Abstract
Background
Patients with atrial fibrillation (AF) treated with oral anticoagulants may be exposed to an increased risk of bleeding events. The HAS‐BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol) score is a simple, well‐established, clinical bleeding‐risk prediction score. Recently, a new algorithm‐based score was proposed, the GARFIELD‐AF (Global Anticoagulant in the Field–AF) bleeding score. We compared HAS‐BLED and GARFIELD‐AF scores in predicting adjudicated bleeding events in a clinical trial cohort of patients with AF taking anticoagulants, in the first external comparative validation of both scores.
Methods and Results
We analyzed patients from the SPORTIF (Stroke Prevention Using an Oral Thrombin Inhibitor in Patients With AF) III and V trials. All patients assigned to the warfarin arm with information to calculate the scores were considered. Outcomes were major, major/clinically relevant nonmajor, and any bleeding. A total of 3550 warfarin‐treated patients were available for analysis. Of these patients, 2519 (71.0%) had a HAS‐BLED score ≥3, whereas based on GARFIELD‐AF median value, 2056 (57.9%) were categorized as “high score.” Both HAS‐BLED and GARFIELD‐AF C‐indexes showed modest predictive value (C‐index [95% confidence interval] for major bleeding, 0.58 [0.56–0.60] and 0.56 [0.54–0.57], respectively); however, GARFIELD‐AF was not predictive of any bleeding. The GARFIELD‐AF bleeding score had a significantly lower sensitivity and a negative reclassification for any bleeding compared with HAS‐BLED, assessed by integrated discrimination improvement and net reclassification improvement (both P<0.001). HAS‐BLED showed a 5% net benefit for any bleeding occurrence.
Conclusions
The algorithm‐based GARFIELD‐AF bleeding score did not show any significant improvement in major and major/clinically relevant nonmajor prediction compared with the simple HAS‐BLED score. For clinical usefulness in prediction of any bleeding, the HAS‐BLED score showed a significant net benefit compared with the GARFIELD‐AF.
Keywords: atrial fibrillation, bleeding risk, clinical risk scores
Subject Categories: Atrial Fibrillation, Prognosis, Anticoagulants, Quality and Outcomes
Clinical Perspective
What Is New?
In anticoagulated patients with atrial fibrillation (AF), both HAS‐BLED and GARFIELD‐AF (Global Anticoagulant in the Field–AF) bleeding scores showed modest predictive ability in predicting most bleeding outcomes.
Use of the algorithm‐based GARFIELD‐AF bleeding score did not show any improvement in prediction of major bleeding and major/clinically relevant nonmajor bleeding compared with the simple HAS‐BLED score.
In predicting the occurrence of any bleeding event, the HAS‐BLED score demonstrated improved net benefit compared with the GARFIELD‐AF bleeding score.
What Are the Clinical Implications?
This study represents the first external independent validation of the GARFIELD‐AF bleeding score, in a clinical trial cohort with adjudicated bleeding outcomes.
In the decision‐making process of prescribing oral anticoagulant therapy in patients with AF, bleeding risk should be evaluated to address modifiable bleeding risk factors and “flag up” the high‐risk patients for early review and follow‐up.
Introduction
The use of oral anticoagulant (OAC) drugs in patients with atrial fibrillation (AF) is highly effective for stroke prevention in AF, but is associated with an increased risk for bleeding events.1, 2, 3 Baseline evaluation and management of bleeding risk, as well as the routine reevaluation during the clinical follow‐up, are pivotal for patients with AF to minimize occurring bleeding events.4, 5
Of the various clinical scores for bleeding risk stratification, the HAS‐BLED score6 is appropriately used to flag up patients for more regular review and follow‐up, as well as drawing attention to modifiable bleeding risk factors.7, 8 The HAS‐BLED score has been shown to be a superior strategy for bleeding risk evaluation compared with an approach only focusing on modifiable bleeding risk factors.9, 10, 11
In recent years, several other bleeding clinical risk scores have been proposed, all of which show modest predictive capacity, despite progressively more complex models.12 More recently, a new bleeding risk score has been proposed to predict major bleeding in patients with AF, derived from the GARFIELD‐AF (Global Anticoagulant in the Field–AF) registry, called the GARFIELD‐AF bleeding score.13
The aim of this article is to compare the predictive value of HAS‐BLED and GARFIELD‐AF bleeding scores for adjudicated bleeding events in a cohort of patients with AF taking warfarin derived from a randomized controlled trial cohort.
Methods
We used the pooled study populations of the SPORTIF (Stroke Prevention Using an Oral Thrombin Inhibitor in Patients With AF) III and V trials. The original protocol and principal results have been previously described.14, 15, 16 In brief, the SPORTIF trials were 2 multicenter phase III clinical trials comparing the efficacy and safety of the direct thrombin inhibitor, ximelagatran, against warfarin in patients with nonvalvular AF. Signed, informed consent was required from each participant in accordance with protocol regulations approved by the local review boards governing research involving human subjects and the Declaration of Helsinki. Deidentified data sets with patient‐level information were obtained directly from AstraZeneca, and all the analyses were performed independently from the company. The analytic methods and study materials could be made available to other researchers for purposes of reproducing the results or replicating the procedures on request to the corresponding author and AstraZeneca. All patients assigned to the warfarin treatment arms and with available data for the clinical variables used to calculate the 2 bleeding prediction scores were included in the present analysis.
