Abstract
Introduction
Regenerative therapy is a developing field in medicine. In the production of cell products for these therapies, hygienic management is even more critical than in the production of a chemical drug. At the same time, however, care is required with the use of decontamination agents, considering their effects on cell viability and characteristics. To date, hydrogen peroxide (H2O2) is most widely used for decontamination in pharmaceutical plants and cell processing facilities.
Methods
In this study, we examined the effects of residual H2O2 in the atmosphere of cell processing units after decontamination on the viability and proliferation of mesenchymal stem cells derived from human bone marrow.
Results
We detected residual H2O2 sufficient to affect cell proliferation and survival even more than 30 h after decontamination ended. Our results suggest a longer time period is required before starting operations after decontamination and that the operating time should be as short as possible.
Conclusions
Here we show the effects of post-decontamination residual H2O2 on the viability and proliferation of mesenchymal stem cells derived from human bone marrow, which may provide us with important information about the hygienic management of cell processing facilities.
Keywords: Hydrogen peroxide (H2O2), Hygienic management, Biological safety cabinet (BSC), Messenchymal stem cells (MSCs)
Abbreviations: H2O2, hydrogen peroxide; CPF, cell processing facility; BSC, biological safety cabinet; MSC, mesenchymal stem cell; HEPA, high efficiency particulate air
Highlights
-
•
Post-decontamination residual H2O2 in BSC affected the proliferation of MSCs for more than 30 h.
-
•
The concentration of H2O2 in water and culture medium was higher than that in the atmosphere.
-
•
Supplementation of culture medium with pyruvic acid canceled the effects of H2O2 on cultured cells.
1. Introduction
Regenerative medicine is currently a focus of global attention, and countries worldwide are actively working to create these products as new treatment modalities through pioneering research and novel discoveries of technologies [1]. In the production of cell products for clinical administration, hygienic management is even more important than in the production of chemical agents. Materials and products in cell/tissue production cannot be sterilized chemically or physically, and contaminating pathological microbes can proliferate during cell processing. In Japan, two laws were established in November 2014 [2], [3], [4], [5]: the Act on the Safety of Regenerative Medicine and the Act on Pharmaceuticals and Medical Devices (PMD Act) were implemented, and legal regulations both in clinical research and in production of regenerative products were prepared. Under these regulations, sanitary control and prevention of contamination in cell processing facilities (CPFs) are major components. Although detailed and global guidance and standards of hygiene management are available regarding the production of chemical agents, no well-established guidance exists for the production of cell/tissue products at the bench in CPF [6]. In most situations, guidelines for good manufacturing practices or others for sterile pharmaceutical products and foods are applied for the production of cell/tissue processing, but each individual person at each facility operates production under their own judgment regarding the specific issues in regenerative products.
In the selection of decontamination agents for CPF, the adverse effects on staff and cells must be considered along with antimicrobial effectiveness. In the regenerative therapy field, because we use various cell types and amounts of cells for different conditions, the types and quantities of agents for decontamination should be selected in accordance with the specific factors involved. Formerly, formaldehyde was generally used for atmospheric decontamination in pharmaceutical facilities. However, with the cancer-causing effects of formaldehyde identified [7], [8] as well as acute toxicities such as mucosal irritation and cutaneous inflammation [9], it was classified as a probable human carcinogen by the World Health Organization (WHO) International Agency for Research on Cancer. Currently, its use is seriously restricted by WHO and other environmental regulations. Thus, the establishment of proper guidance for the sanitation of CPF atmosphere using decontamination agents other than formaldehyde is an emergent issue.
Hydrogen peroxide (H2O2), chlorine disinfectants, and their mixtures now are widely used for decontamination in pharmaceutical plants and CPFs [10], [11]. Above all, H2O2 is mostly a sterilizing agent for sanitation in CPFs [12]. H2O2 is a reactive oxygen species, which can degrade organic compounds. Because it demonstrates broad-spectrum antimicrobial efficacy, including against bacterial spores, viruses, and yeasts, and has insecticidal power, it is applied to sterilize various surfaces [13], such as surgical tools [14], and can also be used as mist for room sterilization [15], [16]. However, highly concentrated H2O2 should be considered hazardous because it is an aggressive oxidizer and will corrode many materials, including human skin [17]. The American Conference of Governmental Industrial Hygienists (ACGIH) has also classified H2O2 as a “known animal carcinogen, with unknown relevance on humans” and has established a permissible exposure limit of 1.0 ppm calculated as an 8-h time-weighted average [18].
