Abstract
Several studies have reported that mesenchymal stromal/stem cells (MSCs) restore neurological damage through their secretion of paracrine factors or their differentiation to neuronal cells. Based on these studies, many clinical trials have been conducted using MSCs for neurological disorders, and their safety and efficacy have been reported. In this review, we provide a brief introduction to MSCs, especially umbilical cord derived-MSCs (UC-MSCs), in terms of characteristics, isolation, and cryopreservation, and discuss the recent progress in regenerative therapies using MSCs for various neurological disorders.
Keywords: Mesenchymal stromal cell, Umbilical cord, Neurological disorders, Cerebral palsy, Neurotrophic factor
Highlights
-
•
MSCs have recently attracted considerable attention because of notable characteristics.
-
•
MSCs restore neurological injury by their differentiation or secretion of paracrine factors.
-
•
Many clinical studies using MSCs to treat brain injuries have been already ongoing.
1. Introduction
Recently, mesenchymal stromal cells (MSCs) have been attracting much attention for their potential to treat neurological disorders [1], [2], [3].
The concept of MSCs introduced by Caplan in 1991 [4], can be traced to investigation of the inherent osteogenic potential associated with bone marrow (BM) [5]. MSCs have now been reported to be isolated from several sources, including BM, umbilical cord blood (UCB), adipose tissue (AD), and the umbilical cord (UC) [3], [6], [7], [8], [9]. Among various sources of MSCs, we focused on the UC because of (1) their abundance and ease of collection, (2) non-invasive process of collection, (3) little ethical controversy, (4) low immunogenicity with significant immunosuppressive ability, and (5) migration ability towards injured sites [3].
Several studies using neurological disorder models have reported improvements after MSCs administration, and clinical studies using MSCs to treat brain injuries have been already conducted [10], [11], [12], [13], [14], [15].
In this review, we characterize UC-MSCs, in terms of characteristics, isolation, and cryopreservation, and discuss the recent progress of regenerative therapies using MSCs in various neurological disorders.
2. Methods to isolate UC-MSCs
There are several protocols used for isolating and culturing UC-MSCs. We review two major methods: explant method and enzymatic digestion method.
2.1. Improved explant method
Collected UCs are manually minced into approximately 1–2 mm3 fragments. These fragments are aligned and seeded regularly on a tissue culture dish. After the tissue fragments attach to the bottom of the dish, culture media is added, slowly and gently to prevent detachment of the fragments [16], [17], [18]. When the fibroblast-like adherent cells growing from the tissues reach 80%–90% confluence in 2–3 weeks, the cells and tissue fragments are detached using trypsin. The culture is then filtered to remove the tissue fragments.
When using the explant method, it is critical that the UC tissue fragments tightly adhere to the dish to obtain MSCs consistently and efficiently. This is because MSCs can only migrate from the adherent UC tissue fragments and not from floating fragments. In fact, we demonstrated that only the adherent part of the cells in UC tissues showed positive CD105 expression [19]. To prevent the floating of tissue fragments from the bottom of the culture dish, we improved the explant method by using a stainless-steel mesh (Cellamigo®; Tsubakimoto Chain Co.). In addition, the incubation time required to reach 80–90% confluence is reduced upon inclusion of the mesh [19].
2.2. Enzymatic digestion method
UCs are minced into small pieces and immersed in media containing enzymes such as collagenase, or a combination of collagenase and hyaluronidase with or without trypsin [16], [20], [21]. Tissues are then incubated with shaking for 2–4 h, washed with media, and then seeded on a tissue culture dish. MSCs are then obtained as described above.
3. Cryopreservation
Long-term cryopreservation of UCs and UC-MSCs is desirable, because the same donor sample may be required multiple times in the future, and because the cells may be further investigated in the future with techniques yet to be devised. Long-term cryopreservation extends the usability of UC-MSCs. The main technique used to prevent damage is a well-studied combination of slow freezing at a controlled rate, and addition of cryoprotectants [22].
As a cryoprotectant, 5–10% dimethyl sulfoxide (DMSO) with animal or human serum is typically used. Ten percent DMSO and various amounts of fetal bovine serum (FBS) with or without culture medium is the common standard cocktail for the cryopreservation of cells in research facilities [23]. There are several reports of cryopreservation of the UC tissue and MSCs, using serum-free and xenogeneic animal free (xeno-free) cryoprotectants [24], [25].
