Abstract
Hallmarks of chronic obstructive pulmonary disease (COPD) include innate inflammation and remodeling of small airways, which begin in early disease, and the development of lung lymphoid follicles (LLF), indicative of adaptive immunity, in more spirometrically severe stages. Common to these processes in all stages is orchestration by dendritic cells (DCs). Recently improved understanding of the analogous lung DC subsets in humans and mice has allowed for better integration and interpretation of the experimental and clinical pathological literature. In this review, we summarize the evidence from human and animal studies to place lung DCs into the context of COPD pathogenesis. We highlight recent studies that demonstrate a potential role for DCs in airway remodeling and that call into question the long-standing belief that intraepithelial DCs actively sample airway lumens. We discuss how DCs drive LLF formation directly and indirectly and also examine the ability of DCs within LLF to instruct downstream effector functions of natural killer cells, CD4+ T cells, and regulatory T cells. Greater awareness of the multifaceted functions of DCs will be essential in the quest to identify new therapeutic modalities to treat COPD.
Keywords: dendritic cells, lung, chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD), currently the third leading cause of death in the United States, and worldwide since 2010 (1), is a chronic, debilitating disease that is increasing rapidly in incidence. The progressive airflow limitation that characterizes COPD results from two major pathological processes: (1) remodeling and narrowing of the small airways; and (2) apoptosis of lung parenchyma cells, leading to emphysema. Dendritic cells (DCs), which serve both as innate lung sentinels and as orchestrators of adaptive immunity, are well equipped to drive both pathological processes from the earliest stages of COPD pathogenesis to end-stage disease.
Understanding of lung DC subsets has improved greatly over the past few years, and there is now consensus that analogous DC subsets exist in humans and mice. All three main subtypes of DCs derive from adult hematopoietic precursors; they are separated into type 1 and type 2 classical/conventional DCs (cDC1 and cDC2, respectively) and plasmacytoid DCs (pDCs) (2). Human cDC1 express CD141 (thrombomodulin, blood dendritic cell antigen-3) and CLEC9A, and the murine equivalents are CD8α+ and CD103+ (3). cDC1 can also express the C-type lectin receptor langerin (4). Development of cDC1 in mice depends on the transcription factor basic leucine zipper ATF-like transcription factor 3 (5). cDC1 are generally found in the mucosa and vascular wall; they are functionally distinct from other DC subsets, because they are able to elicit Th1 responses and uniquely cross-present, apoptotic cell–associated antigens. A comprehensive review of cDC1 was published recently (6). The cDC2 subsets, residing primarily in the lamina propria, are CD1c (blood dendritic cell antigen-1)+ in humans and CD11b+ in mice (2), and the latter are dependent on the transcription factor IFN regulatory factor 4 (7). In the lung, cDC2 are major producers of proinflammatory chemokines, which are responsible for recruiting inflammatory cells (8). These cells have also been shown to have tolerogenic properties, and isolated lung CD1c+ DCs from patients with COPD favored the differentiation of IL-10–secreting CD4+ T cells (9). DCs with a tolerogenic phenotype are important in regulating the immune response; however, it is unclear whether tolerogenic DCs constitute a particular lineage or represent a certain activation state. Tolerogenic DCs have been reviewed recently and are not discussed further in this article (10). pDCs, which are distributed throughout the lung, in the airways, parenchyma, and alveolar septa, are crucial during antiviral responses for their ability to produce type I IFNs. Development of mature murine pDCs depends on the transcription factor E2-2 (11). Table 1 summarizes the human and murine markers used to classify each subset, as well as the production of cytokines and chemokines and key chemokine receptors. The “inflammatory DCs” that are found in inflamed lung tissues and are generally thought to arise from circulating monocyte-derived cells, rather than from a common DC precursor, will not be discussed in this article.
Table 1.
Summary of Lung DC Subsets: Phenotypic Markers, Cytokine/Chemokine Production, and Expression of Key Chemokine Receptors
| Lung DC Subset | Markers (Human) | Marker (Mouse) | Cytokine/Chemokine Production | Chemokine Receptors |
|---|---|---|---|---|
| cDC1 |
|
|
|
|
| cDC2 |
|
|
|
|
| pDC |
|
|
|
|
Definition of abbreviations: BDCA, blood dendritic cell antigen; BST, bone marrow stromal cell antigen; CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; CD, cluster of differentiation; cDC, classical/conventional DCs; CLEC9A, C-type lectin domain family 9 member A; CXCL, C-X-C motif ligand; CX3CR1, C-X3-C motif chemokine receptor 1; DC, dendritic cell; DNGR-1, dendritic cell NK lectin group receptor 1; pDC, plasmacytoid DC; SIRPα, signal regulatory protein α; XCR1, X-C motif chemokine receptor 1.
