Abstract
An efficient study of carbohydrate-protein interactions was achieved using multivalent glycodendrimer library. Different dendrimers with varied peripheral sugar densities and linkers provided an arsenal of potential novel therapeutic agents that could be useful for better specific action and greater binding affinities against their cognate protein receptors. Highly effective click chemistry represents the basic method used for the synthesis of mannosylated dendrimers. To this end, we used propargylated scaffolds of varying sugar densities ranging from 2 to 18 for the attachment of azido mannopyranoside derivatives using copper catalyzed click cycloaddition. Mannopyranosides with short and pegylated aglycones were used to evaluate their effects on the kinetics of binding. The mannosylated dendrons were built using varied scaffolds toward the accelerated and combined “onion peel” strategy These carbohydrates have been designed to fight E. coli urinary infections, by inhibiting the formation of bacterial biofilms, thus neutralizing the adhesion of FimH type 1 lectin present at the tip of their fimbriae against the natural multiantennary oligomannosides of uroplakin 1a receptors expressed on uroepithelial tissues. Preliminary DLS studies of the mannosylated dendrimers to cross- link the leguminous lectin Con A used as a model showed their high potency as candidates to fight the E. coli adhesion and biofilm formation.
Keywords: dendrimers, mannose, click chemistry, E. coli, UTIs, FimH, DLS
1. Introduction
Modern medicine is exploring several alternative strategies to overcome the expending antibiotic resistance problems. One of these goals is to find new ways to fight bacterial infections. Of particular interest are the recurrent urinary tract infections (UTIs). Half of women are affected by UTIs at least once in their lifetime. Hence, UTIs represent an important public health issue because of their frequent occurrence. UTIs are mostly caused by uropathogenic Escherichia coli strains (UPEC) that infest urinary epithelium through their type-1 pili (FimH). At the tips of their pili, E. coli possesses a carbohydrate binding domain called FimH that adhere to mannosylated glycoproteins receptors on the urinary epithelial cells [1,2,3]. In addition, through their quorum sensing molecules, E. coli can infest hosts cell and forms resilient biofilms. Once formed, bacteria become mature and readily acquire drugs resistance.
Besides, as the first defense mechanism against this infectious agent, the innate immune system exploits the structures of mannosides [4,5]. On the other hand, fimbriated E. coli receptor-binding site (FimH) consists of a specific mannose-binding pocket, with a tyrosine gate (Tyr48, Tyr137) [6,7,8] that also recognizes and binds to the mannosides residues of uroplakin-1a, present at the surface of urothelial cells as a premise for bacterial infections causing cystitis and pyelonephritis. Ever since, considerable efforts have been devoted toward the development of new synthetic antiadhesive antagonists that could act as potent competitive inhibitors [9,10,11,12,13]. Since then, synthetic mono-mannopyranosides carrying various aglycones have been reported and their binding to FimH have been fully characterized. Amongst these, heptyl α-d-mannopyranoside (HM) as emerged as one of the strongest monovalent FimH binders described so far [3]. The studies supported that the hydrophobic HM’s aglycone strongly interacted via van der Waals contacts and aromatic stacking with the tyrosine gates residues Tyr148 and Tyr137 [2,8,13].
However, monomeric carbohydrate residues interacting with proteins often occur with low-binding affinities [14,15,16]. Furthermore, multivalent carbohydrate-protein interactions have unequivocally displayed several advantages over monomeric ones, a process commonly used by nature to control a wide variety of cellular processes [17,18,19]. Hence, several glycodendrimers have been designed to address the issue of low affinity carbohydrate-protein interactions [5,20,21,22,23,24,25,26,27]. Mannosylated glycodendrimers were found to be excellent ligands against the leguminous lectin from Canavalia ensiformis (Concanavalin A) and to be excellent ligands toward the fimbriated E. coli K12 with nanomolar affinities [21,26,28,29]. Likewise, the glycoside cluster effect helps to enhance avidity, selectivity and affinity of a multivalent glycoside toward a protein that possess multiple carbohydrate recognition domains (CRDs). This phenomenon was characterized by strong stabilization of carbohydrate-protein interactions through macroscopic cross-linking effects [17,19]. Therefore, mannosylated glycodendrimers constitute excellent lead candidates for the treatment of E. coli infectious agents by the inhibition of bacterial adhesion and biofilm formation on cell surfaces [5,12,30].
To these aim, a new variety of mannosylated dendrimers of different sugar densities and linker’s properties have been developed to prevent infections spreading, by inhibiting E. coli adhesion and biofilm formations. Thus, we report herein a new class of glycodendrimers [5,20,21,22,25,28,31,32] that are built on aromatics, cyclotriphosphazene and pentaerythritol cores using high yielding click chemistry to anchor mannosylated azides onto the above alkyne-functionalized scaffolds. This synthetic strategy provides easy access to biodegradability and biocompatibility to the glycodendrimers. In addition, by using different scaffolds at each layer rather than the identical ones commonly used, we are providing another application of the so called “onion peel” approach. Hence, pegylated tetraethylene glycol spacers have been introduced as aglycones, because of the easy availability, water-solubility, biocompatibility and the variable chain length accessible that should enable good interactions with the multiple FimH binding pili present at the surface of E. coli. The relative affinities of the pegylated mannodendrimers were studied using diffraction light scattering measurements (DLS) in the presence of ConA, toward a better understanding of the multivalent binding interactions and the effect of the linkers. Although ConA is a leguminous lectin, it also binds natural multiantennary mannopyranosides that can also be advantageously replaced by synthetic monomeric derivatives harboring hydrophobic aglycones. Hence, it constitutes an appropriate model to evaluate the relative propensity of mannosylated dendrimers toward E. coli FimH.
2. Results and Discussion
2.1. Scaffold Syntheses
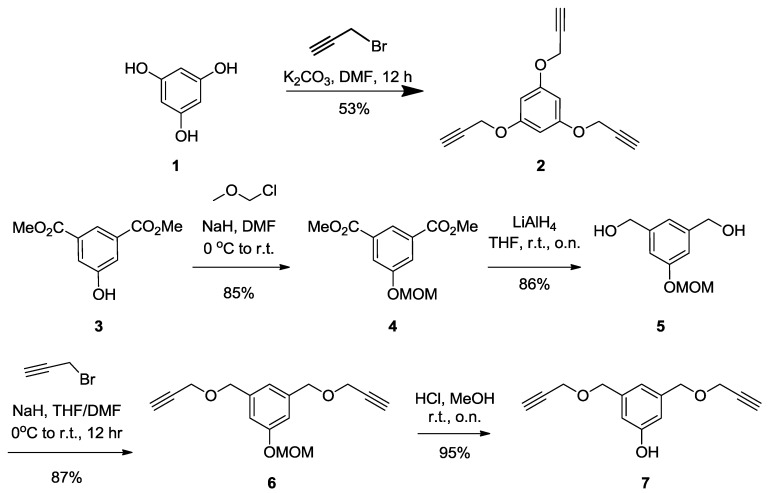
First, the syntheses of different propargylated aromatic scaffolds with valencies of 2 (7) and 3 (2) were achieved in moderate to good yields. Scaffolds 2 and 7 were obtained through propargylation (propargyl bromide, K2CO3, DMF) of polyhydroxylated aromatic cores 1 and 5, respectively. The trimeric phloroglucinol 1 was commercially available and the dimeric compound 5 was synthesized after protection of the hydroxyl group of 5-hydroxy dimethyl isophthalate 3 with a MOM-protecting group (MOMCl, NaH, DMF, 85%), followed by reduction of the intermediate esters of 4 to afford 5 using LiAlH4 in 86% yield (Scheme 1).
Scheme 1.
Synthesis of tris-propagylated 2 and bis-propagylated 7 aromatic scaffolds.
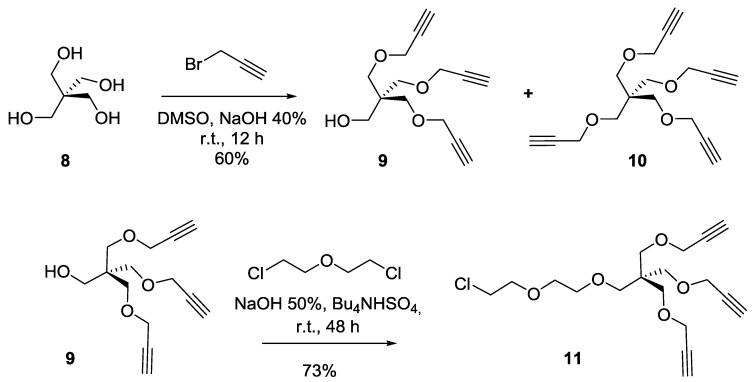
Pentaerythritol 8 was next chosen as template for the synthesis of the desired tris-propargylated core 9 (propargyl bromide, DMSO/NaOH) (Scheme 2). It was accompanied by smaller quantities of the fully tetra-propargylated scaffold 10 which was readily separated by flash chromatography. The reaction mixture gave both 9 and 10 in a 2:1 ratio, respectively. An extended dendron precursor 11 was then obtained from 9 using bis(2-chloroethyl) ether under phase-transfer catalyzed conditions (PTC) (tetrabutylammonium hydrogensulfate (TBAHS), NaOH, r.t.) in 73% yield (Scheme 2).
Scheme 2.
Synthesis of a tripropagylated dendron precursor 11 under PTC conditions.
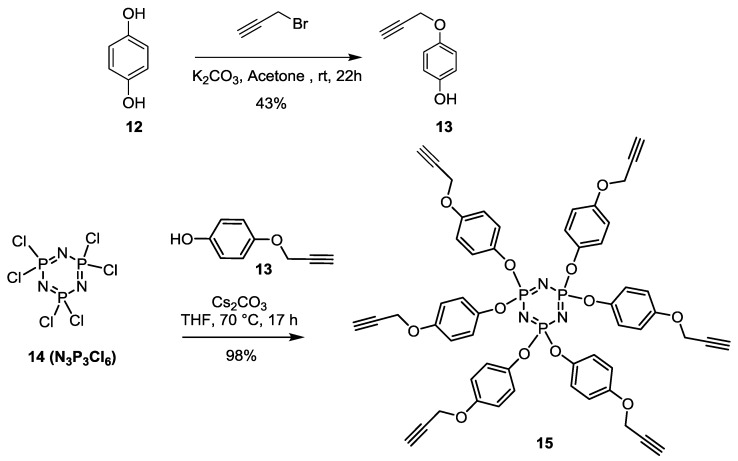
For our next scaffold, harboring six propargylated functionalities, we choose the previously described hexachlorocyclotriphosphazene core 14 (N3P3Cl6) [28,33,34,35]. The choice for this dense hexavalent core was based on previous observations by the Majoral’s group who showed that it was biologically favorable over several other scaffolds and that it can readily form dendrimers of very high generation [36,37,38,39,40]. Toward this goal, diol 12 was monopropargylated (propargyl bromide, K2CO3, acetone, r.t., 12 h) to afford 13 (43%) (Scheme 3) which was treated with 14 under basic conditions (Cs2CO3, THF, 70 °C, 17 h) to provide 15 in essentially quantitative yield (98%). The structural integrity of the fully substituted 15 was compared to its known 1H- and 31P-NMR [28].
Scheme 3.
Synthesis of a hexa-propargylated cyclotriphosphazene core 15.
