Abstract
Bacteria often contain rare deoxy amino sugars which are absent in the host cells. This structural difference can be harnessed for the development of vaccines. Over the last fifteen years, remarkable progress has been made toward the development of novel and efficient protocols for obtaining the rare sugar building blocks and their stereoselective assembly to construct conjugation ready bacterial glycans. In this review, we discuss the total synthesis of a variety of rare sugar containing bacterial glycoconjugates which are potential vaccine candidates.
Keywords: bacterial glycoconjugates, rare deoxy-amino sugars, total synthesis, vaccine candidates, zwitterionic polysaccharides, stereoselective glycosylation
Introduction
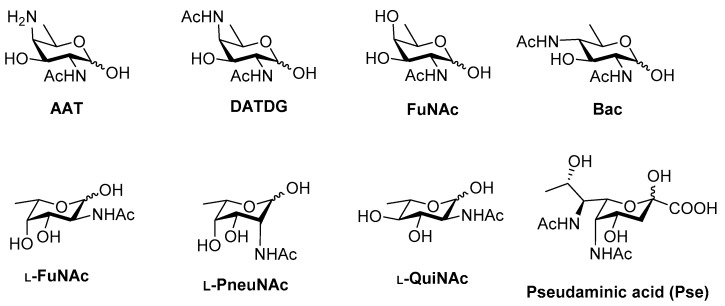
Carbohydrates in the form of glycoconjugates are ubiquitously distributed on the cell surface. By virtue of their position and unique structures, they play key roles in a myriad of vital life processes at the cell–cell interface [1]. For several years, it was believed that glycosylation, the post-translational modification of proteins, is present only in eukaryotes and that it is virtually absent in prokaryotes. However, it is now well established that bacteria and even archaea have glycans present on their surfaces as well. Several bacteria possess capsular polysaccharides (CPS) and other types of carbohydrates such as O-antigens, exopolysaccharrides and teichoic acids, which are immunogenic and hence useful for vaccine development [2]. Currently, several glycoconjugate vaccines are under investigation for various infectious diseases [3]. Very recently, Adamo and co-workers extensively reviewed the progress in the development of antimicrobial glycoconjugate vaccines with special emphasis on targets for future development [4]. An important aspect of bacterial glycoproteins and polysaccharides is that many of them possess unique rare deoxy amino monosaccharides which are virtually absent in humans (Figure 1). More importantly, their presence has been shown to be linked with pathogenesis [5,6,7]. Since these structures are not present in humans, such glycans are potential vaccine candidates and also useful tools for identification, detection and selective targeting of bacteria [8,9,10,11,12]. Over the past 15 years, efficient routes have been developed for the synthesis of rare monosaccharide building blocks starting from simple sugars or via de-novo approaches from amino acid derivatives. These approaches have been categorically reviewed and discussed by Kulkarni and co-workers [13]. In this article, we review the progress made in the chemical assembly of the rare deoxy amino sugars containing oligosaccharides which are potential candidates for future vaccine development.
Figure 1.
Representative examples of rare deoxy amino monosaccharides present in bacteria.
Campylobacter jejuni Heptasaccharide
Campylobacter jejuni is a Gram-negative pathogen. It contains a major non flagellin antigenic glycoprotein designated as PEB3 or Cj0289c [14]. This glycoprotein carries N-linked glycans and have multiple glycosylation sites [15]. The core structure of this glycan was established as α-GalpNAc-(1→4)-α-GalpNAc-(1→4)-[β-Glcp-(1→3)]-α-GalpNAc(1→4)-α-GalpNAc-(1→4)-α-GalpNAc-(1→3)-β-Bacp heptasaccharide. The oligosaccharide contains a rare sugar bacillosamine (2,4-diacetamido-2,4,6-trideoxy-d-glucopyranose), and repeating d-galactosamine building blocks with branched d-glucose. The presence of the N-linked glycan on the surface of C. jejuni has been shown to be crucial for pathogenesis, specifically in enteric adhesion to host cells, which is the first step of virulence [16,17]. C. jejuni causes gastroenteric disorders and is implicated in neuromuscular paralysis as well as Guillian Barre syndrome (GBS) [18]. The biosynthetic pathway of C. jejuni has been delineated by Imperiali and co-workers [19,20,21]. In 2006, Ito and co-workers reported the first chemical synthesis of the heptasaccharide [22]. The synthesis involves stereoselective formation of the repeating unit α-GalpNAc-(1→4)-α-GalpNAc motif, its assembly to construct the branched hexasaccharide Glc1GalNAc5 and coupling with bacillosamine.
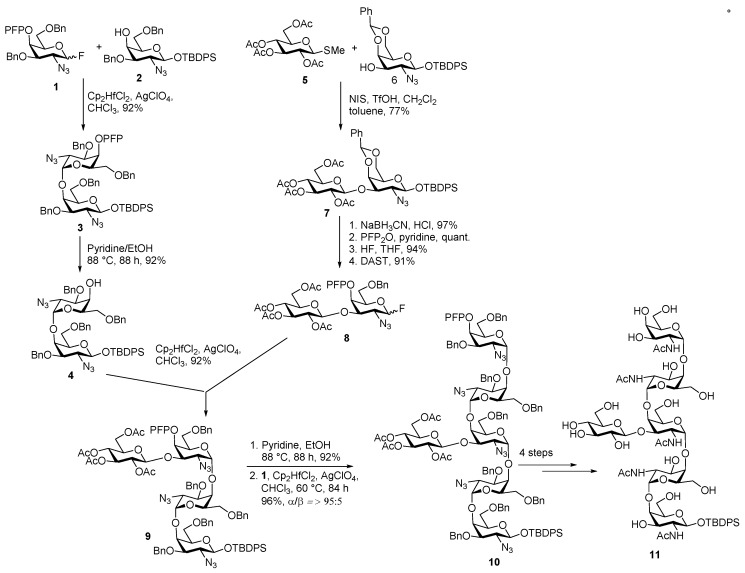
The assembly of C. jejuni hexasaccharide is shown in Scheme 1. Coupling of glycosyl fluoride 1 with 4-OH galactosamine acceptor 2 in the presence of Cp2HfCl2, AgClO4 promoter delivered key disaccharide 3 in a highly stereoselective manner with excellent yield. The α-selectivity observed in this case is a combined effect of the anomeric effect, the remote participation of C4 pentafluoro propionyl (PFP) group and non-participating C2-azido group. Next, the PFP group was selectively removed under mild basic conditions using pyridine/EtOH at elevated temperature to afford disaccharide acceptor 4 in 92% yield. For the construction of the branched structure, glucopyranoside donor 5 was activated by NIS, TfOH activation condition and coupled with acceptor 6 to furnish the desired disaccharide donor (β-Glcp-(1→3)-GalpN3) 7 in 77% yield. Regioselective reductive O4 ring opening of benzylidine acetal, followed by capping of the free 4-OH with PFP group, anomeric desilylation and fluorination afforded glycosyl fluoride 8 in 91% yield. The so formed glycosyl fluoride 8 and disaccharide acceptor 4 were coupled together in the presence of Cp2HfCl2, AgClO4 condition at room temperature to obtain tetrasaccharide 9 in 92% yield. Similar deprotection of PFP group and further elongation furnished glucose branched hexasaccharide. Global deprotection was successfully conducted in a step wise manner to afford oligosaccharide 11 in good yield.
Scheme 1.
Ito’s first-generation synthesis of the heptasaccharide from C. jejuni.
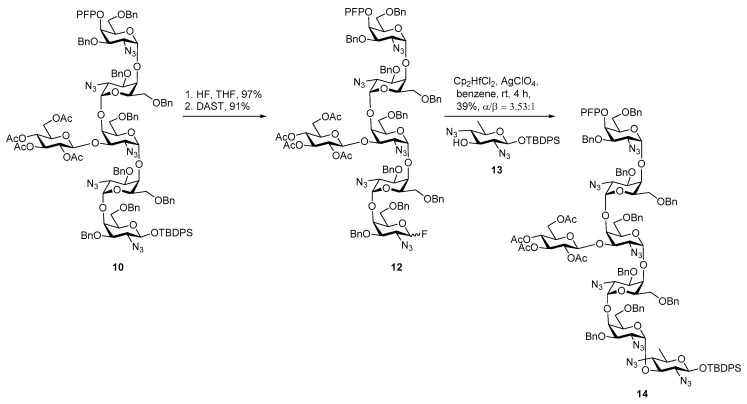
As shown in Scheme 2, the final coupling of hexasaccharide 10 derived glycosyl fluoride 12 with bacillosamine building block 13 turned out to be inefficient, giving 14 in modest yield (39%) and low stereoselectivity (α/β = 3.53:1). Ito and co-workers also successfully coupled the bacillosamine building block with aspargine derivative to obtain Asn-linked Bac derivative [23].
Scheme 2.
Glycosylation of C. jejuni hexasaccharide with bacillosamine.
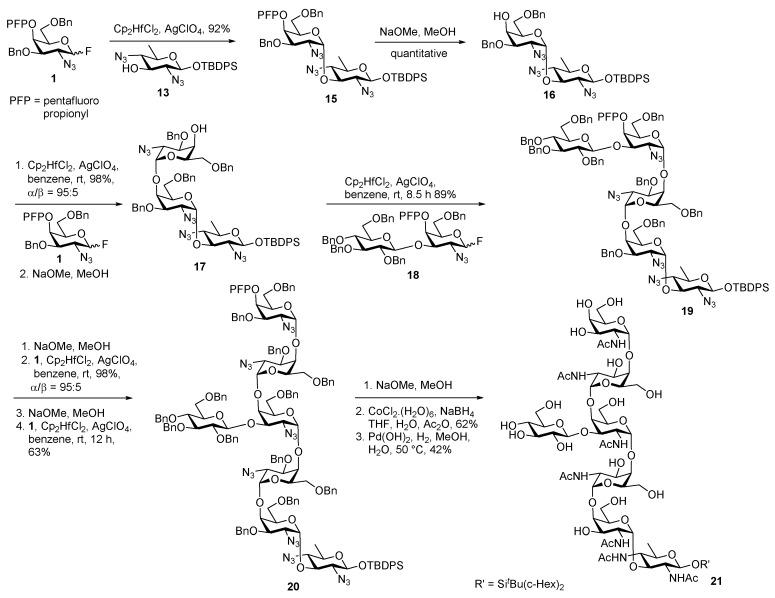
Subsequently, they improved the synthesis of heptasaccharide Glc1GalNAc5Bac1 by employing a linear glycosylation strategy for the assembly of glycan from reducing end to the non-reducing end (Scheme 3) [24]. The route involved stereoselective α glycosylation of di-azido-trideoxyglucose derivative 13 acceptor, with 4-O-PFP protected GalN3 donor 1 (GalN). AgClO4, Cp2HfCl2 promoted glycosylation of donor 1 with bacillosamine acceptor 13 gave α glycoside 15 in 92% yield. Deprotection of PFP group cleanly afforded 4′′-OH acceptor 16 which was glycosylated with galactosyl fluoride donor 1 under similar conditions at room temperature to give the trisaccharide adduct. Further PFP group cleavage using NaOMe, MeOH furnished trisaccharide acceptor 17 in 98% yield. Pentasaccharide 19 was assembled upon glycosylation of fluoro donor 18 with trisaccharide acceptor 17 in excellent yield with clean selectivity. Same deprotection and elongation sequence was carried out twice to get heptasaccharide 20. Functional group deprotection was carried out carefully in a stepwise manner to get the target molecule 21.
Scheme 3.
Ito’s second-generation total synthesis of C. jejuni heptasaccharide.
Zwitterionic Polysaccharides
Zwitterionic polysaccharides (ZPSs) form an important class of immunotherapeutic agents. Several ZPSs appear to stimulate distinct immunological responses that can activate a major histocompatability complex class II (MHCII)-mediated T-cell-dependent immune response in the absence of protein [25,26,27,28,29].
ZPS of Bacteroides fragilis
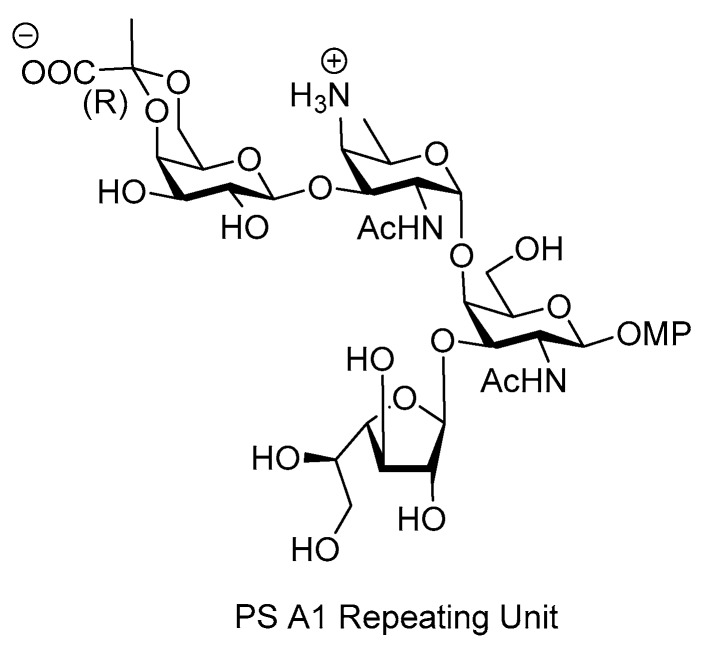
PSA1 ZPS polysaccharide was isolated from the capsule of the commensal bacteria Bacteroides fragilis [30]. It shows anti-inflammatory properties and plays a key role in the development and the maintenance of a balanced mammalian immune system [31,32]. PSA1 stimulates IL-10 secretion, modulates surgical fibrosis [33], inhibits intestinal inflammatory disease caused by Helicobacter hepaticus [34] and protects against central nervous system (CNS) demyelinating disease [35].
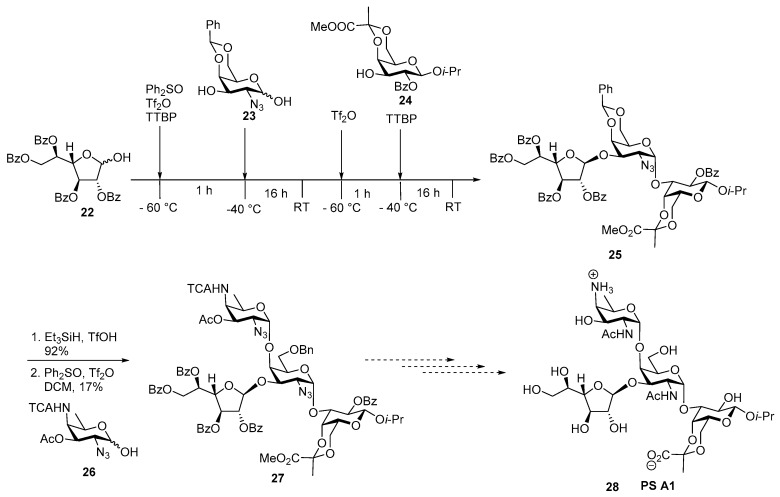
In 2007, van der Marel and co-workers reported a synthesis of the fully protected tetrasaccharide repeating unit of zwitterionic polysaccharide A1 (ZPS A1) as shown in Scheme 4 [36].
Scheme 4.
Van der Marel’s assembly of protected tetrasaccharide repeating unit of zwitterionic polysaccharides (ZPS) A1.
The major difficulties encountered in the assembly of tetrasaccharide are the synthesis of 2-acetamido-4-amino-2,4,6-trideoxy-d-galactopyranose AAT building block and its coupling to d-galactosamine unit through α (1→4) linkage. Trisaccharide 25 was synthesized in a one-pot manner using iterative dehydrative glycosylation conditions. Initially hemiacetal donor 22 was pre-activated by Ph2SO, Tf2O and coupled with acceptor 23 to afford the corresponding disaccharide donor which was again activated under prevalent conditions upon addition of Tf2O and glycosylated with acceptor 24 to furnish the required trisaccharide 25 in 62% yield in one-pot manner. A regioselective triethylsilane reductive ring opening of benzylidene acetal 25 afforded the desired 4-OH acceptor which upon subsequent coupling with the pre-activated AAT donor 26 afforded the tetrasaccharide repeating unit 27 of ZPS A1 in 17% yield. The low yield in the final step could be due to the mismatching reactivity of disarming AAT donor and sterically hindered trisaccharide acceptor.
