Abstract
Background
Sudden cardiac death accounts for the greatest proportion of duty‐related deaths among US firefighters. Increased understanding of the pathoanatomic causes of sudden cardiac death and the risk associated with underlying cardiac pathologies is needed to develop evidence‐based screening recommendations.
Methods and Results
Using autopsy data for duty‐related firefighter fatalities occurring between 1999 and 2014, this retrospective case–control study compared cardiac findings of male firefighters aged 18 to 65 years who died on duty of cardiac‐related causes with those who died of noncardiac trauma‐related causes. Data from 276 cardiac cases and 351 noncardiac trauma controls were analyzed. Among cardiac cases, the most prevalent (82%) underlying pathoanatomic substrate was comorbid coronary heart disease and cardiomegaly/left ventricular hypertrophy. Cardiac cases had a higher prevalence of cardiomegaly (heart weight >450 g), left ventricular hypertrophy (left ventricular wall thickness ≥1.2 cm), and severe coronary artery stenosis (≥75%) than trauma controls (all P<0.001). In multivariate analyses, heart weight >450 g, coronary artery stenosis ≥75%, and evidence of a prior myocardial infarction were strong independent predictors of cardiac death, with odds ratios of 6.1 (95% confidence interval, 3.6–10.4), 9.3 (95% confidence interval, 5.3–16.1), and 6.2 (95% confidence interval, 3.4–11.3), respectively.
Conclusions
The majority of cardiac fatalities had evidence of both coronary heart disease and increased heart mass, and each condition was independently associated with a markedly elevated risk of cardiac death. Targeted screening for coronary heart disease, increased heart mass, and evidence of prior myocardial infarction should be considered to reduce duty‐related cardiac deaths among firefighters.
Keywords: autopsy, cardiomegaly, coronary heart disease, firefighting, left ventricular hypertrophy
Subject Categories: Cardiovascular Disease, Epidemiology
Clinical Perspective
What Is New?
Based on a large, nationally representative sample of firefighter autopsies, 82% of cardiac fatalities in male firefighters (aged 18–65 years) had evidence of both coronary artery disease (≥75% stenosis) and cardiomegaly/left ventricular hypertrophy.
Cardiomegaly, coronary artery stenosis, and prior myocardial infarction were all strong independent predictors of duty‐related cardiac death.
What Are the Clinical Implications?
Firefighters should be screened for both atherosclerotic coronary heart disease and cardiomegaly/left ventricular hypertrophy.
The increased risk of sudden cardiac death should be carefully considered before medically clearing firefighters for duty or return to duty following a cardiac event or significant cardiovascular procedure.
Introduction
Firefighters perform work that is critical to the safety of the public, and their sudden incapacitation may put civilian and firefighter lives, as well as property, in jeopardy. Although firefighters face numerous occupational hazards, the leading cause of duty‐related death among firefighters in the United States is sudden cardiac death (SCD), accounting for ≈42% of duty‐related fatalities annually.1 In the general population2, 3, 4 and the fire service,5, 6 coronary heart disease (CHD) has long been the predominant disease condition associated with SCD. Overt CHD is a strong independent predictor of SCD, conveying a 3‐ to 5‐fold increase in the risk of SCD in a large epidemiological study.7 Among firefighters, a prior diagnosis of CHD or other clinical evidence of arterial–occlusive disease is associated with a 16‐fold increased risk of duty‐related death from CHD after adjustment for comorbid risk factors.6 Importantly, CHD frequently goes undetected, and SCD is often the first manifestation of the disease.2, 3, 4 This is particularly problematic in the case of first responders, whose sudden incapacitation can have ramifications far beyond the individual who experiences a cardiac event.
Left ventricular hypertrophy (LVH) and cardiomegaly have been reported as common secondary findings in SCD attributed to CHD in the general population8, 9, 10, 11 and among firefighters.5, 6, 12, 13 However, the role of LVH and cardiomegaly have not received nearly as much attention as CHD in contributing to sudden cardiac events. LVH has been shown to be a strong independent predictor of SCD in the general population,14, 15 but its prognostic power within the general fire service has not been documented. A recent case–control study determined that cardiomegaly (defined as heart weight >450 g), which was found in 61% of SCD cases, was associated with a 5‐fold increased risk of SCD among young (aged ≤45 years) firefighters.12 Moreover, a review of autopsies revealed that 70% of SCD cases in this young cohort had evidence of LVH.
Prior research has identified occupational16, 17 and individual5, 6, 12 factors associated with an increased risk of cardiac death in the fire service. Careful analysis of autopsy data offer the opportunity to better understand the underlying pathoanatomic features associated with SCD. Although some evidence shows a high prevalence of coexistent CHD and cardiomegaly/LVH in the fire service, these risks have not been quantified, and it is unclear whether increased cardiac mass or hypertrophy contributes to an increased risk of cardiac death beyond that associated with underlying CHD. A greater understanding of the pathoanatomic causes of cardiac death and the risks associated with different underlying cardiac pathologies is needed to improve evidence‐based recommendations to reduce the risk of SCD in the fire service and thereby better ensure public safety. The specific aim of this study was to compare the cardiac findings on autopsy between firefighters who died of duty‐related cardiac causes and those of firefighters who died of noncardiac work‐related trauma.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
This retrospective case–control study included cases and controls selected from duty‐related US firefighter fatalities between 1999 and 2014. The Skidmore College Institutional Review Board determined that the use of records from deceased participants did not meet the definition of human subjects research as defined by the US Department of Health and Human Services and Federal Drug Administration guidelines; therefore, the study was granted an institutional review board exemption.
