Abstract
Animals utilize conserved mechanisms to regulate oxidative stress. The C. elegans SKN-1 protein is homologous to the vertebrate Nrf (NF-E2-related factor) family of cap ’n’ collar (CnC) transcription factors and functions as a core regulator of xenobiotic and oxidative stress responses. The WD40 repeat-containing protein WDR-23 is a key negative regulator of SKN-1 activity. We previously found that the oxidative stress induced by excess iodide can be relieved by loss of function in the BLI-3/TSP-15/DOXA-1 dual oxidase complex. To further understand the molecular mechanism of this process, we screened for new mutants that can survive in excess iodide and identified gain-of-function mutations in skn-1 and loss-of-function mutations in wdr-23. The SKN-1C isoform functions in the hypodermis to affect animal’s response to excess iodide, while the SKN-1A isoform appears to play a minor role. wdr-23(lf) can interact with bli-3 mutations in a manner different from skn-1(gf). Transcriptome studies suggest that excess iodide causes developmental arrest largely independent of changes in gene expression, and wdr-23(lf) could affect the expression of a subset of genes by a mechanism different from SKN-1 activation. We propose that WDR-23 and SKN-1 coordinate with the BLI-3/TSP-15/DOXA-1 dual oxidase complex in response to iodide-triggered oxidative stress.
Keywords: SKN-1, WDR-23, BLI-3 dual oxidase, reactive oxygen species, iodide
Animals utilize similar pathways in response to environmental or endogenous challenges from pathogenic, xenobiotic and oxidative stress (Kensler et al. 2007; Shore and Ruvkun 2013; Blackwell et al. 2015). At the core of the response is the conserved NRF2/Keap1 pathway in mammals or the SKN-1/WDR-23 pathway in C. elegans (Itoh et al. 1997; Itoh et al. 1999; An and Blackwell 2003; Kensler et al. 2007; Choe et al. 2009; Blackwell et al. 2015). When mammalian cells are under stress, Keap1 would dissociate from Nrf2, leading to the nuclear localization of Nrf2 and activation of gene expression that antagonizes the stress. In C. elegans, WDR-23 and SKN-1 appear to play similar roles as those of Keap1 and Nrf2 (Choe et al. 2009; Blackwell et al. 2015). The Nrf2/Keap1 pathway is also implicated in numerous human diseases, including neurodegeneration (Johnson and Johnson 2015), inflammation (Ahmed et al. 2017), cancer (Taguchi and Yamamoto 2017) and cardiovascular diseases (Barancik et al. 2016).
SKN-1 was initially identified as a maternally provided bZIP transcription factor unequally distributed in early embryos to specify the fate of pharyngeal and intestinal cells (Bowerman et al. 1992; Bowerman et al. 1993; Blackwell et al. 1994). SKN-1 is homologous to the vertebrate CNC-group proteins (NF-E2-related factors Nrf1 and Nrf2) with a highly conserved 14-amino-acid transactivator element (“DIDLID”) (Walker et al. 2000) and has a conserved function in regulating oxidative and xenobiotic stress responses by activating the phase II detoxification enzymes (An and Blackwell 2003). In embryos, SKN-1 is expressed in intestine and hypodermis, while in larvae and adults it appears to be highly expressed in the ASI neurons and weakly in intestinal cytoplasm and nuclei (An and Blackwell 2003). Oxidative or heat stress could significantly elevate the level of a SKN-1::GFP fusion protein in intestinal nuclei and enhance the expression of the phase II gene gcs-1 (An and Blackwell 2003).
SKN-1 is a key regulator of the homeostasis of multiple cellular processes. It is required for lipid homeostasis (Steinbaugh et al. 2015), the expression of extracellular collagens for lifespan extension as a consequence of reduced Insulin/IGF-1 INS-18 signaling (Ewald et al. 2015) and the stress response to cuticle damage (Dodd et al. 2018). Mitochondrial proline catabolism can activate SKN-1 to affect lifespan (Zarse et al. 2012) or innate immunity (Tang and Pang 2016), and a mitochondria-associated gain-of-function SKN-1 could mediate a conserved starvation response even with ad lib access to food (Paek et al. 2012). SKN-1 is also involved in mitophagy (Palikaras et al. 2015) and can be activated by the IRE protein sulfenylated by ER- or mitochondria-derived ROS (Hourihan et al. 2016). The crosstalk between SKN-1 and mitochondria appears to be conserved across species (Itoh et al. 2015). SKN-1 activity is regulated by multiple signals (An et al. 2005; Inoue et al. 2005; Kell et al. 2007; Tullet et al. 2008; Wang et al. 2010; Li et al. 2011; Robida-Stubbs et al. 2012; Glover-Cutter et al. 2013; Ruf et al. 2013; Ewald et al. 2015).
WDR-23 is a conserved WD40 repeat-containing protein that interacts with the CUL4-DDB1 ubiquitin ligase to promote ubiquitin proteasome system-mediated degradation of SKN-1 in C. elegans (Choe et al. 2009). wdr-23 loss-of-function mutations can lead to constitutive expression of phase II genes, which is similar to the effect of SKN-1 activation (Hasegawa and Miwa 2010). In mammals, a similar WDR23-DDB1-CUL4-dependent mechanism can repress Nrf2 activity independent of the canonical KEAP1-CUL3 pathway, suggesting that WDR-23-dependent regulation of SKN-1 is conserved (Lo et al. 2017).
Several lines of evidence suggest that SKN-1 and the C. elegans NADPH dual oxidase BLI-3 DUOX1 might act together in response to stress. Manganese (Mn)-induced toxicity requires the activity of BLI-3, while SKN-1 can protect against Mn toxicity (Benedetto et al. 2010). Bacterial or fungal pathogens can trigger BLI-3-dependent ROS generation (Chavez et al. 2009; Zou et al. 2013), which can activate SKN-1 target gene expression (Hoeven et al. 2011; Papp et al. 2012; Van Der Hoeven et al. 2012). Loss of the mammalian mediator of ErbB2-driven cell motility, MEMO-1, could lead to enhanced production of ROS by BLI-3, which stimulates SKN-1 to promote stress resistance and longevity (Ewald et al. 2017). Similarly, a redox co-factor, pyrroloquinoline quinone could activate BLI-3 to produce H2O2 at plasma membrane, the effect of which is transduced by SKN-1, JUN-1 and DAF-16 for lifespan extension (Sasakura et al. 2017). These findings suggest that the BLI-3 dual oxidase activity and the SKN-1 activity are probably coordinated to respond to oxidative stress and maintain ROS homeostasis.
Iodine is a micronutrient essential for life and a key ingredient for the synthesis of thyroid hormones. Insufficient intake of iodide can lead to thyroid hormone deficiency and cause severe hypothyroidism and mental retardation (Nussey and Whitehead 2001). However excess iodide intake has been implicated in autoimmune thyroiditis (Bagchi et al. 1985; Rose et al. 1997; Rose et al. 1999; Teng et al. 2006), hyperthyroidism (Nussey and Whitehead 2001), hypothyroidism (Rose et al. 1999; Teng et al. 2006) and thyroid cancers (Lind et al. 1998; Guan et al. 2009; Blomberg et al. 2012; Dong et al. 2013). The molecular mechanism underlying the pathogenic effects of excess iodide is unclear.
We recently used C. elegans as a model to analyze the xenobiotic effect of excess iodide. We found that excess iodide could cause larval arrest, cuticle shedding defects and premature intestinal autofluorescence, phenotypes that can be reversed when animals were moved to normal growth media (Xu et al. 2014). A screen for mutants that can survive in excess iodide isolated loss-of-function (lf) mutations in bli-3 and tsp-15 (Xu et al. 2014), in which tsp-15 encodes a tetraspanin protein required for BLI-3 activity (Moribe et al. 2004; Moribe et al. 2012). We found that the BLI-3/TSP-15/DOXA-1 dual oxidase complex is required for the xenobiotic effects of excess iodide (Xu et al. 2014), which might involve iodide-induced excessive generation of ROS (Xu et al. 2014).
In this study, we report the identification of novel gain-of-function mutations in skn-1 and loss-of-function mutations in wdr-23 and bli-3/tsp-15/doxa-1 complex and how these genes interact to affect C. elegans cuticle integrity and survival in excess iodide. Besides verifying the known interaction between WDR-23 and SKN-1 in stress responses, we found that wdr-23 can interact with bli-3 and affect gene expression by a mechanism different from SKN-1 activation.
Materials and Methods
Strains
See Supplementary Materials and Methods.
C. elegans survival in excess iodide
The survival assay was performed as described (Xu et al. 2014). In short, five young adults were grown on E. coli OP50-seeded NGM plates with different concentrations of NaI (5 mM, 10 mM or 50 mM). F1 progeny were observed for growth and survival until day 8. For transient transgenic experiments, P0 adult animals injected with transgenes were transferred to OP50-seeded NGM plates with 5 mM NaI and transgene-positive F1 progeny were examined for growth to adults.
Genetic screens and mapping of mutations
See Supplementary Materials and Methods.
Hoechst 33258 staining
Hoechst 33258 staining was performed as described (Moribe et al. 2004; Xu et al. 2014) with minor modifications. Synchronized animals (24 hr post mid-L4) were washed off plates and incubated at 20° with gentle shaking for 15 min with 1 μg/ml Hoechst 33258 (Sigma) diluted in M9. After staining, animals were washed three times with M9 and observed under a Leica DM5000B fluorescence microscope.
RNA interference
L4 animals were fed HT115 (DE3) bacteria expressing dsRNAs on NGM plates with 1 mM IPTG, 0.1 mg/ml Ampicillin (Timmons et al. 2001) with or without 5 mM NaI for 8 days. The progeny were examined under dissecting microscope for survival. The RNAi feeding bacterial strains for wdr-23 and skn-1 were obtained from a whole-genome RNAi library (Kamath et al. 2003), and the inserts were verified by sequencing. The doxa-1 RNAi feeding bacterial strain was described previously (Xu et al. 2014).
Plasmids
See Supplementary Materials and Methods.
