SUMMARY
SETTING:
Four public hospitals in Botswana, a high tuberculosis (TB) burden setting.
OBJECTIVES:
To assess the feasibility and utility of sputum induction in the diagnosis of paediatric TB.
DESIGN:
From 2008 to 2010, children aged ⩽18 years referred for suspected pulmonary TB underwent sputum induction. Confirmed TB was defined as the presence of at least one of the signs and symptoms suggestive of TB and positive Mycobacterium tuberculosis culture. Information on TB-associated symptoms (cough, fatigue, night sweats, low appetite, chest pain, weight loss, haemoptysis and contact with a TB case) was collected for three risk groups: human immunodeficiency virus (HIV) positive children, HIV-negative children aged <3 years and HIV-negative children aged ⩾3 years.
RESULTS:
The median age of the 1394 subjects who underwent sputum induction was 3.8 years (IQR 1.3–8.4); 373 (27%) were HIV-positive, 419 (30%) were HIV-negative and 602 (43%) had unknown HIV status. TB was confirmed in 84 (6.0%); cases were more likely to have weight loss, chest pain or TB household contacts. There were no serious complications attributable to sputum induction during and after the procedure; only 0.8% (9/1174) of patients reported minor complications.
CONCLUSIONS:
In Botswana, paediatric sputum induction was feasible, safe and assisted bacteriological confirmation in a subgroup of children treated for TB.
Keywords: paediatrics, sputum induction, TB, diagnosis, Botswana
RÉSUMÉ
CONTEXTE:
Quatre hôpitaux publics du Botswana, un pays où la tuberculose (TB) constitue un lourd fardeau.
OBJECTIF:
Evaluer la faisabilité et l’utilité de l’expectoration provoquée pour le diagnostic de la TB pédiatrique.
SCHÉMA:
De 2008 à 2010, des enfants âgés de <18 ans référés pour suspicion de TB pulmonaire ont eu une expectoration provoquée. La confirmation de la TB était définie comme la présence d’au moins un des signes ou symptômes suggestifs de TB ainsi qu’une culture positive de Mycobacterium tuberculosis. Des données relatives aux symptômes (toux, fatigue, sueurs nocturnes, anorexie, douleurs thoraciques, perte de poids, hémoptysie et contact avec un patient tuberculeux) ont été recueillies dans trois groupes à risque : enfants au virus de l’immunodéficience humaine (VIH) positif, enfants VIH-négatifs âgés de <3 ans et enfants VIH-négatifs âgés de ⩾3 ans.
RÉSULTATS:
Des 1394 enfants qui ont eu une expectoration provoquée, l’âge médian était de 3,8 ans (IQR 1,3–8,4) ; 373 (27%) étaient VIH-positifs, 419 (30%) étaient VIH-négatifs et pour 602 enfants (43%) le statut était inconnu. Une TB a été confirmée chez 84 enfants (6%); ces cas avaient plus souvent des symptômes tels que perte de poids, douleur thoracique ou contact à domicile avec un patient tuberculeux. L’expectoration provoquée n’a pas entraîné de complications graves ni pendant ni après sa réalisation; seulement 0,8% des enfants se sont plaints de complications minimes.
CONCLUSION:
Au Botswana, la expectoration provoquée pédiatrique s’est avérée faisable et sûre et a contribué à la confirmation bactériologique d’un sous-groupe d’enfants traités pour TB.
RÉSUMÉ
MARCO DE REFERENCIA:
Cuatro hospitales públicos en Botswana, un país con alta carga de morbilidad por tuberculosis (TB).
OBJETIVOS:
Evaluar la factibilidad y la utilidad de la inducción del esputo en el diagnóstico de la TB en los niños.
MÉTODOS:
Del 2008 al 2010 se practicó la inducción del esputo en los niños de <18 años de edad remitidos por presunción diagnóstica de TB pulmonar. Se definió la TB confirmada como la presencia como mínimo de uno de los signos y síntomas indicativos de TB y un cultivo po sitivo de Mycobacterium tuberculosis. Se recogió información sobre los síntomas asociados de la TB (tos, astenia, sudoración nocturna, pérdida del apetito, dolor torácico, pérdida de peso, hemoptisis y contacto con un paciente tuberculoso) en los siguientes tres grupos de riesgo: niños positivos frente al virus de la inmunodeficiencia humana (VIH), niños negativos al VIH de <3 años de edad y niños negativos al VIH de ⩾3 años de edad.
