Abstract
Background
In acute decompensated heart failure, guidelines recommend increasing loop diuretic dose or adding a thiazide diuretic when diuresis is inadequate. We set out to determine the adverse events associated with a diuretic strategy relying on metolazone or high‐dose loop diuretics.
Methods and Results
Patients admitted to 3 hospitals using a common electronic medical record with a heart failure discharge diagnosis who received intravenous loop diuretics were studied in a propensity‐adjusted analysis of all‐cause mortality. Secondary outcomes included hyponatremia (sodium <135 mEq/L), hypokalemia (potassium <3.5 mEq/L) and worsening renal function (a ≥20% decrease in estimated glomerular filtration rate). Of 13 898 admissions, 1048 (7.5%) used adjuvant metolazone. Metolazone was strongly associated with hyponatremia, hypokalemia, and worsening renal function (P<0.0001 for all) with minimal effect attenuation following covariate and propensity adjustment. Metolazone remained associated with increased mortality after multivariate and propensity adjustment (hazard ratio=1.20, 95% confidence interval 1.04–1.39, P=0.01). High‐dose loop diuretics were associated with hypokalemia and hyponatremia (P<0.002) but only worsening renal function retained significance (P<0.001) after propensity adjustment. High‐dose loop diuretics were not associated with reduced survival after multivariate and propensity adjustment (hazard ratio=0.97 per 100 mg of IV furosemide, 95% confidence interval 0.90–1.06, P=0.52).
Conclusions
During acute decompensated heart failure, metolazone was independently associated with hypokalemia, hyponatremia, worsening renal function and increased mortality after controlling for the propensity to receive metolazone and baseline characteristics. However, under the same experimental conditions, high‐dose loop diuretics were not associated with hypokalemia, hyponatremia, or reduced survival. The current findings suggest that until randomized control trial data prove otherwise, uptitration of loop diuretics may be a preferred strategy over routine early addition of thiazide type diuretics when diuresis is inadequate.
Keywords: acute heart failure, cardio‐renal syndrome, diuretics, metolazone, worsening renal function
Subject Categories: Cardiorenal Syndrome, Heart Failure
Clinical Perspective
What Is New?
In this analysis of nearly 14 000 acute decompensated heart failure admissions to 3 hospitals with a common medical record we utilized propensity score adjustment, an epidemiologic method known to reduce confounding by indication, to examine the relationship between both escalating doses of loop diuretics and metolazone with short‐term outcomes and all‐cause mortality.
Notably, the generalizable statistical methodology of propensity scores utilized in this study successfully adjusted away the initial perceived mortality disadvantage associated with increasing doses of loop diuretics, ultimately revealing their safety, even at higher doses
However, propensity adjusted analyses of metolazone use revealed a persistent and significant association with increased mortality, an effect predominantly mediated through short‐term side effects associated with metolazone including hyponatremia, hypokalemia and worsening renal function.
What Are the Clinical Implications?
In the first propensity‐adjusted and large analysis of metolazone use in acute decompensated heart failure, this study strongly calls into question the routine early use of adjuvant metolazone for treatment of acute decompensated heart failure when loop diuretic doses have not yet been optimized.
Introduction
The primary therapeutic objective in the majority of acute decompensated heart failure (ADHF) hospitalizations is relief of congestion.1, 2 Intravenous loop diuretics form the mainstay of decongestive therapy, but unfortunately, resistance to these agents is common.3 Both US and European Heart Failure (HF) guidelines state that when diuresis remains inadequate with loop diuretic therapy, either escalation of loop diuretic dose or the addition of a thiazide diuretic may be considered to intensify the regimen, yet these recommendation are based on limited data to support the relative safety of each appraoch.4, 5, 6 Notably, sequential nephron blockade further limits the ability of the kidney to regulate fluid and electrolyte excretion beyond loop diuretic monotherapy, potentially leading to complications such as hyponatremia, hypokalemia, and worsening renal function (WRF).7, 8, 9, 10
In the current analysis we set out to better understand the relationship between the use of the thiazide‐type diuretic metolazone, high‐dose loop diuretics (HDLDs), and adverse outcomes during and after ADHF therapy. The ideal study design would involve a randomized controlled trial of metolazone powered for all‐cause mortality, but unfortunately such a trial is unlikely to be performed for the foreseeable future. Therefore, our approach was to use epidemiologic methods known to reduce confounding by indication, such as propensity score adjustment, to examine the above associations. Notably, these methods have been previously shown to eliminate the relationship between HDLDs and inferior outcomes, thus providing a positive control to the methodology while directly evaluating the alternative approach of high‐dose loop diuretic use.11
Methods
This is a propensity‐adjusted cohort study of all patients admitted to 3 independent hospitals that all used a common electronic medical record within the Yale Health System (Yale New Haven Hospital, The Hospital of Saint Raphael and Bridgeport Hospital) between January 1, 2013 and September 30, 2015 whose discharge record included documentation of congestive heart failure by International Classification of Diseases, Ninth or Tenth Revision (ICD‐9 or ICD‐10) code. Inclusion required receiving intravenous loop diuretics within the first 7 days and at least 2 creatinine measurements during the admission. In the event of multiple hospitalizations for a single patient, all admissions meeting the inclusion criteria were maintained, particularly given that patients with many readmissions are more likely to receive more aggressive diuretic therapy. Patients without information on race (n=125) to calculate an estimated glomerular filtration rate (eGFR) were excluded resulting in a total of 13 898 unique admissions in 8908 patients.
