Abstract
The formation and recall of long-term memory (LTM) requires neuron activity-induced gene expression. Transcriptome analysis has been used to identify genes that have altered expression after memory acquisition, however, we still have an incomplete picture of the transcriptional changes that are required for LTM formation. The complex spatial and temporal dynamics of memory formation creates significant challenges in defining memory-relevant gene expression changes. The Drosophila mushroom body (MB) is a signaling hub in the insect brain that integrates sensory information to form memories across several different experimental memory paradigms. Here, we performed transcriptome analysis in the MB at two time points after the acquisition of LTM: 1 hr and 24 hr. The MB transcriptome was compared to biologically paired whole head (WH) transcriptomes. In both, we identified more transcript level changes at 1 hr after memory acquisition (WH = 322, MB = 302) than at 24 hr (WH = 23, MB = 20). WH samples showed downregulation of developmental genes and upregulation of sensory response genes. In contrast, MB samples showed vastly different changes in transcripts involved in biological processes that are specifically related to LTM. MB-downregulated genes were highly enriched for metabolic function. MB-upregulated genes were highly enriched for known learning and memory processes, including calcium-mediated neurotransmitter release and cAMP signaling. The neuron activity inducible genes Hr38 and sr were also specifically induced in the MB. These results highlight the importance of sampling time and cell type in capturing biologically relevant transcript level changes involved in learning and memory. Our data suggests that MB cells transiently upregulate known memory-related pathways after memory acquisition and provides a critical frame of reference for further investigation into the role of MB-specific gene regulation in memory.
Keywords: long-term memory, transcriptome analysis, courtship conditioning
Learning and memory can be measured in experimental organisms by observing altered behavior in response to manipulated experiences. The duration of behavioral changes induced by different learning and memory paradigms may be transient or stable (Tully et al. 2003; Hawkins et al. 2006). While the formation of both short-term and long-term memories require similar underlying molecular mechanisms such as calcium- and cAMP-dependent signaling pathways, only long-term memory (LTM) requires gene transcription and de novo protein synthesis (Brunelli et al. 1976; Montarolo et al. 1986; Lee 2015). Many genes have been implicated in LTM formation (Bliim et al. 2016), however, we still know very little about the spatial and temporal dynamics of gene regulation that are required for LTM.
The fruit fly, Drosophila melanogaster, has been a powerful model for the discovery of genes and molecular mechanisms underlying learning and memory (Livingstone et al. 1984; Dudai et al. 1976; Lee 2015). Transcriptome analysis has been used to identify gene expression changes in flies after the acquisition of LTM (Dubnau et al. 2003; Winbush et al. 2012; Bozler et al. 2017; Crocker et al. 2016; Widmer et al. 2018). Several studies have profiled transcript levels in whole heads after memory acquisition (Dubnau et al. 2003; Winbush et al. 2012; Bozler et al. 2017), which has led to the identification of genes that are required for LTM (Dubnau et al. 2003; Bozler et al. 2017). Despite the success of these whole head studies, LTM requires only a subset of neurons that are both spatially and temporally regulated (Cognigni et al. 2018; Pavlowsky et al. 2018; Zhao et al. 2018). As such, cell-type specific analysis of different neuronal subsets involved in LTM will be required to fully elucidate the molecular mechanisms of memory (Johnson et al. 2013).
The mushroom body (MB) is a region of the fly brain that is critical for normal memory (de Belle and Heisenberg 1994; McBride et al. 1999). This synaptically dense structure appears as a pair of neuropils each consisting of ∼2000 neurons with three distinct neuronal subtypes (α/β, α’/β’, and γ) that contribute the formation of 5 distinct lobes α, α’, β, β’, and γ (Lee and Luo 1999). Intrinsic MB neurons, called Kenyon cells (KC), form a hub for integration of sensory information from over 200 olfactory projection neurons, and 20 different types of modulatory dopaminergic neurons (DANs) (Aso et al. 2014). Projection neurons relay olfactory information and synapse with dendrites of the MB neurons in the calyx (Jefferis et al. 2007). DANs synapse on different locations along the axonal MB lobes and correspond to the location of 21 types of MB output neurons (MBON), converging to create a highly structured DAN-KC-MBON compartment (Aso et al. 2014; Cognigni et al. 2018). The coincident activation of DANs and MBONs is thought to be essential in eliciting a behavioral response to conditioning (Cognigni et al. 2018). Different memory paradigms, including olfactory appetitive and aversive conditioning, are known to require distinct, specialized input and output neuron types to produce the corresponding behavioral changes (Liu et al. 2012; Jefferis et al. 2007; Cognigni et al. 2018; Kirkhart and Scott 2015; Owald et al. 2015; Perisse et al. 2016). However, the requirement for KCs is consistent across many different types of memory (de Belle and Heisenberg 1994; McBride et al. 1999). For olfactory memory, it is known that γ KCs are required for STM, α/β KCs play a role in LTM, and α’/β’ in memory consolidation (Blum et al. 2009; Tomchik and Davis 2009; Krashes et al. 2007; Trannoy et al. 2011; Yu et al. 2006). Lobe requirements for courtship conditioning, the assay used in this study, are less well understood and have predominantly been tested during STM. However, it is thought that all lobes play some role in courtship memory, with a known circuit of neurons involving the γ lobe being required for early courtship memory formation (Montague and Baker 2016; Keleman et al. 2012; Zhao et al. 2018; McBride et al. 1999).
Due to its essential role in several forms of memory, the MB is a logical starting point in the search for LTM-dependent gene expression changes. MB-specific transcriptome analysis has led to the discovery of additional genes that are important for LTM (Crocker et al. 2016; Widmer et al. 2018). Crocker et al. used patch-clamp pipets to harvest RNA from specific sets of intrinsic and extrinsic MB neurons 30 min after olfactory avoidance training, revealing a novel role for light-sensing genes in a specific set of MBONs (Crocker et al. 2016). Widmer et al. used targeted DamID (TaDa) to profile RNA polymerase II (polII) binding in intrinsic MB neurons during four 12 hr time windows after memory acquisition (Widmer et al. 2018). This study identified differential polII binding for dozens of genes in each time window. Ten novel genes that are important for LTM were identified by RNAi screening of top candidates that showed differential polII binding 12-72 hr after memory acquisition (Widmer et al. 2018). These studies illustrate the potential of MB-specific transcriptome analysis in revealing novel memory genes. However, the dynamics of gene regulatory changes that occur in the MB after memory acquisition are still not well understood. In the MB, different transcription factors and chromatin modifiers are required for olfactory memory at different temporal stages of memory formation maintenance, but the global effect of these transcription factors is not known (Hirano et al. 2016). Clearly, there is still a lot to learn about the spatial and temporal regulation of gene expression that is required for long term memory.
Courtship conditioning is a well-established learning and memory paradigm that has been commonly used to investigate the molecular mechanisms underlying memory (McBride et al. 1999; Koemans et al. 2017; Kramer et al. 2011; Keleman et al. 2007). Courtship conditioning relies on male courtship behavior being modifiable in response to sexual rejection from a mated unreceptive female (Spieth 1974; Siegel and Hall 1979). After experiencing sexual rejection males show reduced courting attempts with other pre-mated females; an effect which can persist for several days (McBride et al. 1999; Keleman et al. 2007). Courtship memory forms via an enhanced behavioral response to the pheromone cis-vaccenyl-acetate (cVA), which is deposited on females by males during prior mating attempts (Keleman et al. 2012). The MB is required for the acquisition of normal long-term courtship memory (McBride et al. 1999). While courtship conditioning has molecular properties similar to other memory paradigms (Montague and Baker 2016), it is distinct in that it manipulates a complex, naturally occurring behavior with minimal experimental interference (Ejima et al. 2007; Keleman et al. 2012; Montague and Baker 2016). This makes courtship conditioning an attractive model that takes advantage of a robust but ethological form of memory.
Here, we contribute to the emerging picture of LTM-dependent gene regulation by using INTACT (isolation of nuclei tagged in a specific cell type) (Henry et al. 2012) to profile transcript level changes in MBs at two time points after the acquisition of long-term courtship memory. We find a dynamic effect on the regulation of learning and memory genes during LTM formation in MBs. Many known learning and memory genes are transiently upregulated in MBs one-hour after memory acquisition and return to baseline levels after 24 hr. This effect is specific to MBs, as whole head transcriptome analysis did not reveal gene regulatory changes in known memory associated biological pathways. This suggests a high demand for classic learning and memory genes in MBs after the acquisition of courtship memory and highlights the importance of sampling time and cell type in the detection of biologically relevant transcript level changes underlying memory.
Materials and Methods
Fly strains and culture
All Drosophila melanogaster strains were cultured at 25° and 70% humidity on a 12:12 light-dark cycle. Cultures were raised on a standard medium (cornmeal-sucrose-yeast-agar) supplemented by the mold inhibitors methyl-paraben and propanoic acid (Koemans et al. 2017). R14H06-GAL4 flies were generated by the Janelia Farm Flylight project (Jenett et al. 2012) and obtained from Bloomington stock center (Stock #48667) and UAS-unc84::GFP flies were donated by Gilbert L. Henry (Henry et al. 2012). For courtship conditioning assays and transcriptome analysis heterozygotes were generated by crossing UAS-unc84::GFP; R14H06-GAL4 flies to P{CaryP}attP2 (Bloomington stock# 36303). The resulting progeny referred to as MB-unc84 have the genotype UAS-unc84::GFP/+;R14H06-GAL4/attP2. Females used in courtship conditioning were a mixed Canton-S/Oregon-R genetic background generated by J.M. Kramer.
Courtship conditioning and sample collection
Long-term courtship memory was induced as described (Koemans et al. 2017). Newly eclosed MB-unc84 males were collected and individually held in an isolation chamber for four to six days. Males were then trained by introducing a single pre-mated female into the isolation chamber for a period of seven hours. After training, males were separated from females and kept in isolation. Flies being used for RNA-seq analysis were collected one-hour after sexual rejection (1h-AR) and 24-hours after rejection (24h-AR). Naïve flies were also collected, and all flies were collected and flash frozen at the same time of day to avoid any gene regulatory effects due to circadian rhythm. Fly heads were isolated from the abdomen, wings, and legs by vortexing followed quickly by separation through a series of sieves. Heads were then stored at -80° for future processing by INTACT. For each day of courtship conditioning when flies were collected for transcriptome analysis, a subset of naïve and trained males were tested for LTM induction. Statistical significance of courtship suppression was evaluated using a Mann-Whitney U-test.
