Abstract
Background
Little is known about the safety of nicotine replacement therapy (NRT) in smokers hospitalized with coronary heart disease.
Methods and Results
We examined the short‐term safety of NRT use among smokers hospitalized for coronary heart disease in a geographically and structurally diverse sample of US hospitals in the year 2014. We compared smokers who started NRT in the first 2 days of hospitalization with smokers without any exposure to NRT and adjusted for baseline differences through propensity score matching. Outcomes included inpatient mortality, hospital length of stay, and 1‐month readmission. From 270 hospitals, we included 27 459 smokers (mean age, 58 years; 69% men; 56.9% in intensive care unit), of whom 4885 (17.8%) received NRT (97.2% used the nicotine patch, at a median dose of 21 mg/d for 3 days). After propensity matching, covariates were well balanced within each patient group. Among patients with myocardial infarction, compared with patients who did not receive NRT, those who received NRT showed no difference in mortality (2.1% versus 2.3%; P=0.98), mean length of stay (4.4±3.5 versus 4.3±3.3 days; P=0.60), or 1‐month readmission (15.8% versus 14.6%; P=0.31). Results were similar for patients undergoing percutaneous coronary intervention or coronary artery bypass surgery.
Conclusions
Among smokers hospitalized for treatment of coronary heart disease, use of NRT was not associated with any differences in short‐term outcomes. Given the known beneficial effects of NRT in treating nicotine withdrawal, reducing cravings, and promoting smoking cessation after discharge, our findings suggest that NRT is a safe and reasonable treatment option.
Keywords: coronary bypass surgery, myocardial infarction, nicotine Patch, Nicotine Replacement Therapy, percutaneous coronary intervention, safety, smoking
Subject Categories: Quality and Outcomes, Secondary Prevention, Lifestyle, Acute Coronary Syndromes
Clinical Perspective
What Is New?
Prior studies have demonstrated the safety of nicotine replacement therapy among smokers with stable coronary heart disease in outpatient settings; this study is the first to demonstrate that nicotine replacement therapy appears safe among smokers hospitalized for treatment of coronary heart disease, even when started within the first or second hospital day, including among critically ill patients in the intensive care unit.
What Are the Clinical Implications?
Although some caution is always appropriate, our findings should allow clinicians to confidently prescribe nicotine replacement therapy in the hospital to treat withdrawal, reduce cravings, and promote smoking cessation after discharge without concerns for inducing major adverse events.
The immediate cardiovascular effects of nicotine use can include a short‐term increase in heart rate by 10 to 15 beats per minute and an increase in systolic blood pressure of up to 5 to 10 mm Hg.1 Consequently, when nicotine replacement therapy (NRT) was first introduced as a treatment to aid smoking cessation, clinicians worried that NRT would increase myocardial demand and provoke myocardial infarction (MI).1 This concern was later refuted by 3 randomized controlled trials that demonstrated that NRT was safe in smokers with coronary heart disease (CHD), but only in a stable outpatient setting and not among patients hospitalized with CHD.2, 3, 4 However, recognizing that there were still unresolved concerns about the safety of NRT in the hospital, the most recent US Public Health Service Clinical Practice Guidelines (2008) advise that NRT should generally not be used within 2 weeks of an MI.5
However, over the past decade, several small observational studies have suggested that NRT use may be safe in the medical intensive care unit,6 during an admission for acute coronary syndrome,7 or at the time of hospital discharge,8 although another study raised concerns among patients undergoing cardiac surgery.9 However, no large, well‐powered study has examined this question, and none has examined the use of NRT within the first few days of an MI. In addition, recent guidelines from the American College of Cardiology/American Heart Association and the US Preventive Services Task Force provide no recommendations on this issue. Consequently, substantial doubt remains in the minds of many clinicians, and this barrier almost certainly contributes to the low use (20%) of NRT among patients hospitalized with MI.10, 11 Furthermore, because the majority of relapse happens within 2 weeks after hospital discharge,12 it is essential to begin treatment while patients are hospitalized, because this promotes greater NRT use13 and aids in smoking cessation.14
We, therefore, took advantage of data from a large hospital network and evaluated the short‐term safety of NRT among patients admitted for treatment of CHD. We hypothesized that, consistent with prior smaller studies, NRT would have no association with changes (either beneficial or harmful) in short‐term outcomes, even when used within the first few days of an MI or revascularization procedure.
Methods
Design and Setting
We conducted a retrospective cohort study at 282 US hospitals that participated in the Premier Healthcare Inpatient Database Alliance (Premier Inc.) in the year 2014. This database has been previously described.15 In brief, Premier is a geographically and structurally diverse group of US hospitals that captures ≈15% to 20% of inpatient US hospitalizations. Unlike administrative databases that contain only basic sociodemographic, diagnostic, and procedure codes assigned at the time of discharge, Premier also contains date‐stamped hospital service codes for every medication, procedure, diagnostic test, and therapeutic service. Because the data are fully deidentified, the Institutional Review Board at Baystate Medical Center determined that this study did not meet the federal definition of human subject's research and waived the requirement for informed consent.
Data Sharing Statement
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The authors do not determine access to the Premier database. However, researchers interested in reproducing our results may be able to obtain database access directly from Premier Inc.
Population, Characteristics, and Treatments
We included smokers, defined on the basis of an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code of 305.1, which has previously been validated and found to have fair sensitivity but high specificity for active smoking.16 Among these patients, we evaluated those who were admitted with a principal diagnosis of MI (ICD‐9 410.x) or received percutaneous coronary intervention (PCI; ICD‐9 36.06, 36.07, or 36.09) or coronary artery bypass graft (CABG) surgery (ICD‐9 36.1x). We grouped patients into 3 mutually exclusive categories: (1) those with medically managed acute MI, (2) patients who underwent PCI with or without MI, and (3) those who received CABG with or without MI or PCI. This division allowed us to do straightforward and easily interpretable statistical modeling within groups with similar characteristics and outcomes, rather than attempting to combine significantly different populations and outcomes. For patients admitted more than once in the year, we randomly selected a single admission for inclusion in this analysis to allow unbiased assessment of inpatient mortality.
To ensure that NRT exposure preceded outcomes assessment, we included only patients who received NRT in the first 2 days of hospitalization and excluded any patients with death or hospital discharge during the first 2 days. This exposure time frame ensured that all patients were hospitalized for >24 hours and also ensured that nicotine levels were therapeutic, because the nicotine patch achieves peak concentration within 6 hours.17 This also allowed us to evaluate most patients who received NRT, while still allowing adequate observation time for in‐hospital mortality. This exclusion also eliminated elective hospital admissions for planned and uncomplicated PCI, during which patients were only hospitalized for 1 night and would be unlikely to have unstable CHD. More important, because length of stay (LOS) is counted by the number of nights spent in the hospital, LOS=1 is equivalent to 2 days and 1 night in the hospital. Similarly, LOS=3 is equivalent to 4 days and 3 nights spent in the hospital.
We recorded demographic data, such as age, sex, race/ethnicity, and insurance status, for each patient. We included 29 individual chronic comorbidity indicators on the basis of methods developed by Elixhauser et al18 using the software provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality, and also calculated a combined comorbidity score, as described by Gagne et al.19 In addition, we included hospital characteristics, such as size, teaching status, urban or rural population served, and census region.
Because NRT has been associated with increased blood pressure and heart rate, it may have been considered contraindicated among patients who were hemodynamically unstable or who had significant hypertension. Accordingly, we carefully separated ICD‐9 codes for hypertension into 2 categories of complicated and uncomplicated so that we could track and adjust for potential contraindications to the use of NRT among those with more severe hypertension or hypertensive urgency. We consider hypertension to be uncomplicated when given an ICD‐9 code of 401.1, 401.9, 642.00, 642.03, or 642.04 and complicated when given a code of 401.0, 437.2, 642.2x (x=0, 1, 2, 3, 4), 402.00, 402.10, 402.90, 405.09, 405.19, 405.99, 402.01, 402.11, 402.91, 403.00, 403.10, 403.90, 405.01, 405.11, 405.91, 642.10, 642.11, 642.12, 642.13, 642.14, 403.01, 403.11, 403.91, 404.00, 404.10, 404.90, 404.01, 404.11, 404.91, 404.02, 404.12, 404.92, 404.03, 404.13, 404.93, 642.70, 642.71, 642.72, 642.73, 642.74, 642.90, 642.91, 642.92, 642.93, or 642.94. Similarly, we also tracked the use of several critical care therapies in the first 2 days of hospitalization and included them as baseline characteristics. This included recording the use of inotropes, vasopressors, invasive and noninvasive ventilation, intra‐aortic balloon pump, and/or arterial line, as has previously been done.20, 21 We also included location in an intensive care or intermediate care unit as a marker of illness severity. Together, these factors helped us to identify high‐risk subjects who might not be prescribed NRT because of hemodynamic concerns and were important in ensuring that the propensity score adjusted for available baseline differences.