The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR.12 WRF was defined as a ≥20% decrease in eGFR from admission to discharge.13, 14, 15 Any WRF was defined as a ≥20% decrease in estimated glomerular filtration rate from admission to the point of worst eGFR during the hospitalization. Hyponatremia was defined as any sodium level <135 mEq/L and hypokalemia was defined as any serum potassium <3.5 mEq/L. New hyponatremia was defined as a sodium level <135 mEq/L and new hypokalemia was defined as a serum potassium <3.5 mEq/L that developed during the course of the hospitalization (ie, patients with new electrolyte abnormalities who did not meet these criteria at admission). Loop diuretic doses were converted to intravenous furosemide equivalents with 1 mg bumetanide=20 mg torsemide=40 mg furosemide.16, 17 Peak diuretic dose was defined as the highest single intravenous (IV) dose of diuretic during the first 7 days of the hospitalization in IV furosemide equivalents. HDLD use was defined as a peak diuretic dose of >160 mg of IV furosemide equivalents.18 The Elixhauser Comorbidity Index, a marker of in‐hospital mortality, was determined based on ICD‐9 and ICD‐10 diagnostic codes for 30 comorbid conditions.19 All‐cause mortality was determined using the National Death Index.20 This study was approved by the Yale University Institutional Review Board and informed consent was waived. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Propensity Score Development
In this population, combination diuretic therapy using metolazone was at the discretion of the treating physician and therefore the relationship between metolazone and mortality may be confounded by patient factors related to mortality that also affect the decision to use metolazone. A propensity score represents the probability that an individual will receive a given treatment (ie, metolazone) conditional on a set of baseline characteristics. Propensity score adjustment significantly reduces treatment selection bias helping to approximate a randomized controlled trial in the setting of observational data.21 A propensity score was determined using multivariable logistic regression with metolazone use as the dependent variable. Independent variables were comprised of patient and admission characteristics that were associated with metolazone use at a P≤0.2 (Table 1 ) with no additional selection procedures. The 39 independent variables included in the propensity score were age, sex, creatinine, eGFR, blood urea nitrogen, first dose of intravenous diuretic on admission, prior admission with metolazone use, additional admission laboratory data (sodium, chloride, bicarbonate, white blood cell count, hemoglobin, platelet count), arrhythmia, valvular disease, pulmonary circulatory disease, hypertension with and without complications, end‐stage renal disease, renal failure, fluid and electrolyte disorders, chronic obstructive pulmonary disease, diabetes mellitus, hypothyroidism, obesity, liver disease, Elixhauser comorbidity index, hospital of admission and history of prior admission, lymphoma, solid tumor without metastasis, metastatic cancer, rheumatologic disease, coagulopathy, anemia because of blood loss, drug use, neurologic disease, and paralysis. The logistic regression was used to determine the probability of receiving metolazone for each admission, ie, the propensity score, and is presented in Table S1. The adequacy of the propensity score in adjusting for metolazone use was examined by testing for differences in individual covariates between patients who did and did not receive metolazone after stratifying by blocks of the propensity score. The included covariates were well balanced across the metolazone and no metolazone groups within blocks of the propensity score. The propensity score successfully adjusted for major covariates of interest that were found to be highly statistically significantly different between the metolazone groups before adjustment.22 Given that uptitration of loop diuretics is the alternate guideline recommended therapy when lower doses are not effective, as a secondary analysis, a second propensity score was developed using multivariable logistic regression with high‐dose loop diuretic use as the dependent variable. This propensity model was developed similarly to that for metolazone and included the following covariates: metolazone use, baseline diuretic dose, age, arrhythmias, valvular disease, pulmonary circulatory disease, diabetes mellitus, hypertension, obesity, liver disease, lymphoma, malignancy, rheumatoid disease, electrolyte disease, coagulopathy, hospital, readmission, Elixhauser comorbidity index and baseline sodium, chloride, blood urea nitrogen, eGFR, creatinine, white blood cell count, and platelets. This score was also well balanced across high‐ and low‐dose diuretic groups.
Table 1.
Baseline Characteristics of All Admissions and Those With and Without Metolazone Use
| Characteristic | All Admissions | Metolazone Use During Admission | P Value | |
|---|---|---|---|---|
| N=13 898 | No (n=12 850) | Yes (n=1048) | ||
| Demographics | ||||
| Age at encounter, y | 75.0±14.4 | 75.4±14.3 | 69.9±14.3 | <0.001a |
| Male sex, % | 48.4 | 47.7 | 57.5 | <0.001a |
| Black race, % | 16.9 | 16.9 | 18.2 | 0.232 |
| Comorbidities | ||||
| Arrhythmia, % | 63.5 | 62.5 | 75.1 | <0.001a |
| Valvular disease, % | 35.9 | 35.1 | 45.2 | <0.001a |
| Pulmonary circulatory disease, % | 25.1 | 24.3 | 35.1 | <0.001a |
| Peripheral vascular disease, % | 13.9 | 13.9 | 13.7 | 0.830 |
| Hypertension without complications, % | 42.4 | 44.2 | 20.2 | <0.001a |
| Hypertension with complications, % | 38.3 | 36.5 | 60.1 | <0.001a |
| End‐stage renal disease, % | 3.89 | 3.36 | 10.3 | <0.001a |
| Renal failure, % | 39.8 | 37.7 | 66.2 | <0.001a |
| Fluid and electrolyte disorders, % | 42.0 | 40.6 | 59.4 | <0.001a |