Multiple studies have investigated how cigarette smoking or COPD affect lung DC numbers and maturation status (Table 2). The most common approaches include immunohistochemistry of frozen tissue sections or flow cytometry of lung homogenates or bronchoalveolar lavage fluid. Results have been inconsistent, likely because of the limitations inherent in these methodologies. Immunohistochemistry relies on DC identification using only one or two markers, whereas flow cytometry of lung homogenates requires ex vivo processing that could affect surface receptors. In addition, the makeup of cohorts varies greatly among studies, with differences in COPD severity and the use of inhaled corticosteroids likely contributing to conflicting results (12).
Table 2.
Accumulation and Maturation of Human DC Subsets in Response to Smoke and COPD
| Reference | Subjects | Sample | Method | DC Subsets | Observation | Activation Status |
|---|---|---|---|---|---|---|
| Bratke et al. (2008) (63) |
|
BAL fluid | FCM | mDCs (CD11c+, HLA-DR+, lin-) | No difference in percentage; absolute number increased in current smokers | Current smokers had increased expression of CD80 and CD86 |
| Stoll et al. (2014) (14) |
|
BAL fluid | FCM | mDCs (CD11c+, HLA-DR+, lin-) | No difference in percentage; absolute number increased in current smokers with COPD compared to former smokers | Compared to never-smokers, CD86 was increased in current COPD smokers and CD83 (which correlates with duration in tissues) was decreased |
| pDCs (CD123+, HLA-DR+, lin-) | No difference in percentage; absolute number increased in current smokers with COPD compared to former and never-smokers | |||||
| Demedts et al. (2007) (15) |
|
Lung tissue | IHC | Langerhans-type DCs (Langerin+) | Increased in small airways of COPD subjects compared to never smokers and smokers without COPD | |
| Rogers et al. (2008) (64) |
|
Bronchial biopsies | TEM | Identified by TEM | Decreased in current smokers with COPD compared to never-smokers and former smokers with COPD | |
| Tsoumakidou et al. (2009) (65) |
|
Lung tissue | IHC | CD1a+ DCs | No difference between groups | |
| CD83+ DCs | Decreased in all COPD subjects compared to never-smokers and former smokers without COPD | |||||
| Freeman et al. (2009) (16) |
|
Lung tissue | FCM | mDC1: BDCA-1+, HLA-DR+ | No difference in percentage between groups | COPD subjects had increased expression of CD80 and CD83 |
| mDC2: BDCA-3+, HLA-DR+ | No difference in percentage between groups; most abundant subset | COPD subjects had increased expression of CD80 and CD83 | ||||
| pDC: BDCA-2+, CD123+ | No difference in percentage between groups | COPD subjects had increased expression of CD80 and CD40 | ||||
| Vassallo et al. (2010) (17) |
|
Lung tissue | IHC | CD1a+ DCs | No difference between groups | |
| CD83+ DCs | Increased in COPD | |||||
| Van Pottelberge et al. (2010) (66) |
|
Lung tissue | IHC | Langerin+ DCs | Increased in COPD subjects compared to former smokers without COPD | |
| DC-SIGN+ DCs | No difference between groups | |||||
| CD1a+ DCs | No difference between groups | |||||
| BDCA-1+ DCs | Decreased in COPD subjects compared to never-smokers | |||||
| Arellano-Orden et al. (2016) (18) |
|
Lung tissue | FCM | mDC1: BDCA-1+, HLA-DR+ | No difference in percentage between groups | Smokers had decreased CD40 and CD83 compared to non-smokers. No other differences between groups |
| mDC2: BDCA-3+, HLA-DR+ | No difference in percentage between groups; most abundant DC subset | No differences in expression of CD40, CD80, CD83, or CD86 | ||||
| pDC: BDCA-2+, CD123+ | No difference in percentage between groups | No differences in expression of CD40, CD80, CD83, or CD86 | ||||
| IHC | Follicular DCs (CD21+) | Trend toward increase in COPD | ||||
| CD1a+ DCs | Trend toward decrease in COPD | |||||
| Langerin+ DCs | Decreased in COPD |
Definition of abbreviations: BAL, bronchoalveolar lavage; BDCA, blood dendritic cell antigen; CD, cluster of differentiation; COPD, chronic obstructive pulmonary disease; DC, dendritic cell; DC-SIGN, dendritic cell–specific intercellular adhesion molecule-3–grabbing non-integin; FCM, flow cytometry; HLA-DR, human leukocyte antigen - antigen D related; IHC, immunohistochemistry; lin, lineage; mDCs, myeloid DC; pDCs, plasmacytoid DC; TEM, transmission electron microscopy.
The goal of this review was to place these multiple DC subsets into the context of COPD pathogenesis as currently understood, highlighting results from both humans and animal experimental models. We have organized this article around DC actions at specific anatomic sites, which also loosely parallels the evolution of COPD pathogenesis.
DCs in Small Airways and Alveoli
Small airways are the initial and principal site of multiple pathological processes in COPD, including inflammatory cell infiltration, remodeling, and accumulation of mucous exudates. The percentage of small airways containing CD4+ cells, CD8+ cells, B cells, macrophages, and neutrophils in their walls is well known to increase in parallel with spirometrically defined COPD severity (13). Less often appreciated is an increase in DC numbers within the lung (Table 2) (14–18), believed to result from continuous recruitment from the bone marrow.
