Abstract
α-Glucosidase inhibitors (aGIs) have been used as an effective therapy for type-2 diabetes, which remains a global health issue. The aim of this study was to achieve bioactivity-guided isolation, identification and evaluation of hypoglycemic compounds from Euonymus laxiflorus Champ. trunk bark (ELCTB). Eleven active compounds were isolated and identified as walterolactone A/B β-d-pyranoglucoside (1), 1-β-d-glucopyranosyloxy-3,5-dimethoxy-4-hydroxybenzene (9), (−)-gallocatechin (10), schweinfurthinol 9-O-β-d-pyranoglucoside (11), 1-O-(3-methyl)-butenoyl-myo-inositol (12), leonuriside (14), (+)-catechin (19), methyl galloate (20), (−)-catechin (23), and condensed tannins (5 and 18). Of these 11, novel 4 compounds (1, 11, 12, and 14) were found as new α-glucosidase inhibitors. Notably, in vitro results indicated that compounds 1, 5, 10–12, 18, and 19 showed potent activity (IC50 = 0.076−31 µg/mL), and their activities were at a higher level than that of acarbose, a commercial inhibitor (IC50 = 1345 µg/mL). In animal tests, the major inhibitor, condensed tannin (18), demonstrated significant reduction of plasma glucose in mice with no symptoms of diarrhea at the dose of 100 mg/kg bw. The results suggest that Euonymus laxiflorus Champ. is a rich source of bioactive compounds for development as health food or drugs with potent hypoglycemic effect. The results of this study also enriched the current novel biological activities of constituents from Euonymus laxiflorus species.
Keywords: Euonymus laxiflorus Champ., diabetes, α-glucosidase inhibitors, plasma glucose, condensed tannins, natural products
1. Introduction
Natural bioactive products are of great interest due to their beneficial use as health foods or drugs to manage significant numbers of diseases including type-2 diabetes (T2D), a serious current global health issue [1,2]. Several therapies, including the use of α-glucosidase inhibitors (aGIs), have been applied for T2D management [3]. To date, some commercial aGIs have become available for T2D treatment, such as acarbose, miglitol, and voglibose. However, the use of these commercial drugs was reported to cause several side effects, such as diarrhea, flatulence, and abdominal discomfort [4]. Thus, the investigation of natural sources of aGIs for safe use is required.
aGIs could be obtained from various sources, such as herbal extracts [4,5,6,7], biosynthesis via microbial fermentation [2,8,9,10,11,12], or chemical synthesis [13,14]. Herbals were suggested as rich natural sources of aGIs, which may be useful in providing therapy for T2D [4,5]; as such, the bioassay-guided isolation and identification of active aGIs from potent antidiabetic herbals have proven valuable in research.
Euonymus laxiflorus Champ. is distributed in some Asian countries, including China, Vietnam, Cambodia, India, and Myanmar [15]. This herbal species was evaluated as the most potent source of aGIs and α-amylase inhibitors (aAIs) among various medicinal herbals collected in the central highlands of Vietnam in our previous studies [4,16]. The significant effect on the reduction of plasma glucose in diabetic rats of the methanolic extract of Euonymus laxiflorus Champ. trunk bark (ELCTB) was also previously recorded [17]. Recently, some compounds possessing α-amylase inhibitory activity were isolated and identified from the methanolic extract of the trunk bark of Euonymus laxiflorus Champ. [18]; as well, several compounds showing anti-nitro oxide activity were isolated from its leaves [19]. However, no compounds showing α-glucosidase inhibitory property were reported to be isolated and identified from this herbal species.
As part of our ongoing objective to develop Euonymus laxiflorus Champ. as a health food and drug with potent hypoglycemic function, the methanolic extract of ELCTB was conducted to isolate and identify active α-glucosidase inhibitors. The major and most active inhibitor was also tested for its effect on reducing blood glucose in mice.
2. Results and Discussion
2.1. Reclamation of Euonymus laxiflorus Champ. Extracts as a Potent Source of Natural aGIs
The methanol extract of Euonymus laxiflorus Champ. trunk bark (ELCTB) was newly found to be the most potent source of aGIs among 26 samples of indigenous medicinal plants collected in Dak Lak Province, Vietnam [4], showing effective inhibition against various α-glucosidases from rat (IC50 = 360 µg/mL), S. cerevisiae (IC50 = 1.32 µg/mL), and B. stearothermophilus (IC50 = 5.15 µg/mL).
In the comparison, this herbal extract demonstrated comparable or much higher α-glucosidase inhibitory activity compared to those of extracts from other reported herbals also collected in the central highlands of Vietnam due to its smallest IC50 values against α-glucosidases from rat (Table 1).
Table 1.
α-Glucosidase inhibition by some MeOH extracts of medicinal plants collected in Dak Lak.
| Scientific Name of Medicinal Plants | Part Used | IC50 (µg/mL) | Ref. |
|---|---|---|---|
| Euonymus laxiflorus Champ. | Trunk bark | 360 ± 28.9 d,e | Nguyen et al., 2017 [4] |
| Euonymus laxiflorus Champ. | Leaves | 670 ± 40.4 d | Nguyen et al., 2017 [4] |
| Cinnamomum cassia J. S. Presl. | Trunk bark | 1080 ± 103.9 c | Nguyen et al., 2017 [4] |
| Terminalia bellirica | Leaves | 660 ± 63.5 d | Nguyen et al., 2017 [4] |
| Terminalia bellirica | Trunk bark | 410 ± 30.4 d,e | Nguyen et al., 2016 [5] |
| Terminalia corticosa | Trunk bark | 1420 ± 20.2 b,c | Nguyen et al., 2016 [5] |
| Psidium littorale Raddi | Leaves | 250 ± 10.4 e | Nguyen et al., 2018 [6] |
| Dalbergia tonkinensis | Heartwood | 1720 ± 116 b | Nguyen et al., 2018 [7] |
| Dalbergia tonkinensis | Trunk bark | 2910 ± 289 a | Nguyen et al., 2018 [7] |
| Dalbergia tonkinensis | Leaves | 2780 ± 173 a | Nguyen et al., 2018 [7] |
α-Glucosidase from rat was used for testing; results are means ± SD of multi tests (n = 3); coefficient of variation = 12.35314; the means of IC50 values with the different letter are significantly different in comparison based on Duncan′s multiple range test (alpha = 0.01) using SAS version 9.4, Statistical Analysis Software analysis.
The methanol extract of this herbal also possesses significant effect on reducing plasma glucose in diabetic rats [17]. Thus, it was used for bioactivity-guided isolation of active hypoglycemic compounds via several columns, such as Diaion, Octadecylsilane, and preparative HPLC columns in the current report.
2.2. Purification of Active aGIs from MeOH Extract of ELCTB
2.2.1. Separation, Subfractionation and Isolation of Compounds from ELCTB Extract
The methanol extract of Euonymus laxiflorus Champ. trunk bark (ELCTB) was first separated via a Diaion column to obtain five fractions. The crude sample and its fractions were tested for their inhibition against yeast α-glucosidase, and the results are presented in Table 2. The fractions ELCTB-2 (eluted with 40% MeOH) and ELCTB-3 (eluted with 70% MeOH) demonstrated the highest activity with low IC50 and great inhibition (%) values of 2.80 μg/mL, 99%, and 3.50 μg/mL, 98%, respectively. Thus, they were chosen for further separation.
Table 2.
