Abstract
The aim of this study was to identify new potentially active compounds for three protein targets, tropomyosin receptor kinase A (TrkA), N-methyl-d-aspartate (NMDA) receptor, and leucine-rich repeat kinase 2 (LRRK2), that are related to various neurodegenerative diseases such as Alzheimer’s, Parkinson’s, and neuropathic pain. We used a combination of machine learning methods including artificial neural networks and advanced multilinear techniques to develop quantitative structure–activity relationship (QSAR) models for all target proteins. The models were applied to screen more than 13,000 natural compounds from a public database to identify active molecules. The best candidate compounds were further confirmed by docking analysis and molecular dynamics simulations using the crystal structures of the proteins. Several compounds with novel scaffolds were predicted that could be used as the basis for development of novel drug inhibitors related to each target.
Keywords: natural compounds, artificial neural networks, molecular docking, TrkA, NMDA, LRRK2, molecular dynamics, CADD
1. Introduction
A natural product (NP) is generally defined as a chemical compound or substance that is produced by living organisms. NPs can be classified by many criteria and characteristics, such as source, biological function, biosynthetic pathway, physical and chemical properties, etc. Nowadays, NPs find a broad spectrum of applications related to human life, including an important role in medicine. Notably, the use of natural products as medicines has been described throughout human history in terms of substances related to herbal medicines, potions, oils, remedies, and foods. Many of these substances have been discovered by trial and error, and through the years they have become standard products in human lives [1,2].
In the past few decades, natural products have been an important source of potential drug hits and leads [3,4]. However, development efforts in NP drug discovery have demonstrated a certain downturn in recent years [3]. Despite this decline, the vast chemical space of natural products continues to provide abundant structural diversity for discovering novel lead compounds with low molecular weight. Less than 10% of the world’s biodiversity has been explored to find potential biologically active compounds [5,6]. Therefore, numerous lead NPs that may be used against a broad spectrum of diseases await discovery.
Many drugs related to diseases such as malaria, tuberculosis, cancer, human immunodeficiency virus (HIV), inflammatory diseases, cardiac diseases, diabetes, etc. have been derived from NPs [7,8]. One area where natural compounds have also been found to have great research potential and application is neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), schizophrenia, dementia, and neuropathic disorders [9,10,11,12,13]. Recent research in this area has indicated that N-methyl-d-aspartate (NMDA) receptor, leucine-rich repeat kinase 2 (LRRK2), and tropomyosin receptor kinase A (TrkA) have great potential as drug development targets for the above diseases. It has been shown that mutations in LRRK2 are the most common cause of PD [14]. Expression analysis of alpha-synuclein and LRRK2 mRNA levels indicated a significant increase in the temporal cortex of variants of AD patients’ brains as compared with control patients [15]. It is known that AD is characterized by synaptic loss, deposition of Aβ plaques, neurofibrillary tangles, and hyperphosphorylated tau. These changes are associated with NMDA receptor activation and oxidative stress, which ultimately result in AD pathology [16,17]. Moreover, NMDA receptors present in the striatum are crucial for dopamine–glutamate interactions. As such, NMDAs have been frequently used for pharmacological treatment of PD [18] The importance of tyrosine kinase receptors is well known for interactions in neuronal survival, together with nerve growth factors (NGFs). It is now known that NGF is also associated with Alzheimer’s disease and intractable pain, hence it, along with its high-affinity receptor tropomyosin receptor kinase A, is considered to be a new target for therapies being developed to treat these diseases. Anti-NGF antibody and TrkA inhibitors are known to suppress NGF/TrkA signaling. Moreover, it should be mentioned that local anesthetics also possess TrkA inhibitory effects. Therefore, the TrkA receptor plays an important role as a target for treating neurodegenerative diseases [19,20,21].
In the last two decades, statistics have confirmed that natural products can be regarded as an important source for the successful development of new drugs. However, the interest in finding new bioactive NP candidates for neurodegenerative diseases among the main pharmaceutical companies has generally declined. The main reason for this decline is that research in this field is time-consuming, expensive, highly complex, and in many cases ineffective. Therefore, a more effective and rational approach is needed. In this regard, in silico methods and related computer-aided drug design (CADD) methods have been proven throughout the years to be fast, inexpensive, and effective in tackling the above problems [22]. In particular, quantitative structure–activity relationship (QSAR) combined with molecular docking and molecular dynamics could be a powerful tool for development of new lead drug candidates. Usually the standard QSAR models are based on multilinear regression (MLR) methods. However, nonlinear modeling based on machine learning approaches such as artificial neural networks (ANNs) [23,24,25] has become important for QSAR. It has been applied in numerous areas of chemistry and pharmacy [26,27,28]. The mathematical adaptability of ANNs makes them a powerful tool for pattern classification and function/regression approximation [29]. The main advantage of ANNs is their inherent ability to incorporate nonlinear dependencies between dependent and independent variables without using an explicit mathematical function. In contrast with the standard two-dimensional (2D) QSAR approach, molecular docking and molecular dynamics make use of the three-dimensional (3D) structure of the target protein in order to investigate the specific structural characteristics and interactions between the ligand and the protein. The main purpose of molecular docking is to reveal the predominant binding modes of a ligand with the known 3D structure of a receptor. Therefore, the method can identify correct poses of ligands in the binding pocket of a protein and predict the affinity between the ligand and the protein [30,31]. On the other hand, the molecular dynamics approach is a computerized simulation that investigates the actual/physical movements of the atoms and molecules in certain media. Consequently, this method describes patterns, strengths, and properties of protein behavior, drug-receptor interactions, the solvation of molecules, the conformational changes that a protein or molecule may undergo under various conditions, and other events that require the systematic evaluation of molecular properties in dynamic molecular systems [32,33,34].
A non-exhaustive search in the recent scientific literature shows that there are not many works that deal with the exact problem as the present study, i.e., in silico modeling of natural compounds for neurodegenerative diseases. One such work is by Ambure et al., who successfully developed linear discriminant analysis (LDA) related to Alzheimer’s disease [35]. The classification models were used for virtual screening of large numbers of NPs. The best candidates predicted by the LDA classification models were submitted to molecular docking and molecular dynamics. Also, the paper by Corrêa and Fernandes studied the QSAR works related to discovery of potential drugs related to histamine H3R receptor [36]. The H3R is an important target involved in several CNS disorders, such as narcolepsy, attention deficit hyperactivity disorder and schizophrenia. The authors concluded that the QSAR methods are valuable to design better H3R antagonists/inverse agonists. However, pharmacokinetics should also be considered in the models to ensure good CNS penetration. In addition, the reader is also pointed to the review article by Nikolic et al. and the references therein describing works related to discovery of CNS drugs based chemoinformatics, 3D-QSAR and virtual screening techniques [37].
The aim of this study was twofold: (i) to develop potential natural inhibitor hits for multiple protein targets (NMDA, LRRK2, TrkA) related to neurodegenerative diseases (AD, PD), and (ii) to demonstrate the usefulness of a hybrid approach comprising QSAR (machine learning: ANN, MLR, virtual screening) and molecular modeling (molecular docking and molecular dynamics) as applied to the natural compound space. Therefore, we first developed three ANN and three MLR models for the related protein targets. These models were used for further virtual screening of databases of natural compounds to find possible active inhibitors. Then the best candidates were submitted for molecular docking analysis regarding the respective proteins. The best refined candidates from the docking were used in molecular dynamics for further investigation of the molecular interactions. Finally, we identified several possible candidates that passed successfully through the above computational flow.
2. Results and Discussion
2.1. Nonlinear QSAR
Several back-propagation neural network models with different architectures were investigated for each target set. In all our models, the single output was assigned to logIC50 and the asociated experimental values were normalized within the [−1, 1] range. The generalized delta rule was used for updating the weights of the ANN where two adjustable parameters had to be defined prior to the training procedure, namely momentum (alpha) and learning rate (eta). The best ANN models developed for the three sets related to the different targets are shown in Table 1, with their statistical parameters and characteristics. The notations in the headings of Table 1 are as follows: Target, network model name for the respective receptor; alpha, momentum; eta, learning rate; Ntr, number of training data; Nval, number of validation data; epoch, final training epoch; R2tr, coefficient of determination for the training set; R2val, coefficient of determination for the validation set; RMStr, root mean squared error for the training set; RMSval, root mean squared error for the validation set; Architecture, connected network layers and number of neurons within each layer; Input Descriptors, descriptor names in the input layer (independent variables).
Table 1.
