Abstract
Introduction
Anti-tuberculosis agent rifampicin is extensively used for its effectiveness. Possible complications of tuberculosis and prolonged rifampicin treatment include kidney damage; these conditions can lead to reduced efficiency of the affected kidney and consequently to other diseases. Bone marrow-derived mesenchymal stem cells (BMMSCs) can be used in conjunction with rifampicin to avert kidney damage; because of its regenerative and differentiating potentials into kidney cells. This research was designed to assess the modulatory and regenerative potentials of MSCs in averting kidney damage due to rifampicin-induced kidney toxicity in Wistar rats and their progenies. BMMSCs used in this research were characterized according to the guidelines of International Society for Cellular Therapy.
Methods
The rats (male and female) were divided into three experimental groups, as follows: Group 1: control rats (4 males & 4 females); Group 2: rats treated with rifampicin only (4 males & 4 females); and Group 3: rats treated with rifampicin plus MSCs (4 males & 4 females). Therapeutic doses of rifampicin (9 mg/kg/day for 3-months) and MSCs infusions (twice/month for 3-months) were administered orally and intravenously respectively. At the end of the three months, the animals were bred together to determine if the effects would carry over to the next generation. Following breeding, the rats were sacrificed to harvest serum for biochemical analysis and the kidneys were also harvested for histological analysis and quantification of the glomeruli size, for the adult rats and their progenies.
Results
The results showed some level of alterations in the biochemical indicators and histopathological damage in the rats that received rifampicin treatment alone, while the control and stem cells treated group showed apparently normal to nearly normal levels of both bio-indicators and normal histological architecture.
Conclusions
Intravenous administration of MSCs yielded sensible development, as seen from biochemical indicators, histology and the quantitative cell analysis, hence implying the modulatory and regenerative properties of MSCs.
Keywords: Stem cell therapy, Mesenchymal stem cells, Rifampicin, Tuberculosis, Histopathology, Kidney
1. Introduction
The most frequently used and efficacious medication for treating tuberculosis (TB) is rifampicin. Although rifampicin-induced acute kidney malfunction might occur, this is a common complication during therapy, as it occurs in 0.1% of those suffering from TB [45]. Acute interstitial nephritis as an immune-mediated cause of acute kidney malfunction is also related to rifampicin, which can lead to 1.6% mortality [2]. It is still a severe impediment that can proceed to Fanconi syndrome, a proximal renal tubule defect leading to mal-absorption of major elements, such as phosphorus, bicarbonate, sodium, potassium, glucose and amino acid that can lead to numerous signs such as bone pain and fracture, fatigue, and muscular weakness [67]. The epidemiological statistic associated with medication provoked renal damage was placed at 18–27% of all occurrences of acute renal malfunction in the USA hospitals [59]. All the kidney physiological tasks can be disturbed in one way or another due to drug effect on the kidneys. In an event of persistent poor glomerular filtration rate, dialysis and transplantation remains the only cure option. Several reports revealed that rifampicin-induced acute kidney malfunction is a complication when the medication is re-administered or used irregularly [15], [43], [17]. The aftermath of rifampicin-induced acute kidney malfunction is mostly promising upon discontinuation of medication, in which 96% of those suffering from TB can attain complete revitalization within 90 days as at the start of the renal injury [15]; however, to eradicate Mycobacterium tuberculosis from the patient, the medication must be taken for 6 months or more. Yet, the eradication of this disease remains elusive. This has been largely attributed to the ability of Mtb to maintain a latent or dormant infection in a host despite the evidence for a vigorous host immune response [26], [71], [38]. Moreover, many research papers have shown that prolonged use of rifampicin can cause kidney injury [4], [52]. A remarkable elevation in the serum sample of the AST level due to anti-TB medication was reported by Ref. [51]; in patients after receiving treatment by Ref. [6] and in mice treated with rifampicin [65]. A remarkable rise in the serum urea and creatinine levels was reported in patients treated with isoniazid, rifampicin, pyrazinamide, and ethambutol for a period of eight months [70]. In another experiment, a reduction in serum urea levels were recorded in rats treated with isoniazid and rifampicin for a period of one month. Furthermore, an extraordinary elevation in serum urea was reported, but with no meaningful changes in the creatinine level of rats that received rifampicin for a period of 4 weeks. Moreover, an elevation in AST, bilirubin and urea was reported [55], with no remarkable changes in the creatinine level. Histopathological kidney damage such as glomerular injury increased, and mesangial matrix expansion and renal tubule regeneration were observed [55] in albino rats that were treated with rifampicin for a period of 4 weeks via oral gastric tubes [54]. Prolonged rifampicin therapy can also cause hemolysis and subsequently acute kidney failure and can lead to interstitial nephritis (which is due to its direct toxic effect) and is seen as part of pan-nephropathy [40]. Renal lesions were observed and were due to the formation of immune complexes that were detected on capillary glomerular basement membranes using immunofluorescent and electron microscopy. Deterioration in kidney activity appears to be acute when rifampicin is reintroduced [18]. MSCs can be used together with rifampicin to avert kidney damage because they have the ability to home to damaged tissue when injected intravenously. Clinical complications like ischemic acute renal failure (ARF), described by severe regression in the glomerular filtration rate, is usually seen in hospitalized patients and predominantly in multi-organ failure patients. Intravenous administration of Bone marrow mesenchymal stem cells after ARF, was able to histologically locates the injured kidney and considerably boost the restoration of kidney function due to their ability of trans-differentiation into kidney tubular or vascular endothelial cells [47], [37]. A single intra-renal administration of BMMSCs 7 days after ischemia-reperfusion significantly improved renal function and modified renal remodeling. The improvement of renal function was associated with a reduction in extracellular matrix accumulation. In a renal ischemia rat model, administration of MSC was able to decreased tubular dilation that is known to be a typical characteristic of progressive kidney failure [1]. Since physicians are not currently required to monitor renal function during the course of TB treatment unless the patient is at risk for hepatic or renal abnormalities [12]. Here, we want to investigate the therapeutic potentials of transplanted bone marrow-derived mesenchymal stem cells for the rat kidney and their subsequent generation (F1 generation) in managing the toxicity of rifampicin.
2. Methods
2.1. Isolation and culture expansion of mesenchymal stem cells (MSCs)
The isolation of mesenchymal stem cells from 8-weeks old wistar male rats was done according to the methods described by Refs. [20], [50].
2.1.1. Procedure
Anesthesia cocktail of 50 mg/kg ketamine and 10 mg/kg xylazine were injected to the rats. At the back limbs, the femurs and tibias were cut off, the skin and muscles were removed. The dissected femurs and tibias were put in 70% alcohol for a few seconds and later transferred to phosphate buffered saline (Cat. No. P5368, Merck, USA). Within a biosafety hood, the dissected femurs and tibias were then relocated to a 10 cm bowl containing PBS; the individual bone was then held with tweezers, and the two terminals of the bone were cut open using a scissor. Using a 3 ml syringe filled with PBS and 22G needle, the marrow were flushed (2–3 times) to 50 ml collection tube by putting the needle to the open end of the bone. The collected cells were then resuspended by spinning at 200 g (1294 rpm) 40 °C for 5 min. The supernatant was aspirated and the cells were then resuspended in 120 ml MSCs medium, Dulbecco's Modified Eagle's Medium (Cat. No. D5546, Merck, USA), comprising of 10% Fetal Bovine Serum (Cat. No. 12303C, Merck, USA) and 1% Pen-Strep (Cat no. P4333, Merck, USA). A 15 ml suspension having 750,000 cells was seeded into a 75 cm2 culture flask for a total of eight flasks. The culture flasks were kept in a 37 °C and 5% CO2 incubator for 1–2 weeks, and the medium was changed every 3 days.
