Abstract
Background
An association between atrial fibrillation (AF), anxiety, and depression is recognized, but the spectrum of psychological distress remains unclear. We aimed to characterize the severity and predictors of distress associated with AF in a tertiary population and its response to AF management.
Methods and Results
Seventy‐eight patients with symptomatic AF underwent evaluation, including of AF symptom severity, health‐related quality of life, psychological distress, suicidal ideation, and specific personality traits. Twenty participants underwent AF ablation and 58 were managed medically, with repeat assessments at 4, 8, and 12 months. Severe distress (Hospital Anxiety and Depression Scale score, ≥15/42) was identified in 27 of 78 (35%). Independent predictors were a personality marked by vulnerability to stress (Perceived Stress Scale: R 2, 0.54; β=0.7±0.1; t=7.8; P<0.001) and 1 marked by negativity/social inhibition (Type D Personality Scale: R 2, 0.47; β=0.7±0.1; t=6.7; P<0.001). Suicidal ideation was reported by 16 of 78 (20%) and was predicted by personality traits (Perceived Stress Scale score: R 2, 0.35; odds ratio, 1.22±0.06; P<0.001; Type D Personality Scale score: R 2, 0.48; odds ratio, 1.43±0.14; P<0.001). Effective AF ablation (median AF burden 1% [0–1%] over 12 months) was associated with significant reductions in distress (Hospital Anxiety and Depression Scale score, 13.9±1.8 to 4.3±1.8; P<0.05) and prevalence of suicidal ideation (30–5%; P=0.02).
Conclusions
There was a high prevalence of severe psychological distress (35%) and of suicidal ideation (20%) in a tertiary AF population, with personality traits predicting both. Effective AF ablation was associated with significant improvements, suggesting AF itself may be a treatable causative factor of distress.
Keywords: atrial fibrillation, personality, psychological distress, quality of life, suicidal ideation
Subject Categories: Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator
Clinical Perspective
What Is New?
In patients with symptomatic atrial fibrillation managed in a tertiary center, severe psychological distress was present in 35%, reaching the point of suicidal ideation in 20%.
Distress was predicted by the individual pattern of psychological function and, particularly, by a tendency to perceive life situations as stressful and by a personality marked by negativity/social inhibition.
Effective atrial fibrillation ablation was associated with significant reductions in psychological distress and suicidal ideation.
What Are the Clinical Implications?
Presence and severity of psychological distress should be evaluated in patients presenting with symptomatic atrial fibrillation.
Underlying psychological function should be considered as part of this evaluation.
Improvement in psychological distress and reduction in suicidal ideation may be a specific goal of atrial fibrillation ablation.
Introduction
The negative impact of atrial fibrillation (AF) on health‐related quality of life (hrQOL) has been extensively documented,1 with several studies also demonstrating that AF patients may experience significant symptoms of anxiety and depression,2, 3 that themselves may impact on AF symptom severity and hrQOL.4 Other data have indicated that enduring aspects of personality, including stress perception3 and personal negativity,5 may be important to the subjective experience of AF. However, the spectrum of psychological distress severity, key predictors of distress, and response over time to AF management remain unknown.
In this study, we aimed to define the spectrum of psychological distress severity associated with AF, key predictors of severity, and prevalence of suicidal ideation in a population from a tertiary arrhythmia center. We hypothesized that an interplay between presence of AF and enduring personality traits is central to distress severity. We also assessed the response of psychological distress and suicidal ideation to AF management strategies over a 12‐month follow‐up period, hypothesizing that effective rhythm control will lead to significant improvement in both variables.
Methods
Seventy‐eight consecutive consenting patients with symptomatic nonvalvular AF and preserved left ventricular systolic function (left ventricular ejection fraction >50%) aged between 18 and 80 years referred for AF management to a tertiary arrhythmia referral service were recruited over a 4‐month period. This represented 68% of the total population screened and eligible to participate. No patient was referred specifically because of suicidal ideation. Management decisions were taken in a shared decision model, with some patients electing to undergo early ablation and others in concert with the managing electrophysiologist electing to undergo a further period of medical therapy. This study reports on the outcomes of those patients electing a further period of medical treatment (n=58) and those wishing to move directly to ablation (n=20). The primary treating electrophysiologist was not directly involved in study recruitment or psychological assessment. All participants were followed prospectively over a 12‐month period, with study assessments at baseline, 4, 8, and 12 months. In the ablation cohort, the baseline visit was within 4 weeks of the planned AF ablation procedure. The study was approved by the Melbourne Health Human Research Ethics Committee, and all participants provided written informed consent. Data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
All participants underwent detailed assessment at the baseline study visit, which occurred following the initial clinical consultation. Baseline assessment included a transthoracic echocardiogram allowing for detailed assessment of left ventricular diastolic function and an ambulatory sleep study assessment (Somté; Compumedics Ltd, Abbotsford, Vic, Australia). Consenting participants (62 of 78; 80%) underwent implantation of a “Reveal” implanted loop recorder (ILR; Medtronic Inc, Minneapolis, MN) for quantification of AF burden at the commencement of the study.