Isolation of nuclei tagged in a specific cell-type (INTACT)
MB specific transcriptome analysis was accomplished using a described INTACT protocol with several modifications (Henry et al. 2012). Fly heads were suspended in 1 ml of homogenization buffer (25 mM KCl, 5 mM MgCl2, 20 mM tricine, 0.15 mM spermine, 0.5 mM spermidine, 10 mM β-glycerophosphate, 0.25 mM sucrose, RNAsin Plus RNase Inhibitors (Fisher Scientific: PRN2615), 1X Halt protease inhibitors (Thermo Fisher Scientific: 78430), pH 7.8) and ground with a pestle. To disrupt the cell membrane and release nuclei into solution NP40 was added to the homogenate to an end concentration of 0.3% and the solution was Dounce homogenized 6 times using the tight pestle. The 1 ml nuclear extract was passed through a 40 μm cell strainer and a 50 μl input sample was removed. This input fraction is representative of the whole head, containing both MB-specific GFP nuclei and untagged non-MB nuclei. Input fractions were centrifuged to obtain a nuclear pellet which would later be used as a source for whole head RNA sequencing.
Antibody-bound magnetic beads were freshly prepared for each immunopurification by absorbing 1μg of anti-GFP antibody (Invitrogen: G10362) to 60 μl of Protein G Dynabeads (Invitrogen: 10004D) according to the manufacturer’s instructions. To reduce non-specific binding nuclear extracts were pre-cleared by adding 60 μl of beads with no anti-GFP antibody. GFP labeled nuclei were then immunoprecipitated using GFP bound beads for 30 min at 4° with rotation. After washing, these remaining bead-bound nuclei represented the MB-specific fraction that was directly processed for RNA-sequencing.
To investigate the specificity of this protocol we calculated the proportion of INTACT (MB) and input (WH) nuclei with GFP for three independent replicates. Nuclei were labeled with 20mM DRAQ5 (abcam: ab108410) at room temperature for 30 min. Several slides were prepared for each sample and 3 fields of view were captured for each slide using a Zeiss AxioImager Z1 microscope. The average percentage of GFP nuclei was calculated for each biological replicate by manually counting nuclei. The total number of nuclei in WH and MB samples was not significantly different (P = 0.19, Student’s t-test), but the percentage of GFP positive nuclei was drastically different (see results).
Adult brain dissection, staining and confocal microscopy
To observe the expression domain of R14H06-GAL4 in adult brains we crossed this line to UAS-mCD8-GFP (Bloomington stock # 5137) and UAS-unc84::GFP and performed confocal microscopy. Brains were dissected in PBS and fixed with 4% paraformaldehyde for 45 min at room temperature. Counterstaining was performed with nc82 primary antibodies (1:50 dilution – developmental studies hybridoma bank) and DyLight 594 secondary antibodies (1:400 dilution). Brains were mounted in Vectashield (Vector Laboratories) and imaged using a Zeiss LSM 510 duo vario confocal microscope. Confocal projections were captured with 1 μm slices and processed using Image J software (Fiji) and Adobe Photoshop (Schindelin et al. 2012).
RNA isolation and RNA-sequencing
RNA was isolated using a PicoPure RNA Isolation Kit (Invitrogen: KIT0204) for both the WH and MB fractions according to the manufacturer instructions. Sequencing libraries were prepared using the Nugen Ovation Drosophila RNA-Seq System 1-16 (Nugen: NU035032) kit according to instructions. cDNA was sheared to a target size between 200-300 bp using a Covaris S2 sonicator according to the manufacturer’s protocol. Library size was verified using the Agilent Bioanalyzer High Sensitivity DNA Kit and quantified using a Q-bit fluorometer. Libraries were sequenced on an Illumina NextSeq500 using the high output v2 75 cycle kit to a read length of 75 bp with single-end reads at London Regional Genomics Centre.
RNA-seq data analysis
Raw sequence reads were trimmed using Prinseq (version 0.20.4) quality trimming to a minimum base quality score of 30 (error probability of 1 in 1,000 base calls) (Schmieder and Edwards 2011). Trimmed reads were then aligned to the D. melanogaster genome (Ensembl release 88, dm6) using STAR (version 2.5.3a) (Dobin et al. 2013; Aken et al. 2016). To ensure mushroom body specificity of MB samples compared to WH samples, we also aligned reads to the C. elegans unc-84 gene (NC_003284.9). Only uniquely aligned reads with a maximum of four mismatches were used for downstream analysis. Gene counts were obtained using HTSeq-count (version 0.7.1) using the default union settings to generate genic regions (Anders et al. 2015). To identify differentially expressed (DE) genes we used DESeq2 (version 1.18.1) (Love et al. 2014). Cut-off requirements for a gene to be called as DE were q < 0.05 and fold change > 1.3 up or down. Genes mapped to the Y chromosome were removed from the final DE lists. Principal component analysis (PCA) was performed for all samples together, as well as WH and MB samples separately, using the plotPCA function within DESeq2 (Figure S1 A-C). To identify groups of genes with similar trends of transcriptional regulation in response to courtship conditioning we used the ‘stats’ package in R (version 3.3.3) to perform k-means clustering on log2 fold changes (Love et al. 2014; R Core Team 2016).
GO analysis
Gene ontology (GO) analysis was performed using PANTHER (version 13.1) (Ashburner et al. 2000; The Gene Ontology Consortium 2017; Mi et al. 2017). For GO analysis for biological processes of DE genes between MB and WH samples we included all terms with a P < 0.05 (Fisher Exact with FDR multiple test correction). For GO analysis for biological processes of DE genes resulting from courtship conditioning terms were declared significant if they had a p-value of < 0.05 (Binomial test with Bonferroni correction). Results are displayed in ‘hierarchical view’, which groups similar terms together under the most enriched term to remove redundancy (Mi et al. 2017). Further functional analysis of the individual genes associated with each enriched term was provided by FlyBase (Gramates et al. 2017).
Network analysis
Interaction network was generated using the GeneMANIA app in Cytoscape 3.4.0 (Montojo et al. 2014; Su et al. 2014). The network was generated using the following annotated networks: (1) physical interactions - biogrid small scale studies, (2) genetic interactions – biogrid small scale studies, and (3) predicted. No related genes were integrated into the network. Nodes were color annotated using the Cytoscape enhancedGraphics app (Morris et al. 2014). Each node was annotated based on association with relevant gene ontology terms.
Data Availability
Figure S1 contains PCA results of MB and WH RNA sequencing data. Figure S2 contains comparison of DE results between DESeq2 and NOISeq. Table S1 contains read alignment and count data. Table S2 contains DE analysis results for MB specificity. Table S3 contains GO results for DE MB enriched or depleted genes. Table S4 contains DE analysis results for MB and WH specific samples during a time course of LTM. Table S5 contains the results of k-means clustering of DE genes during LTM formation. Table S6 contains GO results for clusters of DE genes identified during LTM formation. Gene expression data are available at GEO with the accession number: GSE115718. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7005842.
Results
MB-unc84 males display normal long-term courtship memory
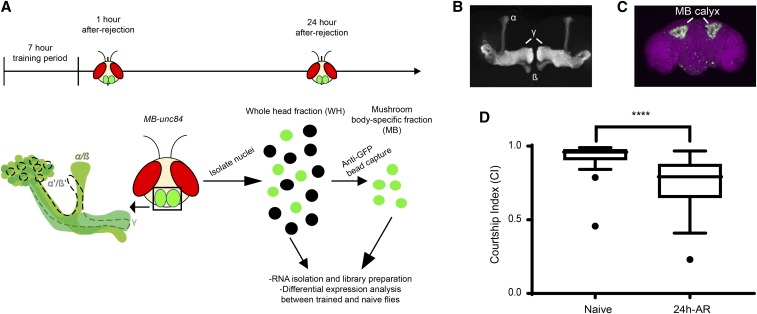
The aim of this study was to identify MB-specific transcript level changes that occur after the acquisition of long-term courtship memory. To achieve this, we used INTACT (Deal and Henikoff 2010; Steiner et al. 2012; Henry et al. 2012) to isolate MB nuclei from fly heads, 1 h and 24 h after courtship conditioning (Figure 1A). We adapted a previously described INTACT protocol that employed a UAS-unc84::GFP transgene (Henry et al. 2012). Unc-84 is a Caenorhabditis elegans nuclear envelope protein, and when coupled with GFP, Unc84::GFP labeled nuclei can be immunoprecipitated from nuclear preparations derived from frozen tissue using an anti-GFP antibody. To drive expression of UAS-unc84::GFP in the MB, we used the R14H06-GAL4 driver line from the Janelia flylight collection (Jenett et al. 2012). This driver is highly specific for the α/β and γ neurons of the mushroom body (Figure 1B), which are required for many forms of memory, including courtship conditioning (Montague and Baker 2016; Zhao et al. 2018; Koemans et al. 2017). R14H06-Gal4 has a higher specificity than many classic MB Gal4 lines, which often have broad expression in the brain (Aso et al. 2009). The expression domain of R14H06-Gal4 is available at (http://flweb.janelia.org/cgi-bin/flew.cgi) and provided here (Figure 1B-C). We generated flies that were heterozygous for both the UAS-unc84 transgene and the R14H06-GAL4 driver, which are hereafter referred to as MB-unc84.
Figure 1.
Schematic of the experimental design and validation of courtship conditioning to induce LTM. A) Long-term memory (LTM) was induced in flies using a previously established seven-hour courtship conditioning protocol (Koemans et al. 2017). Following training, MB-unc84 flies expressing unc84::GFP in the α/β and γ lobes of the MB were collected for INTACT and downstream transcriptome analysis at two time points: one hour and 24 hr after-rejection (AR). RNA was then isolated from the WH and MB-fractions, cDNA libraries prepared, and next-generation sequencing performed. B) Confocal projection of the whole brain showing the expression of UAS-unc84::GFP using R14H06-GAL4. Only a few nuclei outside of the MB calyx are labeled with GFP. MB calyx is indicated. Counterstaining was performed using the nc82 antibody against Bruchpilot. C) Confocal projection showing expression of UAS-mCD8::GFP with R14H06-GAL4 in the α/β and γ lobes of the MB. D) To provide evidence of normal memory in MB-unc84, a subset of flies were collected in parallel with flies used for transcriptome analysis and tested in the courtship conditioning assay. MB-unc84 flies show a decrease in courtship index (CI) at 24h-AR compared to Naïve flies, suggesting normal memory function in this genotype (n = 23 and n =29, respectively, for naïve and trained flies; **** P < 0.001 Mann-Whitney U-test).