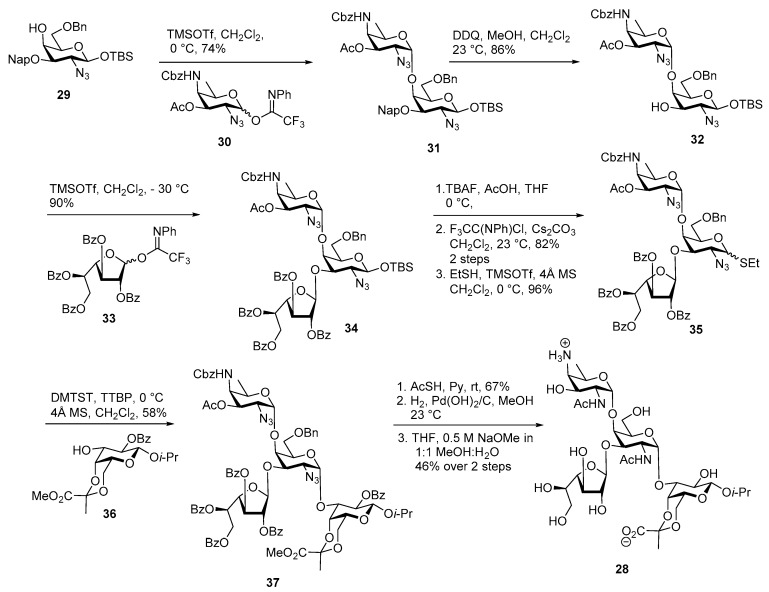
In 2010, Seeberger and co-workers established a successful route to accomplish the total synthesis of ZPS A1 (Scheme 5) tetrasaccharide [37]. After trying several glycosylation routes, they concluded that the coupling of AAT donor with d-galactosamine acceptor has to be performed at the initial stage to get better coupling yields. Accordingly disaccharide 31 was assembled by coupling with AAT imidate donor 30 with d-galactosamine derived acceptor 29 using TMSOTf as a promoter in good yields with α/β ratio of 5:1 [38]. The AAT building block was synthesized via a de novo approach starting from commercially available l-threonine. Oxidative cleavage of 2-napthylmethyl group (Nap) using DDQ in CH2Cl2 and MeOH afforded the disaccharide acceptor 32 in 86% yield. Galactofuranose N-phenyl trifluoroacetimidate 33 was successfully coupled with the corresponding disaccharide acceptor 32 at −30 °C to assemble trisaccharide adduct 34 in 90% yield as a single isomer. The anomeric TBS group was cleaved using TBAF in AcOH to give its corresponding lactol and subsequently converted into N-phenyl trifluoroacetimidate in 82% yield over two steps.
Scheme 5.
First total synthesis of ZPS A1 by Seeberger and co-workers.
The so-formed imidate donor was converted into its corresponding ethyl thioglycoside 35 for further glycosyation. Thioglycoside donor 35 was activated by DMTST, TTBP at 0 °C and coupled with galactose methyl pyruvate acceptor 36 to afford tetrasaccharide 37 in 58% yield as α isomer. Global deprotection was carried out over four steps to obtain tetrasaccharide repeating unit PSA1 of Bacteroides fragilis.
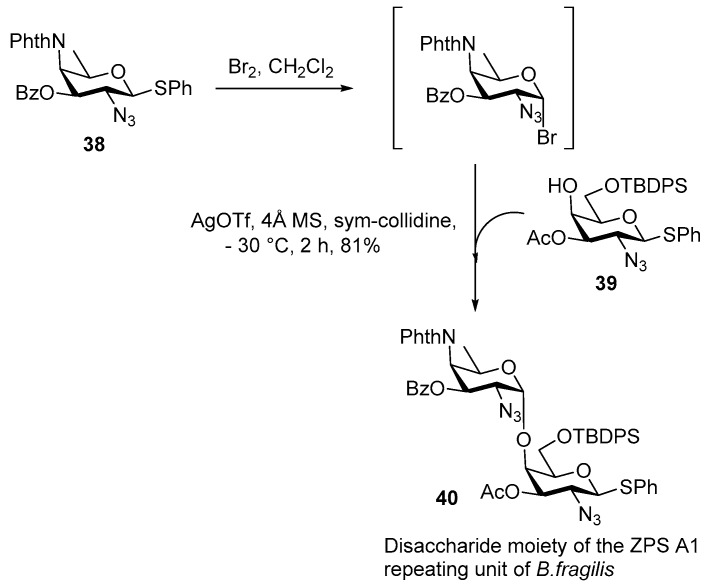
Later on in 2013, Kulkarni and co-workers synthesized the AAT containing key disaccharide of ZPS A1 in an efficient manner [39]. The rare sugar AAT 38 and d-galactosamine acceptor 39 were synthesized from commercially available d-mannose via nucleophilic displacements of 2,4-bis triflates in one pot manner [39].
Thioglycoside AAT derivative 38 (Scheme 6) was converted to its corresponding glycosyl bromide in situ and treated with d-galactosamine acceptor 39 in the presence of AgOTf at −30 °C to give the disaccharide moiety 40 of ZPS A1 in 81% yield as a single α-isomer. The α-stereoselectivity in this case is perhaps arising through the possible formation of a more reactive β-glycosyl triflate using AgOTf and its subsequent SN2 type displacement by the nucleophilic 4-OH acceptor 39.
Scheme 6.
Kulkarni’s synthesis of the rare disaccharide donor unit of ZPS A1.
Andreana and co-workers employed a very novel and unique approach to use PSA1 tetrasaccharide for conjugation with other glycans such as the tumor associated cancer antigens Tn [40] and STn [41] as well as the repeating unit of Streptococcus dysgalactiae [42] 2023 polysaccharide, to construct PSA1 conjugate vaccines. Their studies show that attachment of PSA1 enhances the immune response of the respective glycans.
Very recently, Andreana and co-workers synthesized the PSA1 tetrasaccharide repeating unit with alternating charges on adjacent monosaccharides (Figure 2), employing a linear glycosylation strategy [43]. It is hoped that such a construct would display improved biological activity and its bio-evaluation is awaited.
Figure 2.
Structure of ZPS A1 with alternating charges on adjacent monosaccharides.
ZPS of Streptococcus pneumoniae
Streptococcus pneumoniae polysaccharides are another class of zwitterionic polysaccharides isolated by Wang et al. in 2002 [44]. S. pneumoniae is a Gram positive pathogen and consists of several layers of peptidoglycan to which teichoic acid and lipoteichoic acid are covalently attached. The bacteria causes a variety of life threatening diseases such as severe infection in upper respiratory tract, pneumonia, bacteremia and meningitis, thereby resulting in a high mortality rate [45,46,47]. It consists of a linear polymer of trisaccharide repeating units having a positively charged amino and two negatively charged carboxylic group, two galacturonic acids and a 2-acetamido-4-amino-2,4,6-trideoxygalactose residues. The major challenges in the synthesis of trisaccharides are low reactivity of uronic acid derivatives in glycosylation reactions, incorporation of successive α glycosidic bonds and late stage oxidation. Bundle and co-workers accomplished the first chemical synthesis of its ZPS trisaccharide repeating unit and the corresponding hexasaccharide containing two repeating units [48].
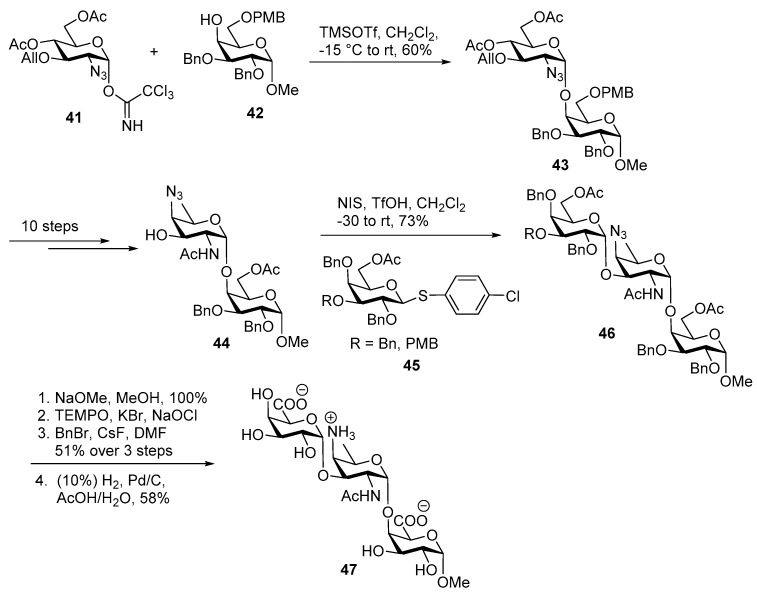
As shown in Scheme 7, glycosylation of glucosamine imidate donor 41 with galactose 4-OH acceptor 42 under TMSOTf activation at −15 °C cleanly afforded α linked disaccharide 43 in 60% yield. After multiple steps, disaccharide 43 was deoxygenated at C6 and the O-allyl group C-3′ was cleaved via PdCl2-catalyzed reaction to afford the disaccharide acceptor 44 in moderate yield. Glycosylation of thioglycoside 45 under NIS/TfOH activation conditions in dichloromethane with acceptor 44 at −30 °C afforded the desired α-linked trisacchride 46 with good selectivity and a trace amount of β linked product was also detected. Global deprotection of 46 via selective deacetylation using NaOMe, MeOH, followed by oxidation of primary alcohol to benzyl esters using TEMPO, KBr, NaOCl and BnBr, CsF, DMF, followed by hydrogenolytic removal of benzyl groups furnished the target trisaccharide 47 in 58% yield.
Scheme 7.
Bundle’s first total synthesis of Streptococcus pneumoniae ZPS trisaccharide.
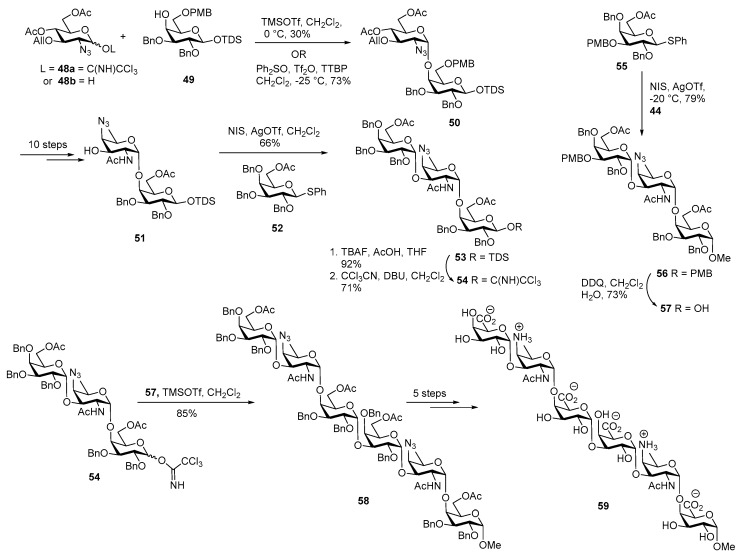
Since the biological studies showed that trisaccharide did not activate T cell, Bundle and co-workers decided to synthesize hexasaccharide containing two repeating units as outlined in Scheme 8 [48]. The glucosamine imidate donor 48a was coupled with galactose 4-OH acceptor 49 in the presence TMSOTf in CH2Cl2 at 0 °C to furnish α-linked disaccharide 50 in a low yield. In this case the 6-OAc group of donor 48a is perhaps offering anchimeric assistance to obtain clean α-selectivity.
Scheme 8.
Bundle’s total synthesis of Streptococcus pneumoniae ZPS hexasaccharide.
Alternatively, by using Gin’s dehydrative glycosylation strategy [49], diphenylsulfoxide and Tf2O mediated lactol activation of 48b at −25 °C with acceptor 49 rapidly furnished only α-linked product 50 in 73% yield. Disaccharide acceptor 51 was synthesized following the same ten steps sequence that was employed for construction of disaccharide 44. Coupling of galactose donor 52 and acceptor 51 using NIS, AgOTf promoter afforded desired trisaccharide 53 in 66% yield. The anomeric TDS group was removed by using TBAF in AcOH, followed by conversion to its corresponding imidate 54 in 71% yield. In parallel, galactose thiodonor 55 was glycosylated with disaccharide acceptor 44 at −20 °C to cleanly afford trisaccharide 56 in good yield. Then PMB group was selectively cleaved by using DDQ, CH2Cl2, H2O to provide trisaccharide acceptor 57 in 73% yield. Having both trisaccharide donor 54 and acceptor 57, imidate donor 54 was activated by TMSOTf promoter to couple with acceptor 57 to obtain hexasaccharide 58 in 85% yield. Global deprotection afforded hexasaccharide repeating unit 59 of Streptococcus pneumoniae.
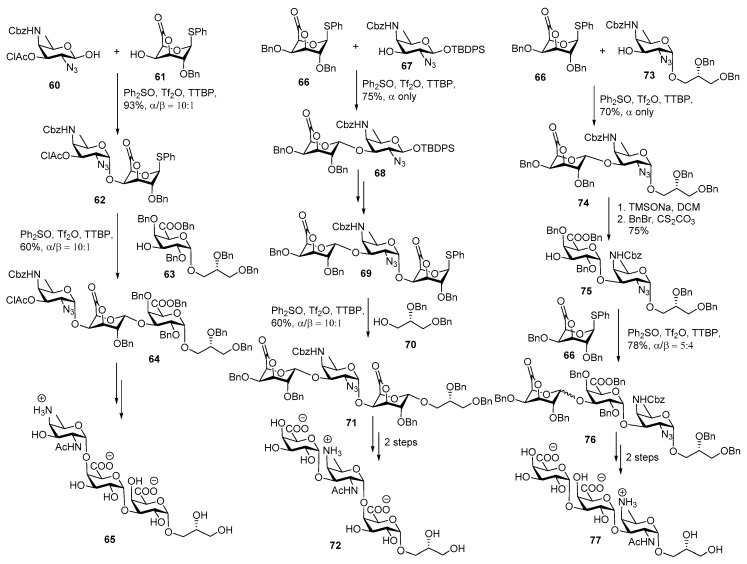
In 2010, Codée and co-workers successfully synthesized all possible trisaccharide repeating units of the type 1 capsular polysaccharide of Streptococcus pneumoniae Sp1 [50]. For the synthesis of the first trisaccharide repeating unit (Scheme 9), the coupling of lactone 61 and 2,4,6-trideoxy-4-amino-d-galactosamine 60 using Ph2SO, Tf2O as a promoter afforded disaccharide 62 in α/β (10:1) ratio. Glycosylation between donor 62 and acceptor 63 using dehydrative glycosylation condition furnished fully protected glycerol capped trisaccharide 64 in 60% yield. The chloroacetyl group was removed, azide was reduced to NHAc, and subsequent debenzylation under hydrogenation conditions delivered the target trisaccharide 65 in 38% yield over two steps. For construction of trisaccharide 72, lactone donor 66 was glycosylated with AAT 3-OH acceptor 67 using in situ generated diphenylsulfonium bistriflate, to afford disaccharide 68 in good yield and excellent stereoselectivity. Then lactone 68 was modified to its corresponding imidate donor through a multiple steps sequence and coupled with lactone acceptor to provide trisaccharide 69. Pre-activation of trisaccharide thio donor 69 using Ph2SO, Tf2O and coupling with glycerol acceptor 70 furnished fully protected trisaccharide 71 in 60% yield (α/β = 10:1).
Scheme 9.
Codée’s synthesis of all the Streptococcus pneumoniae Sp1 ZPS trisaccharides.
The same deprotection strategy was applied to get the trisaccharide 72 in good yield as delineated in scheme 9.
For the synthesis of trisaccharide 77 (Scheme 9), disaccharide 74 was produced in a completely α-selective fashion in 70% yield. Lactone ring was opened using TMSONa and subsequent benzyl ester formation using BnBr and Cs2CO3, afforded disaccharide acceptor 75 in 75% yield over 2 steps. Then lactone donor 66 was coupled with disaccharide acceptor 75 using dehydrative glycosylation conditions to afford trisaccharide 76 in (α/β = 5:4) ratio. Global deprotection was carried out by reduction of azide to NHAc, lactone hydrolysis using TMSONa and hydrogenolysis using H2/Pd to remove benzyl groups to obtain target trisaccharide 77 in good yield.
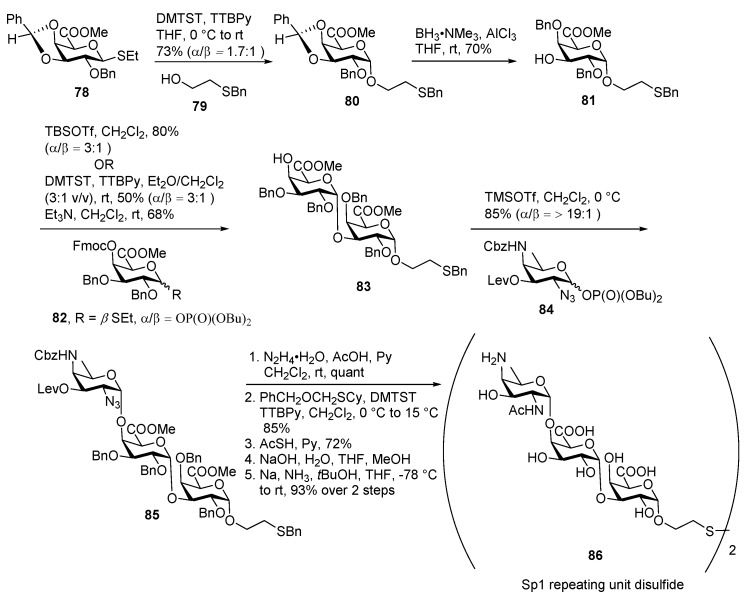
In 2014, Seeberger and co-workers reported a total synthesis of thioether linked trisaccharide repeating unit of Streptococcus pneumoniae Sp1 (Scheme 10) and its immunological characterization for the first time [51]. They positioned the AAT sugar at the non-reducing end to get a more effective immune response.
Scheme 10.
Seeberger’s total synthesis of thioether linked S. pneumoniae Sp1 trisacharide.
Thioether donor 78 was activated by DMTST in THF at 0 °C and coupled with thioether containing alcohol 79 to give glycoside 80 in 73% yield. Regioselective reductive ring opening of the endo-benzylidine ring using BH3·NMe3 and AlCl3 provided 3-OH acceptor 81 in good yield. Coupling of acceptor 81 with glycosyl phosphate donor 82, resulted in disaccharide 83 as a mixture of diastereomers (α/β = 3:1) in 80% yield. To improve the ratio of diastereomers DMTST mediated glycosylation was performed on thio donor by using Et2O as a participating solvent. However, this also presented similar selectivity. Subsequent Fmoc group cleavage delivered disaccharide acceptor 83 in good yield. Trisaccharide 85 was obtained upon glycosylation of AAT phosphate donor 84 with disaccharide acceptor 83 using TMSOTf as a promoter in 85% yield as a single isomer. Fully protected trisaccharide 85 underwent deprotection steps to afford target molecule SP1 repeating unit disulfide 86.
