Abstract
Background
There are limited data on the presentation of Takotsubo cardiomyopathy (TTC) in severe sepsis.
Methods and Results
This was a retrospective cohort study using the National Inpatient Sample database (2007–2013) of all adults with severe sepsis. TTC was identified in patients with severe sepsis using previously validated administrative codes. The primary outcome was in‐hospital mortality, and secondary outcomes included resource utilization and discharge disposition. Regression analysis was performed for the entire cohort and a propensity‐matched sample. An exploratory analysis was performed for predictors of TTC incidence and mortality in TTC. During this 7‐year period, in 7.1‐million hospitalizations for severe sepsis, TTC was diagnosed in 10 746 (0.15%) admissions. TTC was noted more commonly in whites, females, and among 65‐ to 79‐year‐old individuals. TTC was independently associated with lower in‐hospital mortality in severe sepsis (odds ratio, 0.58; 95% confidence interval, 0.51–0.65). This association was more prominent in females (odds ratio, 0.51; 95% confidence interval, 0.44–0.59]) compared with males (odds ratio, 0.69; 95% confidence interval, 0.55–0.85]). Presentation in later years of the study period, middle‐age, female sex, and white race were independent predictors for the diagnosis of TTC. Age ≥80 years, black race, greater comorbidity, and multiorgan dysfunction were independently associated with higher in‐hospital mortality among TTC admissions.
Conclusions
TTC is observed with increasing frequency in severe sepsis and was associated with a significantly lower in‐hospital mortality compared with patients without TTC. Presentation in later years of the study period, middle age, female sex, and white race were independent predictors for the diagnosis of TTC in severe sepsis.
Keywords: apical ballooning syndrome, outcomes research, sepsis, septic shock, shock, stress‐induced cardiomyopathy, Takotsubo cardiomyopathy
Subject Categories: Heart Failure, Cardiomyopathy
Clinical Perspective
What Is New?
Cardiovascular dysfunction in sepsis is associated with worse outcomes; however, there are limited data on the epidemiology and outcomes of Takotsubo cardiomyopathy in severe sepsis.
What Are the Clinical Implications?
In this 7‐year study, using a nationally representative database, we highlight the incidence and predictors of Takotsubo cardiomyopathy in severe sepsis and demonstrate that Takotsubo cardiomyopathy is associated with lower in‐hospital mortality.
Introduction
Takotsubo cardiomyopathy (TTC) was first described in 1990 and has been increasingly recognized in clinical practice.1 Labeled variably as apical‐ballooning syndrome, stress cardiomyopathy, and broken‐heart syndrome, TTC is characterized by transient regional left ventricular systolic dysfunction without obstructive coronary artery disease, precipitated by emotional and physical triggers.1, 2 It is diagnosed most commonly in postmenopausal females, though it does occur in young women and males.1, 3 Postulated pathogenesis includes myocardial stunning, catecholamine toxicity, and impaired myocyte metabolism.4 Among the many proposed diagnostic criteria, TTC is most commonly diagnosed using the Mayo Clinic criteria: (1) transient hypokinesis, akinesias or dyskinesia of the left ventricular midsegments with or without apical involvement; (2) absence of obstructive coronary artery disease or angiographic evidence of acute plaque rupture; (3) new ECG abnormalities or modest elevation in cardiac troponins; and (4) absence of pheochromocytoma or myocarditis.5
Patients admitted to intensive care units are subject to severe physical/emotional stress and catecholamine surges, which can potentially trigger TTC. Sepsis continues to be a leading cause of mortality and morbidity in intensive care units in the United States.6 Multiorgan dysfunction is observed in up to 45% of patients presenting with sepsis and is associated with worse short‐term outcomes and long‐term organ dysfunction.7, 8, 9 Cardiovascular dysfunction in sepsis can manifest as septic cardiomyopathy, TTC, refractory shock, and myocardial injury, resulting in worse mortality and morbidity.10, 11, 12, 13, 14 Despite extensive data on septic cardiomyopathy, there are limited large‐scale epidemiological data on TTC in patients with severe sepsis.1, 15 The aim of this study was to evaluate the trends and outcomes of TTC in patients with severe sepsis from a large US cohort.
Material and Methods
The data used for this study are publicly available with the Healthcare Cost and Utilization Project (HCUP)/National (Nationwide) Inpatient Sample (NIS).16 The data, analytical methods, and study materials have been made available to other researchers for purposes of reproducing the results or replicating the procedure. Please refer to Tables 1 and 2 for detailed International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) codes used in this study.
Table 1.
ICD‐9‐CM Codes Used to Identify Admissions With Severe Sepsis
| Diagnosis | ICD‐9‐CM Code |
|---|---|
| Septicemia | 038.0, 038.10, 038.11, 038.19, 038.2, 038.3, 038.4, 038.40, 038.41, 038.42, 038.43, 038.44, 038.49, 038.8, 038.9 |
| Bacteremia | 790.7 |
| Disseminated fungal infection | 117.9 |
| Disseminated candidal infection | 112.5 |
| Fungal endocarditis | 115.04, 115.14, 115.94 |
| Candidal endocarditis | 112.81 |
| Candidal meningitis | 112.83 |
| Salmonella septicemia | 003.1 |
| Salmonella meningitis | 003.21 |
| Meningococcal septicemia | 036.2 |
| Waterhouse–Friderichsen syndrome | 036.3 |
| Meningococcal meningitis | 036.0 |
| Meningococcal encephalitis | 036.1 |
| Meningococcal endocarditis | 036.42 |
| Septicemic plague | 020.2 |
| Anthrax septicemia | 022.3 |
| Gonococcemia | 098.89 |
| Gonococcal endocarditis | 098.84 |
| Gonococcal meningitis | 098.82 |
| Severe sepsis | 995.92 |
| Septic shock | 785.52 |
ICD‐9‐CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification.
Table 2.
ICD‐9‐CM Codes Used for Acute Organ Dysfunction
| Organ Failure | ICD‐9‐CM Code | Description |
|---|---|---|
| Respiratory | 518.81 | Acute respiratory failure |
| 518.82 | Other pulmonary insufficiency, not elsewhere classified. Includes—acute respiratory distress, acute respiratory insufficiency, adult respiratory distress syndrome NEC | |
| 518.85 | Acute respiratory distress syndrome after shock or trauma | |
| 786.09 | Respiratory distress NOS | |
| 799.1 | Respiratory arrest | |
| 96.7, 96.70, 96.71, 96.72 | Ventilator management | |
| Cardiovascular | 785.5 | Shock without mention of trauma |
| 785.50 | Shock unspecified | |
| 785.59 | Other shock without trauma (includes hypovolemic shock) | |
| 785.51 | Cardiogenic shock | |
| 785.52 | Septic shock | |
| 458.8, 458.9, 796.3 | Hypotension NOS | |
| Renal | 584, 584.5, 584.6, 584.7, 584.8, 584.9 | Acute kidney injury |
| Hepatic | 570 | Acute hepatic failure or necrosis |
| 572.2 | Hepatic encephalopathy | |
| 573.3 | Hepatitis unspecified | |
| 573.4 | Hepatic infarction | |
| Hematologic | 286.6 | Defibrination syndrome |
| 286.7 | Acquired coagulation factor deficiency | |
| 286.9 | Other coagulation defect | |
| 287.4, 287.5 | Thrombocytopenia—secondary or unspecified | |
| Metabolic | 276.2 | Acidosis—metabolic or lactic |
| Neurologic | 293, 293.0, 293.1, 293.8, 293.81, 293.82, 293.83, 293.84, 293.89, 293.9 | Transient organic psychotic conditions |
| 348.1 | Anoxic brain injury | |
| 348.3, 348.30, 348.31, 348.39 | Acute encephalopathy | |
| 780.01 | Coma | |
| 780.09 | Altered consciousness—unspecified | |
| 89.14 | Electroencephalogram |
ICD‐9‐CM indicates International Classification of Diseases, Ninth Revision, Clinical Modification; NEC, not elsewhere classified; NOS, not otherwise specified.