To induce long-term courtship memory, MB-unc84 males were paired with an unreceptive mated female for seven hours. Flies for transcriptome analysis were flash frozen at 1 h and 24 h after this period of sexual rejection - 1h-after rejection (AR) and 24h-AR (Figure 1A). These time points were selected to capture both early and late stages after memory acquisition. We avoided sampling during the rejection period to avoid the direct effect of being paired with a female (Ellis and Carney 2011, 2010). A minimum of four biological replicates was obtained for each time point. In parallel with these collections, we tested a subset of MB-unc84 flies to confirm the induction of normal long-term courtship memory in these cohorts. Indeed, at 24h-AR MB-unc84 males showed a robust reduction in courtship behavior in comparison to naïve males (Figure 1D; P < 0.001 Mann-Whitney U-test). This observed courtship suppression in MB-unc84 flies was in line with expected values from the literature (McBride et al. 1999; Koemans et al. 2017; Keleman et al. 2007), demonstrating that UAS-unc84::GFP expression in the MB does not interfere with normal courtship memory.
INTACT yields high-quality MB-enriched RNA
To provide evidence that our approach could obtain nuclei specific to the MB, we used fluorescent microscopy to measure the proportion of GFP-positive nuclei present in whole head (WH) extracts, compared to INTACT MB samples. WH nuclei obtained from MB-unc84 flies contained 8% GFP positive nuclei (Figure 2A). Note that this is likely an overestimation, as we only analyzed fields of view containing GFP-positive nuclei, which were not present throughout the slide. After immunoprecipitation of nuclei from WH extracts using anti-GFP bound beads, about 90% of nuclei were GFP-positive, indicating a high level of specificity of our INTACT protocol (Figure 2A).
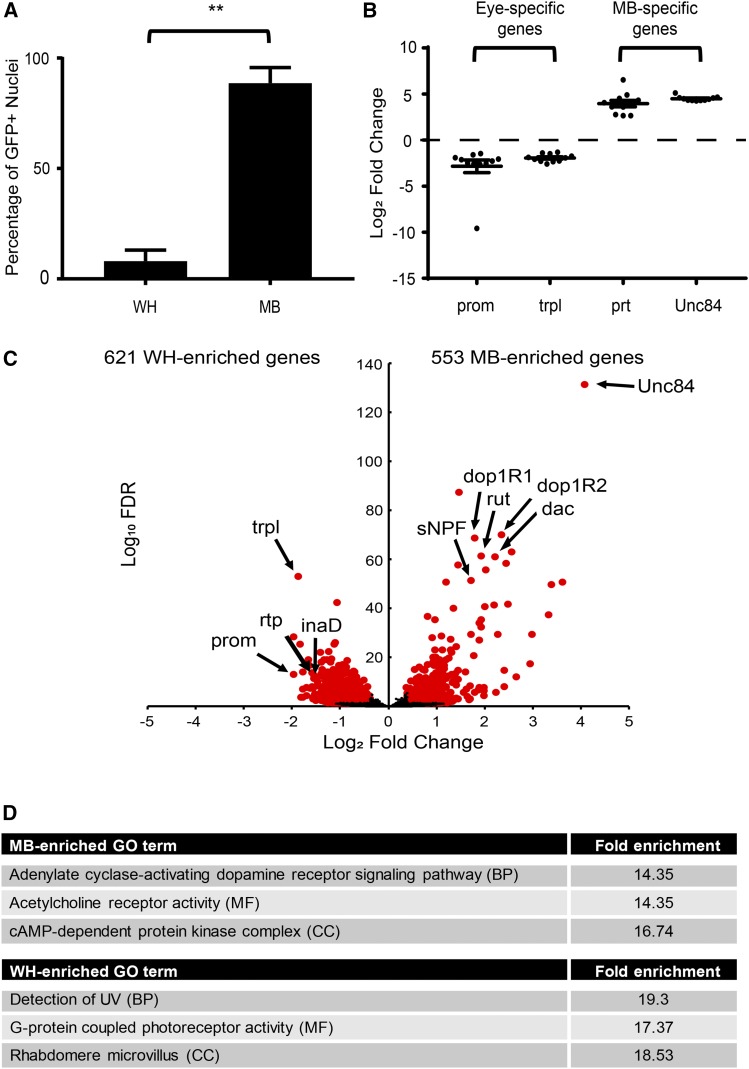
Figure 2.
INTACT yields high-quality MB-enriched RNA. A) Graph showing the average percentage (± SD) of GFP positive nuclei in WH and MB samples obtained using INTACT. The percentage of GFP positive nuclei was determined by counting total nuclei labeled with DRAQ5 (** P < 0.01, Student’s t-test, n = 3). B) Dot plot showing log2 fold changes for a selection of genes with specific expression in the eye (prom, trpl), and the mushroom body (MB) (prt), as well as the nuclear tag unc84. Fold changes were calculated for n = 11 biologically paired WH and MB samples (See Table S1 for details of these samples). The mean and standard error of the mean are indicated. C) Volcano plot showing the results of differential expression (DE) analysis comparing all MB samples (n = 12) to all WH samples (n = 12) (Table S2). 553 and 621 DE genes were significantly enriched in MB and WH, respectively (q < 0.05, fold difference > 1.3). A selection of genes previously known to be enriched in the MB (rut, dac, sNPF, Dop1R1, Dop1R2) and optical lobe (trpl, prom, rtp, inaD), as well as unc84, are highlighted. D) Gene ontology (GO) enrichment analysis was performed for MB-enriched and WH-enriched genes. The most enriched GO terms for biological processes (BP), molecular functions (MF) and cellular components (CC) are displayed for both MB and WH-enriched genes (FDR corrected p-value < 0.05, Fisher exact test, minimum four genes, Table S3).
Next, INTACT was used to extract MB nuclei from MB-unc84 heads at 1h-AR and 24h-AR, as well as from naïve flies matched for age and time-of-day. For each MB sample, we also obtained RNA from nuclei present in the biologically paired WH input for comparison. After verification of RNA quality, sequencing libraries were prepared from both WH and MB samples. Completed libraries were sequenced and reads were aligned to the D. melanogaster genome. Samples that had >10 million genic counts were included for downstream analysis, resulting in a total of 12 MB samples (four naïve, four 1h-AR, four 24h-AR) and 12 WH samples (five naïve, three 1h-AR, four 24h-AR) (Table S1).
To confirm consistent enrichment in MB samples we examined gene expression differences between WH and MB samples. DESeq2 was used to normalize gene counts between all MB and WH samples and genes with less than 50 counts across all samples were removed, leaving a total of 11941 genes with sufficient coverage. Log2 fold changes were then calculated using normalized counts for biologically paired WH and MB samples. For two samples we did not obtain a true biological pair due to failure during sample preparation, resulting in n = 11 samples that were used in this analysis (see Table S1 for details of read depth and biological pairing). As expected, eye-specific genes like prom and trpl were underrepresented in MB-samples, while MB-enriched genes, such as prt and unc84 were overrepresented in the MB samples (Figure 2B). Notably, unc84 expression was highly enriched and highly consistent across all biological replicates suggesting a high degree of consistency in MB-enrichment after INTACT.
To provide further evidence that the nuclei we isolated displayed MB-specific gene expression profiles we performed differential expression analysis between all MB (n = 12) and WH samples (n = 12). We identified 553 and 621 genes (q < 0.05, fold difference > 1.3) that were significantly enriched in either MB or WH samples, respectively (Figure 2C; complete list in Table S2). Many known MB-expressed genes, including rut, dnc, prt, ey, toy, and dac were among the most differentially expressed MB-enriched genes (Livingstone et al. 1984; Noveen et al. 2000; Brooks et al. 2011; Kurusu et al. 2000). In contrast, several eye-specific genes, such as prom, trpl, inaD, and rtp, were among the most differentially expressed WH-enriched genes (Figure 2C). Additionally, we compared MB-enriched genes to cell surface receptors that were found to be characteristically expressed in α/β and γ KC’s when compared to MBONs (Crocker et al. 2016). Indeed, many of these receptors were also found to be enriched in our dataset including: Dop1R2, Dop1R1, Dop2R, 5-HT1B, Oamb, Octβ1R, sNPF, GluRIB, Ir68a, CCKLR-17D1, CCKLR-17D3, GluRIB, and mAChR-A (Table S2). Finally, we examined gene ontology (GO) terms enriched for biological processes (BP), molecular functions (MF), as well as cellular components (CC), among our lists of MB-enriched and WH-enriched genes (Figure 2D, Table S3). The most enriched GO terms for MB-enriched genes were “cAMP-dependent protein kinase complex” (CC) and “adenylate cyclase-activating dopamine receptor signaling pathway” (BP) (Figure 2D), which fits with the known importance of dopaminergic modulatory neurons and cAMP signaling in memory formation in the MB (Aso et al. 2014; Keleman et al. 2012; Blum et al. 2009; Zhang et al. 2015). The most enriched GO term for MF was “acetylcholine receptor activity”, consistent with previous studies which showed that MB KC’s are cholinergic and receive input from cholinergic olfactory projection neurons (Barnstedt et al. 2016; Crocker et al. 2016; Gu 2006). In contrast, the most enriched GO terms for the WH enriched genes were all related to eye function, including “detection of UV” (BP), “G-protein coupled photoreceptor activity” (MF) and “rhabdomere microvillus” (CC) (Figure 2D). Taken together, analysis of genes that are differentially expressed between WH and MB samples revealed a pattern of gene expression that is highly consistent with an effective MB enrichment.