The Seeberger group also showed that a monovalent ST1 trisaccharide with the rare AAT sugar positioned at the non-reducing end induced a strong antibacterial immune response in rabbits and outperformed the ST1 component of the multivalent blockbuster vaccine Prevenar 13, leading to a more efficacious vaccine [52].
Lipoteichoic Acid of Streptococcus pneumoniae
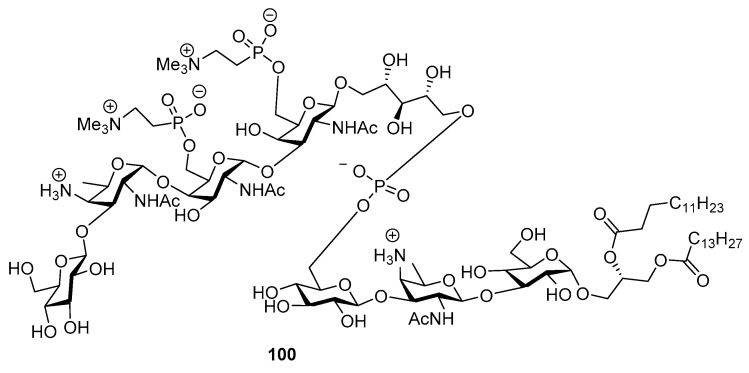
Streptococcus pneumoniae lipoteichoic acid (LTA) is a complex glycophospholipid that consists of nine glycan residues: three glucose, two galactosamine and two 2-acetamino-4-amino-2,4,6-trideoxygalactose (AATDgal) residues that are each differently linked, one ribitol and one diacylated glycerol (DAG) residue (vide infra Figure 3). It’s structural elucidation revealed that pneumococcal LTA of the R6 strain contains phosphodiester inter linked pseudopentasaccharide repeating units carrying each two phosphocholine residues and a glycolipidic core structure comprising a trisaccharide linked to diacylglycerol [53,54,55]. Its unprecedented structure and biological importance makes it more attractive to synthesize. Schmidt and co-workers reported its first chemical synthesis in 2010 [56].
Figure 3.
Structure of lipoteichoic acid of S.pneumoniae.
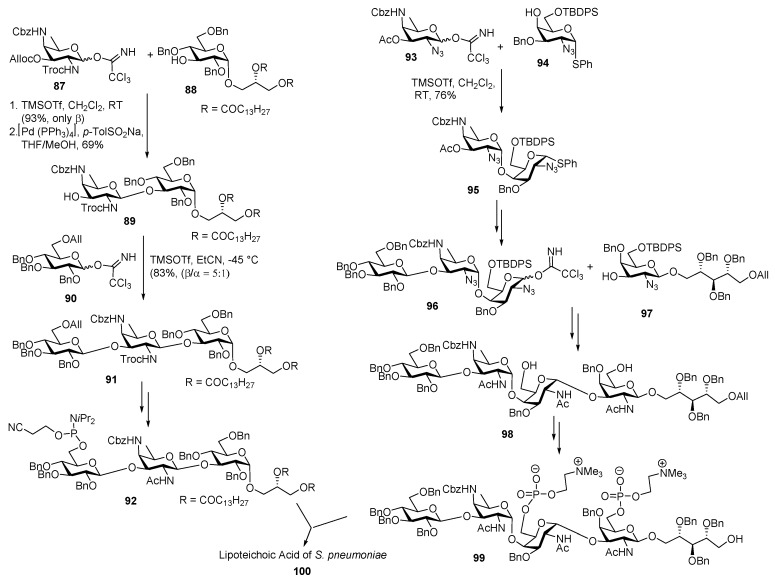
For the synthesis of trisaccharide linked to diacylglycerol, AAT imidate donor 87 was coupled with glycerol derivative acceptor 88 in the presence of TMSOTf promoter to deliver β linked product in high yield. The Alloc group was selectively cleaved using palladium complex to afford disaccharide acceptor 89 in 69% yields. Reaction of 89 with glucose imidate donor 90 was performed under TMSOTf catalysis at −45 °C in propionitrile as a participating solvent to afford the desired β linked trisaccharide intermediate 91 with selectivity of 5:1 β/α ratio. This trisaccharide fragment was also synthesized by the same group, specifically to study its biological property [57]. Phosphorylation of this trisaccharide with bis(diisopropylamino)cyanoethoxyphosphine in the presence of diisopropylammonium tetrazolide furnished phosphorylated trisaccharide 92 in good yield as outlined in Scheme 11.
Scheme 11.
Schmidt’s total synthesis of lipoteichoic acid of Streptococcus pneumoniae.
Pseudopentasaccharide, was constructed from pseudodisaccharide 97 as glycosyl acceptor and trisaccharide 96 as the glycosyl donor. AAT imidate donor 93 was glycosylated with 4-OH galactose acceptor 94 in the presence of TMSOTf promoter in CH2Cl2 at room temperature to obtain α-linked disaccharide 95 (due to NHCbz participation) which was further coupled with glucose donor to give trisaccharide derivative. Then the trisaccharide intermediate was converted to its corresponding imidate donor 96 and glycosylated with disaccharide acceptor 97 to afford pseudopentasaccharide 98 after multiple steps. Then phosphorylation was introduced using cholinoxy-cyanoethoxy-diisopropylaminophosphine using tetrazole as the activator, and then oxidation using tert-butyl hydroperoxide to furnish desired product 99 in high yield. The trisaccharide intermediates 92 and pseudopentasaccharide 99 were treated with tetrazole to deliver phosphite triester which further oxidized with tert-butyl hydroperoxide to give phosphate. The corresponding phosphate upon treatment with dimethylamine led to removal of the cyanoethyl group and formation of the desired phosphodiester dimethylammonium salt, which upon further global deprotection afforded target molecule of lipoteichoic acid of S. pneumoniae 100 (Figure 3).
CPS of Streptococcus pneumoniae Serotype 4
S. pneumoniae serotype 4 (ST4) CPS was discovered in 1931; the structure of its repeating unit was assigned only in 1988 [58,59,60,61,62,63]. This CPS is included in the commercial blockbuster vaccine Prevnar 13. The ST4 polysaccharide consists of a tetrasaccharide repeating unit made up of [3)-β-d-ManpNAc-(1→3)-α-l-FucpNAc-(1→3)-α-d-GalpNAc-(1→4)-α-d-Galp-2,3-(S)-Pyr-(1→]. The presence of N-acetyl sugars, an acid labile trans-2,3-(S)-pyruvate, β-mannoside and α-glycosidic linkages make this molecule a challenging synthetic target. The trisaccharide β-d-ManpNAc-(1→3)-α-l-FucpNAc-(1→3)-α-d-GalpNAc, which lacks the pyruvalated galactose, has already been synthesized [64].
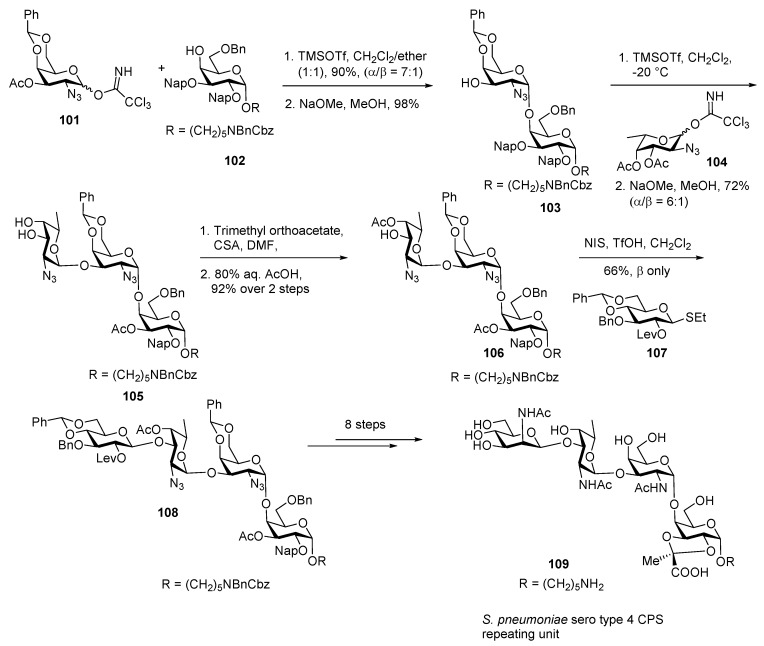
In 2015, Seeberger and co-workers reported its first chemical synthesis as described in Scheme 12 [65]. Disaccharide can be assembled from galactosamine donor 101 with 4-OH acceptor 102 using TMSOTf promoter and ether as participating solvent in 90% yield with α/β ratio 7:1. Acetate group was cleaved using NaOMe, MeOH to afford acceptor 103 in good yield.
Scheme 12.
Seeberger’s total synthesis of capsular polysaccharides (CPS) repeating unit of S. pneumoniae Serotype 4.
Glycosylation of acceptor 103 with l-fucosamine imidate donor 104 in the presence of TMSOTf promoter afforded a trisaccharide, which upon deacetylation delivered diol 105 in 72% yield a diastereomeric mixture (α/β = 6:1). Orthoester formation of diol 105, and selective orthoester opening generated trisaccharide acceptor 106 in excellent yield. The tetrasaccharide 108 was obtained by coupling of trisaccharide acceptor 106 with glucose donor 107 using NIS, TfOH promoter which furnished only β adduct 108 in 66% yield. The gluco configuration of terminal sugar was converted to its corresponding manno configuration and subsequent global deprotection carried out in sequential manner delivered S. pneumoniae serotype 4 repeating unit tetrasaccharide 109.
Streptococcus pneumoniae Serotype 12F CPS
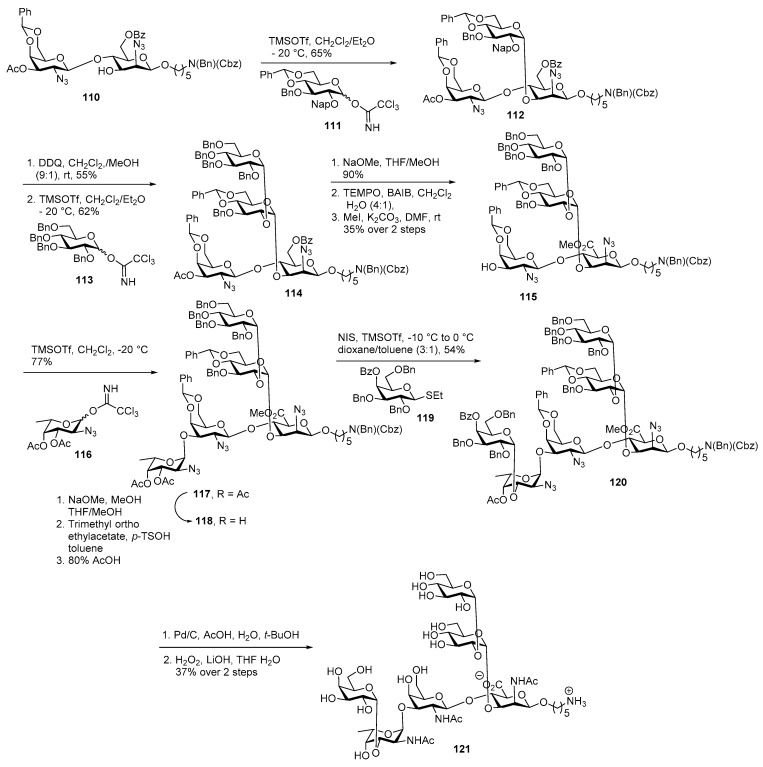
Out of more than ninety, around twenty-three of the S. pneumoniae serotypes are responsible for about 90% of infections worldwide [66]. The licensed polysaccharide vaccine Pneumovax 23 contains serotype 12F. It is not efficacious in young children or elderly people, those at highest risk. The S. pneumoniae serotypes dominates with 85% of pneumococcal disease [67]. This CPS consists of hexasaccharide repeating units containing the [→4)-α-l-FucpNAc-(1→3)-β-d-GalpNAc-(1→4)-β-d-ManpNAcA-(1→] polysaccharide backbone with a disaccharide branch at C3 of β-d-ManpNAcA and C3 of α-l-FucpNAc [68]. In 2017, Seeberger and co-workers reported chemical synthesis of Streptococcus pneumoniae serotype 12F CPS repeating unit employing a stepwise glycosylation route (Scheme 13) [69]. α-Selective glycosylation of disaccharide acceptor 110 with glucose imidate donor 111 using TMSOTf as the activator and diethyl ether as a participating solvent proceeded to produce trisaccharide 112 in 65% yield. Oxidative cleavage of C2 napthylmethyl group using DDQ, in CH2Cl2, MeOH delivered trisaccharide acceptor, which was subsequently treated with glucose imidate donor 113 in the presence of TMSOTf promoter and ether as a co-solvent at −20 °C to furnish tetrasaccharide 114 in 62% yield. 2-Azidomannose moiety of 114 was converted to the corresponding mannosaminuronic acid by cleaving the C6 benzoate ester using sodium methoxide in methanol and selective oxidation of the primary alcohol using BAIB/TEMPO followed by methyl ester formation to furnish tetrasaccharide acceptor 115 in 35% over 2 steps. TMSOTf promoted glycosylation of fucosamine donor 116 with acceptor 115 delivered pentasaccharide 117 as α-isomer by virtue of remote participation of the 4-O-acetate group. Acetate groups were cleaved by using NaOMe, MeOH, followed by orthoester formation and ring opening with 80% AcOH to afford pentasaccharide acceptor 118 in good yield. The desired hexasaccharide 120 was obtained as the α-anomer in 54% yield by coupling galactose building block 119 to pentasaccharide 118 using NIS/TMSOTf in a mixture of toluene/dioxane. Again, the C4 benzoate ester of 119 ensured high selectivity for the desired cis-glycosidic linkage. Global deprotection was carried out carefully to deliver target molecule 121.
Scheme 13.
Seeberger’s total synthesis of S. pneumoniae serotype 12F CPS repeating unit.
CPS of Streptococcus pneumoniae Serotype 5
S. pneumoniae serotype 5 (ST-5) is the fifth most prevalent S. pneumoniae serotypes with different CPS which causes invasive pneumococcal disease among young children globally [70,71]. The ST-5 repeating unit structure was assigned in 1985 [72]. It contains a central N-acetyl l-fucosamine (l-FucNAc) amino sugar that is linked to d-glucose at C4 and to d-glucuronic acid at C3, two rare deoxyamino sugars, the ketoamino sugar 2-acetamido-2,6-dideoxy-d-xylose-hexos-4-ulose (Sugp) and N-acetyl-l-pneumosamine (l-PneuNAc) as shown in Scheme 14. In 2017 Seeberger and co-workers reported chemical synthesis of the reduced form of the pentasaccharide repeating unit of S. pneumoniae serotype 5 [70].
Scheme 14.
Seeberger’s synthesis of S. pneumoniae serotype 5 glycan.
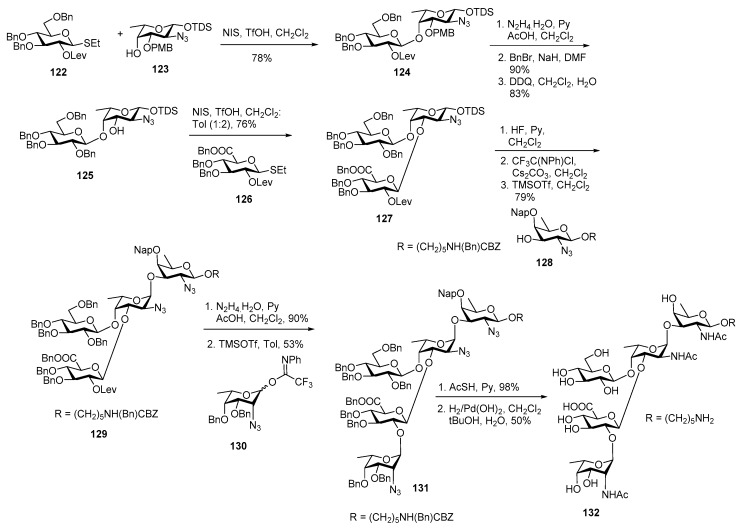
Disaccharide 124 was furnished upon coupling of glucose donor 122 with l-fucosamine 4-OH acceptor 123 in the presence of NIS, TfOH promoter in 78% yield (Scheme 14).
Levulinoyl group was deprotected using N2H4·H2O, subsequently 2′-OH was benzylated and PMB group at O2 position was selectively cleaved to afford disaccharide acceptor 125 in 83% yield. Trisaccharide 127 was assembled from glucuronic donor 126, by coupling with acceptor 125 in the presence of NIS, TfOH promoter in 76% yield. The anomeric TDS group was cleaved using HF·Py to reveal the hemiacetal which was subsequently converted to its corresponding imidate donor which upon further glycosylation with d-fucosamine acceptor 128 furnished tetrasaccharide 129 in 79% yield. Selectively levulinoyl group was removed to obtain tetrasaccharide acceptor which was further coupled with l-pneumosazide donor 130 in toluene as a solvent to obtain fully protected α-linked pentasaccharide 131 in good yield. Global deprotection was carried out to deliver target molecule 132.
ZPS of Shigella sonnei
Shigella are Gram-negative enteroinvasive bacteria generally found in developing countries and industrial areas [73]. It mainly causes shigellosis in humans [74] which continues to be one of the five major diarrheal diseases in children under five [75].