Study Design and Database
The Healthcare Cost and Utilization Project/NIS was used to obtain data for this study duration of 2007–2013. The NIS, the largest all‐payer inpatient care database publicly available in the United States, provides data for a 20% stratified sample of the US community hospitals.17 Information regarding each discharge includes patient demographics, primary payer, hospital characteristics, principal diagnosis, up to 24 additional secondary diagnoses, and procedural diagnoses. No institutional review board approval was sought because of the publically available de‐identified data set used in this research.
Study Population, Variables, and Outcomes
All hospitalizations for severe sepsis as the primary diagnosis in patients aged ≥20 years were included in this study. Severe sepsis was defined using the ICD‐9‐CM codes for severe sepsis or septic shock, septicemia, bacteremia, or fungemia with at least 1 organ dysfunction (Tables 1 and 2).18, 19, 20, 21 This definition of severe sepsis is consistent with the 2001 American College of Chest Physicians/Society of Critical Care Medicine consensus criteria for severe sepsis: consequent organ dysfunction, hypoperfusion, or hypotension.22 Using previously validated algorithms for microbiological cultures, septic patients were classified into “culture‐positive” and “culture‐negative” sepsis.21 Development of TTC in this cohort of severe sepsis was identified using ICD‐9‐CM code 429.83. Demographic characteristics (age, sex, and race), hospital characteristics (teaching status and location, bed size, and region), and primary payer associated with each discharge were identified from the NIS database. Hospitals were divided into tertiles based on the annual volume of severe sepsis discharges (<178, 178–484, or >484 per year). Deyo's modification of Charlson Comorbidity Index was used to identify burden of comorbid diseases.23 Use of mechanical ventilation was identified using ICD‐9‐CM procedure codes 96.70, 96.71, and 96.72.
The primary outcomes of interest were frequency and trends of TTC and its associated in‐hospital mortality. Secondary outcomes included length of stay, hospitalization costs, and discharge disposition in patients with versus without TTC in severe sepsis. Additional secondary outcomes included predictors of TTC and predictors of mortality in patients with TTC in hospital admissions for severe sepsis.
Statistical Analysis
As recommended by the Healthcare Cost and Utilization Project/NIS, survey procedures using discharge weights provided with the NIS database were used to generate national estimates.24 Chi‐square and t tests were used to compare categorical and continuous variables, respectively. Linear regression was used to analyze trends over time. An a priori multivariable regression model was used to assess whether TTC is an independent predictor of in‐hospital mortality in patients with severe sepsis. The model was adjusted for patient age, sex, race, primary payer, Charlson Comorbidity Index, hospital bed size, volume, region, location and teaching status, individual organ dysfunction, use of mechanical ventilation, presence of septic shock, and year of admission. We also performed a supplemental analysis where we used restricted cubic spline transformations of age and year of admission using 5 knots to model the nonlinear relationship with mortality. Because TTC is observed more often in females and in middle‐aged patients,1 we also evaluated for an interaction term between sex and age with the presence of TTC in the model to see whether there was a differential effect of sex/age on in‐hospital mortality attributed to TTC.
All variables had <1% missing values, except race that was missing in 11.1% of the observations. Because patients with and without TTC differed in their baseline characteristics, we performed another sensitivity analysis by using propensity matching for baseline characteristics between the 2 cohorts. We used a multivariable logistic regression to generate a propensity score with TTC as the dependent variable. We then used 1:1 nearest neighbor matching with 0.01 calipers and without replacement to match patients with TTC to those without. The propensity‐matched sample had standardized differences <10% for all baseline characteristics (Figure 1). The final matched cohort had 1945 pairs with a total of 3890 observations. We then used the propensity‐matched sample for logistic regression to assess the impact of TTC on in‐hospital mortality in patients with severe sepsis. A sandwich covariance estimator was used to adjust for correlation between matched pairs in this logistic regression model. All statistical analyses were performed using STATA software (version 14.0; StataCorp LP, College Station, TX).
Figure 1.
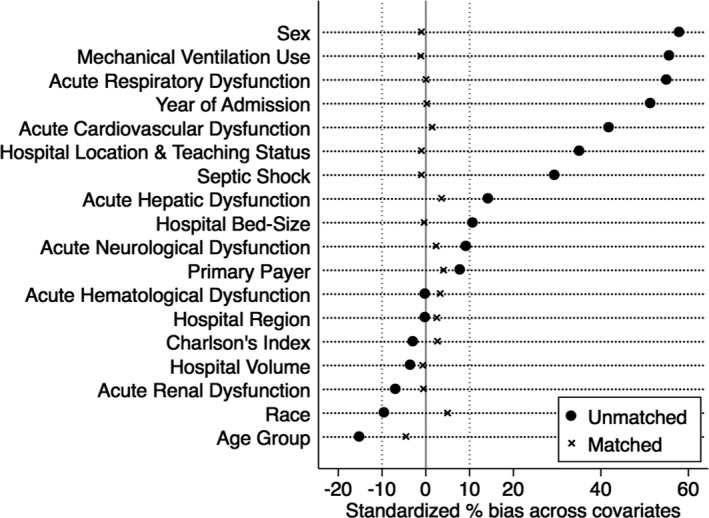
Propensity‐matched sample of patients with severe sepsis with and without Takotsubo cardiomyopathy. All baseline characteristics of demographics, acute organ dysfunction, and hospital characteristics after propensity matching show <10% standardized differences for the cohorts with and without Takotsubo cardiomyopathy in severe sepsis.
