Abstract
Introduction
Using a rat model of nontraumatic early arthritis induced by intra-articular administration of low-dose monoiodoacetic acid (MIA), we transplanted allogeneic chondrocyte sheets and examined the effects on tissue repair.
Methods
MIA (0.2 mg/50 μl) was injected into the right knee of 20 male Wistar rats. Four weeks later, rats were randomly allocated into three groups: Group A was examined 4 weeks after administration of MIA; Group B, 8 weeks after MIA injection and chondrocyte sheet transplantation, and Group C, 8 weeks after MIA injection but without chondrocyte sheet transplantation. Allogeneic chondrocyte sheets were transplanted into the right knee of Group B rats. Pain was assessed as the weight distribution ratio of the damaged to undamaged limb. The OARSI score was used for histological scoring.
Results
The limb weight distribution ratio indicated significantly less pain in Group B. Histological scoring showed significant differences in cartilage repair and inhibition of the progression of cartilage degeneration between Groups B and C, but not between Groups A and B, or Groups A and C.
Conclusions
These findings suggest that, in this rat model of nontraumatic early arthritis induced by low-dose MIA injection, allogeneic chondrocyte sheet transplantation induces cartilage repair and suppresses cartilage degeneration.
Keywords: Osteoarthritis, Chondrocyte sheet, Transplantation, Monoiodoacetic acid (MIA), OARSI score
Abbreviations: AB, Antibiotic-antimycotic solution; Acan, Aggrecan; Col1A1, Collagen type Ⅰalpha 1; Col2A1, Collagen type Ⅱalpha 1; Comp, Cartilage oligomeric matrix protein; DMEM, Dulbecco's modified Eagle's medium; DPBS, Dulbecco's phosphate-buffered saline; EDTA, Ethylenediaminetetraacetic acid; FBS, Fetal bovine serum; IFP, Infrapatellar fat pad; ITGa10, Integrin alpha-10; MIA, Monoiodoacetic acid; Mmp13, Matrix metalloproteinase-13; OA, Osteoarthritis; OARSI, Osteoarthritis research society international; PVDF, Polyvinylidene difluoride; qPCR, Quantitative real-time polymerase chain reaction
Highlights
-
•
Therapeutic effects of allogeneic chondrocyte sheets were examined using an arthritis model of rat induced by low-dose MIA.
-
•
Chondrocyte sheets exhibited sufficient expression of genes important to maintaining a stable cartilage matrix.
-
•
Transplantation of chondrocyte sheets alleviated pain and induced cartilage repair and suppressed cartilage degeneration.
1. Introduction
Osteoarthritis (OA) is one of the most prevalent joint diseases, especially in mature women [1]. The condition is characterized by degenerative changes in articular cartilage, which cause pain and malfunctions as it progresses [2], [3]. Structural changes include loss of articular cartilage, subchondral bone sclerosis, and formation of cysts and osteophytes [4]. The low cellularity and avascular nature of articular cartilage means that its capacity to self-regenerate after injury or degeneration is limited [5]. Mild OA is treated conservatively with oral administration of nonsteroidal anti-inflammatory drugs, intra-articular injection of corticosteroid [6] and/or hyaluronic acid [7], and exercise therapy [8]. However, surgical treatment may be required as the disease progresses. The main treatments are high tibial osteotomy and total knee arthroplasty.
Various arthritis models, such as surgical intervention and intra-articular drug injection, have been used to study cartilage regeneration. The anterior cruciate ligament resection model is the surgical intervention model used most frequently. In this model, anterior cruciate ligament resection triggers cartilage degeneration, subchondral bone sclerosis, and osteophyte formation, which mimic the pathological changes observed in human OA [9], [10], [11].
Intra-articular injection of monoiodoacetic acid (MIA) is one method for inducing OA experimentally. MIA inhibits glyceraldehyde-3-phosphate dehydrogenase activity in chondrocytes and causes cell death and decreased proteoglycan content. The reaction is simple, rapid, and reproducible [12], [13], [14], [15]. Some reports indicate that the OA caused by MIA-induced chemical induction is different from human OA [16], [17]. On the other hand, Sangeetha et al. and others have reported histological and morphological changes in the MIA model similar to those in human OA [18], [19], [20], [21], [22], [23]. Udo et al. [24] reported that the changes caused by MIA depend on the dose and timing, and that low-dose administration is useful for evaluating the pathology of OA, pain mechanisms, and therapeutic effects. Mohan et al. [25] reported that the low-dose MIA-induced arthritis model is suitable for studying nontraumatic OA and the therapeutic effects on cartilage and bone.
We have transplanted chondrocyte sheets into areas showing traumatic changes in an articular cartilage damage model in various animals and have confirmed its effectiveness [26], [27], [28]. The purpose of this study was to determine whether the repair or inhibition of the degeneration of damaged cartilage can be obtained by transplanting allogeneic chondrocyte sheets in a nontraumatic arthritis model of OA induced by intra-articular injection of low-dose MIA.
2. Materials and methods
All procedures using animals in this study were performed in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978) published by the National Institutes of Health, USA, and the Guidelines of Tokai University on Animal Use. This animal experiment was approved by the Animal Committee of Tokai University (permission number 171059).
2.1. Animals
Twenty male Wistar rats (CLEA Japan Inc., Tokyo, Japan) at 8 weeks of age and three male Lewis rats (Charles River Japan Inc., Kanagawa, Japan) at 6 weeks of age were used for the study. The animals were housed under normal conditions for 2 weeks before the start of the experiments to acclimate them to the environment. One to two rats were housed per cage in sterile conditions, and rodent chow and water were allowed ad libitum.
