Abstract
Background
Chronic deposits of advanced glycation end products produced by enzymatic glycation have been suggested as predictors of atherosclerotic‐related disorders. This study aimed to estimate the relationship between advanced glycation end products indicated by skin autofluorescence levels and the risk of cardiovascular and all‐cause mortality based on data from observational studies.
Methods and Results
We systematically searched Medline, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Web of Science databases from their inceptions until November 2017 for observational studies addressing the association of advanced glycation end products by skin autofluorescence levels with cardiovascular and all‐cause mortality. The DerSimonian and Laird random‐effects method was used to compute pooled estimates of hazard ratios and their respective 95% confidence intervals for the risk of cardiovascular and all‐cause mortality associated with levels of advanced glycation end products by skin autofluorescence. Ten published studies were included in the systematic review and meta‐analysis. Higher skin autofluorescence levels were significantly associated with a higher pooled risk estimate for cardiovascular mortality (hazard ratio: 2.06; 95% confidence interval, 1.58–2.67), which might not be important to moderate heterogeneity (I2=34.7%; P=0.163), and for all‐cause mortality (hazard ratio: 1.91; 95% confidence interval, 1.42–2.56) with substantial heterogeneity (I2=60.8%; P=0.0.18).
Conclusions
Our data suggest that skin autofluorescence levels could be considered predictors of all‐cause mortality and cardiovascular mortality in patients at high and very high risk.
Keywords: advanced glycation end products, cardiovascular complications, meta‐analysis, mortality, skin autofluorescence
Subject Categories: Vascular Disease, Risk Factors, Cardiovascular Disease, Biomarkers, Information Technology
Clinical Perspective
What Is New?
This systematic review and meta‐analysis synthesizes the evidence supporting higher levels of skin autofluorescence indicating advanced glycation end products as a predictor of cardiovascular and all‐cause mortality.
Our data confirm that chronic deposits of skin autofluorescence–indicated advanced glycation end products produced by enzymatic glycation in skin are predictors of cardiovascular and all‐cause mortality in patients with diabetes mellitus and cardiovascular and/or renal diseases.
What Are the Clinical Implications?
Our findings highlight that skin autofluorescence levels may be useful as a biomarker of the risk of mortality assessment in patients with diabetes mellitus and cardiovascular and renal diseases.
Advanced glycation end products (AGEs) are a group of heterogeneous cross‐link formations that result from the glycation processes involved in several pathologies including arteriosclerosis and atherosclerosis‐related disorders and particularly complications from micro‐ and macrovascular diabetes mellitus.1 Several pathophysiological pathways by which AGEs contribute to these conditions have been described, including (1) adduct formations in basement membranes that permanently change the extracellular matrix proteins, such as collagen or elastin, and (2) interaction with AGE receptors, known as receptors for advanced glycation end products (RAGEs).2 These receptors alter intracellular signaling cascades and the release of proinflammatory cytokines and, consequently, stimulate oxidative stress in various cells, such as certain neurons and endothelial or smooth muscle cells; all these alterations contribute to the pathological modifications involved in the start of vascular complications.3
From the time of onset of diabetes mellitus, and also for patients with impaired glucose tolerance, high glucose levels are present not only in plasma but also within cells (kidney and retina) and interstitial spaces where the metabolism of glucose is not insulin dependent.4, 5 These elevated glycemic levels result in increased production of AGEs, fostering the development of the vascular complications of diabetes mellitus and chronic kidney disease.6
Conflicting results have been reported from measurement of AGE levels in plasma and concern the ability of AGEs to predict cardiovascular events or cardiovascular mortality, even though most recent works support such a predictive role.7 Changes in levels of plasma RAGEs have also been associated indirectly with increased risk of cardiovascular disease (CVD),8 namely, heart failure9 and coronary heart disease.10
Although the reference standard for measuring AGE levels in tissues is biochemical determination in skin biopsies, skin autofluorescence (SAF) has emerged as a noninvasive, accurate, and practical method for the analysis of AGE levels in skin.11, 12 SAF‐indicated AGE levels are elevated in diabetes mellitus and end stage renal disease, and some studies suggest that they are associated with CVD independent of known CVD risk factors.13, 14, 15 In fact, recent reviews have pointed out a role for SAF levels as new biomarkers of CVD and diabetes mellitus complications.16, 17
To our knowledge, relatively limited evidence exists on the association of AGE levels measured by SAF with cardiovascular and all‐cause mortality, and no meta‐analysis has synthesized this relationship. This systematic review and meta‐analysis aims to estimate the relationship between SAF levels and the risk of cardiovascular and all‐cause mortality based on data from observational studies.
Methods
This study was performed using available data from published literature. The data, analytic methods, and study materials are all available on request by other researchers who want to reproduce the results or replicate the procedures.
This study was reported according to the MOOSE (Meta‐analysis of Observational Studies in Epidemiology) statements18 and followed the recommendations of the Cochrane Collaboration Handbook.19 This systematic review and meta‐analysis was registered through the International Prospective Register of Systematic Reviews (registration no. CRD42017058432).
Search Strategy
We systematically searched Medline (via PubMed), Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and the Web of Science databases from their inceptions until November 2017. Observational studies addressing the association of SAF levels with cardiovascular and all‐cause mortality were eligible. The search expressions are presented in Table S1. The literature search was complemented by screening of references included in the articles that were considered eligible for the systematic review.
Study Selection
Inclusion criteria were as follows: (1) Participants were adults with diabetes mellitus and/or cardiovascular and/or renal disease, (2) study design was longitudinal with prospective data collection, (3) exposure was SAF, and (4) outcome was cardiovascular or/and all‐cause mortality. The criteria for excluding studies were as follows: (1) Reports were not written in English or Spanish; (2) studies included individuals aged <18 years; and (3) publication types were not eligible, such as review articles, editorials, comments, guidelines, or case reports.
When >1 study provided data from the same sample, we considered only the one presenting the most detailed results or providing data for the largest sample size; however, data regarding sample characteristics could be extracted from multiple reports to obtain the most complete information.
The literature search was performed independently by 2 reviewers (I.C.‐R. and C.A.‐B.), and disagreements were solved by consensus or by involving a third researcher (V.M.‐V.).