2.2. Sugar Precursors
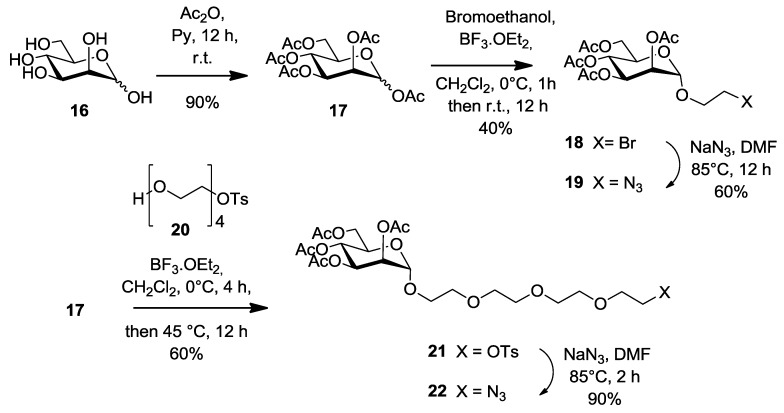
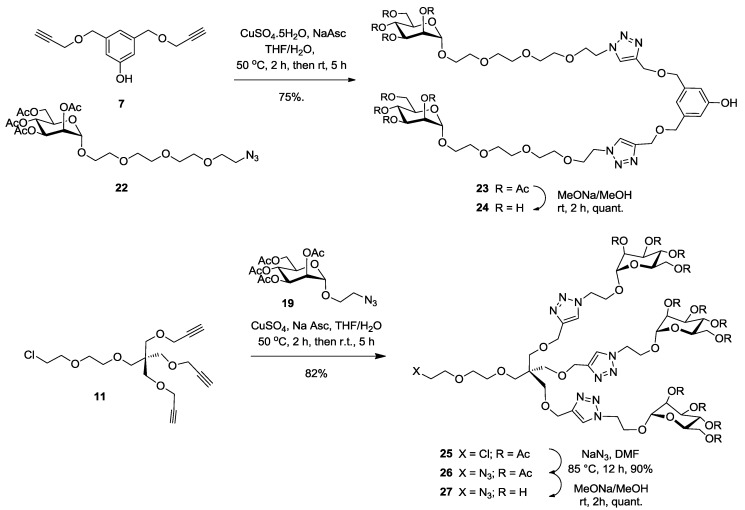
It is well known that the aglyconic moiety of a given carbohydrate can affect the binding affinity and selectivity with lectins. This effect is achieved by either providing better accessibility of the sugar itself into the deep binding pocket of the lectin (linker effect) or by increasing the binding interactions network between neighboring amino acids (pharmacophore effect). Because of the high number of pili at the surface of E. coli, we figured that the linker effect would be a dominant aspect. For comparison purposes, we also built our mannoside residues with a short linker. Thus, known peracetylated mannose 17 was chosen as a starting point after treatment of commercial d-mannose 16 with acetic anhydride in pyridine (Scheme 4). Lewis acid treatment of 17 with bromoethanol in DCM (BF3-Et2O, DCM, 40%) provided known 2-bromoethyl α-d-mannopyranoside 18. Nucleophilic substitution of the bromide by an azide group (NaN3, DMF, 85 °C, 12 h) afforded 2-azidoethyl α-d-mannopyranoside 19 in 60% yield. Similarly, using monotosylated tetraethylene glycol as the aglycone (20), analogous glycosidation of 17 gave tosylate 21 (60%), followed by displacement with azide to give extended mannoside 22 in 90% yield.
Scheme 4.
Synthesis of azido mannoside precursors having a short (19) and a long spacer (22).
2.3. Glycodendron Syntheses
Having the di-(7) and tri-propargylated (11) scaffolds in hands, together with the two family of short (19) and long (22) azido mannosides, we were set for the convergent syntheses of our mannosylated dendrons using the efficacious [1,3]-dipolar copper-catalyzed azide-alkyne cycloaddition (CuAAC) (click chemistry) (Scheme 5). We first combined bis-propargylated scaffold 7 with elongated azido mannoside 22 under typical CuAAC conditions to obtain dimer 23 in 75% yield. Alternatively, the tri-mannosylated dendron was accomplished by treating chloride 11 with the short mannoside 19 under the above click conditions to provide dendron 25 (82%) having an orthogonal chloride functionality at the focal point. Displacement of the chloride by an azide as above gave 26 in 90% yield. Final sugar deprotection using the Zemplén transesterification procedure (NaOMe, MeOH) gave the necessary precursors 24 and 27 in quantitative yields. Completion of the reactions were evidenced by proton NMR spectroscopy, wherein complete disappearance of the propargylic CH signal at δ 2.40 ppm and the appearance of the newly formed singlet of the triazole moiety at δ 7.65 ppm.
Scheme 5.
Synthesis of dimeric (24) and trimeric (27) mannodendrons using click chemistry.
2.4. Glycodendrimer Syntheses
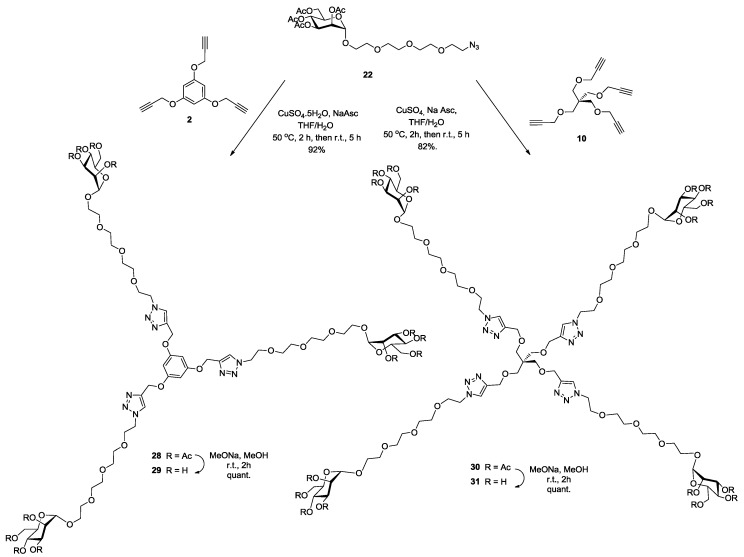
Multivalent binding interactions of glycodendrimers constitute a well-established strategy to enhanced binding efficacies toward their cognate lectins. We chose to prepare several dendrimers harboring from 2 to 18 mannopyranoside moieties to be evaluated as inhibitors against E. coli biofilm formation by using the highly efficient click chemistry. Azide-ending mannoside 22 was anchored to bis-propargylated aromatic scaffold 7 (Scheme 5), tris-propargylated aromatic scaffold 2, tetrapropargylated scaffold 10 (Scheme 6) and hexa-propargylated phosphorus core 15 (Scheme 7) using CuAAC click reaction to afford 23, 28, 30 and 37 in good yields (75%, 92%, 82% and 96%, respectively). Completion of the reaction was evidenced by proton and phosphorus NMR spectroscopy, wherein complete disappearance of the propargylic CH signal and the appearance of the newly formed characteristic singlet of the triazole moieties were observed. Additionally, mass spectral analysis showed the presence of the required molecular ion peak (experimental section). All glycodendrimers were fully deprotected using Zemplén conditions providing the desired glyco-ligands 24, 29, 31 and 38 quantitatively.
Scheme 6.
Synthesis of tri- (29) and tetra-valent (31) pegylated mannosylated clusters using click chemistry on trimer (2) and tetramer (10) scaffolds, respectively.
Scheme 7.
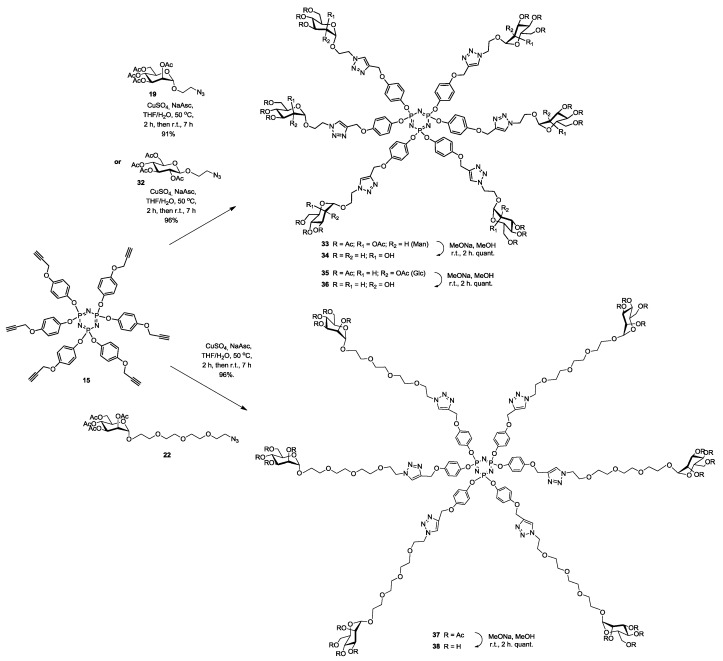
Synthesis of hexavalent phosphorus glycodendrimers 34, 36 and 38 using click chemistry on hexapropagylated core 15 and azido sugars 19, 32 and 22 respectively.
Hexameric mannosylated clusters possessing a short (19) and the long pegylated (22) azido mannosides were anchored onto hexapropargylated phosphorus core 15 using CuAAC click reaction to afford 34 and 38 in good yields of 91% and 96% respectively (Scheme 7). In order to compare the role played by the sugar moiety in the subsequent bioassays, the known short 2-azidoehyl β-d-glucopyranoside 32 [41] was also coupled to the above scaffold 15 under the same reaction conditions to provide 34 also in an excellent 96% yield. Completion of the reactions was again readily evidenced through their respective proton and phosphorus NMR spectroscopy, wherein complete disappearance of the propargylic CH signal and the appearance of the newly formed characteristic singlet of the triazole moieties are showed. Additionally, mass spectral analysis showed the presence of the required molecular ion peak (experimental section). All clusters were fully deprotected using Zemplén reaction (NaOMe, MeOH) to provide the desired glycosylated hexamers 34, 36 and 38 quantitatively.
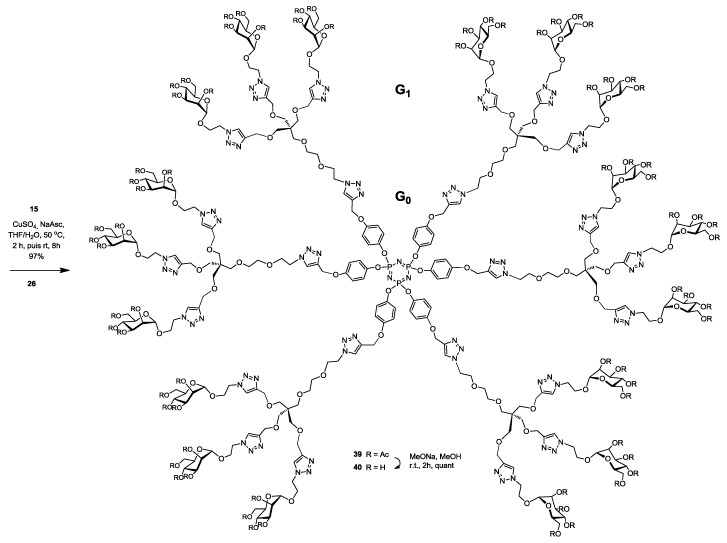
A G1 glycodendrimer 39 possessing 18 mannoside moieties was synthesized in good yield of 97%, by anchoring the azide-ending tri-valent mannosylated dendron 26 to the hexa-propargylated phosphorus core 15 using CuAAC click reaction. Completion of the reaction was evidenced by proton and phosphorus NMR spectroscopy, wherein complete disappearance of the propargylic CH signal δ 2.52 ppm and the appearance of the newly formed characteristic singlet of the triazole moieties δ 7.82 ppm, also the presence of one singlet phosphorus pick at δ 9.54 ppm fully support the homogeneity of the fully substituted cyclophosphazene core. Additionally, mass spectral analysis showed the presence of the required molecular ion peak (experimental section). Compound 39 was fully deprotected by using Zemplén reaction providing the desired G1 glycodendrimer 40 quantitatively (Scheme 8).
Scheme 8.
Synthesis of phosphorus glycodendrimer 40 having 18 α-d-mannopyranoside residues using click chemistry and built around a cyclotriphosphazene core and a pentaerythritol scaffold at the next generation level.
