Abstract
Purpose
The aim of this study was to compare respiratory-triggered (RT) and free breathing (FB) diffusion weighted imaging (DWI) techniques regarding apparent diffusion coefficient (ADC) measurements and repeatability in non-squamous non-small cell lung cancer (NSCLC) measuring the total tumor volume.
Material and Methods
A total of 57 magnetic resonance imaging (MRI) examinations were analyzed. DWI was obtained by a single-shot spin-echo echo-planar imaging sequence, and for each MRI examination 2 consecutive RT and 2 consecutive FB DWI sequences were performed. Two radiologists independently read the images and made measurements. For each tumor the mean ADC value of the whole tumor volume was calculated. The difference in mean ADCs between FB and RT DWI was evaluated using the paired-sample t-test. The repeatability of ADC measurements related to imaging method was evaluated by intra class correlations (ICC) for each of the FB and RT DWI pairs.
Results
There were no significant differences in mean ADCs between FB and RT (Reader 1 p = 0.346, Reader 2 p = 0.583). The overall repeatability of ADC measurement was good for both acquisition methods, with ICCs > 0.9. Subgroup analysis showed somewhat poorer repeatability in small tumors (50 ml or less) and tumors in the lower lung zones for the RT acquisition, with ICC as low as 0.72.
Conclusions
No difference in ADC measurement or repeatability between FB and RT DWI in whole lesion ADC measurements of adenocarcinomas in the lung was demonstrated. The results imply that in this setting the FB acquisition method is accurate and possibly more robust than the RT acquisition technique.
Abbreviations: RT, respiratory-triggered; FB, free breathing; DWI, diffusion weighted imaging; ADC, apparent diffusion coefficient; NSCLC, non-squamous non-small cell lung cancer; MRI, magnetic resonance imaging; ICC, intra class correlations; BH, breath-hold; SNR, signal-to-noise ratio; ROI, region of interest
Key words: Magnetic resonance imaging, Diffusion weighted imaging, non-Small cell lung cancer
1. Introduction
Diffusion-weighted magnetic resonance imaging (DWI) depicts the random Brownian movement of water molecules in biological tissues. The net displacement of these molecules diffusing across an area of tissue per second is the apparent diffusion coefficient (ADC). ADC has proven useful in detection, characterization and treatment response monitoring of malignant diseases [[1], [2], [3], [4]]. In therapy response monitoring of newer targeted anti-cancer therapies, longitudinal non-invasive assessment of the entire tumor volume might be useful [5]. However, DWI is prone to geometric distortions and motion artifacts [6]. Furthermore, susceptibility effects of the air-filled lung parenchyma, as well as cardiac and respiratory motion, make chest DWI extra challenging. Despite these difficulties, DWI of the lungs has been explored for differentiation between central lung tumors and pulmonary atelectasis, differentiating benign from malignant lung nodules [7,8], correlation between ADC and lung cancer grade [[9], [10], [11], [12]], prediction of treatment outcomes, and follow-up monitoring of tumors [2,3,[13], [14], [15]].
There are currently three acquisition methods used for DWI of the lungs, breath-hold (BH), respiratory-triggered (RT) and free-breathing (FB). RT and FB techniques allow better signal-to-noise ratio (SNR) than BH acquisition, and FB is more time efficient than RT. However, concerns have been raised about less detailed anatomical information and less precise ADC quantification because of volume averaging using FB [16]. Several recent lung cancer DWI studies have used RT acquisition [2,3,[10], [11], [12]], but it is still not established which acquisition method that is most robust for measuring ADC. Cui et al evaluated the inter- and intra-observer agreement of ADC measurements in FB, BH, and RT of lung cancer, and found no significant differences between the methods [17], but they focused on the agreement of ADCs on a single slice between different observers and readings at different times of the same DWI scan. The aim of the present study was to compare the RT and FB DWI techniques regarding ADC measurements and repeatability on consecutive scans in non-squamous non-small cell lung cancer (NSCLC) measuring the total tumor volume (3D region of interest).