Autopsy Data/Fire Service Data Extraction
The US Fire Administration (USFA) and the National Fallen Firefighters Foundation (NFFF) collect information on all firefighter deaths associated with firefighting in the United States. In collaboration with the NFFF, all available autopsy data and medical examiner reports for duty‐related firefighter fatalities between 1999 and 2014 were obtained. Based on the USFA's definition of on‐duty fatality, a duty‐related fatality was any injury or illness sustained while on duty that proved fatal, which included illness resulting from a sudden cardiac event or stroke within 24 hours of a training activity or emergency response, regardless of whether or not the firefighter complained of illness while on duty. A portion of these deaths are also reviewed by the National Institute for Occupational Safety and Health (NIOSH), and the resulting reports are made publicly available online. All relevant corresponding NIOSH fatality reports were also obtained. Two study investigators independently reviewed autopsy and other files and extracted anthropometric and cardiac findings using a standardized electronic template. A senior investigator (D.L.S. or S.N.K.) resolved any disagreements between the reviewers.
Body mass index (BMI) was calculated as body mass in kilograms divided by the square of height in meters (kg/m2) using measurements recorded at autopsy. In records in which autopsy height or body mass was missing or deemed inaccurate related to factors such as severe damage to the body, multiple organ procurement, or inclusion of firefighting gear in body mass recorded at autopsy, a BMI was not calculated; however, a BMI value from a NIOSH report was used instead, if available. The firefighters’ job classifications and years of service were obtained from data provided by the USFA. Firefighters who were paid on‐call, part‐time paid, and volunteers were all classified as volunteer firefighters for this study.
Cause of Death and Inclusion Criteria
Fire service authorities generally categorize firefighter fatalities as being due to heart attack (USFA) or SCD (National Fire Protection Association), trauma, asphyxiation, burns, crushing, and other medical causes. Using autopsy data, we verified that these categories were consistent with the medical examiner's cause of death and broadly classified causes of death as being due to noncardiac, cardiac, or other causes. If the cause of death could not be clearly classified as cardiac or noncardiac, the cause of death was identified as indeterminate (eg, vehicle accident that was thought to be due to a sudden cardiac event) and included in the other category. Inclusion criteria for the current study were (1) firefighter duty‐related fatality between 1999 and 2014, as determined by the USFA; (2) autopsy‐verified cardiac or noncardiac cause of death; (3) male sex; (4) age between 18 and 65 years; and (5) a valid heart weight recorded at autopsy. Women were excluded from analyses given the low number of female firefighters (n=25) among available autopsy records. Age was restricted to 18 to 65 years because the prevalence of cardiovascular disease increases with age, and age restrictions are frequently in place for career firefighters.
Fatalities with cause of death classified as cardiac in origin were selected as cases in the present study. Cardiac cases included autopsy underlying causes such as CHD, cardiomegaly/LVH, primary arrhythmia, cardiomyopathies, and valvular disease. It is expected that the vast majority of these cases would be classified as SCD, but the time of death and the onset of symptoms could not be verified in all cases. CHD was considered present if it was noted in the autopsy narrative report or if the percentage of stenosis in at least 1 coronary artery was reported as ≥75%. Cardiomegaly/LVH was considered to be present if it was noted in the autopsy narrative report, if the heart weight was reported as >450 g, or if the left ventricular (LV) wall thickness was reported as ≥1.2 cm. Fatalities with a noncardiac cause of death were selected as noncardiac trauma controls. These deaths included fatalities caused by blunt force trauma, burn injury, smoke inhalation, electrocution, drowning, and gunshot, for which no evidence showed a preceding or simultaneous cardiovascular event.
Statistical Analyses
Continuous characteristics were described using mean±SD and were compared between groups using the t test of independence. Categorical variables were presented as frequencies and percentages and were compared using the χ2 test of association. For categorical variables that were missing values (eg, percentage of stenosis not reported), the missing value was treated as a no based on the presumption that an abnormality would have been recorded. A sensitivity analysis was conducted to determine the effect of setting missing categorical values to no rather than treating them as missing. BMI was categorized as normal (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). Crude and adjusted logistic regression analysis models were utilized to model the probability of having a cardiac death. Variables to be introduced in the multivariate logistic regression model included established risk factors for cardiovascular disease available from the autopsy (age and BMI), the 2 primary study outcome variables (heart weight >450 g and coronary artery with ≥75% stenosis) for the 2 types of heart disease examined, and a variable that has been shown to be predictive of future events (prior myocardial infarction). Results were presented as odds ratios with the corresponding 95% confidence intervals. All tests performed were 2‐tailed using a 0.05 level of significance. SAS v9.3 (SAS Institute) was used for all analyses.
Results
Figure illustrates the selection process for identifying firefighter duty‐related fatalities included in this case–control study. Of the 1644 identified duty‐related deaths between 1999 and 2014, 627 fatalities (276 cardiac cases and 351 noncardiac trauma controls) met the study inclusion criteria. Among the cardiac cases, the underlying cause of death was CHD and cardiomegaly/LVH in 81.9%, CHD alone in 5.4%, cardiomegaly/LVH alone in 5.8%, and causes other than CHD and/or cardiomegaly/LVH in 6.9% of fatalities. The most common causes of death among noncardiac trauma controls were blunt force trauma, accounting for 53.0% of deaths, and smoke inhalation or burns, which individually or in combination caused 35.9% of trauma deaths.
Figure 1.