Statistical and topological parameters of the artificial neural network (ANN) models. LRRK2, leucine-rich repeat kinase 2; NMDA, N-methyl-d-aspartate; TrkA, tropomyosin receptor kinase A; RMS, root mean squared error.
| Target | Alpha | Eta | Ntr | Nval | Epoch | R2tr | R2val | RMStr | RMSval | Architecture | Input Descriptors |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LRRK2 | 0.01 | 0.02 | 81 | 20 | 3525 | 0.788 | 0.565 | 0.195 | 0.381 | 5-5-3-1 | Highest coulombic interaction (AM1); HPSA polar (AM1) part of SASA; max nucleophilic reactivity index (AM1) for C atoms; avg. electrophilic reactivity index (AM1) for C atoms; max electrophilic reactivity index (AM1) for N atoms |
| NMDA | 0.01 | 0.02 | 107 | 26 | 1651 | 0.752 | 0.519 | 0.273 | 0.444 | 5-5-5-1 | Kier and Hall index (order 2); charged (Zefirov) surface area of O atoms; min nucleophilic reactivity index (AM1) for O atoms; highest e-e repulsion (AM1) for C–O bonds; square root of charged (Zefirov) surface area of N atoms |
| TrkA | 0.01 | 0.03 | 121 | 30 | 1641 | 0.781 | 0.798 | 0.216 | 0.230 | 5-5-4-1 | Lowest total interaction (AM1) for N–H bonds; relative number of aromatic bonds; HASA-2/TMSA (AM1); square root of partial charged (AM1) surface area of C atoms; lowest n-n repulsion (AM1) for C–N bonds |
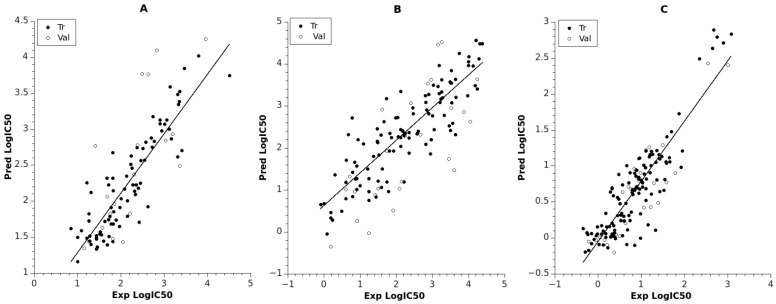
The model for LRRK2 had two hidden layers, with five neurons in the first and three neurons in the second hidden layer. The corresponding RMStr = 0.195 (R2tr = 0.788) for the training set indicated satisfactory prediction, while the large statistical variation of the logIC50 [0.845, 4.505] for the set led to RMSval = 0.381. The linear fit between the predicted and experimental logIC50 is shown in Figure 1A (see also Supplementary Table S1). For this model, the most important descriptors (ANN inputs) are related to the reactivity of the compounds (maximal nucleophilic reactivity index (AM1) for C atoms, average electrophilic reactivity index (AM1) for C atoms, maximal electrophilic reactivity index (AM1) for N atoms) and are derived from molecular orbital theory. Also, the descriptors highest coulombic interaction (AM1) and HPSA (Hydrogen polar surface area) polar (AM1), part of the solvent accessible surface area, play an important role in the electrostatic attraction/repulsion within the intermolecular distances. Moreover, it is noticable that compounds with larger highest coulombic interaction (AM1) values lead to more active compounds, which is reflected by the negative correlation with logIC50 (R = −0.564) for the whole dataset.
Figure 1.
Predicted vs. experimental logIC50 for training and validation sets: (A) ANN model for LRRK2; (B) ANN model for NMDA; (C) ANN model for TrkA. Trend lines used for training data.
The model for NMDA included larger datasets (Ntr = 107, Nval = 26) than the model for LRRK2 (Ntr = 81, Nval = 20). The prediction quality for the training set was RMStr = 0.273 (R2tr = 0.752), while for the validation set it was RMSval = 0.444 (R2val = 0.519), as indicated in Table 1. A linear plot of the experimental and calculated predictions is shown in Figure 1B, and their values are collected in Supplementary Table S2. The model for NMDA had the same number of layers as the model for LRRK2 but included more neurons in the second hidden layer in order to encounter the huge data variability in logIC50 for the whole set (almost 5 log units). The molecular features selected for the inputs of the NMDA network were attributed to (i) compactness/branchness of the compound structure (Kier and Hall index (order 2)); (ii) charged molecular areas (charged (Zefirov) surface area of O atoms, square root of charged (Zefirov) surface area of N atoms); (iii) reactivity of the compounds based on O atoms (minimal nucleophilic reactivity index (AM1) for O atoms, highest e-e repulsion (AM1) for C–O bonds). The descriptor Kier and Hall index (order 2) shows significant negative correlation with logIC50 (R = −0.560), indicating a reverse relation.
The best model related to TrkA had architecture 5-5-4-1 and included the largest numbers of training and validation data points (Ntr = 121, Nval = 30). The model was trained up to 1641 epochs and resulted in RMS of 0.216 and 0.230 for the training and validation set, respectively (see Table 1 and Figure 1C, Supplementary Table S3). This model obtained the highest regression fit (R2tr = 0.781, R2val = 0.798) compared to the other models for the validation set. This maybe due to the lowest statistical variability of the training values logIC50 [−0.346, 3.076] and less structural diversity of the set. Regarding the input descriptors for the TrkA model, they can be attributed to (i) stability of the compound (lowest total interaction (AM1) for N–H bonds, lowest n-n repulsion (AM1) for C–N bonds, relative number of aromatic bonds); and (ii) hydrogen bonding acceptor ability of the molecule (HASA-2/TMSA—Hydrogen Acceptor Surface Area type 2/Total Molecular Solvent Area) (AM1), square root of partial charged (AM1) surface area of C atoms). According to the descriptor Lowest total interaction (AM1) for N–H bonds, larger values contribute to large logIC50 values (R = 0.655). There are eight compounds clustered in the highest range of logIC50, as can be seen from Figure 1C. These compounds are characterized with zero values of lowest total interaction (AM1) for N–H bonds, lowest n-n repulsion (AM1) for C–N bonds, and relative number of aromatic bonds.
2.2. Linear QSAR
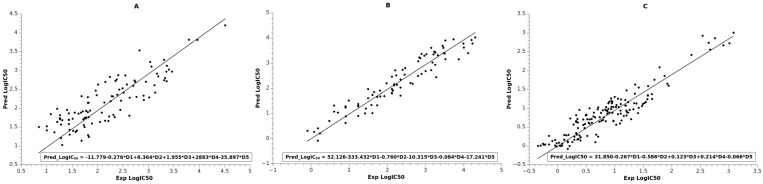
The best multilinear regression (BMLR) algorithm was used to generate several multilinear equations for the dataset with between two and seven descriptors. The best models for each target are presented in Table 2, with their statistical parameters and descriptors. The final model for LRRK2 set had five descriptors. As can be seen from Table 2, the quality of linear equation resulted in R2 = 0.721 and average ABC validation R2pred = 0.725 with Fisher’s statistic F = 49. The plot between predicted and observed logIC50 is shown in Figure 2A, and the values are collected in Supplementary Table S1. The most statistically significant descriptor in the model according to the t-test is Highest coulombic interaction (AM1), which is related to the stability of the compound and its electrostatic interactions. The other descriptor related to the stability of the compound is Maximum bonding contribution of one MO (AM1). The remaining descriptors are related to the reactivity of the compound (Maximum electrophilic reactivity index (AM1) for H atoms, Maximum nucleophilic reactivity index (AM1) for C atoms), and its electrostatic interaction is confined within the negative charge (Relative negative charge—RNCG (QMNEG/QTMINUS) (Zefirov)). It should be noted that the descriptors for this model are similar to the descriptors in the ANN model for LRRK2 (see Table 1).
Table 2.
Best multilinear regression (BMLR) models for logIC50 and their statistical parameters.
| LRRK2 | N = 101, R2 = 0.721, R2cv = 0.683, R2abc = 0.725, s2 = 0.167, F = 49.045 | ||||
| No | B | Errors B | t-Statistics | Descriptor (Di) | |
| 0 | −11.779 | 2.126 | −5.541 | Intercept | |
| 1 | −0.276 | 0.028 | −9.947 | Highest coulombic interaction (AM1) | |
| 2 | 8.364 | 1.087 | 7.697 | Max bonding contribution of one MO (AM1) | |
| 3 | 1.955 | 0.954 | 2.048 | RNCG relative negative charge (QMNEG/QTMINUS) (Zefirov) | |
| 4 | 2883.443 | 389.061 | 7.411 | Max electrophilic reactivity index (AM1) for H atoms | |
| 5 | −35.897 | 8.661 | −4.145 | Max nucleophilic reactivity index (AM1) for C atoms | |
| ABC Validation | |||||
| (AB,C): R2ab = 0.673 | R2ab_cv = 0.607 | R2c_pred = 0.729 | |||
| (BC,A): R2bc = 0.722 | R2bc_cv = 0.662 | R2a_pred = 0.756 | |||
| (CA,B): R2ca = 0.677 | R2ca_cv = 0.567 | R2b_pred = 0.689 | |||
| NMDA | N = 83, R2 = 0.906, R2cv = 0.893, R2abc = 0.901, s2 = 0.123, F = 149.268 | ||||
| 0 | 52.126 | 11.055 | 4.715 | Intercept | |
| 1 | −333.432 | 34.324 | −9.714 | HACA-2/TMSA (Zefirov) | |
| 2 | −0.760 | 0.029 | −26.281 | Kier and Hall index (order 2) | |
| 3 | −10.315 | 0.785 | −13.141 | Average bonding information content (order 0) | |
| 4 | −0.084 | 0.008 | −10.313 | Lowest e-n attraction (AM1) for C–N bonds | |
| 5 | −17.241 | 3.022 | −5.706 | Average valency (AM1) for C atoms | |
| ABC Validation | |||||
| (AB,C): R2ab = 0.905 | R2ab_cv = 0.883 | R2c_pred = 0.894 | |||
| (BC,A): R2bc = 0.920 | R2bc_cv = 0.900 | R2a_pred = 0.884 | |||
| (CA,B): R2ca = 0.888 | R2ca_cv = 0.860 | R2b_pred = 0.926 | |||
| TrkA | N = 151, R2 = 0.866, R2cv = 0.855, R2abc = 0.861, s2 = 0.065, F = 187.251 | ||||
| 0 | 31.850 | 2.351 | 13.545 | Intercept | |
| 1 | −0.267 | 0.017 | −15.942 | Highest coulombic interaction (AM1) for N–H bonds | |
| 2 | −0.586 | 0.041 | −14.412 | Number of F atoms | |
| 3 | 0.123 | 0.010 | 12.637 | Highest e-n attraction (AM1) for C–C bonds | |
| 4 | 0.214 | 0.021 | 10.270 | Charged (Zefirov) surface area of N atoms | |
| 5 | −0.066 | 0.017 | −3.915 | Total point-charge component of molecular dipole (AM1) | |
| ABC Validation | |||||
| (AB,C): R2ab = 0.859 | R2ab_cv = 0.842 | R2c_pred = 0.880 | |||
| (BC,A): R2bc = 0.854 | R2bc_cv = 0.837 | R2a_pred = 0.861 | |||
| (CA,B): R2ac = 0.861 | R2ac_cv = 0.841 | R2b_pred = 0.843 | |||
Figure 2.
