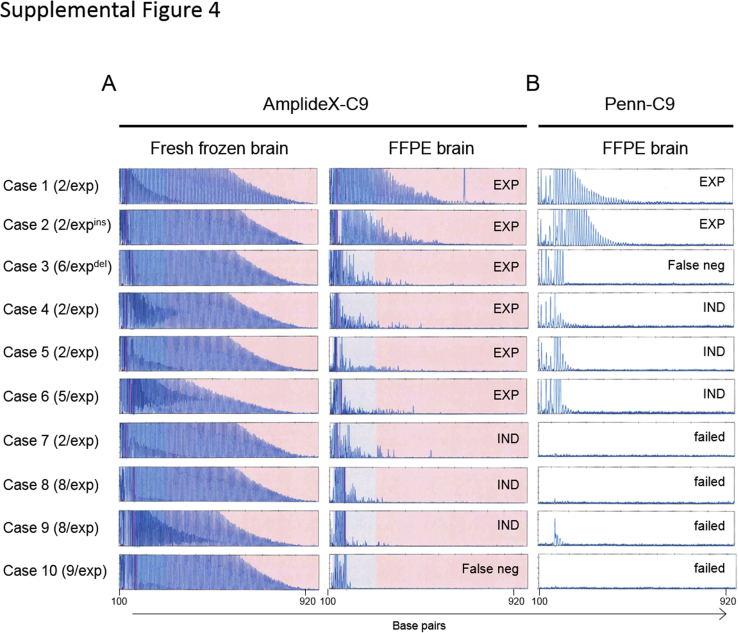
The large size and GC-rich content of the C9orf72 hexanucleotide repeat expansions pose a challenge for detection in any sample type, let alone fragmented DNA from FFPE tissue. Nevertheless, assessing FFPE brain tissue for C9orf72 expansions may be necessary for research or in cases in which testing tissue from an affected family member who died and had an autopsy with no stored frozen tissue could confirm the genetic cause of disease in the family. Although amplification of alleles of less than approximately 12 hexanucleotide repeats by the Penn-C9 sizing reaction using FFPE DNA has been successful (data not shown), RP-PCR with FFPE DNA has been a challenge because of degradation, fragmentation, and/or impurity of DNA from FFPE tissues. The performance of the AmplideX-C9 was evaluated using DNA from FFPE in 10 cases in which DNA was also available from matched DNA from fresh-frozen brain tissue. The results were compared with the Penn-C9 RP-PCR assay (Supplemental Figure S4). AmplideX-C9 was able to generate either a typical saw-tooth pattern or an irregular peak pattern suggestive of a C9orf72 expansion in 6 of 10 cases (Supplemental Figure S4A), whereas an expansion was only detected definitively in two cases using the Penn-C9 RP-PCR with FFPE DNA (Supplemental Figure S4B). The inconsistency of the performance of the C9orf72 assay using FFPE DNA might be caused by various factors, including DNA quality, fixation time, and post-mortem interval, which needs to be further investigated. The results demonstrate that the high sensitivity and robustness of the AmplideX-C9 method increased the amplitude and detection limits of PCR products of FFPE DNA, suggesting that it may improve the likelihood of success for testing the C9orf72 repeat expansion when FFPE tissue is the only source of DNA from patients and/or family members.
Discussion
In this study, the AmplideX-C9 assay, a novel long-read PCR assay for genotyping of C9orf72 alleles and detection of pathogenic hexanucleotide repeat expansions, was evaluated. Its workflow and performance characteristics were compared with the existing laboratory-developed C9orf72 procedure. The laboratory-developed C9orf72 PCR workflow was optimized with approximately 1 to 2 μg of genomic DNA for three separate PCR assays, including a sizing PCR and downstream and upstream RP-PCR amplifications; AmplideX-C9 performed both gene-specific and repeat-primed PCR in a single tube with 40 ng of input DNA. AmplideX-C9 significantly shortened the hands-on time for C9orf72 molecular genetic testing and lowered the required DNA input compared with Penn-C9, thereby permitting testing of samples with limited available DNA and preserving precious banked samples.