| COPD, % | 52.5 | 52.2 | 56.2 | 0.012a |
| Diabetes mellitus without complications, % | 31.3 | 31.1 | 34.5 | 0.020a |
| Diabetes mellitus with complications, % | 11.3 | 10.7 | 19.5 | <0.001a |
| Hypothyroidism, % | 20.6 | 20.4 | 22.8 | 0.063 |
| Obesity, % | 21.4 | 20.6 | 31.7 | <0.001a |
| Liver disease, % | 6.55 | 6.12 | 11.8 | <0.001a |
| Peptic ulcer disease, % | 0.72 | 0.71 | 0.86 | 0.579 |
| HIV, % | 0.41 | 0.40 | 0.48 | 0.724 |
| Lymphoma, % | 2.06 | 1.98 | 2.96 | 0.033a |
| Solid tumor without metastasis, % | 5.55 | 5.73 | 3.34 | 0.001a |
| Metastatic cancer, % | 2.67 | 2.78 | 1.34 | 0.005a |
| Rheumatologic disease, % | 4.94 | 5.07 | 3.44 | 0.019a |
| Coagulopathy, % | 6.32 | 6.11 | 8.97 | <0.001a |
| Anemia because of blood loss, % | 1.92 | 1.98 | 1.15 | 0.057 |
| Anemia because of deficiency, % | 6.20 | 6.16 | 6.77 | 0.424 |
| Alcohol abuse, % | 4.51 | 4.54 | 4.20 | 0.612 |
| Drug use, % | 4.28 | 4.37 | 3.15 | 0.060 |
| Psychoses, % | 1.96 | 1.98 | 1.62 | 0.416 |
| Depression, % | 16.5 | 16.4 | 17.6 | 0.344 |
| Neurologic disease, % | 8.34 | 8.47 | 6.77 | 0.057 |
| Paralysis, % | 1.27 | 1.24 | 0.38 | 0.004a |
| Elixhauser comorbidity index | 6.2±2 | 6.1±2 | 7.2±2 | <0.001a |
| Diuretics | ||||
| First dose of diuretic on admission in intravenous furosemide equivalents, mg | 40 (20,40) | 40 (20,40) | 60 (40,80) | <0.001a |
| Metolazone given on prior admit, % | 4.32 | 2.92 | 21.5 | <0.001a |
| Baseline laboratory values | ||||
| Sodium, mEq/L | 138.0±4.9 | 138.1±4.9 | 136.9±5.0 | <0.001a |
| Potassium, mEq/L | 4.3±0.7 | 4.3±0.7 | 4.3±0.83 | 0.453 |
| Chloride, mEq/L | 100.6±5.9 | 100.8±5.8 | 98.29±6.4 | <0.001a |
| Bicarbonate, mEq/L | 24.5±5.0 | 24.4±5.0 | 24.7±5.2 | 0.171 |
| Blood urea nitrogen, mg/dL | 33.4±22.5 | 32.0±21.1 | 51.2±30.8 | <0.001a |
| Creatinine, mg/dL | 1.60±1.4 | 1.55±1.3 | 2.20±1.5 | <0.001a |
| eGFR, mL/min per 1.73 m2 | 53.3±27 | 54.3±27 | 41.1±28 | <0.001a |
| Glucose, mg/dL | 149.7±77.3 | 149.7±77.5 | 150.4±74.2 | 0.762 |
| White blood cell count, K/mm3 | 10.3±6.9 | 10.3±7.1 | 9.5±5.0 | <0.001a |
| Hemoglobin, g/dL | 11.6±2.3 | 11.6±2.3 | 11.0±2.2 | <0.001a |
| Platelet count, K/mm3 | 231.4±102 | 232.4±102 | 219.8±94.0 | <0.001a |
| Hospitalization | ||||
| Hospital | ||||
| Yale New Haven Hospital, % | 50.5 | 49.2 | 66.9 | <0.001a |
| Saint Raphael Campus, % | 30.2 | 31.0 | 19.7 | |
| Bridgeport Hospital, % | 19.3 | 19.8 | 13.5 | |
| Readmission, % | 35.9 | 34.6 | 51.7 | <0.001a |
Values reported are mean±SD, median (quartile 1 to quartile 3), and percentile. COPD indicates chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate.
Significant P‐value.
Statistical Analysis
Values reported are mean±SD, median (quartile 1 to quartile 3), and percentile. The independent Student t test or the Wilcoxon rank‐sum test was used to compare continuous parameters. The Pearson chi‐square was used to evaluate categorical variables. The primary goal of this analysis was to evaluate the association between metolazone use during the treatment of ADHF and all‐cause mortality using propensity score adjustment. Time‐varying Cox proportional hazards modeling was used to evaluate time‐to‐event associations with all‐cause mortality and to account for multiple admissions per patient. Candidate covariates for multivariable modeling were obtained by screening all baseline variables, and those with a univariate association with mortality at P≤0.2 were entered and retained in the model. Given that in‐patient loop diuretic requirement is strongly related to both metolazone use and outcomes, and that such information is not available at baseline, all adjusted analyses included adjustment for BOTH the propensity score (which includes the first dose of intravenous diuretic in the hospital) and the peak diuretic dose received in the hospital as a representation of loop diuretic requirement. A secondary goal of this analysis was to examine the relationship between metolazone and other relevant outcomes in ADHF including hyponatremia, hypokalemia, and WRF. Logistic and linear regression models were developed and clustered on the individual patient to account for multiple admissions in the same subject. Multivariable models of these outcomes were adjusted for the metolazone propensity score, the peak loop diuretic dose received, baseline characteristics and length of stay. We subsequently determined whether the relationship between metolazone and increased mortality was mediated by a number of these outcomes/side effects. First we constructed separate time‐varying Cox proportional hazard models for hyponatremia, hypokalemia, and WRF to quantify their relationship with mortality. We then examined the effect of the addition of these 3 outcomes (potential mediators) on the magnitude of the hazard ratio for metolazone in the multi‐record Cox proportional hazard model adjusted for the metolazone propensity score and peak loop diuretic dose. Finally, to examine the relationships between HDLD use and mortality, we constructed time‐varying Cox proportional hazards models in a similar fashion to those built for metolazone except adjusted for the HDLD propensity score. We further examined the effect of hyponatremia, hypokalemia, and WRF on the relationship between HDLDs and mortality. Statistical analyses were performed using Stata 14.0 (Statacorp, College Station, TX), and statistical significance was defined as a 2‐sided P<0.05.
Results
Overall, 13 898 admissions in 8908 unique patients met the inclusion criteria. Baseline characteristics of all admissions and those with and without metolazone use are presented in Table 1. In total, 7.5% of all admissions (n=1048) used metolazone in addition to loop diuretics for diuresis. Patients who received metolazone exhibited a number of differences in their presenting characteristics including a greater likelihood of having received metolazone on a prior admission (Table 1).
