Abstract
Background
Blood pressure is determined by the interactions between the heart and arterial properties, and subjects with identical blood pressure may have substantially different hemodynamic determinants. Whether arterial hemodynamic indices quantified by impedance cardiography (ICG), a simple operator‐independent office procedure, independently predict all‐cause mortality in adults from the general population, and specifically among those who do not meet criteria for American College of Cardiology/American Heart Association stage 2 hypertension, is currently unknown.
Methods and Results
We studied 1639 adults aged 18 to 80 years from the general population. We used ICG to measure hemodynamic parameters and metrics of cardiac function. We assessed the relationship between hemodynamic parameters measured at baseline and all‐cause mortality over a mean follow‐up of 10.9 years. Several ICG parameters predicted death. The strongest predictors were total arterial compliance index (standardized hazard ratio=0.38; 95% confidence interval=0.31–0.46; P<0.0001) and indices of cardiac contractility: velocity index (standardized hazard ratio=0.45; 95% confidence interval=0.37–0.55; P<0.0001) and acceleration index (standardized hazard ratio=0.44; 95% confidence interval=0.35–0.55; P<0.0001). These remained independently predictive of death after adjustment for multiple confounders, as well as systolic and diastolic blood pressure. Among subjects without stage 2 hypertension (n=1563), indices of cardiac contractility were independently predictive of death and identified a subpopulation (25% of non‐stage‐2 hypertensives) that demonstrated a high 10‐year mortality risk, equivalent to that of stage 2 hypertensives.
Conclusions
Hemodynamic patterns identified by ICG independently predict mortality in the general population. The predictive value of ICG applies even in the absence of American College of Cardiology/American Heart Association stage 2 hypertension and identifies higher‐risk individuals who are in earlier stages of the hypertension continuum.
Keywords: cardiac function, hemodynamics, impedance cardiography, population‐based, systemic vascular resistance, total arterial compliance
Clinical Perspective
What Is New?
Hemodynamic parameters measured by impedance cardiography (a simple office‐based operator‐independent noninvasive procedure) predict mortality in the general population, even after adjustment for multiple confounders and blood pressure.
Among subjects without stage 2 hypertension, indices of cardiac contractility measured by impedance cardiography are independently predictive of death and identify a proportion (≈1 in 4 adults without stage 2 hypertension) that demonstrate a high 10‐year mortality risk, similar to that of stage 2 hypertensives.
What Are the Clinical Implications?
Parameters measured by impedance cardiography (total arterial compliance index and indices of cardiac contractility) predict mortality in the general population, even after adjustment for multiple confounders and blood pressure.
Subjects without stage 2 hypertension who demonstrate abnormal impedance cardiography indices of cardiac contractility represent a large subpopulation could benefit from more‐aggressive or earlier interventions, which should be addressed in future studies.
Arterial hemodynamic phenomena are increasingly recognized as important determinants of hypertensive target organ damage. Blood pressure (BP) is determined by interactions between the heart and arterial properties. Mean arterial pressure is the product of cardiac output and systemic vascular resistance (SVR), whereas pulse pressure results from the interaction between the stroke volume generated by the left ventricle (LV) and the biophysical properties of conduit arteries.1, 2, 3 It follows that patients with identical BP may have substantially different hemodynamic patterns. An increased mean arterial pressure can result from an increase in cardiac output, SVR, or both. Furthermore, an increase in SVR may not be apparent by BP measurements alone in individuals with relatively low cardiac output. A similar phenomenon can occur with pulse pressure (a key determinant of systolic hypertension), which may be associated with multiple combinations of stroke volume and conduit artery compliance.
Impedance cardiography (ICG) is a simple, operator‐independent, noninvasive method that can approximate stroke volume, allowing for the assessment of cardiac output, systemic SVR, and the stroke volume/pulse pressure ratio (SV/PP; an index of total arterial compliance).3, 4, 5 In addition, ICG provides various indices of LV function/contractility, derived from the rate of change of electrical impedance in the thorax. The new American College of Cardiology (ACC)/American Heart Association (AHA)/American Academy of Physician Assistants/Association of Black Cardiologists/American College of Preventive Medicine/American Geriatrics Society/American Pharmacists Association/American Society of Hypertension/American Society for Preventive Cardiology/National Medical Association/Preventive Cardiovascular Nurses Association Guidelines for the Prevention, Detection, Evaluation, and Management of High BP in Adults6, 7 provide a new definition of hypertension and emphasize early detection and prevention as important objectives for clinical care and further research. Therefore, the identification of adults at increased risk for adverse outcomes, particularly those in earlier stages of the BP continuum, is an important goal. Despite its physiological significance, the value of ICG parameters for prediction of adverse outcomes in hypertensive and nonhypertensive adults in the general population has not been previously investigated.
We aimed to determine whether arterial hemodynamic indices quantified by ICG predict all‐cause mortality in adults from the community and, specifically, among those who do not yet meet criteria for ACC/AHA stage 2 hypertension.
Methods
Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We studied 1639 adults aged 18 to 80 years enrolled in the PREVENCION (Estudio Peruano de Prevalencia de Enfermedades Cardiovasculares, for Peruvian Study of the Prevalence of Cardiovascular diseases) study.8, 9, 10, 11, 12, 13, 14 PREVENCION is a study undertaken in Arequipa, the second largest city in Peru, which occurred in 2 phases: the first phase, which focused on population‐based prevalence estimates of cardiovascular disease/risk factors, and the second phase, which focused on increasing the sample size for prospective analyses regarding the relationship between baseline cardiovascular phenotypes and mortality. Further details regarding the sampling strategy are presented in the Supporting Information (Data S1). The Santa Maria Catholic University Human Research Committee approved all study procedures. All subjects signed a written informed consent. ICG was implemented after enrollment in the parent study had been initiated; therefore, this analysis included a subset of participants, as detailed in the Supporting Information Methods section (Data S1) and Table S1. Subjects with congestive heart failure (n=22) were excluded from this analysis, given that we aimed to assess the value of ICG to detect subclinical changes in ventricular and arterial function.