Bleeding Scores Definition
The HAS‐BLED score was calculated according to the original methods.6 Major bleeding incidence according to HAS‐BLED score, as reported in the original derivation cohort,6 is shown in Table S1. Labile international normalized ratio criterion was defined as a time in therapeutic range <65%. The “impaired liver function” criterion was scored 0, because liver dysfunction was an exclusion criterion from the original SPORTIF trials protocol. A HAS‐BLED score <3 was categorized as “low risk,” whereas a HAS‐BLED score ≥3 was categorized as “high risk.”17 Similarly, the GARFIELD‐AF bleeding score was calculated according to the original proposed scheme.13 According to the median value of the score distribution, patients were categorized as GARFIELD‐AF “high score” (above the median value) and GARFIELD‐AF “low score” (including and below the median value).
Study Outcomes
We considered 3 bleeding end points, which were adjudicated in this trial cohort. Major bleeding outcome was defined by ≥1 of the following criteria: clinically overt bleeding with a concomitant decrease in hemoglobin levels of >2 g/dL or requiring blood transfusion of at least 2 units of whole blood or erythrocytes; or a bleeding episode involving a critical site (intracranial, intraspinal, intraocular, retroperitoneal, pericardial, or nontraumatic intra‐articular bleeding).14 All major bleeding events were centrally adjudicated by a blind independent oversight committee. Major/clinically relevant nonmajor (CRNM) bleeding outcome was defined as all the investigator‐reported major bleeding events, independently of central adjudication. Any bleeding outcome was defined as any bleeding event, both major and minor, that occurred during the study.
Statistical Analysis
Continuous variables were reported as median (interquartile range), whereas categorical variables were expressed as counts and percentages. Differences in survival according to the bleeding risk categories, assessed by an intention‐to‐treat approach, were analyzed using the log‐rank test, and Kaplan‐Meier curve estimates were drafted accordingly. A Cox proportional hazards analysis was used to evaluate the occurrence of the 3 bleeding outcomes according to continuous scores, adjusted for sex and type of AF. C‐indexes were estimated, with exact estimation of 95% confidence interval (CI), according to the method of DeLong et al.18
Discrimination and reclassification abilities were evaluated by the integrated discrimination improvement, the net reclassification improvement, and the median improvement, as described by Pencina et al.19 Integrated discrimination improvement and net reclassification improvement have been calculated using scores as continuous variables and according to a time‐dependent approach, whereas the clinical usefulness and net benefit were estimated using the decision curve analysis, according to the method proposed by Vickers et al.20, 21 In addition, we performed a sensitivity analysis comparing the 2 scores’ predictive performance, using an on‐treatment analysis. A 2‐sided P<0.05 was considered statistically significant. All analyses were performed using SPSS, version 25.0 (IBM, NY) for MacOS and survIDINRI package for R, version 3.3.1 for Windows.
Results
Among the original 7329 patients enrolled in the SPORTIF III and V trials, 3665 (50.0%) were assigned to the warfarin arm. According to data available, a total of 3550 patients (30.5% women; median [interquartile range] age, 72 [66–77] years) were available for this analysis. Baseline characteristics are reported in Table 1. Hypertension was the most prevalent risk factor (3167 patients [89.3%]), whereas previous bleeding was reported in 200 (5.6%) and chronic kidney disease was reported in 918 (25.9%). One fifth (705 patients [19.9%]) of the cohort used concomitant aspirin. Overall, there was a good quality anticoagulation control, with a median (interquartile range) time in therapeutic range of 68.2% (55.1%–79.6%).
Table 1.
Baseline Characteristics
| Characteristics | Value (N=3550) |
|---|---|
| Age, median (IQR), y | 72 (66–77) |
| Female sex, n (%) | 1084 (30.5) |
| BMI, median (IQR), kg/m2 (n=3540) | 28.1 (25.0–31.6) |
| CrCl, median (IQR), mL/min | 79.7 (59.3–102.1) |
| Chronic AF, n (%) (n=3548) | 3167 (89.3) |
| Hypertension, n (%) | 2723 (76.7) |
| Diabetes mellitus, n (%) | 833 (23.5) |
| Coronary artery disease, n (%) | 1574 (44.3) |
| Stroke/TIA, n (%) | 730 (20.6) |
| Heart failure, n (%) | 1324 (37.3) |
| Previous bleeding, n (%) | 200 (5.6) |
| Chronic kidney disease, n (%) | 918 (25.9) |
| Aspirin use, n (%) | 705 (19.9) |
| TTR, median (IQR), % | 68.2 (55.1–79.6) |
AF indicates atrial fibrillation; BMI, body mass index; CrCl, creatinine clearance; IQR, interquartile range; TIA, transient ischemic attack; TTR, time in therapeutic range.
At baseline, the median (interquartile range) HAS‐BLED score was 3 (2–4), with 2519 patients (71.0%) with a HAS‐BLED score ≥3. Using a GARFIELD‐AF median score value, 2056 patients (57.9%) were categorized as high score and 1494 (42.1%) were categorized as low score.