In‐hospital and treatment‐related outcomes of all admissions stratified by metolazone use are presented in Table 2. Loop diuretic requirement both during the hospitalization and at discharge was significantly greater in patients also on metolazone, with a median peak intravenous diuretic dose of 120 mg. However, there was significant heterogeneity in the peak dose of loop diuretic, particularly in those who also were on metolazone, with some patients receiving only minimal doses of loop diuretics in conjunction with metolazone and others on HDLD therapy (Figure 1). On average, patients who received metolazone during their ADHF hospitalization were discharged with lower sodium, potassium and chloride levels as well as lower eGFRs (Table 2). Although patients who received metolazone were more likely to present with electrolyte disturbances and worse renal function (Table 1), the differences at discharge were not merely a reflection of how patients presented, as the median decreases in these electrolytes were also far more pronounced in the metolazone group (Table 2). Admissions using metolazone were also characterized by more hyponatremia, hypokalemia, and WRF at discharge.
Table 2.
In‐Hospital Characteristics of All Admissions by Metolazone Use
| Characteristic | All Admissions | Metolazone Use During Admission | P Value | |
|---|---|---|---|---|
| N=13 898 | No (n=12 850) | Yes (n=1048) | ||
| Diuretics | ||||
| Peak diuretic dose during hospitalization in IV furosemide equivalents, mg | 80 (40,80) | 80 (40,80) | 120 (80 160) | <0.001a |
| Last IV diuretic dose before discharge in IV furosemide equivalents, mg | 60 (40,80) | 40 (40,80) | 80 (40 160) | <0.001a |
| Diuretic dose on day of discharge in IV furosemide equivalents, mg | 40 (0,80) | 40 (0,80) | 80 (0,160) | <0.001a |
| Laboratory findings at discharge | ||||
| Sodium, mEq/L | 138.9±4.28 | 139.0±4.19 | 136.8±4.82 | <0.001a |
| Potassium, mEq/L | 4.11±0.51 | 4.13±0.50 | 3.97±0.53 | <0.001a |
| Chloride, mEq/L | 100.6±5.52 | 101.1±5.18 | 95.0±6.43 | <0.001a |
| Creatinine, mg/dL | 1.53±1.22 | 1.46±1.17 | 2.34±1.50 | <0.001a |
| eGFR, mL/min per 1.73 m2 | 56.0±28.3 | 57.5±28.2 | 38.1±25.1 | <0.001a |
| Hemoglobin, g/dL | 10.9±2.0 | 11.0±2.0 | 10.6±2.0 | <0.001a |
| Median change in laboratory values (discharge—baseline) | ||||
| Sodium, mEq/L | 0 (−2.0, 3.0) | 0 (−2.0, 3.0) | −1 (−3.0, 3.0) | <0.001a |
| Potassium, mEq/L | −0.2 (−0.6, 0.3) | −0.2 (−0.6, 0.3) | −0.3 (−0.8, 0.25) | <0.001a |
| Chloride, mEq/L | 0 (−3.0, 3.0) | 0 (−3.0, 3.0) | −3.0 (−8.0, 1.0) | <0.001a |
| Creatinine, mg/dL | 0 (−0.2, 0.1) | 0 (−0.2, 0.1) | 0.1 (−0.2, 0.5) | <0.001a |
| eGFR, mL/min per 1.73 m2 | 0 (−5.1, 10) | 0.9 (−4.8, 11) | −2.0 (−8.8, 3.8) | <0.001a |
| Hemoglobin, g/dL | −0.5 (−1.4, 0.20) | −0.6 (−1.4, 0.1) | −0.40 (−1.2, 0.3) | <0.001a |
| Outcomes at discharge | ||||
| Hyponatremia, % | 13.5 | 12.3 | 28.0 | <0.001a |
| Hypokalemia, % | 7.25 | 6.55 | 15.7 | <0.001a |
| WRF, % | 14.6 | 13.2 | 32.3 | <0.001a |
| Length of stay, days | 9.29±12.4 | 8.88±12.0 | 14.4±15.3 | <0.001a |
Values reported are mean±SD, median (quartile 1 to quartile 3), and percentile. WRF was defined by a ≥20% decrease in eGFR from admission to discharge. eGFR indicates estimated glomerular filtration rate; IV, intravenous; WRF, worsening renal function.
Significant P‐value.
Figure 1.
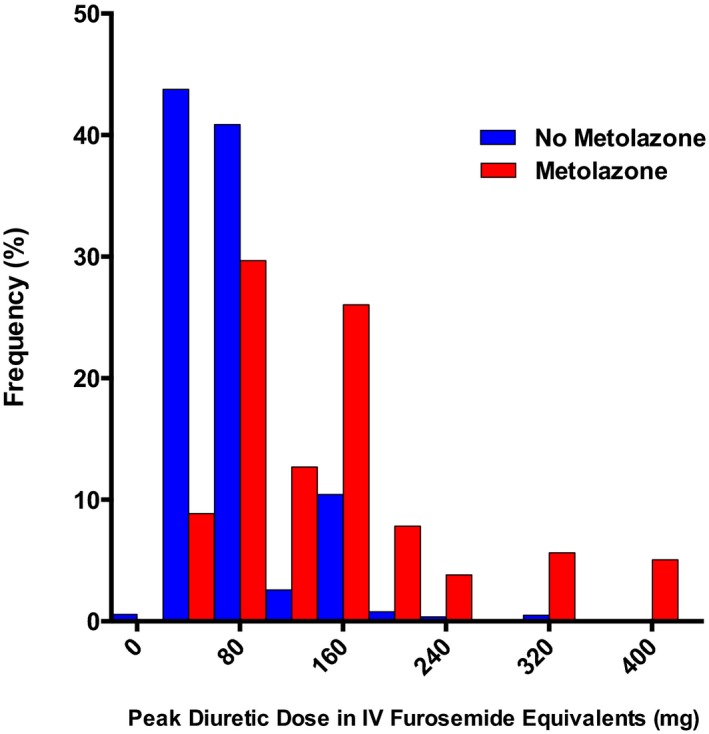
The distribution of peak diuretic dose during admissions with and without use of metolazone. Peak diuretic dose was defined as the highest single intravenous dose of diuretic during the first 7 days of the hospitalization in intravenous furosemide equivalents. IV indicates intravenous.