The AFSS (Atrial Fibrillation Severity Scale)1 was used to quantify symptom severity at baseline. This self‐administered questionnaire includes an instrument to measure the presence and severity of individual symptoms attributable to AF over a 4‐week recall period, with 7 individual symptoms, including palpitation frequency, dyspnea, fatigue, and exercise intolerance, quantified on 5‐point Likert scales and higher values reflecting more‐severe AF symptoms (AFSS symptom score). The Physical Component Summary score of the SF‐36 questionnaire6 was used to quantify physical hrQOL. This 36‐item questionnaire measures 8 key health concepts over a 4‐week recall period, with a normative scoring system applied to generate a profile of individual domain scores and thence derive physical and mental component summary scores, with lower scores denoting poorer life quality.7
Participants also completed validated questionnaires examining domains of personality style3 and the presence of psychological distress. Questionnaires were completed by the study participant at home, after the initial clinical consultation and subsequent recruitment into the study.
Psychological distress, as indicated by the presence of both anxiety and depression symptoms, was quantified by the Hospital Anxiety and Depression Scale.8 This incorporates 14 items specifically designed to evaluate symptoms of anxiety and depression in medical populations, with minimal influence from somatic symptoms that may falsely elevate scores. Cronbach's α for the full scale in cardiac patients is 0.89.9 Items are scored on a 4‐point scale from 0 to 3, and the overall score has previously been taken as a measure of overall psychological distress, with a cutoff of ≥15 used to identify severe distress.9 The Beck Depression Inventory was also administered in order to screen for participants reporting thoughts of self‐harm.10 A definite self‐report of suicidal thoughts, even with an acknowledgement that the participant would not carry these out, was taken as an affirmative response for the purposes of this study. Participants so identified were assessed by a trained clinician and referred as appropriate for further management.
More‐enduring traits were explored at baseline with the Global Measure of Perceived Stress Scale (PSS)11 and the Type D (distressed) Personality Scale (TDPS).12 The PSS measures the degree to which life situations are appraised as stressful. Each item is scored on a 5‐point Likert scale from 0 to 4, with higher total scores indicating greater perceived stress. The Cronbach α coefficient of internal consistency is 0.85, and test‐retest stability is 0.85.3, 11 The Type D Personality denotes a high degree of negative affectivity and social inhibition in the underlying personality. It has been identified as a risk factor for adverse cardiovascular outcomes and is associated with poor hrQOL in AF.5 The TDPS is a 14‐item questionnaire, with each item scored between 0 and 4. It includes 2 subscales, the Negative Affectivity and the Social Inhibition subscales, with the Type D Personality considered to be present based on a score on both of these subscales of ≥10. Cronbach's α coefficient of internal consistency is between 0.86 and 0.88.12
Participants underwent repeat assessment of hrQOL and psychological distress at 4, 8, and 12 months. Of the 312 planned visits, 297 (92%) were completed and 12‐month data were available in 74 (95%). Participants underwent implantable loop recorder interrogation or 24‐hour Holter monitoring at each assessment. After an initial 6‐week blanking period, recurrence of AF after catheter ablation was defined as an episode of documented AF lasting ≥30 seconds, and overall AF burden was determined from implantable loop recorder recordings reviewed at each assessment as the proportion of time spent in AF over the 12‐month study period.
Statistical Analysis
Statistical analysis was performed using commercially available software (STATA version 12.1; StataCorp LP, College Station, TX). Normally distributed data are presented as mean±SD and otherwise as a median with interquartile range. Group means were compared using the Student t test. Statistical significance was set at the 0.05 level.
Relationships between independent variables and continuous outcome variables were tested using general linear models. Association of clinical and personality style variables with psychological distress was tested using simple linear regression, after demonstrating normally distributed residuals with homoscedasticity in their variance. Logistic regression was used to identify associations with suicidal ideation. Because of significant colinearity, the PSS score and TDPS score were subsequently fitted separately within multivariable models, along with the clinical variables that demonstrated an association with the relevant outcome variable on univariable analysis at a significance level of <0.1.
Linear mixed models were used to study temporal change in psychological distress. Participant identifier was included as a random term, whereas clinical group, time, and interaction of group and time were included as fixed terms. The approximate least significant difference method at the 0.05 level was used to estimate the significance of differences within the model.