2.5. Dynamic Light Scattering (DLS) Studies
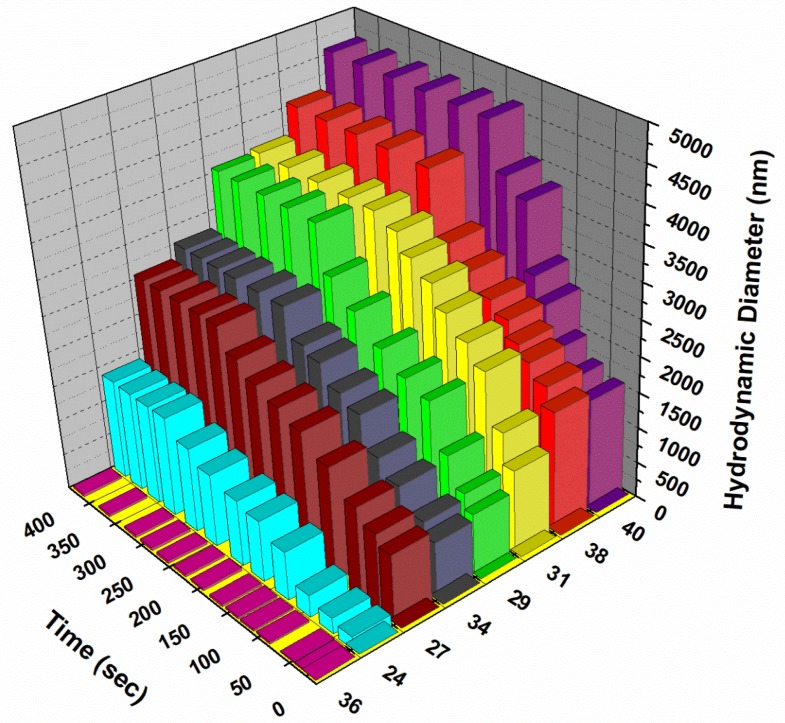
The relative ability of the above glycodendrimers to react with a homotetrameric leguminous lectin such as Concanavalin A (ConA) from Canavalia ensiformis (jack bean) taken as a model and rapidly forming stable cross-linked lattices was monitored using dynamic light scattering (DLS). It was anticipated that the multivalent sugars at the peripheries of the dendrimers would facilitate the multidirectional interactions with its associated tetrameric protein as seen before with several other mannosylated structures [32,42,43,44,45,46,47]. Indeed, these multivalent glycodendrimer–protein complexes were shown to rapidly form large aggregates as a function of time. As seen in Figure 1, the complex resulting from the mannosylated dendrimers 24, 27, 29, 31, 38, 40 and ConA resulted, within 1~3 min to nanometer sizes clusters having sizes ranging from 1400 to 4600 nm. When using the β-d-glucopyranoside dendrimer 36, taken as negative control, no appreciable cross-linked lattice was observed. Clearly, the more highly dense mannodendrimer 40 (18 Man) reacted faster but also formed larger size aggregates than their hexameric cluster counterparts 34 and 38. Interestingly, tetrameric mannoside 31 formed larger aggregates faster than trimer 29 but both smaller clusters plateaued at the same level of ~3000 nm size. Alternatively, tetramer 31 and hexamer 38 formed cross-linked lattices almost equally rapidly but the denser structure 38 formed larger aggregates at the end of the process. As anticipated, hexamer 34, harboring short mannopyranoside aglycone, formed aggregates of smaller size (2530 nm) when compared with hexamer 38 containing the tetraethyleneglycol linker (4090 nm) (see Supplementary Materials). In addition, 38 reached its plateau faster (204 s) in comparison to 34 (230 s), indicating the higher adaptability of the longer spacer to form stable cross-linked lattices.
Figure 1.
3D DLS plots of the cross-linking kinetics of ConA in the presence of mannosylated dendrimers 24, 27, 29, 31, 34, 38 and 40 and the glucosylated dendrimer 36 taken as negative control.
3. Materials and Methods
All reactions in organic medium were performed in standard oven dried glassware under an inert atmosphere of nitrogen using freshly distilled solvents stored over molecular sieves. Solvents were deoxygenated when necessary by bubbling nitrogen through the solution. All reagents were used as supplied without prior purification and obtained from Sigma-Aldrich Chemical Co. (Toronto, ON, Canada) Reactions were monitored by analytical thin-layer chromatography (TLC) using silica gel 60 F254 pre-coated plates (Merck, Darmstadt, Germany) and compounds were visualized by 254 nm light and/or by dipping into a mixture of sulfuric acid and methanol in water or into a mixture of KMin.O4 and K2CO3 in water followed by gentle warming with a heat-gun. Purifications were performed by flash column chromatography using silica gel from Canadian Life Science (60 Å, 40–63 μm) (Peterborough, ON, Canada) with the indicated eluent. 1H-NMR, 13C-NMR and 31P-NMR spectra were recorded at 300 and/or 600 MHz, 75 and/or 150 MHz and 122 and/or 243 MHz, respectively, on a Bruker spectrometer (300 MHz and 600 MHz) (Milton, ON, Canada) and Varian spectrometer (600 MHz) (Milton, ON, Canada). All NMR spectra were measured at 25 °C in indicated deuterated solvents. Proton and carbon chemical shifts (δ) are reported in ppm and coupling constants (J) are reported in Hertz (Hz). The resonance multiplicity in the 1H-NMR spectra are described as “s” (singlet), “d” (doublet), “t” (triplet) and “m” (multiplet) and broad resonances are indicated by “broad.” Residual protic solvent of CDCl3 (1H, 7.27 ppm; 13C, 77.0 ppm (central resonance of the triplet)), D2O (1H, 4.80 ppm and 30.9 ppm for CH3 of acetone for 13C spectra), MeOD (1H, 3.30 ppm and 13C, 49.0 ppm), 85% H3PO4 was used as an external reference for 31P-NMR. Two-dimensional homonuclear correlation 1H-1H COSY and 1H-13C HSQC experiments were used to confirm NMR peak assignments. Letters are used to NMR assignment. Accurate mass measurements (HRMS) were performed on a LC-MSD-TOF instrument from Agilent Technologies (Santa Clara, CA, USA) in positive electrospray mode. Either protonated molecular ions [M + nH]n+ or adducts [M + nX]n+ (X = Na, K, NH4) were used for empirical formula confirmation. Light Scattering-Multiangle Laser Light Scattering (LS-MALLS) detection with performances verified with polystyrene 100 kDa and 2000 kDa were used to determine the number-average molecular weight (Min.) and polydispersity index (MW/Min.). Calculations were performed with Zimm Plot (model). MALDI-TOF MS data were acquired on a Bruker Microflex LRF system (Bruker Daltonics, Billerica, MA, USA) equipped with a Compass 3.1 software platform. Acquisitions were performed in positive ion mode. Reflector mode was utilized for samples below 5 kDa and linear mode for samples above 5 kDa. Samples were dissolved in water (1 mg/mL) and mixed with 10 volumes of α-cyano-4-hydroxycinnamic acid matrix prepared at 10 mg/mL with 50% ACN/H2O 0.1% TFA. A volume of 2 μL was deposited on the target plate and dried. Representative acquisition parameters were: ion source 1, 19.5 kV; ion source 2, 18.05 kV; lens, 7.0 V; pulse ion extraction, 240 ns; detector, 1.905 kV. Approximately 200 laser shot/spectra were obtained with a 60 Hz N2-cartridge-laser set at 337 nm and the laser intensity adjusted according to the signal intensity. Sugar monomers were synthesized following the typical procedures found in literatures with a slight modification as describe bellow.
Dynamic Light Scattering
Particle size distribution (DLS) was measured in water with the help of Zetasizer Nano S90 from Malvern Instruments. Crosslinking studies were carried out in 1 mol/L phosphate buffered saline (PBS) for the plant lectin Concavalin A (Sigma-Aldrich).
General procedure for the Cu(I) azide-alkyne cycloaddition reaction (I) (CuAAc) [28]. Solutions of alkyne (1 equiv.) and azide (1.5 equiv. per alkyne site) were prepared in minimum amount of THF. The resulting solutions were treated with an aqueous solution of CuSO4 (0.3 equiv. per alkyne site) and sodium ascorbate solution in THF (0.35 equiv. per alkyne site). Biphasic mixture was then stirred at 50 °C for 2 h then left at room temperature until completion of the reaction as judged by the complete conversion of the limiting regents (alkyne). After completion of the reaction, EtOAc was added, then Na2SO4 was added to quench the reaction by regenerating the CuSO4·5H2O. The mixture was allowed to stir for 3 min then filtered before the solvent was removed under reduced pressure and the resulting viscous oil was subjected to silica gel column chromatography using the appropriate eluent system (0–10% MeOH in CH2Cl2) to afford the triazole products.
General procedure for the azide substitution (II) [28]. To a solution of the appropriate bromo/tosyl derivatives (1 equiv.) in DMF was added NaN3 (1.5 equiv. per bromide). The reaction mixtures were stirred at 80 °C until completion of reaction as judged by TLC. The excess solvent was next removed under reduced pressure with heating at 60 °C until dryness. The residues were dissolved in EtOAc and washed successively with water and brine. The organic layer was separated and dried on Na2SO4. The residue was subjected to silica gel flash chromatography to afford the desired azido derivative.
General Procedure for De-O-acetylation (III) (Zemplén reaction) [28]. An acetylated glycocluster (0.1 mmol) was dissolved in dry MeOH (3 mL) and a solution of sodium methoxide (1 M in MeOH, 0.5 equiv.) was added. The reaction mixture was stirred at room temperature until the starting material disappeared. The solution was neutralized by the addition of a cationic ion-exchange resin (H+), filtered and washed with MeOH and then the solvent was removed in vacuo. The residue was lyophilized to yield quantitatively the fully deprotected glycoclusters.
Synthesis of2. Benzene-1,3,5-triol (1) [48] (2 g, 15.86 mmol) was dissolved in DMF (10 mL) followed by the addition of K2CO3 (3 g, 14.87 mmol). The mixture was reflux for 30 min, propargyl bromide (8.5 mL, 79.3 mmol, 5 equiv.) was added dropwise, then stirred over night at room temperature. The residue was dissolved in dichloromethane (DCM) and washed with water and brine. The organic layer was dried over Na2SO4, filtered, then concentered under vacuum. The crude product was purified by silica gel flash chromatography using 0–20% EtOAc in Hexane as eluents to afford compound 2 as a white powder (2 g, 53%). Rf = 0.28 (EtOAc/Hexane, 1:4). 1H-NMR (300 MHz, CDCl3) δ 6.27 (s, 3H, k), 4.65 (d, J = 2.4 Hz, 6H, b), 2.53 (t, J = 2.4 Hz, 3H, c).
Synthesis of compound9. To a solution of pentaerythritol 8 (2 g, 14.7 mmol) in dimethylsulfoxide (DMSO, 15 mL) were added an aqueous solution of NaOH (40 wt%, 10 mL). The solution was kept under magnetic stirring at room temperature for 30 min Propargyl bromide (97%, 12 mL, 135.18 mmol) was then added and the solution was kept at room temperature for an additional 24 h. Ethyl ether was added to the reaction mixture then washed with water. The organic layer was dried over Na2SO4. The crude product was purified by silica gel flash chromatography using 0–20% EtOAc in Hexane as eluents to afford compound 9 as a colorless oil (2.2 g, 60%). Rf = 0.25 (EtOAc/Hexane, 1:4). Compound 10 was obtained as a white powder (1.2 g, 28%). Rf = 0.35 (EtOAc/Hexane, 1:4). Tetrapropargylpentaerythritol 10 [49]. 1H-NMR (300 MHz, CDCl3) δ 4.08 (d, J = 2.3 Hz, 8H, OCH2CCH), 3.48 (s, 8H, CH2OCH2CCH), 2.42 (d, J = 2.2 Hz, 4H, OCH2CCH).