2.2. Chondrocyte sheets
2.2.1. Harvest of chondrocytes and fabrication of chondrocyte sheets
To prepare allogeneic cell sheets for transplantation, three 6-week-old male Lewis rats were used. The rats were sacrificed by an overdose of 50% isoflurane. A medial parapatellar incision was made in both legs, each patella was dislocated laterally, and the collateral ligaments and the anterior and posterior cruciate ligaments were severed. The joint capsule on the hip joint side was incised, and the femur was extracted. The articular cartilage tissue was collected with a scalpel from the bilateral femoral head and bilateral femoral condyle. Cells were isolated from the tissue sample using an enzymatic procedure and then incubated in Dulbecco's modified Eagle's medium/F12 (DMEM/F12; Gibco, Grand Island, NY, USA) supplemented with 20% fetal bovine serum (FBS; AusGeneX, Molendinar, Australia), 1% antibiotic–antimycotic solution (AB; Gibco), and 5 mg/ml collagenase type 1 (Worthington Biochemical, Mannheim, Germany) for 3 h at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cell suspension was washed and passed through a 100 μm strainer (BD Falcon, Franklin Lakes, NJ, USA).
The collected cells were seeded at a density of 1 × 104 cells/cm2 in a 150 cm2 culture flask in DMEM/F12 supplemented with 20% FBS and 1% AB, and incubated at 37 °C. After 3 days, 100 μg/ml ascorbic acid (Nissin Pharmaceutical, Yamagata, Japan) was added to the medium, and the medium was replaced every 3 or 4 days. Cells were passaged once when they reached confluence and then cryopreserved.
To fabricate cell sheets, cells were thawed, passaged once, and then seeded onto UpCell® inserts (CellSeed Inc., Tokyo, Japan) at 1 × 104 cells/cm2. Ascorbic acid was added, the medium was replaced every 3 or 4 days, and the cells were cultured for 14 days. To harvest the cells using the characteristics of the UpCell insert, the culture temperature was lowered to <25 °C, which caused the chondrocytes to detach, after which they were preserved for further analysis.
2.2.2. Cell counting
The cell sheets were washed in Dulbecco's phosphate-buffered saline (PBS; Gibco). The sheets were then incubated in TripLE Express® (Gibco) at 37 °C for 40 min and centrifuged at 1500 rpm for 5 min. The cell sheets were resuspended in 0.25 mg/ml collagenase P (Roche, Basel, Switzerland) at 37 °C for up to 5 min and then centrifuged at 1500 rpm for 5 min. Finally, the isolated cells were resuspended in DMEM/F12, and the cells were counted using a trypan blue exclusion assay.
2.2.3. Histological staining
The collected cell sheets were fixed with 20% formalin (Wako Pure Chemical, Japan) and embedded in paraffin wax. The tissue was cut into 3 μm sections, which were then deparaffinized according to standard procedures, and stained with hematoxylin–eosin. The sections were stained with Safranin O in a 0.08% fast green aqueous solution and 0.1% Safranin O aqueous solution. The sections were also stained with a 0.05% toluidine blue solution.
2.2.4. Immunohistochemical staining
Paraffin-embedded tissue was sectioned at 3 μm and deparaffinized using standard procedures. The sections were stained for collagen type I with goat anti-type I collagen-UNLB (Southern Biotech, USA). The sections were washed with PBS and processed using an ImmPRESS Anti-goat Ig Reagent Kit (Vector, USA) and the slides were sealed. Sections for collagen type II staining were deparaffinized and then reacted with Anti-hCL (II) purified IgG (Daiich Fine Chemical, Japan). The sections were washed with PBS and processed using an ImmPRESS Anti-mouse Ig Reaction Kit (Vector), and the slides were sealed.
2.2.5. RNA isolation, cDNA synthesis, and quantitative real-time PCR
Three samples of passage 1 chondrocytes and three samples of cultured cartilage cell sheets were analyzed. The recovered chondrocyte sheets were crushed at 1500 rpm for 3 min using SHAKE Master Neo (Bio Medical Science, Japan). Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer's instructions. Next, first-strand cDNA was synthesized using 1 μg of total RNA and the enzyme, buffer, and primer of a QuantiTect Reverse Transcription Kit (Qiagen) and elongated using a GeneAmp PCR system 9600 (Thermo Fisher Scientific, USA) at 42 °C for 15 min and 95 °C for 3 min. Quantitative real-time polymerase chain reaction (qPCR) was performed using an Applied Biosystems 7300 Real-Time PCR system (Applied Biosystems, USA). The primers were as follows for TaqMan® Gene Expression Assays (Applied Biosystems, USA): Col1A1 (Rn01637087_m1), Col2A1 (Rn01463848_m1), Sox9 (Rn01751070_m1), Acan (Rn00573424_m1), ITGa10 (Rn01533928_m1), Mmp13 (Rn01448194_m1), Lect1 (Rn00578277_m1), Comp (Rn00563255_m1), and Gapdh (Rn01775763_g1). The final reaction volume was 20 μl, and the thermocycling conditions were as follows: 50 °C for 2 min, 95 °C for 10 min, and 95 °C for 15 s and 60 °C for 1 min for 40 cycles. The expression level of the internal control GAPDH was used as a housekeeping gene, and the comparative 2−⊿⊿Ct method was used for analysis.
