Abstract
Rationale: Angioproliferative vasculopathy is a hallmark of pulmonary arterial hypertension (PAH). However, little is known about how endothelial cell (EC) and smooth muscle cell (SMC) crosstalk regulates the angioproliferative vascular remodeling.
Objectives: To investigate the role of EC and SMC interaction and underlying signaling pathways in pulmonary hypertension (PH) development.
Methods: SMC-specific Foxm1 (forkhead box M1) or Cxcr4 knockout mice, EC-specific Foxm1 or Egln1 knockout mice, and EC-specific Egln1/Cxcl12 double knockout mice were used to assess the role of FoxM1 on SMC proliferation and PH. Lung tissues and cells from patients with PAH were used to validate clinical relevance. FoxM1 inhibitor thiostrepton was used in Sugen 5416/hypoxia- and monocrotaline-challenged rats.
Measurements and Main Results: FoxM1 expression was markedly upregulated in lungs and pulmonary arterial SMCs of patients with idiopathic PAH and four discrete PH rodent models. Mice with SMC- (but not EC-) specific deletion of Foxm1 were protected from hypoxia- or Sugen 5416/hypoxia-induced PH. The upregulation of FoxM1 in SMCs induced by multiple EC-derived factors (PDGF-B, CXCL12, ET-1, and MIF) mediated SMC proliferation. Genetic deletion of endothelial Cxcl12 in Egln1Tie2Cre mice or loss of its cognate receptor Cxcr4 in SMCs in hypoxia-treated mice inhibited FoxM1 expression, SMC proliferation, and PH. Accordingly, pharmacologic inhibition of FoxM1 inhibited severe PH in both Sugen 5416/hypoxia and monocrotaline-challenged rats.
Conclusions: Multiple factors derived from dysfunctional ECs induced FoxM1 expression in SMCs and activated FoxM1-dependent SMC proliferation, which contributes to pulmonary vascular remodeling and PH. Thus, targeting FoxM1 signaling represents a novel strategy for treatment of idiopathic PAH.
Keywords: CXCL12/CXCR4, endothelial cells, hyperproliferation, pulmonary vascular remodeling, smooth muscle cells
At a Glance Commentary
Scientific Knowledge on the Subject
The pulmonary vascular niche in pulmonary arterial hypertension (PAH) promotes angioproliferative vasculopathy that involves crosstalk between multiple cell types. Thus, identification of the critical molecular mechanisms mediating vascular cell interaction and cell hyperproliferation is vital to the prevention and treatment of PAH.
What This Study Adds to the Field
Our studies demonstrated the prerequisite role of FoxM1 (forkhead box M1) expression in smooth muscle cells induced by endothelial cell–derived paracrine factors in mediating smooth muscle cell proliferation and pulmonary vascular remodeling. Pharmacologic inhibition of FoxM1 is a potential effective approach for the treatment of PAH in patients.
Pulmonary arterial hypertension (PAH) is a progressive, complex, and devastating disease arising from a variety of pathogenic or genetic causes. Excessive vasoconstriction and abnormal vascular remodeling have been considered as the major factors contributing to the complicated pathogenesis of PAH (1–3). PAH types with different etiologies share several histopathologic features including intima and media thickening, muscularization of distal pulmonary arteries, vascular occlusion, and complex plexiform lesions (4–6), which are largely caused by pulmonary vascular cell hyperproliferation, including endothelial cells (ECs) and smooth muscle cells (SMCs) (7–10). The vascular wall environment in PAH replicates many features of cancer, including elevated growth factors and inflammatory cytokines, which induce and sustain a proliferative vasculopathy that involves the interaction of multiple cell types, such as ECs and SMCs (8, 11, 12). Thus, identification of the critical molecular mechanisms mediating vascular cell hyperproliferation is vital to inhibit and reverse the angioproliferative vascular remodeling in PAH.
FoxM1 (forkhead box M1) is a key regulator of cell cycle progression, which is required for G1/S and G2/M transition, and M-phase progression (13–15). Quiescent cells express a low level of FoxM1, whereas most solid tumors including liver, colon, lung, prostate carcinoma, and glioblastoma express elevated levels of FoxM1 (13–16). FoxM1 is activated after tissue injury, such as inflammatory lung injury and liver injury, and in turn it is required for the reparative processes (17, 18). During development, FoxM1 plays an important role in mediating SMC proliferation, given that constitutive deletion of SMC Foxm1 in mice induces arterial wall defects and premature death immediately after birth (19). Recent studies show that hypoxia-induced human pulmonary artery (PA) SMC proliferation in vitro is mediated by FoxM1, and that FoxM1 promotes expansion of pulmonary arterial smooth muscle cells (PASMCs) (20, 21). However, it is unknown whether FoxM1 mediates pulmonary vascular remodeling and thereby development of pulmonary hypertension (PH) in vivo under hypoxia and nonhypoxia pathologic conditions, in which vascular cell type FoxM1 is required for pulmonary vascular remodeling and how FoxM1 is activated during development of PAH.
In the present study, using cell-specific knockout mouse models, we demonstrate for the first time the critical role of FoxM1 expressed in SMCs but not ECs in mediating pulmonary vascular remodeling and PH. Mechanistically, we show that EC-derived factors associated with PH play an important role in inducing FoxM1 expression in SMCs and resultant SMC hyperproliferation. Thus, EC-SMC crosstalk is a critical determinant of pulmonary vascular remodeling in a FoxM1-dependent manner, and targeting FoxM1 may represent an effective therapeutic strategy to inhibit the angioproliferative pulmonary vascular remodeling in idiopathic PAH (IPAH).
Methods
Animals and Experimental Protocol
Egln1Tie2Cre mice and Foxm1Tie2Cre mice were generated as described previously (17, 22). Foxm1 floxed mice or Cxcr4 floxed mice were bred with Myh11-CreERT2 mice to generate Foxm1iMyh mice or Cxcr4iMyh mice. The animal care and study protocols were approved by the Institutional Animal Care and Use Committees of the University of Illinois at Chicago and Northwestern University.
Human Samples
Human lung tissues were obtained from patients undergoing lung transplantation for IPAH and from unused IPAH-free donor lungs. Informed consent from patients and local ethical approval from the ethics committees of the Hammersmith Hospitals (ref. no. 2001/6003) and Royal Brompton and Harefield Hospitals (ref. no. 01-210) were obtained before tissue collection (22, 23). Clinical data from these patients and healthy control subjects are provided in Supplementary Table E1 in the online supplement (22–24). Human PASMCs and pulmonary endothelial arterial cells (PAECs) were provided by Dr. Roberto Machado (Indiana University) and the Pulmonary Hypertension Breakthrough Initiative.
Primary Cultures of Human PASMCs and PAECs
Primary human PASMCs from normal subjects and patients with IPAH were cultured in SmGM-2 medium supplemented with SmGM-2 SingleQuots. PAECs were cultured in EBM-2 medium supplemented with EGM-2 SingleQuots. All primary cultures were used between passages 6 and 9.
Hemodynamics and Echocardiography
Echocardiography was performed on a VisualSonics Vevo 2100 (FujiFilm VisualSonics Inc.) (22). Right ventricular systolic pressure (RVSP) was measured with a 1.4F (mice) or 3.5F (rats) pressure transducer catheter (Millar Instruments) and analyzed by AcqKnowledge software (Biopac Systems Inc.) (22).