Tripropargylpentaerythritol9 [49]. 1H-NMR (300 MHz, CDCl3) δ 4.13 (d, J = 2.4 Hz, 6H, OCH2CCH), 3.69 (s, 2H, CH2OH), 3.56 (s, 6H, CH2OCH2CCH), 2.42 (t, J = 2.4 Hz, 3H, CCH).
Synthesis of compound11. A solution of pentaerythritol propargyl ether (9) (2 g, 7.9 mmol, 1 equiv.), Bu3NHSO4 (5 g, 14.7 mmol, 1.8 equiv.), Bis(2-chloroethyl) ether (22 mL, 187.61 mmol) in NaOH 50% (30 mL), was stirred at room temperature for 24 h. DCM was added to the reaction mixture and the organic layer was washed successfully with water and brine, then dried over anhydrous sodium sulfate. The crude product was purified by silica gel flash chromatography using 0–20% EtOAc in Hexane as eluents to afford compound 11 as a colorless oil (2.1 g, 73%). Rf = 0.25 (EtOAc/Hexane, 1:4). 1H-NMR (300 MHz, CDCl3) δ 4.11 (d, J = 2.4 Hz, 6H, OCH2CCH), 3.76 (dd, J = 8.8, 3.2 Hz, 2H, CH2-Cl), 3.67–3.56 (m, 6H, OCH2-CH2), 3.52 (s, 6H, CH2OCH2CCH), 3.46 (s, 2H, CH2OCH2), 2.40 (t, J = 2.4 Hz, 3H, CCH).
Monopropagyloxyphenol (13) [50]. Hydroquinone (10.28 g, 93.37 mmol) was dissolved in acetone (150 mL) followed by the addition of K2CO3 (15.42 g, 111.58 mmol). The mixture was reflux for 30 min, propargyl bromide (10.28 mL, 93.50 mmol, 0.98 equiv.) was added dropwise, then stirred over night at room temperature. Residue was dissolved in DCM (100 mL) and washed with water (3 × 50 mL) and brine (3 × 50 mL). The organic layer was dried over Na2SO4, filtered and concentered under vacuum. The crude product was purified by silica gel flash chromatography using 0–30% EtOAc in Hexane as eluents to afford the mono substituted propargyl hydroquinone 13 as a brownish powder (3.11 g, 20.96 mmol, 43%). Rf = 0.35 (EtOAc/Hexane, 3:7). 1H-NMR (300 MHz, CDCl3) δ 6.85 (d, J = 9.1 Hz, 2H, aromatic), 6.78 (d, J = 9.1 Hz, 2H, aromatic), 4.66 (d, J = 1.4 Hz, 2H, CH2), 2.53 (t, J = 2.4 Hz, 1H, ≡CH).
Synthesis of15 [50,51,52,53,54,55]. Cs2CO3 (3.4 g, 10.4 mmol) is added into a reaction mixture of 4-(prop-2-yn-1-yloxy)phenol (13) (6.7 mmol) and N3P3Cl6 (14) (0.6 mmol) in THF (20 mL). The mixture was heated at 40 °C for 17 h, then filtered on Celite and concentrated. The pure 15 was isolated as a white crystal yielding 98% after crystallization in EtOH. 1H-NMR (300 MHz, CDCl3) δ 6.87 (d, J = 9.65 Hz, 6H, OC6H4O), δ 6.80 (d, J = 9.65 Hz, 6H, OC6H4O), 4.65 (d, J = 2.4 Hz, 6H, OCH2), 2.52 (t, J = 2.4 Hz, 3H, CH). 31P-NMR (122 MHz, CDCl3) δ 9.82 (s).
1,2,3,4,6-Penta-O-acetyl-α/β-d-mannopyranose (17). 1,2,3,4,6-penta-O-acetyl-α/β-d-mannopyranose was prepared according to the procedure reported [52] with a slight modification. d-Mannose 16 (3 g, 16.6 mmol), pyridine (40 mL) and acetic anhydride (32 mL, 333 mmol) were stirred at room temperature. After stirring for 12 h, the reaction mixture was diluted with ice-water and extracted with DCM. The combined organic layer was washed with 1 M aqueous HCl, saturated sodium bicarbonate solution (NaHCO3), H2O and Brine. The organic layer was dried over Na2SO4 and the solvent was removed under reduced pressure. The product 17 was obtained as colorless syrup (6 g, 15.4 mmol, 90%) which was a mixture of α and β anomers with a ratio of 3:1. This product was used in the next synthetic step without any further purification. 1H-NMR (300 MHz, CDCl3) δ 6.09 (d, J = 1.8 Hz, 3H, H-1a), 5.86 (d, J = 1.1 Hz, 1H, H-1b), 5.49 (dd, J = 3.3, 1.1 Hz, 1H), 5.38–5.33 (m, 6H), 5.32 (d, J = 2.8 Hz, 1H), 5.26 (t, J = 2.0 Hz, 3H), 5.13 (dd, J = 10.0, 3.3 Hz, 1H), 4.30 (ddd, J = 12.4, 7.5, 5.1 Hz, 5H), 4.18–4.02 (m, 8H), 3.80 (ddd, J = 9.8, 5.3, 2.4 Hz, 1H), 2.22 (s, 3H), 2.18 (s, 9H), 2.17 (s, 9H), 2.10 (s, 3H), 2.09 (s, 12H), 2.05 (s, 12H), 2.01 (s, 12H).
2-Bromoethyl 2,3,4,6-tetra-O-acetyl-α-d-manno-pyranoside (18) [27,51]. Compound 17 (5.37 g, 13.8 mmol) and 2-bromoethanol (0.98 mL, 13.8 mmol) were dissolved in DCM (50 mL). Then, boron trifluoride etherate (5.8 mL, 47.2 mmol) was added to the solution and stirred under a nitrogen atmosphere for 3 h and monitored by TLC (EtOAc/Hexane, 1:1). After addition of DCM (100 mL), the reaction mixture was neutralized by adding saturated sodium bicarbonate solution (100 mL) and the resulting solution was washed with water (2 × 200 mL). The combined organic layers were dried over Na2SO4, filtered and concentrated to dryness under reduced pressure. The resulting oil was then purified using silica gel chromatography (EtOAc/Hexane, 1:1). The relevant fractions were collected, combined and concentrated to dryness under reduced pressure to yield 18 as a colorless powder (2.2 g, 40%). 1H-NMR (300 MHz, CDCl3) δ 5.39–5.18 (m, 4H, H-2, 3 and 4), 4.85 (d, J = 1.6 Hz, 1H, H-1), 4.27 (dd, J = 12.2, 5.3 Hz, 1H, H-6), 4.16–3.98 (m, 2H, H-6, H-5), 3.85 (ddd, J = 10.6, 6.6, 4.0 Hz, 1H, j), 3.72–3.60 (m, 1H, j′), 3.54–3.34 (m, 2H, CH2Br), 2.12 (s, 3H, COCH3), 2.08 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 1.97 (s, 3H, COCH3).
2-Azidoethyl 2,3,4,6-tetra-O-acetyl-α-d-manno-pyranoside (19) [27,51]. Compound 18 (1.40 g, 3.0 mmol) and sodium azide (1.00 g, 15.4 mmol) were dissolved in anhydrous DMF (30 mL) and stirred at 80 °C for 5 h. The reaction mixture was filtered and concentrated to dryness under reduced pressure and further processed as given in general procedure (II) to afford compound 19 (0.75 g, 60%) as white powder. 1H-NMR (300 MHz, CDCl3) δ 5.48–5.11 (m, 3H, H-2, 3 and 4), 4.87 (d, J = 1.6 Hz, 1H, H-1), 4.29 (dd, J = 12.3, 5.3 Hz, 1H, H-6), 4.20–3.99 (m, 2H, H-6′ and 5), 3.87 (m, 1H, j), 3.76–3.60 (m, 1H, j′), 3.56–3.36 (m, 2H, CH2N3), 2.16 (s, 3H, COCH3), 2.11 (s, 3H, COCH3), 2.06–2.03 (s, 3H, COCH3), 2.00 (s, 3H, COCH3).
Monotosylated tetraethylene glycol (20) [53]. To a solution of tetraethylene glycol (26.3 g, 135.4 mmol, 10 equiv.) in THF (60 mL) was added a solution of sodium hydroxide (1.79 g, 44.7 mmol, 3.3 equiv.) dissolved in water (5 mL). The mixture was cooled to 0 °C and toluensulfonyl chloride (2.57 g, 16.54 mmol, 1 equiv.) in THF (5 mL) was added dropwise. The reaction was stirred at 0 °C for 2 h. The solution was poured into water and the aqueous layer was extracted with dichloromethane. The organic layers were washed with water, dried over Na2SO4, filtered and concentrated under reduced pressure to yield 20 as a colorless oil (4.69 g, 97%) yield based on the p-toluenesulfomyl chloride. Rf = 0.25 (in 100% EtOAc). 1H-NMR (300 MHz, CDCl3) δ 7.79 (d, J = 8.0 Hz, 2H, b), 7.33 (d, J = 8.0 Hz, 2H, d), 4.16 (t, J = 4.7 Hz, 2H, a), 3.75–3.55 (m, 14H, e), 2.44 (s, 3H, c).
2-(2-{2-[2-(2-Tosyloxy-ethoxy)-ethoxy]-ethoxy}-ethyl) 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (21) [53]. Into a solution of pentaacetate mannose 17 (1.68 g, 4.31 mmol, 1 equiv.) in anhydrous CH2Cl2 (20 mL) was added boron trifluoride etherate (1.23 mL, 9.93 mmol, 2.3 equiv.) at room temperature under nitrogen atmosphere. The solution was stirred for 4 h before compound 20 (3.76 g, 10.79 mmol, 2.5 equiv.) was added. Glycosylation was completed after stirring at 40 °C overnight. The crude product was washed with NaHCO3 sat, dried over Na2SO4 and concentrated under reduced pressure. Further purification was processed on silica gel flash chromatography using 0–20% EtOAc in Hexane as eluents to afford compound 21 as a colorless oil (1.8 g, 60%). Rf = 0.35 (EtOAc/Hexane, 1:4). 1H-NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.3 Hz, 2H, aromatic), 7.34 (d, J = 8.0 Hz, 2H, aromatic), 5.38–5.25 (m, 3H, H-3, 4 and 2), 4.87 (d, J = 1.6 Hz, 1H, H-1), 4.36–4.19 (m, 2H, H-6), 4.18–4.01 (m, 3H, c and H-5), 3.85–3.75 (m, 1H, j’), 3.73–3.56 (m, 13H, j, i, d, g, f, e), 2.45 (s, 3H, CH3), 2.16 (d, 3H, COCH3), 2.09 (s, 3H, COCH3), 2.03 (s, 3H, COCH3), 1.99 (s, 3H, COCH3).
2-(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl) 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (22) [53]. To a solution of 21 (520 mg, 0.766 mmol, 1 equiv.) in dry DMF (15 mL) under a nitrogen atmosphere were added sodium azide (996 mg, 15.3 mmol, 20 equiv.). After stirring at 80 °C for 2 h, the solution was diluted in EtOAc and further processed as given in general procedure (II) to afford compound 22 as a colorless oil (380 mg, 90%), Rf = 0.4 (EtOAc/Hexane, 1:4). 1H-NMR (300 MHz, CDCl3) δ 5.43–5.24 (m, 3H, H-3, 4 and 2), 4.87 (d, J = 1.6 Hz, 1H, H-1), 4.27 (td, J = 12.6, 5.0 Hz, 1H, H-6), 4.14–4.03 (m, 2H, H-6′ and 5), 3.86–3.76 (m, 1H, j′), 3.71–3.64 (m, 13H, j, i, d, g, e, h, f), 3.39 (t, J = 5.1 Hz, 2H, CH2-N3), 2.15 (s, 3H, COCH3), 2.10 (s, 3H, COCH3), 2.04 (s, 3H, COCH3), 1.99 (s, 3H, COCH3).