2.2.6. Evaluation of cell sheets
We measured cell number and viability and cell sheet thickness, and confirmed the existence of the cartilage matrix in tissue sections. We also used qPCR to quantify the gene expression levels in chondrocyte sheets compared with passage 1 chondrocytes.
2.3. Induction of OA by MIA
We used 20 male Wistar rats at 8 weeks of age. With the animal under anesthesia with isoflurane and oxygen inhalation, both knees were shaved and disinfected. Under sterile conditions, a 1 cm incision was made in the skin via the medial parapatellar approach to expose the patellar tendon. The knee joint was flexed 90°, and MIA (0.2 mg dissolved in 50 μl of physiological saline; Sigma–Aldrich, St. Louis, MO, USA) was injected into the knee joint through a 27 G needle. We considered the rise of joint pressure due to intraarticular administration, so the same volume of saline was injected into the left knee joint using the same method. After injection, the skin was sutured.
2.4. Transplantation of chondrocyte sheets
Four weeks after MIA administration, 20 rats were randomly allocated to three groups. All rats were injected with MIA to induce OA. Group A rats (n = 6) were sacrificed 4 weeks after MIA injection. Group B rats (n = 8) received chondrocyte sheet transplantation 4 weeks after MIA injection and were sacrificed 4 weeks later (i.e., 8 weeks after MIA injection). Group C rats (n = 6) did not receive chondrocyte sheets and were sacrificed 8 weeks after MIA injection. For chondrocyte sheet transplantation, Group B rats were anesthetized with isoflurane and oxygen. Under sterile conditions, both knees were shaved and disinfected, a ∼2 cm skin incision was made to the medial side of the right knee using the parapatellar approach, and the patella was dislocated to the lateral side. Next, the medial side of the knee joint capsule was dissected and the joint was exposed. A chondrocyte sheet that had been cultured for 14 days was transplanted into the femoral condylar region, the dislocation of the patella was reduced, and the joint capsule and skin were sutured.
A sham operation was performed for the joint capsule of the left knee joint of Group B rats and both knee joints of Group C rats.
2.5. Pain evaluation
An incapacitance meter (BrainScience Idea Co., Ltd, Japan) was used to detect changes in the distribution ratio of the damaged limb to the undamaged limb, and the ratio served as the gauge for evaluating pain. This device is used widely to investigate pain and pain-alleviating effects [27], [29], [30]. The measurements were made when the rat's hind legs were both positioned over the platforms and the rat was stationary. The weight distribution of both hind legs was measured 10 times, and the following formula was used to calculate the limb weight distribution ratio (%).
| Limb weight distribution ratio (%) = damaged limb load (g)/(undamaged limb load (g) + damaged limb load (g)) × 100 |
After MIA injection, the weight distribution was measured 14 times on days 1, 7, 14, 21, 28, 29, 31, 35, 38, 42, 45, 49, 52, and 55.
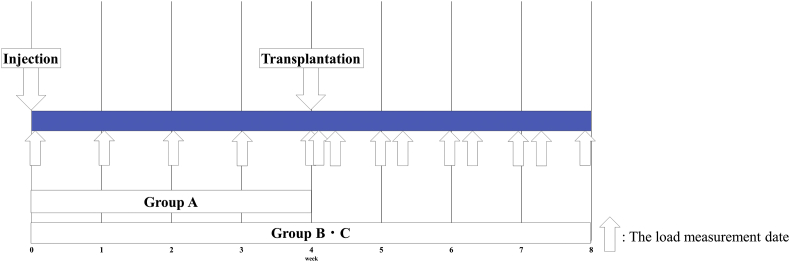
Fig. 1 shows the schedule for MIA administration, transplant surgery, and load measurement.
Fig. 1.
Experimental schedule. Four weeks after administration of MIA, Group A was sacrificed, and Groups B and C were subjected to cell sheet transplantation surgery. At 8 weeks after MIA administration, Group B and C were sacrificed.
2.6. Histological evaluation
Four weeks after MIA injection, six rats in Group A were sacrificed, and 4 weeks after transplantation of chondrocyte sheets (8 weeks after MIA injection), eight rats in Group B and six rats in Group C were sacrificed. The knee joint was exposed using the medial parapatellar approach and the collateral ligaments and anterior and posterior cruciate ligaments were severed. The femur and the tibia were then separated.
The femur and tibia were fixed in 20% formalin (Wako Pure Chemical) for 5–6 days. Each section was degreased and demineralized with acetone solution (Wako Pure Chemical) and ethylenediaminetetraacetic acid (EDTA) solution (Wako Pure Chemical). It was fixed again with 20% formalin, decalcified with EDTA solution, and embedded in paraffin wax. In the rat, the load distribution on the medial side of the femur is higher than on the lateral side [23], [31], [32]; therefore, the section of the medial femoral condyle was cut in the sagittal direction (3 μm sections). The sections were stained with Safranin O, collagen type I staining, and collagen type II staining in the same protocol. Microscopic images were captured under a BZ-9000 Biorevo fluorescence microscope (Keyence, Japan).
Bone samples from Groups A, B, and C were analyzed histologically. We evaluated the distal part of the femoral articular cartilage and the intermediate region of the articular cartilage of the tibia based on the cartilage region determination method of Nomura et al. [33]. The Osteoarthritis Research Society International (OARSI) OA cartilage index system was used to score the histopathology, as established by Pritzker et al. [34]. The scoring system evaluates the grade according to the depth of cartilage damage and the stage according to the extent of damage over the joint surface. The score for grade × stage is calculated and can range from 0 to 24, with higher scores indicating more advanced cartilage degeneration. We show the OARSI score (Table 1). Scoring was performed by three single-blinded examiners.