Linear fit between experimental and observed logIC50 of the BMLR models: (A) LRRK2 set; (B) NMDA set; (C) TrkA set. Trend lines indicated by straight lines. BMLR equations in Table 2 are also incorporated in the boxes.
The next BMLR model for the NMDA set resulted in five descriptors (Table 2). However, in this exercise the full set of 133 data points was not used as it was in the case of the ANN model for NMDA. The reason was that linear modeling did not succeed in producing a statistically significant equation. We then performed leverage analysis of the initial models in order to reduce the number of data points for the BMLR to 83. Most of the reduced compounds contained Br or/and Cl atoms. Thus, the final model had significant quality of fit R2 = 0.906, as shown in Figure 2B (Supplementary Table S2), with Fisher’s statistic F = 149 and average ABC prediction of R2pred = 0.901. The most statistically significant descriptor in the model is the Kier and Hall index, which, together with the descriptor Average bonding information content (order 0), indicates the branching and compactness of the compounds. The Kier and Hall index descriptor also appears in the corresponding ANN model and with the same trend as discussed in Section 2.1. The remaining descriptors can be attributed to the hydrogen acceptor ability (HACA-2/TMSA) and stability (Average valency (AM1) for C atoms, Lowest e-n attraction (AM1) for C–N bonds) of the compounds.
The final BMLR model for the TrkA set resulted in an equation also containing five descriptors, as indicated in Table 2. The model coefficient of determination was R2 = 0.866, with Fisher’s statistic F = 187 and average ABC validation R2pred = 0.861 (see Figure 2C; values for the plot in Supplementary Table S3). It should be noted that this BMLR model had better quality of linear fit compared to the corresponding ANN model (R2tr = 0.781) for the TrkA data. This might be attributed to the superior feature selection of the BMLR model as compared to the ANN descriptor selection. The most statistically significant descriptor in the equation is Highest coulombic interaction (AM1) for N–H bonds, and therefore it is related to the stability of the N–H bonds. The next most significant descriptor in the equation is Number of F atoms. This descriptor reflects the importance of the F atoms, as more than 90% of the compounds in the set include F atoms. The remaining descriptors are related to the electrostatic interactions and stability of the compounds (Charged (Zefirov) surface area of N atoms, Total point-charge component of the molecular dipole (AM1), Highest e-n attraction (AM1) for C–C bonds).
2.3. Virtual Screening of Natural Compounds
In this exploration, we used the models developed in Section 2.1 and Section 2.2 to predict logIC50 values of 13,648 natural compounds extracted from the ZINC database [38]. The compounds were in ascending order according to their predicted logIC50 by both ANN and BMLR models. In this way, the top 100 compounds were further selected by average prediction by both models. During prediction of the compounds, we also used the model’s applicability domain, described in Section 3.5. The resulting three sets of compounds were submitted for molecular docking and molecular dynamics analysis.
2.4. Molecular Modeling Results
The top 40% of compounds predicted by the QSAR models (see Section 2.3) for each biological target were further examined using the molecular modeling techniques. Eighty compounds for NMDA and LRRK2 and 40 for TrkA were studied by molecular docking. In each case, molecular dynamics simulations were further carried out for the three best compounds. The results were compared with the modeling data on known NMDA, LRRK2, and TrkA inhibitors (see Table 3).
Table 3.
Binding energies (kcal/mol) and binding modes of small-molecule ligands to receptors (LRRK2, NMDA, and TrkA).
| No. | Structure | ZINC Code | Binding Energy ΔG, kcal/mol | Ligand Efficiency | Binding Mode, Including H-Bonds (Residue of Amino Acid Group or Atom in a Compound) |
|---|---|---|---|---|---|
| NMDA | |||||
| GNE-5729 |
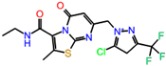
|
==== | −11.3 | −0.42 | Ile116, Val128, Pro129, Phe130, Pro141, Lys143, Tyr144, Thr242, Leu262, Leu263, Val266, Leu270 |
| 1N |
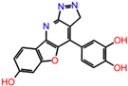
|
ZINC98363705 | −9.8 | −0.39 | Pro129, Phe130, Val131, Glu132, Pro141, Tyr144, Leu263, Val266, Met271, Glu275 |
| 2N |
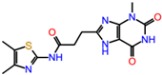
|
ZINC67658347 | −9.7 | −0.40 | Ile128, Pro129, Val131, Glu132, Pro141, Tyr144, Thr242, Gly243, Gly250, Val266, His273 (N...HN) |
| 3N |
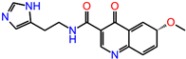
|
ZINC98364250 | −9.0 | −0.39 | Ile128, Pro129, Glu132, Pro141, Tyr144, Thr242, Gly250 (NH...N), Leu263, Val266 |
| LRRK2 | |||||
| PF-06447475 |
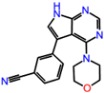
|
==== | −9.0 | −0.39 | Ile30, Glu31, Ala51, Lys53, Glu100 (O...HN), Leu102 (NH...N), Leu151, Asp162 |
| 1L |
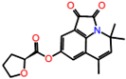
|
ZINC2115150 | −9.0 | −0.36 | Ile30, Val38, Lys53, Glu70, Tyr101, Leu102, Asp162 |
| 2L |
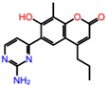
|
ZINC12901845 | −8.5 | −0.37 | Leu102 (NH...O), Ser106 (HO....H2N), Gly105, Asp109, Leu151 |
| 3L |
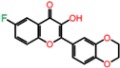
|
ZINC518729 | −8.7 | −0.38 | Ile30, Val38, Tyr101, Leu102 (NH...OH), Gly105, Leu151 |
| TrkA | |||||
| AZ-23 |
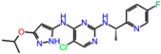
|
ZINC35077985 | −8.7 | −0.32 | Leu516, Val524, Ala542, Phe589, Met592, Gly595, Asp596, Leu657 (O....HN) |
| 1T |
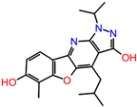
|
ZINC85880015 | −9.80 | −0.38 | Leu516, Gly519, Lys544, Phe589, Met592, Gly595, Leu657, Asp668 |
| 2T |
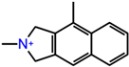
|
ZINC1323099 | −8.6 | −0.54 | Val524, Ala542, Phe589, Met592, Leu657 |
| 3T |
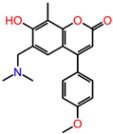
|
ZINC489632 | −9.5 | −0.38 | Leu516 (O... HO), Gly517, Ala542, Lys544, Phe589, Met592 (NH...O), Leu657, Gly667 |
2.4.1. NMDA
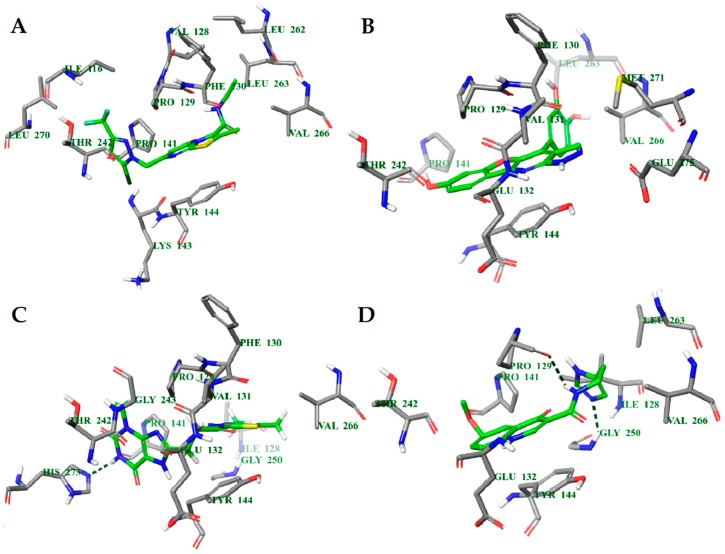
The molecular docking binding energies of the selected compounds span the interval of −11.9 to −2.2 kcal/mol and the ligand efficiencies are between −0.40 and −0.02. The best three compounds by ligand efficiency, compounds 1N, 2N, and 3N, have somewhat smaller binding energies (−9.8, −9.7, and −9.0 kcal/mol, respectively), but similar ligand efficiencies (−0.39, −0.40, and −0.39) compared to those for the known inhibitor GNE-5279 (−11.3 and −0.42 kcal/mol). The binding modes of the three predicted compounds and of the inhibitor GNE-5279 are given in Figure 3.
