Abstract
Background
Aging exponentially increases the incidence of morbidity and mortality of quintessential cardiovascular disease mainly due to arterial proinflammatory shifts at the molecular, cellular, and tissue levels within the arterial wall. Calorie restriction (CR) in rats improves arterial function and extends both health span and life span. How CR affects the proinflammatory landscape of molecular, cellular, and tissue phenotypic shifts within the arterial wall in rats, however, remains to be elucidated.
Methods and Results
Aortae were harvested from young (6‐month‐old) and old (24‐month‐old) Fischer 344 rats, fed ad libitum and a second group maintained on a 40% CR beginning at 1 month of age. Histopathologic and morphometric analysis of the arterial wall demonstrated that CR markedly reduced age‐associated intimal medial thickening, collagen deposition, and elastin fractionation/degradation within the arterial walls. Immunostaining/blotting showed that CR effectively prevented an age‐associated increase in the density of platelet‐derived growth factor, matrix metalloproteinase type II activity, and transforming growth factor beta 1 and its downstream signaling molecules, phospho‐mothers against decapentaplegic homolog‐2/3 (p‐SMAD‐2/3) in the arterial wall. In early passage cultured vascular smooth muscle cells isolated from AL and CR rat aortae, CR alleviated the age‐associated vascular smooth muscle cell phenotypic shifts, profibrogenic signaling, and migration/proliferation in response to platelet‐derived growth factor.
Conclusions
CR reduces matrix and cellular proinflammation associated with aging that occurs within the aortic wall and that are attributable to platelet‐derived growth factor signaling. Thus, CR reduces the platelet‐derived growth factor–associated signaling cascade, contributing to the postponement of biological aging and preservation of a more youthful aortic wall phenotype.
Keywords: aging, arterial remodeling, calorie restriction, proinflammation, rats, vascular smooth muscle cells
Subject Categories: Vascular Biology
Clinical Perspective
What Is New?
Calorie restriction (CR) increases the vascular health span through curbing age‐associated proinflammation within the arterial wall in rats.
CR effectively attenuates age‐associated arterial proinflammatory phenotype shifts at the molecular, cellular, and tissue level in rats.
CR preserve a youthful arterial phenotype in old rats.
What Are the Clinical Implications?
Well‐designed studies could be created in elderly humans without overt cardiovascular disease to confirm that CR can increase vascular health span and life span through postponement of biological aging by maintaining a more youthful aortic wall phenotype in humans.
CR could serve as a clinical approach to attenuate the age‐associated increase in the morbidity and mortality of cardiovascular diseases such as atherosclerosis in humans.
Aging exponentially increases the incidence of morbidity and mortality of quintessential cardiovascular disease mainly due to arterial wall proinflammatory phenotypic shifts at the molecular, cellular, and tissue levels.1, 2, 3 Increases in the local arterial wall proinflammatory molecules, platelet‐derived growth factor (PDGF), matrix metalloproteinase type II (MMP2) and transforming growth factor beta 1 (TGF‐β1), promote cellular and matrix phenotypic shifts that contribute to aortic wall thickening and stiffening that accompany advancing age in mammals, a prominent arterial phenotype of biological aging.4, 5, 6, 7, 8, 9 Interestingly, arterial proinflammatory conditions reduces the threshold or increases the susceptibility for pernicious inflammatory arterial events under hostile conditions such as hyperlipidemia.1, 10
The rate of arterial biological aging or systemic aging can be modulated by changes in lifestyle, for example, food consumption, cessation of smoking, and physical exercise.11, 12, 13, 14, 15 Calorie restriction (CR) effectively reduces the morbidity and mortality of Fischer 344 rats, which is related to a retardation of arterial stiffening, an improvement of arterial distensibility, and an eventual prolongation of the life span.16 Age‐associated increase in arterial stiffening is closely associated with arterial proinflammation.1, 4 Whether and how CR affects the proinflammatory molecular, cellular, and tissue phenotypic shifts within the rat arterial wall with aging, however, remain to be elucidated.
Here, we demonstrate that a 40% CR regimen, beginning at 1 month of age in Fischer 344 rats, substantially retards age‐associated aortic proinflammatory phenotypic shifts in the matrix, cellular, and molecular signaling that underlies intimal and medial thickening, a most prominent clinical phenotype.1, 10, 12 In vitro, CR attenuated the age‐associated vascular smooth muscle cells (VSMC) proinflammatory phenotypic shift with a mitigation of platelet‐derived growth factor (PDGF) signaling. Collectively, the current findings suggest that CR is a practical approach for delaying biological aging and preserving a more youthful aortic wall phenotype.
Material and Methods
All data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The materials that support the findings of this study are available from the corresponding author upon reasonable request.
Rats, Calorie Restriction Protocol, and Aortic Tissue Harvest
Male Fischer 344 (F344) rats were fed ad libitum (AL) or maintained on a 40% calorie restriction (CR) regimen beginning at 1 month of age as described.16, 17 The AL diet was the NIH‐31 standard diet, and the CR diet was a vitamin‐ and mineral‐fortified version of the NIH‐31, 40% less (by weight) than the average AL consumption. Aortic tissue isolated from AL and CR young (n=5, 6‐month‐old,) and old (n=5, 24‐month‐old) F344 rats were harvested as previously described.18 All procedures were approved by the Animal Care and Use Committee of the National Institute on Aging Intramural Research Program in accordance with National Institutes of Health guidelines.
Histology, Immunostaining, and Morphometric Analysis
Hematoxylin and eosin, Picrosirius Red, Verhoeff's Elastic‐van Gieson, and Masson's trichrome stains (American MasterTech Scientific, Inc, Lodi, CA) were performed per the manufacturer's instructions as described previously.5, 6, 18
Immunohistochemistry and immunofluorescence and their analyses were performed according to methods described previously.19 The immunohistochemical analysis was performed using the computerized imaging program MetaMorph (MetaMorph Imaging System, Universal Imaging Corp) using light microscopy.
The sources and description of primary antibodies used are listed in Table.
Table 1.
Primary Antibodies
| Antibody | Species | Titer Blotting | Titer Staining | Sources |
|---|---|---|---|---|
| α‐SMA | M | 1:1000 | 1:20 | Dako Corp, Denmark |
| CD31 | M | 1:50 | Dako Corp, Denmark | |
| MT1‐MMP | R, M | 1:250 | Chemicon Intern. Inc, CA | |
| TIMP2 | R, M | 1:100 | Chemicon Intern. Inc, CA | |
| LTBP‐1 | M | 1:50 | Chemicon Intern. Inc, CA | |
| LAP | M | 1:50 | Chemicon Intern. Inc, CA | |
| p‐SMAD2/3 | R | 1:1000 | 1:50 | Cell signaling, CA |
| SMAD2/3 | R | 1:1000 | Cell signaling, CA | |
| Collagen I | R | 1:1000 | 1:50 | Rockland Immunochemicals, Inc, PA |
| TGF‐β1 | R | 1:200 | 1:50 | Santa Cruz, CA |
| SM22α | G | 1:2000 | Abcam, MA | |
| Myocardin | M | 1:1000 | Abcam, MA | |
| β‐actin | M | 1:10 000 | 1:50 | Sigma‐Aldrich, Inc, MO |
CD31 indicates cluster of differentiation 31; G, goat; LAP, latent associated protein; LTBP‐1, latent TGF binding protein‐1; M, mouse; MT1‐MMP, membrane type 1 matrix metalloproteinase; p‐SMAD2/3, phospho‐mothers against decapentaplegic homolog‐2/3; R, rabbit; SMAD2/3, mothers against decapentaplegic homolog‐2/3; TGF‐β1, transforming growth factor beta 1; TIMP2, tissue inhibitor of metalloproteinases 2; α‐SMA, alpha smooth muscle actin.
