Abstract
Metabolic state is a potent modulator of sleep and circadian behavior, and animals acutely modulate their sleep in accordance with internal energy stores and food availability. Across phyla, hormones secreted from adipose tissue act in the brain to control neural physiology and behavior to modulate sleep and metabolic state. Growing evidence suggests the fat body is a critical regulator of complex behaviors, but little is known about the genes that function within the fat body to regulate sleep. To identify molecular factors functioning in non-neuronal tissues to regulate sleep, we performed an RNAi screen selectively knocking down genes in the fat body. We found that knockdown of Phosphoribosylformylglycinamidine synthase/Pfas (Ade2), a highly conserved gene involved the biosynthesis of purines, sleep regulation and energy stores. Flies heterozygous for multiple Ade2 mutations are also short sleepers and this effect is partially rescued by restoring Ade2 to the Drosophila fat body. Targeted knockdown of Ade2 in the fat body does not alter arousal threshold or the homeostatic response to sleep deprivation, suggesting a specific role in modulating baseline sleep duration. Together, these findings suggest Ade2 functions within the fat body to promote both sleep and energy storage, providing a functional link between these processes.
Keywords: fat body, metabolism, Drosophila, sleep, RNAI screen, purine metabolism
Animals balance nutritional state and energy expenditure in order to achieve metabolic homeostasis (Taguchi and White 2008; Yurgel et al. 2015). In the fruit fly, Drosophila melanogaster, feeding behavior and metabolism are regulated by non-neuronal tissues including the muscle, the adipose-like organ called the fat body, and the gastrointestinal tract (Erion and Sehgal 2013; Itskov and Ribeiro 2013). Similarly, in mammals, endocrine hormones such as ghrelin, leptin, insulin, and glucagon are secreted from the stomach, adipose tissue, and pancreas, to convey nutritional status to the brain regions that regulate sleep and metabolism (Marks et al. 2009; Bruijnzeel et al. 2011; Karra et al. 2013). Dysregulation of non-neuronal hormonal signals leads to a number of metabolic diseases including obesity, diabetes, and insomnia (Arble et al. 2015). Therefore, mechanistic investigation of factors regulating brain-periphery communication is critical to understanding disorders associated with sleep and metabolism.
Adipose tissue senses overall nutrient levels in the animal and modulates hunger-induced behaviors through controlling energy storage and secreting factors that act on the nervous system to affect behavior (Xu et al. 2011; Sassu et al. 2012; Musselman et al. 2013). The insect fat body is central to the control of energy homeostasis. It is the primary site of glycogen and triglyceride storage, and the main detoxification organ in the fly, thereby exhibiting functions analogous to the mammalian liver and adipose tissue (Arrese and Soulages 2010). Genome-wide transcriptome analysis has identified many genes that are upregulated during starvation, including metabolic enzymes, cytochromes, metabolite transporters, kinases, and proteins involved in lipid metabolism (Grönke et al. 2005). Even though the primary function of many of these genes in the regulation of energy storage has been studied in detail, little is known about how they may impact sleep and other behaviors.
Many of the genes and transmitters required for metabolic regulation of sleep and feeding in mammals are conserved in Drosophila (Allada and Siegel 2008; Padmanabha and Baker 2014), and numerous conserved factors have been identified that regulate sleep and metabolic function (Arble et al. 2015; Yurgel et al. 2015). The GAL4/UAS system, in combination with genome-wide RNAi libraries, allow for selectively decreasing gene expression in the fly fat body and then measuring the effects on sleep (Brand and Perrimon 1993; Dietzl et al. 2007). Growing evidence suggests that the fat body regulates complex behaviors including sleep (Lazareva et al. 2007; Kim et al. 2017; Umezaki et al. 2018), yet the molecular basis through which the fat body regulates sleep remains poorly understood.
Here, we sought to identify sleep regulators in the fat body by selectively decreasing the expression of genes that have been previously identified to be upregulated in starved flies and then measuring their effects on sleep (Grönke et al. 2005). We identified that Phosphoribosylformylglycinamidine synthase (Ade2), a highly conserved gene involved in the biosynthesis of purines, is required for normal sleep in flies. Flies deficient for Ade2 are short-sleepers and have reduced triglyceride stores, suggesting that loss of Ade2 impairs energy storage and inhibits sleep. Disruption of Ade2 in the fat body does not disrupt arousal threshold and homeostatic response to sleep deprivation, suggesting a specific role in modulating baseline sleep duration. These findings provide a novel factor that functions in the fat body to regulate sleep, and support growing evidence that a non-neuronal metabolic tissue is critical for the proper regulation of sleep.
Methods
Fly Stocks
Flies were grown and maintained on standard food (Bloomington Recipe, Genesee Scientific). Flies were kept in incubators (Powers Scientific; Dros52) at 25° on a 12:12 LD cycle with humidity set to 55–65%. The background control line used in this study is w1118 fly strain, and all experimental flies were outcrossed 6-8 generations into this background, unless already in this background. The following fly strains were ordered from Bloomington Stock Center, w1118(5905; Levis et al. 1985),CG-GAL4 (7011; Asha et al. 2003), r4-GAL4(33832; Lee and Park 2004), and hmlΔ3-GAL4 (30141; Goto et al. 2003). Ade23-20, Ade21-6, and UAS-Ade2 were obtained from D. Clark and have been previously characterized (Holland et al. 2011). Drosophila lines used in the RNAi screen originate from the TRiP collection (Brand and Perrimon 1993; Ni et al. 2009) and are described in (Table 1).
Table 1. RNAi lines used for fat body screen.
| BL Stock # | Total Sleep | Day Sleep | Night sleep | Waking activity | Total bout | ABL | ||
|---|---|---|---|---|---|---|---|---|
| FBgn0012034 | 41917 | AcCOAs | 1030.63 | 339.06 | 691.56 | 1.05 | 27.50 | 39.46 |
| FBgn0000052 | 36686 | ade2 | 722.50 | 67.50 | 655.00 | 0.88 | 17.67 | 42.63 |