The ZPS of S. sonnei has a disaccharide repeating unit made up of two uncommon aminosugars, a 2-acetamido-2-deoxy-l-altruronic acid (residue A) and a 2-acetamido-4-amino-2,4,6-trideoxy-d-galactopyranose (AAT, residue B) which are 1,2-trans linked to one another [76]. Mulard and co-workers accomplished chemical synthesis of all the ZPS oligosaccharides of Shigella sonnei [76].
Synthesis of Disaccharide AB-Pr (1)
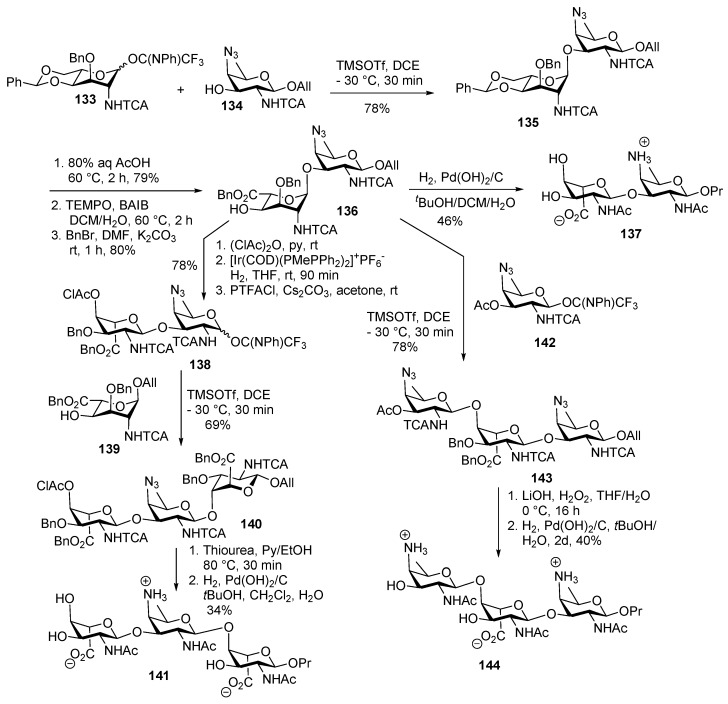
AAT acceptor 134 and l-altrosaminyl donor 133 were coupled in the presence of TMSOTf at −30 °C to furnish disaccharide 135 (Scheme 15). Benzylidene cleavage followed by regioselective 6-OH oxidation by TEMPO, BAIB and benzyl ester protection of free acid using BnBr, NaHCO3 provided the disaccharide acceptor 136 in 80% yield over 2 steps. All benzyl groups were removed using H2/Pd(OH)2/C to deliver target disaccharide 137 in moderate yield.
Scheme 15.
Mulard’s total synthesis of ZPS of Shigella sonnei.
Free 4′-OH of 136 was protected with chloroacetyl group, and subsequent cleavage of anomeric O-allyl group furnished the corresponding hemiacetal which was treated with PTFACl, Cs2CO3 to deliver disaccharide imidate donor 138 in 78% yield over 2 steps. For the synthesis of trisaccharides ABA′-Pr (2) and B′AB-Pr (3) disaccharide acceptor 136 and donor 138 were used. Uronate acceptor 139 was glycosylated with disaccharide imidate donor 138 at −30 °C to obtain fully protected trisaccharide 140 as a sole product. Chloroacetyl group was cleaved using thiourea, pyridine followed by hydrogenolysis to afford trisaccharide 141. TMSOTf-mediated coupling of acceptor 136 with the AAT donor 142 resulted in trisaccharide 143 in 78% yield. Global deprotection was carried out to deliver trisaccharide 144 in good yield over 2 steps.
Phosphorylated ZPS of Providencia alcalifaciens O22
Ovchinnikova and co-workers isolated a new phosphorylated O-polysaccharide from P. alcalifaciens O22 and proposed its structure as -4)-(d-GroAN-2-P-3-)-β-d-GalNAc-(1−4)-β-d-Gal-(1−3)-β-d-Fuc-NAc4N-(1- (Scheme 16) [77].
Scheme 16.
Kulkarni’s one-pot synthesis of ZPS of P. alcalifaciens O22.
PA O22 is a Gram negative and rod-shaped bacterium belonging to the family of Enterobacteriaceae. This pathogen is particularly known to cause diarrhea in children, travelers [78,79] and mainly involved in pericarditis [80], endocarditis [81], meningitis [82], and ocular [83] infections. These species are isolated from sputum, urine, perineum, axilla, stool, blood, and wound specimens of humans as well as from other animals and from soil and water sources [84].
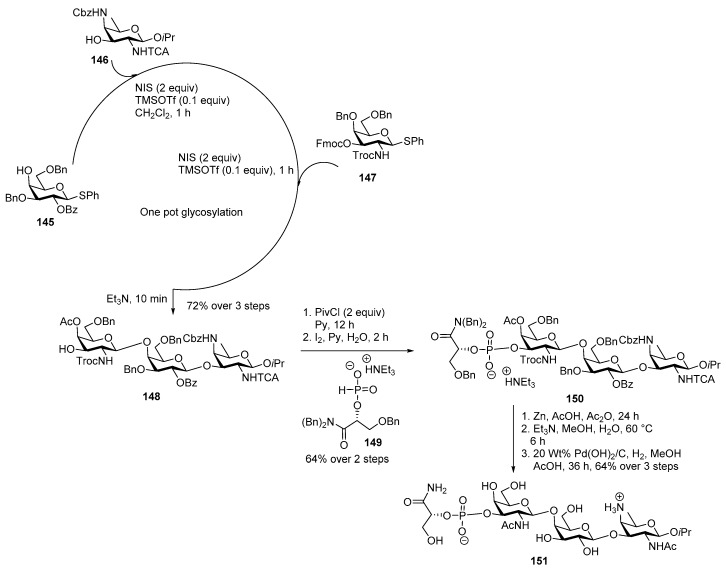
The O-glycan shows a zwitterionic character because of phosphate group and free amino group of AAT residue. P. alcalifaciens O22 polysaccharide repeating unit has a number of synthetic challenges. The main difficult tasks for the synthesis of trisaccharide are the construction of orthogonally protected rare sugar AAT building block and phosphorylation of the secondary alcohol adjacent to the amide functionality in the d-glyceramide unit. Kulkarni and co-workers reported first total synthesis of trisaccharide 151 in one-pot manner in highly regioselective fashion [85]. Coupling of 4-OH thiophenyl galactoside donor 145 and 3-OH AAT acceptor 146 using NIS, TMSOTf promoter led to the formation of 4′-OH disaccharide as a single α isomer. Addition of galactose donor 147 in the same pot in the presence of NIS, TMSOTf cleanly delivered fully protected trisaccharide. When Et3N was added in the same pot, Fmoc group was cleaved to afford 3-OH′′ trisaccharide 148 in 72% yield over three steps, in a one pot manner. H-phosphonate 149 was further coupled with trisaccharide 148 in the presence of pivaloyl chloride and pyridine followed by oxidation with I2 to furnish the phosphorylated trisaccharide 150 in 64% yield over two steps. In global deprotection first, NHTCA and NHTroc were reduced to NHAc by using Zn, AcOH, and Ac2O, followed by hydrolysis of esters using Et3N, MeOH, H2O at 60 °C and concomitant hydrogenolysis using H2, Pd(OH)2/C, and a drop of AcOH in MeOH to obtain target molecule 151 in 64% yield over three steps.
Staphylococcus aureus Type 5 Capsular Polysaccharide
Serotype 5 and 8 capsular polysaccharides predominate among 12 known serotypes of the S. aureus capsular polysaccharides [86]. Staphylococcus aureus type 5 causes skin and soft tissue infections (SSTIs) which can lead to invasive disease with bacteremia, sepsis of endocarditis [87]. The capsular polysaccharide of S. aureus type 5 is a causative agent of infections in newborns, surgical patients, and immunocompromised individuals [88]. The chemical composition of both serotypes CP5 and CP8 are →4)-β-d-ManpNAcA-(1→4)-α-l-FucpNAc(3-OAc)-(1→3)-β-d-Fucp-NAc-(1→ and →3)-β-d-ManpNAcA(4-OAc)-(1→3)-α-l-Fucp-NAc-(1→3)-β-d-FucpNAc-(1→, respectively [89]. Both the serotypes share a common disaccharide which only differs in the acetyl ester position and an anomeric linkage. It has been reported that a bivalent vaccine of S. aureus capsular polysaccharide types 5 and 8 conjugated to Pseudomonas aeruginosa exotoxin A (rEPA) gave 60% protection, but failed to provide long-term protection for end stage renal disease patients, who are often affected by these infections [90,91]. The major challenges in the synthesis of both trisaccharides are efficient synthesis of rare monosaccharides, such as d- and l-fucosamines, stereocontrolled installation of glycosidic linkages having a 1,2-cis-configuration including a N-acetyl-β-d-mannosaminuronic acid (β-d-ManpNAcA) and N-acetyl-α-l-fucosamine (α-l-FucpNAc) glycosides and having to retain acetyl esters in the target compounds.
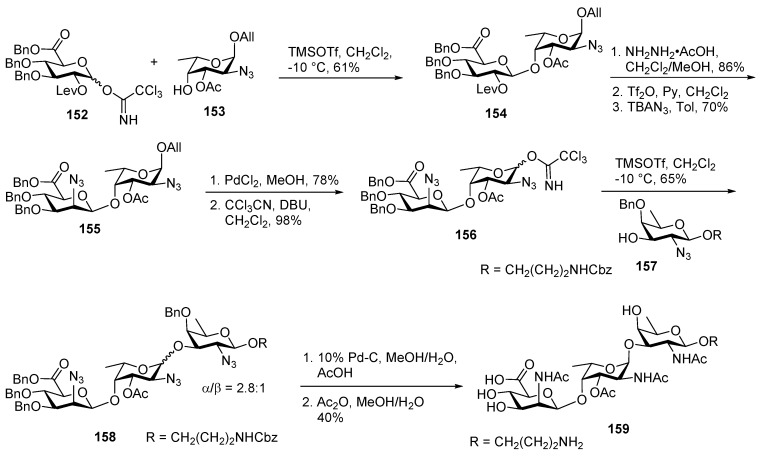
Adamo and co-workers reported the first total synthesis of Staphylococcus aureus type 5 in 2012 as described in Scheme 17 [92]. They employed a glucuronate donor for glycosylation and subsequent gluco to manno epimerization in the disaccharide to construct the key disaccharide which was glycosylated with the rare sugar d-fucosamine. Accordingly, disaccharide 154 was assembled via TMSOTf promoted glycosylation of glucorunic imidate donor 152 and fucosamine 4-OH acceptor 153 at −10 °C. The levulinoyl group was orthogonally deprotected using hydrazine acetic acid, followed by triflation of free 2′-OH and C2 inversion by TBAN3 to fashion the β-manno derivative 155 in 70% yield over 2 steps. PdCl2 catalyzed anomeric deallylation, followed by conversion of the corresponding hemiacetal to its imidate donor 156 and its subsequent coupling with fucosamine 3-OH acceptor 157 in the presence of TMSOTf at −10 °C afforded trisaccharide 158 as a diastereomeric mixture (α/β = 2.8:1). The α-isomer was separated from the unwanted β-isomer at this stage and was subjected to global deprotection. Azide groups were converted to acetamide and simultaneous deprotection of trisaccharide 158 was carried out by hydrogenation with 10% Pd-C, followed by treatment with acetic anhydride in MeOH to furnish target molecule 159.
Scheme 17.
Adamo’s first total synthesis of Staphylococcus aureus type 5 repeating unit.
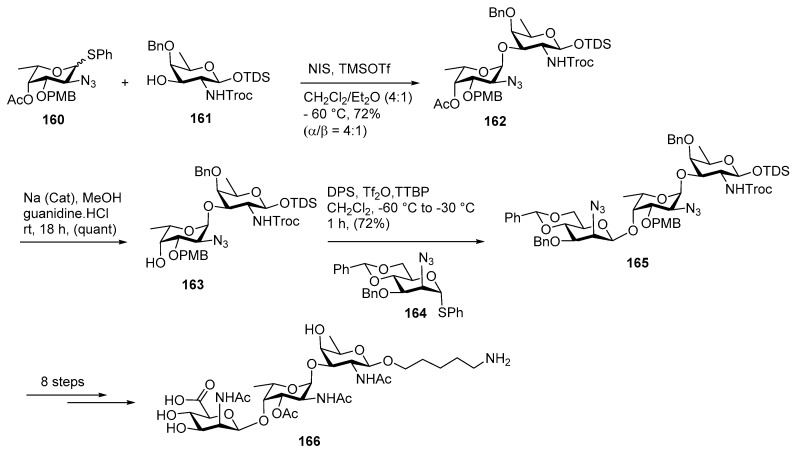
In 2015, Boons and co-workers reported second synthesis of Staphylococcus aureus type 5 polysaccharide (Scheme 18) [93]. Their strategy involves assembly of the glycan from the reducing end to the non-reducing end and a direct β-mannosylation using a mannosyl donor and subsequent late stage oxidation. The assembly of l-fucosamine donor 160 and d-fucosamine 3-OH acceptor 161 in the presence of NIS, TMSOTf as promoter and Et2O as participating solvent afforded α-linked glycoside 162 in 72% yield with (α/β = 4:1) ratio. The α-isomer was separated by using silica gel column chromatography at this stage. The acetyl ester was removed using NaOMe, MeOH to provide disaccharide acceptor 163 which was further glycosylated with mannosamine donor 164 using DPS/Tf2O promoter to deliver β-mannoside product 165 in an excellent yield of 72%. The orthogonally protected trisaccharide 165 was subsequently coupled with amino propyl linker at the late stage and further global deprotection provided target molecule 166 in good yield.
Scheme 18.
Boons’ total synthesis of Staphylococcus aureus type 5 repeating unit.
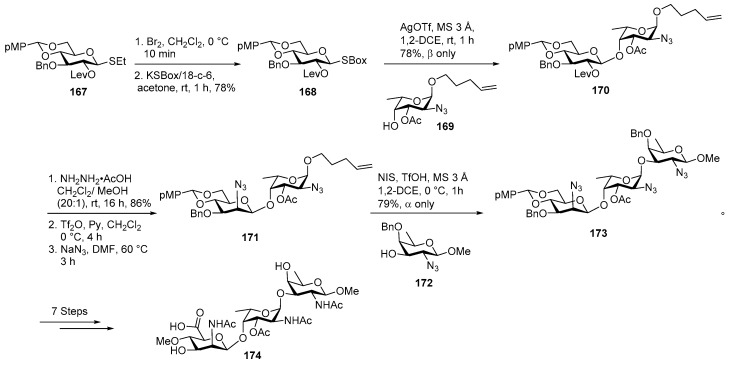
Recently, in 2015, Demchenko and co-workers reported third total synthesis of CP5 serotype polysaccharide by taking advantage of the best of both routes, as shown in Scheme 19 [94]. Notably, this route did not encounter any α/β mixtures in glycosylation with the rare sugar. Thioethylglycoside 167 was treated with Br2, CH2Cl2 at 0 °C and converted to its corresponding glycosyl bromide which upon treatment with KSBox in the presence of 18-crown-6 in acetone yielded building block 168 in 78% yield over 2 steps. AgOTf promoted orthogonal glycosylation of glucose donor 168 with l-fucosamine 4-OH acceptor 169 delivered n-pentenyl disaccharide 170 in 78% yield as a β isomer. In disaccharide 170, C-2′ stereocenter was epimerized to give its corresponding manno configured derivative. For that purpose, O2-levulinoyl group was selectively cleaved using NH2NH2.AcOH, followed by triflation using Tf2O, pyridine and treatment with NaN3 in DMF at 60 °C afforded 171 in 81% yield over 2 steps.
Scheme 19.
Demchenko’s total synthesis of Staphylococcus aureus type 5 repeating unit.
d-fucosamine 3-OH acceptor 172 was coupled with disaccharide donor 171, by activation of O-n-pentenyl leaving group in the presence of NIS, TfOH as a promoter in 1,2-DCE at 0 °C to obtain desired trisaccharide 173 in 79% yield as α anomer. Global deprotection and functional group modification was carried out to obtain target trisaccharide 174.
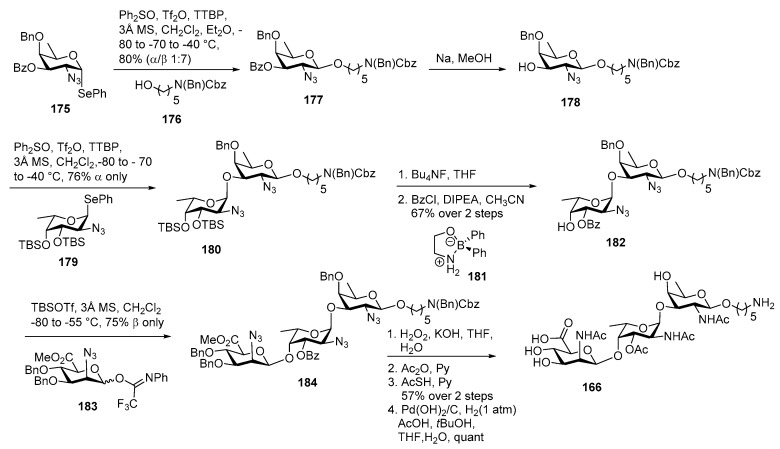
In 2017, Codée and co-workers reported fourth total synthesis S. aureus type 5 CPS repeating unit as outlined in Scheme 20 [95]. Galactosamine donor 175 was coupled with linker derivative 176 in presence of Ph2SO, Tf2O condition to afford desired product 177 in 80% yield as a diastereomeric mixture (α/β = 1:7) ratio. Benzoate group was removed using Zemplén condition to afford acceptor 178 in good yield. For the construction of α-glycosidic linkage between the l-FucN3 and d-FucN3 moieties, the more reactive 3,4-di-O-TBS donor 179 was glycosylated with acceptor 178 in the presence of Ph2SO, Tf2O at lower temperature to obtain disaccharide 180 as a single anomer in 76% yield. TBS ethers were removed, followed by regioselective benzoylation of the C3-O′ position, using Taylor’s diphenylborinate catalyst [96] 181 to give disaccharide acceptor 182 in 67% yield over two steps.