Gene expression changes after memory acquisition
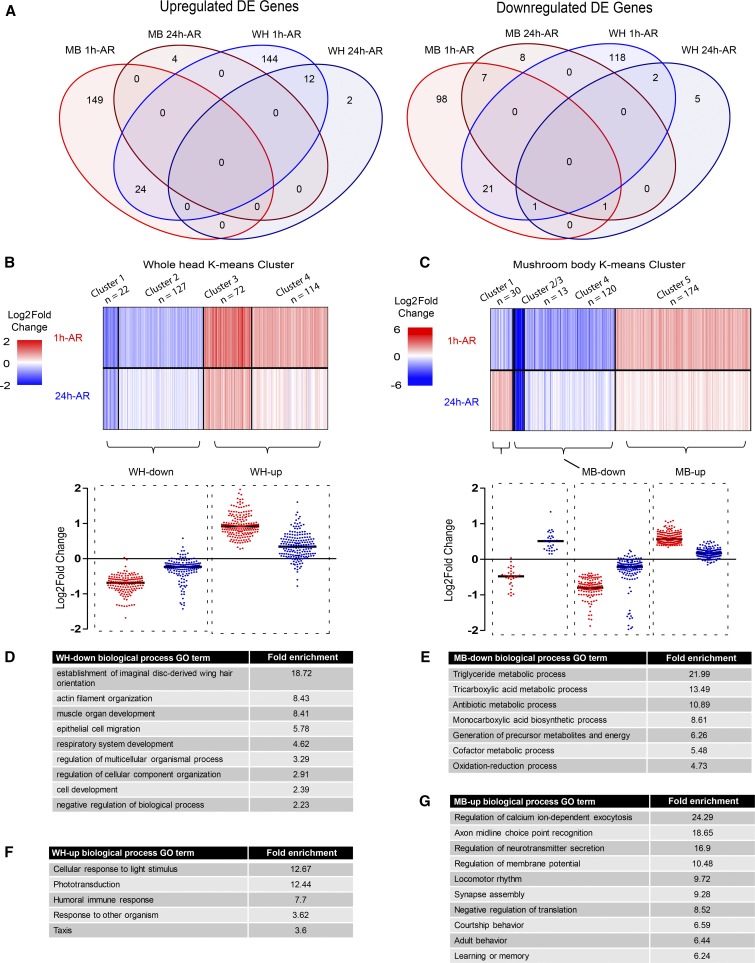
Next, we used DESeq2 to identify genes that were differentially expressed (DE) in response to courtship conditioning by comparing 1h-AR and 24h-AR to naïve flies. DE analysis was performed on normalized counts for both WH and MB samples after genes with less than 50 mean counts across all samples were removed, which left 8730 and 8561 genes, respectively, with sufficient coverage. For both WH and MB samples we observed more DE genes at 1h-AR than at 24h-AR (n = 322/23, n = 302/20, for WH and MB sample 1h-AR/24h-AR, respectively). There was some overlap in DE genes between 1h-AR and 24h-AR, and between WH and MB samples, however, most DE genes identified in WH and MB samples were different (Figure 3A). To investigate trends in gene expression after courtship conditioning we compiled a list of all DE genes that were differentially expressed in at least one of the three pairwise comparisons: 1h-AR vs. naïve, 24h-AR vs. naïve, and 1h-AR vs. 24h-AR (Table S4). This led to the identification of 332 and 342 DE genes for WH and MB samples, respectively. For each tissue, we performed k-means clustering on log2 fold changes at 1h-AR and 24h-AR (Figure 3B and 3C, Table S5). In WH samples, four clusters were identified with two distinct trends: cluster 1 and 2 (n = 22 and 127) contained genes that were downregulated at 1h-AR and either reduced or not changed at 24h-AR (WH-down, Figure 3B and Table S5). Cluster 3 and 4 (n = 72 and 114) contained genes that were upregulated at 1h-AR and either less upregulated or not changed at 24h-AR (WH-up - Figure 3B and Table S5). For MB samples k-means clustering revealed five clusters with three distinct expression trends. Cluster 1 (n = 30) contained genes that were downregulated at 1h-AR and upregulated 24h-AR. Clusters 2, 3, and 4 (n = 2, 13 and 120, respectively) contained genes that were downregulated at 1h-AR and either downregulated or not changed at 24h-AR (MB-down - Figure 3C and Table S5). Cluster 5 (n = 174) contained genes that were upregulated at 1h-AR and either upregulated or not changed at 24h-AR (MB-up - Figure 3C and Table S5). This clustering allowed us to identify gene groups with similar expression trends and emphasized the relatively strong effect of sexual rejection at 1h-AR.
Figure 3.
Differential expression and clustering analysis of MB and WH RNA sequencing. A) Venn diagram showing overlap between MB and WH differentially expressed (DE) genes (q < 0.05, fold difference 1.3 up or down) for both upregulated and downregulated genes (File S4). (B-C) Cluster analysis of WH and MB DE genes identified by DESeq2 (q < 0.05, fold difference 1.3 up or down). Log2 fold change data were obtained for significant DE genes at both one hour, as well as 24-hour time points and clustered using k-means. Heatmap shows the individual log2 fold changes for each gene. Dot plot shows log2 fold changes for genes with similar expression trends (File S5). (B) Four clusters were identified for WH DE genes, with two distinct trends. Cluster 1 and 2 were downregulated at both time-points (WH-down) and cluster 3 and 4 were upregulated at both time-points (WH-up). C) Five clusters were identified for MB DE genes with three distinct trends. Cluster 1 was downregulated 1h-AR and upregulated 24h-AR. Cluster 2, 3 and 4 were downregulated at both time-points (MB-down). Cluster 5 was upregulated at both time-points (MB-up). (D-G) GO enrichment analysis for biological processes using PANTHER (P < 0.05, Binomial test with Bonferroni correction, minimum 5 genes, sorted by hierarchical view). The top GO terms, heading each GO hierarchical cluster are displayed, sorted by fold enrichment (Table S6), for (D) WH-down, (E) MB-down, (F) WH-up, (G) MB-up.
Courtship conditioning is associated with MB-specific downregulation of metabolic genes
To investigate the functions of genes that are differentially expressed in response to courtship conditioning, we first performed GO enrichment analysis for gene clusters with similar expression trends. For WH-down genes (n = 149, Figure 3B) we observed, almost exclusively, enrichment of GO terms related to development, for example, “metamorphosis”, “cell differentiation”, and “cell migration” (Figure 3D and Table S6). For MB-down genes (n = 135, Figure 3C) we observed enrichment only of GO terms related to metabolism (Figure 3E and Table S6). In fact, over half (n = 73) of the MB-specific downregulated genes are annotated with the term “metabolic processes” (Table S6). Notably, there was no overlap in enriched GO terms between WH-down and MB-down genes. The highly specific effect of courtship conditioning on the regulation of metabolic genes in the MB is very interesting as metabolic changes are known to be important for the formation of LTM and in response to synaptic activity (Bas-Orth et al. 2017; Segarra‐Mondejar et al. 2018; Goyal et al. 2014; Plaçais et al. 2017; Tadi et al. 2015). We see MB-specific downregulation of genes encoding mitochondrial proteins involved in the Krebs cycle (Mdh1, Acon, Ldh, ScsβA) and the electron transport chain (blw, ATPsynβ, ATPsynγ, ND-51) (Table S5). This downregulation of genes involved in oxidative glucose metabolism suggests a shift toward aerobic glycolysis, known as the Warburg effect, where cells favor non-oxidative glucose metabolism despite the presence of oxygen (Chen et al. 2015). This type of metabolism is seen in mammalian neurons in response to synaptic activity and LTM formation (Bas-Orth et al. 2017; Segarra‐Mondejar et al. 2018; Tadi et al. 2015). Aerobic glycolysis may serve to protect neurons against oxidative damage and has been suggested as a mechanism to provide precursor molecules that are required for synaptogenesis (Bas-Orth et al. 2017; Segarra‐Mondejar et al. 2018; Goyal et al. 2014; Tadi et al. 2015).
Courtship conditioning is associated with MB-specific upregulation of synaptic proteins and learning and memory pathways
For WH-up genes (n= 186, Figure 3B) all enriched GO terms were related to biological responses, such as “cellular response to light stimulus”, “humoral immune response”, “response to other organism”, and “taxis” (Figure 3F and Table S6). Indeed, 62 of the WH-up genes were annotated with the term “response to stimulus” (Table S6). GO terms related to biological response were also enriched for MB-up genes (n = 174, Figure 3C). There were 5 enriched GO terms common to WH-up and MB-up genes (“response to light stimulus”, “response to abiotic stimulus”, “response to stimulus”, “response to external stimulus”, and “taxis”) (Table S6). Yet the MB-up gene group showed many more enriched GO terms - 181 compared to 15 for WH-up - suggesting a high level of functional relatedness in this gene group. Using annotated protein-protein and genetic interactions, we identified a network of 54 MB-up genes (Figure 4). This network was comprised of genes encoding ion channels, transcription factors, RNA binding proteins, and genes with functional annotations related to synapse formation, synaptic signaling, behavior, and learning and memory (Figure 4). Interestingly, some of the most enriched GO categories that were unique for MB-up genes were related to synaptic plasticity (e.g., “regulation of calcium ion-dependent exocytosis”), behavior (e.g., “courtship behaviour”), and memory (“e.g. “learning or memory”) (Figure 3G). Taken together, these results suggest that MB-up genes encode a highly interactive group of proteins with biological relevance to learning and memory.
Figure 4.
Network analysis of genes that are upregulated in the MB in response to courtship conditioning. Of 178 genes in the MB-up group, 54 form a single network based on a subset protein-protein and genetic interactions that are annotated in geneMANIA (see methods). Each node is color coded to represent selected gene ontology annotations.