The multivariable model used to determine the propensity score representing the probability of receiving metolazone is presented in Table S1. There was good discrimination between patients who did and did not receive metolazone as evidenced by the area under the receiver operating curve of 0.83 (95% confidence interval [CI] 0.82–0.84). Not surprisingly, 2 of the strongest predictors of metolazone use were the initial dose of IV diuretic in the hospital and prior metolazone use. Older age and worse renal function were also strongly predictive of receiving metolazone (Table S1). The propensity score successfully adjusted for important covariates, including age, Elixhauser comorbidity index, hemoglobin, sodium, creatinine, and baseline diuretic dose, as evidenced by similar values of these covariates across deciles of the propensity score for metolazone use (Figure 2).
Figure 2.
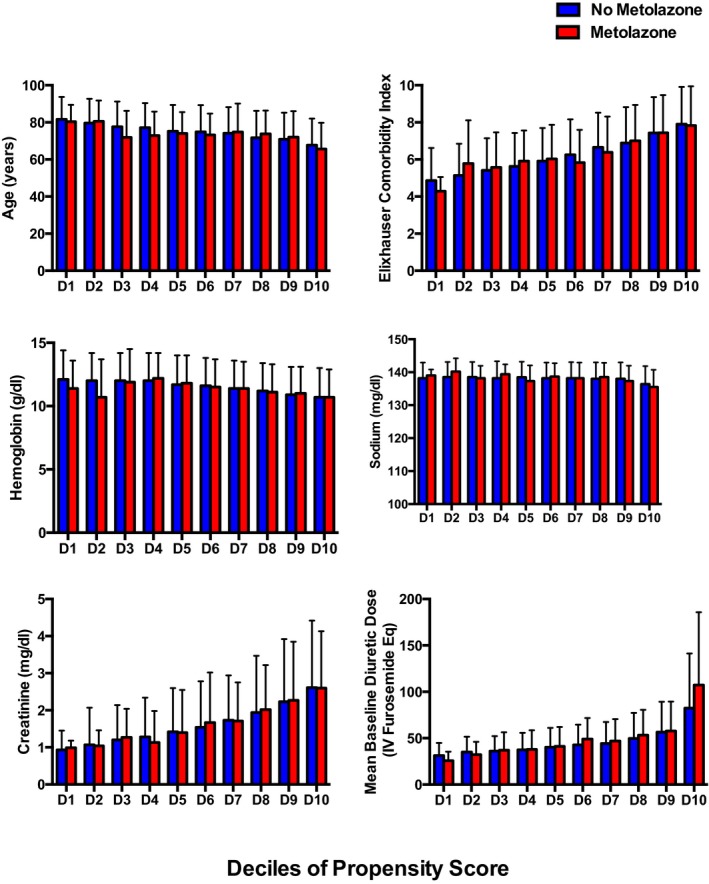
Patients were stratified into deciles of the propensity score and within each decile, patients who received metolazone were compared with those who did not. The 6 variables shown represent a combination of important confounders and disease severity indicators. Selection bias was significantly minimized with implementation of the propensity score as shown above. Eq indicates equivalents; IV intravenous.
Metolazone and Risk of In‐Hospital Adverse Events
Both the unadjusted and propensity adjusted analyses of metolazone use and outcomes both during the hospitalization and at discharge are presented in Figure 3 and Table S2. Metolazone was strongly and significantly associated with its previously reported side effects: hyponatremia (both incident and discharge), hypokalemia (incident and discharge), and WRF (both any and at discharge). Following adjustment for the propensity to receive metolazone and peak loop diuretic dose, the association between metolazone and hyponatremia, hypokalemia, and WRF remained with minimal attenuation (Figure 3, Table S2). Furthermore, additional extensive adjustment for baseline characteristics in addition to the metolazone propensity score and peak loop diuretic dose only served to strengthen the significant associations between metolazone and these outcomes at discharge (Figure 3, Table S2).
Figure 3.