Data Extraction and Quality Assessment
The following data were extracted from the original reports: (1) year of publication, (2) study characteristics (country and length of follow‐up), (3) sample characteristics (type of population; sample size; male sex percentage; age and body mass index distribution; current smoker status percentage; and prevalence of diabetes mellitus, hypertension, and preexisting CVD), (4) SAF levels as the predictor variable, and (5) cardiovascular or all‐cause mortality as the outcome variable. In addition, the following covariates included in the studies were extracted: levels of high‐sensitivity C‐reactive protein (hs‐CRP), systolic and diastolic blood pressure, hemoglobin, HbA1c, albumin, creatinine, total cholesterol, LDL (low‐density lipoprotein), and triglycerides.
The Quality in Prognosis Studies (QUIPS) tool20 was used to evaluate the risk of bias in 6 domains: study participation (sampling bias), study attrition (attrition bias), prognostic factor measurement, outcome measurement (ascertainment bias), study confounding, and statistical analysis and reporting. Studies were considered to have low risk of bias if they satisfied 5 or all 6 domains, moderate risk of bias if they satisfied 3 or 4 of the 6 domains, or high risk of bias if they satisfied 1 or 2 of the 6 domains.
Data extraction and quality assessment were independently performed by 2 researchers (I.C.‐R. and C.A.‐B.), and inconsistencies were resolved by consensus or by involving a third researcher (V.M.‐V.).
Statistical Analysis
The DerSimonian and Laird random‐effects21 method was used to compute pooled estimates of hazard ratios (HRs) and respective 95% confidence intervals (95% CIs) for the risk of cardiovascular and all‐cause mortality associated with SAF levels. The lowest SAF levels measured as continuous or categorical variables reported from included studies were considered as the reference level to calculate pooled HR estimates. The heterogeneity of results across studies was evaluated using the I2 statistic and was categorized as might not be important (0–40%), may represent moderate (30–60%), may represent substantial (50–90%), and considerable (75–100%) heterogeneity.18 In addition, the corresponding P values were considered.
When a study reported several statistical models, only the one including the largest number of additional covariates was considered. For each HR estimate, the lnHR was calculated by converting it to the natural log scale.
Sensitivity analyses (systematic reanalysis while removing studies one at a time) were conducted to assess the robustness of the summary estimates. Results of the sensitivity analyses were considered meaningful when the resulting estimates were modified beyond the CIs of the original summary estimate. In addition, sensitivity analyses provided insight into whether any particular study accounted for a large proportion of heterogeneity among the correlation pooled estimations, based on the change in I2 values (and associated categories reported previously).
Subgroup analyses were performed based on the type of target population used to estimate the risk of mortality associated with SAF levels (diabetes mellitus, cardiovascular, or renal patients). In addition, subgroup analyses were performed based on whether or not patients received hemodialysis. For subgroup analyses, at least 3 studies in each group were needed.
Random‐effects metaregression was used to evaluate whether results differed according to the length of follow‐up; percentage of male participants; mean age of participants; prevalence of diabetes mellitus; body mass index; percentage of current smokers; prevalence of hypertension and preexisting CVD; and levels of baseline SAF, hs‐CRP, systolic and diastolic blood pressure, hemoglobin, HbA1c, albumin, creatinine, total cholesterol, LDL and triglycerides, as these could be considered major sources of heterogeneity.22
Finally, publication bias was evaluated through visual inspection of funnel plots and by using the method proposed by Egger.23 The trim‐and‐fill computation was used to assess the effect of publication bias on the interpretation of results.24 Statistical analyses were performed using StataSE software v14 (StataCorp).
Results
Systematic Review
Of the 56 full‐text articles reviewed, only 10 studies25, 26, 27, 28, 29, 30, 31, 32, 33, 34 met the eligibility criteria (Figure 1). Seven studies25, 27, 28, 30, 33, 34 quantified the risk for all‐cause mortality, and 8 samples from 7 studies25, 26, 27, 29, 32, 34 quantified the risk for cardiovascular mortality. The studies were conducted in 4 European countries25, 26, 27, 28, 30, 31, 32, 33, 34 and 1 Asian country.29 The reports were published between 2005 and 2015 (Table 1).
Figure 1.
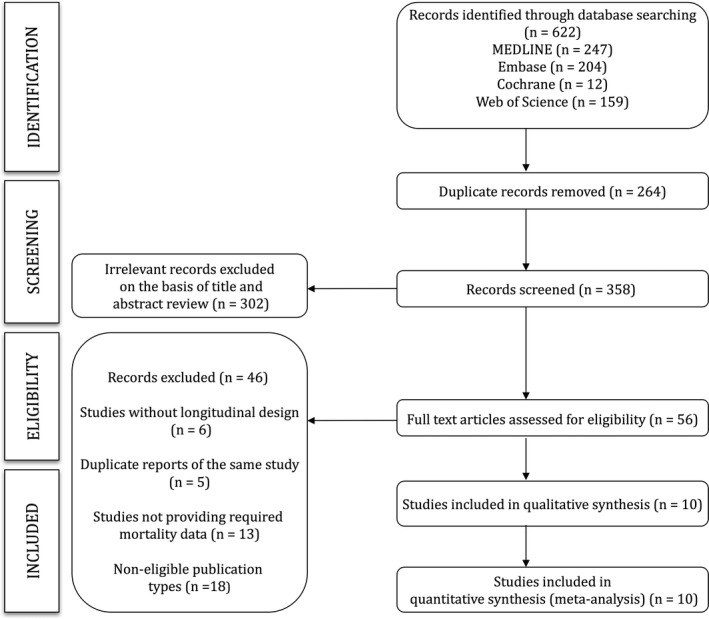
Literature search: CONSORT (Consolidated Standards of Reporting Trials) diagram.
Table 1.