The overall concordance between Penn-C9 and AmplideX-C9 was 99.1%, with only two discordant samples, which were only detectable by the Penn-C9 RvRP-PCR, which is not surprising given this reaction was specifically designed for this scenario. Because the current AmplideX PCR/CE C9orf72 kit amplifies only the downstream sequences of the C9orf72 repeat expansion region, which contains hot-spots of indels and sequence variations, inclusion of a bidirectional assay to detect the C9orf72 expansion (like the Penn-C9 RvRP-PCR) would improve the clinical sensitivity of AmplideX-C9 if it has been fully validated as an LDP. From a workflow perspective, a reverse direction amplification could be done upfront in all cases, as done with Penn-C9, or alternatively added as a reflex amplification only in cases that are homozygous for a normal repeat allele (ie, in cases in which two separate normal alleles are not detected). However, because the homozygosity rate in nonexpanded individuals is approximately 30%, it may be more efficient to include the extra amplification up front. Follow-up Southern blot is possible, but it is labor intensive and primarily used as a research tool and in cases with indeterminate results.
Genotype data generated by AmplideX-C9 were robust, with high peak amplitudes and allele sizing information that was accurate and of high resolution (from 1 to 140 repeats), even with low DNA input or in a 25% mixture with control DNA. The AmplideX-C9 assay also demonstrated improved success using DNA from FFPE tissue. The detection of indels in expansion alleles was also improved using the AmplideX-C9 compared with the Penn-C9 method. Indels were identified in 27.5% (39 of 142) of expanded alleles compared with only 8.5% (12 of 142) of expanded alleles using the Penn-C9 method, suggesting that the frequency of indels on the expanded allele is much higher than previously thought. Consequently, the clinical implication of the sequence variations on the expansion alleles may not be fully recognized or studied. Although the cause and effect of indels is yet unknown, it has been suggested to be attributable to instability of the C9orf72 expansion locus, which merits further study given the higher frequency identified in this study.25 Although rare, homozygous expansion cases (ie, no normal-sized alleles) have been reported.27 With AmplideX-C9, the high-amplitude normal allele visible above the saw-tooth pattern is absent in a tested homozygous expansion case (data not shown), suggesting that it is missing a normal allele. If desired, the gene-specific primers from the Asuragen, Inc., kit can be used alone without the repeat primer to confirm absence of a normal allele. Thus, to detect a homozygous expansion using AmplideX-C9, one must pay attention to the peak amplitudes rather than just the expansion pattern, unlike in an assay where the sizing amplification is separate from the repeat-primed assay, such as Penn-C9. If suspicion for homozygous expansion is raised, the Asuragen, Inc., gene-specific primers can be used, as described, to confirm the suspicion and show that no normal allele product is amplified.
The high sensitivity and resolution of the AmplideX-C9 assay also allowed detection of potential mosaicism, which is visualized by heterogeneous saw-tooth patterns. Heterogeneity likely reflects sequence variation outside of pure G4C2 repetition; however, the underlying composition of the mosaicism needs to be further investigated to determine its sequence or origin. Furthermore, because of its ability to detect and size expansions of up to approximately 140 repeats, the AmplideX-C9 assay is theoretically able to detect mosaicism of smaller expansions that are not able to be distinguished from larger expansions with the Penn-C9 assay. Somatic mosaicism of repeat expansions has been previously reported in trinucleotide repeat expansion diseases, including fragile X syndrome, caused by a CGG trinucleotide repeat expansion in the 5′ untranslated region of the FMR1 gene,28 and spinocerebellar ataxia type 1, caused by a CAG repeat expansion in the coding region of the ataxin-1 gene.29 It is still not clear if there is any association between indels/sequence variations/mosaicism and clinical-pathologic phenotypes. This long-read PCR technology will be a valuable tool to enable the detection of indels that were missed by other methods, providing more accurate variation data to perform correlation analyses.
