Abstract
This study has developed a sensitive and simple ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry method for the simultaneous determination of corydaline, dehydrocorydaline, tetrahydropalmatine, protopine, palmatine, tetrahydroberberine, columbamine, berberine, coptisine and berberrubine in beagle dog plasma after the oral administration of the Corydalis yanhusuo W.T. Wang and Yuanhuzhitong tablets. Chromatographic separation was achieved on an Agilent Eclipse Plus C18 RRHD column (1.8 µm, 50 × 2.1 mm) using a gradient elution program with a mobile phase consisting of acetonitrile and water containing 0.1% formic acid at a flow rate of 0.3 mL/min. A tandem mass spectrometric detection was conducted by multiple reaction monitoring (MRM) mode via an electrospray ionization source in the positive mode. The calibration curves of all analytes showed good linear (r2 > 0.9800). The intra-day and inter-day precisions were less than 15% and the accuracies were within ±15%. The extraction recoveries conformed to the acceptable range. And there was no interference of endogenous substances in the sensitive assay method. All analytes were proven to be stable during sample storage and analysis procedures. The pharmacokinetic study indicated that the Yuanhuzhitong tablets could get a better absorption than Corydalis yanhusuo W.T. Wang.
Keywords: ultra-high performance liquid chromatography-electrospray ionization-tandem mass spectrometry, Corydalis yanhusuo W.T. Wang, Yuanhuzhitong tablets, pharmacokinetics
1. Introduction
Yuanhuzhitong tablet (YHZT), formally incorporated into Chinese Pharmacopoeia and Chinese National Essential Medicine List, consists of two herbs including 223 g of Radix Angelicae dahuricae and 445 g of vinegar-processed Corydalis yanhusuo W.T. Wang (C. yanhusuo) [1]. As a classic Chinese Patent Drug, YHZT has been frequently applied to alleviate various pains including hypochondriac pain, headache, stomachache and dysmenorrhea [1]. Chemically, alkaloids and coumarins are the main active constituents of YHZT, which possess a variety of pharmacological actions, such as analgesia, spasmolysis, anti-inflammatory, antianxiety and vasodilatation [2]. The vinegar-quenching processing technology has been utilized for the extraction of the active ingredient of C. yanhusuo due to a better solubility and to increase the curative effect [3]. The processing method of vinegar C. yanhusuo has a long application history in traditional Chinese medicines (TCM). Previous study showed that the alkaloid content of C. yanhusuo was higher with vinegar in its decoction process than that without it [4]. TCM is often composed of two or more herbs to obtain synergistic effects or to reduce possible adverse reactions [5,6,7]. Clinical practice and previous pharmacological studies have proven that Radix Angelicae dahuricae could enhance the analgesic effect of C. yanhusuo [8].
C. yanhusuo, which is also called yuanhu in China, is a key constituent herb in YHZT. It is one of the most significant dried medicinal herbs used in TCM [9]. C. yanhusuo was recorded in the Pharmacopoeia of the People’s Republic of China in 2015 [1]. It has been used for centuries for analgesic, sedation, anti-arrhythmic. It has been studied more and more nowadays since the recent discovery of its cancer pain relief effect [10,11,12,13,14]. A number of its constituents have been isolated and reported, including alkaloids, aliphatic acid, ecdysterone, etc [15]. Alkaloids were identified as the main effective components in C. yanhusuo according to the pharmacological study results. Meanwhile, tetrahydropalmatine is the one alkaloid that has been selected as a phytochemical marker for the quality control of C. yanhusuo in the Chinese Pharmacopoeia [1]. Tetrahydropalmatine, corydaline, tetrahydrobeberine and berberine have a significant analgesic effect [16,17]. Dehydrocorydaline and coptisine have the effect of anti-inflammatory and anti-coronary heart disease [18]. Tetrahydrobeberine have protection on cardiac cerebral vessel and antipsychotic effects [19]. Prptopine exhibits significant antihepatotoxic effects [20]. Palmatine, berberine and berberrubine are reported to possess antibacterial activity [21].
For a better understanding of pharmacokinetic behavior of alkaloids in the animal and human body, a sensitive analytical method needs to be established to quantitatively analyze the alkaloids in C. yanhusuo and YHZT. Several methods have been reported, including high-performance liquid chromatography with ultra-violet detection (HPLC-UV) [22,23], high-performance liquid chromatography with DAD detection (HPLC-DAD) [24], and high-performance liquid chromatography electrospray ionization-tandem mass spectrometry (HPLC-ESI-MS/MS) [25] for quantitative analysis of alkaloids in C. yanhusuo extract. The effect of the co-administration of C. yanhusuo and Radix Angelicae dahuricae on pharmacokinetic behavior of tetrahydropalmatine was investigated and the result showed that the plasma concentration of tetrahydropalmatine was higher in the combination pair group than that in the C. yanhusuo single herb group [26]. Many pharmacokinetic studies have been reported about the alkaloids from C. yanhusuo extract in rat plasma [27,28,29].
In this article, a new UHPLC-ESI-MS/MS method has been developed for simultaneous analysis of 10 alkaloids in beagle dog plasma after the oral administration of C. yanhusuo extraction and YHZT. It is the first article that analyse 10 alkaloids from C. yanhuso in biological fluids in a single run. According to the calculation, the dosage of C. yanhusuo extract and YHZT for beagle dog could be confirmed accurately. In the fasting state, the beagle dogs were given the equal amount of the two medicines. The absorption of alkaloids in beagle dog plasma was compared and their pharmacokinetic characteristics were elucidated, which would hopefully establish the basic groundwork for the further study and clinical application of C. yanhusuo and YHZT.
2. Results
2.1. Optimization of UHPLC-ESI-MS/MS Condition
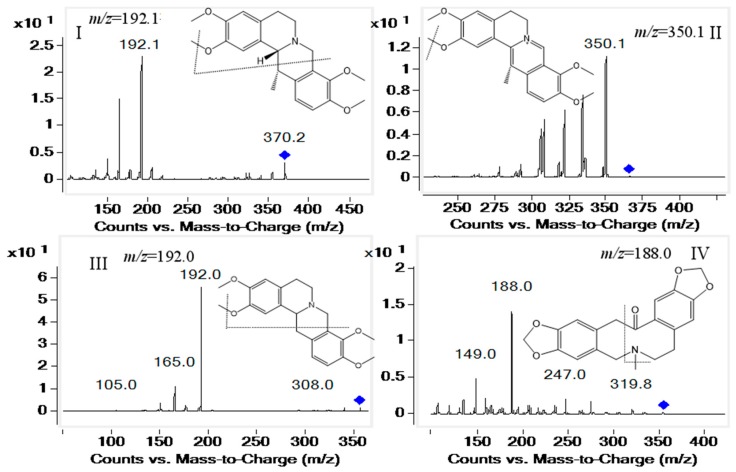
In present study, a sensitive and rapid UHPLC-ESI-MS/MS method was established to simultaneously quantify 10 alkaloids in beagle dog plasma. The alkaloids can be analysed by positive ESI mass spectrometry. Consequently, ESI operated in positive mode was preferentially selected as the ionization source for the compounds. Owing to the fact biological samples are complex, the MRM mode was selected to increase the specificity of the detection method. As Figure 1 shows, the intense peaks were observed at m/z 370.2→192.1 for corydaline (I), m/z 366.1→350.1 for dehydrocorydaline (II), m/z 356.0→192.0 for tetrahydropalmatine (III), m/z 354.1→188.0 for protopine (IV), m/z 352.1→336.2 for palmatine (V), m/z 340.1→176.1 for tetrahydroberberine (VI), m/z 339.2→323.2 for columbamine (VII), m/z 336.2→320.1 for berberine (VIII), m/z 320.2→292.2 for coptisine (IX), m/z 322.2→307.2 for berberrubine (X) and m/z 181.2→124.0 for theophyline (I.S.), respectively. The mobile phases consisting of 0.1% formic acid/water (A) and acetonitrile (B) could achieved a satisfactory separation effect. The flow rate was 0.3 mL/min and the column temperature was 35 °C Under the selected conditions, all compounds were separated rapidly in 10.5 min without interference from other components in beagle dog plasma.
Figure 1.