Results
Overall, the study cohort, with a mean age of 60±9 years, a male predominance, a body mass index (BMI) of 30±5 kg/m2, and a prevalence of hypertension, diabetes mellitus, and vascular disease of 46%, 15%, and 21%, respectively, and a median CHA2DS2Vasc score of 1 (0–3) was broadly representative of patients referred for management to tertiary arrhythmia centers (Table 1), rather than of the broader community population with AF. Left ventricular diastolic function was well preserved, but there was significant left atrial structural remodeling manifest with an indexed left atrial volume of 47.2±15.2, consistent with a high arrhythmia burden. Of the total population, 58 (74%) had a paroxysmal pattern of AF and 20 (25%) had persistent AF at baseline. A total of 35 of the 78 patients in the study cohort (45%) were identified as manifesting the Type D Personality. Groups undergoing medical management and AF ablation were well matched at the time of the baseline study visit for important clinical variables (Table 1), with only a higher prevalence of sotalol therapy in the AF ablation cohort.
Table 1.
Characteristics of the Study Population at the Time of the Baseline Study Visit
| AF Med Mx (n=58) | AF Ablation (n=20) | P Value | Overall (n=78) | |
|---|---|---|---|---|
| Age, y | 60±10 | 57±13 | 0.242 | 59±10 |
| Male, n (%) | 43 (74) | 15 (75) | 0.323 | 58 (74) |
| BMI, kg/m2 | 30.1±5.7 | 29.2±3.7 | 0.519 | 29.9±5.2 |
| Hypertension, n (%) | 28 (48) | 8 (40) | 0.524 | 36 (46) |
| Diabetes mellitus, n (%) | 11 (19) | 2 (10) | 0.263 | 13 (17) |
| Stroke/TIA, n (%) | 4 (7) | 1 (5) | 0.768 | 5 (6) |
| Vascular disease, n (%) | 12 (21) | 4 (20) | 0.948 | 16 (21) |
| OSA, n (%) | 12 (21) | 4 (20) | 0.954 | 16 (21) |
| CHA2DS2Vasc | 1 (0–3) | 1 (0–2) | 0.691 | 1 (0–3) |
| E/E′ | 8.6±3.0 | 7.3±1.9 | 0.089 | 8.3±2.8 |
| LA volume, mL/m2 | 47.5±16.5 | 46.3±11.0 | 0.761 | 47.2±15.2 |
| Peak positive LA strain rate, s−1 | 1.02±0.36 | 0.98±0.21 | 0.681 | 1.0±0.3 |
| Chronic anticoagulation, n (%) | 32 (55) | 13 (65) | 0.671 | 45 (58) |
| β‐blocker, n (%) | 20 (34) | 7 (35) | 0.967 | 27 (35) |
| Calcium‐channel blocker, n (%) | 7 (12) | 2 (10) | 0.804 | 9 (12) |
| Digoxin, n (%) | 15 (26) | 4 (20) | 0.601 | 19 (24) |
| Flecainide, n (%) | 11 (19)a | 2 (10) | 0.357 | 13 (17) |
| Sotalol, n (%) | 15 (26)b | 10 (50) | 0.047 | 25 (32) |
AF indicates atrial fibrillation; BMI, body mass index; LA, left atrium; OSA, obstructive sleep apnea; TIA, transient ischemic attack.
Increased to 31% by 12‐month study visit.
Increased to 39% by 12‐month study visit.
At baseline, the AF ablation cohort reported an hrQOL that was nonsignificantly lower than that reported by the cohort managed medically (SF‐36 Physical Component Summary score: 40.3±2.2 versus 43.2±1.3; P=0.244), whereas severity of symptoms associated with AF episodes over a 4‐week recall period was significantly higher in the ablation group compared with the group undergoing medical management (AFSS symptom score: 16.7±1.8 versus 12.9±1.3; P=0.023).
Spectrum and Predictors of Psychological Distress in AF
At baseline assessment, the median Hospital Anxiety and Depression Scale score in the overall population was 10/42 (4–17), with significant psychological distress (Hospital Anxiety and Depression Scale score, ≥15) identified in 27 of 78 (35%) overall. Severity of psychological distress correlated directly with severity of AF symptoms (r=0.66; adjusted R 2=0.43; β=0.64±0.09; t=7.4; P<0.001) and inversely with the physical domain of hrQOL (r=−0.44; adjusted R 2=0.19; β=−0.36±0.09; t=−4.2; P<0.001).
Variables associated with higher levels of psychological distress were younger age, elevated BMI, and both measures of personality style (Table 2). Both the total Type D Personality score and presence of the Type D Personality, as defined by a score on both subscales of ≥10, were associated with greater levels of distress. A paroxysmal compared with a persistent pattern of AF was not significantly associated with distress.
Table 2.