| FBgn0000078 | 57561 | Amy-D | 1025.42 | 351.67 | 673.75 | 0.92 | 29.83 | 36.56 |
| FBgn0036449 | 25926 | bmm | 1057.33 | 392.00 | 665.33 | 1.16 | 30.82 | 36.44 |
| FBgn0039241 | 53332 | CG11098 | 979.06 | 284.06 | 695.00 | 0.66 | 22.88 | 46.56 |
| FBgn0039649 | 34885 | CG11198 | 1136.25 | 460.31 | 675.94 | 1.06 | 24.25 | 55.81 |
| FBgn0034721 | 38214 | CG11298 | 1212.50 | 503.75 | 708.75 | 1.07 | 23.50 | 52.07 |
| FBgn0039299 | 57194 | CG11854 | 1085.00 | 402.92 | 682.08 | 1.20 | 32.67 | 35.62 |
| FBgn0039649 | 51476 | CG11899 | 899.06 | 245.00 | 654.06 | 0.75 | 33.38 | 28.42 |
| FBgn0039330 | 53379 | CG11909 | 1085.63 | 419.38 | 666.25 | 0.78 | 29.19 | 41.18 |
| FBgn0035228 | 40936 | CG12091 | 1040.31 | 345.63 | 694.69 | 0.86 | 27.75 | 38.68 |
| FBgn0027842 | 33635 | CG12891 | 1060.00 | 397.50 | 662.50 | 0.97 | 30.20 | 41.02 |
| FBgn0036419 | 58240 | CG13482 | 1051.88 | 355.31 | 696.56 | 0.93 | 19.88 | 54.49 |
| FBgn0034404 | 50676 | CG15101 | 879.00 | 204.50 | 674.50 | 0.71 | 27.20 | 33.02 |
| FBgn0025803 | 35341 | CG17299 | 936.25 | 262.19 | 674.06 | 0.80 | 26.88 | 37.55 |
| FBgn0035108 | 35188 | CG18374 | 979.09 | 299.55 | 679.55 | 0.98 | 30.64 | 34.73 |
| FBgn0034382 | 44510 | CG18609 | 967.19 | 267.67 | 696.33 | 1.07 | 24.40 | 42.31 |
| FBgn0029823 | 57739 | CG3011 | 1095.00 | 415.00 | 680.00 | 0.76 | 28.78 | 41.68 |
| FBgn0031645 | 43179 | CG3036 | 830.00 | 168.75 | 661.25 | 0.86 | 23.25 | 37.89 |
| FBgn0039361 | 31150 | CG31092 | 991.25 | 325.31 | 665.94 | 1.51 | 32.81 | 37.64 |
| FBgn0038463 | 35245 | CG3534 | 866.25 | 233.13 | 633.13 | 0.93 | 33.43 | 28.72 |
| FBgn0023507 | 53355 | CG3835 | 1031.50 | 351.50 | 680.00 | 0.89 | 31.20 | 34.95 |
| FBgn0034664 | 61330 | CG4377 | 907.22 | 223.89 | 683.33 | 0.67 | 27.56 | 35.32 |
| FBgn0032349 | 55629 | CG4779 | 1084.06 | 385.94 | 698.13 | 1.23 | 22.88 | 50.50 |
| FBgn0035950 | 60091 | CG5288 | 1025.94 | 329.38 | 696.56 | 0.72 | 27.31 | 39.24 |
| FBgn0039493 | 35486 | CG5889 | 963.13 | 276.33 | 692.33 | 1.30 | 25.73 | 39.07 |
| FBgn0029831 | 33932 | CG5966 | 828.93 | 179.29 | 649.64 | 0.85 | 29.36 | 29.72 |
| FBgn0034247 | 60372 | CG6484 | 1200.00 | 477.00 | 707.00 | 1.09 | 17.40 | 75.96 |
| FBgn0036030 | 60086 | CG6767 | 1177.00 | 484.00 | 693.00 | 0.83 | 24.40 | 52.88 |
| FBgn0033385 | 38305 | CG8055 | 1043.24 | 367.65 | 675.59 | 0.85 | 27.41 | 39.16 |
| FBgn0034003 | 57404 | CG8094 | 963.13 | 300.94 | 662.19 | 0.75 | 32.25 | 30.98 |
| FBgn0022073 | 36667 | CG8846 | 1013.13 | 311.88 | 701.25 | 0.72 | 23.50 | 44.63 |
| FBgn0031689 | 53892 | Cyp28d1 | 1056.56 | 368.44 | 688.13 | 0.80 | 26.75 | 42.65 |
| FBgn0015714 | 33887 | Cyp6a17 | 975.50 | 311.00 | 664.50 | 1.10 | 32.80 | 32.07 |
| FBgn0000473 | 64008 | Cyp6a2 | 1011.79 | 335.71 | 676.07 | 0.96 | 25.07 | 43.28 |
| FBgn0037249 | 27565 | EIF-S10 | 869.69 | 180.31 | 689.38 | 0.68 | 23.30 | 37.87 |
| FBgn0033465 | 56864 | Etf-QO | 887.14 | 281.43 | 605.71 | 0.90 | 32.71 | 30.25 |
| FBgn0263773 | 63980 | FOK | 1040.45 | 353.64 | 686.82 | 0.73 | 28.91 | 39.11 |
| FBgn0030013 | 31118 | GIIIspla2 | 985.00 | 318.75 | 666.25 | 1.02 | 32.56 | 32.29 |
| FBgn0030484 | 36717 | GstT4 | 858.00 | 165.00 | 693.00 | 0.71 | 23.30 | 38.88 |
| FBgn0001565 | 28991 | hlc | 925.83 | 238.08 | 673.33 | 0.90 | 21.92 | 44.75 |
| FBgn0001208 | 29540 | Hn | 812.81 | 187.19 | 625.63 | 1.49 | 25.69 | 35.12 |
| FBgn0264785 | 34717 | hph | 885.00 | 195.94 | 689.06 | 1.27 | 24.13 | 39.14 |
| FBgn0034329 | 42599 | IM1 | 1041.00 | 360.00 | 681.00 | 0.86 | 22.90 | 47.18 |
| FBgn0025583 | 28788 | IM2 | 875.31 | 237.50 | 637.81 | 1.63 | 25.06 | 36.49 |
| FBgn0038465 | 57814 | IRC | 938.13 | 306.47 | 628.24 | 1.08 | 37.94 | 28.36 |
| FBgn0001301 | 31251 | Kelch 1 | 1042.50 | 347.50 | 695.00 | 0.73 | 26.29 | 42.49 |
| FBgn0001301 | 55612 | Kelch 2 | 1025.31 | 334.38 | 690.94 | 0.95 | 29.00 | 37.14 |
| FBgn0034140 | 60400 | Limostatin | 1077.81 | 401.25 | 676.56 | 0.95 | 25.00 | 45.52 |
| FBgn0017581 | 28357 | LK6 1 | 1044.17 | 380.83 | 663.33 | 0.88 | 32.92 | 33.61 |
| FBgn0017581 | 35352 | LK6 2 | 905.94 | 196.25 | 709.69 | 0.65 | 22.56 | 42.63 |
| FBgn0030608 | 32846 | lsd2 | 1088.62 | 397.76 | 690.86 | 1.46 | 26.86 | 53.74 |
| FBst0056039 | 56039 | lsp1 | 996.00 | 311.50 | 684.50 | 0.82 | 27.20 | 38.87 |
| FBst0031603 | 31603 | luc | 975.32 | 292.62 | 682.70 | 1.18 | 27.20 | 38.49 |
| FBgn0033296 | 62252 | MALA7 | 1039.06 | 396.56 | 642.50 | 1.03 | 31.25 | 38.96 |
| FBgn0033297 | 55193 | MALA8 | 1061.25 | 383.33 | 677.92 | 0.91 | 25.83 | 43.76 |
| FBgn0032381 | 55346 | MALB1 | 914.69 | 246.88 | 667.81 | 0.69 | 31.44 | 30.48 |
| FBgn0032382 | 62253 | MALB2 | 920.63 | 254.69 | 665.94 | 0.80 | 28.69 | 35.34 |
| FBgn0029870 | 31157 | Marf-RNAi | 953.13 | 302.19 | 650.94 | 1.03 | 30.75 | 32.58 |
| FBgn0027579 | 63587 | mino | 965.50 | 305.50 | 660.00 | 0.89 | 30.30 | 34.35 |
| FBgn0010222 | 62268 | Nmdmc | 1063.13 | 366.88 | 696.25 | 0.84 | 26.38 | 41.24 |
| FBgn0017558 | 28635 | pdk | 1064.29 | 374.64 | 689.64 | 1.03 | 23.86 | 46.59 |
| FBgn0000489 | 27569 | pkaC3 | 1093.75 | 392.81 | 700.94 | 0.93 | 27.19 | 43.02 |
| FBgn0027601 | 55272 | pudgy | 1036.25 | 339.38 | 696.88 | 0.69 | 24.00 | 45.53 |
| FBgn0016715 | 57766 | Reg2 | 1094.58 | 407.92 | 686.67 | 1.05 | 22.50 | 53.33 |
| FBgn0031971 | 43213 | sirup | 955.63 | 282.19 | 673.44 | 0.99 | 31.63 | 31.87 |
| FBgn0024289 | 34556 | Sodh1 | 913.75 | 242.50 | 671.25 | 1.26 | 27.31 | 36.86 |
| FBgn0014031 | 51935 | spat | 922.00 | 281.50 | 640.50 | 0.93 | 31.80 | 29.83 |
| FBgn0035147 | 44496 | UDP | 1020.00 | 328.75 | 691.25 | 0.94 | 28.75 | 38.73 |
| FBgn0030904 | 33949 | upd2 1 | 898.18 | 328.00 | 634.00 | 1.01 | 34.27 | 31.10 |
| FBgn0030904 | 33988 | upd2 2 | 860.63 | 203.13 | 657.50 | 0.96 | 21.81 | 44.19 |
Sleep analysis
The Drosophila Activity Monitor System (DAMS) detects activity by monitoring infrared beam crossings for each animal (Pfeiffenberger et al. 2010a). These data were used to calculate sleep information by extracting immobility bouts of 5 min using the Drosophila Counting Macro (Pfeiffenberger et al. 2010b; Garbe et al. 2015). For all experiments, flies were kept on 12:12 LD cycle. 5-7 day old female flies were briefly anesthetized with CO2 and placed into plastic tubes containing standard food. All flies were given 24 hr to recover after being anesthetized. Activity was recorded for 24 hr on food (ZT0-ZT24).