BP and Biochemical Measurements
As previously described,8, 9 BP was measured between 7:00 and 10:00 am using a mercury sphygmomanometer with the auscultatory method, according to recommendations from the seventh report of the Joint National Committee for the Diagnosis, Evaluation, and Treatment of High BP.15
Venous blood was obtained after at least 8 hours of fasting. Total cholesterol, glucose, and triglycerides were measured in serum using enzymatic automated methods (Cobas Mira analyzer; Roche, Basel, Switzerland). Low‐density lipoprotein‐cholesterol and high‐density lipoprotein‐cholesterol were measured by direct methods using the same analyzer. Diabetes mellitus was defined as fasting blood glucose ≥126 mg/dL or pharmacological treatment with glucose‐lowering drugs. Glomerular filtration rate was estimated with the Modification of Diet in Renal Disease equation.16
Hemodynamic Assessments
As further described in the Supporting Information, ICG assessments were performed between 7:00 and 10 :00am after a resting period of at least 5 minutes with a BioZ ICG device (CardioDynamics, San Diego, CA), which utilizes validated algorithms for estimation of stroke volume and cardiac output.17 Four dual ICG sensors were placed on the subject, 2 above the base of the neck and just below each ear and 1 on either side of the thorax in the midaxillary line at the level of the xiphoid process (Figure 1). ICG provides measures of cardiac function (cardiac output, cardiac index, stroke volume, and stroke volume index), in addition to indices of LV chamber contractility (velocity index, acceleration index, pre‐ejection period, LV ejection time, and the systolic time ratio [ie, pre‐ejection period divided by LV ejection time]).3, 4 The definition of these indices and the units of measurement are shown in Table S2.
Figure 1.
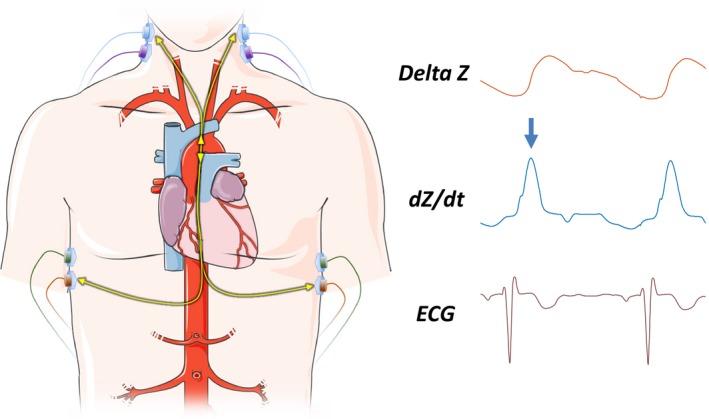
The impedance (Z) waveform originates from changes in the electrical impedance of the thorax. Velocity index describes the maximum deflection of the first derivative of the Z waveform, shown as the dZ/dt waveform (arrow). For reference, the ECG tracing is also shown. Landmarks in the ICG waveform can be used to identify the time of aortic valve opening and closure and therefore the ejection time. The ECG can then be used to compute the pre‐ejection time (LVET) and the systolic time ratio (PEP/LVET). ICG indicates impedance cardiography; LVET, left ventricle ejection time; PEP, pre‐ejection period.
Assessment of 10‐Year All‐Cause Mortality
All‐cause mortality was ascertained by review of the national death registry, a government‐run centralized electronic database of death record information.
Statistical Analysis
We assessed the general clinical characteristics of the study sample, including hemodynamic parameters measured by ICG, according to hypertension stage. We used chi‐square tests for categorical variables and ANOVA for continuous variables, with post‐hoc pair‐wise comparisons with Bonferroni correction for alpha error. Log transformation was done, when appropriate, to improve normality in the distribution of values.
Prognostic value of various parameters was assessed using Cox (proportional hazards) regression. We obtained standardized hazard ratios (HRs) and 95% confidence intervals (CIs), which represent the relative risk per 1‐SD increase in the predictor, allowing for a more‐direct comparison between the various predictors. Our study had 85% power to detect significant associations for which a standardized effect size of 0.24 (corresponding to standardized HRs >1.10 or <0.91), at α=0.05. Statistical significance was defined as a 2‐tailed P<0.05. Statistical analyses were performed using the Matlab statistics and machine learning toolbox (The Mathworks, Inc, Natwick, MA) and SPSS for Windows (v22; SPSS, Inc, Chicago, IL).
Results
Table 1 shows the general characteristics of the study population. The sample consisted of 704 subjects without hypertension, 471 subjects with ACC/AHA stage 1‐hypertension, and 464 subjects with ACC/AHA stage 2‐hypertension (total=1639). There was a progressive increase in age, body mass index, total cholesterol,low‐density lipoprotein‐cholesterol, fasting glucose, and prevalence of diabetes mellitus and impaired fasting glucose, from nonhypertension to stage 2 hypertension, with a progressive decrease in estimated glomerular filtration rate. Serum triglycerides were lower in nonhypertensive subjects compared with either stage 1 or 2 hypertension.
Table 1.