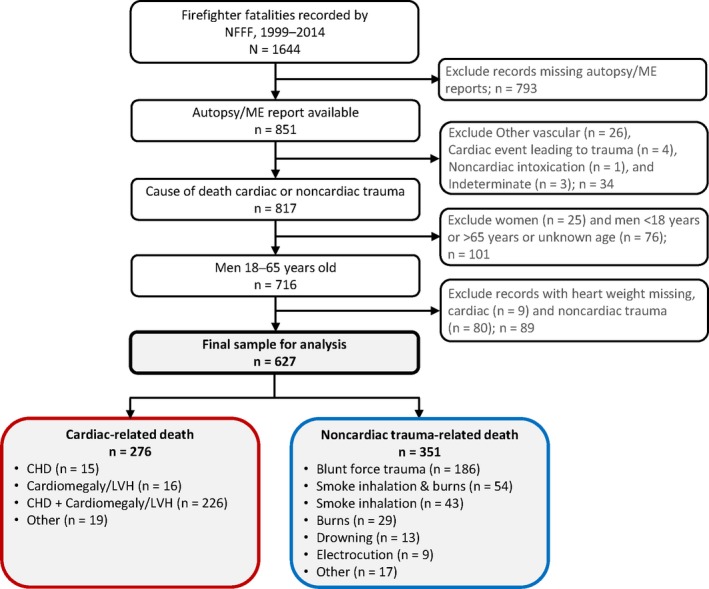
Process for identifying study population: US firefighters with underlying cause of death determined to be cardiac or noncardiac in origin by autopsy or medical records. CHD indicates coronary heart disease; LVH, left ventricular hypertrophy; ME, medical examiner; NFFF, National Fallen Firefighters Foundation.
A comparison of descriptive characteristics between cardiac cases and noncardiac trauma controls is presented in Table 1. Cardiac cases were significantly older than noncardiac trauma controls (48.7±9.1 versus 39.4±12.1 years, respectively; P<0.001). Cardiac cases were slightly taller and heavier on average, with higher mean BMI, than noncardiac trauma controls (P<0.05). There was also a significant association between obesity group and type of fatality (P=0.017), with a higher percentage of obese firefighters among the cardiac cases than the noncardiac controls. More than 93% of victims in each fatality group were white; ethnicity was not associated with type of fatality (P=0.952). On average, cardiac cases had 6 more years of service than noncardiac trauma controls (P<0.001). Job classification was associated with type of fatality (P<0.001), with cardiac cases having a higher proportion of volunteer and lower proportion of wildland firefighters compared with noncardiac trauma controls.
Table 1.
Descriptive Characteristics of Cardiac Cases and Noncardiac Trauma Controls
| Variablea | Cardiac Cases (n=276) | Noncardiac Trauma Controls (n=351) | P Value |
|---|---|---|---|
| Age, y (n=276; n=351) | 48.7±9.1 | 39.4±12.1 | <0.001 |
| Height, m (n=265; n=292) | 1.81±0.07 | 1.79±0.07 | 0.030 |
| Body mass, kg (n=261; n=288) | 104.5±22.0 | 99.8±22.3 | 0.013 |
| BMI, kg/m2 (n=260; n=285) | 31.8±5.8 | 30.8±6.1 | 0.046 |
| Normal (<25.0) | 26 (10.0) | 45 (15.8) | 0.017 |
| Overweight (25.0–29.9) | 80 (30.8) | 104 (36.5) | |
| Obese (≥30) | 154 (59.2) | 136 (47.7) | |
| Ethnicity | |||
| White | 247 (93.9) | 316 (93.2) | 0.952 |
| Black | 14 (5.3) | 20 (5.9) | |
| Other | 2 (0.8) | 3 (0.9) | |
| Years of service (n=256; n=325) | 18.9±11.5 | 12.9±10.9 | <0.001 |
| Job classification | |||
| Career | 105 (38.0) | 160 (45.6) | <0.001 |
| Volunteer | 162 (58.7) | 159 (45.3) | |
| Wildland | 9 (3.3) | 32 (9.1) | |
Values are reported as mean±SD or n (%). BMI indicates body mass index.
Sample size indicates number of records with a numeric or yes/no value for cardiac cases and noncardiac trauma controls, respectively.
Table 2 summarizes the comparison of autopsy findings between cardiac cases and noncardiac trauma controls. Heart weight was documented for all records, but LV wall thickness was not reported for 48% of noncardiac trauma controls and 33% of cardiac cases. Cardiac cases had significantly heavier hearts compared with noncardiac trauma controls (difference in mean values ≈120 g; P<0.001), with 77% of cardiac cases versus 33% of controls having cardiomegaly (defined as heart weight >450 g; P<0.001). Similarly, LV wall thickness and the prevalence of LVH (defined as LV wall thickness ≥1.2 cm) were significantly greater among cardiac cases than noncardiac trauma controls (P<0.001). Although not the cause of death, ≈60% of noncardiac trauma controls had evidence of a structurally enlarged heart, as defined by a heart weight >450 g and/or LV wall thickness ≥1.2 cm, with nearly a third of these participants meeting both criteria. All reported CHD‐related outcome measures were significantly higher among cardiac cases than noncardiac trauma controls (all P<0.001). Autopsies from the majority (78%) of cardiac cases included a measure of the degree of coronary artery stenosis, whereas <30% of autopsies from noncardiac trauma controls reported percentage of stenosis. The presence of at least 1 coronary artery with ≥75% stenosis was reported in 165 (59.8%) cardiac cases, and among these cases, 58 had double‐vessel disease, 27 had triple‐vessel disease, and 4 had quadruple‐vessel disease. Plaque rupture and intracoronary thrombus were identified only in cardiac cases, with 16% of cardiac cases having evidence of an intracoronary thrombus, definitively establishing the cause of death as myocardial infarction. More than 50% of cardiac cases had evidence of a prior myocardial infarction at autopsy compared with 6.6% of noncardiac trauma controls (P<0.001).
Table 2.