Characteristics of Studies Included in the Systematic Review and Meta‐analysis
| Study | Study Characteristics | Population Characteristics | Predictor Variable | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country | Length of Follow‐up, y | Type of Target Population | n | Male Sex, n (%) | Age, y, Mean±SD | DM, n (%) | BMI, Kg/m2, Mean±SD | Current Smoker, n (%) | Hypertension, n (%) | Pre‐CVD, n (%) | SAF, AU, Mean±SD | Deaths, n (%) | |
| Arsov et al, 201325 | Macedonia | 3.0 | ESRD patients | 169 | 104 (61.0) | 56.0±13.0 | 41 (24.0) | 23.2±4.8 | 17 (10.0) | 34 (18.0) | 12 (29.0) | 3.2±0.9 |
All‐cause: 49 (28.9) Cardiovascular: 32 (18.9) |
| de Vos et al, 201426 | Netherlands | 5.1 | PAD patients | 252 | 183 (73.0) | 66.0±11.0 | 74 (29.0) | 27.0±4.0 | 127 (50.0) | 229 (91.0) | 112 (44.0) | 2.8±0.7 | Cardiovascular: 62 (24.6) |
| Fraser et al, 201427 | UK | 3.6 | Stage 3 CKD patients | 1707 | 671 (39.3) | 73.0±9.0 | 284 (16.6) | 29.0±5.1 | 79 (4.6) | 1495 (87.6) | 580 (34.0) | 2.7±0.7 |
All‐cause: 170 (10.0) Cardiovascular: 69 (4.0) |
| Gerrits et al, 201228 | Netherlands | 4.9 | ESRD patients | 105 | 68 (64.8) | 65.1±14.6 | 23 (22.0) | 24.9±5.1 | 15 (14.0) | N/A | 53 (50.0) | 3.2±0.9 |
All‐cause: 69 (65.7) Cardiovascular: 34 (32.4) |
| Kimura et al, 201429 | Japan | 6.0 | ESRD patients | 128 | 59 (46.1) | 65.1±11.6 | 44 (34.3) | 22.1±3.3 | N/A | N/A | 39 (30.5) | 2.3±0.9 |
All‐cause: 42 (32.8) Cardiovascular:19 (14.8) |
| Lutgers et al, 200930 | Netherlands | 3.0 | Type 2 DM patients | 967 | 454 (47.0) | 66.0±11.0 | 967 (100.0) | 29.0±5.0 | 184 (19.0) | N/A | 377 (39.0) | 2.8±0.8 |
All‐cause: 86 (8.9) Cardiovascular:44 (4.6) |
| Meerwaldt et al, 200531 | Netherlands | 3.0 | ESRD patients | 109 | 68 (62.4) | 57.0±16.0 | 23 (21.0) | 23.9±3.4 | N/A | 41 (37.6) | N/A | 2.4±0.7 |
All‐cause: 42 (38.5) Cardiovascular: 25 (22.9) |
| Meerwaldt et al, 200732 | Netherlands | 5.0 | Type 2 DM patients | 69 | 45 (65.0) | 61.0±13.0 | 69 (100.0) | 24.4±1.2 | 10 (14.5) | 33 (47.8) | N/A | 2.1±0.3 | Cardiovascular: 23 (33.3) |
| Type 1 DM patients | 48 | 23 (48.0) | 45.0±15.0 | 48 (100.0) | 22.8±1.6 | 11 (22.9) | 15 (31.2) | N/A | 1.6±0.5 | Cardiovascular: 11 (22.9) | |||
| Nongnuch and Davenport, 201533 | UK | 2.5 | Stage 5 CKD patients | 332 | 213 (64.2) | 67.5±18.2 | 139 (41.9) | N/A | 123 (37.0) | 205 (61.7) | 107 (32.2) | 3.3±0.9 | All‐cause: 74 (22.3) |
| Siriopol et al, 201534 | Romania | 2.5 | ESRD patients | 304 | 135 (44.4) | 56.7±14.4 | 56 (18.4) | N/A | 47 (15.5) | 273 (89.8) | 104 (34.2) | 3.6±0.8 |
All‐cause: 57 (18.8) Cardiovascular: 24 (7.9) |
AU indicates arbitrary units; BMI, body mass index; CKD, chronic kidney disease; DM, diabetes mellitus; ESRD, end‐stage renal disease; N/A, not available; PAD, peripheral artery disease; pre‐CVD, preexisting cardiovascular disease; SAF, skin autofluorescence (indicating advanced glycation end products).
The age of the included participants ranged between 45.0 and 67.5 years, with sample sizes ranging from 48 to 1707 participants. The studies included patients with renal disease,25, 27, 28, 29, 31, 33, 34 peripheral artery disease,26 and diabetes mellitus.30, 32 The percentage of male participants ranged from 39.3% to 73.0%, and the percentage of current smokers ranged from 10.0% to 50.0%. Most studies included a normal weight population,25, 28, 29, 31, 32 and only 3 included an overweight population.26, 27, 31 The prevalence of hypertension ranged from 18.0% to 91.0%, and the prevalence of preexisting CVD ranged from 29.0% to 50.0%.
Studies that included patients with diseases other than diabetes mellitus (ie, focused on renal and peripheral arterial disease patients) showed a prevalence of diabetes mellitus that ranged from 16.6% to 41.9%. Only 2 studies focused on patients with diabetes mellitus specified the type of diabetes mellitus.30, 32 On baseline measurements, SAF levels ranged from 1.6 to 3.6 arbitrary units.32, 34 All studies estimated SAF using an AGE Reader device (DiagnOptics). The biochemical (hs‐CRP, hemoglobin, HbA1c, albumin, creatinine, total cholesterol, LDL, and triglycerides) and vascular (systolic and diastolic blood pressure) covariates of the included studies are shown in Table 2. Most studies reported models adjusted for several covariates (Table S2).
Table 2.