Protein, glucose, glycogen and triglyceride measurements
Assays for quantifying triglyceride, glycogen, free glucose and protein content of flies were performed as previously described (Mikoluk et al. 2018). Two bodies from female flies aged 3-5 days were homogenized in buffer containing 50 mM Tris-HCl, pH 7.4, 140mM NaCl, 0.1% Triton-X, 1X protease inhibitor cocktail (Roche). Triglyceride concentration was measured using the Infinity Triglyceride Reagent (ThermoFisher), and protein concentrations were measuring using a BCA Protein Assay Kit (Pierce Scientific). Total glucose levels were determined using the Glucose Oxidase Reagent (Pointe Scientific) in samples previously treated with 8mg/mL amyloglucosidase (Sigma) in 0.2M Sodium Citrate buffer, pH 5.0. Free glucose was measured in samples not treated with amyloglucosidase and then glycogen concentrations were determined by subtracting the free glucose from total glucose concentration. Free glucose, glycogen and triglyceride concentrations were standardized to the total protein content of each sample.
Sleep deprivation
Five-seven day old fruit flies were loaded into the DAM System and allowed to acclimate for 24 hr. Following acclimation, day sleep (ZT0-ZT12) was measured in undisturbed flies. Flies were then sleep deprived by mechanical stimulation every 2-3 min for 12 hr throughout the night time (ZT12-24). The mechanical stimulus was applied using a vortexer (Fisher Scientific, MultiTube Vortexer) and a repeat cycle relay switch (Macromatic, TR63122). Sleep rebound was measured the following day from ZT0-ZT12.
Arousal Threshold
Arousal threshold was measured using the Drosophila Arousal Tracking system (DART), as previously described (Faville et al. 2015). In brief, individual female flies were loaded in plastic tubes (Trikinectics, Waltham, MA) and placed on plastic trays containing vibrating motors. Arousal threshold was tested with sequentially increasing vibration intensities, from 0 to 1.2 g, in 0.3 g (200 ms) increments, with an inter-stimulus delay of 15 s, once per hour over 24 hours starting at ZT0. Flies were recorded continuously using a USB-webcam (Logitech) at 1 frame per second. The vibrational stimulus and video tracking parameters, and data analysis were performed using the DART interface developed in Matlab (MathWorks, Natick, MA).
Statistical Analysis
The experimental data are presented as means ± SEM. Unless otherwise noted a one-way (ANOVA) followed by Tukey’s post-hoc test was used for comparisons between two or more genotypes and one treatment. Unpaired t-test was used for comparisons between two genotypes. For arousal threshold experiment, the non-parametric Mann Whitney U-test was used to compare two genotypes. For two or more genotypes, a Kruskal-Wallis test followed by Dunn’s post hoc test was used. All statistical analyses were performed using InStat software (GraphPad Software 6.0) with a 95% confidence limit (P < 0.05).
Data Availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7121264.
Results
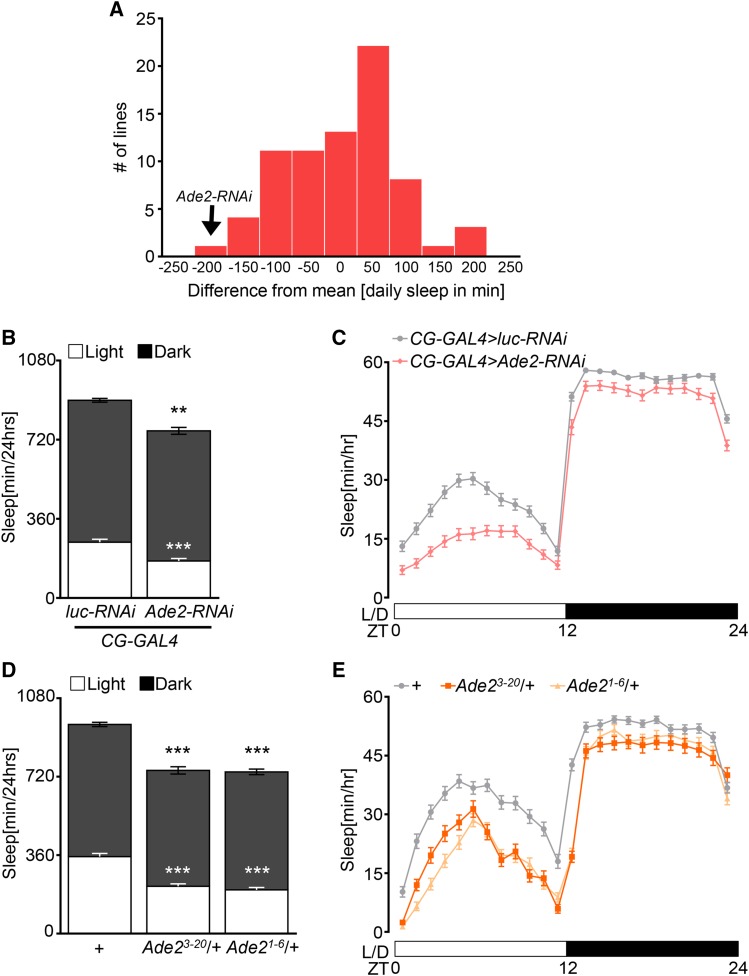
To identify genes expressed in adipose tissue that regulate sleep, we performed an RNAi screen by assaying the TRiP RNAi collection to selectively knock down genes in the fat body (Brand and Perrimon 1993; Ni et al. 2009). A total of 113 genes previously reported to be upregulated in whole flies during starvation (Grönke et al. 2005) were selectively knocked down in the fat body using the GAL4 driver CG-GAL4 (Asha et al. 2003), and female flies were then assayed for sleep (Figure 1A) in the Drosophila Activity Monitors (Pfeiffenberger et al. 2010a). Flies with RNAi targeted to the fat body were compared to controls expressing RNAi targeted to luciferase (CG-GAL4 > luc-RNAi). Knockdown of Ade2 in the fat body (CG-GAL4 > Ade2-RNAi) resulted in a loss of over 200 min of sleep, while a knockdown of the glucose transporter CG6484 (CG-GAL4 > CG6484-RNAi) and CG6767, a kinase involved in purine/pyrimidine metabolism, (CG-GAL4 > CG6767-RNAi) resulted in increased sleep (Table 1). We chose to focus analysis on the role of Ade2 in sleep regulation because of the robust sleep loss phenotype identified in the screen. To verify these results, we retested the effects of Ade2 knockdown on sleep. In agreement with the screen, sleep was reduced in Ade2 knockdown flies (CG-GAL4 > Ade2-RNAi) compared to control flies with CG-GAL4 driving RNAi targeted to luciferase (CG-GAL4 > luc-RNAi) (Figure 1B). Quantification of sleep throughout the 24 hr testing period revealed that fat body specific knockdown of Ade2 results in sleep loss with significant reductions during both the day and night periods, suggesting Ade2 is required for both day and nighttime sleep (Figure 1B,C).
Figure 1.
Ade2 functions in the fat body to promote sleep. (A) Histogram showing the distribution of sleep over 24 hr from fat body-specific knockdown of genes previously reported to be upregulated during starvation. Daily sleep is depicted as the difference between the mean of a group of ∼80 viable lines tested. Black arrow indicates the Ade2-RNAi control line. (B) Knock down of Ade2 in the fat body (CG-GAL4 > UAS-Ade2-RNAi; n = 84) results in a significant reduction in sleep during daytime (white; P < 0.0001, t = 6.241) and nighttime (black; P = 0.0004, t = 3.614) compared to control flies (CG-GAL4 > UAS-luc-RNAi; n = 115). Unpaired t-test. (C) Sleep profile of hourly sleep averages over a 24 hr experiment. White/black bars represent lights on and off, respectively. ZT denotes Zeitgeber time. Sleep is reduced in flies expressing Ade2-RNAi in the fat body (pink) compared to control (gray). (D) Sleep is significantly reduced in Ade23-20/+ mutants (n = 66) and Ade21-6/+ (n = 90) during daytime (P < 0.0001 for all groups) and nighttime (P = 0.0002 and p = 0.004) compared to w1118 control (n = 110). One-way ANOVA, Light, F(2, 261) = 11.20; Dark, F(2, 263)=45.91. (E) Sleep profile of hourly sleep for Ade23-20/+ mutants (dark orange), Ade21-6/+ (light orange), and w1118 control (gray). All columns are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
The CG-GAL4 driver is expressed in both the hemocytes and fat body.(Yoshida et al. 2001; Asha et al. 2003). To identify the sleep-regulating cell-type, we knocked down Ade2 using an additional fat-body GAL4 driver line, r4-GAL4, which exclusively labels the fat body (Lee and Park 2004). Targeting Ade2-RNAi with r4-GAL4 decreased both daytime and nighttime sleep compared to control flies expressing luciferase (r4-GAL4 > luc-RNAi), phenocopying knockdown with CG-GAL4 (Fig S1A). Conversely, knocking down Ade2 in adult hemocytes using hmlΔ3-GAL4 did not affect sleep (Fig S1B, (Goto et al. 2003)), supporting the notion that Ade2 functions in the fat body to promote sleep.