α-Glucosidase inhibitory activity of methanol extract of Euonymus laxiflorus Champ. trunk bark (ELCTB), and its fraction and subfraction separation via Diaion and ODS (Octadecylsilane) columns, respectively.
| Fractions/Subfractions | % Gradient of Solvent in Water | α-Glucosidase Inhibitory Activity | |
|---|---|---|---|
| IC50 (μg/mL) | Inhibition (%) * | ||
| Separation of ELCTB via a Diaion column with MeOH in H2O | |||
| ELCTB | 7.16 ± 0.21 | 98 ± 2.5 | |
| ELCTB-1 | 0 | 21.75 ± 1.32 | 67 ± 3.3 |
| ELCTB-2 | 40 | 2.80 ± 0.03 | 99 ± 3.1 |
| ELCTB-3 | 70 | 3.50 ± 0.12 | 98 ± 1.4 |
| ELCTB-4 | 100 | 18.50 ± 1.39 | 97 ± 2.3 |
| ELCTB-5 | 100% EA | - | - |
| Separation of ELCTB-2 via an ODS column with MeOH in H2O | |||
| ELCTB-2.1 | 0 | 6.20 ± 0.43 | 97 ± 3.5 |
| ELCTB-2.2 | 5 | 85.30 ± 4.03 | 97 ± 2.9 |
| ELCTB-2.3 | 10 | 26.03 ± 1.83 | 98 ± 1.8 |
| ELCTB-2.4 | 15 | 14.96 ± 0.78 | 98 ± 3.0 |
| ELCTB-2.5 | 20 | 6.56 ± 0.45 | 97 ± 2.0 |
| ELCTB-2.6 | 25 | 6.92 ± 0.73 | 99 ± 1.3 |
| ELCTB-2.7 | 30 | 72.95 ± 3.93 | 98 ± 1.0 |
| ELCTB-2.8 | 35 | 6.42 ± 0.41 | 98 ± 2.8 |
| ELCTB-2.9 | 40 | 10.75 ± 0.63 | 95 ± 3.7 |
| ELCTB-2.10 | 100 | - | - |
| Separation of ELCTB-3 via an ODS column with ACN in H2O | |||
| ELCTB-3.1 | 10 | 1.12 ± 0.03 | 100 ± 2.3 |
| ELCTB-3.2 | 15 | 6.21 ± 0.05 | 97 ± 2.0 |
| ELCTB-3.3 | 20 | 2.48 ± 0.07 | 97 ± 2.4 |
| ELCTB-3.4 | 25 | 1.64 ± 0.04 | 99 ± 3.6 |
| ELCTB-3.5 | 30 | 1.95 ± 0.08 | 97 ± 2.7 |
| ELCTB-3.6 | 35 | 1.89 ± 0.0 | 100 ± 2.0 |
| ELCTB-3.7 | 40 | 4.87 ± 0.12 | 99 ± 3.0 |
| ELCTB-3.8 | 45 | 4.34 ± 0.21 | 99 ± 3.4 |
| ELCTB-3.9 | 50 | 7.506 ± 0.32 | 96 ± 3.8 |
| ELCTB-3.10 | 100 | - | - |
| Acarbose | 1239 ± 78 | 64 ± 2.4 | |
(-): no α-glucosidase inhibition; (*): the inhibition of samples and acarbose were tested at their concentration of 150 μg/mL and 2500 μg/mL, respectively; EA: ethyl acetate.
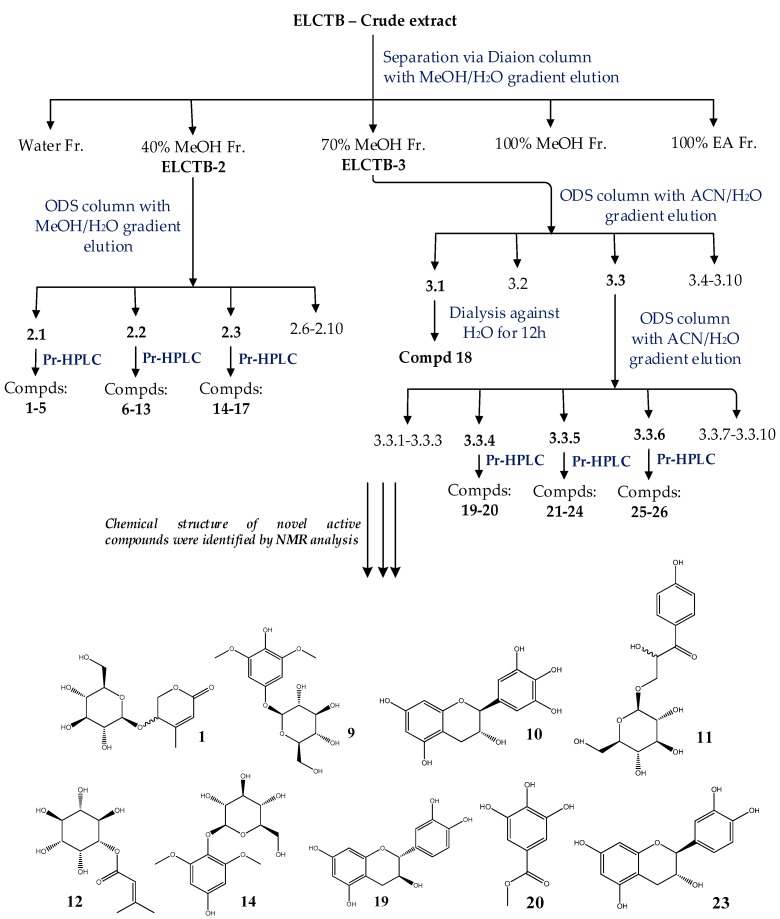
Fractions ELCTB-2 and ELCTB-3 were loaded onto ODS columns and eluted with MeOH and ACN in H2O, respectively, to obtain 10 subfractions from each fraction, and their α-glucosidase inhibitory activity was tested. As shown in Table 2, subfraction ELCTB-2.1 showed good activity (IC50 = 6.20 µg/mL, max inhibition = 97%). Similarly, subfraction ELCTB-3.1 demonstrated the highest activity (IC50 = 1.12 µg/mL, max inhibition = 100%) among the 10 subfractions separated from ELCTB-3. Thus, these 2 potent subfractions were chosen for the isolation of active compounds. The other three fractions: ELCTB-2.2, ELCTB-2.3, and ELCTB-3.3 were also considered for further purification due to their good TLC separation profiles. From these five subfractions (ELCTB-2.1, ELCTB-2.2, ELCTB-2.3, ELCTB-3.1, and ELCTB-3.3), a total of 26 compounds were isolated by utilizing preparative HPLC. The purification process is summarized in Figure 1.
Figure 1.
Flow chart on the purification and identification of active compounds from ELCTB extract. ACN: acetonitrile; ODS: octadecylsilane; Compds: compounds; Pr-HPLC: preparative high-performance liquid chromatography. (1): Walterolactone A/B β-d-pyranoglucoside; (9): 1-β-d-glucopyranosyloxy-3,5-dimethoxy-4-hydroxybenzene; (10): (−)-gallocatechin; (11): schweinfurthinol 9-O-β-d-pyranoglucoside; (12): 1-O-(3-methyl)-butenoyl-myo-inositol; (14): leonuriside; (19): (+)-catechin; (20): methyl galloate; (23): (−)-catechin.