Results
In the period from 2007 to 2013, there were an estimated 7.1 million (95% confidence interval [CI], 6.9–7.3) admissions with severe sepsis in this stratified sample from 20% of US nonfederal hospitals. A causative organism was identifiable in 50.28% of all admissions (culture positive), and the rest were culture negative. TTC was identified in 10 746 (95% CI, 10 049–11 444) admissions during this period that comprised 0.15% of all severe sepsis admissions. There was a progressive increase in frequency of TTC from 0.02% in 2007 to 0.25% in 2013 (P<0.001; Figure 2). Baseline clinical characteristics of patients with and without TTC in severe sepsis are summarized in Table 3. Mean (±SD) age was 65.6±14.6 versus 67.7±16.2 years (P<0.001) in those with and without TTC. Frequency of female sex, ages between 45 and 79 years, and white race were more frequent in patients with TTC. Diagnosis of TTC with severe sepsis was more frequent in large‐sized and urban teaching hospitals. Patients with TTC had a higher frequency of a pre‐existing anxiety disorder and, during the acute event, more likely to develop respiratory, hepatic, and neurological dysfunction. TTC patients also had a greater incidence of septic shock and the need for mechanical ventilation and intra‐aortic balloon pumping. Diagnostic coronary angiography without the need for coronary interventions was performed in 32% of admissions with TTC, suggestive of the absence of obstructive coronary artery disease. Only 2% of admissions with severe sepsis without TTC received diagnostic coronary angiography.
Figure 2.
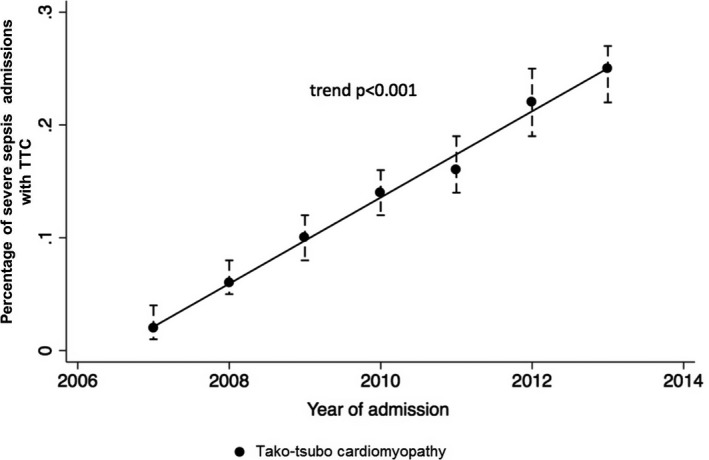
Trends of Takotsubo cardiomyopathy. Represented as estimated percentage of cases with 95% confidence interval; P value for trend: P<0.001. TTC indicates Takotsubo cardiomyopathy.
Table 3.
Baseline Characteristics of Severe Sepsis Patients With and Without TTC
| Characteristic | Severe Sepsis With TTC% (N=10 746) | Severe Sepsis Without TTC% (N=7 052 712) | P Value |
|---|---|---|---|
| Age group, y | |||
| 20 to 44 | 7.7 | 9.0 | <0.001 |
| 45 to 64 | 35.9 | 30.1 | |
| 65 to 79 | 38.6 | 33.2 | |
| ≥80 y | 17.7 | 27.7 | |
| Sex | |||
| Female | 75.7 | 49.1 | <0.001 |
| Race | |||
| White | 77.9 | 69.5 | <0.001 |
| Black | 8.0 | 15.4 | |
| Hispanic | 7.5 | 8.9 | |
| Asian | 3.2 | 2.7 | |
| Native American | 0.6 | 0.6 | |
| Others | 2.7 | 2.7 | |
| Primary payer | |||
| Medicare | 62.5 | 66.6 | 0.003 |
| Medicaid | 11.0 | 10.4 | |
| Private | 19.8 | 17.0 | |
| Uninsured | 3.8 | 3.3 | |
| No charge | 0.3 | 0.3 | |
| Others | 2.3 | 2.3 | |
| Hospital teaching status and locationa | |||
| Rural | 4.7 | 9.7 | <0.001 |
| Urban nonteaching | 28.2 | 41.6 | |
| Urban teaching | 67.1 | 48.7 | |
| Hospital bed sizea | |||
| Small | 8.7 | 10.9 | 0.003 |
| Medium | 22.8 | 25.0 | |
| Large | 68.5 | 64.1 | |
| Hospital volumea | |||
| Small | 33.0 | 33.2 | 0.1 |
| Medium | 36.9 | 34.2 | |
| Large | 30.1 | 32.6 | |
| Hospital regiona | |||
| Northeast | 18.5 | 18.5 | <0.001 |
| Midwest | 26.2 | 21.7 | |
| South | 31.4 | 38.5 | |
| West | 23.9 | 21.2 | |
| Charlson Comorbidity Index | |||
| 0 to 3 | 72.3 | 71.7 | 0.001 |
| 4 to 6 | 21.6 | 20.8 | |
| ≥7 | 6.0 | 7.5 | |
| Smoking history | 19.9 | 15.8 | <0.001 |
| Anxiety disorder | 7.0 | 4.3 | <0.001 |
| Hypertension | 48.9 | 52.8 | <0.001 |
| Mechanical ventilation | 57.4 | 30.5 | <0.001 |
| Intra‐aortic balloon pumping | 4.7 | 0.4 | <0.001 |
| Angiography use | 32.4 | 2.1 | <0.001 |
| Septic shock | 45.2 | 31.0 | <0.001 |
| Acute respiratory dysfunctionb | 75.2 | 48.5 | <0.001 |
| Acute cardiovascular dysfunctionb | 63.8 | 43.4 | <0.001 |
| Acute renal dysfunctionb | 51.1 | 54.6 | <0.001 |
| Acute hepatic dysfunctionb | 9.3 | 5.5 | <0.001 |
| Acute hematological dysfunctionb | 19.0 | 18.6 | 0.6 |
| Acute neurological dysfunctionb | 24.7 | 20.1 | <0.001 |
All values are represented as percentage. NIS indicates National Inpatient Sample; TTC, Takotsubo cardiomyopathy.
Refer to text and the NIS database classification for details.
Refer to Table 2 for acute organ dysfunction details.
Mortality and Morbidity Outcomes
Unadjusted in‐hospital mortality was not different between those with and without TTC (20.6% versus 22.3%; P=0.06; Table 4). After multivariable adjustment TTC patients had lower in‐hospital mortality (odds ratio [OR], 0.58; 95% CI, 0.51–0.65; P<0.001; Table 5). This lower adjusted in‐hospital mortality was still observed when restricting cubic spline transformations of age and year of admission using 5 knots to model the nonlinear relationship with mortality and in the regression model (OR, 0.58; 95% CI, 0.51–0.66; P<0.001) and using the propensity‐matched sample (OR, 0.52; 95% CI, 0.44–0.61; P<0.001; Table S1). Patients with TTC had a longer duration of hospitalization, higher hospitalization costs, and were more frequently to be discharged to a skilled nursing facility and home with home healthcare assistance as compared with those without TTC (Table 4).
Table 4.