To confirm that the sleep loss phenotype observed with Ade2 knockdown was not due to off-target effects of RNAi, we assayed sleep in Ade2 mutant Drosophila. Two Ade2 mutants, Ade23-20 and Ade21-6, have been generated by P-element excision (Holland et al. 2011). For Ade21-6, the deletion includes the transcription start site and part of the first coding exon resulting in a null allele (Holland et al. 2011). While both alleles are homozygous lethal, heterozygous flies are viable. Flies heterozygous for Ade23-20 or Ade21-6 sleep less than w1118 flies (the background control strain) during the day and night, phenocopying results obtained with RNAi knockdown in the fat body (Figure 1D). Further, a sleep profile analysis shows sleep loss is reduced throughout the day and night confirming the sleep phenotype observed in RNAi knockdown flies (Figure 1E).
Reduced sleep can be accounted for by a reduction in the total number of sleep bouts, shortened duration of individual sleep bouts, or a combination of both (Garbe et al. 2015). RNAi knockdown of Ade2 (CG-GAL4 > UAS-Ade2-RNAi) resulted in reduced sleep bout length compared to control flies (Fig S1C), while total sleep bout number was reduced during the day and increased during the night (Fig S1D). Similarly, average sleep bout length was significantly reduced in both Ade2 mutants (Ade23-20 and Ade21-6) compared to controls, suggesting that both Ade2-RNAi and mutant flies present a less consolidated sleep pattern (Fig S1E). No difference in sleep bout number was detected during the daytime in flies heterozygous for the Ade23-20 or Ade21-6 mutations compared to w1118 controls, while a significant increase in sleep bout number was detected for both heterozygous mutants during the night (Fig S1F). Taken together, these experiments suggest that the short sleeping phenotype of Ade2 deficient flies primarily derives from the shortening of sleep bouts.
To determine whether the sleep phenotype might be explained by generalized changes in locomotor activity, we analyzed waking activity (beam breaks/minute) in Ade2 knockdown flies and flies heterozygous for each mutation. Waking activity did not significantly differ between CG-GAL4 > Ade2-RNAi flies compared to control flies, though CG-GAL4 > Ade2-RNAi trended toward increased waking activity during the day and the night (Fig S1G). Waking activity was significantly increased in Ade21-6 heterozygous flies during daytime and nighttime compared to w1118 controls, while Ade23-20/+ had a significant increase in daytime but not nighttime waking activity (Fig S1H). Together, these findings suggest that disruption of Ade2 function induces hyperactivity, in addition to shortening sleep phenocopying starved flies.
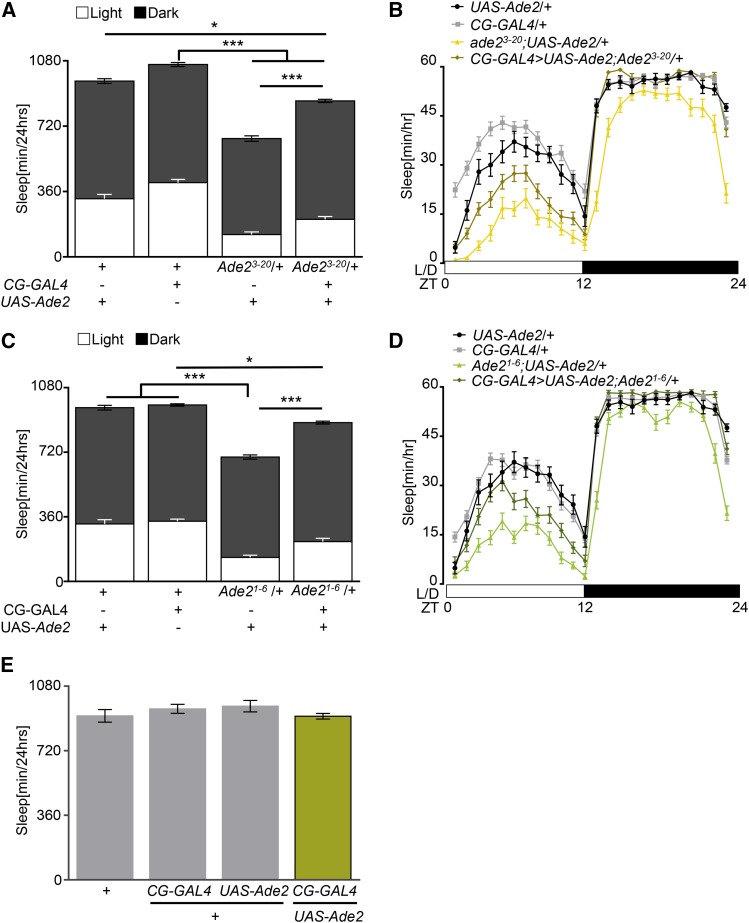
To verify that expression of Ade2 in the fat body is sufficient for normal sleep, we selectively restored Ade2 to the fat body in the background of Ade2 heterozygous flies and measured sleep. Heterozygous flies with Ade2 restored to the fat body (CG-GAL4 > UAS-Ade2 Ade23-20/+) slept more than Ade23-20/+ heterozygous mutants harboring the UAS-Ade2 transgene without the GAL4 (UAS-Ade2; Ade23-20/+) (Figure 2A,B). However, fat body expression did not fully rescue sleep, as rescue flies slept less than control flies harboring CG-GAL4 or UAS-Ade2 transgenes alone. Therefore, restoration of Ade2 to the fat body partially restores sleep to Ade23-20 mutant flies. Similarly, restoring Ade2 to the fat body of flies heterozygous for the Ade21-6 mutation (CG-GAL4 > UAS-Ade2; Ade21-6/+) partially restores sleep, with rescue flies sleeping significantly more than UAS-Ade2; Ade23-20/+ heterozygous flies, but less than flies harboring CG-GAL4 transgene alone (Figure 2C,D). Expression of Ade2 in the fat body of flies heterozygous for Ade23-20 or Ade21-6 rescued both average sleep bout length during nighttime and sleep bout number during the day and nighttime to control levels (Fig S2A-D). Similarly, the hyperactivity phenotype seen in Ade23-20 and Ade21-6 heterozygous mutant flies was restored in rescue flies, and these flies did not differ from heterozygous controls (Fig S2E, S2F). To determine whether upregulation of Ade2 in the fat body is sufficient to promote sleep, we overexpressed Ade2 in the fat body of wildtype flies (CG-GAL4 > UAS-Ade2). Sleep in these flies did not differ from transgenic controls harboring CG-GAL4 or UAS-Ade2 alone (Figure 2E). Taken together, these findings suggest Ade2 expression in the fat body is necessary for normal sleep, but enhanced Ade2 expression is not sufficient to increase sleep.
Figure 2.