Factors Associated With Psychological Distress and Suicidal Ideation by Univariable Analysis
| HADS Score | ||||
|---|---|---|---|---|
| Adjusted R 2 | β | t | P Value | |
| Clinical group (PAF vs PersAF) | 0.02 | −3.3±2.1 | −1.6 | 0.126 |
| AF burden, % | −0.01 | −0.02±0.03 | −0.6 | 0.543 |
| Age, y | 0.06 | −0.2±0.09 | −2.4 | 0.020 |
| Sex | 0.01 | 3.1±2.3 | 1.4 | 0.180 |
| BMI, kg/m2 | 0.05 | 0.4±0.2 | 2.2 | 0.028 |
| CHA2DS2Vasc score | −0.01 | −0.4±0.5 | −0.7 | 0.475 |
| LA volume, mL/m2 | −0.01 | −0.05±0.06 | −0.7 | 0.473 |
| LVMI, g/m2 | −0.01 | −0.02±0.04 | −0.4 | 0.692 |
| E/E′ | −0.01 | −0.3±0.3 | −0.9 | 0.354 |
| PSS score | 0.53 | 0.7±0.08 | 9.2 | <0.001 |
| TDPS score | 0.40 | 0.7±0.1 | 7.0 | <0.001 |
| Type D personality diagnosis | 0.13 | 6.2±1.8 | 3.4 | 0.001 |
| Suicidal Ideation | ||||
|---|---|---|---|---|
| Pseudo R 2 | Odds Radio | z | P Value | |
| Clinical group (PAF vs PersAF) | 0.001 | 1.0±0.6 | 0.1 | 0.953 |
| AF burden, % | 0.002 | 1.0±0.01 | −0.3 | 0.751 |
| Age, y | 0.001 | 1.0±0.02 | 0.4 | 0.712 |
| Sex | 0.01 | 1.8±1.0 | 1.0 | 0.322 |
| BMI, kg/m2 | 0.09 | 1.1±0.06 | 2.6 | 0.009 |
| CHA2DS2Vasc score | 0.01 | 1.2±0.2 | 1.1 | 0.268 |
| LA volume, mL/m2 | 0.007 | 1.0±0.02 | 0.8 | 0.428 |
| LVMI, g/m2 | 0.002 | 1.0±0.01 | 0.5 | 0.633 |
| E/E′ | 0.004 | 1.1±0.1 | 0.6 | 0.524 |
| PSS score | 0.28 | 1.2±0.05 | 4.1 | <0.001 |
| TDPS score | 0.40 | 1.3±0.1 | 4.2 | <0.001 |
| Type D personality diagnosis | 0.15 | 7.4±4.4 | 3.4 | 0.001 |
AF indicates atrial fibrillation; BMI, body mass index; HADS, Hospital Anxiety and Depression Scale; LA, left atrium; LVMI, left ventricular mass index; PSS, Perceived Stress Scale; TDPS, Type D Personality score.
In a multivariable analysis including PSS score, age, and BMI, a tendency to perceive of life events as stressful (PSS score) was the only independent predictor of distress (R 2 for the model, 0.54; β=0.72±0.11; t=7.8; P<0.001). In a second model substituting TDPS score for PSS score, a personality marked by negativity and social inhibition (TDPS score) was independently predictive (R 2 for the model, 0.47; β=0.73±0.09; t=6.7; P<0.001), along with younger age (Table 3). In a third model involving presence of the Type D Personality as a dichotomous variable, this was again an independent predictor of distress (R 2 for the model, 0.29; β=6.14±1.81; t=3.5; P=0.001), along with both younger age and an elevated BMI (Table 3).
Table 3.
Factors Associated With Psychological Distress (Total HADS Score) by Multivariable Analysis
| Variables Included in the Model | Model 1: PSS Score | Model 2: TDPS Score | Model 3: Type D Personality Diagnosis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | t | P Value | β | t | P Value | β | t | P Value | |
| Age | −0.10±0.07 | −1.44 | 0.153 | −0.22±0.07 | −3.11 | 0.003 | −0.21±0.08 | −2.54 | 0.013 |
| BMI, kg/m2 | −0.04±0.15 | −0.24 | 0.809 | −0.05±0.16 | −0.32 | 0.747 | 0.37±0.17 | 2.12 | 0.037 |
| Personality variable | 0.71±0.09 | 7.83 | <0.001 | 0.74±0.11 | 6.65 | 0.004 | 6.12±1.76 | 3.48 | 0.001 |
Personality style variables included individually in different models given their strong colinearity. Model 1: adjusted R 2=0.54. Model 2: adjusted R 2=0.47. Model 3: adjusted R 2=0.26. BMI indicates body mass index; HADS, Hospital Anxiety and Depression Scale; PSS, Perceived Stress Scale; TDPS, Type D Personality score.