Over a mean of 1.56 (SD, 0.38) years of follow‐up, 127 major bleeding (2.29 per 100 patient‐years), 168 major/CRNM bleeding (3.03 per 100 patient‐years), and 1450 any bleeding (26.2 per 100 patient‐years) outcomes were recorded.
Kaplan‐Meier analyses showed that patients with a HAS‐BLED score ≥3 had a higher cumulative risk of major bleeding and major/CRNM bleeding outcomes compared with patients with a HAS‐BLED score <3 (Figure 1, top panels).
Figure 1.
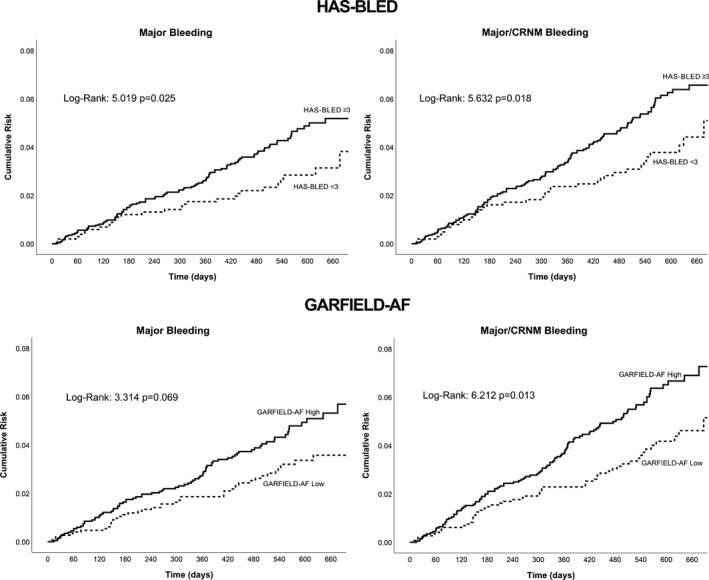
Kaplan‐Meier curves for major and major/clinically relevant nonmajor (CRNM) bleeding for GARFIELD‐AF (Global Anticoagulant in the Field–Atrial Fibrillation) bleeding and HAS‐BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol) scores.
For GARFIELD‐AF risk categories, the high‐score category showed a nonsignificant trend for major bleeding in the higher‐risk category compared with the low‐score category (P=0.069), whereas for the major/CRNM bleeding outcome, the high‐score category had a significantly greater risk than the low‐score category (P=0.013) (Figure 1, bottom panels). For the “any bleeding” outcome, patients with a HAS‐BLED score ≥3 had a higher cumulative risk compared with those with a HAS‐BLED score <3 (P<0.001), but no significant difference was found according to the GARFIELD‐AF bleeding risk categories (P=0.250) (Figure 2).
Figure 2.
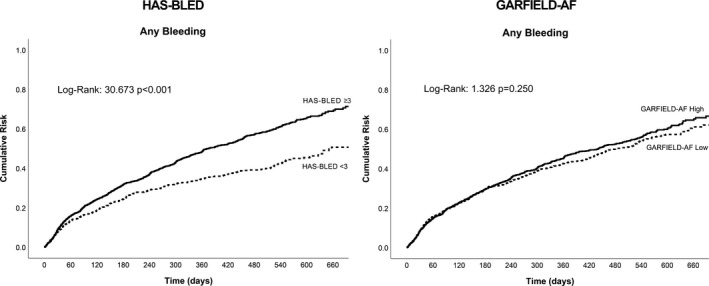
Kaplan‐Meier curves for any bleeding for GARFIELD‐AF (Global Anticoagulant in the Field–Atrial Fibrillation) bleeding and HAS‐BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol) scores.
Survival and Predictive Analysis
Using a Cox regression model (Table 2), adjusted for sex and type of AF, we found that the continuous HAS‐BLED score was significantly associated with the occurrence of all the 3 bleeding outcomes, with an increase in relative risks ranging from 13% to 31% (for any bleeding and major bleeding, respectively) for each score point. The GARFIELD‐AF bleeding score was only significantly associated with the major bleeding outcome (hazard ratio, 1.39; 95% CI, 1.04–1.86).
Table 2.
Survival and Predictive Analysis for Bleeding Outcomes for GARFIELD‐AF Bleeding and HAS‐BLED Scores
| Variable | HAS‐BLED | GARFIELD‐AF | ||
|---|---|---|---|---|
| HR (95% CI) | C‐Index (95% CI) | HR (95% CI) | C‐Index (95% CI) | |
| Major bleeding | 1.31 (1.14–1.51) | 0.58 (0.56–0.60) | 1.39 (1.04–1.86) | 0.56 (0.54–0.57) |
| Major/CRNM bleeding | 1.23 (1.09–1.39) | 0.56 (0.54–0.58) | 1.23 (0.99–1.54) | 0.57 (0.55–0.58) |
| Any bleeding | 1.13 (1.09–1.18) | 0.55 (0.53–0.57) | 1.05 (0.98–1.13) | 0.51 (0.49–0.53) |
CI indicates confidence interval; CRNM, clinically relevant nonmajor; GARFIELD‐AF, Global Anticoagulant in the Field–Atrial Fibrillation; HAS‐BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol; HR, hazard ratio.