RESULTADOS:
Se practicó la inducción del esputo en 1394 niños. La mediana de la edad fue 3,8 años (IQR 1,3–8,4), 373 fueron positivos al VIH (27%), 419 negativos al VIH (30%) y en 602 se desconocía su situación frente al virus (43%). Se estableció el diagnóstico de TB confirmada en 84 niños (6,0%); fue más probable que los casos confirmados presentaran pérdida de peso, dolor torácico y contactos tuberculosos en el hogar. No se observaron complicaciones graves atribuibles a la inducción del esputo durante el procedimiento ni después del mismo; solo 0,8% de los niños (9/1174) refirieron complicaciones menores.
CONCLUSIÓN:
Se demostró que en Botswana la inducción del esputo es factible y segura y el procedimiento contribuyó a la confirmación bacteriológica en un subgrupo de niños tratados por TB.
ALTHOUGH paediatric cases comprise up to 20% of tuberculosis (TB) notifications in developing countries, children are often neglected in health system responses to TB.1–4 The burden of TB disease among children is estimated at 490 000 cases and 64 000 deaths per year.5 However, paediatric statistics may substantially underestimate the true incidence of TB, estimated at 0.3–1.4 million cases annually,4,5 in part due to the difficulty in diagnosing TB in children.3 The majority of paediatric TB cases are smear-negative due to the typically paucibacillary presentation in children and their inability to produce sputum on demand. Although microbiological confirmation is ideal for intrathoracic TB, TB culture facilities are often unavailable.6 Most paediatric TB diagnoses are clinical, relying on practical but imperfect algorithms based on history of exposure, signs and symptoms.
Diagnosis of childhood TB is particularly challenging in human immunodeficiency virus (HIV) co-infected children.7 In Botswana, where the national prevalence of HIV is 17.6%, and 5% in children aged <15 years, TB incidence has nearly tripled in the last two decades.8,9 Although Botswana has the world’s sixth highest TB notification rate (536 per 100000 population in 2008),9 robust paediatric diagnostic services have historically been unavailable.
Sputum induction may increase the diagnostic yield in paediatric TB, but data from settings similar to ours are scarce.10 University-affiliated hospitals in Cape Town, South Africa, and Kampala, Uganda, have achieved culture positivity rates of up to 30%.11–14 Although a successful paediatric sputum induction programme at a primary facility in the Western Cape was recently reported,15 we are unaware of published paediatric sputum induction data from smaller facilities, or from African settings other than South Africa. We report the results of a programme that introduced paediatric sputum induction to Botswana’s public hospitals in Gaborone, Francistown, Serowe and Maun. The findings have implications for the feasibility of rolling out paediatric sputum induction in similar settings, the utility of sputum induction for microbiological diagnosis in paediatric TB suspects and the development of locally relevant diagnostic algorithms.
STUDY POPULATION AND METHODS
Study setting
The study was conducted in four large public hospitals across Botswana. Sites were chosen based on high TB incidence in surrounding catchment areas. A series of steps established each new site: first, a respiratory therapist developed a sputum induction training curriculum. At each site, hospital management was briefed on the project objectives and equipment for sputum induction, and a work space was organised. A core sputum induction team, consisting of a respiratory therapist, a nurse, a health care auxiliary and a paediatrician, hosted a 2-day workshop describing the pathophysiology of paediatric TB and provided hands-on training by conducting sputum induction among paediatric TB suspects. A local nurse was then designated to coordinate the scheduling and continued education for sputum induction in each hospital.
Sputum induction technique and sample processing
After a history was taken and physical examination was performed, patients received a standard dose of salbutamol nebuliser for 3–5 min as prophylaxis against broncho-constriction. Nebulised hypertonic saline (3–5%) was inhaled by mask until the patient produced a wet cough (typically 5–15 min). Suction at 20 mmHg pressure was applied through a suction catheter inserted into the nasopharynx or oropharynx if sputum was being coughed and expectorated orally until >2 ml of sputum was collected. Only one specimen was collected per patient, as at the time there was evidence that one induced sputum sample may have the same yield as three gastric aspirates.11 Specimens were labelled, dated and stored at a temperature of 4°C (Appendix).
Samples were transported at least twice weekly to the regional laboratory for acid-fast bacilli smear and to the National TB Reference Laboratory for repeat smear using concentrated fluorescence microscopy, culture and drug susceptibility testing. Samples arriving >1 week after collection were discarded due to concerns about bacterial contamination. Sputum specimens were decontaminated using sodium hydroxide and mucolysed with N-acetyl-L-cysteine.16 Samples for culture were vortexed for 30 s, centrifuged at 3000× g for 15 min and inoculated onto Löwenstein Jensen media at 37°C for up to 8 weeks.16
Definitions
Microbial confirmation was defined as at least one positive culture with confirmed Mycobacterium tuberculosis speciation from sputum. Confirmed TB was defined as the presence of at least one sign or symptom suggestive of TB and microbiological confirmation.6 Age was stratified consistent with other childhood TB studies.17–19
Data collection
TB signs and symptoms, including HIV status, were recorded for each TB suspect. Care givers were encouraged to have children tested if HIV status was unknown and to return for the sputum induction results. If a child did not return for follow-up and was diagnosed with confirmed TB, the care givers were contacted by telephone. If this was unsuccessful, a home visit was arranged. Patient demographics, clinical characteristics and any complications were captured in a standardised form. Information was entered into a secure electronic database.