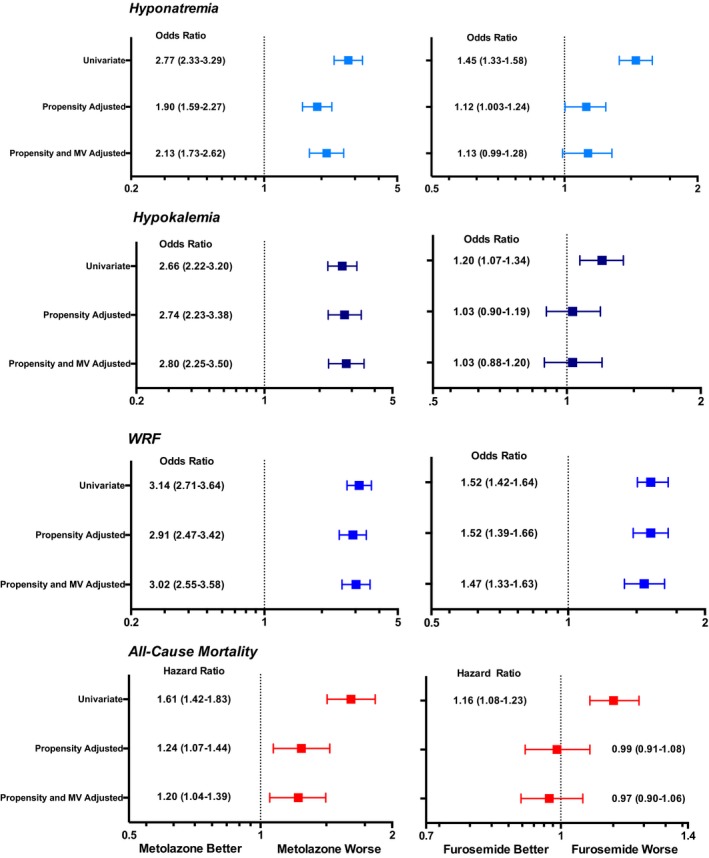
The unadjusted, propensity adjusted, and propensity plus multivariable adjusted relationships between metolazone use and hyponatremia, hypokalemia, worsening renal function (WRF), and all‐cause mortality as well as the relationships between increasing doses of loop diuretic are presented above. All metolazone propensity‐adjusted analyses are also adjusted for peak‐loop diuretic dose received in the hospital in intravenous furosemide equivalents as a representation of loop diuretic requirement. Multivariable models included adjustment for age at encounter, race, sex, arrhythmia, valvular disease, pulmonary circulatory disease, hypertension with and without complications, chronic obstructive pulmonary disease, diabetes mellitus with and without complications, hypothyroidism, renal failure, electrolyte disease, neurologic disease, paralysis, liver disease, lymphoma, malignancy with and without metastasis, rheumatologic disease, coagulopathy, obesity, anemia secondary to blood loss, drug abuse, Elixhauser comorbidity index, prior metolazone use, readmission, as well as baseline laboratory values including sodium, chloride, bicarbonate, blood urea nitrogen, white blood cell count, hemoglobin, platelets, glomerular filtration rate and length of stay. WRF was defined as a ≥20% decrease in estimated glomerular filtration rate from admission to discharge. Hyponatremia was defined as a sodium level <135 mEq/L and hypokalemia was defined as a serum potassium <3.5 mEq/L. All hazard ratios reported for furosemide are for every 100 mg of intravenous furosemide. MV indicates multivariable; WRF, worsening renal function.
Metolazone and Risk of All‐Cause Mortality
Over a median follow‐up of 423 days from a subject's first admission, 31.7% of the population died (n=2827). Metolazone use was significantly associated with increased mortality (hazard ratio [HR]=1.61; 95% CI 1.42–1.83, P<0.001, Figure 3). Following adjustment for the metolazone propensity score and peak diuretic dose, thereby approximating a quasi‐randomized experiment, metolazone remained independently and highly significantly associated with mortality (HR=1.24; 95% CI 1.07–1.44, P=0.004; Figure 3). Extensive adjustment for baseline characteristics and length of stay did not alter the strong and independent relationship between metolazone and decreased survival, convincingly supporting a potential causal link between metolazone and mortality. We then examined if the effect on all‐cause mortality of metolazone was mediated through the adverse in‐hospital outcomes that were strongly and independently linked. Further adjustment of the survival model for the 3 putative adverse effects of metolazone (WRF, hypokalemia, and hyponatremia) resulted in a substantial decrease in the strength of the HR for metolazone, consistent with much of the adverse survival effect of metolazone being mediated through these short‐term outcomes (HR=1.06; 95% CI 0.91–1.23, P=0.448).
High‐Dose Loop Diuretics and Adverse Outcomes
Using a similar approach, we next examined the impact of high doses of loop diuretics on outcomes in our cohort adjusting for the propensity to receive HDLDs. This propensity score also demonstrated good discrimination between patients who did and did not receive HDLDs as evidenced by the area under the receiver operating curve of 0.92 (95% CI 0.91–0.93). Similar to metolazone, increasing doses of loop diuretic were significantly associated with hyponatremia, hypokalemia, and WRF (Figure 3, Table S2). Following adjustment for the propensity score, the relationship between higher doses of loop diuretics and both hyponatremia and hypokalemia as well as incident hyponatremia and incident hypokalemia dramatically attenuated (Figure 3, Table S2). The relationship between HDLDs and WRF was strong and minimally attenuated after adjustment regardless of whether WRF occurred during the hospitalization or only at time of discharge.
Higher loop diuretic dose demonstrated a strong univariate association with increased mortality (HR=1.15 per 100 mg of IV furosemide, 95% CI 1.08–1.23, P<0.001; Figure 3). Following adjustment for the propensity score, there was no significant relationship between the higher peak inpatient diuretic dose and survival (HR=0.99 per 100 mg of IV furosemide, 95% CI 0.91–1.08, P=0.82; Figure 3). However, with extensive adjustment for baseline characteristics as well as potential mediators of adverse outcomes (hyponatremia, hypokalemia, and WRF), increasing doses of loop diuretics demonstrated a trend toward improved survival (HR=0.93 per 100 mg of IV furosemide, 95% CI 0.85–1.006, P=0.07).
Metolazone Use in Patients on High‐Dose Loop Diuretics
Given the heterogeneity in dose of loop diuretic used in conjunction with adjuvant thiazide, as an exploratory analysis, we examined the relationship between metolazone, its mediators and mortality in only those patients on high‐dose loop diuretics (>160 mg intravenous furosemide equivalents; n=469). Remarkably, amongst patients receiving HDLDs (of which 51% received metolazone) there was no relationship with mortality even in the unadjusted analysis (HR=1.10, 95% CI 0.73–1.39; P=0.95). The lack of an association between metolazone and mortality persisted following adjustment for the metolazone propensity score, baseline characteristics, and potential mediators of the adverse effect of metolazone (WRF, hypokalemia, and hyponatremia), with the point estimate now favoring a protective effect, although this was not significant. (HR=0.73, 95% CI 0.50–1.08, P=0.11).
Discussion
The principal finding of this study is that in a large and diverse real‐world population, using statistical methodology to reduce the influence of confounding by indication, adjuvant metolazone use during ADHF was independently and strongly associated with hyponatremia, hypokalemia, WRF, and decreased long‐term survival. This risk associated with metolazone appeared to be largely mediated through these in‐hospital acute adverse events. However, when the use of HDLDs were subjected to the same analytic methodology, there was no risk for hyponatremia, hypokalemia, or mortality. In light of the existing randomized trial data to support the safety of aggressive loop diuretic dosing,23 and the currently presented findings suggesting a mortality disadvantage with metolazone as well as an increased risk of electrolyte disturbances and WRF, routine escalation of loop diuretic doses may be the preferred approach for the management of ADHF until adequately powered trials are available to more definitively inform this question.