2.2. Immunophenotyping of MSCs
The characterization of bone marrow-derived mesenchymal stem cells was done to determine the presence of mesenchymal stem cells surface antigens (CD90, CD29, CD106, CD54, CD105 and CD271) and the lack of or little appearance of hematopoietic markers (CD45 and CD34) before the commencement of the various experimentations. The antibodies against CD90, CD29, CD106, CD54, CD105, CD271, CD45 and CD34 (Biolegend & novusbio) conjugated fluorescein isothiocyanate or phycoerythrin) were used. All cells at eight passages at ±80% confluence were detached using trypsin (0.1% trypsin/EDTA; Gibco) for 5–10 min at 37 °C; the cells were amassed into a 15 ml collecting tube and centrifuged at 200 g (1294 rpm), at 4 °C for 5 min. After aspirating the supernatant, the cells were then washed twice with PBS and resuspended in 5 ml media (PBS containing 2% FBS) and passed through a cell strainer; counted to a final volume of 200,000 cells/tube, the tubes were centrifuged at 1000 rpm for 5 min, and the media was aspirated and cells resuspended in 100 μl PBS. Primary antibodies at appropriates concentrations (10 μl) were added to the following labeled tubes: Tube 1, cells only; Tube 2, CD90; Tube 3, CD29; Tube 4, CD106; Tube 5, CD45; Tube 6, CD54; Tube 7, CD34; Tube 8, CD105; and Tube 9, CD271, which were incubated at 4 °C for 30 min. The cells were washed twice with PBS (500 μl) and centrifuged at 1000 rpm for 5 min; then, the supernatant was discarded by inverting the tube. The cells were further resuspended in 500 μl of PBS, and the samples were acquired for flow cytometry. The samples were analyzed using FACSAria III with FACSDiva 6.1.2 software.
2.3. Tri-lineage differentiation and staining
The differentiating capabilities of the cells into osteoblast, adipocytes and chondrocytes was ascertained.
2.3.1. Osteogenesis differentiation (for 24-well tissue culture plates)
The 24-well tissue culture plates were coated as follows; Vitronectin and collagen were diluted with 1X PBS to yield final concentrations of 12 μg/mL for each Extracellular molecule (ECM). A 0.5 mL of the vitronectin/collagen mixture was added to each well of a 24-well plate and incubated overnight at room temperature. On the following day, the vitronectin/collagen combination was aspirated from the wells and the wells were washed with 1X PBS.
2.3.2. Cell plating
The cell suspension in mesenchymal stem cell expansion medium at a concentration of 60,000 cells per well were seeded in the vitronectin/collagen coated 24-well culture plates with 1 mL capacity for each well. After that the cells were incubated at 37° C in a 5% CO2 humidified incubator overnight. At 100% confluent of the cells, the medium was carefully removed from each well and 1 mL osteogenesis induction medium was added. This medium change tallies to differentiation day 1. The medium was changed with fresh osteogenesis induction medium every 2–3 days for 14–17 days. The osteocytes were fixed and later stained with Alizarin Red Solution after 14–17 days of differentiation.
2.3.3. Alizarin red staining protocol
The medium was cautiously removed from each well and the osteocytes were fixed by incubating in iced cold 70% ethanol for 1 h at room temperature. The alcohol was cautiously removed and the cells wash twice (5–10 min each) with water. The water was removed and adequate Alizarin red solution added to cover the wells (500 μL–1 mL per well in a 24 well plate), and then incubated at room temperature for the next 30 min. After 30 min incubation, the Alizarin Red Solution was aspirated and the wells were rinsed four times with 1 mL water. A 1.5 mL water was added to each well to stop the cells from dehydrating and the plate was observed and image obtained. Osteocytes having calcium deposits appeared orange red by the Alizarin Red Solution.
2.4. Adipogenesis differentiation
The cells were suspended in mesenchymal stem cell expansion medium, at a density of 60,000 cells per well were plated in the vitronectin/collagen coated 24-well culture plate with 1 mL volume per well. Later the cells were incubated at 37° C in a 5% CO2 humidified incubator overnight. After reaching a confluence of 100%, the medium was cautiously removed from all the well and replaced with 1 mL adipogenesis induction medium. This medium change corresponds to differentiation day 1. The fresh adipogenesis induction or maintenance medium were replaced every 2–3 days for 21 days, according to the differentiation schedule. Lipid droplets can be detected by microscopic examination as early as 5 days into differentiation period. Adipocytes were later fixed and the lipid droplets were stained with oil red O solution after 21 days of differentiation.
2.4.1. Oil red O staining protocol
The medium was cautiously removed from all the well and the adipocytes were fixed by incubating in 4% paraformaldehyde for 30–40 min at room temperature. The paraformaldehyde was cautiously removed and cells were washed three times (5–10 min each) with 1X PBS. The water was removed and adequate oil red O solution added to cover the wells (500 μL–1 mL per well in a 24 well plate), and was incubated at room temperature for 50 min. After 50 min, the oil red solution was aspirated and the wells were rinsed four times with 1 mL water. The cell nuclei were stained with 0.5 ml hematoxylin solution for 5–15 min. Adipocytes containing lipid droplets were stained red by the oil red solution while the cell nuclei were stained black/blue from the hematoxylin.
2.5. Chondrogenesis differentiation
The cell monolayer from the basal cultures that were expanded in the stempro MSC SFC, mesenpro medium (standard growth medium containing DMEM + 10% FBS) to certify the mid-log growth confluence (80%) were observed. The depleted medium, including some floating cells were removed from the culture flask. A 10 mL Dulbecco's phosphate-buffered saline (DPBS) was added to wash the cells monolayer. The DPBS was aspirated and 7 mL of pre-warmed trypsine EDTA was added to entirely cover the culture surface. The culture flask was incubated for 8 min at 36 °C until the cells were fully detached. The detached cells were pipetted into a single cell solution and confirmed on inverted microscope. The cell suspensions were removed from the flask and centrifuge at 1000 rpm for 10 min to pellet the cells. The cell sustainability and total cell calculation was done using trypan blue stain using haemocytometer. The pellets were resuspended in a standard growth medium containing DMEM + 10 FBS to seeding density of 1.6 × 107 cells/mL. A 5-μL droplet of cell solution was seeded in the center of multi-well plate to generate micromass cultures for 2 h under humid condition. The warmed chondrogenesis media was added to the culture dish incubated at 37 °C incubator with 5% CO2, and the cultures were re-feed with fresh medium every 2–3 days. The chondrogenic pellets after 14 day days of cultivation were stained using Toluidine blue stain.
2.5.1. Toluidine blue staining
The depleted chondrocyte differentiating medium was aspirated from the culture plates after 14 days of differentiation, the plate was washed with DPBS and sufficient neutral buffer formalin (10%) was added to cover the cellular monolayer. The formalin was cautiously removed after 30 min, Toluidine blue Stain was added for 2–3 min, the stain was then aspirated and cells were rinsed with distilled water for three times. The cells were visualized under the microscope and images were acquired and analyzed. Synthesis of proteoglycans by chondrocytes is indicated by blue staining of the cells.
2.6. Animal study
2.6.1. Animal ethical statement
All animal protocols were done according to the certified procedures Institutional Animal Care and Use Committee (IACUC), University Putra Malaysia.
2.6.2. Test and treated group
From an accredited supplier we procured for this investigation, wistar rats male and female weighing 200–250 g. After getting permission from the Institutional Animal Care and Use Committee/Animal Utilization Protocol, University Putra Malaysia (R036/2015). The rats were housed in the animal facility of the Faculty of Medicine and Health Sciences, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia, in hygienic polypropylene cages under normal conditions of temperature (25 ± 2 °C) and 12 h light/12 h dark cycle and were nourished with a standard diet and water ad libitum. The rats were divided into three experimental groups; Group 1: control rats (4 males & 4 females); Group 2: rats treated with rifampicin only (4 males & 4 females); Group 3: rats treated with rifampicin plus MSCs (4 males & 4 females). The prepared therapeutic doses of rifampicin (9 mg/kg/day for 3-months) and MSCs infusions (twice/month for 3-months) were administered orally and intravenously respectively.
2.6.3. Rifampicin treatment
Therapeutic dosages of rifampicin (9 mg/kg/day) were given to the rats [4], via oral gavage, daily over 90 days. The rats in all the groups were observed thoroughly, looking for any behavior difference, feeding and drinking habits, as well as body weight and general morphological transformations.
2.6.4. Mesenchymal stem cells treatment
Mesenchymal stem cells (MSCs) were infused into the rats based on the created group, while under an anesthesia dosage comprising a cocktail of 50 mg/kg ketamine and 10 mg/kg xylazine. A 100 μl (2.5 × 105 cells) cell suspension in PBS was injected intravenously into the tail vein of each rat (twice/month for 3-months) [56].