General Characteristics of Study Subjects
| ACC/AHA Blood Pressure Group | P Value | |||
|---|---|---|---|---|
| Nonhypertensive | Stage 1 Hypertension | Stage 2 Hypertension | ||
| Mean (95% CI) n=704 | Mean (95% CI) n=471 | Mean (95% CI) n=464 | ||
| Age, y | 41 (40–42) | 50.4 (48.9–51.9) | 62.3 (60.5–64.2) | <0.0001a, b, c |
| Male sex | 308 (43.75%) | 272 (57.75%) | 202 (43.53%) | <0.0001 |
| Body mass index, kg/m2 | 25 (24.7–25.3) | 26.4 (26.1–26.8) | 28.5 (28.1–28.9) | <0.0001a, b, c |
| Body surface area, m2 | 1.74 (1.72–1.75) | 1.81 (1.79–1.83) | 1.82 (1.81–1.84) | <0.0001a, b |
| Systolic blood pressure, mm Hg | 106 (105–107) | 120 (118–121) | 140 (139–142) | <0.0001a, b, c |
| Diastolic blood pressure, mm Hg | 70.2 (69.8–70.5) | 81.3 (80.8–81.9) | 85.7 (85.1–86.3) | <0.0001a, b, c |
| Pulse pressure, mm Hg | 35 (34.3–35.7) | 37.2 (36.2–38.1) | 52.8 (51.4–54.1) | <0.0001a, b, c |
| Total cholesterol, mg/dL | 191 (189–194) | 199 (196–203) | 211 (208–215) | <0.0001a, b, c |
| LDL‐cholesterol, mg/dL | 111 (109–114) | 117 (114–120) | 125 (122–128) | <0.0001a, b, c |
| HDL‐cholesterol, mg/dL | 46.7 (46–47.4) | 46.3 (45.4–47.2) | 46.4 (45.5–47.3) | 0.80 |
| Triglycerides mg/dL | 137 (132–142) | 167 (159–174) | 174 (166–182) | <0.0001a, b |
| Fasting glucose, mg/dL | 79.1 (78–80.2) | 81.3 (79.9–82.7) | 87.2 (85.7–88.6) | <0.0001a, b, c |
| Impaired fasting glucose | 18 (2.56%) | 26 (5.52%) | 40 (8.62%) | <0.0001 |
| Diabetes mellitus | 21 (2.98%) | 23 (4.88%) | 56 (12.07%) | <0.0001 |
| Serum creatinine, mg/dL | 0.751 (0.739–0.764) | 0.792 (0.777–0.808) | 0.817 (0.801–0.833) | <0.0001a, b |
| Estimated GFR, mg/dL/1.73 m2 | 96.8 (95.1–98.5) | 91 (89.1–93) | 80.7 (79–82.4) | <0.0001a, b, c |
| Antihypertensive medication use | 0 (0.00%) | 0 (0.00%) | 285 (61.42%) | <0.0001 |
| Aspirin use | 6 (0.86%) | 13 (2.79%) | 24 (5.21%) | <0.0001 |
| Current smoking | 151 (21.45%) | 84 (17.83%) | 55 (11.85%) | 0.0001 |
| Peripheral arterial disease | 0 (0.00%) | 3 (0.64%) | 3 (0.65%) | 0.10 |
Nonhypertensive: BP <13 080 mm Hg. Stage 1 hypertension: systolic BP 130 to 139 or diastolic BP 80 to 89 mm Hg; stage II hypertension: systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg, or pharmacological treatment for hypertension. ACC indicates American College of Cardiology; AHA, American Heart Association; BP, blood pressure; CI, confidence interval; GFR, glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Post‐hoc pair‐wise comparison different between nonhypertensive and stage 1 hypertension.
Post‐hoc pair‐wise comparison different between nonhypertensive and stage 2 hypertension.
Post‐hoc pair‐wise comparison different between stage 1 and 2 hypertension.
ICG Patterns in Hypertension Groups
Table 2 shows a comparison of ICG parameters between subjects without hypertension and those with ACC/AHA stage 1 and 2 hypertension. Stage 2 hypertension, but not stage 1 hypertension, was associated with an increase in heart rate and a reduction in stroke volume, stroke volume index, and cardiac index compared with subjects without hypertension. In contrast, SVR, SVR index, total arterial compliance (TAC), and TAC index (measures of resistive and pulsatile arterial properties) progressively increased from nonhypertension to stage 2 hypertension. Similarly, measures of cardiac contractility (velocity index and acceleration index) and the systolic time ratio (pre‐ejection period/LV ejection time) progressively decreased from nonhypertension to stage 2 hypertension. Very similar results were observed when subjects taking antihypertensive medications were excluded from the analysis (Table S3).
Table 2.