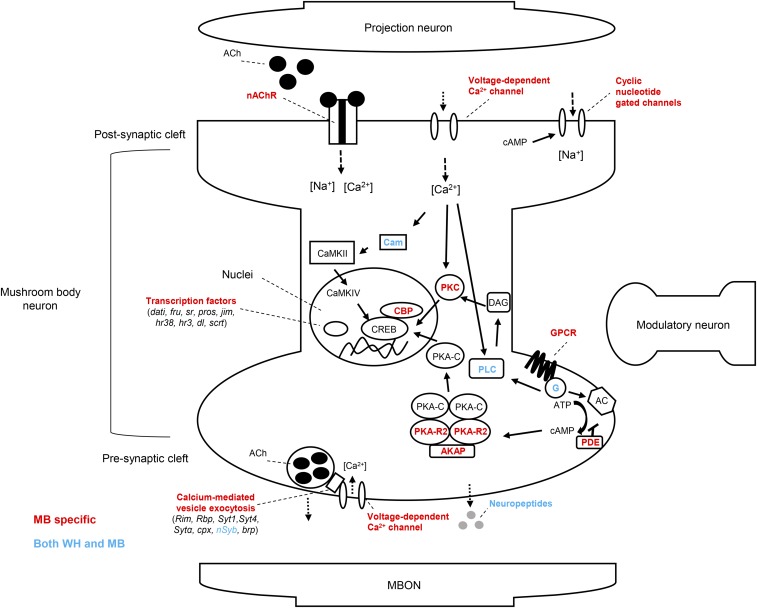
Next, we manually curated the MB-up gene group to illustrate how they may be represented in memory-relevant molecular pathways in MB KCs (Figure 5). During learning and memory formation KCs receive olfactory input from over 200 olfactory projection neurons (PNs) that synapse with the dendrites of the calyx (Caron et al. 2013). Olfactory signals are reinforced to form memories by sensory signals from modulatory dopaminergic neurons (DANs), which synapse at discrete locations along the axons of the MB lobes (Aso et al. 2014). In courtship conditioning, the primary olfactory signal is thought to be the pheromone cVA, which is deposited on females by males during mating (Everaerts et al. 2010). Courtship memory is formed when cVA is paired with sexual rejection, which is conveyed to the MB γ lobe via a specific class of DANs (Keleman et al. 2012). Long-term courtship memory is also dependent on the production of the hormone ecdysone, which can also act as an input signal to KCs (Ishimoto et al. 2009; Ishimoto et al. 2013). Olfactory PNs are cholinergic and are thought to stimulate KCs through activation of nicotinic acetylcholine receptors (nAChRs), which are ligand-gated channels that induce calcium influx into KCs (Christiansen et al. 2011; Yasuyama et al. 2002; Campusano et al. 2007). Calcium influx is required for downstream signaling associated with synaptic plasticity and memory formation (Lisman 1994). Among MB-up genes, we noted several genes involved in receiving olfactory signals and mediating downstream calcium dependent signaling (Figure 5). These included genes encoding three nAChR subunits (nAChRα1, nAChRα6, nAChRβ1), the acetylcholinesterase (Ace) involved in acetylcholine recycling, the voltage-gated calcium channel Ca-β, the calcium-activated signaling proteins PLC and PKC, and the calcium-binding messenger calmodulin (Cam) (Widmann et al. 2016; Pavot et al. 2015; Campusano et al. 2007). Many MB-up genes also encode proteins involved in receiving modulatory signals, and in the cAMP signaling pathway that is activated by these signals during memory formation (Figure 5). Notably, we identified MB-specific upregulation of four G-protein coupled receptors (GPCR). These included oamb, hec, and SIFaR, all known to be involved in male courtship behavior (Li et al. 2011; Sellami and Veenstra 2015; Zhou et al. 2012), and DopEcR, an atypical GPCR that responds to both dopamine and ecdysone, and is essential for cAMP signal activation during courtship memory (Ishimoto et al. 2013). We identified five MB-up genes encoding components of the heterotrimeric G-protein complex (Gαq, Gβ13F, Gγ30A, Gαo, Gγ1), which acts directly downstream GPCRs to induce adenylate cyclase activity and production of cAMP (Livingstone et al. 1984; Levin et al. 1992). Several downstream cAMP signaling components were also upregulated specifically in the MB, including regulatory subunits of protein kinase A (PKA-R2), the PKA anchoring protein (Akap200), cAMP-gated ion channels (Ih, Cngl), and the CREB-binding protein, nej, a histone acetyltransferase that is thought to be involved in LTM-associated gene expression (Alarcón et al. 2004; Hirano et al. 2016). Thus, many MB-up genes are directly related to receiving and processing the signals that induce courtship memory.
Figure 5.
Schematic representation of molecular pathways underlying memory in the mushroom body. Manually curated diagram of memory-relevant molecular pathways in MB Kenyon cells which were identified to be differentially expressed in the MB-up gene group (shown in red). Calcium-dependent, cholinergic, and cAMP signaling pathways are among the molecular pathways represented. Additionally, genes encoding proteins involved in calcium-mediated presynaptic neurotransmitter release, as well as differentially expressed transcription factors are shown.
KC axons provide presynaptic output to 21 MBONs (Aso et al. 2014). Several MB-up genes encode proteins involved in calcium-mediated presynaptic neurotransmitter release, including the synaptic vesicle docking proteins RIM and RBP, the synaptotagmins (Syt1, Syt4, Sytα), components of the SNARE complex (cpx and nSyb), the presynaptic calcium channel cacophony, and the active zone marker brp (Figure 5) (Deitcher et al. 1998; Kittel and Heckmann 2016; Huntwork and Littleton 2007; Liu et al. 2011; Knapek et al. 2011). We also observed upregulation of two neuropeptides, Nplp2, and sNPF. sNPF is has been shown to act synergistically with ACh in communicating to MBONs in the context of olfactory memory formation (Barnstedt et al. 2016). Thus, many MB-up genes are involved in pre-synaptic neurotransmission and it can be inferred that these genes may play a role in transmitting memory signals to MBONs (Figure 5).
Finally, we also observed upregulation of many genes encoding transcription factors and RNA binding proteins. RNA binding proteins like stau and Orb2 are thought to be involved in LTM formation through localized regulation of translation at synapses (Dubnau et al. 2003; Khan et al. 2015). Some of the transcription factors in the MB-up group have known roles in courtship behavior, such as dati, fru and pros (Grosjean et al. 2007; Schinaman et al. 2014; Manoli et al. 2005). Interestingly, we identified MB-specific upregulation of sr and Hr38, which are transcription factors that have been proposed as markers of neuron activation in insects (Fujita et al. 2013; Lutz and Robinson 2013; Chen et al. 2016).
Discussion
Understanding transcriptional changes that are required in neurons to mediate LTM is an important challenge in neuroscience. Many studies have identified gene expression changes after memory acquisition in Drosophila (Winbush et al. 2012; Bozler et al. 2017; Dubnau et al. 2003; Crocker et al. 2016) and this approach has been used to identify new genes involved in memory formation (Bozler et al. 2017; Dubnau et al. 2003; Crocker et al. 2016). However, we still understand very little about the spatial and temporal requirement for transcription in LTM. When are critical memory genes activated and in which neurons? Here, we used MB-specific transcriptional profiling to identify changes in transcript levels that occur in response to courtship conditioning, an ethological memory paradigm that is commonly used in Drosophila. This analysis revealed gene expression changes in established learning and memory pathways that occurred for the most part at 1 hr after courtship rejection, but not after 24 hr. Importantly, canonical memory related pathways were only differentially regulated in the MB and not in biologically paired WH samples. These results suggest that memory related biological processes are transiently upregulated in the MB after memory acquisition, illustrating the importance of sampling time, as well as cell type, in the identification of biologically relevant gene regulation in LTM and offer a valuable list of candidate genes for further investigation.
In our study, we compared males that experienced sexual rejection to naïve socially isolated males. Although samples were collected at least one hour after exposure to a female fly, it is impossible to conclusively differentiate between transcript level changes that occur because of sexual rejection - and long-term memory formation - and changes that might happen in response to any social interaction. Previous studies have looked at gene expression changes that occur in whole heads in response to courtship, male-male interactions, and mating (Ellis and Carney 2011, 2010). As could be expected, in WH samples we do observe a significant overlap with those studies (36 genes, 1.5-fold enrichment, P < 0.001). This suggests that some gene expression changes in whole heads represent general responses to social interactions. In contrast, we see no significant overlap between genes identified in these social interaction studies and genes that we observe to be changed in the MB after courtship conditioning (6 genes, 0.26-fold enrichment, P > 0.05). This is consistent with the fact that the MB is not required for normal male-female interactions like courtship behavior or mating as MB-ablated flies reproduce normally and even show normal learning in response to sexual rejection (de Belle and Heisenberg 1994; McBride et al. 1999). Therefore, it is reasonable to suggest that MB-specific gene expression changes we observed are likely related to memory acquisition.
One major limitation of this study is that we have not functionally or technically validated the DE genes. As with any genomic dataset, there will be false positives. However, several factors suggest that many of the DE genes identified here have a high potential to be true positives and that our overall conclusions are not affected by the presence of false positives. First, we have used several biological replicates as well as a sophisticated software package, DESeq2, which performs among the highest in several measures of DE analysis quality (true positive rate, accuracy, positive predictive value, accuracy) when compared to other available software tools in an independent study (Costa-Silva et al. 2017). Second, we have compared results obtained with DESeq2 to NOISeq, another DE analysis program with high ranking quality measures (Tarazona et al. 2015; Costa-Silva et al. 2017). Although the list of DE genes does differ between the two programs, there is a high degree of overlap in the specific genes, resulting in a very similar enrichment of GO terms for genes that are differentially expressed 1h-AR (Figure S2). Third, some memory induced transcript changes observed here have been seen in other studies, providing indirect technical validation of our dataset. For example, stau was shown to be upregulated in whole heads after olfactory conditioning (Dubnau et al. 2003). We also see a significant overlap of genes upregulated 24 hr after rejection in Winbush et al. (3 genes, 50-fold enrichment, P < 0.001), where WH transcript levels were also measured 24 hr after courtship memory acquisition (Winbush et al. 2012). Finally, genes that are upregulated in the MB after memory acquisition (from both DESeq2 and NOIseq) show a remarkable correlation with known memory pathways. From post-synaptic receptors, to signaling pathways, to presynaptic neurotransmitter release mechanisms, nearly all known aspects of memory related synaptic plasticity are accounted for (Figures 3-5). In the MB-up group, there are 13 established learning and memory genes (Table S6), thus, functional validation of our dataset is available from the literature.
In general, other Drosophila memory transcriptome studies have not observed such a profound effect on known memory related genes and pathways (Crocker et al. 2016; Dubnau et al. 2003; Bozler et al. 2017; Winbush et al. 2012). This is likely due to both the sampling time and cell type we investigated. Certainly, memory specific transcriptional signals would be diluted in whole head analysis (Winbush et al. 2012; Bozler et al. 2017; Dubnau et al. 2003). In comparison to other MB specific transcriptome analyses, we do not see overlap in DE genes identified by Widmer et al. who profiled MB gene expression for 72 h after olfactory memory acquisition using DamID (Widmer et al. 2018). The lack of overlap may be due to differences in the Gal4 driver used targeting a different subset of MB neurons, the different memory paradigm (appetitive olfactory conditioning), or the differences in sampling time. Whereas we have obtained a snapshot view of mRNA transcript levels at two specific time points, Widmer et al. collected cumulative changes occurring over four 12-hour periods using the powerful DAM-ID method, which tracks transcription by indirectly measuring the association of polII with DNA. Crocker et al. used cell-specific patch clamping to investigate gene expression from MB neurons labeled by the c739-Gal4 and NP1131-Gal4 drivers, 30 min after memory acquisition. However, they identified very few differentially expressed genes in these neurons, which as they explain, is likely due to pooling of many samples that were conditioned with different odors (Crocker et al. 2016). The fact that we observed many expected memory genes and pathways to be induced in the MB suggests that we have serendipitously captured a critical time point for gene regulation in the formation of long-term courtship memory. Additionally, it stands to reason that the genes we identified that have not been previously associated with known memory pathways may represent novel mechanisms, which could be further investigated.