Predictive analysis (Table 2), performed according to C‐indexes, indicated that both scores only had modest predictive value, for the 3 bleeding outcomes (HAS‐BLED, C‐indexes 0.55–0.58; GARFIELD‐AF C‐indexes 0.56–0.57 for major and major/CRNM bleeding, but nonsignificant for any bleeding). No significant differences were found between the 2 scores for their respective C‐indexes for major and major/CRNM bleeding.
Discrimination and Reclassification Analysis
On the basis of integrated discrimination improvement analyses, the GARFIELD‐AF bleeding score showed a nonsignificant decrease in the averaged sensitivity for major bleeding (−0.2%, P=0.318) and a nonsignificant increase in the averaged sensitivity for major/CRNM bleeding (0.1%, P=0.746) in comparison with the HAS‐BLED score (Table 3). Using net reclassification improvement, there was a nonsignificant negative reclassification against HAS‐BLED for major bleeding (−4.2%, P=0.448) and a nonsignificant positive reclassification for major/CRNM bleeding (3.3%, P=0.756). The GARFIELD‐AF bleeding score had a significantly lower sensitivity and a negative reclassification for any bleeding compared with HAS‐BLED, assessed by both integrated discrimination improvement (−1.1%, P<0.001) and net reclassification improvement (−8.7%, P<0.001). This demonstrated that, overall, the median improvement of the GARFIELD‐AF bleeding score was reduced almost in 2% compared with the HAS‐BLED score (−1.6%, P<0.001).
Table 3.
IDI, NRI, and Median Improvement Between GARFIELD‐AF Bleeding and HAS‐BLED Scores
| Variable | GARFIELD‐AF vs HAS‐BLED | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| IDI | 95% CI | P Value | NRI | 95% CI | P Value | Median Improvement | 95% CI | P Value | |
| Major bleeding | −0.002 | −0.007/0.003 | 0.318 | −0.042 | −0.189/0.087 | 0.448 | −0.002 | −0.010/0.005 | 0.308 |
| Major/CRNM bleeding | 0.001 | −0.005/0.007 | 0.746 | 0.033 | −0.094/0.129 | 0.756 | 0.001 | −0.007/0.009 | 0.378 |
| Any bleeding | −0.011 | −0.019/−0.005 | <0.001 | −0.087 | −0.131/−0.056 | <0.001 | −0.016 | −0.030/−0.001 | <0.001 |
CI indicates confidence interval; CRNM, clinically relevant nonmajor; GARFIELD‐AF, Global Anticoagulant in the Field–Atrial Fibrillation; HAS‐BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
We also tested the clinical usefulness and net benefit of the 2 scores using decision curve analyses (Figure 3). For major bleeding and major/CRNM bleeding, the curves corresponding to both models overlapped, suggesting no apparent net benefit of one model over the other. Only a slight higher net benefit was observed for HAS‐BLED for the major bleeding outcome. However, the HAS‐BLED score graphically demonstrated a net benefit of ≈5% over the GARFIELD‐AF bleeding score for any bleeding.
Figure 3.
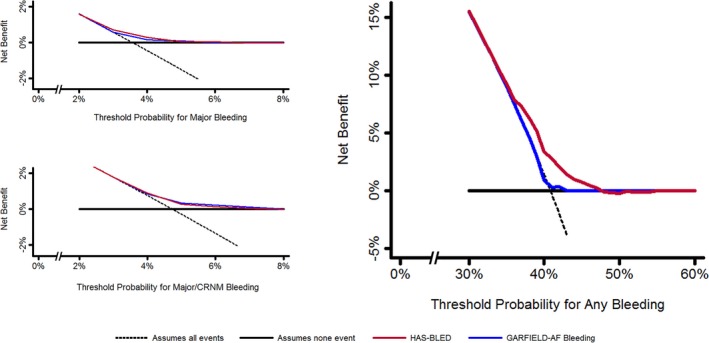
Decision curve analysis according to GARFIELD‐AF (Global Anticoagulant in the Field–Atrial Fibrillation) bleeding and HAS‐BLED (Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol) scores. CRNM indicates clinically relevant nonmajor.
Sensitivity Analysis
Throughout the entire follow‐up period, a total of 804 (22.6%) of patients interrupted warfarin treatment. A sensitivity analysis on the predictive ability of HAS‐BLED and GARFIELD‐AF bleeding scores based only on patients who continued treatment was then performed (Table 4).
Table 4.
Predictive Analysis for Bleeding Outcomes in On‐Treatment Cohort
| Variable | C‐Index (95% CI) | P Value for Comparison | |
|---|---|---|---|
| HAS‐BLED | GARFIELD‐AF | ||
| Major bleeding | 0.60 (0.53–0.68) | 0.55 (0.47–0.63) | 0.352 |
| Major/CRNM bleeding | 0.59 (0.53–0.66) | 0.57 (0.50–0.65) | 0.694 |
| Any bleeding | 0.56 (0.54–0.58) | 0.50 (0.48–0.53) | <0.001 |
CI indicates confidence interval; CRNM, clinically relevant nonmajor; GARFIELD‐AF, Global Anticoagulant in the Field–Atrial Fibrillation; HAS‐BLED, Hypertension, Abnormal renal and liver function, Stroke, Bleeding, Labile INRs, Elderly, Drugs or alcohol.