In this study, we first examined the effects of residual H2O2 after decontamination on the viability and proliferation of cells. Although the effects of low concentration of H2O2 in the atmosphere will be different depending on the cell types, in this study, we selected MSCs as cell type, which can be a representative example because they are insulated from the influence of other conditions, such as operation technique, cell lot, and all.
2. Materials and methods
2.1. Experimental design
The effects of residual H2O2 on cell growth after decontamination were evaluated by exposing human MSCs in a biological safety cabinet (BSC) in an experimental vinyl chamber within a cleanroom. After the decontamination with H2O2 vapor, we waited until the atmospheric concentration of H2O2 adequately fell down. And then MSCs were exposed in the BSC for some periods, and their proliferation rates were evaluated.
2.2. Construction of a vinyl chamber in clean room
The chamber was composed of vinyl sheets (PVC Film Achilles flare FU-RE04; Achilles Corporation, Tokyo, Japan), including a BSC and H2O2 generator (DryDeco mobby MHPE-0411TK02; Taikisha Ltd., Tokyo, Japan) to produce H2O2 mist. It was constructed within a clean room to prevent H2O2 from leaking out and to keep the atmospheric concentration of H2O2 constant. The size of chamber was 9 m3.
2.3. Measurement of H2O2 concentration in water and in atmosphere
Concentrations of H2O2 dissolved in water were measured using water analysis products (DIGITALPACKTEST-MULTI DPM-MT and PACKTEST WAK-H2O2; Kyoritsu Chemical-Check Lab Corp., Tokyo, Japan) for H2O2 (high concentration, 3–700 mg/L; low concentration, 0.05–5 mg/L).
Atmospheric concentrations of H2O2 were measured by H2O2 sensors (DragerPolytron700, 8317610; Drager Sensor H2O2 HC, 6809675, for 30–300 mg/L and Drager Sensor H2O2LC, 6809705 for 3–30 mg/L). We also used a detector tube (gas detector tube measurement system GV-110S and H2O2 detector tube No. 32; Gastec Corporation, Kanagawa, Japan) to confirm that H2O2 concentration was lower than 1.0 mg/L.
2.4. Cell preparation and cell culture
MSCs derived from human bone marrow were purchased from PromoCell GmbH (Heidelberg, Germany, Cat. C12974, Lot 4031804.5) and cultured in Dulbecco's Modified Eagle Medium (Nacalai Tesque, Kyoto, Japan) containing 10% fetal bovine serum (Nacalai Tesque, Kyoto, Japan) and 1% antibiotic-antimycotic mixed stock solution (100×), including penicillin 10,000 units/mL, streptomycin 10,000 μg/mL, and amphotericin-B 25 μg/mL (Nacalai Tesque). At experiment start, MSCs subcultured in three passages were seeded at a density of 2.2 × 105 cells/100 mm dish and cultured in a highly humidified incubator maintained with 5% CO2 at 37 °C. H2O2 for supplementation in culture medium was purchased form Fujifilm Wako Pure Chemical Corporation (Osaka, Japan), and sodium pyruvate solution was from Nacalai Tesque.
2.5. Cell calculation
To quantify the proliferation rate of cultured MSCs, detached MSCs were stained with trypan-blue solution. Cell numbers were counted using Countess® II FL (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.6. Statistical analyses
Statistical analyses were carried out using standard Student t tests, and error bars indicate standard deviation. A P value ≤ 0.05 was considered to represent a statistically significant difference.