Transgene experiments
See Supplementary Materials and Methods.
qRT-PCR
Synchronized animals at the L1 larval stage were allowed to recover on OP50-seeded NGM plates with or without 5 mM NaI for 8 hr and subsequently washed three times with H2O. RNA was extracted using TRIzol (Invitrogen) and chloroform-isopropanol purification and treated with DNase I (NEB). RNA concentration and quality were measured with a NanoDrop 1000 spectrophotometer (Thermo Fisher). cDNAs were prepared using the Maxima First Strand cDNA Synthesis Kit for qRT-PCR (Thermo Fisher). mRNA levels were quantified from three biological replicates using Maxima SYBR Green (Thermo Fisher) fluorescence on a LightCycler 96 Instrument (Roche). After a pre-incubation step (95° for 10 min), two-step amplification was performed using 40 cycles of denaturation (95° for 15 s) and annealing (60° for 45 s). Target gene expression levels were normalized to that of the reference gene tba-1. Primers for constructs and qRT-PCR experiments are listed in Table S7.
Transcriptome analyses
See Supplementary Materials and Methods.
Statistics
P values were determined by two-tailed unpaired Student’s t-test for comparisons between two samples and Bonferroni test with one-way ANOVA for comparisons of more than two samples.
*: P < 0.05; **: P < 0.01; ***: P < 0.001.
Data Availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Gene expression data are available at GEO with the accession number: GSE117222. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6983384.
Results
Loss-of-function mutations in wdr-23 and gain-of-function mutations in skn-1 can promote C. elegans survival in excess iodide
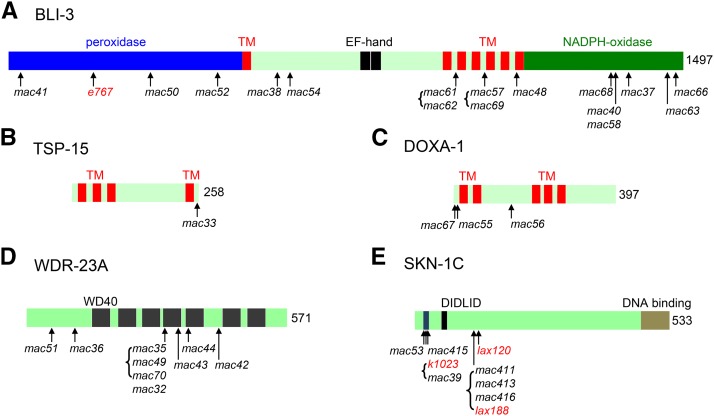
In a previous screen for mutants that can survive in excess iodide (5 mM NaI) (Xu et al. 2014) (Table S1, Screen 1), we isolated four lf mutations in bli-3 (mac37, mac38, mac40, mac41) and one lf mutation in tsp-15 (mac33) (Table S1, Table S2 and Figure 1). The lf nature of the tsp-15(mac33) mutation was further confirmed by transgene rescue experiments in this study (Table S3), showing that wild-type tsp-15 transgenes nearly abolished the survival of tsp-15(mac33lf) mutants in excess iodide.
Figure 1.
Protein domain structures and positions of mutations in BLI-3 (A), TSP-15 (B), DOXA-1 (C), WDR-23A (D) and SKN-1C (E). Protein domains are labeled. In (E), “DIDLID” is conserved between SKN-1C and mammalian Nrf2. TM, transmembrane domain. Brackets indicate that the enclosed mutations cause identical amino acid changes.
From Screen 1 (Table S1), we also isolated six mutations (mac32, mac35, mac36, mac42, mac43 and mac44) that form a distinct complementation group on Chr. I. SNP mapping and candidate gene analyses indicate that they affect wdr-23 (Table S2 and Figure 1). wdr-23 encodes a WD40 repeat-containing protein that functions with the CUL-4/DDB-1 ubiquitin ligase to negatively regulate the activity of SKN-1 (Choe et al. 2009). Both wdr-23(mac32) homozygous animals and animals fed RNAi targeting wdr-23 can survive in excess iodide, while wdr-23(mac32) heterozygous animals failed to (Table 1). We could rescue the survival-promoting effect of the wdr-23(mac32) mutation using either wdr-23a or wdr-23b isoform transgenes (Table S3), suggesting that these mutations cause loss of function in wdr-23.
Table 1. The survival of different mutants and wild-type animals treated with RNAi in excess iodide.
| Genotype | Survival in 5 mM NaI |
|---|---|
| WT | No |
| control RNAi | No |
| wdr-23(mac32) | Yes |
| wdr-23(mac32)/+ | No |
| wdr-23(RNAi) | Yes |
| skn-1(mac39) | Yes |
| skn-1(mac39)/+ | Yes |
| skn-1(lax120gf) | Yes |
| skn-1(lax120gf)/+ | Yes |
| skn-1(zu135lf) | No |
| skn-1(RNAi) | No |
| doxa-1(mac55) | Yes |
| doxa-1(mac55)/+ | No |
| doxa-1(RNAi) | Yes |
We mapped the last mutation (mac39) in Screen 1 to Chr. IV within a region containing skn-1. Knowing that WDR-23 is a negative regulator of SKN-1 (Choe et al. 2009), we took skn-1 as a candidate and indeed identified a missense mutation that causes an R43C amino acid change (Table S1, Table S2 and Figure 1) on the SKN-1C isoform in mac39 mutants.
Both mac39 heterozygous and homozygous animals can survive in excess iodide, while the skn-1(zu135lf) (Bowerman et al. 1992) homozygous mutants and animals fed RNAi targeting skn-1 failed to (Table 1). In addition, both heterozygous and homozygous animals of the previously identified skn-1(lax120gf) mutation (Paek et al. 2012) can survive in excess iodide (Table 1). Based on these findings, we propose that mac39 causes a gain of function (gf) in skn-1.
Additional screens isolated novel lf mutations in bli-3, doxa-1 and wdr-23 and gf mutations in skn-1
To identify more genes and mutations involved in animal’s response to excess iodide, we performed additional screens (Table S1) for mutants that can survive in 5 mM NaI potentially as homozygotes (Screen 2 for F2 mutants) or heterozygotes (Screen 3 for F1 mutants).
In total, 33 independent mutants were isolated. Genetic and sequence analyses identified 16 lf mutations in bli-3 (mac48, mac50, mac52, mac54, mac57, mac58, mac59, mac60, mac61, mac62, mac63, mac64, mac65, mac66, mac68 and mac69), three lf mutations in wdr-23 (mac49, mac51 and mac70), five gf mutations in skn-1 (mac53, mac411, mac413, mac415 and mac416) and three lf mutations in doxa-1 (mac55, mac56 and mac67). (Figure 1, Tables S1 and S2). We are currently investigating the genetic changes in six remaining isolates that can survive in excess iodide as heterozygotes (Table S1).
Among the nine wdr-23 mutants, mac35, mac49 and mac70 cause an identical D312N amino acid change in the 4th WD40 domain. The same mutation was previously described in the xrep-1(k1011lf) (wdr-23) mutant that exhibits constitutive expression of phase II enzymes (Hasegawa and Miwa 2010). mac32 and mac43 cause a D313N change and a G331R change in the 4th WD40 domain, respectively, and mac44 causes a G361R change in the 5th WD40 domain (Table S2 and Figure 1). mac36, mac42 and mac51 cause nonsense mutations at residues R130, W413 and Q80 (Figure 1 and Table S2), respectively.
All skn-1 mutants can survive in excess iodide as heterozygotes or homozygotes. Using skn-1c as the reference isoform, the amino acid change (G39D) in skn-1(mac53) (Table S2 and Figure 1) is one amino acid away from the R41C change caused by skn-1(k1023gf) (Tang and Choe 2015) and three amino acids away from the R43C change caused by skn-1(mac39gf), suggesting functional importance of a potential domain in SKN-1C that contains these amino acid residues. The skn-1(mac415) mutation causes the same R41C change in SKN-1C as that by skn-1(k1023gf) (Tang and Choe 2015). The mac411, mac413, mac416 mutations cause an E147K amino acid change identical to the previously described skn-1(lax188gf) mutation (Paek et al. 2012). Therefore, we isolated new skn-1 gf mutations as well as mutations that were previously described.
The new screens also isolated mutations in doxa-1 (mac55, mac56, mac67) (Table S1). doxa-1 encodes an ortholog of the mammalian dual oxidase maturation factor (Moribe et al. 2012). Both mac55 homozygous animals (Table 1) and animals fed RNAi targeting doxa-1 (Table 1) (Xu et al. 2014) can survive in excess iodide, while mac55 heterozygous animals failed to (Table 1). The survival-promoting effect of mac55 could be rescued by wild-type doxa-1 transgenes (Table S3), suggesting that mac55 and the other two mutations cause loss of function.
SKN-1C functions in the hypodermis (epidermis) to promote animal survival in excess iodide
skn-1 is predicted to express four isoforms, skn-1a, skn-1b, skn-1c and skn-1d (Blackwell et al. 2015), among which skn-1c has been extensively studied. In larvae and adults, skn-1c is apparently expressed in ASI neurons and weakly in intestine (An and Blackwell 2003). skn-1c functions in intestine to regulate a variety of biological processes (Blackwell et al. 2015). SKN-1C expression has also been observed in hypodermis using skn-1c transgenes (Wu et al. 2016) and in hypodermis, pharynx and body-wall muscles based on the expression of SKN-1C target genes (Hasegawa et al. 2008; Paek et al. 2012).
To identify the tissue(s) in which skn-1(gf) mutations promote the survival in excess iodide, we performed phenocopy experiments by introducing skn-1c transgenes to wild-type animals. Stable skn-1c(wt) cDNA transgenes under control of a skn-1c endogenous promoter (An and Blackwell 2003) (Fig. S1A) (Figure 2A), an intestine-specific nhx-2 promoter (Nehrke 2003) (Figure 2A) or ges-1 promoter (Egan et al. 1995), a hypodermis-specific dpy-7 promoter (Gilleard et al. 1997) (Figure 2A) or a body-wall muscle-specific myo-3 promoter (Okkema et al. 1993), all failed to promote the survival (Table S4).