2.2.2. Evaluation and Identification of Active aGIs
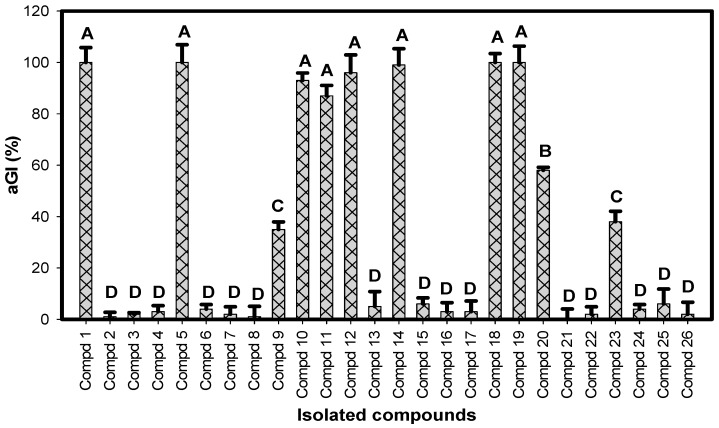
The 26 isolated compounds were primarily tested for their inhibition against α-glucosidase at the same concentration of 250 µg/mL; the α-glucosidase inhibition, aGI (%), is presented in Figure 2. Eight compounds: 1, 5, 10, 11, 12, 14, 18, and 19, demonstrated potent activity (90–100%, ranked at level A), and three compounds: 9, 20, and 23, showed activity of 38–60% (ranked at level B–C based on Duncan’s Multiple Range Test (alpha = 0.01)).
Figure 2.
Evaluation of α-glucosidase inhibition (aGI (%)) of isolated compounds. All compounds were tested at a concentration of 250 µg/mL. Results are means ± SD of multi tests (n = 3). Coefficient of variation (%) = 21.72221. Mean values with the different letters are significantly different based on Duncan’s Multiple Range Test (alpha = 0.01).
The chemical structures of the active compounds (1, 5, 9, 10, 11, 12, 14, 18, 19, 20, and 23) were identified via analysis of their NMR data, including 1H-MNR, 13C-NMR, COSY, HSQC, and HMBC coupled with the comparison of those of reported compounds. These active compounds were identified as walterolactone A/B β-d-pyranoglucoside (1) [18]; 1-β-d-glucopyranosyloxy-3,5-dimethoxy-4-hydroxybenzene (9) [20]; (−)-gallocatechin (10) [21]; schweinfurthinol 9-O-β-d-pyranoglucoside (11) [18]; 1-O-(3-methyl)-butenoyl-myo-inositol (12) [18]; leonuriside (14) [22,23]; (+)-catechin (19) [21]; methyl galloate (20) [24]; (−)-catechin (23) [21]; and condensed tannin (5 and 18) [18]. The NMR data of all these identified compounds are presented in the materials and methods section.
Compounds 5 & 18 were confirmed as condensed tannins by using total phenolic determination, biological assays [25], and comparison of their 13C-NMR data with those of reported condensed tannins [18,26,27]. These 2 compounds were pre-confirmed as tannins since they contained ≥94% total phenolic acid and also exhibited tested biological activities, including antioxidant activity (≥96%), α-amylase inhibition (100%), and protease inhibition (≥95%) at their tested concentration of 1 mg/mL. In addition, the 13C-NMR data on these compounds (materials and methods section) were similar to those of condensed tannin ELC3.1-d isolated and identified from the same herbal species [18], and also closely similar to those of other reported condensed tannins, including persimmon tannin [26], and condensed tannins from Delonix regia [27]. Thus, compounds 5 & 18 were confirmed as condensed tannins.
Compounds 1, 11, and 12 were newly isolated and identified as new compounds from the same medicinal plant in the previous report by Nguyen et al. [18]. However, the α-glucosidase inhibitory activity of these compounds was investigated for the first time in this study. They were determined as new aGIs based on the current literature review, and all their NMR spectrums, including 1H-MNR, 13C-NMR, DEPT135, COSY, HSQC, and HMBC were presented in the supplementary materials section (Figures S1–S21). Compound 14, leonuriside, was reported to possess several bioactive properties, including α-amylase inhibition [18], COX-1 inhibition [24], and potent anti-NO [22]. However, the α-glucosidase inhibition of leonuriside had not been reported before; this compound was also determined as a new aGI.
Other known phenolic compounds: 5, 9, 10, 18–20, and 23, have been reported to exhibit numerous beneficial bioactivities, including antimicrobial, cytotoxic [28], antiviral [29], antioxidant [30,31], anti-inflammatory [32], and enzyme inhibitory activities [18,20,33,34], as well as exercising a relaxation effect [35]. The results indicate that Euonymus laxiflorus Champ. is a rich potent source of hypoglycemic compounds, and possesses many other valuable biological activities.
2.3. Comparision of α-Glucosidase Inhibitory Activity of Identified Compounds
To screen the most active α-glucosidase inhibitors, all the isolated inhibitors were tested for their activity at various concentrations; the activity was then expressed as IC50 (μg/mL) value. As shown in Table 3, compounds 1, 5, 14, 18, and 19 demonstrated the most effective inhibition due to their smallest IC50 values (0.076–0.926 μg/mL). These inhibitors also showed great maximum inhibition (99–100% at 250 μg/mL). Compounds 10, 11, and 12 were also found as potent inhibitors, which possessed low IC50 values (11.9–31.6 μg/mL) and high maximum inhibition (87–96%). Compounds 9 and 23 showed weak activity (≤38%). Overall, these novel inhibitors had their activity ranked sequentially based on Duncan’s Multiple Range Test (alpha = 0.01): 9 & 23 ≤ acarbose ≤ 20 ≤ 10, 11 & 12 ≤ 1, 5, 14, 18 & 19.
Table 3.
α-Glucosidase inhibitory activity of isolated compounds.
| No. | Compound | IC50 (μg/mL) | Maximum Inhibition (%) |
|---|---|---|---|
| 1 | Walterolactone A/B β-d-pyranoglucoside | 0.907 ± 0.102 e | 100 ± 5.8 a |
| 5 | Condensed tannin-ELCTB-2.1.2 | 0.083 ± 0.004 e | 100 ± 6.9 a |
| 9 | 1-β-d-Glucopyranosyloxy-3,5-dimethoxy-4-hydroxybenzene | UD | 35 ± 2.9 c |
| 10 | (−)-Gallocatechin | 11.9 ± 1.674 d | 93 ± 2.9 a |
| 11 | Schweinfurthinol 9-O-β-d-pyranoglucoside | 31.6 ± 0.924 b | 87 ± 4.0 a |
| 12 | 1-O-(3-Methyl)-butenoyl-myo-inositol | 27.1 ± 1.212 c | 96 ± 6.9 a |
| 14 | Leonuriside | 0.926 ± 0.043 e | 99 ± 6.4 a |
| 18 | Condensed tannin-ELCTB-3.1. | 0.076 ± 0.008 e | 100 ± 6.2 a |
| 19 | (+)-Catechin | 0.113 ± 0.008 e | 100 ± 5.9 a |
| 20 | Methyl galloate | 110 ± 1.732 a | 58 ± 1.2 b |
| 23 | (−)-Catechin | UD | 38 ± 4.0 c |
| Acarbose (positive control) | 1345 ± 89 | 65 ± 2.7 | |
| Coefficient of variation (%) | 6.510292 | 8.746013 |
All compounds were tested at concentrations in the range of 0.122–7.81 μg/mL (compounds 1 and 14), 0.0152–0.977 μg/mL (compounds 5, 18, and 19), 31.25–250 μg/mL (compounds 9, 20, and 23), 1.95–250 μg/mL (compounds 10, 11 and 12), 156.25–2500 μg/mL (acarbose), and the maximum inhibition was recorded at the compounds concentration of 7.81 μg/mL (compounds 1 and 14), 0.977 μg/mL (compounds 5, 18, and 19), 250 μg/mL (compounds 9, 10, 11, 12, 20, and 23), and 2500 μg/mL (acarbose); the means of IC50, and maximum inhibition values with the different letters in the same column are significantly different in comparison based on Duncan’s multiple range test (alpha = 0.01) using SAS version 9.4, Statistical Analysis Software. UD: unable to determine.