Table 1.
OARSI osteoarthritis cartilage histopathology scoring.
| Grade (key feature) | Stage % Involvement (surface, area, volume) |
|||
|---|---|---|---|---|
| Stage 1 (<10%) | Stage 2 (10–25%) | Stage 3 (25–50%) | Stage 4 (>50%) | |
| Grade 1 (surface intact) | 1 | 2 | 3 | 4 |
| Grade 2 (surface discontinuity) | 2 | 4 | 6 | 8 |
| Grade 3 (vertical fissures, clefts) | 3 | 6 | 9 | 12 |
| Grade 4 (erosion) | 4 | 8 | 12 | 16 |
| Grade 5 (denudation) | 5 | 10 | 15 | 20 |
| Grade 6 (deformation) | 6 | 12 | 18 | 24 |
| Score = grade × stage | ||||
2.7. Statistical analysis
The results are expressed as the mean ± standard deviation (SD). Gene expression levels in the cell sheets were analyzed using the Mann–Whitney U test. A p-value < 0.05 was considered to indicate a significant difference; however, changes in gene expression <2-fold are considered to be within the physiological limit and, therefore, such changes were considered to be unchanged despite being statistically significant. The damaged limb weight distribution ratio and histological assessment as measured by the OARSI score were evaluated using analysis of variance. The Tukey–Kramer test was used for post hoc testing. A p-value < 0.05 was deemed to be significant.
3. Results
3.1. Sheet thickness, cell count, and tissue evaluation of chondrocyte sheets
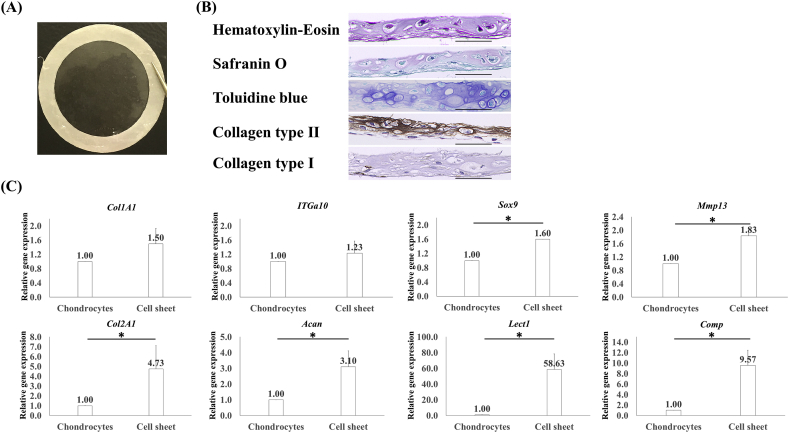
The macroscopic and microscopic findings for cell sheets are shown in Fig. 2A and B. Chondrocyte sheets contained cells at a high density, and these cells were easily harvested and manipulated without tearing after 14 days of culture. Chondrocyte sheets contained an average 1.32 ± 0.28 × 106 cells and had a survival rate of 81.3% ± 2.4% and an average thickness of 29.53 ± 2.23 μm. The sheets formed a thick structure with integrated layers, which were examined histologically and by staining the cartilage matrix with Safranin O and toluidine blue.
Fig. 2.
Macroscopic and histological findings, and gene expression in cell sheets. (A) Representative images of a cell sheet attached to a white polyvinylidene membrane. (B) Hematoxylin–eosin, Safranin O, toluidine blue, and collagen type II staining of the cartilage matrix. Collagen type I did not stain (×40, scale bar = 50 μm). (C) The expression of Col1A1 and ITGa10 increased, but there was no significant difference (p > 0.05). Expression of Sox9 and Mmp13 increased significantly but not more than 2-fold (*p < 0.05, <2-fold). Expression of Col2A1, Acan, Lect1, and Comp increased significantly by >2-fold (*p < 0.05, >2-fold).
3.2. qPCR of chondrocyte sheets
Gene expression in three chondrocyte sheets was evaluated and compared with that in passage 1 chondrocytes (Fig. 2C). The relative gene expression of chondrocytes was set at 1.0; the relative values were 1.5 times for Col1A1, 4.73 times for Col2A1, 1.6 times for Sox9, 3.10 times for Acan, 1.23 times for ITGa10, 1.83 times for Mmp13, 58.63 times for Lect1, and 9.57 times for Comp. This analysis confirmed an increase in the expression of all genes analyzed.