RNAscope In Situ Hybridization Assay
To determine FOXM1 mRNA expression in SMCs, we used a single-plex RNAscope in situ hybridization assay (Advanced Cell Diagnostics) combined with anti–α-SMA (smooth muscle actin) immunofluorescent staining on lung sections from patients with IPAH and normal subjects.
Immunofluorescent Staining
Human lung sections from patients with IPAH and healthy donors were dewaxed and dehydrated, followed by antigen retrieval and proteinase digestion. After blocking, the sections were incubated with anti-FoxM1 and anti–α-SMA antibodies, then fluorescence-conjugated secondary antibodies, and mounted with media containing DAPI.
Statistical Analysis
Statistical significance was determined by one-way ANOVA with a Tukey post hoc analysis that calculates P values corrected for multiple comparisons using Prism 7 (Graphpad Software, Inc.). Two-group comparisons were analyzed by the unpaired two-tailed Student’s t test for equal variance or the Welch t test for unequal variance. P less than 0.05 denoted the presence of a statistically significant difference. All bars in dot plot figures represent mean. All bar graphs represent mean ± SD.
An expanded Materials and Methods section is provided in the online supplement.
Results
Prominent Expression of FoxM1 in Lung Tissues from Patients with IPAH and Rodent PH Models
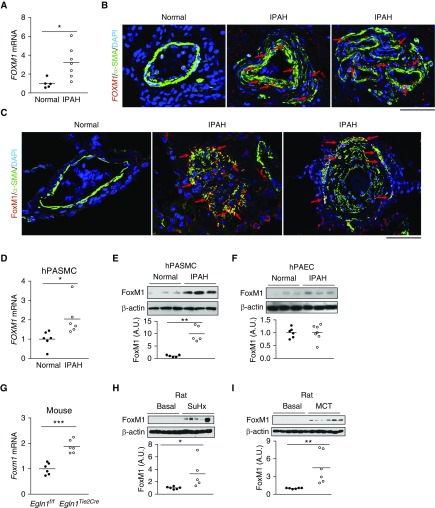
First, we quantified FOXM1 mRNA expression in lung tissues from healthy donors and patients with IPAH by qRT-PCR analysis. FOXM1 was markedly increased in IPAH lung tissues (Figure 1A). In situ RNA hybridization (RNAscope) assay demonstrated prominent FOXM1 expression in SMCs of the vascular lesions in IPAH lungs (Figure 1B; see Figure E1). Accordingly, immunofluorescent staining showed that FoxM1 expression was markedly induced in the medial layer of patients with IPAH, which mainly contains SMCs (Figure 2C; see Figure E2A). We next observed that FoxM1 and its downstream target genes CDC25C and CCNB1 were highly induced in PASMCs but not PAECs from patients with IPAH. (Figures 1D–1F; see Figure E2B). We also evaluated FoxM1 expression in lung tissues from mouse and rat PH models. Induction of FoxM1 was prominent in lungs of mice challenged with Sugen 5416/hypoxia (SuHx) (see Figure E3A) and Egln1Tie2Cre mice (22), a mouse model of severe PH resembling the pathology seen in IPAH (including obliterative pulmonary vascular remodeling) (Figure 1G). Similarly, SuHx rats and monocrotaline (MCT)-exposed rats exhibited increased expression of FoxM1 as well as its target genes Ccna2 and Cenpf (Figures 1H and 1I; see Figures E3B–E3E). These data demonstrate that FoxM1 is highly induced in PASMCs and lung tissues of patients with IPAH and PH animal models.
Figure 1.
Activation of FoxM1 (forkhead box M1) signaling in lungs of patients with idiopathic pulmonary arterial hypertension (IPAH) and pulmonary hypertension mice and rats. (A) qRT-PCR analysis demonstrating upregulation of FOXM1 mRNA expression in human IPAH lungs. (B) Representative micrographs showing prominent FOXM1 mRNA expression in smooth muscle cells (SMCs) of remodeled vessel (middle) and plexiform lesion (right) of patients with IPAH. RNA expression was fluorescently labeled by RNAscope reagent (RNA in situ hybridization) (red). SMCs were immunostained with anti–α-SMA (green). Arrows point to FoxM1-expressing SMCs. (C) Immunofluorescent staining demonstrating marked increase of FoxM1 protein expression in SMCs of vascular lesions in patients with IPAH. Lung tissue sections were immunostained with anti-FoxM1 (red) and anti–α-SMA (green). Arrows point to FoxM1-expressing SMCs. (D and E) Upregulation of FoxM1 in pulmonary arterial smooth muscle cells isolated from patients with IPAH analyzed by qRT-PCR and Western blotting. The intensity of FoxM1 protein band was normalized to the loading control of β-actin. (F) Similar levels of FoxM1 expression in pulmonary endothelial arterial cells from patients with IPAH and normal subjects. (G) Induced FoxM1 expression in lungs of Egln1Tie2Cre mice. Lung tissues were collected from Egln1Tie2Cre mice and Egln1f/f littermate control animals at the age of 2 months. (H) Western blotting analysis showing increased FoxM1 protein levels in Sugen 5416/hypoxia rats compared with basal. (I) Increased FoxM1 protein expression in monocrotaline-exposed rats compared with basal rats. *P < 0.05, **P < 0.01, and ***P < 0.001. (A) Welch t test. (D–I) Student’s t test. Horizontal bars in dot plot panels represent the mean. Scale bars, 50 μm. A.U. = arbitrary units; MCT = monocrotaline; hPAEC = human pulmonary endothelial arterial cell; hPASMC = human pulmonary arterial smooth muscle cell; SMA = smooth muscle actin; SuHx = Sugen 5416/hypoxia.
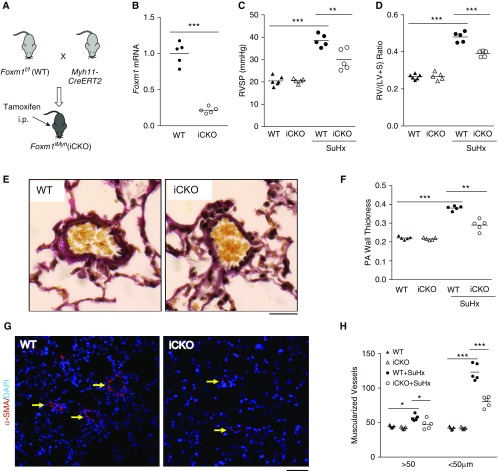
Figure 2.
Foxm1 disruption in smooth muscle cells (SMCs) inhibits Sugen 5416/hypoxia (SuHx)-induced pulmonary hypertension in mice. (A) A diagram showing the strategy for generating tamoxifen-inducible SMC-specific knockdown of Foxm1 in mice (iCKO). (B) Tamoxifen treatment induced loss of FoxM1 expression in SMCs (α-SMA+) isolated from iCKO mice compared with wild-type (WT) mice. (C) Hemodynamic measurement showing that iCKO mice had decreased right ventricular systolic pressure compared with Foxm1f/f (WT) mice after SuHx treatment. (D) Attenuation of RV hypertrophy in iCKO mice compared with WT mice after SuHx treatment. (E) Representative micrographs of Russel-Movat pentachrome staining showing increased medial thickness in SuHx WT mice compared with SuHx iCKO mice. (F) Quantification of pulmonary artery wall thickness. Pulmonary arteries with a diameter of 20–200 μm were quantified. (G and H) Muscularization of distal pulmonary vessels was markedly inhibited in SuHx iCKO mice compared with SuHx WT mice. Lung sections were immunostained with anti–α-SMA (red). Arrows indicate α-SMA+ distal pulmonary vessels. α-SMA+ vessels were quantified in 100 fields per sample. *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Student’s t test. (C, D, F, and H) One-way ANOVA. Horizontal bars in dot plot panels represent the mean. Scale bars: (E) 100 μm; (G) 50 μm. Foxm1 = forkhead box M1; PA = pulmonary artery; RV/(LV + S) = right ventricle versus left ventricle plus septum; RVSP = right ventricular systolic pressure; SMA = smooth muscle actin.