Using pharmacy charges, we identified patients who were dispensed any form of NRT, including the nicotine patch, gum, lozenge, and inhaler. We excluded patients who received varenicline or bupropion at any point in the hospitalization because these medications were used only rarely10 and have separate safety concerns. We measured the average daily dose of nicotine patch (the most common therapy), but were unable to track how often the other ad libitum NRT products were used.
Outcome Measures
We evaluated 3 primary outcomes: all‐cause inpatient mortality, total hospital LOS, and 1‐month readmission among survivors. To ensure that any outcomes occurred after exposure to NRT and to avoid immortal time bias, we excluded patients with a hospital LOS=1 (equivalent to 2 days.). We also included total hospital cost as an outcome of interest because this is a useful marker to overall resource use.22 Because cost and LOS were highly skewed, we winsorized both outcomes at the 1st or 99th percentile, depending on their distribution.23 Because the Premier database is deidentified, readmission was known only if it occurred to the same hospital as the primary event, and only the month of readmission was recorded. This factor may have reduced the incidence of this outcome, because potential readmission to other hospitals was unknown.
Statistical Analysis
We calculated descriptive statistics for patients and hospitals using percentages for categorical variables and means, SDs, or quartiles (median and 25th and 75th percentiles) for continuous variables. We compared characteristics of patients with and without NRT treatment using absolute standardized differences rather than P values (Table 1, footnote).24, 25 Within each patient diagnosis group, we examined the association between receipt of NRT and unadjusted outcomes using generalized estimating equations models to account for clustering of patients within hospitals, using logit link for binary outcomes and identity link for continuous outcomes. To obtain stable estimates from hierarchical models, we excluded hospitals with <10 eligible patients in each patient group.
Table 1.
Patient Characteristics and Unadjusted Outcomes in All Cohorts
| Description | PCIMI | |||
|---|---|---|---|---|
| Total | No NRT | Early NRT | Absolute Standardized Differences | |
| n (%) | n (%) | n (%) | % | |
| Patients | 16 785 (100) | 13 716 (81.7) | 3069 (18.3) | |
| Group | ||||
| PCI | 4169 (24.8) | 3362 (24.5) | 807 (26.3) | |
| MI+PCI | 12 616 (75.2) | 10 354 (75.5) | 2262 (73.7) | 4.1 |
| Age, y | ||||
| Median (IQR) | 57 (50–64) | 57 (51–65) | 56 (49–62) | |
| Mean (SD) | 57.4 (10.5) | 57.8 (10.6) | 55.9 (9.7) | 18.3 |
| Sex | ||||
| Male | 11 718 (69.8) | 9617 (70.1) | 2101 (68.5) | |
| Female | 5067 (30.2) | 4099 (29.9) | 968 (31.5) | 3.6 |
| Race/ethnicity | 16.9 | |||
| White | 12 155 (72.4) | 9775 (71.3) | 2380 (77.5) | |
| Black | 1889 (11.3) | 1623 (11.8) | 266 (8.7) | |
| Hispanic | 840 (5) | 745 (5.4) | 95 (3.1) | |
| Other | 1901 (11.3) | 1573 (11.5) | 328 (10.7) | |
| Marital status | 7.7 | |||
| Married | 7200 (42.9) | 5920 (43.2) | 1280 (41.7) | |
| Single | 7544 (44.9) | 6081 (44.3) | 1463 (47.7) | |
| Other | 2041 (12.2) | 1715 (12.5) | 326 (10.6) | |
| Insurance payer | 18 | |||
| Medicare | 5673 (33.8) | 4748 (34.6) | 925 (30.1) | |
| Medicaid | 2641 (15.7) | 2006 (14.6) | 635 (20.7) | |
| Managed care | 4410 (26.3) | 3603 (26.3) | 807 (26.3) | |
| Commercial‐indemnity | 1140 (6.8) | 961 (7) | 179 (5.8) | |
| Self‐pay | 1864 (11.1) | 1504 (11) | 360 (11.7) | |
| Other | 1057 (6.3) | 894 (6.5) | 163 (5.3) | |
| Gagne combined comorbidity score | ||||
| Median (IQR) | 0 (0–2) | 0 (0–2) | 0 (0–2) | |
| Mean (SD) | 1.1 (2.2) | 1.1 (2.2) | 1.1 (2.1) | 1.4 |
| Comorbidities | ||||
| Congestive heart failure | 3352 (20) | 2761 (20.1) | 591 (19.3) | 2.2 |
| Valvular disease | 1371 (8.2) | 1129 (8.2) | 242 (7.9) | 1.3 |
| Pulmonary circulation disease | 524 (3.1) | 431 (3.1) | 93 (3) | 0.7 |
| Peripheral vascular disease | 2152 (12.8) | 1754 (12.8) | 398 (13) | 0.5 |
| Hypertension with complications | 2066 (12.3) | 1738 (12.7) | 328 (10.7) | 6.2 |
| Hypertension without complications | 10 181 (60.7) | 8273 (60.3) | 1908 (62.2) | 3.8 |
| Paralysis | 159 (0.9) | 137 (1) | 22 (0.7) | 3.1 |
| Other neurological disorders | 692 (4.1) | 552 (4) | 140 (4.6) | 2.7 |
| Chronic pulmonary disease | 4653 (27.7) | 3593 (26.2) | 1060 (34.5) | 18.2 |
| Diabetes mellitus | 5270 (31.4) | 4353 (31.7) | 917 (29.9) | 4 |
| Hypothyroidism | 1076 (6.4) | 908 (6.6) | 168 (5.5) | 4.8 |
| Renal failure | 1475 (8.8) | 1266 (9.2) | 209 (6.8) | 8.9 |
| Liver disease | 272 (1.6) | 202 (1.5) | 70 (2.3) | 6 |
| AIDS | 37 (0.2) | 30 (0.2) | 7 (0.2) | 0.2 |
| Lymphoma | 47 (0.3) | 43 (0.3) | 4 (0.1) | 3.9 |
| Metastatic cancer | 58 (0.3) | 48 (0.3) | 10 (0.3) | 0.4 |
| Solid tumor without metastasis | 139 (0.8) | 119 (0.9) | 20 (0.7) | 2.5 |
| Rheumatoid arthritis/collagen vascular | 310 (1.8) | 258 (1.9) | 52 (1.7) | 1.4 |
| Obesity | 3178 (18.9) | 2589 (18.9) | 589 (19.2) | 0.8 |
| Weight loss | 260 (1.5) | 220 (1.6) | 40 (1.3) | 2.5 |
| Chronic blood loss anemia | 66 (0.4) | 59 (0.4) | 7 (0.2) | 3.5 |
| Deficiency anemias | 1504 (9) | 1279 (9.3) | 225 (7.3) | 7.2 |
| Alcohol abuse | 1190 (7.1) | 881 (6.4) | 309 (10.1) | 13.3 |
| Drug abuse | 1136 (6.8) | 880 (6.4) | 256 (8.3) | 7.4 |
| Psychoses | 581 (3.5) | 449 (3.3) | 132 (4.3) | 5.4 |
| Depression | 1520 (9.1) | 1150 (8.4) | 370 (12.1) | 12.1 |
| Early treatments/procedures (day 0, 1, or 2) | ||||
| ICU/CVICU/intermediate care | 9675 (57.6) | 7989 (58.2) | 1686 (54.9) | 6.7 |
| Vasodilators | 6508 (38.8) | 5315 (38.7) | 1193 (38.9) | 0.2 |
| NIV | 529 (3.1) | 431 (3.1) | 98 (3.2) | 0.3 |
| IMV | 982 (5.8) | 878 (6.4) | 104 (3.4) | 14 |
| Vasopressors | 1944 (11.6) | 1654 (12.1) | 290 (9.4) | 8.4 |
| Arterial line | 302 (1.8) | 268 (1.9) | 34 (1.1) | 6.9 |
| IABP | 478 (2.8) | 428 (3.1) | 50 (1.6) | 9.8 |
| Inotropes | 872 (5.2) | 775 (5.6) | 97 (3.2) | 12.1 |
| Hospital size, beds | 10.8 | |||
| ≤200 | 1873 (11.2) | 1536 (11.2) | 337 (11) | |