2.6.5. Blood withdrawal
Under an anesthesia dosage containing a cocktail of 50 mg/kg ketamine and 10 mg/kg xylazine. Blood was withdrawn from the parent rat and F1 generation at the end of the rifampicin and bone marrow mesenchymal stem cells treatment through cardiac puncture and jugular vein into plain sample tubes. The blood was allowed to stand for 2 h, and afterward centrifuged at 2000g for 20 min to separate serum from the blood cells.
2.6.6. Organ harvesting
The animal was positioned down in a procedure station, and a longitudinal opening was made in the abdomen; the kidney was cut off and washed completely with a normal saline solution. Sections of the kidney from the parent and F1 generation were dipped in a 10% neutral buffered formalin solution for histology assessment.
2.6.7. Biochemical analysis
Kidney function enzyme and other bio-indicators like aspartate aminotransferase (AST), urea and creatinine famous to be essential indicators in kidney damage profile were assessed using completely an automated clinical chemistry analyzer (Hitachi 902, Japan).
2.6.8. Tissue processing and immunohistochemical staining
The kidney tissues were secure in 10% neutral buffered formalin to conserve the tissue and avert autolysis and putrefaction. The tissue was dried in diverse dilutions of alcohol and finally put in xylene, with each step of dehydration lasting for approximately 2–3 h. The xylene suspensions permeated the tissue and then substituted the alcohol. The tissues were set in paraffin, which substituted the xylene in the tissue gaps. The tissue was then put in a para-phenol block to become chill and generate a solid block with tissue embedded in it. The block was laid on the microtome, the cut sections were collected up and put in water, and a glass slide was placed underneath the tissues. The paraffin fragment on the slide was dried for a night so that it will adhere to the slide; the paraffin was later positioned in a dish. The sections were stain with hematoxylin and eosin, which give rise to blue and red color difference. The sections on the glass slide were placed into the hematoxylin stain, into differentiating solutions, and into the eosin stain and then dehydrated. The slides were mounted for viewing. A plastic mounting media was placed over the stain sections, which were covered by a cover slip. The tissue sections were observed under the microscope [19].
2.7. Statistical analysis
Results are expressed as the mean ± SE following an analysis via one-way ANOVA to assess significant differences between groups and with Turkey's HSD test to examine significant difference at P < 0.05.
3. Results
3.1. Animal grouping and physiological examination
Wistar rats of both sexes were used in this experiment, therapeutic doses of rifampicin (9 mg/kg/day) were administered to the rats via oral gavage [4], as well as bone marrow mesenchymal stem cells at a volume of 100-μl (2.5 × 105 cells) intravenously [56] daily for 90 days. The rats in different groups were all fine without any negative sign in behavioral changes, no alterations in feeding, drinking habits, and also no body weight and other general morphological changes. To determine the effects of prolong rifampicin treatment on the subsequent generation; the rats were bred to get the F1 generation. The rats were grouped as follows: group 1 male; group 2 female; group 3 male F1 generations and group 4 female F1 generations. At the end of the breeding, blood serum was obtain from all the rats according to the grouping, for analysis of kidney enzymes and bio-indicators like aspartate aminotransferase (AST), as well as urea and creatinine also known to be important markers in kidney damage were estimated using fully an automated clinical chemistry analyzer (Hitachi 902, Japan).
Physiological changes in control and treated groups were normal, as the rats were constantly observed closely, looking for any behavioral change, feeding and drinking habits, as well as body weight and general morphological changes.
3.2. Morphological features, immunophenotyping and trilineage differentiation MSCs
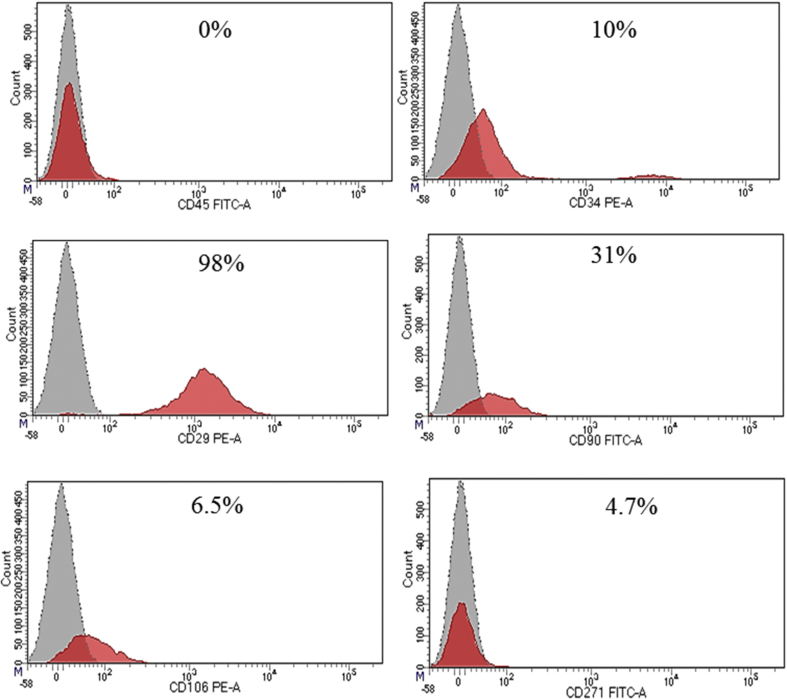
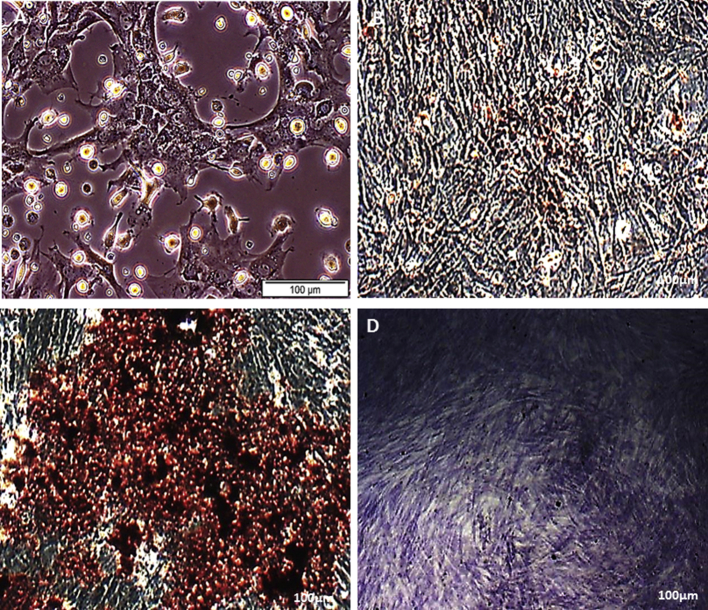
Fig. 1 presents the appearances structure; typically spindle displayed by bone marrow derived mesenchymal stem cells that were isolated from Wistar rats at day 7 before passage. The immunophenotyping categorization of the cells at 8 passage expressed the following surface markers as examined using FACSAria III with FACSDiva 6.12 software, as either positive for MSCs (CD90, CD29, CD106, CD54, CD105 and CD271) and lacked or with little expression of hematopoietic markers (CD45 and CD34) as negative for MSCs (Fig. 2) and the cells were further tested to ascertained their tri-lineage potentials by their differentiation into osteoblast, adipocytes and chondrocytes (Fig. 3).
Fig. 1.
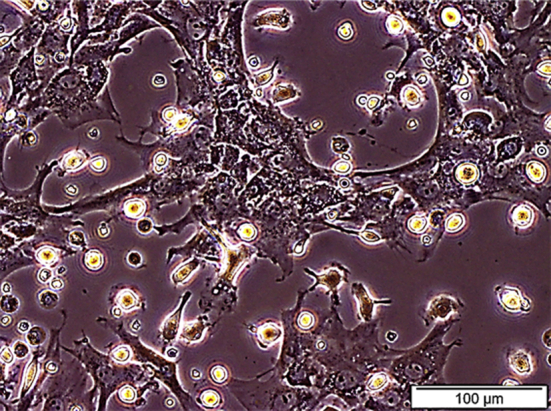
Morphological features of bone marrow derived mesenchymal stem cells isolated from Wistar rat, showing the spindle-shaped morphology of the cells at day 7 before passage.
Fig. 2.