Analysis
We assessed the diagnostic sensitivity and specificity of signs, symptoms and demographic variables.19 To explore information on TB-associated symptoms,19 we carried out logistic regression and classification and regression trees, with bagging20 to address imbalances in outcome class distributions. For the purposes of analysis, we considered the outcome in three risk groups: HIV-infected children (all ages), non-HIV-infected children aged <3 years and non-HIVinfected children aged >3 years.
Statistical comparisons were performed using χ2 test, Fisher’s exact or the Wilcoxon rank-sum test as appropriate. Analyses used Stata Statistical Software: Release 12 (Stata Corp, College Station, TX, USA) and R version 2.14 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Findings were considered statistically significant when P < 0.05.
Ethics
Approval was granted by the Botswana Ministry of Health, the Princess Marina Hospital, the Nyangabgwe Referral Hospital, Gaborone, Botswana, the US Centers for Diseases Control and Prevention, At lanta, GA, USA, the Baylor College of Medicine, Houston, TX, USA, and the University of Pennsylvania, Philadelphia, PA, USA.
RESULTS
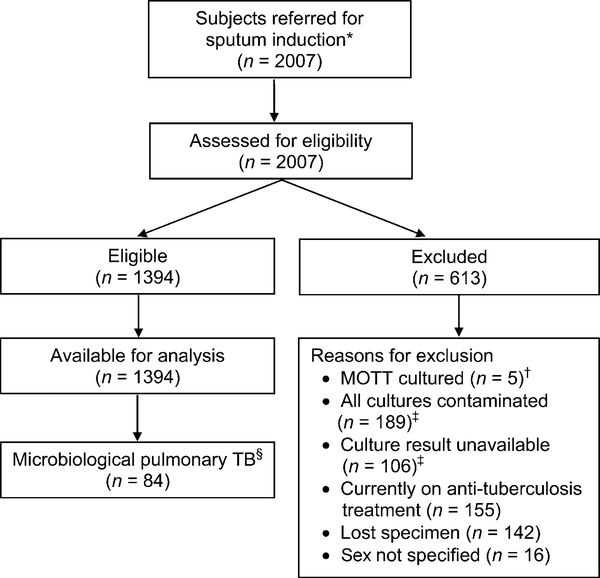
Following the successful rollout of sputum induction in Botswana, 2007 subjects were referred for the procedure; 1394 (69%) were eligible for analysis (Figure 1). Of these, 84 (6%) were diagnosed with confirmed TB. Study subjects did not differ by demographic or HIV status; however, culture-positive TB was more common among older children (Table 1).
Figure 1.
Flow diagram of all children evaluated for TB. * Subjects were referred due to suspicion of pulmonary TB; referral was at the discretion of a primary health care nurse or doctor. † Children for whom MOTT were cultured as the sole organism (in one or more cultures) were classified as MOTT. Children who grew M. tuberculosis from one culture and MOTT from another were classified as TB. ‡ Subjects were included in the study cohort if at least one culture result was available and uncontaminated. An unavailable result meant that there was no laboratory report even when a sample was taken from the patient and sent to the laboratory. This may have been due to the fact that cultures were not performed or, if performed, the results were not recorded or reported to the clinicians. § Microbiological confirmation defined as positive for TB using culture. MOTT = mycobacteria other than TB; TB = tuberculosis.
Table 1.
Demographics of children who underwent sputum induction in Botswana by TB status
| Cohort by microbiological status*† |
|||
|---|---|---|---|
| Variable | Total cohort (n = 1394) n (%) | AFB-positive (n = 47) n (%) | TB culture-positive (n = 84) n (%) |
| Sex | |||
| Female | 651 (47) | 23 (4) | 41 (6) |
| Male | 743 (53) | 24 (3) | 43 (6) |
| Age, years | |||
| Median [IQR] | 3.8 [1.3–8.4] | 5.9 [1.3–11.2] | 5.9 [2.1–10.1] |
| <3 | 613 (44) | 16 (3) | 26 (4) |
| 3–4 | 173 (12) | 3 (2) | 11 (6) |
| 5–9 | 320 (23) | 9 (3) | 23 (7) |
| 10–17 | 252 (18) | 16 (6) | 21 (8) |
| Unknown | 36 (3) | 3 (8) | 3 (8) |
| HIV status | |||
| Positive | 373 (27) | 13 (3) | 22 (6) |
| Negative | 419 (30) | 15 (4) | 28 (7) |
| Unknown | 602 (43) | 19 (3) | 34 (6) |
Data for each variable were not captured for all subjects. Proportion calculated as total positive by the diagnostic modality with the characteristic/total number with the characteristic for whom the diagnostic modality was captured (%).