2.4. The Effect of Condensed Tannin (CT) on Reducing Plasma Glucose in a Mouse Model
Condensed tannin-ELCTB-3.1 (CT) was isolated at a large amount (~2000 mg) and showed efficient inhibition against α-glucosidase. Thus, this major inhibitor was conducted to test its effect on reducing plasma glucose in mice. To evaluate the effect of the samples on reducing plasma glucose in animals, sucrose and starch tolerance tests were used [11,36]. In this study, starch (3 g/kg bw) was chosen for the assay, since it is abundant in cereals, the daily food of people in Asian countries. Therefore, in the case that the compound (CT) showed significant effect on the reducing plasma glucose in mice, it may show potent hypoglycemic effect in humans.
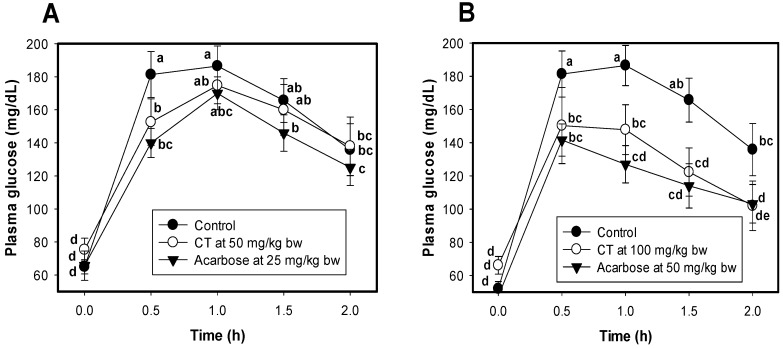
Two doses of isolated CT (50 and 100 mg/kg bw) were orally administered to mice to evaluate the effect on their plasma glucose level. CT at the dose of 50 mg/kg bw showed significant reduction of plasma glucose in mice at 0.5 h after CT administration; thereafter, the hypoglycemic effect showed less significant difference than that of the control group (mice administered water only) (Figure 3A). On the other hand, the effect of CT on the reduction of plasma of mice at the dose of 100 mg/kg bw was clearly observed from 0.5 to 2 h after CT administration (Figure 3B). Notably, the effect of CT at 100 mg/kg bw was comparable to that of acarbose at 50 mg/kg bw (Figure 3B), and much higher than that of acarbose at 25 mg/kg bw (Figure 3A). The mice showing symptoms of diarrhea during the tests were recorded. CT at all treated doses on mice led to no symptoms of diarrhea in mice, while mice with this illness symptom were recorded at 20% and 50% of the mice treated with acarbose doses of 25 and 50 mg/kg bw, respectively.
Figure 3.
The effect of condensed tannin-ELCTB-3.1.1 and acarbose on reducing plasma glucose in ICR (Institute of Cancer Research) mice. Condensed tannin-ELCTB-3.1.1 and acarbose at the doses of 50 mg/kg bw and 25 mg/kg bw (A), 100 mg/kg bw and 50 mg/kg bw (B), respectively, were administered to mice (n = 8). Distilled water was administered to mice in the control groups (●, n = 8). Blood of mice was sampled and measured at 0.5, 1, 1.5, and 2 h after loading the compound or acarbose. Means of blood glucose level with the different letters in the same figure are significantly different based on Duncan’s Multiple Range Test (alpha = 0.05). CV: coefficient of variation.
Natural products such as tea and coffee with high polyphenolic compounds content [37], were reported to show good effect on the reduction of plasma glucose in mice [38], as well as on postprandial plasma glucose in healthy humans [37]. Some herbal extracts rich in condensed tannins [6,17,39] were also tested for their anti-hyperglycemic effects in diabetic rats. Psidium littorale Raddi leaf extract at the dose of 150 mg/kg bw reduced fasting plasma glucose levels in streptozotocin-induced diabetic rats [6]. The methanolic extract of Euonymus laxiflorus Champ. trunk bark (the crude sample of CT) showed significant reduction of blood glucose in diabetic rats at the dose of 200 mg/kg bw, comparable to that of acarbose at 120 mg/kg bw [17]. The pinhão coat extract, and the A. mearnsii tannin also demonstrated significant effectiveness in diminishing the post-prandial glycemic levels in rats at their doses of 250 mg/kg bw after starch administration [39]. In this study, Euonymus laxiflorus Champ. condensed tannins demonstrated significant effect on reducing plasma glucose at their low doses of 50, and 100 mg/kg bw.
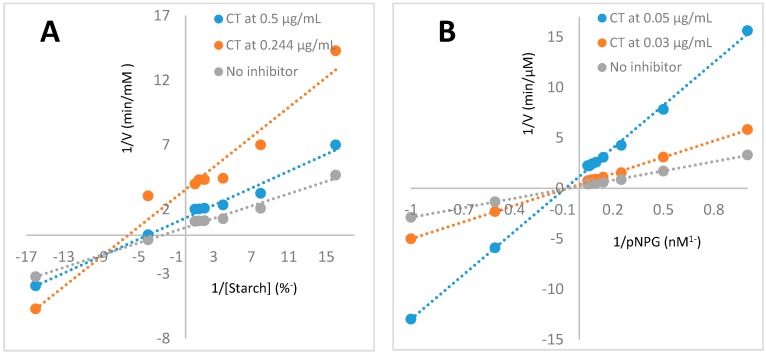
Condensed Tannins possess vast beneficial bioactivities, including cardio-protective, antioxidative, antitumor, antiviral, antibacterial, immune-modulatory, anti-inflammatory activities, antiobesity, antidiabetic [40,41], and hypoglycemic effects [39]. Euonymus laxiflorus Champ. condensed tannins (condensed tannin-ELCTB-3.1) were found to possess potent hypoglycemic effect in this study. It is well known that starch, a polysaccharide, is degraded by amylases to dextrin oligomers; these oligomers are then further degraded to α-d-glucoses by α-glucosidase. These monomeric may enter the blood circulation via intestinal epithelial absorption. Therefore, the use of α-amylase and α-glucosidase inhibitors may block or slow down this process, leading to the hypoglycemic effect after meals. Euonymus laxiflorus Champ. condensed tannins (ELC-CT) showed effective inhibition both on α-amylase [16] and α-glucosidase, since this resulted in their significant hypoglycemic effect. To determine the inhibition mode of ELC-CT, three concentrations of ELC-CT: 0.5, 0.244, 0 µg/mL, and 0.05, 0.03, 0 µg/mL against α-amylase, and α-glucosidase, respectively, were tested. According to the Lineweaver-Burk plots of enzymatic inhibition kinetics of ELC-CT against α-amylase (Figure 4A), and α-glucosidase (Figure 4B), ELC-CT was determined as mix (non-competitive-uncompetitive) inhibition against α-amylase, and non-competitive inhibition against α-glucosidase via comparison to the typical Lineweaver-Burk plots [42].