Bone marrow-derived mesenchymal stem cells isolated from a Wistar rat at passage 8 expressed the following surface markers if positive for MSC (CD90, CD29, CD106, CD54, CD105 and CD271) and lack the expression of hematopoietic markers (CD45 and CD34) if negative for MSC, as analyzed using FACSAria III with FACSDiva 6.1.2 software.
Fig. 3.
Control bone marrow derived mesenchymal stem cells (A), Osteoblast detection in bone marrow derived mesenchymal stem cells using Alizarin Red Staining; after 21 days osteogenic induction. Exhibiting extracellular calcium deposits, osteogenic induced MSCs (B). Adipocyte detection in bone marrow derived mesenchymal stem cells using oil Red O Staining; after 21 days adipogenic induction. Exhibiting oil red O staining of extensive intracellular fat deposits, adipogenic induced MSCs (C). Chondrogenic detection using Tuludine blue staining in bone marrow derived mesenchymal stem cells; after 21 days chondrogenic induction. Exhibiting the staining of glycosaminoglycans/proteoglycans in cartilage matrix, chondrogenic induced MSCs (D).
3.3. Biochemical analysis
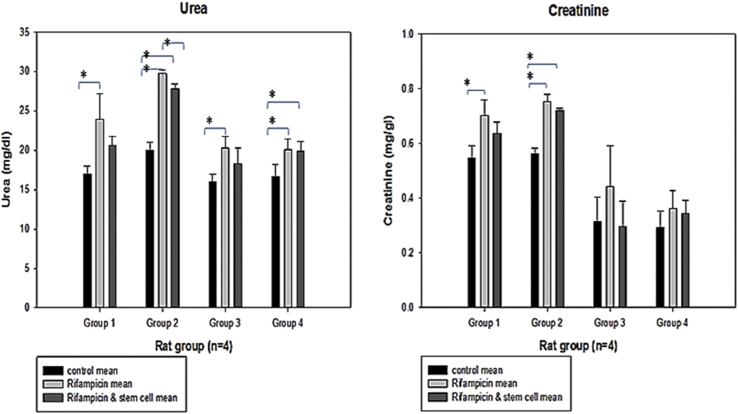
Fig. 4 shows the mean ± SE concentration of urea and creatinine in the serum of rats and their progeny treated with rifampicin, rifampicin plus bone marrow mesenchymal stem cells and the control group. For the analysis of urea concentration in the male rats, the biochemistry result for urea showed a significant difference between the control and rifampicin treated rats, with no significant differences between the rifampicin treated and also rifampicin plus stem cells treated rats group 1. However, in the female rats there was a significant difference between control and rifampicin treated rats, control and rifampicin plus stem cells treated and also between rifampicin treated rats and rifampicin plus stem cell treated rats group 2. Also in the male F1 generation rats there was a significant difference in the urea levels between control and rifampicin treated, with no any significant difference between control and rifampicin plus stem cells treated rats and also between rifampicin and rifampicin plus stem cells treated rats group 3. There was also a significant difference in the female F1 generation rats between control and rifampicin treated rats and also between control and rifampicin plus stem cells treated rats group 4 (Fig. 4).
Fig. 4.
Mean ± SE of the concentrations (mg/dL) of urea and creatinine in the serum of rats and their progeny treated with rifampicin, rifampicin and bone marrow mesenchymal stem cells and the control group, respectively. *Significant difference from the control at P < 0.05. Group 1, male; Group 2, female; Group 3, male F1 generation; Group 4, female F1 generation.
The creatinine concentration was analysed, and the biochemistry result male rats revealed a significant difference between control and rifampicin treated rats, with no any significant difference between rifampicin treated and rifampicin plus stem cells and also no significant difference between control and rifampicin plus stem cells treated rats in group 1. However, in the female rats there was a significant difference between control and rifampicin treated rats and also between control and rifampicin plus stem cell treated rats, with no significant difference between rifampicin treated and rifampicin plus stem cell treated rats in group 2. Remarkably, there were no significant differences in male F1 generation and female F1 generation rats in group 3 and 4 (Fig. 4).
3.4. Histological and quantitative histopathological examination
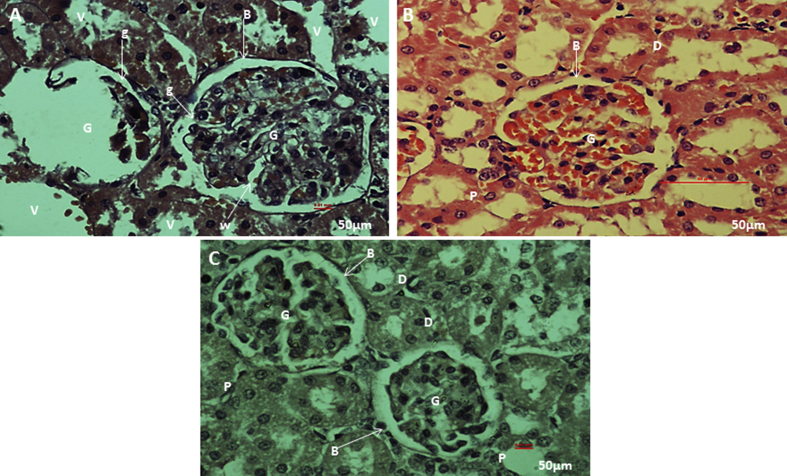
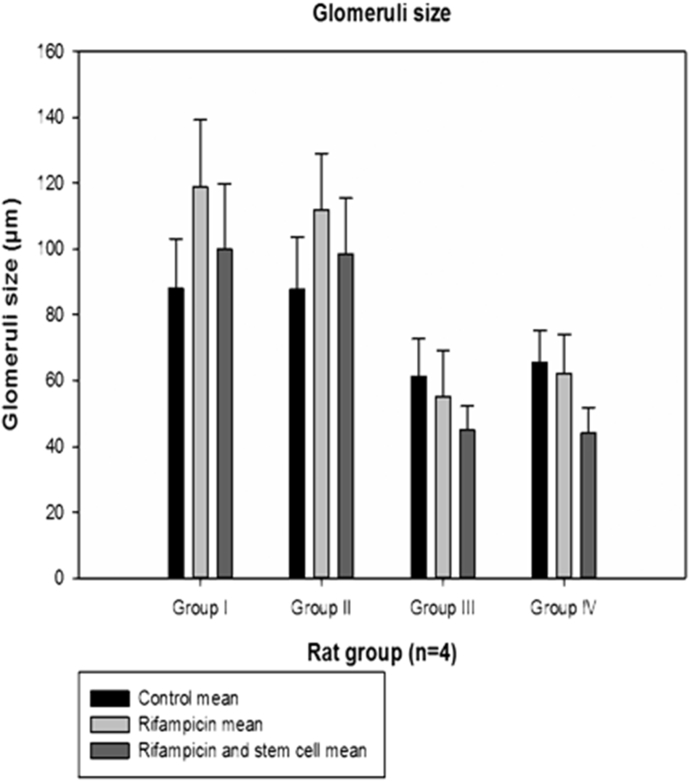
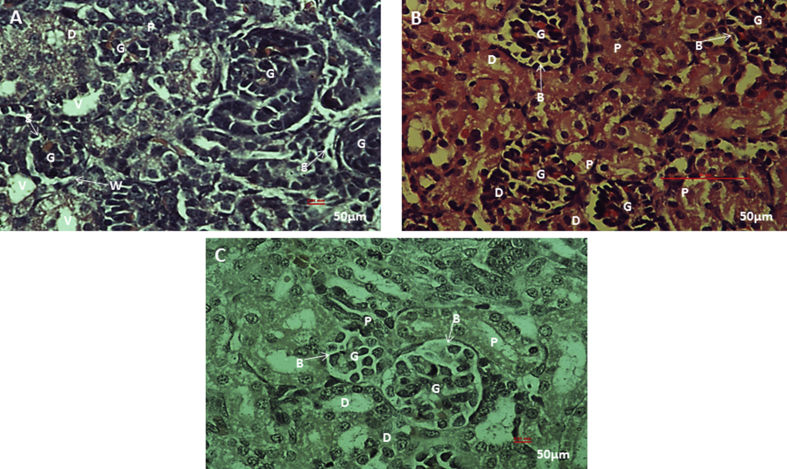
From the histological observations, rifampicin treated kidney section (Fig. 5A), showed some architectural damages like; cell necrosis in glomeruli (G), malpighian renal corpuscle with shrunken glomeruli (g) and widened bowman's space (W), cytoplasm lining of the tubular cells exhibit vacuolation (V), cellular debris dislodged in tubular lamina (C). While rifampicin plus stem cells treated kidney tissue (Fig. 5B), and section of kidney from the control (Fig. 5C), showed apparently normal histological architecture of renal corpuscle with well-formed glomerular tuft (G) surrounded by Bowman's space (B), renal tubules; proximal tubules (P) and distal convoluted tubules (D), showed apparently normal histological architecture. Quantitative histopathological examination revealed a decrease in size of glomeruli, due to shrinkage in kidney tissue of rifampicin treated rat. While in the control and rifampicin plus stem cells treated kidney tissue, the size of the glomeruli are apparently normal (Fig. 7).