Product ion mass spectot of corydaline (I), dehydrocorydaline (II), tetrahydropalmatine (III), protopine (IV), palmatine (V), tetrahydroberberine (VI), columbamine (VII), berberine (VIII), coptisine (IX), berberrubine (X) and theophyline (I.S.) in the postive mode.
2.2. Method Validation
2.2.1. Selectivity
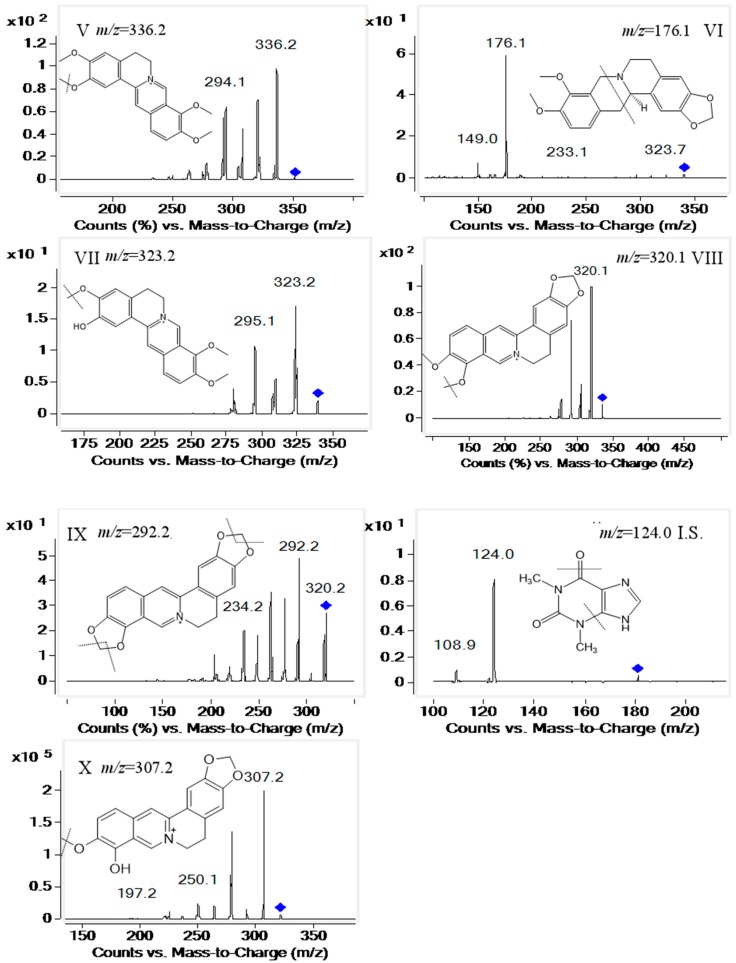
The selectivity of the method was evaluated by analyzing drug-free beagle dog plasma sample, a beagle dog plasma sample taken at 0.75 h after administration of 0.150 g/kg Yuanhuzhitong Tablets, a beagle dog plasma sample taken at 0.75 h after oral administration of 0.048 g/kg C. yanhusuo extraction and LLOQ sample (10 alkaloids and I.S. spiked in blank plasma). The retention time of I-X was 6.57 min, 9.24 min, 5.67 min, 4.38 min, 8.84 min, 6.28 min, 7.10 min, 8.26 min, 8.13 min and 5.58 min, respectively. The retention time of I.S. was 1.52 min. The results are shown in Figure 2. In the selectivity test, there was no endogenous interference in the blank plasma samples at the mass transitions of the alkaloids at the same retention times.
Figure 2.
Representative MRM chromatograms of corydaline (I), dehydrocorydaline (II), tetrahydropalmatine (III), protopine (IV), palmatine (V), tetrahydroberberine (VI), columbamine (VII), berberine (VIII), berberrubine (IX), coptisine (X) and theophyline (I.S.) in the beagle dog plasma: (1) drug-free beagle dog plasma sample; (2) A beagle dog plasma sample taken 0.75 h after administration of 0.150 g/kg Yuanhuzhitong Tablets; (3) A beagle dog plasma sample taken 0.75 h after administration of 0.048 g/kg C. yanhusuo extraction; (4) LLOQ sample (10 alkaloids and I.S. in blank plasma).
2.2.2. Linearity and LLOQs
Table 1 shows the regression equations, linear ranges, and LLOQ for the determination of the alkaloids in beagle dog plasma. All 10 alkaloids exhibited good linearity, and all r2 of the linear equations were higher than 0.9800. The linear calibration ranges were 0.54–557.0 ng/mL for I, 0.25–257.5 ng/mL for II, 0.50–507.0 ng/mL for III, 0.53–540.0 ng/mL for IV, 0.51–525.0 ng/mL for V, 0.41–420.0 ng/mL for VI, 0.39–400.0 ng/mL for VII, 0.53–540.0 ng/mL for VIII, 0.49–500.0 ng/mL for IX and 0.22–230.0 ng/mL for X, respectively. Accordingly, the concentrations can be determined by linear regression within the calibration range, which are sufficient for a pharmacokinetic study of 10 constituents following oral administration of C. yanhusuo and YHZT, respectively.
Table 1.
The regression equations, linear ranges, and LLOQ for the determination of the alkaloids in the beagle dog plasma. (n = 7).
| Compound | Regression Equation | r 2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| corydaline | Y = 1.395 × 10−3X + 1.874 × 10−3 | 0.9938 | 0.54–557.0 | 0.54 |
| dehydrocorydaline | Y = 9.668 × 10−4X + 3.035 × 10−3 | 0.9912 | 0.25–257.5 | 0.25 |
| tetrahydropalmatine | Y = 1.368 × 10−3X − 2.608 × 10−4 | 0.9810 | 0.50–507.0 | 0.50 |
| protopine | Y = 4.086 × 10−4X − 3.221 × 10−5 | 0.9870 | 0.53–540.0 | 0.53 |
| palmatine | Y = 4.409 × 10−4X − 7.801 × 10−5 | 0.9868 | 0.51–525.0 | 0.51 |
| tetrahydroberberine | Y = 6.810 × 10−4X + 1.381 × 10−4 | 0.9801 | 0.41–420.0 | 0.41 |
| columbamine | Y = 4.941 × 10-4X − 1.679 × 10−4 | 0.9936 | 0.39–400.0 | 0.39 |
| berberine | Y = 1.667 × 10−3X + 6.950 × 10−3 | 0.9888 | 0.53–540.0 | 0.53 |
| coptisine | Y = 3.263 × 10−4X + 5.393 × 10−3 | 0.9934 | 0.49–500.0 | 0.49 |
| berberrubine | Y = 1.623 × 102X − 2.685 × 10−3 | 0.9816 | 0.22–230.0 | 0.22 |
2.2.3. Precision and Accuracy
The accuracy and precision experiments were performed with three levels of QCs (HQC, MQC, LQC) and LLOQ. The results indicated that the method was reliable and reproducible for the determination of beagle dog plasma samples. The results are listed in Table 2. The RSD of intra-day and inter-day precision were within 15%, and the accuracy within reasonable range. These results conformed to the requirements that defined by regulatory guidelines.
Table 2.