Zymography
Zymography was performed according to modified protocols, as previously reported.5, 6, 7, 18 Sodium dodecyl sulfate polyacrylamide gel electrophoresis zymography was performed according to the manufacturer's protocol (ThermoFisher). In situ zymography was performed to localize net in situ activities of matrix metalloproteinases (MMPs). Fluorescein isothiocyanate–labeled dye‐quenched gelatin, intramolecularly quenched (Molecular Probes, Eugene, OR) was used as a substrate. Fresh aortic fragments were immersed in ornithine carbamyl transferase (Tissue‐Tek, Torrance, CA) and quick‐frozen in a block of dry ice. Aortic segments were then cut into 10‐μm sections and coated with a drop of the liquid mixture (fluorescein isothiocyanate–labeled dye‐quenched gelatin [500 ng/mL] mixed with the reaction buffer [0.05 mol/L Tris‐HCl, 0.15 mol/L NaCl, 5 mmol/L CaCl2, and 0.2 mmol/L NaN3, pH 7.6]). Slides were protected from light in a humidified incubator for 48 hours at 37°C. Negative controls were created as described without the addition of the fluorescein isothiocyanate‐labeled dye‐quenched gelatin.
Immunoblotting
Immunoblotting of supernatant from thoracic aortae homogenates or VSMC lysates were performed as previously described.5, 6, 19, 20 Primary antibodies used for Western blotting are listed in Table.
Cultured VSMCs
VSMCs were enzymatically isolated as previously described.5, 6, 19, 20 Briefly, young and old AL and CR F344 rat thoracic aortae (n=3 animals/group) were rinsed in Hanks balanced salt solution containing 50 μg/mL penicillin, 50 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (ThermoFisher). After digestion for 30 minutes in 2 mg/mL collagenase I solution (Worthington Biomedical, Freehold, NJ) at 37°C, the adventitia and intima were removed from the aortic medial layer, which was then placed in complete medium (Dulbecco's modified Eagle's medium containing 10% fetal bovine serum [FBS] and 50 μg/mL penicillin, 50 μg/mL streptomycin) for 2 hours. The arterial media was further digested with 2 mg/mL collagenase II/0.5 mg/mL elastase (Sigma) for 90 minutes at 37°C, and the isolated cells were washed and plated in complete medium. In all cases, >95% of cells stained positive for α‐smooth muscle actin (α‐SMA).
Early passages (p3‐p5) rat VSMCs were cultured both with and without PDGF‐BB (0, 10, and 25 ng/mL) for 24 hours and then were lysed for Western blot analysis.
TGF‐β1 ELISA
The supernatants from both young and old VSMCs were treated with or without PDGF‐BB and collected and analyzed with a TGF‐β1 ELISA kit based on the manufacturer's instruction (R&D Systems, MN). The optical density of each well was read using a VICTOR X Multilabel Plate Reader, set to 450 nm (PerkinElmer, Inc, Waltham, MA).
Silencing of PDGFβ Gene in VSMCs
VSMCs were cultured to about 80% confluence in 6‐well plates in Dulbecco's modified Eagle's medium/10% fetal bovine serum/1% nonessential amino acid medium. PDGFβ small interfering RNAs (Target sequence: GGUGAGAAAGAUCGAAAUU; J‐081176‐09‐0002) and scrambled control siRNA (Target sequence: UGGUUUACAUGUCGACUAA, D‐001810‐01‐05) were purchased from Dharmacon (Chicago, IL). Small interfering RNAs were delivered via Dharmafect 2 transfection reagent according to the manufacturer's instructions (Dharmacon, Chicago, IL). After 24 hours, cell medium was replaced with fresh culture medium and cells were harvested at 48 hours after transfection for further mRNA and protein analyses.
Wound Healing Assay
A wound healing assay was performed as previously described.9 Briefly, both AL and CR rat VSMCs were seeded into 12‐well plates and grown to confluence. A pipette tip was used to scratch the surface of the monolayer, creating a wound. After washing, the cells were treated with PDGF‐BB (10 ng/mL) or vehicle control or saline solution for 24 hours. Images were captured at 0 and 24 hours. Quantitative analysis of the percent closure of the wound was performed using Image J (National Institutes of Health). The levels of wound‐healing capacity (percent closure) were evaluated by the percentage of the healed cell area to the original damaged area.
Statistical Analysis
All measurements were carried out blindly for age and diet group. All results are expressed as the mean±SEM. Statistical analysis was performed with one or two‐way ANOVA, when appropriate, to determine the effects of age, diet, and their interaction, followed by Bonferroni post hoc tests. When the interaction was not statistically significant, it was removed from the model to determine the overall effects of age or diet. When repeated measurements over time were taken on the same sample, a 2‐way ANOVA with repeated measures was used to account for the nonindependence within the samples. A probability value (P) <0.05 was regarded as significant. All statistical analysis was carried out with GraphPad Prism (V6.0).
Results
CR Retards Age‐Associated Arterial Phenotypic Shifts
Aortic lumen area and intimal medial wall thickness were increased in old versus young AL rats (Figure 1A and 1B). Markedly fragmented/degraded elastin lamina determined by the percentage of decrease in elastin fraction were also observed in old versus young AL aortae (Figure 2A and 2B). Increases in the abundance of collagen (red) was also observed in old AL aortae in the intima, media, and adventitia (Figure 3A and 3B). Immunostaining of α‐SMA, a cellular marker of VSMCs, indicated that the number of intimal VSMCs was markedly increased in old versus young AL rats (Figure 4A and 4B). CR significantly retarded age‐associated arterial remodeling, including lumen area, intimal wall thickening, disruption/degradation of the elastin laminae, and collagen deposition (Figures 1, 2, 3 through 4).
Figure 1.
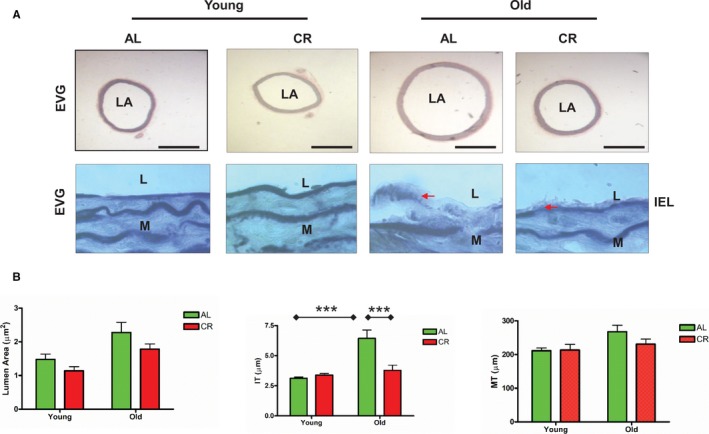
CR retards age‐associated aortic wall remodeling. A, Representative photomicrographs of paraffin sections of thoracic aortae from both young and old F344 rats with an AL or a CR diet stained with EVG (upper panels: EVG stain elastin as dark blue, ×50); and representative photomicrographs of paraffin sections of thoracic aortae from both young and old Fischer 344 rats fed an AL or a CR diet stained with EVG (elastin laminae as dark blue, ×1000). Arrow indicates eroded or broken IEL. Bar scale, 200 μm. B, Data shown as mean±SEM (n=5 animals/group). Left panel: LA was analyzed with a 2‐way ANOVA showing no age×diet interaction (P>0.05), after removing this term from the model, the overall main age (P<0.05) and diet (P<0.01) effects were significant. Middle panel of IT was also done with a 2‐way ANOVA followed by a Bonferroni comparisons test: P<0.001 for age×diet interaction. Post hoc comparisons: old vs young within AL group and AL vs CR in old group were reported (***P<0.001). Right Panel of MT: In a 2‐way ANOVA, there were no age×diet interactions (P>0.05), but overall main age effect was reported (P<0.05). AL indicates ad libitum; CR, calorie restriction; EVG, Elastic‐van Gieson; IEL, intimal elastin laminae; IT, intimal thickness; L, lumen; LA, lumen area; M, media; MT, media thickness.
Figure 2.