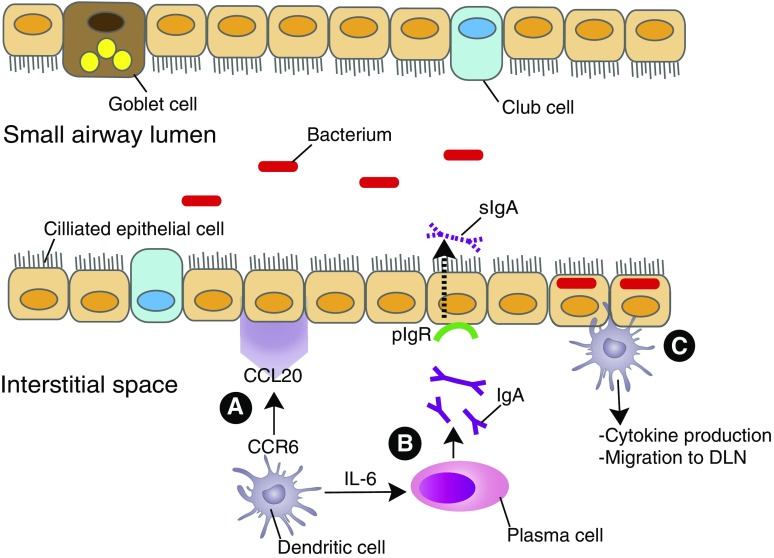
Among the several chemokine receptors implicated in DC recruitment to the lungs, the most prominent is CCR6 (19) (Figure 1). The only known endogenous CCR6 ligand, CCL20, is produced by epithelial cells and fibroblasts (20). Total lung CCL20 messenger RNA is significantly higher in patients with COPD compared with never-smokers and smokers without COPD (15). In cigarette smoke (CS)–exposed mice, CCR6 deficiency attenuated recruitment of DCs and other inflammatory cells, compared with littermate control animals (20, 21), and partially protected against emphysema, as measured by a significantly lower mean linear intercept and destructive index (21).
Figure 1.
Recruitment and role of DCs in small airways. (A) Beginning in the small airways, CCR6+ DCs are recruited to the site of inflammation via CCL20, expressed by epithelial cells or fibroblasts. (B) Activated DCs secrete IL-6, which drives activated B cells to turn into IgA-producing plasma cells. (C) In healthy lung tissue, pIgR is necessary to transport dimeric IgA across epithelial cells to the airway lumen; there, it is cleaved to generate sIgA, which prevents binding of microbes and neutralizes their toxins. In chronic obstructive pulmonary disease, decreased pIgR leads to decreased sIgA and a corresponding increase in commensal bacteria localized within the epithelium. Bacterial “invasion” could trigger DCs to initiate additional immune responses. CCL, C-C motif chemokine ligand; CCR, C-C motif chemokine receptor; DC, dendritic cell; DLN, draining lymph node; pIgR, polymeric Ig receptor; sIgA, secretory IgA.
Interestingly, reduced airway fibrosis has also been seen in CCR6–/– mice after IL-1β adenoviral delivery (22), suggesting a link between airway remodeling and DCs. On the basis of the correlation of IL-1β levels in both serum (23) and lung parenchyma (24) with clinical COPD severity, IL-1β has been used to study key features of airway remodeling in COPD. IL-1β delivery resulted in both DC localization surrounding the airways and in airway wall fibrosis, which was mediated by DC-driven T-cell immune responses, and was reduced significantly by DC depletion (22). The disparity between this finding and the lack of effect of CCR6 deficiency in protecting against airway wall remodeling after CS exposure (21) may relate to greater stimulus intensity in the adenoviral model. IL-1β can induce increased CCL20 production by both murine and human fibroblasts (25) via transforming growth factor-β activation (24).
DCs also provide immune surveillance of the small airways, important in health but potentially contributing to COPD progression. Two complementary studies using mice expressing the CD11c-EYFP transgene in combination with two-photon microscopy elucidated behavioral differences between intraepithelial DCs (putatively, CD103+) and subepithelial DCs (putatively, CD11b+), as well as differences between the former depending on airway versus alveolar location. The first study showed that although airway intraepithelial DCs exhibited active probing movements that occasionally extended into the lumen, they unexpectedly did not take up antigen (26). The second study likewise found that airway DCs rarely sent processes into the lumen, but that in alveoli, intraepithelial DCs actively scanned and took up antigen (27). These studies do not support the current belief that the location of intraepithelial DCs guarantees sampling from the airway lumen. However, it remains possible that in COPD, epithelial damage might facilitate sampling by intraepithelial DCs.