3. Results
3.1. Decontamination with H2O2 in the working unit in CPF and its concentration in the atmosphere
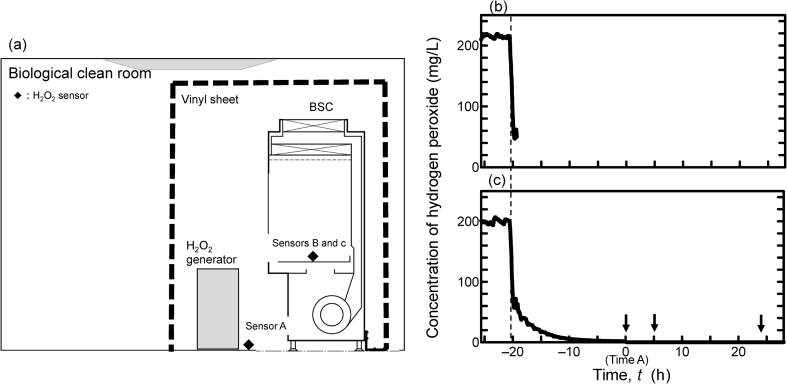
The effects of residual H2O2 on cell growth after decontamination were evaluated using human MSCs in an experimental vinyl chamber installed within a cell processing clean room. As shown in Fig. 1a, two types of H2O2 sensors, for high concentration (30–300 mg/L; sensors A and B) and for low concentration (3–30 mg/L; sensor c), were set in the chamber. Sensors A and B were used during generation of H2O2, and sensor c was used for concentration monitoring after generation of H2O2 was stopped.
Fig. 1.
The construction of experimental chamber in clean room and H2O2 concentrations. (a) Schematic illustration of the experimental environments. An experimental chamber with vinyl sheet was set in a biological clean room including a BSC and H2O2 generator. Filled squares indicate H2O2 sensors, and the dotted line indicates the vinyl sheet. (b) Time profile of H2O2 concentration. Curved line shows the concentration of H2O2 in the vinyl sheet measured by the sensor A in Fig. 1 during the decontamination. (c) Line shows the concentration of H2O2 in the BSC measured by sensor B and c in Fig. 1 during and after decontamination. The minimum detection limit of the sensors was 3 mg/L. Time A: t = 0 is defined as the time point at which H2O2 concentration reached 1 mg/L, as measured by a detection tube. The dotted line indicates the end of 5 h of decontamination. Each arrow indicates the time when the respective dish was uncovered in the subsequent experiments.
In the chamber, H2O2 was generated to keep the concentration in the atmosphere at approximately at 200 mg/L for 5 h. During this time, the BSC continued running, and the H2O2 of the atmosphere within the cabinet was kept about 200 mg/L, just below the concentration outside the cabinet. For concentrations during and after the generation of H2O2, values detected by sensors are shown in Fig. 1. After stopping the generation, the cracking unit was started, and H2O2 concentrations at sensors A and B were rapidly degraded to 50 mg/L. However, after that, the decrease rate of H2O2 became slower and slower. It was not until 25 h and 40 min after stopping generation of H2O2 that the sensitive detector tube showed 1.0 mg/L, which is the time-weighted average threshold limit value of H2O2 by ACGIH [18]. At that time point, defined as Time A, we started the clean room ventilation and removed the vinyl sheets.
To confirm the efficacy of H2O2 against microbes, biological indicators covered with over 1 × 106 of spore bacteria were placed at three points in the BSC before H2O2 generation. We could identify no growth in any of the biological indicators after a 7-day incubation at 55 °C (data not shown).
3.2. Concentrations of integrated H2O2 in the water exposed to atmosphere
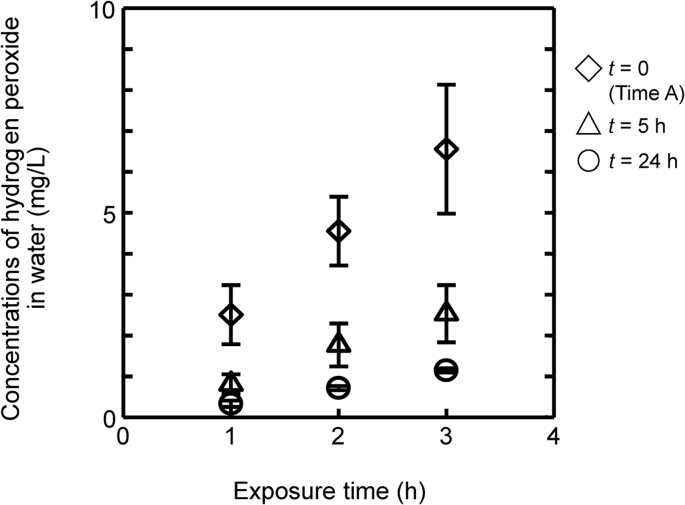
To investigate how much H2O2 residual in the atmosphere after decontamination could be integrated into water, 100-mm dishes filled with distilled water to 10 mm depth were exposed covered or uncovered in the BSC from Time A, 5 h and 24 h after Time A, respectively. Dishes were retrieved after 1, 2, or 3 h of exposure in the cabinet, and the concentrations of H2O2 in the water of dishes were measured.