47/1394 (3%) were AFB-positive, 84/1394 (6%) were TB culture-positive. TB = tuberculosis; AFB = acid-fast bacilli; IQR = interquartile range; HIV = human immunodeficiency virus.
Table 2 shows signs and symptoms. Confirmed TB was associated with each of the following: weight loss, chest pain, household TB suspect and household TB contacts. Table 3 indicates the value of individual variables used to diagnose confirmed TB within the relevant risk groups. Although fever for >14 days trended towards higher sensitivity in the HIV-infected group than in the HIV-negative group, this failed to reach statistical significance. Similarly, fatigue and chest pain appeared more sensitive in the HIV-infected than the HIV-negative group, but did not reach statistical significance. Multivariate modelling using parametric and non-parametric approaches failed to identify any variables significantly associated with confirmed TB.
Table 2.
TB symptoms and signs of 1394 children who underwent sputum induction by TB status
| Proportion among those with variable captured* n/N (%) | TB diagnostic modality* |
Positive microbiological TB status† |
||||
|---|---|---|---|---|---|---|
| AFB-positive‡ (n = 47) n/N (%) | TB culturepositive† (n = 84) n/N (%) | Those without symptom | Those with symptom | P value | ||
| Symptom | ||||||
| Weight loss | 678/1264 (54) | 25/44 (57) | 50/73 (68) | 23/586 (4) | 50/678 (7) | 0.01 |
| Cough >14 days | 1052/1355 (78) | 35/46 (76) | 65/79 (82) | 14/303 (5) | 65/1052 (6) | 0.31 |
| Fever >14 days | 540/1339 (40) | 24/46 (52) | 41/81 (51) | 40/799 (5) | 41/540 (8) | 0.05 |
| Night sweats >14 days | 581/1328 (44) | 22/45 (49) | 42/78 (54) | 36/747 (5) | 42/581 (7) | 0.06 |
| Fatigue >14 days | 380/1324 (29) | 21/46 (46) | 24/77 (31) | 53/944 (6) | 24/380 (6) | 0.62 |
| Poor appetite >14 days | 458/1349 (34) | 22/46 (48) | 31/80 (39) | 49/891 (6) | 31/458 (7) | 0.35 |
| Chest pain >14 days§ | 229/1014 (23) | 10/32 (31) | 29/59 (41) | 19/361 (5) | 18/143 (13) | 0.04 |
| Haemoptysis | 35/1260 (3) | 1/43 (2) | 1/76 (1) | 75/1225(6) | 1/35 (3) | 0.42 |
| Medications | ||||||
| Recent antibiotics¶ | 497/1258 (40) | 18/44 (41) | 35/75 (47) | 40/761 (5) | 35/497 (7) | 0.19 |
| Previously on anti-tuberculosis treatment | 142/1327 (11) | 8/44 (18) | 6/75 (8) | 69/1185 (6) | 6/142 (4) | 0.44 |
| Household data | ||||||
| Number of persons at home, median [IQR] | 6 [4–9] | 7 [4–8] | 7 [5–9] | 0.49 | ||
| Household TB contact# | 562/1309 (43) | 24/42 (57) | 42/77 (55) | 35/747 (5) | 42/562 (7) | 0.03 |
| Household contact or suspect | 671/1174 (57) | 28/39 (72) | 51/69 (74) | 18/503 (4) | 51/671 (8) | 0.004 |
| ⩾5 persons in home | 750/1039 (72) | 24/33 (73) | 48/60 (80) | 12/289 (4) | 48/750 (6) | 0.16 |
| Contact with death on anti-tuberculosis treatment | 223/1258 (18) | 7/42 (17) | 18/77 (23) | 59/1035 (6) | 18/223 (8) | 0.18 |
| Patient disposition | ||||||
| In-patient/hospitalised | 420/1104 (38) | 15/36 (42) | 20/64 (31) | 44/684 (6) | 20/420 (5) | 0.25 |
Data for each variable were not recorded for all subjects. The percentage indicates those who were positive using the microbiological technique, those who had the symptom or those for whom the symptom was recorded.