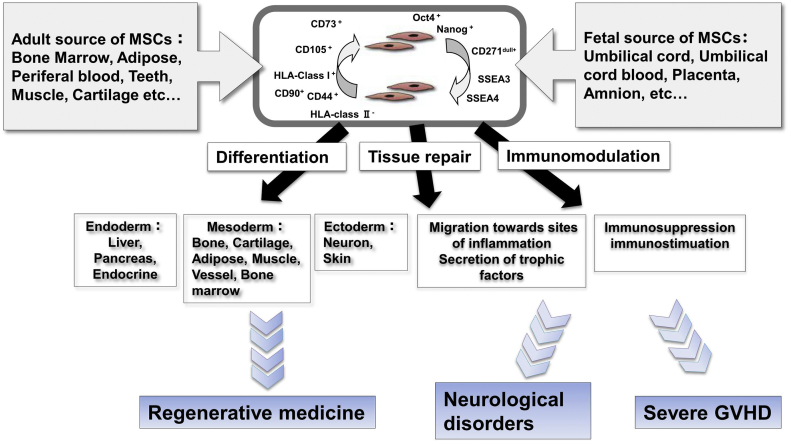
4. Characteristics of MSCs
MSCs and characteristics of MSCs are defined by criteria that form the basis for their use as therapeutic agents (Fig. 1).
Fig. 1.
Characteristics of MSCs.
4.1. Criteria for MSCs; biomarkers and differentiation potentials
The International Society for Cellular Therapy proposed minimal criteria for defining human MSCs [26], [27]. Firstly, MSCs must be plastic-adherent when maintained in standard culture conditions. Secondly, MSC must express CD105, CD73, and CD90, but not CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules. Thirdly, MSCs must differentiate into adipocytes, chondroblasts, and osteoblasts in vitro. UC-MSCs as well as MSCs derived from other sources meet these criteria [24], [28].
4.2. Immunosuppressive properties
Immunosuppressive and immunomodulatory effects have now become the most popular property of MSCs for their clinical use [29]. Defect of HLA-class II expression in UC-MSCs can theoretically rescue them from immune recognition by CD4+ T cells [30]. Moreover, MSCs don't express co-stimulatory surface antigens, CD40, CD80, and CD86, which activate T-cells [31]. Thus, MSCs can escape activated T cells and exert immunomodulation. The immunomodulation may be resulted from soluble factors such as indoleamine 2,3-dioxygenase (IDO), PGE2, galectin-1, and HLA-G5 [23]. On the other hand, several studies have reported that UC-MSCs may display immunosuppressive properties only after exposure to inflammatory cytokines and/or activated T-cells, a process called licensing or priming [32], [33], [34]. With these anti-inflammatory actions, MSCs could be good therapeutic candidates for neurological disorders accompanying inflammation.
4.3. Migration ability
MSCs are reported to exert migratory action similar to those of leukocytes with respect to cytokine responsiveness and the ability for transendothelial migration [35], and this migration capacity towards injured sites is one of the important factors in MSCs-based transplant therapy. In addition, Teo GS et al. reported that, BM-MSCs preferentially adhere to and migrate across tumor necrosis factor-α-activated endothelium in a vascular cell adhesion molecule-1 (VCAM-1) and G-protein-coupled receptor signaling-dependent manner and transmigrate into inflammatory lesion like leukocytes [36]. We actually reported the migration ability of UC-MSCs towards injured neural cells with glucose depletion in vitro [28]. Considering the treatment of neurological diseases, the passage of the MSCs through the blood–brain barrier (BBB) is a critical issue. Lin MN et al. reported phosphatidylinositol 3-kinase (PI3K)/Akt and Rho/ROCK (Rho kinase) pathways are involved in MSCs migration through human brain microvascular endothelial cell monolayers [37]. In addition, Matsushita T et al. revealed MSCs transmigrate across the brain microvascular endothelial cells (BMECs) monolayers through transiently formed intercellular gaps between the BMECs [38]. The migration ability of MSCs and the elucidation of mechanisms broaden the possibility of cellular therapy of neurological diseases.