Concentrations of H2O2 in the water of dishes are shown in Fig. 1b and c. When the dishes were placed uncovered in the cabinet at Time A, the H2O2 concentrations in the water were 2.5, 4.6, and 6.6 mg/L after 1, 2, and 3 h of exposure, respectively, even though the concentration in the air flow in cabinet was no more than 1.0 mg/L. For the same measurements performed with dishes placed at 5 h after Time A, each concentration was 0.8, 1.6, and 2.5 mg/L, respectively, and at 24 h after Time A, they were 0.3, 0.7, and 2.5 mg/L, respectively (Fig. 2). These results suggest that the residual H2O2 in the BSC was integrated into the water exposed to the air flow even after some substantive waiting time and that this integration rate depended on the exposure period.
Fig. 2.
Concentrations of H2O2 in water. Uncovered dishes of distilled water at 10 mm of depth were placed in the BSC for 1, 2, and 3 h from 0, 5, and 24 h after Time A, respectively. Vertical bars indicate the standard deviations (n = 3).
3.3. The correlation of H2O2 concentration dissolved in the medium with the proliferation of MSCs
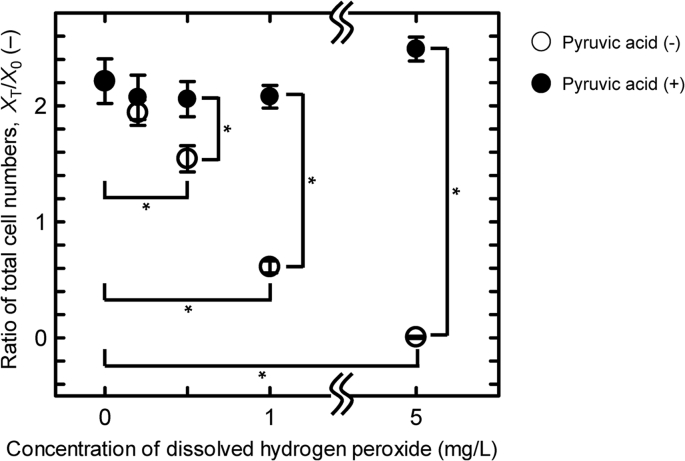
To elucidate the effects on cell growth of H2O2 dissolved in the culture medium, we observed cell proliferation following addition of known doses of liquid H2O2 directly in the medium. MSCs were seeded in the medium supplemented with H2O2 at the concentrations of 0, 0.1, 0.2, 0.5, 1.0, and 5.0 mg/L, respectively, and cultured in the CO2 incubator for 48 h. Cell proliferation and survival rates were significantly reduced dose dependently with more than 0.5 mg/L of H2O2 (Fig. 3), and MSCs were killed off with 5.0 mg/L of H2O2. These results, together with those in Fig. 2, suggest that during operation of the BSC, residual H2O2 could dissolve in the culture medium and affect later cell proliferation.
Fig. 3.
Ratio of total cell numbers of MSCs cultured in the medium mixed with H2O2. MSCs were cultured for 48 h in the medium supplemented with H2O2 at a concentration of 0, 0.2, 0.5, 1.0, and 5.0 mg/L, respectively, with (filled circle) or without (open circle) pyruvic acid. Vertical bars indicate the standard deviations (n = 3). Statistical significance based on the student's t-test (*P < 0.01).