In conclusion, AmplideX-C9, a commercially available, novel, long-read PCR/CE assay, is a sensitive, robust, and efficient method for detection and characterization of the C9orf72 hexanucleotide repeat expansion. Compared with standard repeat-primed C9orf72 genotyping procedures, like Penn-C9, it has the added advantage of being able to detect indels on expanded C9orf72 alleles and to identify repeat mosaicism with increased sensitivity, which will serve as an important research tool for further study of structural variations in C9orf72.
Acknowledgments
We thank the patients and family members who participated in research and made these studies possible and our many colleagues at the University of Pennsylvania who helped to recruit and evaluate subjects, collect biosamples, and perform a thorough diagnostic neuropathological evaluation of the autopsy cases.
Footnotes
Supported by NIH grants P01-AG-017586 (V.M.V.D.) and R44-NS089423 (V.M.V.D.) and ALS Association grant 16-LGCA-285 (V.M.V.D.). Eran Bram and Kristen Culp (Asuragen Inc.) provided reagents and technical consultation.
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.jmoldx.2018.07.001.
Supplemental Data
Supplemental Figure S1.
The pile-up peak is detected in cases with large expansion in C9orf72. Representative capillary electropherograms for samples without (A) and with (B) C9orf72 G4C2 repeat expansion using AmplideX-C9. PCR products were separated on an ABI 3130xl Genetic Analyzer and visualized by GeneMapper software version 3.7. The vertical pink bars indicate off-scale peaks. High-molecular-weight pile-up peaks are indicated by red arrows, which are not present in the cases without C9orf72 expansion. G4C2 repeat sizes are indicated in each electropherogram. exp, expansion; expdel, expansion with deletion.
Supplemental Figure S2.
The repeat-primed (RP) profiles of the AmplideX-C9 assay and the sequences of the representative cases carrying the C9orf72 expansion with insertions/deletions (indels) downstream of the expansion region. The 22-bp region with frequent indels in the downstream region of the hexanucleotide repeat region, (GGGGCC)n, is shown in the red boxed area in the top reference sample. G4C2 repeat sizes are indicated in each electropherogram. exp, expansion; expdel, expansion with deletion.
Supplemental Figure S3.
Detection of insertions/deletions in C9orf72 alleles by size determination using the PCR product profiles. Unusual size of amplicons indicates insertion or deletion between the binding sites of the forward and reverse primers for the Penn-C9 and AmplideX-C9 assays. The vertical pink bars indicate off-scale peaks. RP-PCR, repeat-primed PCR. G4C2 repeat sizes are indicated in each electropherogram. exp, expansion; expdel, expansion with deletion.
Supplemental Figure S4.
Comparison of the sensitivity between AmplideX-C9 and Penn-C9 in detecting the C9orf72 expansion using formalin-fixed, paraffin-embedded (FFPE) DNA. Genomic DNA was extracted from paired fresh-frozen brain and FFPE tissue from 10 representative cases carrying the C9orf72 expansion. Allele sizes for each case are indicated in the parentheses. Expansions without sequence variation (exp), with an insertion (expins), or with a deletion (expdel) are noted. All samples were tested with AmplideX-C9 (A) and Penn-C9 repeat-primed PCR assays (B). The result for the fresh-frozen brain for Penn-C9 is not shown, but all had the expected saw-tooth pattern for a C9orf72 expansion. Genotypes, with the number of repeats on the normal allele, are indicated for each case on the left. The result interpretation for each FFPE electropherogram is indicated: expansion (EXP), false negative (False neg), indeterminate (IND), or no amplification (failed).