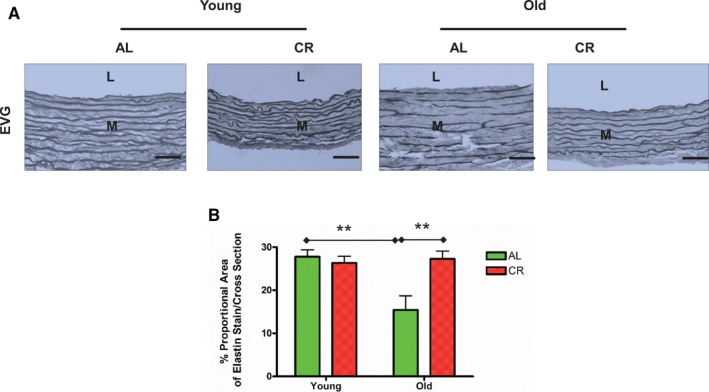
CR prevents age‐associated increases in elastin lamina fragmentation/degradation in the aortic walls. A, Representative photomicrographs of paraffin sections thoracic aortae from both young and old F344 rats with an AL or a CR diet stained with EVG (dark blue) (×400). Bar scale, 10 μm. B, Elastin fraction shown as mean±SEM (n=5 animals/group). The data were analyzed with a 2‐way ANOVA: P<0.01 for age×diet interaction. Pairwise comparisons: old vs young within AL group and AL vs CR in old group were shown (**P<0.01). AL indicates ad libitum; CR, calorie restriction; EVG, Elastica‐van Gieson; L, lumen; M, media.
Figure 3.
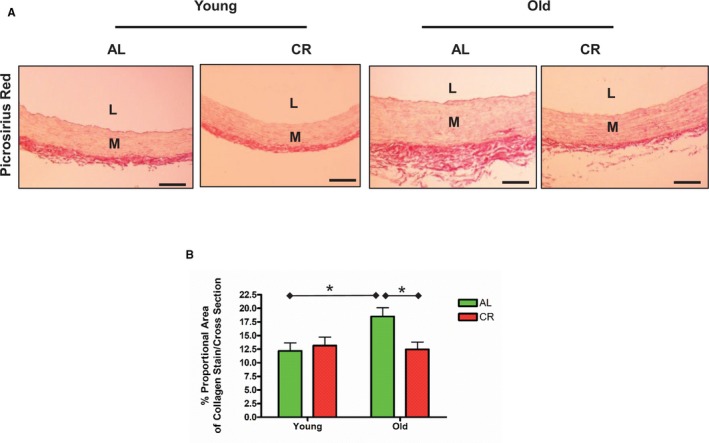
CR retards age‐associated increases in collagen deposition within the aortic walls. A, Representative photomicrographs of paraffin thoracic aortic sections of both young and old F344 rats with an AL or a CR diet stained with Sirius Red for collagen (red), observed using conventional light microscopy (×200). n=5 rats/group, respectively. Bar scale, 20 μm. B, Collagen density shown as mean±SEM (n=5 animals/group). The data were analyzed with a 2‐way ANOVA: P<0.05 for age×diet interaction. Pairwise comparison: old vs young within AL group and AL vs CR in old group were shown (*P<0.05). AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media.
Figure 4.
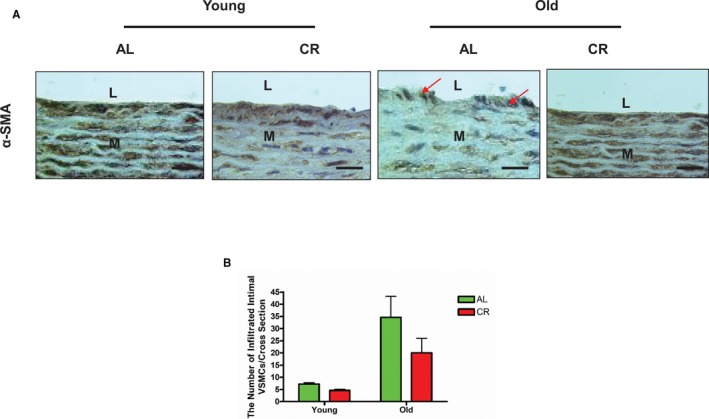
CR affects age‐associated increases in VSMC infiltration in intima within the aortic walls. A, Representative photomicrographs of paraffin sections thoracic aortae from both young and old F344 rats with an AL or a CR diet, stained with an antibody against α‐SMA (brown, ×400) showing infiltrated intimal VSMCs (arrowhead). n=5 rats/group. Scale bar, 20 μm. B, The numbers of infiltrated VSMC presented as mean±SEM (n=5 animals/group). The data were analyzed with a 2‐way ANOVA and showed that there was no age×diet interaction (P>0.05); but an overall main age effect (P<0.001) was reported. AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; VSMC, vascular smooth muscle cells; α‐SMA, alpha‐smooth muscle actin.
CR Diminishes Age‐Associated Proinflammatory Molecular Phenotypic Shifts
PDGF‐BB
Expression of PDGF‐BB (brown), a potent regulatory molecule of VSMC phenotypes,21, 22, 23, 24, 25 in the aortic wall was markedly increased in old AL versus young AL (Figure 5A and 5B). Further, aortic wall intima‐media gradient of PDGF‐BB was also markedly increased in old AL compared with young AL rats (Figure 5A and 5B). Importantly, CR reduced the age‐associated increase in PDGF protein abundance and gradient (Figure 5A and 5B).
Figure 5.
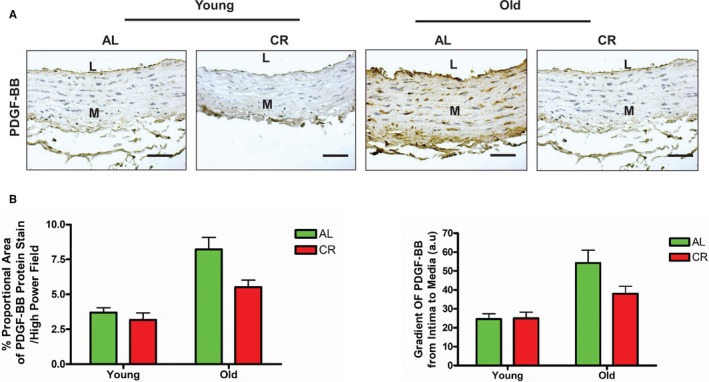
CR inhibits PDGF expression within the aortic wall with aging. A, Representative of photomicrographs of immunostaining of PDGF‐BB on paraffin sections of the rat thoracic aortae (brown, upper panels, ×200). Scale bar, 10 μm. B, PDGF‐BB density or gradient shown as mean±SEM (n=3–5 animals/group). The data were analyzed with a 2‐way ANOVA and showed no age×diet interaction (P>0.05), but the overall main age effect (P<0.001) and diet effect (P<0.05) were reported in percent proportional area of PDGF‐BB and in the gradient of PDGF‐BB. AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; PDGF‐BB, platelet derived growth factor.
MMP2 activation
MMP2 potently degrades elastin fibers and various types of degenerated collagen.1, 2 Gelatin zymography demonstrated that activated aortic gelatinases, including MMP 2/9 (green) in situ were increased in the old AL versus young AL aortae (Figure 6, upper panels), and that this age effect was substantially reduced in CR rats (Figure 6, upper right panel). Interestingly, activated MMP2/9 in old AL was substantially reduced by the MMP2 inhibitor, the recombinant human tissue inhibitor of metalloproteinases 2 (rhTIMP2) (lower middle panel), suggesting that activated MMP2 is a predominant gelatinase in the aged aortic wall.
Figure 6.
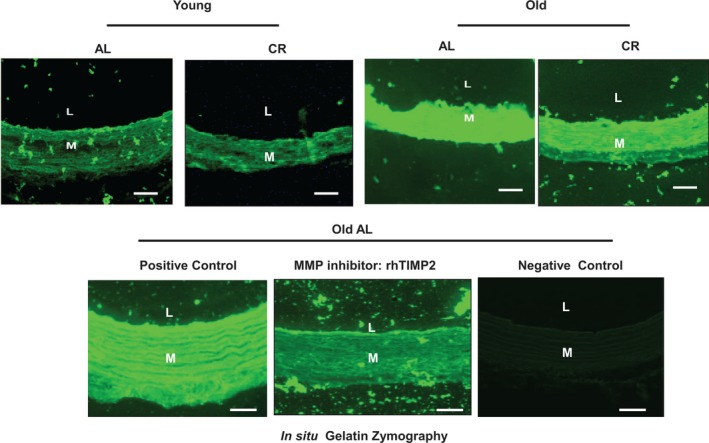
CR reduces aortic MMP‐2 activation in situ within the aortic wall with aging. Representative photomicrographs of in situ thoracic aortic gelatin zymogram from both young and old AL and CR F344 rats with or without MMP inhibitor: recombinant human TIMP2 (rhTIMP2) (500 ng/mL) (activated MMP, green color). Scale bar, 20 μm. AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; MMP, matrix metalloproteinase; rhTIMP2, recombinant human tissue inhibitor of metalloproteinases 2.