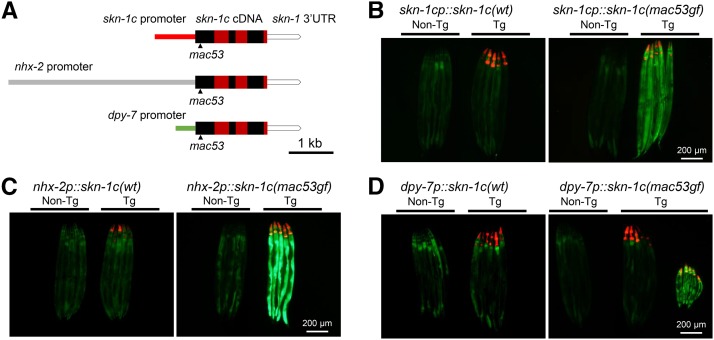
Figure 2.
skn-1c(mac53gf) transgenes under control of skn-1c or nhx-2 promoters can increase the gst-4p::GFP (dvIs19) reporter expression. (A) Structures of transgenes for phenocopying the survival-promoting effect of skn-1c(gf) mutations. The coding exons of skn-1c are delineated as alternating red and black boxes. (B, C, D) Left panels. skn-1c(wt) transgenes did not increase the expression of the gst-4p::GFP reporter. Non-transgenic (Non-Tg) animals are on left. Transgenic animals (Tg, red fluorescence in pharynxes expressed from the pCFJ90 co-injection marker) are on right. (B, C, D) Right panels, skn-1c(mac53gf) transgenes. Non-transgenic and transgenic animals are indicated on left and right, respectively. (D) Right panel, Tg: dpy-7p::skn-1c(mac53gf) transgenes did not increase the expression of the gst-4p::GFP reporter in most cases (animals on the left). Occasionally the transgene caused a strong Dpy phenotype with increased reporter expression in the intestine (animals on the right).
We next examined whether the skn-1c(mac53gf) cDNA transgene could promote the survival in excess iodide. We established stable skn-1c(mac53gf) transgenic lines using the two intestine-specific promoters (nhx-2p and ges-1p) and the body-wall muscle-specific myo-3 promoter. However, these transgenic animals could not survive in excess iodide (Table S4), suggesting that skn-1(gf) might not function in intestine or muscle to promote the survival.
Surprisingly, we failed to establish stable skn-1c(mac53gf) lines using the skn-1c promoter or the hypodermis-specific dpy-7 promoter (Table S4), probably due to the toxicity of hypodermis-specific skn-1c(gf) overexpression. Such toxicity is only obvious in the F2 generation as we could generate abundant viable skn-1(gf) transgene-positive F1 animals (Table 2).
Table 2. Hypodermis-specific skn-1c(gf) expression can promote animal survival in excess iodide.
| Transient transgene | Experiment (No. injected WT P0) | No. survived Tg F1 adults (No. Tg F1) | Percentage |
|---|---|---|---|
| skn-1cp::skn-1c(wt) (skn-1c endogenous promoter) | 1 (15) | 0(28) | 0.00% |
| 2 (15) | 0(70) | 0.00% | |
| 3 (15) | 0(34) | 0.00% | |
| skn-1cp::skn-1c(mac53gf) (skn-1c endogenous promoter) | 1 (20) | 37(129) | 28.68% |
| 2 (20) | 31(153) | 20.26% | |
| 3 (20) | 52(181) | 28.73% | |
| 4 (20) | 28(158) | 17.72% | |
| dpy-7p::skn-1c(wt) (hypodermis promoter) | 1 (15) | 0(37) | 0.00% |
| 2 (15) | 0(21) | 0.00% | |
| 3 (15) | 0(26) | 0.00% | |
| dpy-7p::skn-1c(mac53gf) (hypodermis promoter) | 1 (15) | 31(46) | 67.39% |
| 2 (15) | 23(63) | 36.51% | |
| 3 (15) | 49(74) | 66.22% |
To overcome the toxicity of stable skn-1c(gf) transgenes under control of the skn-1c or the dpy-7 promoter, we examined the survival of skn-1c(mac53gf) transgene-positive (based on co-injection marker expression) F1 progeny in excess iodide. Here, we found that numerous transgene-positive F1 animals could grow into adults in excess iodide (Table 2) and the dpy-7 promoter appears to be more robust than the skn-1c promoter. Therefore, skn-1c(gf) can function in the hypodermis to promote the survival.
Since we isolated lf mutations in the bli-3/tsp-15/doxa-1 complex in the same screens, we tested whether these genes function in the hypodermis as well. Indeed, stable tsp-15 cDNA transgenes under control of the dpy-7 promoter could strongly rescue the survival-promoting effect of the tsp-15(mac33lf) mutation (Table S3), suggesting that the bli-3/tsp-15/doxa-1 complex also functions in the hypodermis to affect the survival in excess iodide.
skn-1c(mac53gf) transgenes can activate the expression of SKN-1C target gene gst-4
To test whether the failure of intestine-specific skn-1c(gf) transgene expression in promoting the survival might be caused by a lack of activated SKN-1C target gene expression, we introduced skn-1c(mac53gf) transgenes to the dvIs19 transgenic animals (Link and Johnson 2002). The dvIs19 transgene expresses GFP under control of the gst-4 promoter and is used as a reliable reporter for SKN-1C activation.
We found that skn-1c(mac53gf) transgenes, under control of either skn-1c promoter or the intestine-specific nhx-2 promoter, can significantly increase GFP expression in the intestines of transgene-positive F1 progeny (Figure 2B and 2C, right panels), while skn-1c(wt) transgenes have no obvious effect (Figure 2B and 2C, left panels). The nhx-2 promoter appears to cause a more robust GFP expression than the skn-1c promoter does.
Under control of the dpy-7 promoter, the skn-1c(mac53gf) transgene resulted in two distinct groups of F1 transgenic progeny. In most cases, the transgenic animals have normal size with normal intestinal GFP expression and can grow into adults in excess iodide (Figure 2D, right panel, animals on the left under Tg). Occasionally, we found transgenic animals with a strong Dpy phenotype and an apparent increase in intestinal GFP expression that failed to grow in excess iodide (Figure 2D, right panel, animals on the right under Tg). The mechanism underlying these distinct phenotypes might be related to the difference in levels, temporal stages or leakiness of the transgene expression. These results together suggest that intestinal activation of SKN-1C is not sufficient for animal survival in excess iodide.
The SKN-1A isoform can weakly promote animal survival in excess iodide
The skn-1(gf) mutations that we isolated affect SKN-1A and SKN-1C isoforms, but not SKN-1B or SKN-1D isoforms (Fig. S1). SKN-1A is expressed in most tissues (An and Blackwell 2003; Bishop and Guarente 2007; Staab et al. 2014) and associated with ER to mediate transcriptional activation of proteasome subunit genes upon proteasome disruption (Lehrbach and Ruvkun 2016). Oxidative and ER stress can increase skn-1a expression (Glover-Cutter et al. 2013).
To examine whether skn-1a plays a role in animal’s response to excess iodide, we generated transgenic lines (Table S4) with skn-1a(wt) or skn-1a(mac53gf) cDNA under control of the skn-1a (Staab et al. 2014) or skn-1c promoter (An and Blackwell 2003) (Fig. S1A). All transgenic lines failed to survive in excess iodide (Table S4). However, we consistently found escapers in the skn-1a(mac53gf) lines controlled by the skn-1a promoter (Table S4). To verify this finding, we examined the survival of F1 skn-1a transgenic animals in excess iodide (Table S5). The results suggest that both skn-1a(wt) and skn-1a(gf) transgenes under control of either skn-1c or skn-1a promoter could weakly promote the survival.
To examine whether skn-1a transgenes might affect skn-1c target gene expression, we introduced these transgenes to the dvIs19 animals. It appears that the skn-1a(wt) or skn-1a(gf) transgenes under control of the skn-1a promoter could weakly activate the GFP expression (Fig. S1B, two left panels), while these transgenes under control of the skn-1c promoter failed to do so (Fig. S1B, two right panels). These results suggest that both skn-1a(wt) and skn-1a(gf) are capable of weakly activating SKN-1C target gene expression. Furthermore, skn-1a(wt) might carry an activity similar to skn-1a(gf) in promoting animal survival in excess iodide. The underlying mechanism remains to be understood.
skn-1 is required for the survival of bli-3, tsp-15, doxa-1 and wdr-23 lf mutants in excess iodide
Since skn-1(zu135lf) and skn-1(RNAi) animals could not survive to adults in excess iodide (Table 1), we examined whether skn-1 is epistatic to bli-3, tsp-15, doxa-1 or wdr-23. We generated double mutants carrying the skn-1(zu135lf) mutation and one of two or more independently isolated mutations in these other genes. Except for bli-3(e767lf); skn-1(zu135lf) double mutants, which were too sick for survival test, all other double mutants grew similarly as skn-1(zu135lf) single mutants under normal condition but failed to survive to adults in excess iodide (Table 3). Therefore, skn-1 is required for mutations in the other four genes to promote animal survival in excess iodide.
Table 3. skn-1 is required for the survival of bli-3, tsp-15, doxa-1 and wdr-23 lf mutants in excess iodide.
| Genotype | Growth in 5 mM NaI | |
|---|---|---|
| skn-1(lf) | zu135/nT1[qIs51] (heterozygous) | Larval arrest |
| zu135 (homozygous) | Larval arrest | |
| bli-3(lf); skn-1(lf) | e767; zu135/nT1[qIs51] | ND |
| e767; zu135 | ND | |
| mac52; zu135/nT1[qIs51] | Adult | |
| mac52; zu135 | Larval arrest | |
| mac40; zu135/nT1[qIs51] | Adult | |
| mac40; zu135 | Larval arrest | |
| mac66; zu135/nT1[qIs51] | Adult | |
| mac66; zu135 | Larval arrest | |
| tsp-15(lf); skn-1(lf) | sv15; zu135/nT1[qIs51] | Adult |
| sv15; zu135 | Larval arrest | |
| mac33; zu135/nT1[qIs51] | Adult | |
| mac33; zu135 | Larval arrest | |
| doxa-1(lf); skn-1(lf) | mac55; zu135/nT1[qIs51] | Adult |
| mac55; zu135 | Larval arrest | |
| mac67; zu135/nT1[qIs51] | Adult | |
| mac67; zu135 | Larval arrest | |
| wdr-23(lf); skn-1(lf) | mac32; zu135/nT1[qIs51] | Adult |
| mac32; zu135 | Larval arrest | |
| mac35; zu135/nT1[qIs51] | Adult | |
| mac35; zu135 | Larval arrest | |
wdr-23(lf) and skn-1(gf) interact with bli-3(lf) differentially to affect animal survival in high concentration of NaI
To further understand the interactions of bli-3 with skn-1 and wdr-23, we chose two independent alleles of each of the three genes and generated double mutants. We examined the survival of single or double mutants in 10 mM or 50 mM NaI. Higher concentrations of iodide might cause more severe oxidative stress, which can be used for detecting additive or synergistic genetic interactions (Table 4).