Autopsy Findings of Cardiac Cases and Noncardiac Trauma Controls
| Characteristica | Cardiac Cases | Noncardiac Trauma Controls | P Value |
|---|---|---|---|
| Structure‐related measures | |||
| Heart weight, g (n=276; n=351) | 551±125 | 430±90 | <0.001 |
| Heart weight >450 g | 213 (77.2) | 114 (32.5) | <0.001 |
| Heart weight >550 g | 120 (43.5) | 32 (9.1) | <0.001 |
| LV wall thickness, cm (n=182; n=184) | 1.7±0.4 | 1.5±0.3 | <0.001 |
| LV wall thickness ≥1.2 cm | 175 (63.4) | 161 (45.9) | <0.001 |
| LV wall thickness ≥1.4 cm | 162 (58.7) | 127 (36.2) | <0.001 |
| Heart size abnormality noted (n=275; n=351) | 181 (65.8) | 74 (21.1) | <0.001 |
| Increased wall thickness noted (n=276; n=347) | 143 (51.8) | 50 (14.4) | <0.001 |
| Valve abnormalities noted (n=274; n=351) | 40 (14.6) | 12 (3.4) | <0.001 |
| CHD‐related measures | |||
| Coronary artery with ≥50% stenosis (n=214; n=103) | 202 (73.2) | 65 (18.5) | <0.001 |
| Coronary artery with ≥75% stenosis (n=214; n=103) | 165 (59.8) | 29 (8.3) | <0.001 |
| Multivessel disease, ≥50% stenosis (n=201; n=98) | 138 (50.0) | 22 (6.3) | <0.001 |
| Multivessel disease, ≥75% stenosis (n=201; n=98) | 89 (32.3) | 12 (3.4) | <0.001 |
| Coronary artery atherosclerosis noted (n=273; n=348) | 246 (90.1) | 131 (37.6) | <0.001 |
| Calcified plaque (n=276; n=349) | 113 (40.9) | 22 (6.3) | <0.001 |
| Plaque rupture (n=276; n=351) | 13 (4.7) | 0 (0.0) | <0.001 |
| Intracoronary thrombosis (n=276; n=349) | 45 (16.3) | 0 (0.0) | <0.001 |
| Evidence of prior MI (n=276; n=351) | 143 (51.8) | 23 (6.6) | <0.001 |
Values are reported as mean±SD or n (%). CHD indicates coronary heart disease; LV, left ventricular; MI, myocardial infarction.
Sample size indicates number of records with a numeric or yes/no value for cardiac cases and noncardiac trauma controls, respectively.
The results of logistic regression modeling are summarized in Table 3. As seen in the crude models, age and obesity status are both associated with an increased risk of duty‐related cardiac death. In age‐adjusted models, all structure‐ and CHD‐related outcome measures were associated with an increased risk of duty‐related cardiac death. Odds of cardiac death were ≈5‐fold higher with cardiomegaly and 2‐fold higher with LVH, and they increased slightly with increasing severity of the structural abnormality. The presence of at least 1 coronary artery with ≥50% stenosis increased the odds of cardiac death by ≈8.5‐fold, and ≥75% stenosis increased odds of cardiac death by ≈12‐fold. Odds of cardiac death were ≈6‐fold higher with the presence of calcified plaque. Evidence of a prior myocardial infarction was associated with an 11‐fold higher risk of cardiac death. BMI was not associated with elevated odds of cardiac death after adjusting for age.
Table 3.
Logistic Regression Models
| Variable | Crude Model OR (95% CI) | Age‐Adjusted Model OR (95% CI) |
|---|---|---|
| Descriptive measures | ||
| Age, y | 1.08 (1.06–1.10) | … |
| BMI, kg/m2 | 1.029 (1.000–1.059) | 1.018 (0.987–1.050) |
| Structure‐related measures | ||
| Heart weight, g | 1.012 (1.009–1.014) | 1.010 (1.008–1.012) |
| Heart weight >450 g | 7.0 (4.9–10.1) | 5.2 (3.6–7.6) |
| Heart weight >550 g | 7.7 (5.0–11.8) | 5.4 (3.4–8.4) |
| LV wall thickness, cm | 7.8 (3.9–15.6) | 6.3 (3.2–12.6) |
| LV wall thickness ≥1.2 cm | 2.0 (1.5–2.8) | 1.9 (1.4–2.7) |
| LV wall thickness ≥1.4 cm | 2.5 (1.8–3.5) | 2.3 (1.6–3.3) |
| Heart size abnormality noted | 7.2 (5.0–10.3) | 5.7 (3.9–8.4) |
| Increased wall thickness noted | 6.4 (4.4–9.4) | 5.3 (3.5–7.9) |
| Valve abnormalities noted | 4.8 (2.5–9.4) | 3.7 (1.9–7.6) |
| CHD‐related measures | ||
| Coronary artery with ≥50% stenosis | 12.0 (8.2–17.5) | 8.6 (5.8–12.7) |
| Coronary artery with ≥75% stenosis | 16.5 (10.5–25.9) | 12.2 (7.7–19.4) |
| Multivessel disease, ≥50% stenosis | 15.0 (9.1–24.5) | 11.0 (6.6–18.2) |
| Multivessel disease, ≥75% stenosis | 13.4 (7.2–25.2) | 9.5 (5.0–18.1) |
| Coronary artery atherosclerosis noted | 15.1 (9.6–23.7) | 10.2 (6.2–16.7) |
| Calcified plaque | 10.4 (6.3–17.0) | 6.4 (3.8–10.7) |
| Evidence of prior MI | 15.3 (9.4–24.9) | 10.8 (6.5–17.7) |
BMI indicates body mass index; CHD, coronary heart disease; CI, confidence interval; LV, left ventricular; MI, myocardial infarction; OR, odds ratio.