SMC Foxm1 Deletion in Adult Mice Protects from SuHx-induced PH
To address the cell-specific role of FoxM1 in the pathogenesis of PH, we generated mouse models with EC- or SMC-specific disruption of Foxm1. Given that constitutive deletion of Foxm1 in SMC induces neonatal death (19), we used an inducible deletion strategy to obtain adult mutant mice by breeding Foxm1 floxed mice into the genetic background of Myh11Cre-ERT2 mice (Foxm1iMyh) (25) (Figure 2A). Tamoxifen treatment induced approximately 75% decrease in Foxm1 expression in α-SMA+ cells isolated from adult Foxm1iMyh mice (Figure 2B). To determine the role of SMC FoxM1 in the pathogenesis of PH, both Foxm1f/f (wild-type) and Foxm1iMyh mice were kept in a 10% O2 chamber for 3 weeks with weekly injections of Sugen 5416 (26). Foxm1f/f mice after SuHx treatment exhibited elevated RVSP, whereas Foxm1iMyh mice exhibited a marked decrease in RVSP compared with Foxm1f/f mice (Figure 2C). Foxm1iMyh mice also showed decreased weight ratio of right ventricular (RV) versus left ventricular plus septum, indicative of RV hypertrophy, compared with Foxm1f/f mice (Figure 2D). Further examination of pulmonary pathology demonstrated that SuHx-induced increases in pulmonary vessel wall thickness seen in Foxm1f/f mice was attenuated in Foxm1iMyh mice (Figures 2E and 2F). Muscularization of distal pulmonary arterioles was also markedly decreased in Foxm1iMyh mice compared with Foxm1f/f mice (Figures 2G and 2H). Foxm1iMyh mice also exhibited marked decreases of RVSP and RV hypertrophy following hypoxia exposure compared with Foxm1f/f mice (see Figures E4A and E4B). These data suggest that FoxM1 in SMCs contributes to the development of SuHx-induced PH in mice.
To investigate the role of EC FoxM1 in the pathogenesis of PH, we used previously characterized Tie2Cre-mediated EC Foxm1 knockout mice (17). Foxm1Tie2Cre mice did not show any differences regarding both RVSP and RV hypertrophy compared with Foxm1f/f mice following hypoxia challenge (see Figures E4C and E4D), indicating that EC FoxM1 is not involved in hypoxia-induced PH in mice.
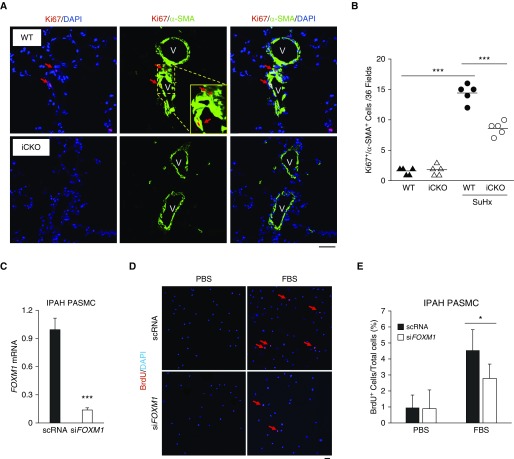
FoxM1 Is Required for SMC Proliferation In Vivo during PH Development
To determine whether FoxM1 mediates SMC proliferation in vivo, we assessed cell proliferation by immunostaining for Ki67 in SuHx-challenged mice. Foxm1iMyh mice had a marked decrease in the number of Ki67+/α-SMA+ cells compared with that of Foxm1f/f mice (Figures 3A and 3B). Moreover, knockdown of FoxM1 in PASMCs from patients with IPAH reduced cell proliferation (Figures 3C and 3D). Taken together, these data suggest that FoxM1 is required for SMC proliferation in PH.
Figure 3.
FoxM1 (forkhead box M1) is required for smooth muscle cell (SMC) proliferation in the pathogenesis of pulmonary hypertension. (A) In vivo cell proliferation assessment by anti-Ki67 immunostaining showing decrease of SMC proliferation in Foxm1iMyh (iCKO) mice exposed to SuHx compared with wild-type mice. Lung cryosections were immunostained with anti-Ki67 (red) and anti–α-SMA (green). Arrows indicate proliferating SMCs (Ki67+/α-SMA+ cells). V = vessel. (B) Quantification of proliferating SMCs. Ki67+/α-SMA+ cells were quantified in 36 fields/sample. (C) siRNA-mediated knockdown of FoxM1 expression in pulmonary arterial smooth muscle cells (PASMCs). (D) Representative micrographs of anti-BrdU immunostaining of PASMCs from patients with idiopathic pulmonary arterial hypertension. Proliferating cells were labeled with BrdU and immunostained with anti-BrdU (red). Nuclei were counterstained with DAPI (blue). Arrows point to BrdU+ cells. (E) Quantification demonstrating that siRNA-mediated FOXM1 knockdown resulted in inhibition of pulmonary arterial hypertension PASMC proliferation. *P < 0.05 and *** P < 0.001. (C and E) Student’s t test. (B) One-way ANOVA. Horizontal bars in dot plot panels represent the mean. Scale bars, 50 μm (A and D). BrdU = 5-bromo-2′-deoxyuridine; FBS = fetal bovine serum; IPAH = idiopathic pulmonary arterial hypertension; PBS = phosphate-buffered saline; scRNA = scrambled siRNA; siFOXM1 = siRNA against FoxM1; SMA = smooth muscle actin; SuHx = Sugen 5146/hypoxia; WT = wild type.
Endothelium-derived Factors Induce FoxM1-dependent Proliferation of SMCs
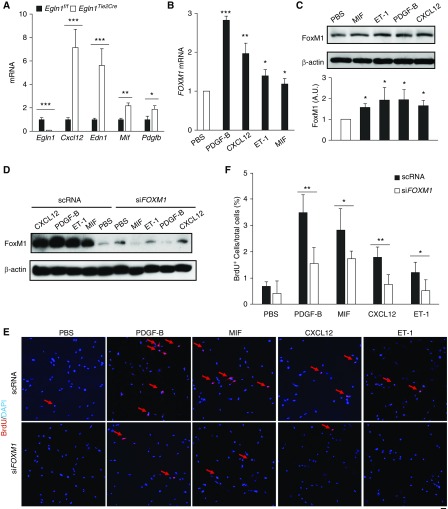
Recent studies indicate that dysregulation of endothelium-derived factors modulates the vascular niche and affects SMC proliferation during pulmonary vascular remodeling (27, 28). We hypothesized that EC-derived factors might induce FoxM1 expression in SMCs, and in turn, activate SMC proliferation during development of PH. Because Egln1Tie2Cre mice demonstrate severe PH resembling the pathology seen in IPAH (including obliterative pulmonary vascular remodeling) (22), ECs were isolated from the lungs of Egln1f/f and Egln1Tie2Cre mice to address this hypothesis. qRT-PCR analysis revealed that ECs from Egln1Tie2Cre mice expressed higher levels of Cxcl12, Edn1, Mif, and Pdgfb than ECs from Egln1f/f mice (Figure 4A). PAECs isolated from patients with IPAH also showed induced expression of CXCL12, PDGFB, and MIF (see Figure E5). Treatment of human PASMCs with recombinant PDGF-B, CXCL12, ET-1, or MIF induced marked increases of FOXM1 expression (Figures 4B and 4C). Knockdown of FOXM1 blocked the proliferative effect of CXCL12, ET-1, PDGF-B, and MIF on SMC proliferation (Figures 4D–4F). These data suggest that FoxM1 is a common transcription factor in SMCs induced by various endothelium-derived factors mediating SMC proliferation and pulmonary vascular remodeling.