Recent studies provide a unifying view of how such damage to the small airway epithelium may occur, by loss of the “immune exclusion” of microbes provided physiologically by secretory IgA (sIgA). Transport of sIgA into the lumen requires that airway epithelial cells express the polymeric Ig receptor (pIgR). Expression of pIgR decreases in COPD (28) (Figure 1), resulting in focal deficiency of sIgA on the mucosa of both large and small airways that correlates with spirometric disease severity (28). Although both CD103+ and CD11b+ murine lung DCs can induce IgA production in B cells (29), in the absence of pIgR, excess IgA accumulates in the subepithelial areas, which has been shown in COPD (30).
Because even healthy lungs have a bacterial microbiome, loss of epithelial barrier integrity in COPD exposes lung DCs to microbial antigens. pIgR–/– mice developed airway remodeling and emphysematous changes as they aged, even in the absence of CS exposure. They also had significantly more airways in which bacteria localized within the epithelium, relative to wild-type mice, whereas the percentage of airways with luminal bacteria did not differ (31). On the basis of these murine findings, even if DCs do not actively sample antigen in the healthy human airway lumen (26, 27), during the progression of small airway damage that initiates COPD they likely will be exposed to bacteria. Human monocyte-derived DCs were activated (as measured by induction of CD83, CD40, and CD86, plus increased proinflammatory cytokine production) by exposure in vitro to nonpathogenic bacteria, and, to a greater degree, by the Proteobacteriaciae (Haemophilus and Moraxella) common in COPD (32). Recognition by lung cDCs of bacterial invasion of terminal airway epithelium may be a key early step by which these cell types initiate lung inflammation that persists even after smoking cessation.
In addition, cigarette smokers with and without COPD have reduced lower airway activity of the cystic fibrosis transmembrane conductance regulator, compared with healthy nonsmokers (33). Decreased cystic fibrosis transmembrane conductance regulator expression results in increased epithelial and endothelial cell permeability (34). It also leads to airway surface liquid (ASL) volume depletion, resulting in impaired mucociliary transport (35), as well as a decrease in ASL pH. Increased acidity inhibits bacterial killing by the mixture of antimicrobial factors present in normal ASL. Both loss of bacterial killing by ASL and increased cell permeability could contribute to bacterial invasion of the terminal airway epithelium, lung DC activation, and sustained lung inflammation.
DCs in Tertiary Lymphoid Follicles
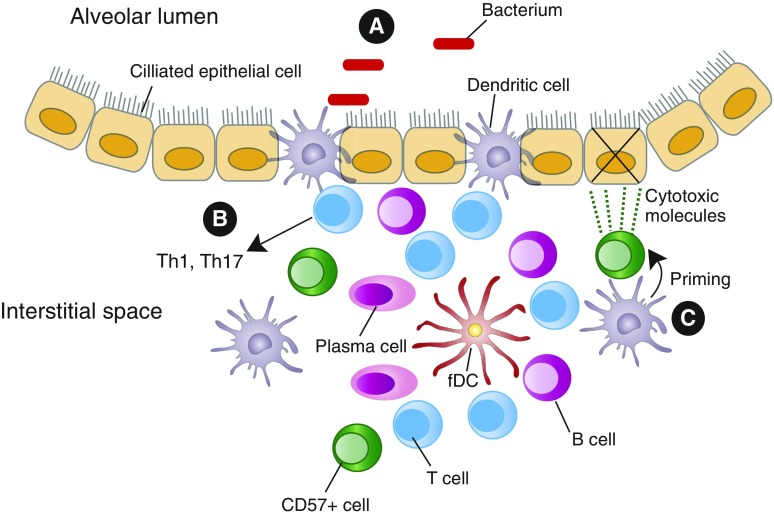
After encountering antigens, cDCs migrate to draining lymph nodes to activate naive T cells. However, as COPD severity progresses, cross-talk with other innate immune cells and initiation of adaptive immune responses can also occur within the lungs, as demonstrated by lung lymphoid follicles (LLF) containing germinal centers (13), which are involved in B-cell affinity maturation and isotype switching (36). Lung DCs can contribute crucially to the initial development of tertiary lymphoid tissues, including LLF (Figure 2). Unlike encapsulated lymph nodes (which require lymphoid inducer cells), LLF can be triggered by cDC expression of lymphotoxin α/β (37, 38), which induces differentiation of follicular DCs (FDC) from ubiquitous perivascular precursors (39). FDC retain antigen and present them to B cells, especially those ingested as immune complexes. FDC are also a source of B-cell activating factor, which has been associated with LLF accumulation in COPD (40, 41), and are an abundant source of CXCL13, the chemokine essential for colocalization of B cells with T follicular helper cells, essential for isotype class switching. Lung homogenates from patients with COPD with LLF (identified by immunohistochemistry) contained more CXCL13 than did those from patients with COPD without LLF, and CXCL13 concentrations correlated with LLF density (42).
Figure 2.