Table 4. The survival of single and double mutants in 10 or 50 mM NaI.
| Genotype | Alleles | Survival in 10 mM NaI | Survival in 50 mM NaI |
|---|---|---|---|
| bli-3(lf) | e767 | Yes | No |
| mac40 | Yes | No | |
| skn-1(gf) | lax120 | Yes | No |
| mac53 | Yes | No | |
| wdr-23(lf) | mac32 | Yes | No |
| mac35 | Yes | No | |
| bli-3(lf); skn-1(gf) | e767; mac53 | Yes | No |
| e767; lax120 | Yes | No | |
| mac40; mac53 | Yes | Yes | |
| mac40; lax120 | Yes | Yes | |
| bli-3(lf) wdr-23(lf) First group | e767 mac32 | Yes | No |
| e767 mac35 | Yes | No | |
| mac40 mac32 | Yes | No | |
| mac40 mac35 | Yes | No | |
| bli-3(lf) wdr-23(lf) Second group | mac40 mac36 | Yes | No |
| mac40 mac42 | No | No | |
| mac40 mac44 | Yes | No | |
| mac40 mac51 | Yes | No |
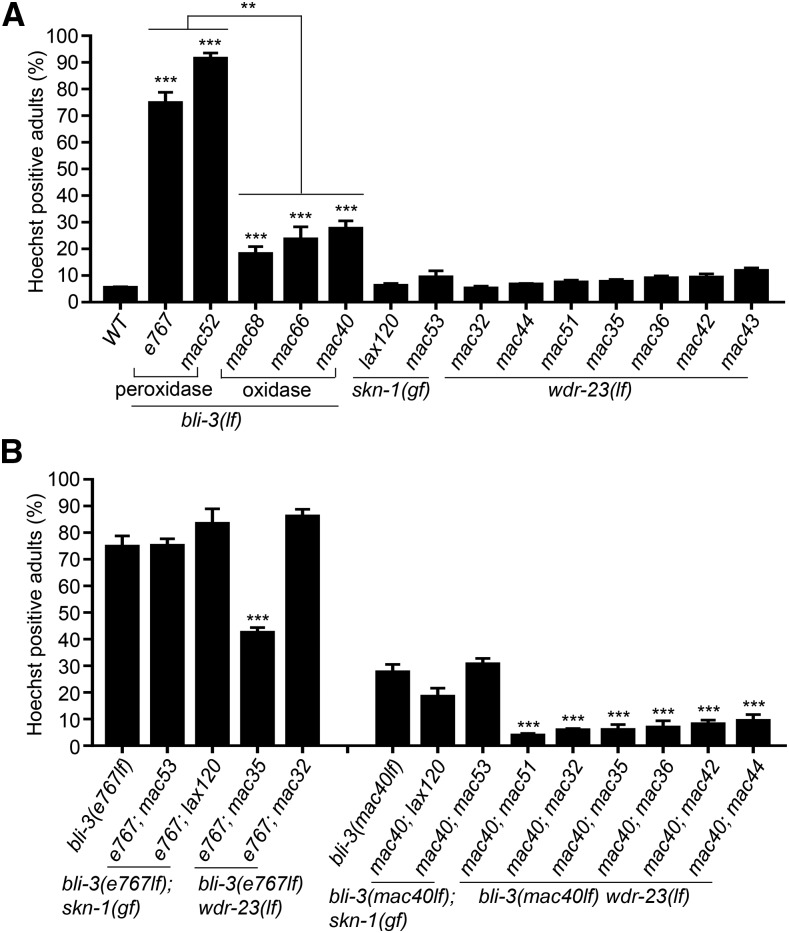
We found that all single and double mutants can survive in 10 mM NaI (Table 4), suggesting that at this concentration iodide does not generate a lethal oxidative stress. We next tested 50 mM NaI, in which all single mutants failed to survive (Table 4). Interestingly, bli-3(lf); skn-1(gf) double mutants exhibited split phenotypes: the two bli-3(e767lf); skn-1(gf) double mutants failed to survive in 50 mM NaI, while the two bli-3(mac40lf); skn-1(gf) double mutants could survive. Different from bli-3(lf); skn-1(gf), all four bli-3(lf) wdr-23(lf) double mutants we initially generated failed to survive (Table 4, First group). Therefore, skn-1(gf) and wdr-23(lf) can interact with bli-3(mac40lf) differentially.
To examine whether other wdr-23 alleles might interact with bli-3(mac40lf) in a similar manner, we generated new bli-3(mac40lf) wdr-23(lf) double mutants that include four more wdr-23 missense or nonsense mutations (Table S2). All these bli-3(mac40lf) wdr-23(lf) double mutants also failed to survive in 50 mM NaI (Table 4, Second group).
In all double mutants, only bli-3(mac40lf) wdr-23(mac42lf) could not survive in 10 mM NaI. We speculate that an unknown defect(s) in the double mutants that is not derived from oxidative stress contributes to the inviability, since single mutants of either mutation could survive in 10 mM NaI.
skn-1(gf) and wdr-23(lf) interact with bli-3 differentially to affect the cuticle integrity
A critical function of the BLI-3 dual oxidase is to catalyze the crosslinking of tyrosyl residues of the cuticle collagens. The process involves the BLI-3 NADPH oxidase domain, the BLI-3 peroxidase domain and the peroxidase MLT-7 (Edens et al. 2001; Meitzler and Ortiz De Montellano 2009; Thein et al. 2009; Meitzler et al. 2010; Moribe and Mekada 2013). To examine whether skn-1 or wdr-23 interacts with bli-3 to affect cuticle formation, we tested the cuticle integrity of mutants by staining with the fluorescent nuclear dye Hoechst 33258 (Thein et al. 2009; Xu et al. 2014).
An examination of bli-3 single mutants suggests that the peroxidase domain and the oxidase domain affect cuticle integrity differentially: the peroxidase domain mutations (mac52 and e767) resulted in cuticles more defective than the oxidase domain mutations did (mac68, mac66, mac40) (Figure 3A). None of the skn-1(gf) or wdr-23(lf) single mutants has apparently defective cuticles (Figure 3A), suggesting that these two genes are not directly involved in cuticle formation.
Figure 3.
Cuticle integrity of single and double mutants based on Hoechst 33258 staining. (A) Percentage of Hoechst-positive single mutants. (B) Percentage of Hoechst-positive double mutants compared to single mutants. Results are from four biological replicates (n = 100 for each). Comparisons were made with WT or between genotypes. Error bar: Mean ± SE. Statistics: Bonferroni test with one-way ANOVA. *: P < 0.05; **: P < 0.01; ***: P < 0.001.
An examination of double mutants suggests that skn-1(gf) mutations do not apparently alter the cuticle defects caused by mutations affecting either the peroxidase domain or oxidase domain of BLI-3 (Figure 3B). Surprisingly, wdr-23(lf) mutations affect the cuticles in a bli-3 allele-specific manner: they uniformly and strongly suppress the cuticle defects of the bli-3(mac40lf) (oxidase domain) mutants but not that of bli-3(e767lf) (peroxidase domain) mutants (Figure 3B).
skn-1(gf) and wdr-23(lf) similarly affect the expression of most, but not all target genes
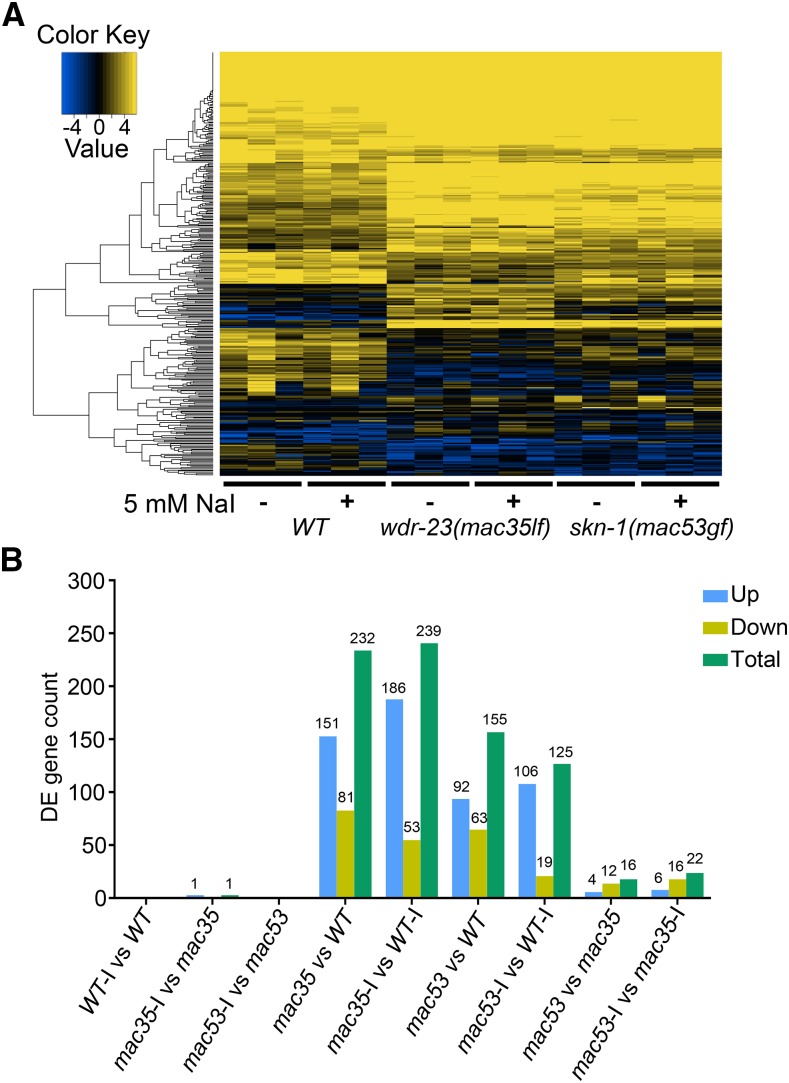
To understand what downstream genes of skn-1 and wdr-23 might be involved in promoting animal survival in excess iodide, we performed RNA-Seq on synchronized wild-type, skn-1(mac53gf) and wdr-23(mac35lf) larvae grown with or without excess iodide and analyzed their transcriptomes.