Table 4 presents results from the multivariate logistic regression analysis after further adjustment. The presence of ≥75% stenosis was the strongest predictor of cardiac death, with odds increased ≈9‐fold. Cardiomegaly and evidence of a prior myocardial infarction were associated with approximately a 6‐fold increase in the risk of cardiac death. In addition, age was associated with an increased risk of cardiac death.
Table 4.
Multivariate Logistic Regression Model
| Characteristic | Model 1 OR (95% CI) | Model 2 OR (95% CI) |
|---|---|---|
| Age, y | 1.03 (1.01–1.05) | 1.02 (0.99–1.04) |
| BMI, kg/m2 | 0.94 (0.90–0.98) | 0.88 (0.83–0.93) |
| Heart weight >450 g | 6.07 (3.55–10.39) | ··· |
| Heart weight, g | ··· | 1.01 (1.01–1.02) |
| Coronary artery with ≥75% stenosis | 9.27 (5.33–16.10) | 9.70 (5.46–17.25) |
| Evidence of prior MI | 6.23 (3.44–11.28) | 5.25 (2.79–9.86) |
BMI indicates body mass index; CI, confidence interval; MI, myocardial infarction; OR, odds ratio.
The sensitivity analysis found that the approach of treating missing categorical values as no abnormality rather than missing led to different sample sizes and prevalences and thus different odds ratios and 95% confidence intervals; however, the findings of significantly elevated risk were comparable for both approaches and led to the same interpretation of results.
Discussion
This case–control study retrospectively examined all available autopsy records of US firefighter fatalities between 1999 and 2014 and compared the cardiac findings, chiefly coronary artery pathology, cardiac mass, and LV wall thickness, among firefighters who died of duty‐related cardiac causes with those who died of duty‐related noncardiac trauma‐related causes. The primary finding of this study was that severe CHD, cardiomegaly, and evidence of a prior myocardial infarction were strong independent predictors of duty‐related cardiac death, with odds ≈9‐fold higher, 6‐fold higher, and 6‐folder higher, respectively, in multivariate analyses. Compared with firefighters who died from trauma‐related causes, cardiac fatalities had a significantly higher prevalence of myocardial scarring, severe coronary artery stenosis, and cardiomegaly.
Although it is intuitive that cardiac‐related deaths would have greater evidence of CHD, this is the first firefighter fatality study to report on the extent of coronary artery stenosis as documented on the autopsy for both cardiac cases and controls. In individuals with CHD, cardiac death may be precipitated by plaque rupture or plaque erosion leading to thrombus formation and myocardial infarction. Although 90% of cardiac cases had evidence of atherosclerosis noted at autopsy, an intracoronary thrombus was identified at autopsy in only 16.3% of the cases. This relatively small proportion of cardiac deaths with an identified thrombus is consistent with the widely divergent frequency of 4% to 76% reported in prior studies.18, 19, 20 Calcified plaque was found in 40% of cardiac cases and conveyed a 6‐fold increased risk of duty‐related cardiac death after adjustment for age in this study. It is unlikely, however, that this increased risk was related to rupture of a vulnerable plaque and thrombus formation because few cases with calcified plaque also showed evidence of plaque rupture, and calcium likely confers stability rather than vulnerability to plaques.21 Rather, the presence of calcified plaque likely represents an increased global burden of atherosclerosis. In ≈50% of SCD victims with CHD, autopsies reveal only stable plaques and chronic changes.2, 22 In these cases, the mechanism of SCD may be ventricular arrhythmia triggered by acute ischemia or related to structural alterations secondary to chronic ischemia or prior myocardial infarction.2, 23 The majority of cardiac cases in this study had structural changes that would have predisposed them to ventricular arrhythmia, along with an absence of an intracoronary thrombus, making arrhythmia the most likely mechanism of death in most of these cases.
Severe coronary artery stenosis (≥75%) was found in 60% of cardiac cases, with more than half of these cases (32% of all cardiac cases) having severe stenosis in multiple vessels. Autopsy studies among the general population have consistently reported both severe and diffuse coronary artery stenosis among SCD victims. In fact, several studies have reported that >95% of SCD victims had at least 1 coronary artery with ≥75% stenosis, and the majority of victims had multivessel disease, with the prevalence of 3‐ or 4‐vessel disease as high as 60%.19, 24, 25, 26, 27 The lower prevalence of severe stenosis in the current study compared with the aforementioned studies may suggest that the multiple stressors associated with firefighting can trigger a sudden cardiac event in individuals with a lower overall burden of disease. The 8.6‐fold increased risk of cardiac death associated with the presence of at least 50% stenosis in univariate analyses supports this interpretation. Alternatively, the lower prevalence of severe stenosis may reflect a lack of standardization or differences in autopsy procedures rather than actual pathological differences.28 The present study used available autopsy reports from deaths that occurred across many jurisdictions within the United States, and the level of detail among reports was highly variable, whereas research studies that quantified coronary stenosis typically employed standardized autopsy procedures that involved extensive analyses that may not be routinely performed at autopsy.26, 27
Autopsy data in this study revealed a high prevalence of cardiomegaly, with 63.4% of cardiac cases having a heart weight >450 g and nearly as many (58.7%) having a heart weight >550 g. In the present study, cardiomegaly conveyed a 6‐fold increased risk of duty‐related cardiac death among firefighter fatalities after adjustment for age, BMI, severe coronary artery stenosis, and evidence of a prior myocardial infarction. In univariate analyses, LVH, defined as ventricular wall thickness ≥1.2 cm, was associated with ≈2‐fold increased risk of cardiac death. The finding of elevated risk of SCD among firefighters with increased heart mass or wall thickness is consistent with findings from a study investigating cardiac death in a cohort of young firefighters. Yang et al12 found that the odds of duty‐related SCD in firefighters aged ≤45 years increased nearly 5‐fold in the presence of cardiomegaly. In population‐based studies, LVH has been shown to be a strong independent predictor of cardiovascular mortality, including SCD,14, 15 and the risk of SCD increased with increasing LV mass.14 In addition, LVH is associated with an increased risk of ventricular arrhythmias in the absence of CHD, with a graded and continuous relationship between LV mass or wall thickness and occurrence and complexity of ventricular arrhythmia.29 Although not completely understood, mechanisms by which increased heart mass or wall thickness increase the risk of SCD have been proposed. Maladaptive changes in myocardial architecture, such as interstitial fibrosis, may alter the propagation of electrical impulses and predispose to ventricular arrhythmias, which may be provoked by factors such as acute myocardial ischemia, neuroendocrine fluctuations, electrolyte disturbances, and ventricular wall stress,30, 31 with acute myocardial ischemia proposed as having the most important proarrhythmic association with LVH.31 Firefighting involves multiple stressors, including strenuous physical work, heat stress and dehydration, and activation of the sympathetic nervous system, that may lead to ischemia, electrolyte disturbances, and ventricular wall stress, all of which could trigger a sudden cardiac event in a firefighter with cardiac enlargement.32, 33, 34, 35