Ade2 expression in the fat body partially rescues sleep loss. (A) Fat body rescue of Ade23-20/+ (CG-GAL4 > UAS-Ade2;Ade23-20/+; n = 43) partially restores total sleep compared to CG-GAL4/+ (n = 69, P < 0.0001) control and UAS-Ade2/+ (n = 31, P = 0.014). Total sleep duration in rescue flies is significantly increased compared to Ade23-20/+;UAS-Ade2 mutant control (n = 30, P < 0.0001). One-way ANOVA, F(3, 169)=37.76. (B) Sleep profile of hourly sleep averages over a 24 hr experiment for Ade23-20 rescue (gold) compared with Ade23-20/+;UAS-Ade2 (yellow), CG-GAL4/+ (gray), and UAS-Ade2/+ (black) controls. White/black bars represent lights on and off. ZT denotes Zeitgeber time. (C) Total sleep is significantly increased in flies expressing Ade2 in the fat body of Ade21-6 mutants (n = 37) compared to Ade21-6/+;UAS-Ade2 mutant controls (n = 39, P < 0.0001). Rescue flies are significantly different than control CG-GAL4/+ (gray, n = 79, P = 0.010). Ade21-6/+;UAS-Ade2 mutants have reduced sleep compared to UAS-Ade2/+ (n = 31, P < 0.0001) and CG-GAL4/+ (P < 0.0001) controls. One-way ANOVA, F(3, 182)= 41.94. (D) Sleep profile of hourly sleep averages over a 24 hr experiment for Ade21-6 rescue (dark green) compared with Ade21-6/+;UAS-Ade2 (light green), CG-GAL4/+ (gray), and UAS-Ade2/+ (black) controls. (E) Total sleep did not differ between flies overexpressing Ade2 in the fat body (CG-GAL4 > UAS-Ade2; n = 64) compared to w1118 (n = 55, P > 0.99), CG-GAL4/+(n = 32, P = 0.74), or UAS-Ade2/+ (n = 31, P = 0.51) controls. One-way ANOVA, F(3,178)=0.43. All columns are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
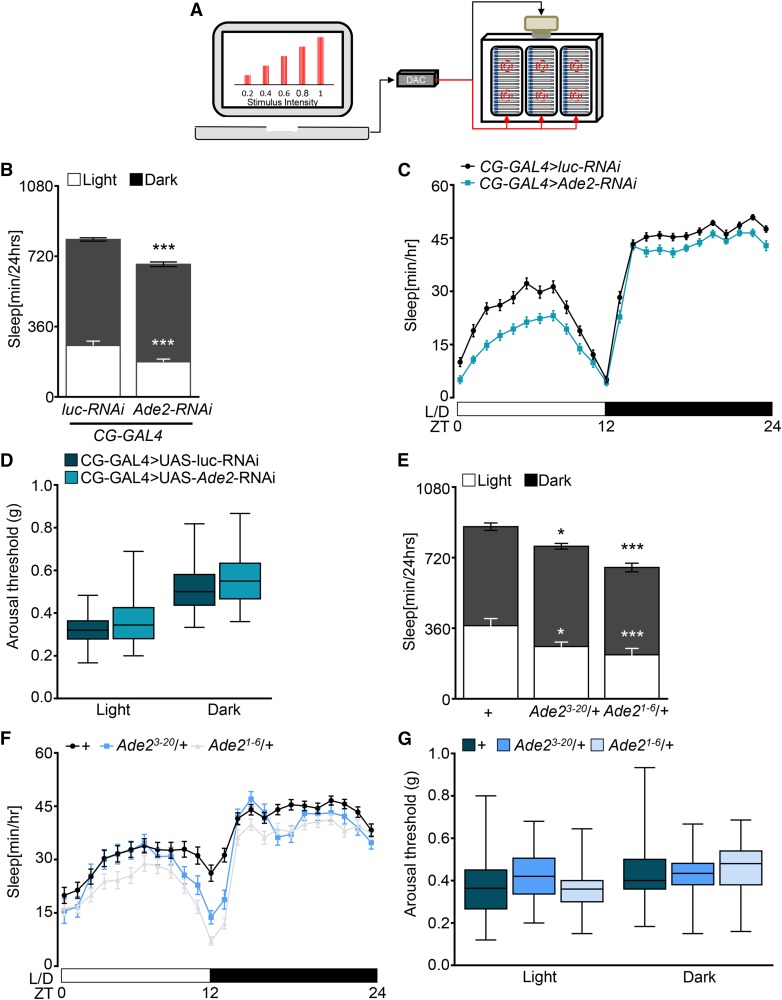
Sleep is associated with an elevated arousal threshold where animals are less responsive to environmental stimuli (Campbell and Tobler 1984; Hendricks et al. 2000; Shaw et al. 2000). To determine whether Ade2 regulates arousal threshold, we measured sleep in the Drosophila ARousal Tracking system (DART, Figure 3A) (van Alphen et al. 2013; Faville et al. 2015). Briefly, the system allows for automated video-tracking combined with controlled application of a vibration stimulus. The response of sleeping animals to the vibration is used to determine the arousal threshold (Figure 3A). In agreement with infrared-based recordings, video-monitoring in the DART system confirmed reduced sleep in CG-GAL4 > Ade2-RNAi flies with Ade2 knocked down in the fat body (Figure 3B,C). No differences in arousal threshold were detected between Ade2 knockdown and control flies during the daytime or nighttime suggesting Ade2 affects sleep duration, but not sleep-associated changes in arousal (Figure 3D). Similarly, video monitoring in the DART system confirmed sleep duration was reduced in Ade23-20 and Ade21-6 heterozygous flies, but no effect on arousal threshold was detected during the day or night (Figure 3E-G). Together, these results suggest that arousal threshold is not altered in Ade2 deficient flies.
Figure 3.
Arousal threshold is normal in Ade2-RNAi and Ade2 mutants. (A) The Drosophila Arousal Tracking (DART) software records fly movement while simultaneously controlling mechanical stimuli via a digital analog converter (DAC). Mechanical stimuli are delivered to three platforms, each housing twenty flies under the control of two motors. Mechanical stimuli of increased strength were used to assess arousal threshold (shown on the computer screen). Arousal thresholds were determined hourly, starting at ZT = 0. (B) Video-tracking analysis of sleep. Sleep during daytime (white, P < 0.0001, t = 6.11) and nighttime (black, P < 0.0001, t = 5.47) is significantly reduced in flies expressing Ade2-RNAi in the fat body (CG-GAL4 > UAS-Ade2-RNAi; n = 105) compared to control (CG-GAL4 > UAS-luc-RNAi; n = 114). Unpaired t-test. (C) Sleep profile over a 24 period. White/black bars represent lights on and off. ZT denotes Zeitgeber time. Ade2 knock down flies (turquoise) sleep less than control (black). (D) Arousal threshold during dayttime (P = 0.06) and nighttime (P = 0.07) does not differ between CG-GAL4 > UAS-Ade2-RNAi (turquoise; n = 79) and control (dark green;n = 85). Mann-Whitney U; Light 2783; Dark, 2807. (E) Video-tracking analysis shows reduced sleep during daytime (P = 0.045) and nighttime (P = 0.034) in Ade23-20/+ (n = 46) compared to w111 control flies (n = 87). Ade21-6/+ mutant flies (n = 80, P < 0.0001) sleep significantly less than control flies during day and night (P < 0.0001). One way-ANOVA, Light, F(2,210)=15.11; Dark, F(2,210)=17.94 (F) Sleep profile representative of data in (E). w1118 control flies (black) slept more than Ade23-20/+ (blue) and Ade21-6/+ (gray) mutants. (G) Arousal threshold does not differ between w1118 control (dark green n = 71) and Ade23-20/+ (blue, n = 29) and Ade21-6/+ (light blue; n= 63) during light and dark. Kruskall Wallis; Light, 6.87; Dark, 3.02. All columns are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
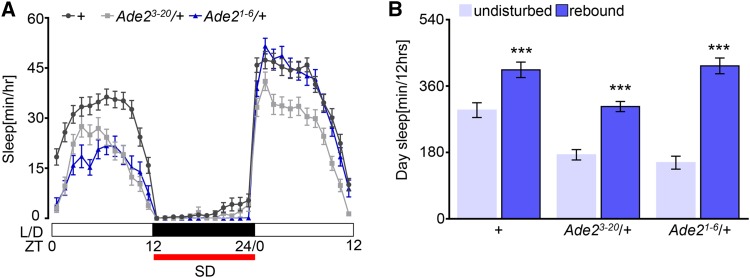
Growing evidence suggests that independent neural mechanisms regulate sleep under undisturbed conditions and the homeostatic sleep rebound following deprivation (Seidner et al. 2015; Liu et al. 2016). To determine if sleep homeostasis is intact in Ade2 deficient flies, we sleep deprived flies by mechanical shaking for 12-hours throughout the night, and measured sleep during the following day. The sleep deprivation protocol resulted in in significant sleep rebound in flies heterozygous for the Ade23-20 and Ade21-6 mutations, similar to controls (Figure 4A,B). Together, these results suggest Ade2 is dispensable for homeostatic sleep rebound.
Figure 4.