The polyacrylamide gel electrophoresis gelatin zymography further confirmed that activated MMP2 (Figure 7A, lowest band) was markedly increased within the aortic wall in old versus young AL rats, and this effect was also markedly diminished in the CR rats (Figure 7A and 7B).
Figure 7.
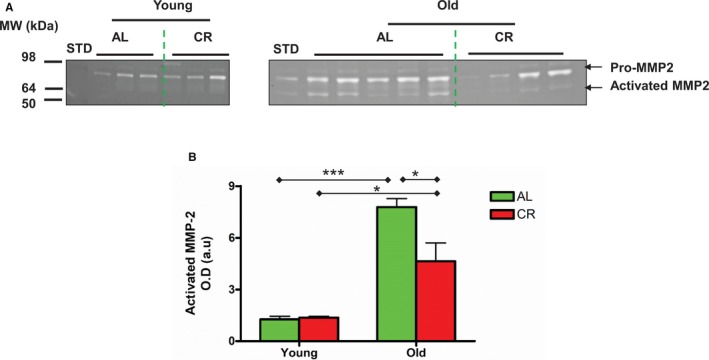
CR reduces aortic MMP‐2 activation in the aortic wall with aging. A, PAGE gelatin zymogram of lysates of intact thoracic aortae. B, Densitometric analysis of activated MMP‐2. Data shown as mean±SEM (n=3–5 animals/group). The data were analyzed with a 2‐way ANOVA showing a marginally significant age×diet interaction (P=0.06), age effect (P<0.001), and diet effect (P<0.001). Pairwise comparison: old vs young within AL or CR group, respectively, and AL vs CR in old group were reported (*P<0.05; ***P<0.001). AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; MMP‐2, matrix metalloproteinase type II.
The age‐associated increase in activation of MMP2 is mediated via an imbalance of its activator, membrane type 1 MMPs (MT1‐MMP), and its inhibitor, TIMP2.8, 18 Immunostaining analysis demonstrated that the expression of aortic membrane type 1 MMP (brown) and TIMP2 (brown) was substantially increased in old versus young AL, but this effect was markedly reduced in CR rats (Figure 8A through 8C). Importantly, the ratio of MT1‐MMP to TIMP2 was substantially increased in AL rats with aging but this effect was mitigated in CR rats (Figure 8C, right panel).
Figure 8.
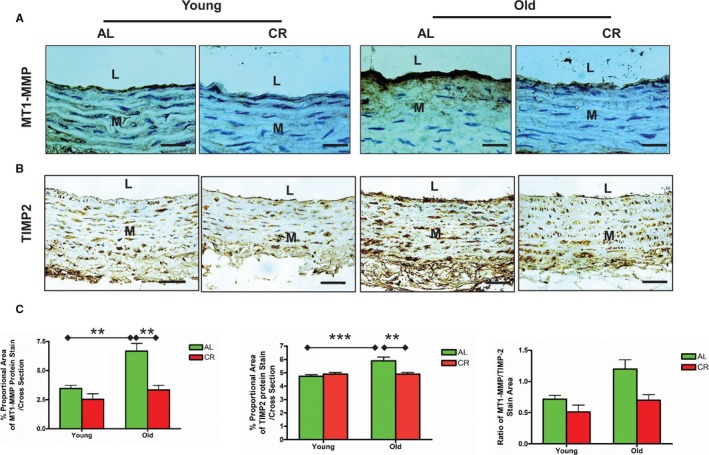
CR decreases MT1‐MMP and TIMP2 protein expression within the aged aortic wall. A, Immunostaining for MT1‐MMP (×400). Bar scale, 10 μm. B, Immunostaining for TIMP2 (×400). Bar scale, 10 μm. C, Morphometric analysis for MT1‐MMP and TIMP2 immunostaining target to total aortic area ratio/cross section. Data shown as mean±SEM (n=5 animals/group). Left panel: The data of MT1‐MMP density was analyzed with a 2‐way ANOVA showing age×diet interaction (P<0.05). Pairwise comparisons (old vs young within AL group; AL vs CR in old group) were reported (**P<0.01). Middle panel: In 2‐way ANOVA of TIMP2 density, age×diet interaction (P<0.01) was found. Pairwise comparison: old vs young within AL group and AL vs CR in old group were reported (**P<0.01; ***P<0.001). Right panel: In a 2‐way ANOVA of the ratio of MT1‐MMP/TIMP2, age×diet interaction was not found (P>0.05), but overall main age effect (P<0.05) and diet effect (P<0.05) were reported. AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; MMP‐2, matrix metalloproteinase type II; MT1‐MMP, membrane type 1 matrix metalloproteinase; TIMP2, tissue inhibitor of metalloproteinases 2.
TGF‐β1 activation
TGF‐β1, a potent profibrogenic cytokine, is activated to mediate collagen gene expression, and plays a central role in extracellular matrix remodeling with aging via its downstream signaling molecules phospho‐mothers against decapentaplegic homolog‐2/3 (p‐SMAD‐2/3).1, 5 Consequently, collagen deposition is increased in the aging arterial wall.1, 5, 26 Notably, activated TGF‐β1 is released through stepwise cleavage of latent TGF binding protein‐1 (LTBP‐1) and latent associate protein (LAP) by MMPs.5, 26 Immunostaining (brown) (Figure 9A and 9B) and immunoblotting (Figure 9C and 9D) demonstrated that activated TGF‐β1 was increased within the aged aortic wall of AL. There was reduced TGF‐β1 expression in old CR versus AL aortae (Figure 9A through 9D). Immunofluorescence staining (red) also demonstrated that arterial wall LTBP‐1 (Figure 10A) and LAP (Figure 10B) expression increased with aging in AL, and that expression of both was reduced in old CR versus AL aortae (Figure 10A and 10B).
Figure 9.
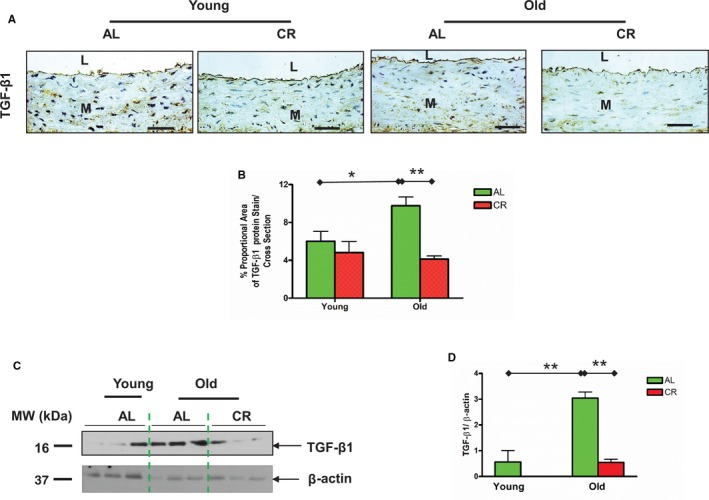
CR diminishes the age‐associated TGF‐β1 increase in the aortic wall. A, Immunostaining for TGF‐β1 (×400). Bar scale, 10 μm. B, Quantification of TGF‐β1 protein staining proportion shown mean±SEM (n=3 animals/group). Two‐way ANOVA followed by Bonferroni post hoc test showed that an age×diet interaction (P<0.05), pairwise comparison (old vs young within AL group; AL vs CR in old group) was reported (*P<0.05; **P<0.01). C, Immunoblotting TGF‐β1 from lysates of thoracic aortic tissue. D, Densitometric analysis of TGF‐β1. Data shown as mean±SEM (n=3 animals/group). One‐way ANOVA of TGF‐β1 abundance (P<0.05) followed by Bonferroni post hoc test, pairwise comparison (old vs young within AL group; AL vs CR in old group) was reported (**P<0.01). AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; TGF‐β1, transforming growth factor beta 1.