2. Materials and methods
This prospective single institution study was approved by the local ethics committee and informed consent was obtained from each patient.
2.1. Patients
20 patients with histologically or cytologically documented metastatic (Stage IV) NSCLC, all adenocarcinomas, were enrolled in the study between September 2014 and April 2017. Two patients, in whom the lesion proved to be too small to measure (<2 ml), were excluded. The remaining 18 patients were 8 females and 10 males aged between 51 and 77 years with median age 69 years. DWI was performed before, during and after medical treatment at 4 occasions with at least 1 week apart.
Four patients did not participate in the full study program. Two magnetic resonance imaging (MRI) examinations were incomplete due to technical problems, and 8 MRI examinations were excluded due to the tumors becoming too small in size (<2 ml). A total of 57 MRI examinations were included.
2.2. MRI protocol
All examinations were performed on a 1.5 T MR scanner (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) with body coil (Body 18) and spine coil. DWI was obtained by a single-shot spin-echo echo-planar imaging sequence (TR = 6000 ms, TE = 53 ms, slice thickness = 5 mm, slice gap = 1 mm and in-plane voxel size 2.4 × 2.4 mm) with diffusion-encoding gradient pulses applied in the x, y, and z axes with b = 100 s/mm2 (NEX = 2) and b = 750 s/mm2 (NEX = 5). The FB and RT acquisitions had identical parameters.
For each MRI examination 2 consecutive RT and 2 consecutive FB DWI sequences were performed. For the RT examinations a 2D prospective acquisition correction (PACE) technique was used with a liver dome scout (acquisition window = 1000 ms, scout TR = 150 ms, accept window = 2 mm and accept position = 10%). Conventional images were additionally acquired using an axial T2-weighted BLADE turbo spin-echo sequence over the tumor and a coronal T2-weighted sequence over the thorax.
2.3. Image analysis and ADC measurements
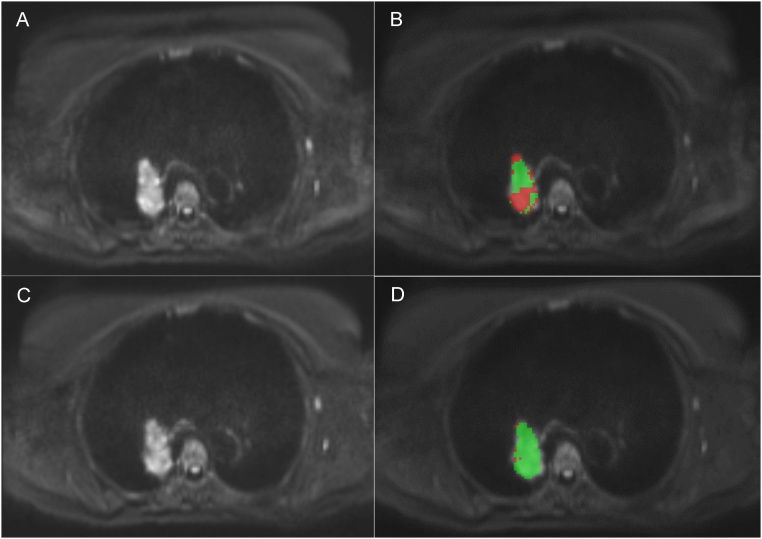
Segmentation and analysis of tumors were undertaken on a stand-alone installation of OncoTreat (Siemens Healthineers, Erlangen, Germany, released research prototype), where parametric images for ADCs were automatically generated out of the two sets of DWI images. Two radiologists with full knowledge of the study objectives independently read the images and made measurements. In order to reduce inaccuracies in ADC measurements resulting from partial volume averaging effect, lesions smaller than 2 ml were excluded. In patients with multiple lesions only the largest lesion was measured. With T2 weighted images as aid, a 3D region of interest (ROI) was drawn on the b = 750 s/mm2 images following the inner rim of the whole tumor volume as illustrated in Fig. 1. For each tumor mean ADC value with standard deviation was calculated. ROIs were drawn only in the first respiratory-triggered (RT1) and free-breathing (FB1) acquisition, and then reused in the second respiratory-triggered (RT2) and free-breathing (FB2) acquisition, respectively. The location was determined (upper, mid and lower zones) using the carina to define the lower limit of the upper lung zone, the pulmonary veins to define the lower limit of the mid lung zone and the hemidiaphragms to define the lower limit of the lower lung zone on axial imaging.