The primary treatment for ADHF is intravenous loop diuretic, and in most patients, this therapy at modest doses is adequate to improve symptoms.24 However, compensatory sodium reabsorption that occurs in the distal convoluted tubule, the site of action of thiazide diuretics, is an important cause for diuretic resistance.25 The addition of a thiazide or thiazide‐like diuretic (ie, metolazone) to loop diuretics, often referred to as sequential nephron blockade, inhibits compensatory distal tubular sodium reabsorption, thereby enhancing natriuresis.26 Furthermore, unlike loop diuretics, thiazides do not directly stimulate renin secretion at the macula densa and therefore do not activate the renin angiotensin aldosterone system to the same extent.27 Physiologically this seems like an attractive approach to decongestion, one that is supported in the current heart failure practice guidelines, despite no randomized trials comparing combination diuretic therapy with a thiazide to loop diuretics alone.4 In fact, most studies examining metolazone use in HF are small, single‐center retrospective analyses without a control arm, published over 25 years ago, that focused on end points like urinary volume and sodium concentration as opposed to clinical outcomes.8, 10, 28, 29 Nevertheless, the cumulative existing evidence in only 350 patients does illustrate that metolazone produces a 3‐fold increase in natriuresis, increases weight loss and improves diuresis in patients who were previously not suitable for hospital discharge.7, 8, 9, 10, 28, 29, 30
Of course, these benefits of thiazides do not come without a cost; by blocking sequential nephron segments, the kidney's ability to deploy diuretic breaking and prevent overdiuresis is impaired.27 As a result, the sequelae of overzealous diuresis like WRF, as we found here, are to be expected. Furthermore, because of their location of action, thiazides directly cause hyponatremia and lead to a disproportionate loss of potassium, thus predisposing to hypokalemia, which affected >30% of patients in this study who received metolazone.29 As a result of these mechanisms, thiazides can either benefit patients by accelerating decongestion in the face of diuretic resistance or harm patients via electrolyte depletion and WRF. We are therefore left with clinical equipoise as to whether thiazides should be used early in ADHF, and if so, when and in which subset of patients.
This treatment question often presents itself in the setting of a patient who is not responding to traditional doses of diuretics with the therapeutic options including increasing loop diuretics to higher doses versus the addition of a thiazide diuretic. Although there is a lack of randomized data to specifically answer this question, the DOSE (Diuretic Optimization Strategies Evaluation) trial provides support for the safety and efficacy of intravenous HDLDs. In this 308 ADHF patient population, patients randomized to intravenous HDLD received an average of 773 mg of intravenous furosemide in 72 hours and experienced greater fluid and weight loss, a greater incidence of WRF, but without any worsening in death or rehospitalization.23 In our study using propensity score adjustment to reduce confounding by indication, we found similar findings with a greater incidence of WRF but no survival disadvantage. The application of these identical epidemiologic methods to metolazone revealed a persistent significant mortality disadvantage. Therefore, when faced with a choice of loop diuretic escalation versus employing a therapy that may carry a mortality risk mediated by its side effect profile, our results would suggest the safer therapy may be the dose escalation of loop diuretics.
That is not to say that there may be situations when the addition of a thiazide diuretic is appropriate or when the potential benefits outweigh the known risks as shown in this study. Patients on the highest doses of diuretic therapy in this study who went on to receive metolazone did not appear to suffer the same mortality disadvantage. To the contrary, when the analysis was adjusted for the mediators of metolazone's mortality impact, there was a trend toward a benefit of metolazone. Although this subgroup analysis was highly exploratory and should be interpreted as hypothesis‐generating only, it does call for further investigation into which patients may in fact benefit from combination diuretic therapy.
Limitations
There are limitations that must be considered when interpreting these results. First, although propensity score adjustment significantly reduces confounding and was developed to simulate a quasi‐randomized study design for the purposes of causal inference, this study was not a randomized controlled trial and uncontrolled confounding most likely remains. Although we have adjusted our analysis more expansively than prior published reports, bias may still occur as the propensity score cannot adjust for unmeasured confounders. Furthermore, given the retrospective nature of this study, causality is impossible to demonstrate. Secondly, physicians were not masked to electrolytes or measures of renal function and may have altered treatment decisions in response to these data. Still, our focus was on the presence of known side effects of metolazone at discharge, so regardless of physician treatment decisions, the physician in charge of the patient still felt despite the presence of electrolyte abnormalities or WRF that the patient was suitable for discharge. Third, although our data were generated from both academic and community hospital environments, all hospitals were located in the same state and health system, potentially limiting the generalizability of the results. Fourth, given the similarities of this data set to claims data, we lacked access to all granular data and clinical information within an individual patient's chart that could significantly affect mortality like blood pressure, ejection fraction and fluid and weight loss. We relied on diagnostic codes incorporated in the Elixhauser Comorbidity Index which has been validated in congestive heart failure.31 Finally, as this analysis was restricted to metolazone, these results may not be generalizable to all thiazide diuretics. As a result of the above limitations, our findings serve to primarily initiate further investigation into combination diuretic therapy with metolazone.
Conclusions
During the treatment of ADHF, metolazone use is independently associated with increased mortality. This survival disadvantage appears to be mediated predominantly by the hyponatremia, hypokalemia, and WRF metolazone induces. Importantly, treatment of ADHF with high doses of loop diuretics, a clinical alternative to the addition of a thiazide diuretic, demonstrates no survival disadvantage. These data suggest combination diuretic therapy with metolazone is not without risk and may not be appropriate in all patients. Further research is necessary to establish which patients, are most likely to benefit from combination diuretic therapy. Until these data are available, based on the current findings in the context of prior published studies, maximizing loop diuretic dose, rather than early introduction of adjuvant metolazone, may be the preferred strategy in clinical practice.