Fig. 5.
Histological examination of kidney tissue from the parent rats. Photomicrograph section of rifampicin treated kidney showing cell necrosis in glomeruli (G), malpighian renal corpuscle with shrunken glomeruli (g) and widened bowman's space (W), cytoplasm of the lining tubular cells exhibit vacuolation (V), cellular debris dislodged in tubular lamina (C) (H&E stain) (A), photomicrograph section of rifampicin plus stem cell treated kidney showing apparently normal histological architecture of renal corpuscle with well-formed glomerular tuft (G) surrounded by Bowman's space (B), Renal tubules; proximal tubules (P) and distal convoluted tubules (D), show apparently normal histological architecture (H&E stain) (B). Photomicrograph section of kidney from the control showing normal architecture of malpighian renal corpuscle with well-formed glomerular tuft (G) surrounded by bowman's space (B). Renal tubules; proximal tubules (P) and distal convoluted tubules, (D) show apparently normal histological architecture (H&E stain) (C).
Fig. 7.
Mean glomeruli size of rats and their progenies after 4-months treatment with rifampicin plus rifampicin with bone marrow-derived mesenchymal stem cells. Mean ± SE of the glomeruli size (μm) of rats and their progenies treated with rifampicin, rifampicin plus bone-derived mesenchymal stem cells and the control group. Group 1 male; Group II female; Group III male F1 generation; Group IV female F1 generation.
From the histological observations, rifampicin treated kidney section (Fig. 6 A), showed some architectural damages like; cell necrosis in glomeruli (G), malpighian renal corpuscle with shrunken glomeruli (g) and widened bowman's space (W), cytoplasm lining of the tubular cells exhibit vacuolation (V), cellular debris dislodged in tubular lamina (C). While rifampicin plus stem cells treated kidney tissue (Fig. 6B), and section of kidney from the control (Fig. 6C), showed apparently normal histological architecture of renal corpuscle with well-formed glomerular tuft (G) surrounded by Bowman's space (B), renal tubules; proximal tubules (P) and distal convoluted tubules (D), showed apparently normal histological architecture. Quantitative histopathological examination revealed a decrease in size of glomeruli, due to shrinkage in kidney tissue of rifampicin treated rat. While in the control and rifampicin plus stem cells treated kidney tissue, the size of the glomeruli are apparently normal (Fig. 7). The histopathological changes that were seen in the kidney of adult rats due to rifampicin treatment of tuberculosis were also noticed in the kidney of F1 generation rats, but with some level of regenerative improvement in rats kidney treated with rifampicin plus stem cells. Thus, suggesting the therapeutic potentials of bone marrow derived mesenchymal stem cells.
Fig. 6.
Histological examination of kidney tissue from F1 generation rats. Photomicrograph section of kidney of progeny F1 from rifampicin treated rats showing cell necrosis in glomeruli (G), malpighian renal corpuscle with shrunken glomeruli (g) and widened bowman's space (W). Cytoplasm of the lining tubular cells exhibit vacuolation (V), cellular debris dislodged in tubular lamina (C) (H&E stain) (A). Photomicrograph section of kidney of progeny F1 from rifampicin plus stem cell treated rats showing apparently normal histological architecture of renal corpuscle with well-formed glomerular tuft (G) surrounded by Bowman's space (B). Renal tubules; proximal tubules and distal convoluted tubules, show apparently normal histological architecture (H&E stain) (B). Photomicrograph section of kidney of progeny from the control showing normal architecture of renal corpuscle with well-formed glomerular tuft (G) surrounded by Bowman's space (B). Renal tubules; proximal tubules (P) and distal convoluted tubules (D) (H&E stain) (C).
4. Discussion
Prolong rifampicin treatment due to tuberculosis have been reported to cause damage to kidney. The competency of BMMSCs to move and engraft to spot of damage area, regardless of tissue type make it a good source of therapeutic material. Therefore, administration of bone marrow derived mesenchymal stem cells with rifampicin can help in averting organ damage caused by the drug. Wistar rats of both sexes were used in this experiment, therapeutic doses of rifampicin (9 mg/kg/day) were administered to the rats via oral gavage [4], as well as bone marrow mesenchymal stem cells at a volume of 100-μl (2.5 × 105 cells twice per month) intravenously [56] daily for 90 days. The rats in different groups were all fine without any negative sign in behavioral changes, no alterations in feeding, drinking habits, and also no body weight and other general morphological changes. To determine the effects of prolong rifampicin treatment on the subsequent generation; the rats were bred to get the F1 generation, and the rifampicin and stem cells treatment was continue for another one month. The rats were grouped as follows: group 1 male; group 2 female; group 3 male F1 generations and group 4 female F1 generations. At the end of the breeding, blood serum was obtain from all the rats according to the grouping, for analysis of kidney enzymes and bio-indicators like aspartate aminotransferase (AST) and urea and creatinine that are known to be important markers in kidney damage, were estimated using fully an automated clinical chemistry analyzer (Hitachi 902, Japan). Furthermore, kidney tissues from the adults and F1 generation were processed for histology analysis. The kidney is regarded as the chief structure for the biological purification of wastes (particularly urea) from the blood and excretes these wastes, which encompass some vital bio-indicators, and water in the urine. Therefore, any alteration in these bio-indicators (urea, creatinine and AST, which are known to be satisfactory indicators of kidney injury) due to rifampicin treatment, compared with the reference value, is considered damage to the kidney. A sequence of investigations of the entire metabolic panel conducted following 2 months of treatment with isoniazid, rifampicin and ethambutol showed an AST of 26 IU/L and a creatinine of 2.45 mg/dL with an estimated glomerular filtration rate (eGFR) of 32 (using the Modification of Diet in Renal Disease-MDRD method) [2]. However, 14 days earlier, the level of creatinine was 1.03 mg/dL with an eGFR of 92, while the reference creatinine value was 0.65 mg/dL with an eGFR of 123. This shows that the administration of anti-tuberculosis drugs, such as rifampicin, significantly increases the level of biochemical indicators associated with kidney function and the eGFR. Furthermore, prolonged rifampicin treatment can also lead to mild anemia with a hemoglobin count of 10.3 g/dL and a hematocrit of 31.3% [2]; this can also lead to severe serum glucose values above the normal limits. Other damage due to rifampicin treatment include inflammation and necrosis in the kidney cells, acute kidney injury [14], acute interstitial nephritis [2], acute renal failure [13], [18], [57]. The considerable elevation in the concentration of urea from the serum of rats that received rifampicin treatment in our study is similar to what was reported earlier by Refs. [46], [60], [70]. Similar findings were reported by Refs. [4], [52] respectively, which showed a significant increase in the level creatinine and blood urea nitrogen due to prolonged rifampicin treatment. Furthermore, [66]; reported a significantly high level of creatinine and blood urea nitrogen following rifampicin induction compared with non-rifampicin-treated rats. Insignificant alterations in serum creatinine concentration due to rifampicin treatment in rats can be attributed to circumstances in which the concentration of serum creatinine can only rise significantly when virtually half of the nephrons in the kidney are completely injured or ruined [8]. The only way to prevent this damage due to rifampicin treatment is to stop the medication, and this can lead to drug resistance.