The HAS‐BLED score modestly significantly predicted all the bleeding outcomes, with a slightly higher predictive capacity. The GARFIELD‐AF bleeding score did not predict major bleeding and any bleeding outcomes but had marginal predictive capacity for major/CRNM bleeding in this on‐treatment analysis.
Comparing C‐indexes, the HAS‐BLED score had a significantly higher predictive capacity for the any bleeding outcome (P<0.001).
Discussion
In this analysis, derived from a large international randomized controlled trial, we showed that the HAS‐BLED score had modest predictive capacity for all the bleeding outcomes, but was significantly associated with all bleeding outcomes. Conversely, the GARFIELD‐AF bleeding score showed modest predictive capacity for major and major/CRNM bleeding outcomes but did not predict the any bleeding outcome. Second, when comparing the more complex GARFIELD‐AF bleeding score with the simple HAS‐BLED score, there was no significant advantage in terms of reclassification and net benefit for major bleeding and major/CRNM bleeding; however, the HAS‐BLED score demonstrated a net benefit of ≈5% over the GARFIELD‐AF bleeding score for the any bleeding outcome. Finally, the on‐treatment cohort analysis shows that the HAS‐BLED score significantly predicted all the bleeding outcomes studied, whereas the GARFIELD‐AF score only marginally predicted major/CRNM bleeding.
Incident bleeding events among patients with AF taking anticoagulants are a feared complication when prescribing OACs,22 and overestimating patients’ bleeding risk leads to underprescription of OACs.23 Data from the ORBIT‐AF (Outcomes Registry for Better Informed Treatment of AF) study show that history of bleeding was one of the most prevalent reasons for not prescribing OACs,24 as well as for OAC discontinuation.25
The impact of bleeding events, irrespective of the type, can be strongly relevant in the clinical course and decision making for patients with AF. Major bleeding events can lead to a significant risk of death and major adverse outcomes, both short‐ and long‐term.26, 27 Also, all bleeding events are associated with an impaired quality of life.28 Irrespective of type, patients experiencing a bleeding event are more likely to discontinue OAC treatment,29 which is associated with an increased risk for clinically significant events.30
Nonetheless, a high risk of bleeding should not be a reason to withhold OAC prescription.8 Guidelines on management of AF recommend assessing baseline bleeding risk to evaluate specific interventions to control and reduce this risk.4 Also, bleeding risk assessment should be routinely repeated at follow‐up visits and modifiable risks should be managed appropriately.5
Thus far, the HAS‐BLED score has been validated in several cohorts and is able to predict major bleeding in various clinical settings.31, 32, 33, 34 Several studies reported about the comparison between HAS‐BLED and other bleeding risk scores in vitamin K antagonist‐treated cohorts.31, 35, 36, 37, 38, 39 These studies all demonstrate a modest predictive capacity for these clinical bleeding risk scores, although most analyses reported that the HAS‐BLED score performs best.31, 36, 38 In particular, high‐risk patients (HAS‐BLED score ≥3) have a significantly higher risk of major bleeding, as demonstrated in several studies.31, 40 In a Spanish real‐world cohort, for example, a HAS‐BLED score ≥3 demonstrated significant predictive ability (C‐index [95% CI], 0.68 [0.65–0.71]).31 Similar data, derived from the Loire Valley AF Project, showed that patients with a HAS‐BLED score ≥3 reported a higher rate of major bleeding, with a significantly increased risk (hazard ratio, 3.57; 95% CI, 2.59–4.92) compared with patients with a HAS‐BLED score of 2 and a HAS‐BLED score of 0 to 1.40
The GARFIELD‐AF bleeding score has been recently derived from the GARFIELD‐AF registry study.13 In the original derivation cohort, the GARFIELD‐AF bleeding score performed modestly (C‐index, 0.66; 95% CI, 0.62–0.69), with marginal improvement compared with the HAS‐BLED score (C‐index, 0.64; 95% CI, 0.61–0.68).13 When applied in an external validation cohort, derived from the ORBIT‐AF registry study, the new model performed less well (C‐index, 0.61; 95% CI, 0.59–0.63 for the 3‐year major bleeding risk).13 Even if the GARFIELD‐AF bleeding score is intended to be used as a continuous score, data from the derivation cohort showed that on stratifying according to the median value, those patients in the high‐score group had a higher cumulative incidence, compared with those patients in the low‐score group.13 In addition, the statistical model used to derive the score cannot be easily compiled at the patient's bedside or in outpatient clinics, and the model proposed does not consider some established bleeding risk factors and strong predictors.41 This aspect likely affects the predictive abilities of the GARFIELD‐AF bleeding score in identifying any bleeding occurrence.