| 201–400 | 5717 (34.1) | 4545 (33.1) | 1172 (38.2) | |
| ≥401 | 9195 (54.8) | 7635 (55.7) | 1560 (50.8) | |
| Rural/urban | 0.7 | |||
| Urban | 14 309 (85.2) | 11 791 (86.0) | 2518 (82.1) | |
| Rural | 2476 (14.7) | 1925 (14.0) | 551 (17.9) | |
| Hospital region | 5.7 | |||
| Northeast | 2623 (15.6) | 2104 (15.3) | 519 (16.9) | |
| Midwest | 3256 (19.4) | 2636 (19.2) | 620 (20.2) | |
| West | 1163 (6.9) | 965 (7.0) | 198 (6.4) | |
| South | 9743 (58.0) | 8011 (58.4) | 1732 (56.4) | |
| Teaching status | 2.5 | |||
| Nonteaching | 8726 (52.0) | 7052 (51.4) | 1674 (54.5) | |
| Teaching | 8059 (48.0) | 6664 (48.6) | 1395 (45.4) | |
| Outcomes—PCIMI Group | GEE P Valuea | |||
| In‐hospital mortality | 246 (1.5) | 231 (1.7) | 15 (0.5) | <0.001 |
| LOS, d | ||||
| Median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | |
| Mean (SD) | 3.8 (3.7) | 3.9 (3.9) | 3.6 (3.1) | |
| Winsorized at 99th percentile, mean (SD) | 3.8 (3.0) | 3.6 (2.6) | 0.002 | |
| Winsorized at 99th percentile, survivors, mean (SD) | 3.7 (2.8) | 3.7 (2.9) | 3.5 (2.5) | |
| All‐cause readmission among survivors (≈1 mo) | 1490 (9.0) | 1210 (9) | 280 (9.2) | 0.79 |
| Cost, US $ | ||||
| Median (IQR) | 14 705 (11 258–20 336) | 14 768 (11 235–20 620) | 14 498 (11 362–19 114) | |
| Mean (SD) | 18 427 (15 025) | 18 681 (15 701) | 17 295 (11 463) | |
| Winsorized at 1st and 99th percentiles, mean (SD) | 18 208 (11 746) | 17 110 (9905) | <0.001 | |
| Description | CABG | |||
|---|---|---|---|---|
| Total | No NRT | Early NRT | Absolute Standardized Differences | |
| n (%) | n (%) | n (%) | % | |
| Patients | 6155 (100) | 5177 (84.1) | 978 (15.9) | |
| Principle diagnosis of myocardial infarction | 2337 (38.0) | 1914 (37.0) | 423 (43.2) | 12.8 |
| Age, y | ||||
| Median (IQR) | 60 (54–67) | 61 (54–67) | 58 (52–64) | |
| Mean (SD) | 60.2 (9.9) | 60.6 (10.0) | 57.9 (9.1) | 28.5 |
| Sex | ||||
| Male | 4649 (75.5) | 3915 (75.6) | 734 (75.1) | |
| Female | 1506 (24.5) | 1262 (24.4) | 244 (24.9) | 1.3 |
| Race/ethnicity | 19.3 | |||
| White | 4672 (75.9) | 3887 (75.1) | 785 (80.3) | |
| Black | 550 (8.9) | 497 (9.6) | 53 (5.4) | |
| Hispanic | 274 (4.5) | 246 (4.8) | 28 (2.9) | |
| Other | 659 (10.7) | 547 (10.6) | 112 (11.5) | |
| Marital status | 13.8 | |||
| Married | 2898 (47.1) | 2449 (47.3) | 449 (45.9) | |
| Single | 2526 (41) | 2083 (40.2) | 443 (45.3) | |
| Other | 731 (11.9) | 645 (12.5) | 86 (8.8) | |
| Insurance payer | 25.8 | |||
| Medicare | 2533 (41.2) | 2182 (42.1) | 351 (35.9) | |
| Medicaid | 931 (15.1) | 707 (13.7) | 224 (22.9) | |
| Managed care | 1432 (23.3) | 1230 (23.8) | 202 (20.7) | |
| Commercial‐indemnity | 483 (7.8) | 412 (8) | 71 (7.3) | |
| Self‐pay | 427 (6.9) | 347 (6.7) | 80 (8.2) | |
| Other | 349 (5.7) | 299 (5.8) | 50 (5.1) | |
| Gagne combined comorbidity score | ||||
| Median (IQR) | 2 (0–4) | 2 (0–4) | 2 (0–4) | |
| Mean (SD) | 2.3 (2.4) | 2.2 (2.5) | 2.3 (2.5) | 3.2 |
| Comorbidities | ||||
| Congestive heart failure | 1654 (26.9) | 1392 (26.9) | 262 (26.8) | 0.2 |
| Valvular disease | 1062 (17.3) | 903 (17.4) | 159 (16.3) | 3.2 |
| Pulmonary circulation disease | 342 (5.6) | 288 (5.6) | 54 (5.5) | 0.2 |
| Peripheral vascular disease | 1409 (22.9) | 1183 (22.9) | 226 (23.1) | 0.6 |
| Hypertension with complications | 928 (15.1) | 773 (14.9) | 155 (15.8) | 2.5 |
| Hypertension without complications | 4195 (68.2) | 3523 (68.1) | 672 (68.7) | 1.4 |
| Paralysis | 103 (1.7) | 91 (1.8) | 12 (1.2) | 4.4 |
| Other neurological disorders | 291 (4.7) | 240 (4.6) | 51 (5.2) | 2.7 |
| Chronic pulmonary disease | 2726 (44.3) | 2180 (42.1) | 546 (55.8) | 27.7 |
| Diabetes mellitus | 2586 (42) | 2170 (41.9) | 416 (42.5) | 1.3 |
| Hypothyroidism | 459 (7.5) | 385 (7.4) | 74 (7.6) | 0.5 |
| Renal failure | 751 (12.2) | 642 (12.4) | 109 (11.1) | 3.9 |
| Liver disease | 146 (2.4) | 111 (2.1) | 35 (3.6) | 8.6 |
| AIDS | 8 (0.1) | 5 (0.1) | 3 (0.3) | 4.7 |
| Lymphoma | 24 (0.4) | 19 (0.4) | 5 (0.5) | 2.2 |
| Metastatic cancer | 6 (0.1) | 6 (0.1) | 4.8 | |
| Solid tumor without metastasis | 60 (1) | 51 (1) | 9 (0.9) | 0.7 |
| Rheumatoid arthritis/collagen vascular | 88 (1.4) | 73 (1.4) | 15 (1.5) | 1 |
| Obesity | 1488 (24.2) | 1250 (24.1) | 238 (24.3) | 0.4 |
| Weight loss | 202 (3.3) | 170 (3.3) | 32 (3.3) | 0.1 |
| Chronic blood loss anemia | 74 (1.2) | 66 (1.3) | 8 (0.8) | 4.5 |
| Deficiency anemias | 1100 (17.9) | 911 (17.6) | 189 (19.3) | 4.5 |
| Alcohol abuse | 517 (8.4) | 383 (7.4) | 134 (13.7) | 20.6 |
| Drug abuse | 340 (5.5) | 244 (4.7) | 96 (9.8) | 19.8 |
| Psychoses | 247 (4) | 192 (3.7) | 55 (5.6) | 9.1 |
| Depression | 637 (10.3) | 523 (10.1) | 114 (11.7) | 5 |
| Early treatments/procedures (day 0, 1, or 2) | ||||
| ICU/CVICU/intermediate care | 3940 (64) | 3380 (65.3) | 560 (57.3) | 16.5 |
| Vasodilators | 2537 (41.2) | 2182 (42.1) | 355 (36.3) | 12 |
| NIV | 357 (5.8) | 312 (6) | 45 (4.6) | 6.4 |
| IMV | 2650 (43.1) | 2403 (46.4) | 247 (25.3) | 45.3 |
| Vasopressors | 2636 (42.8) | 2379 (46) | 257 (26.3) | 41.9 |
| Arterial line | 701 (11.4) | 627 (12.1) | 74 (7.6) | 15.3 |
| IABP | 392 (6.4) | 357 (6.9) | 35 (3.6) | 14.9 |
| Inotropes | 1281 (20.8) | 1180 (22.8) | 101 (10.3) | 34 |
| Hospital size, beds | 4.5 | |||
| ≤200 | 327 (5.3) | 282 (5.4) | 45 (4.6) | |
| 201–400 | 1866 (30.3) | 1559 (30.1) | 307 (31.4) | |
| ≥401 | 3962 (64.4) | 3336 (64.4) | 626 (64) | |
| Rural/urban | 3.9 | |||
| Urban | 5346 (86.9) | 4503 (87.0) | 843 (86.2) | |
| Rural | 809 (13.1) | 674 (13.0) | 135 (13.8) | |
| Hospital region | 17.3 | |||
| Northeast | 886 (14.4) | 725 (14.0) | 161 (16.5) | |
| Midwest | 1048 (17.0) | 930 (18.0) | 118 (12.1) | |
| West | 384 (6.2) | 326 (6.3) | 58 (5.9) | |
| South | 3837 (62.3) | 3196 (61.7) | 641 (65.5) | |
| Teaching status | 16.6 | |||
| Nonteaching | 2831 (46.0) | 2351 (45.4) | 480 (49.1) | |
| Teaching | 3324 (54.0) | 2826 (54.6) | 498 (50.9) | |
| Outcomes—CABG Group | GEE P Valuea | |||
| In‐hospital mortality | 103 (1.7) | 92 (1.8) | 11 (1.1) | 0.14 |
| LOS, d | ||||
| Median (IQR) | 8 (6–11) | 8 (6–11) | 9 (7–12) | |
| Mean (SD) | 9.6 (6.3) | 9.5 (6.5) | 10.4 (5.4) | |