Figure 4.
Lineweaver-Burk plots of enzymatic inhibition kinetics of ELC-CT against α-amylase (A), and α-glucosidase (B).
These results indicated that ELC-CT did not combine with enzymes in the active sites of both enzymes (α-amylase, and α-glucosidase); ELC-CT combined with α-glucosidase to produce dead-end enzyme-inhibitor complex regardless of the substrate (pNPG) was bound; while some ELC-CT molecules could combine with α-amylase-starch complex, but did not combine with free amylase, and some ELC-CT molecules could combine with both α-amylase-starch complex, and free amylase to block the amylase activity [42].
According to the results of this study and the previous report [17], suggest that Euonymus laxiflorus Champ. is a rich source of condensed tannins, which could be developed as health food with potent hypoglycemic effect.
3. Materials and Methods
3.1. Materials
The methanolic extract of Euonymus laxiflorus Champ. trunk bark was obtained from the previous study [4]. Saccharomyces cerevisiae (yeast) α-glucosidase and acarbose were purchased from Sigma Chemical Co., St. Louis City, MO, USA; p-nitrophenyl glucopyranoside (pNPG) was obtained from Sigma Aldrich, 3050 Spruce Street, St. Louis, MO, USA. All the solvents and common chemicals were obtained at their highest grade.
3.2. Biological Activity Assays
3.2.1. α-Glucosidase Inhibitory Activity Determination
The α-glucosidase inhibitory activity was closely detected following the method described in detail by Nguyen et al. (2018) [12]. The mixture of 50 μL α-glucosidase, 50 μL sample solutions, 100 μL buffer was pre-incubated at 37 °C for 20 min; the reaction then started when 50 μL of p-nitrophenyl glucopyranoside (10 mmol/L) was added to the mixture. After incubation at the same temperature for 30 min, the reaction was stopped by adding 100 μL Na2CO3 solution (1 mol/L) to the reaction mixture; the absorbance of this final mixture was then measured at 410 nm (A). The control group also underwent the same described method with the use of 50 μL buffer instead of 50 μL sample solutions; the absorbance was recorded at 410 nm (B). The aGI activity (%) was calculated using the following equation:
| aGI (%) = (A − B)/ A × 100. |
The inhibition was also expressed as IC50 value determined as per the previous study [2]. The enzyme and the samples were prepared in 0.1 mol/L potassium phosphate buffer (pH 7). The purified compounds were tested at concentrations in the range of 0.015–250 μg/mL and the IC50 plots for all tested compounds (1, 5, 10, 11, 12, 14, 18, 19, and 20), and acarbose were presented in the supplementary materials section (Figures S22–S31). The corresponding % inhibition at each concentration of all tested compounds were also recorded in Figure S32.
3.2.2. Experimental Animal Protocol
Animals: seven week-old male ICR (Institute of Cancer Research) mice were purchased from The National Laboratory Animal Center (No. 128, Sec. 2, Academia Rd., Nangang Dist., Taipei City 11529, Taiwan) and tested in accordance with the approval and guidelines of the Institutional Animal Care and Use Committee of the National Research Institute of Chinese Medicine, Ministry of Health and Welfare (IACUC No. 104-706-1, 29 December 2014). Forty ICR mice were randomly divided into 5 groups (8 mice/group), including a control group orally administered with distilled water, and 4 experimental groups orally administered with 50 mg CT/kg bw, 100 mg CT/kg bw, 25 mg acarbose/kg bw, and 50 mg acarbose/kg bw, respectively. Distilled water was used to prepare the condensed tannins and acarbose solutions.
Assay: The effect of condensed tannin-ELCTB-3.1.1 on the reduction of plasma glucose in mice was performed according to the experimental animal protocol described by Nguyen et al. (2017) [11] with the use of starch solution (3 g/kg bw) instead of sucrose solution administered to ICR mice. All the mice groups were fasted overnight (16 h), and then orally administered water (control group), CT or acarbose (4 experimental groups); thereafter (20 min) starch solution (3 g/kg bw) was orally administered to mice; blood was then sampled and measured after 0.5, 1.0, 1.5, and 2.0 h.
3.2.3. Determination of Enzymatic Inhibition Modes of Isolated Condensed Tannin
The inhibition mode of condensed tannin was determined by performing as the reported assay with minor modification [10]. Enzyme kinetics of the isolated condensed tannin was determined using the α-glucosidase and α-amylase inhibitory activity assay mentioned above. The concentration range used was 2–18 mmol/L pNPG, and 0.0625–2% starch for the α-glucosidase, and α-amylase inhibitory activity assays, respectively. The inhibition modes of the sample were determined by analyzing the Vmax and comparing the Lineweaver-Bur plots of condensed tannin to the standard typical Lineweaver-Bur plots [42].
3.3. Purification and Identification Procedures of the Active aGIs
Methanolic extract of ELCTB (40 g) was primarily fractionated via a Diaion column with successive eluting with distilled water, 40% MeOH, 70% MeOH, 100% MeOH, and 100% ethyl acetate to obtain 5 fractions: ELCTB-1 (20.97 g), ELCTB-2 (9.12 g), ELCTB-3 (4.67 g), ELCTB-4 (1.46 g), ELCTB-5 (1.63 g), respectively.
ELCTB-2 (4.5 g) was loaded onto an ODS column and 10 subfractions were obtained with the elution of a gradient mobile phase of MeOH in H2O (0–100%, v/v). The 3 subfractions: ELCTB-2.1 (1.8 g), ELCTB-2.2 (0.5 g), and ELCTB-2.3 (0.25 g) were obtained by eluting with 0%, 5%, and 10% MeOH in H2O, respectively. All of these subfractions were further separated by injecting them into a preparative HPLC (Pr-HPLC) (preparative Cosmosil 5C18-AR-II column equipped with a 250 × 20 mm i.d. and a UV detector (Nacalai Tesque, Inc., Kyoto, Japan) at 210 and 254 nm) for the isolation of 17 compounds (1–17) via elution with 8% ACN (compounds 1–5), 5% ACN (compounds 5–17).
ELCTB-3 (4.5 g) was also loaded onto the same ODS column and then eluted by ACN in the H2O mobile phase with the gradient of 10–100%, v/v, to obtain 10 subfractions (ELCTB-3.1-ELCTB-3.10). Two subfractions: ELCTB-3.1 (1.8 g), and ELCTB-3.3 (0.6 g) were collected by eluting with 10% and 20% ACN, respectively. ELCTB-3.3 (0.6 g) was further separated via the same column and eluted with ACN in H2O mobile phase with a gradient of 0–100%, v/v, and 10 subfractions (ELCTB-3.3.1-ELCTB-3.3.10) were obtained. Of these, 3 subfractions: ELCTB-3.3.4, ELCTB-3.3.5, and ELCTB-3.3.6, eluted with 10%, 13%, and 16% ACN, respectively, were injected into the Pr-HPLC for the isolation of 8 compounds (19–26) by the elution with 14% ACN (19, 20, 25, 26), and 22% MeOH (21–24). Compound 18 was obtained from subfraction ELCTB-3.1 via dialysis. The purification process is summarized in Figure 1.