Clinical Outcomes of TTC in Severe Sepsis
| Outcome | Severe Sepsis With TTC% (N=10 746) | Severe Sepsis Without TTC% (N=7 052 712) | P Value |
|---|---|---|---|
| In‐hospital mortality | 20.6 | 22.3 | 0.06 |
| Median length of stay, days | 12 (7–20) | 8 (4–15) | <0.001 |
| Median hospitalization costs (US dollars) | 115 625 (63 901–227 979) | 57 652 (27 374–127 229) | <0.001 |
| Discharge disposition | |||
| Home | 20.4 | 22.5 | 0.02 |
| Skilled nursing facility | 39.0 | 36.0 | 0.005 |
| Transferred to other hospitals | 3.6 | 4.1 | 0.2 |
| Home with home health care | 15.7 | 14.2 | 0.03 |
| Against medical advice | 0.1 | 0.5 | 0.01 |
| Unknown | 0.3 | 0.2 | 0.1 |
Represented as percentage or median (interquartile range). TTC indicates Takotsubo cardiomyopathy.
Table 5.
Multivariate Analysis for In‐Hospital Mortality in Severe Sepsis
| Characteristic | Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Takotsubo cardiomyopathy | 0.58 | 0.51 | 0.65 | <0.001 |
| Age group, y | ||||
| 20 to 44 | Reference groupa | |||
| 45 to 64 | 1.48 | 1.44 | 1.51 | <0.001 |
| 65 to 79 | 2.26 | 2.19 | 2.34 | <0.001 |
| ≥80 y | 4.03 | 3.90 | 4.19 | <0.001 |
| Sex | ||||
| Female | 1.06 | 1.05 | 1.07 | <0.001 |
| Year of admission | ||||
| 2007 | Reference groupa | |||
| 2008 | 0.95 | 0.90 | 1.00 | 0.09 |
| 2009 | 0.79 | 0.75 | 0.84 | <0.001 |
| 2010 | 0.71 | 0.67 | 0.75 | <0.001 |
| 2011 | 0.63 | 0.60 | 0.68 | <0.001 |
| 2012 | 0.51 | 0.48 | 0.53 | <0.001 |
| 2013 | 0.47 | 0.44 | 0.49 | <0.001 |
| Race | ||||
| White | Reference groupa | |||
| Black | 1.05 | 1.03 | 1.07 | <0.001 |
| Hispanic | 0.99 | 0.96 | 1.02 | 0.56 |
| Asian | 1.03 | 0.97 | 1.08 | 0.35 |
| Native American | 0.96 | 0.88 | 1.04 | 0.29 |
| Others | 0.98 | 0.95 | 1.03 | 0.46 |
| Primary payer | ||||
| Medicare | Reference groupa | |||
| Medicaid | 1.08 | 1.05 | 1.10 | <0.001 |
| Private | 1.08 | 1.05 | 1.10 | <0.001 |
| Uninsured | 1.30 | 1.24 | 1.36 | <0.001 |
| No charge | 1.22 | 1.08 | 1.38 | 0.002 |
| Others | 1.34 | 1.25 | 1.44 | <0.001 |
| Hospital teaching status and locationb | ||||
| Rural | Reference groupa | |||
| Urban nonteaching | 0.89 | 0.85 | 0.92 | <0.001 |
| Urban teaching | 1.04 | 0.99 | 1.10 | 0.10 |
| Hospital bed sizeb | ||||
| Small | Reference groupa | |||
| Medium | 1.04 | 0.99 | 1.09 | 0.09 |
| Large | 1.15 | 1.10 | 1.20 | <0.001 |
| Hospital volumeb | ||||
| Small | Reference groupa | |||
| Medium | 0.90 | 0.87 | 0.93 | <0.001 |
| Large | 0.79 | 0.75 | 0.82 | <0.001 |
| Hospital regionb | ||||
| Northeast | Reference groupa | |||
| Midwest | 0.68 | 0.65 | 0.72 | <0.001 |
| South | 0.82 | 0.79 | 0.86 | <0.001 |
| West | 0.78 | 0.75 | 0.82 | <0.001 |
| Charlson Comorbidity Index | ||||
| 0 to 3 | Reference groupa | |||
| 4 to 6 | 1.31 | 1.29 | 1.33 | <0.001 |
| ≥7 | 2.52 | 2.47 | 2.58 | <0.001 |
| Mechanical ventilation | 1.76 | 1.72 | 1.79 | <0.001 |
| Septic shock | 1.34 | 1.31 | 1.36 | <0.001 |
| Culture negative sepsis | 1.74 | 1.72 | 1.76 | <0.001 |
| Acute respiratory dysfunctionc | 3.32 | 3.27 | 3.39 | <0.001 |
| Acute cardiovascular dysfunctionc | 2.0 | 1.95 | 2.04 | <0.001 |
| Acute renal dysfunctionc | 1.33 | 1.31 | 1.35 | <0.001 |
| Acute hepatic dysfunctionc | 2.22 | 2.17 | 2.26 | <0.001 |
| Acute hematological dysfunctionc | 1.36 | 1.34 | 1.39 | <0.001 |
| Acute neurological dysfunctionc | 1.18 | 1.16 | 1.20 | <0.001 |
All values are represented as odds ratio (95% confidence interval).
The first category in variables with >2 categories was used as a reference group to develop odds ratios for the remaining categories was has been labeled as “Reference group.”
Refer to text and the NIS (National Inpatient Sample) database classification for details.
Refer to Table 2 for acute organ dysfunction details.
There was a significant interaction between TTC and sex (P=0.02) for in‐hospital mortality, such that relative mortality was higher in males (OR, 0.69; 95% CI, 0.55–0.85) compared with (OR, 0.51; 95% CI, 0.44–0.59). Baseline characteristics and outcomes for TTC patients stratified by sex are summarized in Table 6 and Figure 3. Male patients with TTC were significantly younger than females. Male patients had a higher Charlson Comorbidity Index and were more likely to require mechanical ventilation and develop multiorgan failure.
Table 6.