Homeostatic recovery sleep is not altered in Ade2 mutants. (A) Sleep profile for hourly sleep for 36 hr. Flies are undisturbed on the first 12 hr (ZT0-ZT12), lights on (white bars). In the subsequent night (black bars, ZT12-ZT24), flies are mechanically sleep deprived (red), and rebound is measured in the next 12 hr during the light period (ZT0-ZT12). ZT denotes Zeitgeber time. (B) w1118 control (n = 78, P = 0.0001), Ade23-20/+ (n = 51, P = 0.0002), and Ade21-6/+ mutants (n = 50, P < 0.0001) significantly rebound after sleep deprivation (purple) compared to undisturbed day (light purple). Two-way ANOVA, F(2, 352)=8.75. All columns are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
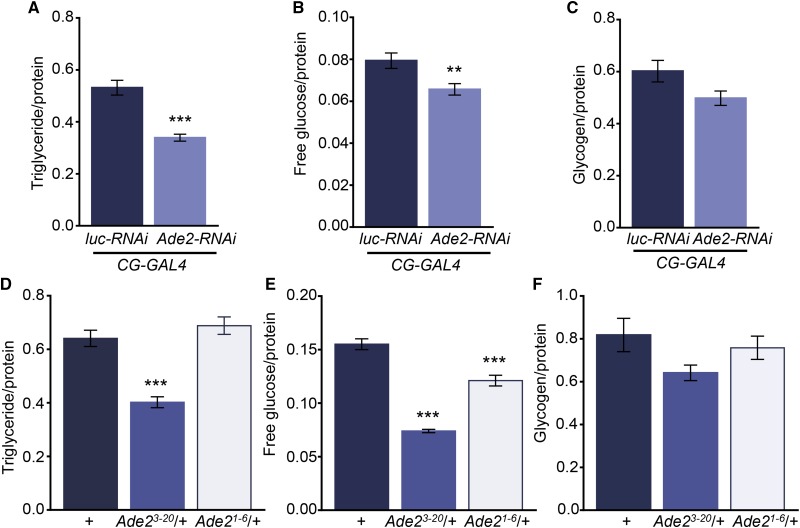
Flies suppress sleep and increase waking activity in response to starvation and when energy stores are depleted (Lee and Park 2004; Keene et al. 2010; Murakami et al. 2016). Therefore, we reasoned that the loss of sleep in Ade2 mutants may be due to reduced energy storage. To determine if energy stores are dysregulated in Ade2 mutants, we measured whole-body triglycerides, glycogen, and free glucose levels in fed Ade2 loss of function flies using colorimetric assays (Mikoluk et al. 2018). Knockdown of Ade2 selectively in the fat body (CG-GAL4 > Ade2-RNAi) resulted in reduced triglycerides and free glucose levels (Figure 5A-C) without affecting glycogen levels. Similarly, triglyceride and free glucose levels were reduced in Ade23-20 heterozygote flies, while glycogen levels were unaffected (Figure 5D-F). In Ade21-6 heterozygous flies, only free glucose levels are reduced (Figure 5E). Taken together, these findings suggest Ade2 function in the fat body is required for the normal storage of triglycerides and free glucose, supporting the notion that the reduced sleep may be caused metabolic changes that place the fly in a starvation-like state.
Figure 5.
Triglycerides and free glucose are altered in Ade2 knock down and mutants. (A-C) Triglyceride (A, P < 0.001, t = 0.56) and free glucose levels (B, P = 0.007, t = 0.25) are reduced in flies with Ade2 knock down in the fat body (CG-GAL4 > UAS-Ade2-RNAi; n = 13) compared to control flies (CG-GAL4 > UAS-luc-RNAi; n = 15), while there are no significant differences in glycogen levels (C, n = 13, P = 0.053, t = 2.025). Unpaired t-test. (D-F) Triglyceride (D, n = 14, P < 0.0001) and free glucose levels (E, P < 0.0001) are reduced in Ade23-20/+ mutants (n = 14) compared to w1118 controls (n = 14), while there are no significant differences in glycogen levels (F, P = 0.75). Ade21-6 mutants (n = 15) show a reduction in free glucose levels (E, P < 0.0001) compared to w1118 controls. Triglyceride (D, P = 0.47) and glycogen (F, P = 0.09) stores do not differ between Ade21-6 and control flies. One-way ANOVA; TAG, F(2,40)=28.59; Free Glucose, F(2,40)=90.30; Glycogen, F(2,40)=2.45. All columns are mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001.
Discussion
To our knowledge, this study represents the first genetic screen for Drosophila sleep regulators that specifically examine the role of non-neuronal tissue in sleep regulation. The fat body is critical for regulating energy storage in Drosophila and has been implicated in many behaviors including sleep regulation, courtship, circadian rhythms, and feeding (Lazareva et al. 2007; Xu et al. 2011; Kim et al. 2017; Thimgan et al. 2010). While the genetic examination of many behaviors, including sleep, have predominantly focused on investigating the neural regulation of behavior, the contribution of the fat body to behavioral regulation is less understood (Iijima et al. 2009; Xu et al. 2011; Sassu et al. 2012). A complete understanding of behavior will require systematic investigation of the role of the fat body and other non-neuronal tissues in behavioral regulation.
The fat body-specific screen for sleep regulators described here identified that Ade2 functions within this tissue type and is critical for normal sleep. These findings add to a growing body of literature suggesting that the adipose tissue is a critical regulator of sleep in both mammals and invertebrates (Laposky 2005; Thimgan et al. 2010; Arble et al. 2015). In inbred fly lines derived from wild caught Drosophila, sleep duration positively associated with whole body triglyceride levels, and Drosophila selected for starvation resistance have elevated fat body stores and prolonged sleep (Harbison et al. 2004; Masek et al. 2014; Slocumb et al. 2015; Brown et al. 2018). Similarly, flies mutant for the triglyceride lipase gene brummer have elevated triglyceride stores and an enhanced homeostatic sleep response, while mutants for the perilipin-like protein lipid storage droplet 2 (lsd2) have reduced triglyceride stores and a lowered homeostatic sleep response (Thimgan et al. 2010). Together, these findings suggest triglycerides, and perhaps energy stores more generally, are required for normal sleep in flies.
The role of adipose tissue in sleep regulation appears to be conserved across phyla. In mammals, leptin is secreted from adipocytes in response to nutritional state and acts on hypothalamic circuits in the brain to decrease feeding as well as increase energy expenditure (Adamantidis and de Lecea 2008; Dardeno et al. 2010). In addition, sleep is disrupted in leptin-deficient mice, suggesting a role for this adipose-derived hormone in regulating sleep (Laposky 2005). Supporting these findings, our RNAi screen found reduced sleep in flies with fat body knockdown of upd2, a proposed Drosophila ortholog of mammalian leptin (Rajan and Perrimon 2012). The identification of Ade2 as well as a number of additional candidate sleep regulators suggest a central role for the fat body in sleep regulation.
Ade2 encodes a phosphoribosylformylglycinamidine synthase that plays a critical role in purine synthesis in nearly all living organisms (Chaudhary et al. 2004). Mutations in Ade2, or other components of the de novo purine biosynthesis pathway, have been implicated in the arrest of cell growth as well as reduced fertility and lifespan, suggesting broad biological functions of this gene (Tiong and Nash 1990; Malmanche and Clark 2004). For example, flies heterozygous for Ade2 mutations develop necrosis as pupae, suggesting haploinsufficiency may result in physiological abnormalities (Holland et al. 2011). The possibility that targeted disruption of Ade2 in the fat body disrupts development of this organ, or its function in energy storage, is supported by our findings that whole-body triglycerides and/or free glucose levels are reduced in Ade2-deficient flies. In addition, it is possible that reduced levels of purines themselves contribute to the sleep phenotype. In both flies and mammals, adenosine promotes sleep, and the accumulation of adenosine during periods of wakefulness is associated with increased sleep drive (Jones 2009; Nall et al. 2016). Therefore, it is possible that a reduction in adenosine or other purinerginic signaling contributes to the sleep loss phenotype.
Our findings suggest that overexpression of Ade2 in an otherwise wild type fly does not promote sleep, suggesting Ade2 is essential for normal sleep, rather than variation in expression levels of this gene regulating amounts of sleep. In addition, flies with either mutation in the Ade2 gene have a partial restoration of sleep, which suggests Ade2 may function in the brain or other tissue to regulate sleep. Ade2 is ubiquitously expressed and it is possible that the haploinsufficiency may be caused by dysregulated purinergic signaling or developmental abnormalities in additional brain regions. In flies, many neural circuits have been found to regulate sleep including the central complex, circadian neurons, and gustatory neurons (Donlea et al. 2011; Linford et al. 2012; Liu et al. 2012; Guo et al. 2016). In addition, more recent work has revealed a critical role for non-neuronal tissue, such as glia, in sleep regulation (Chen et al. 2015; Farca Luna et al. 2017). Targeted disruption of Ade2 in additional cell types may help reveal novel insights into Ade2 function.