3.4. The effects of residual H2O2 in the cabinet on the proliferation of MSCs
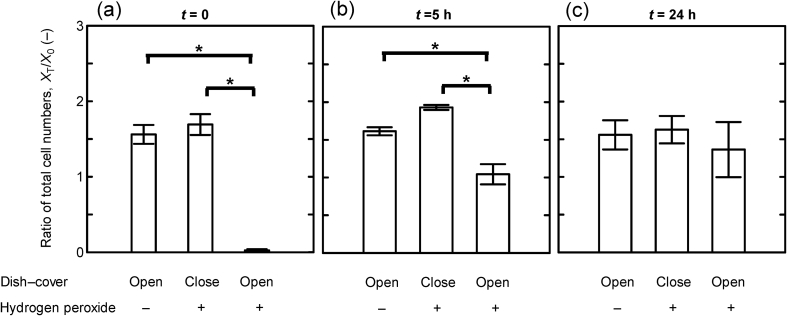
To examine the effects of residual H2O2 in BSC on the proliferation of cells, we seeded defrosted MSCs in 100-mm dishes at a concentration of 5000 cells/cm2, i.e., 275,000 cells/dish. After 24 h of incubation, these dishes were placed covered or uncovered for an hour in the cabinet decontaminated with H2O2 or in the cabinet not exposed to H2O2. After that, MSCs in each dish were cultured in CO2 incubator for 48 h and their viability assessed. When the MSCs were placed uncovered in the BSC at Time A, almost all the MSCs were killed off after 48-h culture. However, when the dish was covered, the proliferation rate of MSCs was not significantly different from those in an uncovered dish in the cabinet not exposed to H2O2. When MSCs were placed in the cabinet after 5 h of waiting time from Time A, the proliferation rate of cells in the uncovered dish was also significantly reduced compared to that of cells in the covered dish or not exposed to residual H2O2. The 24 h of waiting time from Time A, i.e., 50 h from stopping decontamination, could almost entirely cancel the effects of residual H2O2 on the cell proliferation rate (Fig. 4). These results suggest that H2O2 remaining in the equipment could affect cell proliferation and survival for more than 30 h after decontamination.
Fig. 4.
Ratio of total cell numbers of MSCs exposed to the atmosphere after decontamination. MSCs were cultured for 72 h after exposure to the atmosphere in the BSC for 1 h from the indicated points (t = 0 (a), 5 (b), and 24 (c) hours after Time A, respectively). At each time, cell dishes were placed uncovered in the not-decontaminated BSC (left bar), covered in the decontaminated BSC (middle bar), or uncovered in the decontaminated BSC with H2O2 (right bar). Vertical bars indicate the standard deviations (n = 3). Statistical significance based on the student's t-test (*P < 0.01).
3.5. Cancellation of H2O2 toxicity by pyruvic acid
We also examined the effects of pyruvic acid, which has been reported to protect cells against reactive oxygen species such as H2O2 [9], [19]. MSCs were cultured in the medium supplemented with 1 mM of pyruvic acid along with liquid H2O2 at concentrations of 0, 0.1, 0.2, 0.5, 1.0, and 5.0 mg/L, respectively, and their proliferation evaluated. As shown with filled circles in Fig. 3, the proliferation of MSCs cultured in medium including pyruvic acid was not affected even by 5.0 mg/L of H2O2. Thus, the effects of residual H2O2 after decontamination can be avoided by a deoxidizing composition of medium as well as the waiting time after decontamination.
4. Discussion
Sterilization of BSCs in the clean room is now mainly achieved with wiping using decontamination agents. However, because fungi or bacterial spores trapped in HEPA filters and other equipment can diffuse into the atmosphere, we should develop a safe and effective way to decontaminate the whole environmental space, based on valid evidence. We studied the effects of residual H2O2 on the cell viability and proliferation, and our results will provide some important information in achieving new methods of decontamination for the entire space.
We detected residual H2O2 that was sufficient to affect cell proliferation and survival at more than 30 h after stopping H2O2 generation. Today, in cell/tissue engineering fields as well as in pharmaceuticals, H2O2 vapor is gaining in importance as a decontamination agent of cell processing equipment, especially of cell processing isolators [20]. Some studies have reported that the effects on microbes of some fixed concentrations of H2O2 are influenced by the atmospheric humidity [21], [22]. Because H2O2 is highly stable in liquid form, it can be easily condensed in water, so that we have to consider that exposure to a low concentration of H2O2 in atmosphere can mean a higher concentration in culture medium. It is probable that cells processed in isolator systems have been damaged because of residual H2O2 without noticed, because cells from organs have their own individual features and strict specifications cannot be applied across all cell/tissue products. Although clean rooms and isolator systems in cell culture engineering have been widely developed, H2O2-inactivation time for changeover has not established yet. More data are needed to establish guidance for the use of clean room and isolators for cell processing.