Lung DCs in LLF. Lung DCs can contribute to the formation of LLF. In addition to draining lymph nodes, LLF provide a microenvironment where DCs interact with other cells. (A) Antigen uptake may also be occurring in LLF, where many DCs, including CD103+/Langerin+ DCs, directly interface with the alveolar spaces (52). (B) CD103+ DCs drive Th1 and Th17 responses in chronic obstructive pulmonary disease, whereas IL-17 production can initiate development of new LLF. (C) Signals from DCs could prime CD57+ NK cells to become cytotoxic and kill epithelial cells. CD, cluster of differentiation; fDC, follicular DCs; LLF, lung lymphoid follicles; NK, natural killer; Th, T helper cell.
DCs could also promote LLF formation indirectly by secreting IL-23, which can induce various populations of innate lymphoid cells and conventional T cells to produce IL-17 (43). In addition to driving CXCL13 production, IL-17 is central to an alternative pathway of LLF development, dependent on CXCL12 and independent of CXCL13 (44). IL-17 was shown to be required for initiation, but not maintenance, of LLF in neonatal mice that received LPS (45), and LLF development was impaired in CS-exposed IL-17a–/– mice (46). In a mouse model of elastase-induced emphysema, IL-23–/– mice were protected from emphysematous changes compared with wild-type mice, but LLF formation was not assessed (47). Defining the contribution of DCs to LLF development in humans is an important goal.
Another constituent of LLF in COPD is CD57+ cytotoxic lymphocytes, most likely natural killer (NK) cells, which may contribute to emphysema, the other process leading to impaired air flow and gas exchange. The density of CD57+ cells within LLF of patients with COPD was significantly increased compared with that of control subjects (48). Similarly, the percentage of LLF containing apoptotic cells was also higher in patients with COPD. Cytotoxic lymphocytes isolated from human lung tissue using magnetic beads against another NK cell marker (CD56) were more cytotoxic against autologous lung cells in patients with COPD, compared with smokers without COPD (49). Importantly, purified lung CD8+ T cells from the same subjects did not significantly kill autologous epithelial cells in this assay, implying a central role for NK cells in direct emphysema induction. To become cytotoxic, NK cells require additional signals, termed priming. In vivo murine studies have shown that DCs are required for NK priming to viral and bacterial pathogens (50). In a mouse model of acute CS exposure, the percentage of primed lung NK cells (measured by CD69 expression) was increased in CS-exposed mice compared with air-exposed mice (51). Confocal microscopy also showed that NKs made physical contact with lung CD11c+ DCs and that this contact was mediated by DC expression of CCR4. NK priming was diminished in CS-exposed CCR4–/– mice, as were the number of contacts between DCs and NKs (51). Hence, dysregulated DC-mediated priming of cytotoxic NK cells could contribute to emphysema pathogenesis (Figure 2).
Just as in injured small airways, antigen uptake may be occurring in COPD in LLF adjacent to alveoli. A study using computerized, three-dimensional reconstructions to analyze human lung tissue found that the vast majority of LLF directly interfaced the alveolar lumen (52). Furthermore, in COPD tissues, an increased number of langerin+ DCs were observed in the interface between LLF and the alveolar lumen, compared with lungs from never-smokers and smokers without COPD. Many such DCs simultaneously contacted both the alveolar surface and lymphocytes within the LLF (52) (Figure 2). The langerin receptor is commonly expressed by CD103+ DCs (4), which in mice have been shown to elicit predominantly Th1 and Th17 responses (53). Similarly, coincubation of CD1a+ human lung DCs isolated from subjects with advanced emphysema, but not from control subjects, induced peripheral blood CD4+ T cells to express IFN-γ and IL-17A (54). Interestingly, CD1a+ lung DCs from control subjects drove differentiation of CD4+ T cells into regulatory T cells (TReg), as evidenced by increased expression of CD25 and Foxp3, but CD1a+ lung DCs from subjects with emphysema did not (54). This finding supports the findings of several studies showing reduced TReg in the lungs of patients with emphysema (55–58). Although one study reported TReg to be increased within the LLF of patients with moderate COPD (59), that finding is not incompatible with the idea that loss of TReg numbers or function could be associated with progression to severe COPD. Moreover, TReg have been shown to limit LLF formation (60); therefore, the inability of DCs to drive TReg differentiation in severe COPD might contribute to the increased numbers of lymphoid follicles at later stages of the disease.