Remodeling of the myocardium subsequent to an infarct is associated with an enhanced arrhythmogenic response to ischemia.4 This study found that evidence of a prior myocardial infarction conveyed approximately a 6‐fold increase in the risk of duty‐related cardiac death. Diagnosed CHD is one of the strongest predictors of SCD in both the general population7 and among firefighters;6 and, among firefighters with diagnosed CHD, those with evidence of myocardial damage were more likely to experience a fatal outcome related to a duty‐related CHD event.5 Evidence of a prior myocardial infarction was noted in ≈50% of cardiac deaths in this study, which is consistent with values of 40% to 70% reported in the literature. The enhanced arrhythmogenic response associated with this healed tissue presents another potential pathway for cardiac death in a large proportion of cases in this study.
It is widely accepted that CHD is the most common substrate underlying SCD, accounting for ≈80% of SCD in the Western world,3, 4 yet considerable evidence, including data from this study, demonstrates that increased cardiac mass or LVH, each a vulnerable substrate, often coexist with CHD.10, 11, 27 It is possible that cardiac enlargement, solely or more frequently as a synergistic substrate with CHD in a multifactorial etiology involving acute ischemia and enhanced electrical instability, is a substrate underlying SCD in a large proportion of deaths that do not have clear evidence of an acute myocardial infarction but are attributed to CHD in the absence of any other lethal disease. The potential primary causal role of cardiac enlargement in cardiac death is supported by the study finding that heart weight >450 g was associated with a considerable escalation of risk of duty‐related cardiac death (odds ratio: 6.1) beyond that attributed to CHD in multivariate models. Prior research has also demonstrated the predictive power of LVH independent of CHD using echocardiographic measurements of LV mass.36 Although these findings do not oppose an overlap in pathways leading to cardiac death for the 2 substrates, they suggest that LVH has pathways distinct from CHD. This study, however, provides specific information on only the underlying substrates among cardiac cases and does not delineate the mechanism of cardiac death.
Several factors known to be potential triggers of SCD in susceptible individuals, such as strenuous physical activity, psychological stress, and environmental pollutants, are inherent to the job of firefighting.34, 37 However, in the absence of myocardial instability in a vulnerable substrate, such as CHD or cardiac enlargement, transient acute stressors are often not sufficient to produce SCD. It is notable that SCD is the first manifestation of the disease in as much as 50% of all SCDs due to CHD.3, 38 Given the substantially higher risk of cardiac death associated with CHD and cardiac enlargement, screening measures to detect cardiac enlargement and CHD among firefighters are critical to reduce cardiac deaths in the fire service. Importantly, regression of LVH through targeted treatment has been shown to reduce the risk of cardiovascular events.39, 40, 41 Results from this study showing that cardiomegaly and LVH were fairly common among noncardiac trauma controls, who were aged <40 years on average, corroborate the findings of Yang et al12 and suggest that screening at a relatively young age may be justified for firefighters at risk of cardiomegaly.
Obesity is an established risk factor for cardiovascular disease and was recently shown to be a major driver of LV mass among firefighters,13 but in the univariate analysis, BMI was not found to be a predictor of cardiac death. There are 2 likely explanations. First, obesity conveys much of its SCD risk via CHD and cardiomegaly. Second, it is possible that obese firefighters are also at increased risk of traumatic fatalities because their body size makes them more susceptible to becoming physically trapped or because they cannot egress a building as quickly during rapidly changing fire conditions. Importantly, there was a high prevalence of obesity among both the cardiac cases and trauma controls compared with population studies in the US fire service.42, 43, 44, 45 In fact, in the multivariate analysis, obesity was associated with an increased risk of death in the trauma controls.
Although the use of all available autopsy records from duty‐related deaths of US firefighters over a 15‐year period made this study less prone to selection bias than previous firefighter fatality studies, some limitations exist. The level of detail for the cardiovascular system provided in the autopsy reports varied considerably, particularly among noncardiac trauma controls, for which a large percentage of records were missing quantitative values for LV wall thickness and/or degree of coronary stenosis. The study took the approach that if a structural abnormality or severe stenosis were evident, it would have been recorded, and that may have resulted in odds ratios and prevalence estimates that were not identical to those obtained when treating values as “missing”; however, study findings were consistent for both approaches. The use of an absolute cutoff point of 450 g rather than an indexed value to define cardiomegaly may be considered a limitation because body size affects heart weight; however, both methods are practiced among pathologists. This study relied on information available from autopsy reports, thus it is not possible to explore the effect of risk factors, such as smoking or hypertension, on measures of CHD or cardiomegaly/LVH. Future research that combines information from medical records and autopsy reports is needed to provide additional insights into duty‐related cardiac death in this occupational group.