Covariates of Studies Included in the Systematic Review and Meta‐analysis
| Study | hs‐CRP, mg/L, Mean±SD | SBP, mm Hg, Mean±SD | DBP, mm Hg, Mean±SD | Hemoglobin, g/dL, Mean±SD | HbA1c, %, Mean±SD | Albumin, g/L, Mean±SD | Creatinine, mg/dL, Mean±SD | TC, mg/dL, Mean±SD | LDL‐C, mg/dL, Mean±SD | Triglycerides, mg/dL, Mean±SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Arsov et al, 201325 | 12.6±42.0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| de Vos et al, 201426 | N/A | 140.0±125.5 | 80±60.7 | N/A | 6.8±4.4 | N/A | N/A | N/A | N/A | N/A |
| Fraser et al, 201427 | N/A | 134. 0±18.0 | 72. 0±11.0 | 13.2±125.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Gerrits et al, 201228 | N/A | 146. 0±24.0 | 65. 0±20.0 | 12.6±1.3 | N/A | 38.0±5.0 | 9.9±2.7 | 146.9±34.8 | N/A | N/A |
| Kimura et al, 201429 | 1.4±1.7 | N/A | N/A | 10.1±1.6 | N/A | 37.0±0.4 | 10.4±3.4 | 148.3±N/A | 81.2±26.6 | 108.2±61.2 |
| Lutgers et al, 200930 | N/A | 146±±20.0 | 81±10.0 | N/A | 7.0±1.3 | N/A | 1.1±0.2 | 201.1±38.7 | 112.1±34.8 | 203.7±115.1 |
| Meerwaldt et al, 200531 | 4.8±5.6 | N/A | N/A | N/A | N/A | 38.3±2.6 | 11.1±2.7 | 204.9±N/A | 123.7±30.9 | 212.6±79.7 |
| Meerwaldt et al, 200732 | ||||||||||
| Type 2 DM patients | N/A | N/A | N/A | N/A | 8.2±0.9 | N/A | 1.0±0.2 | 196.1±N/A | 123.7±38.7 | 168.3±70.8 |
| Type 1 DM patients | N/A | N/A | N/A | N/A | 7.9±1.0 | N/A | 1.0±0.1 | 203.3±N/A | 123.7±27.1 | 146.1±70.8 |
| Nongnuch and Davenport, 201533 | 15.1±27.2 | N/A | N/A | 10.9±1.4 | 11.7±15.0 | 38.9±4.9 | N/A | 158.5±42.5 | N/A | N/A |
| Siriopol et al, 201534 | 4.3±36.5 | N/A | N/A | 11.3±1.6 | N/A | 47.6±64.0 | N/A | 185.0±275.6 | N/A | 160.0±449.1 |
DBP indicates diastolic blood pressure; DM, diabetes mellitus; HbA1c, glycated hemoglobin A1c; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; N/A, not available; SBP, systolic blood pressure; TC, total cholesterol.
Study Quality
As assessed by the QUIPS tool (Table S3), 70% of the studies had a low risk of bias and 30% had a high risk of bias. The study attrition domain showed a high risk of bias in 90% of the studies. Conversely, 100% of the studies showed a low risk of bias in the statistical analysis and reporting domain, and no study scored a high risk of bias in the study participation, statistical analysis and reporting, and study confounding domains.
Meta‐analyses
Higher SAF levels were significantly associated with higher pooled risk estimates for cardiovascular mortality (HR: 2.06; 95% CI, 1.58–2.67) and all‐cause mortality (HR: 1.91; 95% CI, 1.42–2.56). Heterogeneity in the HR estimates was not important to moderate for the risk of cardiovascular mortality (I2=34.7%; P=0.163) and substantial for the risk of all‐cause mortality (I2=60.8%; P=0.0.18; Figure 2).
Figure 2.
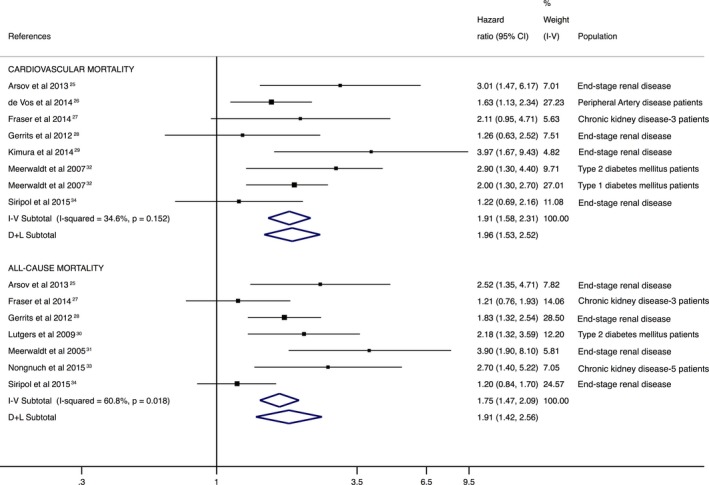
Forest plot including pooled hazard ratios for cardiovascular and all‐cause mortality using skin autofluorescence–indicated advanced glycation end products as predictors. CI indicates confidence interval; D+L, DerSimonian and Laird; I‐V, inverse variance.
Sensitivity Analysis
The pooled HR estimate was not significantly modified in magnitude or direction when individual study data were removed from the analysis one at a time (eg, HRs of 1.86–2.08 for cardiovascular mortality and 1.74–2.09 for all‐cause mortality). Heterogeneity was modified from not important to moderate after removing data from Meerwaldt et al (I2=43.5%)32 and Fraser et al (I2=43.6%)27 for cardiovascular mortality. In addition, heterogeneity was modified from substantial to moderate only after removing data from Siriopol et al (I2=47.0%).34
Subgroup Analysis and Metaregression
When analyses were performed based on the type of target population, there were only enough studies to perform an analysis in renal patients. The risk of mortality associated with higher SAF levels in renal patients was 2.23 (95% CI, 1.31–3.82; I2=54.0%) for cardiovascular mortality and 1.88 (95% CI, 1.34–2.64; I2=65.4%) for all‐cause‐mortality (Figure 3).
Figure 3.