4.4. Tissue repair properties
Neurorestorative and neuroprotective effects as a tissue repair property of MSCs can be mainly characterized by two mechanisms of action: (1) neurogenic differentiation and cell replacement, and (2) secretion of neurotrophic factors. Regarding the former, it has been reported transplantation of UC-MSCs can significantly alleviate ischemic injury, and the rescue arises from differentiation of transplanted cells into neurons and astrocytes [11]. On the other hand, concerning the latter point, it has been reported the paracrine effects of UC-MSCs on nerve regeneration, showing that UC-MSCs secret neurotrophic factors and that UC-MSCs-conditioned medium enhances Schwann cell viability and proliferation via increases in nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) expression [39]. We also found that UC-MSCs which secrete neurotrophic factors such as BDNF and hepatocyte growth factor (HGF), but not nerve growth factor (NGF), attenuate brain injury [40]. BDNF has been reported to improve hypomyelination via Erk phosphorylation or TrkB signaling [41], [42], and HGF has been reported to influence the development and growth of oligodendrocytes, as well as the proliferation of myelin-forming Schwann cells [43], and also reduces gliosis by suppressing MCP-1 induction [44]. These BDNF and HGF are reported to activate the phosphatidylinositol 3-kinase/Akt and MAP-kinase pathways which lead to neurorestorative, anti-apoptotic and neurogenic effects [45], [46], [47], [48], [49]. In addition to the anti-inflammatory effect mentioned above, these neurogenic differentiation ability and the paracrine effects of UC-MSCs are expected to contribute toward their use as therapeutics for neurological injuries.
5. Clinical application of MSCs for neurological disorders
Based on the mechanisms suggested by basic experiments, clinical trials using MSCs for neurological disorders have been increasing in number and the recent clinical reports are summarized in Table 1.
Table 1.
Summary of recent clinical trials using mesenchymal stromal cells for neurological disorders.
| Reference | Disease | Source | Number of patients | Mean age (range), year | Route of administration | Number of cells | Number of treatments | Results | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Liu X et al. (2017) [62] | Cerebral palsy | BM | 35 | 4.12 (6m-11) | IT | 1 × 106/kg at 3–4 days interval | 4 | Motor functional recovery after 12 months | Nausea, fever, head ache |
| Vaquero J et al. (2017) [51] | Spinal cord injury | BM | 12 | 42.2 (34–59) | IT | 3 × 107 cells at 3-months interval | 4 | Motor functional recovery after 12 months | Head ache, puncture pain |
| Petrou P et al. (2016) [56] | ALS | BM | 20 | 20–75 | IT | 1.0–2.0 × 106 cells | 1 | Motor functional recovery after 6 months | Nausea, fever, head ache |
| IM | 2.4–4.8 × 107 cells | ||||||||
| Satti H et al. (2016) [50] | Spinal cord injury | BM | 9 | 31.6 (24–38) | IT | 1.2 × 106/kg | 2 or 3 | Only safety assessment | No |
| Oh K et al. (2015) [55] | ALS | BM | 8 | 45.7 (29–62) | IT | 1.2 × 106/kg at 26 days interval | 2 | Decline in the ALSFRS-R score was not accelerated during 6 months | No serious adverse events |
| Mendonça MV et al. (2014) [53] | Spinal cord injury | BM | 14 | 35.7 (18–65) | Direct injection into the lesion following laminectomy and durotomy | 1 × 107 cells | 1 | Motor functional recovery after 6 months | low-intensity pain at the incision site |
| Tsai YA et al. (2017) [59] | SCA | AD | 7 | 21–66 | IV | 1 × 106/kg | 1 | Possible efficacy | No |
| Staff NP et al. (2016) [57] | ALS | AD | 27 | 36–75 | IT | 1 × 107, 5 × 107, 5 × 107 × 2, 1 × 108, 1 × 108 × 2 cells | 1 or 2 | No efficacy | Temporary back and leg pain in the highest dose |
| Hur JW et al. (2016) [54] | Spinal cord injury | AD | 14 | 41.9 (20–66) | IT | 9 × 107 cells at 1-month interval | 2 | Motor (5/14) and sensory (10/14) functional recovery after 8 months | Nausea, vomit, head ache |
| Okur SC et al. (2018) [64] | Cerebral palsy | UC | 1 | 6 | IT | 1 × 106/kg | 4 | Motor functional recovery after 18 months | Back pain |
| IV | 1 × 106/kg | ||||||||
| Wang et al. (2015) [15] | Cerebral palsy | UC | 16 (8 twins) | 6.29 (3–12) | IT | 1–1.5 × 107 cells at 3–5 days interval | 4 | Motor functional recovery after 1 & 6 months | No |
| Cheng H et al. (2014) [52] | Spinal cord injury | UC | 10 | 35.3 (19–57) | IT | 2 × 107 cells at 10 days interval | 2 | Motor functional recovery (7/10) after 6 months | No |
| Jin et al. (2013) [60] | SCA | UC | 16 | 39.9 (21–56) | IT + IV | IV: 4 × 107 cells | 4 | Motor functional recovery after 6 months | No |
| IT: 2 × 107 cells at 7-day interval | |||||||||
| Wang et al. (2013) [14] | Traumatic brain injury | UC | 20 | 27.5 (5–48) | IT | 1 × 107 cells at 5–7 day interval | 4 | Motor functional recovery after 6 months | No |
| Huang L et al. (2018) [63] | Cerebral palsy | UCB | 27 | 7.3 (3–12) | IV | 5 × 107 cells at 7 days interval | 4 | Gross motor and comprehensive functional recovery after 3, 6,12, 24 months | No |
IV, intravenous injection; IT, intrathecal injection; IM, intramuscular injection; BM, bone marrow; AD, Adipose; UC, Umbilical cord; UCB, Umbilical cord blood; ALS, amyotrophic lateral sclerosis; SCA, spinocerebellar ataxia.