The reason H2O2 vapor remained so long in the atmosphere in the BSC may be mainly that a non-negligible amount of H2O2 was absorbed onto the high efficiency particulate air (HEPA) filter attached to the BSC, which flowed down into the culture dish along with the blowing air from the filter. How much H2O2 can be absorbed in a HEPA filter is unknown and should be our next area of focus. In our data in Fig. 1, H2O2 concentration in atmosphere in the BSC was a little lower than that outside of the BSC during the generation of H2O2, and this gap might represent absorbed and accumulated H2O2 in the HEPA filter or in other parts of the BSC throughout the decontamination period.
In the case of a covered culture dish or flask (data not shown), H2O2 in the atmosphere had little effect on cell proliferation, even though the inside gas was exchanged effectively and maintained gas balance with the outer air. This fact suggests that in the event of an uncovered dish, the surface of the culture medium was contacting the air flow including H2O2 but at a very low concentration and was continuously blending and condensed into the culture medium. On the other hand, because the ventilation velocity of the covered dish or flask is slower than the dissolving speed of H2O2 into culture medium, the inside H2O2 concentration would have become lower and lower. When we use containers with a wide contact area with the outer air such as a culture dish, we invest in shortening the operating time.
Vapor phase H2O2 has effective broad-spectrum antimicrobial properties and inactivates bacteria, fungi, viruses, and highly resistant spores by its oxidizing functions [15], [23]. However, several endogenous or exogenous molecules in the organisms, such as cysteine, glutathione, methionine, ascorbic acid, and α-keto acids, including pyruvic acid, alter oxidation of proteins and important cellular components caused by H2O2 and reactive oxygen species [24]. The presence of catalase or other peroxidases in these organisms can increase tolerance in the presence of lower H2O2 concentrations. In this case, higher concentrations and longer contact times would be required for sporicidal activity, and the amount of residual H2O2 in the atmosphere or in the detail of device is non-negligible.
Pyruvic acid is a main player in energy metabolism in cells. It supplies energy to cells through the citric acid cycle (also known as the Krebs cycle) under aerobic conditions and produces lactate when oxygen is lacking. In the citric acid cycle, pyruvic acid is carboxylated into oxaloacetate, which reduces NADH to NAD. Thus, pyruvic acid is an excellent scavenger of oxidant species through its own decarboxylation [19]. By supplementing culture medium with pyruvic acid, the effect of H2O2 on cultured cells in culture medium can be canceled without undermining its antiseptic effects in the atmosphere.
Acknowledgement
Some methodological suggestions in constructing simulation chamber were kindly provided by Mr. Matsuhisa Kameyama.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Shi Y., Inoue H., Wu J.C., Yamanaka S. Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov. 2016;16:115–130. doi: 10.1038/nrd.2016.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobita M., Konomi K., Trashima Y., Kimura K., Taoka M., Kaminota M. Japan's challenges of translational regenerative medicine: Act on the safety of regenerative medicine. Regen Ther. 2016;4:78–81. doi: 10.1016/j.reth.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maeda D., Yamaguchi T., Ishizuka T., Hirata M., Tanaka K., Sato D. Regulatory frameworks for gene and cell therapies in Japan. Adv Exp Med Biol. 2015;871:147–162. doi: 10.1007/978-3-319-18618-4_8. [DOI] [PubMed] [Google Scholar]
- 4.Tsukamoto A., Abbot S., Kabyk L., Dewitt N., Schaffer D., Werener M. Challenging regeneration to transform medicine. Stem Cells Transl Med. 2016;5:1–7. doi: 10.5966/sctm.2015-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusakabe T. Regulatory perspectives of Japan. Biologicals. 2015;43:422–424. doi: 10.1016/j.biologicals.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Iwayama D., Yamato M., Tsubokura T., Takahashi M., Okano T. Cell/tissue processing information system for regenerative medicine. J Tissue Eng Regen Med. 2016;10:908–915. doi: 10.1002/term.1869. [DOI] [PubMed] [Google Scholar]