Precision and accuracy of the determination of the 10 alkaloids in the beagle dog plasma (n = 18, six replicates per day for three days).
| Compound | Spiked Concentration (ng/mL) | Measured Concentration (ng/mL) | Accuracy (RE%) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) |
|---|---|---|---|---|---|
| corydaline | 0.5 | 0.53 ± 0.03 | −7.50 | 10.90 | 6.00 |
| 1.1 | 0.96 ± 0.07 | −10.75 | 8.22 | 2.53 | |
| 34.8 | 32.87 ± 1.69 | −5.56 | 5.18 | 4.82 | |
| 445.6 | 432.80 ± 18.00 | −2.86 | 3.99 | 5.24 | |
| dehydrocorydaline | 0.3 | 0.21 ± 0.04 | −12.10 | 12.10 | 12.40 |
| 0.5 | 0.43 ± 0.05 | −4.95 | 4.43 | 8.30 | |
| 16.1 | 14.59 ± 1.45 | −9.34 | 9.94 | 10.13 | |
| 206.0 | 196.20 ± 8.40 | −4.75 | 3.81 | 6.79 | |
| tetrahydropalmatine | 0.5 | 0.45 ± 0.03 | −6.90 | 8.80 | 12.10 |
| 1.0 | 0.93 ± 0.05 | −5.82 | 5.00 | 7.95 | |
| 31.7 | 30.04 ± 1.50 | −5.18 | 5.19 | 2.72 | |
| 405.6 | 401.50 ± 20.26 | −3.84 | 4.15 | 4.22 | |
| protopine | 0.5 | 0.48 ± 0.02 | −11.90 | 9.20 | 8.80 |
| 1.1 | 0.94 ± 0.04 | −5.96 | 4.97 | 1.53 | |
| 33.8 | 31.54 ± 0.88 | −6.56 | 2.76 | 2.96 | |
| 432.0 | 403.30 ± 14.58 | −6.64 | 3.61 | 3.64 | |
| palmatine | 0.5 | 0.47 ± 0.02 | −9.60 | 7.00 | 12.00 |
| 1.0 | 0.91 ± 0.11 | −12.32 | 10.51 | 7.09 | |
| 32.8 | 30.56 ± 1.48 | −6.83 | 5.02 | 3.42 | |
| 420.0 | 401.90 ± 12.13 | −4.29 | 2.94 | 3.54 | |
| tetrahydroberberine | 0.4 | 0.39 ± 0.03 | −12.90 | 7.79 | 7.30 |
| 0.8 | 0.78 ± 0.06 | −4.46 | 7.57 | 4.67 | |
| 26.3 | 24.17 ± 1.80 | −7.92 | 7.66 | 5.50 | |
| 336.0 | 306.40 ± 24.86 | −8.81 | 8.02 | 8.80 | |
| columbamine | 0.4 | 0.38 ± 0.02 | −10.50 | 11.40 | 13.70 |
| 0.8 | 0.76 ± 0.04 | −3.19 | 5.04 | 4.61 | |
| 25.0 | 24.02 ± 1.41 | −3.92 | 6.19 | 2.35 | |
| 320.0 | 295.20 ± 7.17 | −7.74 | 2.16 | 3.91 | |
| berberine | 0.5 | 0.42 ± 0.05 | −14.40 | 11.20 | 14.90 |
| 1.1 | 0.87 ± 0.09 | −13.22 | 10.66 | 3.28 | |
| 33.8 | 32.04 ± 0.74 | −5.07 | 2.40 | 1.35 | |
| 432.0 | 411.60 ± 17.07 | −4.73 | 4.22 | 3.60 | |
| coptisine | 0.5 | 0.47 ± 0.04 | −5.90 | 8.80 | 13.40 |
| 1.0 | 0.92 ± 0.06 | −7.71 | 6.13 | 7.29 | |
| 31.3 | 30.28 ± 0.89 | −3.10 | 2.78 | 3.97 | |
| 400.0 | 389.40 ± 9.20 | −2.66 | 2.30 | 2.78 | |
| berberrubine | 0.2 | 0.18 ± 0.04 | −16.09 | 19.40 | 18.20 |
| 0.5 | 0.41 ± 0.02 | −8.70 | 5.93 | 6.70 | |
| 14.4 | 13.48 ± 1.15 | −6.18 | 7.89 | 12.47 | |
| 184.0 | 185.50 ± 10.41 | 0.83 | 5.15 | 8.30 |
2.2.4. Extraction Recovery and I.S.-Normalized Matrix Factor
The results of extraction recovery was shown in Table 3. The selected extraction solvent had a good extraction efficiency. The mean recoveries of 10 alkaloids were in the range of 79.09–98.55% at the concentrations of LQC, MQC, HQC samples and the mean recovery of the I.S. was 90.41 ± 6.24%. The IS-normalized matrix factor of QC samples were showed in Table 3. These results illustrated that the developed method had no matrix effect and was reliable for bioanalysis.
Table 3.
IS-normalized matrix factor and extraction recovery for the 10 alkaloids in the beagle dog plasma. (n = 6).
| Compound | Spiked Concentration (ng/mL) | IS-Normalized Matrix Factor | Extraction Recovery | |
|---|---|---|---|---|
| Mean (%) | RSD (%) | |||
| corydaline | 1.1 | 0.91 | 79.36 | 9.903 |
| 34.8 | 0.97 | 85.91 | 6.930 | |
| 445.6 | 1.00 | 79.11 | 9.320 | |
| dehydrocorydaline | 0.5 | 1.00 | 84.12 | 8.781 |
| 16.1 | 1.04 | 88.02 | 8.180 | |
| 206.0 | 1.14 | 86.84 | 5.280 | |
| tetrahydropalmatine | 1.0 | 0.94 | 90.72 | 9.730 |
| 31.7 | 0.98 | 87.25 | 12.68 | |
| 507.0 | 1.01 | 87.96 | 10.15 | |
| protopine | 1.1 | 1.15 | 84.33 | 12.67 |
| 33.8 | 1.08 | 91.22 | 13.75 | |
| 432.0 | 1.09 | 83.46 | 4.350 | |
| palmatine | 1.0 | 0.94 | 91.21 | 11.01 |
| 32.8 | 0.87 | 87.29 | 7.730 | |
| 420.0 | 0.74 | 84.82 | 7.230 | |
| tetrahydroberberine | 0.8 | 0.89 | 89.17 | 12.30 |
| 26.3 | 0.88 | 88.48 | 5.243 | |
| 336.0 | 0.92 | 93.00 | 8.870 | |
| columbamine | 0.8 | 0.96 | 96.58 | 11.31 |
| 25.0 | 0.91 | 91.61 | 11.58 | |
| 320.0 | 0.94 | 84.66 | 5.910 | |
| berberine | 1.1 | 0.90 | 90.06 | 6.380 |
| 33.8 | 0.94 | 84.58 | 5.520 | |
| 432.0 | 0.97 | 87.94 | 7.270 | |
| coptisine | 1.0 | 0.95 | 91.75 | 9.640 |
| 31.3 | 0.88 | 88.93 | 7.880 | |
| 400.0 | 0.95 | 85.60 | 6.890 | |
| berberrubine | 0.5 | 0.94 | 88.41 | 7.790 |
| 14.4 | 0.97 | 77.09 | 12.86 | |
| 184.0 | 0.91 | 98.55 | 5.000 | |
2.2.5. Stability
The stability of the 10 alkaloids in beagle dog plasma was investigated under a variety of storage and process conditions. Table 4 summarizes the results of freeze and thaw, long-term, room temperature, and post-preparation stability experiments (The QC samples were kept in the auto-sampler at 4 °C for 12 h.). The measured concentrations were all within acceptable limits (±15% of the nominal concentrations) during the entire validation. The results of stability indicated that the 10 alkaloids were stable under conditions assessed in this method.
Table 4.
Stability data of 10 alkaloids in the beagle dog plasma. (n = 6).
| Compound | Spiked Concentration (ng/mL) | Stability (% RE a) | |||
|---|---|---|---|---|---|
| Short-Term | Long-Term | Three Freeze-Thaw | Post-Preparation | ||
| corydaline | 1.1 | 3.86 | 4.90 | −2.72 | 2.30 |
| 34.8 | −4.30 | −4.52 | −4.83 | −4.36 | |
| 445.6 | −1.88 | −4.45 | −3.63 | −4.56 | |
| dehydrocorydaline | 0.5 | 8.52 | 2.85 | 3.90 | 3.87 |
| 16.1 | 4.15 | 5.16 | 10.41 | 13.40 | |
| 206.0 | 3.50 | 2.97 | 1.85 | 1.05 | |
| tetrahydropalmatine | 1.0 | −4.16 | −4.47 | −4.77 | −4.36 |
| 31.7 | −1.92 | −4.85 | −4.76 | 2.09 | |
| 507.0 | −2.69 | −3.38 | −3.36 | −4.54 | |
| protopine | 1.1 | −4.47 | −4.48 | −6.91 | −4.40 |
| 33.8 | −2.86 | −4.78 | −3.96 | −3.63 | |
| 432.0 | −4.96 | −4.93 | −4.38 | −3.92 | |
| palmatine | 1.0 | −4.06 | 2.25 | −2.08 | −4.98 |
| 32.8 | −3.35 | −4.59 | −3.35 | −3.35 | |
| 420.0 | −3.83 | −3.95 | −2.02 | −2.78 | |
| tetrahydroberberine | 0.8 | −2.54 | −2.59 | −4.69 | −3.54 |
| 26.3 | −3.38 | −4.95 | −3.22 | −4.74 | |
| 336.0 | −3.32 | −3.04 | −3.92 | −4.51 | |
| columbamine | 0.8 | −3.44 | −3.46 | −3.06 | −1.75 |
| 25.0 | −2.54 | −3.94 | −4.49 | −3.14 | |
| 320.0 | −3.99 | −4.88 | −4.29 | −4.42 | |
| berberine | 1.1 | −4.14 | −4.51 | −3.91 | −3.47 |
| 33.8 | −1.87 | −4.50 | −4.62 | −4.47 | |
| 432.0 | −2.88 | −4.28 | −3.62 | −4.08 | |
| coptisine | 1.0 | −5.42 | −4.76 | −3.90 | −1.88 |
| 31.3 | −3.34 | −4.31 | −1.37 | −4.31 | |
| 400.0 | −4.14 | −2.34 | −2.09 | −4.09 | |
| berberrubine | 0.5 | −12.10 | −6.88 | −7.75 | −11.52 |
| 14.4 | 10.68 | −3.02 | −13.61 | −5.61 | |
| 184.0 | 1.39 | −2.87 | 1.67 | 0.89 | |
a RE is expressed as (measured concentration/freshly prepared concentration−1) × 100%.