Figure 3.
Calculated binding modes of ligands in the active site of NMDA (PDB ID: 5TP9): (A) compound GNE-5729, (B) compound 1N, (C) compound 2N, (D) compound 3N. The amino acid residues of NMDA are colored gray (carbon), blue (nitrogen), red (oxygen), and white (hydrogen). Hydrogen bonds formed between compounds and residues of NMDA are represented by green dashed lines.
In order to elaborate the ligand–enzyme interactions further, molecular dynamics simulations of 50 ns were carried out for all four compounds. The root mean standard deviation (RMSD) of the ligand and protein was stable between 1 and 4 Å, except for compound 2N which had change at about 35 ns (see Supplementary Figure S1). Therefore, only the first 30 ns was taken into account in further data analysis for this compound.
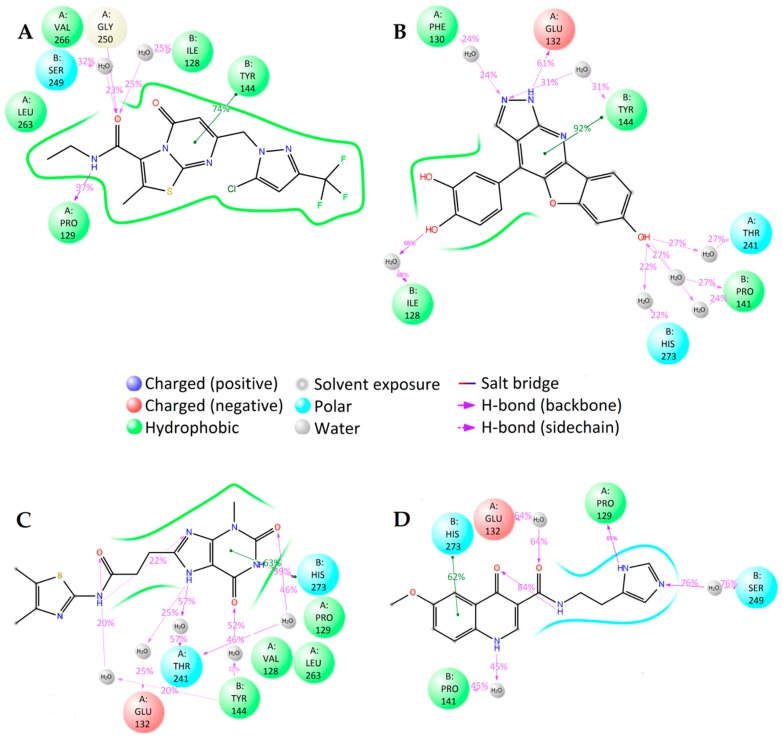
There are notable differences in the calculated binding of ligand compounds to NMDA. The molecular dynamics calculated contacts of compound 3N are similar to known inhibitor GNE-5279 (Figure 4A,D and Supplementary Figure S2A,D), involving strong hydrogen bonding with PRO129 and hydrophobic interactions around TYR144 of the protein. The binding pictures for compounds 1N and 2N are very different, being directed primarily by hydrophobic interactions and bonding through water molecules (Figure 4B,C and Supplementary Figure S2B,C).
Figure 4.
2D summary of molecular dynamics calculated contacts between NMDA and compounds (A) GNE-5729, (B) 1N, (C) 2N, and (D) 3N.
The interactions between the ligands and the NMDA protein were also analyzed with the MM-GBSA method (Supplementary Table S4). The total binding energy for the studied compounds is smaller than that of the known NMDA inhibitor GNE-5279. Nevertheless, the ligand efficiency for compound 2N is still too high to suggest it as a potential new inhibitor.
2.4.2. LRRK2
The binding energies of the compounds selected from ANN results for molecular docking were between −9.7 and −5.9 kcal/mol and the respective ligand efficiencies in the interval −0.38 to −0.17. The best three compounds by ligand efficiency, compounds 1L, 2L, and 3L, have very similar binding energies (−9.0, −8.5, and −8.7 kcal/mol, respectively) and ligand efficiencies (−0.36, −0.37, and −0.38, respectively) compared to those for the known inhibitor PF-06447475 (−9.0 and −0.39 kcal/mol, respectively) (Table 3). The binding modes of these three compounds and the inhibitor PF-06447475 are given in Figure 5. Again, molecular dynamics simulations of 50 ns were carried out for all four compounds. The root mean standard deviation (RMSD) of the ligand and protein was stable between 0.8 and 3.6 Å for all compounds (Supplementary Figure S3). To examine the stability of the molecular dynamics simulations in time, we carried out additional runs of 20, 40, and 60 ns for the ligand 1L (see Supplementary Figures S5 and S6). The RMSDs of ligand and protein positions and binding histograms obtained by multiple runs demonstrate the stability of the simulations and congruency of the results.
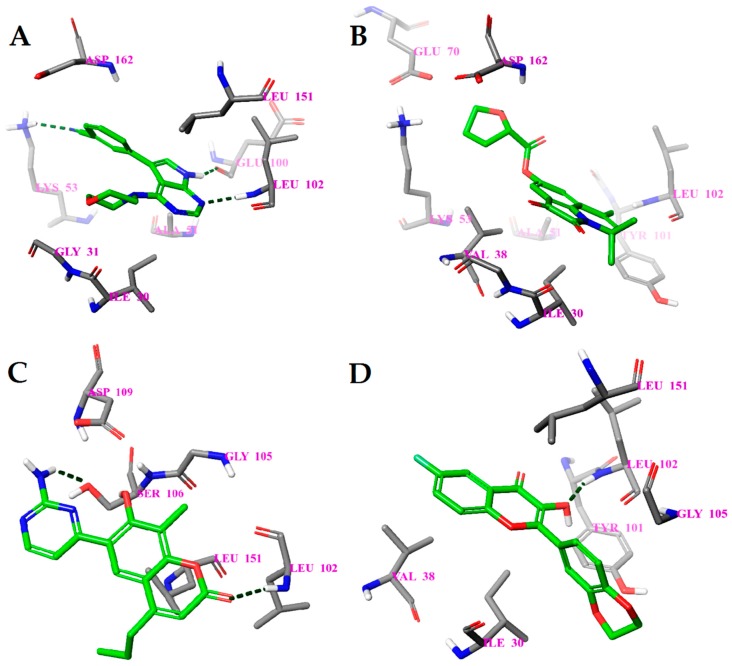
Figure 5.
Calculated binding modes of (A) compound PF-06447475, (B) compound 1L, (C) compound 2L, and (D) compound 3L in the active site of LRRK2 (PDB ID: 4U8Z). The amino acid residues of LRRK2 are colored gray (carbon), blue (nitrogen), red (oxygen), and white (hydrogen). Hydrogen bonds formed between compound and residues of LRRK2 are represented by green dashed lines.
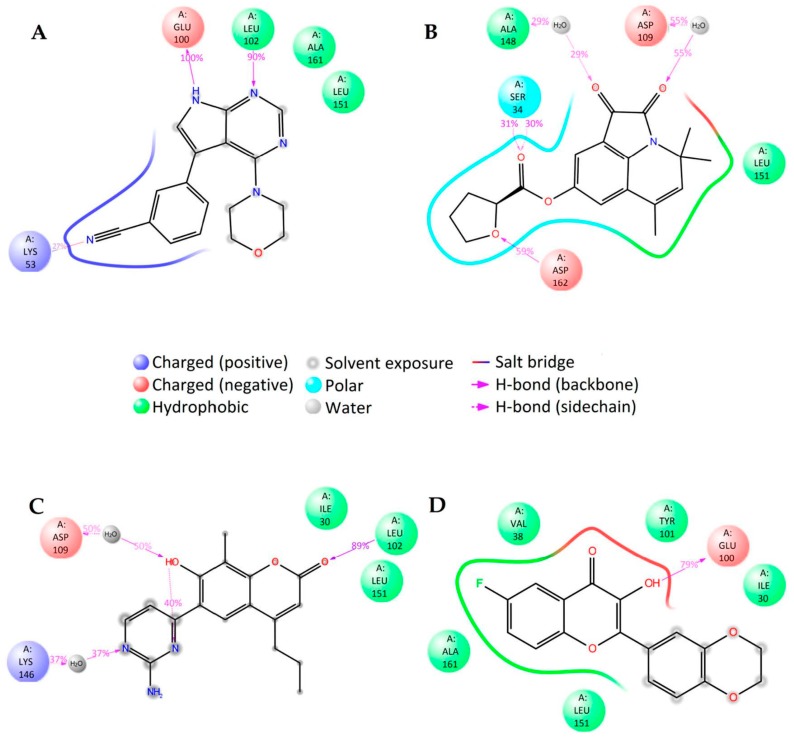
In the case of LRRK2, molecular dynamics simulations indicate some similarities in the binding of different ligands. The molecular dynamics calculated contacts of compounds 2L and 3L include strong hydrogen bonding between ligand and peptide links at amino acid residues GLU100 and/or LEU102, similarly to known inhibitor PF-06447475 (Figure 6A,C,D and Supplementary Figure S4A,C,D). The molecular dynamics simulated binding picture of compound 1L is different, with two relatively strong hydrogen bonds at the SER34 and ASP162 residues of the LRRK2 protein (Figure 6B and Supplementary Figure S4B). Nevertheless, as it is located in the active site of the enzyme, this compound may also act as an inhibitor. Furthermore, when the interactions between the ligands and the LRRK2 protein were calculated using the MM-GBSA method (Supplementary Table S5), the compound 1L gave significantly better interaction energy (−100.04 kcal/mol) and ligand efficiency (−4.00) than the known inhibitor PF-06447475 (−72.41 kcal/mol and −3.15, respectively). Consequently, this compound (9,11,11-trimethyl-2,3-dioxo-1-azatricyclo[6.3.1.044,12]dodeca-4(12),5,7,9-tetraen-6-yloxolane-2-carboxylate) is predicted as a potential new strong LRRK2 inhibitor.