Spectrum and Predictors of Suicidality in AF
Suicidal ideation was identified at baseline in 16 of 78 (20%) participants. Severity of AF‐related symptoms (AFSS symptom score, 18.9±6.6 versus 9.7±8.0; P<0.001) and psychological distress (Hospital Anxiety and Depression Scale score, 20.1±8.2 versus 9.4±6.9; P<0.001) were markedly higher and hrQOL was markedly lower (Physical Component Summary score, 37.6±7.6 versus 44.3±10.3; P=0.022) in those with suicidal ideation.
An elevated BMI, each of the PSS and TDPS scores, and the Type D Personality were associated with the presence of suicidal ideation (Table 2). Personality variables remained independent predictors on multivariable analysis (PSS score: R 2 for the model, 0.35; TDPS Score: R 2 for the model, 0.49; Type D Personality: R 2 for the model, 0.28; Table 4), with increasing BMI remaining predictive only in the model with the Type D Personality diagnosis (Table 4).
Table 4.
Factors Associated With Suicidal Ideation by Multivariable Analysis
| Model 1: PSS Score | Model 2: Type D Score | Model 3: Type D Personality Diagnosis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | z | P Value | Odds Ratio | z | P Value | Odds Ratio | z | P Value | |
| BMI, kg/m2 | 1.05±0.07 | 0.67 | 0.501 | 0.97±0.08 | −0.42 | 0.677 | 1.16±0.08 | 2.3 | 0.024 |
| Personality variable | 1.22±0.06 | 3.77 | <0.001 | 1.43±0.14 | 3.75 | <0.001 | 12.5±9.1 | 3.4 | 0.001 |
Personality style variables included individually in different models given their strong colinearity. Model 1: pseudo R 2=0.35; P<0.001. Model 2: pseudo R 2=0.49; P<0.001. Model 3: pseudo R 2=0.28; P<0.001. BMI indicates body mass index; PSS, Perceived Stress Scale; TDPS, Type D Personality score.
Response to AF Ablation
Over 12 months, AF burden measured by implanted loop recorders in the group undergoing medical management was 39% (3–100%). Conversely, over 12 months following catheter ablation, 80% remained free from AF and AF burden over 12 months was significantly lower than with medical management, with a median burden of 1% (0–2%; P<0.001). No repeat ablation procedures were performed during the study period. In the group undergoing medical management, therapeutic decisions over this time period were made in concert by the patient and their treating electrophysiologist, who was not involved in the psychological or other study assessments and with neither aware of the scores on the testing instruments for AF symptom severity or psychological distress. In this group, flecainide use increased from 19% to 31% and sotalol use increased from 26% to 39%.
There was significant between‐group divergence in psychological distress over 12 months (Figure), with significant improvement following AF ablation (13.9±1.8 to 4.3±1.8; P<0.05), but not with medical management (11.0±1.1 to 10.6±1.1; P>0.05). This paralleled a significant increase hrQOL in the cohort undergoing AF ablation as reflected in the Physical Component Summary score of the SF‐36 questionnaire (40.3±2.2 to 52.7±2.1; P<0.05), with no significant change in the group undergoing medical therapy (43.2±1.3 to 43.5±1.3; P>0.05). Consistent with these data, prevalence of suicidal ideation fell significantly only after AF ablation, falling from 6/20 (30%) to 1/20 (5%; P=0.021). There was no significant change with medical management of AF, between 9/58 (16%) at baseline and 14/58 (24%) at 12 months (P=0.642).
Figure 1.
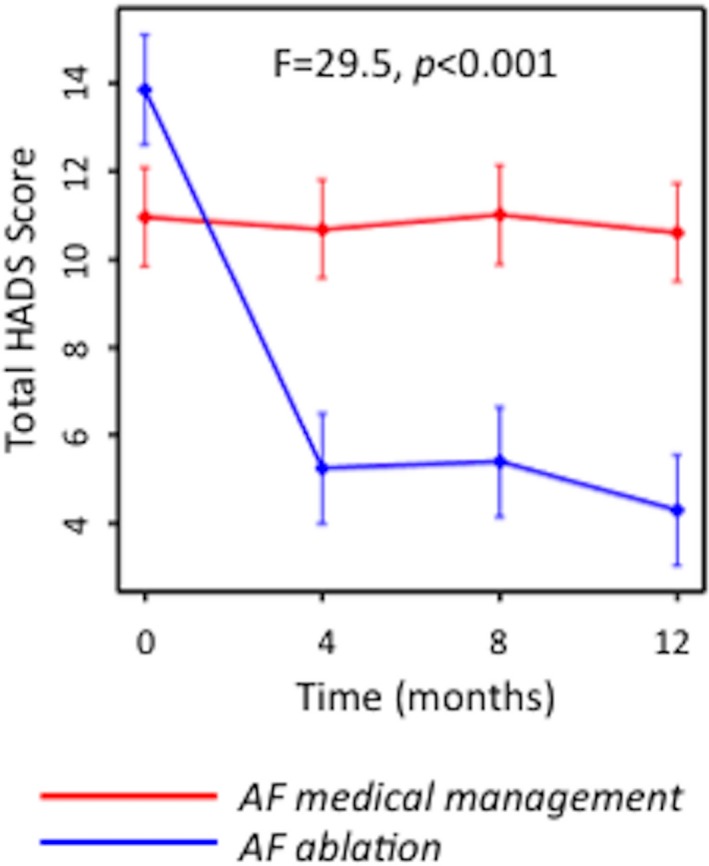
Temporal change over 12 months in psychological distress (HADS score) with AF ablation or medical management. Significant change was observed only in the group undergoing effective catheter ablation of AF. AF indicates atrial fibrillation; HADS, Hospital Anxiety and Depression Scale.