The ability of the scores to correctly identify patients more likely to experience a bleeding event allows us to properly flag up those high‐risk patients to treat the modifiable bleeding risks and to help schedule those patients for early review and follow‐up (eg, 4 weeks rather than 4–6 months)8, 42 to ultimately minimize the occurrence of any bleeding event throughout the long‐term observation.
This study represents the first external independent validation of the new prognostic score, in a clinical trial cohort with adjudicated bleeding outcomes. Our data clearly showed that irrespective of the type of bleeding, the GARFIELD‐AF bleeding score performed only modestly in predicting bleeding outcomes and was not predictive of any bleeding. At the cost of reduced ease and practicality, the GARFIELD‐AF also did not show improvement in the discrimination or reclassification of bleeding risk. In the prediction of any bleeding outcome, the HAS‐BLED score showed a significant net benefit compared with the GARFIELD‐AF score, using decision curve analysis. This article provides needed evidence in the context of reassuring clinicians that the use of an established (and largely validated) simple score (ie, HAS‐BLED) remains best, instead of using complex new scores with limited additional advantages.
Limitations
This study is mainly limited by its post hoc retrospective nature. Given that the study cohort was derived from a randomized controlled trial, all bleeding risk factors were recorded and managed, probably resulting in a lower rate of bleeding events compared with the real‐life populations. Furthermore, the exclusion of patients with liver disease from the original cohort, as well as the exclusive use of warfarin as an OAC treatment, may somewhat limit the generalizability of our results.
Conclusions
The algorithm‐based GARFIELD‐AF bleeding score did not show any significant improvement in bleeding risk prediction for major and major/CRNM bleeding compared with the simple HAS‐BLED score. For clinical usefulness in prediction of any bleeding, the HAS‐BLED score showed a significant net benefit compared with the GARFIELD‐AF score. The use of a simple, easy to compile, and accurate score, such as the HAS‐BLED score, is pivotal to streamline the bleeding risk evaluation, favor the management of modifiable bleeding risk factors, and ultimately reduce the occurrence of any bleeding event.
Disclosures
Proietti has received a small consulting fee from Boheringer Ingelheim. Lip has served as consultant for Bayer/Janssen, BMS/Pfizer, Biotronik, Medtronic, Boehringer Ingelheim, Microlife, and Daiichi‐Sankyo; and has been a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Microlife, Roche, and Daiichi‐Sankyo. No fees were received personally. The remaining authors have no disclosures to report.
Supporting information
Table S1. Major bleeding rates according to HAS‐BLED score as reported in the original derivation cohort1
Acknowledgments
AstraZeneca provided data sets for the analysis. AstraZeneca was never involved in any stage of manuscript drafting and preparation.
(J Am Heart Assoc. 2018;7:e009766 DOI: 10.1161/JAHA.118.009766.)
References
- 1. Lip GYH, Freedman B, de Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: past, present and future comparing the guidelines and practical decision‐making. Thromb Haemost. 2017;117:1230–1239. [DOI] [PubMed] [Google Scholar]
- 2. Lamberts M, Staerk L, Olesen JB, Fosbøl EL, Hansen ML, Harboe L, Lefevre C, Evans D, Gislason GH. Major bleeding complications and persistence with oral anticoagulation in non‐valvular atrial fibrillation: contemporary findings in real‐life Danish patients. J Am Heart Assoc. 2017;6:e004517 DOI: 10.1161/JAHA.116.004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lip GYH, Andreotti F, Fauchier L, Huber K, Hylek E, Knight E, Lane D, Levi M, Marin F, Palareti G, Kirchhof P. Bleeding risk assessment and management in atrial fibrillation patients: executive summary of a position document from the European Heart Rhythm Association [EHRA], endorsed by the European Society of Cardiology [ESC] working group on thrombosis. Thromb Haemost. 2011;106:997–1011. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener H‐C, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GYH, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 5. Chao T‐F, Lip GYH, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Liao J‐N, Chung F‐P, Chen T‐J, Chen S‐A. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow‐up and Delta HAS‐BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118:768–777. [DOI] [PubMed] [Google Scholar]
- 6. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJGM, Lip GYH. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. [DOI] [PubMed] [Google Scholar]