However, since mesenchymal stem cells can differentiate and regenerate damage tissues, they were administered along with rifampicin and were able to restore the elevated kidney bio-indicators to their normal range in both the parents and the offspring compared with the control group, as shown in Fig. 3, Fig. 4, Fig. 5 above. This may be due to the immune-modulatory properties of MSCs in the repair process of damage organs as reported [21], [24], [28], [34], [35], [39]. Furthermore, the mechanism of action of bone marrow mesenchymal stem cells can be attributed to their competency to move and engraft to the site of damage, regardless of tissue type, thus making BMMSCs a good source of therapeutic material. Growth factors such as chemokines released by damaged cells and/or a reaction to immune cells are mainly responsible for the movement of MSCs to the spot of injury [58]. Moreover, receptor tyrosine kinase growth factors such as platelet-derived growth factor (PDGF) or insulin-like growth factor 1 (IGF-1) and chemokines such as C—C chemokine receptor type 2 (CCR2), C—C chemokine receptor type 3 (CCR3), C—C chemokine receptor type 4 (CCR4) or C—C chemokine receptor type 5 (CCL5) also regulate MSCs mobility as determined by in vitro migration assays [69]. It was reported that stem cells derived from bone marrow or its progenitor can treat inflammation and organ injury-related diseases [5], [7], [22], [29], [33]. Nephritis can also be attributed to the unfavorable side effects of rifampicin [49]. There are also some rare instances of acute renal failure (ARF) as a result of rifampicin treatment [70]. Urea and creatinine elevation can be linked to diminished kidney activity as revealed in our histological analysis showing degeneration and necrosis in the glomeruli and renal tubules as a result of the toxicity of rifampicin to the kidney. This can lead to low efficiency of the kidney in filtering waste products such as urea and creatinine from the blood [9]. The kidney is the ultimate collective route for the removal of drugs along with their metabolites and is always prone to high levels of materials known to be potentially toxic. Drugs along with their metabolites are usually absorbed in a selective manner and distilled by the renal tubule before removal from the urine. This makes the intracellular concentration high, especially at the renal medulla, which is known to have a small vasculature compared with the renal cortex [3]. Our histological analysis revealed deformation and a decreased glomerular count. There is also hyaline droplet deposition with tubular dilation in some tubular cells, similar to what was reported by Ref. [68]. Diffuse mesangial proliferative glomerulonephritis was observed in biopsies of individuals consuming rifampicin accompanied by sub-acute infective endocarditis [11]; however, interstitial nephritis and/or acute necrosis of the tubule were also reported after rifampicin treatment [53]. The renal damage process is assumed to be due to the allergic response to rifampicin and its metabolites resulting in allergic interstitial nephritis. [27]; reported that samples from renal biopsy hardly reveal intense mononuclear cells infiltration, and sometimes, the actual image is a critical, dispersed or central necrotic tubule that exhibits moderate interstitial alterations. Tubular damage can occur when the immune complexes are accumulated in the blood vessels or interstitium, leading to glomerular endotheliosis and reducing the functionality of the kidney [48]. This may likely cause kidney failure due to prolonged rifampicin treatment of tuberculosis, as is clearly seen in our findings. [44]; reported that rat offspring from parents that receive antibiotic treatment reveal some symptoms of tubular vacuolation and necrosis, and these symptoms are more pronounced in the newborn rats, although some signs of tubular restoration are also seen at 10 and 20 days after treatment. Our result regarding the rifampicin plus stem cell-treated kidney showed an apparently normal histological architecture of renal corpuscle with a well-formed glomerular tuft surrounded by Bowman's space. Renal tubules, proximal tubules and distal convoluted tubules showed an apparently normal histological architecture both in the parents as well as the offspring. Furthermore, a quantitative histopathological image evaluation of kidney changes in rifampicin-treated rats, rifampicin plus stem cells-treated rats, and the control group was performed. Histopathological impairment was evaluated based on the quantifiable dimension of the glomeruli. There was a gradual increase in the dimension of the glomeruli and some form of histological alterations in the rifampicin-treated group, while the rifampicin plus stem cells-treated group showed almost normal architecture. The rats in the group treated with rifampicin presented with an increasing amount of glomeruli damage, whereas the rifampicin plus stem cells-treated group presented a normal dimension of glomeruli compared with the control. Transplanted MSCs can differentiate and move to the renal tubular epithelium during an early stage of damage, in a C57BL/6 mouse model of ischemia-reperfusion (I/R) kidney, and the specialized donor cells were able to replenish the empty area of the dead cells and promoted the preservation of structural intactness and the tissue restoration activity [42]. It was noticed that intravenous administration of BMMSCs after ARF histologically targeted the injured kidney and considerably boosted the restoration of kidney function due to their ability of transdifferentiation into kidney tubular or vascular endothelial cells [47], [37], and a single intrarenal administration of BMMSCs 7 days after ischemia-reperfusion significantly improved renal function and modified renal remodeling. In a renal ischemia rat model, the administration of MSCs decreased tubular dilation, which is known to be a typical characteristic of progressive kidney failure [1]. It was reported that one intravenous administration of MSCs caused beta-pancreatic islet restoration, thereby averting kidney impairment in streptozotocin-induced type 1 diabetes in C57BL/6 mice [23] and reduced hyperglycemia and glycosuria, which lasted for 60 days post injection. Moreover, the diabetic mice that received MSCs therapy revealed histologically normal glomeruli and decreased albuminuria. There was a reduction in blood glucose levels and mesangial thickening and macrophage infiltration diminishing due to MSCs administration, indicating the MSCs ability to amending kidney lesions in patients with diabetes mellitus as investigated by Lee and his colleagues in immunodeficient mice with type 2 diabetes elicited through multiple low dosages of streptozotocin [41]. There was also decreased kidney injury and kidney function was safeguarded in rats with modified 5/6 nephrectomy that receive one intravenous injection of MSCs a day after nephrectomy [16]. Rats that received a single dosage of bone marrow-derived MSC 11 weeks after kidney transplantation showed reduced interstitial fibrosis, tubular atrophy, T-cell and macrophage infiltration, and the expression of inflammatory cytokines [25]. The processes likely to be responsible for restorative or regenerative mechanisms induced by MSCs treatment in renal damage were analyzed by Refs. [61], [62]; and they reported that rat BMMSCs where transitorily engrafted in an injured kidney tissue, caused by ischaemic-reperfusion (IR) injury and were able to apply therapeutic impact on kidney functionality and tubular injury by releasing of anti-apoptotic, pro-mitogenic and vascular factors. Their result showed that, there was a decline in interleukin 1β, tumor necrosis factor α and interferon γ in the kidney of animals that received BMMSCs transfusion combined with upregulation of anti-inflammatory cytokines and growth factors like IL10, basic fibroblast growth factor (bFGF) transforming growth factor α and anti-apoptotic Bcl-2 [61]. Clinical complications like ischemic acute renal failure (ARF), described by severe regression in the glomerular filtration rate, is usually seen in hospitalized patients and predominantly in multiorgan failure patients. Intravenous administration of BMMSCs after ARF, was able to histologically locates the injured kidney and considerably boost the restoration of kidney function due to their ability of transdifferentiation into kidney tubular or vascular endothelial cells [47], [37].
Reduction in inflammatory and profibrotic cytokines levels in animals that received MSCs infusion was linked to an increase in anti-inflammatory cytokines IL-10, suggesting that the therapeutic impact of the cells may be primarily connected to the paracrine immunomodulatory potentials as well. The idea of kidney-protection induced by MSCs through a local paracrine action was proven by report that repetitive administration of BMMSCs conditioned medium to mice with cisplatin-induced ARF reduced kidney damage, apoptosis and prolong animal existence or survival [10]. The perception linking soluble factors to be responsible for MSCs kidney-protective impact was proved by in vitro document revealing that BMMSCs co-cultured, but actually split with cisplatin-injured proximal tubular cells, provoked mitogenic and antiapoptotic impacts on tubular cells [31]. The growth factors such as insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) that are really accountable for BMMSCs kidney restorative action in animals with ARF were verified by gene-silencing experimentations [31], [63]. Transplantation of murine BMMSCs in which IGF-1 was knocked down via siRNA restricted cell-protection on kidney function and tubular injury in mice with cisplatin-induced ARF [31]. Also [63]; reported that VEGF silencing decrease the efficacy of BMMSCs of rat on kidney revival and survival in IR injury model.