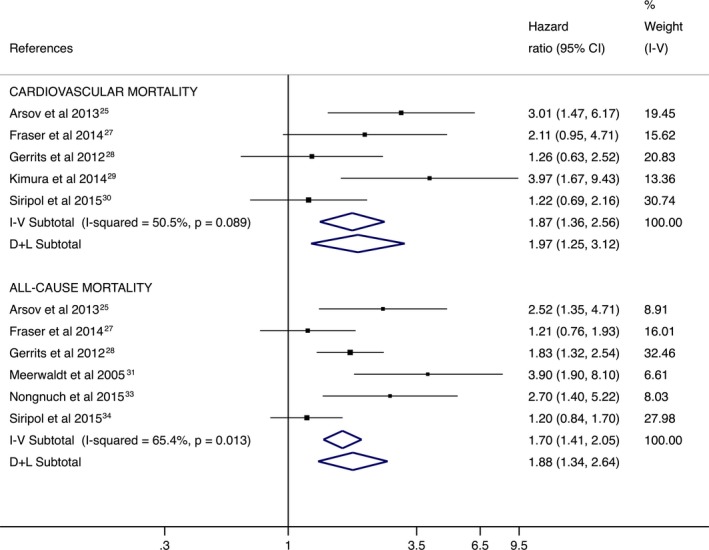
Forest plot including the pooled hazard ratios for cardiovascular and all‐cause mortality in renal patients using skin autofluorescence–indicated advanced glycation end products as predictors. CI indicates confidence interval; D+L, DerSimonian and Laird; I‐V, inverse variance.
For hemodialysis treatment status analysis, the risk of cardiovascular mortality associated with higher SAF levels was 1.97 (95% CI, 1.11–3.49; I2=62.4%) for hemodialysis patients and 1.95 (95% CI, 1.56–2.45; I2=0.0%) in nonhemodialysis patients. Nevertheless, for the risk of all‐cause mortality, there were only enough studies to perform an analysis in hemodialysis patients (HR: 2.08; 95% CI, 1.41–3.06; Figure 4).
Figure 4.
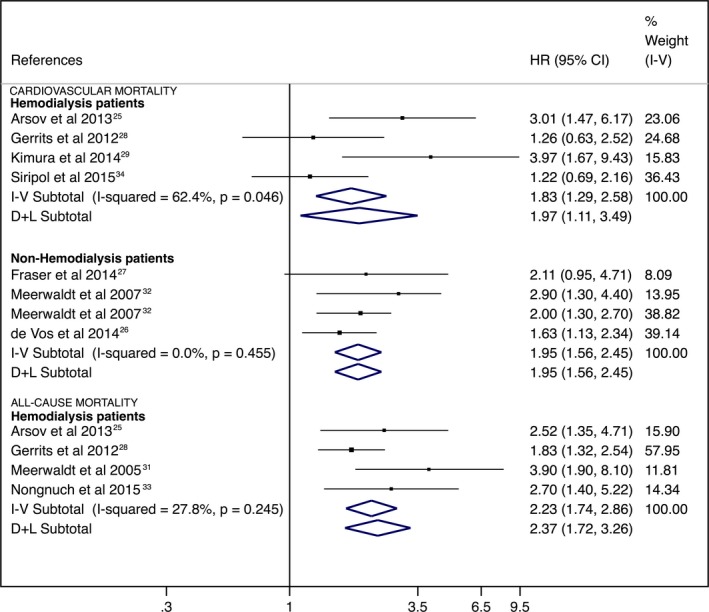
Forest plot including the pooled hazard ratios for cardiovascular and all‐cause mortality in hemodialysis and nonhemodialysis patients using skin autofluorescence–indicated advanced glycation end products as predictors. CI indicates confidence interval; D+L, DerSimonian and Laird; HR, hazard ratio; I‐V, inverse variance.
The random‐effects metaregression model showed that no covariate was related to the pooled HR estimates (Table S4). For cardiovascular mortality, heterogeneity was modified from not important to moderate when metaregression models were based on levels of albumin (I2=42.6%), total cholesterol (I2=44.2%), and triglycerides (I2=59.7%) and from not important to substantial when models were based on hs‐CRP levels (I2=61.8%). Furthermore, for all‐cause mortality, heterogeneity was modified from substantial to not important when metaregression models were based on percentage of male participants (I2=1.4%), body mass index (I2=21.8%), hypertension prevalence (I2=0.0%), and levels of hs‐CRP (I2=26.7%), systolic blood pressure (I2=0.0%), albumin (I2=16.8%), and triglycerides (I2=0.0%). heterogeneity for all‐cause mortality was modified from substantial to moderate when metaregression models were based on length of follow‐up (I2=49.4%), diabetes mellitus prevalence (I2=36.4%), percentage of current smokers (I2=38.5%), preexisting CVD prevalence (I2=38.8%), baseline SAF levels (I2=48.3%), and creatinine levels (I2=41.4%).
Publication Bias
Evidence of publication bias was found by funnel plot asymmetry and the Egger test for the cardiovascular mortality estimate (P=0.056) and for the all‐cause mortality estimate (P=0.007; Figure S1). Moreover, trim‐and‐fill computation showed that 3 studies were needed to remove publication bias from both cardiovascular mortality (P=0.909) and all‐cause mortality (P=0.698; Figure S2).
Discussion
This systematic review and meta‐analysis provides a synthesis of the evidence suggesting that higher SAF levels could be a predictor of cardiovascular and all‐cause mortality. Our data confirm the association between chronic deposits of AGEs produced by enzymatic glycation in skin and mortality, suggesting that SAF may be used as a tissue biomarker of cardiovascular and all‐cause mortality risk in patients with diabetes mellitus, CVD, and renal disease.
Previous studies in which AGE levels were estimated in plasma have reported ambiguous results regarding the association between AGE levels and mortality.35, 36 Conversely, the results of this meta‐analysis show that SAF is consistently associated with mortality. This may be due to the levels of AGEs in long‐lived proteins placed in skin over time, which is a chronic indicator of AGE levels,37 whereas in blood plasma, we can find AGEs produced in a short period of time, which may not be directly related to chronic outcomes.
The crucial role of chronic hyperglycemia in the development and progression of atherosclerosis and CVD events in patients with diabetes mellitus or renal disease is not debatable.38, 39, 40 However, the identification of AGEs in the atherosclerotic plaques of nondiabetic patients with coronary artery disease have magnified the importance of AGEs and oxidative stress in accelerating atherosclerosis.41 AGEs from long‐lived proteins such as collagens42 predict future progression of microvascular disease, progression of carotid intima‐media thickness, left ventricular mass, and the severity of coronary artery calcium.43 Accordingly, the results of our study demonstrate that levels of SAF are associated with cardiovascular events and cardiovascular mortality. Moreover, the homogeneity in the results of the longitudinal studies included in this meta‐analysis argues in favor of the validity of our findings.