Synthesis of23. Into a solution of bispropargylated core (7) (30 mg, 0.13 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 2-(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl) 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (22) (220 mg, 0.39 mmol, 3 equiv.), Na-ascorbate (18 mg, 0.09 mmol, 0.7 equiv.) and CuSO4·5H2O (19 mg, 0.08 mmol, 0.6 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 23 (136 mg, 75%) as a white powder; Rf = 0.35 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.74 (s, 2H, H-triazole), 6.77 (s, 1H, HP-aromatic), 6.74 (s, 2H, HO-aromatic), 5.30 (dd, J = 10.0, 3.4 Hz, 2H, H-3), 5.25 (d, J = 9.9 Hz, 2H, H-4), 5.23–5.20 (m, 2H, H-2), 4.82 (s, 2H, H-1), 4.61 (s, 4H, b), 4.52–4.49 (m, 4H, c), 4.47 (s, 4H, m), 4.24 (dd, J = 12.2, 4.8 Hz, 2H, H-6), 4.05 (dd, J = 12.3, 4.1 Hz, 2H, H-6′), 4.03–3.99 (m, 2H, H-5), 3.83 (t, J = 4.9 Hz, 4H, d), 3.77–3.73 (m, 2H, j), 3.61 (m, 2H, j′), 3.60 (s, 4H, i), 3.58 (s, 16H, g, f, e, h), 2.11 (s, 6H, COCH3), 2.05 (s, 6H, COCH3, 1.99 (s, 6H, COCH3), 1.94 (s, 6H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 170.6 (C=O), 169.9 (C=O), 169.8 (C=O), 169.6 (C=O), 156.8 (COH), 144.7 (C-triazole), 139.5 (Cm-aromatic), 123.8 (CH-triazole), 118.4 (Cp-aromatic), 113.9 (CO-aromatic), 97.5 (C-1), 71.9 (Cm), 70.5 (Cd), 70.4 (Cg), 70.3 (Cf), 69.8 (Ce), 69.4 (Ch), 69.4 (Cj), 69.3 (C-2), 68.9 (C-3), 68.2 (C-4), 67.2 (C-5), 65.9 (Ci), 63.4 (Cb), 62.2 (C-6), 50.1 (Cc), 20.8 (COCH3), 20.6 (COCH3), 20.6 (COCH3). ESI+-HRMS: [M + Na]+ calcd for C56H86N6NaO29, 1329.5331; found: 1329.5395.
Synthesis of24. Compound 23 (50 mg, 0.036 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 24 (38 mg, quant.) was obtained as a colorless solid. 1H-NMR (600 MHz, D2O) δ 8.04 (s, 2H), 6.89 (s, 1H), 6.82 (s, 2H), 4.84 (s, 2H), 4.70 (s, 4H), 4.66–4.59 (m, 4H), 4.57 (s, 4H), 3.98–3.94 (m, 4H), 3.93–3.80 (m, 4H), 3.80–3.72 (m, 4H), 3.67–3.60 (m, 12H), 3.60–3.55 (m, 8H), 3.55–3.52 (m, 4H). 13C-NMR (150 MHz, D2O) δ 215.4, 155.9, 143.9, 139.3, 125.5, 120.1, 114.7, 99.8, 72.7, 71.7, 70.5, 69.9, 69.7, 69.6, 69.5, 69.4, 69.4, 68.7, 66.7, 66.3, 62.4, 60.9, 49.9, 30.2. ESI+-HRMS: [M + Na] + calcd for C40H70N6NaO21, 993.4486; found: 993.4545.
Synthesis of25. Into a solution of tripropargyl pentaerythritol core (11) (125 mg, 0.35 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 2-azidoethyl 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (19) (745 mg, 1.42 mmol, 5.1 equiv.), Na-ascorbate (70 mg, 0.29 mmol, 1.1 equiv.) and CuSO4·5H2O (78 mg, 0.31 mmol, 0.9 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 25 (480 mg, 82%) as a white powder; Rf = 0.3 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (300 MHz, CDCl3) δ 7.70 (s, 3H, HB-triazole), 5.31–5.19 (m, 9H, H-3, 4 and 2), 4.81 (d, J = 1.3 Hz, 3H, H-1), 4.61 (m, 6 H, i), 4.59 (s, 6H, h), 4.21 (dd, J = 12.3, 5.1 Hz, 3H, H-6), 4.13 (dt, J = 10.4, 4.9 Hz, 3H, j′), 4.04 (dd, J = 12.3, 2.3 Hz, 3H, H-6′), 3.91 (dt, J = 10.6, 5.2 Hz, 3H, j), 3.74 (m, 2H, d), 3.61 (m, 7 H, H-5, f and CH2-Cl), 3.57–3.53 (m, 2H, e), 3.49 (s, 6H, g′), 3.44 (s, 2H, g), 2.13 (s, 9H, COCH3), 2.09 (s, 9H, COCH3), 2.04 (s, 9H, COCH3), 1.99 (s, 9H, COCH3).
Synthesis of26. To a solution of compound 25 (480 mg, 0.29 mmol, 1.0 equiv.) in dry DMF (2 mL) under a nitrogen atmosphere were added sodium azide (186 mg, 2.9 mmol, 10 equiv.). After stirring at 80 °C for 2 h, the solution was diluted in EtOAc and further processed as given in general procedure (II) to afford compound 26 as a white powder (430 mg, 90%). 1H-NMR (300 MHz, CDCl3) δ 7.65 (s, 3H, HB-triazole), 5.28–5.09 (m, 9H, H-3, 4 and 2), 4.76 (d, J = 1.1 Hz, 3H, H-1), 4.6–4.57 (m, 6H, i), 4.53 (s, 6H, h), 4.15 (dd, J = 12.4, 5.1 Hz, 3H, H-6), 4.07 (dt, J = 10.5, 5.7 Hz, 3H, j’), 3.98 (dd, J = 12.3, 2.3 Hz, 3H, H-6′), 3.86 (dt, J = 10.4, 5.1 Hz, 3H, j), 3.63–3.59 (m, 2H, d), 3.56–3.53 (m, 5H, H-5, f), 3.49 (m, 2H, e), 3.44 (s, 6H, g′), 3.39 (s, 2H, g), 3.33–3.27 (m, 2H, c, CH2-N3), 2.07 (s, 9H, COCH3), 2.03 (s, 9H, COCH3), 1.98 (s, 9H, COCH3), 1.94 (s, 9H, COCH3). 13C-NMR (75 MHz, CDCl3) δ 170.6 (C=O), 170.0 (C=O), 169.6 (C=O), 169.1 (C=O), 145.6 (C-triazole), 123.7 (CHB-triazole), 97.6 (C-1), 77.2 (Cd), 71.0 (Cf), 70.3 (Ce), 70.0 (Cg), 69.2 (C-2), 69.1 (C-3), 68.9 (C-4), 66.3 (C-5), 65.7 (Cj), 64.8 (Ch), 62.2 (C-6), 51.8 (Ci), 50.8 (Cc), 49.5 (Cq), 20.8 (COCH3), 20.7 (COCH3).
Synthesis of27. Compound 26 (200 mg, 0.02 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 27 (150 mg, quant.) was obtained as a white powder. 1H-NMR (300 MHz, MeOD) δ 8.00 (s, 3H), 4.69–4.60 (m, 9H), 4.55 (s, 6H), 4.11 (dt, J = 10.3, 4.9 Hz, 3H), 3.88 (dt, J = 5.8, 4.6 Hz, 3H), 3.81–3.71 (m, 6H), 3.65 (ddd, J = 18.4, 7.4, 5.7 Hz, 18H), 3.58–3.49 (m, 4H), 3.46 (s, 6H), 3.40 (dd, J = 9.5, 4.3 Hz, 6H), 3.21 (dd, J = 7.6, 5.1 Hz, 4H). 13C-NMR (75 MHz, MeOD) δ 146.08, 126.31, 101.33, 79.41, 74.58, 72.26, 72.06, 71.69, 71.35, 70.99, 70.1, 68.1, 66.9, 65.2, 62.4, 51.8, 51.4, 50.1, 49.9, 49.6, 49.3, 49.0, 49.0, 48.7, 48.4, 46.5, 31.3, 24.6. ESI+-HRMS: [M + 2H]2+ calcd for C42H72N12O23, 556.2418; found: 556.2470.
Synthesis of28. Into a solution of trispropargylated core (2) (25 mg, 0.104 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 2-(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl) 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (22) (281 mg, 0.5 mmol, 5 equiv.), Na-ascorbate (22 mg, 0.11 mmol, 0.5 equiv.) and CuSO4·5H2O (23 mg, 0.09 mmol, 0.9 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 28 (188 mg, 92%) as a white powder; Rf = 0.32 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.83 (s, 3H, H-triazole), 6.28 (s, 3H, k) 5.32 (dd, J = 10.0, 2.8 Hz, 3H, H-3), 5.27 (t, J = 10.0 Hz, 3H, H-4), 5.25 (dd, J = 3.0, 1.7 Hz, 3H, H-2), 5.12 (s, 6H, b), 4.85 (d, J = 1.4 Hz, 3H, H-1), 4.55 (t, J = 4.9 Hz, 6H, c), 4.27 (dd, J = 12.4, 5.1 Hz, 3H, H-6), 4.07 (d, J = 12.8 Hz, 3H, H-6′), 4.03 (dd, J = 5.7, 3.5 Hz, 3H, H-5), 3.89 (t, J = 4.9 Hz, 6H, d), 3.82–3.73 (m, 3H, j), 3.65 (m, 3H, j′), 3.63 (s, 6H, i), 3.61 (s, 24H, g, e, f, h), 2.13 (s, 9H, COCH3), 2.07 (s, 9H, COCH3), 2.01 (s, 9H, COCH3), 1.96 (s, 9H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 170.8 (C=O), 170.1 (C=O), 170.0 (C=O), 169.8 (C=O), 160.3 (COCb-aromatic), 143.7 (C-triazole), 124.2 (CH-triazole), 97.8 (C-1), 95.1 (Ck-aromatic), 70.8 (Cd), 70.7 (Cg), 70.6 (Cf), 70.1 (Ce), 69.7 (Ch), 69.6 (Cj), 69.5 (C-2), 69.2 (C-3), 68.5 (C-4), 67.5 (C-5), 66.2 (Ci), 62.5 (Cb), 62.1 (C-6), 50.4 (Cc), 21.0 (COCH3), 20.9 (COCH3), 20.8 (COCH3), 20.8 (COCH3). MALDI-TOF: [M + H]+ calcd for C81H118N9O42: 1888.737; found, 1888.789.
Synthesis of29. Compound 28 (100 mg, 0.05 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 29 (74 mg, quant.) was obtained as a white solid. 1H-NMR (600 MHz, MeOD) δ 8.16 (s, 3H), 6.37 (s, 3H), 5.19 (s, 6H), 4.65–4.63 (m, 8H), 3.95–3.92 (m, 4H), 3.88–3.87 (m, 2H), 3.82 (m, 10.0, 3.1 Hz, 4H), 3.77–3.71 (m, 4H), 3.68–3.54 (m, 23H). 13C-NMR (150 MHz, MeOD) δ 159.9, 143.3, 125.4, 100.1, 95.5, 72.9, 70.9, 70.3, 69.9, 69.8, 69.8, 68.9, 66.9, 66.4, 61.1, 50.2, 48.5, 47.9, 47.9, 47.8, 47.8, 47.8. MALDI-TOF: [M]+ calcd for C57H92N9O30: 1382.592; found, 1382.801.