3.3. Pain evaluation
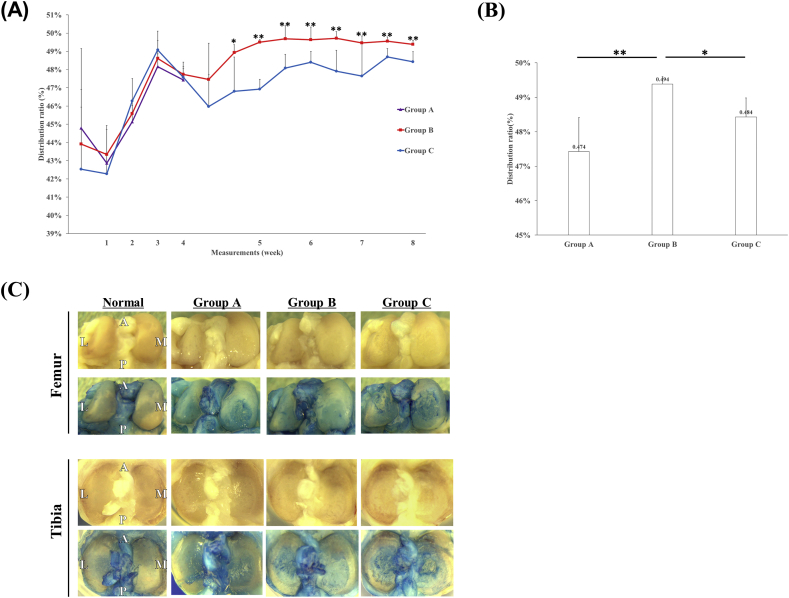
The normal limb weight distribution ratio is 50%. Fig. 3A shows the changes in the weight distribution ratio with time after MIA injection in each group. Up to 4 weeks after MIA injection, the ratio did not differ significantly between any groups. Fig. 3B shows the limb weight distribution ratio on the last day was 47.4% ± 1.0% in Group A, 49.4% ± 0.2% in Group B, and 48.4% ± 0.6% in Group C; the value was significantly higher in Group B than in the other two groups.
Fig. 3.
Time course after administration of MIA, limb weight distribution ratio on the last day of each group, and macroscopic findings for femoral condylar articular cartilage. (A) The limb weight distribution ratio decreased transiently after administration of MIA and after transplantation surgery. At 28 days after MIA administration, the ratios did not differ significantly (p > 0.05) between any group: 47.4% ± 1.0% in Group A, 47.7% ± 0.3% in Group B, and 47.6% ± 0.6% in Group C. (B) On the last day, the limb weight distribution ratio was significantly higher in Group B than in the other two groups: 47.4% ± 1.0% in Group A, 49.4% ± 0.2% in Group B, and 48.4% ± 0.6% in Group C (**p < 0.01 for Group A vs B; *p < 0.05 for Group B vs C; p > 0.05 for Group A vs C). (C) The upper panel shows unstained samples, and the lower panel shows samples stained with indigo carmine. Compared with undamaged tissue, Group A showed damage to the medial condyle, and Group B showed less damage. More damage was observed in the lateral condylar region in Group C.
3.4. Macroscopic evaluation
No signs of infection such as swelling or redness of the knee joint were observed macroscopically throughout the entire study. Fig. 3C shows the macroscopic findings for femoral condylar and tibial articular cartilage in each group compared to normal. Normal femoral condylar articular cartilage did not stain with indigo carmine and its surface was smooth. In Group A, damage was observed in the medial condyle. Minor injury was observed in Group B, and severe injury was observed in the medial and lateral condyles in Group C. Tibial articular cartilage damage was observed in all groups as for the femur, but there was less evidence of erosion in Group B than in Group C.
3.5. Histological evaluation
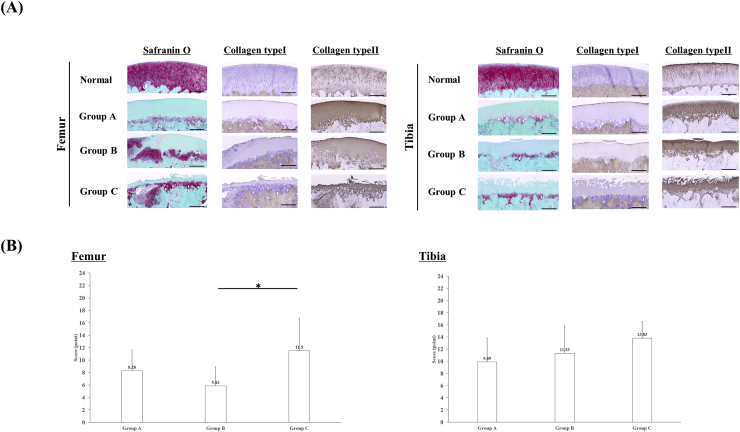
Fig. 4A shows the histological findings for femoral condylar articular cartilage and tibial articular cartilage. Safranin O staining of the femur and tibia showed loss of chondrocytes and decreased proteoglycan staining in Group A, but the superficial cartilage layer was stable. Similar findings were observed in Group C, but thinning of the articular cartilage was also observed. By contrast, in Group B, the superficial cartilage layer and partial staining were retained in the femur, and only mild damage was found in the surface cartilage layer of the tibia. Immunostaining showed collagen type II staining in all intact cartilage, but collagen type I staining was observed only in the superficial regions of cartilage in normal, Group A, and Group B.
Fig. 4.
Histological findings in the femur and tibia, and OARSI scores. (A) Undamaged femoral condylar Safranin O staining was observed in Group A with loss of chondrocytes and proteoglycans. Group B showed a cartilage layer and some Safranin O staining. Group C showed erosion and thinning of the cartilage layer. No peeling of the transplanted cartilage sheet or uninitiated parts were observed in Group B. Safranin O staining of the normal tibia and loss of chondrocytes and proteoglycans were observed in Group A, as seen in the femur. Compared with the femur in Group B, although the cartilage layer remained, slight erosion was observed and this tissue did not stain with Safranin O in Group A. In Group C, erosion and thinning of the cartilage layer were observed (×20, scale bar = 50 μm). Immunostaining of the femur showed collagen type II staining in all intact cartilage, but collagen type I staining was observed only in the superficial regions of cartilage in normal, Group A, and Group B. Immunostaining of the tibia showed both collagen type I and collagen type II staining (×20, scale bar = 50 μm). (B) The OARSI score for the femur differed significantly between Groups B and C but not between Groups A and B, or Groups A and C (*p < 0.05, Group B vs C; p > 0.05, Group A vs B, and Group A vs C). The OARSI score for the tibia did not differ significantly between the three groups.