Three pathophysiological mechanisms are known by which AGEs may cause cardiovascular events and cardiovascular mortality: (1) AGEs can affect the physiological properties of cardiac proteins in the extracellular matrix by creating cross‐links, and excessive cross‐linking caused by the accumulation of AGEs weakens the flexibility of the matrix proteins and produces stiffness in vascular walls44; (2) they can affect vascular function by influencing both endothelial function and vascular compliance, which occurs because AGEs reduce nitric oxide vasodilator levels45 and favor the production of a potent vasoconstrictor, endothelin‐146; and (3) they can cause multiple vascular and myocardial changes through interaction with RAGEs. RAGEs mediate the induction of fibrosis through the increase of TGF‐β (transforming growth factor β)47 and influence calcium metabolism in cardiac myocytes.48 In addition, RAGE interaction may induce atherosclerosis, thrombosis, and vasoconstriction.49
Some limitations of this study that could compromise our results should be stated. First, the reasons for death included in the definition of all‐cause mortality could vary across the studies included; therefore, misclassification bias could potentially affect our estimates of the associations between SAF levels and the risk of mortality. Second, there was evidence of significant publication bias with the Egger test, and results from studies that are not published could have modified the results of our meta‐analysis. Traditionally, publication bias is thought of as the editorial tendency to publish significant results to the detriment of research reporting a nonsignificant relationship between the variables being analyzed. Moreover, in this study, we cannot neglect that SAF measurements have not been included as a cardiovascular risk factor until recently, and this can be a source of publication bias publication. To better understand the effect of publication bias on the interpretation of results, the trim‐and‐fill computation was used. Finally, although we extracted the most fully adjusted risk estimates, our results might still be threatened by residual confounding. Conversely, this meta‐analysis has several strengths: Two searches were performed across several electronic databases to ensure that all suitable studies were identified. Regarding SAF measurements, all included studies used the same device—the AGE Reader—to perform measurements.
Conclusions
In summary, our data support that SAF levels could be considered predictors of all‐cause mortality and cardiovascular mortality in patients at high and very high risk. Although the appropriate use of our results should be understood in each particular clinical context, our data suggest that clinicians should consider the level of SAF when they assess the risk of cardiovascular and all‐cause mortality. Notwithstanding, our data highlighted the need for more research to establish an optimal level of SAF in different populations to evaluate the appropriateness of including this biomarker as a routine assessment for cardiovascular risk in the usual clinical practice of patients at different CVD risk levels.
Future Perspectives
It would be of particular interest to study the clinical performance of SAF for predicting cardiovascular events and mortality risk in patients at moderate risk and in the general population. Furthermore, it would be important to understand the increased value of using SAF levels as predictors of CVD above and beyond the risk estimates that are already in use in clinical practice.50, 51, 52, 53 The definitive establishment of SAF as a biomarker of CVD and mortality will be well established once all the conventional consensus criteria are fulfilled.54, 55
Sources of Funding
Cavero‐Redondo and Martínez‐Hortelano are supported by a grant from the Universidad de Castilla‐La Mancha (FPU13/01582 and PREDUCLM16/14, respectively). Soriano‐Cano is supported by a grant from Spanish Ministry of Economy, Industry, and Competitiveness (Fi 17/332). Garrido‐Miguel and Berlanga‐Macías are supported by a grant from the Spanish Ministry of Education, Culture, and Sport (FPU15/03847 and FPU16/02380, respectively).
Disclosures
None.
Supporting information
Table S1. Search Strategy for Medline
Table S2. Covariates Used for Adjusting the Data Reported by the Included Studies
Table S3. Study Quality Assessed by the Quality in Prognosis Studies (QUIPS) Tool
Table S4. Random Effect Metaregression Model
Figure S1. Assessment of potential publication bias by the Egger test.
Figure S2. Assessment of potential publication bias by the Egger test after trim and fill.
Acknowledgments
The support of the Iberian Network on Arterial Structure, Central Hemodynamics, and Cognition is gratefully acknowledged.
(J Am Heart Assoc. 2018;7:e009833 DOI: 10.1161/JAHA.118.009833.)
References
- 1. Yamagishi SI, Nakamura K, Imaizumi T. Advanced glycation end products (AGEs) and diabetic vascular complications. Curr Diabetes Rev. 2005;1:93–106. [DOI] [PubMed] [Google Scholar]
- 2. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end‐products: a review. Diabetologia. 2001;44:129–146. [DOI] [PubMed] [Google Scholar]
- 3. Takeuchi M, Takino JI, Yamagishi SI. Involvement of the toxic AGEs (TAGE)‐RAGE system in the pathogenesis of diabetic vascular complications: a novel therapeutic strategy. Curr Drug Targets. 2010;11:1468–1482. [DOI] [PubMed] [Google Scholar]
- 4. Jansson PA, Fowelin JP, von Schenck HP, Smith UP, Lönnroth PN. Measurement by microdialysis of the insulin concentration in subcutaneous interstitial fluid. Importance of the endothelial barrier for insulin. Diabetes. 1993;42:1469–1473. [DOI] [PubMed] [Google Scholar]
- 5. Castillo C, Bogardus C, Bergman R, Thuillez P, Lillioja S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J Clin Invest. 1994;93:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. [DOI] [PubMed] [Google Scholar]