Synthesis of30. Into a solution of tetrapropargyl pentaerythritol core (10) (67 mg, 0.23 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 2-(2-{2-[2-(2-Azido-ethoxy)-ethoxy]-ethoxy}-ethyl) 2,3,4,6-tetra-O-acetyl-α-d-mannopyranoside (22) (790 mg, 1.4 mmol, 6 equiv.), Na-ascorbate (65 mg, 0.32 mmol, 1.4 equiv.) and CuSO4·5H2O (70 mg, 0.28 mmol, 1.2 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 30 as a white powder (484 mg, 82%). Rf = 0.27 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.68 (s, 4H, H-triazole), 5.32 (dd, J = 10.0, 3.4 Hz, 4H, H-3), 5.26 (t, J = 10.0 Hz, 4H, H-2), 5.24 (dd, J = 3.2, 1.6 Hz, 4H, H-4), 4.85 (s, 4H, H-1), 4.52 (s, 8H, b), 4.51 (t, J = 5.5 Hz, 8H, c), 4.27 (dd, J = 12.2, 4.9 Hz, 4H, H-6), 4.07 (dd, J = 12.2, 2.2 Hz, 4H, H-6′), 4.04 (ddd, J = 9.8, 4.8, 2.3 Hz, 4H, H-5), 3.87 (t, J = 5.3 Hz, 8H, d), 3.81–3.75 (m, 4H, j), 3.66 (dd, J = 5.8, 3.7 Hz, 4H, j′), 3.64–3.63 (m, 8H, i), 3.62–3.58 (m, 32H, g, f, e, h), 3.45 (s, 8H, k), 2.13 (s, 12H, COCH3), 2.08 (s, 12H, COCH3), 2.01 (s, 12H, COCH3), 1.96 (s, 12H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 170.7 (C=O), 170.0 (C=O), 169.9 (C=O), 169.7 (C=O), 145.1 (C-triazole), 123.6 (CH-triazole), 97.7 (C-1), 70.7 (Cd), 70.6 (Cg), 70.5 (Cf), 69.9 (Ce), 69.6 (Ch), 69.5 (Cj), 69.5 (C-2), 69.2 (Cq), 69.1 (C-3), 68.4 (C-4), 67.4 (C-5), 66.1 (Ci), 64.9 (Cb), 62.4 (C-6), 50.1 (Cc), 45.3 (Ck), 20.9 (COCH3), 20.8 (COCH3), 20.7 (COCH3), 20.7 (COCH3). ESI+-HRMS: [M + 2H]2+ calcd for C105H162N12O56, 1244.5021; found: 1244.0172.
Synthesis of31. Compound 30 (100 mg, 0.038 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 31 (70 mg, quant.) was obtained as a colorless solid. 1H-NMR (600 MHz, D2O) δ 8.01 (s, 4H), 4.87 (s, 4H), 4.60 (t, J = 4.9 Hz, 8H), 4.52 (s, 8H), 3.95 (d, J = 4.8 Hz, 12H), 3.90–3.86 (m, 4H), 3.85 (dd, J = 7.4, 3.7 Hz, 4H), 3.81 (dd, J = 9.1, 3.2 Hz, 4H), 3.75 (dd, J = 12.2, 5.4 Hz, 4H), 3.72–3.65 (m, 12H), 3.65–3.60 (m, 24H), 3.60–3.57 (m, 16H), 3.40 (s, 8H). 13C-NMR (150 MHz, D2O) δ 144.1, 125.3, 99.9, 72.7, 70.5, 69.9, 69.9, 69.7, 69.6, 69.5, 69.5, 68.7, 68.1, 66.7, 66.3, 63.5, 60.9, 49.9, 44.6. ESI+-HRMS: [M + 2H]2+ calcd for C73H130N12O40, 907.4248; found: 907.4258.
Synthesis of33. Into a solution of hexapropargylated core (15) (30 mg, 0.03 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 19 (152 mg, 0.29 mmol, 9.6 equiv.), Na-ascorbate (12 mg, 0.06 mmol, 1.98 equiv.) and CuSO4·5H2O (14 mg, 0.05 mmol, 1.8 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 33 as a white powder (97 mg, 91%); Rf = 0.3 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.83 (s, 6H, H-triazole), 6.85 (d, J = 9.0 Hz, 12H, aromatic), 6.79 (d, J = 9.1 Hz, 12H, aromatic), 5.31–5.19 (m, 18H, H-2, 3 and 4), 5.16–5.08 (m, 12H, Ar-OCH2), 4.82 (s, 6H, H-1), 4.64–4.54 (m, 12H, Man-OCH2), 4.21 (dd, J = 12.3, 5.1 Hz, 6H, CHH′N), 4.16–4.09 (m, 6H, H-6), 4.04 (dd, J = 12.3, 2.2 Hz, 6H, H-6′), 3.90 (dt, J = 10.4, 5.1 Hz, 6H, CHH′N), 3.65–3.58 (m, 6H, H-5), 2.13 (s, 18H, COCH3), 2.09 (s, 18H, COCH3), 1.98 (s, 18H, COCH3), 1.97 (s, 18H, COCH3). 13C-NMR (151 MHz, CDCl3) δ 170.8 (C=O), 170.2 (C=O), 170.2 (C=O), 169.9 (C=O), 155.7 (CO-aromatic), 145.1 (C-triazole), 144.5 (Cp-aromatic), 124.5 (CH-triazole), 122.2 (Cm-aromatic), 115.7 (CO-aromatic), 97.9 (C-1), 77.2 (C-2), 69.6 (C-3), 69.5 (C-4), 69.3 (C-5), 66.6 (Cj), 66.2 (Cb), 62.7 (C-6), 50.0 (Ci), 21.1 (COCH3), 21.0 (COCH3), 20.9 (COCH3). 31P-NMR (243 MHz, CDCl3) δ 9.84. ESI+-HRMS: [M + 3H]3+ calcd for C150H183N21O72P3, 1174.34752; found: 1174.6753.
Synthesis of34. Compound 33 (97 mg, 0.03 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 34 (69 mg, quant.) was obtained as a white solid. 1H-NMR (600 MHz, D2O) δ 7.80 (s, 6H), 6.56 (s, 24H), 4.84 (s, 18H), 4.65 (s, 6H), 4.36 (s, 12H), 3.87 (s, 6H), 3.74 (s, 6H), 3.71–3.50 (m, 24H), 3.05 (d, J = 3.2 Hz, 6H). 13C-NMR (151 MHz, D2O) δ 154.9, 143.8, 143.1, 125.1, 121.6, 115.5, 99.5, 72.8, 70.5, 69.9, 66.4, 65.2, 61.3, 60.6, 49.8. 31P-NMR (243 MHz, CDCl3) δ 10.16. ESI+-HRMS: [M + 3H]3+ calcd for C102H135N21O48P3, 838.26603; found: 838.5901.
Synthesis of35. Into a solution of hexapropargylated core (15) (50 mg, 0.049 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added 32 (185 mg, 0.44 mmol, 6 equiv.), Na-ascorbate (20 mg, 0.1 mmol, 0.1 equiv.) and CuSO4·5H2O (22 mg, 0.28 mmol, 0.09 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 35 as a white powder (171 mg, 96%); Rf = 0.2 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.74 (s, 6H, H-triazole), 6.92–6.74 (m, 24H, aromatic), 5.16 (t, J = 9.5 Hz, 6H, H-3), 5.11 (d, J = 6.3 Hz, 12H, b), 5.05 (t, J = 9.7 Hz, 6H, H-4), 4.98 (dd, J = 9.6, 8.0 Hz, 6H, H-2), 4.60 (dt, J = 14.4, 4.0 Hz, 6H, j), 4.53–4.47 (m, 12H, j′, H-1), 4.24 (dt, J = 8.4, 4.5 Hz, 12H, i, H-6), 4.13 (dd, J = 12.3, 2.2 Hz, 6H, H-6′), 3.93 (ddd, J = 10.6, 8.8, 3.5 Hz, 6H, i′), 3.70 (ddd, J = 10.0, 4.6, 2.4 Hz, 6H, H-5), 2.07 (s, 18H, COCH3), 2.01 (s, 18H, COCH3), 1.97 (s, 18H, COCH3), 1.92 (s, 18H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 169.6 (C=O), 169.1 (C=O), 168.4 (C=O), 168.4 (C=O), 154.2 (CO-aromatic), 143.6 (C-triazole), 142.7 (Cp-aromatic), 123.3 (CH-triazole), 120.9 (Cm-aromatic), 114.3 (CO-aromatic), 99.5 (C-1), 71.5 (C-2), 70.9 (C-3), 69.9 (C-4), 67.2 (C-5), 66.7 (Cj), 61.2 (Cb), 60.7 (C-6), 48.9 (Ci), 19.7 (COCH3), 19.7 (COCH3), 19.6 (COCH3), 19.5 (COCH3). 31P-NMR (243 MHz, CDCl3) δ 9.72. MALDI-TOF: [M]+ calcd for C150H179N21O72P3: 3519.020; found, 3519.489.
Synthesis of36. Compound 35 (100 mg, 0.03 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 36 (78 mg, quant.) was obtained as a white powder. 1H-NMR (600 MHz, MeOD) δ 8.05 (s, 6H), 6.70 (dd, J = 40.7, 9.1 Hz, 24H), 5.02 (s, 12H), 4.51 (t, J = 5.0 Hz, 12H), 4.26 (d, J = 7.9 Hz, 6H), 4.17–4.07 (m, 6H), 4.01–3.84 (m, 6H), 3.75 (dd, J = 12.1, 1.9 Hz, 6H), 3.55 (dd, J = 12.1, 5.8 Hz, 6H), 3.31 (t, J = 9.0 Hz, 6H), 3.27–3.22 (m, 6H), 3.18 (d, J = 9.6 Hz, 6H), 3.11 (dd, J = 9.2, 8.0 Hz, 6H). 13C-NMR (150 MHz, MeOD) δ 156.3, 145.2, 144.2, 126.5, 122.7, 116.6, 103.8, 77.3, 77.1, 74.3, 70.9, 68.9, 62.4, 62.1, 51.4, 49.4, 49.0, 24.2. 31P-NMR (243 MHz, MeOD) δ 10.25. MALDI-TOF: [M + H]+ calcd for C102H132N21O48P3: 2512.778; found, 2512.851.
Synthesis of37. Into a solution of hexapropargylated core (15) (25 mg, 0.025 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added compound 22 (140 mg, 0.25 mmol, 10 equiv.), Na-ascorbate (10 mg, 0.05 mmol, 2.1 equiv.) and CuSO4·5H2O (10.8 mg, 0.04 mmol, 1.8 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 37 as a white powder (101 mg, 96%); Rf = 0.22 (with 4% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.85 (s, 6H, H-triazole), 6.81 (dd, J = 29.7, 9.0 Hz, 24H, aromatic), 5.33 (dd, J = 10.0, 3.4 Hz, 6H, H-3), 5.28 (t, J = 10.0 Hz, 6H, H-2), 5.25 (dd, J = 3.2, 1.6 Hz, 6H, H-4), 5.13 (d, J = 7.6 Hz, 12H, b), 4.86 (s, 6H, H-1), 4.54 (t, J = 5.1 Hz, 12H, c), 4.28 (dd, J = 12.2, 4.9 Hz, 6H, H-6), 4.08 (dd, J = 12.3, 2.2 Hz, 6H, H-6′), 4.05 (ddd, J = 9.7, 4.8, 2.3 Hz, 6H, H-5), 3.88 (t, J = 5.2 Hz, 12H, d), 3.81–3.76 (m, 6H, j), 3.67–3.65 (m, 6H, j′), 3.63 (d, J = 3.7 Hz, 12H, i), 3.61 (d, J = 7.6 Hz, 48H, g, f, e, h), 2.14 (s, 18H, COCH3), 2.09 (s, 18H, COCH3), 2.02 (s, 18H, COCH3), 1.98 (s, 18H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 170.7 (C=O), 170.0 (C=O), 169.9 (C=O), 169.7 (C=O), 155.3 (CO-aromatic), 144.6 (C-triazole), 143.6 (Cp-aromatic), 124.2 (CH-triazole), 121.8 (Cm-aromatic), 115.3 (CO-aromatic), 97.7 (C-1), 70.7 (Cd), 70.6 (Cg), 70.5 (Cf), 69.9 (Ce), 69.6 (Ch), 69.5 (Cj), 69.4 (C-2), 69.1 (C-3), 68.4 (C-4), 67.4 (C-5), 66.1 (Ci), 62.4 (Cb), 62.3 (C-6), 50.2 (Cc), 20.9 (COCH3), 20.8 (COCH3), 20.7 (COCH3), 20.7 (COCH3). 31P-NMR (243 MHz, CDCl3) δ 9.68. ESI+-HRMS: [M + 2H]2+ calcd for C186H254N21O90P3, 2158.2604; found: 2158.2588.