- 7. Lip GYH. Assessing bleeding risk with the HAS‐BLED score: balancing simplicity, practicality, and predictive value in bleeding‐risk assessment. Clin Cardiol. 2015;38:562–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lip GYH, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14:1711–1714. [DOI] [PubMed] [Google Scholar]
- 9. Chao T‐F, Lip GYH, Lin Y‐J, Chang S‐L, Lo L‐W, Hu Y‐F, Tuan T‐C, Liao J‐N, Chung F‐P, Chen T‐J, Chen S‐A. Major bleeding and intracranial hemorrhage risk prediction in patients with atrial fibrillation: attention to modifiable bleeding risk factors or use of a bleeding risk stratification score? A nationwide cohort study. Int J Cardiol. 2018;254:157–161. [DOI] [PubMed] [Google Scholar]
- 10. Guo Y, Zhu H, Chen Y, Lip GYH. Comparing bleeding risk assessment focused on modifiable risk factors only versus validated bleeding risk scores in atrial fibrillation. Am J Med. 2018;131:185–192. [DOI] [PubMed] [Google Scholar]
- 11. Esteve‐Pastor MA, Rivera‐Caravaca JM, Shantsila A, Roldán V, Lip GYH, Marín F. Assessing bleeding risk in atrial fibrillation patients: comparing a bleeding risk score based only on modifiable bleeding risk factors against the HAS‐BLED score: the AMADEUS Trial. Thromb Haemost. 2017;117:2261–2266. [DOI] [PubMed] [Google Scholar]
- 12. Zulkifly H, Lip GYH, Lane DA. Bleeding risk scores in atrial fibrillation and venous thromboembolism. Am J Cardiol. 2017;120:1139–1145. [DOI] [PubMed] [Google Scholar]
- 13. Fox KAA, Lucas JE, Pieper KS, Bassand J‐P, Camm AJ, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Hacke W, Kayani G, Oto A, Mantovani LG, Misselwitz F, Piccini JP, Turpie AGG, Verheugt FWA, Kakkar AK; GARFIELD‐AF Investigators . Improved risk stratification of patients with atrial fibrillation: an integrated GARFIELD‐AF tool for the prediction of mortality, stroke and bleed in patients with and without anticoagulation. BMJ Open. 2017;7:e017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Halperin JL. Ximelagatran compared with warfarin for prevention of thromboembolism in patients with nonvalvular atrial fibrillation: rationale, objectives, and design of a pair of clinical studies and baseline patient characteristics (SPORTIF III and V). Am Heart J. 2003;146:431–438. [DOI] [PubMed] [Google Scholar]
- 15. Olsson SB. Stroke prevention with the oral direct thrombin inhibitor ximelagatran compared with warfarin in patients with non‐valvular atrial fibrillation (SPORTIF III): randomised controlled trial. Lancet. 2003;362:1691–1698. [DOI] [PubMed] [Google Scholar]
- 16. Albers GW, Diener H‐C, Frison L, Grind M, Nevinson M, Partridge S, Halperin JL, Horrow J, Olsson SB, Petersen P, Vahanian A. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690–698. [DOI] [PubMed] [Google Scholar]
- 17. Camm AJ, Kirchhof P, Lip GYH, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey J‐YY, Ponikowski P, Rutten FH, Vahanian A, Auricchio A, Bax J, Ceconi C, Dean V, Filippatos G, Funck‐Brentano C, Hobbs R, Kearney P, McDonagh T, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Vardas PE, Widimsky P, Agladze V, Aliot E, Balabanski T, Blomstrom‐Lundqvist C, Capucci A, Crijns H, Dahlf B, Folliguet T, Glikson M, Goethals M, Gulba DC, Ho SY, Klautz RJM, Kose S, McMurray J, Perrone Filardi P, Raatikainen P, Salvador MJ, Schalij MJ, Shpektor A, Sousa J, Stepinska J, Uuetoa H, Zamorano JL, Zupan I. Guidelines for the management of atrial fibrillation. Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 18. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19. Pencina MJ, D'Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172; discussion 207–212. [DOI] [PubMed] [Google Scholar]
- 20. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raparelli V, Proietti M, Cangemi R, Lip GY, Lane DA, Basili S. Adherence to oral anticoagulant therapy in patients with atrial fibrillation focus on non‐vitamin K antagonist oral anticoagulants. Thromb Haemost. 2017;117:209–218. [DOI] [PubMed] [Google Scholar]
- 23. Bungard TJ, Ghali WA, Teo KK, McAlister FA, Tsuyuki RT. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. [DOI] [PubMed] [Google Scholar]
- 24. O'Brien EC, Holmes DN, Ansell JE, Allen LA, Hylek E, Kowey PR, Gersh BJ, Fonarow GC, Koller CR, Ezekowitz MD, Mahaffey KW, Chang P, Peterson ED, Piccini JP, Singer DE. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) registry. Am Heart J. 2014;167:601–609.e1. [DOI] [PubMed] [Google Scholar]
- 25. O'Brien EC, Simon DN, Allen LA, Singer DE, Fonarow GC, Kowey PR, Thomas LE, Ezekowitz MD, Mahaffey KW, Chang P, Piccini JP, Peterson ED. Reasons for warfarin discontinuation in the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Am Heart J. 2014;168:487–494. [DOI] [PubMed] [Google Scholar]
- 26. Gómez‐Outes A, Lagunar‐Ruíz J, Terleira‐Fernández A‐I, Calvo‐Rojas G, Suárez‐Gea ML, Vargas‐Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508–2521. [DOI] [PubMed] [Google Scholar]