3.6. Histological scoring
Fig. 4B shows the OARSI scores for the femur and tibia in the three groups. The score for the femur was significantly lower (indicating less damage) in Group B (5.83 ± 3.02 points) than in Group A (8.28 ± 4.87 points) and Group C (11.5 ± 4.5 points). For the tibia sites, the scores did not differ significantly between groups: 9.89 ± 5.09 points in Group A, 11.33 ± 4.57 points in Group B, and 13.83 ± 2.26 in Group C.
4. Discussion
We have previously investigated defects created in articular cartilage of various animals such as rats [26], rabbits [27], and mini pigs [28], and we have transplanted chondrocyte sheets to confirm the efficiency of articular cartilage repair. The purpose of this study was to investigate the application of allogeneic chondrocyte sheets in a rat model of nontraumatic early arthritis.
The prepared chondrocyte sheets had a survival rate of 80% or more, sufficient thickness, abundant extracellular matrix, and significant increase in the expression (>2-fold) of Col2A1, Acan, Lect1, and Comp. Collagen type II and aggrecan are the major components of cartilage matrix and are essential for the maintenance of normal chondrocytes [35]. Collagen type II is resistant to tensional force [36]. Aggrecan is also involved in providing resistance to joint loading by forming a complex through interactions with proteins such as hyaluronic acid [37]. Lect1 encodes a chondromodulin I protein, and Miura et al. [38] reported that it has an antiangiogenic effect in cartilage tissue and promotes cartilage formation. The STAT signaling pathway regulates transcription of multiple genes that regulate cell proliferation and differentiation, including osteoblast differentiation, and bone-related factors such as alkaline phosphatase and collagen type X [39], [40]. Chondromodulin I inhibits the STAT signaling pathway [41]. Klinger et al. [42] reported that chondromodulin I strongly suppresses chondrocyte hypertrophy and endochondral ossification and helps to stabilize cartilage repair tissue.
Cartilage oligomeric matrix protein (COMP) is a noncollagenous protein that is important for multicellular networks and is known to stimulate chondrocyte proliferation and cartilage formation [43], [44]. Some have reported interactions between COMP and collagen types I and II, and that these interactions play a role in maintaining the stability of the collagen network [45], [46]. Although COMP is found in various tissues, it is most abundant in tissues that bear mechanical loading such as tendons and cartilage [47]. Our data suggest that chondrocyte sheets exhibit sufficient expression of genes important to maintaining a stable cartilage matrix.
Using the limb load distribution ratio as an index of pain, we observed a significant pain-alleviating effect in the rats that received transplanted chondrocyte sheets. Our results are similar to those reported for transplantation of allogeneic chondrocyte sheets in a rabbit articular cartilage defect model at 4 weeks after transplantation reported by Ito et al. [27] and 12 weeks after transplantation reported by Tani et al. [30].
Macroscopic observations showed no inflammatory findings in the knee such as swelling or reddening. In addition, the transplanted group (Group B) showed evidence of suppression of cartilage degeneration compared with the nontransplanted groups (Groups A and C), which did not receive transplanted sheets.
Histologically, the adhesion of the margin and bottom of the cell sheet was good, and tissue was significantly repaired on the femur sites. On the tibia sites, there was no significant difference between groups that received or did not receive chondrocyte sheet transplantation. However, there was a tendency toward less progression of cartilage degeneration in Group B. In this study, the cell sheet was transplanted into the femoral condylar aspect, and significant cartilage repair was obtained on the femur side. These findings suggest that degeneration of the tibia sites may have been suppressed by a paracrine effect.
Udo et al. [24] also used an MIA arthritis model, but our macroscopic findings and histological scoring of Groups A and C were more favorable in our study. As noted earlier, the effects of MIA depend on the timing and dose. Although there are no reports of the residual time in joints, Udo et al. reported the progress of cartilage degeneration for 12 weeks after low-dose MIA injection. Future studies should extend the observation period and investigate the effectiveness of chondrocyte sheet transplantation. We did not measure inflammatory factors in the synovium or infrapatellar fat pad (IFP) in this study. Udo et al. observed decreased inflammation in the IFP and reversible changes 7 days after low-dose MIA injection. Synovitis and cartilage degeneration are typical features of knee joint OA, and it is necessary to confirm the effectiveness of chondrocyte sheet transplantation against synovitis.
5. Conclusion
In this study, we transplanted allogeneic chondrocyte sheets into rats with OA induced by low-dose MIA injection. Transplantation of cell sheets alleviated pain, as shown by a higher limb weight ratio in the group that received transplanted sheets. Histological findings showed less degeneration in rats that received transplanted sheets. These data suggest that, in this nontraumatic early arthritis model, transplantation of allogeneic chondrocyte sheets reduced the effects of OA, induced cartilage repair, and inhibited the progression of articular cartilage degeneration.
Declarations of interest
M.S. is one of the inventors on the patent (WO2006093151) submitted by CellSeed Inc. for the manufacturing process of chondrocyte sheets. M.S. receives research funds from CellSeed Inc.