Fig. 1.
Respiratory-triggered (A, B) and free breathing (C, D) axial diffusion weighted images (b = 750 s/mm2) of a tumor in the right lung without and with a region of interest. The respiratory-triggered acquisition time was approximately 9 min and the free breathing acquisition time was 2:26 min.
2.4. Statistical analysis
The difference in mean ADCs between FB and RT DWI was evaluated by using the paired-sample t-test.
Interobserver agreement was determined for FB1 and RT1 using intraclass correlation coefficients (ICC).
ICCs were calculated for each of the FB and RT DWI pairs, to evaluate the repeatability of the ADC measurements. An ICC greater than 0.75 was considered to represent good agreement. To further assess the robustness of the methods the 95% limits of agreement between the ADCs measured on the first and second acquisition were obtained according to the Bland-Altman method for both readers [4,18], and were expressed as percentages of mean ADCs.
3. Results
In total 57 lesions were analyzed. Tumor sizes ranged from 2 to 297 ml with a median volume of 14 ml, 35 lesions had a volume of 50 ml or less and 22 lesions were larger than 50 ml. 28 of the lesions were located in the upper lung zone, 15 in the mid lung zone and 14 in the lower lung zone. Mean ADCs by acquisition method are summarized in Table 1. There were no significant differences in mean ADCs between FB and RT in the first acquisition for Reader 1 (p = 0.346) or for Reader 2 (p = 0.583).
Table 1.
Mean ADCs by acquisition method. Difference evaluated by paired sample t-test.
| Free-breathing | Respiratory-triggered | p | ||
|---|---|---|---|---|
| Reader 1 | First acquisition | 1251 +/− 253 | 1270 +/− 248 | 0.346 |
| Second acquisition | 1285 +/− 249 | 1303 +/− 280 | ||
| Reader 2 | First acquisition | 1286 +/− 256 | 1296 +/− 238 | 0.583 |
| Second acquisition | 1308 +/− 250 | 1345 +/− 295 |
Data are +/−SD.
Interobserver agreements were good for both acquisition methods with ICCs > 0.9, FB1 0.97 (0.95–0.99) and RT1 0.97 (0.94–0.98).
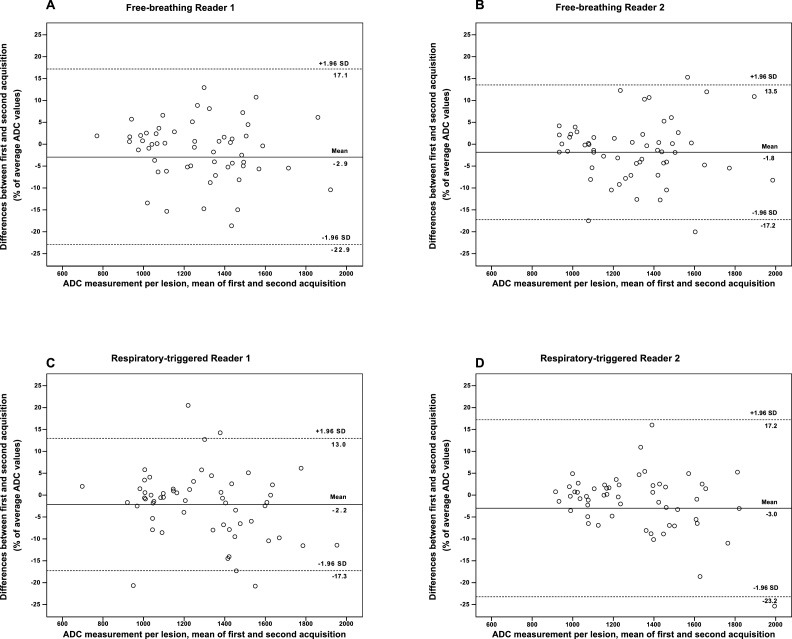
The overall repeatability of ADC measurement was good for both acquisition methods, with ICCs > 0.9 (Table 2). The 95% limits of agreement between ADCs measured on repeated DWI (expressed as percentage of the mean) was 15.2% (RT) and 20.0% (FB) for Reader 1, and 20.2% (RT) and 15.4% (FB) for Reader 2, illustrated in Fig. 2.