We found that animals of the same genotype exhibit largely similar gene expression profiles with or without excess iodide, as shown in the gene expression heat map (Figure 4A) and the numbers of differentially expressed genes (DEGs) (Figure 4B). The gene expression profiles of skn-1(mac53gf) and wdr-23(mac35lf) mutants are apparently different from that of wild type, while a subtle but visible difference is also seen between them (Figure 4A). Fewer genes are altered in skn-1(mac53gf) mutants compared to wdr-23(mac35lf) mutants (Figure 4B). Direct comparison of skn-1(mac53gf) and wdr-23(mac35lf) identified 16 (without iodide) and 22 (with iodide) DEGs (Figure 4B).
Figure 4.
Transcriptome analyses of DEGs in wild-type and mutant animals grown with or without excess iodide. (A) Heat map of differentially expressed genes (DEGs). Results are from three biological replicates. (B) Numbers of DEGs from pairwise comparisons. Iodide treatment is indicated as “-I”.
Gene ontology (GO) analyses revealed that many of the DEGs in skn-1 and/or wdr-23 mutants belong to signaling pathways that regulate metabolism and defense response, with glutathione metabolic process being the top pathway in each mutant (Fig. S2 and S3). Cellular process, metabolic process and single-organism process are the top three GO subterms that contain the highest percentage of DEGs in each mutant (Fig. S4).
A few GO subterms are differentially affected between wdr-23(mac35lf) and skn-1(mac53gf) mutants. For example, innate immune response is one biological process that is significantly affected by wdr-23(mac35lf) but not by skn-1(mac53gf) (Fig. S3A). Glutathione and alkyl or aryl transferase activities are the two molecular functions that are significantly different between skn-1(mac53gf) and wdr-23(mac35lf) mutants (Fig. S3B).
skn-1(gf) and wdr-23(lf) can affect gene expression differentially
We compared the DEGs in each mutant (Fig. S5). The list of shared genes indicates that gst and ugt genes are the most represented antioxidant gene categories among the up-regulated groups (Result S1). Interestingly, wdr-23 is significantly up-regulated in each mutant, suggesting an autoregulation of wdr-23 expression by the SKN-1/WDR-23 pathway (Result S1).
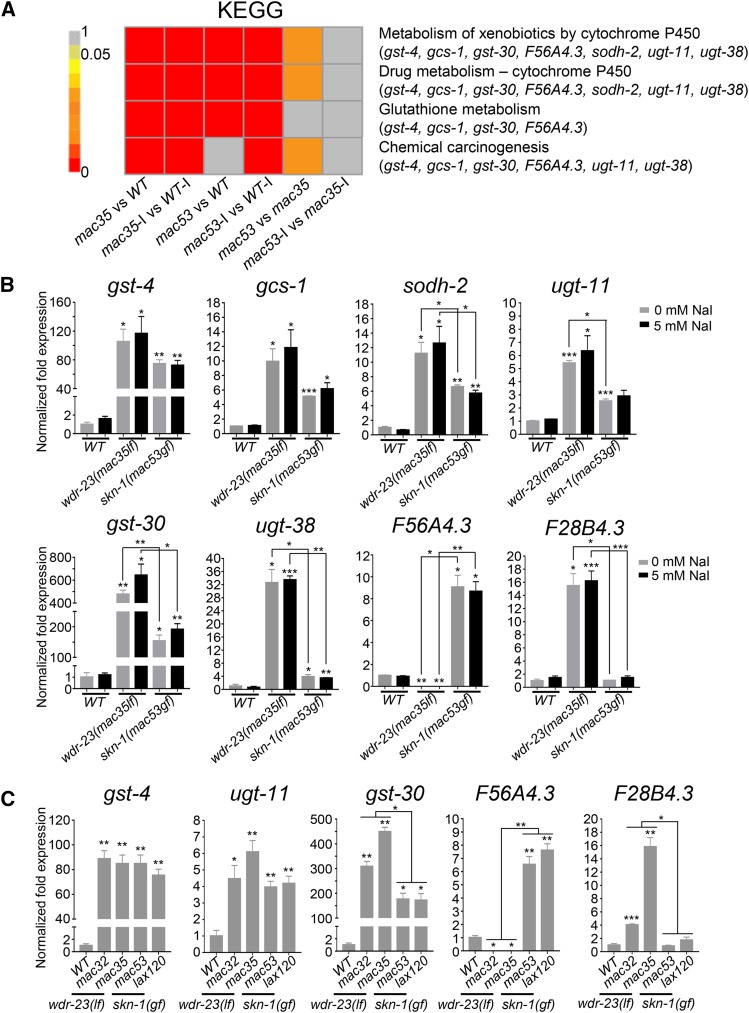
Compared to wild type, the top four KEGG pathways affected by skn-1(mac53gf) and wdr-23(mac35lf) include glutathione metabolism, cytochrome P450-related drug and xenobiotics metabolisms, and chemical carcinogenesis (Figure 5A). We selected seven genes with the highest differential expression in these four comparisons to verify their expression by qRT-PCR. The increased expression of the first six genes (gst-4, gcs-1, sodh-2, ugt-11, gst-30 and ugt-38) is verified in both skn-1(mac53gf) and wdr-23(mac35lf) mutants (Figure 5B). Compared to wdr-23(mac35lf), the expression of sodh-2, ugt-11, gst-30 and ugt-38 is less increased in skn-1(mac53gf) mutants (Figure 5B).
Figure 5.
qRT-PCR verification of altered gene expression. (A) Top four pathways and seven candidate genes that are altered in both wdr-23(lf) and skn-1(gf) mutants based on KEGG analysis. Among the candidate genes, gst-30,F56A4.3 and ugt-38 are also differentially expressed between skn-1(mac53gf) and wdr-23(mac35lf) based on RNA-Seq. (B) qRT-PCR examination of the eight skn-1/wdr-23 target genes in different genotypes treated with or without excess iodide. (C) qRT-PCR examination of the expression of five skn-1/wdr-23 target genes in independent mutants of skn-1 or wdr-23. Results are from three biological replicates. Reference gene: tba-1. Comparisons were made with wild type or between genotypes. Error bars: Mean ± SE. Statistics: two-tailed unpaired Student’s t-test. *: P < 0.05; **: P < 0.01; ***: P < 0.001.
The seventh gene, F56A4.3, exhibits a completely different expression: its expression is significantly increased in skn-1(mac53gf) mutants but essentially abolished in wdr-23(mac35lf) mutants (Figure 5B). F56A4.3 encodes a glutathione S-transferase with unknown function (www.wormbase.org).
We examined whether the expression of other genes is also differentially affected by skn-1(gf) and wdr-23(lf). An examination of DEGs between skn-1(gf) and wdr-23(lf) mutants (Result S2) identified F28B4.3: qRT-PCR indicates that its expression is significantly increased in wdr-23(mac35lf) but remains unaltered in skn-1(mac53gf) mutants (Figure 5B).
To determine whether the differential effects on gene expression by skn-1(mac53gf) and wdr-23(mac35lf) are allele-specific, we examined the expression of a subset of these genes in skn-1(lax120gf) and wdr-23(mac32lf) mutants (Figure 5C). The expression of these genes exhibits similar patterns in the two independent mutants of skn-1 or wdr-23 (Figure 5C), suggesting that skn-1(gf) and wdr-23(lf) can affect the expression of a subset of genes, e.g., F56A4.3 and F28B4.3, by a mechanism different from the one used by their shared gst and ugt target genes.
Discussion
In this study, we isolated multiple mutations affecting C. elegans skn-1, wdr-23 and the bli-3/tsp-15/doxa-1 complex by screening for surviving mutants in excess iodide. We suggest that WDR-23 and SKN-1 interact with the BLI-3/TSP-15/DOXA-1 complex to regulate animal’s response to oxidative stress. We also suggest that WDR-23 loss of function can affect BLI-3 activity and some gene expression independent of SKN-1 activation.
SKN-1/WDR-23 and BLI-3/TSP-15/DOXA-1 affect C. elegans survival in excess iodide by a conserved mechanism
SKN-1 and BLI-3 are involved in C. elegans response to pathogens (Hoeven et al. 2011; Tang and Pang 2016), oxidative stress (Ewald et al. 2017), manganese toxicity (Benedetto et al. 2010) and ROS-related lifespan extension (Sasakura et al. 2017). The BLI-3/TSP-15/DOXA-1 complex is also required for the formation of C. elegans cuticles by generating H2O2, which is utilized by the BLI-3 peroxidase domain (Edens et al. 2001) and the peroxidase MLT-7 for crosslinking cuticle proteins (Thein et al. 2009). The concurrent involvement of SKN-1 and BLI-3 in multiple cellular processes suggests functional crosstalk between these two molecules and/or the pathways. It is unclear whether and how the oxidase and peroxidase activities of BLI-3, the activation of SKN-1, and the ROS production are coordinated in vivo.
Our screening for recessive and dominant mutations that can promote animal survival in excess iodide identified lf mutations in the bli-3/tsp-15/doxa-1 complex and wdr-23 and gf mutations in skn-1. It is plausible that the reduced ROS generation in bli-3/tsp-15/doxa-1 lf mutants and the activation of antioxidant gene expression in wdr-23(lf) or skn-1(gf) mutants would attenuate the oxidative stress caused by excess iodide, a strong inducer of ROS in C. elegans and mammals (Many et al. 1992; Golstein and Dumont 1996; Corvilain et al. 2000; Vitale et al. 2000; Yao et al. 2012; Serrano-Nascimento et al. 2014; Xu et al. 2014). Consistent with this hypothesis, we found that the antioxidants ascorbic acid (vitamin C) and N-acetylcysteine (NAC) can antagonize the toxic effect of excess iodide (Table S6).
Recent studies found that excess iodide could increase Nrf2 expression in rat thyroid (Wang et al. 2017) and activate the Nrf2 pathway in human skin cells (Ben-Yehuda Greenwald et al. 2017). Therefore, it is a conserved mechanism that the BLI-3/TSP-15/DOXA-1 dual oxidase complex and the Nrf2/SKN-1 pathway are both involved in the response to oxidative stress induced by excess iodide.