Figure 6.
2D summary of the molecular dynamics calculated contacts between compounds (A) PF-06447475, (B) 1L, (C) 2L, (D) 3L and LRRK2.
2.4.3. TrkA
The binding energies of the 40 compounds selected from ANN results for molecular docking were between −11.9 and −3.5 kcal/mol and the respective ligand efficiencies in the interval −0.5 to −0.16. The best three compounds (1T, 2T, and 3T) had significantly better ligand efficiency than the known TrkA inhibitor AZ-23 (Table 1). In particular, compound 2T, with almost the same binding energy as compound AZ-23 (−8.6 vs. −8.7 kcal mol), had much higher ligand efficiency (−0.54 vs. −0.32). The binding modes of the three selected best inhibitor candidates and the inhibitor AZ-23 are given in Figure 7. Molecular dynamics simulations of 50 ns were also carried out for all four compounds. The observed root mean standard deviations (RMSDs) of the ligand and protein were stable between 1 and 4.5 Å for all compounds, indicating the stability of the respective complexes (Supplementary Figure S7).
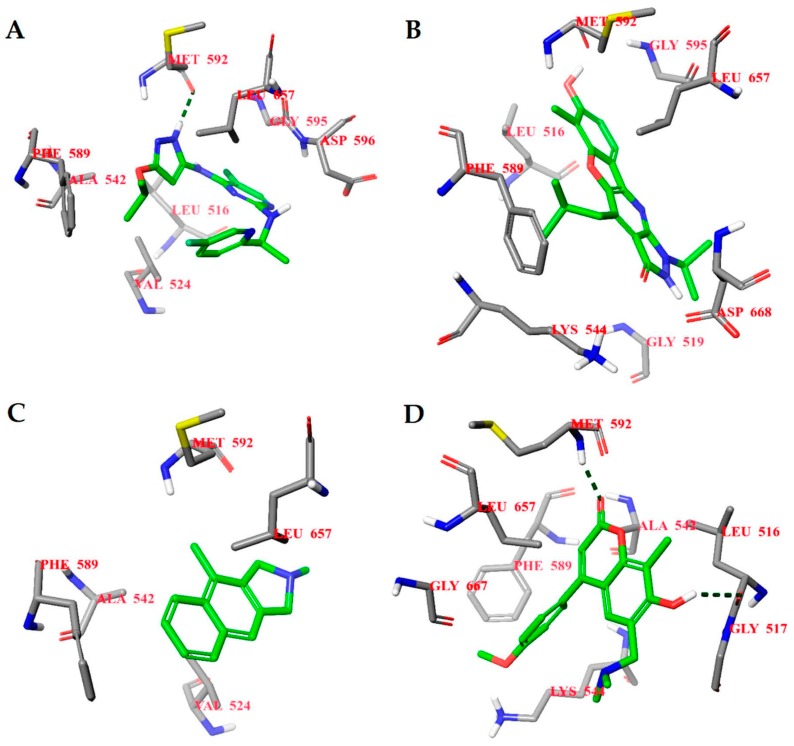
Figure 7.
Calculated binding modes of (A) compound AZ-23, (B) compound 1T, (C) compound 2T, and (D) compound 3T in the active site of TrkA (PDB ID: 4AOJ). The amino acid residues of TrkA are colored gray (carbon), blue (nitrogen), red (oxygen), and white (hydrogen). Hydrogen bonds formed between compound and residues of TrkA are represented by green dashed lines.
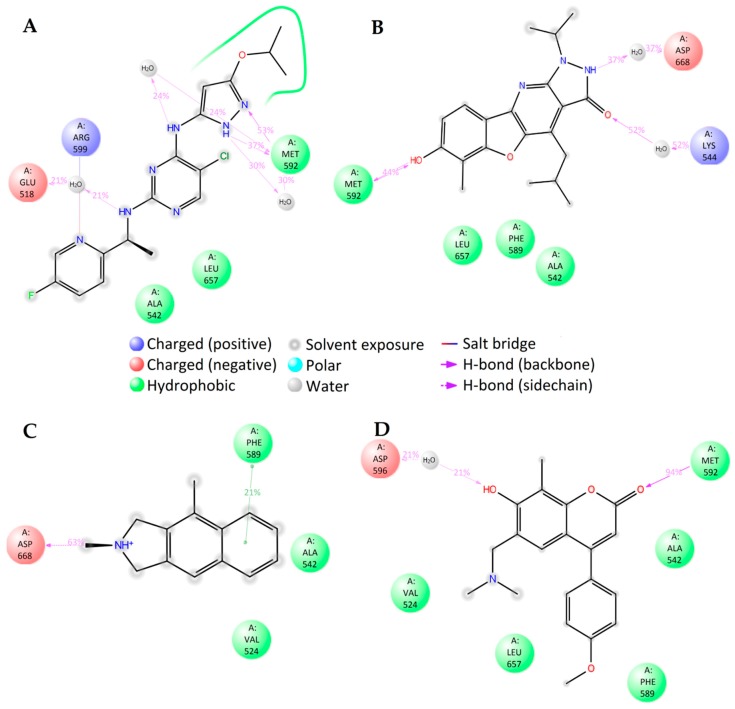
The molecular dynamics simulations of TrkA with different ligands show close similarity in the binding of compounds 1T, 3T, and known inhibitor compound AZ-23. The main directing interactions are the strong hydrogen bonding at amino acid residue MET592 and hydrophobic interactions around PHE589, LEU657, and VAL524 residues (Figure 8A,B,D and Supplementary Figure S8A,B,D). The binding mode of positively charged compound 1T was very different, as expected, involving the hydrogen bonding between the NH+ group of the ligand and the ASP668 residue of the protein (Figure 7C and Supplementary Figure S8C).
Figure 8.
2D summary of molecular dynamics calculated contacts between compounds (A) AZ-23, (B) 1T, (C) 2T, and (D) 3T and TrkA.
An analysis of the interactions between the studied ligands and the TrkA protein using the MM-GBSA method (Supplementary Table S6) gives close ligand efficiencies for compounds 2T and 3T and inhibitor AZ-23 (−2.69, −2.79, and −2.61, respectively). Thus these two compounds can be tested as potential new TrkA inhibitors.
3. Materials and Methods
3.1. Data for Building QSAR Models
The data for building the current QSAR models were extracted from the ChEMBL database [39]. The measured inhibitory concentration IC50[nM] (or log IC50) was used as a dependent variable in all QSAR models. Several criteria were applied for the preparation of the three datasets related to TrkA, NMDA, and LRRK2 proteins: (i) where possible, newer experimental data were preferable; (ii) preferably the same (or similar) experimental protocol for activity measurements was used; (iii) where possible, data were obtained by a single laboratory, author, or group; (iv) diverse structural compounds with low molecular weight were used; and (v) statistical range of the measured activity for each set was a minimum 2 to 3 logarithmic units. Thus, 151, 133, and 101 compound datasets were prepared for TrkA, NMDA, and LRRK2, respectively. The data collected are shown in Supplementary Tables S1, S2 and S3).
3.2. Geometry Optimization and Descriptor Generation
The two-dimensional molecular structures of the compounds obtained in Section 3.1 were converted into three-dimensional structures using Open Babel [40]. Conformational search was carried out by the CMol3D program of FQSARModel [41,42] for the dataset structures, where random conformations were constructed by means of the stochastic proximity embedding algorithm [43], followed by optimization based on MMFF94s force field [44] to improve their quality. Thereafter, all geometries were optimized as random vacuum conformer with the minimum potential energy using MOPAC 6.0 [45]. The quantum-mechanical semiempirical calculation in the form of AM1 [46] energy minimization was subsequently applied with a gradient of 0.01 kcal/Å as a stop criterion. The following mopac keywords were used for the optimization procedure: AM1, VECTORS, BONDS, PI, POLAR, PRECISE, ENPART, EF, MMOK, NOINTER, GNORM = 0.05, XYZ.
For a given compound structure, it was possible to generate a large number (>600) of molecular descriptors [47] using the descriptor calculator in the FQSARModel program applied on the 3D structures obtained by MOPAC6. These features can be generally classified into 2 categories: structural and quantum-chemical descriptors. The first group is further divided into constitutional, topological, geometric, and electrostatic subgroups. The constitutional descriptors are simple fragment additive and mostly reflect the general properties of the compound’s structure. The topological descriptors were calculated using graph theory applied to the scheme of atom connections of the structure. The second main group included electronic and quantum chemical classes of descriptors. The best few descriptors found in Section 3.3 were used as independent variable inputs for the developed models.