Figure 4.
Induction of FoxM1 (forkhead box M1)-dependent smooth muscle cell proliferation by multiple endothelial cell–derived factors. (A) qRT-PCR analysis demonstrating that endothelial cells isolated from Egln1Tie2Cre mouse lungs express high levels of multiple pulmonary hypertension–associated factors including Cxcl12, Edn1, Mif, and Pdgfb compared with that from wild-type (Egln1f/f) mouse lungs. (n = 3 per group). (B and C) Upregulation of FoxM1 expression in human pulmonary arterial smooth muscle cells (PASMCs) by treatment with various recombinant proteins including PDGF-B, CXCL12, ET-1, and MIF. PASMCs were lysed for qRT-PCR at 8 hours post-treatment and Western blotting analyses at 16 hours post-treatment. (D) Western blotting analysis demonstrating FOXM1 siRNA (siFOXM1)-mediated knockdown of FOXM1 in human PASMCs. scRNA = scrambled FOXM1 siRNA. (E and F) FoxM1-dependent human PASMC proliferation. Representative micrographs showing reduced smooth muscle cell proliferation in FoxM1-deficient human PASMCs following treatment with recombinant PDGF-B, MIF, CXCL12, and ET-1. Proliferating cells were labeled with BrdU and immunostained with anti-BrdU (red). Nuclei were counterstained with DAPI (blue). Arrows point to BrdU+ cells. Quantification showing that siRNA-mediated FOXM1 knockdown resulted in inhibition of SMC proliferation following treatment with various angiogenic factors. *P < 0.05, **P < 0.01, and ***P < 0.001. (A) Student’s t test. (B and C) One-way ANOVA. (F) Welch t test. Scale bar, 50 μm (E). A.U. = arbitrary units; BrdU = 5-bromo-2′-deoxyuridine; PBS = phosphate-buffered saline.
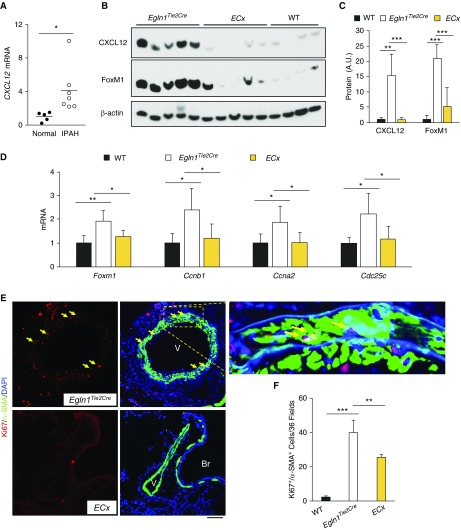
Because Cxcl12 was markedly induced in lung ECs from Egln1Tie2Cre mice (see Figure E6A) and patients with IPAH (see Figure E5), as well as in lung tissues from patients with IPAH (Figure 5A), we next aimed to determine whether endothelium-derived Cxcl12 is important in activating FoxM1 expression in SMCs and promoting pulmonary vascular remodeling in vivo. We generated Egln1/Cxcl12Tie2Cre double-knockout mice by breeding Egln1Tie2Cre mice into the genetic background of Cxcl12 floxed mice (29). Cxcl12 expression is completely normalized in Egln1/Cxcl12Tie2Cre lungs compared with that in Egln1Tie2Cre lungs (Figures 5B and 5C; see Figure E6B). Accordingly, we observed inhibited expression of FoxM1 and its target genes essential for cell cycle progression including Ccnb1, Ccna2, and Cdc25c in Egln1/Cxcl12Tie2Cre lungs (Figures 5B–5D). Immunostaining revealed marked inhibition of SMC proliferation in Egln1/Cxcl12Tie2Cre lungs, which was consistent with decreased FoxM1 expression in PASMCs isolated from Egln1/Cxcl12Tie2Cre mice (Figures 5E and 5F; see Figure E6C). Thus, EC-derived Cxcl12 secondary to Egln1 deficiency plays a critical role in activating FoxM1 expression and resulting SMC proliferation in vivo.
Figure 5.
Role of endothelial Cxcl12 in activating FoxM1 (forkhead box M1)-dependent smooth muscle cell proliferation in vivo. (A) qRT-PCR analysis demonstrating increased CXCL12 expression in lung tissues of patients with IPAH compared with healthy donors. (B and C) Western blotting and densitometric analysis demonstrating inhibited FoxM1 expression in the lungs of Egln1/Cxcl12Tie2Cre double-knockout mice (ECx) compared with Egln1Tie2Cre mice. At 3.5 months of age, mouse lungs were collected for Western blotting analysis. These data demonstrated that endothelial cell–derived Cxcl12 secondary to Egln1 deficiency induced FoxM1 expression in vivo. (D) qRT-PCR analysis showing normalization of FoxM1 and its downstream genes essential for cell cycle progression, Ccnb1, Ccna2, and Cdc25c in ECx lungs compared with Egln1Tie2Cre lungs. n = 4 wild-type, 5 Egln1Tie2Cre, and 5 ECx. (E and F) In vivo cell proliferation assessment by anti-Ki67 immunostaining showing decreased smooth muscle cell proliferation in ECx lungs compared with Egln1Tie2Cre lungs. Lung cryosections were immunostained with anti-Ki67 (red) and anti–α-smooth muscle actin (green). Nuclei were counterstained with DAPI. Arrows point to proliferating smooth muscle cells. n = 4/each group. *P < 0.05, **P < 0.01, and ***P < 0.001. (A) Welch t test. (C, D, and F) One-way ANOVA. Scale bar, 50 μm. A.U. = arbitrary units; Br = bronchiole; IPAH = idiopathic pulmonary arterial hypertension; SMA = smooth muscle actin; V = vessel; WT = wild type.