In flies, starvation results in increased waking activity in addition to reduced sleep duration. We find that waking activity is significantly increased, or trends toward an increase, in Ade2-deficient flies, indicating that the mutant phenotype recapitulates the hyperactivity induced by starvation. Further, our analysis suggests that Ade2 is required for normal sleep under baseline conditions, while it is dispensable for sleep homeostasis. Mechanically sleep depriving flies throughout the night results in a rebound the following day, a response that is unaffected in Ade2-deficient flies. These data support the notion that independent genetic mechanisms underlie regulation of sleep in undisturbed conditions and during sleep rebound. Along these lines, distinct neural circuits have been identified regulating baseline sleep and recovery sleep in Drosophila (Seidner et al. 2015; Liu et al. 2016). Similarly, arousal threshold is unaffected in Ade2 mutant flies suggesting the quality of the sleep is not dysregulated, but rather the observed phenotype is specific to sleep. Together, these findings suggest Ade2 regulates baseline sleep duration, but may be dispensable for regulating sleep depth and homeostasis.
A growing body of evidence suggests that the levels of energy storage molecules are critical for normal sleep regulation. Starvation potently reduces whole-body triglycerides and glycogen in Drosophila and is associated with reduced sleep (Schwasinger-Schmidt et al. 2012). In addition, sleep duration and triglycerides are enhanced in flies selected for starvation resistance (Masek et al. 2014). Therefore, it is possible that loss of Ade2 in the fat body induces a chronic starvation-like state, where animals suppress their sleep. Alternatively, the fat body may regulate sleep through a mechanism that is independent of signaling energy stores.
Taken together, the confirmed role of Ade2 and the identification of additional candidate genes that function within the fat body to either promote or inhibit sleep, supports a central role for the fat body in sleep regulation. The fat body may regulate sleep directly through controlling circulating nutrients that are sensed by the brain or by hormonal communication. In both flies and mammals, sleep-modulating neurons directly sense glucose, raising the possibility that circulating glucose levels regulate sleep (Varin et al. 2015; Manière et al. 2016). The identification of genes that function within the fat body, combined with genetic technology for in vivo imaging of sleep circuits, will allow for investigation of how non-neuronal gene regulation modulates sleep circuits within the brain. The identification of Ade2, and other candidate regulators of sleep, provides a platform for investigating the role of periphery-brain communication in sleep regulation.
Acknowledgments
We thank Burczyk/Faville/Kottler LTD for the DART system. We are grateful to Dr. Denise Clark (University of New Brunswick) for generously providing Ade2 mutant Drosophila. This grant was support by NIH award 1R01NS085252 to ACK.
Footnotes
Supplemental material available at Figshare: https://doi.org/10.25387/g3.7121264.
Communicating editor: A. Bashirullah
Literature Cited
- Adamantidis A., de Lecea L., 2008. Sleep and metabolism: shared circuits, new connections. Trends Endocrinol. Metab. 19: 362–370. 10.1016/j.tem.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Allada R., Siegel J. M., 2008. Unearthing the phylogenetic roots of sleep. Curr. Biol. 18: R670–R679. 10.1016/j.cub.2008.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen B., Yap M. H. W., Kirszenblat L., Kottler B., van Swinderen B., 2013. A dynamic deep sleep stage in Drosophila. J. Neurosci. 33: 6917–6927. 10.1523/JNEUROSCI.0061-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble D. M., Bass J., Behn C. D., Butler M. P., Challet E., et al. , 2015. Impact of Sleep and Circadian Disruption on Energy Balance and Diabetes : A Summary of Workshop Discussions. Sleep (Basel) 38: 1849–1860. 10.5665/sleep.5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese E., Soulages J., 2010. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 55: 207–225. 10.1146/annurev-ento-112408-085356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha H., Nagy I., Kovacs G., Stetson D., Ando I., et al. , 2003. Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N., 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Brown E. B., Torres J., Bennick R. A., Rozzo V., Kerbs A., et al. , 2018. Variation in sleep and metabolic function is associated with latitude and average temperature in Drosophila melanogaster. Ecol. Evol. 8: 4084–4097. 10.1002/ece3.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel A. W., Corrie L. W., Rogers J. A., Yamada H., 2011. Effects of insulin and leptin in the ventral tegmental area and arcuate hypothalamic nucleus on food intake and brain reward function in female rats. Behav. Brain Res. 219: 254–264. 10.1016/j.bbr.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. S., Tobler I., 1984. Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8: 269–300. 10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- Chaudhary K., Darling J. A., Fohl L. M., Sullivan W. J., Donald R. G. K., et al. , 2004. Purine salvage pathways in the apicomplexan parasite Toxoplasma gondii. J. Biol. Chem. 279: 31221–31227. 10.1074/jbc.M404232200 [DOI] [PubMed] [Google Scholar]
- Chen W. F., Maguire S., Sowcik M., Luo W., Koh K., et al. , 2015. A neuron-glia interaction involving GABA transaminase contributes to sleep loss in sleepless mutants. Mol. Psychiatry 20: 240–251. 10.1038/mp.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardeno T. A., Chou S. H., Moon H.-S., Chamberland J. P., Fiorenza C. G., et al. , 2010. Leptin in Human Physiology and Therapeutics. Front. Neuroendocrinol. 31: 377–393. 10.1016/j.yfrne.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G., Chen D., Schnorrer F., Su K.-C., Barinova Y., et al. , 2007. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. 10.1038/nature05954 [DOI] [PubMed] [Google Scholar]
- Donlea J., Leahy A., Thimgan M. S., Suzuki Y., Hughson B. N., et al. , 2012. foraging alters resilience/vulnerability to sleep disruption and starvation in Drosophila. Proc. Natl. Acad. Sci. USA 109: 2613–2618. 10.1073/pnas.1112623109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J. M., Thimgan M. S., Suzuki Y., Gottschalk L., Shaw P. J., 2011. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332: 1571–1576. 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion R., Sehgal A., 2013. Regulation of insect behavior via the insulin-signaling pathway. Front. Physiol. 4: 353 10.3389/fphys.2013.00353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farca Luna A. J., Perier M., Seugnet L., 2017. Amyloid Precursor Protein in Drosophila Glia Regulates Sleep and Genes Involved in Glutamate Recycling. J. Neurosci. 37: 4289–4300. 10.1523/JNEUROSCI.2826-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faville R., Kottler B., Goodhill G., Shaw P. J., van Swinderen B., 2015. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci. Rep. 5: 8454 10.1038/srep08454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe D., Bollinger W., Vigderman A., Masek P., Gertowski J., et al. , 2015. Context-specific comparison of sleep acquisition systems in Drosophila. Biol. Open 4: 1558–1568. 10.1242/bio.013011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A., Kadowaki T., Kitagawa Y., 2003. Drosophila hemolectin gene is expressed in embryonic and larval hemocytes and its knock down causes bleeding defects. Dev. Biol. 264: 582–591. [DOI] [PubMed] [Google Scholar]
- Grönke S., Mildner A., Fellert S., Tennagels N., Petry S., et al. , 2005. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 1: 323–330. 10.1016/j.cmet.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Guo F., Yu J., Jung H. J., Abruzzi K. C., Luo W., et al. , 2016. Circadian neuron feedback controls the Drosophila sleep–activity profile. Nature 536: 292–297. 10.1038/nature19097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison S. T., Yamamoto A. H., Fanara J. J., Norga K. K., Mackay T. F. C., 2004. Quantitative trait loci affecting starvation resistance in Drosophila melanogaster. Genetics 166: 1807–1823. 10.1534/genetics.166.4.1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., et al. , 2000. Rest in Drosophila is a sleep-like state. Neuron 25: 129–138. 10.1016/S0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Holland C., Lipsett D. B., Clark D. V., 2011. A link between impaired purine nucleotide synthesis and apoptosis in Drosophila melanogaster. Genetics 188: 359–367. 10.1534/genetics.110.124222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima K., Zhao L., Shenton C., Iijima-Ando K., 2009. Regulation of energy stores and feeding by neuronal and peripheral CREB activity in Drosophila. PLoS One 4: e8498 10.1371/journal.pone.0008498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskov P. M., Ribeiro C., 2013. The dilemmas of the gourmet fly: The molecular and neuronal mechanisms of feeding and nutrient decision making in Drosophila. Front. Neurosci. 7: 12 10.3389/fnins.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. E., 2009. Glia, Adenosine, and Sleep. Neuron 61: 156–157. 10.1016/j.neuron.2009.01.005 [DOI] [PubMed] [Google Scholar]