Baseline Characteristics and Outcomes of Male and Female Patients With Takotsubo Cardiomyopathy
| Baseline Characteristics and Outcomes | Male Sex % (N=2613) | Female Sex % (N=8133) | P Value |
|---|---|---|---|
| Baseline characteristics | |||
| Year of admission | |||
| 2007 | 0.9 | 2.2 | 0.08 |
| 2008 | 4.2 | 5.7 | |
| 2009 | 7.6 | 9.4 | |
| 2010 | 12.0 | 13.6 | |
| 2011 | 18.5 | 16.8 | |
| 2012 | 23.2 | 23.9 | |
| 2013 | 33.7 | 28.5 | |
| Age group, y | |||
| 20 to 44 | 11.2 | 6.7 | <0.001 |
| 45 to 64 | 41.3 | 34.2 | |
| 65 to 79 | 34.7 | 39.9 | |
| ≥80 | 12.9 | 19.2 | |
| Race | |||
| White | 74.0 | 79.2 | 0.11 |
| Black | 8.8 | 7.7 | |
| Hispanic | 9.2 | 6.7 | |
| Asian | 4.3 | 2.8 | |
| Native American | 0.2 | 0.8 | |
| Other | 3.4 | 2.5 | |
| Primary payer | |||
| Medicare | 56.5 | 64.4 | 0.004 |
| Medicaid | 10.0 | 11.4 | |
| Private | 24.7 | 18.2 | |
| Uninsured | 5.2 | 3.4 | |
| No charge | 0.4 | 0.3 | |
| Others | 3.2 | 2.3 | |
| Hospital teaching status and locationa | |||
| Rural | 3.6 | 5.1 | <0.001 |
| Urban nonteaching | 21.7 | 30.3 | |
| Urban teaching | 74.8 | 64.6 | |
| Hospital bed sizea | |||
| Small | 9.0 | 8.6 | 0.29 |
| Medium | 20.3 | 23.5 | |
| Large | 70.7 | 67.9 | |
| Hospital volumea | |||
| Small | 30.1 | 33.9 | 0.17 |
| Medium | 40.1 | 35.9 | |
| Large | 29.8 | 30.2 | |
| Hospital regiona | |||
| Northeast | 18.5 | 18.5 | 0.97 |
| Midwest | 26.9 | 25.9 | |
| South | 31.4 | 31.4 | |
| West | 23.2 | 24.2 | |
| Charlson Comorbidity Index | |||
| 0 to 3 | 68.8 | 72.5 | 0.01 |
| 4 to 6 | 24.1 | 20.8 | |
| ≥7 | 7.1 | 5.7 | |
| Smoking history | 22.0 | 19.3 | 0.18 |
| Anxiety disorder | 5.1 | 7.6 | 0.05 |
| Hypertension | 45.9 | 49.8 | 0.11 |
| Mechanical ventilation | 62.7 | 55.7 | 0.006 |
| Septic shock | 48.9 | 44.0 | 0.06 |
| Acute respiratory dysfunctionb | 78.5 | 74.2 | 0.05 |
| Acute cardiovascular dysfunctionb | 68.1 | 62.5 | 0.02 |
| Acute renal dysfunctionb | 56.5 | 49.4 | 0.005 |
| Acute hepatic dysfunctionb | 9.4 | 9.4 | 0.99 |
| Acute hematological dysfunctionb | 22.0 | 18.0 | 0.04 |
| Acute neurological dysfunctionb | 23.5 | 25.1 | 0.43 |
| Outcomes | |||
| In‐hospital mortality | 23.6 | 19.7 | 0.05 |
| Discharge disposition | |||
| Home | 21.3 | 20.1 | 0.54 |
| Skilled nursing facility | 35.7 | 40.1 | 0.07 |
| Transferred to other hospitals | 4.8 | 3.3 | 0.11 |
| Home with home health care | 14.1 | 16.2 | 0.26 |
| Against medical advice | 0.0 | 0.3 | 0.25 |
| Unknown | 0.4 | 0.4 | 0.97 |
All values are represented as percentage.
Refer to text and the NIS (National Inpatient Sample) database classification for details.
Refer to Table 2 for acute organ dysfunction details.
Figure 3.
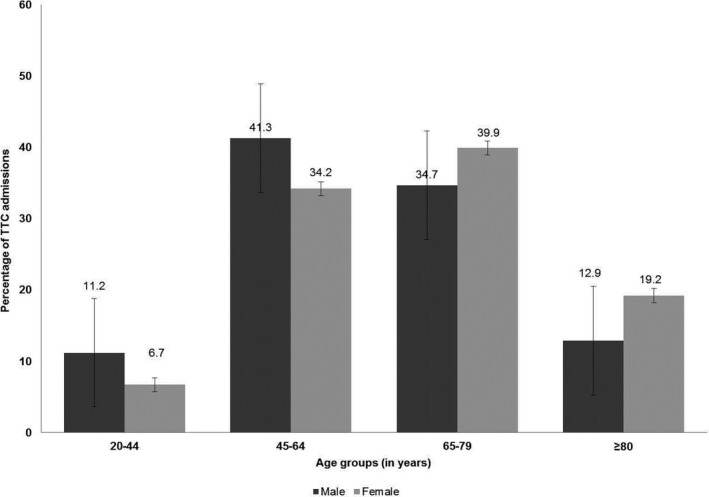
Age distribution for male and female patients with Takotsubo cardiomyopathy complicating severe sepsis. TTC indicates Takotsubo cardiomyopathy.
Predictors of Development and Mortality in TTC
In an exploratory analysis in admissions with TTC, multivariate logistic regression analysis was used to evaluate predictors of development of TTC and mortality in admissions with TTC (Table 7). Baseline characteristics and comorbidities were used to evaluate predictors of the development of TTC. Presentation in later years of the study period, middle aged (45–79 years), female sex, and white race were independent predictors for the diagnosis of TTC in severe sepsis. In severe sepsis admissions with TTC, age ≥80 years, black race, greater comorbidity, and multiorgan dysfunction were independently associated with in‐hospital mortality (Table 7).
Table 7.
Multivariable Predictors of Development of and Mortality in TTC
| Parameter | Incidence of TTC (N=10 746) | Mortality in TTC (N=2214) | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Year of admission | ||||
| 2007 | Reference groupa | |||
| 2008 | 2.2 (1.4–3.6) | 0.002 | 2.2 (0.8–6.4) | 0.13 |
| 2009 | 3.6 (2.2–5.8) | <0.001 | 1.6 (0.6–4.4) | 0.37 |
| 2010 | 5.2 (3.3–8.1) | <0.001 | 1.5 (0.6–3.9) | 0.43 |
| 2011 | 6.0 (3.9–9.3) | <0.001 | 1.3 (0.5–3.3) | 0.63 |
| 2012 | 8.5 (5.4–13.5) | <0.001 | 1.1 (0.4–2.8) | 0.88 |
| 2013 | 9.3 (6.0–14.4) | <0.001 | 1.0 (0.4–2.6) | 0.98 |
| Age group, y | ||||
| 20 to 44 | Reference groupa | |||
| 45 to 64 | 1.4 (1.2–1.7) | <0.001 | 1.4 (0.8–2.4) | 0.19 |
| 65 to 79 | 1.6 (1.3–1.9) | <0.001 | 1.8 (1.1–3.3) | 0.04 |
| ≥80 | 0.9 (0.7–1.2) | 0.41 | 2.9 (1.6–5.6) | 0.001 |
| Sex | ||||
| Female | 3.6 (3.2–4.0) | <0.001 | 0.8 (0.6–1.1) | 0.10 |
| Race | ||||
| White | Reference groupa | |||
| Black | 0.4 (0.3–0.5) | <0.001 | 1.6 (1.1–2.4) | 0.03 |
| Hispanic | 0.7 (0.6–0.8) | <0.001 | 1.4 (0.9–2.1) | 0.15 |
| Asian | 0.9 (0.7–1.2) | 0.39 | 0.6 (0.3–1.4) | 0.21 |
| Native American | 0.7 (0.4–1.3) | 0.30 | 0.7 (0.2–3.4) | 0.65 |
| Other | 0.7 (0.6–0.9) | 0.04 | 1.7 (0.9–3.3) | 0.11 |
| Primary payer | ||||
| Medicare | Reference groupa | |||
| Medicaid | 1.1 (0.9–1.3) | 0.41 | 1.1 (0.7–1.7) | 0.76 |
| Private | 1.1 (1.0–1.3) | 0.13 | 0.9 (0.6–1.2) | 0.46 |
| Uninsured | 1.3 (1.1–1.6) | 0.03 | 0.9 (0.5–1.9) | 0.98 |
| No charge | 1.1 (0.5–2.4) | 0.86 | 1.7 (0.3–8.9) | 0.55 |
| Others | 1.2 (0.9–1.6) | 0.22 | 0.3 (0.1–1.1) | 0.08 |
| Hospital teaching status and locationb | ||||
| Rural | Reference groupa | |||
| Urban nonteaching | 1.2 (0.9–1.6) | 0.16 | 0.7 (0.4–1.3) | 0.27 |
| Urban teaching | 2.2 (1.7–3.0) | <0.001 | 1.0 (0.6–1.8) | 0.92 |
| Hospital bed sizeb | ||||
| Small | Reference groupa | |||
| Medium | 1.1 (0.9–1.4) | 0.36 | 1.1 (0.7–1.8) | 0.66 |
| Large | 1.3 (1.0–1.6) | 0.05 | 1.2 (0.7–1.9) | 0.53 |
| Hospital volumeb | ||||
| Small | Reference groupa | |||
| Medium | 1.2 (1.1–1.5) | 0.01 | 0.8 (0.6–1.1) | 0.21 |
| Large | 1.1 (0.8–1.5) | 0.59 | 0.7 (0.4–1.2) | 0.16 |
| Hospital regionb | ||||
| Northeast | Reference groupa | |||
| Midwest | 1.1 (0.9–1.3) | 0.53 | 0.8 (0.6–1.1) | 0.22 |