2.3. Pharmacokinetic Studies
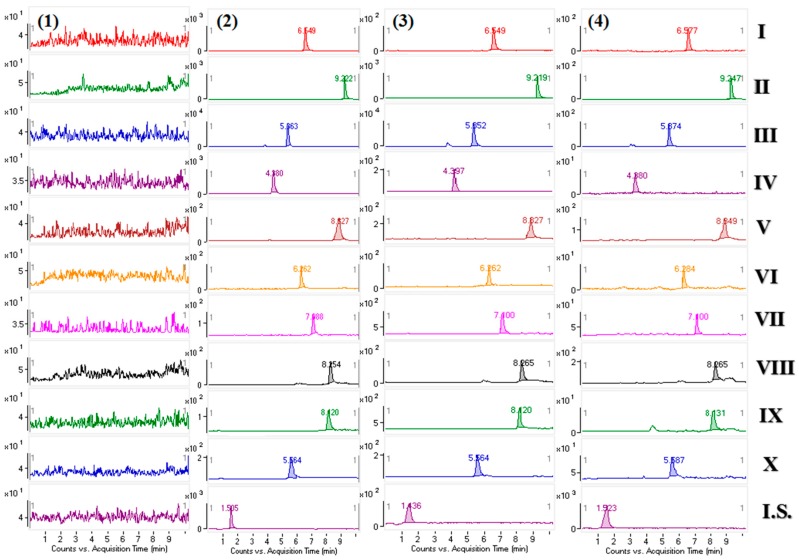
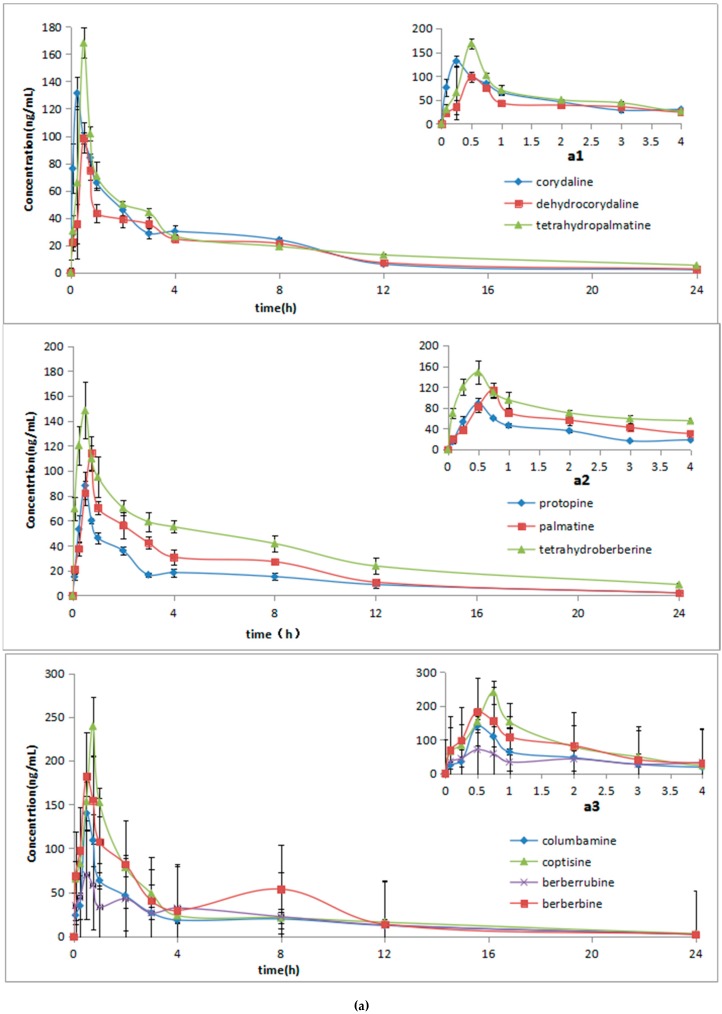
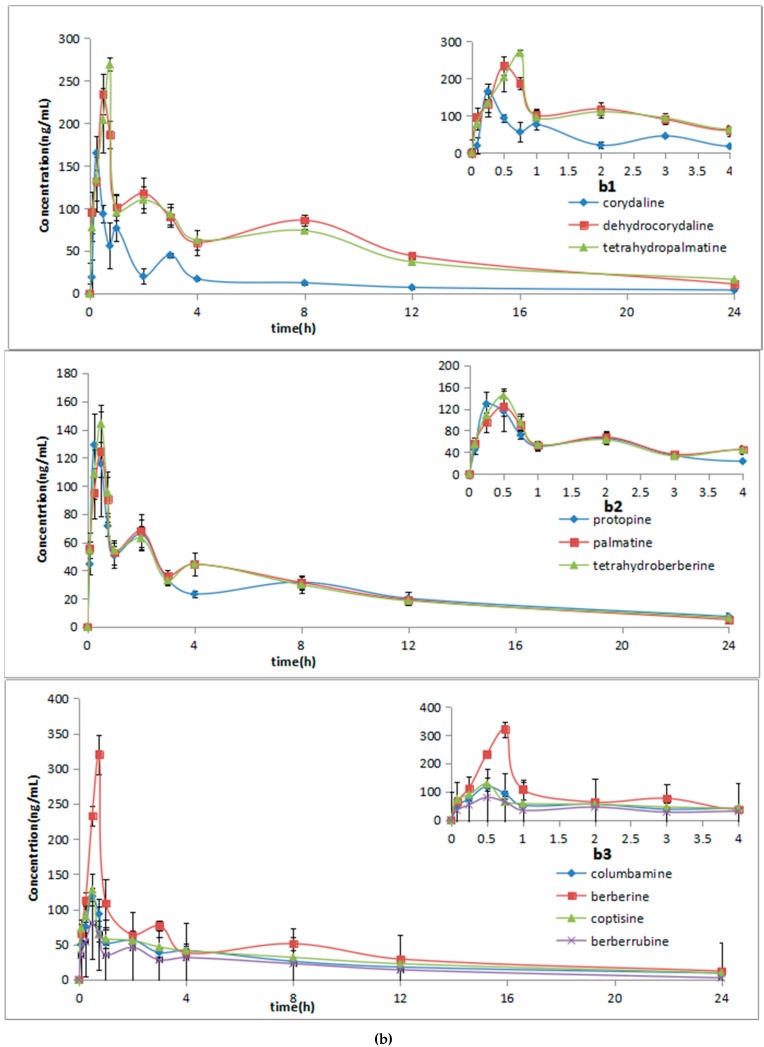
An UHPLC-ESI-MS/MS method for simultaneous determination of 10 alkaloids of C. yanhusuo extract and YHZT in beagle dog plasma was applied to the plasma sample analysis in the pharmacokinetic study. After oral administration, the main plasma concentration-time profiles of the 10 alkaloids were showed in Figure 3. Pharmacokinetic information of palmatine, tetrahydroberberine, columbamine, coptisine and berberrubine from C. yanhusuo extraction and YHZT were firstly obtained from beagle plasma. The concentrations versus time profiles were analyzed by a non-compartmental model. The results are listed in Table 5. The pharmacokinetic study indicated that the YHZT can get a better satisfactory absorbtion than C. yanhusuo.