The chemical structures of the isolated compounds were identified via analysis of their NMR data, coupled with the comparison of those of reported compounds. The 1H and 13C-NMR spectra, and 2D-NMR spectra (COSY, HMQC, HMBC, and NOESY), were recorded in MeOH-d4 on a Bruker AVX NMR spectrometer (Bruker, Karlsruhe, Germany) operating at 600 MHz for 1 to 12 h and 150 MHz for 13C using the MeOH-d4 solvent peak as internal standard (δH 3.317, δC 49.1 ppm).
3.4. Characteristics and NMR Data of Identified Compounds
Compound 1: Walterolactone A/B β-d-pyranoglucoside was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 5.86 (q, J = 1.8 Hz), 4.57 (dd, J = 12.0, 3.0 Hz), 4.42 (dd, J = 12.0, 3.0 Hz), 4.41 (d, J = 7.8 Hz), 4.40 (t, J = 3.0 Hz), 3.88 (dd, J = 12.0, 1.8 Hz), 3.67 (dd, J = 12.0, 5.4 Hz), 3.36 (t, J = 9.0 Hz), 3.29 (m, 2 H), 3.17 (dd, J = 9.0, 7.8 Hz), 2.10 (d, J = 1.8 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 166.1, 158.6, 118.9, 103.0, 78.2, 78.0, 74.8, 71.5, 71.0, 70.3, 62.7, 20.4.
Compound 9: 1-β-d-glucopyranosyloxy-3,5-dimethoxy-4-hydroxybenzene was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 6.5 (s, 2H), 4.75 (d, J = 7.2 Hz), 3.91 (dd, J = 12.0, 2.4 Hz), 3.81 (s, 6 H), 3.66 (dd, J = 12.0, 6.6 Hz), 3.4 ~3.5 (m, 3 H). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 152.4, 149.4, 131.0, 103.8, 96.6, 78.2, 77.9, 74.9, 62.7and 56.8.
Compound 10: (−)-Gallocatechin was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 6.40 (s, 2H), 5.92 (d, J = 2.4 Hz), 5.85 (d, J = 2.4 Hz), 4.52 (d, J = 7.2 Hz), 3.96 (m), 2.8 (dd, J = 15.6, 5.4 Hz), 2.49 (dd, J = 15.6, 7.8 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 157.8, 157.6, 156.8, 146.9, 134.0, 131.5, 107.2, 100.8, 96.3, 95.5, 82.9, 68.7, 28.1.
Compound 11: Schweinfurthinol 9-O-β-d-pyranoglucoside or 1-(4-hydroxyphenyl)-2,3-dihydroxypropan-1-one 3-O-β-d-pyranoglucoside was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 7.93 (d, J = 7.2 Hz, 2H), 6.85 (d, J = 7.2 Hz, 2H), 5.28 (dd, J = 6.6, 3.6 Hz), 4.25 (d, J = 7.8 Hz), 4.18 (dd, J = 11.4, 3.6 Hz), 3.84 (dd, J = 12.0, 1.8 Hz), 3.73 (dd, J = 11.4, 6.6 Hz), 3.65 (dd, J = 12.0, 6.0 Hz), 3.32 (overlapped), 3.28 (t, J = 9.0 Hz), 3.26 (m), 3.19 (dd, J = 9.0, 7.2 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 198.6, 162.8, 132.5, 127.5, 116.6, 105.0, 78.0, 77.8, 75.1, 74.3, 73.8, 71.5, 62.6.
Compound 12: 1-O-(3-methyl)-butenoyl-myo-inositol or Myo-inositol 1-O-3,3-dimethylacrylate was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 5.81 (brs), 4.62 (dd, J = 10.2, 2.4 Hz), 4.05 (t, J = 2.4 Hz), 3.82 (dd, J = 10.2, 9.6 Hz), 3.63 (t, J = 9.0 Hz), 3.41 (dd, J = 9.0, 2.4 Hz), 3.22 (dd, J = 9.6, 9.0 Hz), 2.16 (d, J = 1.2 Hz), 1.92 (d, J = 1.2 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 167.7, 158.7, 117.0, 76.6, 75.0, 74.1, 73.1, 72.0, 71.8, 27.4, 20.4.
Compound 14: Leonuriside was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 6.13 (s, 2H), 4.66 (d, J = 7.2 Hz), 3.79 (s, 6 H), 3.78 (dd, J = 12.0, 2.4 Hz), 3.67 (dd, J = 12.0, 5.4 Hz), 3.4~3.5 (m, 2 H), 3.36 (overlapped), 3.19 (m). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 155.9, 154.7, 129.6, 94.5, 106.1, 78.2, 77.7, 75.7, 65.2 and 56.8.
Compounds 5 & 18: condensed tannins were obtained as brow yellow amorphous powder: 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 161.0, 155.0, 144.5, 138.5, 132.0, 122.0–118.0, 116.3–114.0, 107.0, 102.0, 97.0, 76.0, 35.5 and 34.0).
Compound 19 (+)-Catechin was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 6.83 (d, J = 1.8 Hz), 6.76 (d, J = 7.8 Hz), 6.71 (dd, J = 7.8, 1.8 Hz), 5.93 (d, J = 2.4 Hz), 5.85 (d, J = 2.4 Hz), 4.46 (d, J = 7.8 Hz), 3.98 (m), 2.83 (dd, J = 16.2, 5.4 Hz), 2.49 (dd, J = 16.2, 8.4 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 157.7, 157.5, 156.8, 146.2, 132.2, 120.1, 116.2, 115.3, 100.9, 96.3, 95.5, 82.8, 68.7, 28.5.
Compound 20: Methyl galloate was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 7.05 (s, 2H), 3.72 (s, 3H). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 167.7, 145.9, 138.8, 121.0, 109.5, 52.0.
Compound 23: (−)-Catechin was obtained as a white amorphous powder. 1H-NMR data (600 MHz, MeOH-d4, δH ppm): 6.83 (d, J = 1.8 Hz), 6.76 (d, J = 7.8 Hz), 6.71 (dd, J = 7.8, 1.8 Hz), 5.93 (d, J = 2.4 Hz), 5.85 (d, J = 2.4 Hz), 4.46 (d, J = 7.8 Hz), 3.98 (m), 2.83 (dd, J = 16.2, 5.4 Hz), 2.49 (dd, J = 16.2, 8.4 Hz). 13C-NMR data (150 MHz, MeOH-d4, δC ppm): 157.7, 157.5, 156.8, 146.2, 132.2, 120.1, 116.2, 115.3, 100.9, 96.3, 95.5, 82.8, 68.7, 28.5.
3.5. Statistical Analysis
Statistical Analysis Software (SAS) version 9.4, provided by SAS Institute Taiwan Ltd., Minsheng East Road, Section 2, Taipei, Taiwan 149-8, was used to analyze the differences between the means of inhibition, and blood glucose level via Duncan’s Multiple Range Test (alpha = 0.01 or 0.05). All tests were repeated in triplicate.