Discussion
In a population referred to a tertiary arrhythmia center, prevalence of severe psychological distress was 35% and that of suicidal ideation was 20%. This is the first study to suggest that AF may lead to severe psychological distress and also to suicidal ideation in a significant proportion of symptomatic patients. Whereas organic factors, including younger age and an elevated BMI, were associated with greater distress, personality style was the predominant predictor of distress severity and suicidal ideation. Importantly, however, effective catheter ablation of AF was associated with significant improvement in both psychological distress and reported suicidal ideation, indicating a complex interplay between enduring personality traits and AF, with AF itself potentially a causative and treatable factor in patients with personality‐based vulnerability.
Given that the interaction of psychological factors with clinical outcomes across a range of cardiovascular conditions has become better appreciated, increasing attention has been given to the interaction between these factors and human AF. Compared with both healthy and disease‐control2 cohorts, prevalence of anxiety and depression is elevated in those with AF, with anxiety the dominant affective response.3 This study extends these observations to highlight that not only do 35% experience significant psychological distress, but also that 20% report suicidal ideation. This prevalence is greatly in excess of the 9% lifetime prevalence13 and the 2% 12‐month prevalence14 previously identified in large population studies, but very similar to the 17% rate reported in patients with symptomatic congestive heart failure15 and the 21% rate in those with coronary artery disease undergoing active management.16 Prevalence of 5% after successful rhythm control with catheter ablation observed in this study population is more in line with these population prevalence rates.
Several groups have studied the impact of psychology on the patient experience of AF, in isolation from consideration of clinical or organic cardiac factors. Presence of anxiety and depression have been associated with more‐severe AF‐related symptoms and a lower hrQOL,2, 4 and several personality traits, including anxiety sensitivity,9 stress perception,3 somatization,17 negative affectivity and social inhibition,5, 18 and optimism,9 have been associated with the subjective experience of AF. The current study reinforces the centrality of enduring personality‐based vulnerabilities in this subjective experience. These appear to be the key predictors of psychological distress and even of suicidal ideation, largely subsuming the influence of clinical variables.
The influence of the Type D Personality on cardiovascular disease prevalence and outcomes has been a particular focus of previous research.19, 20, 21 In the current study, prevalence of Type D Personality was 45%, comparable to the 32%5 and 54%18 rates reported in previous studies of the Type D Personality in AF and higher than the prevalence reported in the general population of between 18% and 39%.21 Previous researchers have identified the Type D Personality as a predictor of suicidal ideation in a variety of populations, independent of demographic or socioeconomic variables22, 23 and independent of cardiovascular comorbidities.22 In this context, the current study suggests that identification of the Type D Personality might play an important role in clinical AF management, in risk stratification for distress and suicidal ideation.
It has been previously suggested that personal psychology and presence of psychological distress might, through an influence on the sympathetic nervous system, systemic and regional inflammation, and endothelial function, be factors contributing to AF development,24 and it is likely that psychological factors and a distressed personality would increase the likelihood of detecting AF episodes. A personality marked by chronic tension has been associated with increased risk of incident AF in men,25 the Type D Personality has specifically been associated with an increased risk of incident AF following cardiac surgery,26 depression is associated with increased risk of recurrent AF after electrical cardioversion and catheter ablation,27 and yoga has been demonstrated not only to improve hrQOL and reduce psychological distress in AF, but also even to reduce the frequency of symptomatic and asymptomatic AF episodes recorded by external monitoring.24 Neurohormonal activation and inflammation certainly play a role in atrial remodeling in AF,28 and chronic stress may activate such physiological systems.29 With the active intervention in the current study directed only at the arrhythmia and with it leading to marked improvements in levels of psychological distress, these data now highlight the complexity of the interaction between personal psychology, AF, and distress. Indeed, following effective ablation, there was significant reduction both in distress and in prevalence of suicidal ideation, suggesting that distress is an important consequence of AF in vulnerable patients, and one that may be ameliorated by effective rhythm management.