Comparison of ICG Parameters Between Nonhypertensive, Stage 1 Hypertensive, and Stage 2 Hypertensive Subjects in the Study Sample
| ACC/AHA Blood Pressure Group | P Value | |||
|---|---|---|---|---|
| Nonhypertensive | Stage 1 Hypertension | Stage 2 Hypertension | ||
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Stroke volume, mL | 80.4 (79–81.9) | 83.2 (81.3–85) | 77.2 (75.5–78.9) | <0.0001a, b |
| Stroke index, mL/m2 | 47.4 (46.7–48) | 47 (46.3–47.8) | 43.7 (43–44.5) | <0.0001a, b |
| Cardiac output, mL/min | 5.01 (4.93–5.1) | 5.22 (5.11–5.32) | 4.99 (4.89–5.09) | 0.0029b, c |
| Cardiac index, mL m2/min | 2.91 (2.87–2.95) | 2.91 (2.86–2.96) | 2.76 (2.71–2.8) | <0.0001a, b |
| SVR, dyn s/cm5 | 1132 (1112–1153) | 1183 (1156–1209) | 1389 (1358–1420) | <0.0001a, b, c |
| SVR index, dyn s m2/cm5 | 1954 (1924–1985) | 2124 (2083–2165) | 2517 (2468–2566) | <0.0001a, b, c |
| TAC, mL/mm Hg | 2.03 (1.98–2.08) | 1.88 (1.82–1.94) | 1.37 (1.33–1.41) | <0.0001a, b, c |
| TAC index, mL m2/mm Hg | 1.175 (1.147–1.203) | 1.049 (1.018–1.079) | 0.757 (0.735–0.779) | <0.0001a, b, c |
| Velocity index, 1/100 s | 59.2 (57.8–60.6) | 52 (50.5–53.5) | 45.7 (44.4–47.1) | <0.0001a, b, c |
| Acceleration index, 1/100 s2 | 104 (100.9–107) | 88.8 (85.6–92) | 77.4 (74.6–80.2) | <0.0001a, b, c |
| Systolic time ratio | 0.248 (0.244–0.252) | 0.235 (0.231–0.24) | 0.223 (0.218–0.227) | <0.0001a, b, c |
| Pre‐ejection period, ms | 85.7 (84.7–86.7) | 84.9 (83.7–86.2) | 80.8 (79.7–82) | <0.0001a, b |
| LV ejection time, ms | 338 (335–342) | 353 (349–357) | 355 (350–359) | <0.0001a, c |
Nonhypertensive: BP <13 080 mm Hg. Stage 1 hypertension: systolic BP 130 to 139 or diastolic BP 80 to 89 mm Hg; stage II hypertension: systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg, or pharmacological treatment for hypertension. ACC indicates American College of Cardiology; AHA, American Heart Association; BP, blood pressure; CI, confidence interval; ICG, impedance cardiography; LV, left ventricular; SVR, systemic vascular resistance; TAC, total arterial compliance.
Post‐hoc pair‐wise comparison different between nonhypertensive and stage 2 hypertension.
Post‐hoc pair‐wise comparison different between stage 1 and 2 hypertension.
Post‐hoc pair‐wise comparison different between nonhypertensive and stage 1 hypertension.
ICG Parameters as Predictors of Mortality in Nonadjusted Analyses
There were 156 deaths during a mean follow‐up of 10.9 years. Figure 2 presents results of unadjusted Cox models assessing ICG parameters as predictors of mortality. All ICG parameters, with the exception of LV ejection time, were significant predictors of death in unadjusted models (Figure 2, left panel). The strongest predictors of mortality were measures of conduit artery function: TAC (standardized HR=0.40; 95% CI=0.33–0.48; P<0.0001) and TAC index (standardized HR=0.38; 95% CI=0.31–0.46; P<0.0001), velocity index (standardized HR=0.45; 95% CI=0.37–0.55; P<0.0001), and acceleration index (standardized HR=0.44; 95% CI=0.35–0.55; P<0.0001). Figure S1 shows 10‐year Kaplan–Meier survival curves for quartiles of these ICG parameters.
Figure 2.
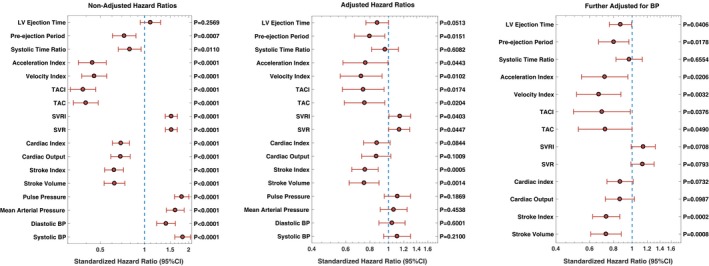
ICG parameters as predictors of all‐cause mortality in unadjusted proportional hazards regression models (left panel) and models adjusted for age, sex, body mass index, LDL‐cholesterol, HDL‐cholesterol, triglycerides, fasting plasma glucose, diabetes mellitus, serum creatinine, and smoking history (middle panel) and after further adjustment for systolic and diastolic blood pressure (right panel). BP indicates blood pressure; CI, confidence interval; LV, left ventricular; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index; TAC, total arterial compliance; TACI, total arterial compliance index.
Other predictors of included a lower stroke volume (standardized HR=0.62; 95% CI=0.53–0.73; P<0.0001), a lower stroke volume index (standardized HR=0.62; 95% CI=0.53–0.73; P<0.0001), a lower cardiac output (standardized HR=0.69; 95% CI=0.59–0.80; P<0.0001), a lower cardiac index (standardized HR=0.69; 95% CI=0.60–0.79; P<0.0001), a lower pre‐ejection period (standardized HR=0.73; 95% CI=0.61–0.87; P=0.0007), and a lower systolic time ratio (standardized HR=0.79; 95% CI=0.66–0.95; P=0.011). In contrast, greater SVR (standardized HR=1.52; 95% CI=1.39–1.67; P<0.0001), SVR index (standardized HR=1.52; 95% CI=1.38–1.68; P<0.0001), heart rate (standardized HR=1.20; 95% CI=1.04–1.39; P=0.014), and BP (particularly systolic and pulse pressure) were associated with increased mortality.
Of note, the inverse of the standardized HR for TAC index (risk associated with a 1‐SD reduction in the value) was significantly greater (2.63; 95% CI=2.17–3.23) and did not overlap with the standardized HR for pulse pressure (1.79; 95% CI=1.60–2.01), indicating that knowledge of stroke volume in conjunction with pulse pressure provides greater risk stratification than that provided by pulse pressure alone.