3.3. Development of QSAR Models
3.3.1. ANN Modeling
In the present study, we developed a fully connected neural network with a back-propagation algorithm of the error [48]. The ANNs constructed were used in the building of the nonlinear models for all activities. Training of the net (optimization of the weights) was performed by generalized delta rule. A hyperbolic activation function was applied for the neurons’ transformations. In order to find important descriptors as inputs to the net, a sensitivity analysis was performed on a preselected descriptor space, based on the lowest root mean squared error (RMS) or Pearson’s correlation coefficient (R) with respect to logIC50. The descriptor space was formed after applying the following criteria to reduce the total descriptor space: (i) all descriptors with variance less than 10−4 were excluded, (ii) descriptors that did not have indicated Pearson correlation coefficient R > 0.2 with respect to the property were excluded, and (iii) certain chemically irrelevant descriptors were inspected. Further, all the remaining descriptors were correlated with the property in order to extract (and use as inputs to the net) the best few with highest coefficient of determination (R2). Prior to this procedure, the descriptors were normalized according to their variation (distance between minimum and maximum values) and standard deviation. The main reason for such selection is that the descriptors can be explained by/related to the mechanistic picture behind the property interaction in a way similar to the multilinear regression models. For instance, a positive correlation in MLR would suggest that with increased descriptor value, the property value would also increase.
To find an optimal ANN architecture, we followed the common principle of generality of ANN prediction [49] i.e., seek the lowest possible number of neurons for the smallest structure. Several ANN models with different architectures were built for each logIC50 related to the different training sets for the NMDA, TrkA, and LRRK2 proteins. In addition, we monitored the RMS (or Pearson’s correlation coefficient R, or R2) for each architecture (regarding the hidden units in the hidden layer). This procedure was done in order to select the topology with the lowest RMS (highest R). The number of layers was chosen to be three- to fourfold based on common practice (usually not more than 2 hidden layers) for the QSAR ANN modeling and by taking into account the number of data points to reduce the chance of overfitting during the training stage. The whole ANN training procedure was performed by the ANN module in FQSARModel.
Validation of the ANN models was carried out by using training and validation (selection) sets. These subsets were constructed to reflect the distribution of the experimental property values of the whole dataset. The chosen validation sets included each third or fifth data point of the total data in a set (see Section 3.1, Supplementary S1). In this way, the validation set was used to train the network to avoid overfitting by stopping the training procedure prematurely when the RMSval started to increase.
3.3.2. MLR Modeling
The best multilinear regression (BMLR) method [50] was used to find the best correlation models from selected non-collinear descriptors. This approach gradually builds multiple regression equations by searching the first few descriptors among a large descriptor space, such as that obtained in Section 3.2. Thus, BMLR selects the best 2-parameter regression equations, the best 3-parameter regression equations, etc., based on the highest R2 and F values in a stepwise regression procedure. The result obtained by BMLR is the “best” linear representation of the activity (logIC50) in a given descriptor pool.
An ABC validation test [51] was applied to estimate the predictivity of the MLR equations developed, taking into account the property data distribution. The ABC method consists of sorting the data in ascending order according to the observed (experimental) values and forming 3 subsets (A, B, C): the first, fourth, seventh, etc., data points comprised the first subset, A; the second, fifth, eighth, etc., comprised the second subset, B; and the third, sixth, ninth, etc., comprised the third subset, C. Then the three training sets were prepared as combinations of any two subsets. Subsequently, the tested MLR model was rebuilt for each of the training sets (AB, AC, and BC), with the same descriptors but with other optimized regression coefficients. Further, these three models, AB, AC, and BC, were used to predict the property values for the C, B, and A subsets, respectively. The prediction was assessed based on the coefficient of determination of R2 between the predicted and observed property values. The final result was estimated by the average square correlation coefficient by the three “external” sets C, B, and A, R2pred. If the average R2pred is close to the R2 of the model, it indicates satisfactory prediction of that model. In addition to the ABC validation, the standard leave-one-out cross-validation (R2cv) for the MLR model was also used.
3.4. Molecular Modeling
3.4.1. Targets
In our study we used the LRRK2 homology model MST3 crystal structure, which was obtained from the Protein Data Bank (PDB) (ID: 4U8Z) [52]. The structural model was measured by X-ray diffraction with resolution 1.63 Å. The structure of the human GluN1/GluN2A ligand-binding domain (LBD) was obtained from the Protein Data Bank (ID: 5TP9). The crystal structure of LBD of NMDA was measured by X-ray diffraction with resolution 2.4 Å [53]. The crystal structure of TrkA was obtained from the Protein Data Bank (ID: 4AOJ), with resolution 2.75 Å measured by X-ray diffraction. This structure is actually a trimer of TrkA, with individual protein molecules denoted as chain A, chain B, and chain C. For modeling purposes, only a single TrkA molecule is needed, thus just chain A was used [54]. Raw crystal structures were corrected and hydrogen atoms were automatically added to the protein using Schrödinger’s Protein Preparation Wizard of Maestro 10.7 [55,56,57]. Water molecules were removed from the crystal structure.
Small molecule structures. The two-dimensional chemical structures of ligands were downloaded using the ZINC [38] tool and the database. For ligand structure preparation we used Ligprep from the Schrödinger suite [58]. Ligprep used the OPLS2005 force field in all ligand preparation steps. Generation of all possible states and ionization states was enumerated for each ligand using Epik at a pH of 7.0 ± 2. Stereoisomers were determined from 3D structure. PDB files for the molecular docking procedure were created from lowest energy conformers for each ligand.
3.4.2. Molecular Docking
AutoDock Vina 1.1.2 [59] was used for the docking studies to determine binding modes and binding energies of ligands to the receptor. Schrödinger’s Glide Grid Generation was used to identify the binding interface between the co-crystallized ligand and receptor for each structure [60]. The active site was surrounded by a grid box with a size of 20 × 20 × 20 points and spacing of 1.000 Å. The settings used for the iterated local search global optimizer, based on mutation and local optimization steps accepted or rejected with a Metropolis criterion in Vina, were 9 modes, 1 central processing unit, and energy range of 1 kcal/mol. Other settings were used as default.
3.4.3. Molecular Dynamics
The molecular dynamic simulations were carried out using the Desmond simulation package of Schrödinger [61]. In all runs, the NPT (isothermal–isobaric) ensemble was applied with a temperature of 300 K and pressure of 1 bar. The simulation length was 50 ns, with relaxation time 1 ps. The force field parameters for each simulation were according to OPLS_2005 [62]. The long-range electrostatic interactions were calculated using the particle mesh Ewald (PME) method [63]. The cutoff radius in Coloumb interactions was 9.0 Å. The water molecules were described using a simple point charge model (SPC) [64]. The Martyna–Tuckerman–Klein chain coupling scheme [65] with a coupling constant of 2.0 ps was used for pressure control and the Nosé–Hoover chain coupling scheme for temperature control. Nonbonded forces were calculated using an r-RESPA integrator, where the short-range forces were updated every step and the long-range forces were updated every 3 steps. The trajectories were saved at 4.8 ps intervals for analysis. To analyze the behavior and interactions between the ligands and protein, we used the Simulation Interactions Diagram tool implemented in the Desmond molecular dynamics package.
3.5. Virtual Screening of Database of Natural Compounds
In our study, we screened the ZINC [38] database for potential natural agonists/antagonists by utilizing the nonlinear and linear QSAR models developed in Section 2. We extracted nearly 17,000 compounds collected in the natural product database subsection of ZINC. The collections were attributed to AnalytiCon Discovery and IBScreen providers [66,67] within ZINC sets. We further refined the NPs by applying general criteria as follows: (i) removal of duplicates, (ii) removal of compounds with unclear structural connectivity, (iii) removal of compounds with molecular weight >600 amu, and (iv) removal of compounds without purchasable information. In this way, the number of NPs was reduced to 13,648. These compounds were further submitted to geometric optimization and descriptor calculation as described in Section 3.2. With the availability of the molecular descriptors, the ANN and MLR QSAR models were then employed to predict NPs with low inhibitory concentration. However, it is important for a predictive QSAR model that certain limits are defined for future predictions of compounds, i.e., applicability domain (AD). We defined the applicability domain of the general ANN and BMLR models quantitatively, proceeding from the minimum and maximum descriptor values for the corresponding training sets in Section 3.1. Our practice showed that predictions of new external compounds (with descriptor Dix) are reasonable to be bound within the descriptor interval [Dimin,Dimax] augmented by ±|Dimax − Dimin| × 0.3, where Dimin,Dimax are the minimum and maximum descriptor values for the training set for the ith descriptor (shown in square brackets above). This condition has to be simultaneously fulfilled for all Dix descriptors so that the QSAR models give realistic predictions.
4. Conclusions
In this study we have identified several potential candidates for further research on drugs to treat neurodegenerative diseases related to LRRK2, NMDA, and TrkA proteins. These inhibitors are based on natural products and have passed through a full cycle of in silico research from QSAR modeling through virtual screening, molecular docking, and molecular dynamics. We have also demonstrated that NPs are still a viable source of drug research despite the recent decline in this area.