Of the total cohort, 84/1394 (6%) were microbiologically diagnosed with TB (culture).
Of those AFB-positive, 17/47(36%) were also TB culture-positive.
To ensure validity, we limited the analysis of ‘chest pain’ to children aged ⩾5 years.
Antibiotics for the current complaint.
Household contact with either cough or weight loss.
Table 3.
Individual variables documented at presentation in relevant risk groups to diagnose microbiologically confirmed pulmonary TB in children (n = 84)
| Individual variables at presentation | Low-risk Non-HIV-infected* aged ⩾3 years |
High-risk |
||||
|---|---|---|---|---|---|---|
| Non-HIV-infected aged <3 years |
HIV-infected aged <3 years |
|||||
| Sensitivity % | Specificity % | Sensitivity % | Specificity % | Sensitivity % | Specificity % | |
| Weight loss† | 75 | 54 | 70 | 43 | 67 | 41 |
| Cough > 14 days‡ | 88 | 25 | 100 | 25 | 81 | 16 |
| Fever >14 days§ | 41 | 63 | 55 | 66 | 64 | 59 |
| Night sweats >14 days¶ | 59 | 60 | 40 | 62 | 65 | 56 |
| Fatigue >14 days# | 25 | 72 | 18 | 75 | 50 | 65 |
| Poor appetite >14 days | 41 | 66 | 36 | 68 | 48 | 65 |
| Chest pain >14 days** | 27 | 75 | 33 | 89 | 58 | 76 |
| Haemoptysis†† | 0 | 94 | 0 | 99 | 5 | 97 |
| Household contact‡‡ | 56 | 45 | 55 | 59 | 43 | 71 |
| Combined symptoms | ||||||
| ⩾2 symptoms | 94 | 23 | 100 | 24 | 86 | 19 |
| ⩾3 symptoms | 71 | 42 | 82 | 40 | 86 | 36 |
| Death of a family member on anti-tuberculosis treatment | 31 | 81 | 27 | 85 | 18 | 82 |
All patients diagnosed with TB were offered HIV testing as part of Botswana’s National HIV and TB guidelines—subjects with unknown HIV status were not included in this analysis.
Unexplained weight loss indicates ⩾5% reduction in weight compared with the highest weight recorded in last 3 months, reported by the parent/care giver.
Persistent or non-remitting cough for >2 weeks.
Persistent (>2 weeks) and unexplained fever (>38°C) as reported by the parent/care giver.
Sweating in excess of other children the same age as reported by the care giver.
Perceived decrease in playfulness or activity as reported by the parent/care giver.
As reported by the child or care giver.
Presence of blood in the sputum (not haematemesis or a nose bleed).
Exposure to an adult TB index case in the household in the preceding 24 months.
HIV = human immunodeficiency virus; TB = tuberculosis.
The procedure was contra-indicated in 1.5% (20/1294) of patients due to wheeze (n = 16, 80%), severe respiratory distress (n = 2, 10%), thrombocytopaenia (n = 1, 5%) and epistaxis (n = 1, 5%). Adverse events data were captured in 84% (1174/1394) of the patients (Table 4); there were no serious events and only 9/1174 (0.8%) patients had minor events. Table 5 summarises the clinical features of children for whom mycobacteria other than tuberculosis were cultured; outcomes were unknown.
Table 4.
Frequency and description of adverse events of subjects undergoing sputum induction (n = 1174)
| Adverse event | Events n |
|---|---|
| Vomiting | 6 |
| Nose bleed | 5 |
| Increased coughing | 2 |
| Oxygen desaturations (to 90% post procedure) | 1 |
| Wheeze | 1 |
| Total | 15 adverse events* |
A total of 9 patients experienced adverse events: 6 experienced two events (5 vomiting and nose bleed, 1 vomiting and wheeze).
Table 5.
Clinical characteristics of children with MOTT (n = 5)
| Subject | Sex | Age | HIV status | PPD test | MOTT species | Signs and symptoms |
|---|---|---|---|---|---|---|
| 1 | Female | 7 years | Unknown | Negative | Unknown | Night sweats, persistent unremitting cough and intermittent pyrexia >2 weeks. No household TB contacts |
| 2 | Male | 2 months | Unknown | Negative | M. fortuitum | Persistent unremitting cough, intermittent pyrexia, night sweats, reduced playfulness >2 weeks. Positive household TB contact |
| 3 | Female | 11 years | Unknown | Negative | M. intracellulare | Asymptomatic, referred for sputum induction as positive household TB contact. Contact had persistent cough, weight loss and was on anti-tuberculosis treatment |
| 4 | Female | 6 years | Unknown | Negative | M. gordoniae | Persistent unremitting cough, night sweats >2 weeks and recurrent chest pain. No positive household TB contacts |
| 5 | Male | 6 years | Positive | Positive | Unknown | Weight loss and positive household TB contact |
PPD >10 mm was considered positive if non-HIV-infected or >5 mm if HIV-infected or severely malnourished.