References
- 1.Irwin D.J., Cairns N.J., Grossman M., McMillan C.T., Lee E.B., Van Deerlin V.M., Lee V.M., Trojanowski J.Q. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015;129:469–491. doi: 10.1007/s00401-014-1380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: from genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belzil V.V., Katzman R.B., Petrucelli L. ALS and FTD: an epigenetic perspective. Acta Neuropathol. 2016;132:487–502. doi: 10.1007/s00401-016-1587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Mossevelde S., van der Zee J., Cruts M., Van Broeckhoven C. Relationship between C9orf72 repeat size and clinical phenotype. Curr Opin Genet Dev. 2017;44:117–124. doi: 10.1016/j.gde.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Suh E., Lee E.B., Neal D., Wood E.M., Toledo J.B., Rennert L., Irwin D.J., McMillan C.T., Krock B., Elman L.B., McCluskey L.F., Grossman M., Xie S.X., Trojanowski J.Q., Van Deerlin V.M. Semi-automated quantification of C9orf72 expansion size reveals inverse correlation between hexanucleotide repeat number and disease duration in frontotemporal degeneration. Acta Neuropathol. 2015;130:363–372. doi: 10.1007/s00401-015-1445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., Kouri N., Wojtas A., Sengdy P., Hsiung G.Y., Karydas A., Seeley W.W., Josephs K.A., Coppola G., Geschwind D.H., Wszolek Z.K., Feldman H., Knopman D.S., Petersen R.C., Miller B.L., Dickson D.W., Boylan K.B., Graff-Radford N.R., Rademakers R. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renton A.E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J.R. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zee J., Gijselinck I., Dillen L., Van Langenhove T., Theuns J., Engelborghs S. A pan-European study of the C9orf72 repeat associated with FTLD: geographic prevalence, genomic instability, and intermediate repeats. Hum Mutat. 2013;34:363–373. doi: 10.1002/humu.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Tortosa E., Gallego J., Guerrero-Lopez R., Marcos A., Gil-Neciga E., Sainz M.J., Diaz A., Franco-Macias E., Trujillo-Tiebas M.J., Ayuso C., Perez-Perez J. C9ORF72 hexanucleotide expansions of 20-22 repeats are associated with frontotemporal deterioration. Neurology. 2013;80:366–370. doi: 10.1212/WNL.0b013e31827f08ea. [DOI] [PubMed] [Google Scholar]
- 10.Ratti A., Corrado L., Castellotti B., Del Bo R., Fogh I., Cereda C., Tiloca C., D'Ascenzo C., Bagarotti A., Pensato V., Ranieri M., Gagliardi S., Calini D., Mazzini L., Taroni F., Corti S., Ceroni M., Oggioni G.D., Lin K., Powell J.F., Soraru G., Ticozzi N., Comi G.P., D'Alfonso S., Gellera C., Silani V., Consortium S. C9ORF72 repeat expansion in a large Italian ALS cohort: evidence of a founder effect. Neurobiol Aging. 2012;33:2528.e7–2528.e14. doi: 10.1016/j.neurobiolaging.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Beck J., Poulter M., Hensman D., Rohrer J.D., Mahoney C.J., Adamson G., Campbell T., Uphill J., Borg A., Fratta P., Orrell R.W., Malaspina A., Rowe J., Brown J., Hodges J., Sidle K., Polke J.M., Houlden H., Schott J.M., Fox N.C., Rossor M.N., Tabrizi S.J., Isaacs A.M., Hardy J., Warren J.D., Collinge J., Mead S. Large C9orf72 hexanucleotide repeat expansions are seen in multiple neurodegenerative syndromes and are more frequent than expected in the UK population. Am J Hum Genet. 2013;92:345–353. doi: 10.1016/j.ajhg.