Figure 3.
The mean ± SD plasma concentration-time profiles of the 10 alkaloids in the beagle dog plasma after oral administration of C. yanhusuo extract (a) and Yuanhuzhitong Tablets (b), respectively. The mean ± SD plasma concentration-time profiles of the 10 alkaloids in the beagle dog plasma after oral administration of C. yanhusuo extract (a1,a2,a3) and Yuanhuzhitong Tablets (b1,b2,b3) from 0 to 4 h, respectively (n = 6).
Table 5.
Pharmacokinetic parameters of the 10 alkaloids in the beagle dog after oral administration of C. yanhusuo (A) and YHZT (B), respectively. (mean ± SD, n = 6).
| Compounds | Cmax (ng/mL) | Tmax (h) | t1/2 (h) | AUC0→t (ng·h/mL) | AUC0→∞ (ng·h/mL) | |
|---|---|---|---|---|---|---|
| (A) | corydaline | 138 ± 24.5 | 0.29 ± 0.10 | 4.90 ± 0.70 | 358.34 ± 39.02 | 401.61 ± 48.38 |
| dehydrocorydaline | 99.7 ± 12.3 | 0.54 ± 0.10 | 5.83 ± 1.75 | 342.28 ± 9.30 | 403.19 ± 30.18 | |
| tetrahydropalmatine | 169 ± 55.3 | 0.54 ± 0.10 | 9.09 ± 2.17 | 424.21 ± 20.94 | 591.20 ± 50.58 | |
| protopine | 89.4 ± 8.49 | 0.95 ± 0.13 | 5.75 ± 0.81 | 275.69 ± 18.43 | 349.18 ± 38.33 | |
| palmatine | 116 ± 13.9 | 0.95 ± 051 | 4.45 ± 0.71 | 397.05 ± 30.16 | 465.14 ± 34.69 | |
| tetrahydroberberine | 154 ± 24.9 | 0.50 ± 0.16 | 7.06 ± 1.35 | 711.63 ± 56.12 | 1044.40 ± 68.18 | |
| columbamine | 144 ± 11.01 | 0.54 ± 0.10 | 5.67 ± 1.95 | 404.21 ± 15.57 | 506.82 ± 40.75 | |
| berberine | 187 ± 23.4 | 0.54 ± 0.10 | 5.00 ± 0.90 | 593.56 ± 23.76 | 673.64 ± 29.62 | |
| coptisine | 242 ± 58.2 | 0.79 ± 0.10 | 5.89 ± 1.07 | 631.90 ± 86.03 | 748.34 ± 68.39 | |
| berberrubine | 72.1 ± 1.9 | 0.54 ± 0.10 | 5.01 ± 0.61 | 422.88 ± 23.26 | 440.26 ± 19.33 | |
| (B) | corydaline | 165 ± 21.3 * | 0.29 ± 0.10 | 11.24 ± 1.49 | 361.474 ± 43.83 | 435.90 ± 58.28 |
| dehydrocorydaline | 187 ± 23.8 * | 0.54 ± 0.10 | 5.61 ± 1.05 | 1306.88 ± 148.77 * | 1399.25 ± 142.40 * | |
| tetrahydropalmatine | 277 ± 23.7 * | 0.71 ± 0.10 | 9.34 ± 0.26 | 1268.11 ± 61.64 * | 1462.37 ± 98.46 * | |
| protopine | 139 ± 24.2 * | 0.38 ± 0.14 | 7.77 ± 0.81 | 601.27 ± 66.95 * | 683.25 ± 67.68 * | |
| palmatine | 127 ± 14.6 | 0.50 ± 0.16 | 6.09 ± 0.66 | 638.87 ± 43.52 * | 684.56± 60.57 * | |
| tetrahydroberberine | 145 ± 12.0 | 0.54 ± 0.10 | 7.65 ± 1.14 | 637.16 ± 59.08 | 718.80 ± 98.29 | |
| columbamine | 120 ± 83 * | 0.54 ± 0.10 | 11.73 ± 2.92 | 611.24 ± 53.13 * | 755.66 ± 95.47 * | |
| berberine | ± 28.0 * | 0.71 ± 0.10 | 8.48 ± 1.92 | 988.42 ± 116.18 * | 1140.97 ± 91.92 * | |
| coptisine | 133 ± 15.1 * | 0.46 ± 0.10 | 9.58 ± 1.81 | 685.60 ± 60.34 | 824.82 ± 106.82 | |
| berberrubine | 86.0 ± 6.7 * | 0.54 ± 0.10 | 5.05 ± 0.48 | 445.44 ± 27.44 | 464.11 ± 25.40 |
The Cmax value, AUC0→t and AUC0→∞ compared with C. yanhusuo group * p < 0.05.
3. Discussion
3.1. Selection of Extraction Method
Choosing an appropriate sample preparation method is the key to simultaneously and accurately analyzing the target compound [24]. Liquid–liquid extraction (LLE) and protein precipitation (PPT) are the most frequently used methods for sample preparations. PPT is evaluated for its convenient operation and high recovery rate, but it is not suitable for the low content of the analytes. Ethyl acetate, ether, dichloromethane and acetone were tested as the extraction solvent, the results showed that 3 mL ethyl acetate could achieve the aim of both high extraction recovery and weak matrix factor. The remaining extraction solvents could not achieve a satisfactory effect.
3.2. Selection of I.S.
It was critical to select an appropriate I.S. for the analysis of the whole biological samples. During the selection of I.S., biphenyl diester was found to possess a weak polarity and a long retention time under this mobile phase. The polarity of theophylline is similar to the alkaloid components, which could effectively shorten the analysis time. In addition, the result showed that theophylline had no direct interference in the analysis.
3.3. Pharmacokinetic Studies
In this article, a new UHPLC-ESI-MS/MS method was developed for simultaneous determination of 10 alkaloids in beagle dog plasma after the oral administration of C. yanhusuo extraction and YHZT. YHZT is composed of two herbs including Radix Angelicae dahuricae and Corydalis yanhusuo W.T. Wang (vinegar-process). Through the comparison of the above two groups, the following conclusions can be obtained. Firstly, the tmax of the two groups was shorter than 1h, which could be attributed that the active ingredient of C. yanhusuo and YHZT known as alkaloids belonging to quaternary ammonium alkaloids, are all dissolved easily in blood. It also showed that these alkaloids entered the blood through the absorption in stomach [30]. Secondly, we can see the Cmax of corydaline, dehydrocorydaline, tetrahydropalmatine, protopine, berberine and berberruine in YHZT group were higher remarkably than those in the C. yanhusuo group (p < 0.05). The explanation could be that the extraction rate of alkaloids in YHZT group was higher than that in C. yanhusuo group due to the processing method with vinegar as mentioned in Introduction. Thirdly, the AUC0→∞ and AUC0→t of dehydrocorydaline, tetrahydropalmatine, protopine, palmatine, columbamine and berberine in YHZT group are higher remarkably than that of the C. yanhusuo group (p < 0.05). It indicated that the bioavailability of YHZT group was higher than that of the C. yanhusuo group. Moreover, the t1/2 of corydaline, protopine, columbamine, berberine and coptisine in YHZT group is longer than these in the C. yanhusuo group. It indicated that the elimination time of YHZT is longer than C. yanhusuo. Thus, it had a guidance for clinical medication. Finally, following oral administration of C. yanhusuo extraction and YHZT, multiple-peaks phenomenon was observed on the mean plasma concentration curves of 10 analytes, and this phenomenon could be attributed to distribution, re-absorption and enterohepatic circulation [31].