- 7.Bassig B.A., Zhang L., Vermeulen R., Tang X., Li G., Lan Q. Comparison of hematological alterations and markers of B-cell activation in workers exposed to benzene, formaldehyde and trichloroethylene. Carcinogenesis. 2016;37:692–700. doi: 10.1093/carcin/bgw053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lan Q., Smithe M.T., Tang X., Guo W., Vermeulen R., Zhang L. Chromosome-wide aneuploidy study of cultured circulating myeloid progenitor cells from workers occupationally exposed to formaldehyde. Carcinogenesis. 2015;36:160–167. doi: 10.1093/carcin/bgu229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzio E., Soliman K.F.A. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci Lett. 2003;337:77–80. doi: 10.1016/s0304-3940(02)01327-7. [DOI] [PubMed] [Google Scholar]
- 10.Ali S., Muzslay M., Bruce M., Jeanes A., Moore G., Wilson A. Efficacy of two hydrogen peroxide vapour aerial decontamination systems for enhanced disinfection of meticillin-resistant Staphylococcus aureus, Klebsiella pneumoniae and Clostridium difficile in single isolation rooms. J Hosp Infect. 2016;93:70–77. doi: 10.1016/j.jhin.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Barbut F., Menuet D., Verachten M., Girou E. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol. 2009;30:507–514. doi: 10.1086/597232. [DOI] [PubMed] [Google Scholar]
- 12.Mcdonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha A.K., Haque M.F., Karmaker S., Mohanta M.K. Antibacterial effects of some antiseptics and disinfectants. J Life Earth Sci. 2009;3–4:19–21. [Google Scholar]
- 14.Rutala W.A., Weber D.J. Disinfection and sterilization in health care facilities: what clinicians need to know. Clin Infect Dis. 2004;39:702–709. doi: 10.1086/423182. [DOI] [PubMed] [Google Scholar]
- 15.Rogers J.V., Sabourin C.L.K., Choi Y.W., Richter W., Riggs K.B., Chang J. Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator. J Appl Microbiol. 2005;99:739–748. doi: 10.1111/j.1365-2672.2005.02686.x. [DOI] [PubMed] [Google Scholar]
- 16.Falagas M.E., Thomaidis P.C., Kotsantis I.K., Sgouros K., Samonis G., Karageorgopoulos D.E. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hosp Infect. 2011;78:171–177. doi: 10.1016/j.jhin.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Gagnaire F., Marignac B., Hecht G., Héry M. Sensory irritation of acetic acid, hydrogen peroxide, peroxyacetic acid and their mixture in mice. Ann Occup Hyg. 2002;46:97–102. doi: 10.1093/annhyg/mef005. [DOI] [PubMed] [Google Scholar]
- 18.Mastrangelo G., Zanibellato R., Fadda E., Lange J.H., Scoizzato L., Rylander R. Exposure to hydrogen peroxide and eye and nose symptoms among workers in a beverage processing plant. Ann Occup Hyg. 2009;53:161–165. doi: 10.1093/annhyg/men077. [DOI] [PubMed] [Google Scholar]
- 19.Lopalco A., Dalwadi G., Niu S., Schowen R.L., Douglas J., Stella V.J. Mechanism of decarboxylation of pyruvic acid in the presence of hydrogen peroxide. J Pharmacol Sci. 2016;105:705–713. doi: 10.1002/jps.24653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackler S.E. Barrier isolation technology can improve life sciences cleanroom applications. Am Pharmacol Rev. 2001:142–145. [Google Scholar]
- 21.Imai K., Watanabe S., Ohima Y., Kokubo M., Akers J.E. A new approach to decontamination of isolators and clean rooms by vapor phase hydrogen peroxide. Pharm Tech Japan. 2006;22:967–972. [Google Scholar]
- 22.Unger-Bimczok B., Kottke V., Hertel C., Rauschnabel J. The influence of humidity, hydrogen peroxide concentration, and condensation on the inactivation of geobacillus stearothermophilus spores with hydrogen peroxide vapor. J Pharm Innov. 2008;3:123–133. [Google Scholar]
- 23.Heckert R.A., Best M., Jordan L.T., Dulac G.C., Eddington D.L., Sterritt W.G. Efficacy of vaporized hydrogen peroxide against exotic animal viruses. Appl Environ Microbiol. 1997;63:3916–3918. doi: 10.1128/aem.63.10.3916-3918.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Omar M.A. vol. 34. Elsevier Inc.; 2009. pp. 265–298. (Profiles of drug substances, excipients and related methodology). [DOI] [PubMed] [Google Scholar]