Limitations
This was a prospective but observational and nonrandomized study in which all eligible patients undergoing AF management in a tertiary arrhythmia service were invited to participate. Assignment to treatment group was made independently by treating electrophysiologists who were not involved in psychological assessments, with many patient, drug, and disease factors contributing to this decision.30 Nevertheless, treatment groups were well matched at baseline for most important clinical variables that might impact on levels of psychological distress. With the study population drawn from a tertiary referral arrhythmia center, its results may not be generalizable to populations managed in less‐specialized settings. The representation of female participants was lower than that of male participants, potentially compromising its power to demonstrate female sex as an important predictive variable.
Whereas the current study suggests that extent of distress in an individual patient is influenced by their individual psychological and personality‐based characteristics, other factors such as pre‐existing health beliefs might also be expected to have an influence, and these were not systematically explored. As noted, study assessments were undertaken distinct from the clinical management provided by the same group of clinical electrophysiologists for both participant groups. Responses to questionnaires and the perceived well‐being of study participants may have been influenced by the extent and quality of interaction between caregivers and patients, with an influence on study assessments from the health beliefs of each participant and their beliefs around likely future outcomes.
Conclusion
In a tertiary AF population, significant psychological distress is observed in 35% and suicidal ideation may be reported by up to 20%. A personality marked both by vulnerability to the perception of life events as stressful and by negativity or social inhibition is a key predictor of distress. Significant reduction in levels of psychological distress and prevalence of suicidal ideation follow effective AF ablation, suggesting that distress is a consequence of AF itself interacting with personality‐based vulnerabilities.
Sources of Funding
Dr Walters is supported by a Postgraduate Research Scholarship from the National Health and Medical Research Council and the National Heart Foundation of Australia. Professors Sanders, Kistler and Kalman are supported by Practitioner Fellowships from the National Health and Medical Research Council of Australia.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e005502 DOI: 10.1161/JAHA.117.005502.)
References
- 1. Dorian P, Jung W, Newman D, Paquette M, Wood K, Ayers GM, Camm J, Akhtar M, Lüderitz B. The impairment of health‐related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol. 2000;36:1303–1309. [DOI] [PubMed] [Google Scholar]
- 2. Thrall G, Lip GY, Carroll D, Lane D. Depression, anxiety, and quality of life in patients with atrial fibrillation. Chest. 2007;132:1259–1264. [DOI] [PubMed] [Google Scholar]
- 3. Lane DA, Langman CM, Lip GY, Nouwen A. Illness perceptions, affective response, and health‐related quality of life in patients with atrial fibrillation. J Psychosom Res. 2009;66:203–210. [DOI] [PubMed] [Google Scholar]
- 4. Charitakis E, Barmano N, Walfridsson U, Walfridsson H. Factors predicting arrhythmia‐related symptoms and health‐related quality of life in patients referred for radiofrequency ablation of atrial fibrillation: an observational study (the SMURF Study). JACC Clin Electrophysiol. 2017;3:494–502. [DOI] [PubMed] [Google Scholar]
- 5. Son YJ, Song EK. The impact of type D personality and high‐sensitivity C‐reactive protein on health‐related quality of life in patients with atrial fibrillation. Eur J Cardiovasc Nurs. 2012;11:304–312. [DOI] [PubMed] [Google Scholar]
- 6. Ware J, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 7. McHorney CA, Ware JE, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. [DOI] [PubMed] [Google Scholar]
- 8. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. [DOI] [PubMed] [Google Scholar]
- 9. Ong L, Cribbie R, Harris L, Dorian P, Newman D, Mangat I, Nolan R, Irvine J. Psychological correlates of quality of life in atrial fibrillation. Qual Life Res. 2006;15:1323–1333. [DOI] [PubMed] [Google Scholar]
- 10. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories‐1A and ‐2 in psychiatric outpatients. J Pers Assess. 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 11. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 12. Denollet J. DS14: standard assessment of negative affectivity, social inhibition, and type D personality. Psychosom Med. 2005;67:89–97. [DOI] [PubMed] [Google Scholar]