This study found that cardiomegaly, severe coronary stenosis, and prior infarction all convey an independently increased risk of duty‐related cardiac death; therefore, the presence of these pathologic conditions carries a substantially greater risk than the presence of any condition alone. These findings indicate that screening for CHD, cardiac enlargement, and structural heart damage should be considered for firefighters. Moreover, taking actionable measures to mitigate the risk of cardiac death following identification of any of these conditions is imperative to reduce duty‐related cardiac deaths in the fire service.
Sources of Funding
Smith, Haller, Korre, Fehling, Sampani, Christophi, and Kales were supported by Federal Emergency Management Agency Assistance to Firefighters Grant EMW‐2013‐FP‐00749. Grossi Porto was supported by a scholarship from the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico–CNPq–Brazil.
Disclosures
Dr Kales reports serving as a paid expert witness, an independent medical examiner, or both in workers compensation and disability cases, including cases involving firefighters. Dr Smith reports serving as a consultant in cases involving medical evaluations and firefighter fatalities. The remaining authors have no disclosures to report.
Acknowledgements
The authors express their appreciation to Dr Thomas Rowland for providing a review of the article.
(J Am Heart Assoc. 2018;7:e009446 DOI: 10.1161/JAHA.118.009446.)
References
- 1. Fahy RF, LeBlanc PR, Molis JL. Firefighter Fatalities in the United States—2016. Quincy, MA: National Fire Protection Association; 2017. [PubMed] [Google Scholar]
- 2. Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. 2012;125:620–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myerburg RJ, Interian A Jr, Mitrani RM, Kessler KM, Castellanos A. Frequency of sudden cardiac death and profiles of risk. Am J Cardiol. 1997;80:10F–19F. [DOI] [PubMed] [Google Scholar]
- 4. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. [DOI] [PubMed] [Google Scholar]
- 5. Geibe JR, Holder J, Peeples L, Kinney AM, Burress JW, Kales SN. Predictors of on‐duty coronary events in male firefighters in the United States. Am J Cardiol. 2008;101:585–589. [DOI] [PubMed] [Google Scholar]
- 6. Kales SN, Soteriades ES, Christoudias SG, Christiani DC. Firefighters and on‐duty deaths from coronary heart disease: a case control study. Environ Health. 2003;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cupples LA, Gagnon DR, Kannel WB. Long‐ and short‐term risk of sudden coronary death. Circulation. 1992;85:I11–I18. [PubMed] [Google Scholar]
- 8. Dean JH, Gallagher PJ. Cardiac ischemia and cardiac hypertrophy. An autopsy study. Arch Pathol Lab Med. 1980;104:175–178. [PubMed] [Google Scholar]
- 9. Jimenez RA, Myerburg RJ. Sudden cardiac death. Magnitude of the problem, substrate/trigger interaction, and populations at high risk. Cardiol Clin. 1993;11:1–9. [PubMed] [Google Scholar]
- 10. Burke AP, Farb A, Liang YH, Smialek J, Virmani R. Effect of hypertension and cardiac hypertrophy on coronary artery morphology in sudden cardiac death. Circulation. 1996;94:3138–3145. [DOI] [PubMed] [Google Scholar]
- 11. Tavora F, Zhang Y, Zhang M, Li L, Ripple M, Fowler D, Burke A. Cardiomegaly is a common arrhythmogenic substrate in adult sudden cardiac deaths, and is associated with obesity. Pathology. 2012;44:187–191. [DOI] [PubMed] [Google Scholar]
- 12. Yang J, Teehan D, Farioli A, Baur DM, Smith D, Kales SN. Sudden cardiac death among firefighters ≤45 years of age in the United States. Am J Cardiol. 2013;112:1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Korre M, Porto LG, Farioli A, Yang J, Christiani DC, Christophi CA, Lombardi DA, Kovacs RJ, Mastouri R, Abbasi S, Steigner M, Moffatt S, Smith D, Kales SN. Effect of Body Mass Index on Left Ventricular Mass in Career Male Firefighters. Am J Cardiol. 2016;118:1769–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. [DOI] [PubMed] [Google Scholar]
- 15. Reinier K, Dervan C, Singh T, Uy‐Evanado A, Lai S, Gunson K, Jui J, Chugh SS. Increased left ventricular mass and decreased left ventricular systolic function have independent pathways to ventricular arrhythmogenesis in coronary artery disease. Heart Rhythm. 2011;8:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farioli A, Yang J, Teehan D, Baur DM, Smith DL, Kales SN. Duty‐related risk of sudden cardiac death among young US firefighters. Occup Med (Lond). 2014;64:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kales SN, Soteriades ES, Christophi CA, Christiani DC. Emergency duties and deaths from heart disease among firefighters in the United States. N Engl J Med. 2007;356:1207–1215. [DOI] [PubMed] [Google Scholar]
- 18. Davies MJ. Pathological view of sudden cardiac death. Br Heart J. 1981;45:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Davies MJ. Anatomic features in victims of sudden coronary death. Coronary artery pathology. Circulation. 1992;85:I19–I24. [PubMed] [Google Scholar]
- 20. Virmani R, Roberts WC. Sudden cardiac death. Hum Pathol. 1987;18:485–492. [DOI] [PubMed] [Google Scholar]