Figure 10.
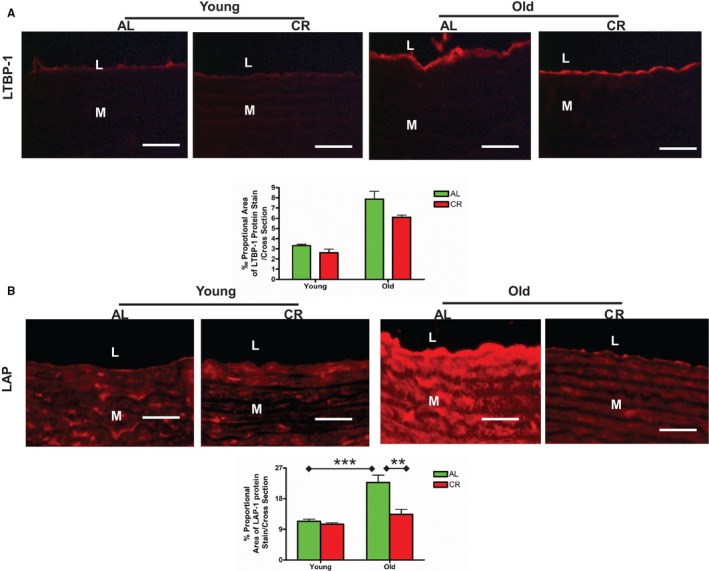
CR attenuates latent TGF‐β1 associated protein expression within aging aortic walls. A, Representative immunofluorescence staining for LTBP‐1 (upper panels, ×400, red) and quantification of the LTBP‐1 staining proportion (lower panel). Data shown as mean±SEM (n=3 animals/group). Two‐way ANOVA followed by Bonferroni post hoc test showed no age×diet interaction (P>0.05). Scale bar, 20 μm. B, Representative immunofluorescence staining for LAP (upper panels, ×400, red) and morphometric analysis of the LAP protein fraction. Data shown as mean±SEM (n=3 animals/group). Two‐way ANOVA followed by Bonferroni post hoc test, indicated an age×diet interaction was reported (P<0.05); pairwise comparison (old vs young within AL group; AL vs CR in old group) was presented (**P<0.01; ***P<0.001). Scale bar, 20 μm. AL indicates ad libitum; CR, calorie restriction; L, lumen; LAP, latent associated protein; LTBP‐1, latent TGF binding protein‐1; M, media.
Immunostaining (Figure 11A) and immunoblotting analyses (Figure 11B) indicated that the expression of p‐SMAD‐2/3 localization in the nuclei of old AL aortae was reduced by CR in old rats.
Figure 11.
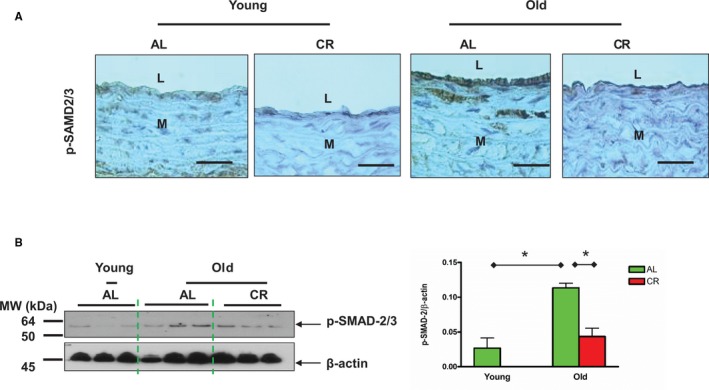
CR decreases SMAD‐2/3 phosphorylation within aged aortic walls. A, Immunostaining for p‐SMAD‐2/3 (brown, upper panels, ×400). Scale bar, 20 μm. B, Immunoblotting for p‐SMAD‐2/3 (left side of panel). Densitometric analysis of p‐SMAD‐2/3 (right side). Data presented as mean±SEM (n=3 animals/group). One‐way ANOVA showed significance was found (P<0.05); pairwise comparison (old vs young within AL group; AL vs CR in old group) was presented (*P<0.05). AL indicates ad libitum; CR, calorie restriction; L, lumen; M, media; p‐SMAD‐2/3, phospho‐mothers against decapentaplegic homolog‐2/3.
CR Modifies the Age‐Associated VSMC Phenotypic Shifts
VSMC phenotypes can exhibit plasticity in response to modulation by the age‐associated alterations in the proinflammatory niche, characterized by a loss of contractility and abnormal proliferation and migration.7, 9, 27 These VSMC phenotypic shifts are driven by a coordinated repression/activation of contractile markers SM22α, α‐SMA, and myocardin.28, 29 Notably, myocardin is a key specific transcriptional coactivator of α‐SMA.29
Immunoblotting analyses of VSMC lysates from AL and CR rats demonstrated that aging modifies SM22α, α‐SMA and myocardin proteins in VSMCs. However, CR also modifies the age‐associated phenotypic protein shifts in VSMCs (Figure 12A and 12B).
Figure 12.
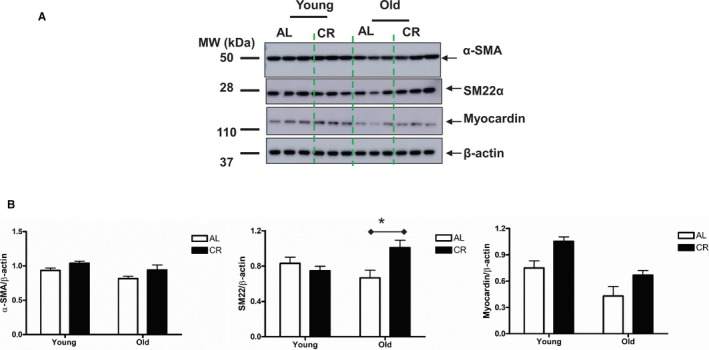
CR modifies the phenotype of VSMCs isolated from old rats. A, Immunoblotting for α‐SMA, SM22α, and myocardin. B, Densitometric analysis. Data shown as mean±SEM (n=3–6 independent experiments). Left panel: α‐SMA protein abundance was analyzed with a 2‐way ANOVA followed by a Bonferroni comparisons test and showed no age×diet interaction (P>0.05), but an overall main age effect (P<0.05) and diet effect (P<0.05) were reported; Middle panel: In a 2‐way ANOVA of SM22α, an age×diet interaction was found (P<0.05), pairwise comparison (AL vs CR in old group) was presented (*P<0.05). Right Panel: In a 2‐way ANOVA analysis of myocardin protein abundance: an age×diet interaction was not found (P>0.05), but an overall main age effect (P<0.01) and diet effect (P<0.01) were reported. AL indicates ad libitum; CR, calorie restriction; α‐SMA, alpha smooth muscle actin.
CR Modifies Age‐Associated VSMC Proinflammation Through PDGF Signaling
The wound healing assay is a validated approach to measure characteristics of migration, proliferation, and proinflammation of synthetic VSMCs in vitro by mimicking the formation of the neointima or intimal thickening found in injured arterial walls in vivo.30 The current study Figure 5 demonstrated that CR significantly inhibited the age‐associated increases in PDGF‐BB expression. The wound healing assay showed that PDGF‐BB was involved in the wound healing processes of VSMCs (Figure 13A and 13B). Interestingly, a significant confluency reduction in the healing area was seen in response to PDGF‐BB treatment when comparing VSMCs from old CR versus AL rats (Figure 13A, right columns and 13B, right panel).
Figure 13.