| Winsorized at 99th percentile, mean (SD) | 9.2 (4.4) | 9.0 (4.4) | 10.0 (4.3) | <0.001 |
| Winsorized at 99th percentile, survivors, mean (SD) | 9.2 (4.3) | 9.0 (4.3) | 10 (4.3) | |
| All‐cause readmission among survivors (≈1 mo) | 700 (11.6) | 579 (11.4) | 121 (12.5) | 0.2 |
| Cost, US $ | ||||
| Median (IQR) | 35 414 (27 944–47 363) | 35 058 (27 222–47 410) | 36 603 (29 229–47 254) | |
| Mean (SD) | 41 327 (25 034) | 41 349 (25 879) | 41 210 (19 990) | |
| Winsorized at 1st and 99th percentiles, mean (SD) | 40 768 (20 812) | 40 726 (21 238) | 40 996 (18 403) | 0.56 |
| Description | Medical MI | |||
|---|---|---|---|---|
| Total | No NRT | Early NRT | Absolute Standardized Differences | |
| n (%) | n (%) | n (%) | % | |
| Patients | 4519 (100) | 3681 (81.5) | 838 (18.5) | |
| Age, y | ||||
| Median (IQR) | 61 (53–69) | 61 (53–70) | 60 (52–67) | |
| Mean (SD) | 61.0 (12.6) | 61.3 (12.9) | 60.0 (11.4) | 11 |
| Sex | ||||
| Male | 2725 (60.3) | 2211 (60.1) | 514 (61.3) | |
| Female | 1794 (39.7) | 1470 (39.9) | 324 (38.7) | 2.6 |
| Race/ethnicity | 15.7 | |||
| White | 3089 (68.4) | 2468 (67) | 621 (74.1) | |
| Black | 779 (17.2) | 657 (17.8) | 122 (14.6) | |
| Hispanic | 179 (4) | 152 (4.1) | 27 (3.2) | |
| Other | 472 (10.4) | 404 (11) | 68 (8.1) | |
| Marital status | 8.1 | |||
| Married | 1664 (36.8) | 1358 (36.9) | 306 (36.5) | |
| Single | 2372 (52.5) | 1914 (52) | 458 (54.7) | |
| Other | 483 (10.7) | 409 (11.1) | 74 (8.8) | |
| Insurance payer | 11.3 | |||
| Medicare | 2334 (51.6) | 1906 (51.8) | 428 (51.1) | |
| Medicaid | 744 (16.5) | 593 (16.1) | 151 (18) | |
| Managed care | 704 (15.6) | 582 (15.8) | 122 (14.6) | |
| Commercial‐indemnity | 194 (4.3) | 149 (4) | 45 (5.4) | |
| Self‐pay | 318 (7) | 257 (7) | 61 (7.3) | |
| Other | 225 (5) | 194 (5.3) | 31 (3.7) | |
| Gagne combined comorbidity score | ||||
| Median (IQR) | 2 (0–5) | 2 (0–5) | 2 (1–4) | |
| Mean (SD) | 2.7 (2.8) | 2.8 (2.8) | 2.7 (2.7) | 4.7 |
| Comorbidities | ||||
| Congestive heart failure | 1823 (40.3) | 1518 (41.2) | 305 (36.4) | 10 |
| Valvular disease | 733 (16.2) | 616 (16.7) | 117 (14) | 7.7 |
| Pulmonary circulation disease | 361 (8) | 291 (7.9) | 70 (8.4) | 1.6 |
| Peripheral vascular disease | 912 (20.2) | 746 (20.3) | 166 (19.8) | 1.1 |
| Hypertension with complications | 1220 (27) | 1025 (27.8) | 195 (23.3) | 10.5 |
| Hypertension without complications | 2408 (53.3) | 1937 (52.6) | 471 (56.2) | 7.2 |
| Paralysis | 95 (2.1) | 83 (2.3) | 12 (1.4) | 6.1 |
| Other neurological disorders | 363 (8) | 298 (8.1) | 65 (7.8) | 1.3 |
| Chronic pulmonary disease | 2074 (45.9) | 1629 (44.3) | 445 (53.1) | 17.8 |
| Diabetes mellitus | 1684 (37.3) | 1408 (38.3) | 276 (32.9) | 11.1 |
| Hypothyroidism | 398 (8.8) | 326 (8.9) | 72 (8.6) | 0.9 |
| Renal failure | 998 (22.1) | 854 (23.2) | 144 (17.2) | 15 |
| Liver disease | 187 (4.1) | 154 (4.2) | 33 (3.9) | 1.2 |
| AIDS | 8 (0.2) | 8 (0.2) | 6.6 | |
| Lymphoma | 17 (0.4) | 16 (0.4) | 1 (0.1) | 6 |
| Metastatic cancer | 61 (1.3) | 51 (1.4) | 10 (1.2) | 1.7 |
| Solid tumor without metastasis | 99 (2.2) | 87 (2.4) | 12 (1.4) | 6.8 |
| Rheumatoid arthritis/collagen vascular | 112 (2.5) | 96 (2.6) | 16 (1.9) | 4.7 |
| Obesity | 797 (17.6) | 659 (17.9) | 138 (16.5) | 3.8 |
| Weight loss | 229 (5.1) | 183 (5) | 46 (5.5) | 2.3 |
| Chronic blood loss anemia | 44 (1) | 40 (1.1) | 4 (0.5) | 6.9 |
| Deficiency anemias | 866 (19.2) | 721 (19.6) | 145 (17.3) | 5.9 |
| Alcohol abuse | 483 (10.7) | 338 (9.2) | 145 (17.3) | 24.1 |
| Drug abuse | 481 (10.6) | 379 (10.3) | 102 (12.2) | 5.9 |
| Psychoses | 279 (6.2) | 212 (5.8) | 67 (8) | 8.8 |
| Depression | 610 (13.5) | 478 (13) | 132 (15.8) | 7.9 |
| Early treatments/procedures (day 0, 1, or 2) | ||||
| ICU/CVICU/intermediate care | 2009 (44.5) | 1663 (45.2) | 346 (41.3) | 7.9 |
| Vasodilators | 1031 (22.8) | 845 (23) | 186 (22.2) | 1.8 |
| NIV | 329 (7.3) | 273 (7.4) | 56 (6.7) | 2.9 |
| IMV | 314 (6.9) | 281 (7.6) | 33 (3.9) | 15.9 |
| Vasopressors | 318 (7) | 288 (7.8) | 30 (3.6) | 18.4 |
| Arterial line | 43 (1) | 35 (1) | 8 (1) | 0 |
| IABP | 51 (1.1) | 43 (1.2) | 8 (1) | 2.1 |
| Inotropes | 164 (3.6) | 153 (4.2) | 11 (1.3) | 17.5 |
| Hospital size, beds | 6.5 | |||
| ≤200 | 380 (8.4) | 313 (8.5) | 67 (8) | |
| 201–400 | 1611 (35.6) | 1291 (35.1) | 320 (38.2) | |
| ≥401 | 2528 (55.9) | 2077 (56.4) | 451 (53.8) | |
| Rural/urban | 1.9 | |||
| Urban | 3785 (83.8) | 3105 (84.3) | 680 (81.1) | |
| Rural | 734 (16.2) | 576 (15.6) | 158 (18.8) | |
| Hospital region | 6.8 | |||
| Northeast | 634 (14.0) | 510 (13.8) | 124 (14.8) | |
| Midwest | 869 (19.2) | 720 (19.6) | 149 (17.8) | |
| West | 268 (5.9) | 225 (6.1) | 43 (5.1) | |
| South | 2748 (60.8) | 2226 (60.5) | 522 (62.3) | |
| Teaching status | 4.6 | |||
| Nonteaching | 2117 (46.8) | 1696 (46.1) | 421 (50.2) | |
| Teaching | 2402 (53.1) | 1985 (53.9) | 417 (49.8) | |
| Outcomes—Medical MI | GEE P Valuea | |||
| In‐hospital mortality | 189 (4.2) | 169 (4.6) | 20 (2.4) | 0.005 |
| LOS, d | ||||
| Median (IQR) | 3 (2–5) | 3 (2–6) | 3 (2–5) | |
| Mean (SD) | 4.8 (4.5) | 4.8 (4.6) | 4.4 (3.8) | |
| Winsorized at 99th percentile, mean (SD) | 4.7 (3.8) | 4.7 (3.9) | 4.4 (3.5) | 0.02 |
| Winsorized at 99th percentile, survivors, mean (SD) | 4.6 (3.7) | 4.7 (3.8) | 4.3 (3.4) | |
| All‐cause readmission among survivors (≈1 mo) | 686 (15.8) | 560 (15.9) | 126 (15.4) | 0.57 |
| Cost, US $ | ||||
| Median (IQR) | 8491 (5821–13 303) | 8586 (5822–13 721) | 8154 (5815–11 822) | |
| Mean (SD) | 12 162 (13 399) | 12 497 (13 915) | 10 691 (10 726) | |
| Winsorized at 1st and 99th percentiles | 11 807 (10 504) | 12 113 (10 837) | 10 464 (8776) | 0.0005 |
CABG indicates coronary artery bypass graft; CVICU, cardiovascular ICU; GEE, generalized estimating equation; IABP, intra‐aortic balloon pump; ICU, intensive cardiac unit; IMV, invasive mechanical ventilation; IQR, interquartile range; LOS, length of stay; MI, myocardial infarction; NIV, noninvasive ventilation; NRT, nicotine replacement therapy; PCI, percutaneous coronary intervention; PCIMI, PCI with or without MI.