ICG Parameters as Independent Predictors of Mortality
Figure 2 presents results of adjusted Cox models assessing ICG parameters are predictors of mortality. In models adjusted for age, sex, body mass index, low‐density lipoprotein‐cholesterol, high‐density lipoprotein‐cholesterol, triglycerides, fasting plasma glucose, diabetes mellitus, serum creatinine, and smoking history (Figure 2, middle panel), significant independent predictors of death included stroke volume, stroke volume index, TAC and TAC index, and measures of cardiac contractility (velocity index and acceleration index).
Further adjustment for systolic and diastolic BP did not attenuate these relationships (Figure 2, right panel). In these adjusted models, stroke volume (standardized HR=0.73; 95% CI=0.61–0.88; P=0.0008), stroke volume index (standardized HR=0.73; 95% CI=0.62–0.86; P=0.0002), velocity index (standardized HR=0.67; 95% CI=0.51–0.87; P=0.003) and acceleration index (standardized HR=0.72; 95% CI=0.54–0.95; P=0.021), TAC (standardized HR=0.72; 95% CI=0.52–0.99; P=0.049) and TAC index (standardized HR=0.70; 95% CI=0.49–0.98; P=0.038), as well as longer pre‐ejection and ejection periods and higher heart rate, were associated with increased mortality.
Figure S1 shows Kaplan–Meier survival curves for quartiles of velocity index, acceleration index, and TAC and TAC index.
ICG for Risk Stratification Among Subjects Without ACC/AHA Stage 2 Hypertension
Among subjects without stage 2 hypertension, there were 75 deaths. Table S4 presents results of nonadjusted and adjusted Cox models assessing ICG parameters as predictors of mortality in subjects without ACC/AHA stage 2 hypertension (ie, those with either normal BP, elevated BP, or ACC/AHA stage 1 hypertension). In unadjusted analyses, all ICG parameters were predictive of all‐cause death, including stroke volume, stroke volume index, cardiac output, cardiac index, SVR and SVR index, TAC and compliance index, as well as the systolic time ratio, pre‐ejection period, and ejection time. The strongest predictors of death in unadjusted models were indices of cardiac contractility: velocity index (standardized HR=0.32; 95% CI=0.23–0.44; P<0.0001) and acceleration index (standardized HR=0.31; 95% CI=0.22–0.44; P<0.0001). In models adjusted for age, sex, body mass index, low‐density lipoprotein‐cholesterol, high‐density lipoprotein‐cholesterol, triglycerides, fasting plasma glucose, diabetes mellitus, serum creatinine and smoking history, velocity index (standardized HR=0.61; 95% CI=0.42–0.88; P=0.0008) and acceleration index (standardized HR=0.62; 95% CI=0.41–0.94; P=0.023), and well as the systolic time ratio and pre‐ejection period, were independent predictors of death. Further adjustment for systolic and diastolic BP did not appreciably attenuate these relationships (Table S4).
Figure 3 shows 10‐year Kaplan–Meier survival curves for quartiles of acceleration index and velocity index among subjects without ACC/AHA stage 2 hypertension in the sample. Superimposed on those curves is the Kaplan–Meier survival curve for subjects with ACC/AHA stage 2 hypertension (blue curve). As can be appreciated, subjects who were not classified as stage 2 hypertension who were in the lower quartile of velocity index and acceleration index demonstrated survival curves comparable with that of stage 2 hypertensive subjects.
Figure 3.
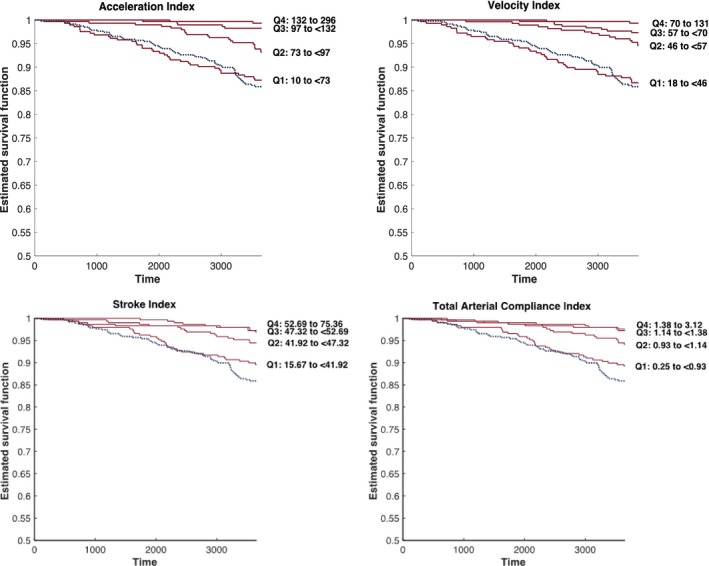
Kaplan–Meier survival curves for quartiles of acceleration index and velocity index among subjects without ACC/AHA stage 2 hypertension (red curves). Superimposed on these curves is the Kaplan–Meier survival curve for subjects with ACC/AHA stage 2 hypertension (blue curve). ACC indicates American College of Cardiology; AHA, American Heart Association.
Discussion
We report that hemodynamic indices of arterial and cardiac function quantified by ICG, an easily applicable, operator‐independent, noninvasive technique, independently predict ≈10‐year all‐cause mortality in adults from the community. The strongest predictors of mortality were TAC (which reflects conduit artery compliance/stiffness) and subclinical indices of cardiac contractility (velocity index and acceleration index). We further demonstrate that ICG identifies an important proportion (≈25%) of individuals without stage 2 hypertension that exhibit a high 10‐year mortality risk, similar to that of stage 2 hypertensives. Our findings have implications for our understanding of the role of ventricular‐arterial function as a prognostic factor in the general population.