4. Conclusions
Eleven hypoglycemic compounds were isolated and identified from the methanolic extract of Euonymus laxiflorus Champ. trunk bark. Of these, four novel compounds of walterolactone A/B β-d-pyranoglucoside (1), schweinfurthinol 9-O-β-d-pyranoglucoside (11), 1-O-(3-methyl)-butenoyl-myo-inositol (12), and leonuriside (14) were determined as new α-glucosidase inhibitors. The results of in vitro tests indicated that most of the isolated compounds (1, 5, 10–12, 17, and 18) showed much higher activity than that of acarbose. Codensed tannin (18) demonstrated significant reduction of blood glucose in mice at the dose of 100 mg/kg bw. The results could enrich the current biological activities of constituents isolated from Euonymus laxiflorus Champ. species, and also suggest that this medicinal plant is a valuable source of bioactive compounds for development as health food or drugs with potent hypoglycemic effect.
Acknowledgments
This work was supported in part by a grant from the Ministry of Science and Technology, Taiwan (MOST 106-2320-B-032-001-MY3), Ministry of Education, Taiwan (TKU 0657010), the Ministry of Science and Technology, Taiwan (MOST104-2320-B-077-006-MY3), and Ministry of Health and Welfare, Taiwan (MOHW107-NRICM-B-315-143002), Ministry of Science and Technology, Vietnam (MOST. NVQG-2018/19).
Supplementary Materials
The following are available online, including 32 figures (Figure S1–S32).
Authors Contributions
Conceived the study: S.-L.W., V.B.N. and A.D.N. Designed the study: V.B.N., S.-L.W., A.D.N. and T.H.N. Performed the experiments: V.B.N., T.H.N., M.T.N., Z.-H.L., C.T.D., T.N.T. and Q.V.N. Contributed reagents/materials/analysis tools: S.-L.W., A.D.N., Y.-H.K. and V.B.N. Analyzed data and wrote the paper: V.B.N. and S.L.W.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds including Walterolactone A/B β-d-pyranoglucoside, and condensed tannin-ELCTB-3.1 (compound 18) isolated from ELCTB extract are available from the authors.
References
- 1.Nguyen V.B., Nguyen T.H., Doan C.T., Tran T.N., Nguyen A.D., Kuo Y.H., Wang S.L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Molecules. 2018;23:1124. doi: 10.3390/molecules23051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen V.B., Nguyen A.D., Wang S.L. Utilization of fishery processing by-product squid pens for α-glucosidase inhibitors production by Paenibacillus sp. Mar. Drugs. 2017;15:274. doi: 10.3390/md15090274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMelo E.B., Gomes A., Carvalha I. α-and β-Glucosidase inhibitors: Chemical structure and biological activity. J. Tetrahedr. 2006;62:10277–10302. [Google Scholar]
- 4.Nguyen V.B., Nguyen Q.V., Nguyen A.D., Wang S.L. Screening and evaluation of α-glucosidase inhibitors from indigenous medicinal plants in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2017;43:3599–3612. doi: 10.1007/s11164-016-2434-x. [DOI] [Google Scholar]
- 5.Nguyen Q.V., Nguyen V.B., Eun J.B., Wang S.L., Nguyen D.H., Tran T.N., Nguyen A.D. Anti-oxidant and antidiabetic effect of some medicinal plants belong to Terminalia species collected in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2016;42:5859–5871. doi: 10.1007/s11164-015-2409-3. [DOI] [Google Scholar]
- 6.Nguyen Q.V., Wang S.L., Nguyen A.D. In vitro α-glucosidase and α-amylase inhibition, and in vivo anti-hyperglycemic effects of Psidium littorale Raddi leaf extract. Res. Chem. Intermed. 2018;44:1745–1753. doi: 10.1007/s11164-017-3195-x. [DOI] [Google Scholar]
- 7.Nguyen V.B., Wang S.-L., Nhan N.T., Nguyen T.H., Nguyen N.P.D., Nghi D.H., Cuong N.M. New records of potent in-vitro antidiabetic properties of Dalbergia tonkinensis heartwood and the bioactivity-guided isolation of active compounds. Molecules. 2018;23:1589. doi: 10.3390/molecules23071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen V.B., Wang S.L. Reclamation of marine chitinous materials for the production of α-glucosidase inhibitors via microbial conversion. Mar. Drugs. 2017;15:350. doi: 10.3390/md15110350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S.L., Su Y.C., Nguyen V.B., Nguyen A.D. Reclamation of shrimp heads for the production of α-glucosidase inhibitors by Staphylococcus sp. TKU043. Res. Chem. Intermed. 2018;44:4929–4937. doi: 10.1007/s11164-018-3345-9. [DOI] [Google Scholar]
- 10.Hsu C.H., Nguyen V.B., Nguyen A.D., Wang S.L. Conversion of shrimp heads to α-glucosidase inhibitors via co-culture of Bacillus mycoides TKU040 and Rhizobium sp. TKU041. Res. Chem. Intermed. 2017;44:4597–4607. doi: 10.1007/s11164-018-3266-7. [DOI] [Google Scholar]
- 11.Nguyen V.B., Nguyen A.D., Kuo Y.H., Wang S.L. Biosynthesis of α-glucosidase inhibitors by a newly isolated bacterium, Paenibacillus sp. TKU042 and its effect on reducing plasma glucose in mouse model. Int. J. Mol. Sci. 2017;18:700. doi: 10.3390/ijms18040700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen V.B., Wang S.L. New novel α-glucosidase inhibitors produced by microbial conversion. Process Biochem. 2018;65:228–232. doi: 10.1016/j.procbio.2017.11.016. [DOI] [Google Scholar]
- 13.Wang G., Peng Z., Wang J., Li X., Li J. Synthesis, in vitro evaluation and molecular docking studies of novel triazine-triazole derivatives as potential α-glucosidase inhibitors. Eur. J. Med. Chem. 2017;125:423–429. doi: 10.1016/j.ejmech.2016.09.067. [DOI] [PubMed] [Google Scholar]
- 14.Ghani U. Re-exploring promising a-glucosidase inhibitors for potential development into oral anti-diabetic drugs: Finding needle in the haystack. Eur. J. Med. Chem. 2015;103:133–162. doi: 10.1016/j.ejmech.2015.08.043. [DOI] [PubMed] [Google Scholar]
- 15.Flora of China. [(accessed on 10 May 2018)]; Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012808.