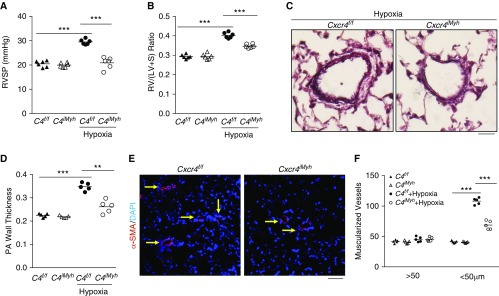
Cxcr4 Deletion in SMCs Protects against Hypoxia-induced PH
Given that CXCR4 is the well-known receptor for Cxcl12, we further investigated the role of SMC CXCR4 in the development of PH. We generated mice with tamoxifen-induced SMC-specific disruption of Cxcr4 (Cxcr4iMyh) by breeding Cxcr4 floxed mice (30) with Myh11-CreERT2 mice. Cxcr4iMyh mice were protected from hypoxia-induced PH, evident by inhibited hypoxia-induced increases in RVSP, RV versus left ventricular plus septum ratio, vessel wall thickness, and muscularization of distal pulmonary vessels (Figures 6A–6E). qRT-PCR analysis demonstrated FoxM1 was markedly induced in pulmonary vascular SMCs from hypoxia-challenged wild-type mice but not Cxcr4iMyh mice (see Figure E7).
Figure 6.
Smooth muscle cell Cxcr4 mediates smooth muscle cell proliferation and pulmonary hypertension development. (A and B) Inhibition of hypoxia-induced pulmonary hypertension in mice with tamoxifen-induced smooth muscle cell–specific disruption of Cxcr4 (C4iMyh) in contrast to wild-type (WT) littermates. Three weeks following hypoxia challenge, right ventricular systolic pressure was assessed and then RV/(LV + S) ratio was calculated for determination of right ventricular hypertrophy. (C and D) Quantitative evaluation of Russel-Movat pentachrome staining showing attenuation of vascular remodeling (pulmonary artery [PA] wall thickness) in hypoxia-challenged Cxcr4iMyh mice compared with Cxcr4f/f WT mice. PAs with a diameter of 20–200 μm were quantified. (E and F) Quantification of α-SMA+ vessels demonstrating inhibited muscularization of distal pulmonary vessels in hypoxia-challenged Cxcr4iMyh mice compared with WT mice. After 3 weeks of exposure to hypoxia, mouse lungs were collected and processed for immunostaining with anti–α-SMA antibody (red). Nuclei were counterstained with DAPI (blue). Arrows point to muscularized distal pulmonary vessels. **P < 0.01 and ***P < 0.001. (A, B, D, and F) One-way ANOVA. Scale bars: (C) 100 μm; (E) 50 μm. RV/(LV + S) = right ventricle versus left ventricle plus septum; RVSP = right ventricular systolic pressure; SMA = smooth muscle actin.
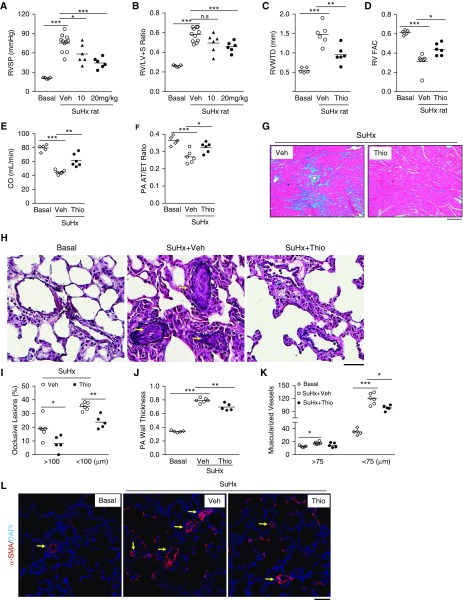
Pharmacologic Inhibition of FoxM1 Inhibits Severe PH in SuHx Rats
We next set out to assess the therapeutic potential of blocking FoxM1 in rat PH models. Given that thiostrepton, a Food and Drug Administration approved drug for veterinary medicine, has been demonstrated to be a FoxM1 inhibitor by inhibiting FoxM1 binding to its genomic target site (31), we first aimed to determine the efficacy of thiostrepton in SuHx rats. Treatment with thiostrepton inhibited SuHx-induced expression of FoxM1 and its target genes (see Figures E8A–E8D), indicating effective inhibition of FoxM1 by thiostrepton. Accordingly, SuHx-induced increase in RVSP was inhibited in thiostrepton-treated SuHx rats at 10 or 20 mg/kg (Figure 7A). RV hypertrophy induced by SuHx was only markedly reduced by 20 mg/kg of thiostrepton treatment. However, 20 mg/kg of thiostrepton treatment in SuHx rats did not affect systemic blood pressure, heart rate, or body weight compared with vehicle groups (see Figures E8E–E8G). Thus, we focused on evaluating the effects of 20 mg/kg of thiostrepton treatment on PH.
Figure 7.
Thiostrepton (Thio) inhibition of FoxM1 (forkhead box M1) inhibits pulmonary hypertension in Sugen 5416/hypoxia (SuHx) rats. (A) Reduction of right ventricular systolic pressure by Thio treatment in SuHx rats compared with vehicle (Veh)-treated SuHx rats. Three weeks post-SuHx challenge, the rats were returned to normoxia and treated with Thio (10 mg/kg, or 20 mg/kg, i.p. daily) or DMSO (Veh) for 3 weeks. (B) Right ventricle (RV) hypertrophy was markedly decreased by higher dose of Thio (20 mg/kg) treatment in SuHx rats. (C–F) Echocardiography revealing reduced RV wall thickness, improved RV contractility, and enhanced cardiac output and pulmonary artery (PA) diastolic function as evidenced by normalization of pulmonary arterial acceleration time/ejection time ratio in Thio-treated SuHx rats compared with respective control animals. Thio, 20 mg/kg treatment. (G) Trichrome staining demonstrating marked inhibition of RV fibrosis (blue) by Thio treatment in SuHx rats. (H–J) Russel-Movat pentachrome staining demonstrating reduced PA occlusion lesions and wall thickness. Arrows indicate occlusive vascular lesions. PAs in a diameter of 20–200 μm were quantified. (K and L) Reduction of distal pulmonary vessel muscularization by Thio treatment. Sections of rat lung tissues immunostained with anti–α-smooth muscle actin antibody (red). Nuclei counterstained with DAPI (blue). Arrows indicate muscularized distal vessels. *P < 0.05, **P < 0.01, and ***P < 0.001. (A–F, J, and K) One-way ANOVA. (I) Student’s t test. Scale bars: (G) 100 μm; (H and L) 50 μm. CO = cardiac output; n.s. = not significant; PA AT/ET = pulmonary arterial acceleration time/ejection time; RVFAC = right ventricular fractional area change; RVWTD = right ventricle wall thickness in diastole; RV/(LV + S) = right ventricle versus left ventricle plus septum; RVSP = right ventricular systolic pressure; SMA = smooth muscle actin.
Echocardiography analysis demonstrated that thiostrepton treatment (20 mg/kg) reduced RV wall thickness, total pulmonary vascular resistance, and increased RV fraction area change, indicative of RV contractility, as well as cardiac output (Figures 7C–7E; see Figures E9A and E9B). PA function was preserved, evident by restored pulmonary arterial acceleration time/ejection time ratio (Figure 7F; see Figure E9C). Trichrome staining revealed reduced RV fibrosis in thiostrepton-treated rat lungs compared with vehicle control animals (Figure 7G). Thiostrepton treatment also attenuated pulmonary vascular remodeling including wall thickening, formation of occlusive lesions, and muscularization of distal pulmonary arterials in SuHx rats (Figures 7H–7L). The reduction of vascular remodeling by thiostrepton treatment coincided with decreased expression of the cell proliferation marker, PCNA (see Figure E8B), and with SMC proliferation (see Figures E9D and E9E). Together, these data suggest that blocking FoxM1 inhibits severe PH and prevents RV fibrosis and RV failure in SuHx rats.