Many studies in mouse have profiled transcriptional changes in the hippocampus in response to fear conditioning and other memory paradigms (Zovkic et al. 2014; Vogel-Ciernia et al. 2013; Tadi et al. 2015). Consistent with our observations, these studies show many gene expression changes 30 min after memory acquisition, and not at later time points (Peixoto et al. 2015). In general, however, these studies do not identify widespread differential expression of canonical learning and memory pathways as we do in the fly MB (Zovkic et al. 2014; Vogel-Ciernia et al. 2013; Tadi et al. 2015). In mouse, across many different studies, fear conditioning consistently invokes strong activation of immediate early genes such as c-Fos, which are known to be induced in response to neuron firing (Mayford and Reijmers 2016). In insects, neuron activity induced genes have been more elusive, however, two genes, Hr38 and sr, are consistently upregulated in response to a variety of neuronal activation stimuli in flies and other insects (Fujita et al. 2013; Lutz and Robinson 2013; Chen et al. 2016; Adhikari et al. 2018). It is very interesting that we observe these two genes to be specifically activated in the MB in response to sexual rejection. No other Drosophila memory-related transcriptome study has identified induction of these genes (Bozler et al. 2017; Winbush et al. 2012; Dubnau et al. 2003; Crocker et al. 2016; Widmer et al. 2018), except for Crocker et al. who did identify Hr38 induction in the MB α/β cells at 30 min after memory acquisition, albeit with a borderline q-value (0.058) (Crocker et al. 2016). This suggests that our MB-specific analysis, coupled with an appropriate sampling time, has revealed a parallel mechanism to mammals that has not previously been observed in flies, where the induction of neuron activity induced genes is observed following memory acquisition.
In the future, it will be important to further refine the cell types and sampling times to fully understand transcriptional dynamics associated with memory formation. Indeed, even by focusing on less than 2000 MB cells, the actual circuit involved in the formation and long-term maintenance of the memory is likely composed of far fewer cells. The specific circuits that are required for courtship memory and other memory forms are being elucidated rapidly (Montague and Baker 2016; Zhao et al. 2018) and tools are now becoming available to label these cell populations for genomic analysis (Southall et al. 2013; Henry et al. 2012; Crocker et al. 2016). It is likely that further focus on more discrete cell populations will be required to fully understand gene activation in LTM.
Acknowledgments
This work was funded by a Natural Science and Engineering Research Council of Canada Discovery Grant, the Canada Research Chairs Program, and the Canadian Foundation for Innovation. We thank the Bloomington Drosophila Stock center the Janelia Farm Research Campus, and G.L. Henry for providing Drosophila stocks. Thanks to David Carter and the London Regional Genomics Center for help with sequencing and to Robert Cumming for help with interpretation of our transcriptome data.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.7005842.
Communicating editor: H. Lipshitz
Literature Cited
- Adhikari P., Orozco D., Randhawa H., Wolf F. W., 2018. Mef2 Induction of the Immediate Early Gene Hr38/Nr4a Is Terminated by Sirt1 to Promote Ethanol Tolerance. Genes Brain Behav. e12486 10.1111/gbb.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aken B. L., Ayling S., Barrell D., Clarke L., Curwen V., et al. , 2016. The Ensembl Gene Annotation System. Database : The Journal of Biological Databases and Curation 2016: baw093 10.1093/database/baw093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón J. M., Malleret G., Touzani K., Vronskaya S., Ishii S., et al. , 2004. Chromatin Acetylation, Memory, and LTP Are Impaired in CBP+/− Mice: A Model for the Cognitive Deficit in Rubinstein-Taybi Syndrome and Its Amelioration. Neuron 42: 947–959. 10.1016/j.neuron.2004.05.021 [DOI] [PubMed] [Google Scholar]
- Anders S., Pyl P. T., Huber W., 2015. HTSeq-A Python Framework to Work with High-Throughput Sequencing Data. Bioinformatics 31: 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Ball C., Blake J. A., Botstein D., Butler H., et al. , 2000. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 25: 25–29. 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y., Grübel K., Busch S., Friedrich A. B., Siwanowicz I., et al. , 2009. The Mushroom Body of Adult Drosophila Characterized by GAL4 Drivers. J. Neurogenet. 23: 156–172. 10.1080/01677060802471718 [DOI] [PubMed] [Google Scholar]
- Aso Y., Hattori D., Yu Y., Johnston R. M., Iyer N. A., et al. , 2014. The Neuronal Architecture of the Mushroom Body Provides a Logic for Associative Learning. eLife 3: e04577 10.7554/eLife.04577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstedt, Oliver, David Owald, Johannes Felsenberg, Ruth Brain, John Paul Moszynski, Clifford B. Talbot, Paola N. Perrat, and Scott Waddell, 2016 Memory-Relevant Mushroom Body Output Synapses Are Cholinergic. Neuron 89: 1237–1247. 10.1016/j.neuron.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bas-Orth C., Yan W. T., Lau D., Bading H., 2017. Synaptic Activity Drives a Genomic Program That Promotes a Neuronal Warburg Effect. J. Biol. Chem. 292: 5183–5194. 10.1074/jbc.M116.761106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Belle J. S., Heisenberg M., 1994. Associative Odor Learning in Drosophila Abolished by Chemical Ablation of Mushroom Bodies. Science 263: 692–5. 10.1126/science.8303280 [DOI] [PubMed] [Google Scholar]
- Bliim, Nicola, Iryna Leshchyns’ka, Vladimir Sytnyk, and Michael Janitz, 2016 Transcriptional Regulation of Long-Term Potentiation. Neurogenetics 17 (4): 201–10. 10.1007/s10048-016-0489-x [DOI] [PubMed]
- Blum, Allison L, Wanhe Li, Mike Cressy, and Josh Dubnau, 2009 Short- and Long-Term Memory in Drosophila Require CAMP Signaling in Distinct Neuron Types. Curr. Biol. 19: 1341–1350. 10.1016/j.cub.2009.07.016 [DOI] [PMC free article] [PubMed]
- Bozler J., Kacsoh B. Z., Chen H., Theurkauf W. E., Weng Z., et al. , 2017. A Systems Level Approach to Temporal Expression Dynamics in Drosophila Reveals Clusters of Long Term Memory Genes. PLoS Genet. 13: e1007054 10.1371/journal.pgen.1007054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks E. S., Greer C. L., Romero-Calderón R., Serway C. N., Grygoruk A., et al. , 2011. A Putative Vesicular Transporter Expressed in Drosophila Mushroom Bodies That Mediates Sexual Behavior May Define a Neurotransmitter System. Neuron 72: 316–329. 10.1016/j.neuron.2011.08.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli M., Castellucci V., Kandel E. R., 1976. Synaptic Facilitation and Behavioral Sensitization in Aplysia: Possible Role of Serotonin and Cyclic AMP. Science 194: 1178–1181. 10.1126/science.186870 [DOI] [PubMed] [Google Scholar]
- Campusano J. M., Su H., Jiang S. A., Sicaeros B., O’Dowd D. K., 2007. NAChR-Mediated Calcium Responses and Plasticity InDrosophila Kenyon Cells. Dev. Neurobiol. 67: 1520–1532. 10.1002/dneu.20527 [DOI] [PubMed] [Google Scholar]
- The Gene Ontology Consortium , 2017. Expansion of the Gene Ontology Knowledgebase and Resources. Nucleic Acids Res. 45: D331–D338. 10.1093/nar/gkw1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron S., Ruta V., Abbott L. F., Axel R., 2013. Random Convergence of Olfactory Inputs in the Drosophila Mushroom Body. Nature 497: 113–117. 10.1038/nature12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Rahman R., Guo F., Rosbash M., 2016. Genome-Wide Identification of Neuronal Activity-Regulated Genes in Drosophila. eLife 5: e19942 10.7554/eLife.19942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Qian Y., Wu S., 2015. The Warburg Effect: Evolving Interpretations of an Established Concept. Free Radic. Biol. Med. 79: 253–263. 10.1016/j.freeradbiomed.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen F., Zube C., Andlauer T. F. M., Wichmann C., Fouquet W., et al. , 2011. Presynapses in Kenyon Cell Dendrites in the Mushroom Body Calyx of Drosophila. J. Neurosci. 31: 9696–9707. 10.1523/JNEUROSCI.6542-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cognigni, Paola, Johannes Felsenberg, and Scott Waddell, 2018 Do the Right Thing: Neural Network Mechanisms of Memory Formation, Expression and Update in Drosophila. Curr. Opin. Neurobiol. 49: 51–58. 10.1016/j.conb.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Silva J., Domingues D., Lopes F. M., 2017. RNA-Seq Differential Expression Analysis: An Extended Review and a Software Tool. PLoS One 12: e0190152 10.1371/journal.pone.0190152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A., Guan X.-J., Murphy C. T., Murthy M., 2016. Cell-Type-Specific Transcriptome Analysis in the Drosophila Mushroom Body Reveals Memory-Related Changes in Gene Expression. Cell Reports 15: 1580–1596. 10.1016/j.celrep.2016.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal R. B., Henikoff S., 2010. A Simple Method for Gene Expression and Chromatin Profiling of Individual Cell Types within a Tissue. Dev. Cell 18: 1030–1040. 10.1016/j.devcel.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitcher D. L., Ueda A., Stewart B. A., Burgess R. W., Kidokoro Y., et al. , 1998. Distinct Requirements for Evoked and Spontaneous Release of Neurotransmitter Are Revealed by Mutations in the Drosophila Gene Neuronal-Synaptobrevin. J. Neurosci. 18: 2028–2039. 10.1523/JNEUROSCI.18-06-02028.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., et al. , 2013. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J., Chiang A. S., Grady L., Barditch J., Gossweiler S., et al. , 2003. The Staufen/Pumilio Pathway Is Involved in Drosophila Long-Term Memory. Curr. Biol. 13: 286–296. 10.1016/S0960-9822(03)00064-2 [DOI] [PubMed] [Google Scholar]
- Dudai Y., Jan Y. N., Byers D., Quinn W. G., Benzer S., 1976. Dunce, a Mutant of Drosophila Deficient in Learning. Proc. Natl. Acad. Sci. USA 73: 1684–1688. 10.1073/pnas.73.5.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejima A., Smith B. P. C., Lucas C., van der Goes van Naters W., Miller C. J., et al. , 2007. Generalization of Courtship Learning in Drosophila Is Mediated by Cis-Vaccenyl Acetate. Curr. Biol. 17: 599–605. 10.1016/j.cub.2007.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. L., Carney G. E., 2010. Mating Alters Gene Expression Patterns in Drosophila Melanogaster Male Heads. BMC Genomics 11: 558 10.1186/1471-2164-11-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L. L., Carney G. E., 2011. Socially-Responsive Gene Expression in Male Drosophila Melanogaster Is Influenced by the Sex of the Interacting Partner. Genetics 187: 157–169. 10.1534/genetics.110.122754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts C., Farine J. P., Cobb M., Ferveur J. F., 2010. Drosophila Cuticular Hydrocarbons Revisited: Mating Status Alters Cuticular Profiles. PLoS One 5: e9607 10.1371/journal.pone.0009607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Nozomi, Yuka Nagata, Takumi Nishiuchi, Makoto Sato, Masafumi Iwami, and Taketoshi Kiya, 2013 Visualization of Neural Activity in Insect Brains Using a Conserved Immediate Early Gene, Hr38. Curr. Biol. 23: 2063–2070. 10.1016/j.cub.2013.08.051 [DOI] [PubMed]