Scheme 20.
Codée’s total synthesis of Staphylococcus aureus type 5 repeating unit.
For the introduction of the mannosaminuronic unit, 2-azidomannuronate donor 183 was activated by TBSOTf and coupled with disaccharide acceptor 182 at lower temperature to furnish fully protected trisaccharide 184 in 75% yield as a β anomer. Functional group deprotection was carried out sequentially to deliver target molecule 166.
Staphylococcus aureus Type 8 Capsular Polysaccharide
Demchenko and co-workers reported the first total synthesis of Staphylococcus aureus type 8 capsular polysaccharide in 2015 [97]. It is a Gram positive, cluster forming, bacteria and one of the highest among all bacterial pathogens [98]. It causes infection in surgical patients, trauma and burn patients, patients receiving an implant, newborns, and dialysis patients with high mortality rates frequently ensuing.
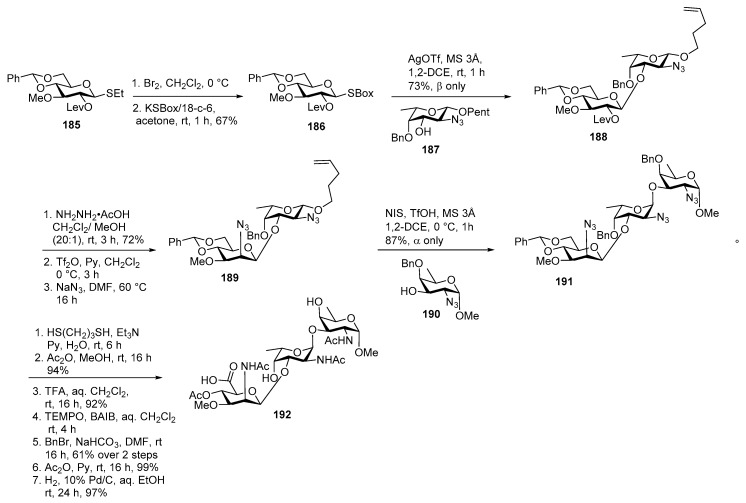
As shown in Scheme 21 ethane thiodonor 185 was converted to its glycosyl bromide upon treatment with Br2, CH2Cl2, followed by reaction with KSBox to convert into its glucose SBox donor 186 in 67% yield over 2 steps. SBox group was activated by AgOTf in 1,2-DCE at rt to orthogonally glycosylate donor 186 with l-fucosamine 3-OH acceptor 187 to afford n-pentenyl disaccharide 188 as a sole product. Then C-2′ stereocenter was inverted by selective levulinoyl group deprotection, followed by triflation of free OH and inversion with NaN3, DMF at 60 °C to furnish disaccharide 189 in excellent yield.
Scheme 21.
Demchenko’s total synthesis of Staphylococcus aureus type 8 repeating unit.
Then the corresponding disaccharide donor 189 was activated by NIS, TfOH and coupled with d-fucosamine 3-OH acceptor 190 to produce α linked trisaccharide 191 in 87% yield. The target trisaccharide 192 was accomplished by sequential deprotection of functional groups.
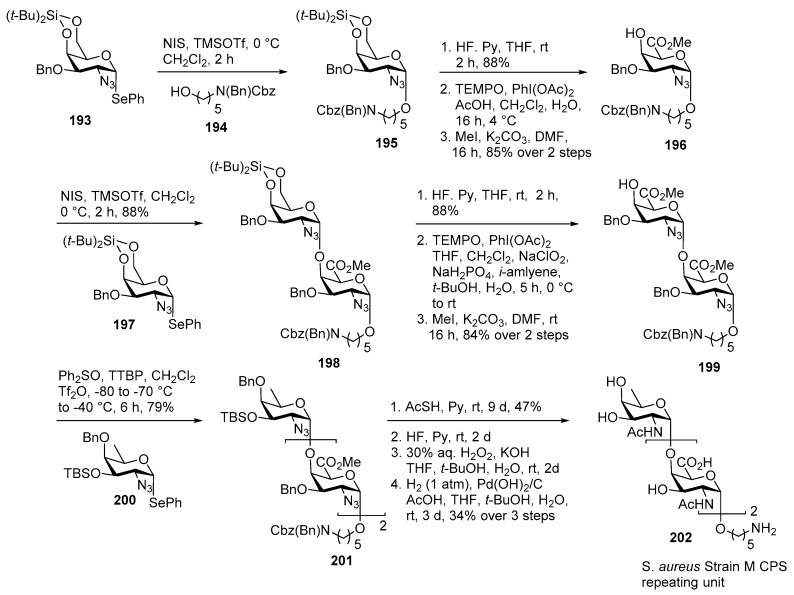
Staphylococcus aureus Strain M Capsular Polysaccharide
Staphylococcus aureus is a Gram-positive pathogen which is implicated in the infections of the skin, lungs, and joints and can cause life-threatening conditions such as endocarditis or toxic shock syndrome [99]. The capsular polysaccharide (CPS) consists of rare N-acetylgalactosaminuronic acid (GalNAcA) and N-acetylfucosamine (FucNAc) units [100]. The synthetic challenges are installation of cis-glycosidic linkages and synthesis of the rare monosaccharide derivatives. In 2017, Codee and co-workers reported the first total synthesis of Staphylococcus aureus Strain M capsular polysaccharide as delineated in Scheme 22 [99].
Scheme 22.
Codée’s total synthesis of Staphylococcus aureus Strain M repeating unit.
Glycosylation of selenoglycoside donor 193 with aminopentanol 194 proceeded smoothly, leading to the expected α-linked product 195 exclusively. Removal of the di-tertiary butyl silyl (DTBS) group, using HF in pyridine, followed by regioselective oxidation of the primary alcohol using the TEMPO/PhI(OAc)2 system furnished acceptor 196 in 85% over 2 steps. Coupling of DTBS protected donor 197 with acceptor 196 in the presence of NIS, TMSOTf promoter furnished disaccharide 198 in 88% yield as a sole anomer. The α-selectivity is a result of the well-known steric effect offered by DTBS group. DTBS group was removed using HF in pyridine, further primary alcohol was oxidized by using one-pot TEMPO/PhI(OAc)2–Pinnick oxidation protocol to give disaccharide acceptor 199 in good yield.
The final glycosylation was performed using azido-fucoside donor 200 with disaccharide acceptor 199 using Ph2SO/Tf2O as promoter at a lower temperature to give fully protected trisaccharide 201 in 79% yield with complete stereoselectivity. Deprotection of the S. aureus strain M repeating unit commenced with the AcSH-mediated conversion of the azides to their corresponding acetamido units, next the TBS ether was removed using HF in pyridine, and the methyl esters were saponified to give the corresponding diacid. Finally, catalytic hydrogenolysis of the benzyl carbamate and ethers provided fully deprotected target trisaccharide 202 in 34% yield over 3 steps.
Neisseria meningitidis Pilin Glycans
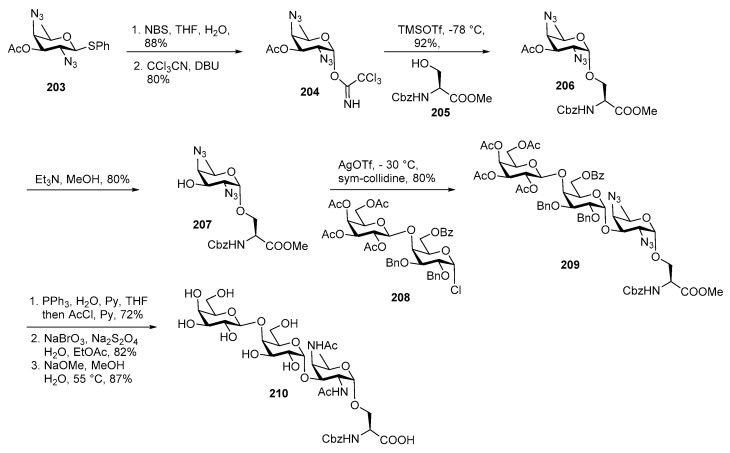
Neisseria meningitidis is a Gram negative and round shaped bacterium. Meningitis is a highly contagious disease which involves inflammation of the protective membranes (meninges) of the brain and spinal cord [101]. It causes a life-threatening sepsis called meningitidis in case of children and young adults and has a high mortality rate. Thus, novel and more effective vaccines are required to control the periodic outbreak of this deadly disease. In 1995, Stimson and co-workers isolated a pilin attached trisaccharide and proposed its structure as Gal-(β1→4)-Gal(α1→3)-2,4-diacetimido-2,4,6-trideoxyhexose. Using mass spectrometry, they inferred that this trisaccharide contains a rare sugar called 2,4-diacetamido-2,4,6-trideoxyhexose (DADTH) which is α linked to l-serine but couldn’t define its stereochemistry at C-4 [102]. Kulkarni and co-workers reported the first total synthesis of both (C4 axial ‘DATDG’ and C4 equatorial ‘Bacillosamine’) the α-l-serine linked trisaccharide of N. meningitidis [103,104]. The major synthetic challenges for construction of this trisaccharide are synthesis of rare, deoxyamino glycans (bacillosamine and DATDG) and incorporation of two consecutive α glycosidic bonds.
Total synthesis of DATDG containing trisaccharide is outlined in Scheme 23, Coupling of DATDH donor with primary alcohol of amino acid in a stereoselective fashion is a difficult task. As shown in Scheme 23, thioglycoside derivative 203 was converted to its corresponding hemiacetal upon treatment with NBS, THF, H2O and the so formed hemiacetal was treated with trichloroacetonitrile and DBU to afford imidate donor 204 in good yield.
Scheme 23.
Kulkarni’s total synthesis of DATDG containing pilin glycan of N. meningitidis.
Glycosylation of imidate donor 204 with l-serine derived acceptor 205 in the presence of TMSOTf promoter, and THF as a participating solvent at −78 °C cleanly afforded α linked product 206 in 92% yield. Then acetate group was selectively removed upon treatment with Et3N, MeOH to deliver acceptor 207 in excellent yield. Glycosyl chloride 208 was activated by AgOTf at −30 °C and coupled with acceptor 207 to furnish trisaccharide 209 in 80% with clean selectivity. Global deprotection was done in 3 steps, involving Staudinger reduction of azides, followed by N-acetylation, then oxidative debenzylation and de-O-acetylation to afford the target molecule 210 in 51% overall yield.
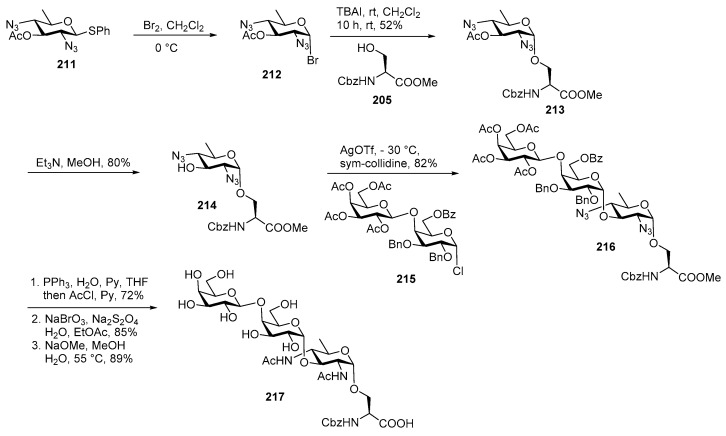
For the synthesis of the other trisaccharide [104,105], the bacillosamine building block 211 was converted to the corresponding glycosyl bromide in situ and treated with acceptor 205 in the presence of TBAI to obtain α linked product 213 in 52% yield (Scheme 24). In this case the in-situ anomeriazation conditions offered clean α-selectivity whereas solvent participation was not very effective. The acetate group was cleaved to afford acceptor 214 which was further coupled with glycosyl chloride 215 in the presence of AgOTf at −30 °C to produce trisaccharide 216 in 82% yield.
Scheme 24.
Kulkarni’s total synthesis of Bac containing pilin glycan of N. meningitidis.
Similar global deprotection steps carried out as previous trisaccharide furnished target trisaccharide 217 in good yield.
Bacillus cereus Ch HF-PS
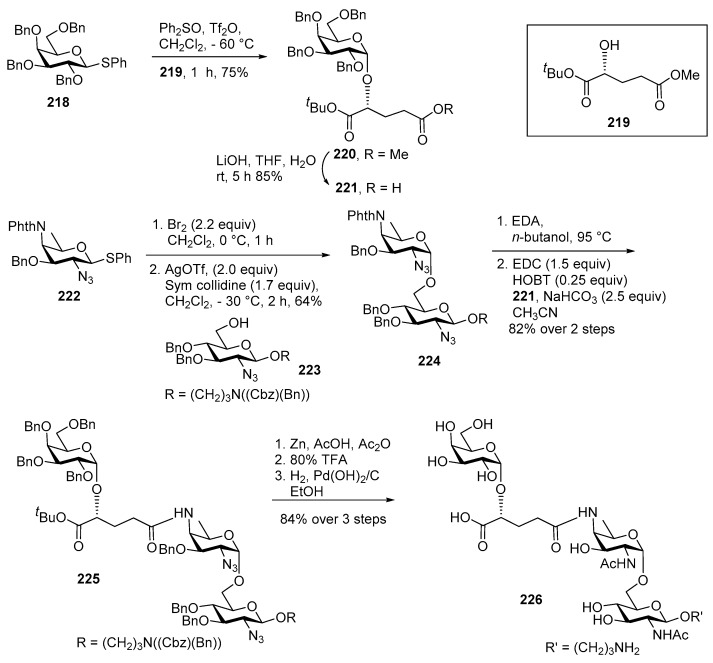
Bacillus cereus is an aerobic, Gram-positive, spore-forming bacterium [101]. Recently, Guérardel and co-workers isolated B. cereus ATCC 14579 and proposed its structure as →6)-Gal(α1−2)(2-R-hydroxyglutar-5-ylamido)Fuc2NAc4N(α1−6)GlcNAc(β1→, [106]. B. cereus can cause serious opportunistic infections such as wound infections, bacteremia, septicaemia, meningitis, pneumonia, infections of the central nervous system, endocarditis, pericarditis, respiratory infections, and peripheral infections [107,108,109,110]. It is also associated with keratitis, panopthalmitis, and other ocular ophthalmic infections which usually result in the loss of the eye [111,112,113]. B. cereus infections are difficult to treat, as the bacterium is resistant to several antibiotics including penicillin, ampicillin, cephalosporins, and trimethoprim [114]. The synthesis of this trisaccharide is more attractive because of its unique structure and immunogenic potential. The major synthetic challenges for constructing this trisaccharide are synthesis of the orthogonally protected rare sugar AAT building block and construction of two consecutive 1,2-cis linkages.
Kulkarni and co-workers reported its first total synthesis in 2014 [115]. Trisaccharide 226 was assembled in [2 + 1] glycosylation manner as outlined in Scheme 25. Galactose thio donor 218 was activated by Ph2SO, Tf2O, at −60 °C and coupled with d-glutaric derivative 219 to furnish α linked product 220 in 75% yield. Then methyl ester was selectively hydrolyzed under mild basic condition to afford acid 221 in excellent yield. For the synthesis of the reducing part disaccharide, first AAT donor 222 was converted to its glycosyl bromide in situ and then treated with glucosamine 6-OH acceptor 223 in the presence of AgOTf promoter at −30 °C, to afford disaccharide 224 as a single anomer. Here the α-stereoselectivity is probably arising due to the possible formation of a more reactive β-glycosyl triflate using AgOTf and its subsequent SN2 type displacement by the nucleophilic 6-OH acceptor 223. Then phthalimide group was selectively cleaved using ethylene diamine in n-BuOH at an elevated temperature to obtain free amine which was subsequently coupled with acid 221 using EDC.HCl, HOBT to give the fully protected trisaccharide 225 in 82% yield over 2 steps. Global deprotection via reduction of azide to NHAc, hydrolysis of tert-butyl esters, followed by removal of benzyl ethers using H2/Pd(OH)2 furnished the target trisaccharide 226.
Scheme 25.
Kulkarni’s first total synthesis of Bacillus cereus Ch HF-PS.