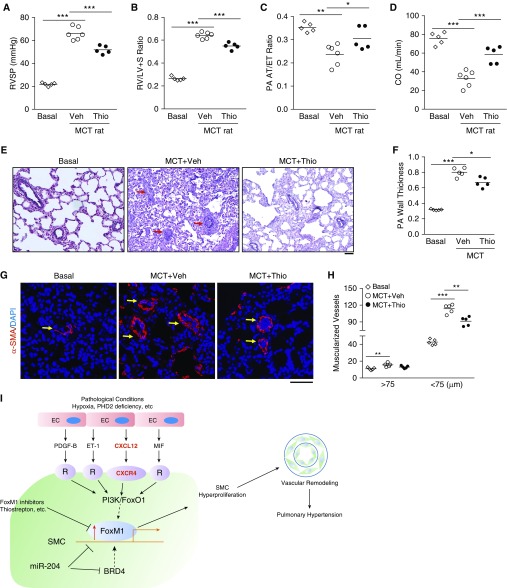
FoxM1 Inhibition Suppresses MCT-induced PH in Rats
We also evaluated the therapeutic potential of targeting FoxM1 in MCT-exposed rats. Thiostrepton treatment in MCT rats significantly reduced FoxM1 expression (see Figures E10A–E10C) and PH phenotype, because RVSP, RV versus left ventricular plus septum ratio, and total pulmonary vascular resistance were reduced in thiostrepton-treated MCT rats compared with vehicle control animals (Figures 8A and 8B; see Figure E10D). There was an improvement in cardiac output and PA function following thiostrepton treatment (Figures 8C and 8D). The vascular lesions, wall thickness of pulmonary vessels, and distal pulmonary arterial muscularization as well as the number of proliferative SMCs were greatly attenuated by thiostrepton treatment (Figures 8G and 8H; see Figures E10E and E10F). Together, these data demonstrate that targeting FoxM1 attenuates MCT-induced PH in rats.
Figure 8.
Thiostrepton (Thio) treatment inhibits monocrotaline (MCT)-induced pulmonary hypertension in rats. (A) Hemodynamic assessment of right ventricular systolic pressure (RVSP). Two weeks post-MCT challenge, the rats were treated with either Thio (20 mg/kg, i.p., daily) or vehicle (Veh) for 2 weeks. RVSP was measured at 4 weeks post-MCT challenge. (B) Reduced right ventricular hypertrophy by Thio treatment in MCT rats compared with vehicle-treated MCT rats. (C and D) Echocardiography assessment of pulmonary arterial (PA) acceleration time/ejection time ratio indicating that Thio treatment preserved PA diastolic function and cardiac output. (E and F) Representative micrographs of Russel-Movat pentachrome staining and quantification showing reduced wall thickening in Thio-treated rats compared with vehicle group. Arrows point to PA. PAs with a diameter of 20–200 μm were quantified. (G and H) Muscularization of distal pulmonary vessels reduced by Thio treatment in MCT rats. Sections of rat lung tissues immunostained with anti–α-smooth muscle actin antibody (red). Nuclei were counterstained with DAPI (blue). Arrows indicate muscularized distal pulmonary vessels. (I) A scheme showing the concept that upregulation of FoxM1 expression in smooth muscle cells (SMCs) induced by endothelial cell (EC)-derived factors through EC-SMC crosstalk mediates SMC proliferation leading to pulmonary vascular remodeling and development of pulmonary hypertension. Under various pathologic conditions (e.g., hypoxia and PHD2 deficiency) ECs release multiple paracrine factors, such as PDGF-B, ET-1, CXCL12, and MIF, which act to induce FoxM1 expression in SMCs likely through PI3K/FoxO1 signaling; activation of FoxM1 in SMCs results in SMC proliferation, which leads to pulmonary vascular remodeling and pulmonary hypertension. Other studies also show BRD4 and miR-204 regulates FoxM1 expression in SMCs. Thus, pharmacologic inhibition of FoxM1 may represent a novel therapeutic strategy to inhibit pulmonary vascular remodeling for treatment of IPAH. *P < 0.05, **P < 0.01, and ***P < 0.001. (A–D, F, and H) One-way ANOVA. Scale bars: (E) 100 μm; (G) 50 μm. CO = cardiac output; FoxM1 = forkhead box M1; PA AT/ET = pulmonary arterial acceleration time/ejection time; R = receptor; RV/(LV + S) = right ventricle versus left ventricle plus septum; SMA = smooth muscle actin.
Discussion
In this study we demonstrate that FoxM1 is markedly upregulated in PASMCs and lungs of patients with IPAH and various rodent models of PH. Genetic disruption of Foxm1 in SMCs but not ECs in mice protects from hypoxia- or SuHx-induced PH, supporting the crucial role of smooth muscle FoxM1 in the pathogenesis of pulmonary vascular remodeling and PH. We also observe that endothelial-derived factors, such as CXCL12, PDGF-B, ET-1, and MIF, induce SMC proliferation in a FoxM1-dependent manner. Deletion of Cxcl12 in ECs or its cognate receptor Cxcr4 in SMCs blocks FoxM1 induction, along with attenuation of pulmonary vascular remodeling and PH. Furthermore, pharmacologic inhibition of FoxM1 with thiostrepton inhibits PH in both SuHx- and MCT-challenged rats. Thus, targeting FoxM1 may represent a novel approach for inhibiting pulmonary vascular remodeling and effectively treating PAH including IPAH. Our data also provide strong evidence about the role of EC-SMC crosstalk in the pathogenesis of pulmonary vascular remodeling and PH (Figure 8H).
We demonstrate that FoxM1 is markedly induced in PASMCs and lungs of patients with IPAH and four rodent PH models and also in PASMCs by various factors released from ECs. Decreased cell proliferation was observed in FoxM1-deficient SMCs in vitro and in vivo and in PH rats treated with thiostrepton, and there was a significant reduction of pulmonary vascular remodeling in SuHx-challenged Foxm1iMyh mice and in PH rats treated with thiostrepton. However, we observed no marked changes of cell apoptosis under these conditions (see Figure E11). These data strongly support our hypothesis that elevated FoxM1 expression in SMCs mediates SMC proliferation, which contributes to pulmonary vascular remodeling and PH development. Consistent with our observations, recent studies show that FOXM1 is upregulated in PASMCs isolated from patients with IPAH and PH rats (21, 32), and that FoxM1 mediates PASMC expansion in vitro (20, 21). Using tamoxifen-inducible SMC-specific Foxm1 knockout mice, our work for the first time demonstrates the causal role of increased FoxM1 expression in the mechanisms of SMC proliferation in vivo and pulmonary vascular remodeling.
Although EC proliferation is evident in patients with PAH and PH rodent models, it seems that endothelial FoxM1 is dispensable for the pathogenesis of PH. It is also possible that endothelial dysfunction but not EC hyperproliferation per se plays a pathogenic role in the development of PH. A recent study, for example, has shown that forced EC apoptosis induces spontaneous PH in mice (33). Our study shows that dysfunctional ECs can regulate FoxM1 expression in SMCs in a paracrine manner and thereby contribute to the pathogenesis of PH. The cellular and molecular mechanisms of PAH are complex and multifaceted, involving cell types within the vascular wall and also other cells, such as immune cells. Although the triggers for the initiation of PH remain unknown, one current hypothesis is that EC dysfunction is the initiating event during PAH development (34, 35). Dysfunctional ECs produce several regulatory mediators, such as ET-1 (35), IL-6 (36), fibroblast growth factor-2 (28), and transforming growth factor-β (37), resulting in excessive vasoconstriction and SMC proliferation.