4. Materials and Methods
4.1. Materials
Corydaline (151118), dehydrocorydaline (16051707), tetrahydropalmatine (151123), protopine (17062106), palmatine (140821), tetrahydroberberine (151111), columbamine (141108), berberine (141128), coptisine (140430), berberrubine (140407) and theophyline (17011102) with over 98% purity were purchased from Chengdu Pufei De Biotech Co. Ltd. (Chengdu, Sichuan Province, China). HPLC grade methanol and acetonitrile were purchased from Dikma Technologies Inc (Beijing, China). All other reagents including ethyl acetate, ether, dichloromethane and acetone belonged to analytical grade. Ultra-pure water was prepared by using a Milli-Q water purification system (Millipore, Molsheim, France). The blank plasma samples were achieved from the blood of healthy beagle dogs. Yuanhuzhitong tablet (20151201) was purchased from Sunflower Pharmaceutical Industry (Harbin, China). Corydalis yanhusuo W.T. Wang was obtained from the Panan Traditional Chinese Crude Drug Market, located in Zhejiang Province, China, and it was identified by Professor Zhenyue Wang of Heilongjiang University of Chinese Medicine in May, 2017.
4.2. UHPLC-ESI-MS/MS Conditions
The UHPLC-ESI-MS/MS was performed on an Agilent 1290 ultra-high performance liquid chromatography (UHPLC) system and an Agilent 6430 QQQ-MS mass spectrometer with an electrospray ionization (ESI) source interface (Agilent Technologies, Santa Clara, CA, USA). The UHPLC-ESI-MS/MS was performed on an Agilent Eclipse Plus C18 RRHD column (1.8 µm, 50 × 2.1 mm). In order to obtain the maximum sensitivity of the MRM, parameters such as fragmentor, collision energy and the nitrogen flow rate were optimized. The results showed in the Table 6. The mobile phases used 0.1% formic acid/water (A) and acetonitrile (B) as follows: 0–7.5 min, 22–32% (B); 7.5–8.5 min, 32–43% (B); 8.5–10.5 min, 43–55% (B). The flow rate was 0.3 mL/min. The column temperature was 35 °C. 5 μL sample solution was injected with a needle wash process. High-purity N2 was used as the nebulizing gas, and N2 was used as drying gas at a flow rate of 11 L/min. The mass spectrometer was operated in positive-ion mode at a capillary voltage of 4500 V. The source temperature was kept at 100 °C. The desolvation temperature was kept at 350 °C.
Table 6.
Quantitative, Qualifier ions and MS parameters of eight alkaloids and I.S.
| Compound | Ion Pair (m/z) | Qualifier Ion (m/z) | Fragment (V) | CE (V) | Polarity |
|---|---|---|---|---|---|
| corydaline | 370.2→192.1 | 165.1 | 170 | 30 | Positive |
| dehydrocorydaline | 366.1→350.1 | 334.1 | 140 | 30 | Positive |
| tetrahydropalmatine | 356.0→192.0 | 165.0 | 159 | 27 | Positive |
| protopine | 354.1→188.0 | 149.0 | 170 | 30 | Positive |
| palmatine | 352.1→336.2 | 294.1 | 155 | 40 | Positive |
| tetrahydroberberine | 340.1→176.1 | 149.0 | 168 | 40 | Positive |
| columbamine | 339.2→323.2 | 295.1 | 160 | 29 | Positive |
| berberine | 336.2→320.1 | 292.2 | 136 | 30 | Positive |
| coptisine | 320.2→292.2 | 292.2 | 167 | 29 | Positive |
| berberrubine | 322.2→307.2 | 250.1 | 160 | 29 | Positive |
| theophyline (I.S.) | 181.2→124.0 | 108.9 | 120 | 14 | Positive |
The cell accelerator volltage value of the compounds are 5V.
4.3. Preparation of Standard and QC Solutions
A stock standard solution with compound I–X mixing together was prepared in methanol with a concentration of 223.0, 206.0, 200.0, 113.0, 216.0, 200.0, 202.0, 201.0, 200.0 and 230.0 µg/mL, respectively. Theophyline (I.S.) was prepared with methanol at concentration of 1050.0 ng/mL. A series of calibration standards solutions were prepared at seven different concentration levels, I (557.0, 139.3, 34.8, 8.7, 2.2, 1.1 and 0.5 ng/mL), II (257.5, 64.4, 16.1, 4.0, 1.0, 0.5 and 0.3 ng/mL), III (507.0, 126.75, 31.68, 7.920, 1.980, 0.9902 and 0.4951 ng/mL ), IV (540.0, 135.0, 33.8, 8.5, 2.1, 1.1 and 0.5 ng/mL), V (525.0, 131.3, 32.8, 8.2, 2.1, 1.0 and 0.5 ng/mL), VI (420.0, 105.0, 26.3, 6.6, 1.6, 0.8 and 0.4 ng/mL), VII (400.0, 100.0, 25.0, 6.3, 1.6, 0.8 and 0.4 ng/mL), VIII (540.0, 135.0, 33.8, 8.4, 2.1, 1.1 and 0.5 ng/mL), IX (500.0, 125.0, 31.3, 7.8, 2.0, 1.0 and 0.5 ng/mL) and X (230.0, 57.5, 14.4, 3.6, 0.9, 0.4 and 0.2 ng/mL) respectively. Three different concentration levels of the QC samples were prepared for this assay, high QC (445.6/206.0/405.6/432.0/420.0/336.0/320.0/432.0/400.0/184.0 ng/mL), medium QC (34.8/16.1/31.7/33.8/32.8/26.3/25.0/33.8/31.3/14.4 ng/mL), low QC (1.1/0.5/1.0/1.1/1.0/0.9/0.8/1.0/1.0/0.4 ng/mL), and LLOQ (0.5/0.3/0.5/0.5/0.5/0.4/0.4/0.5/0.5/0.2 ng/mL), for I–X, respectively. All above samples were kept at 4 °C until use.
4.4. Preparation of C. yanhusuo Extract and YHZT
After soaking for 24 h, the C. yanhusuo was extracted twice with 60% ethanol (1:4, w/v) at 75 °C, 3 h for the first time and 2 h for the second time. The combined decoction was filtered and it was concentrated. Then, the paste rate is calculated. The contents of C. yanhusuo extract for I–X were 0.73, 1.50, 1.12, 0.92, 1.51, 1.03, 1.01, 0.81, 0.89 and 0.47 mg/g, respectively.
According to Chinese Pharmacopoeia, the dosage of C. yanhusuo medicinal materials for human is 10 g [1]. Specific surface area of human to dog equivalent dose ratio of body surface area conversion betwwen the human and dog is 0.32. The dosage of C. yanhusuo medicinal materials for dog is 0.27 g/kg. After a series of extraction and concentration operations, the extraction ratio of C. yanhusuo medicinal materials is 18%. Thus, the dosage of C. yanhusuo extract for beagle dog is 0.0486 g/kg.
The formulation illustration of YHZT was prescribed in the Chinese Pharmacopoeia [1]. 1000 Yuanhuzhitong tablets were made of 223 g of Radix Angelicae dahuricae and 445 g of vinegar-processed Corydalis yanhusuo W.T. Wang medicinal materials by a series of extraction and concentration operations. Each tablet was equivalent to 0.445 g C.yanhusuo medicinal materials. And according to Chinese Pharmacopoeia, the weight of the tablet core is 0.25 g [1]. Therefore, every gram YHZT powders was equivalent to 1.78 g C.yanhusuo medicinal materials. After taking off the Sugar coating, the tablets were lapped into powder. Then, the YHZT powder was weighed precisely. As mentioned above, the dosage of C. yanhusuo medicinal materials for beagle dog is 0.27 g/kg. It was equivalent to 0.15 g YHZT powders.
4.5. Biosample Preparation
A liquid-liquid extraction method was selected for the beagle dog plasma sample preparation. Fifty µL of internal standard solution (1050 ng/mL), 100 µL aliquot of plasma samples, and 100 µL of methanol were pipetted into a 10 mL glass tube. The mixture solution was vortex-mixed in glass tubes for 60 s. Then it was extracted with 3 mL ethyl acetate, vortex-mixed for 120 s, centrifuged at 877× g for 5 min. The organic layer was transferred into another spotless tube. Then, it was dried under a flow of nitrogen at 40 °C. The residue was re-dissolved by 100 µL mobile phase, vortex-mixed for 120 s, filtered by a 0.22 μm nylon 66 membrane. A 5 µL aliquot was injected into the UHPLC-ESI-MS/MS system.