Most of these clinical studies were performed in adults, and trials focusing on cerebral palsy were performed in children. With regard to the source of MSCs, most of the BM and AD are autologous, whereas the UC are allogeneic. Autologous transplantation is desirable considering the possibility of transplant rejection, but it depends on the sources and administrative timing. Regarding sources, it is difficult to isolate autologous MSCs derived from BM and AD in infants and children compared to the autologous MSCs from UC and UCB, because sampling of BM and AD is an invasive procedure. As for administrative timing, as it takes 3–6 months to mass culture MSCs and to confirm that they pass quality tests such as infection tests and chromosome tests, it is impossible to administer autologous MSCs in the acute phase of neurological injuries. On the other hand, allogeneic MSCs can be ordered as a preparation and administered in the acute to subacute phase.
As for the administration route of MSCs, intravenous injection is usual for graft versus host disease, liver disease, and heart disease, whereas local injection is used for arthrosis, and so on, but in clinical studies targeting neurological disorders, most studies were performed using intrathecal injection [3].
5.1. Spinal cord injury
A recent study reported the safety of intrathecal injection of autologous BM-MSCs in nine patients with spinal cord injury [50]. In this study, patient received two or three injections with a median of 1.2 × 106 cells/kg and no treatment-related adverse event was observed. Vaquero J et al. reported that variable improvement was found in the patients, and that mean values of BDNF, glial-derived neurotrophic factor, ciliary neurotrophic factor, and neurotrophin 3 and 4 showed slight increases compared with basal levels after subarachnoid administrations of BM-MSCs [51]. In a study using UC-MSCs study, it was proved that transplantation of allogeneic UC-MSCs has advantages in neurofunctional recovery in comparison with rehabilitation therapy and self-healing alone [52]. Other reports using BM, AD-MSCs showed the feasibility of MSCs administration in spinal cord injury patients, and subjects displayed variable improvements especially in motor functional recovery [53], [54].
5.2. Amyotrophic lateral sclerosis (ALS)
Oh K et al. reported two repeated intrathecal injections of autologous BM-MSCs were safe and feasible for ALS patients throughout the duration of the 12-month follow-up period [55]. In addition, there is a report showing intrathecal and intramuscular administration of BM-MSCs secreting neurotrophic factors (MSC-NTF) in patients with ALS is safe and indicates possible clinical benefits [56]. On the other hand, Staff NP et al. reported the safety of intrathecal autologous AD-MSCs treatment for ALS patients, but the results didn't directly address the efficacy of MSCs therapy [57]. It has been reported that therapeutic effectiveness of intrathecal administration of MSCs was related with the level of neurotrophic factor and anti-inflammatory cytokines in ALS patients and that the potential therapeutic effect of MSCs would not be long-lasting because MSCs gradually disappear over time in cerebrospinal fluid, therefore multiple administration of MSCs would be needed to sustain therapeutic effects [55], [58].