Conclusions
Because DCs can directly produce profibrogenic cytokines and can indirectly propagate airway inflammation, both likely contributing to airway remodeling, modulating DC recruitment and function remains an attractive therapeutic approach to limiting COPD progression. Identifying which specific DC subsets are involved in pathogenic processes could allow for a tailored approach, which would block the unwanted DC subset without impeding protective or beneficial DCs. Ultimately, a better understanding of the role(s) of DCs in COPD progression is needed for the development of much-needed novel treatments for this disease.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2016-0272TR on October 21, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010 Lancet 20123802095–2128.[Published erratum appears in Lancet 381:628.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guilliams M, Ginhoux F, Jakubzick C, Naik SH, Onai N, Schraml BU, Segura E, Tussiwand R, Yona S. Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol. 2014;14:571–578. doi: 10.1038/nri3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al. Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulin LF, Reyal Y, Uronen-Hansson H, Schraml BU, Sancho D, Murphy KM, Håkansson UK, Moita LF, Agace WW, Bonnet D, et al. DNGR-1 is a specific and universal marker of mouse and human Batf3-dependent dendritic cells in lymphoid and nonlymphoid tissues. Blood. 2012;119:6052–6062. doi: 10.1182/blood-2012-01-406967. [DOI] [PubMed] [Google Scholar]
- 6.van der Aa E, van Montfoort N, Woltman AM. BDCA3+CLEC9A+ human dendritic cell function and development. Semin Cell Dev Biol. 2015;41:39–48. doi: 10.1016/j.semcdb.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Schlitzer A, McGovern N, Teo P, Zelante T, Atarashi K, Low D, Ho AW, See P, Shin A, Wasan PS, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim TH, Lee HK. Differential roles of lung dendritic cell subsets against respiratory virus infection. Immune Netw. 2014;14:128–137. doi: 10.4110/in.2014.14.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsoumakidou M, Tousa S, Semitekolou M, Panagiotou P, Panagiotou A, Morianos I, Litsiou E, Trochoutsou AI, Konstantinou M, Potaris K, et al. Tolerogenic signaling by pulmonary CD1c+ dendritic cells induces regulatory T cells in patients with chronic obstructive pulmonary disease by IL-27/IL-10/inducible costimulator ligand. J Allergy Clin Immunol. 2014;134:944–954.e8. doi: 10.1016/j.jaci.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 10.Takenaka MC, Quintana FJ.Tolerogenic dendritic cells Semin Immunopathol[online ahead of print] 19 Sep 2016; DOI: 10.1007/s00281-016-0587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Condon TV, Sawyer RT, Fenton MJ, Riches DW. Lung dendritic cells at the innate-adaptive immune interface. J Leukoc Biol. 2011;90:883–895. doi: 10.1189/jlb.0311134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lommatzsch M, Kraeft U, Troebs L, Garbe K, Bier A, Stoll P, Klammt S, Kuepper M, Bratke K, Virchow JC. Fluticasone impact on airway dendritic cells in smokers: a randomized controlled trial. Respir Res. 2013;14:114. doi: 10.1186/1465-9921-14-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 14.Stoll P, Heinz AS, Bratke K, Bier A, Garbe K, Kuepper M, Virchow JC, Lommatzsch M. Impact of smoking on dendritic cell phenotypes in the airway lumen of patients with COPD. Respir Res. 2014;15:48. doi: 10.1186/1465-9921-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demedts IK, Bracke KR, Van Pottelberge G, Testelmans D, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Accumulation of dendritic cells and increased CCL20 levels in the airways of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:998–1005. doi: 10.1164/rccm.200608-1113OC. [DOI] [PubMed] [Google Scholar]
- 16.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:1179–1188. doi: 10.1164/rccm.200904-0552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vassallo R, Walters PR, Lamont J, Kottom TJ, Yi ES, Limper AH. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir Res. 2010;11:45. doi: 10.1186/1465-9921-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arellano-Orden E, Calero-Acuña C, Moreno-Mata N, Gómez-Izquierdo L, Sánchez-López V, López-Ramírez C, Tobar D, López-Villalobos JL, Gutiérrez C, Blanco-Orozco A, et al. Cigarette smoke decreases the maturation of lung myeloid dendritic cells. PLoS One. 2016;11:e0152737. doi: 10.1371/journal.pone.0152737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osterholzer JJ, Ames T, Polak T, Sonstein J, Moore BB, Chensue SW, Toews GB, Curtis JL. CCR2 and CCR6, but not endothelial selectins, mediate the accumulation of immature dendritic cells within the lungs of mice in response to particulate antigen. J Immunol. 2005;175:874–883. doi: 10.4049/jimmunol.175.2.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Ma R, Murray LA, Tsui P, Lou J, Marks JD, Baron JL, et al. TGF-β-dependent dendritic cell chemokinesis in murine models of airway disease. J Immunol. 2015;195:1182–1190. doi: 10.4049/jimmunol.1500348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracke KR, D’Hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, Brusselle GG. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol. 2006;177:4350–4359. doi: 10.4049/jimmunol.177.7.4350. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Goodsell A, Ma R, Moermans C, McKnelly KJ, Baron JL, Krummel MF, et al. A critical role for dendritic cells in the evolution of IL-1β-mediated murine airway disease. J Immunol. 2015;194:3962–3969. doi: 10.4049/jimmunol.1403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh B, Arora S, Khanna V. Association of severity of COPD with IgE and interleukin-1β. Monaldi Arch Chest Dis. 2010;73:86–87. doi: 10.4081/monaldi.2010.303. [DOI] [PubMed] [Google Scholar]