Glycan of Yersinia enterocolitica
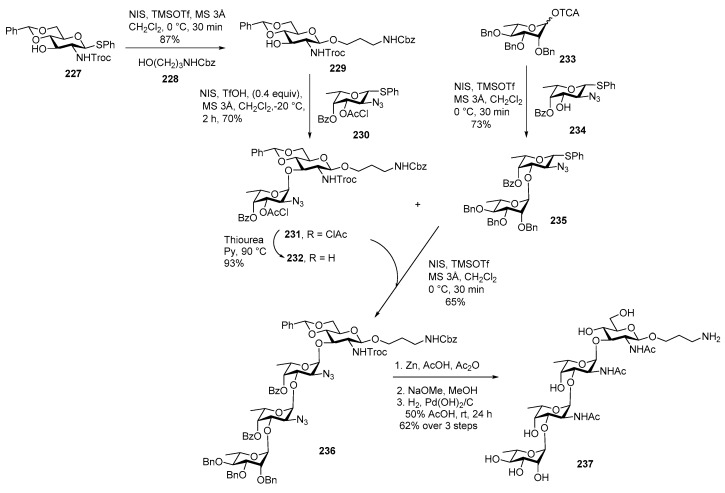
It is a Gram negative bacteria from species of Yersinia genus [116]. It mainly causes enterocolitis, acute diarrhoea, mesenteric lymphadentis and pseudoappendicitis [117]. This polysaccharide was isolated in 2012 [118] and the structure of its repeating unit was elucidated as →2)-α-l-Rhap-(1→3)-α-l-FucpNAc-(1→3)-α-l-FucpNAc-(1→3)-β-d-GlcpNAc-(1 [116]. The key challenges encountered in the synthesis of this tetrasaccharide are synthesis of appropriately protected l-fucosamine building block and installation of consecutive α-linkages. The synthesis of tetrasaccharide was accomplished in [2 + 2] glycosylation route by Kulkarni and co-workers (Scheme 26) [119]. Regioselective glycosylations of glucosamine donor 227 and linker derivative acceptor 228 using NIS, TMSOTf promoter at 0 °C afforded glucosamine 3-OH acceptor 229 in 87% yield. This acceptor was further coupled with l-fucosamine donor 230 in the presence of NIS, TMSOTf promoter at −20 °C to cleanly afford α linked product 231 in 70% yield. The clean α-selectivity in this case can be attributed to the remote participation of axial C4-OBz group. The chloroacetyl group was selectively removed using thiourea in pyridine at 90 °C to afford disaccharide acceptor 232. For the synthesis of the non-reducing end disaccharide, imidate donor 233 was coupled with l-fucosamine 3-OH acceptor 234 in the presence of TMSOTf to give 235 in 73% yield. Crucial coupling of disaccharide donor 235 and acceptor 232 in the presence of NIS, TMSOTf in CH2Cl2 delivered 236 in 65% yield. Global deprotection of tetrasaccharide 236 was achieved in 3 steps. Conversion of azide and NHTroc to NHAc using Zn, AcOH, Ac2O, debenzoylation using NaOMe, MeOH and debenzylation and benzylidine deprotection using H2/Pd(OH)2 smoothly delivered target tetrasaccharide 237.
Scheme 26.
Kulkarni’s total synthesis of glycan of Yersinia enterocolitica.
P. chlororaphis Subsp. Aureofaciens Strain M71 Glycan
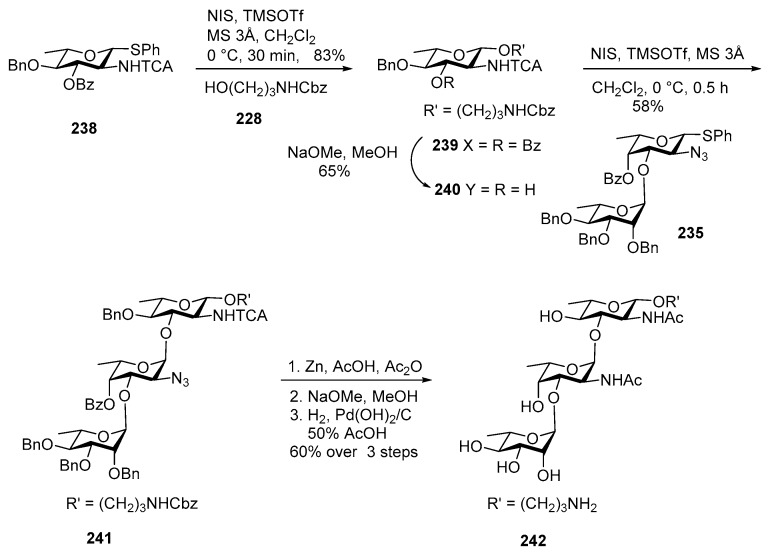
This compound was isolated from the root of a tomato plant by the mild acid hydrolysis of the lipopolysaccharide from P. chlororaphis subsp. aureofaciens strain M71 and its structure was elucidated as →2)-α-l-Rhap-(1→3)-α-l-FucpNAc-(1→3)-β-l-Quip-NAc-(1→ [120].
Scheme 27 describes synthesis of trisaccharide 243 [119]. Quinovosamine 238 was glycosylated with amino acceptor 228 to give coupling product 239 in 83% yield. Debenzoylation of 239 using NaOMe, MeOH furnished acceptor 240 in good yield. The same non-reducing end disaccharide 235 was used for coupling with acceptor 240 by using NIS, TMSOTf promoter to afford fully protected trisaccharide 241 in 58% yield. Global deprotection accomplished in similar manner to afford trisaccharide 242. Although compound 242 may not be useful for vaccine development the approach can be utilized for synthesizing similar l-sugar containing bacterial glycans.
Scheme 27.
Kulkarni’s synthesis of P. chlororaphis subsp. aureofaciens strain M71 glycan.
Plesiomonas shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen
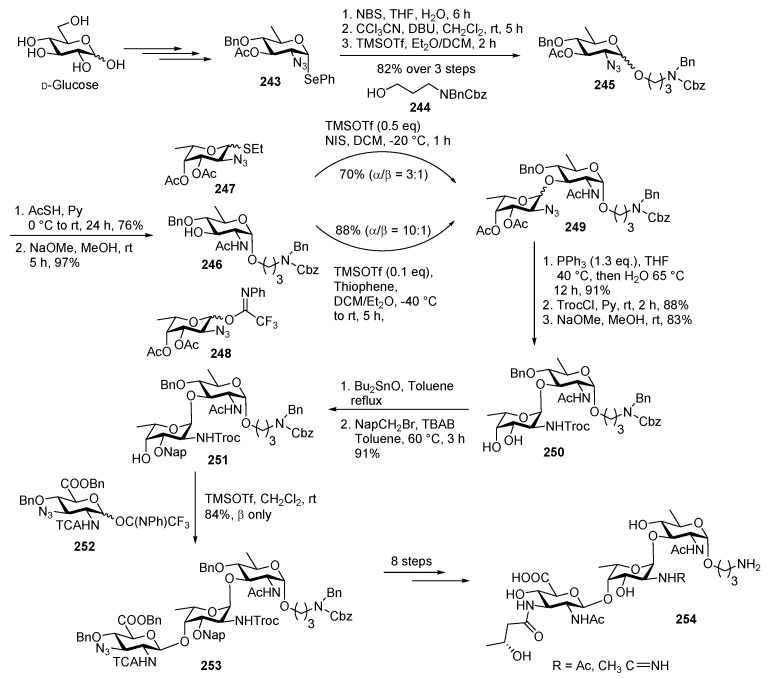
Plesiomonas shigelloides is a Gram-negetive bacterium and a potential useful vaccine for travellers of subtropical and tropical regions [121]. It mainly involves in a variety of extra-intestinal infections such as sepsis, meningitis in case of children and patients with underlying diseases and causes high mortality rate [122]. This bacterium is also a common cause of severe travellers’ diarrhea [122,123]. The proposed structure of this trisaccharide was [→4)-β-d-GlcpNAc3NHbA-(1→4)-α-l-FucpAm3OAc-(1→3)-α-d-QuipNAc-(1→] [123]. This oligosaccharide contains rare functional groups i.e., N-acetyl substituted aminodideoxyhexoses, O-acetyl substituted diaminodideoxyuronic acid, rare acetamidino (Am) and d-3-hydroxybutyryl (Hb) groups [123].
Very recently, Seeberger, Yin and co-workers reported the first total synthesis of the target glycan [124]. The d-quinovosamine building block 243 was synthesized from commercially available d-glucose by a multistep route. As shown in Scheme 28, quinovosamine donor 243 was converted to its corresponding hemiacetal and treated with trichloroacetonitrile and DBU to give imidate which was subsequently coupled with linker acceptor 244 using TMSOTf promoter to afford linker derivative product 245 in 82% yield over 3 steps. Then azide was reduced to NHAc, and acetyl group was selectively removed using NaOMe, MeOH to afford quinovosamine acceptor 246 in 97% yield. Coupling of thioglycoside donor 247 using NIS, TMSOTf promoter with acceptor 246 at −20 °C led to an α/β mixture of disaccharide 249 in a ratio of 3:1. TMSOTf promoted glycosylation of imidate donor 248 in the presence of blended solvent system containing CH2Cl2, Et2O, and thiophene afforded disaccharide 249 with better stereoselectivity in a ratio of 10:1. Azide was reduced to amine using Staudinger method, at elevated temperature and the free amine was trocylated using TrocCl, Py. The acetate groups were removed using Zémplen deacetylation condition to give diol 250 in 83% yield. Then 3-OH was protected with napthylmethyl group using tin chemistry to deliver disaccharide acceptor 251 in 91% yield over 2 steps. Trisaccharide 253 was furnished upon glycosylation of imidate donor 252 with disaccharide acceptor 251 in 84% yield with complete β streoselectivity. Global deprotection of all functional group was carried out over several steps to accomplish target molecule 254 in good yield.
Scheme 28.
Synthesis of P. shigelloides serotype 51 aminoglycoside trisaccharide antigen.
Pseudomonas aeruginosa 1244 Pilin
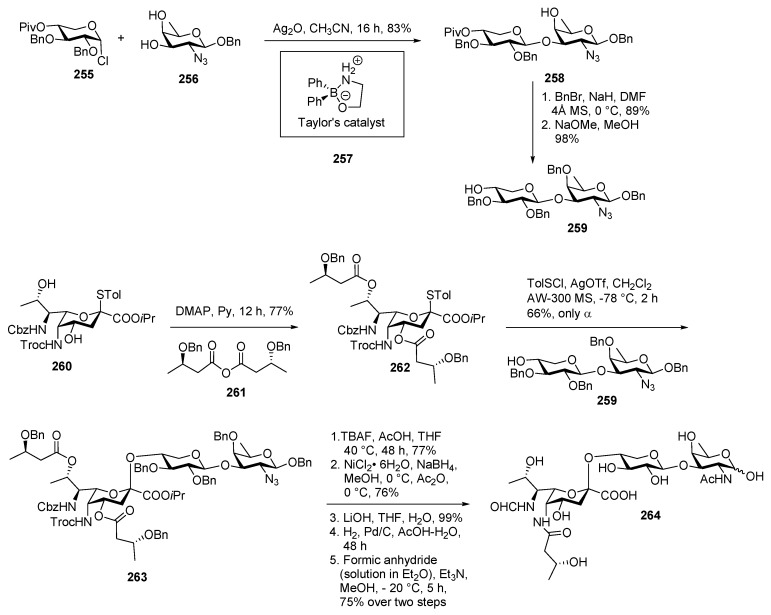
Pseudaminic acid (Pse)-containing glycans and glycoconjugates play critical roles in bacteria [125,126]. Thus, the development of an efficient synthesis of Pse and its derivatives is of great value. P. aeruginosa 1244 pilin is a Gram-negative pathogen and well known for its antibiotic resistance and biofilm formation [127]. This bacteria has been shown to play an important role in immunogenicity. Cystic fibrosis type of infection in immunocompromised patients caused by P. aeruginosa is life threatening [128]. The structure of the pilin glycan 264, was elucidated by Castric et al. as α-5NβOHC47NFmPse-(2→4)-β-Xyl-(1→3)-FucNAc in 2001 [129]. The pilin in P. aeruginosa 1244 is glycosylated with trisaccharide 264 at the C-terminal Ser148 residue through β-glycosidic linkage to the d-fucosamine [130]. In 2017, Li and co-workers reported the first total synthesis of P. aeruginosa 1244 pilin trisaccharide 264 through α-selective glycosylation of the Pse glycosyl donors as outlined in Scheme 29 [131]. They used de novo approach starting from l-allo threonine for the synthesis of pseudaminic acid and its relative functionalized derivatives.
Scheme 29.
Li’s synthesis of Pseudomonas aeruginosa 1244 Pilin glycan.
Disaccharide 258 was obtained by using Taylor’s glycosylations method [132]. 2-aminoethyl diphenylborinate 257 catalyzed the glycosylation of xylosyl chloride 255 and d-fucosamine acceptor 256 regioselectively and stereoselectively to give disaccharide 258 with absolute β (1→3) linkage in 83% yield. Free 4′-OH was benzylated, followed and the pivaloyl group was cleaved in the presence of NaOMe, MeOH to afford acceptor 259 in 98% yield. For the synthesis of Pse derivative 262, the 3-benzyloxy butyrate group was installed onto both O4 and O8 of diol 260 simultaneously using the corresponding anhydride 261 in the presence of pyridine and DMAP in 77% yield. Pse glycosyl donor 262 was coupled with disaccharide acceptor 259 in the presence of TolSCl, AgOTf promoter in CH2Cl2 at −78 °C to provide the desired 263 only as axial anomer in 66% yield. Functional group deprotection was carried out in step wise manner via (1) TBAF-mediated Troc deprotection, (2) O4-to-N5 acyl transfer, (3) azide to amine reduction using a nickel boride reagent, (4) in situ acetylation by Ac2O, and (5) mild saponification to obtain an acid which was treated with H2 and Pd/C in HOAc–H2O to remove the Cbz group and five benzyl groups, and the desired formyl group was next installed onto the released N7 by freshly prepared formic anhydride at −20 °C to deliver final product P. aeruginosa 1244 pilin trisaccharide 264.
Summary and Outlook
Bacteria contain rare deoxy amino sugars which are absent in host cells. This difference in their glycan structures can be exploited for vaccine development. The rare sugars which are present in bacteria are however not available commercially. Tremendous progress has been achieved in recent years toward the development of novel protocols for procurement of the rare sugar building blocks and their stereoselective assembly to synthesize structurally complex bacterial glycans. These advances offer pure and structurally well-defined and linker-attached glycans which are ready for conjugation with carrier proteins. Recent advances in one-pot and automated assembly of glycans [133] would is expected to expedite procurement of bacterial glycans. The immunological studies carried out with these glycoconjugates are expected to lead to identification of new epitopes for vaccine development.