Accounting for patient clustering within hospitals using GEE.
Our primary analysis evaluated the independent association of NRT with outcomes in a propensity‐matched cohort. Within each patient diagnosis group, we developed a hierarchical nonparsimonious propensity model with a random intercept for the hospital to predict receipt of NRT. These models included every defined and reported variable, which included patient demographics, comorbidities, early critical care therapies, hospital characteristics, and significant interactions between all factors.26 We then used a greedy match algorithm to pair each treated patient with a patient who did not receive NRT with similar propensity score24 and assessed balance between the matched samples using absolute standardized differences. We then used multivariable conditional logistic regression models for inpatient mortality and 1‐month readmission outcomes in the matched cohort. For LOS and cost, we used identity link models. All models accounted for the propensity match. Among the medically managed MI group, we also adjusted for the few residual imbalanced factors between groups after propensity matching. Analyses were performed using SAS, version 9.4 (SAS Institute, Inc, Cary, NC) and STATA (StataCorp 2013; Stata Statistical Software: Release 13; StataCorp LP, College Station, TX).
Results
We identified 36 675 admissions for smokers admitted with medically managed MI, PCI±MI, or CABG. After exclusions, we evaluated a total of 27 459 unique admissions for unique smokers from 270 hospitals (Figure 1). Of these individuals, 4885 (17.8%) received some form of NRT in the first 2 days of hospitalization. The most common prescription was the nicotine patch (17.6% of patients) at a median daily dose of 21 mg/d for a median of 3 days. Before matching, baseline characteristics in all 3 patient groups showed multiple, clinically significant differences across treatment categories. When compared with patients without NRT, patients who received NRT were generally younger, were more likely to be white, and had higher rates of complicated hypertension, chronic obstructive pulmonary disease, drug use, and alcohol use (Table 1). There were also differences in unadjusted outcomes (Table 1), which changed only minimally when patients with late‐start NRT were included in the control group (Table 2).
Figure 1.
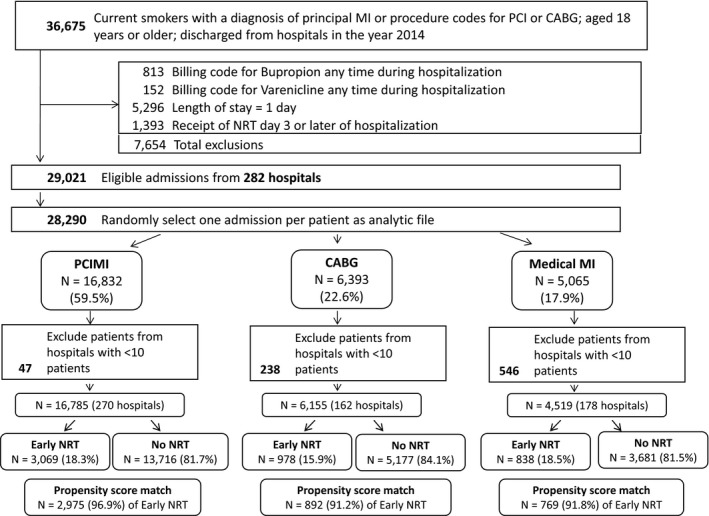
Patient selection flow chart. CABG indicates coronary artery bypass graft; MI, myocardial infarction; NRT, nicotine replacement therapy; PCI, percutaneous coronary intervention; PCIMI, PCI with or without MI.
Table 2.
Unadjusted Outcomes When Including Late NRT Starts in the Control Group
| Description | PCIMI | CABG | Medical MI | |||
|---|---|---|---|---|---|---|
| No/Late NRT | Early NRT | No/Late NRT | Early NRT | No/Late NRT | Early NRT | |
| (14 368 [82.4]) | (3071 [17.6]) | (5596 [85.2]) | (972 [14.8]) | (3867 [82.2]) | (838 [17.8]) | |
| In‐hospital mortality, n (%) | 242 (1.7) | 15 (0.5) | 98 (1.7) | 11 (1.1) | 172 (4.4) | 20 (2.4) |
| LOS, median (IQR), da | 3 (2–4) | 3 (2–4) | 8 (6–11) | 9 (7–12) | 3 (2–6) | 3 (2–5) |
| All‐cause readmission among survivors (≈1 mo), n (%) | 1289 (9.1) | 287 (9.4) | 621 (11.3) | 121 (12.6) | 575 (15.6) | 124 (15.2) |
| Cost, median (IQR), $b | 14 936 (11 318–20 976) | 14 493 (11 355–19 136) | 35 363 (27 427–47 784) | 36 653 (29 301–47 318) | 8746 (5913–13 980) | 8210 (5826–12 011) |
CABG indicates coronary artery bypass graft; IQR, interquartile range; LOS, length of stay; MI, myocardial infarction; NRT, nicotine replacement therapy; PCIMI, percutaneous coronary intervention with or without MI.
Measure winsorized at 99th percentile.
Measure winsorized at 1st and 99th percentiles.
The distributions of propensity scores with overlap between groups are shown in Figure 2. For patients with medically managed MI, PCI±MI, and CABG, the areas under the receiver operating curve to distinguish likelihood of NRT receipt were 0.762, 0.740, and 0.816, respectively, for each propensity score. We successfully matched 91.8%, 91.2%, and 96.9%, respectively, of the patients who were treated with NRT to those not treated for a total of 769, 2975, and 892 matched pairs for each patient group, respectively (Figure 1).
Figure 2.
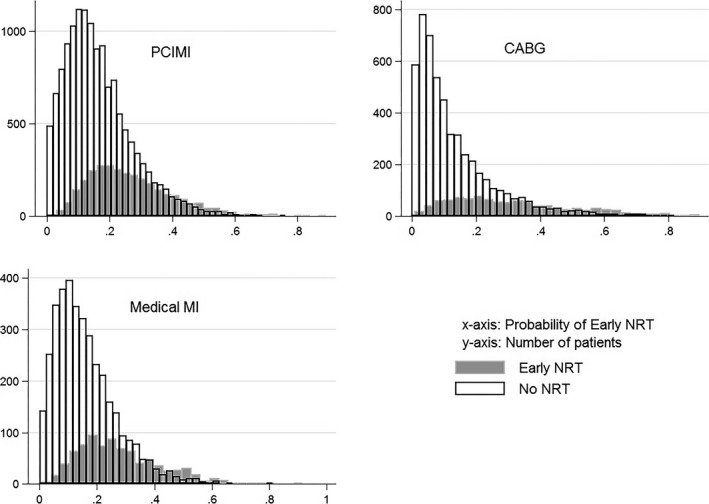
Propensity score distributions and overlap by receipt of nicotine replacement therapy (NRT) by patient group. CABG indicates coronary artery bypass graft; MI, myocardial infarction; PCIMI, percutaneous coronary intervention with or without MI.
Patient characteristics after matching are shown in Table 3. In general, the mean age ranged from 56 to 60 years, with 59% to 75% men, and 75% to 79% white, depending on the group. After matching, all covariates were well balanced, with absolute standardized differences of <10% among all groups, suggesting mostly similar baseline characteristics, except among patients with medically managed MI. These patients had persistent baseline differences in the frequency of pulmonary circulation disorders, hypertension with complications, and renal failure, but in all 3 of these comorbidities there was a higher prevalence of these disorders among patients treated with NRT.
Table 3.