Synthesis of38. Compound 37 (100 mg, 0.023 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 38 (77 mg, quant.) was obtained as a white solid. 1H-NMR (300 MHz, D2O) δ 7.96 (s, 6H, H-triazole), 6.71 (s, 24H, aromatic), 4.99 (s, 12H, b), 4.82 (s, 6H, H-1), 4.49 (s, 12H, c), 3.93–3.75 (m, 36H, H-2,3,4,5,6 and 6′), 3.74–3.38 (m, 84H, d, e, f, g, h, i, j). 31P-NMR (122 MHz, D2O) δ 10.11. ESI+-HRMS: [M + 2H]2+ calcd for C138H206N21O66P3, 1653.6321; found: 1653.6342.
Synthesis of39. Into a solution of hexapropargylated core (15) (26 mg, 0.026 mmol, 1.0 equiv.) in a mixture of THF/H2O (4 mL, 3:1, v/v), was added (26) (400 mg, 0.24 mmol, 9.6 equiv.), Na-ascorbate (11 mg, 0.06 mmol, 2.1 equiv.) and CuSO4·5H2O (12 mg, 0.05 mmol, 1.8 equiv.). The mixture was stirred at 50 °C for 2 h, then at room temperature for 5 h. Upon completion of the reaction, EtOAc was added to the reaction mixture and further processed as given in general procedure (I) to afford compound 39 as a white powder (272 mg, 97%); Rf = 0.2 (with 5% MeOH in CH2Cl2 as eluents). 1H-NMR (600 MHz, CDCl3) δ 7.82 (s, 6H, HA-triazole), 7.69 (s, 18H, HB-triazole), 6.82 (dd, J = 32.8, 8.8 Hz, 24H, aromatic), 5.22 (t, J = 10.0 Hz, 18H, H-3), 5.19 (d, J = 3.2 Hz, 12H, H-4), 5.17 (s, 12H, H-2), 5.09 (s, 12H, b), 4.79 (s, 18H, H-1), 4.59 (dd, J = 10.5, 5.7 Hz, 36H, i), 4.54 (s, 36H, h), 4.49 (t, J = 5.0 Hz, 12H, c), 4.19 (dd, J = 12.3, 5.0 Hz, 18H, H-6), 4.14–4.07 (m, 18H, j′), 4.02 (dd, J = 12.3, 1.8 Hz, 18H, H-6′), 3.89 (m, 18H, j), 3.86 (t, J = 5.2 Hz, 12H, d), 3.62–3.59 (m, 18H, H-5), 3.55–3.48 (m, 24H, e, f), 3.46 (s, 36H, g′), 3.40 (s, 36H, g), 2.11 (s, 54H, COCH3), 2.06 (s, 54H, COCH3), 2.01 (s, 54H, COCH3), 1.96 (s, 54H, COCH3). 13C-NMR (150 MHz, CDCl3) δ 170.6 (C=O), 169.9 (C=O), 169.6 (C=O), 169.1 (C=O), 155.4 (CO-aromatic), 145.5 (CB-triazole), 144.6 (CA-triazole), 143.5 (Cp-aromatic), 124.2 (CAH-triazole), 123.7 (CBH-triazole), 121.8 (Cm-aromatic), 115.3 (CO-aromatic), 97.5 (C-1), 71.0 (Cf), 70.3 (Ce), 69.7 (Cg), 69.4 (Cd), 69.2 (C-2), 69.1 (C-3), 68.9 (C-4), 68.8 (C-5), 66.3 (Cj), 65.7 (Cb), 64.8 (Ch), 62.2 (C-6), 50.3 (Ci), 49.5 (Cc), 45.3 (Cq), 20.8 (COCH3), 20.7 (COCH3), 20.7 (COCH3), 20.7 (COCH3). 31P-NMR (243 MHz, CDCl3) δ 9.54. MALDI-TOF: [M + H]+ calcd for C450H607N75O222P3: 10705.772; found, 10706.833.
Synthesis of40. Compound 39 (200 mg, 0.02 mmol) and sodium methoxide (16 μL from 1 M solution in MeOH) in 3 mL of methanol were stirred for 4 h and the mixture was treated following the general procedure (III) described above. Deprotected compound 40 (150 mg, quant.) was obtained as a white solid. 1H-NMR (600 MHz, D2O) δ 8.05 (s, 1H), 7.92 (s, 3H), 6.74 (dd, J = 42.4, 7.8 Hz, 4H), 5.09 (s, 2H), 4.75 (s, 3H), 4.61–4.51 (m, 6H), 4.45 (s, 6H), 4.02 (s, 3H), 3.83 (s, 6H), 3.71 (d, J = 10.8 Hz, 3H), 3.63 (ddd, J = 25.4, 15.8, 7.5 Hz, 8H), 3.36 (dd, J = 75.3, 44.3 Hz, 12H), 3.11–3.02 (m, 3H). 13C-NMR (150 MHz, D2O) δ 215.7, 154.9, 144.3, 143.8, 142.9, 125.3, 125.1, 121.7, 115.8, 99.5, 72.8, 70.5, 69.9, 69.7, 68.9, 68.7, 68.3, 66.3, 65.4, 63.6, 61.4, 60.6, 50.1, 49.9, 44.8, 30.2, 29.7, 29.6, 29.4, 29.3, 29.2. 31P-NMR (243 MHz, D2O) δ 10.06. MALDI-TOF: [M + H]+ calcd for C306H463N75O150P3: 7681.011; found, 7681.383.
4. Conclusions
The synthesis of a new library of glycodendrimers possessing pegylated sugars in the aglycones with different valences from 2 to 18, has been achieved in satisfying yields provided by the highly efficient click chemistry. The above set of assay is relevant in demonstrating the relative binding abilities of these glycodendrimers for their respective lectins. In DLS measurements, the use of kinetics of mannodendrimers-protein aggregation in presence of the mannose-specific lectin ConA gave useful preliminary results. We noticed that, highly substituted glycodendrimers demonstrated a higher capacity to crosslink ConA by forming insoluble complexes within a short time frame. Hence, the results obtained herein suggest that dendrimers harboring pegylated mannosides provided gradual and functional binding interactions with a model mannose-binding protein such as ConA. Perhaps, more importantly, is the fact that the synthetic strategy that we recently coined “onion peel strategy” offers advantages over classical ones [32,47,54,55,56,57]. Indeed, by choosing the cyclotriphosphazene core having six functional groups available (an A6 core) together with an AB3 pentaerythritol scaffold at the next generation, provided a dendrimeric architecture from which, a total of 18 surface groups can be achieved by a convergent synthesis in a short synthetic sequence leading to a G1 generation. Ongoing activities, aimed at measuring the inhibition of biofilm formation from uropathogenic E. coli, are underway.
Acknowledgments
This work was supported by grants from the Natural Science and Engineering Research Council of Canada (NSERC) to R.R. including a Canadian Research Chair and the Fonds du Québec—Nature et Technologies to R.R.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
C.S. performed the experiments, analyzed the NMR data and DLS measurements and wrote the paper. T.C.S. and L.M.S. contributed equally to this work. A.A. co-directed the student C.S. and proofread the paper. R.R. conceived the paper, designed the thematic, wrote and finalized the paper.
Funding
This research was funded by Natural Science and Engineering Research Council of Canada (NSERC) and the Fonds du Québec—Nature et Technologies to R.R.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Firon N., Ashkenazi S., Mirelman D., Ofek I., Sharon N. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 1987;55:472–476. doi: 10.1128/iai.55.2.472-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanwetswinkel S., Volkov A.N., Sterckx Y.G., Garcia-Pino A., Buts L., Vranken W.F., Bouckaert J., Roy R., Wyns L., van Nuland N.A. Study of the structural and dynamic effects in the FimH adhesin upon alpha-d-heptyl mannose binding. J. Med. Chem. 2014;57:1416–1427. doi: 10.1021/jm401666c. [DOI] [PubMed] [Google Scholar]
- 3.Wellens A., Lahmann M., Touaibia M., Vaucher J., Oscarson S., Roy R., Remaut H., Bouckaert J. The tyrosine gate as a potential entropic lever in the receptor-binding site of the bacterial adhesin FimH. Biochemistry. 2012;51:4790–4799. doi: 10.1021/bi300251r. [DOI] [PubMed] [Google Scholar]
- 4.Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Touaibia M., Roy R. Glycodendrimers as anti-adhesion drugs against type 1 fimbriated E. coli uropathogenic infections. Mini Rev. Med. Chem. 2007;7:1270–1283. doi: 10.2174/138955707782795610. [DOI] [PubMed] [Google Scholar]
- 6.Rabbani S., Krammer E.M., Roos G., Zalewski A., Preston R., Eid S., Zihlmann P., Prevost M., Lensink M.F., Thompson A., et al. Mutation of Tyr137 of the universal Escherichia coli fimbrial adhesin FimH relaxes the tyrosine gate prior to mannose binding. IUCrJ. 2017;4:7–23. doi: 10.1107/S2052252516016675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung C.S., Bouckaert J., Hung D., Pinkner J., Widberg C., DeFusco A., Auguste C.G., Strouse R., Langermann S., Waksman G., et al. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 2002;44:903–915. doi: 10.1046/j.1365-2958.2002.02915.x. [DOI] [PubMed] [Google Scholar]
- 8.Touaibia M., Krammer E.M., Shiao T.C., Yamakawa N., Wang Q., Glinschert A., Papadopoulos A., Mousavifar L., Maes E., Oscarson S., et al. Sites for Dynamic Protein-Carbohydrate Interactions of O- and C-Linked Mannosides on the E. coli FimH Adhesin. Molecules. 2017;22:1101. doi: 10.3390/molecules22071101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight S.D., Bouckaert J. Structure, function and assembly of type 1 fimbriae. Top. Curr. Chem. 2009;288:67–107. doi: 10.1007/128_2008_13. [DOI] [PubMed] [Google Scholar]
- 10.Schaechter M. Escherichia coli and Salmonella 2000: The view from here. Microbiol. Mol. Biol. Rev. 2001;65:119–130. doi: 10.1128/MMBR.65.1.119-130.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mulvey M.A., Schilling J.D., Martinez J.J., Hultgren S.J. Bad bugs and beleaguered bladders: Interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl α-d-mannopyranoside. J. Infect. Dis. 1979;139:329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- 13.Papadopoulos A., Shiao T.C., Roy R. Diazo transfer and click chemistry in the solid phase syntheses of lysine-based glycodendrimers as antagonists against Escherichia coli FimH. Mol. Pharm. 2012;9:394–403. doi: 10.1021/mp200490b. [DOI] [PubMed] [Google Scholar]
- 14.Mammen M., Choi S.-K., Whitesides G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors-Mammen-1998-Angewandte Chemie International Edition-Wiley Online Library. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Lee R.T., Lee Y.C. Affinity enhancement by multivalent lectin—carbohydrate interaction. Glycoconj. J. 2000;17:543–551. doi: 10.1023/A:1011070425430. [DOI] [PubMed] [Google Scholar]
- 16.Kitov P.I., Shimizu H., Homans S.W., Bundle D.R. Optimization of tether length in nonglycosidically linked bivalent ligands that target sites 2 and 1 of a Shiga-like toxin. J. Am. Chem. Soc. 2003;125:3284–3294. doi: 10.1021/ja0258529. [DOI] [PubMed] [Google Scholar]
- 17.Kiessling L.L., Gestwicki J.E., Strong L.E. Synthetic multivalent ligands as probes of signal transduction. Angew. Chem. Int. Ed. Engl. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy R. Syntheses and some applications of chemically defined multivalent glycoconjugates. Curr. Opin. Struct. Biol. 1996;6:692–702. doi: 10.1016/S0959-440X(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 19.Roy R. A decade of glycodendrimer chemistry. TIGGS. 2003;15:291–310. doi: 10.4052/tigg.15.291. [DOI] [Google Scholar]