- 13. Nock MK, Borges G, Bromet EJ, Alonso J, Angermeyer M, Beautrais A, Bruffaerts R, Chiu WT, de Girolamo G, Gluzman S, de Graaf R, Gureje O, Haro JM, Huang Y, Karam E, Kessler RC, Lepine JP, Levinson D, Medina‐Mora ME, Ono Y, Posada‐Villa J, Williams D. Cross‐national prevalence and risk factors for suicidal ideation, plans and attempts. Br J Psychiatry. 2008;192:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borges G, Nock MK, Haro Abad JM, Hwang I, Sampson NA, Alonso J, Andrade LH, Angermeyer MC, Beautrais A, Bromet E, Bruffaerts R, de Girolamo G, Florescu S, Gureje O, Hu C, Karam EG, Kovess‐Masfety V, Lee S, Levinson D, Medina‐Mora ME, Ormel J, Posada‐Villa J, Sagar R, Tomov T, Uda H, Williams DR, Kessler RC. Twelve‐month prevalence of and risk factors for suicide attempts in the World Health Organization World Mental Health Surveys. J Clin Psychiatry. 2010;71:1617–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lossnitzer N, Müller‐Tasch T, Löwe B, Zugck C, Nelles M, Remppis A, Haass M, Rauch B, Jünger J, Herzog W, Wild B. Exploring potential associations of suicidal ideation and ideas of self‐harm in patients with congestive heart failure. Depress Anxiety. 2009;26:764–768. [DOI] [PubMed] [Google Scholar]
- 16. Nascimento ER, Maia AC, Soares‐Filho G, Nardi AE, Cardoso A. Predictors of suicidal ideation in coronary artery disease. Compr Psychiatry. 2015;57:16–20. [DOI] [PubMed] [Google Scholar]
- 17. Gehi AK, Sears S, Goli N, Walker TJ, Chung E, Schwartz J, Wood KA, Guise K, Mounsey JP. Psychopathology and symptoms of atrial fibrillation: implications for therapy. J Cardiovasc Electrophysiol. 2012;23:473–478. [DOI] [PubMed] [Google Scholar]
- 18. Jeong HK, Cho JG, Lee KH, Park HW, Kim MR, Lee KJ, Jang SY, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Hong YJ, Kim JH, Ahn Y, Jeong MH, Park JC. Determinants of quality of life in patients with atrial fibrillation. Int J Cardiol. 2014;172:e300–e302. [DOI] [PubMed] [Google Scholar]
- 19. Denollet J, Sys SU, Stroobant N, Rombouts H, Gillebert TC, Brutsaert DL. Personality as independent predictor of long‐term mortality in patients with coronary heart disease. Lancet. 1996;347:417–421. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen SS, Lemos PA, van Vooren PR, Liu TK, Daemen J, Erdman RA, Smits PC, Serruys PW, van Domburg RT. Type D personality predicts death or myocardial infarction after bare metal stent or sirolimus‐eluting stent implantation: a Rapamycin‐Eluting Stent Evaluated at Rotterdam Cardiology Hospital (RESEARCH) registry substudy. J Am Coll Cardiol. 2004;44:997–1001. [DOI] [PubMed] [Google Scholar]
- 21. Mols F, Denollet J. Type D personality in the general population: a systematic review of health status, mechanisms of disease, and work‐related problems. Health Qual Life Outcomes. 2010;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Michal M, Wiltink J, Till Y, Wild PS, Münzel T, Blankenberg S, Beutel ME. Type‐D personality and depersonalization are associated with suicidal ideation in the German general population aged 35‐74: results from the Gutenberg Heart Study. J Affect Disord. 2010;125:227–233. [DOI] [PubMed] [Google Scholar]
- 23. Yoon DH, Kim SJ, Lee JH, Kim PM, Park DH, Ryu SH, Yu J, Ha JH. The relationship between type D personality and suicidality in low‐income, middle‐aged adults. Psychiatry Investig. 2015;12:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakkireddy D, Atkins D, Pillarisetti J, Ryschon K, Bommana S, Drisko J, Vanga S, Dawn B. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. 2013;61:1177–1182. [DOI] [PubMed] [Google Scholar]
- 25. Eaker ED, Sullivan LM, Kelly‐Hayes M, D'Agostino RB, Benjamin EJ. Tension and anxiety and the prediction of the 10‐year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham Offspring Study. Psychosom Med. 2005;67:692–696. [DOI] [PubMed] [Google Scholar]
- 26. Kelpis T. Prevalence of “distressed” personality in patients with coronary artery disease and its correlation with morbidity after coronary surgery. Hellenic J Cardiol. 2013;54:362–367. [PubMed] [Google Scholar]
- 27. Efremidis M, Letsas KP, Lioni L, Giannopoulos G, Korantzopoulos P, Vlachos K, Dimopoulos NP, Karlis D, Bouras G, Sideris A, Deftereos S. Association of quality of life, anxiety, and depression with left atrial ablation outcomes. Pacing Clin Electrophysiol. 2014;37:703–711. [DOI] [PubMed] [Google Scholar]
- 28. Aviles RJ, Martin DO, Apperson‐Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 29. Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185:320–326. [DOI] [PubMed] [Google Scholar]
- 30. Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haïssaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jaïs P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow‐up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. [DOI] [PubMed] [Google Scholar]