Table 2.
Repeatability of ADC measurements for the first and second acquisition of free-breathing and respiratory-triggered techniques, respectively, for each reader evaluated by intraclass correlation coefficients (ICC) and 95% limits of agreement.
| Reader 1 |
Reader 2 |
|||
|---|---|---|---|---|
| Free-breathing | Respiratory-triggered | Free-breathing | Respiratory-triggered | |
| ICC | 0.94 (0.90-0.97) | 0.96 (0.92-0.97) | 0.95 (0.92−0.97) | 0.90 (0.82−0.94) |
| 95% limit of agreementa | 20.0 | 15.2 | 15.4 | 20.2 |
Data in parenthesis are 95% confidence interval.
Expressed as percentage of the mean.
Fig. 2.
Bland-Altman plots show reproducibility of ADC measurement for Reader 1 (A, C) and Reader 2 (B, D) with repeated free-breathing (A, B) and respiratory-triggered (C, D) imaging. X-axes show means of ADCs measured on the first and second acquisition, and y-axes show differences between ADCs on each set as a percentage of their mean. Dashed lines = 95% limits of agreement.
A subgroup analysis of small tumors (50 ml or less) was performed. The repeatability of ADC measurements in this group also showed good agreement between the first and second acquisition with ICCs >0.9 (Table 3), except for the respiratory-triggered acquisition for Reader 2 with ICC 0.80 (0.60–0.90).
Table 3.
Subgroup analysis of small tumors (<50 ml). Repeatability of ADC measurements in the first and second acquisition evaluated by intraclass correlation coefficients (ICC).
| Reader 1 | Reader 2 | |
|---|---|---|
| Free-breathing | 0.90 (0.80−0.95) | 0.93 (0.86−0.96) |
| Respiratory-triggered | 0.92 (0.82−0.96) | 0.80 (0.60−0.90) |
Data in parenthesis are 95% confidence interval.
A subgroup analysis of the lesions grouped by location was also performed. The repeatability of ADC differed in the different subgroups (Table 4), with good agreement between the first and second acquisition for both methods in the upper and middle lungs (ICCs >0.9). In the lower lungs the repeatability of ADC measurements showed less agreement between the first and second acquisition, especially for the respiratory-triggered acquisition with ICC as low as 0.72 (0.16–0.91).
Table 4.
Subgroup analysis of tumors by location. Repeatability of ADC measurements in the first and second acquisition evaluated by intraclass correlation coefficients (ICC).
| Upper lunga |
Mid lungb |
Lower lungc |
||||
|---|---|---|---|---|---|---|
| Reader 1 | Reader 2 | Reader 1 | Reared 2 | Reader 1 | Reader 2 | |
| Free-breathing | 0.98 (0.96−0.99) | 0.98 (0.96−0.99) | 0.91 (0.73−0.97) | 0.93 (0.79−0.98) | 0.90 (0.68−0.97) | 0.93 (0.78−0.98) |
| Respiratory-triggered | 0.98 (0.96−0.99) | 0.99 (0.97−0.99) | 0.93 (0.79−0.98) | 0.96 (0.90−0.99) | 0.89 (0.66−0.97) | 0.72 (0.16−0.91) |
Data in parenthesis are 95% confidence interval.