MOTT = mycobacteria other than tuberculosis; HIV = human immunodeficiency virus; PPD = purified protein derivative; TB = tuberculosis.
DISCUSSION
Sputum induction as a modality for TB diagnosis in children was successfully introduced in four Botswana hospitals in this first phase of a national scale-up. Previous sputum induction projects in resourcec onstrained settings have examined the efficacy of the procedure at tertiary academic institutions or in clinical trials.11,21 This project, however, focused on implementing sputum induction in national health care facilities, including non-academic settings.
Previous studies from resource-constrained settings have shown variable diagnostic yield of TB in children and a number of these focused on hospitalised children.11–14 Hatherill et al. compared the diagnostic yield of TB from sputum induction and gastric lavage among children in an out-patient community setting, where the crude diagnostic yield for sputum induction was 5.8% compared to 6.2% for gastric lavage.21 A Ugandan in-patient study of sputum induction yielded 12% positive smears and 30% positive cultures for M. tuberculosis.13 Zar et al. showed a 9% yield with sputum induction compared to a 6% yield from gastric lavage in in-patients.12 In comparison, our sputum induction yield of 6% was comparable to those of community-based studies,21 and lower than in studies focusing on hospitalised children.11–14
We found no significant differences in TB yield between in-patient and out-patient groups. Compared to our cohort, Zar et al.’s hospitalised child cohort were of similar age but were likely more ill, with 38% HIV-infected and 27% requiring supplemental oxygen.11Hospitalised children who are more ill in such settings are likely to have more severe TB disease and hence a higher diagnostic yield from sputum induction.
We found that symptom-based screening using weight loss, fever, household TB contact or suspect and chest pain correlated with confirmed TB. The sensitivity of cough as a diagnostic symptom remained low across the various risk groups. Hatherill et al. found an association between confirmed TB and cough of >2 weeks or weight loss, but none with household TB contacts.21
History of a household contact had a sensitivity of 59% for confirmed TB in high-risk children who were non-HIV-infected and aged <3 years. As relatively few children with household TB exposure will be missed on symptom-based clinical screening, a positive household TB contact should be incorporated into national TB diagnostic algorithms. For TB household contacts, Marais et al. reported a lower sensitivity across all risk groups and lower sensitivity (69%) and specificity (54%) among non-HIV-infected children aged <3 years.19 While TB-HIV prevalence was high in both Marais et al.’s study and ours, our study represents a larger cohort in a setting with more extensive highly active antiretroviral therapy coverage (93% in Botswana vs. 55% in South Africa in 2010).22
This study has some limitations. This analysis of retrospective data limits conclusions to a documented patient population; those referred may not represent all patients presenting to facilities. Although we used a standardised data collection form, the completeness of the data presented here may be higher than what would be captured under operational conditions. Our diagnostic yield of 6% was low, probably due to the low diagnostic yield seen in children generally,1–4 the low pre-test probability of many who were referred and the fact that sputum induction was performed only once for each subject. Refining the clinical criteria to better target future sputum induction procedures may reduce programme costs.
Programme challenges included lost sputum samples, contaminated sputum cultures and unavailable culture results. As a result of high staff turnover, there were continuously new health care workers requiring training in sputum induction. As children were often referred by their primary doctor, accessing accurate clinical and radiological data was often a challenge, as was post-sputum induction clinical outcome follow-up. Due to low HIV testing coverage, 43% of those in our cohort had unknown HIV status. Furthermore, we did not assess for extra-pulmonary TB disease and we were unable to differentiate common intra-thoracic disease entities and their associated symptoms or yields, as accurate radiological data were not routinely available. Finally, our definition of a TB case required microbiological confirmation, which is achievable in only 30–50% of children with pulmonary TB, which may have concealed actual clinical information from identification in our multivariate analyses.
CONCLUSION
Sputum induction can be performed successfully at referral and secondary hospitals in Botswana and has potential for further rollout in Botswana and similar settings. Findings from this multi-site implementation project suggest that sputum induction should preferentially be performed on children who meet a number of clinical and demographic criteria for TB. To improve the method towards better yield and costeffectiveness, future studies should consider prediction modelling using TB outcomes informed by both confirmed and carefully selected empirical diagnoses. Future studies should explore the optimal timing and number of samples needed, and elucidate whether samples should be collected using a combination of available techniques (e.g., sputum induction and gastric aspirate).