5.3. Spinocerebellar ataxia (SCA)
There are two clinical trials to assess the feasibility and efficacy of allogeneic MSCs therapy in patients with SCA. Tsai YA et al. reported the safety and possible efficacy (scale for assessment and rating of ataxia and sensory organization testing scores, metabolite ratios on the brain magnetic resonance spectroscopy, and brain glucose metabolism) during the 1-year follow-up after intravenous administration of allogeneic AD-MSCs [59]. On the other hand, Jin J.L et al. reported intravenous and intrathecal infusion of allogeneic UC-MSCs was performed with no serious transplant-related adverse events and the majority of patients showed improved Berg Balance Scale (BBS) and International Cooperative Ataxia Rating Scale (ICARS) scores continuing for at least 6 months [60].
5.4. Cerebral palsy
Recently, stem cells are emerging as a new treatment and possible cure for cerebral palsy [61]. Liu X et al. investigated whether BM-MSCs and BM-mononuclear cells (MNC) have any difference of curative effect for the treatment of spastic cerebral palsy. The results indicated that BM-MSCs transplantation for the treatment of cerebral palsy is safe and feasible, and can improve gross and fine motor function significantly compared with the results of BM-MNC treatment [62]. In addition, both of UC-MSCs and UCB-MSCs transplantation in children with cerebral palsy were reported to be safe and effective in improving gross motor function [15], [63]. These reports suggest transplantation of MSCs in subjects with cerebral palsy is safe and may promote neurological improvements.
6. Conclusion
MSCs for neurological disorders are expected as a new cell therapy to be effective by combining with rehabilitation and other medication therapy suggested by recent clinical trials.
Considering the suppression of inflammation, administration of allogeneic third-party MSCs in the acute to subacute period is considered desirable, whereas administration of autologous MSCs is sufficiently effective in the chronic phase possibly resulting from neurotrophic and neurorestorative effects rather than immunosuppressive effects in recent clinical trials. Therefore, it is important to investigate the appropriate administration protocol of MSCs considering the sources and timing, and also there are many problems to be solved including the number of MSCs to be administered, the kinds of medium used for culturing MSCs, the number of culture passages, and the preservation state until use. Further studies and clinical use in the near future will extend our knowledge of MSCs.
Conflicts of interest
The authors confirm that this article content has no conflict of interest.
Acknowledgements
Declared none.
Footnotes
This article is written by the recipient of The JSRM Young Investigator's Awards (Clinical Researches) 2018.
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Peng W., Sun J., Sheng C., Wang Z., Wang Y., Zhang C. Systematic review and meta-analysis of efficacy of mesenchymal stem cells on locomotor recovery in animal models of traumatic brain injury. Stem Cell Res Ther. 2015;6:47. doi: 10.1186/s13287-015-0034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang K.A., Lee J.H., Suh Y.H. Therapeutic potential of human adipose-derived stem cells in neurological disorders. J Pharmacol Sci. 2014;126(4):293–301. doi: 10.1254/jphs.14R10CP. [DOI] [PubMed] [Google Scholar]
- 3.Nagamura-Inoue T., Mukai T. Umbilical cord is a rich source of mesenchymal stromal cells for cell therapy. Curr Stem Cell Res Ther. 2016;11(8):634–642. doi: 10.2174/1574888x10666151026115017. [DOI] [PubMed] [Google Scholar]
- 4.Caplan A.I. Mesenchymal stem cells. J Orthop Res: Off Publ Orthop Res Soc. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 5.Friedenstein A.J., Piatetzky S., II, Petrakova K.V. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16(3):381–390. [PubMed] [Google Scholar]
- 6.Gnecchi M., Melo L.G. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 7.Bieback K., Kluter H. Mesenchymal stromal cells from umbilical cord blood. Curr Stem Cell Res Ther. 2007;2(4):310–323. doi: 10.2174/157488807782793763. [DOI] [PubMed] [Google Scholar]