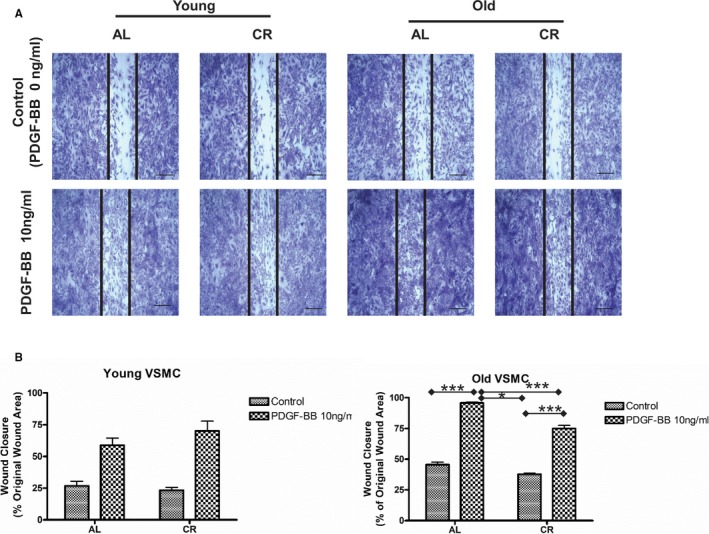
CR attenuates old VSMC wound‐healing capabilities. A, Representative photomicrographs of the wound healing assay of both young and old VSMCs isolated from AL and CR aortae treated with or without PDGF‐BB (10 ng/mL) for 24 hours. Scale bar, 200 μm. B, The percentage of wound healing. Data shown as mean±SEM (n=4 independent experiments/group). Left panel: Young VSMC: Two‐way ANOVA with repeated measures demonstrated that there was no PDGF‐BB treatment×diet interaction (P>0.05), but an overall PDGF‐BB treatment effect (P<0.01) was reported. Right panel: Old VSMC: Two‐way ANOVA with repeated measures followed by Bonferroni post hoc test demonstrated that a PDGF‐BB treatment×diet interaction (P<0.01); pairwise comparisons (PDGF‐BB vs control within AL and CR groups, respectively; and AL vs CR in PDGF treatment group) were reported (*P<0.05; ***P<0.001). AL indicates ad libitum; CR, calorie restriction; PDGF‐BB, platelet derived growth factor; VSMC, vascular smooth muscle cells.
Furthermore, PDGF‐BB treatment markedly increased TGF‐β1 activation, as measured by ELISA (Figure 14A), and p‐SMAD‐2/3 (Figure 14B) in both young and old AL VSMCs in a dose‐dependent manner.
Figure 14.
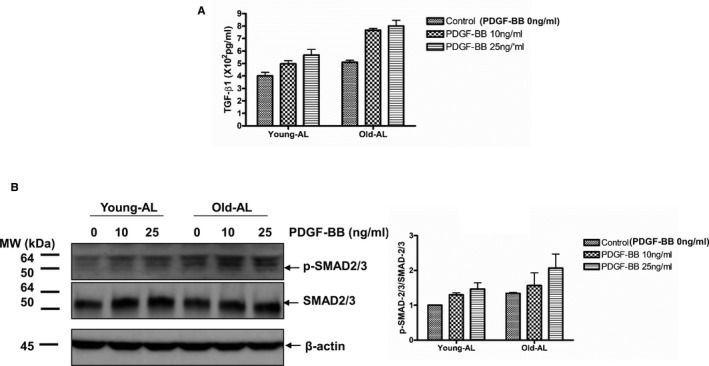
PDGF‐BB profibrogenic signaling in AL VSMCs. A, Quantification of activated TGF‐β1 secreted from both young and old AL VSMCs determined by an ELISA. Data shown as mean±SEM (n=3, each group contained VSMCS isolated from 3 different rats). Two‐way ANOVA followed by Bonferroni post hoc test, demonstrated that no PDGF‐BB treatment×diet interaction (P>0.05) was found, but an overall PDGF‐BB treatment (P<0.001) and age effect (P<0.001) were reported. B, Left panels: Immunoblotting p‐SMAD‐2/3, SMAD‐2/3, and β‐actin of cellular lysates from both young and old AL VSMCs treated with or without PDGF‐BB for 24 hours (n=3, each group contained VSMCS isolated from 3 different rats). Right panel: Densitometric analysis. Data shown as mean±SEM. Two‐way ANOVA demonstrated that there was no PDGF‐BB treatment×diet interaction, but an overall PDGF‐BB treatment effect (P<0.05) and age effect (P<0.05) were reported. AL indicates ad libitum; CR, calorie restriction; PDGF‐BB, platelet derived growth factor; SMAD, mothers against decapentaplegic homolog‐2/3; TGF‐β1, transforming growth factor beta 1; VSMC, vascular smooth muscle cells.
Importantly, polymerase chain reaction analysis demonstrated that PDGF‐β mRNA abundance was increased in old versus young AL control VSMCs; and silencing PDGF‐β markedly decreased PDGF‐β mRNA levels in both young and old AL VSMCs (Figure 15A). Both treated young AL and old AL showed significant decrease as compared with the AL control, respectively, but still significant increase when compared with the old CR control (Figure 15A). However, silencing the PDGF‐β gene in old AL significantly reduced the amount of PDGF‐BB and p‐SMAD proteins toward the levels of old CR. It also reduced the amount of TGF‐β1 seen in old AL, down to the level of old CR (Figure 15B).
Figure 15.
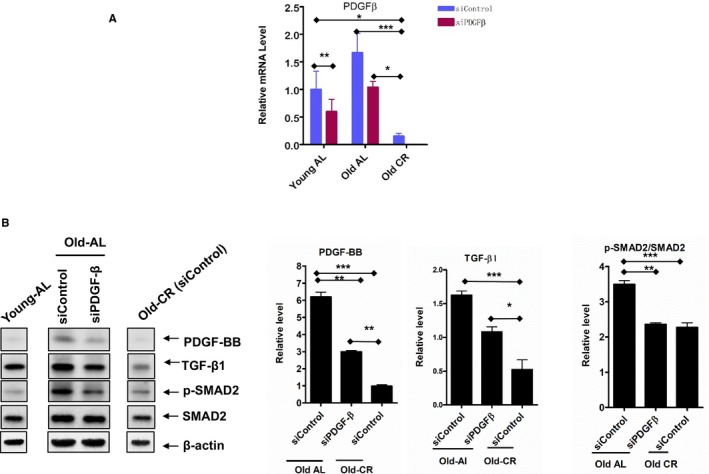
PDGF silences profibrogenic signaling in VSMCs. A, PDGF‐β‐silencing in both young and old AL and CR VSMCs measured with PCR (n=3 independent experiments). One‐way ANOVA followed by Bonferroni post hoc test, *P<0.05, **P<0.01, ***P<0.001. B, Representative immunoblotting PDGF‐BB, p‐SMAD‐2/3, SMAD‐2/3, and β‐actin (left panel) of cellular lysates from old AL and CR VSMCs with or without PDGF silencing, and average data (right panel) of PDGF‐BB, TGF‐β1, and p‐SMAD‐2/3/SMAD‐2/3 (n=3 independent experiments). One‐way ANOVA followed by Bonferroni post hoc test, *P<0.05, **P<0.01, ***P<0.001. AL indicates ad libitum; CR, calorie restriction; PDGF, platelet derived growth factor; SMAD, mothers against decapentaplegic homolog‐2/3; TGF‐β1, transforming growth factor beta 1; VSMC, vascular smooth muscle cells.
Discussion
With advancing age, adverse arterial remodeling occurs due to an increase in the proinflammation.1, 2, 31 Intimal thickening, collagen deposition, and elastin fragmentation are reliable indications of adverse arterial remodeling, which are closely associated with an increase in the prevalence and severity of age‐associated quintessential cardiovascular disease such as atherosclerosis due to either a reduction of threshold or an enhancement of susceptibility to hostile stimuli, for example, hyperlipidemia.1, 3, 31 Findings from both experimental animal models and human studies indicate that CR has numerous beneficial effects on arterial structural and functional remodeling and eventually decelerates biological aging.14, 15, 16, 32, 33, 34, 35, 36, 37, 38, 39 Our current study further documents that CR effectively prevents the age‐associated arterial wall proinflammation at the molecular, cellular, and tissue levels, and preserves a more youthful phenotype in rats.