- 7. Hanssen NMJ, Scheijen JLJM, Jorsal A, Parving HH, Tarnow L, Rossing P, Stehouwer CDA, Schalkwijk CG. Higher plasma methylglyoxal levels are associated with incident cardiovascular disease in individuals with type 1 diabetes: a 12‐year follow‐up study. Diabetes. 2017;66:2278–2283. [DOI] [PubMed] [Google Scholar]
- 8. Nin JW, Jorsal A, Ferreira I, Ferreira I, Schalkwijk CG, Prins MH, Parving HH, Tarnow L, Rossing P, Stehouwer CDA. Higher plasma soluble receptor for advanced glycation end products (sRAGE) levels are associated with incident cardiovascular disease and all‐cause mortality in type 1 diabetes: a 12‐year follow‐up study. Diabetes. 2010;59:2027–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lazo M, Halushka MK, Shen L, Maruthur N, Rebholz CM, Rawlings AM, Hoogeveen RC, Brinkley TE, Ballantyne CM, Astor BC, Selvin E. Soluble receptor for advanced glycation end products and the risk for incident heart failure: the Atherosclerosis Risk in Communities Study. Am Heart J. 2015;170:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Falcone C, Bozzini S, D'Angelo A, Matrone B, Colonna A, Benzi A, Paganini EM, Falcone R, Pelissero G. Plasma levels of soluble receptor for advanced glycation end products and coronary atherosclerosis: possible correlation with clinical presentation. Dis Markers. 2013;35:135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Meerwaldt R, Links T, Graaff R, Thorpe SR, Baynes JW, Hartog J, Gans R, Smit A. Simple noninvasive measurement of skin autofluorescence. Ann N Y Acad Sci. 2005;1043:290–298. [DOI] [PubMed] [Google Scholar]
- 12. Januszewski AS, Sachithanandan N, Karschimkus C, O′Neal DN, Yeung CK, Alkatib N, Jenkins AJ. Non‐invasive measures of tissue autofluorescence are increased in type 1 diabetes complications and correlate with a non‐invasive measure of vascular dysfunction. Diabet Med. 2012;29:726–733. [DOI] [PubMed] [Google Scholar]
- 13. Mulder DJ, van Haelst PL, Graaff R, Gans RO, Zijlstra F, Smit AJ. Skin autofluorescence is elevated in acute myocardial infarction and is associated with the one‐year incidence of major adverse cardiac events. Neth Heart J. 2009;17:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noordzij MJ, Lefrandt JD, Loeffen EA, Saleem BR, Meerwaldt R, Lutgers HL, Smit AJ, Zeebregts CJ. Skin autofluorescence is increased in patients with carotid artery stenosis and peripheral artery disease. Int J Cardiovasc Imaging. 2012;28:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Vos LC, Noordzij MJ, Mulder DJ, Smit AJ, Lutgers HL, Dullaart RP, Kamphuisen PW, Zeebregts CJ, Lefrant JD. Skin autofluorescence as a measure of advanced glycation end product deposition is elevated in peripheral artery disease. Arterioscler Thromb Vasc Biol. 2013;33:131–138. [DOI] [PubMed] [Google Scholar]
- 16. Yamagishi S, Fukami K, Matsui T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: a novel marker of vascular complications in high‐risk patients for cardiovascular disease. Int J Cardiol. 2015;185:263–268. [DOI] [PubMed] [Google Scholar]
- 17. Bos DC, de Ranitz‐Greven WL, de Valk HW. Advanced glycation end products, measured as skin autofluorescence and diabetes complications: a systematic review. Diabetes Technol Ther. 2011;13:773–779. [DOI] [PubMed] [Google Scholar]
- 18. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) Group . Meta‐analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JPT, Green S. Selecting studies and collecting data Cochrane Handbook of Systematic Reviews of Interventions, Version 5.1.0. Cochrane Collaboration; 2011. Available at: http://www.cochrane-handbook.org. (updated Mar 2011). Accessed February 28, 2018. [Google Scholar]
- 20. Hayden JA, van der Windt DA, Cartwright JL, Côte P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158:280–286. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Kacker R. Random‐effects model for meta‐analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. [DOI] [PubMed] [Google Scholar]
- 22. Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. [DOI] [PubMed] [Google Scholar]
- 23. Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta‐analysis. BMJ. 2001;323:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 25. Arsov S, Trajceska L, Oeveren W, Smit AJ, Dzekova P, Stegmayr B, Sikole A, Rakhorst G, Graaff R. Increase in skin autofluorescence and release of heart‐type fatty acid binding protein in plasma predicts mortality of hemodialysis patients. Artif Organs. 2013;37:e114–e122. [DOI] [PubMed] [Google Scholar]
- 26. de Vos LC, Mulder DJ, Smit AJ, Dullaart RP, Kleefstra N, Lijfering WM, Kamphuisen PW, Zeebregts CJ, Lefrandt JD. Skin autofluorescence is associated with 5‐year mortality and cardiovascular events in patients with peripheral artery disease. Arterioscler Thromb Vasc Biol. 2014;34:933–938. [DOI] [PubMed] [Google Scholar]
- 27. Fraser SD, Roderick PJ, McIntyre NJ, Harris S, McIntyre CW, Fluck RJ, Taal MW. Skin autofluorescence and all‐cause mortality in stage 3 CKD. Clin J Am Soc Nephrol. 2014;9:1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gerrits EG, Lutgers HL, Smeets GH, Groenier KH, Smit AJ, Gans RO, Bilo HJ. Skin autofluorescence: a pronounced marker of mortality in hemodialysis patients. Nephron Extra. 2012;2:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kimura H, Tanaka K, Kanno M, Watanabe K, Hayashi Y, Asahi K, Suzuki H, Sato K, Sakaue M, Terawaki H, Nakayama M, Miyata T, Watanabe T. Skin autofluorescence predicts cardiovascular mortality in patients on chronic hemodialysis. Ther Apher Dial. 2014;18:461–467. [DOI] [PubMed] [Google Scholar]
- 30. Lutgers HL, Gerrits EG, Graaff R, Links TP, Sluiter WJ, Gans RO, Bilo HJ, Smit AJ. Skin autofluorescence provides additional information to the UK Prospective Diabetes Study (UKPDS) risk score for the estimation of cardiovascular prognosis in type 2 diabetes mellitus. Diabetologia. 2009;52:789–797. [DOI] [PubMed] [Google Scholar]