BP is determined by the interaction between the LV and the load imposed by systemic arteries (arterial load). There are 2 broad components of arterial pressure: mean arterial pressure, which is, in turn, determined by cardiac output and total peripheral resistance (provided largely by the microcirculation), and pulsatile changes around mean pressure, which depend on the interaction between LV ejection and the pulsatile input impedance of the systemic vasculature. The resistive component of arterial load can be computed simply as the ratio of mean arterial pressure/cardiac output. Pulsatile load is complex, time varying, and best assessed with detailed modeling of aortic pressure‐flow relations.2, 18, 19 A readily available index of pulsatile arterial load, mainly related to TAC, is the ratio of SV/PP.
Previous studies have identified relationships between the ratio of echocardiographic SV/PP and cardiovascular events in populations with stage 2 hypertension, diabetes mellitus, or in elderly men.20, 21, 22, 23 In 1 study, TAC assessed by echocardiography did not predict mortality in a primary prevention clinical setting, although only 42 deaths occurred in that cohort.24 To our knowledge, only 1 study has assessed the relationship between SV/PP and incident risk in the general population.25 Lilly et al reported a significant relationship between SV/PP (where SV was measured with cardiac magnetic resonance imaging) and cardiovascular events in the MESA [Multiethnic Study of Atherosclerosis]). Our study is the first to assess the value of SV/PP measured by ICG, a simple, inexpensive, and operator‐independent technique, to predict all‐cause mortality in the general population. We demonstrate that greater total SV/PP ratio and SVR were independently associated with all‐cause death, independently of BP. Our findings support the concept that alternations in the biophysical properties of conduit vessels are independently related to the risk of all‐cause death. Our findings are also consistent with previous studies demonstrating that increased carotid‐femoral pulse wave velocity (a measure of aortic wall stiffness) is predictive of cardiovascular events and mortality in healthy and at‐risk populations.26 However, we note that SV/PP and carotid‐femoral pulse wave velocity are not interchangeable. SV/PP is dependent on the compliance of the entire arterial tree, which is dependent on arterial wall stiffness and arterial size and is composed of variable contributions from both large and muscular arteries.1, 2, 3 Our data also indicate that SV/PP is a more‐robust predictor of mortality than SVR, given that, in contrast to SV/PP, the latter did not remain a significant independent predictor of death in subjects without established stage 2 hypertension.
Subclinical Cardiac Dysfunction and Mortality
We demonstrate that ICG parameters of cardiac contractility are independently predictive of all‐cause death in subjects from the general population. Impaired LV contractility may increase mortality through several pathways, including progression to heart failure or sudden cardiac death. In addition, abnormal LV contractility could indicate the presence of underlying subclinical coronary artery disease or genetic polymorphisms that confer an increased risk of death. Whereas our study is the first to assess ICG parameters as predictors of mortality, few previous studies assessed the prognostic value of LV function assessed with cardiac imaging. In unselected adults from the community, subclinical LV dysfunction assessed with echocardiography has been shown to predict mortality in some, but not all, studies. In the Rotterdam Study, myocardial contractility assessed with echocardiography did not show an independent association with all‐cause mortality.27 However, a more‐recent analysis in the same cohort showed that a lower fractional shortening was associated with an increased risk of sudden cardiac death.28 Furthermore, subclinical abnormalities in LV function assessed by echocardiography have been shown to predict all‐cause death in the Framingham Heart Study29 and among Olmsted County residents.30 Similarly, regional myocardial dysfunction detected by echocardiography predicted cardiovascular death in the Strong Heart Study,31 whereas regional myocardial dysfunction measured with cardiac magnetic resonance imaging predicted coronary artery disease–related death in the MESA cohort.32 Whereas these previous studies are valuable, the application of noninvasive imaging is limited by cost and widespread availability. ICG, on the other hand, is a quick, simple, inexpensive, operator‐independent office‐based procedure, which, in principle, carries a cost and complexity similar to a 12‐lead ECG. This is an obvious advantage for the assessment of cardiovascular risk in the general population, including high‐ and low‐resource settings.
Strengths and Limitations
Our study should be interpreted in the context of its strengths and limitations. Strengths of our study include the large, population‐based sample inclusive of subjects from a wide age range and the prospective design with long‐term mortality data. In addition to pathophysiological insights regarding the importance of ventricular and arterial function as predictors of death, our study demonstrates the value of a very easily applicable technique, which can be widely utilized in office settings. Our study also has limitations. We did not assess nonfatal events. We ascertained death from the National Death Index and thus were unable to adjudicate cause of death and assess disease‐specific mortality. This will be a focus of future studies. We did not compare the predictive value of ICG with that of noninvasive imaging (such as echocardiography or cardiac magnetic resonance imaging). We did not assess whether ICG predicts mortality independent of other subclinical measures of target organ damage, such as microalbuminuria.
We only assessed resting hemodynamic indices in the office, which may not represent 24‐hour patterns. BP among subjects with stage 1 and 2 hypertension, according to the new guidelines, were found to be lower than those recently reported in the US population,33 and thus our results may not extrapolate to the US population. It is also important to note that our findings using the BioZ ICG device may not be directly extrapolated to other ICG devices that utilize different hardware and/or processing algorithms, and that device‐specific validation for hemodynamic measurements is required. Finally, whereas ICG parameters were found to be independently predictive of 10‐year all‐cause death, its incorporation into multivariable risk scores was not assessed in the present study and should be the focus of future studies.