Aging increases intimal thickening associated with an increase of the phenotypic shifts of VSMCs from a contractile to a synthetic state and increases PDGF‐BB signaling. The shift of aging VSMCs from a quiescent/contractile phenotype to an active synthetic phenotype is in response to proinflammation, a key molecular and cellular event that accelerates arterial intimal thickening, which is associated with PDGF signaling.21, 22, 24, 25, 40 The contractile proteins SM22α, α‐SMA, and myocardin are diminished in AL VSMCs with aging while PDGF is increased. Notably, these contractile protein changes with age are alleviated, while the PDGF gene, protein and its signaling are markedly decreased in CR cells. These findings suggest that CR decelerates the age associated intimal thickening mainly via a retardation of the phenotypic shifts of VSMCs and their PDGF‐BB signaling.
VSMC infiltration is a feature of intimal thickening in the aged arterial wall, which is associated with PDGF signaling. PDGF‐BB is not only a potent mitogen, but also a strong chemoattractant of VSMCs, creating a permissive tissue microenvironment for VSMC migration and invasion, promoting formation of a thickened intima in vivo.1, 2, 3, 8, 41, 42 Prior findings indicate that PDGF‐BB treatment induces invasion and migration of old VSMCs in vitro.8, 9 The capacity of VSMC to repopulate a damaged area in vitro, a model for the cellular process of thickening intimal or neointimal formation, is markedly increased after PDGF‐BB treatment.43, 44 Current findings demonstrate that the exaggerated VSMCs proliferation/migration is significantly decreased in old CR versus AL cells in response to PDGF‐BB treatment. Taken together, these findings support the idea that CR retards intimal cellularity in old arterial wall, mainly through suppressing PDGF‐BB signaling, consequently decelerating arterial intimal biological aging (intimal thickening).
Collagen deposition is another feature of the aged arterial wall. We show that aging increases PDGF‐BB, LTBP‐1, LAP expression, and TGF‐β1 activation; and their downstream signaling molecules p‐SMAD‐2/3 in the arterial wall of AL rats. Activated MMP2 is a potent activator of LTBP‐1 and LAP cleaving into active TGF‐β1, contributing to collagen production in VSMCs.5 CR decreases extracellular matrix deposition in vivo, and this effect is associated with a decrease in PDGF‐BB, TGF‐β1 and MMP2 activation. Specifically, the current in vitro studies demonstrate that PDGF signaling plays a causal role in TGF‐β1 activation and p‐SMAD‐2/3 expression. CR, through the inhibition of PDGF‐mediated fibrogenic TGF‐β1 signaling and decreased arterial collagen deposition, decelerates arterial wall aging. Notably, the degree of collagen deposition within the arterial wall is not only dependent upon production but may also depend upon a clearance of collagen by autophagy.45, 46
The structural and functional integrity of the elastin network is a key to arterial health. Competent elastin networks not only buffer the mechanical stress burden on the heart but also maintain arterial elasticity and decrease arterial cell stress response with aging.6, 27 Our results demonstrate that CR prevents elastin fiber network breakdown, and this effect is linked to the reduction of the MMP2/TGF‐β1/fibrosis loop signaling.47
CR increases fractional aortic expansion, improving age‐related aortic distensibility while decreasing aortic pulse wave velocity, thus retarding aortic stiffening in old rats.16 These beneficial effects of CR on age‐related arterial dysfunction are closely related to the alleviation of collagen deposition and the preservation of elastin network integrity.16 Further, TGF‐β1 is not only a powerful fibrogenic molecule but also is a powerful pro‐stiffening molecule to VSMCs.4 TGF‐β1 reinforces arterial rigidity through an enhancement of the stiffness of VSMCs with aging.4 Thus, CR improves both age‐associated arterial and VSMCs stiffening that is associated with a reduction of TGF‐β1.
CR may attenuate the age‐associated increase in the prevalence and severity of atherosclerosis through an attenuation of proinflammation in the elderly. Atherosclerosis is described as “accelerated arterial aging,” characterized by a mass inflammatory fibroproliferative response of VSMCs.10, 48, 49, 50, 51 PDGF‐BB, TGF‐β1, p‐SMAD‐2/3 and collagen deposition are all involved in the initiation and progression of atherosclerosis.5, 26, 40, 41, 52, 53
In summary, CR retards arterial aging by attenuating the age‐associated proinflammatory phenotypic shifts in molecules, cells, and tissues. CR alleviates the age‐associated effects on the arterial wall, including arterial thickening, fibrosis, and elastin network breakdown, through a mitigation of the PDGF‐directed signaling cascade. These arterial effects are the cornerstone of the health span and life span promoted by CR since age may be best measured by the biological aging rate of the arteries.54 Taken together, CR is a practical approach for decelerating biological aging and prolonging a more youthful aortic phenotype.
Sources of Funding
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Heath.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009112 DOI: 10.1161/JAHA.118.009112.)
References
- 1. Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension. 2015;65:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang M, Shah AM. Age‐associated pro‐inflammatory remodeling and functional phenotype in the heart and large arteries. J Mol Cell Cardiol. 2015;83:101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu W, Kim BC, Wang M, Huang J, Isak A, Bexiga NM, Monticone R, Ha T, Lakatta EG, An SS. TGFbeta1 reinforces arterial aging in the vascular smooth muscle cell through a long‐range regulation of the cytoskeletal stiffness. Sci Rep. 2018;8:2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, Monticone R, Lakatta EG. Matrix metalloproteinase 2 activation of transforming growth factor‐beta1 (TGF‐beta1) and TGF‐beta1‐type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. [DOI] [PubMed] [Google Scholar]
- 6. Wang M, Zhang J, Telljohann R, Jiang L, Wu J, Monticone RE, Kapoor K, Talan M, Lakatta EG. Chronic matrix metalloproteinase inhibition retards age‐associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Angiotensin II activates matrix metalloproteinase type II and mimics age‐associated carotid arterial remodeling in young rats. Am J Pathol. 2005;167:1429–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang M, Takagi G, Asai K, Resuello RG, Natividad FF, Vatner DE, Vatner SF, Lakatta EG. Aging increases aortic MMP‐2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. [DOI] [PubMed] [Google Scholar]
- 9. Wang M, Fu Z, Wu J, Zhang J, Jiang L, Khazan B, Telljohann R, Zhao M, Krug AW, Pikilidou M, Monticone RE, Wersto R, Van Eyk J, Lakatta EG. MFG‐E8 activates proliferation of vascular smooth muscle cells via integrin signaling. Aging Cell. 2012;11:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Head T, Daunert S, Goldschmidt‐Clermont PJ. The aging risk and atherosclerosis: a fresh look at arterial homeostasis. Front Genet. 2017;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cicero AFG, Fogacci F, Colletti A. Food and plant bioactives for reducing cardiometabolic disease risk: an evidence based approach. Food Funct. 2017;8:2076–2088. [DOI] [PubMed] [Google Scholar]
- 12. Jakovljevic DG. Physical activity and cardiovascular aging: physiological and molecular insights. Exp Gerontol. 2018;109:67–74. [DOI] [PubMed] [Google Scholar]
- 13. Rossman MJ, Kaplon RE, Hill SD, McNamara MN, Santos‐Parker JR, Pierce GL, Seals DR, Donato AJ. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am J Physiol Heart Circ Physiol. 2017;313:H890–H895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Redman LM, Smith SR, Burton JH, Martin CK, Il'yasova D, Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metab. 2018;27:805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmet I, Tae HJ, de Cabo R, Lakatta EG, Talan MI. Effects of calorie restriction on cardioprotection and cardiovascular health. J Mol Cell Cardiol. 2011;51:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up‐regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A. 2006;103:19908–19912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase‐2 in aortic remodeling during aging. Hypertension. 2002;39:865–873. [DOI] [PubMed] [Google Scholar]
