The Fox (forkhead box) proteins belong to a large family of evolutionary conserved transcription factors (TFs) in the winged helix/forkhead DNA-binding domain. The Fox family includes more than 55 distinct mammalian members grouped into 19 subfamilies (FoxA–FoxS) according to their sequence homology within the DNA-binding domain. Although all Fox proteins share this unique DNA-binding domain, distinct protein domains apart from conserved DNA-binding domain, expression patterns, and post-translational modifications contribute to the divergent functions of Fox family members. The Fox proteins regulate a wide spectrum of biological processes including cell proliferation, apoptosis, differentiation, and resistance to DNA damage (1). As a consequence, either a loss or gain of Fox function can alter cell fate and promote various human pathologies including cancer and cardiovascular diseases. The best-studied Fox proteins involved in the above-mentioned pathologies are FoxM, FoxO, and FoxP (1).
Among these, FoxM1 is crucial for G1-S and G2-M cell cycle phase progression and mitotic spindle integrity. FoxM1 is highly expressed in proliferating cells including embryonic tissues, adult tissues that have high proliferation index (2), and all solid tumors (3). In contrast, activation of the FoxO family of proteins (FoxO1, FoxO3A, FoxO4, and FoxO6) is associated with cell cycle arrest and the induction of apoptosis (4), and thus, the function of FoxO proteins is often reduced in proliferating cells.
Accumulating evidence suggests that Fox subfamilies FoxM1 and FoxOs are correlated with various biological processes associated with the development of pulmonary hypertension (PH), such as PH initiation and progression. This editorial highlights the complex regulatory mechanisms of FoxM1 and FoxOs in PH, their critical roles in the pathogenesis of PH, their potential as therapeutic targets, and questions remaining to be addressed concerning these issues.
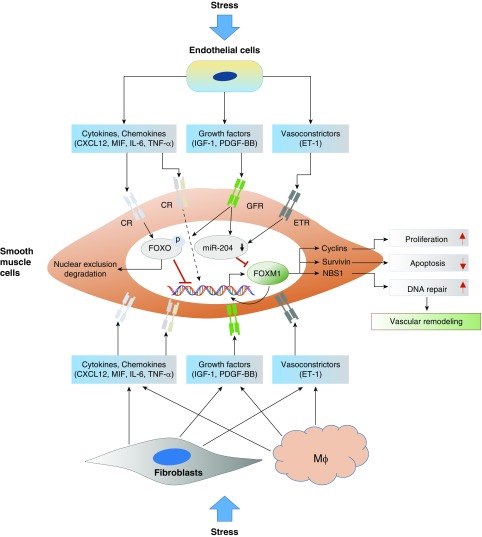
Pulmonary hypertension (PH) is a deadly disease characterized by vasoconstriction and abnormal remodeling of pulmonary vessels, leading to a progressive increase in pulmonary artery pressure, which culminates in right ventricular failure and premature death (5). Observations across species and animal models have demonstrated that numerous stimuli and pathologic conditions (sheer stress, hypoxia, oxidative stress, infection, and others) can cause deregulation of normal cellular processes in the pulmonary vasculature, which culminates in abnormalities in proliferation, differentiation, inflammation, and cell death programs, which all together contribute to the development of PH. The initiation process can involve a variety of growth factor, integrin, cytokine, and other ligands, which initiate different signaling cascades, ultimately converging on a common program targeting the activity of certain TFs. Multiple TFs have been implicated in PH development (6). In this issue of the Journal, Dai and colleagues (pp. 788–802) identify another TF, FoxM1, as playing a key role in PH development (7). They demonstrate that FoxM1 is markedly upregulated in the lungs of patients with idiopathic pulmonary arterial hypertension (PAH) and various rodent models of PH. These observations are consistent with another recently published manuscript by Bourgeois and colleagues, also demonstrating that FoxM1 is overexpressed in pulmonary artery smooth muscle cells (SMCs) from patients with idiopathic PAH (8). In elegant genetic disruption studies, the authors found that deletion of FoxM1 in SMCs, but not in endothelial cells, in mice protects from hypoxia or sugen/hypoxia-induced PH. Similarly, there is also ample evidence implicating FoxOs, mainly FoxO1 isoform, in PH. Indeed, targeted depletion of FoxO1 specifically in SMCs, both in vitro and in vivo, is sufficient to induce pulmonary vascular remodeling and PH and also synergizes with the hypertensive effects of hypoxia, resulting in more severe PH (9) and suggesting a causative role of SMC FoxO1 in PH (9) (Figure 1).