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Redondo A., Dols-Icardo O., Rojas-Garcia R., Esteban-Perez J., Cordero-Vazquez P., Munoz-Blanco J.L. Analysis of the C9orf72 gene in patients with amyotrophic lateral sclerosis in Spain and different populations worldwide. Hum Mutat. 2013;34:79–82. doi: 10.1002/humu.22211. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., Lin Z., Chen X., Cao B., Wei Q., Ou R., Zhao B., Song W., Wu Y., Shang H.F. Large C9orf72 repeat expansions are seen in Chinese patients with sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2016;38:217.e15–217.e22. doi: 10.1016/j.neurobiolaging.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Xi Z., Zinman L., Grinberg Y., Moreno D., Sato C., Bilbao J.M. Investigation of c9orf72 in 4 neurodegenerative disorders. Arch Neurol. 2012;69:1583–1590. doi: 10.1001/archneurol.2012.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuytemans K., Bademci G., Kohli M.M., Beecham G.W., Wang L., Young J.I., Nahab F., Martin E.R., Gilbert J.R., Benatar M., Haines J.L., Scott W.K., Zuchner S., Pericak-Vance M.A., Vance J.M. C9ORF72 intermediate repeat copies are a significant risk factor for Parkinson disease. Ann Hum Genet. 2013;77:351–363. doi: 10.1111/ahg.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao B., Guo J.F., Wang Y.Q., Yan X.X., Zhou L., Liu X.Y., Zhang F.F., Zhou Y.F., Xia K., Tang B.S., Shen L. C9orf72 mutation is rare in Alzheimer's disease, Parkinson's disease, and essential tremor in China. Front Cell Neurosci. 2013;7:164. doi: 10.3389/fncel.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dols-Icardo O., Garcia-Redondo A., Rojas-Garcia R., Sanchez-Valle R., Noguera A., Gomez-Tortosa E., Pastor P., Hernandez I., Esteban-Perez J., Suarez-Calvet M., Anton-Aguirre S., Amer G., Ortega-Cubero S., Blesa R., Fortea J., Alcolea D., Capdevila A., Antonell A., Llado A., Munoz-Blanco J.L., Mora J.S., Galan-Davila L., Rodriguez De Rivera F.J., Lleo A., Clarimon J. Characterization of the repeat expansion size in C9orf72 in amyotrophic lateral sclerosis and frontotemporal dementia. Hum Mol Genet. 2014;23:749–754. doi: 10.1093/hmg/ddt460. [DOI] [PubMed] [Google Scholar]
- 18.van Blitterswijk M., DeJesus-Hernandez M., Niemantsverdriet E., Murray M.E., Heckman M.G., Diehl N.N., Brown P.H., Baker M.C., Finch N.A., Bauer P.O., Serrano G., Beach T.G., Josephs K.A., Knopman D.S., Petersen R.C., Boeve B.F., Graff-Radford N.R., Boylan K.B., Petrucelli L., Dickson D.W., Rademakers R. Association between repeat sizes and clinical and pathological characteristics in carriers of C9ORF72 repeat expansions (Xpansize-72): a cross-sectional cohort study. Lancet Neurol. 2013;12:978–988. doi: 10.1016/S1474-4422(13)70210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubers A., Marroquin N., Schmoll B., Vielhaber S., Just M., Mayer B., Hogel J., Dorst J., Mertens T., Just W., Aulitzky A., Wais V., Ludolph A.C., Kubisch C., Weishaupt J.H., Volk A.E. Polymerase chain reaction and Southern blot-based analysis of the C9orf72 hexanucleotide repeat in different motor neuron diseases. Neurobiol Aging. 2014;35:1214.e1–1214.e6. doi: 10.1016/j.neurobiolaging.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 20.Van Mossevelde S., van der Zee J., Gijselinck I., Sleegers K., De Bleecker J., Sieben A., Vandenberghe R., Van Langenhove T., Baets J., Deryck O., Santens P., Ivanoiu A., Willems C., Baumer V., Van den Broeck M., Peeters K., Mattheijssens M., De Jonghe P., Cras P., Martin J.J., Cruts M., De Deyn P.P., Engelborghs S., Van Broeckhoven C., Belgian Neurology Consortium Clinical evidence of disease anticipation in families segregating a C9orf72 repeat expansion. JAMA Neurol. 2017;74:445–452. doi: 10.1001/jamaneurol.2016.4847. [DOI] [PubMed] [Google Scholar]