Acknowledgements
The authors thank all nursing staff, doctors, health care auxiliaries, laboratory staff and the management at the Baylor College of Medicine and the Botswana–Baylor Children’s Clinical Centre of Excellence, Gaborone, Botswana, and all participating institutions for supporting this project. They also thank the children and their parents for participating in the project. This publication was made possible through core services and support from the Penn Center for AIDS Research, a National Institutes for Health funded programme (P30 AI 045008). The sputum induction project was supported by the US President’s Emergency Plan for AIDS Relief grant cooperative agreement number 1U2G/PS001881–03 through the US Centre for Disease Control–Botswana.
The views and opinions expressed in the article are solely those of the authors and do not necessarily reflect those of the US Agency for International Development nor those of the US Government.
APPENDIX
Sputum induction in children
Precaution: Only health care workers (HCWs) who have been trained in proper, safe techniques should perform sputum induction.
Patients should be under observation at all times during sputum induction
- Sputum induction should not be performed in children with:
- —Acute (active) asthma
- —Any signs of moderate to severe respiratory distress
- —Wheezing
- —Abnormal vital signs
- —Epistaxis
- —Pneumothorax
- —Fractured ribs or other chest trauma
- —Recent eye surgery
Infection control
As sputum induction produces coughing, it is likely that infectious droplets, if present, will be expelled into the air. Sputum induction should therefore be performed in a well-ventilated area.
The HCW should wear an N95 respirator throughout the procedure and disposable gloves when handling sputum.
If outside, determine the wind direction before beginning the procedure. The patient must always be located downwind of the HCW.
If inside, open doors and windows and use fans to direct airflow away from the HCW.
Nebulisers and tubing should be sterilised after every use (with precept or glutaraldehyde) for 15–20 min.
Preparations for the procedure: pre-operative fasting requirements
The child should have fasted for 6 h before the procedure.
Do not perform sputum induction if the child has eaten within 3 h before the procedure. Medication with a small amount of liquid is acceptable.
Procedure preparations: area setup
Set up area ahead of time to minimise the anxiety level of the child.
Keep syringes and needles out of sight.
Preload the equipment with salbutamol and normal saline.
Keep a suction catheter or Yankaeur nearby in case of vomiting.
Fill out laboratory request forms.
Procedure preparation: patient positioning
Have the care giver hold the child during the procedure. If this is not possible, an assistant should hold the child.
Position the child in the upright or semi-upright position.
Hold infants supine in the feeding position.
Stand or sit where you can clearly observe the child and all of the equipment.
Procedure
Run correct dosage of salbutamol in normal saline (0.9% sodium chloride [NaCl] solution) for 3–5 min.
Add hypertonic saline (3–5% NaCl solution) to the solution.
- Continue nebulisation for at least 10 min.
- —If the child coughs during this time and produces a specimen, the procedure is completed.
- —If the child does not produce a specimen within 10 min, insert the suction catheter, nasopharyngeal airway, or oropharyngeal airway to stimulate a cough.
When there is adequate sputum in the oronasopharyngeal area, insert the catheter from the sputum trap (either alone or through an airway).
Apply vacuum until at least 2 ml of sputum is collected in the sputum trap. Start at 15–20 kPa pressure and increase only if needed.
Ensure that the sputum collection container is tightly sealed and labelled.
After the procedure
Monitor the child for several minutes. If pulse oximetry is below baseline or there are signs of respiratory distress, give oxygen and suction excess sputum from the airway.
Educate the care giver that coughing may be more frequent within 24 h of the procedure.
Send samples immediately to the laboratory. Refrigerate all samples that are sent for culture.
Keep samples out of direct sunlight.
Stop the procedure if the child has any of the following:
Respiratory distress, including increased respiratory rate, wheezing, laboured breathing, chest wall
retractions, nasal fl aring or cyanosis
Profuse sweating
Nausea or vomiting
Light-headedness, dizziness or loss of consciousness
Equipment required:
N95 respirator for the HCW
Ultrasonic nebuliser machine*
Sterile hypertonic saline (3–5%)
Salbutamol and 0.9% NaCl solution (normal saline)
Suction machine and catheter or Yankaeur
Pulse oximetry machine
Oxygen cylinder
Goggles
Small volume nebuliser
The compressor
Infra-red light
Sputum traps
Disinfectant (precept or glutaraldehyde)
Disposable gloves
Completed laboratory request form with patient details
Sterile sputum collection container identified with the patient details
Footnotes
Conflict of interest: none declared.
Some ultrasonic nebulisers have a loading volume and should not run dry. If it does run dry, a safety mechanism will shut the machine off.