Acknowledgments
We thank the Science and Engineering Research Board, Department of Science and Technology (Grant No. EMR/2014/000235), ISF-UGC joint research program framework (Grant No.2253) and A.B. thanks UGC-New Delhi and IRCC-IIT Bombay for a fellowship.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Varki A. Biological Roles of Oligosaccharides: All of the Theories Are Correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishat S., Andreana P. Entirely Carbohydrate-Based Vaccines: An Emerging Field for Specific and Selective Immune Responses. Vaccines. 2016;4:19. doi: 10.3390/vaccines4020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avci F.Y., Li X., Tsuji M., Kasper D.L. A Mechanism for Glycoconjugate Vaccine Activation of the Adaptive Immune System and Its Implications for Vaccine Design. Nat. Med. 2011;17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micoli F., Costantino P., Adamo R. Potential Targets for next Generation Anti-Microbial Glycoconjugate Vaccines. FEMS Microbiol. Rev. 2018;42:388–423. doi: 10.1093/femsre/fuy011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dube D.H., Champasa K., Wang B. Chemical Tools to Discover and Target Bacterial Glycoproteins. Chem. Commun. 2011;47:87–101. doi: 10.1039/C0CC01557A. [DOI] [PubMed] [Google Scholar]
- 6.Longwell S.A., Dube D.H. Deciphering the Bacterial Glycocode: Recent Advances in Bacterial Glycoproteomics. Curr. Opin. Chem. Biol. 2013;17:41–48. doi: 10.1016/j.cbpa.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adibekian A., Stallforth P., Hecht M.L., Werz D.B., Gagneux P., Seeberger P.H. Comparative Bioinformatics Analysis of the Mammalian and Bacterial Glycomes. Chem. Sci. 2011;2:337–344. doi: 10.1039/C0SC00322K. [DOI] [Google Scholar]
- 8.Morelli L., Poletti L., Lay L. Carbohydrates and Immunology: Synthetic Oligosaccharide Antigens for Vaccine Formulation. Eur. J. Org. Chem. 2011;2011:5723–5777. doi: 10.1002/ejoc.201100296. [DOI] [Google Scholar]
- 9.Fernández-Tejada A., Cañada F.J., Jiménez-Barbero J. Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem. Eur. J. 2015;21:10616–10628. doi: 10.1002/chem.201500831. [DOI] [PubMed] [Google Scholar]
- 10.Seeberger P.H., Werz D.B. Synthesis and Medical Applications of Oligosaccharides. Nature. 2007;446:1046–1051. doi: 10.1038/nature05819. [DOI] [PubMed] [Google Scholar]
- 11.Clark E.L., Emmadi M., Krupp K.L., Podilapu A.R., Helble J.D., Kulkarni S.S., Dube D.H. Development of Rare Bacterial Monosaccharide Analogs for Metabolic Glycan Labeling in Pathogenic Bacteria. ACS Chem. Biol. 2016;11:3365–3373. doi: 10.1021/acschembio.6b00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dumont A., Malleron A., Awwad M., Dukan S., Vauzeilles B. Click-Mediated Labeling of Bacterial Membranes through Metabolic Modification of the Lipopolysaccharide Inner Core. Angew. Chem. Int. Ed. 2012;51:3143–3146. doi: 10.1002/anie.201108127. [DOI] [PubMed] [Google Scholar]
- 13.Emmadi M., Kulkarni S.S. Recent Advances in Synthesis of Bacterial Rare Sugar Building Blocks and Their Applications. Nat. Prod. Rep. 2014;31:870–879. doi: 10.1039/C4NP00003J. [DOI] [PubMed] [Google Scholar]
- 14.Wacker M., Linton D., Hitchen P.G., Nita-Lazar M., Haslam S.M., North S.J., Panico M., Morris H.R., Dell A., Wren B.W. N-Linked Glycosylation in Campylobacter Jejuni and Its Functional Transfer into E. coli. Science. 2002;298:1790–1793. doi: 10.1126/science.298.5599.1790. [DOI] [PubMed] [Google Scholar]
- 15.Young N.M., Brisson J.R., Kelly J., Watson D.C., Tessier L., Lanthier P.H., Jarrell H.C., Cadotte N., St. Michael F., Aberg E., et al. Structure of the N-Linked Glycan Present on Multiple Glycoproteins in the Gram-Negative Bacterium, Campylobacter Jejuni. J. Biol. Chem. 2002;277:42530–42539. doi: 10.1074/jbc.M206114200. [DOI] [PubMed] [Google Scholar]
- 16.Szymanski C.M., Burr D.H., Guerry P. Campylobacter Protein Glycosylation Affects Host Cell Interactions. Infect. Immun. 2002;70:2242–2244. doi: 10.1128/IAI.70.4.2242-2244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Vliet A.H.M., Ketley J.M. Pathogenesis of Enteric Campylobacter Infection. J. Appl. Microbiol. 2001;90:45S–56S. doi: 10.1046/j.1365-2672.2001.01353.x. [DOI] [PubMed] [Google Scholar]
- 18.Nachamkin I., Allos B.M., Ho T. Campylobacter Species and Guillain-Barre Syndrome. Clin. Microbiol. Rev. 1998;11:555–567. doi: 10.1128/cmr.11.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glover K.J., Weerapana E., Imperiali B. In Vitro Assembly of the Undecaprenylpyrophosphate-Linked Heptasaccharide for Prokaryotic N-Linked Glycosylation. Proc. Natl. Acad. Sci. USA. 2005;102:14255–14259. doi: 10.1073/pnas.0507311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weerapana E., Glover K.J., Chen M.M., Imperiali B. Investigating Bacterial N-Linked Glycosylation: Synthesis and Glycosyl Acceptor Activity of the Undecaprenyl Pyrophosphate-Linked Bacillosamine. J. Am. Chem. Soc. 2005;127:13766–13767. doi: 10.1021/ja054265v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glover K.J., Weerapana E., Numao S., Imperiali B. Chemoenzymatic Synthesis of Glycopeptides with PglB, a Bacterial Oligosaccharyl Transferase from Campylobacter Jejuni. Chem. Biol. 2005;12:1311–1316. doi: 10.1016/j.chembiol.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishiwata A., Ohta S., Ito Y. A Stereoselective 1,2-Cis Glycosylation toward the Synthesis of a Novel N-Linked Glycan from the Gram-Negative Bacterium, Campylobacter Jejuni. Carbohydr. Res. 2006;341:1557–1573. doi: 10.1016/j.carres.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Amin M.N., Ishiwata A., Ito Y. Synthesis of Asparagine-Linked Bacillosamine. Carbohydr. Res. 2006;341:1922–1929. doi: 10.1016/j.carres.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Amin M.N., Ishiwata A., Ito Y. Synthesis of N-Linked Glycan Derived from Gram-Negative Bacterium, Campylobacter Jejuni. Tetrahedron. 2007;63:8181–8198. doi: 10.1016/j.tet.2007.05.126. [DOI] [Google Scholar]
- 25.Cobb B.A., Kasper D.L. Zwitterionic Capsular Polysaccharides: The New MHCII-Dependent Antigens. Cell. Microbiol. 2005;7:1398–1403. doi: 10.1111/j.1462-5822.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 26.Mazmanian S.K., Kasper D.L. The Love-Hate Relationship between Bacterial Polysaccharides and the Host Immune System. Nat. Rev. Immunol. 2006;6:849–858. doi: 10.1038/nri1956. [DOI] [PubMed] [Google Scholar]
- 27.Avci F.Y., Kasper D.L. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol. 2010;28:107–130. doi: 10.1146/annurev-immunol-030409-101159. [DOI] [PubMed] [Google Scholar]
- 28.Cobb B.A., Wang Q., Tzianabos A.O., Kasper D.L. Polysaccharide Processing and Presentation by the MHCII Pathway. Cell. 2004;117:677–687. doi: 10.1016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cobb B.A., Kasper D.L. Characteristics of Carbohydrate Antigen Binding to the Presentation Protein HLA-DR. Glycobiology. 2008;18:707–718. doi: 10.1093/glycob/cwn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumann H., Tzianabos A.O., Brisson J.R., Kasper D.L., Jennings H.J. Structural Elucidation of Two Capsular Polysaccharides from One Strain of Bacteroides Fragilis Using High-Resolution NMR Spectroscopy. Biochemistry. 1992;31:4081–4089. doi: 10.1021/bi00131a026. [DOI] [PubMed] [Google Scholar]
- 31.Wang Q., McLoughlin R.M., Cobb B.A., Charrel-Dennis M., Zaleski K.J., Golenbock D., Tzianabos A.O., Kasper D.L. A Bacterial Carbohydrate Links Innate and Adaptive Responses through Toll-Like Receptor 2. J. Exp. Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazmanian S.K., Cui H.L., Tzianabos A.O., Kasper D.L. An Immunomodulatory Molecule of Symbiotic Bacteria Directs Maturation of the Host Immune System. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Perez B., Chung D.R., Sharpe A.H., Yagita H., Kalka-Moll W.M., Sayegh M.H., Kasper D.L., Tzianabos A.O. Modulation of Surgical Fibrosis by Microbial Zwitterionic Polysaccharides. Proc. Natl. Acad. Sci. USA. 2005;102:16753–16758. doi: 10.1073/pnas.0505688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazmanian S.K., Round J.L., Kasper D.L. A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 35.Ochoa-Repáraz J., Mielcarz D.W., Wang Y., Begum-Haque S., Dasgupta S., Kasper D.L., Kasper L.H. A Polysaccharide from the Human Commensal Bacteroides Fragilis Protects against CNS Demyelinating Disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- 36.Van den Bos L.J., Boltje T.J., Provoost T., Mazurek J., Overkleeft H.S., van der Marel G.A. A Synthetic Study towards the PSA1 Tetrasaccharide Repeating Unit. Tetrahedron Lett. 2007;48:2697–2700. doi: 10.1016/j.tetlet.2007.02.067. [DOI] [Google Scholar]
- 37.Pragani R., Seeberger P.H. Total Synthesis of the Bacteroides Fragilis Zwitterionic Polysaccharide A1 Repeating Unit. J. Am. Chem. Soc. 2011;133:102–107. doi: 10.1021/ja1087375. [DOI] [PubMed] [Google Scholar]
- 38.Pragani R., Seeberger P.H. De Novo Synthesis of a 2-Acetamido-4-Amino-2,4,6-Trideoxy-d-Galactose (AAT) Building Block for the Preparation of the Zwitterionic Polysaccharide A1 (PS A1) Repeating Subunit of Bacteroides Fragilis. Org. Lett. 2010;12:1624–1627. doi: 10.1021/ol1003912. [DOI] [PubMed] [Google Scholar]
- 39.Emmadi M., Kulkarni S.S. Orthogonally Protected d-Galactosamine Thioglycoside Building Blocks via Highly Regioselective, Double Serial and Double Parallel Inversions of β-d-Thiomannoside. Org. Biomol. Chem. 2013;11:4825–4830. doi: 10.1039/c3ob40935j. [DOI] [PubMed] [Google Scholar]
- 40.De Silva R.A., Wang Q., Chidley T., Appulage D.K., Andreana P.R. Immunological Response from an Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc. 2009;131:9622–9623. doi: 10.1021/ja902607a. [DOI] [PubMed] [Google Scholar]
- 41.Shi M., Kleski K.A., Trabbic K.R., Bourgault J.-P., Andreana P.R. Sialyl-Tn A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc. 2016;138:14264–14272. doi: 10.1021/jacs.6b05675. [DOI] [PubMed] [Google Scholar]
- 42.Ghosh S., Nishat S., Andreana P.R. Synthesis of an Aminooxy Derivative of the Tetrasaccharide Repeating Unit of Streptococcus dysgalactiae 2023 Polysaccharide for a PS A1 Conjugate Vaccine. J. Org. Chem. 2016;81:4475–4484. doi: 10.1021/acs.joc.6b00195. [DOI] [PubMed] [Google Scholar]
- 43.Eradi P., Ghosh S., Andreana P.R. Total Synthesis of Zwitterionic Tetrasaccharide Repeating Unit from Bacteroides fragilis ATCC 25285/NCTC 9343 Capsular Polysaccharide PS A1 with Alternating Charges on Adjacent Monosaccharides. Org. Lett. 2018 doi: 10.1021/acs.orglett.8b01829. [DOI] [PubMed] [Google Scholar]
- 44.Choi Y.H., Roehrl M.H., Kasper D.L., Wang J.Y. A Unique Structural Pattern Shared by T-Cell-Activating and Abscess-Regulating Zwitterionic Polysaccharides. Biochemistry. 2002;41:15144–15151. doi: 10.1021/bi020491v. [DOI] [PubMed] [Google Scholar]
- 45.Zangwill K.M., Vadheim C.M., Vannier A.M., Hemenway L.S., Greenberg D.P., Ward J.I. Epidemiology of Invasive Pneumococcal Disease in Southern California: Implications for the Design and Conduct of a Pneumococcal Conjugate Vaccine Efficacy Trial. J. Infect. Dis. 1996;174:752–759. doi: 10.1093/infdis/174.4.752. [DOI] [PubMed] [Google Scholar]
- 46.Jedrzejas M.J. Pneumococcal Virulence Factors: Structure and Function. Microbiol. Mol. Biol. Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowy F.D. Staphylococcus Aureus Infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 48.Wu X., Cui L., Lipinski T., Bundle D.R. Synthesis of Monomeric and Dimeric Repeating Units of the Zwitterionic Type 1 Capsular Polysaccharide from Streptococcus Pneumoniae. Chem. Eur. J. 2010;16:3476–3488. doi: 10.1002/chem.200902460. [DOI] [PubMed] [Google Scholar]
- 49.Boebel T.A., Gin D.Y. Sulfoxide Covalent Catalysis: Application to Glycosidic Bond Formation. Angew. Chem. Int. Ed. 2003;42:5874–5877. doi: 10.1002/anie.200352761. [DOI] [PubMed] [Google Scholar]
- 50.Christina A.E., Van Den Bos L.J., Overkleeft H.S., Van Der Marel G.A., Codée J.D.C. Galacturonic Acid Lactones in the Synthesis of All Trisaccharide Repeating Units of the Zwitterionic Polysaccharide Sp1. J. Org. Chem. 2011;76:1692–1706. doi: 10.1021/jo102363d. [DOI] [PubMed] [Google Scholar]
- 51.Schumann B., Pragani R., Anish C., Pereira C.L., Seeberger P.H. Synthesis of Conjugation-Ready Zwitterionic Oligosaccharides by Chemoselective Thioglycoside Activation. Chem. Sci. 2014;5:1992–2002. doi: 10.1039/C3SC53362J. [DOI] [Google Scholar]
- 52.Schumann B., Reppe K., Kaplonek P., Wahlbrink A., Anish C., Witzenrath M., Pereira C.L., Seeberger P.H. Development of an Efficacious, Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus pneumoniae Serotype 1. ACS Cent. Sci. 2018;4:357–361. doi: 10.1021/acscentsci.7b00504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fischer W., Behr T., Hartmann R., Peter-Katalinic J., Egge H. Teichoic Acid and Lipoteichoic Acid of Streptococcus Pneumoniae Possess Identical Chain Structures: A Reinvestigation of Teichoid Acid (C Polysaccharide) Eur. J. Biochem. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 54.Greenberg J.W., Fischer W., Joiner K.A. Influence of Lipoteichoic Acid Structure on Recognition by the Macrophage Scavenger Receptor. Infect. Immun. 1996;64:3318–3325. doi: 10.1128/iai.64.8.3318-3325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischer W. Pneumococcal Lipoteichoic and Teichoic Acid. Microb. Drug Resist. 1997;3:309–325. doi: 10.1089/mdr.1997.3.309. [DOI] [PubMed] [Google Scholar]
- 56.Pedersen C.M., Figueroa-Perez I., Lindner B., Ulmer A.J., Zähringer U., Schmidt R.R. Total Synthesis of Lipoteichoic Acid of Streptococcus Pneumoniae. Angew. Chem. Int. Ed. 2010;49:2585–2590. doi: 10.1002/anie.200906163. [DOI] [PubMed] [Google Scholar]
- 57.Pedersen C.M., Figueroa-Perez I., Boruwa J., Lindner B., Ulmer A.J., Zähringer U., Schmidt R.R. Synthesis of the Core Structure of the Lipoteichoic Acid of Streptococcus Pneumoniae. Chem. Eur. J. 2010;16:12627–12641. doi: 10.1002/chem.201001204. [DOI] [PubMed] [Google Scholar]
- 58.Jansson P.E., Lindberg B., Lindquist U. Structural Studies of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1981;95:73–80. doi: 10.1016/S0008-6215(00)85296-9. [DOI] [PubMed] [Google Scholar]
- 59.Jones C., Currie F. The Pneumococcal Polysaccharide S4: A Structural Re-Assessment. Carbohydr. Res. 1988;184:279–284. doi: 10.1016/0008-6215(88)80031-4. [DOI] [PubMed] [Google Scholar]
- 60.Higginbotham J.D., Heidelberger M. The Specific Capsular Polysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1972;23:165–173. doi: 10.1016/S0008-6215(00)88021-0. [DOI] [PubMed] [Google Scholar]
- 61.Jow Y.L., Heidelberger M. Note Linkage of Pyruvyl Groups in the Specific Capsular Poiysaccharide of Pneumococcus Type IV. Carbohydr. Res. 1976;52:255–258. doi: 10.1016/s0008-6215(00)85971-6. [DOI] [PubMed] [Google Scholar]
- 62.Jones C. A Novel Method for the Determination of the Stereochemistry of Pyruvate Acetal Substituents Applied to the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1990;198:353–357. doi: 10.1016/0008-6215(90)84305-E. [DOI] [PubMed] [Google Scholar]
- 63.Jones C., Currie F., Forster M.J. Nmr and Conformational Analysis of the Capsular Polysaccharide from Streptococcus Pneumoniae Type 4. Carbohydr. Res. 1991;221:95–121. doi: 10.1016/0008-6215(91)80051-N. [DOI] [PubMed] [Google Scholar]
- 64.Horito S., Lorentzen J.P., Paulsen H. Bausteine von Oligosacchariden, LXXVII. Synthese Einer Trisaccharideinheit Des Kapselpolysaccharides VonStreptococcus Pneumoniae Typ 4. Liebigs Ann. Chem. 1986;1986:1880–1890. doi: 10.1002/jlac.198619861108. [DOI] [Google Scholar]
- 65.Pereira C.L., Geissner A., Anish C., Seeberger P.H. Chemical Synthesis Elucidates the Immunological Importance of a Pyruvate Modification in the Capsular Polysaccharide of Streptococcus Pneumoniae Serotype 4. Angew. Chem. Int. Ed. 2015;54:10016–10019. doi: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
- 66.Lund E., Henrichsen J. Laboratory Diagnosis, Serology and Epidemiology of Streptococcus Pneumoniae. Methods Microbiol. 1978;12:241–262. [Google Scholar]