- Goyal M. S., Hawrylycz M., Miller J. A., Snyder A. Z., Raichle M. E., 2014. Aerobic Glycolysis in the Human Brain Is Associated with Development and Neotenous Gene Expression. Cell Metab. 19: 49–57. 10.1016/j.cmet.2013.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates L. S., Marygold S. J., Dos Santos G., Urbano J. M., Antonazzo G., et al. , 2017. FlyBase at 25: Looking to the Future. Nucleic Acids Res. 45: D663–D671. 10.1093/nar/gkw1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean Y., Guenin L., Bardet H. M., Ferveur J. F., 2007. Prospero Mutants Induce Precocious Sexual Behavior in Drosophila Males. Behav. Genet. 37: 575–584. 10.1007/s10519-007-9152-5 [DOI] [PubMed] [Google Scholar]
- Gu H., 2006. Cholinergic Synaptic Transmission in Adult Drosophila Kenyon Cells In Situ. J. Neurosci. 26: 265–272. 10.1523/JNEUROSCI.4109-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. D., Kandel E. R., Bailey C. H., 2006. Molecular Mechanisms of Memory Storage in Aplysia. Biol. Bull. 210: 174–191. https://doi.org/210/3/174. [DOI] [PubMed] [Google Scholar]
- Henry G. L., Davis F. P., Picard S., Eddy S. R., 2012. Cell Type-Specific Genomics of Drosophila Neurons. Nucleic Acids Res. 40: 9691–9704. 10.1093/nar/gks671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Ihara K., Masuda T., Yamamoto T., Iwata I., et al. , 2016. Shifting Transcriptional Machinery Is Required for Long-Term Memory Maintenance and Modification in Drosophila Mushroom Bodies. Nat. Commun. 7: 13471 10.1038/ncomms13471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork S., Littleton J. T., 2007. A Complexin Fusion Clamp Regulates Spontaneous Neurotransmitter Release and Synaptic Growth. Nat. Neurosci. 10: 1235–1237. 10.1038/nn1980 [DOI] [PubMed] [Google Scholar]
- Ishimoto H., Sakai T., Kitamoto T., 2009. Ecdysone Signaling Regulates the Formation of Long-Term Courtship Memory in Adult Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 106: 6381–6386. 10.1073/pnas.0810213106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H., Wang Z., Rao Y., Chun F. W., Kitamoto T., 2013. A Novel Role for Ecdysone in Drosophila Conditioned Behavior: Linking GPCR-Mediated Non-Canonical Steroid Action to CAMP Signaling in the Adult Brain. PLoS Genet. 9: e1003843 10.1371/journal.pgen.1003843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis G. S., Potter C. J., Chan A. M., Marin E. C., Rohlfing T., et al. , 2007. Comprehensive Maps of Drosophila Higher Olfactory Centers: Spatially Segregated Fruit and Pheromone Representation. Cell 128: 1187–1203. 10.1016/j.cell.2007.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenett A., Rubin G. M., Ngo T. T., Shepherd D., Murphy C., et al. , 2012. A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Reports 2: 991–1001. 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. R., Atallah J., Plachetzki D. C., 2013. The Importance of Tissue Specificity for RNA-Seq: Highlighting the Errors of Composite Structure Extractions. BMC Genomics 14: 586 10.1186/1471-2164-14-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleman, K., S. Krüttner, M. Alenius, and B. J. Dickson, 2007. Function of the Drosophila CPEB Protein Orb2 in Long-Term Courtship Memory. Nat. Neurosci. 10: 1587–1593. 10.1038/nn1996 [DOI] [PubMed] [Google Scholar]
- Keleman, K., E. Vrontou, S. Krüttner, J. Y. Yu, A. Kurtovic-Kozaric et al. , 2012. Dopamine Neurons Modulate Pheromone Responses in Drosophila Courtship Learning. Nature 489: 145–149. 10.1038/nature11345 [DOI] [PubMed] [Google Scholar]
- Khan M. R., Li L., Pérez-Sánchez C., Saraf A., Florens L., et al. , 2015. Amyloidogenic Oligomerization Transforms Drosophila Orb2 from a Translation Repressor to an Activator. Cell 163: 1468–1483. 10.1016/j.cell.2015.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkhart C., Scott K., 2015. Gustatory Learning and Processing in the Drosophila Mushroom Bodies. J. Neurosci. 35: 5950–5958. 10.1523/JNEUROSCI.3930-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel R. J., Heckmann M., 2016. Synaptic Vesicle Proteins and Active Zone Plasticity. Front. Synaptic Neurosci. 8: 1–8. 10.3389/fnsyn.2016.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapek S., Sigrist S., Tanimoto H., 2011. Bruchpilot, A Synaptic Active Zone Protein for Anesthesia-Resistant Memory. J. Neurosci. 31: 3453–3458. 10.1523/JNEUROSCI.2585-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koemans T. S., Oppitz C., Donders R. A. T., van Bokhoven H., Schenck A., et al. , 2017. Drosophila Courtship Conditioning As a Measure of Learning and Memory. J. Vis. Exp. (124), e55808 10.3791/55808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Kochinke K., Oortveld M. A., Marks H., Kramer D., et al. , 2011. Epigenetic Regulation of Learning and Memory by Drosophila EHMT/G9a. PLoS Biol. 9: e1000569 10.1371/journal.pbio.1000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M. J., Keene A. C., Leung B., Armstrong J. D., Waddell S., 2007. Sequential Use of Mushroom Body Neuron Subsets during Drosophila Odor Memory Processing. Neuron 53: 103–115. 10.1016/j.neuron.2006.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu M., Nagao T., Walldorf U., Flister S., Gehring W. J., et al. , 2000. Genetic Control of Development of the Mushroom Bodies, the Associative Learning Centers in the Drosophila Brain, by the Eyeless, Twin of Eyeless, and Dachshund Genes. Proc. Natl. Acad. Sci. USA 97: 2140–2144. 10.1073/pnas.040564497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., 2015. Global and Local Missions of CAMP Signaling in Neural Plasticity, Learning, and Memory. Front. Pharmacol. 6: 1–7. 10.3389/fphar.2015.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L., 1999. Mosaic Analysis with a Repressible Cell Marker for Studies of Gene Function in Neuronal Morphogenesis. Neuron 22: 451–461. 10.1016/S0896-6273(00)80701-1 [DOI] [PubMed] [Google Scholar]
- Levin L. R., Han P. L., Hwang P. M., Feinstein P. G., Davis R. L., et al. , 1992. The Drosophila Learning and Memory Gene Rutabaga Encodes a Ca2+ Calmodulin-Responsive Adenylyl Cyclase. Cell 68: 479–489. 10.1016/0092-8674(92)90185-F [DOI] [PubMed] [Google Scholar]
- Li Y., Hoxha V., Lama C., Dinh B. H., Vo C. N., et al. , 2011. The Hector G-Protein Coupled Receptor Is Required in a Subset of Fruitless Neurons for Male Courtship Behavior. PLoS One 6: e28269 10.1371/journal.pone.0028269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., 1994. The CaM Kinase II Hypothesis for the Storage of Synaptic Memory. Trends Neurosci. 17: 406–412. 10.1016/0166-2236(94)90014-0 [DOI] [PubMed] [Google Scholar]
- Liu C., Plaa̧ais P. Y., Yamagata N., Pfeiffer B. D., Aso Y., et al. , 2012. A Subset of Dopamine Neurons Signals Reward for Odour Memory in Drosophila. Nature 488: 512–516. 10.1038/nature11304 [DOI] [PubMed] [Google Scholar]
- Liu K. S. Y., Siebert M., Mertel S., Knoche E., Wegener S., et al. , 2011. RIM-Binding Protein, a Central Part of the Active Zone, Is Essential for Neurotransmitter Release. Science 334: 1565–1569. 10.1126/science.1212991 [DOI] [PubMed] [Google Scholar]
- Livingstone M. S., Sziber P. P., Quinn W. G., 1984. Loss of Calcium Calmodulin Responsiveness in Adenylate-Cyclase of Rutabaga, a Drosophila Learning Mutant. Cell 37: 205–215. 10.1016/0092-8674(84)90316-7 [DOI] [PubMed] [Google Scholar]
- Love M. I., Huber W., Anders S., 2014. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 15: 550 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C. C., Robinson G. E., 2013. Activity-Dependent Gene Expression in Honey Bee Mushroom Bodies in Response to Orientation Flight. J. Exp. Biol. 216: 2031–2038. 10.1242/jeb.084905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli D. S., Foss M., Villella A., Taylor B. J., Hall J. C., et al. , 2005. Male-Specific Fruitless Specifies the Neural Substrates of Drosophila Courtship Behaviour. Nature 436: 395–400. 10.1038/nature03859 [DOI] [PubMed] [Google Scholar]
- Mayford M., Reijmers L., 2016. Exploring Memory Representations with Activity-Based Genetics. Cold Spring Harb. Perspect. Biol. 8: a021832 10.1101/cshperspect.a021832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride S. M., Giuliani G., Choi C., Krause P., Correale D., et al. , 1999. Mushroom Body Ablation Impairs Short-Term Memory and Long-Term Memory of Courtship Conditioning in Drosophila Melanogaster. Neuron 24: 967–977. 10.1016/S0896-6273(00)81043-0 [DOI] [PubMed] [Google Scholar]
- Mi H., Huang X., Muruganujan A., Tang H., Mills C., et al. , 2017. PANTHER Version 11: Expanded Annotation Data from Gene Ontology and Reactome Pathways, and Data Analysis Tool Enhancements. Nucleic Acids Res. 45: D183–D189. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague S. A., Baker B. S., 2016. Memory Elicited by Courtship Conditioning Requires Mushroom Body Neuronal Subsets Similar to Those Utilized in Appetitive Memory. PLoS One 11: e0164516 10.1371/journal.pone.0164516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montarolo P. G., Goelet P., Castellucci V. F., Morgan J., Kandel E. R., et al. , 1986. A Critical Period for Macromolecular Synthesis in Long-Term Heterosynaptic Facilitation in Aplysia. Science 234: 1249–1254. 10.1126/science.3775383 [DOI] [PubMed] [Google Scholar]
- Montojo J., Zuberi K., Rodriguez H., Bader G. D., Morris Q., 2014. GeneMANIA: Fast Gene Network Construction and Function Prediction for Cytoscape. F1000 Res. 3: 1–7. 10.12688/f1000research.4572.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. H., Kuchinsky A., Ferrin T. E., Pico A. R., 2014. EnhancedGraphics: A Cytoscape App for Enhanced Node Graphics. F1000 Res. 3: 147 10.12688/f1000research.4460.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noveen A., Daniel A., Hartenstein V., 2000. Early Development of the Drosophila Mushroom Body: The Roles of Eyeless and Dachshund. Development 127: 3475–3488. [DOI] [PubMed] [Google Scholar]
- Owald, David, Johannes Felsenberg, Clifford B. Talbot, Gaurav Das, Emmanuel Perisse, Wolf Huetteroth, and Scott Waddell, 2015 Activity of Defined Mushroom Body Output Neurons Underlies Learned Olfactory Behavior in Drosophila. Neuron 86: 417–427. 10.1016/j.neuron.2015.03.025 [DOI] [PMC free article] [PubMed]
- Pavlowsky A., Schor J., Plaçais P. Y., Preat T., 2018. A GABAergic Feedback Shapes Dopaminergic Input on the Drosophila Mushroom Body to Promote Appetitive Long-Term Memory. Curr. Biol. 28: 1783–1793.e4. 10.1016/j.cub.2018.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavot, P., E. Carbognin, and J.-R. Martin, 2015 PKA and CAMP/CNG Channels Independently Regulate the Cholinergic Ca2+-Response of Drosophila Mushroom Body Neurons. eNeuro 2: ENEURO.005414.2015 10.1523/ENEURO.0054-14.2015 [DOI] [PMC free article] [PubMed]
- Peixoto L. L., Wimmer M. E., Poplawski S. G., Tudor J. C., Kenworthy C. A., et al. , 2015. Memory Acquisition and Retrieval Impact Different Epigenetic Processes That Regulate Gene Expression. BMC Genomics 16 (Suppl 5): S5 10.1186/1471216416S5-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisse, Emmanuel, David Owald, Oliver Barnstedt, Clifford B B. Talbot, Wolf Huetteroth, and Scott Waddell, 2016 Aversive Learning and Appetitive Motivation Toggle Feed-Forward Inhibition in the Drosophila Mushroom Body. Neuron 90: 1086–1099. 10.1016/j.neuron.2016.04.034 [DOI] [PMC free article] [PubMed]
- Plaçais P. Y., De Tredern É., Scheunemann L., Trannoy S., Goguel V., et al. , 2017. Upregulated Energy Metabolism in the Drosophila Mushroom Body Is the Trigger for Long-Term Memory. Nat. Commun. 8: 15510 10.1038/ncomms15510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2016. “R: A Language and Environment for Statistical Computing.” Vienna, Austria: R foundation for statistical computing. https://www.r-project.org/.