Patient Characteristics in Propensity‐Matched Cohorts
| Characteristics | PCIMI | CABG | Medical MI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No NRT | Early NRTa | ASD | No NRT | Early NRTa | ASD | No NRT | Early NRTa | ASD | |
| Patients, n (%) | 2975 (50) | 2975 (50) | 892 (50) | 892 (50) | 769 (50) | 769 (50) | |||
| Group | |||||||||
| PCI | 26.4 | 25.9 | |||||||
| MI+PCI | 73.6 | 74.1 | 1.1 | ||||||
| Age, mean (SD), y | 56.1 (10.3) | 56 (9.8) | 1 | 58.4 (9.5) | 58.2 (9.2) | 2.1 | 60.2 (12.4) | 60.2 (11.6) | 0.1 |
| Female sex | 30.7 | 31.5 | 1.9 | 25.3 | 24.4 | 2.1 | 41.7 | 39.9 | 3.7 |
| Race/ethnicity | 5.3 | 1.4 | 6.6 | ||||||
| White | 79.4 | 77.3 | 79.6 | 79.4 | 76.5 | 74 | |||
| Black | 8.2 | 8.8 | 5.8 | 5.9 | 13.4 | 14.2 | |||
| Hispanic | 2.8 | 3.2 | 2.9 | 3.1 | 3 | 3.1 | |||
| Other | 9.6 | 10.7 | 11.7 | 11.5 | 7.2 | 8.7 | |||
| Marital status | 4.4 | 4 | 8.6 | ||||||
| Married | 44 | 41.9 | 47.5 | 47.1 | 41.1 | 37.1 | |||
| Single | 46.2 | 47.5 | 44.5 | 43.8 | 51.2 | 54.1 | |||
| Other | 9.8 | 10.6 | 8 | 9.1 | 7.7 | 8.8 | |||
| Insurance payer | 4.2 | 4.9 | 4.9 | ||||||
| Medicare | 29.6 | 30.2 | 36 | 37.1 | 49.9 | 51.9 | |||
| Medicaid | 21.2 | 20.1 | 21.3 | 20.5 | 17.8 | 17.6 | |||
| Managed care | 25.9 | 26.7 | 21.1 | 21.7 | 15.6 | 15.1 | |||
| Commercial‐indemnity | 5.9 | 5.8 | 8.6 | 7.7 | 4.7 | 4.7 | |||
| Self‐pay | 12.4 | 11.8 | 7.3 | 7.6 | 8.1 | 7 | |||
| Other | 4.9 | 5.4 | 5.7 | 5.3 | 3.9 | 3.8 | |||
| Gagne combined comorbidity score, mean (SD) | 1 (2.1) | 1.1 (2.1) | 0.7 | 2.4 (2.5) | 2.3 (2.5) | 2.9 | 2.5 (2.6) | 2.7 (2.7) | 4.7 |
| Comorbidities | |||||||||
| Congestive heart failure | 18 | 19.1 | 2.9 | 26 | 27.1 | 2.5 | 38.5 | 37.1 | 3 |
| Valvular disease | 8.1 | 7.9 | 1 | 16.1 | 16.7 | 1.5 | 12.1 | 14.4 | 6.9 |
| Peripheral vascular disease | 12.1 | 12.9 | 2.4 | 23.1 | 23.4 | 0.8 | 18.1 | 20.8 | 6.9 |
| Hypertension with complications | 9.4 | 10.7 | 4.2 | 17.3 | 15.4 | 5.2 | 18.5 | 23.8 | 13.1 |
| Hypertension without complications | 63.5 | 62.1 | 3.1 | 66.6 | 68.9 | 5 | 58.4 | 56 | 4.7 |
| Chronic pulmonary disease | 35 | 33.8 | 2.6 | 56.6 | 54.7 | 3.8 | 53.8 | 52.8 | 2.1 |
| Diabetes mellitus | 29.6 | 30.1 | 1 | 41.9 | 42.4 | 0.9 | 30.8 | 33.8 | 6.4 |
| Renal failure | 6.5 | 6.8 | 1.4 | 13.5 | 11.8 | 5.1 | 14.2 | 17.9 | 10.3 |
| Obesity | 20.8 | 19.3 | 4 | 24.2 | 23.8 | 1.1 | 17.4 | 16.4 | 2.8 |
| Deficiency anemias | 7 | 7.2 | 0.5 | 18.5 | 19.8 | 3.4 | 17.4 | 17.4 | 0 |
| Alcohol abuse | 9.7 | 9.1 | 2.1 | 12.6 | 11.8 | 2.4 | 16.3 | 12.9 | 9.6 |
| Drug abuse | 8.7 | 7.9 | 2.8 | 9.5 | 8.4 | 3.9 | 10 | 11.4 | 4.6 |
| Depression | 12.5 | 11.5 | 3.1 | 11.3 | 10.9 | 1.4 | 15.1 | 16 | 2.5 |
| Early treatmentsa | |||||||||
| ICU/intermediate care | 56.2 | 55.1 | 1.1 | 59.3 | 57.4 | 3.9 | 40.6 | 41.2 | 1.3 |
| Vasodilators | 41.8 | 39.1 | 3.3 | 36 | 36.9 | 1.9 | 23.3 | 22.2 | 2.5 |
| NIV | 3.4 | 3.2 | 1.4 | 4 | 4.7 | 3.3 | 6.4 | 7 | 2.6 |
| IMV | 2.8 | 3.4 | 0.6 | 26.1 | 27.2 | 2.5 | 3.5 | 4.3 | 4 |
| Vasopressors | 9.7 | 9.3 | 0 | 27.5 | 27.7 | 0.5 | 4.4 | 3.8 | 3.3 |
| Arterial line | 1.1 | 1.1 | 2 | 7.7 | 8 | 0.8 | 0.8 | 1 | 2.7 |
| IABP | 1.5 | 1.5 | 5.6 | 3 | 3.9 | 4.9 | 0.9 | 0.8 | 1.4 |
| Inotropes | 2.8 | 3.2 | 2.1 | 10.3 | 11.2 | 2.9 | 1 | 1.4 | 3.5 |
| Hospital size, beds | 1.4 | 3.6 | 0.8 | ||||||
| ≤200 | 11.1 | 10.8 | 4.7 | 4.4 | 7.8 | 7.9 | |||
| 201–400 | 38.1 | 37.8 | 32.3 | 30.9 | 37.7 | 38 | |||
| ≥401 | 50.8 | 51.4 | 63 | 64.7 | 54.5 | 54.1 | |||
| Hospital region | 3.4 | 2.6 | 8.7 | ||||||
| Northeast | 16.2 | 17 | 16.4 | 16.8 | 13.3 | 15.1 | |||
| Midwest | 21.4 | 20.2 | 12.1 | 12.8 | 16.2 | 17.9 | |||
| West | 6.2 | 6.5 | 5.9 | 5.8 | 4.5 | 5.2 | |||
| South | 56.2 | 56.3 | 65.6 | 64.6 | 65.9 | 61.8 | |||
| Urban hospital | 81.6 | 82.3 | 1.1 | 86.2 | 86.3 | 1.6 | 81.9 | 81.9 | 0.5 |
| Teaching hospital | 45.4 | 45.6 | 2.8 | 52.8 | 52.3 | 2 | 53.8 | 50.7 | 4.5 |
Data are given as percentage of each group unless otherwise indicated. ASDs are the ratio between the absolute differences in means of the 2 populations divided by mean variance of the total population. When sample sizes are large, ASDs are preferred to P values because P values are commonly <0.05 because of high statistical power, without necessarily being reflective of important clinical differences. ASD values >10% are generally considered clinically important (≈7% nonoverlap) between populations. ASD indicates absolute standardized difference; CABG, coronary artery bypass graft; IABP, intra‐aortic balloon pump; ICU, intensive care unit; IMV, invasive mechanical ventilation; MI, myocardial infarction; NIV, noninvasive ventilation; NRT, nicotine replacement therapy; PCI, percutaneous coronary intervention; PCIMI, PCI with or without MI.
Treatment on day 0, 1, or 2.
Outcomes are shown in Table 4 for all 3 propensity‐matched patient groups. Overall, depending on subgroup, in‐hospital mortality ranged from 0.5% to 2.3%, mean hospital LOS ranged from 3.5 to 10 days, 1‐month readmission ranged from 8.9% to 15.8%, and cost ranged from $10 428 to $42 118. Unadjusted models show significantly lower overall mortality, hospital LOS, readmission, and total costs for patients treated with NRT (Table 1). However, in the propensity‐matched cohort, we found no differences in any outcomes in all 3 patient groups for patients treated versus not treated with NRT (Table 4 and Figure 3).
Table 4.