- 8.In't Anker P.S., Scherjon S.A., Kleijburg-van der Keur C., de Groot-Swings G.M., Claas F.H., Fibbe W.E. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22(7):1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- 9.Gruber H.E., Deepe R., Hoelscher G.L., Ingram J.A., Norton H.J., Scannell B. Human adipose-derived mesenchymal stem cells: direction to a phenotype sharing similarities with the disc, gene expression profiling, and coculture with human annulus cells. Tissue Eng Part A. 2010;16(9):2843–2860. doi: 10.1089/ten.TEA.2009.0709. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Q., Zhang Z., Zhang S., Yang H., Zhang X., Pan J. Human umbilical cord mesenchymal stem cells protect against ischemic brain injury in mouse by regulating peripheral immunoinflammation. Brain Res. 2015;1594:293–304. doi: 10.1016/j.brainres.2014.10.065. [DOI] [PubMed] [Google Scholar]
- 11.Ding D.C., Shyu W.C., Chiang M.F., Lin S.Z., Chang Y.C., Wang H.J. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27(3):339–353. doi: 10.1016/j.nbd.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Zhu L.H., Bai X., Zhang N., Wang S.Y., Li W., Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13–21. doi: 10.1016/j.brainres.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 13.Forostyak S., Jendelova P., Sykova E. The role of mesenchymal stromal cells in spinal cord injury, regenerative medicine and possible clinical applications. Biochimie. 2013;95(12):2257–2270. doi: 10.1016/j.biochi.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Cheng H., Dai G., Wang X., Hua R., Liu X. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84. doi: 10.1016/j.brainres.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Wang X., Hu H., Hua R., Yang J., Zheng P., Niu X. Effect of umbilical cord mesenchymal stromal cells on motor functions of identical twins with cerebral palsy: pilot study on the correlation of efficacy and hereditary factors. Cytotherapy. 2015;17(2):224–231. doi: 10.1016/j.jcyt.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 16.He H., Nagamura-Inoue T., Tsunoda H., Yuzawa M., Yamamoto Y., Yorozu P. Stage-specific embryonic antigen 4 in Wharton's jelly-derived mesenchymal stem cells is not a marker for proliferation and multipotency. Tissue Eng Part A. 2014;20(7–8):1314–1324. doi: 10.1089/ten.TEA.2013.0333. [DOI] [PubMed] [Google Scholar]
- 17.Majore I., Moretti P., Stahl F., Hass R., Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2011;7(1):17–31. doi: 10.1007/s12015-010-9165-y. [DOI] [PubMed] [Google Scholar]
- 18.Marmotti A., Mattia S., Bruzzone M., Buttiglieri S., Risso A., Bonasia D.E. Minced umbilical cord fragments as a source of cells for orthopaedic tissue engineering: an in vitro study. Stem Cell Int. 2012;2012 doi: 10.1155/2012/326813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori Y., Ohshimo J., Shimazu T., He H., Takahashi A., Yamamoto Y. Improved explant method to isolate umbilical cord-derived mesenchymal stem cells and their immunosuppressive properties. Tissue Eng Part C Methods. 2015;21(4):367–372. doi: 10.1089/ten.TEC.2014.0385. [DOI] [PubMed] [Google Scholar]
- 20.Kikuchi-Taura A., Taguchi A., Kanda T., Inoue T., Kasahara Y., Hirose H. Human umbilical cord provides a significant source of unexpanded mesenchymal stromal cells. Cytotherapy. 2012;14(4):441–450. doi: 10.3109/14653249.2012.658911. [DOI] [PubMed] [Google Scholar]
- 21.Salehinejad P., Alitheen N.B., Ali A.M., Omar A.R., Mohit M., Janzamin E. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Dev Biol Anim. 2012;48(2):75–83. doi: 10.1007/s11626-011-9480-x. [DOI] [PubMed] [Google Scholar]
- 22.Meryman H.T. Cryoprotective agents. Cryobiology. 1971;8(2):173–183. doi: 10.1016/0011-2240(71)90024-1. [DOI] [PubMed] [Google Scholar]
- 23.Seshareddy K., Troyer D., Weiss M.L. Method to isolate mesenchymal-like cells from Wharton's Jelly of umbilical cord. Meth Cell Biol. 2008;86:101–119. doi: 10.1016/S0091-679X(08)00006-X. [DOI] [PubMed] [Google Scholar]
- 24.Shimazu T., Mori Y., Takahashi A., Tsunoda H., Tojo A., Nagamura-Inoue T. Serum- and xeno-free cryopreservation of human umbilical cord tissue as mesenchymal stromal cell source. Cytotherapy. 2015;17(5):593–600. doi: 10.1016/j.jcyt.2015.03.604. [DOI] [PubMed] [Google Scholar]
- 25.Al-Saqi S.H., Saliem M., Quezada H.C., Ekblad A., Jonasson A.F., Hovatta O. Defined serum- and xeno-free cryopreservation of mesenchymal stem cells. Cell Tissue Bank. 2015;16(2):181–193. doi: 10.1007/s10561-014-9463-8. [DOI] [PubMed] [Google Scholar]