Tumors in the upper lung, above the carina.
Tumors in the mid lung, between the carina and the pulmonary veins.
Tumors in the lower lung, below the pulmonary veins.
4. Discussion
This study demonstrated no significant difference of ADC measurements obtained with respiratory-triggered technique compared to free-breathing technique in lung adenocarcinomas when measuring the total tumor volume (3D).
There are currently three available imaging methods used for DWI of the lungs, breath-holding, respiratory triggered and free breathing. When using the BH technique it is difficult to obtain high SNR, with consequently less accurate ADC [19]. Prior studies have evaluated lung tumors with DWI acquired by RT [2,3,7,11,12]. The advantages of RT are less motion artifacts and better SNR, which presumably would give more robust and accurate ADC values. The disadvantage of the RT technique is its longer exam time compared to FB technique, which can be problematic in lung cancer patients with breathing disabilities.
Our studied cohort was homogenous with all tumors being adenocarcinomas. The total tumor ADC was measured using a 3D ROI, and both the RT and FB sequence were consecutively performed twice.
We found no significant difference between the different techniques, and the repeatability of the ADC measurements, as well as the interobserver agreement was good for both techniques. The repeatability of the ADC measurements ranged from 15.2% to 20.2%, in line with a prior study analyzing repeatability of FB DWI [20], and ICCs determining the interobserver agreement were > 0.9 in concordance with a study analyzing the interobserver agreement for RT and FB acquisition techniques using 2D ROIs [17].
Our hypothesis was that the FB technique would be less robust compared to the RT technique, since it does not compensate for breathing motion. Since ROI placement is observer dependent we choose to measure inter-observer variability for the first acquisition and the reuse the first ROI on the second acquisition. Any difference in data between the first and second acquisition could thereby not depend on an observer-dependent ROI placement and should instead depend on the different techniques used. Interestingly, no significant difference in mean ADCs between FB and RT in the first acquisition was found.
ADC measurements are influenced by the selection of b-values, the ADC calculation method and the acquisition method [4,21,22]. A sufficient SNR is essential to ensure high precision in ADC measurements [19]. Previous studies have shown ADC values of approximately 1.0–1.4 × 10−3 mm2/s in lung adenocarcinomas, which increase as a response to treatment [9,13,23]. Similarly to a study by Bernardin et al [24], we therefore choose a high b-value of 750 s/mm2. To further ensure high SNR we used fewer slices to only investigate the tumors, not the entire lungs. Finally, the low b value was 100 s/mm2 to reduce the effects of perfusion on the ADC estimate [25]. The ADC values in our cohort were in keeping with values in earlier studies of lung tumors [9,13,23].
Even though the tumors in this study were histologically homogenous they had more diverse size (2–297 ml) than lesions in other studies [17,24]. Since the tumors varied so greatly in size, we performed a subgroup analysis. Similarly to an earlier study [24], we found a trend towards lower repeatability of ADC measures of the smaller lesions when compared to the whole cohort, though only in the RT acquisition. The improved repeatability with lesion size is to be expected as partial volume effects are reduced with increasing size of the lesion.
We also performed a subgroup analysis of lesions by location and somewhat surprisingly we found lower repeatability of ADC measurements in the RT acquisitions of the lesions in the lower lung, with ICC as low as 0.72 compared to 0.98 in the upper lung and 0.93 in the mid lung respectively. The FB technique showed good repeatability in all three subgroups with ICCs >0.9. If the respiratory triggering does not fully compensate for the motion in the lower lungs the much longer RT acquisition time (approximately 9–10 min compared to FB acquisition time 2:26 min) might explain these results. This might also be a reason to why we found no significant difference in ADC measurement or repeatability between FB and RT DWI in the whole data set.