- 21. Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. [DOI] [PubMed] [Google Scholar]
- 22. Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116:1887–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345:1473–1482. [DOI] [PubMed] [Google Scholar]
- 24. Farb A, Tang AL, Burke AP, Sessums L, Liang Y, Virmani R. Sudden coronary death. Frequency of active coronary lesions, inactive coronary lesions, and myocardial infarction. Circulation. 1995;92:1701–1709. [DOI] [PubMed] [Google Scholar]
- 25. Liberthson RR, Nagel EL, Hirschman JC, Nussenfeld SR, Blackbourne BD, Davis JH. Pathophysiologic observations in prehospital ventricular fibrillation and sudden cardiac death. Circulation. 1974;49:790–798. [DOI] [PubMed] [Google Scholar]
- 26. Perper JA, Kuller LH, Cooper M. Arteriosclerosis of coronary arteries in sudden, unexpected deaths. Circulation. 1975;52:III27–III33. [PubMed] [Google Scholar]
- 27. Warnes CA, Roberts WC. Sudden coronary death: relation of amount and distribution of coronary narrowing at necropsy to previous symptoms of myocardial ischemia, left ventricular scarring and heart weight. Am J Cardiol. 1984;54:65–73. [DOI] [PubMed] [Google Scholar]
- 28. Korre M, Sampani K, Porto LGG, Farioli A, Yang J, Christiani DC, Christophi CA, Lombardi DA, Kovacs RJ, Mastouri R, Abbasi S, Steigner M, Moffatt S, Smith D, Kales SN. Cardiac enlargement in US firefighters: prevalence estimates by echocardiography, cardiac magnetic resonance and autopsies. J Clin Exp Cardiol. 2016;7:7. [Google Scholar]
- 29. Ghali JK, Kadakia S, Cooper RS, Liao YL. Impact of left ventricular hypertrophy on ventricular arrhythmias in the absence of coronary artery disease. J Am Coll Cardiol. 1991;17:1277–1282. [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee S, Bavishi C, Sardar P, Agarwal V, Krishnamoorthy P, Grodzicki T, Messerli FH. Meta‐analysis of left ventricular hypertrophy and sustained arrhythmias. Am J Cardiol. 2014;114:1049–1052. [DOI] [PubMed] [Google Scholar]
- 31. Wolk R. Arrhythmogenic mechanisms in left ventricular hypertrophy. Europace. 2000;2:216–223. [DOI] [PubMed] [Google Scholar]
- 32. Kales SN, Smith DL. Firefighting and the heart: implications for prevention. Circulation. 2017;135:1296–1299. [DOI] [PubMed] [Google Scholar]
- 33. Smith DL, Barr DA, Kales SN. Extreme sacrifice: sudden cardiac death in the US Fire Service. Extrem Physiol Med. 2013;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith DL, DeBlois JP, Kales SN, Horn GP. Cardiovascular strain of firefighting and the risk of sudden cardiac events. Exerc Sport Sci Rev. 2016;44:90–97. [DOI] [PubMed] [Google Scholar]
- 35. Hunter AL, Shah AS, Langrish JP, Raftis JB, Lucking AJ, Brittan M, Venkatasubramanian S, Stables CL, Stelzle D, Marshall J, Graveling R, Flapan AD, Newby DE, Mills NL. Fire simulation and cardiovascular health in firefighters. Circulation. 2017;135:1284–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cooper RS, Simmons BE, Castaner A, Santhanam V, Ghali J, Mar M. Left ventricular hypertrophy is associated with worse survival independent of ventricular function and number of coronary arteries severely narrowed. Am J Cardiol. 1990;65:441–445. [DOI] [PubMed] [Google Scholar]
- 37. Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Doyle JT, Kannel WB, McNamara PM, Quickenton P, Gordon T. Factors related to suddenness of death from coronary disease: combined Albany‐Framingham studies. Am J Cardiol. 1976;37:1073–1078. [DOI] [PubMed] [Google Scholar]
- 39. Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction—A LIFE review. J Electrocardiol. 2014;47:630–635. [DOI] [PubMed] [Google Scholar]
- 40. Verdecchia P, Angeli F, Borgioni C, Gattobigio R, de Simone G, Devereux RB, Porcellati C. Changes in cardiovascular risk by reduction of left ventricular mass in hypertension: a meta‐analysis. Am J Hypertens. 2003;16:895–899. [DOI] [PubMed] [Google Scholar]
- 41. Wachtell K, Okin PM, Olsen MH, Dahlof B, Devereux RB, Ibsen H, Kjeldsen SE, Lindholm LH, Nieminen MS, Thygesen K. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive therapy and reduction in sudden cardiac death: the LIFE Study. Circulation. 2007;116:700–705. [DOI] [PubMed] [Google Scholar]
- 42. Baur DM, Christophi CA, Tsismenakis AJ, Cook EF, Kales SN. Cardiorespiratory fitness predicts cardiovascular risk profiles in career firefighters. J Occup Environ Med. 2011;53:1155–1160. [DOI] [PubMed] [Google Scholar]
- 43. Durand G, Tsismenakis AJ, Jahnke SA, Baur DM, Christophi CA, Kales SN. Firefighters’ physical activity: relation to fitness and cardiovascular disease risk. Med Sci Sports Exerc. 2011;43:1752–1759. [DOI] [PubMed] [Google Scholar]
- 44. Poston WS, Haddock CK, Jahnke SA, Jitnarin N, Tuley BC, Kales SN. The prevalence of overweight, obesity, and substandard fitness in a population‐based firefighter cohort. J Occup Environ Med. 2011;53:266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Poston WSC, Jitnarin N, Haddock CK, Jahnke SA, Day RS. Accuracy of self‐reported weight, height and BMI in US firefighters. Occup Med (Lond). 2014;64:246–254. [DOI] [PubMed] [Google Scholar]