The facilitators responsible for the useful benefits of MSCs have been identify to be molecules like vascular endothelial growth factor, insulin-like growth factor-1, interleukin-10, basic fibroblast growth factor, and transforming growth factor-a (MSC secretome) [64], [32]. Even though MSCs have the capacity to localize to damaged spot and act in a paracrine and endocrine style, the renal effects can be determined by the mode of infusion. The main vital breakthrough in these findings shows the useful outcome was not associated with trans-differentiation of infusion cells into tubular epithelial cells alone; however it happened via differentiation-independent paths. Infused MSCs can be localized to the damage kidney to produce anti-inflammatory, anti-apoptotic, mitogenic, and pro-angiogenic impacts in the damage kidney giving rise to fewer damage and quicker restoration function of the kidney [64], [30]. Even though our study did not involve the lineage tracing of transplanted MSCs into the kidney cells, but previous researchers have confirmed the trans-differentiation of MSCs into kidney cells as reported by Refs. [47], [37]; after acute renal failure [42], [61], [62], after ischaemic-reperfusion (IR) kidney respectively.
5. Conclusion
It can be concluded that rifampicin-induced kidney toxicity remains a severe harmful consequence for patients receiving anti-tuberculosis treatment, but the co-administration of bone marrow derived mesenchymal stem cells with rifampicin restored renal biochemical indicators to their normal levels; moreover, the histological architecture of the kidney was restored.
Conflict of interest
The authors declare no conflicts of interest regarding the publication of this paper.
Author contributions
Lawal Danjuma conducted the experiment, collected the data, designed the figures and wrote the manuscript; Mok Pooi Ling, Rukman Awang Hamat, Akon Higuchi, Murugan A Munusamy, KB Swamy, K. Murugan, Nataraja Seenivasan, Mariappan Rajan, Arivudai Nambi, Kiruthiga Vijayaraman analyzed the data; Suresh Kumar collected, edited, and analyzed the data, designed this work, and co-wrote and edited the manuscript.
Acknowledgements
This work was supported by the Putra Grant, Universiti Putra Malaysia, Malaysia (9436400), and the IBS Putra grant, Universiti Putra Malaysia, Malaysia (9470200).
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Alfarano C., Roubeix C., Chaaya R., Ceccaldi C., Calise D., Mias C. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transpl. 2012 Sep 1;21(9):2009–2019. doi: 10.3727/096368912X640448. [DOI] [PubMed] [Google Scholar]
- 2.Alexandria B., Barbara S., Naveen P. Rifampicin-induced nephrotoxicity in a tuberculosis patient. J Clin Tubercul Other Mycobact Dis. 2015;1:13–15. doi: 10.1016/j.jctube.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson J.K. Drugs and renal insufficient. Medicine. 2003;1:103–109. [Google Scholar]
- 4.Awodele O., Akintonwa A., Osunkalu V.O., Coker H.A.B. Modulatory activity of antioxidants against the toxicity of rifampicin in vivo. Rev Inst Med Trop São Paulo. 2010;52(1):43–46. doi: 10.1590/s0036-46652010000100007. [DOI] [PubMed] [Google Scholar]
- 5.Baksh D., Song L., Tuan R.S. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8:301–316. doi: 10.1111/j.1582-4934.2004.tb00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balamurugan K., Vanithakumari G., Indra N. Effect of rifampicin on certain biochemical parameter in the liver of albino rats. Internet J Toxicol. 2009;6(1) [Google Scholar]
- 7.Barry F.P., Murphy J.M. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya H., Lun L., Gomez G.D. Biochemical effects to toxicity of CCL4 on rosy barbs (Puntius conchonius) Our Nat. 2005;3:20–25. [Google Scholar]
- 9.Bihorac A., Ozener C., Akoglue E., Kullu S. Tetracycline induced acute interstitial nephritis as a cause of acute renal failure. Nephron. 1999;81(1):72–75. doi: 10.1159/000045249. [DOI] [PubMed] [Google Scholar]
- 10.Bi B., Schmitt R., Israilova M., Nishio H., Cantley L.G. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007 Jul 26;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 11.Boulton-Jones J.M., Sissons J.G.P., Evans D.J., Peters D.K. Renal lesions of subacute infective endocarditis. Br Med J. 1974;2:11–14. doi: 10.1136/bmj.2.5909.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDD Treatment of tuberculosis. MMWR (Morb Mortal Wkly Rep) June 20, 2003;52(RR11):1–77. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5211a1.htm?mobile=nocontent2015 [Google Scholar]
- 13.Cengiz U., Inci G., Fahrettin K., Ramazan Y., Mustapha O. Acute renal failure due to rifampicin treatment. Nephron. 1994;67:367–368. doi: 10.1159/000187999. [DOI] [PubMed] [Google Scholar]
- 14.Chang C., Yen-Fu C., Vin-Cent W., Chin-Chung S., Chih-Hsin L., Jann-Yuan W. Acute kidney injury due to anti-tuberculosis drugs: a five-year experience in an aging population. BMC Infect Dis. 2014;14:23. doi: 10.1186/1471-2334-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiba S., Tsuchiya K., Sakashita H., Ito E., Inase N. Rifampicin-induced acute kidney injury during the initial treatment for pulmonary tuberculosis: a case report and literature review. Intern Med. 2013;52(21):2457–2460. doi: 10.2169/internalmedicine.52.0634. [DOI] [PubMed] [Google Scholar]
- 16.Choi S., Park M., Kim J., Hwang S., Park S., Lee Y. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:52–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 17.Costiniuk C.T., McCarthy A.E., Talreja H., Zimmerman D., Liu T.T., Owen E. Acute renal failure and disseminated intravascular coagulation associated with rifampin in tuberculosis treatment. Int J Tuberc Lung Dis. 2011;15(3):421. [PubMed] [Google Scholar]
- 18.Covic V., Goldsmith D.J., Segall L., Stoicescu C., Lungu S., Volovat C. Rifampicin-induced acute renal failure: a series of 60 patients. Nephrol Dial Transplant. 1998;13(4):924–929. doi: 10.1093/ndt/13.4.924. [DOI] [PubMed] [Google Scholar]
- 19.Culling C.F.K. 3rd ed. Trowbridge an Esher Publishers Redwood Burn Limited; 1974. Handbook of histopathological and histochemical techniques; pp. 712–730. [Google Scholar]
- 20.De Hemptinne I., Vermeiren C., Maloteaux J.M., Hermans E. Induction of glial glutamate transporters in adult mesenchymal stem cells. J Neurochem. 2004;91:155–166. doi: 10.1111/j.1471-4159.2004.02709.x. [DOI] [PubMed] [Google Scholar]
- 21.Di Nicola M., Carlo-Stella C., Magni M., Milanesi M., Longoni P.D., Matteucci P. Human bone marrow stromal cells suppress t-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M., Hofmann T.J., Horwitz E.M. Bone marrow mesenchymal cells: biological properties and clinical applications. J Biol Regul Homeost Agents. 2001;15:28–37. [PubMed] [Google Scholar]
- 23.Ezquer F.E., Ezquer M.E., Parrau D.B., Carpio D., Yanez A.J., Conget P.A. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–640. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Frank M.H., Sayegh M.H. Immunomodulatory functions of mesenchymal stem cells. Lancet. 2004;363:1411–1412. doi: 10.1016/S0140-6736(04)16134-5. [DOI] [PubMed] [Google Scholar]
- 25.Franquesa M., Herrero E., Torras J., Ripoll E., Flaquer M., Goma M. Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev. 2012;21:3125–3135. doi: 10.1089/scd.2012.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomez J.E., McKinney J.D. M. tuberculosis persistence, latency, and drug tolerance. Tuberculosis. 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Grunfeld J.P., Kleinknecht D., Droz D. Diseases of the kidney. Little Brown; Boston: 1993. Acute interstitial nephritis; pp. 1331–1353. [Google Scholar]