Our data provide direct evidence that ECs from Egln1Tie2Cre mice display increased expression of multiple proproliferative factors including CXCL12, PDGF-B, ET-1, and MIF. All these factors can induce marked expression of SMC FoxM1 and resulting proliferation. We recently show that CXCL12 can induce FoxM1 expression in ECs via PI3K/FoxO1 signaling (38). It is possible that CXCL12-induced FoxM1 expression in SMCs is also through the same mechanism. Intriguingly, PDGF-B, ET-1, and MIF can also activate PI3K signaling (39–41). PI3K activation inactivates FoxO and thereby activates FoxM1 expression (38, 42). Thus, the PI3K/FoxO signaling is likely the common mechanisms mediating FoxM1 expression induced by these paracrine factors from ECs (Figure 8I). Future studies are warranted to investigate this possibility. Apart from the paracrine effects of ECs on the regulation of FoxM1 expression in PASMCs, FoxM1 expression in PAH SMCs is also regulated by miR-204 (43) and BRD4 (44). Previous study also shows that FoxM1 can be upregulated in hypoxic SMCs (20). These data together demonstrate that FoxM1 is a common effector molecule in mediating SMC proliferation and pulmonary vascular remodeling in response to hypoxia and nonhypoxia pathologic stimuli.
Elevated plasma CXCL12 is associated with an unfavorable prognosis in patients with PAH, and in hypoxic rodent lungs (45, 46). Our data also demonstrate a marked increase of CXCL12 expression in lungs of patients with IPAH. The cellular source of excessive CXCL12 in PAH could be ECs, given the prominent CXCL12 immunostaining in the lung of patients with PAH (47) and our finding that CXCL12 expression is completely normal in Egln1/Cxcl12Tie2Cre lungs. Previous studies using the CXCR4 inhibitor, AMD3100, have implicated the role of CXCR4 signaling in the context of PH (48–51). Given that Cxcr4−/− mice are embryonic lethal (52), the cell-specific role of CXCR4 in the pathogenesis of PH had been unexplored in vivo. Here we used an inducible genetic deletion strategy and observed attenuated pulmonary vascular remodeling and PH in Cxcr4iMyh mice following hypoxia challenge. Thus, we have demonstrated an essential role of endothelial CXCL12 and SMC CXCR4 in mediating FoxM1-dependent SMC proliferation and pulmonary vascular remodeling. Our studies provide clear evidence of EC-SMC crosstalk in the pathogenesis of PH.
The present studies have shown that targeting FoxM1 with thiostrepton effectively inhibits PH in both SuHx- and MCT-challenged rats. Using genetic and pharmacologic approaches, our studies provide unequivocal evidence that targeting SMC FoxM1 may be an effective approach to inhibit SMC proliferation and pulmonary vascular remodeling in patients with PAH. Consistently, a recent study also showed an important role of FoxM1 in promoting SMC expansion and pharmacologic inhibition of FoxM1 attenuates PAH in SuHx and MCT rats (21).
Notably, our studies have also demonstrated an important role of thiostrepton treatment in reducing RV fibrosis. It is possible that fibroblast FoxM1 is also markedly induced in RV during PH development, which promotes fibroblast proliferation in the process of RV fibrosis. In this case, thiostrepton could also inhibit fibroblast proliferation and thereby repress RV fibrosis. It is also possible that the reduced RV fibrosis is attributable to decreased severity of the PH phenotype in thiostrepton-treated SuHx rats. Further studies are warranted to address the mechanisms that regulate this important finding.
In summary, our studies demonstrate a pathogenic role of SMC FoxM1 in mediating SMC proliferation and pulmonary vascular remodeling, and provide clear evidence of EC-SMC crosstalk in the pathogenesis of PH. We also show that pharmacologic inhibition of FoxM1 inhibits severe PH in rodent models. These studies suggest that FoxM1 inhibition could be an effective strategy to pursue for the treatment of PAH in humans.
Acknowledgments
Acknowledgment
The authors thank Dr. John Wharton from the Centre for Pharmacology and Therapeutics, Department of Medicine, Imperial College of London, Hammersmith Hospital, London, United Kingdom for providing lung tissues from patients with idiopathic pulmonary arterial hypertension and unused donors. Data/tissue samples provided the Pulmonary Hypertension Breakthrough Initiative. Funding for the Pulmonary Hypertension Breakthrough Initiative is provided under an NHLBI R24 grant (R24HL123767) and by the Cardiovascular Medical Research and Education Fund.
Footnotes
Supported in part by NIH grants R01HL123957, R01HL125350, R01HL133951, and P01HL077806 (Project 3) (Y.-Y.Z.) and K99HL138278 (Z.D.).
Author Contributions: Z.D. and Y.-Y.Z. conceived the experiments. Z.D., M.M.Z., Y.P., H.J., N.M., Z.Q., and X.Z. designed and performed experiments, and analyzed the data. Z.D. and Y.-Y.Z. analyzed and interpreted the data. Z.D. wrote the manuscript. Y.-Y.Z. supervised the project and revised the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201709-1835OC on April 17, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119:2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. J Am Coll Cardiol. 2015;65:1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 3.Sutendra G, Michelakis ED. Pulmonary arterial hypertension: challenges in translational research and a vision for change. Sci Transl Med. 2013;5:208sr5. doi: 10.1126/scitranslmed.3005428. [DOI] [PubMed] [Google Scholar]
- 4.Tuder RM, Stacher E, Robinson J, Kumar R, Graham BB. Pathology of pulmonary hypertension. Clin Chest Med. 2013;34:639–650. doi: 10.1016/j.ccm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinovitch M. Pathobiology of pulmonary hypertension. Annu Rev Pathol. 2007;2:369–399. doi: 10.1146/annurev.pathol.2.010506.092033. [DOI] [PubMed] [Google Scholar]
- 7.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8:443–455. doi: 10.1038/nrcardio.2011.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guignabert C, Tu L, Le Hiress M, Ricard N, Sattler C, Seferian A, et al. Pathogenesis of pulmonary arterial hypertension: lessons from cancer. Eur Respir Rev. 2013;22:543–551. doi: 10.1183/09059180.00007513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension. From cancer biology to new pulmonary arterial hypertension therapeutics. Targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson AAR, Lawrie A. Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol Med. 2017;23:31–45. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Rai PR, Cool CD, King JAC, Stevens T, Burns N, Winn RA, et al. The cancer paradigm of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;178:558–564. doi: 10.1164/rccm.200709-1369PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 13.Wierstra I. The transcription factor FOXM1 (Forkhead box M1): proliferation-specific expression, transcription factor function, target genes, mouse models, and normal biological roles. Adv Cancer Res. 2013;118:97–398. doi: 10.1016/B978-0-12-407173-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 14.Costa RH. FoxM1 dances with mitosis. Nat Cell Biol. 2005;7:108–110. doi: 10.1038/ncb0205-108. [DOI] [PubMed] [Google Scholar]