Sources of Funding
This work was supported by the National Institutes of Health, K23HL128933 (Brisco‐Bacik), K23HL114868, R01HL128973, and 4L30HL115790 (Testani), and K23DK097201 (Wilson).
Disclosures
None.
Supporting information
Table S1. Multivariable Model of Metolazone Use Utilized for Propensity Score Determination
Table S2. Relationships Between Metolazone and Loop Diuretic Use With Incident Hyponatremia, Incident Hypokalemia, and Any WRF During Heart Failure Hospitalization
(J Am Heart Assoc. 2018;7:e009149 DOI: 10.1161/JAHA.118.009149.)
Contributor Information
F. Perry Wilson, Email: francis.p.wilson@yale.edu.
Jeffrey M. Testani, Email: Jeffrey.testani@yale.edu.
References
- 1. Adams K, Fonarow G, Emerman C, LeJemtel T, Costanzo M, Abraham W, Berkowitz R, Galvao M, Horton D. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. [DOI] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119:S3–S10. [DOI] [PubMed] [Google Scholar]
- 3. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, Tang WHW. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. 2014;7:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL; American College of Cardiology Foundation and American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;62:e240–e327. [DOI] [PubMed] [Google Scholar]
- 5. Heart Failure Society of A , Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. [DOI] [PubMed] [Google Scholar]
- 6. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol (Engl Ed). 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- 7. Rosenberg J, Gustafsson F, Galatius S, Hildebrandt PR. Combination therapy with metolazone and loop diuretics in outpatients with refractory heart failure: an observational study and review of the literature. Cardiovasc Drugs Ther. 2005;19:301–306. [DOI] [PubMed] [Google Scholar]
- 8. Channer KS, McLean KA, Lawson‐Matthew P, Richardson M. Combination diuretic treatment in severe heart failure: a randomised controlled trial. Br Heart J. 1994;71:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng TM, Konopka E, Hyderi AF, Hshieh S, Tsuji Y, Kim BJ, Han SY, Phan DH, Jeng AI, Lou M, Elkayam U. Comparison of bumetanide‐ and metolazone‐based diuretic regimens to furosemide in acute heart failure. J Cardiovasc Pharmacol Ther. 2013;18:345–353. [DOI] [PubMed] [Google Scholar]
- 10. Kiyingi A, Field MJ, Pawsey CC, Yiannikas J, Lawrence JR, Arter WJ. Metolazone in treatment of severe refractory congestive cardiac failure. Lancet. 1990;335:29–31. [DOI] [PubMed] [Google Scholar]
- 11. Yilmaz MB, Gayat E, Salem R, Lassus J, Nikolaou M, Laribi S, Parissis J, Follath F, Peacock WF, Mebazaa A. Impact of diuretic dosing on mortality in acute heart failure using a propensity‐matched analysis. Eur J Heart Fail. 2011;13:1244–1252. [DOI] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Testani JM, Mccauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio‐renal interactions. Cardiology. 2010;116:206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, Tang WH, Testani JM. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. 2016;22:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin‐converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–189. [DOI] [PubMed] [Google Scholar]
- 17. Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–609. [DOI] [PubMed] [Google Scholar]
- 18. Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic‐associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, Saunders LD, Beck CA, Feasby TE, Ghali WA. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43:1130–1139. [DOI] [PubMed] [Google Scholar]
- 20. Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. Am J Epidemiol. 2009;170:515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo S, Fraser MW. Propensity Score Analysis: Statistical Methods and Applications, 2nd ed Los Angeles: SAGE Publications, Inc; 2015. [Google Scholar]
- 22. Connors AF Jr, Speroff T, Dawson NV, Thomas C, Harrell FE Jr, Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ Jr, Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA. 1996;276:889–897. [DOI] [PubMed] [Google Scholar]
- 23. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, Lewinter MM, Deswal A, Rouleau JL, Ofili EO. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. [DOI] [PubMed] [Google Scholar]
- 25. Rao V, Planavsky N, Hanberg JS, Ahmed T, Brisco‐Bacik MA, Wilson FP, Jacoby D, Chen M, Wilson Tang WH, Cherney DZI, Ellison DH, Testani J. Compensatory distal reabsorption drives diuretic resistance in human heart failure. J Am Soc Nephrol. 2017;28:3414–3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jentzer J, DeWald T, Hernandez A. Combination of loop diuretics with thiazide‐type diuretics in heart failure. J Am Coll Cardiol. 2010;56:1527–1534. [DOI] [PubMed] [Google Scholar]
- 27. Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96:132–143. [DOI] [PubMed] [Google Scholar]
- 28. Tilstone WJ, Dargie H, Dargie EN, Morgan HG, Kennedy AC. Pharmacokinetics of metolazone in normal subjects and in patients with cardiac or renal failure. Clin Pharmacol Ther. 1974;16:322–329. [DOI] [PubMed] [Google Scholar]
- 29. Steinmuller ST, Puschett JB. Effects of metolazone in man: comparison with chlorothiazide. Kidney Int. 1972;1:169–181. [DOI] [PubMed] [Google Scholar]
- 30. Moranville MP, Choi S, Hogg J, Anderson AS, Rich JD. Comparison of metolazone versus chlorothiazide in acute decompensated heart failure with diuretic resistance. Cardiovasc Ther. 2015;33:42–49. [DOI] [PubMed] [Google Scholar]
- 31. Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD‐9 and ICD‐10 administrative databases. BMC Health Serv Res. 2008;8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariable Model of Metolazone Use Utilized for Propensity Score Determination
Table S2. Relationships Between Metolazone and Loop Diuretic Use With Incident Hyponatremia, Incident Hypokalemia, and Any WRF During Heart Failure Hospitalization