- Schinaman J. M., Giesey R. L., Mizutani C. M., Lukacsovich T., Sousa-Neves R., 2014. The KRÜPPEL-Like Transcription Factor DATILÓGRAFO Is Required in Specific Cholinergic Neurons for Sexual Receptivity in Drosophila Females. PLoS Biol. 12: e1001964 10.1371/journal.pbio.1001964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., et al. , 2012. Fiji: An Open Source Platform for Biological Image Analysis. Nat. Methods 9: 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R., Edwards R., 2011. Quality Control and Preprocessing of Metagenomic Datasets. Bioinformatics 27: 863–864. 10.1093/bioinformatics/btr026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra‐Mondejar M., Casellas‐Díaz S., Ramiro‐Pareta M., Müller‐Sánchez C., Martorell‐Riera A., et al. , 2018. Synaptic Activity‐induced Glycolysis Facilitates Membrane Lipid Provision and Neurite Outgrowth. EMBO J. 37 e97368 10.15252/embj.201797368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami, Azza, and Jan A. Veenstra, 2015 SIFamide Acts on Fruitless Neurons to Modulate Sexual Behavior in Drosophila Melanogaster. Peptides 74: 50–56. 10.1016/j.peptides.2015.10.003 [DOI] [PubMed]
- Siegel R. W., Hall J. C., 1979. Conditioned Responses in Courtship Behavior of Normal and Mutant Drosophila. Proc. Natl. Acad. Sci. USA 76: 3430–3434. 10.1073/pnas.76.7.3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall, Tony D., Katrina S. Gold, Boris Egger, Catherine M. Davidson, Elizabeth E. Caygill, Owen J. Marshall, and Andrea H. Brand, 2013 Cell-Type-Specific Profiling of Gene Expression and Chromatin Binding without Cell Isolation: Assaying RNA Pol II Occupancy in Neural Stem Cells. Dev. Cell. 26: 101–112. 10.1016/j.devcel.2013.05.020 [DOI] [PMC free article] [PubMed]
- Spieth H. T., 1974. Courtship Behavior in Drosophila. Annu. Rev. Entomol. 19: 385–405. 10.1146/annurev.en.19.010174.002125 [DOI] [PubMed] [Google Scholar]
- Steiner F. A., Talbert P. B., Kasinathan S., Deal R. B., Henikoff S., 2012. Cell-Type-Specific Nuclei Purification from Whole Animals for Genome-Wide Expression and Chromatin Profiling. Genome Res. 22: 766–777. 10.1101/gr.131748.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, G, J Morris, B Demchak, and G Bader, 2014 Biological Network Exploration With Cytoscape 3. Curr. Protoc. Bioinformatics. 47: 8.13.1–8.13.24 10.1002/0471250953.bi0813s47 [DOI] [PMC free article] [PubMed]
- Tadi M., Allaman I., Lengacher S., Grenningloh G., Magistretti P. J., 2015. Learning-Induced Gene Expression in the Hippocampus Reveals a Role of Neuron -Astrocyte Metabolic Coupling in Long Term Memory. PLoS One 10: e0141568 10.1371/journal.pone.0141568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona S., Furió-Tarí P., Turrà D., Di Pietro A., Nueda M. J., et al. , 2015. Data Quality Aware Analysis of Differential Expression in RNA-Seq with NOISeq R/Bioc Package. Nucleic Acids Res. 43 10.1093/nar/gkv711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomchik S. M., Davis R. L., 2009. Dynamics of Learning-Related CAMP Signaling and Stimulus Integration in the Drosophila Olfactory Pathway. Neuron 64: 510–521. 10.1016/j.neuron.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trannoy S., Redt-Clouet C., Dura J. M., Preat T., 2011. Parallel Processing of Appetitive Short- and Long-Term Memories in Drosophila. Curr. Biol. 21: 1647–1653. 10.1016/j.cub.2011.08.032 [DOI] [PubMed] [Google Scholar]
- Tully T., Bourtchouladze R., Scott R., Tallman J., 2003. Targeting the CREB Pathway for Memory Enhancers. Nat. Rev. Drug Discov. 2: 267–277. 10.1038/nrd1061 [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A., Matheos D. P., Barrett R. M., Kramár E. A., Azzawi S., et al. , 2013. The Neuron-Specific Chromatin Regulatory Subunit BAF53b Is Necessary for Synaptic Plasticity and Memory. Nat. Neurosci. 16: 552–561. 10.1038/nn.3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmann A., Artinger M., Biesinger L., Boepple K., Peters C., et al. , 2016. Genetic Dissection of Aversive Associative Olfactory Learning and Memory in Drosophila Larvae. PLoS Genet. 12: e1006378 10.1371/journal.pgen.1006378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer Y. F., Bilican A., Bruggmann R., Sprecher S. G., 2018. Regulators of Long-Term Memory Revealed by Mushroom Body-Specific Gene Expression Profiling in Drosophila Melanogaster. Genetics 209: 1167–1181. 10.1534/genetics.118.301106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winbush A., Reed D., Chang P. L., Nuzhdin S. V., Lyons L. C., et al. , 2012. Identification of Gene Expression Changes Associated with Long-Term Memory of Courtship Rejection in Drosophila Males. G3 (Bethesda) 2: 1437–1445. 10.1534/g3.112.004119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D., Akalal D. B., Davis R. L., 2006. Drosophila Alpha/Beta Mushroom Body Neurons Form a Branch-Specific, Long-Term Cellular Memory Trace after Spaced Olfactory Conditioning. Neuron 52: 845–855. 10.1016/j.neuron.2006.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuyama K., Meinertzhagen I. A., Schürmann F. W., 2002. Synaptic Organization of the Mushroom Body Calyx in Drosophila Melanogaster. J. Comp. Neurol. 445: 211–226. 10.1002/cne.10155 [DOI] [PubMed] [Google Scholar]
- Zhang J., Tanenhaus A. K., Davis J. C., Hanlon B. M., Yin J. C. P., 2015. Spatio-Temporal in Vivo Recording of DCREB2 Dynamics in Drosophila Long-Term Memory Processing. Neurobiol. Learn. Mem. 118: 80–88. 10.1016/j.nlm.2014.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Lenek D., Dag U., Dickson B. J., Keleman K., 2018. Persistent Activity in a Recurrent Circuit Underlies Courtship Memory in Drosophila. eLife 7 10.7554/eLife.31425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C., Huang H., Kim S. M., Lin H., Meng X., et al. , 2012. Molecular Genetic Analysis of Sexual Rejection: Roles of Octopamine and Its Receptor OAMB in Drosophila Courtship Conditioning. J. Neurosci. 32: 14281–14287. 10.1523/JNEUROSCI.0517-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovkic I. B., Paulukaitis B. S., Day J. J., Etikala D. M., David Sweatt J., 2014. Histone H2A.Z Subunit Exchange Controls Consolidation of Recent and Remote Memory. Nature 515: 582–586. 10.1038/nature13707 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Figure S1 contains PCA results of MB and WH RNA sequencing data. Figure S2 contains comparison of DE results between DESeq2 and NOISeq. Table S1 contains read alignment and count data. Table S2 contains DE analysis results for MB specificity. Table S3 contains GO results for DE MB enriched or depleted genes. Table S4 contains DE analysis results for MB and WH specific samples during a time course of LTM. Table S5 contains the results of k-means clustering of DE genes during LTM formation. Table S6 contains GO results for clusters of DE genes identified during LTM formation. Gene expression data are available at GEO with the accession number: GSE115718. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7005842.