- 15.Kalin TV, Ustiyan V, Kalinichenko VV. Multiple faces of FoxM1 transcription factor: lessons from transgenic mouse models. Cell Cycle. 2011;10:396–405. doi: 10.4161/cc.10.3.14709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halasi M, Gartel AL. FOX(M1) news: it is cancer. Mol Cancer Ther. 2013;12:245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y-Y, Gao X-P, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. doi: 10.1172/JCI27154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA. 2002;99:16881–16886. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ustiyan V, Wang I-C, Ren X, Zhang Y, Snyder J, Xu Y, et al. Forkhead box M1 transcriptional factor is required for smooth muscle cells during embryonic development of blood vessels and esophagus. Dev Biol. 2009;336:266–279. doi: 10.1016/j.ydbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raghavan A, Zhou G, Zhou Q, Ibe JC, Ramchandran R, Yang Q, et al. Hypoxia-induced pulmonary arterial smooth muscle cell proliferation is controlled by forkhead box M1. Am J Respir Cell Mol Biol. 2012;46:431–436. doi: 10.1165/rcmb.2011-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. J Mol Med (Berl) 2018;96:223–235. doi: 10.1007/s00109-017-1619-0. [DOI] [PubMed] [Google Scholar]
- 22.Dai Z, Li M, Wharton J, Zhu MM, Zhao Y-Y. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y-Y, Zhao YD, Mirza MK, Huang JH, Potula H-HSK, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119:2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonnet S, Provencher S, Guignabert C, Perros F, Boucherat O, Schermuly RT, et al. Translating research into improved patient care in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:583–595. doi: 10.1164/rccm.201607-1515PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth A, Benyó Z, Lukasova M, Leutgeb B, Wettschureck N, Gorbey S, et al. G12-G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat Med. 2008;14:64–68. doi: 10.1038/nm1666. [DOI] [PubMed] [Google Scholar]
- 26.Ciuclan L, Bonneau O, Hussey M, Duggan N, Holmes AM, Good R, et al. A novel murine model of severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2011;184:1171–1182. doi: 10.1164/rccm.201103-0412OC. [DOI] [PubMed] [Google Scholar]
- 27.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl. S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Izikki M, Guignabert C, Fadel E, Humbert M, Tu L, Zadigue P, et al. Endothelial-derived FGF2 contributes to the progression of pulmonary hypertension in humans and rodents. J Clin Invest. 2009;119:512–523. doi: 10.1172/JCI35070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie Y, Waite J, Brewer F, Sunshine MJ, Littman DR, Zou YR. The role of CXCR4 in maintaining peripheral B cell compartments and humoral immunity. J Exp Med. 2004;200:1145–1156. doi: 10.1084/jem.20041185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hegde NS, Sanders DA, Rodriguez R, Balasubramanian S. The transcription factor FOXM1 is a cellular target of the natural product thiostrepton. Nat Chem. 2011;3:725–731. doi: 10.1038/nchem.1114. [DOI] [PubMed] [Google Scholar]
- 32.Yu J, Wilson J, Taylor L, Polgar P. DNA microarray and signal transduction analysis in pulmonary artery smooth muscle cells from heritable and idiopathic pulmonary arterial hypertension subjects. J Cell Biochem. 2015;116:386–397. doi: 10.1002/jcb.24987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldthorpe H, Jiang J-Y, Taha M, Deng Y, Sinclair T, Ge CX, et al. Occlusive lung arterial lesions in endothelial-targeted, fas-induced apoptosis transgenic mice. Am J Respir Cell Mol Biol. 2015;53:712–718. doi: 10.1165/rcmb.2014-0311OC. [DOI] [PubMed] [Google Scholar]
- 34.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 35.Sakao S, Tatsumi K, Voelkel NF. Endothelial cells and pulmonary arterial hypertension: apoptosis, proliferation, interaction and transdifferentiation. Respir Res. 2009;10:95. doi: 10.1186/1465-9921-10-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricard N, Tu L, Le Hiress M, Huertas A, Phan C, Thuillet R, et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation. 2014;129:1586–1597. doi: 10.1161/CIRCULATIONAHA.113.007469. [DOI] [PubMed] [Google Scholar]
- 37.Sakao S, Taraseviciene-Stewart L, Wood K, Cool CD, Voelkel NF. Apoptosis of pulmonary microvascular endothelial cells stimulates vascular smooth muscle cell growth. Am J Physiol Lung Cell Mol Physiol. 2006;291:L362–L368. doi: 10.1152/ajplung.00111.2005. [DOI] [PubMed] [Google Scholar]
- 38.Huang X, Dai Z, Cai L, Sun K, Cho J, Albertine KH, et al. Endothelial p110γPI3K mediates endothelial regeneration and vascular repair after inflammatory vascular injury. Circulation. 2016;133:1093–1103. doi: 10.1161/CIRCULATIONAHA.115.020918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fantauzzo KA, Soriano P. PI3K-mediated PDGFRα signaling regulates survival and proliferation in skeletal development through p53-dependent intracellular pathways. Genes Dev. 2014;28:1005–1017. doi: 10.1101/gad.238709.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. 2007;26:5046–5059. doi: 10.1038/sj.onc.1210318. [DOI] [PubMed] [Google Scholar]
- 41.Schorlemmer A, Matter ML, Shohet RV. Cardioprotective signaling by endothelin. Trends Cardiovasc Med. 2008;18:233–239. doi: 10.1016/j.tcm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 43.Courboulin A, Paulin R, Giguère NJ, Saksouk N, Perreault T, Meloche J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011;208:535–548. doi: 10.1084/jem.20101812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res. 2015;117:525–535. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 45.McCullagh BN, Costello CM, Li L, O’Connell C, Codd M, Lawrie A, et al. Elevated plasma CXCL12α is associated with a poorer prognosis in pulmonary arterial hypertension. PLoS One. 2015;10:e0123709. doi: 10.1371/journal.pone.0123709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costello CM, McCullagh B, Howell K, Sands M, Belperio JA, Keane MP, et al. A role for the CXCL12 receptor, CXCR7, in the pathogenesis of human pulmonary vascular disease. Eur Respir J. 2012;39:1415–1424. doi: 10.1183/09031936.00044911. [DOI] [PubMed] [Google Scholar]
- 47.Toshner M, Voswinckel R, Southwood M, Al-Lamki R, Howard LSG, Marchesan D, et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2009;180:780–787. doi: 10.1164/rccm.200810-1662OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Inhibition of the SDF-1/CXCR4 axis attenuates neonatal hypoxia-induced pulmonary hypertension. Circ Res. 2009;104:1293–1301. doi: 10.1161/CIRCRESAHA.109.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gambaryan N, Perros F, Montani D, Cohen-Kaminsky S, Mazmanian M, Renaud JF, et al. Targeting of c-kit+ haematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur Respir J. 2011;37:1392–1399. doi: 10.1183/09031936.00045710. [DOI] [PubMed] [Google Scholar]
- 50.Farkas D, Kraskauskas D, Drake JI, Alhussaini AA, Kraskauskiene V, Bogaard HJ, et al. CXCR4 inhibition ameliorates severe obliterative pulmonary hypertension and accumulation of C-kit+ cells in rats. PLoS One. 2014;9:e89810. doi: 10.1371/journal.pone.0089810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu L, Hales CA. Effect of chemokine receptor CXCR4 on hypoxia-induced pulmonary hypertension and vascular remodeling in rats. Respir Res. 2011;12:21. doi: 10.1186/1465-9921-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]