In conclusion, our study demonstrates that hemodynamic patterns identified by ICG independently predict all‐cause mortality in the general population. Predictive value of ICG applies even in the absence of ACC/AHA stage 2 hypertension and identifies high‐risk individuals who are in earlier stages of the hypertension continuum. ICG may be useful to identify candidates for earlier and/or more‐aggressive therapeutic interventions in high‐risk individuals. This strategy should be addressed in future studies.
Sources of Funding
Chirinos is supported by NIH grants R56HL‐124073‐01A1 and R01 HL 121510‐01A1.
Disclosures
Chirinos has received consulting honoraria from BMS, OPKO Healthcare, Fukuda Denshi, Microsoft, Merck, Ironwood Pharmaceuticals, Sanifit, and Vital Labs. He received research grants from National Institutes of Health, American College of Radiology Network, Fukuda‐Denshi, Bristol‐Myers Squibb, Microsoft, and CVRx Inc. and device loans from AtCor Medical. Chirinos is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of Heart Failure and Preserved Ejection Fraction. Other authors have no disclosures.
Supporting information
Data S1. Supplemental methods.
Figure S1. Kaplan–Meier survival curves for quartiles of velocity index, acceleration index, total arterial compliance, and total arterial compliance index.
Table S1. General Characteristics of Subjects Who Were Included vs Not Included in This Subanalysis, Because of Availability of ICG Data
Table S2. Physiologic Indices Derived From Impedance Cardiography (ICG)
Table S3. Comparison of ICG Parameters Between Nonhypertensive, Stage 1 Hypertensive, and Stage 2 Hypertensive Subjects in the Study Sample, After Exclusion of Hypertensive Subjects Receiving Antihypertension Medications
Table S4. ICG Parameters as Predictors of All‐Cause Mortality in Unadjusted and Adjusted Proportional Hazards Regression Models Among Subjects Without ACC/AHA Stage 2 Hypertension
(J Am Heart Assoc. 2018;7:e009259 DOI: 10.1161/JAHA.118.009259.)
References
- 1. Nichols WW, O'Rourke M, Vlachopolous C. Mcdonald's Blood Flow in Arteries. Theoretical, Experimental and Clinical Principles. London, UK: Hodder Arnold; 2011. [Google Scholar]
- 2. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 2: arterial pressure‐flow and pressure‐volume relations in humans. Hypertension. 2010;56:563–570. [DOI] [PubMed] [Google Scholar]
- 3. Yancy C, Abraham WT. Noninvasive hemodynamic monitoring in heart failure: utilization of impedance cardiography. Congest Heart Fail. 2003;9:241–250. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg P, Yancy CW. Noninvasive assessment of hemodynamics: an emphasis on bioimpedance cardiography. Curr Opin Cardiol. 2000;15:151–155. [DOI] [PubMed] [Google Scholar]
- 5. Chemla D, Hebert JL, Coirault C, Zamani K, Suard I, Colin P, Lecarpentier Y. Total arterial compliance estimated by stroke volume‐to‐aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–H505. [DOI] [PubMed] [Google Scholar]
- 6. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199–2269. [DOI] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 8. Medina‐Lezama J, Chirinos JA, Zea Diaz H, Morey O, Bolanos JF, Munoz‐Atahualpa E, Chirinos‐Pacheco J. Design of PREVENCION: a population‐based study of cardiovascular disease in Peru. Int J Cardiol. 2005;105:198–202. [DOI] [PubMed] [Google Scholar]
- 9. Medina‐Lezama J, Zea‐Diaz H, Morey‐Vargas OL, Bolanos‐Salazar JF, Postigo‐Macdowall M, Paredes‐Diaz S, Corrales‐Medina F, Valdivia‐Ascuna Z, Cuba‐Bustinza C, Villalobos‐Tapia P, Munoz‐Atahualpa E, Chirinos‐Pacheco J, Raij L, Chirinos JA. Prevalence and patterns of hypertension in Peruvian Andean Hispanics: the PREVENCION study. J Am Soc Hypertens. 2007;1:216–225. [DOI] [PubMed] [Google Scholar]
- 10. Medina‐Lezama J, Zea‐Diaz H, Morey‐Vargas OL, Bolanos‐Salazar JF, Munoz‐Atahualpa E, Postigo‐MacDowall M, Corrales‐Medina F, Valdivia‐Ascuna Z, Cuba‐Bustinza C, Paredes‐Diaz S, Villalobos‐Tapia P, Chirinos‐Pacheco J, Goldberg RB, Chirinos JA. Prevalence of the metabolic syndrome in Peruvian Andean Hispanics: the PREVENCION study. Diabetes Res Clin Pract. 2007;78:270–281. [DOI] [PubMed] [Google Scholar]
- 11. Chirinos JA, David R, Bralley JA, Zea‐Diaz H, Munoz‐Atahualpa E, Corrales‐Medina F, Cuba‐Bustinza C, Chirinos‐Pacheco J, Medina‐Lezama J. Endogenous nitric oxide synthase inhibitors, arterial hemodynamics, and subclinical vascular disease: the PREVENCION study. Hypertension. 2008;52:1051–1059. [DOI] [PubMed] [Google Scholar]