- 24.Brand OJ, Somanath S, Moermans C, Yanagisawa H, Hashimoto M, Cambier S, Markovics J, Bondesson AJ, Hill A, Jablons D, et al. Transforming growth factor-β and interleukin-1β signaling pathways converge on the chemokine CCL20 promoter. J Biol Chem. 2015;290:14717–14728. doi: 10.1074/jbc.M114.630368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J, Goodsell A, Publicover J, Reichardt L, Jablons D, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest. 2011;121:2863–2875. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veres TZ, Voedisch S, Spies E, Tschernig T, Braun A.Spatiotemporal and functional behavior of airway dendritic cells visualized by two-photon microscopy Am J Pathol 2011179603–609.[Published erratum appears in Am J Pathol 179:2674.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation to T cells in the lung. J Exp Med. 2012;209:1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du RH, Richmond BW, Blackwell TS, Jr, Cates JM, Massion PP, Ware LB, Lee JW, Kononov AV, Lawson WE, Blackwell TS, et al. Secretory IgA from submucosal glands does not compensate for its airway surface deficiency in chronic obstructive pulmonary disease. Virchows Arch. 2015;467:657–665. doi: 10.1007/s00428-015-1854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki Y, Suda T, Furuhashi K, Shibata K, Hashimoto D, Enomto N, Fujisawa T, Nakamura Y, Inui N, Nakamura H, et al. Mouse CD11bhigh lung dendritic cells have more potent capability to induce IgA than CD103+ lung dendritic cells in vitro. Am J Respir Cell Mol Biol. 2012;46:773–780. doi: 10.1165/rcmb.2011-0329OC. [DOI] [PubMed] [Google Scholar]
- 30.Ladjemi MZ, Lecocq M, Weynand B, Bowen H, Gould HJ, Van Snick J, Detry B, Pilette C. Increased IgA production by B-cells in COPD via lung epithelial interleukin-6 and TACI pathways. Eur Respir J. 2015;45:980–993. doi: 10.1183/09031936.00063914. [DOI] [PubMed] [Google Scholar]
- 31.Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS, Gleaves L, Abdolrasulnia R, Polosukhina D, Clark PE, et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun. 2016;7:11240. doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen JM, Steen-Jensen DB, Laursen JM, Søndergaard JN, Musavian HS, Butt TM, Brix S. Divergent pro-inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS One. 2012;7:e31976. doi: 10.1371/journal.pone.0031976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown MB, Hunt WR, Noe JE, Rush NI, Schweitzer KS, Leece TC, Moldobaeva A, Wagner EM, Dudek SM, Poirier C, et al. Loss of cystic fibrosis transmembrane conductance regulator impairs lung endothelial cell barrier function and increases susceptibility to microvascular damage from cigarette smoke. Pulm Circ. 2014;4:260–268. doi: 10.1086/675989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brusselle GG, Demoor T, Bracke KR, Brandsma CA, Timens W. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J. 2009;34:219–230. doi: 10.1183/09031936.00150208. [DOI] [PubMed] [Google Scholar]
- 37.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hämmerling G, Garbi N, Sutter G, et al. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–2601. doi: 10.1084/jem.20091472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–2349. doi: 10.1084/jem.20090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krautler NJ, Kana V, Kranich J, Tian Y, Perera D, Lemm D, Schwarz P, Armulik A, Browning JL, Tallquist M, et al. Follicular dendritic cells emerge from ubiquitous perivascular precursors. Cell. 2012;150:194–206. doi: 10.1016/j.cell.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, Rios A, Jahn A, Sauleda J, Divo M, et al. B cell-activating factor. An orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:695–705. doi: 10.1164/rccm.201501-0107OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seys LJ, Verhamme FM, Schinwald A, Hammad H, Cunoosamy DM, Bantsimba-Malanda C, Sabirsh A, McCall E, Flavell L, Herbst R, et al. Role of B cell-activating factor in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:706–718. doi: 10.1164/rccm.201501-0103OC. [DOI] [PubMed] [Google Scholar]
- 42.Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, et al. CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1194–1202. doi: 10.1164/rccm.201208-1543OC. [DOI] [PubMed] [Google Scholar]