| South | 0.9 (0.8–1.1) | 0.17 | 0.9 (0.7–1.3) | 0.57 |
| West | 1.3 (1.1–1.5) | 0.003 | 0.9 (0.6–1.3) | 0.66 |
| Charlson Comorbidity Index | ||||
| 0 to 3 | Reference groupa | |||
| 4 to 6 | 1.0 (0.9–1.2) | 0.54 | 1.3 (0.9–1.7) | 0.07 |
| ≥7 | 0.9 (0.8–0.9) | 0.02 | 1.6 (1.1–2.5) | 0.03 |
| Mechanical ventilation | ··· | ··· | 1.5 (1.1–2.2) | 0.02 |
| Septic shock | ··· | ··· | 1.1 (0.8–1.5) | 0.62 |
| Acute respiratory dysfunctionc | ··· | ··· | 2.3 (1.5–3.5) | <0.001 |
| Acute cardiovascular dysfunctionc | ··· | ··· | 1.9 (1.4–2.7) | <0.001 |
| Acute renal dysfunctionc | ··· | ··· | 1.3 (0.9–1.6) | 0.09 |
| Acute hepatic dysfunctionc | ··· | ··· | 1.7 (1.2–2.5) | 0.003 |
| Acute hematological dysfunctionc | ··· | ··· | 1.4 (1.1–1.9) | 0.02 |
| Acute neurological dysfunctionc | ··· | ··· | 1.2 (0.9–1.6) | 0.13 |
All values are represented as odds ratio (95% confidence interval). CI indicates confidence interval; OR, odds ratio; TTC, Takotsubo cardiomyopathy.
The first category in variables with >2 categories was used as a reference group to develop odds ratios for the remaining categories was has been labeled as “Reference group.”
Refer to text and the NIS (National Inpatient Sample) database classification for details.
Refer to Table 2 for acute organ dysfunction details.
Discussion
To the best of our knowledge, this is the first large‐scale study evaluating the epidemiology of TTC in severe sepsis. TTC comprised 0.15% of the total admissions with severe sepsis, with a steady increase in frequency from 0.02% in 2007 to 0.25% in 2013. Severe sepsis admissions with TTC were frequent in middle‐aged females and had higher severity of illness. In this cohort of severe sepsis, a diagnosis of TTC was associated with a significantly lower in‐hospital mortality compared with patients without TTC, being more prominent in females.
Epidemiology of TTC
Sepsis has long been known to be a trigger for TTC. In a previous study using the NIS database, El‐Sayed et al noted 7% of TTC cases to be associated with sepsis.25 In a Japanese database of 3747 TTC patients, 2.8% of the cases were noted to be associated with sepsis.26 In a recent systematic review, Cappelletti et al documented 26 published cases of TTC in septic patients.27 These data highlight the current paucity of data on the epidemiology of TTC in sepsis and clinical outcomes. Using validated ICD‐9‐CM algorithms, this study addresses the knowledge gap in epidemiology and outcomes of TTC in sepsis.25, 28 Templin et al, in a multinational registry of 1750 patients, noted 90% of TTC patients to be women, with 79% being aged >50 years.1 Our study in severe sepsis patients is consistent with respect to age, with 92% patients being aged >45 years. However, a notable difference was a lower incidence of female sex with only 76% being women. The lower frequency of women with TTC in this study compared with other epidemiological data can be explained by the differences in epidemiology of severe sepsis, given that nearly 50% of all patients with severe sepsis are women.28 Potentially, differences in the nature of the triggering event may also account for this difference. A physical trigger was present in only 36% of the Templin et al study, whereas severe sepsis and the processes of care in the intensive care unit served as potential physical triggers for our entire study population.
Frequency of TTC steadily increased from 2007 to 2013 in this nationally representative population. This can be explained by multiple hypotheses. First, this study duration coincided with the evolution of point‐of‐care cardiac ultrasonography in critical illness, resulting in the higher application and likely greater diagnosis of TTC at the bedside.29 Second, the initial TTC criteria were proposed in 2004 with a subsequent revision in 2008 that potentially resulted in the further unification of clinical definitions and higher awareness in treating physicians.2, 5 The first reported series of TTC in sepsis was in 2005, providing additional awareness.30 Third, there has been a growing interest in cardiovascular outcomes in sepsis in recent years, potentially influencing clinical diagnostic and management pathways.9, 11, 12 Finally, epidemiology of sepsis has changed over these years, likely reflecting an increase in illness severity and thereby triggering higher rates of TTC.21
Mortality With TTC in Severe Sepsis
Whereas unadjusted mortality in the 2 groups was not different, we observed lower adjusted mortality in septic patients with TTC (OR, 0.58). In the a priori designed multivariate analysis using clinical and statistically relevant covariates, we noted that adjusting for sepsis and mechanical ventilation had the highest influence on mortality. This may be attributed to several factors. TTC patients were younger, more likely to be admitted to large‐volume teaching hospitals, and had lower baseline comorbidity, characteristics associated with better survival.31 In the United States, absolute mortality from TTC is much higher in patients with sepsis (20.6% in this study) than other patient types with TTC (4%).32 In an unselected critical ill population, mortality was 15%, which is similar to that noted in this cohort of severe sepsis patients.33 This interaction between sepsis and TTC is worthy of further mechanistic studies, similar to previous literature on septic cardiomyopathy.34 TTC shares many common attributes with septic cardiomyopathy, and one can speculate that it may share similar mechanisms and confer survival advantage in left ventricular systolic dysfunction in sepsis.35 Conversely, the lower mortality in TTC patients may have occurred because of ascertainment bias given that the sickest septic patients with TTC may have died before a diagnosis was made.