- 12. Pastorius CA, Medina‐Lezama J, Corrales‐Medina F, Bernabe‐Ortiz A, Paz‐Manrique R, Salinas‐Najarro B, Khan ZA, Takahashi J, Toshima G, Zea‐Diaz H, Postigo‐Macdowall M, Chirinos‐Pacheco J, Ibanez F, Chirinos DA, Saif H, Chirinos JA. Normative values and correlates of carotid artery intima‐media thickness and carotid atherosclerosis in Andean‐Hispanics: the PREVENCION study. Atherosclerosis. 2010;211:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medina‐Lezama J, Pastorius CA, Zea‐Diaz H, Bernabe‐Ortiz A, Corrales‐Medina F, Morey‐Vargas OL, Chirinos DA, Muñoz‐Atahualpa E, Chirinos‐Pacheco J, Chirinos JA; PREVENCION Investigators . Optimal definitions for abdominal obesity and the metabolic syndrome in Andean Hispanics: the PREVENCION study. Diabetes Care. 2010;33:1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Medina‐Lezama J, Morey‐Vargas OL, Zea‐Diaz H, Bolanos‐Salazar JF, Corrales‐Medina F, Cuba‐Bustinza C, Chirinos‐Medina DA, Chirinos JA. Prevalence of lifestyle‐related cardiovascular risk factors in Peru: the PREVENCION study. Rev Panam Salud Publica. 2008;24:169–179. [DOI] [PubMed] [Google Scholar]
- 15. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 16. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 17. Van De Water JM, Miller TW, Vogel RL, Mount BE, Dalton ML. Impedance cardiography: the next vital sign technology? Chest. 2003;123:2028–2033. [DOI] [PubMed] [Google Scholar]
- 18. Chirinos JA. Deep phenotyping of systemic arterial hemodynamics in HFpEF (part 1): physiologic and technical considerations. J Cardiovasc Transl Res. 2017;10:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chirinos JA, Segers P. Noninvasive evaluation of left ventricular afterload: part 1: pressure and flow measurements and basic principles of wave conduction and reflection. Hypertension. 2010;56:555–562. [DOI] [PubMed] [Google Scholar]
- 20. de Simone G, Roman MJ, Koren MJ, Mensah GA, Ganau A, Devereux RB. Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension. 1999;33:800–805. [DOI] [PubMed] [Google Scholar]
- 21. Lind L, Andren B, Sundstrom J. The stroke volume/pulse pressure ratio predicts coronary heart disease mortality in a population of elderly men. J Hypertens. 2004;22:899–905. [DOI] [PubMed] [Google Scholar]
- 22. Mohty D, Pibarot P, Echahidi N, Poirier P, Dagenais GR, Dumesnil JG. Reduced systemic arterial compliance measured by routine Doppler echocardiography: a new and independent predictor of mortality in patients with type 2 diabetes mellitus. Atherosclerosis. 2012;225:353–358. [DOI] [PubMed] [Google Scholar]
- 23. Fagard RH, Pardaens K, Staessen JA, Thijs L. The pulse pressure‐to‐stroke index ratio predicts cardiovascular events and death in uncomplicated hypertension. J Am Coll Cardiol. 2001;38:227–231. [DOI] [PubMed] [Google Scholar]
- 24. Haluska BA, Jeffries L, Carlier S, Marwick TH. Measurement of arterial distensibility and compliance to assess prognosis. Atherosclerosis. 2010;209:474–480. [DOI] [PubMed] [Google Scholar]
- 25. Lilly SM, Jacobs D, Bluemke DA, Duprez D, Zamani P, Chirinos J. Resistive and pulsatile arterial hemodynamics and cardiovascular events: the Multiethnic Study of Atherosclerosis. J Am Heart Assoc. 2014;3:e001223 DOI: 10.1161/JAHA.114.001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension . Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kardys I, Deckers JW, Stricker BH, Vletter WB, Hofman A, Witteman JC. Echocardiographic parameters and all‐cause mortality: the Rotterdam Study. Int J Cardiol. 2009;133:198–204. [DOI] [PubMed] [Google Scholar]
- 28. Niemeijer MN, Leening MJ, van den Berg ME, Hofman A, Franco OH, Deckers JW, Rijnbeek PR, Stricker BH, Eijgelsheim M. Subclinical abnormalities in echocardiographic parameters and risk of sudden cardiac death in a general population: the Rotterdam Study. J Card Fail. 2016;22:17–23. [DOI] [PubMed] [Google Scholar]
- 29. Wang TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation. 2003;108:977–982. [DOI] [PubMed] [Google Scholar]
- 30. Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 31. Cicala S, de Simone G, Roman MJ, Best LG, Lee ET, Wang W, Welty TK, Galloway JM, Howard BV, Devereux RB. Prevalence and prognostic significance of wall‐motion abnormalities in adults without clinically recognized cardiovascular disease: the Strong Heart Study. Circulation. 2007;116:143–150. [DOI] [PubMed] [Google Scholar]
- 32. Yan RT, Bluemke D, Gomes A, Burke G, Shea S, Liu K, Bahrami H, Sinha S, Wu C, Fernandes V, McClelland R, Lima JA. Regional left ventricular myocardial dysfunction as a predictor of incident cardiovascular events: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2011;57:1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation. 2018;137:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.
Figure S1. Kaplan–Meier survival curves for quartiles of velocity index, acceleration index, total arterial compliance, and total arterial compliance index.
Table S1. General Characteristics of Subjects Who Were Included vs Not Included in This Subanalysis, Because of Availability of ICG Data
Table S2. Physiologic Indices Derived From Impedance Cardiography (ICG)
Table S3. Comparison of ICG Parameters Between Nonhypertensive, Stage 1 Hypertensive, and Stage 2 Hypertensive Subjects in the Study Sample, After Exclusion of Hypertensive Subjects Receiving Antihypertension Medications
Table S4. ICG Parameters as Predictors of All‐Cause Mortality in Unadjusted and Adjusted Proportional Hazards Regression Models Among Subjects Without ACC/AHA Stage 2 Hypertension