Acknowledgements
This research was supported by the Project Focused on Developing Key Evaluation Technology: Manufacturing Technology for Industrialization in the Field of Regenerative Medicine (No. 14525207) and by the Project Focused on Developing Key Evaluation Technology: Evaluation for Industrialization in the Field of Regenerative Medicine (No. 15667006 to M.S.) from the Japan Agency for Medical Research and Development.
We are grateful to the Support Center for Medical Research and Education, Tokai University.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Contributor Information
Naoki Takatori, Email: n.tori001@tsc.u-tokai.ac.jp.
Masato Sato, Email: sato-m@is.icc.u-tokai.ac.jp.
References
- 1.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metabol. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q., Vaillancourt F., Côté V., Fahmi H., Lavigne P., Afif H. Alterations of metabolic activity in human osteoarthritic osteoblasts by lipid peroxidation end product 4-hydroxynonenal. Arthritis Res Ther. 2006;8:R159. doi: 10.1186/ar2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldring S.R., Goldring M.B. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 4.Bailey A.J., Mansell J.P., Sims T.J., Banse X. Biochemical and mechanical properties of subchondral bone in osteoarthritis. Biorheology. 2004;41:349–358. [PubMed] [Google Scholar]
- 5.Paget J. The classics. II. Healing of cartilage. Sir James Paget, Bart, M.D., London, member of the Royal Society. Clin Orthop Relat Res. 1969;64:7–8. [PubMed] [Google Scholar]
- 6.Conaghan P.G., Dickson J., Grant R.L. Care and management of osteoarthritis in adults: summary of NICE guidance. BMJ. 2008;336:502–503. doi: 10.1136/bmj.39490.608009.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asari A., Miyauchi S., Matsuzaka S., Ito T., Kominami E., Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;l61:125–135. doi: 10.1679/aohc.61.125. [DOI] [PubMed] [Google Scholar]
- 8.Cifuentes D.J., Rocha L.G., Silva L.A., Brito A.C., Rueff-Barroso C.R., Porto L.C. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthr Cartil. 2010;18:1088–1095. doi: 10.1016/j.joca.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Ameye L.G., Young M.F. Animal models of osteoarthritis: lessons learned while seeking the “Holy Grail”. Curr Opin Rheumatol. 2006;18:537–547. doi: 10.1097/01.bor.0000240369.39713.af. [DOI] [PubMed] [Google Scholar]
- 10.Hayami T., Pickarski M., Zhuo Y., Wesolowski G.A., Rodan G.A., Duong L.T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and minisectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Setton L.A., Elliott D.M., Mow V.C. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthr Cartil. 1999;7:2–14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 12.Van der Kraan P.M., Vitters E.L., van de Putte L.B., van den Berg W.B. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989;135:1001–1014. [PMC free article] [PubMed] [Google Scholar]
- 13.Beyreuther B., Callizot N., Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9:R14. doi: 10.1186/ar2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams V.S. Intraarticular hyaluronic acid supplementation in the horse: the role of molecular weight. J Equine Vet Sci. 2007;27:298–303. [Google Scholar]
- 15.AL-Saffar F.J., Ganabadi S., Yaakub H., Fakurazi S. Collagenase and sodium iodoacetate-induced experimental osteoarthritis model in Sprague Dawley rats. Asian J Sci Res. 2009;2:167–179. [Google Scholar]
- 16.Teeple E., Jay G.D., Elsaid K.A., Fleming B.C. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15:438–446. doi: 10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuyinu E.L., Narayanan G., Nair L.S., Laurencin C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. 2016;11:19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernihough J., Gentry C., Malcangio M., Fox A., Rediske J. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112:83–93. doi: 10.1016/j.pain.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Bove S.E., Calcaterra S.L., Brooker R.M., Huber C.M., Guzman R.E., Juneau P.L. Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthr Cartil. 2003;11:821–830. doi: 10.1016/s1063-4584(03)00163-8. [DOI] [PubMed] [Google Scholar]
- 20.Ivanavicius S.P., Ball A.D., Heapy C.G., Westwood F.R., Murray F., Read S.J. Structural pathology in a rodent model of osteoarthritis is associated with neuropathic pain: increased expression of ATF-3 and pharmacological characterization. Pain. 2007;128:272–282. doi: 10.1016/j.pain.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Piscaer T.M., Waarsing J.H., Kops N., Pavljasevic P., Verhaar J.A.N., van Osch G.J.V.M. In vivo imaging of cartilage degeneration using μCT-arthrography. Osteoarthr Cartil. 2008;16:1011–1017. doi: 10.1016/j.joca.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Koh Y.H., Hong S.H., Kang H.S., Chung C.Y., Koo K.H., Chung H.W. The effects of bone turnover rate on subchondral trabecular bone structure and cartilage damage in the osteoarthritis rat model. Rheumatol Int. 2009;30:1165–1171. doi: 10.1007/s00296-009-1118-x. [DOI] [PubMed] [Google Scholar]