SKN-1/WDR-23 and BLI-3/TSP-15/DOXA-1 likely function in the hypodermis to affect C. elegans survival in excess iodide
In C. elegans, skn-1 and wdr-23 are expressed in intestine, hypodermis and other tissues (An and Blackwell 2003; Hasegawa et al. 2008; Choe et al. 2009; Hasegawa and Miwa 2010; Paek et al. 2012; Wu et al. 2016) and the anti-stress functions of SKN-1C have been primarily associated with its intestinal expression (Blackwell et al. 2015). bli-3 is also expressed in intestine and hypodermis (Edens et al. 2001; Van Der Hoeven et al. 2015), while tsp-15 appears to be expressed primarily in hypodermis (Moribe et al. 2004). We could phenocopy the survival-promoting effect of skn-1c(gf) mutation or rescue that of tsp-15(lf) mutation by hypodermis-specific expression of skn-1c(gf) or tsp-15(wt) transgenes, respectively (Tables 2 and S3), suggesting that SKN-1/WDR-23 and BLI-3/TSP-15/DOXA-1 function in the hypodermis to affect the oxidative stress effect of excess iodide.
How C. elegans takes in iodide is unknown. In mammals, iodide uptake is mediated by Na(+)/I(-) symporter (NIS), an integral plasma membrane glycoprotein expressed in multiple tissues including thyroid, the lacrimal sac and nasolacrimal duct, salivary glands, choroid plexus, stomach, intestine, lactating breast, kidney, placenta and ovary (Ravera et al. 2017). We previously found that RNAi targeting two C. elegans genes similar to NIS did not apparently affect animal survival in excess iodide (Xu et al. 2014). It is possible that iodide is absorbed by the intestine and then transported to the hypodermis in C. elegans. Alternatively, iodide might gain access to the hypodermis directly via microscopic openings on the cuticle. The detailed mechanism remains to be understood.
SKN-1C is the primary SKN-1 isoform responsible for promoting animal survival in excess iodide
The SKN-1C isoform normally resides in the cytoplasm and enters the nucleus in response to stress signals (An and Blackwell 2003; Blackwell et al. 2015). The SKN-1A isoform might be associated with mitochondria (Paek et al. 2012) to mediate starvation response. It is also associated with ER (Lehrbach and Ruvkun 2016) to respond to proteasome dysfunction signals. The skn-1 gf mutations we identified affect both A and C isoforms (Fig. S1). Our transgene experiments suggest that SKN-1C is the major isoform that promotes animal survival, while SKN-1A might play a minor role. Hypodermic overexpression of SKN-1C(gf) might be highly toxic, which explains why we failed to obtain any stable transgenic lines using the skn-1c promoter or the hypodermis-specific dpy-7 promoter and suggests that a highly regulated SKN-1C activity in hypodermis is essential for development and survival.
In WormBase (www.wormbase.org), five WDR-23 isoforms are annotated. The mutations we isolated affect all wdr-23 isoforms (Figure 1D and Fig. S6). Previous studies found that the WDR-23A isoform is associated with outer mitochondrial membranes, while the WDR-23B isoform is localized exclusively in the nucleus (Staab et al. 2013; Staab et al. 2014). We found that wdr-23a and wdr-23b transgenes can strongly rescue the phenotype of wdr-23(lf) mutants with a similar efficiency (Table S3), which is consistent with the previous finding that a functional difference of the two WDR-23 isoforms was not detected in transgenic experiments (Staab et al. 2013).
The peroxidase domain and oxidase domain of BLI-3 are functionally divergent
BLI-3 is the only functional dual oxidase in C. elegans that contains an N-terminal peroxidase domain and a C-terminal oxidase domain (Edens et al. 2001; Donko et al. 2005; Bedard et al. 2007). It is also the only NADPH oxidase in C. elegans (Bedard et al. 2007). Studies of missense mutations affecting the peroxidase domain or the oxidase domain of BLI-3 found that peroxidase mutations do not or only weakly affect infection-induced H2O2 production, while an oxidase mutation (Moribe et al. 2012) has an apparently stronger effect (Chavez et al. 2009; Van Der Hoeven et al. 2015). The oxidase domain mutation could also reduce the lifespan and make C. elegans more susceptible to pathogens, while the peroxidase mutations do not or only weakly do so (Chavez et al. 2009; Van Der Hoeven et al. 2015).
In our study, BLI-3 peroxidase domain mutations impair the cuticle integrity more severely than oxidase mutations do (Figure 3A), suggesting a functional difference of these two domains that is consistent with previous findings (Chavez et al. 2009; Van Der Hoeven et al. 2015). We previously found that ROS overproduction caused by excess iodide in C. elegans can be partially suppressed by both the bli-3(e767lf) (peroxidase) and bli-3(mac40lf) (oxidase) mutations (Xu et al. 2014), suggesting that BLI-3 peroxidase domain mutations also impact the oxidase domain. Therefore, the peroxidase domain [that consumes ROS to crosslink tyrosyl residues of collagens] and the oxidase domain [that generates ROS] likely interact and also function differentially to affect cuticle formation, ROS generation and the response to oxidative stress or pathogens.
WDR-23 might carry out a function independent of SKN-1 activation
wdr-23(lf) and skn-1(gf) interact with bli-3(lf) differentially. First, only bli-3(mac40lf); skn-1(gf) double mutants could survive in 50 mM NaI, while other double mutants, including all bli-3(lf) wdr-23(lf) and bli-3(e767lf); skn-1(gf) mutants, could not (Table 4). Second, wdr-23(lf) mutations could strongly suppress the cuticle defects of only bli-3(mac40lf) mutants but not that of bli-3(e767lf) mutants, while skn-1(gf) mutations do not suppress the cuticle defects of either bli-3(lf) mutants (Figure 3). These findings imply that WDR-23 might suppress BLI-3 activity. Since WDR-23 inhibits SKN-1 activation by promoting proteasome-mediated SKN-1 degradation (Choe et al. 2009), it is plausible that WDR-23 might inhibit BLI-3 or a protein required for BLI-3 activity by a similar mechanism. Future studies are warranted to test this hypothesis.
Our transcriptome analyses suggest that excess iodide does not apparently alter gene expression in wild-type or mutant C. elegans (Figure 4). The survival-promoting effect of skn-1(gf) and wdr-23(lf) mutations likely involve the activation of antioxidant gene expression (Fig. S2, S3, S4 and Result S1), similar to what previous studies have found (Blackwell et al. 2015). However, skn-1(gf) and wdr-23(lf) can differentially affect the expression of a subset of genes. For example, wdr-23(lf) causes higher expression of four genes that are up-regulated in both wdr-23(lf) and skn-1(gf) mutants (Figure 5B and 5C, sodh-2, ugt-11, gst-30, ugt-38). The expression of F56A4.3 is abolished in wdr-23(lf) mutants but significantly increased in skn-1(gf) mutants (Figure 5B and 5C), while the expression of F28B4.3 is significantly increased in wdr-23(lf) mutants but unaltered in skn-1(gf) mutants (Figure 5B and 5C). These differences are not allele-specific for wdr-23 or skn-1, suggesting a potentially novel mechanism underlying these effects of WDR-23 loss of function and SKN-1 gain of function. The biological significance of this differentiation remains unclear.
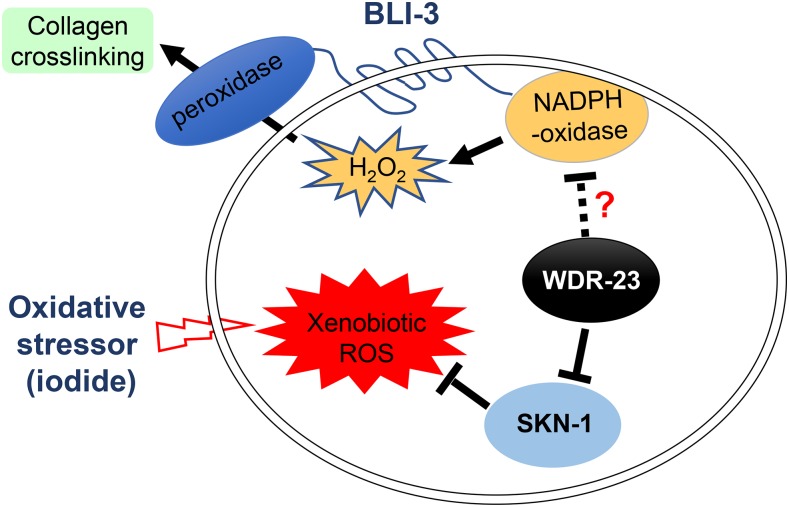
We generated a working model to describe the known and potential interactions among SKN-1, WDR-23 and BLI-3 (Figure 6). In this model, the H2O2 generated by BLI-3 for crosslinking cuticle collagens also contributes to the oxidative stress caused by xenobiotic stressors such as iodide. Besides its canonical role as a SKN-1 negative regulator, WDR-23 might also suppress BLI-3 activity, a possibility supported by the genetic interaction between wdr-23(lf) and bli-3(lf) (Figure 3 and Table 4).
Figure 6.
A working model describing the known and potential interactions among SKN-1, WDR-23 and BLI-3. In this model, BLI-3 contributes to oxidative stress by generating H2O2 used for crosslinking cuticle collagens. Besides inhibiting the activation of SKN-1, WDR-23 might also suppress BLI-3 by an unknown mechanism.
In summary, we found that the oxidative stress triggered by excess iodide can be suppressed by defects in the BLI-3/TSP-15/DOXA-1 complex or the activation of SKN-1 either by skn-1(gf) or wdr-23(lf) mutations. We provide further genetic and molecular evidence supporting the consensus that WDR-23 can function as a negative regulator of SKN-1 in activating antioxidant gene expression and also suggest that WDR-23 could interact with BLI-3 and affect some gene expression by a mechanism(s) different from the prior one. Our findings should facilitate the understanding of animal’s response to oxidative stress. Future studies are warranted for elucidating the underlying molecular mechanism.
Acknowledgments
We thank members of the Ma laboratory for suggestions. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The study is supported by a MOST grant (2016YFC1201805) and Natural Science Foundation of China grants (No. 31371253, No. 31571045) to LM and a Fundamental Research Fund for the Central Universities of Central South University (2015zzts093) to ZX. ZX and LM designed the experiments. ZX performed the experiments with assistance of YH, YD, YC, HH, SH and QN. ZX and LM analyzed the data. ZX, QP, DM and LM wrote the manuscript. The authors declare no conflict of interest.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.6983384.