- Karra E., O’Daly O. G., Choudhury A. I., Yousseif A., Millership S., et al. , 2013. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J. Clin. Invest. 123: 3539–3551. 10.1172/JCI44403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene A. C., Duboué E. R., McDonald D. M., Dus M., Suh G. S. B., et al. , 2010. Clock and cycle limit starvation-induced sleep loss in drosophila. Curr. Biol. 20: 1209–1215. 10.1016/j.cub.2010.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. H., Shin M., Jung S. H., Kim Y. J., Jones W. D., 2017. A fat-derived metabolite regulates a peptidergic feeding circuit in Drosophila. PLoS Biol. 15: e2000532 10.1371/journal.pbio.2000532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laposky A. D., 2005. Altered sleep regulation in leptin-deficient mice. AJP Regul. Integr. Comp. Physiol. 290: R894–R903. 10.1152/ajpregu.00304.2005 [DOI] [PubMed] [Google Scholar]
- Lazareva A. A., Roman G., Mattox W., Hardin P. E., Dauwalder B., 2007. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 3: 0115–0122. 10.1371/journal.pgen.0030016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G., Park J. H., 2004. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167: 311–323. 10.1534/genetics.167.1.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Hazelrigg T., Rubin G. M., 1985. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science 229: 558–561. 10.1126/science.2992080 [DOI] [PubMed] [Google Scholar]
- Linford N. J., Chan T. P., Pletcher S. D., 2012. Re-patterning sleep architecture in Drosophila through gustatory perception and nutritional quality. PLoS Genet. 8: e1002668 10.1371/journal.pgen.1002668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Liu S., Kodama L., Driscoll M. R., Wu M. N., 2012. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr. Biol. 22: 2114–2123. 10.1016/j.cub.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu Q., Tabuchi M., Wu M., 2016. Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit. Cell 165: 1347–1360. 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmanche N., Clark D. V., 2004. Drosophila melanogaster Prat, a purine de novo synthesis gene, has a pleiotropic maternal-effect phenotype. Genetics 168: 2011–2023. 10.1534/genetics.104.033134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manière G., Ziegler A. B., Geillon F., Featherstone D. E., Grosjean Y., 2016. Direct Sensing of Nutrients via a LAT1-like Transporter in Drosophila Insulin-Producing Cells. Cell Reports 17: 137–148. 10.1016/j.celrep.2016.08.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks D. R., Tucker K., Cavallin M. A., Mast T. G., Fadool D. A., 2009. Awake Intranasal Insulin Delivery Modifies Protein Complexes and Alters Memory, Anxiety, and Olfactory Behaviors. J. Neurosci. 29: 6734–6751. 10.1523/JNEUROSCI.1350-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P., Reynolds L. A., Bollinger W. L., Moody C., Mehta A., et al. , 2014. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J. Exp. Biol. 217: 3122–3132. 10.1242/jeb.103309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoluk C., Nagengast A. A., DiAngelo J. R., 2018. The splicing factor transformer2 (tra2) functions in the Drosophila fat body to regulate lipid storage. Biochem. Biophys. Res. Commun. 495: 1528–1533. 10.1016/j.bbrc.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K., Yurgel M. E., Stahl B. A., Masek P., Mehta A., et al. , 2016. Translin Is Required for Metabolic Regulation of Sleep. Curr. Biol. 26: 972–980. 10.1016/j.cub.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musselman L. P., Fink J. L., Ramachandran P. V., Patterson B. W., Okunade A. L., et al. , 2013. Role of fat body lipogenesis in protection against the effects of caloric overload in drosophila. J. Biol. Chem. 288: 8028–8042. 10.1074/jbc.M112.371047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nall A. H., Shakhmantsir I., Cichewicz K., Birman S., Hirsh J., et al. , 2016. Caffeine promotes wakefulness via dopamine signaling in Drosophila. Sci. Rep. 6: 20938 10.1038/srep20938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J. Q., Liu L. P., Binari R., Hardy R., Shim H. S., et al. , 2009. A drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 182: 1089–1100. 10.1534/genetics.109.103630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabha D., Baker K. D., 2014. Drosophila gains traction as a repurposed tool to investigate metabolism. Trends Endocrinol. Metab. 25: 518–527. 10.1016/j.tem.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C., Lear B. C., Keegan K. P., Allada R., 2010a Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb. Protoc. 2010: pdb.prot5518 10.1101/pdb.prot5518 [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger C., Lear B. C., Keegan K. P., Allada R., 2010b Processing sleep data created with the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010: pdb.prot5520 10.1101/pdb.prot5520 [DOI] [PubMed] [Google Scholar]
- Rajan A., Perrimon N., 2012. Drosophila Cytokine Unpaired 2 Regulates Physiological Homeostasis by Remotely Controlling Insulin Secretion. Cell 151: 123–137. 10.1016/j.cell.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassu E. D., McDermott J. E., Keys B. J., Esmaeili M., Keene A. C., et al. , 2012. Mio/dChREBP coordinately increases fat mass by regulating lipid synthesis and feeding behavior in Drosophila. Biochem. Biophys. Res. Commun. 426: 43–48. 10.1016/j.bbrc.2012.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwasinger-Schmidt T. E., Kachman S. D., Harshman L. G., 2012. Evolution of starvation resistance in Drosophila melanogaster: Measurement of direct and correlated responses to artificial selection. J. Evol. Biol. 25: 378–387. 10.1111/j.1420-9101.2011.02428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidner G., Robinson J., Wu M., Worden K., Masek P., et al. , 2015. Identification of Neurons with a Privileged Role in Sleep Homeostasis in Drosophila melanogaster. Curr. Biol. 25: 2928–2938. 10.1016/j.cub.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J., Cirelli C., Greenspan R. J., Tononi G., 2000. Correlates of sleep and waking in Drosophila melanogaster. Science 287: 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Slocumb M. E., Regalado J. M., Yoshizawa M., Neely G. G., Masek P., et al. , 2015. Enhanced sleep is an evolutionarily adaptive response to starvation stress in Drosophila. PLoS One 10: e0131275 10.1371/journal.pone.0131275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A., White M. F., 2008. Insulin-Like Signaling, Nutrient Homeostasis, and Life Span. Annu. Rev. Physiol. 70: 191–212. 10.1146/annurev.physiol.70.113006.100533 [DOI] [PubMed] [Google Scholar]
- Thimgan M. S., Suzuki Y., Seugnet L., Gottschalk L., Shaw P. J., 2010. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 8: e1000466 10.1371/journal.pbio.1000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong S. Y. K., Nash D., 1990. Genetic analysis of the adenosine3 (Gart) region of the second chromosome of Drosophila melanogaster. Genetics 124: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezaki Y., Hayley S. E., Chu M. L., Seo H. W., Shah P., et al. , 2018. Feeding-State-Dependent Modulation of Temperature Preference Requires Insulin Signaling in Drosophila Warm-Sensing Neurons. Curr. Biol. 28: 779–787.e3. 10.1016/j.cub.2018.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varin C., Rancillac A., Geoffroy H., Arthaud S., Fort P., et al. , 2015. Glucose Induces Slow-Wave Sleep by Exciting the Sleep-Promoting Neurons in the Ventrolateral Preoptic Nucleus: A New Link between Sleep and Metabolism. J. Neurosci. 35: 9900–9911. 10.1523/JNEUROSCI.0609-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., DiAngelo J. R., Hughes M. E., Hogenesch J. B., Sehgal A., 2011. The circadian clock interacts with metabolic physiology to influence reproductive fitness. Cell Metab. 13: 639–654. 10.1016/j.cmet.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Inoue Y. H., Hirose F., Sakaguchi K., Matsukage A., et al. , 2001. Over-expression of DREF in the Drosophila wing imaginal disc induces apoptosis and a notching wing phenotype. Genes Cells 6: 877–886. 10.1046/j.1365-2443.2001.00473.x [DOI] [PubMed] [Google Scholar]
- Yurgel M. E., Masek P., DiAngelo J., Keene A. C., 2015. Genetic dissection of sleep–metabolism interactions in the fruit fly. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 201: 869–877. 10.1007/s00359-014-0936-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables. Supplemental material available at Figshare: https://doi.org/10.25387/g3.7121264.