- 27. Steinberg BA, Simon DN, Thomas L, Ansell J, Fonarow GC, Gersh BJ, Kowey PR, Mahaffey KW, Peterson ED, Piccini JP; Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF) Investigators and Patients . Management of Major Bleeding in Patients With Atrial Fibrillation Treated With Non‐Vitamin K Antagonist Oral Anticoagulants Compared With Warfarin in Clinical Practice (from Phase II of the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation [ORBIT‐AF II]). Am J Cardiol. 2017;119:1590–1595. [DOI] [PubMed] [Google Scholar]
- 28. Wang K, Li H, Kwong WJ, Antman EM, Ruff CT, Giugliano RP, Cohen DJ, Magnuson EA; ENGAGE AF‐TIMI 48 Trial Investigators . Impact of spontaneous extracranial bleeding events on health state utility in patients with atrial fibrillation: results from the ENGAGE AF‐TIMI 48 Trial. J Am Heart Assoc. 2017;6:e006703 DOI: 10.1161/JAHA.117.006703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien EC, Holmes DN, Thomas L, Fonarow GC, Kowey PR, Ansell JE, Mahaffey KW, Gersh BJ, Peterson ED, Piccini JP, Hylek EM. Therapeutic strategies following major, clinically relevant nonmajor, and nuisance bleeding in atrial fibrillation: findings from ORBIT‐AF. J Am Heart Assoc. 2018;7:e006391 DOI: 10.1161/JAHA.117.006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Proietti M, Romiti GF, Romanazzi I, Farcomeni A, Staerk L, Nielsen PB, Lip GYH. Restarting oral anticoagulant therapy after major bleeding in atrial fibrillation: a systematic review and meta‐analysis. Int J Cardiol. 2018;261:84–91. [DOI] [PubMed] [Google Scholar]
- 31. Roldán V, Marín F, Fernández H, Manzano‐Fernandez S, Gallego P, Valdés M, Vicente V, Lip GYH. Predictive value of the HAS‐BLED and ATRIA bleeding scores for the risk of serious bleeding in a “real‐world” population with atrial fibrillation receiving anticoagulant therapy. Chest. 2013;143:179–184. [DOI] [PubMed] [Google Scholar]
- 32. Gallego P, Roldán V, Torregrosa JM, Gálvez J, Valdés M, Vicente V, Marín F, Lip GYH. Relation of the HAS‐BLED bleeding risk score to major bleeding, cardiovascular events, and mortality in anticoagulated patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:312–318. [DOI] [PubMed] [Google Scholar]
- 33. Omran H, Bauersachs R, Rübenacker S, Goss F, Hammerstingl C. The HAS‐BLED score predicts bleedings during bridging of chronic oral anticoagulation: results from the national multicentre BNK Online bRiDging REgistRy (BORDER). Thromb Haemost. 2012;108:65–73. [DOI] [PubMed] [Google Scholar]
- 34. Smith JG, Wieloch M, Koul S, Braun OÖ, Lumsden J, Rydell E, Ohman J, Scherstén F, Svensson PJ, van der Pals J. Triple antithrombotic therapy following an acute coronary syndrome: prevalence, outcomes and prognostic utility of the HAS‐BLED score. EuroIntervention. 2012;8:672–678. [DOI] [PubMed] [Google Scholar]
- 35. Olesen JB, Lip GYH, Hansen PR, Lindhardsen J, Ahlehoff O, Andersson C, Weeke P, Hansen ML, Gislason GH, Torp‐Pedersen C. Bleeding risk in “real world” patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. J Thromb Haemost. 2011;9:1460–1467. [DOI] [PubMed] [Google Scholar]
- 36. Senoo K, Proietti M, Lane DA, Lip GYH. Evaluation of the HAS‐BLED, ATRIA and ORBIT bleeding risk scores in atrial fibrillation patients on warfarin. Am J Med. 2016;129:600–607. [DOI] [PubMed] [Google Scholar]
- 37. Proietti M, Senoo K, Lane DA, Lip GYH. Major bleeding in patients with non‐valvular atrial fibrillation: impact of time in therapeutic range on contemporary bleeding risk scores. Sci Rep. 2016;6:24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Apostolakis S, Lane DA, Guo Y, Buller H, Lip GYH. Performance of the HEMORR(2)HAGES, ATRIA, and HAS‐BLED bleeding risk‐prediction scores in patients with atrial fibrillation undergoing anticoagulation: the AMADEUS (evaluating the use of SR34006 compared to warfarin or acenocoumarol in patients with atrial fibrillation) study. J Am Coll Cardiol. 2012;60:861–867. [DOI] [PubMed] [Google Scholar]
- 39. Rivera‐Caravaca JM, Roldán V, Esteve‐Pastor MA, Valdés M, Vicente V, Lip GYH, Marín F. Importance of time in therapeutic range on bleeding risk prediction using clinical risk scores in patients with atrial fibrillation. Sci Rep. 2017;7:12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lip GYH, Banerjee A, Lagrenade I, Lane DA, Taillandier S, Fauchier L. Assessing the risk of bleeding in patients with atrial fibrillation: the loire valley atrial fibrillation project. Circ Arrhythmia Electrophysiol. 2012;5:941–948. [DOI] [PubMed] [Google Scholar]
- 41. Lip GYH, Lane DA. Assessing bleeding risk in atrial fibrillation with the HAS‐BLED and ORBIT scores: clinical application requires focus on the reversible bleeding risk factors. Eur Heart J. 2015;36:3265–3267. [DOI] [PubMed] [Google Scholar]
- 42. Lip GYH, Lane DA. Matching the NOAC to the patient: remember the modifiable bleeding risk factors. J Am Coll Cardiol. 2015;66:2282–2284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Major bleeding rates according to HAS‐BLED score as reported in the original derivation cohort1