- 16.Nguyen V.B., Nguyen Q.V., Nguyen A.D., Wang S.L. Porcine pancreatic α-amylase inhibitors from Euonymus laxiflorus Champ. Res. Chem. Intermed. 2017;43:259–269. doi: 10.1007/s11164-016-2619-3. [DOI] [Google Scholar]
- 17.Nguyen Q.V., Nguyen N.H., Wang S.L., Nguyen V.B., Nguyen A.D. Free radical scavenging and antidiabetic activities of Euonymus laxiflorus champ extract. Res. Chem. Intermed. 2017;43:5615–5624. doi: 10.1007/s11164-017-2951-2. [DOI] [Google Scholar]
- 18.Nguyen V.B., Wang S.L., Nguyen A.D., Vo T.P.K., Zhang L.J., Nguyen Q.V., Kuo Y.H. Isolation and identification of novel α-amylase inhibitors from Euonymus laxiflorus Champ. Res. Chem. Intermed. 2018;44:1411–1424. doi: 10.1007/s11164-017-3175-1. [DOI] [Google Scholar]
- 19.Liu L.M., Cheng S.F., Shieh P.C., Lee J.C., Chen J.J., Ho C.T., Kuo S.C., Kuo D.H., Huang L.J., Way T.D. The methanol extract of Euonymus laxiflorus, Rubia lanceolata and Gardenia jasminoides inhibits xanthine oxidase and reduce serum uric acid level in rats. Food Chem. Toxicol. 2014;70:179–184. doi: 10.1016/j.fct.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 20.Shao J.H., Chen J., Zhao C.C., Shen J., Liu W.Y., Gu W.Y., Li K.H. Insecticidal and α-glucosidase inhibitory activities of chemical constituents from Viburnum fordiae Hance. Nat. Prod. Res. 2018;27:1–6. doi: 10.1080/14786419.2018.1466130. [DOI] [PubMed] [Google Scholar]
- 21.Nomizu K., Hashida K., Makino R., Ohara S. Antioxidants from steamed used tea leaves and their reaction behavior. Biosci. Biotechnol. Biochem. 2008;72:1682–1689. doi: 10.1271/bbb.70762. [DOI] [PubMed] [Google Scholar]
- 22.Shrestha S., Lee D.Y., Park J.H., Cho J.G., Lee D.S., Li B., Kim Y.C., Kim G., Bang M.H., Baek N.I. Phenolic components from Rhus parviflora fruits and their inhibitory effects on lipopolysaccharide-induced nitric oxide production in RAW 264.7 macrophages. Nat. Prod. Res. 2013;27:2244–2247. doi: 10.1080/14786419.2013.814050. [DOI] [PubMed] [Google Scholar]
- 23.Li X., Liu Z., Zhang X.F., Wang L.J., Zheng Y.N., Yuan C.C., Sun G.Z. Isolation and characterization of phenolic compounds from the leaves of Salix matsudana. Molecules. 2008;13:1530–1537. doi: 10.3390/molecules13081530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo P., Anderson J.D., Bozell J.J., Zivanovic S. The effect of solvent composition on grafting gallic acid onto chitosan via carbodiimid. Carbohydr. Polym. 2016;140:171–180. doi: 10.1016/j.carbpol.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Schofield P., Mbugua D.M., Pell A.N. Analysis of condensed tannins: A review. Anim. Feed. Sci. Technol. 2001;91:21–40. doi: 10.1016/S0377-8401(01)00228-0. [DOI] [Google Scholar]
- 26.Li C., Leverence R., Trombley J.D., Xu S., Yang J., Tian Y., Reed J.D., Hagerman A.E. High molecular weight persimmon (Diospyros kaki) proanthocyanidin: A highly galloylated, a-linked tannin with an unusual flavonol terminal unit, myricetin. J. Agric. Food Chem. 2010;58:9033–9042. doi: 10.1021/jf102552b. [DOI] [PubMed] [Google Scholar]
- 27.Chai W.M., Shi Y., Feng H.L., Qiu L., Zhou H.C., Deng Z.W., Yan C.L., Chen Q.X. NMR, HPLC-ESI-MS, and MALDI-TOF MS analysis of condensed tannins from Delonix regia (Bojer ex Hook.) Raf. and their bioactivities. J. Agric. Food Chem. 2012;60:5013–5022. doi: 10.1021/jf300740d. [DOI] [PubMed] [Google Scholar]
- 28.Sadik G., Islam R., Rahman M.M., Khondkar P., Rashid M.A., Sarker S.D. Antimicrobial and cytotoxic constituents of Loranthus globosus. Fitoterapia. 2003;74:308–311. doi: 10.1016/S0367-326X(03)00041-8. [DOI] [PubMed] [Google Scholar]
- 29.Kwon D.H., Choi W.J., Lee C.H., Kim J.H., Kim M.B. Flavonoid Compound Having an Antiviral Activity. 7,998,937. U.S. Patent. 2011 Aug 16;
- 30.Lianda R.L.P., Santana L.D., Echevarria A., Castro R.N. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. J. Braz. Chem. Soc. 2012;23:618–627. doi: 10.1016/j.phytochem.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Jing H., Xing Y.F., Huang B., Zhang Y.Z., Zeng C.M. Tea catechins induce the conversion of preformed lysozyme amyloid fibrils to amorphous aggregates. J. Agric. Food Chem. 2009;57:11391–11396. doi: 10.1021/jf902664f. [DOI] [PubMed] [Google Scholar]
- 32.Ngoumfo R.M., Ngounou G.E., Tchamadeu C.V., Qadir M.I., Mbazoa C.D., Begum A., Ngninzeko F.N., Lontsi D., Choudhary M.I. Inhibitory effect of macabarterin, a polyoxygenated ellagitannin from Macaranga barteri, on human neutrophil respiratory burst activity. J. Nat. Prod. 2008;71:1906–1910. doi: 10.1021/np8004634. [DOI] [PubMed] [Google Scholar]
- 33.Rawat P., Khan M.F., Kumar M., Tamarkar A.K., Srivastava A.K., Arya K.R., Maurya R. Constituents from fruits of Cupressus sempervirens. Fitoterapia. 2010;81:162–166. doi: 10.1016/j.fitote.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J., Zhou X.W., Chen X.B., Wang Q.X. α-Glucosidase inhibitory constituents from Toona sinensis. Chem. Nat. Compd. 2009;45:244–246. doi: 10.1007/s10600-009-9289-y. [DOI] [Google Scholar]
- 35.Sanae F., Miyaichi Y., Kizu H., Hayashi H. Effects of catechins on vascular tone in rat thoracic aorta with endothelium. Life Sci. 2002;71:2553–2562. doi: 10.1016/S0024-3205(02)02080-5. [DOI] [PubMed] [Google Scholar]
- 36.Yusoff N.A., Ahmad M., al Hindi B., Widyawati T., Yam M.F., Mahmud R., Razak K.N.A., Asmawi M.Z. Aqueous extract of Nypa fruticans wurmb. Vinegar alleviates postprandial hyperglycemia in normoglycemic rats. Nutrients. 2015;7:7012–7026. doi: 10.3390/nu7085320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryans J.A., Judd P.A., Ellis P.R. The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans. J. Am. Coll. Nutr. 2007;26:471–478. doi: 10.1080/07315724.2007.10719638. [DOI] [PubMed] [Google Scholar]
- 38.Igarashi M., Satoh T., Yamashita H., Wantanabe K. Black tea inhibits small intestinal α-glucosidase activity in db/db mouse. Jpn. J. Complem. Altern. Med. 2014;11:25–33. doi: 10.1625/jcam.11.25. [DOI] [Google Scholar]
- 39.da Silva S.M., Koehnlein E.A., Bracht A., Castoldi R., de Morais G.R., Baesso M.L., Peralta R.A., de Souza C.G.M., de Sa-Nakanishi A.B., Peralta R.M. Inhibition of salivary and pancreatic α-amylases by a pinhão coat (Araucaria angustifolia) extract rich in condensed tannin. Food Res. Int. 2014;56:1–8. doi: 10.1016/j.foodres.2013.12.004. [DOI] [Google Scholar]
- 40.Ogawa S., Yazaki Y. Tannins from acacia mearnsii de wild. bark: Tannin determination and biological activities. Molecules. 2018;23:837. doi: 10.3390/molecules23040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumari M., Jain S. Tannins: An antinutrient with positive effect to manage diabetes. Res. J. Recent Sci. 2012;1:70–73. [Google Scholar]
- 42.Palmer T. Understanding Enzymes. 3rd ed. Ellis Horwood Limited; Chichester, UK: 1991. p. 155. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.