- 31. Meerwaldt R, Hartog JW, Graaff R, Huisman RJ, Links TP, den Hollander NC, Thorpe SR, Baynes JW, Navis G, Gans RO, Smit AJ. Skin autofluorescence, a measure of cumulative metabolic stress and advanced glycation end products, predicts mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3687–3693. [DOI] [PubMed] [Google Scholar]
- 32. Meerwaldt R, Lutgers HL, Links TP, Graaff R, Baynes JW, Gans RO, Smit AJ. Skin autofluorescence is a strong predictor of cardiac mortality in diabetes. Diabetes Care. 2007;30:107–112. [DOI] [PubMed] [Google Scholar]
- 33. Nongnuch A, Davenport A. Skin autofluorescence advanced glycosylation end products as an independent predictor of mortality in high flux haemodialysis and haemodialysis patients. Nephrology. 2015;20:862–867. [DOI] [PubMed] [Google Scholar]
- 34. Siriopol D, Hogas S, Veisa G, Mititiuc I, Volovat C, Apetrii M, Onofriescu M, Busila I, Oleniuc M, Covic A. Tissue advanced glycation end products (AGEs), measured by skin autofluorescence, predict mortality in peritoneal dialysis. Int Urol Nephrol. 2015;47:563–569. [DOI] [PubMed] [Google Scholar]
- 35. Schwedler SB, Metzger T, Schinzel R, Wanner C. Advanced glycation end products and mortality in hemodialysis patients. Kidney Int. 2002;62:301–310. [DOI] [PubMed] [Google Scholar]
- 36. Roberts MA, Thomas MC, Fernando D, Macmillan N, Power DA, Ierino FL. Low molecular weight advanced glycation end products predict mortality in asymptomatic patients receiving chronic haemodialysis. Nephrol Dial Transplant. 2006;21:1611–1617. [DOI] [PubMed] [Google Scholar]
- 37. Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type I diabetes mellitus and collagen‐linked fluorescence. N Engl J Med. 1986;314:403–408. [DOI] [PubMed] [Google Scholar]
- 38. Arici M, Walls J. End‐stage renal disease, atherosclerosis, and cardiovascular mortality: is C‐reactive protein the missing link? Kidney Int. 2001;59:407–414. [DOI] [PubMed] [Google Scholar]
- 39. Chen SC, Tseng CH. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud. 2013;10:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Aronson D, Rayfield EJ. How hyperglycemia promotes atherosclerosis: molecular mechanisms. Cardiovasc Diabetol. 2002;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanauchi M, Tsujimoto N, Hashimoto T. Advanced glycation end products in nondiabetic patients with coronary artery disease. Diabetes Care. 2001;24:1620–1623. [DOI] [PubMed] [Google Scholar]
- 42. Nishizawa Y, Koyama H, Inaba M. AGEs and cardiovascular diseases in patients with end‐stage renal diseases. J Ren Nutr. 2012;22:128–133. [DOI] [PubMed] [Google Scholar]
- 43. Araszkiewicz A, Naskret D, Zozulinska‐Ziolkiewicz D, Pilacinski S, Uruska A, Grzelka A, Wegner M, Wierusz‐Wysocka B. Skin autofluorescence is associated with carotid intima‐media thickness, diabetic microangiopathy, and long‐lasting metabolic control in type 1 diabetic patients. Results from Poznan Prospective Study. Microvasc Res. 2015;98:62–67. [DOI] [PubMed] [Google Scholar]
- 44. Smit AJ, Lutgers HL. The clinical relevance of advanced glycation endproducts (AGE) and recent developments in pharmaceutics to reduce AGE accumulation. Curr Med Chem. 2004;11:2767–2784. [DOI] [PubMed] [Google Scholar]
- 45. Sanders DB, Kelley T, Larson D. The role of nitric oxide synthase/nitric oxide in vascular smooth muscle control. Perfusion. 2000;15:97–104. [DOI] [PubMed] [Google Scholar]
- 46. Quehenberger P, Bierhaus A, Fasching P, Muellner C, Klevesath M, Hong M, Stier G, Schleicher E, Speiser W, Nawroth PP. Endothelin 1 transcription is controlled by nuclear factor‐kappaB in AGE‐stimulated cultured endothelial cells. Diabetes. 2000;49:1561–1570. [DOI] [PubMed] [Google Scholar]
- 47. Striker LJ, Striker GE. Administration of AGEs in vivo induces extracellular matrix gene expression. Nephrol Dial Transplant. 1996;11:62–65. [DOI] [PubMed] [Google Scholar]
- 48. Petrova R, Yamamoto Y, Muraki K, Yonekura H, Sakurai S, Watanabe T, Li H, Takeuchi M, Makita Z, Kato I, Takasawa S, Okamoto H, Imaizumi Y, Yamamoto H. Advanced glycation endproduct‐induced calcium handling impairment in mouse cardiac myocytes. J Mol Cell Cardiol. 2002;34:1425–1431. [DOI] [PubMed] [Google Scholar]
- 49. Bucala R, Makita Z, Vega G, Grundy S, Koschinsky T, Cerami A, Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci USA. 1994;91:9441–9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sehestedt T, Jeppesen J, Hansen TW, Rasmussen S, Wachtell K, Ibsen H, Torp‐Pedersen C, Olsen MH. Thresholds for pulse wave velocity, urine albumin creatinine ratio and left ventricular mass index using SCORE, Framingham and ESH/ESC risk charts. J Hypertens. 2012;30:1928–1936. [DOI] [PubMed] [Google Scholar]
- 51. Sehestedt T, Jeppesen J, Hansen TW, Watchtell K, Ibsen H, Torp‐Pedersen C, Hildebrandt P, Olsen MH. Risk prediction is improved by adding markers of subclinical organ damage to SCORE. Eur Heart J. 2010;31:883–891. [DOI] [PubMed] [Google Scholar]
- 52. Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njøstad I, Oganov RG, Thomsen T, Tunstall‐Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten‐year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 53. D'Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 54. Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, Go AS, Harrell FE Jr, Hong Y, Howard BV, Howard VJ, Hsue PY, Kramer CM, McConnell JP, Normand SL, O'Donnell CJ, Smith SC Jr, Wilson PW. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhalidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt‐Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O'Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search Strategy for Medline
Table S2. Covariates Used for Adjusting the Data Reported by the Included Studies
Table S3. Study Quality Assessed by the Quality in Prognosis Studies (QUIPS) Tool
Table S4. Random Effect Metaregression Model
Figure S1. Assessment of potential publication bias by the Egger test.
Figure S2. Assessment of potential publication bias by the Egger test after trim and fill.