- 26.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz E.M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F.C. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 28.Mukai T., Nagamura-Inoue T., Shimazu T., Mori Y., Takahashi A., Tsunoda H. Neurosphere formation enhances the neurogenic differentiation potential and migratory ability of umbilical cord-mesenchymal stromal cells. Cytotherapy. 2016;18(2):229–241. doi: 10.1016/j.jcyt.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Nagamura-Inoue T., He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cell. 2014;6(2):195–202. doi: 10.4252/wjsc.v6.i2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krampera M., Glennie S., Dyson J., Scott D., Laylor R., Simpson E. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 31.Weiss M.L., Anderson C., Medicetty S., Seshareddy K.B., Weiss R.J., VanderWerff I. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26(11):2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 32.Valencic E., Piscianz E., Andolina M., Ventura A., Tommasini A. The immunosuppressive effect of Wharton's jelly stromal cells depends on the timing of their licensing and on lymphocyte activation. Cytotherapy. 2010;12(2):154–160. doi: 10.3109/14653240903493417. [DOI] [PubMed] [Google Scholar]
- 33.Deuse T., Stubbendorff M., Tang-Quan K., Phillips N., Kay M.A., Eiermann T. Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transpl. 2011;20(5):655–667. doi: 10.3727/096368910X536473. [DOI] [PubMed] [Google Scholar]
- 34.Batsali A.K., Kastrinaki M.C., Papadaki H.A., Pontikoglou C. Mesenchymal stem cells derived from Wharton's Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther. 2013;8(2):144–155. doi: 10.2174/1574888x11308020005. [DOI] [PubMed] [Google Scholar]
- 35.Nitzsche F., Muller C., Lukomska B., Jolkkonen J., Deten A., Boltze J. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells. 2017;35(6):1446–1460. doi: 10.1002/stem.2614. [DOI] [PubMed] [Google Scholar]
- 36.Teo G.S., Ankrum J.A., Martinelli R., Boetto S.E., Simms K., Sciuto T.E. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-alpha-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30(11):2472–2486. doi: 10.1002/stem.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin M.N., Shang D.S., Sun W., Li B., Xu X., Fang W.G. Involvement of PI3K and ROCK signaling pathways in migration of bone marrow-derived mesenchymal stem cells through human brain microvascular endothelial cell monolayers. Brain Res. 2013;1513:1–8. doi: 10.1016/j.brainres.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Matsushita T., Kibayashi T., Katayama T., Yamashita Y., Suzuki S., Kawamata J. Mesenchymal stem cells transmigrate across brain microvascular endothelial cell monolayers through transiently formed inter-endothelial gaps. Neurosci Lett. 2011;502(1):41–45. doi: 10.1016/j.neulet.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 39.Guo Z.Y., Sun X., Xu X.L., Zhao Q., Peng J., Wang Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res. 2015;10(4):651–658. doi: 10.4103/1673-5374.155442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukai T., Mori Y., Shimazu T., Takahashi A., Tsunoda H., Yamaguchi S. Intravenous injection of umbilical cord-derived mesenchymal stromal cells attenuates reactive gliosis and hypomyelination in a neonatal intraventricular hemorrhage model. Neuroscience. 2017;355:175–187. doi: 10.1016/j.neuroscience.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Xiao J., Wong A.W., Willingham M.M., van den Buuse M., Kilpatrick T.J., Murray S.S. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro Signals. 2010;18(3):186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- 42.Peckham H., Giuffrida L., Wood R., Gonsalvez D., Ferner A., Kilpatrick T.J. Fyn is an intermediate kinase that BDNF utilizes to promote oligodendrocyte myelination. Glia. 2016;64(2):255–269. doi: 10.1002/glia.22927. [DOI] [PubMed] [Google Scholar]
- 43.Yan H., Rivkees S.A. Hepatocyte growth factor stimulates the proliferation and migration of oligodendrocyte precursor cells. J Neurosci Res. 2002;69(5):597–606. doi: 10.1002/jnr.10323. [DOI] [PubMed] [Google Scholar]