- 21.Galimberti D., Arosio B., Fenoglio C., Serpente M., Cioffi S.M., Bonsi R., Rossi P., Abbate C., Mari D., Scarpini E. Incomplete penetrance of the C9ORF72 hexanucleotide repeat expansions: frequency in a cohort of geriatric non-demented subjects. J Alzheimers Dis. 2014;39:19–22. doi: 10.3233/JAD-131172. [DOI] [PubMed] [Google Scholar]
- 22.Cooper-Knock J., Shaw P.J., Kirby J. The widening spectrum of C9ORF72-related disease: genotype/phenotype correlations and potential modifiers of clinical phenotype. Acta Neuropathol. 2014;127:333–345. doi: 10.1007/s00401-014-1251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akimoto C., Volk A.E., van Blitterswijk M., Van den Broeck M., Leblond C.S., Lumbroso S. A blinded international study on the reliability of genetic testing for GGGGCC-repeat expansions in C9orf72 reveals marked differences in results among 14 laboratories. J Med Genet. 2014;51:419–424. doi: 10.1136/jmedgenet-2014-102360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rollinson S., Bennion Callister J., Young K., Ryan S.J., Druyeh R., Rohrer J.D., Snowden J., Richardson A., Jones M., Harris J., Davidson Y., Robinson A., Ealing J., Johnson J.O., Traynor B., Mead S., Mann D., Pickering-Brown S.M. Small deletion in C9orf72 hides a proportion of expansion carriers in FTLD. Neurobiol Aging. 2015;36:1601.e1–1601.e5. doi: 10.1016/j.neurobiolaging.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordin A., Akimoto C., Wuolikainen A., Alstermark H., Forsberg K., Baumann P., Pinto S., de Carvalho M., Hubers A., Nordin F., Ludolph A.C., Weishaupt J.H., Meyer T., Grehl T., Schweikert K., Weber M., Burkhardt C., Neuwirth C., Holmoy T., Morita M., Tysnes O.B., Benatar M., Wuu J., Lange D.J., Bisgard C., Asgari N., Tarvainen I., Brannstrom T., Andersen P.M. Sequence variations in C9orf72 downstream of the hexanucleotide repeat region and its effect on repeat-primed PCR interpretation: a large multinational screening study. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18:256–264. doi: 10.1080/21678421.2016.1262423. [DOI] [PubMed] [Google Scholar]
- 26.Filipovic-Sadic S., Sah S., Chen L., Krosting J., Sekinger E., Zhang W., Hagerman P.J., Stenzel T.T., Hadd A.G., Latham G.J., Tassone F. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem. 2010;56:399–408. doi: 10.1373/clinchem.2009.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fratta P., Poulter M., Lashley T., Rohrer J.D., Polke J.M., Beck J., Ryan N., Hensman D., Mizielinska S., Waite A.J., Lai M.C., Gendron T.F., Petrucelli L., Fisher E.M., Revesz T., Warren J.D., Collinge J., Isaacs A.M., Mead S. Homozygosity for the C9orf72 GGGGCC repeat expansion in frontotemporal dementia. Acta Neuropathol. 2013;126:401–409. doi: 10.1007/s00401-013-1147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves T.F., dos Santos J.M., Goncalves A.P., Tassone F., Mendoza-Morales G., Ribeiro M.G., Kahn E., Boy R., Pimentel M.M., Santos-Reboucas C.B. Finding FMR1 mosaicism in fragile X syndrome. Expert Rev Mol Diagn. 2016;16:501–507. doi: 10.1586/14737159.2016.1135739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus-Perrotta C., Lagalwar S. Expansion, mosaicism and interruption: mechanisms of the CAG repeat mutation in spinocerebellar ataxia type 1. Cerebellum Ataxias. 2016;3:20. doi: 10.1186/s40673-016-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.