An important observation is the differences in characteristics for males and females with TTC in this study. Despite being predominantly a disease of female sex, there was higher frequency of males, and males were younger but with higher comorbidities. Consistent with other studies, we showed higher severity of illness and a trend toward worse outcomes in males as compared with females with severe sepsis.36, 37 Importantly, this study noted improving mortality in patients with TTC with every subsequent year during the study period. This could be explained by multiple hypothesis: (1) With a greater understanding of sepsis pathophysiology, there has been a shift away from elements of the early goal‐directed therapy, such as blood transfusions and inotropes, that may adversely affect mortality38, 39; (2) greater emphasis on cardiovascular outcomes in sepsis and use of bedside point‐of‐care ultrasonography in critical care11, 12; and (3) evolution in the diagnosis and management of TTC since the unifying criteria were published in 2005.5 Alternately, as with other administrative databases, this could potentially represent potential overdiagnoses resulting in decreased severity of illness of included patients.
Limitations
This study has certain important limitations. Use of an administrative database and ICD‐9‐CM codes is associated with selection and informational biases. However, the previous validation of these ICD‐9‐CM codes partially mitigates these shortcomings.21 The lack of detailed echocardiographic and coronary angiography data that are used to define TTC can result in significant overlap with septic cardiomyopathy. However, the dramatic “apical ballooning” observed in TTC and the global left ventricular systolic dysfunction of septic cardiomyopathy are readily distinguishable on echocardiography.11, 12 Additionally, coronary angiography is infrequently performed (≈5%) in septic patients because of concomitant hemodynamic instability and kidney injury.40 It is possible that the increasing incidence of TTC could reflect a greater diagnosis and awareness, as highlighted in the discussion previously, but alternately could represent a false‐positive diagnosis. However, our data are consistent with national trends of both primary and secondary diagnosis of TTC from other groups, making a false‐positive diagnosis less likely.41 Our data sources did not allow us to capture processes of care—especially intravenous fluid therapy, vasopressor and inotrope use, and extent of positive pressure used in mechanical ventilation—all of which could potentially influence the development or worsening of TTC. The recent iteration of the NIS database after its redesign in 2012 limits volume assessments both at hospital and state level.42 Because the NIS data are limited to duration of hospital stay, we are unable to comment on the long‐term outcomes, including recovery of ventricular function, in this cohort. The absence of detailed echocardiographic data prevents us from evaluating the occurrence of nonapical variants of TTC in this population.
Conclusions
In conclusion, despite the inherent limitations of the study, it provides novel insights into the evolving epidemiology and outcomes of TTC in severe sepsis. TTC is observed with increasing frequency in patients with severe sepsis and was associated with a significantly lower in‐hospital mortality compared with patients without TTC. Presentation in later years of the study period, middle age, female sex, and white race were independent predictors for the diagnosis of TTC in severe sepsis. Further dedicated studies evaluating the pathophysiology, clinical implications, and diagnosis of TTC in severe sepsis and septic shock are needed to optimize management and outcomes in these patients.
Author Contributions
Study design, literature review, data analysis, statistical analysis: Vallabhajosyula, Deshmukh, Sakhuja. Data management, data analysis, drafting manuscript: Vallabhajosyula, Sakhuja. Access to data: Vallabhajosyula, Deshmukh, Kashani, Prasad, Sakhuja. Manuscript revision, intellectual revisions, mentorship: Deshmukh, Kashani, Prasad, Sakhuja. Final approval: Vallabhajosyula, Deshmukh, Kashani, Prasad, Sakhuja.
Disclosures
None.
Supporting information
Table S1. Multivariate Analysis for In‐Hospital Mortality in Severe Sepsis (Restricted Cubic Spline Analysis)
(J Am Heart Assoc. 2018;7:e009160 DOI: 10.1161/JAHA.118.009160.)
These findings were presented in part as a poster at the American Thoracic Society International Conference, May 19 to 24, in Washington, DC.
References
- 1. Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschöpe C, Schultheiss H‐P, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Böhm M, Erbel R, Cuneo A, Kuck K‐H, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun‐Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KEJ, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Lüscher TF. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–938. [DOI] [PubMed] [Google Scholar]
- 2. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS, Rihal CS. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST‐segment elevation myocardial infarction. Ann Intern Med. 2004;141:858–865. [DOI] [PubMed] [Google Scholar]
- 3. Patel SM, Chokka RG, Prasad K, Prasad A. Distinctive clinical characteristics according to age and gender in apical ballooning syndrome (Takotsubo/stress cardiomyopathy): an analysis focusing on men and young women. J Card Fail. 2013;19:306–310. [DOI] [PubMed] [Google Scholar]
- 4. Komamura K, Fukui M, Iwasaku T, Hirotani S, Masuyama T. Takotsubo cardiomyopathy: pathophysiology, diagnosis and treatment. World J Cardiol. 2014;6:602–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako‐tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408–417. [DOI] [PubMed] [Google Scholar]
- 6. Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 7. Jentzer JC, Vallabhajosyula S, Khanna AK, Chawla LS, Busse LW, Kashani KB. Management of refractory vasodilatory shock. Chest. 2018;154:416–426. [DOI] [PubMed] [Google Scholar]
- 8. Kotecha A, Vallabhajosyula S, Coville HH, Kashani K. Cardiorenal syndrome in sepsis: a narrative review. J Crit Care. 2018;43:122–127. [DOI] [PubMed] [Google Scholar]
- 9. Vallabhajosyula S, Sakhuja A, Geske JB, Kumar M, Kashyap R, Kashani K, Jentzer JC. Clinical profile and outcomes of acute cardiorenal syndrome type‐5 in sepsis: an eight‐year cohort study. PLoS One. 2018;13:e0190965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kotecha AA, Vallabhajosyula S, Apala DR, Frazee E, Iyer VN. Clinical outcomes of weight‐based norepinephrine dosing in underweight and morbidly obese patients: a propensity‐matched analysis. J Intensive Care Med. 2018. Available at: https://journals.sagepub.com/doi/abs/10.1177/0885066618768180. Accessed September 9, 2018. [DOI] [PubMed] [Google Scholar]
- 11. Vallabhajosyula S, Jentzer JC, Geske JB, Kumar M, Sakhuja A, Singhal A, Poterucha JT, Kashani K, Murphy JG, Gajic O, Kashyap R. New‐onset heart failure and mortality in hospital survivors of sepsis‐related left ventricular dysfunction. Shock. 2018;49:144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vallabhajosyula S, Kumar M, Pandompatam G, Sakhuja A, Kashyap R, Kashani K, Gajic O, Geske JB, Jentzer JC. Prognostic impact of isolated right ventricular dysfunction in sepsis and septic shock: an 8‐year historical cohort study. Ann Intensive Care. 2017;7:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallabhajosyula S, Rayes HA, Sakhuja A, Murad MH, Geske JB, Jentzer JC. Global longitudinal strain using speckle‐tracking echocardiography as a mortality predictor in sepsis: a systematic review. J Intensive Care Med. 2018. Available at: http://journals.sagepub.com/doi/abs/10.1177/0885066618761750?journalCode=jica. Accessed September 9, 2018. [DOI] [PubMed] [Google Scholar]
- 14. Vallabhajosyula S, Sakhuja A, Geske JB, Kumar M, Poterucha JT, Kashyap R, Kashani K, Jaffe AS, Jentzer JC. Role of admission troponin‐T and serial troponin‐T testing in predicting outcomes in severe sepsis and septic shock. J Am Heart Assoc. 2017;6:e005930 DOI: 10.1161/JAHA.117.005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164:66–71.e1. [DOI] [PubMed] [Google Scholar]
- 16. HCUP‐NIS . Healthcare Cost and Utilization Project (HCUP) Databases. Rockville, MD: Agency for Healthcare Research and Quality; 2018. [PubMed]
- 17. Introduction to the HCUP Nationwide Inpatient Sample 2009. Available at: http://www.hcup-us.ahrq.gov/db/nation/nis/NIS_2009_INTRODUCTION.pdf. Accessed January 18, 2015.