- 44.Kadoyama K., Funakoshi H., Ohya W., Nakamura T. Hepatocyte growth factor (HGF) attenuates gliosis and motoneuronal degeneration in the brainstem motor nuclei of a transgenic mouse model of ALS. Neurosci Res. 2007;59(4):446–456. doi: 10.1016/j.neures.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura T., Nishizawa T., Hagiya M., Seki T., Shimonishi M., Sugimura A. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342(6248):440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- 46.Zeng W., Ju R., Mao M. Therapeutic potential of hepatocyte growth factor against cerebral ischemia. Exp Therapeut Med. 2015;9(2):283–288. doi: 10.3892/etm.2014.2133. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang J., Deguchi K., Ohta Y., Liu N., Zhang X., Tian F. Strong neurogenesis, angiogenesis, synaptogenesis, and antifibrosis of hepatocyte growth factor in rats brain after transient middle cerebral artery occlusion. J Neurosci Res. 2011;89(1):86–95. doi: 10.1002/jnr.22524. [DOI] [PubMed] [Google Scholar]
- 48.Reichardt L.F. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond Ser B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollock K., Dahlenburg H., Nelson H., Fink K.D., Cary W., Hendrix K. Human mesenchymal stem cells genetically engineered to overexpress brain-derived neurotrophic factor improve outcomes in huntington's disease mouse models. Mol Ther. 2016;24(5):965–977. doi: 10.1038/mt.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satti H.S., Waheed A., Ahmed P., Ahmed K., Akram Z., Aziz T. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. 2016;18(4):518–522. doi: 10.1016/j.jcyt.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Vaquero J., Zurita M., Rico M.A., Bonilla C., Aguayo C., Fernandez C. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. 2017;19(3):349–359. doi: 10.1016/j.jcyt.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H., Liu X., Hua R., Dai G., Wang X., Gao J. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. 2014;12:253. doi: 10.1186/s12967-014-0253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mendonca M.V., Larocca T.F., de Freitas Souza B.S., Villarreal C.F., Silva L.F., Matos A.C. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5(6):126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hur J.W., Cho T.H., Park D.H., Lee J.B., Park J.Y., Chung Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh K.W., Moon C., Kim H.Y., Oh S.I., Park J., Lee J.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6):590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrou P., Gothelf Y., Argov Z., Gotkine M., Levy Y.S., Kassis I. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73(3):337–344. doi: 10.1001/jamaneurol.2015.4321. [DOI] [PubMed] [Google Scholar]
- 57.Staff N.P., Madigan N.N., Morris J., Jentoft M., Sorenson E.J., Butler G. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology. 2016;87(21):2230–2234. doi: 10.1212/WNL.0000000000003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H.Y., Kim H., Oh K.W., Oh S.I., Koh S.H., Baik W. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: an investigator-initiated trial and in vivo study. Stem Cells. 2014;32(10):2724–2731. doi: 10.1002/stem.1770. [DOI] [PubMed] [Google Scholar]
- 59.Tsai Y.A., Liu R.S., Lirng J.F., Yang B.H., Chang C.H., Wang Y.C. Treatment of spinocerebellar ataxia with mesenchymal stem cells: a phase I/IIa clinical study. Cell Transpl. 2017;26(3):503–512. doi: 10.3727/096368916X694373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jin J.L., Liu Z., Lu Z.J., Guan D.N., Wang C., Chen Z.B. Safety and efficacy of umbilical cord mesenchymal stem cell therapy in hereditary spinocerebellar ataxia. Curr Neurovascular Res. 2013;10(1):11–20. doi: 10.2174/156720213804805936. [DOI] [PubMed] [Google Scholar]
- 61.Novak I., Walker K., Hunt R.W., Wallace E.M., Fahey M., Badawi N. Concise review: stem cell interventions for people with cerebral palsy: systematic review with meta-analysis. Stem Cells Transl Med. 2016;5(8):1014–1025. doi: 10.5966/sctm.2015-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X., Fu X., Dai G., Wang X., Zhang Z., Cheng H. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J Transl Med. 2017;15(1):48. doi: 10.1186/s12967-017-1149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L., Zhang C., Gu J., Wu W., Shen Z., Zhou X. A randomized, placebo-controlled trial of human umbilical cord blood mesenchymal stem cell infusion for children with cerebral palsy. Cell Transplant. 2018;27(2):325–334. doi: 10.1177/0963689717729379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okur S.C., Erdogan S., Demir C.S., Gunel G., Karaoz E. The effect of umbilical cord-derived mesenchymal stem cell transplantation in a patient with cerebral palsy: a case report. Int J Stem Cells. 2018;11(1):141–147. doi: 10.15283/ijsc17077. [DOI] [PMC free article] [PubMed] [Google Scholar]