- 23.Naveen S.V., Ahmad R.E., Hui W.J., Suhaeb A.M., Murali M.R., Shanmugam R. Histology, glycosaminoglycan level and cartilage stiffness in monoiodoacetate-induced osteoarthritis: comparative analysis with anterior cruciate ligament transection in rat model and human osteoarthritis. Int J Med Sci. 2014;11:97–105. doi: 10.7150/ijms.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udo M., Muneta T., Tsuji K., Ozeki N., Nakagawa Y., Ohara T. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: proposed model-specific scoring systems. Osteoarthr Cartil. 2016;24:1284–1291. doi: 10.1016/j.joca.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Mohan G., Perilli E., Kuliwaba J.S., Humphries J.M., Parkinson I.H., Fazzalari N.L. Application of in vivo micro-computed tomography in the temporal characterisation of subchondral bone architecture in a rat model of low-dose monosodium iodoacetate-induced osteoarthritis. Arthritis Res Ther. 2011;13:R210. doi: 10.1186/ar3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takaku Y., Murai K., Ukai T., Ito S., Kokubo M., Satoh M. In vivo cell tracking by bioluminescence imaging after transplantation of bioengineered cell sheets to the knee joint. Biomaterials. 2014;35:2199–2206. doi: 10.1016/j.biomaterials.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 27.Ito S., Sato M., Yamato M., Mitani G., Kutsuna T., Nagai T. Repair of articular cartilage defect with layered chondrocyte sheets and cultured synovial cells. Biomaterials. 2012;33:5278–5286. doi: 10.1016/j.biomaterials.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 28.Ebihara G., Sato M., Yamato M., Mitani G., Kutsuna T., Nagai T. Cartilage repair in transplanted scaffold-free chondrocyte sheets using a minipig model. Biomaterials. 2012;33:3846–3851. doi: 10.1016/j.biomaterials.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 29.Mihara M., Higo S., Uchiyama Y., Tanabe K., Saito K. Different effects of high molecular weight sodium hyaluronate and NSAID on the progression of the cartilage degeneration in rabbit OA model. Osteoarthr Cartil. 2007;15:543–549. doi: 10.1016/j.joca.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Tani Y., Sato M., Maehara M., Nagashima H., Yokoyama M., Yokoyama M. The effects of using vitrified chondrocyte sheets on pain alleviation and articular cartilage repair. J Tissue Eng Regen Med. 2017;11:3437–3444. doi: 10.1002/term.2257. [DOI] [PubMed] [Google Scholar]
- 31.Mapp P.I., Sagar D.R., Ashraf S., Burston J.J., Suri S., Chapman V. Differences in structural and pain phenotypes in the sodium monoiodoacetate and meniscal transection models of osteoarthritis. Osteoarthr Cartil. 2013;21:1336–1345. doi: 10.1016/j.joca.2013.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashraf S., Mapp P.I., Walsh D.A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2001;63:2700–2710. doi: 10.1002/art.30422. [DOI] [PubMed] [Google Scholar]
- 33.Nomura M., Sakitani N., Iwasawa H., Kohara Y., Takano S., Wakimoto Y. Thinning of articular cartilage after joint unloading or immobilization. An experimental investigation of the pathogenesis in mice. Osteoarthr Cartil. 2017;25:727–736. doi: 10.1016/j.joca.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Pritzker K.P., Gay S., Jimenez S.A., Ostergaard K., Pelletier J.P., Revell P.A. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Pan T., Wu D., Cai N., Chen R., Shi X., Li B. Alpha-Mangostin protects rat articular chondrocytes against IL-1β-induced inflammation and slows the progression of osteoarthritis in a rat model. Int Immunopharmacol. 2017;52:34–43. doi: 10.1016/j.intimp.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Buckwalter J.A., Mankin H.J. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 37.Huang K., Wu L.D. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36:1149–1160. doi: 10.1177/147323000803600601. [DOI] [PubMed] [Google Scholar]
- 38.Miura S., Kondo J., Kawakami T., Shukunami C., Aimoto S., Tanaka H. Synthetic disulfide-bridged cyclic peptides mimic the anti-angiogenic actions of chondromodulin-I. Cancer Sci. 2012;103:1311–1318. doi: 10.1111/j.1349-7006.2012.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikami Y., Asano M., Honda M.J., Takagi M. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol. 2010;223:123–133. doi: 10.1002/jcp.22017. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Eliezer M., Phillip M., Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32:235–244. doi: 10.1007/s12020-007-9025-y. [DOI] [PubMed] [Google Scholar]
- 41.Mera H., Kawashima H., Yoshizawa T., Ishibashi O., Ali M.M., Hayami T. Chondromodulin-1 directly suppresses growth of human cancer cells. BMC Cancer. 2009;9:166. doi: 10.1186/1471-2407-9-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klinger P., Surmann-Schmitt C., Brem M., Swoboda B., Distler J.H., Carl H.D. Chondromodulin 1 stabilizes the chondrocyte phenotype and inhibits endochondral ossification of porcine cartilage repair tissue. Arthritis Rheum. 2011;63:2721–2731. doi: 10.1002/art.30335. [DOI] [PubMed] [Google Scholar]
- 43.Xu K., Zhang Y., Ilalov K., Carlson C.S., Feng J.Q., Di Cesare P.E. Cartilage oligomeric matrix protein associates with granulin–epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J Biol Chem. 2007;282:11347–11355. doi: 10.1074/jbc.M608744200. [DOI] [PubMed] [Google Scholar]
- 44.Kipnes J., Carlberg A.L., Loredo G.A., Lawler J., Tuan R.S., Hall D.J. Effect of cartilage oligomeric matrix protein on mesenchymal chondrogenesis in vitro. Osteoarthr Cartil. 2003;11:442–454. doi: 10.1016/s1063-4584(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 45.Rosenberg K., Olsson H., Morgelin M., Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 46.Thur J., Rosenberg K., Nitsche D.P., Pihlajamaa T., Ala-Kokko L., Heinegard D. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 47.Mann H.H., Ozbek S., Engel J., Paulsson M., Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]