The calibration standards and the QC samples were prepared under the same conditions as the plasma samples, which collected from the beagle dog after oral administration C. yanhusuo and YHZT powders. The spiked samples were handled by the following steps. 50 µL of internal standard solution (1050 ng/mL), 100 µL aliquot of blank plasma samples, and 100 µL of calibration standards or the QC methanol solution were pipetted into a 10 mL glass tube. And the mixture solution was vortex-mixed in glass tubes for 60 s. Then it was extracted with 3 mL ethyl acetate, vortex-mixed for 120 s, centrifuged at 877× g for 5 min. The organic layer was transferred into another spotless tube. Then, it was dried under a flow of nitrogen at 40 °C. The residue was re-dissolved by 100 µL mobile phase, vortex-mixed for 120 s, filtered by a 0.22 μm nylon66 membrane. The 5 µL samples was detected by the UHPLC-ESI-MS/MS system.
4.6. Method Validation
Validation procedures were accomplished by the US Food and Drug Administration (FDA) guidelines for bioanalytical method validation, http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf [32]. Parameters such as recovery, linearity, accuracy, precision, selectivity, matrix effect and stability of samples were investigated.
4.6.1. Selectivity
In order to rule out the interference from endogenous matrix compounds with 10 analytes and I.S., method specificity was determined in beagle dog plasma. Six different blank plasma were obtained from different beagle dog plasma, and spiked with the alkaloids and I.S. at LLOQ level, then analyzed by UHPLC-MS/MS system. The response of co-eluting interferences was evaluated by the comparison of the drug free plasma chromatograms, the blank plasma chromatograms which spiked with analytes and I.S., and the plasma samples from the beagle dogs after oral administration of the C. yanhusuo extract and YHZT.
4.6.2. Linearity and LLOQ
Each calibration curve was assessed by plotting the peak area ratio of the analytes and I.S. (Y) versus each nominal concentration (X) of the analytes. The calibration model was selected based on the analysis of the data by linear regression with weighting factors (1/x2). The LLOQ was determined as the lowest concentration point of the calibration curve with sufficient precision and the accuracy. The LLOQ could be quantified with S/N, which should be bigger than 10. Six replicate samples were used for evaluation.
4.6.3. Precision and Accuracy
The precision and accuracy of the intra-day and inter-day were determined by the analysis of LLOQ, LQC, MQC and HQC samples (n = 6) on the one day and on three different days, respectively. Accuracy was evaluated as the relative error (RE) of the measured mean value. And the precision was determined as the RSD of the measured concentration. The concentration of each sample was calculated via a calibration curve that constructed on the same day. The criteria for precision and accuracy of evaluation are as follows: the accuracy should be less than 15% of the actual value for QC samples and the RSD should no more than 15%. The RSD of LLOQ samples should not exceed 20%.
4.6.4. Extraction Recovery and I.S.-Normalized Matrix Factor
The extraction efficiency of the ten analytes was investigated by the comparison of six replicates beagle dog plasma samples at low, medium and high QC levels. The relative recoveries of the 10 analytes and the I.S. were determined by comparing the peak areas of an extracted sample against a post-extraction spiked sample and calculated by the ratio of the peak responses. The matrix factor was evaluated by comparing the absolute peak areas of blank matrix samples spiked after extraction with analytes to pure solution of the analytes. The I.S.-normalized matrix factor was calculated by the matrix factor ratio of the analyte to the internal standard.
4.6.5. Stability
The stability test included three freeze-thaw cycles stability (−40 to 23 °C), room temperature stability (storage for 4 h at ambient temperature), long-term sample storage stability (−20 °C for two weeks) and ready-to-injection of extracted sample stability (4 °C for 12 h). LQC, MQC, HQC samples with five replicates at each level were kept at the above conditions and analyzed against freshly prepared calibrators as the reference.
4.7. Application to Pharmacokinetic Studies
Animal experimental procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, and the study was approved by the Animal Ethics Committee of the Institution. Twelve healthy male beagle dogs (body weight 12 ± 2 kg) were obtained from Shenyang Kangping Institute of Medical Laboratory Animals (SCXK (Liao) 2014-0003). Then it was randomly divided into two groups. All experimental procedures were approved by the ethics committees of Harbin Medical University. The blood samples of beagle dog were achieved at specific time points after oral administration of C. yanhusuo extraction with a dose of 0.0486 g/kg and YHZT powders with a dose of 0.15 g/kg. The beagle dogs were fasted overnight before experiment, and had free water supply even during the experiment. For animals, after oral administration of C. yanhusuo extraction and YHZT powders, 0.5 mL blood samples were withdrawn from the forearm vein into heparinized tubes at 0, 0.083, 0.25, 0.5, 0.75, 1.0, 2.0, 3.0, 4.0, 8.0, 12.0 and 24.0 h. Blood samples were centrifuged at 4582× g for 6 min and the upper layer plasma were kept at −20 °C until detection.
4.8. Data Analysis
In order to determine the pharmacokinetics parameters of 10 alkaloids, all data were processed by non-compartmental analysis using the DAS 2.0 software package (Chinese Pharmacological Society, Shanghai, China). The plasma concentration at different times was expressed as mean ± SD, and the mean concentration-time curves were plotted. The corresponding pharmacokinetic parameters (the elimination half-life, t1/2; area under the peak area-time curve from time zero to last sampling time AUC0→t and AUC0→∞) were tested. The elimination rate constant (Ke) was calculated by linear regression of the terminal points in a semi-log plot of the plasma concentration against time. The elimination half-life was calculated using the formula t1/2 = 0.693/Ke. The area under plasma concentration-time curve (AUC0→t) to the last measurable plasma concentration (Ct) was estimated by using the linear trapezoidal rule. The area under the plasma concentration-time curve to time infinity (AUC0→∞) was calculated as AUC0→∞ = AUC0→t + Ct/Ke. Moreover, the maximum observed plasma concentration (Cmax) and the time of first occurrence of Cmax (time for maximal concentration, Tmax) were also determined.
5. Conclusions
In the present article, a reliable and precise UHPLC-ESI-MS/MS method has been developed for the simultaneous quantification of 10 alkaloids in beagle dog plasma after oral administration of C. yanhusuo extraction and YHZT. This paper indicated that the YHZT could get a better absorbtion than C. yanhusuo. It will lay the foundation for the future study of C. yanhusuo.
Author Contributions
All authors contributed to this work as follows: C.Y. conceived and designed the experiments; B.C. and J.Y. performed the experiments; B.C., J.Y., Z.W., C.W., H.D. and Y.R. analyzed the data; J.Y., Z.W., C.Y. contributed reagents/materials/analysis tools; B.C., J.Y. and C.Y. wrote the paper. C.Y. and Z.W. polished the paper. J.Y. and C.Y. acquired funding for the research. All authors approved the final manuscript.
Funding
This work was supported by The Scientific Research Project of National Natural Science Foundation of China (No.81573551, 81603418), Harbin Science and Technology Bureau (No. 2015RQQXJ077).
Conflicts of Interest
The authors have declared no conflicts of interest.
Footnotes
Sample Availability: Samples of the corydaline, dehydrocorydaline, tetrahydropalmatine, protopine, palmatine, tetrahydroberberine, columbamine, berberine, coptisine and berberrubine are available from the authors.