The topical scientific literature indicates that there has been little exploration of NPs as potential drug candidates for neurodegenerative diseases using hybrid combinations of in silico techniques. This is especially true for CADD related to protein targets such as NMDA, LRRK2, and TrkA and small natural ligands. There are, however, many studies related to the above diseases and in silico modeling, but they do not concentrate on finding hit compounds in the NP space. Therefore, we also showed here that in silico techniques can be applied in the large NP space and can obtain potential hits quickly. A similar work by Ambure et al. [35] also indicated that this type of in silico modeling could lead to fast and reliable results. Their study was based on a classification QSAR problem, while our modeling is based on a regression problem. In this way, our QSAR models could predict more precisely in the range of IC50 values as compared to Ambure et al.’s classification models. Notably, our approach enabled to detect new scaffolds of the compounds structure. The chemical modification of compounds based on these scaffolds can lead to novel drug candidates against the pharmacological targets studied. A natural continuation of the present work, therefore, is experimental confirmation (in vitro/in vivo) of the best hit candidates found herein and their further structural optimization.
Supplementary Materials
The following are available online at http://www.mdpi.com/1420-3049/23/8/1847/s1: Table S1. LRRK2 dataset. Predicted and experimental logIC50 values for ANN and BMLR models. Table S2. NMDA dataset. Predicted and experimental logIC50 values for ANN and BMLR models. Table S3. TrkA dataset. Predicted and experimental logIC50 values for ANN and BMLR models. Table S4. Binding free energies (in kcal/mol) of NMDA—ligand complexes calculated using the MM/GBSA method. Table S5. Binding free energies (in kcal/mol) of LRRK2—ligand complexes calculated using the MM/GBSA method. Table S6. Binding free energies (in kcal/mol) of TrkA—ligand complexes calculated using the MM/GBSA method. Figure S1. RMSDs of atomic positions for the compounds (A) GNE-5729, (B) 1N, (C) 2N, and (D) 3N (in red) and the receptor NMDA (in blue) of 50 ns molecular dynamics simulations using Desmond code. Figure S2. Molecular dynamics calculated contacts between compounds (A) GNE-5729, (B) 1N, (C) 2N, and (D) 3N and NMDA. Figure S3. RMSDs of atomic positions for the compounds (A) PF-06447475, (B) 1L, (C) 2L, and (D) 3L (in red) and the receptor LRRK2 (in blue) of 50 ns molecular dynamics simulations using Desmond code. Figure S4. Molecular dynamics calculated contacts between compounds (A) PF-06447475, (B) 1L, (C) 2L, and (D) 3L and LRRK2. Figure S5. RMSD of the atomic positions for the compounds 1L (in red) and the receptor LRRK2 (in blue) of the 20 ns (A), 40 ns (B) and 60 ns (C) molecular dynamics simulations using Desmond code. Figure S6. Molecular dynamics calculated contacts between compound 1L and LRRK2 (A) −20 ns, (B) −40 ns, (C) −60 ns. Figure S7. RMSDs of atomic positions for the compounds (A) AZ-23, (B) 1T, (C) 2T, and (D) 3T (in red) and the receptor TrkA (in blue) of 50 ns molecular dynamics simulations using Desmond code. Figure S8. Molecular dynamics calculated contacts between compounds (A) AZ-23, (B) 1T, (C) 2T, and (D) 3T and TrkA.
Author Contributions
Conceptualization, D.A.D. and M.K.; methodology, D.A.D. and L.I.; software, D.A.D. and L.I.; formal analysis, D.A.D., M.K., and L.I.; investigation, D.A.D., L.I., and M.K.; data curation, D.A.D. and L.I.; writing–original draft preparation, D.A.D., M.K., and L.I.
Funding
This research was funded by the EU European Regional Development Fund through the Center of Excellence in Molecular Cell Engineering (Project No. 2014-2020.4.01.15-0013), Estonia; and Estonian Research Council grant PUT-582.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds in Table 3 are available from the authors.
References
- 1.Hicks S. Desert Plants and People. 1st ed. Naylor Co.; San Antonio, TX, USA: 1966. p. 75. [Google Scholar]
- 2.Dias D.A., Urban S., Roessner U. A Historical Overview of Natural Products in Drug Discovery. Metabolites. 2012;2:303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra B.B., Tiwari V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011;46:4769–4807. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 4.Cragg G.M., Newman D.J. Biodiversity: A continuing source of novel drug leads. Pure Appl. Chem. 2005;77:7–24. doi: 10.1351/pac200577010007. [DOI] [Google Scholar]
- 5.Cragg G.M., Newman D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta. 2013;1830:3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 7.Choudhary S., Singh P.K., Verma H., Singh H., Silakari O. Success stories of natural product-based hybrid molecules for multi-factorial diseases. Eur. J. Med. Chem. 2018;151:62–97. doi: 10.1016/j.ejmech.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Braicu C., Mehterov N., Vladimirov B., Sarafian V., Nabavi S.M., Atanasov A.G., Berindan-Neagoe I. Nutrigenomics in cancer: Revisiting the effects of natural compounds. Semin. Cancer Biol. 2017;46:84–106. doi: 10.1016/j.semcancer.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Dehhaghi M., Mohammadipanaha F., Guillemin G.J. Myxobacterial natural products: An under-valued source of products for drug discovery for neurological disorders. Neuro Toxicol. 2018;66:195–203. doi: 10.1016/j.neuro.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Srivastav S., Fatima M., Mondal A.C. Important medicinal herbs in Parkinson’s disease pharmacotherapy. Biomed. Pharmacother. 2017;92:856–863. doi: 10.1016/j.biopha.2017.05.137. [DOI] [PubMed] [Google Scholar]
- 11.Dey A., Bhattacharya R., Mukherjee A., Pandey D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotech. Adv. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Houghton P.J., Howes M.-J. Natural Products and Derivatives Affecting Neurotransmission Relevant to Alzheimer’s and Parkinson’s Disease. Neurosignals. 2005;14:6–22. doi: 10.1159/000085382. [DOI] [PubMed] [Google Scholar]
- 13.Court J.A., Piggot M.A., Lloyd S., Cookson N., Ballard C.G., McKeith I.G., Perry R.H., Perry E.K. Nicotine binding in human striatum: elevation in schizophrenia and reductions in dementia with Lewy bodies, Parkinson’s disease and Alzheimer’z disease and in relation to neuroleptic medication. Neuroscience. 2000;98:79–87. doi: 10.1016/S0306-4522(00)00071-3. [DOI] [PubMed] [Google Scholar]
- 14.Bae J.R., Lee B.D. Function and dysfunction of leucine-rich repeat kinase 2 (LRRK2): Parkinson’s disease and beyond. BMB Rep. 2015;48:243–248. doi: 10.5483/BMBRep.2015.48.5.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linnertz C., Lutz M.W., Ervin J.F., Allen J., Miller N.R., Welsh-Bohmer K.A., Roses A.D., Chiba-Falek O. The genetic contributions of SNCA and LRRK2 genes to Lewy Body pathology in Alzheimer’s disease. Hum. Mol. Genet. 2014;23:4814–4821. doi: 10.1093/hmg/ddu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y., Li P., Feng J., Wu M. Dysfunction of NMDA receptors in Alzheimer’s disease. Neurol. Sci. 2016;37:1039–1047. doi: 10.1007/s10072-016-2546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou O., Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2014;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 18.Hallett P.J., Standaert D.G. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol. Ther. 2004;102:155–174. doi: 10.1016/j.pharmthera.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Moises T., Wüller S., Saxena S., Senderek J., Weis J., Krüttgen A. Proteasomal inhibition alters the trafficking of the neurotrophin receptor TrkA. Biochem. Biophys. Res. Commun. 2009;387:360–364. doi: 10.1016/j.bbrc.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Hirose M., Kuroda Y., Murata E. NGF/TrkA Signaling as a Therapeutic Target for Pain. Pain Pract. 2016;16:175. doi: 10.1111/papr.12342. [DOI] [PubMed] [Google Scholar]
- 21.Santos N.A., Martins N.M., Sisti F.M., Fernandes L.S., Ferreira R.S., Queiroz R.H., Santos A.C. The neuroprotection of cannabidiol against MPP+-induced toxicity in PC12 cells involves TrkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol. In Vitro. 2015;30:231–240. doi: 10.1016/j.tiv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Rollinger J.M., Langer T., Stuppner H. Strategies for Efficient Lead Structure Discovery from Natural Products. Curr. Med. Chem. 2006;13:1491–1507. doi: 10.2174/092986706777442075. [DOI] [PubMed] [Google Scholar]
- 23.Goll S., Jurs P.C. Prediction of vapor pressures of hydrocarbons and halohydrocarbons from molecular structure with a computational neural network model. Chem. Inf. Comput. Sci. 1999;39:1081. doi: 10.1021/ci9900433. [DOI] [Google Scholar]