- 18. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. [DOI] [PubMed] [Google Scholar]
- 19. Kumar G, Kumar N, Taneja A, Kaleekal T, Tarima S, McGinley E, Jimenez E, Mohan A, Khan RA, Whittle J, Jacobs E, Nanchal R; Milwaukee Initiative in Critical Care Outcomes Research (MICCOR) Group of Investigators . Nationwide trends of severe sepsis in the 21st century (2000–2007). Chest. 2011;140:1223–1231. [DOI] [PubMed] [Google Scholar]
- 20. Sakhuja A, Kumar G, Gupta S, Mittal T, Taneja A, Nanchal RS. Acute kidney injury requiring dialysis in severe sepsis. Am J Respir Crit Care Med. 2015;192:951–957. [DOI] [PubMed] [Google Scholar]
- 21. Gupta S, Sakhuja A, Kumar G, McGrath E, Nanchal RS, Kashani KB. Culture‐negative severe sepsis: nationwide trends and outcomes. Chest. 2016;150:1251–1259. [DOI] [PubMed] [Google Scholar]
- 22. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 23. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 24. Healthcare Cost and Utilization Project . Nationwide Inpatient Sample Redesign Report. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- 25. El‐Sayed AM, Brinjikji W, Salka S. Demographic and co‐morbid predictors of stress (Takotsubo) cardiomyopathy. Am J Cardiol. 2012;110:1368–1372. [DOI] [PubMed] [Google Scholar]
- 26. Isogai T, Yasunaga H, Matsui H, Tanaka H, Ueda T, Horiguchi H, Fushimi K. Out‐of‐hospital versus in‐hospital Takotsubo cardiomyopathy: analysis of 3719 patients in the Diagnosis Procedure Combination database in Japan. Int J Cardiol. 2014;176:413–417. [DOI] [PubMed] [Google Scholar]
- 27. Cappelletti S, Ciallella C, Aromatario M, Ashrafian H, Harding S, Athanasiou T. Takotsubo cardiomyopathy and sepsis. Angiology. 2017;68:288–303. [DOI] [PubMed] [Google Scholar]
- 28. Angus DC, Linde‐Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. [DOI] [PubMed] [Google Scholar]
- 29. Levitov A, Frankel HL, Blaivas M, Kirkpatrick AW, Su E, Evans D, Summerfield DT, Slonim A, Breitkreutz R, Price S, McLaughlin M, Marik PE, Elbarbary M. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients—part II: cardiac ultrasonography. Crit Care Med. 2016;44:1206–1227. [DOI] [PubMed] [Google Scholar]
- 30. Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296–302. [DOI] [PubMed] [Google Scholar]
- 31. Burke LG, Frakt AB, Khullar D, Orav E, Jha AK. Association between teaching status and mortality in US hospitals. JAMA. 2017;317:2105–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murugiah K, Wang Y, Desai NR, Spatz ES, Nuti SV, Dreyer RP, Krumholz HM. Trends in short‐ and long‐term outcomes for Takotsubo cardiomyopathy among Medicare fee‐for‐service beneficiaries, 2007 to 2012. JACC Heart Fail. 2016;4:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Champion S, Belcour D, Vandroux D, Drouet D, Gauzere BA, Bouchet B, Bossard G, Djouhri S, Jabot J, Champion M, Lefort Y. Stress (Tako‐tsubo) cardiomyopathy in critically‐ill patients. Eur Heart J Acute Cardiovasc Care. 2015;4:189–196. [DOI] [PubMed] [Google Scholar]
- 34. Landesberg G, Jaffe AS, Gilon D, Levin PD, Goodman S, Abu‐Baih A, Beeri R, Weissman C, Sprung CL, Landesberg A. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014;42:790–800. [DOI] [PubMed] [Google Scholar]
- 35. Micek ST, McEvoy C, McKenzie M, Hampton N, Doherty JA, Kollef MH. Fluid balance and cardiac function in septic shock as predictors of hospital mortality. Crit Care. 2013;17:R246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murakami T, Yoshikawa T, Maekawa Y, Ueda T, Isogai T, Sakata K, Nagao K, Yamamoto T, Takayama M. Gender differences in patients with Takotsubo cardiomyopathy: multi‐center registry from Tokyo CCU Network. PLoS One. 2015;10:e0136655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schneider B, Athanasiadis A, Stollberger C, Pistner W, Schwab J, Gottwald U, Schoeller R, Gerecke B, Hoffmann E, Wegner C, Sechtem U. Gender differences in the manifestation of Tako‐tsubo cardiomyopathy. Int J Cardiol. 2013;166:584–588. [DOI] [PubMed] [Google Scholar]
- 38. Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal‐directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. [DOI] [PubMed] [Google Scholar]
- 39. Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC. A randomized trial of protocol‐based care for early septic shock. N Engl J Med. 2014;370:1683–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Allou N, Brulliard C, Valance D, Esteve JB, Martinet O, Corradi L, Cordier C, Bouchet B, Allyn J. Obstructive coronary artery disease in patients hospitalized for severe sepsis or septic shock with concomitant acute myocardial infarction. J Crit Care. 2016;32:159–164. [DOI] [PubMed] [Google Scholar]
- 41. Khera R, Light‐McGroary K, Zahr F, Horwitz PA, Girotra S. Trends in hospitalization for Takotsubo cardiomyopathy in the United States. Am Heart J. 2016;172:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khera R, Krumholz HM. With great power comes great responsibility: big data research from the national inpatient sample. Circ Cardiovasc Qual Outcomes. 2017;10:e003846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Multivariate Analysis for In‐Hospital Mortality in Severe Sepsis (Restricted Cubic Spline Analysis)