There are limitations to this study. Since ROIs were drawn in the first RT and FB acquisition and then reused in the second RT and FB acquisition, respectively, small patient movements between the acquisitions might influence the accuracy of the ROI placement and give less accurate ADC values in the second acquisitions. However, the aim was to test the repeatability for the different techniques without any interobserver variability and our results showed no statistical difference. Although only adenocarcinomas were examined, the high variability in tumor size and ADCs would allow the results to be used for other lung tumors.
In conclusion, no difference in ADC measurement or repeatability between FB and RT DWI in whole lesion (3D) ADC measurements of adenocarcinomas in the lung was demonstrated. Furthermore subgroup analysis of small tumors and tumors in the lower lung showed a trend towards lower repeatability in the RT technique than in the FB technique. The results of our study imply that in this setting the FB acquisition method is accurate and possibly more robust than the RT acquisition technique.
Role of the funding source
The study was funded by Roche and software supported by Siemens. The funding sources had no influence on study design, collection and interpretation of data or manuscript preparation.
Declarations of interest and source of funding
A Carlberg is employed by Siemens. For the remaining authors no conflicts of interest were declared.
Acknowledgement
The study was conducted at Karolinska University Hospital (Solna, Sweden) with funding from Roche (Basel, Switzerland) and software support from Siemens Healthineers (Erlangen, Germany). The funding sources had no influence on study design, collection and interpretation of data or manuscript preparation.
Contributor Information
Signe Swerkersson, Email: signe.swerkersson@capiostgoran.se.
Oscar Grundberg, Email: oscar.grundberg@sll.se.
Karl Kölbeck, Email: karl.kolbeck@sll.se.
Andreas Carlberg, Email: andreas.carlberg@siemens-healthineers.com.
Sven Nyrén, Email: sven.nyren@sll.se.
Mikael Skorpil, Email: mikael.skorpil@ki.se.
References
- 1.Charles-Edwards E.M., deSouza N.M. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–143. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reischauer C., Froehlich J.M., Pless M. Early treatment response in non-small cell lung cancer patients using diffusion-weighted imaging and functional diffusion maps–a feasibility study. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu J., Li W., Zhang Z. Prediction of early response to chemotherapy in lung cancer by using diffusion-weighted MR imaging. Scientific World J. 2014;2014:135841. doi: 10.1155/2014/135841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taouli B., Beer A.J., Chenevert T. Diffusion-weighted imaging outside the brain: consensus statement from an ISMRM-sponsored workshop. J. Magn. Reson. Imaging. 2016;44(3):521–540. doi: 10.1002/jmri.25196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padhani A.R., Liu G., Koh D.M. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Bihan D., Poupon C., Amadon A. Artifacts and pitfalls in diffusion MRI. J. Magn. Reson. Imaging. 2006;24(3):478–488. doi: 10.1002/jmri.20683. [DOI] [PubMed] [Google Scholar]
- 7.Liu H., Liu Y., Yu T. Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur. Radiol. 2010;20(4):807–815. doi: 10.1007/s00330-009-1629-6. [DOI] [PubMed] [Google Scholar]
- 8.Çakır Ç., Gençhellaç H., Temizöz O. Diffusion weighted magnetic resonance imaging for the characterization of solitary pulmonary lesions. Balkan Med. J. 2015;32(4):403–409. doi: 10.5152/balkanmedj.2015.15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koyama H., Ohno Y., N Aoyama. Comparison of STIR turbo SE imaging and diffusion-weighted imaging of the lung: capability for detection and subtype classification of pulmonary adenocarcinomas. Eur. Radiol. 2010;20(4):790–800. doi: 10.1007/s00330-009-1615-z. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Liu Y., Yu T. Evaluation of apparent diffusion coefficient associated with pathological grade of lung carcinoma, before therapy. J. Magn. Reson. Imaging. 2015;42(3):595–601. doi: 10.1002/jmri.24823. [DOI] [PubMed] [Google Scholar]