Communicating editor: S. Lee
Literature Cited
- Ahmed S. M., Luo L., Namani A., Wang X. J., Tang X., 2017. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta 1863: 585–597. 10.1016/j.bbadis.2016.11.005 [DOI] [PubMed] [Google Scholar]
- An J. H., Blackwell T. K., 2003. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 17: 1882–1893. 10.1101/gad.1107803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An J. H., Vranas K., Lucke M., Inoue H., Hisamoto N., et al. , 2005. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc. Natl. Acad. Sci. USA 102: 16275–16280. 10.1073/pnas.0508105102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagchi N., Brown T. R., Urdanivia E., Sundick R. S., 1985. Induction of autoimmune thyroiditis in chickens by dietary iodine. Science 230: 325–327. 10.1126/science.4048936 [DOI] [PubMed] [Google Scholar]
- Barancik M., Gresova L., Bartekova M., Dovinova I., 2016. Nrf2 as a key player of redox regulation in cardiovascular diseases. Physiol. Res. 65: S1–S10. [DOI] [PubMed] [Google Scholar]
- Bedard K., Lardy B., Krause K. H., 2007. NOX family NADPH oxidases: not just in mammals. Biochimie 89: 1107–1112. 10.1016/j.biochi.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Ben-Yehuda Greenwald M., Frusic-Zlotkin M., Soroka Y., Ben-Sasson S., Bianco-Peled H., et al. , 2017. A novel role of topical iodine in skin: Activation of the Nrf2 pathway. Free Radic. Biol. Med. 104: 238–248. 10.1016/j.freeradbiomed.2017.01.011 [DOI] [PubMed] [Google Scholar]
- Benedetto A., Au C., Avila D. S., Milatovic D., Aschner M., 2010. Extracellular dopamine potentiates mn-induced oxidative stress, lifespan reduction, and dopaminergic neurodegeneration in a BLI-3-dependent manner in Caenorhabditis elegans. PLoS Genet. 6: e1001084 10.1371/journal.pgen.1001084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop N. A., Guarente L., 2007. Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447: 545–549. 10.1038/nature05904 [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Bowerman B., Priess J. R., Weintraub H., 1994. Formation of a monomeric DNA binding domain by Skn-1 bZIP and homeodomain elements. Science 266: 621–628. 10.1126/science.7939715 [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Steinbaugh M. J., Hourihan J. M., Ewald C. Y., Isik M., 2015. SKN-1/Nrf, stress responses, and aging in Caenorhabditis elegans. Free Radic. Biol. Med. 88: 290–301. 10.1016/j.freeradbiomed.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg M., Feldt-Rasmussen U., Andersen K. K., Kjaer S. K., 2012. Thyroid cancer in Denmark 1943–2008, before and after iodine supplementation. Int. J. Cancer 131: 2360–2366. 10.1002/ijc.27497 [DOI] [PubMed] [Google Scholar]
- Bowerman B., Draper B. W., Mello C. C., Priess J. R., 1993. The maternal gene skn-1 encodes a protein that is distributed unequally in early C. elegans embryos. Cell 74: 443–452. 10.1016/0092-8674(93)80046-H [DOI] [PubMed] [Google Scholar]
- Bowerman B., Eaton B. A., Priess J. R., 1992. skn-1, a maternally expressed gene required to specify the fate of ventral blastomeres in the early C. elegans embryo. Cell 68: 1061–1075. 10.1016/0092-8674(92)90078-Q [DOI] [PubMed] [Google Scholar]
- Chavez V., Mohri-Shiomi A., Garsin D. A., 2009. Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect. Immun. 77: 4983–4989. 10.1128/IAI.00627-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe K. P., Przybysz A. J., Strange K., 2009. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol. Cell. Biol. 29: 2704–2715. 10.1128/MCB.01811-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvilain B., Collyn L., van Sande J., Dumont J. E., 2000. Stimulation by iodide of H(2)O(2) generation in thyroid slices from several species. Am. J. Physiol. Endocrinol. Metab. 278: E692–E699. 10.1152/ajpendo.2000.278.4.E692 [DOI] [PubMed] [Google Scholar]
- Dodd W., Tang L., Lone J. C., Wimberly K., Wu C. W., et al. , 2018. A Damage Sensor Associated with the Cuticle Coordinates Three Core Environmental Stress Responses in Caenorhabditis elegans. Genetics 208: 1467–1482. 10.1534/genetics.118.300827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W., Zhang H., Zhang P., Li X., He L., et al. , 2013. The changing incidence of thyroid carcinoma in Shenyang, China before and after universal salt iodization. Med. Sci. Monit. 19: 49–53. 10.12659/MSM.883736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donko A., Peterfi Z., Sum A., Leto T., Geiszt M., 2005. Dual oxidases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360: 2301–2308. 10.1098/rstb.2005.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edens W. A., Sharling L., Cheng G., Shapira R., Kinkade J. M., et al. , 2001. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154: 879–891. 10.1083/jcb.200103132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan C. R., Chung M. A., Allen F. L., Heschl M. F., Van Buskirk C. L., et al. , 1995. A gut-to-pharynx/tail switch in embryonic expression of the Caenorhabditis elegans ges-1 gene centers on two GATA sequences. Dev. Biol. 170: 397–419. 10.1006/dbio.1995.1225 [DOI] [PubMed] [Google Scholar]
- Ewald C. Y., Hourihan J. M., Bland M. S., Obieglo C., Katic I., et al. , 2017. NADPH oxidase-mediated redox signaling promotes oxidative stress resistance and longevity through memo-1 in C. elegans. eLife 6: e19493 10.7554/eLife.19493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald C. Y., Landis J. N., Porter Abate J., Murphy C. T., Blackwell T. K., 2015. Dauer-independent insulin/IGF-1-signalling implicates collagen remodelling in longevity. Nature 519: 97–101. 10.1038/nature14021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleard J. S., Barry J. D., Johnstone I. L., 1997. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol. Cell. Biol. 17: 2301–2311. 10.1128/MCB.17.4.2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover-Cutter K. M., Lin S., Blackwell T. K., 2013. Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet. 9: e1003701 10.1371/journal.pgen.1003701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golstein J., Dumont J. E., 1996. Cytotoxic effects of iodide on thyroid cells: difference between rat thyroid FRTL-5 cell and primary dog thyrocyte responsiveness. J. Endocrinol. Invest. 19: 119–126. 10.1007/BF03349847 [DOI] [PubMed] [Google Scholar]
- Guan H., Ji M., Bao R., Yu H., Wang Y., et al. , 2009. Association of high iodine intake with the T1799A BRAF mutation in papillary thyroid cancer. J. Clin. Endocrinol. Metab. 94: 1612–1617. 10.1210/jc.2008-2390 [DOI] [PubMed] [Google Scholar]
- Hasegawa K., Miwa J., 2010. Genetic and cellular characterization of Caenorhabditis elegans mutants abnormal in the regulation of many phase II enzymes. PLoS One 5: e11194 10.1371/journal.pone.0011194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K., Miwa S., Isomura K., Tsutsumiuchi K., Taniguchi H., et al. , 2008. Acrylamide-responsive genes in the nematode Caenorhabditis elegans. Toxicol. Sci. 101: 215–225. 10.1093/toxsci/kfm276 [DOI] [PubMed] [Google Scholar]
- Hoeven R. v., McCallum K. C., Cruz M. R., Garsin D. A., 2011. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 7: e1002453 10.1371/journal.ppat.1002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hourihan J. M., Moronetti Mazzeo L. E., Fernandez-Cardenas L. P., Blackwell T. K., 2016. Cysteine Sulfenylation Directs IRE-1 to Activate the SKN-1/Nrf2 Antioxidant Response. Mol. Cell 63: 553–566. 10.1016/j.molcel.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Hisamoto N., An J. H., Oliveira R. P., Nishida E., et al. , 2005. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 19: 2278–2283. 10.1101/gad.1324805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Chiba T., Takahashi S., Ishii T., Igarashi K., et al. , 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236: 313–322. 10.1006/bbrc.1997.6943 [DOI] [PubMed] [Google Scholar]
- Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., et al. , 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13: 76–86. 10.1101/gad.13.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Ye P., Matsumiya T., Tanji K., Ozaki T., 2015. Emerging functional cross-talk between the Keap1-Nrf2 system and mitochondria. J. Clin. Biochem. Nutr. 56: 91–97. 10.3164/jcbn.14-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Johnson J. A., 2015. Nrf2–a therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 88: 253–267. 10.1016/j.freeradbiomed.2015.07.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath R. S., Fraser A. G., Dong Y., Poulin G., Durbin R., et al. , 2003. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421: 231–237. 10.1038/nature01278 [DOI] [PubMed] [Google Scholar]
- Kell A., Ventura N., Kahn N., Johnson T. E., 2007. Activation of SKN-1 by novel kinases in Caenorhabditis elegans. Free Radic. Biol. Med. 43: 1560–1566. 10.1016/j.freeradbiomed.2007.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler T. W., Wakabayashi N., Biswal S., 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47: 89–116. 10.1146/annurev.pharmtox.46.120604.141046 [DOI] [PubMed] [Google Scholar]
- Lehrbach N. J., Ruvkun G., 2016. Proteasome dysfunction triggers activation of SKN-1A/Nrf1 by the aspartic protease DDI-1. eLife 5: e17721 10.7554/eLife.17721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Matilainen O., Jin C., Glover-Cutter K. M., Holmberg C. I., et al. , 2011. Specific SKN-1/Nrf stress responses to perturbations in translation elongation and proteasome activity. PLoS Genet. 7: e1002119 10.1371/journal.pgen.1002119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind P., Langsteger W., Molnar M., Gallowitsch H. J., Mikosch P., et al. , 1998. Epidemiology of thyroid diseases in iodine sufficiency. Thyroid 8: 1179–1183. 10.1089/thy.1998.8.1179 [DOI] [PubMed] [Google Scholar]
- Link C. D., Johnson C. J., 2002. Reporter transgenes for study of oxidant stress in Caenorhabditis elegans. Methods Enzymol. 353: 497–505. 10.1016/S0076-6879(02)53072-X [DOI] [PubMed] [Google Scholar]