References
- 1.Keshavjee S, Harrington M, Gonsalves G, Chesire L, Farmer PE. Time for zero deaths from tuberculosis. Lancet 2011; 378: 1449–1450. [DOI] [PubMed] [Google Scholar]
- 2.Nelson LJ, Wells CD. Global epidemiology of childhood tu-berculosis. Int J Tuberc Lung Dis 2004; 8: 636–647. [PubMed] [Google Scholar]
- 3.Marais BJ, Schaaf HS. Childhood tuberculosis: an emerging and previously neglected problem. Infect Dis Clin North Am 2010; 24: 727–749. [DOI] [PubMed] [Google Scholar]
- 4.du Cros P, Nyang’wa B, Gale M, et al. Counting children: com-paring reporting for paediatric HIV and tuberculosis. Bull World Health Organ 2011; 89: 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Global tuberculosis report, 2012. WHO/HTM/TB/2012.6. Geneva, Switzerland: WHO, 2012. http://www.who.int/tb/publications/global_report/gtbr12_main.pdf Accessed January 2014. [Google Scholar]
- 6.Graham SM, Ahmed T, Amanulla F, et al. Evaluation of tuber-culosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 2012; 205 (Suppl 2): S199–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards DJ, Kitetele F, Van Rie A. Agreement between clinical scoring systems used for the diagnosis of pediatric tuberculosis in the HIV era. Int J Tuberc Lung Dis 2007; 11: 263–269. [PubMed] [Google Scholar]
- 8.Central Statistics Office, Ministry of Health Botswana. The 2008 Botswana AIDS Impact Survey III (BAIS III). Gaborone, Botswana: MoH, 2008. [Google Scholar]
- 9.Botswana National Tuberculosis Programme. Annual tubercu-losis and leprosy report, 2006–2008. Gaborone, Botswana: Ministry of Health, Republic of Botswana, 2010. [Google Scholar]
- 10.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuber-culosis in children: new advances. Expert Rev Anti Infect Ther 2010; 8: 277–288. [DOI] [PubMed] [Google Scholar]
- 11.Zar HJ, Hanslo D, Apolles P, Swingler G, Hussey G. Induced sputum versus gastric lavage for microbiological confirmation of pulmonary tuberculosis in infants and young children: a prospective study. Lancet 2005; 365: 130. [DOI] [PubMed] [Google Scholar]
- 12.Zar HJ, Tannebaum E, Hanslo D, Hussey G. Sputum induc-tion as a diagnostic tool for community-acquired pneumonia in infants and young children from a high HIV prevalence area. Pediatr Pulmonol 2003; 36: 58–62. [DOI] [PubMed] [Google Scholar]
- 13.Iriso R, Mudido PM, Karamagi C, Whalen C. The diagnosis of childhood tuberculosis in an HIV-endemic setting and the use of induced sputum. Int J Tuberc Lung Dis 2005; 9: 716–726. [PubMed] [Google Scholar]
- 14.Qureshi UA, Gupta AK, Mahajan B, et al. Microbiological diagnosis of pulmonary tuberculosis in children: comparative study of induced sputum and gastric lavage. Indian J Pediatr 2011; 78: 1429–1430. [DOI] [PubMed] [Google Scholar]
- 15.Moore HA, Apolles P, de Villiers PJT, Zar HJ. Sputum induction for microbiological diagnosis of childhood pulmonary tuberculosis in a community setting. Int J Tuberc Lung Dis 2011; 15: 1185–1190. [DOI] [PubMed] [Google Scholar]
- 16.American Thoracic Society/Centers for Disease Control and Prevention. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 2000; 161: 1376–1395. [DOI] [PubMed] [Google Scholar]
- 17.Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N. Diver-sity of disease in childhood pulmonary tuberculosis. Ann Trop Paed 2005; 25: 79–86. [DOI] [PubMed] [Google Scholar]
- 18.Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis 2006; 10: 732–738. [PubMed] [Google Scholar]
- 19.Marais BJ, Gie RP, Hesseling AC, et al. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 2006; 118: e1350–1359. [DOI] [PubMed] [Google Scholar]
- 20.Breiman L Bagging predictors. Machine Learning 1996; 24: 123–140. [Google Scholar]
- 21.Hatherill M, Hawkridge T, Zar HJ, et al. Induced sputum or gastric lavage for community based diagnosis of childhood pulmonary tuberculosis? Arch Dis Child 2009; 94: 195–201. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization/Joint United Nations Programme on HIV/AIDS/United Nations International Childrens’ Emergency Fund. Global HIV/AIDS response. Annex 4. Geneva, Switzerland: WHO, 2011. http://www.who.int/hiv/data/en/ Accessed December 2013. [Google Scholar]