Outcomes in Propensity‐Matched Cohorts by Diagnosis and Use of NRT
| Variable | PCIMI | CABG | Medical MI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No NRT | Early NRT | P Valuea | No NRT | Early NRT | P Valuea | No NRT | Early NRT | P Valueb | |
| Patients, n (%) | 2975 (50) | 2975 (50) | 892 (50) | 892 (50) | 769 (50) | 769 (50) | |||
| In‐hospital mortality, n (%) | 26 (0.9) | 14 (0.5) | 0.06 | 20 (2.2) | 11 (1.2) | 0.099 | 16 (2.1) | 18 (2.3) | 0.98 |
| LOS, mean (SD), dc | 3.5 (2.7) | 3.6 (2.6) | 0.91 | 9.6 (4.5) | 10.0 (4.4) | 0.06 | 4.3 (3.3) | 4.4 (3.5) | 0.60 |
| All‐cause readmission among survivors (≈1 mo), n (%) | 289 (9.8) | 263 (8.9) | 0.22 | 91 (10.4) | 111 (12.6) | 0.16 | 119 (15.8) | 110 (14.6) | 0.31 |
| Cost, mean (SD), US $d | 17 243 (10 475) | 17 085 (9855) | 0.55 | 42 118 (23 111) | 41 078 (18 529) | 0.28 | 10 502 (8731) | 10 428 (8814) | 0.57 |
CABG indicates coronary artery bypass graft; LOS, length of stay; MI, myocardial infarction; NRT, nicotine replacement therapy; PCIMI, percutaneous coronary intervention with or without MI.
Accounting for propensity match.
Accounting for propensity match and adjustment for imbalanced factors.
Measure winsorized at 99th percentile (number [percentage] of patients affected: PCI, 145 [0.9%]; CABG, 60 [1%]; medical MI, 37 [0.8%]).
Measure winsorized at 1st and 99th percentiles (number [percentage] of patients affected: PCI, 197 [1.1%] and 167 [1%]; CABG, 67 [1.1%] and 61 [1%]; medical MI, 51 [1.1%] and 45 [1%], respectively).
Figure 3.

Odds ratios for mortality and 1‐month all‐cause readmission for each group in propensity‐matched cohort. All models account for propensity match. Models for medical myocardial infarction (MI) group are further adjusted for unbalanced factors. CABG indicates coronary artery bypass graft; NRT, nicotine replacement therapy; PCIMI, percutaneous coronary intervention with or without MI.
Discussion
In this large pharmacoepidemiologic study of US smokers hospitalized with acute CHD, using robust analytic techniques and high statistical power, we found that starting NRT in the first 2 days of the hospitalization was not associated with any significant change in inpatient mortality, hospital LOS, readmission, or hospital costs. These findings were consistent across clinically diverse groups, including those with MI and those who underwent CABG or PCI. In addition, more than half of the population received care in an intensive care unit, >25% of patients undergoing CABG remained intubated postoperatively, and a sizable portion (in all patient groups) required vasopressors, vasodilators, inotropes, and/or mechanical circulatory support. Despite a high level of acuity, MI among many patients, and possible hemodynamic instability, the use of NRT products starting during the first 2 days of hospitalization was not associated with any significant differences (harm or benefit) in outcomes among smokers hospitalized with acute CHD.
This study provides the strongest data available to address clinical uncertainty about the safety of NRT when used as a replacement for smokers hospitalized with acute MI or for treatment of CHD. The uncertainty stems from an absence of randomized controlled trials that included this specific population, in whom nicotine has a theoretical potential for causing harm because of its adrenergic and vasospastic properties. This is in contrast to the use of NRT in smokers with stable CHD seen in the outpatient setting, where randomized controlled trials have established NRT's safety.2, 3, 4 Although NRT has never clearly been associated with harm in the setting of acute CHD, prior concerns were extrapolated from physiologic data in nonsmokers, in short‐term pharmacologic and physiologic experimental settings, or in case reports or small case series, despite the potential pitfalls in using this kind of data to make clinical decisions. Instead, our well‐powered study joins the majority of observational and randomized trials that have demonstrated a lack of any association between NRT use and morbidity, including patients at hospital discharge for CAD, in the medical intensive care unit, and among the general population.6, 7, 8, 27 Furthermore, recent physiologic studies have called into question the universally held belief that NRT is consistently associated with an increase in blood pressure and heart rate, particularly when it is used to replace cigarette smoking during a short‐term hospitalization.28, 29 Although NRT does have minor adverse effects30 and some caution will always be appropriate, it appears that for the general population of smokers (both inpatient and outpatient), the use of NRT is unlikely to lead to important adverse cardiovascular effects.
We believe our findings have significant implications for tobacco treatment among patients hospitalized with CHD for several reasons. First, and most important, our findings suggest that physicians can prescribe NRT to alleviate nicotine withdrawal symptoms without concern for inducing adverse events. Although nicotine withdrawal is underrecognized in the hospital, nicotine withdrawal is common, peaks within 1 week of smoking cessation, and includes symptoms of irritability, anxiety, difficulty concentrating, restlessness, and depressed mood.31 Because these symptoms are largely relieved with NRT, increased use of NRT should improve quality of care and patient experience for many smokers,32, 33, 34 and should also help avoid nicotine withdrawal‐associated delirium.35, 36 Second, because inpatient NRT use has been associated with greater outpatient NRT use13 and NRT is a well‐accepted and generally effective treatment for smoking cessation,37, 38 greater use of NRT in the inpatient setting should positively influence long‐term smoking cessation rates.14 Third, because the Joint Commission now considers prescription of pharmacotherapy for smoking cessation (of which the vast majority is NRT) a standard of care for both inpatient use and at hospital discharge,39 and patient motivation to quit smoking is high after a hospitalization, we believe that hospitals can pursue quality initiatives40 to ensure that all hospitalized smokers are offered and prescribed smoking cessation medications to improve smoking cessation outcomes and to meet Joint Commission performance measures. Fourth, we believe that there is now enough combined evidence that guideline writers should consider adding statements about the lack of harm with NRT in smokers with acute CHD.
Strengths of this study include its large size, geographic diversity, high acuity population, and use of robust statistical techniques with excellent covariate balance after propensity matching. These minimize concern for confounding. Nonetheless, as an observational study, it may have residual confounding because of important unmeasured covariates, which limits the causal inference that can be made. For example, NRT prescription at hospital discharge and NRT use at home were not available in Premier, but would be useful to understand the risk of NRT on readmission. In addition, usual smoking habits (cigarettes per day), dual tobacco product use, such as e‐cigarettes, and usual blood nicotine levels were not available and could have potentially influenced the dose of NRT that was prescribed by clinicians. If patients were consistently underdosed or overdosed, this could affect the safety of NRT in the short‐term setting. Future research would do well to explore issues related to NRT dose and safety. Last, because Premier only records the day of NRT administration, a more detailed analysis by hours of exposure was not possible. Thus, for inpatient mortality, it is possible that we excluded some patients with NRT‐related mortality if they died within the first 2 days. However, we think this is unlikely to be the case because unadjusted mortality among the 5296 patients excluded was 1.6% versus 6.2% for early NRT versus no NRT.
The other main limitation is that we were unable to examine smoking cessation outcomes and long‐term outcomes, such as 1‐year mortality, although we did evaluate 30‐day readmission. Similarly, we were unable to examine intermediate outcomes, like recurrent MI, or new‐onset hypertensive urgency, stroke, or arrhythmias that occurred after initiation of NRT. This occurred because ICD‐9 codes do not have an associated date of onset, so it was not possible to determine if a stroke (for example) occurred before or after the administration of NRT. However, if these complications had occurred with any frequency as a result of NRT use, we believe such complications would have manifested themselves in difference in LOS or total hospital costs between groups, which were not seen in this study.
Although the prevalence of smoking is declining nationwide, it is important for clinicians to remember that smoking is still the leading cause of preventable death in the United States and is responsible for >480 000 deaths per year.41 Smoking remains a critical risk factor for MI, with ≈50% of patients with ST‐segment–elevation MI being smokers.42 Although treatments such as NRT and varenicline are both readily available and effective,14, 43 they remain highly underused both during hospitalization and after discharge.10, 44 These findings suggest that physicians and hospitals have a large opportunity to improve the care of smokers with acute CHD.
Conclusions
For years, clinicians have worried about the potential toxicity of NRT in patients hospitalized with acute cardiac disease. Although a randomized trial would be the most definitive way to answer this question, our results should significantly reduce concerns about the safety of NRT among hospitalized patients with CHD. Further research should focus on identifying effective strategies that increase delivery of NRT and other smoking cessation therapies so that all smokers receive effective treatments to help them quit smoking permanently.
Sources of Funding
Drs Pack, Lagu, and Lindenauer were each supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD) under award numbers 1K23HL135440, 1K01HL114745, and 1K24HL132008, respectively.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e009424 DOI: 10.1161/JAHA.118.009424)
The abstract of this work was presented at the American Heart Association's Scientific Sessions, November 11 to 15, 2017, in Anaheim, CA.