- Lo J. Y., Spatola B. N., Curran S. P., 2017. WDR23 regulates NRF2 independently of KEAP1. PLoS Genet. 13: e1006762 10.1371/journal.pgen.1006762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Many M. C., Mestdagh C., van den Hove M. F., Denef J. F., 1992. In vitro study of acute toxic effects of high iodide doses in human thyroid follicles. Endocrinology 131: 621–630. [DOI] [PubMed] [Google Scholar]
- Meitzler J. L., Brandman R., Ortiz de Montellano P. R., 2010. Perturbed heme binding is responsible for the blistering phenotype associated with mutations in the Caenorhabditis elegans dual oxidase 1 (DUOX1) peroxidase domain. J. Biol. Chem. 285: 40991–41000. 10.1074/jbc.M110.170902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzler J. L., Ortiz de Montellano P. R., 2009. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) “peroxidase” domains: insights into heme binding and catalytic activity. J. Biol. Chem. 284: 18634–18643. 10.1074/jbc.M109.013581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moribe H., Konakawa R., Koga D., Ushiki T., Nakamura K., et al. , 2012. Tetraspanin is required for generation of reactive oxygen species by the dual oxidase system in Caenorhabditis elegans. PLoS Genet. 8: e1002957 10.1371/journal.pgen.1002957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moribe H., Mekada E., 2013. Co-occurrence of tetraspanin and ROS generators: Conservation in protein cross-linking and other developmental processes. Worm 2: e23415 10.4161/worm.23415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moribe H., Yochem J., Yamada H., Tabuse Y., Fujimoto T., et al. , 2004. Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J. Cell Sci. 117: 5209–5220. 10.1242/jcs.01403 [DOI] [PubMed] [Google Scholar]
- Nehrke K., 2003. A reduction in intestinal cell pHi due to loss of the Caenorhabditis elegans Na+/H+ exchanger NHX-2 increases life span. J. Biol. Chem. 278: 44657–44666. 10.1074/jbc.M307351200 [DOI] [PubMed] [Google Scholar]
- Nussey S., Whitehead S., 2001. Endocrinology: An Integrated Approach. BIOS Scientific Publishers, Oxford. [PubMed] [Google Scholar]
- Okkema P. G., Harrison S. W., Plunger V., Aryana A., Fire A., 1993. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics 135: 385–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek J., Lo J. Y., Narasimhan S. D., Nguyen T. N., Glover-Cutter K., et al. , 2012. Mitochondrial SKN-1/Nrf mediates a conserved starvation response. Cell Metab. 16: 526–537. 10.1016/j.cmet.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palikaras K., Lionaki E., Tavernarakis N., 2015. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 521: 525–528. 10.1038/nature14300 [DOI] [PubMed] [Google Scholar]
- Papp D., Csermely P., Soti C., 2012. A role for SKN-1/Nrf in pathogen resistance and immunosenescence in Caenorhabditis elegans. PLoS Pathog. 8: e1002673 10.1371/journal.ppat.1002673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravera S., Reyna-Neyra A., Ferrandino G., Amzel L. M., Carrasco N., 2017. The Sodium/Iodide Symporter (NIS): Molecular Physiology and Preclinical and Clinical Applications. Annu. Rev. Physiol. 79: 261–289. 10.1146/annurev-physiol-022516-034125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robida-Stubbs S., Glover-Cutter K., Lamming D. W., Mizunuma M., Narasimhan S. D., et al. , 2012. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 15: 713–724. 10.1016/j.cmet.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Rasooly L., Saboori A. M., Burek C. L., 1999. Linking iodine with autoimmune thyroiditis. Environ. Health Perspect. 107: 749–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N. R., Saboori A. M., Rasooly L., Burek C. L., 1997. The role of iodine in autoimmune thyroiditis. Crit. Rev. Immunol. 17: 511–517. [PubMed] [Google Scholar]
- Ruf V., Holzem C., Peyman T., Walz G., Blackwell T. K., et al. , 2013. TORC2 signaling antagonizes SKN-1 to induce C. elegans mesendodermal embryonic development. Dev. Biol. 384: 214–227. 10.1016/j.ydbio.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakura H., Moribe H., Nakano M., Ikemoto K., Takeuchi K., et al. , 2017. Lifespan extension by peroxidase and dual oxidase-mediated ROS signaling through pyrroloquinoline quinone in C. elegans. J. Cell Sci. 130: 2631–2643. 10.1242/jcs.202119 [DOI] [PubMed] [Google Scholar]
- Serrano-Nascimento C., da Silva Teixeira S., Nicola J. P., Nachbar R. T., Masini-Repiso A. M., et al. , 2014. The acute inhibitory effect of iodide excess on sodium/iodide symporter expression and activity involves the PI3K/Akt signaling pathway. Endocrinology 155: 1145–1156. 10.1210/en.2013-1665 [DOI] [PubMed] [Google Scholar]
- Shore D. E., Ruvkun G., 2013. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 23: 409–420. 10.1016/j.tcb.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab T. A., Evgrafov O., Knowles J. A., Sieburth D., 2014. Regulation of synaptic nlg-1/neuroligin abundance by the skn-1/Nrf stress response pathway protects against oxidative stress. PLoS Genet. 10: e1004100 10.1371/journal.pgen.1004100 Erratum in PLoS Genet. 2014 Apr; 10(4): e1004361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab T. A., Griffen T. C., Corcoran C., Evgrafov O., Knowles J. A., et al. , 2013. The conserved SKN-1/Nrf2 stress response pathway regulates synaptic function in Caenorhabditis elegans. PLoS Genet. 9: e1003354 10.1371/journal.pgen.1003354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbaugh M. J., Narasimhan S. D., Robida-Stubbs S., Moronetti Mazzeo L. E., Dreyfuss J. M., et al. , 2015. Lipid-mediated regulation of SKN-1/Nrf in response to germ cell absence. eLife 4: e07836 10.7554/eLife.07836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Yamamoto M., 2017. The KEAP1–NRF2 System in Cancer. Front. Oncol. 7: 85 10.3389/fonc.2017.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Pang S., 2016. Proline catabolism modulates innate immunity in Caenorhabditis elegans. Cell Reports 17: 2837–2844. 10.1016/j.celrep.2016.11.038 [DOI] [PubMed] [Google Scholar]
- Tang L., Choe K. P., 2015. Characterization of skn-1/wdr-23 phenotypes in Caenorhabditis elegans; pleiotrophy, aging, glutathione, and interactions with other longevity pathways. Mech. Ageing Dev. 149: 88–98. 10.1016/j.mad.2015.06.001 [DOI] [PubMed] [Google Scholar]
- Teng W., Shan Z., Teng X., Guan H., Li Y., et al. , 2006. Effect of iodine intake on thyroid diseases in China. N. Engl. J. Med. 354: 2783–2793. 10.1056/NEJMoa054022 [DOI] [PubMed] [Google Scholar]
- Thein M. C., Winter A. D., Stepek G., McCormack G., Stapleton G., et al. , 2009. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J. Biol. Chem. 284: 17549–17563. 10.1074/jbc.M900831200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L., Court D. L., Fire A., 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263: 103–112. 10.1016/S0378-1119(00)00579-5 [DOI] [PubMed] [Google Scholar]
- Tullet J. M., Hertweck M., An J. H., Baker J., Hwang J. Y., et al. , 2008. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132: 1025–1038. 10.1016/j.cell.2008.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven R., Cruz M. R., Chavez V., Garsin D. A., 2015. Localization of the dual oxidase BLI-3 and characterization of its NADPH oxidase domain during infection of Caenorhabditis elegans. PLoS One 10: e0124091 10.1371/journal.pone.0124091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven R., McCallum K. C., Garsin D. A., 2012. Speculations on the activation of ROS generation in C. elegans innate immune signaling. Worm 1: 160–163. 10.4161/worm.19767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale M., Di Matola T., D’Ascoli F., Salzano S., Bogazzi F., et al. , 2000. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 141: 598–605. 10.1210/endo.141.2.7291 [DOI] [PubMed] [Google Scholar]
- Walker A. K., See R., Batchelder C., Kophengnavong T., Gronniger J. T., et al. , 2000. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap’n’Collar-related basic leucine zipper proteins. J. Biol. Chem. 275: 22166–22171. 10.1074/jbc.M001746200 [DOI] [PubMed] [Google Scholar]
- Wang J., Robida-Stubbs S., Tullet J. M., Rual J. F., Vidal M., et al. , 2010. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 6: e1001048 10.1371/journal.pgen.1001048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liang X., Abeysekera I. R., Iqbal U., Duan Q., et al. , 2017. Activation of the Nrf2-Keap 1 Pathway in Short-Term Iodide Excess in Thyroid in Rats. Oxid. Med. Cell. Longev. 2017: 4383652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Deonarine A., Przybysz A., Strange K., Choe K. P., 2016. The Skp1 Homologs SKR-1/2 Are Required for the Caenorhabditis elegans SKN-1 Antioxidant/Detoxification Response Independently of p38 MAPK. PLoS Genet. 12: e1006361 10.1371/journal.pgen.1006361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Luo J., Li Y., Ma L., 2014. The BLI-3/TSP-15/DOXA-1 dual oxidase complex is required for iodide toxicity in Caenorhabditis elegans. G3 (Bethesda) 5: 195–203. 10.1534/g3.114.015982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X., Li M., He J., Zhang G., Wang M., et al. , 2012. Effect of early acute high concentrations of iodide exposure on mitochondrial superoxide production in FRTL cells. Free Radic. Biol. Med. 52: 1343–1352. 10.1016/j.freeradbiomed.2012.02.002 [DOI] [PubMed] [Google Scholar]
- Zarse K., Schmeisser S., Groth M., Priebe S., Beuster G., et al. , 2012. Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 15: 451–465. 10.1016/j.cmet.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou C. G., Tu Q., Niu J., Ji X. L., Zhang K. Q., 2013. The DAF-16/FOXO transcription factor functions as a regulator of epidermal innate immunity. PLoS Pathog. 9: e1003660 10.1371/journal.ppat.1003660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Gene expression data are available at GEO with the accession number: GSE117222. Supplemental material available at Figshare: https://doi.org/10.25387/g3.6983384.