Figure 1.
Role of Fox (forkhead box) transcription factors in pulmonary hypertension. Numerous stresses can activate pulmonary vascular cells including endothelial cells, fibroblasts, and resident and recruited macrophages. Activated vascular cells produce increased amounts of cytokines, chemokines, growth factors, and vasoconstrictors, leading to increased FoxM1 gene expression in smooth muscle cells via reduced FoxO activity, which is a negative transcriptional regulator of FoxM1 gene expression; reduced levels of miR-204, also a negative regulator of FoxM1 mRNA stability and translation; and open FoxM1 chromatin structure, via phosphorylating histones and epigenetic regulators by signal-activated kinases. Increased FoxM1 levels and activity result in smooth muscle cell proliferation, apoptosis resistance, and genome stability by activating genes involved in cell proliferation, antiapoptosis, and DNA repair. CR = cytokine receptor; CXCL12 = chemokine ligand 12; ET-1 = endothelin-1; ETR = endothelin receptor; GFR = growth factor receptor; IGF-1 = insulin-like growth factor-1; Mϕ = macrophage; MIF = macrophage migration inhibitory factor; NBS1 = Nijmegen breakage syndrome 1; PDGF-BB = platelet-derived growth factor-BB; TNF-α = tumor necrosis factor-α.
Open Questions and Remaining Challenges
Is FoxM1 a critical integrator of multiple signaling pathways driving PH?
Dai and colleagues demonstrate that endothelial-derived factors such as CXCL12, PDGFβ, ET-1, and MIF are capable of inducing FoxM1 expression in SMCs and of causing SMC proliferation in a FoxM1-dependent manner (7). In addition to the apparent direct stimulatory effects of cytokines and growth factors on FoxM1 gene transcription are findings demonstrating the epigenetic- and TF-mediated regulation of FoxM1. Raghavan and colleagues demonstrated that hypoxia induces FoxM1 gene expression via HIF-2α, whereas Bourgeois and colleagues demonstrated miR-204–mediated regulation of FoxM1 in pulmonary artery SMCs (8, 10). Further, it has been shown that growth factors and cytokines, which are activated in patients with PH, lead to activation of AKT or JNKs, which then leads to the phosphorylation and nuclear exclusion of FoxOs, and thus upregulation of FoxM1 (9) (Figure 1). Although this study shows a role for endothelial-derived factors in controlling FoxM1 production, it is clear that other cell–cell interactions in the vessel wall may also regulate SMC FoxM1 expression. Future studies should evaluate the role of FoxM1 in inflammatory cells and fibroblasts, as well as the interactions between these cells and SMCs (Figure 1).
What are the consequences of FoxM1 deregulation?
Dai and colleagues showed that FoxM1 upregulation leads to SMC hyperproliferation, and subsequently vascular remodeling. In addition, Bourgeois and colleagues showed that FoxM1 deficiency reduces resistance to apoptosis through diminished DNA repair mechanisms and increased survivin expression (8). Recent studies suggest mitochondrial, metabolic, and inflammatory remodeling as critical pathogenetic components of PH (11). FoxM1 being a target gene of FoxOs (major metabolic regulators among the Fox family members) (12), as well as an interacting partner of various TFs, such as NFκB, β-catenin, and STAT3, suggests a better understanding of the regulation of FoxM1 in the above-mentioned remodeling processes could provide interesting insights into PH development. Identification of FoxM1-FoxO axis target genes and gene networks in PH vascular cells will be very important.
Is FoxM1 a potential therapeutic target for PH?