References
- 1. Benowitz NL, Gourlay SG. Cardiovascular toxicity of nicotine: implications for nicotine replacement therapy. J Am Coll Cardiol. 1997;29:1422–1431. [DOI] [PubMed] [Google Scholar]
- 2. Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, Sherman SE, Cleveland M, Antonuccio DO, Hartman N, McGovern PG. The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med. 1996;335:1792–1798. [DOI] [PubMed] [Google Scholar]
- 3. Tzivoni D, Keren A, Meyler S, Khoury Z, Lerer T, Brunel P. Cardiovascular safety of transdermal nicotine patches in patients with coronary artery disease who try to quit smoking. Cardiovasc Drugs Ther. 1998;12:239–244. [DOI] [PubMed] [Google Scholar]
- 4. Working Group for the Study of Transdermal Nicotine in Patients With Coronary Artery Disease. Nicotine replacement therapy for patients with coronary artery disease. Arch Intern Med. 1994;154:989–995. [PubMed] [Google Scholar]
- 5. Fiore MC, Jaen MC, Baker TB. Treating tobacco use and dependence: 2008 update: clinical practice guideline. Am J Prev Med. 2008;35:158–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cartin‐Ceba R, Warner DO, Hays JT, Afessa B. Nicotine replacement therapy in critically ill patients: a prospective observational cohort study. Crit Care Med. 2011;39:1635–1640. [DOI] [PubMed] [Google Scholar]
- 7. Meine TJ, Patel MR, Washam JB, Pappas PA, Jollis JG. Safety and effectiveness of transdermal nicotine patch in smokers admitted with acute coronary syndromes. Am J Cardiol. 2005;95:976–978. [DOI] [PubMed] [Google Scholar]
- 8. Woolf KJ, Zabad MN, Post JM, McNitt S, Williams GC, Bisognano JD. Effect of nicotine replacement therapy on cardiovascular outcomes after acute coronary syndromes. Am J Cardiol. 2012;110:968–970. [DOI] [PubMed] [Google Scholar]
- 9. Paciullo CA, Short MR, Steinke DT, Jennings HR. Impact of nicotine replacement therapy on postoperative mortality following coronary artery bypass graft surgery. Ann Pharmacother. 2009;43:1197–1202. [DOI] [PubMed] [Google Scholar]
- 10. Pack QR, Priya A, Lagu TC, Pekow PS, Rigotti NA, Lindenauer PK. Smoking cessation pharmacotherapy among smokers hospitalized for coronary heart disease. JAMA Intern Med. 2017;177:1525–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. May FC, Stocks N, Barton C. Identification of barriers that impede the implementation of nicotine replacement therapy in the acute cardiac care setting. Eur J Cardiovasc Prev Rehabil. 2008;15:646–650. [DOI] [PubMed] [Google Scholar]
- 12. Colivicchi F, Mocini D, Tubaro M, Aiello A, Clavario P, Santini M. Effect of smoking relapse on outcome after acute coronary syndromes. Am J Cardiol. 2011;108:804–808. [DOI] [PubMed] [Google Scholar]
- 13. Regan S, Reyen M, Richards AE, Lockhart AC, Liebman AK, Rigotti NA. Nicotine replacement therapy use at home after use during a hospitalization. Nicotine Tob Res. 2012;14:885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ortega F, Vellisco A, Marquez E, Lopez‐Campos JL, Rodriguez A, de los Angeles Sanchez M, Barrot E, Cejudo P. Effectiveness of a cognitive orientation program with and without nicotine replacement therapy in stopping smoking in hospitalised patients. Arch Bronconeumol. 2011;47:3–9. [DOI] [PubMed] [Google Scholar]
- 15. Lindenauer PK, Pekow P, Gao S, Crawford AS, Gutierrez B, Benjamin EM. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144:894–903. [DOI] [PubMed] [Google Scholar]
- 16. Wiley LK, Shah A, Xu H, Bush WS. ICD‐9 tobacco use codes are effective identifiers of smoking status. J Am Med Inform Assoc. 2013;20:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benowitz NL, Hukkanen J, Jacob P III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;192:29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 19. Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64:749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lagu T, Pekow PS, Shieh MS, Stefan M, Pack QR, Kashef MA, Atreya AR, Valania G, Slawsky MT, Lindenauer PK. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. 2016;9:e002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lagu T, Lindenauer PK, Rothberg MB, Nathanson BH, Pekow PS, Steingrub JS, Higgins TL. Development and validation of a model that uses enhanced administrative data to predict mortality in patients with sepsis. Crit Care Med. 2011;39:2425–2430. [DOI] [PubMed] [Google Scholar]
- 22. Lagu T, Krumholz HM, Dharmarajan K, Partovian C, Kim N, Mody PS, Li SX, Strait KM, Lindenauer PK. Spending more, doing more, or both? An alternative method for quantifying utilization during hospitalizations. J Hosp Med. 2013;8:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weichle T, Hynes DM, Durazo‐Arvizu R, Tarlov E, Zhang Q. Impact of alternative approaches to assess outlying and influential observations on health care costs. Springerplus. 2013;2:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Glob Forum. 2012. Paper 335. Available at: http://support.sas.com/resources/papers/proceedings12/335-2012.pdf. Accessed August 8, 2018. [Google Scholar]
- 26. D'Agostino RB Jr. Propensity scores in cardiovascular research. Circulation. 2007;115:2340–2343. [DOI] [PubMed] [Google Scholar]
- 27. Mills EJ, Thorlund K, Eapen S, Wu P, Prochaska JJ. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta‐analysis. Circulation. 2014;129:28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sobieraj DM, White WB, Baker WL. Cardiovascular effects of pharmacologic therapies for smoking cessation. J Am Soc Hypertens. 2013;7:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva AP, Scholz J, Abe TO, Pinheiro GG, Gaya PV, Pereira AC, Santos PC. Influence of smoking cessation drugs on blood pressure and heart rate in patients with cardiovascular disease or high risk score: real life setting. BMC Cardiovas Disord. 2016;16:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mills EJ, Wu P, Lockhart I, Wilson K, Ebbert JO. Adverse events associated with nicotine replacement therapy (NRT) for smoking cessation: a systematic review and meta‐analysis of one hundred and twenty studies involving 177,390 individuals. Tob Induc Dis. 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res. 2007;9:315–327. [DOI] [PubMed] [Google Scholar]
- 32. Foulds J. Use of nicotine replacement therapy to treat nicotine withdrawal syndrome and aid temporary abstinence. Int J Clin Pract. 2010;64:292–294. [DOI] [PubMed] [Google Scholar]
- 33. Pfaff KA, El‐Masri MM, Fox‐Wasylyshyn SM. Comparing the psychological stress between non‐smoking patients and smoking patients who experience abrupt smoking cessation during hospitalization for acute myocardial infarction: a pilot study. Can J Cardiovasc Nurs. 2009;19:26–32. [PubMed] [Google Scholar]
- 34. West R, Shiffman S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: a systematic review. Psychopharmacology. 2001;155:115–122. [DOI] [PubMed] [Google Scholar]
- 35. Lucidarme O, Seguin A, Daubin C, Ramakers M, Terzi N, Beck P, Charbonneau P, du Cheyron D. Nicotine withdrawal and agitation in ventilated critically ill patients. Crit Care. 2010;14:R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park H, Kim KW, Yoon IY. Smoking cessation and the risk of hyperactive delirium in hospitalized patients: a retrospective study. Can J Psychiatry. 2016;61:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. [DOI] [PubMed] [Google Scholar]
- 38. Suissa K, Lariviere J, Eisenberg MJ, Eberg M, Gore GC, Grad R, Joseph L, Reynier PM, Filion KB. Efficacy and safety of smoking cessation interventions in patients with cardiovascular disease: a network meta‐analysis of randomized controlled trials. Circ Cardiovasc Qual Outcomes. 2017;10:e002458. [DOI] [PubMed] [Google Scholar]
- 39. Fiore MC, Adsit R. Will hospitals finally “do the right thing”? Providing evidence‐based tobacco dependence treatments to hospitalized patients who smoke. Jt Comm J Qual Patient Saf. 2016;42:207–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Slattery C, Freund M, Gillham K, Knight J, Wolfenden L, Bisquera A, Wiggers J. Increasing smoking cessation care across a network of hospitals: an implementation study. Implement Sci. 2016;11:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fiore MC, Jaén CR, Baker TB et al. Treating Tobacco Use and Dependence: 2008 Update. Quick Reference Guide for Clinicians. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service. April 2009. [Google Scholar]
- 42. Lloyd A, Steele L, Fotheringham J, Iqbal J, Sultan A, Teare MD, Grech ED. Pronounced increase in risk of acute ST‐segment elevation myocardial infarction in younger smokers. Heart. 2017;103:586–591. [DOI] [PubMed] [Google Scholar]
- 43. Eisenberg MJ, Windle SB, Roy N, Old W, Grondin FR, Bata I, Iskander A, Lauzon C, Srivastava N, Clarke A, Cassavar D, Dion D, Haught H, Mehta SR, Baril JF, Lambert C, Madan M, Abramson BL, Dehghani P; EVITA Investigators . Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133:21–30. [DOI] [PubMed] [Google Scholar]
- 44. Pagidipati NJ, Hellkamp A, Thomas L, Gulati M, Peterson ED, Wang TY. Use of prescription smoking cessation medications after myocardial infarction among older patients in community practice. JAMA Cardiol. 2017;2:1040–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]