- 28.Horwitz E.M., Gordon P.L., Koo W.K., Marx J.C., Neel M.D., McNall R.Y. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci. 2002 Jun 25;99(13):8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horwitz E.M. Stem cell plasticity: the growing potential of cellular therapy. Arch Med Res. 2003;34:600–606. doi: 10.1016/j.arcmed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys B.D., Bonventre J.V. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 31.Imberti B., Morigi M., Tomasoni S., Rota C., Corna D., Longaretti L. Insulin-like growth factor-1 sustains stem cell–mediated renal repair. J Am Soc Nephrol. 2007 Nov 1;18(11):2921–2928. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- 32.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10:709–716. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Koc O.N., Lazarus H.M. Mesenchymal stem cells: heading into the clinic. Bone Marrow Transplant. 2001;27:235–239. doi: 10.1038/sj.bmt.1702791. [DOI] [PubMed] [Google Scholar]
- 34.Koc O.N., Day J., Nieder M., Gerson S.L., Lazarus H.M., Krivit W. Allogeneic mesenchymal stem cell infusion for treatment of metachromatic leukodystrophy (MLD) and hurler syndrome (MPS-IH) Bone Marrow Transplant. 2002;30:215–222. doi: 10.1038/sj.bmt.1703650. [DOI] [PubMed] [Google Scholar]
- 35.Krampera M., Cosmi L., Angeli R., Pasini A., Liotta F., Andreini A. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 37.Lange C., Tögel F., Ittrich H., Clayton F., Nolte-Ernsting C., Zander A.R. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005 Oct 1;68(4):1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 38.Lawal D., Mok P.L., Rukman A.H., Akon H., Abdullah A.A., Marlina Genomic plasticity between human and mycobacterial DNA: a review. Tuberculosis. 2017;107(2017):38–47. doi: 10.1016/j.tube.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485–489. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 40.Lee H.A. Drug induced diseases, drug related disease and the kidney. Br Med J. 1979;2:104–107. doi: 10.1136/bmj.2.6182.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee R.H., Seo M.J., Reger R.L., Spees J.L., Pulin A.A., Olson S.D. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103:17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K., Han Q., Yan X., Liao L., Zhao R.C. Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem Cells Dev. 2010;19:1267–1275. doi: 10.1089/scd.2009.0196. [DOI] [PubMed] [Google Scholar]
- 43.Luzzati R., Giacomazzi D., Franchi F., Barcobello M., Vento S. Life-threatening, multiple hypersensitivity reactions induced by rifampicin in one patient with pulmonary tuberculosis. South Med J. 2007;100(8):854–856. doi: 10.1097/SMJ.0b013e3180f60a57. [DOI] [PubMed] [Google Scholar]
- 44.Machado A.L.D., Brandão A.A.H., da Silva C.M.O.M., da Rocha R.F. Influence of tetracycline in the hepatic and renal development of rat's offspring. Braz Arch Biol Technol Int J. 2003;46(1):47–51. [Google Scholar]
- 45.Manika K., Tasiopoulou K., Vlogiaris L., Lada M., Papaemmanouil S., Zarogoulidis K. Rifampicin-associated acute renal failure and hemolysis: a rather uncommon but severe complication. Ren Fail. 2013;35(8):1179–1181. doi: 10.3109/0886022X.2013.815567. [DOI] [PubMed] [Google Scholar]
- 46.Miller G., McGarity G.J. Tetracycline-induced renal failure after dental treatment. JADA. 2009;140(1):56–60. doi: 10.14219/jada.archive.2009.0018. [DOI] [PubMed] [Google Scholar]
- 47.Morigi M., Imberti B., Zoja C., Corna D., Tomasoni S., Abbate M. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004 Jul 1;15(7):1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 48.Muthukumar T., Jayakumar M., Fernando E.M., Muthusethupthi M.A. Acute renal failure due to rifampicin. A study of 25 patients. Am J Kidney Dis. 2002;40:690–696. doi: 10.1053/ajkd.2002.35675. [DOI] [PubMed] [Google Scholar]
- 49.Plumb D.C. 3rd ed. Iowa State University Press/Ames; Minnesota: 1999. Veterinary drug handbook; pp. 654–656. [Google Scholar]
- 50.Porter R.M., Huckle W.R., Goldstein A.S. Effect of dexamethasone withdrawal on osteoblastic differentiation of bone marrow stromal cells. J Cell Biochem. 2003;90:13–22. doi: 10.1002/jcb.10592. [DOI] [PubMed] [Google Scholar]
- 51.Rana S.V., Pal R., Vaiphei K., Ola R.P., Singh K. Hepatoprotection by carotenoids in isoniazid- rifampicin induced hepatic injury in rats. Biochem Cell Biol NBC Res Press. 2010;88(5):819–834. doi: 10.1139/o10-023. [DOI] [PubMed] [Google Scholar]
- 52.Rekha V.V., Santha T., Jawahar M.S. Rifampicin induced renal toxicity during retreatment of patients with pulmonary tuberculosis. J Assoc Physician India. 2005;53:811–813. [PubMed] [Google Scholar]
- 53.Salih S.B., Kharal M., Qahtani M., Dahneem L., Nohair S. Acute interstitial nephritis induced by intermittent use of rifampicin in patient with brucellosis. Saudi J Kidney Dis Transpl. 2008;19(3):450–452. [PubMed] [Google Scholar]
- 54.Santhosh S., Sini T.K., Anandan R., Mathew P.T. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur J Pharmacol. 2007;572:69–73. doi: 10.1016/j.ejphar.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 55.Shabana M.B., Hania M., Soheir E.M.K., Elemam Marwa G. Influence of rifampicin and tetracycline administration on some biochemical and histological parameters in albino rats. J Basic Appl Zool. 2012;65(5):299–308. [Google Scholar]
- 56.Shirley H.J.M., Haitsman Jack J., Claudia C.D.S., Yupu D., Patrick F.H.L., Arthur S.S. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. [DOI] [PubMed] [Google Scholar]
- 57.Singh N.P., Ganguli A., Prakash A. Drug-induced kidney diseases. JAPI. 2003;51:975–976. [PubMed] [Google Scholar]
- 58.Spaeth E., Klopp A., Dembinski J., Andreeff M., Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15:730–738. doi: 10.1038/gt.2008.39. [DOI] [PubMed] [Google Scholar]
- 59.Taber S.S., Pasko D.D. The epidemiology of drug-induced disorders: the kidney. Expert Opin Drug Saf. 2008;7(6):679–690. doi: 10.1517/14740330802410462. [DOI] [PubMed] [Google Scholar]
- 60.Tasduq S.A., Kaiser P., Sharma S.C., Johri R.K. Potentiation of isoniazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: a toxicity profile study. Hepatol Res. 2007;37:845–853. doi: 10.1111/j.1872-034X.2007.00129.x. [DOI] [PubMed] [Google Scholar]
- 61.Togel F., Hu Z., Weiss K., Isaac J., Lange C., Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005 Jul;289(1):F31–42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 62.Tögel F., Weiss K., Yang Y., Hu Z., Zhang P., Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007 May;292(5):F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- 63.Tögel F., Cohen A., Zhang P., Yang Y., Hu Z., Westenfelder C. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009 Apr 1;18(3):475–486. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Togel F.E., Westenfelder C. Kidney protection and regeneration following acute injury: progress through stem cell therapy. Am J Kidney Dis. 2012;60:1012–1022. doi: 10.1053/j.ajkd.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 65.Upadhyay G., Kumar A., Singh M.P. Effect of Silymarin on pyrogallol-and rifampicin-induced hepatotoxicity in mouse. Eur J Pharmacol. 2007;565:190–201. doi: 10.1016/j.ejphar.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Viswanatha Swamy A.H.M., Kulkarni Rucha V., Koti B.C., Gadad P.C., Thippeswamy A.H.M., Aparna G. Hepatoprotective effect of Cissus quadrangularis stem extract against rifampicin-induced hepatotoxicity in rats. Indian J Pharm Sci. 2012;74(2):183–187. doi: 10.4103/0250-474X.103859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X.B., Zhu X.C., Huang X.Y., Ye W.J., Wang L.X. Fanconi syndrome due to prolonged use of low-dose adefovir. J Res Med Sci Off J Isfahan Univ Med Sci. 2015;20(4):416–419. [PMC free article] [PubMed] [Google Scholar]
- 68.Warner S.D. Nephrotic syndrome in rhesus monkeys. Abstracts of cases presented at the 25th Annual meeting American college of veterinary pathologists. Vet Pathol. 1975;12:61–73. [PubMed] [Google Scholar]
- 69.Yagi H., Soto-Gutierrez A., Parekkadan B., Kitagawa Y., Tompkins R.G., Kobayashi N. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yanardag H., Caner M., Gunes Y., Uygun S. Acute hemolysis and oligoanuric acute renal failure caused by interrupted. Internet J Nephrol. 2005;2(1) [Google Scholar]
- 71.Young D.B., Gideon H.P., Wilkinson R.J. Eliminating latent tuberculosis. Trends Microbiol. 2009;17:183–188. doi: 10.1016/j.tim.2009.02.005. [DOI] [PubMed] [Google Scholar]