- 20.Roy R., Touaibia M. 3.36-Application of Multivalent Mannosylated Dendrimers in Glycobiology. In: Kamerling H., editor. Comprehensive Glycoscience. Elsevier; Oxford, UK: 2007. pp. 821–870. [Google Scholar]
- 21.Touaibia M., Wellens A., Shiao T.C., Wang Q., Sirois S., Bouckaert J., Roy R. Mannosylated G(0) dendrimers with nanomolar affinities to Escherichia coli FimH. Chem. Med. Chem. 2007;2:1190–1201. doi: 10.1002/cmdc.200700063. [DOI] [PubMed] [Google Scholar]
- 22.Touaibia M., Shiao T.C., Papadopoulos A., Vaucher J., Wang Q., Benhamioud K., Roy R. Tri- and hexavalent mannoside clusters as potential inhibitors of type 1 fimbriated bacteria using pentaerythritol and triazole linkages. Chem. Commun. 2007:380–382. doi: 10.1039/B612471B. [DOI] [PubMed] [Google Scholar]
- 23.Nagahori N., Lee R.T., Nishimura S., Page D., Roy R., Lee Y.C. Inhibition of adhesion of type 1 fimbriated Escherichia coli to highly mannosylated ligands. Chembiochem Eur. J. Chem. Biol. 2002;3:836–844. doi: 10.1002/1439-7633(20020902)3:9<836::AID-CBIC836>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Pieters R.J. Intervention with bacterial adhesion by multivalent carbohydrates. Med. Res. Rev. 2007;27:796–816. doi: 10.1002/med.20089. [DOI] [PubMed] [Google Scholar]
- 25.Imberty A., Chabre Y.M., Roy R. Glycomimetics and glycodendrimers as high affinity microbial anti-adhesins. Chem. Eur. J. 2008;14:7490–7499. doi: 10.1002/chem.200800700. [DOI] [PubMed] [Google Scholar]
- 26.Sleiman M., Varrot A., Raimundo J.M., Gingras M., Goekjian P.G. Glycosylated asterisks are among the most potent low valency inducers of Concanavalin A aggregation. Chem. Commun. 2008;48:6507–6509. doi: 10.1039/B814816C. [DOI] [PubMed] [Google Scholar]
- 27.Chabre Y.M., Papadopoulos A., Arnold A.A., Roy R. Synthesis and solvodynamic diameter measurements of closely related mannodendrimers for the study of multivalent carbohydrate-protein interactions. Beilstein J. Org. Chem. 2014:1524–1535. doi: 10.3762/bjoc.10.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touaibia M., Roy R. First synthesis of “Majoral-Type” glycodendrimers bearing covalently bound alpha-d-mannopyranoside residues onto a hexachlocyclotriphosphazene core. J. Org. Chem. 2008;73:9292–9302. doi: 10.1021/jo801850f. [DOI] [PubMed] [Google Scholar]
- 29.Pagé D., Roy R. Synthesis of divalent α-d-mannopyranosylated clusters having enhanced binding affinities towards concanavalin A and pea lectins. Bioorg. Med. Chem. Lett. 1996;6:1765–1770. doi: 10.1016/0960-894X(96)00312-5. [DOI] [Google Scholar]
- 30.Sharon N., Ofek I. Safe as mother’s milk: Carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 2000;17:659–664. doi: 10.1023/A:1011091029973. [DOI] [PubMed] [Google Scholar]
- 31.Bock V.D., Hiemstra H., van Maarseveen J.H. CuI-Catalyzed Alkyne–Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006:51–68. doi: 10.1002/ejoc.200500483. [DOI] [Google Scholar]
- 32.Bagul R.S., Hosseini M., Shiao T.C., Saadeh N.K., Roy R. Heterolayered hybrid dendrimers with optimized sugar head groups for enhancing carbohydrate—Protein interactions. Polym. Chem. 2017;8:5354–5366. doi: 10.1039/C7PY01044C. [DOI] [Google Scholar]
- 33.Chabre Y.M., Roy R. Design and Creativity in Synthesis of Multivalent Neoglycoconjugates. Adv. Carbohydr. Chem. Biochem. 2010;63:165–393. doi: 10.1016/S0065-2318(10)63006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chabre Y.M., Roy R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013;42:4657–4708. doi: 10.1039/c3cs35483k. [DOI] [PubMed] [Google Scholar]
- 35.Chabre Y.M., Roy R. Recent trends in glycodendrimer syntheses and applications. Curr. Top. Med. Chem. 2008;8:1237–1285. doi: 10.2174/156802608785848987. [DOI] [PubMed] [Google Scholar]
- 36.Caminade A.M., Ouali A., Laurent R., Turrin C.O., Majoral J.P. The dendritic effect illustrated with phosphorus dendrimers. Chem. Soc. Rev. 2015;44:3890–3899. doi: 10.1039/C4CS00261J. [DOI] [PubMed] [Google Scholar]
- 37.Caminade A.-M., Turrin C.-O., Majoral J.-P. Biological properties of phosphorus dendrimers. New J. Chem. 2010;34:1512–1524. doi: 10.1039/c0nj00116c. [DOI] [Google Scholar]
- 38.Wang L., Yang Y.-X., Shi X., Mignani S., Caminade A.-M., Majoral J.-P. Cyclotriphosphazene core-based dendrimers for biomedical applications: An update on recent advances. J. Mater. Chem. B. 2018;6:884–895. doi: 10.1039/C7TB03081A. [DOI] [PubMed] [Google Scholar]
- 39.Caminade A.M., Turrin C.O., Majoral J.P. Biological properties of water-soluble phosphorhydrazone dendrimers. Braz. J. Pharm. Sci. 2013;49:34–43. doi: 10.1590/S1984-82502013000700004. [DOI] [Google Scholar]
- 40.Caminade A.M., Hameau A., Majoral J.P. The specific functionalization of cyclotriphosphazene for the synthesis of smart dendrimers. Dalton Trans. 2016;45:1810–1822. doi: 10.1039/C5DT03047A. [DOI] [PubMed] [Google Scholar]
- 41.Park S., Shin I. Carbohydrate microarrays for assaying galactosyltransferase activity. Org. Lett. 2007;9:1675–1678. doi: 10.1021/ol070250l. [DOI] [PubMed] [Google Scholar]
- 42.Pratt J., Roy R., Annabi B. Concanavalin-A-induced autophagy biomarkers requires membrane type-1 matrix metalloproteinase intracellular signaling in glioblastoma cells. Glycobiology. 2012;22:1245–1255. doi: 10.1093/glycob/cws093. [DOI] [PubMed] [Google Scholar]
- 43.Papp I., Dernedde J., Enders S., Riese S.B., Shiao T.C., Roy R., Haag R. Multivalent presentation of mannose on hyperbranched polyglycerol and their interaction with concanavalin A lectin. Chembiochem Eur. J. Chem. Biol. 2011;12:1075–1083. doi: 10.1002/cbic.201000718. [DOI] [PubMed] [Google Scholar]
- 44.Roy R., Trono M.C., Giguère D. Effects of Linker Rigidity and Orientation of Mannoside Cluster for Multivalent Interactions with Proteins. ACS Symp. Ser. 2005;896:137–150. [Google Scholar]
- 45.Dam T.K., Roy R., Page D., Brewer C.F. Negative cooperativity associated with binding of multivalent carbohydrates to lectins. Thermodynamic analysis of the “multivalency effect”. Biochemistry. 2002;41:1351–1358. doi: 10.1021/bi015830j. [DOI] [PubMed] [Google Scholar]
- 46.Dam T.K., Roy R., Das S.K., Oscarson S., Brewer C.F. Binding of multivalent carbohydrates to concanavalin A and Dioclea grandiflora lectin. Thermodynamic analysis of the “multivalency effect”. J. Biol. Chem. 2000;275:14223–14230. doi: 10.1074/jbc.275.19.14223. [DOI] [PubMed] [Google Scholar]
- 47.Bagul R.S., Hosseini M.M., Shiao T.C., Roy R. “Onion peel” glycodendrimer syntheses using mixed triazine and cyclotriphosphazene scaffolds. Can. J. Chem. 2017;95:975–983. doi: 10.1139/cjc-2017-0220. [DOI] [Google Scholar]
- 48.Wang G.N., Andre S., Gabius H.J., Murphy P.V. Bi- to tetravalent glycoclusters: Synthesis, structure-activity profiles as lectin inhibitors and impact of combining both valency and headgroup tailoring on selectivity. Org. Biomol. Chem. 2012;10:6893–6907. doi: 10.1039/c2ob25870f. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J., Zhu X., Kang E.T., Neoh K.G. Design and synthesis of star polymers with hetero-arms by the combination of controlled radical polymerizations and click chemistry. Polymer. 2007;48:6992–6999. doi: 10.1016/j.polymer.2007.10.004. [DOI] [Google Scholar]
- 50.Cavero E., Zablocka M., Caminade A.-M., Majoral J.P. Design of Bisphosphonate-Terminated Dendrimers. Eur. J. Org. Chem. 2010;2010:2759–2767. doi: 10.1002/ejoc.200901291. [DOI] [Google Scholar]
- 51.Lindhorst T.K., Kötter S., Krallmann-Wenzel U., Ehlers S. Trivalent α-D-mannoside clusters as inhibitors of type-1 fimbriaemediated adhesion of Escherichia coli: structural variation and biotinylation. J. Chem. Soc. Perkin Trans. 1. 2001:823–831. doi: 10.1039/b009786l. [DOI] [Google Scholar]
- 52.Yin L., Chen Y., Zhang Z., Yin Q., Zheng N., Cheng J. Biodegradable micelles capable of mannose-mediated targeted drug delivery to cancer cells. Macromol. Rapid Commun. 2015;36:483–489. doi: 10.1002/marc.201400650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S., Moussodia R.-O., Sun H.-J., Leowanawat P., Muncan A., Nusbaum C.D., Chelling K.M., Heiney P.A., Klein M.L., André S., Roy R., Gabius H.-J., Percec P. Mimicking biological membranes with programmable glycan ligands self-assembled from amphiphilic Janus glycodendrimers. Angew. Chem. Int. Ed. Engl. 2014;53:10899–10903. doi: 10.1002/anie.201403186. [DOI] [PubMed] [Google Scholar]
- 54.Abbassi L., Chabre Y.M., Kottari N., Arnold A.A., André S., Josserand J., Gabius H.-J., Roy R. Multifaceted glycodendrimers with programmable bioactivity through convergent, divergent and accelerated approaches using polyfunctional cyclotriphosphazenes. Polym. Chem. 2015;6:7666–7683. doi: 10.1039/C5PY01283J. [DOI] [Google Scholar]
- 55.Sharma R., Naresh K., Chabre Y.M., Rej R., Saadeh N.K., Roy R. “Onion peel” dendrimers: A straightforward synthetic approach towards highly diversified architectures. Polym. Chem. 2014;5:4321–4331. doi: 10.1039/C4PY00218K. [DOI] [Google Scholar]
- 56.Sharma R., Zhang I., Abbassi L., Rej R., Maysinger D., Roy R. A fast track strategy toward highly functionalized dendrimers with different structural layers: An “onion peel approach”. Polym. Chem. 2015;6:1436–1444. doi: 10.1039/C4PY01761G. [DOI] [Google Scholar]
- 57.Sharma R., Kottari N., Chabre Y.M., Abbassi L., Shiao T.C., Roy R. A highly versatile convergent/divergent “onion peel” synthetic strategy toward potent multivalent glycodendrimers. Chem. Commun. 2014;50:13300–13303. doi: 10.1039/C4CC06191H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.