- 24.Tetteh J., Suzuki T., Metcalfe E., Howells S. QSPR for for the estimation of boiling point and flash point using a radial basis function neural network. J. Chem. Inf. Comput. Sci. 1999;39:491. doi: 10.1021/ci980026y. [DOI] [Google Scholar]
- 25.Zupan J., Gasteiger J. Neural Networks for Chemists: An Introduction. VCH-Verlag; Weinheim, Germany: 1993. pp. 213–228. [Google Scholar]
- 26.Burns J.A., Whitesides G. Feed-forward neural networks in chemistry: mathematical systems for classification and pattern recognition. Chem. Rev. 1993;93:2583. doi: 10.1021/cr00024a001. [DOI] [Google Scholar]
- 27.Katritzky A.R., Dobchev D.A., Fara D.C., Karelson M. QSAR studies on 1-phenylbenzimidazoles as inhibitors of the platelet-derived growth factor. Bioorg. Med. Chem. 2005;13:6598. doi: 10.1016/j.bmc.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 28.Katritzky A.R., Dobchev D.A., Fara D.C., Hur E., Tämm K., Kurunczi L., Karelson M., Varnek A., Solov’ev V.P. Skin Permeation Rate as a Function of Chemical Structure. J. Med. Chem. 2006;49:3305. doi: 10.1021/jm051031d. [DOI] [PubMed] [Google Scholar]
- 29.Dobchev D.A., Pillai G., Karelson M. In silico machine learning methods in drug development. Curr. Top. Med. Chem. 2014;14:1913. doi: 10.2174/1568026614666140929124203. [DOI] [PubMed] [Google Scholar]
- 30.Morris G.M., Lim-Wilby M. Molecular docking. Methods Mol Biol. 2008;443:365–382. doi: 10.1007/978-1-59745-177-2_19. [DOI] [PubMed] [Google Scholar]
- 31.Meng X.-Y., Zhang H.-X., Mezei M., Cui M. Molecular Docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided. Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Vivo M., Masetti M., Bottegoni G., Cavalli A. Role of molecular dynamics and related methods in drug Discovery. J. Med. Chem. 2016;59:4035–4061. doi: 10.1021/acs.jmedchem.5b01684. [DOI] [PubMed] [Google Scholar]
- 33.Mortier J., Rakers C., Bermudez M., Murgueitio M.S., Riniker S., Wolber G. The impact of molecular dynamics on drug design: applications for the characterization of ligand-macromolecule complexes. Drug Discov. Today. 2015;20:686–702. doi: 10.1016/j.drudis.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Kerrigan J.E. Molecular dynamics simulations in drug design. Methods Mol. Biol. 2013;993:95–113. doi: 10.1007/978-1-62703-342-8_7. [DOI] [PubMed] [Google Scholar]
- 35.Ambure P., Bhat J., Puzyn T., Roy K. Identifying natural compounds as multi-target-directed ligands against Alzheimer’s disease: an in silico approach. J. Biomol. Struct. Dyn. 2018;23:1. doi: 10.1080/07391102.2018.1456975. [DOI] [PubMed] [Google Scholar]
- 36.Corrêa M., Dos Santos F. Qsar modeling of histamine H3R antagonists/inverse agonists as future drugs for neurodegenerative diseases. Curr. Neuropharmacol. 2017;17:1. doi: 10.2174/1570159X15666170818100644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikolic K., Mavridis L., Djikic T., Vucicevic J., Agbaba D., Yelekci K., Mitchell J. Drug design for CNS diseases: Polypharmacological profiling of compounds using cheminformatic, 3D-QSAR and virtual screening methodologies. Front. Neurosci. 2016;10:256. doi: 10.3389/fnins.2016.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ZINC Database. [(accessed on 10 May 2018)]; Available online: http://zinc15.docking.org/
- 39.ChEMBL Database. [(accessed on 19 May 2018)]; Available online: https://www.ebi.ac.uk/chembldb/
- 40.O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminformatics. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karelson M., Dobchev D.A., Karelson G., Tamm T., Tämm K., Nikonov A., Mutso M., Merits A. Fragment-based development of HCV protease inhibitors for the treatment of hepatitis C. Curr. Comput. Aided Drug Des. 2012;8:55–61. doi: 10.2174/157340912799218516. [DOI] [PubMed] [Google Scholar]
- 42.Tammiku-Taul J., Park R., Jaanson K., Luberg K., Dobchev D.A., Kananovich D., Noole A., Mandel M., Kaasik A., Lopp M., et al. Indole-like Trk receptor antagonists. Eur. J. Med. Chem. 2016;121:541–552. doi: 10.1016/j.ejmech.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Xu H., Izrailev D., Agrafiotis D.K. Conformational sampling by self-organization. J. Chem. Inf. Comput. Sci. 2003;43:1186–1191. doi: 10.1021/ci0340557. [DOI] [PubMed] [Google Scholar]
- 44.Halgren T.A. MMFF VI. MMFF94s option for energy minimization studies. J. Comput. Chem. 1999;20:720–729. doi: 10.1002/(SICI)1096-987X(199905)20:7<720::AID-JCC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.MOPAC6. [(accessed on 10 May 2018)]; Available online: http://openmopac.net/
- 46.Dewar M.J.S., Zoebisch E.G., Healy E.F., Stewart J.J.P. Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J. Am. Chem. Soc. 1985;107:3902–3909. doi: 10.1021/ja00299a024. [DOI] [Google Scholar]
- 47.Karelson M. Molecular Descriptors in QSAR/QSPR. Wiley-Interscience; New York, NY, USA: 2000. [Google Scholar]
- 48.Haykin S. Neural Networks. A Comprehensive Foundation. Pearson Ed; London, UK: 1999. [Google Scholar]
- 49.Baskin I., Palyulin V., Zefirov N. Neural networks in building QSAR models. Methods Mol. Biol. 2009;458:133. [PubMed] [Google Scholar]
- 50.Katritzky A.R., Lobanov V., Karelson M. Correlation of Boiling Points with Molecular Structure. 1. A Training Set of 298 Diverse Organics and a Test Set of 9 Simple Inorganics. J. Phys. Chem. 1996;100:10400. doi: 10.1021/jp953224q. [DOI] [Google Scholar]
- 51.Karelson M., Karelson G., Tamm T., Tulp I., Jänes J., Tämm K., Lomaka A., Savchenko D., Dobchev D.A. QSAR study of pharmacological permeabilities. Arkivoc. 2009;2:218–238. [Google Scholar]
- 52.Henderson J.L., Kormos B.L., Hayward M.M., Coffman K.J., Jasti J., Kurumbail R.G., Wager T.T., Verhoest P.R., Noell G.S., Chen Y., et al. Discovery and preclinical profiling of 3-[4-(morpholin-4-yl)-7H-pyrrolo[2,3-d]pyrimidin-5-yl]benzonitrile (PF-06447475), a highly potent, selective, brain penetrant, and in vivo active LRRK2 kinase inhibitor. J. Med. Chem. 2015;58:419–432. doi: 10.1021/jm5014055. [DOI] [PubMed] [Google Scholar]
- 53.Villemure E., Volgraf M., Jiang Y., Wu G., Ly C.Q., Yuen P.W., Lu A., Luo X., Liu M., Zhang S., et al. GluN2A-Selective Pyridopyrimidinone Series of NMDAR Positive Allosteric Modulators with an Improved in Vivo Profile. ACS Med. Chem. Lett. 2017;8:84–89. doi: 10.1021/acsmedchemlett.6b00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang T., Lamb M.L., Block M.H., Davies A.M., Han Y., Hoffmann E., Ioannidis S., Josey J.A., Liu Z., Lyne P.D., et al. Discovery of Disubstituted Imidazo[4,5-B]Pyridines and Purines as Potent Trka Inhibitors. ACS Med. Chem. Lett. 2012;3:705. doi: 10.1021/ml300074j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 56.Schrödinger Suite 2016-3 Protein Preparation Wizard. [(accessed on 10 May 2018)]; Available online: https://www.schrodinger.com/protein-preparation-wizard.
- 57.Irwin J.J., Sterling T., Mysinger M.M., Bolstad E.S., Coleman R.G. ZINC: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012;52:1757–1768. doi: 10.1021/ci3001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schrödinger Release 2018-1, LigPrep. Schrödinger, LLC; New York, NY, USA: 2018. [Google Scholar]
- 59.Trott O., Olson A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schrödinger Release 2018-1, Glide. Schrödinger, LLC; New York, NY, USA: 2018. [Google Scholar]
- 61.Bowers K.J., Chow D.E., Xu H., Dror R.O., Eastwood M.P., Gregersen B.A., Klepeis J.L., Kolossvary I., Moraes M.A., Sacerdoti F.D., et al. Scalable algorithms for molecular dynamics simulations on commodity clusters; Proceedings of the ACM/IEEE SC 2006 conference; Tampa, FL, USA. 11–17 November 2006; p. 43. [Google Scholar]
- 62.Banks J.L., Beard H.S., Cao Y., Cho A.E., Damm W., Farid R., Felts A.K., Halgren T.A., Mainz D.T., Maple J.R., et al. Integrated Modeling Program, Applied Chemical Theory (IMPACT) J. Comput. Chem. 2005;26:1752. doi: 10.1002/jcc.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toukmaji A.Y., Board J.A., Jr. Ewald summation techniques in perspective: a survey. Comput. Phys. Comm. 1996;95:73–92. doi: 10.1016/0010-4655(96)00016-1. [DOI] [Google Scholar]
- 64.Zielkiewicz J. Structural properties of water: comparison of the SPC, SPCE, TIP4P, and TIP5P models of water. J. Chem. Phys. 2006;124:109901. doi: 10.1063/1.2178809. [DOI] [PubMed] [Google Scholar]
- 65.Martyna G.J., Klein M.L. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992;97:2635. doi: 10.1063/1.463940. [DOI] [Google Scholar]
- 66.AC Discovery. [(accessed on 10 May 2018)]; Available online: http://www.ac-discovery.com.
- 67.IBScreen. [(accessed on 10 May 2018)]; Available online: http://www.ibscreen.com.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.