The studies from both Dai and Bourgeois demonstrate that inhibition of FoxM1 with thiostrepton significantly improved established PH in both hypoxia+SU5416 and monocrotaline-challenged rats. Furthermore, FoxO activators or HDAC (histone deacetylase) inhibitors can indirectly target FoxM1. It may be that the beneficial effects of FoxM1 inhibition in vivo are the result of a cumulative effect on numerous signaling pathways. Because of the critical role of FoxM1 in normal cell proliferation, maintenance of genome stability, and DNA damage repair, it will be essential to specifically deliver FoxM1 inhibitors to the pulmonary vasculature to reduce/avoid the potential adverse effect of loss of FoxM1 activity in proliferating normal cells.
Conclusions
FoxM1 is implicated in SMC proliferation, which contributes to pulmonary vascular remodeling and PH. Thus, targeting FoxM1 signaling represents a novel strategy for treatment of PAH. However, a deeper understanding of the upstream and downstream molecular mechanisms mediating FoxM1 control of PH (e.g., FoxM1 regulation and role in different subgroups of PH, the mechanisms driving FoxM1 regulation, and the molecular and cellular effects driven by FoxM1 in PH-vascular cells) is essential for developing tailored therapeutic concepts focusing on FoxM1 inhibition.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.201804-0702ED on April 25, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lam EW, Brosens JJ, Gomes AR, Koo CY. Forkhead box proteins: tuning forks for transcriptional harmony. Nat Rev Cancer. 2013;13:482–495. doi: 10.1038/nrc3539. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. Forkhead box M1 transcription factor: a novel target for cancer therapy. Cancer Treat Rev. 2010;36:151–156. doi: 10.1016/j.ctrv.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halasi M, Gartel AL. FOX(M1) news--it is cancer. Mol Cancer Ther. 2013;12:245–254. doi: 10.1158/1535-7163.MCT-12-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25) Suppl:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 6.Pullamsetti SS, Perros F, Chelladurai P, Yuan J, Stenmark K. Transcription factors, transcriptional coregulators, and epigenetic modulation in the control of pulmonary vascular cell phenotype: therapeutic implications for pulmonary hypertension (2015 Grover Conference series) Pulm Circ. 2016;6:448–464. doi: 10.1086/688908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai Z, Zhu MM, Peng Y, Jin H, Machireddy N, Qian Z, et al. Endothelial and smooth muscle cell interaction via FoxM1 signaling mediates vascular remodeling and pulmonary hypertension Am J Respir Crit Care Med 2018198788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourgeois A, Lambert C, Habbout K, Ranchoux B, Paquet-Marceau S, Trinh I, et al. FOXM1 promotes pulmonary artery smooth muscle cell expansion in pulmonary arterial hypertension. J Mol Med (Berl) 2018;96:223–235. doi: 10.1007/s00109-017-1619-0. [DOI] [PubMed] [Google Scholar]
- 9.Savai R, Al-Tamari HM, Sedding D, Kojonazarov B, Muecke C, Teske R, et al. Pro-proliferative and inflammatory signaling converge on FoxO1 transcription factor in pulmonary hypertension. Nat Med. 2014;20:1289–1300. doi: 10.1038/nm.3695. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan A, Zhou G, Zhou Q, Ibe JC, Ramchandran R, Yang Q, et al. Hypoxia-induced pulmonary arterial smooth muscle cell proliferation is controlled by forkhead box M1. Am J Respir Cell Mol Biol. 2012;46:431–436. doi: 10.1165/rcmb.2011-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, Ježek P, Li M, Zhang H, et al. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid Redox Signal. 2018;28:230–250. doi: 10.1089/ars.2017.7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengupta A, Kalinichenko VV, Yutzey KE. FoxO1 and FoxM1 transcription factors have antagonistic functions in neonatal cardiomyocyte cell-cycle withdrawal and IGF1 gene regulation. Circ Res. 2013;112:267–277. doi: 10.1161/CIRCRESAHA.112.277442. [DOI] [PMC free article] [PubMed] [Google Scholar]