References
- 1.China Pharmacopoeia Committee . Pharmacopoeia of the People’s Prpublic of China. China Chemical Industry Press; Beijing, China: 2015. p. 139. [Google Scholar]
- 2.Zhang J., Jin Y., Dong J., Xiao Y.S., Feng J.T., Zhang X.L. Systematic screening and characterization of tertiary and quaternary alkaloids from corydalis yanhusuo W.T. Wang using ultra-performance liquid chromatography–quadrupoletime-of-flight mass spectrometry. Talanta. 2009;78:513–522. doi: 10.1016/j.talanta.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Ma Z.J., Li X.D., Gu X.Z., Cheng L.P., Mao S.J. Effects of different types and standard of processing vinegaron inherent constituents in rhizoma of Corydalis yanhusuo. China J. Chinese Mater. Med. 2006;31:465–467. [PubMed] [Google Scholar]
- 4.Wu H., Waldbauer K., Tang L., Xie L., McKinnon R., Yang H., Xu H., Kopp B. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo) Molecules. 2014;19:11487–11504. doi: 10.3390/molecules190811487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Q., Yue P.F., Wu B., Hu P.Y., Wu Z.F., Yang M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J. Ethnopharmacol. 2011;137:221–225. doi: 10.1016/j.jep.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Yang S.S., Zhang K.R., Lin X., Miao Y.Q., Meng L.K., Chen W., Tang X. Pharmacokinetic comparisons of single herb extract of Fufang Danshen preparation with different combinations of its constituent herbs in rats. Pharm. Biomed. Anal. 2012;67:77–85. doi: 10.1016/j.jpba.2012.03.058. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Y.H., Tong L., Zhou S.P., Sun H., Bi K.S., Zhang B.L. Simultaneous determinationof active flavonoids and alkaloids of Tang-Min-Ling-Pill in rat plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr. B. 2012;904:51–58. doi: 10.1016/j.jchromb.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Y.Y., Yu B.Y. Comparative studies on chemistry and pharmacodynamics of the compatibility of Yuanhu Zhitong prescription. Chin. Pharm. Univ. 2003;34:461–464. [Google Scholar]
- 9.Chen D.D., Zhou P., Bai G.G., Li X., Chen J.W. Discussion of HPLC fingerprint of traditional Chinese medicine of Corydalis yanhusuo and its preparation. China J. Chin. Mater. Med. 2015;40:2470–2473. [PubMed] [Google Scholar]
- 10.Zhou Y., Gao X., Wu C., Wu Y. Bioaccessibility and safety assessment of trace elements from decoction of “Zhebawei” herbal medicines by in vitro digestion method. Trace Elem. Med. Biol. 2014;28:173–178. doi: 10.1016/j.jtemb.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang L., Zhang Y., Wang Z., Tong N., Kweon T.D., Vo B.C., Wang X., Zhang J.Y., Chung A., Alachkar X., et al. The antinociceptive properties of the Corydalis yanhusuo extract. PLoS ONE. 2016;11:e0162875. doi: 10.1371/journal.pone.0162875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L., Ling H., Li L., He M. Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction. J. Pharm. Pharmacol. 2007;59:695–701. doi: 10.1211/jpp.59.5.0010. [DOI] [PubMed] [Google Scholar]
- 13.Ling H., Wu L., Li L. Corydalis yanhusuo, rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother. Res. 2006;20:448–453. doi: 10.1002/ptr.1875. [DOI] [PubMed] [Google Scholar]
- 14.Cheng X.Y., Shi Y. Analysis of chemical constituents of anti-myocardial ischemia fraction of Corydalis yanhusuo. China J. Chin. Mater. Med. 2008;33:1717–1719. [PubMed] [Google Scholar]
- 15.Hu T., Zhang X., Ma S., Cheng Y., Yao X. Chemical Constituents from Corydalis yanhusuo. China J. Chin. Mater. Med. 2009;34:1917–1920. [PubMed] [Google Scholar]
- 16.Guo Z., Man Y., Wang X., Jin H., Sun X., Su X., Hao J., Mi W. Levo-tetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci. Rep. 2014;4:3905. doi: 10.1038/srep03905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J., Liang L., Zhang Q., Li X., Fu Z. Preclinical pharmacokinetics, tissue distribution and excretion studies of a potential analgesics-corydaline using an ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;30:70–76. doi: 10.1016/j.jchromb.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Ishiguro K., Ando T., Maeda O., WSTANABE O., Goto H. Dehydrocorydaline inhibits elevated mitochondrial membrane potential in lipopolysaccharide-stimulated macrophages. Int. Immunopharmacol. 2011;11:1362–1367. doi: 10.1016/j.intimp.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Lapish C.C., Belardetti F., Ashby D.M., Ahn S., Butts K.A., So K., Macrae C.M., Hynd J.J., Miller J.J., Phillips A.G. A preclinical assessment of D, L-govadine as a potential antipsychotic and cognitive enhancer. Int. J. Neuropsychopharmacol. 2011;15:1441–1455. doi: 10.1017/S146114571100157X. [DOI] [PubMed] [Google Scholar]
- 20.Still P.C., Yi B., González-Cestari T.F., Pan L., Pavlovicz R.E., Chai H.B., Ninh T.N., Soejarto D.D., McKay D.B., Kinghorm A.D. Alkaloids from Microcos paniculata with cytotoxic and nicotinic receptor antagonistic activities. J. Nat. Prod. 2013;76:243–249. doi: 10.1021/np3007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Severina I.I., Muntyan M.S., Lewis K., Skulachev V.P. transfer of cationic antibacterial agents berberine, palmatine, and benzalkonium through bimolecular planar phospholipid film and staphylococcus aureus membrane. Iubmb. Life. 2001;52:321–324. doi: 10.1080/152165401317291183. [DOI] [PubMed] [Google Scholar]
- 22.Wei X., Shen H., Wang L., Meng Q., Li W. Analyses of total alkaloid extract of corydalis yanhusuo by comprehensive RP × RP liquid chromatography with pH difference. Anal. Method. Chem. 2016;2016:9752735. doi: 10.1155/2016/9752735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng X.Y., Yue S., Zhen S.L., Sun H., Jin W. HPLC-MS analysis of ethanol extract of Corydalis yanhusuo and simultaneous determination of eight protoberberine quaternary alkaloids by HPLC-DAD. J. Chromatogr. Sci. 2010;48:441–444. doi: 10.1093/chromsci/48.6.441. [DOI] [PubMed] [Google Scholar]
- 24.Ding B., Zhou T., Fan G., Hong Z., Wu Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/MS and LC-DAD. J. Pharm. Biomed. Anal. 2007;45:219–226. doi: 10.1016/j.jpba.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Li Q.Y., Li K.T., Sun H., Jin W., Shi J.W., Shi Y. LC-MS/MS determination and pharmacokinetic study of dehydrocorydaline in rat plasma after oral administration of dehydrocorydaline and Corydalis yanhusuo extract. Molecules. 2014;19:16312–16326. doi: 10.3390/molecules191016312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong Z.Y., Le J., Lin M., Fan G.R., Chai Y.F., Yin X.P., Wu Y.T. Comparative studies on pharmacokinetic fates of tetrahydropalmatine enantiomers in different chemical environments in rats. Chirality. 2008;20:119–124. doi: 10.1002/chir.20507. [DOI] [PubMed] [Google Scholar]
- 27.Ma H., Wang Y., Guo T., He Z., Chang X., Pu X. Simultaneous determination of tetrahydropalmatine, protopine, and palmatine in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2009;49:440–446. doi: 10.1016/j.jpba.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Gao Y., Hu S., Zhang M., Li L., Lin Y. Simultaneous determination of four alkaloids in mice plasma and brain by LC-MS/MS for pharmacokinetic studies after administration of Corydalis Rhizoma and Yuanhu Zhitong extracts. J. Pharm. Biomed. Anal. 2014;92:6–12. doi: 10.1016/j.jpba.2013.12.037. [DOI] [PubMed] [Google Scholar]
- 29.Cheng X.Y., Shi Y., Sun H., Jin W., Zheng S.L., Li K.T., Yang S. Identification and analysis of absorbed components in rat plasma after oral administration of active fraction of Corydalis yanhusuo by LC-MS/MS. Acta Pharm. Sin. B. 2009;44:167–174. [PubMed] [Google Scholar]
- 30.Murata S., Ueda S., Shimojo F., Tokunaga Y., Hata T., Ohnishi N. In vivo performance of time-controlled explosion system (TES) in GI physiology regulated dogs. Int. J. Pharm. 1998;161:161–168. doi: 10.1016/S0378-5173(97)00347-5. [DOI] [Google Scholar]
- 31.Yang C., Wang S., Guo X., Sun J., Lin L., Wu L. Simultaneous determination of seven anthraquinones in rat plasma by Ultra High Performance Liquid Chromatography-tandem Mass Spectrometry and pharmacokinetic study after oral administration of Semen Cassiae extract. J. Ethnopharmacol. 2015;169:305–313. doi: 10.1016/j.jep.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration. [(accessed on 1 September 2013)]; Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM368107.pdf.