- 11.Usuda K., Zhao X.T., Sagawa M. Diffusion-weighted imaging (DWI) signal intensity and distribution represent the amount of cancer cells and their distribution in primary lung cancer. Clin. Imaging. 2013;37(2):265–272. doi: 10.1016/j.clinimag.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Yang R.M., Li L., Wei X.H. Differentiation of central lung cancer from atelectasis: comparison of diffusion-weighted MRI with PET/CT. PLoS One. 2013;8(4):e60279. doi: 10.1371/journal.pone.0060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Q., Wu N., Ouyang H. Diffusion-weighted magnetic resonance imaging of lung cancer at 3.0 T: a preliminary study on monitoring diffusion changes during chemoradiation therapy. Clin. Imaging. 2012;36(2):98–103. doi: 10.1016/j.clinimag.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Okuma T., Matsuoka T., Yamamoto A. Assessment of early treatment response after CT-guided radiofrequency ablation of unresectable lung tumours by diffusion-weighted MRI: a pilot study. Br. J. Radiol. 2009;82(984):989–994. doi: 10.1259/bjr/13217618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yabuuchi H., Hatakenaka M., Takayama K. Non-small cell lung cancer: detection of early response to chemotherapy by using contrast-enhanced dynamic and diffusion-weighted MR imaging. Radiology. 2011;261(2):598–604. doi: 10.1148/radiol.11101503. [DOI] [PubMed] [Google Scholar]
- 16.Kartalis N., Loizou L., Edsborg N. Optimising diffusion-weighted MR imaging for demonstrating pancreatic cancer: a comparison of respiratory-triggered, free-breathing and breath-hold techniques. Eur. Radiol. 2012;22(10):2186–2192. doi: 10.1007/s00330-012-2469-3. [DOI] [PubMed] [Google Scholar]
- 17.Cui L., Yin J.B., Hu C.H. Inter- and intraobserver agreement of ADC measurements of lung cancer in free breathing, breath-hold and respiratory triggered diffusion-weighted MRI. Clin. Imaging. 2016;40(5):892–896. doi: 10.1016/j.clinimag.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. [PubMed] [Google Scholar]
- 19.Jones D.K., Horsfield M.A., Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42(32):515–525. [PubMed] [Google Scholar]
- 20.Weller A., Papoutsaki M.V., Waterton J.C. Eur. Radiol. 2017;27(11):4552–4562. doi: 10.1007/s00330-017-4828-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S.Y., Lee S.S., Byun J.H. Malignant hepatic tumors: short-term reproducibility of apparent diffusion coefficients with breath-hold and respiratory-triggered diffusion-weighted MR imaging. Radiology. 2010;255(3):815–823. doi: 10.1148/radiol.10091706. [DOI] [PubMed] [Google Scholar]
- 22.Jones D.K. Precision and accuracy in diffusion tensor magnetic resonance imaging. Top. Magn. Reson. Imaging. 2010;21(2):87–99. doi: 10.1097/RMR.0b013e31821e56ac. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., Zhang J., Chen Y. Relationship between apparent diffusion coefficient and tumour cellularity in lung cancer. PLoS One. 2014;9(6):e99865. doi: 10.1371/journal.pone.0099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernardin L., Douglas N.H., Collins D.J. Diffusion-weighted magnetic resonance imaging for assessment of lung lesions: repeatability of the apparent diffusion coefficient measurement. Eur. Radiol. 2014;24(2):502–511. doi: 10.1007/s00330-013-3048-y. [DOI] [PubMed] [Google Scholar]
- 25.A. Ogura, K. Hayakawa, T. Miyati, et al., Imaging parameter effects in apparent diffusion coefficient determination of magnetic resonance imaging, Eur J Radiol, 77 (1) 2011 185-188. [DOI] [PubMed]