- 19. Jiang L, Wang M, Zhang J, Monticone RE, Telljohann R, Spinetti G, Pintus G, Lakatta EG. Increased aortic calpain‐1 activity mediates age‐associated angiotensin II signaling of vascular smooth muscle cells. PLoS One. 2008;3:e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP‐1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. [DOI] [PubMed] [Google Scholar]
- 21. Yang XP, Pei ZH, Ren J. Making up or breaking up: the tortuous role of platelet‐derived growth factor in vascular ageing. Clin Exp Pharmacol Physiol. 2009;36:739–747. [DOI] [PubMed] [Google Scholar]
- 22. Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann N Y Acad Sci. 2000;902:39–51; discussion 51‐32 [DOI] [PubMed] [Google Scholar]
- 23. Ouyang L, Zhang K, Chen J, Wang J, Huang H. Roles of platelet‐derived growth factor in vascular calcification. J Cell Physiol. 2018;233:2804–2814. [DOI] [PubMed] [Google Scholar]
- 24. Motegi S, Garfield S, Feng X, Sardy M, Udey MC. Potentiation of platelet‐derived growth factor receptor‐beta signaling mediated by integrin‐associated MFG‐E8. Arterioscler Thromb Vasc Biol. 2011;31:2653–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ha JM, Yun SJ, Kim YW, Jin SY, Lee HS, Song SH, Shin HK, Bae SS. Platelet‐derived growth factor regulates vascular smooth muscle phenotype via mammalian target of rapamycin complex 1. Biochem Biophys Res Commun. 2015;464:57–62. [DOI] [PubMed] [Google Scholar]
- 26. Jiang L, Zhang J, Monticone RE, Telljohann R, Wu J, Wang M, Lakatta EG. Calpain‐1 regulation of matrix metalloproteinase 2 activity in vascular smooth muscle cells facilitates age‐associated aortic wall calcification and fibrosis. Hypertension. 2012;60:1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wheeler JB, Mukherjee R, Stroud RE, Jones JA, Ikonomidis JS. Relation of murine thoracic aortic structural and cellular changes with aging to passive and active mechanical properties. J Am Heart Assoc. 2015;4:e001744 DOI: 10.1161/JAHA.114.001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chistiakov DA, Sobenin IA, Orekhov AN, Bobryshev YV. Human miR‐221/222 in physiological and atherosclerotic vascular remodeling. Biomed Res Int. 2015;2015:354517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lagna G, Ku MM, Nguyen PH, Neuman NA, Davis BN, Hata A. Control of phenotypic plasticity of smooth muscle cells by bone morphogenetic protein signaling through the myocardin‐related transcription factors. J Biol Chem. 2007;282:37244–37255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Louis SF, Zahradka P. Vascular smooth muscle cell motility: from migration to invasion. Exp Clin Cardiol. 2010;15:e75–e85. [PMC free article] [PubMed] [Google Scholar]
- 31. Wang M, Khazan B, Lakatta EG. Central arterial aging and angiotensin II signaling. Curr Hypertens Rev. 2010;6:266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301:H1205–H1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castello L, Froio T, Cavallini G, Biasi F, Sapino A, Leonarduzzi G, Bergamini E, Poli G, Chiarpotto E. Calorie restriction protects against age‐related rat aorta sclerosis. FASEB J. 2005;19:1863–1865. [DOI] [PubMed] [Google Scholar]
- 34. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti‐oxidative, pro‐angiogenic, and anti‐inflammatory effects and promotes anti‐aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–H306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA, Seals DR. Life‐long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fontana L, Meyer TE, Klein S, Holloszy JO. Long‐term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lefevre M, Redman LM, Heilbronn LK, Smith JV, Martin CK, Rood JC, Greenway FL, Williamson DA, Smith SR, Ravussin E; Pennington Ct . Caloric restriction alone and with exercise improves CVD risk in healthy non‐obese individuals. Atherosclerosis. 2009;203:206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vaiserman A, Lushchak O. Implementation of longevity‐promoting supplements and medications in public health practice: achievements, challenges and future perspectives. J Transl Med. 2017;15:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zeng L, Li Y, Yang J, Wang G, Margariti A, Xiao Q, Zampetaki A, Yin X, Mayr M, Mori K, Wang W, Hu Y, Xu Q. XBP 1‐deficiency abrogates neointimal lesion of injured vessels via cross talk with the PDGF signaling. Arterioscler Thromb Vasc Biol. 2015;35:2134–2144. [DOI] [PubMed] [Google Scholar]
- 41. Alvarez RH, Kantarjian HM, Cortes JE. Biology of platelet‐derived growth factor and its involvement in disease. Mayo Clin Proc. 2006;81:1241–1257. [DOI] [PubMed] [Google Scholar]
- 42. Fu Z, Wang M, Gucek M, Zhang J, Wu J, Jiang L, Monticone RE, Khazan B, Telljohann R, Mattison J, Sheng S, Cole RN, Spinetti G, Pintus G, Liu L, Kolodgie FD, Virmani R, Spurgeon H, Ingram DK, Everett AD, Lakatta EG, Van Eyk JE. Milk fat globule protein epidermal growth factor‐8: a pivotal relay element within the angiotensin II and monocyte chemoattractant protein‐1 signaling cascade mediating vascular smooth muscle cells invasion. Circ Res. 2009;104:1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lee HH, Paudel KR, Kim DW. Terminalia chebula fructus inhibits migration and proliferation of vascular smooth muscle cells and production of inflammatory mediators in RAW 264.7. Evid Based Complement Alternat Med. 2015;2015:502182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vazquez‐Padron RI, Lasko D, Li S, Louis L, Pestana IA, Pang M, Liotta C, Fornoni A, Aitouche A, Pham SM. Aging exacerbates neointimal formation, and increases proliferation and reduces susceptibility to apoptosis of vascular smooth muscle cells in mice. J Vasc Surg. 2004;40:1199–1207. [DOI] [PubMed] [Google Scholar]
- 45. LaRocca TJ, Gioscia‐Ryan RA, Hearon CM Jr, Seals DR. The autophagy enhancer spermidine reverses arterial aging. Mech Ageing Dev. 2013;134:314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11:2092–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang M, Spinetti G, Monticone RE, Zhang J, Wu J, Jiang L, Khazan B, Telljohann R, Lakatta EG. A local proinflammatory signalling loop facilitates adverse age‐associated arterial remodeling. PLoS One. 2011;6:e16653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bianucci R, Loynes RD, Sutherland ML, Lallo R, Kay GL, Froesch P, Pallen MJ, Charlier P, Nerlich AG. Forensic analysis reveals acute decompensation of chronic heart failure in a 3500‐year‐old Egyptian dignitary. J Forensic Sci. 2016;61:1378–1381. [DOI] [PubMed] [Google Scholar]
- 49. Gabrovsky AN, O'Neill KD, Gerszten E. Paleopathology of cardiovascular diseases in South American mummies. Int J Cardiol. 2016;223:101–107. [DOI] [PubMed] [Google Scholar]
- 50. McGill HC Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72:1307S–1315S. [DOI] [PubMed] [Google Scholar]
- 51. Shin DH, Oh CS, Hong JH, Kim Y, Lee SD, Lee E. Paleogenetic study on the 17th century Korean mummy with atherosclerotic cardiovascular disease. PLoS One. 2017;12:e0183098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Toffoli B, Pickering RJ, Tsorotes D, Wang B, Bernardi S, Kantharidis P, Fabris B, Zauli G, Secchiero P, Thomas MC. Osteoprotegerin promotes vascular fibrosis via a TGF‐beta1 autocrine loop. Atherosclerosis. 2011;218:61–68. [DOI] [PubMed] [Google Scholar]
- 53. Wang M, Wang HH, Lakatta EG. Milk fat globule epidermal growth factor VIII signaling in arterial wall remodeling. Curr Vasc Pharmacol. 2013;11:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Seals DR, Kaplon RE, Gioscia‐Ryan RA, LaRocca TJ. You're only as old as your arteries: translational strategies for preserving vascular endothelial function with aging. Physiology (Bethesda). 2014;29:250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]