- 43.Hwang JY, Randall TD, Silva-Sanchez A. Inducible bronchus-associated lymphoid tissue: taming inflammation in the lung. Front Immunol. 2016;7:258. doi: 10.3389/fimmu.2016.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleige H, Ravens S, Moschovakis GL, Bölter J, Willenzon S, Sutter G, Häussler S, Kalinke U, Prinz I, Förster R. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med. 2014;211:643–651. doi: 10.1084/jem.20131737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roos AB, Sandén C, Mori M, Bjermer L, Stampfli MR, Erjefält JS. IL-17A is elevated in end-stage chronic obstructive pulmonary disease and contributes to cigarette smoke-induced lymphoid neogenesis. Am J Respir Crit Care Med. 2015;191:1232–1241. doi: 10.1164/rccm.201410-1861OC. [DOI] [PubMed] [Google Scholar]
- 47.Fujii U, Miyahara N, Taniguchi A, Waseda K, Morichika D, Kurimoto E, Koga H, Kataoka M, Gelfand EW, Cua DJ, et al. IL-23 is essential for the development of elastase-induced pulmonary inflammation and emphysema. Am J Respir Cell Mol Biol. 2016;55:697–707. doi: 10.1165/rcmb.2016-0015OC. [DOI] [PubMed] [Google Scholar]
- 48.Olloquequi J, Montes JF, Prats A, Rodríguez E, Montero MA, García-Valero J, Ferrer J. Significant increase of CD57+ cells in pulmonary lymphoid follicles of COPD patients. Eur Respir J. 2011;37:289–298. doi: 10.1183/09031936.00201509. [DOI] [PubMed] [Google Scholar]
- 49.Freeman CM, Stolberg VR, Crudgington S, Martinez FJ, Han MK, Chensue SW, Arenberg DA, Meldrum CA, McCloskey L, Curtis JL. Human CD56+ cytotoxic lung lymphocytes kill autologous lung cells in chronic obstructive pulmonary disease. PLoS One. 2014;9:e103840. doi: 10.1371/journal.pone.0103840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolberg VR, Martin B, Mancuso P, Olszewski MA, Freeman CM, Curtis JL, Chensue SW. Role of CC chemokine receptor 4 in natural killer cell activation during acute cigarette smoke exposure. Am J Pathol. 2014;184:454–463. doi: 10.1016/j.ajpath.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori M, Andersson CK, Svedberg KA, Glader P, Bergqvist A, Shikhagaie M, Löfdahl CG, Erjefält JS. Appearance of remodelled and dendritic cell-rich alveolar-lymphoid interfaces provides a structural basis for increased alveolar antigen uptake in chronic obstructive pulmonary disease. Thorax. 2013;68:521–531. doi: 10.1136/thoraxjnl-2012-202879. [DOI] [PubMed] [Google Scholar]
- 53.Furuhashi K, Suda T, Hasegawa H, Suzuki Y, Hashimoto D, Enomoto N, Fujisawa T, Nakamura Y, Inui N, Shibata K, et al. Mouse lung CD103+ and CD11bhigh dendritic cells preferentially induce distinct CD4+ T-cell responses. Am J Respir Cell Mol Biol. 2012;46:165–172. doi: 10.1165/rcmb.2011-0070OC. [DOI] [PubMed] [Google Scholar]
- 54.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 55.Freeman CM, McCubbrey AL, Crudgington S, Nelson J, Martinez FJ, Han MK, Washko GR, Jr, Chensue SW, Arenberg DA, Meldrum CA, et al. Basal gene expression by lung CD4+ T cells in chronic obstructive pulmonary disease identifies independent molecular correlates of airflow obstruction and emphysema extent. PLoS One. 2014;9:e96421. doi: 10.1371/journal.pone.0096421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13:567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 57.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barceló B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agustí AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31:555–562. doi: 10.1183/09031936.00010407. [DOI] [PubMed] [Google Scholar]
- 59.Plumb J, Smyth LJ, Adams HR, Vestbo J, Bentley A, Singh SD. Increased T-regulatory cells within lymphocyte follicles in moderate COPD. Eur Respir J. 2009;34:89–94. doi: 10.1183/09031936.00100708. [DOI] [PubMed] [Google Scholar]
- 60.Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE, Foster PS, King C, Steptoe RJ, Mazzone SB, et al. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J Immunol. 2015;194:4567–4576. doi: 10.4049/jimmunol.1400909. [DOI] [PubMed] [Google Scholar]
- 61.Boltjes A, van Wijk F. Human dendritic cell functional specialization in steady-state and inflammation. Front Immunol. 2014;5:131. doi: 10.3389/fimmu.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bratke K, Klug M, Bier A, Julius P, Kuepper M, Virchow JC, Lommatzsch M. Function-associated surface molecules on airway dendritic cells in cigarette smokers. Am J Respir Cell Mol Biol. 2008;38:655–660. doi: 10.1165/rcmb.2007-0400OC. [DOI] [PubMed] [Google Scholar]
- 64.Rogers AV, Adelroth E, Hattotuwa K, Dewar A, Jeffery PK. Bronchial mucosal dendritic cells in smokers and ex-smokers with COPD: an electron microscopic study. Thorax. 2008;63:108–114. doi: 10.1136/thx.2007.078253. [DOI] [PubMed] [Google Scholar]
- 65.Tsoumakidou M, Koutsopoulos AV, Tzanakis N, Dambaki K, Tzortzaki E, Zakynthinos S, Jeffery PK, Siafakas NM. Decreased small airway and alveolar CD83+ dendritic cells in COPD. Chest. 2009;136:726–733. doi: 10.1378/chest.08-2824. [DOI] [PubMed] [Google Scholar]
- 66.Van Pottelberge GR, Bracke KR, Demedts IK, De Rijck K, Reinartz SM, van Drunen CM, Verleden GM, Vermassen FE, Joos GF, Brusselle GG. Selective accumulation of langerhans-type dendritic cells in small airways of patients with COPD. Respir Res. 2010;11:35. doi: 10.1186/1465-9921-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]