- 67.Robbins J.B., Austrian R., Lee C.-J., Rastogi S.C., Schiffman G., Henrichsen J., Makela P.H., Broome C.V., Facklam R.R., Tiesjema R.H., et al. Considerations for Formulating the Second-Generation Pneumococcal Capsular Polysaccharide Vaccine with Emphasis on the Cross-Reactive Types within Groups. J. Infect. Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 68.Heidelberger M., Avery O.T. The Soluble Specific Subtance of Pneumococcus. J. Exp. Med. 1923;38:73–79. doi: 10.1084/jem.38.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seeberger P.H., Pereira C.L., Govindan S. Total Synthesis of a Streptococcus Pneumoniae Serotype 12F CPS Repeating Unit Hexasaccharide. Beilstein J. Org. Chem. 2017;13:164–173. doi: 10.3762/bjoc.13.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lisboa M.P., Khan N., Martin C., Xu F.-F., Reppe K., Geissner A., Govindan S., Witzenrath M., Pereira C.L., Seeberger P.H. Semisynthetic Glycoconjugate Vaccine Candidate against Streptococcus Pneumoniae Serotype 5. Proc. Natl. Acad. Sci. USA. 2017;114:11063–11068. doi: 10.1073/pnas.1706875114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson H.L., Deloria-Knoll M., Levine O.S., Stoszek S.K., Hance L.F., Reithinger R., Muenz L.R., O’Brien K.L. Systematic Evaluation of Serotypes Causing Invasive Pneumococcal Disease among Children under Five: The Pneumococcal Global Serotype Project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.How M.J., Brimacombe J.S., Stacey M. The Pneumococcal Polysaccharides. Adv. Carbohydr. Chem. 1964;19:303–358. doi: 10.1016/s0096-5332(08)60285-4. [DOI] [PubMed] [Google Scholar]
- 73.Niyogi S.K. Shigellosis. J. Microbiol. 2005;43:133–143. [PubMed] [Google Scholar]
- 74.Barry E.M., Pasetti M.F., Sztein M.B., Fasano A., Kotloff K.L., Levine M.M. Progress and Pitfalls in Shigella Vaccine Research. Nat. Rev. Gastroenterol. Hepatol. 2013;10:245–255. doi: 10.1038/nrgastro.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kenne L., Lindberg B., Petersson K., Katzenellenbogen E., Romanowska E. Structural Studies of the O-Specific Side-Chains of the Shigella Sonnei Phase I Lipopolysaccharide. Carbohydr. Res. 1980;78:119–126. doi: 10.1016/S0008-6215(00)83665-4. [DOI] [Google Scholar]
- 76.Pfister H.B., Mulard L.A. Synthesis of the Zwitterionic Repeating Unit of the O-Antigen from Shigella Sonnei and Chain Elongation at Both Ends. Org. Lett. 2014;16:4892–4895. doi: 10.1021/ol502395k. [DOI] [PubMed] [Google Scholar]
- 77.Ovchinnikova O.G., Kocharova N.A., Bialczak-Kokot M., Shashkov A.S., Rozalski A., Knirel Y.A. Structure of the O-Polysaccharide of Providencia Alcalifaciens O22 Containing d-Glyceramide 2-Phosphate. Eur. J. Org. Chem. 2012;2012:3500–3506. doi: 10.1002/ejoc.201200318. [DOI] [Google Scholar]
- 78.Yoh M., Matsuyama J., Ohnishi M., Takagi K., Miyagi H., Mori K., Park K.S., Ono T., Honda T. Importance of Providencia Species as a Major Cause of Travellers’ Diarrhoea. J. Med. Microbiol. 2005;54:1077–1082. doi: 10.1099/jmm.0.45846-0. [DOI] [PubMed] [Google Scholar]
- 79.Shah M.M., Odoyo E., Larson P.S., Apondi E., Kathiiko C., Miringu G., Nakashima M., Ichinose Y. First Report of a Foodborne Providencia Alcalifaciens Outbreak in Kenya. Am. J. Trop. Med. Hyg. 2015;93:497–500. doi: 10.4269/ajtmh.15-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simon C., Dieli M., Brucato A., Pedrotti P., Brambilla P., Curri S.F., Senni M., Pericotti S., Suter F., Ferrazzi P. Images in Cardiovascular Medicine. Bacterial Pericarditis Due to Providencia Stuartii: An Atypical Case of Relapsing Pericarditis. Circulation. 2010;122:e401–e403. doi: 10.1161/CIRCULATIONAHA.110.943118. [DOI] [PubMed] [Google Scholar]
- 81.Krake P.R., Tandon N. Infective Endocarditis Due to Providenca Stuartii. South. Med. J. 2004;97:1022–1023. doi: 10.1097/01.SMJ.0000141308.19657.BA. [DOI] [PubMed] [Google Scholar]
- 82.Sipahi O.R., Bardak-Ozcem S., Ozgiray E., Aydemir S., Yurtseven T., Yamazhan T., Tasbakan M., Ulusoy S. Meningitis Due to Providencia Stuartii. J. Clin. Microbiol. 2010;48:4667–4668. doi: 10.1128/JCM.01349-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koreishi A.F., Schechter B.A., Karp C.L. Ocular Infections Caused by Providencia Rettgeri. Ophthalmology. 2006;113:1463–1466. doi: 10.1016/j.ophtha.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 84.O’Hara C.M., Brenner F.W., Miller J.M. Classification, Identification, and Clinical Significance of Proteus, Providencia, and Morganella. Clin. Microbiol. Rev. 2000;13:534–546. doi: 10.1128/CMR.13.4.534-546.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Podilapu A.R., Kulkarni S.S. Total Synthesis of Repeating Unit of O-Polysaccharide of Providencia Alcalifaciens O22 via One-Pot Glycosylation. Org. Lett. 2017;19:5466–5469. doi: 10.1021/acs.orglett.7b02791. [DOI] [PubMed] [Google Scholar]
- 86.O’Riordan K., Lee J.C. Staphylococcus Aureus Capsular Polysaccharides. Clin. Microbiol. Rev. 2004;17:218–234. doi: 10.1128/CMR.17.1.218-234.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Daum R.S., Spellberg B. Progress toward a Staphylococcus Aureus Vaccine. Clin. Infect. Dis. 2012;54:560–567. doi: 10.1093/cid/cir828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del Rio A., Cervera C., Moreno A., Moreillon P., Miró J.M. Patients at Risk of Complications of Staphylococcus Aureus Bloodstream Infection. Clin. Infect. Dis. 2009;48:S246–S253. doi: 10.1086/598187. [DOI] [PubMed] [Google Scholar]
- 89.Jones C. Revised Structures for the Capsular Polysaccharides from Staphylococcus Aureus Types 5 and 8, Components of Novel Glycoconjugate Vaccines. Carbohydr. Res. 2005;340:1097–1106. doi: 10.1016/j.carres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 90.Fattom A.I., Schneerson R., Szu S.C., Vann W.F., Shiloach J., Karakawa W.W., Robbins J.B. Synthesis and Immunologic Properties in Mice of Vaccines Composed of Staphylococcus aureus Type 5 and Type 8 Capsular Polysaccharides Conjugated to Pseudomonas aeruginosa Exotoxin A. Infect. Immun. 1990;58:2367–2374. doi: 10.1128/iai.58.7.2367-2374.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fattom A.I., Sarwar J., Ortiz A., Naso R. A Staphylococcus Aureus Capsular Polysaccharide (CP) Vaccine and CP-Specific Antibodies Protect Mice against Bacterial Challenge. Infect. Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Danieli E., Proietti D., Brogioni G., Romano M.R., Cappelletti E., Tontini M., Berti F., Lay L., Costantino P., Adamo R. Synthesis of Staphylococcus Aureus Type 5 Capsular Polysaccharide Repeating Unit Using Novel L-FucNAc and D-FucNAc Synthons and Immunochemical Evaluation. Bioorg. Med. Chem. 2012;20:6403–6415. doi: 10.1016/j.bmc.2012.08.048. [DOI] [PubMed] [Google Scholar]
- 93.Gagarinov I.A., Fang T., Liu L., Srivastava A.D., Boons G.J. Synthesis of Staphylococcus Aureus Type 5 Trisaccharide Repeating Unit: Solving the Problem of Lactamization. Org. Lett. 2015;17:928–931. doi: 10.1021/acs.orglett.5b00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yasomanee J.P., Visansirikul S., Pornsuriyasak P., Thompson M., Kolodziej S.A., Demchenko A.V. Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 5 to Study Chemical Activation and Conjugation of Native CP5. J. Org. Chem. 2016;81:5981–5987. doi: 10.1021/acs.joc.6b00910. [DOI] [PubMed] [Google Scholar]
- 95.Hagen B., Ali S., Overkleeft H.S., Van der Marel G.A., Codée J.D.C. Mapping the Reactivity and Selectivity of 2-Azidofucosyl Donors for the Assembly of N-Acetylfucosamine-Containing Bacterial Oligosaccharides. J. Org. Chem. 2017;82:848–868. doi: 10.1021/acs.joc.6b02593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee D., Taylor M.S. Borinic Acid-Catalyzed Regioselective Acylation of Carbohydrate Derivatives. J. Am. Chem. Soc. 2011;133:3724–3727. doi: 10.1021/ja110332r. [DOI] [PubMed] [Google Scholar]
- 97.Visansirikul S., Yasomanee J.P., Pornsuriyasak P., Kamat M.N., Podvalnyy N.M., Gobble C.P., Thompson M., Kolodziej S.A., Demchenko A.V. A Concise Synthesis of the Repeating Unit of Capsular Polysaccharide Staphylococcus Aureus Type 8. Org. Lett. 2015;17:2382–2384. doi: 10.1021/acs.orglett.5b00899. [DOI] [PubMed] [Google Scholar]
- 98.Ala’Aldeen D.A.A., Hiramatsu K. Staphylococcus Aureus: Molecular and Clinical Aspects. Elsevier; New York, NY, USA: 2004. [Google Scholar]
- 99.Hagen B., Van Dijk J.H.M., Zhang Q., Overkleeft H.S., Van Der Marel G.A., Codée J.D.C. Synthesis of the Staphylococcus Aureus Strain M Capsular Polysaccharide Repeating Unit. Org. Lett. 2017;19:2514–2517. doi: 10.1021/acs.orglett.7b00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Murthy S., Melly M.A., Harris T.M., Hellerqvist C.G., Hash J.H. The Repeating Sequence of the Capsular Polysaccharide of Staphylococcus Aureus M. Carbohydr. Res. 1983;117:113–123. doi: 10.1016/0008-6215(83)88080-X. [DOI] [PubMed] [Google Scholar]
- 101.WHO Fact Sheets: Meningococcal Meningitis. [(accessed on 9 August 2018)]; Available online: http://www.who.int/mediacentre/factsheets/fs141/en/index.html.
- 102.Stimson E., Virji M., Makepeace K., Dell A., Morris H.R., Payne G., Saunders J.R., Jennings M.P., Barker S., Panico M., et al. Meningococcal pilin: A glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol. Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 103.Emmadi M., Kulkarni S.S. Expeditious Synthesis of Bacterial, Rare Sugar Building Blocks to Access the Prokaryotic Glycome. Org. Biomol. Chem. 2013;11:3098–3102. doi: 10.1039/c3ob40615f. [DOI] [PubMed] [Google Scholar]
- 104.Emmadi M., Kulkarni S.S. Synthesis of Orthogonally Protected Bacterial, Rare-Sugar and d-Glycosamine Building Blocks. Nat. Protoc. 2013;8:1870–1889. doi: 10.1038/nprot.2013.113. [DOI] [PubMed] [Google Scholar]
- 105.Emmadi M., Kulkarni S.S. Total Synthesis of the Bacillosamine Containing α-l-Serine Linked Trisaccharide of Neisseria Meningitidis. Carbohydr. Res. 2014;399:57–63. doi: 10.1016/j.carres.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 106.Candela T., Maes E., Garénaux E., Rombouts Y., Krzewinski F., Gohar M., Guérardel Y. Environmental and Biofilm-Dependent Changes in a Bacillus Cereus Secondary Cell Wall Polysaccharide. J. Biol. Chem. 2011;286:31250–31262. doi: 10.1074/jbc.M111.249821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Public Health Agency of Canada. [(accessed on 9 August 2018)]; Available online: http://www.phac-aspc.gc.ca/labbio/res/psds-ftss/bacillus-cereus-eng.php.
- 108.Logan N.A., Rodrigez-Diaz M. Bacillus Spp. and Related Genera. In: Gillespie S.H., Hawkey P.M., editors. Principles and Practice of Clinical Bacteriology. 2nd ed. John Wiley and Sons Ltd.; West Sussex, UK: 2006. pp. 139–158. [Google Scholar]
- 109.Rosovitz M.J., Voskuil M.I., Chambliss G.H. Bacillus. In: Collier L., Balows A., Sussman M., Balows A., Duerden B.I., editors. Topley & Wilson’s Microbiology and Microbial Infection: Systematic Bacteriology. 9th ed. Hodder Education Publishers; London, UK: 1998. pp. 709–729. [Google Scholar]
- 110.Drobniewski F.A. Bacillus Cereus and Related Species. Am. Soc. Microbiol. 1993;6:324–338. doi: 10.1128/CMR.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pinna A., Sechi L.A., Zanetti S., Usai D., Delogu G., Cappuccinelli P., Carta F. Bacillus Cereus Keratitis Associated with Contact Lens Wear. Ophthalmology. 2001;108:1830–1834. doi: 10.1016/S0161-6420(01)00723-0. [DOI] [PubMed] [Google Scholar]
- 112.Cowan C.L., Madden W.M., Hatem G.F., Merritt J.C. Endogenous Bacillus Cereus Panophthalmitis. Ann. Ophthalmol. 1987;19:65–68. [PubMed] [Google Scholar]
- 113.O’Day D.M., Smith R.S., Gregg C.R., Turnbull P.C.B., Head W.S., Ives J.A., Ho P.C. The Problem of Bacillus Species Infection with Special Emphasis on the Virulence of Bacillus Cereus. Ophthalmology. 1981;88:833–838. doi: 10.1016/S0161-6420(81)34960-4. [DOI] [PubMed] [Google Scholar]
- 114.Ginsburg A.S., Salazar L.G., True L.D., Disis M.L. Fatal Bacillus Cereus Sepsis Following Resolving Neutropenic Enterocolitis during the Treatment of Acute Leukemia. Am. J. Hematol. 2003;72:204–208. doi: 10.1002/ajh.10272. [DOI] [PubMed] [Google Scholar]
- 115.Podilapu A.R., Kulkarni S.S. First Synthesis of Bacillus Cereus Ch HF-PS Cell Wall Trisaccharide Repeating Unit. Org. Lett. 2014;16:4336–4339. doi: 10.1021/ol5021527. [DOI] [PubMed] [Google Scholar]
- 116.Skurnik M., Toivonen S. Identification of Distinct Lipopolysaccharide Patterns among Yersinia Enterocolitica and Y. Enterocolitica-Like Bacteria. Biochem. 2011;76:823–831. doi: 10.1134/S0006297911070133. [DOI] [PubMed] [Google Scholar]
- 117.Fàbrega A., Vila J. Yersinia Enterocolitica: Pathogenesis, Virulence and Antimicrobial Resistance. Enferm. Infecc. Microbiol. Clin. 2012;30:24–32. doi: 10.1016/j.eimc.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 118.Beczała A., Duda K.A., Skurnik M., Holst O. The Structure of the O-Specific Polysaccharide of the Lipopolysaccharide from Yersinia Enterocolitica Serotype O:50 Strain 3229. Carbohydr. Res. 2012;359:97–101. doi: 10.1016/j.carres.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 119.Sanapala S.R., Kulkarni S.S. Expedient Route to Access Rare Deoxy Amino L-Sugar Building Blocks for the Assembly of Bacterial Glycoconjugates. J. Am. Chem. Soc. 2016;138:4938–4947. doi: 10.1021/jacs.6b01823. [DOI] [PubMed] [Google Scholar]
- 120.Pieretti G., Puopolo G., Carillo S., Zoina A., Lanzetta R., Parrilli M., Evidente A., Corsaro M.M. Structural Characterization of the O-Chain Polysaccharide from an Environmentally Beneficial Bacterium Pseudomonas Chlororaphis Subsp. Aureofaciens Strain M71. Carbohydr. Res. 2011;346:2705–2709. doi: 10.1016/j.carres.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 121.Michael Janda J., Abbott S.L., McIver C.J. Plesiomonas Shigelloides Revisited. Clin. Microbiol. Rev. 2016;29:349–374. doi: 10.1128/CMR.00103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stock I. Plesiomonas Shigelloides: An Emerging Pathogen with Unusual Properties. Rev. Med. Microbiol. 2004;15:129–139. doi: 10.1097/00013542-200410000-00002. [DOI] [Google Scholar]
- 123.Maciejewska A., Lukasiewicz J., Niedziela T., Szewczuk Z., Lugowski C. Structural Analysis of the O-Specific Polysaccharide Isolated from Plesiomonas Shigelloides O51 Lipopolysaccharide. Carbohydr. Res. 2009;344:894–900. doi: 10.1016/j.carres.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 124.Qin C., Schumann B., Zou X., Pereira C.L., Tian G., Hu J., Seeberger P.H., Yin J. Total Synthesis of a Densely Functionalized Plesiomonas Shigelloides Serotype 51 Aminoglycoside Trisaccharide Antigen. J. Am. Chem. Soc. 2018;140:3120–3127. doi: 10.1021/jacs.8b00148. [DOI] [PubMed] [Google Scholar]
- 125.Morrison M.J., Imperiali B. The Renaissance of Bacillosamine and Its Derivatives: Pathway Characterization and Implications in Pathogenicity. Biochemistry. 2014;53:624–638. doi: 10.1021/bi401546r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lewis A.L., Desa N., Hansen E.E., Knirel Y.A., Gordon J.I., Gagneux P., Nizet V., Varki A. Innovations in Host and Microbial Sialic Acid Biosynthesis Revealed by Phylogenomic Prediction of Nonulosonic Acid Structure. Proc. Natl. Acad. Sci. USA. 2009;106:13552–13557. doi: 10.1073/pnas.0902431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boucher H.W., Talbot G.H., Bradley J.S., Edwards J.E., Gilbert D., Rice L.B., Scheld M., Spellberg B., Bartlett J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 128.Ramphal R., Sadoff J.C., Pyle M., Silipigni J.D. Role of Pili in the Adherence of Pseudomonas Aeruginosa to Injured Tracheal Epithelium. Infect. Immun. 1984;44:38–40. doi: 10.1128/iai.44.1.38-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Castric P., Cassels F.J., Carlson R.W. Structural Characterization of the Pseudomonas Aeruginosa 1244 Pilin Glycan. J. Biol. Chem. 2001;276:26479–26485. doi: 10.1074/jbc.M102685200. [DOI] [PubMed] [Google Scholar]
- 130.Comer J.E., Marshall M.A., Blanch V.J., Deal C.D., Castric P. Identification of the Pseudomonas Aeruginosa 1244 Pilin Glycosylation Site. Infect. Immun. 2002;70:2837–2845. doi: 10.1128/IAI.70.6.2837-2845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu H., Zhang Y., Wei R., Andolina G., Li X. Total Synthesis of Pseudomonas Aeruginosa 1244 Pilin Glycan via de Novo Synthesis of Pseudaminic Acid. J. Am. Chem. Soc. 2017;139:13420–13428. doi: 10.1021/jacs.7b06055. [DOI] [PubMed] [Google Scholar]
- 132.Gouliaras C., Lee D., Chan L., Taylor M.S. Regioselective Activation of Glycosyl Acceptors by a Diarylborinic Acid-Derived Catalyst. J. Am. Chem. Soc. 2011;133:13926–13929. doi: 10.1021/ja2062715. [DOI] [PubMed] [Google Scholar]
- 133.Kulkarni S.S., Wang C.-C., Sabbavarapu N.M., Podilapu A.R., Liao P.-H., Hung S.-C. “One-Pot” Protection, Glycosylation, and Protection−Glycosylation Strategies of Carbohydrates. Chem. Rev. 2018 doi: 10.1021/acs.chemrev.8b00036. [DOI] [PubMed] [Google Scholar]